Abstract
Site-specific recombination by phages λ and P22 is carried out by multiprotein-DNA complexes. Integration host factor (IHF) facilitates λ site-specific recombination by inducing DNA bends necessary to form an active recombinogenic complex. Mutants lacking IHF are over 1,000-fold less proficient in supporting λ site-specific recombination than wild-type cells. Although the attP region of P22 contains strong IHF binding sites, in vivo measurements of integration and excision frequencies showed that infecting P22 phages can perform site-specific recombination to its maximum efficiency in the absence of IHF. In addition, a plasmid integration assay showed that integrative recombination occurs equally well in wild-type and ihfA mutant cells. P22 integrative recombination is also efficient in Escherichia coli in the absence of functional IHF. These results suggest that nucleoprotein structures proficient for recombination can form in the absence of IHF or that another factor(s) can substitute for IHF in the formation of complexes.
Bacteriophage P22 is a lambdoid phage which infects Salmonella typhimurium. P22 can integrate into and excise out of its host chromosome via site-specific recombination. Both integration and excision reactions require the phage-encoded int gene (29). Mutations of P22 affecting excision (xis mutants) are also known (32). Both the int and xis genes of P22 have been sequenced (15).
Comparison of the deduced amino acid sequence of P22 Int with other site-specific recombinases reveals that it is a member of the λ integrase family (1, 5, 22). The Int proteins of λ and P22 are composed of two domains. The catalytic domain binds to the core region of the phage recombination site, attP, where the actual recombination reactions occur. The smaller amino-terminal domain binds to arm-type sequences which are located on either site of the core within the attP (20, 30). The active components of λ integrative and excisive recombination are nucleosome-like structures, called intasomes, in which DNA is folded around several molecules of Int and integration host factor (IHF) (2, 8, 23, 26, 27). It has been demonstrated that one monomer of λ integrase can simultaneously occupy both a core-type binding site and an arm-type binding site (11, 16). Formation of these bridges is facilitated by IHF, which binds to specific sequences and imparts a substantial bend to the DNA (3, 25, 27, 31).
The attP regions of P22 and λ are also similar in that both contain arm regions, known as the P and P′ arms, which contain Int arm-type binding sites and IHF binding sites (15, 30). However, the arrangement, spacing, and orientation of the Int and IHF binding sites are distinct (30). The attP region of λ contains two Int arm-type binding sites on the P arm and three on the P′ arm. The P arm contains two IHF binding sites, and the P′ arm contains a single site. The attP region of P22 contains three Int arm-type binding sites on the P arm and two sites on the P′ arm. In addition, IHF binding sites, called H and H′, are located on each arm of the P22 attP. Leong et al. (14) showed that the Escherichia coli IHF can recognize and bind to these P22 IHF binding sites in vitro. It was also shown that the maximum amount of P22 integrative recombination occurred in the presence of E. coli IHF in vitro, whereas in its absence, recombination was detectable but depressed (30). However, the requirement for IHF or other possible accessory proteins during P22 site-specific recombination in vivo has not been tested. In this study, we assessed the role of IHF in P22 integration and excision in vivo.
MATERIALS AND METHODS
Bacterial strains, media, chemicals, and enzymes.
The bacterial strains used in this study are listed in Table 1. S. typhimurium JG1148 was constructed by transduction of the ihfA::tet insertion from NH560 into MS1868 by selection for tetracycline resistance. The Tetr cassette was inserted at the Lys-15 codon of the S. typhimurium ihfA gene (10a). E. coli JG1242 was constructed by transduction of the Tn10-ihfAΔ82 allele from KL1299 (7) and selection for tetracycline resistance.
TABLE 1.
