Abstract
Purpose
Children diagnosed with craniopharyngioma are vulnerable to adverse health outcomes. Characterization of body mass index (BMI), physical function, and cardiopulmonary fitness in those treated with proton radiotherapy (PRT) will serve to design interventions to improve outcomes.
Methods
Ninety-four children with craniopharyngioma completed physical function testing prior to PRT and annually for 5 years. For each outcome, age- and sex-specific z-scores were calculated using normative values. Participants with z-scores > 1.5 or < − 1.5 were classified as impaired. Those with z-scores > 2.0 or < − 2.0 were classified as significantly impaired. Descriptive statistics were used to describe study outcomes and change in prevalence of impairments from 2 to 5 years after treatment.
Results
Nearly half of participants [45.2%, 95% confidence interval (CI) 39.4, 51.0] had mean BMI z-scores > 1.5 at baseline, with prevalence increasing to 66.7% (95% CI 61.5, 71.9) at 5 years. More than half of participants (54.2%, 95% CI 48.4, 60.0) had knee extension strength z-scores < − 1.5 at baseline, with prevalence increasing to 81.3% (95% CI 77.7, 84.9) at 5 years. BMI and knee extension strength had the largest proportion of participants impaired at both 2 and 5 years (53.2% and 62.3%, respectively). Resting heart rate had the highest proportion of participants not impaired at 2 years but became impaired at 5 years (26.6%).
Conclusions
Children with craniopharyngioma have BMI and fitness abnormalities at diagnosis and continue 5 years after treatment. This cohort may benefit from interventions designed to improve BMI, strength, and resting indicators of cardiopulmonary fitness.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11060-022-04116-2.
Keywords: Physical function, Fitness, Craniopharyngioma, Children
Introduction
Children diagnosed with craniopharyngioma have a high rate of survival, with over 95% surviving 5 years or longer [1]. The sellar/suprasellar location of this tumor and current treatments leave survivors vulnerable to a spectrum of adverse outcomes including body mass index (BMI) [2] and fitness impairments [3, 4]. Hormone deficiencies [2] and metabolic disorders [2] are common. While replacement therapy can normalize hormonal function, these children have persistent problems with obesity. Weight gain is both organic [5] and behavioral [6], manifested as elevated BMI, increased waist circumference, and an abnormal waist to height ratio [7]. In long-term survivors of childhood cancer, particularly among those with untreated growth hormone deficiency, BMI abnormalities are associated with muscle weakness and suboptimal cardiorespiratory fitness [8].
Obesity, weakness, and poor cardiorespiratory fitness during childhood are a problematic constellation of impairments, predisposing individuals to long-term chronic physical and psychosocial health conditions which are associated with poor adaptive physical function in the general population [9, 10]. These are major concerns in long-term survivors of childhood craniopharyngioma [11, 12]. Previous studies among children with craniopharyngioma are limited to obesity rates [13], cross-sectional methodology [4], or description of cohorts treated with conventional photon-based radiation therapy [14]. Because most children diagnosed with craniopharyngioma are now treated with proton radiotherapy (PRT), a modality designed to reduce late sequalae by limiting radiation exposure in healthy tissue [15], fewer or less severe sequelae are expected.
Hypothalamic function influences BMI and hunger through hormonal regulation of insulin, leptin and ghrelin [13], and cardiorespiratory fitness, by regulation of the parasympathetic and sympathetic nervous activity [16]. Sparing tissue not involved with the tumor could help improve these outcomes. Limited, but affirmative results among children with other brain tumor histologies indicate reduced doses of radiation to the hypothalamus result in better endocrine outcomes with PRT.10 To our knowledge, no study has characterized BMI, muscle strength, and cardiorespiratory fitness among children with craniopharyngioma treated with PRT. Our goal was to describe the proportion of participants that have impaired BMI, muscle strength, adaptive physical function, and cardiorespiratory fitness, as well as describe the proportion of participants that change or maintain their impairment status between 2 and 5 years after treatment. The findings from this study will inform monitoring guidelines, caregiver education, and development of targeted interventions.