Characteristics of the bacterial strains used in this study
| Strain | Genotype | Source or reference |
|---|---|---|
| S. typhimurium | ||
| MS1868 | leuA414 hsdSB endE40 | 10 |
| NH560 | ihfA::tet | N. P. Higgins |
| JG1148 | MS1868 ihfA::tet | This work |
| JG1151 | MS1868 ihfA::tet ihfB::cat | 11 |
| E. coli | ||
| MC4100 | araD139 Δ(argF-lac)U169 rpsL150 relA1 flbB301 deoC1 ptsF25 rbsR | M. Casadaban |
| KL1299 | galK strA Tn10-ihfAΔ82 | H. Nash |
| JG1242 | MC4100 Tn10-ihfAΔ82 | This work |
The media and buffers used have been described previously (12). Luria-Bertani (LB) medium and Tris-EDTA (TE) buffer were made as described by Maniatis et al. (18). All antibiotics were purchased from Sigma and used at the following concentrations: ampicillin, 100 μg/ml; kanamycin, 50 μg/ml; and chloramphenicol, 20 μg/ml. Arabinose was obtained from Sigma and added to a final concentration of 1%. T4 DNA ligase and all restriction endonucleases were obtained from Gibco BRL. Pfu DNA polymerase was obtained from Stratagene.
Plasmid constructions.
Plasmids containing attP sites of phages λ and P22 were derivatives of pJK4 (18a), which contains oriR6K and a gene conferring resistance to kanamycin (Kanr). The λ attP site was cloned as a 267-bp PCR fragment (λ positions 27569 to 27836) (4) into the unique NlaIV site of pJK4 to create plasmid pRA102. The P22 attP site was inserted into pJK4 by cloning a 1,034-bp PCR fragment (positions +725 to −291) (14) into the NlaIV site of pJK4. In addition, a copy of lacPUV5 O+Z+Y+ from λplac5 UV5 (24) was also inserted into the plasmid. The fragment carrying the lac genes was obtained by digestion of phage DNA with KpnI and SphI (λ positions 18568 and 23946) (4). It was purified and cloned into the unique AvaII site of pJK4 to form plasmid pRA113. The attP-containing fragments of both plasmids contain all of the sequences necessary for λ or P22 integrative recombination in vitro (28, 30).
The P22 int gene was cloned by PCR amplification of the gene from bacteriophage P22 xis 2B (21) by using oligodeoxyribonucleotides P22 Int-Top and P22 Int-Bot (Table 2). The amplified DNA contains P22 sequences from position 62 to position 1216 (15). The oligodeoxyribonucleotides introduced KpnI and PstI sites adjacent to the DNA encoding the translation initiation and termination codons of Int, respectively. PCR DNA was digested with KpnI and PstI and ligated into pBAD33 DNA cleaved with the same enzymes to form the plasmid pIntP22. The DNA upstream of the DNA encoding the translation initiation codon contains the natural ribosome binding site for P22 int. The lambda int gene was cloned into pBAD33 as follows (33). Oligodeoxyribonucleotides (IntXis1 and IntXis2 [Table 2]) complementary to sequences 5′ of the xis gene and 3′ of the int gene were used for PCR amplification of the int and xis genes of λ vir. The resultant fragment contained an SphI site encoded by the IntXis2 primer downstream of the C terminus of int. The fragment was digested with XmnI (position 29015 in xis) (4) and SphI, and then the fragment was cloned into pBAD33 DNA digested with SmaI and SphI. The construct contains part of xis and the natural translation start signal upstream of int. The int genes of both pIntP22 and pBMS10 were sequenced to ensure that no PCR-induced errors occurred.
TABLE 2.
Sequences of synthetic oligonucleotides for amplification of P22 integrase attachment sites and cloning of int genes
| Primer | Sequencea | Positionb |
|---|---|---|
| B+ | dTCCGAATTCATGAGGTTGTACATAAGTGA | +57 to +49 ataA |
| B− | dTCCGAATTCGTGCGTAATAAATGAC | −175 to −156 ataA |
| P+ | dCAGAATTCATATGGAACGCGAATGGAAGATGC | +725 to +707 attP |
| P− | dTCCGAATTCGGAAAGGTCTGAAG | −291 to −274 attP |
| P22 Int-Top | dCGGGGTACCGAGGAACTGAAATGTCACT | +1216 to +1198 P22 int |
| P22 Int-Bot | dAACTGCAGTACTTACGTATTATTCGTGCC | +62 to +42 P22 int |
| IntXis1 | dCGGCCTTAAGAACGCACATTAACGCCTCTGAAACG | 29080 to 29107 λ int |
| IntXis2 | dCGACGCATGCTCATTATTTGATTTCAATTTTGTCC | 27809 to 27833 λ int |
Integration and excision assays.
To measure integration, MS1868 or JG1151 cells were grown in LB medium at 37°C to mid-exponential phase, P22 phage were added to a multiplicity of infection of 20 to 25, and the cell and phage mixtures were spread on EBU plates (17) which were previously seeded with 109 PFU of P22 H5 (c2) (17). Nonlysogens are killed by P22 H5, and only true P22 lysogens form light green colonies.
Spontaneous excision was measured by growing lysogenic bacteria overnight in LB medium followed by treatment with chloroform and centrifugation. Supernatants were diluted and spotted on a lawn of MS1868 to measure plaque formation. Excision of mid-exponential-phase cells cultivated in LB medium was induced by adding mitomycin to a final concentration of 2 μg/ml. Cells were incubated at 37°C with aeration. After lysis, cell debris was centrifuged, and supernatants were diluted and spotted against a lawn of MS1868 to measure plaque formation.
Plasmid integration assays.
We developed a second integration assay based on integration of a suicide plasmid containing the P22 attP site. Integration of the attP-containing plasmid pRA113 was used to measure the relative frequencies of integrative recombination in various host backgrounds (described above). The plasmid DNA was mixed with an equal amount of pCKR101 (12) DNA, which carries an ampicillin resistance gene and a colE1 origin of replication. Cells containing pIntP22 or pBAD33 were electroporated with 1 μl of the plasmid mixture and grown for 1 h at 37°C in LB medium supplemented with 1% arabinose. Dilutions were plated on LB agar plates supplemented with 1% arabinose, chloramphenicol, and either kanamycin or ampicillin. Because the strains lack Pi protein, which is required for replication of pRA113, kanamycin-resistant colonies can only arise by integration of the plasmid into the host chromosome. The relative frequencies of integration are expressed as the ratio of chloramphenicol- and kanamycin-resistant colonies (which contain pIntP22 and have integrated pRA113) to chloramphenicol- and ampicillin-resistant colonies (which contain pIntP22 and pCKR101). Assays for integration performed with λ Int were done as described for the P22 integration assay, except that cells contained pBMS10, which expresses λ Int under PBAD control, and pRA102 carrying λ attP was used as the integration indicator plasmid.
RESULTS AND DISCUSSION
Isolation of a P22 derivative which does not require IHF for lytic growth.
The ihfA and ihfB genes encode the α and β subunits, respectively, of IHF. Phage P22 forms smaller plaques on hosts carrying either ihfA or ihfB mutations than on a wild-type host (12, 13). To measure the extent of the IHF effect on lytic growth of P22, we compared single burst sizes of P22 on a wild-type strain and an IHF-deficient strain. The single burst size represents the number of progeny phage particles which are produced from a single host cell infected by a single phage. Infection of MS1868 (LT2 leuA414 hsdSB endE40) with P22 resulted in a single burst size of 196 PFU per cell, whereas infection of an IHF-deficient host, JG1151 (MS1868 ihfA::tet ihfB::cam) (12, 13), decreased the burst size to 6 PFU per cell. This indicates that P22 does not grow lytically as well in an IHF-deficient strain as in a wild-type strain, which is consistent with an observation by Henthorn and Friedman (10). To avoid complications due to IHF effects on the lytic growth of P22, a derivative of P22 able to grow lytically in an IHF-deficient strain was isolated as a mutant allele which formed normal plaques on JG1151 (Table 1). The mutant, designated P22 goh5, which plaques with equal efficiency on both strains, was used in integration and excision assays in vivo.