Methods
Participants
Participants were enrolled on the RT2CR protocol (NCT01419067) at St. Jude Children’s Research Hospital (SJCRH, n = 72) or the University of Florida Health Proton Therapy Institute (UFHPTI, n = 22) between 8/22/2011 and 1/19/2016. The median age at enrollment was 9.21 years (range 0.88–20.13 years). Following baseline assessments performed at SJCRH, participants were treated with PRT at UFHPTI using passive scattering methods and 0.5 cm clinical target volume margin. The prescribed dose was 54 Gy (RBE) in 30 fractions. Surgery prior to PRT was individualized and performed at referring centers or enrollment sites. Upon completion of PRT, patients were followed at SJCRH at regular intervals (± 3 months) which included 5 years of annual assessments and measures contained in this report. Written informed consent was obtained from all participants and the protocol was approved by the SJCRH Institutional Review Board. All performance assessments were completed by master’s level, American College of Sports Medicine certified Exercise Specialists using standardized instructions. All staff participate in yearly inter-rater reliability and are trained to encourage participants during effort-dependent tests.
Demographic and clinical characteristics
Demographic and clinical characteristics were recorded for each participant and included sex, race, and surgical approach (craniotomy vs. no craniotomy). Surgical approach was dichotomized into two groups (craniotomy and no craniotomy). Patients who received one or two craniotomies, or multiple approaches that included a craniotomy were categorized in the craniotomy group. Patients who received an endoscopic resection, ommaya-closed, ommaya-open, a transsphenoidal, or had no surgery were categorized in the no craniotomy group. Participants were determined to have diabetes insipidus if they required permanent treatment with desmopressin prior to baseline assessment [17].
Anthropometrics
Height and weight were measured without shoes using a wall mounted stadiometer (SECA, Hanover, MD, USA) in centimeters (cm), and on an electronic scale (Scale-tronix, White Plains, NY, USA) in kilograms (kg), respectively. BMI was calculated as weight in kg divided by height in meters squared. Sex- and age-adjusted BMI z-scores were calculated using the 2000 Center of Disease Control (CDC) growth charts [18]. Waist circumference was measured using a Gulick tape measure at the smallest point between the xyphoid process and the umbilicus. The waist to height ratio (WHR) was calculated by dividing waist circumference by height.
Flexibility and strength
Low back and hamstring flexibility was assessed using a Flex-Tester sit and reach box (Novel Products, Inc., Rockton, IL, USA). Participants sat with their hips at ninety degrees and their knees extended. Bending only at their hips, participants reached forward as far as possible. The test was repeated, and the best distance to the 0.5 cm was recorded [19]. Hand grip strength was assessed using a Jamar hand grip dynamometer (Performance Health, Warrenville, IL, USA). With the elbow in ninety degrees of flexion and the wrist and shoulder in neutral, participants squeezed until peak force was reached. The better of two trials for each hand was documented in kg [20]. Knee extension strength was measured with a Biodex System IV Dynamometer (Biodex Medical Systems, Shirley, New York City, NY, USA). Participants sat in a supported position with the hips at 95 degrees of flexion and the knees in 60 degrees of flexion. Three trials of maximum isometric strength, alternating extension and flexion, were performed for 5 s each, with 5 s of rest in between. Peak isometric extension normalized for body weight for each leg was recorded in newtons (N)/kg [21].
Balance
Balance was measured using the Sensory Organization Test (SOT; Neurocom Smart Equitest, Natus Medical Inc., Pleasanton, CA, USA). Participants stood upright on a force plate with a visual surround, and experienced six 20 s perturbations during which the percentage of time spent inside a 12.5 degree sway envelope was recorded [22]. These included (1) eyes open, (2) eyes closed, (3) eyes open—vision sway referenced (the visual surround moved in concert with anterior–posterior sway), (4) eyes open—proprioception sway referenced (the force plate moved in concert with anterior–posterior sway), (5) eyes closed—proprioception sway referenced, and (6) eyes open—vision and proprioception sway referenced. The composite score (out of 100 possible) for all six conditions was used for analysis.