P22 can integrate into the ataA site efficiently in the absence of IHF.
To determine if IHF plays an important architectural role during P22 integration in vivo, we measured the lysogenization frequencies of wild-type P22 and P22 goh5 in MS1868 and in JG1151. If IHF binding to the H and/or H′ sites on the P22 attP region is required, the lysogenization frequency of P22 in the IHF-deficient host JG1151 would be lower than that of the wild-type strain, MS1868. As shown in Table 3, the percent lysogeny of JG1151 upon infection of both P22 wild-type phage and P22 goh5 was the same as that of MS1868. This indicates that IHF in S. typhimurium is not an absolute requirement for the P22 integration process in vivo.
TABLE 3.
Frequencies of lysogeny by P22 and P22 goh5
| Host | Phage | % Lysogenya |
|---|---|---|
| MS1868 (IHF+) | P22 wild type | 13.6 ± 6.3 |
| P22 goh5 | 25.2 ± 3.3 | |
| JG1151 (IHF−) | P22 wild type | 19.2 ± 21.0 |
| P22 goh5 | 13.7 ± 9.5 |
Expressed as the number of lysogens divided by the number of viable cells upon phage infection multiplied by 100. Values represent the average of three independent assays.
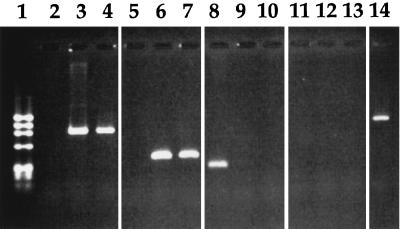
We then tested whether the P22 prophages were inserted into the specific bacterial attachment site, ataA, in IHF-deficient JG1151 (P22) lysogens by using PCR. Oligonucleotides B+ and B− were designed to amplify the ataA-containing region of the S. typhimurium genome, and oligonucleotides P+ and P− were designed to amplify the attP region of the phage P22 DNA (Table 2). DNA fragments containing attL or attR can be amplified from a P22 lysogen when appropriate primer pairs were used for PCRs: P+ and B− for the attL fragment and P− and B+ for the attR fragment. DNA fragments containing the attP region of P22 can be amplified from P22 phage DNA or from chromosomal DNA of multiple P22 lysogens. Chromosomal DNA templates which were purified from MS1868 nonlysogens, MS1868(P22) lysogens, or JG115 (P22) lysogens were amplified by PCR. Both MS1868(P22) and JG1151(P22) generated fragments containing the attL and attR regions (Fig. 1, lanes 3 and 4 and 6 and 7). No fragments equivalent to the ataA-containing fragments were amplified when templates were purified from P22 lysogens (Fig. 1, lanes 9 and 10). The MS1868 nonlysogen only produced the ataA-containing fragment (Fig. 1, lanes 2, 5, and 8). This result shows that the P22 phage integrated into the same ataA site in the IHF-deficient strain as in the wild-type S. typhimurium strain. None of the three strains produced the attP-containing fragments (Fig. 1, lanes 11 to 13), which indicated those lysogens contained a single prophage.
FIG. 1.
PCR analysis to detect P22 prophage integrated into the ataA site of S. typhimurium. PCR products were analyzed by electrophoresis on a 1.5% agarose gel. The nucleotide sequences of primers are shown in Table 2. Primer sets P+ and B−, P− and B+, B+ and B, and P+ and P− were used to amplify the 918-bp attL-containing fragments, 362-bp attR-containing fragments, 246-bp ataA-containing fragments, and 1,034-bp attP-containing fragments, respectively (Table 2). DNA templates were purified from MS1868 (lanes 2, 5, 8, and 11), MS1868(P22) lysogen (lanes 3, 6, 9, and 12), JG1151(P22) lysogen (lanes 4, 7, 10, and 13), and P22 (lane 14). Lane 1 contains molecular size markers, which are φX174 DNA digested with restriction enzyme HaeIII. Samples were amplified with primers P+ and B− (lanes 2 to 4), P− and B+ (lanes 5 to 7), B+ and B− (lanes 8 to 10), and P+ and P− (lanes 11 to 14).
We used a second assay based on integration of a plasmid containing P22 attP into the host ataA site. The strains carry pIntP22, a pBAD33 (9) derivative containing a copy of P22 int under PBAD control, the pACYC184 origin of replication, and a gene encoding resistance to chloramphenicol. Integration of the plasmid pRA113 was used to measure the relative frequencies of integrative recombination in various host backgrounds. It contains oriR6K, lacPuv5 O+ Z+ Y+, a copy of P22 attP, and a cassette conferring resistance to kanamycin. Because the strains lack Pi protein, which is required for replication of pRA113, kanamycin-resistant colonies can only arise by integration of the plasmid into the host chromosome. The relative frequencies of integration are expressed as the ratio of chloramphenicol- and kanamycin-resistant colonies (which contain pIntP22 and have integrated pRA113) to chloramphenicol- and ampicillin-resistant colonies (which contain pIntP22 and pCKR101).
The results in Table 4 show that the integration frequency of pRA113 is the same in both the wild type and a strain carrying an ihfA::tet allele (JG1148 ihfA::tet). We also obtained the same result with strain NH560, which contains the original ihfA::tet allele (data not shown). These results are consistent with the results from phage infections and further support the conclusion that integration requires Int but not IHF. We did not detect integration in a strain that contains the pBAD33 vector, indicating that integration is site specific.
TABLE 4.
Plasmid integration assaysa
| Strain | Plasmid carrying attPb | Presence of IHF | Int typec | Plasmid integration frequency (Kan/Amp)d |
|---|---|---|---|---|
| S. typhimurium | ||||
| MS1868 | P22 | + | <2 × 10−7 | |
| MS1868 | + | P22 | 7.4 × 10−2 | |
| JG1148 | − | <4 × 10−7 | ||
| JG1148 | − | P22 | 4.0 × 10−2 | |
| MS1868 | λ | + | <4 × 10−6 | |
| MS1868 | + | λ | 2.4 × 10−1 | |
| JG1148 | − | <2 × 10−5 | ||
| JG1148 | − | λ | 4.4 × 10−4 | |
| E. coli | ||||
| MC4100 | P22 | + | <1 × 10−5 | |
| MC4100 | + | P22 | 4.2 × 10−2 | |
| JG1242 | − | <1 × 10−6 | ||
| JG1242 | − | P22 | 1.2 × 10−2 | |
| MC4100 | λ | + | <3 × 10−6 | |
| MC4100 | + | λ | 3.1 × 10−1 | |
| JG1242 | − | <2 × 10−6 | ||
| JG1242 | − | λ | 5.5 × 10−5 |
Assays were performed as described in Materials and Methods.
The P22 attP site is carried on plasmid pRA113. The λ attP site is carried on plasmid pRA102.
P22 Int is expressed from pIntP22, which has a copy of P22 int cloned downstream of PBAD in pBAD33. λ Int is expressed from pBMS10, which has a copy of λ int cloned downstream of PBAD in pBAD33.
Values are expressed as the ratio of Camr Kanr colonies (which contain pBAD33, pIntP22, or pBMS10 and an integrated copy of pRA113 or pRA 102) to Camr Ampr colonies (which contain pBAD33, pIntP22, or pBMS10 and pCKR101). The results are averages of three experiments.
We also tested P22 attP integration in isogenic E. coli strains containing wild-type and mutant ihfA genes. Integration occurred at the same frequency in both types of cells (Table 4). In contrast, integration of a plasmid containing λ attP into the attB site of E. coli and the presumed attB site in S. typhimurium showed a marked dependence on IHF. Integration was decreased by approximately 5,000- and 500-fold in strains carrying mutant ihfA alleles.