Adaptive physical function
Gross and fine motor skills were assessed using the Bruininks-Oseretsky Test of Motor Proficiency 2nd Edition Short Form (BOT-SF) [23]. This standardized subset of tests include fine motor precision, fine motor integration, manual dexterity, bilateral coordination, balance, running speed and agility, upper-limb coordination, and strength. Raw scores are converted to a standard score based on age, sex, and the type of pushup performed (knee or full pushup). Standard scores have a mean of 50 and a SD of 10. The BOT-SF has excellent test–retest reliability (intraclass correlation coefficient [ICC] = 0.80–0.99) [24].
Metabolic and cardiopulmonary exercise testing
Resting metabolic rate (RMR) was measured via indirect calorimetry (UltimaCardio2; MGC Diagnostics, St. Paul, MN, USA). Participants fasted for 8 h prior to assessment, and rested supine for 20 min, wearing a face mask attached to a low flow pneumotach. The RMR [kilocalories (kcals)] was calculated using the modified Weir equation [25]. This test was performed on a separate day from all other performance testing.
A ramping maximal cardiopulmonary exercise test was also completed using a modified Balke treadmill protocol [26]. Participants walked 3–3.5 miles per hour, depending on ability, starting at 0% incline. Every minute, the incline increased by 1% until the patient reached maximal exertion. While the treadmill was the preferred method of testing, some participants were tested with a leg ergometer using a ten watt per minute ramping protocol because of small stature, younger age, or balance deficits. Participants wore a 12-lead electrocardiogram, automated blood pressure (BP) monitor, and a pulse oximeter for the duration of the test and into recovery. The BP and heart rate (HR) were recorded pre-test, at three-minute intervals during the test, immediately post-test, and into recovery. Breath-by-breath gas exchange was evaluated continuously, using the mid 5 of 7 breaths, to assess peak oxygen uptake (peak volume of oxygen; peak VO2; milliliters/kg/min) [27, 28]. The test was stopped at the request of the participant, at maximal exertion, or for any safety concerns.
Statistical analysis
Descriptive statistics were used to characterize study participants. For each outcome, age and sex specific z-scores were calculated using established normative values. Participants with z-scores < − 1.5 (or > 1.5 for WHR, BMI, resting systolic BP, resting HR, and peak VO2 were classified as impaired; participants with z-scores < − 2.0 (or > 2.0 for the measures listed above) were classified as significantly impaired). Missing outcome data from each functional test were reviewed by two authors (RP, MW); those whose medical status precluded test completion were also classified as impaired. All other missing outcome data were omitted from analyses. To compare change in impairment status after conclusion of treatment, each outcome was classified as impaired or not impaired at 2 and 5 years and compared as the following: not impaired at 2 or 5 years, not impaired at 2 but impaired at 5 years, impaired at 2 and 5 years, or impaired at 2 but not impaired at 5 years. A sensitivity analysis examined the impact of excluding participants who started treatment over 1 year after diagnosis (n = 22) and those who did not complete all timepoints. A second sensitivity analysis was done using z-scores that were calculated using means that were adjusted for hypothyroid medication (yes or no), growth hormone (yes or no) and corticosteroids (yea or no). Finally, a supplemental analysis was done calculating the z-scores of those in the craniotomy surgery group and no craniotomy group separately. Data were analyzed with SAS version 9.4 (SAS institute, Cary NC, USA).”
Results
Participants
The characteristics of participants are described in Table 1. The mean age at diagnosis was 9.0 (SD 4.52), 55.3% were female, and 64.9% were white. More than half of participants (61.7%) had a craniotomy. Tanner stages for participants in each time point are presented in (Online Resource Table 10). The sensitivity analysis, which removed patients who had treatment more than 1 year after diagnosis, did not substantially change the characteristics of the cohort (Online Resource Table 1).
Table 1.