Prophage excision of P22 lysogen can proceed without IHF.
To determine whether IHF plays a role in P22 excision in vivo, the number of phage particles produced spontaneously after overnight cultivation and upon mitomycin treatment of lysogens was measured. MS1868(P22) produced more phage particles than the IHF-deficient lysogen, JG1151(P22), under both conditions (Table 5). However, these values could represent the effects of IHF on the two different processes—excisive recombination of the prophage and the efficiency of lytic growth of the excised P22 phage. The fact that MS1868(P22 goh5) and JG1151(P22 goh5) produced the same level of viable phages indicates that excision can occur with similar efficiencies in these lysogens. Taken together, we conclude that the amount of excisive recombination of P22 was not affected by the absence of IHF in vivo.
TABLE 5.
Phage yields upon induction of P22 lysogens
| Host | Phage | Phage yield (PFU/ml)a
|
|
|---|---|---|---|
| Spontaneousb | Mitomycin inducedc | ||
| MS1868 (IHF+) | P22 wild type | (1.6 ± 0.7) × 105 | (2.1 ± 1.6) × 1011 |
| P22 goh5 | (1.7 ± 0.9) × 105 | (2.5 ± 1.6) × 1011 | |
| JG1151 (IHF−) | P22 wild type | (4.4 ± 2.3) × 103 | (6.9 ± 7.9) × 107 |
| P22 goh5 | (2.6 ± 0.9) × 105 | (4.2 ± 2.0) × 1011 | |
Values represent the average of yields from induction of three independently isolated single lysogens.
Is IHF in S. typhimurium involved in the site-specific recombination of bacteriophage P22?
The site-specific recombination systems of phages P22 and λ are similar in that they are carried out by related integrases and that the attP regions carry several Int arm-type binding sites and IHF binding sites (14, 30). IHF binding sites on the attP region of P22 were shown to be recognized by E. coli IHF (14). In addition, E. coli IHF stimulates recombination of P22 in vitro (30). We wanted to test whether these two IHF binding sites are functional during the site-specific recombination of P22 in vivo. The results showed that neither the integration nor excision processes were affected by the absence of IHF in vivo under the conditions used in this study. In contrast, λ integration frequencies decreased dramatically in the absence of IHF (Table 5) (19).
These results do not necessarily indicate that the two IHF binding sites on the P22 attP site are dispensable. Although P22 site-specific recombination can proceed without IHF, there may be certain physiological conditions under which IHF facilitates site-specific recombination of P22. In addition, the function of IHF in P22 site-specific recombination may be substituted for by other DNA-bending proteins in the host. It is possible that such a protein could be made by both E. coli and S. typhimurium, because integration occurs in both hosts in the absence of IHF. Goodman et al. (6) reported that the closely related protein HU can partially compensate for the function of IHF in λ site-specific recombination under some circumstances. We observed no difference in integration and excision frequencies of phage P22 in vivo in a strain deficient in HU production (data not shown). Further studies and comparison of the intasome structures of the two phages will help us to elucidate the structural and functional relationship of the multiple protein-DNA complexes.
ACKNOWLEDGMENTS
We thank R. Kazmierczak, S. Maloy, M. Surber, and B. Swalla for comments on the manuscript and J. Slauch, W. Metcalf, B. Swalla, and W. Reznikoff for strains.
This work was supported by KOSEF grant 951-0502-014-2 from Korea Science and Engineering Foundation and NIH grant GM28717.
REFERENCES
- 1.Argos P, Landy A, Abremski K, Egan B J, Haggard-Ljungquist E, Hoess R H, Kahn M L, Kalionis B, Narayana S V L, Pierson III L S, Sternberg N, Leong J M. The integrase family of site-specific recombinases: regional similarities and global diversity. EMBO J. 1986;5:433–440. doi: 10.1002/j.1460-2075.1986.tb04229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Better M, Lu C, Williams R C, Echols H. Site-specific DNA condensation and pairing mediated by the Int protein of bacteriophage λ. Cell. 1982;32:161–168. doi: 10.1073/pnas.79.19.5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Craig N L, Nash H A. E. coli integration host factor binds to specific sites in DNA. Cell. 1984;39:707–716. doi: 10.1016/0092-8674(84)90478-1. [DOI] [PubMed] [Google Scholar]
- 4.Daniels D, Schroeder J, Szybalski W, Sanger F, Coulson A, Hong G, Hill D, Petersen G, Blattner F. Complete annotated lambda sequence. In: Hendrix R W, Roberts J W, Stahl F W, Weisberg R A, editors. Lambda II. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1983. pp. 519–676. [Google Scholar]
- 5.Esposito D, Scocca J J. The integrase family of tyrosine recombinases: evolution of a conserved active site domain. Nucleic Acids Res. 1997;25:3605–3614. doi: 10.1093/nar/25.18.3605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goodman S D, Nicholson S C, Nash H A. Deformation of DNA during site-specific recombination of bacteriophage 1: replacement of IHF protein by HU protein or sequence directed bends. Proc Natl Acad Sci USA. 1992;89:11910–11914. doi: 10.1073/pnas.89.24.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Granston A E, Nash H N. Characterization of a set of integration host factor mutants deficient for DNA binding. J Mol Biol. 1993;234:45–59. doi: 10.1006/jmbi.1993.1562. [DOI] [PubMed] [Google Scholar]
- 8.Griffith J D, Nash H A. Genetic rearrangement of DNA induces knots with a unique topology: implications for the mechanism of synapsis and crossing-over. Proc Natl Acad Sci USA. 1985;82:3124–3128. doi: 10.1073/pnas.82.10.3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Henthorn K S, Friedman D I. Identification of related genes in phages φ80 and P22 whose products are inhibitory for phage growth in Escherichia coli IHF mutants. J Bacteriol. 1995;177:3185–3190. doi: 10.1128/jb.177.11.3185-3190.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10a.Higgins, N. P. Personal communication.
- 11.Kim S, Moitoso de Vargas L, Nunes-Düby S E, Landy A. Mapping of a higher order protein-DNA complex: two kinds of long-range interaction in λ attL. Cell. 1990;63:773–780. doi: 10.1016/0092-8674(90)90143-3. [DOI] [PubMed] [Google Scholar]
- 12.Lee E C, MacWilliams M P, Gumport R I, Gardner J F. Genetic analysis of Escherichia coli integration host factor interactions with its bacteriophage λ H′ recognition site. J Bacteriol. 1991;173:609–617. doi: 10.1128/jb.173.2.609-617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee E C, Hales L M, Gumport R I, Gardner J F. The isolation and characterization of mutants of the integration host factor (IHF) of Escherichia coli with altered, expanded DNA-binding specificities. EMBO J. 1992;11:305–313. doi: 10.1002/j.1460-2075.1992.tb05053.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leong J M, Nunes-Düby S E, Lesser C F, Youderian P, Susskind M M, Landy A. The φ80 and P22 attachment sites: primary structure and interaction with Escherichia coli integration host factor. J Biol Chem. 1985;260:4468–4477. [PubMed] [Google Scholar]
- 15.Leong J M, Nunes-Düby S E, Oser A B, Lesser C F, Youderian P, Susskind M M, Landy A. Structural and regulatory divergence among site-specific recombination genes of lambdoid phage. J Mol Biol. 1986;189:603–616. doi: 10.1016/0022-2836(86)90491-2. [DOI] [PubMed] [Google Scholar]
- 16.MacWilliams M P, Gumport R I, Gardner J F. Genetic analysis of the bacteriophage λ attL nucleoprotein complex. Genetics. 1996;143:1069–1079. doi: 10.1093/genetics/143.3.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maloy S R, Stewart V J, Taylor R K. Genetic analysis of pathogenic bacteria: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1996. [Google Scholar]
- 18.Maniatis T, Fritsch E F, Sambrook J. Molecular cloning: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1982. [Google Scholar]
- 18a.Metcalf, W. Unpublished data.