Demographics and clinical characteristics
| Participants (n = 94) | |
|---|---|
| Sex, n (%) | |
| Female | 51 (54.26%) |
| Male | 43 (45.74%) |
| Race, n (%) | |
| White | 61 (64.89%) |
| Other | 33 (35.11%) |
| Age at diagnosis, mean (SD) | 9.0 (4.52) |
| Surgical approach, n (%) | |
| Craniotomy | 58 (61.70%) |
| No craniotomy | 36 (38.30%) |
| CSF shunt placed, n (%) | |
| Yes | 32 (34.04%) |
| No | 62 (65.96%) |
| Diabetes insipidus, n (%) | |
| Yes | 56 (59.57%) |
| No | 38 (40.43%) |
SD standard deviation, n number; % percentage; SD standard deviation
Fitness impairments
Mean (± SD) age and sex specific z-scores for each outcome are shown in Table 2. Mean BMI was consistently elevated at each time point with z-scores ranging from 1.3±1.1 at baseline to 1.7±0.8 at 5 years. The WHR z-scores were also elevated throughout the study period (baseline, 1.2±1.4; 5 years, 1.7±1.7). Mean peak VO2 was lower than expected at baseline (− 0.9±0.9) and improved over time but was still lower than expected at 5 years (− 0.5±0.9). On average, hand grip and knee extension strength were more impaired than all other fitness measures. Hand grip strength declined from baseline (− 1.6±1.8) to 6 months (− 1.9±1.8), recovered to baseline levels, but was still impaired at 5 years (− 1.4±1.6). Mean knee extension strength z-score was − 2.1±1.0 at baseline and declined to − 2.9±1.4 by 5 years. Adjusting the z-scores for medication use yielded similar results (Online Resource Table 2). Those who received at least one craniotomy had worse outcomes compared to those who did not (Online Resource Table 3a, b). Tables 3 and 4 show the percentage of participants with fitness impairments more than 1.5 SD (3) and 2.0 SD (4) below (or above, for WHR, BMI, resting systolic BP, and HR) age and sex specific normative values. Nearly half of participants (45.2%, 95% confidence internal [CI] 39.4, 51.0) had a BMI more than 1.5 SD above the mean at baseline and this frequency increased to 66.7% (95% CI 51.5, 71.9) by 5 years. The percentage of participants with resting systolic BP 1.5 SD above the mean decreased from baseline (50%, 95% CI 44.1, 55.9) to 5 years (34.6%, 95% CI 29.3, 39.9). The percentage of participants with resting HR more than 1.5 SD above the mean increased from baseline to 5 years (baseline, 26.2%, 95% CI 21.7, 30.7; 5 years, 43.2%, 95% CI 37.5, 48.9). Over half of participants (54.2%, 95% CI 48.4, 60.0) had impaired knee extension strength at 1.5 SD below the mean and this frequency increased to 81.3% (95% CI 77.7, 84.9) by 5 years. Trends in percentages (> 2 SD) were similar for more severe impairment for all measures.
Table 2.
Age- and sex-specific z-scores (Mean and SD) for physical fitness measures in participants with craniopharyngioma during a 5-year follow-up period
| Baseline (n = 84) | 6-months (n = 73) | 1 year (n = 76) | 2 years (n = 89) | 3 years (n = 91) | 4 years (n = 83) | 5 years (n = 81) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | Mean | SD | |
| Anthropometrics | ||||||||||||||