- 19.Miller H I, Mozola M A, Friedman D I. int-h:: an int mutation of phage λ that enhances site-specific recombination. Cell. 1980;20:721–729. doi: 10.1016/0092-8674(80)90318-9. [DOI] [PubMed] [Google Scholar]
- 20.Moitoso de Vargas N, Pargellis C A, Hasan N M, Bushman E W, Landy A. Autonomous DNA binding domains of λ integrase recognize two different sequence families. Cell. 1988;54:923–929. doi: 10.1016/0092-8674(88)90107-9. [DOI] [PubMed] [Google Scholar]
- 21.Numrych T E, Gumport R I, Gardner J F. Characterization of the bacteriophage lambda excisionase (Xis) protein: the C-terminus is required for Xis-integrase cooperativity but not for DNA binding. EMBO J. 1992;11:3797–3806. doi: 10.1002/j.1460-2075.1992.tb05465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nunes-Düby S E, Kwon H J, Tirumalai R S, Ellenberger T, Landy A. Similarities and differences among 105 members of the Int family of site-specific recombinases. Nucleic Acids Res. 1998;26:391–406. doi: 10.1093/nar/26.2.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pollock T J, Nash H A. Knotting of DNA caused by a genetic rearrangement: evidence for a nucleosome-like structure in site-specific recombination of bacteriophage lambda. J Mol Biol. 1983;170:1–18. doi: 10.1016/s0022-2836(83)80224-1. [DOI] [PubMed] [Google Scholar]
- 24.Reznikoff W S, Abelson J N. The lac promoter. In: Miller J H, Reznikoff W S, editors. The operon. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1978. pp. 221–243. [Google Scholar]
- 25.Rice P A, Yang S-W, Mizuuchi K, Nash H A. Crystal structure of an IHF-DNA complex: a protein-induced DNA U-turn. Cell. 1996;87:1295–1306. doi: 10.1016/s0092-8674(00)81824-3. [DOI] [PubMed] [Google Scholar]
- 26.Richet E, Abcarian P, Nash H A. Synapsis of attachment sites during lambda integrative recombination involves capture of a naked DNA by a protein-DNA complex. Cell. 1988;52:9–17. doi: 10.1016/0092-8674(88)90526-0. [DOI] [PubMed] [Google Scholar]
- 27.Robertson C A, Nash H A. Bending of the bacteriophage lambda attachment site by Escherichia coli integration host factor. J Biol Chem. 1988;263:3554–3557. [PubMed] [Google Scholar]
- 28.Ross W, Landy A. Bacteriophage lambda int protein recognizes two classes of sequence in the phage att site: characterization of the arm-type sites. Proc Natl Acad Sci USA. 1982;79:7724–7728. doi: 10.1073/pnas.79.24.7724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith H O, Levine M. A phage P22 gene controlling integration of prophage. Virology. 1967;31:297–316. doi: 10.1016/0042-6822(67)90164-x. [DOI] [PubMed] [Google Scholar]
- 30.Smith-Mungo L, Chan I T, Landy A. Structure of the P22 att site: conservation and divergence in the motif of recombinogenic complexes. J Biol Chem. 1994;269:20798–20805. [PubMed] [Google Scholar]
- 31.Snyder U K, Thompson J F, Landy A. Phasing of protein-induced DNA bends in a recombination complex. Nature. 1989;341:255–257. doi: 10.1038/341255a0. [DOI] [PubMed] [Google Scholar]
- 32.Susskind M M, Botstein D. Molecular genetics of bacteriophage P22. Microbiol Rev. 1978;42:385–413. doi: 10.1128/mr.42.2.385-413.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Swalla, B. M. Unpublished results.