| Waist to height ratio | 1.2 | 1.4 | 1.5 | 1.4 | 1.4 | 1.6 | 1.3 | 1.5 | 1.4 | 1.5 | 1.6 | 1.5 | 1.7 | 1.7 |
| Body mass index | 1.3 | 1.1 | 1.5 | 0.9 | 1.5 | 1.0 | 1.5 | 0.9 | 1.6 | 0.9 | 1.7 | 0.8 | 1.7 | 0.8 |
| Cardiopulmonary fitness | ||||||||||||||
| Resting systolic blood pressurea | 0.9 | 5.9 | 1.5 | 5.9 | 2.3 | 4.7 | 1.1 | 4.6 | 1.1 | 4.7 | 0.8 | 4.6 | 1.1 | 4.2 |
| Resting heart rate | 0.8 | 1.6 | 0.6 | 1.7 | 0.8 | 1.8 | 0.8 | 1.7 | 0.8 | 1.8 | 1.0 | 1.9 | 1.2 | 1.6 |
| Peak oxygen uptake | − 0.9 | 0.9 | − 0.8 | 1.0 | − 0.8 | 1.0 | − 0.8 | 0.9 | − 0.6 | 0.8 | − 0.5 | 0.9 | − 0.5 | 0.9 |
| Metabolism | ||||||||||||||
| Resting metabolic rate | − 0.6 | 0.7 | − 0.7 | 0.7 | − 0.6 | 0.8 | − 0.2 | 0.7 | − 0.5 | 0.7 | − 0.4 | 0.8 | − 0.4 | 0.8 |
| Flexibility | ||||||||||||||
| Sit and reach | -0.5 | 1.2 | -0.3 | 1.0 | -0.2 | 1.1 | -0.2 | 1.2 | -0.4 | 1.2 | -0.6 | 1.2 | -0.7 | 1.3 |
| Dorsiflexion ROM | 0.3 | 1.7 | 0.5 | 1.3 | 0.2 | 1.4 | -0.1 | 1.7 | -0.3 | 1.5 | -0.5 | 1.6 | -0.3 | 1.4 |
| Muscular strength | ||||||||||||||
| Hand grip strength | − 1.6 | 1.8 | − 1.9 | 1.8 | − 1.6 | 1.6 | − 1.4 | 1.8 | − 1.3 | 1.7 | − 1.4 | 2.0 | − 1.4 | 1.6 |
| Knee extension strength | − 2.1 | 1.0 | − 2.6 | 1.5 | − 2.6 | 1.3 | − 2.5 | 1.4 | − 2.7 | 1.4 | − 2.9 | 1.5 | − 2.9 | 1.4 |
| Balance | ||||||||||||||
| Sensory organization test | − 0.5 | 2.1 | 0.0 | 2.0 | − 0.1 | 2.0 | − 0.6 | 2.2 | − 1.0 | 2.0 | − 1.0 | 2.2 | − 1.2 | 2.7 |
| Adaptive physical function | ||||||||||||||
| BOT-SF standardized score | − 1.0 | 0.9 | − 0.9 | 1.0 | − 0.8 | 0.9 | − 1.0 | 0.9 | − 0.9 | 0.9 | − 1.0 | 1.1 | − 0.9 | 1.1 |
Some participants were removed from the model due to missing some or all tests at each time point: Baseline, (n = 10) < 4 years old; 6-months, (n = 8) < 4 years old, (n = 13) no scheduled visit; 1 year (n = 6) < 4 years old, (n = 11) no scheduled visit, (n = 1) no show; 2 years, (n = 1) < 4 years old, (n = 4) no scheduled visit; 3 years, (n = 2) no scheduled visit, (n = 1) no show; 4 years, (n = 9) no scheduled visit, (n = 1) off study, (n = 1) COVID-19 cancellation; 5 years, (n = 6) no scheduled visit, (n = 5) COVID-19 cancellation, (n = 1) no show, (n = 1) off study
BOT-SF Bruininks-Oseretsky test of motor proficiency short form, n Number, ROM Range of motion, SD Standard deviation
aFour participants were taking blood pressure medications at the time of assessment and were well controlled
Table 3.
Percentage of participants with craniopharyngioma whose performance on physical fitness measures is 1.5 SD below (*or above) age- and sex-specific normative values during a 5-year follow-up period
| Baseline (n = 84) | 6-months (n = 73) | 1 year (n = 76) | 2 years (n = 89) | 3 years (n = 91) | 4 years (n = 83) | 5 years (n = 81) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Anthropometrics | ||||||||||||||
| Waist to height ratio* | 33.7 | (28.5, 38.9) | 43.1 | (37.4, 48.8) | 44.6 | (38.8, 50.4) | 40.4 | (34.8, 46.0) | 40 | (34.4, 45.6) | 49.4 | (43.5, 55.3) | 46.3 | (40.5, 52.1) |
| Body mass index* | 45.2 | (39.4, 51.0) | 63 | (57.5, 68.5) | 61.8 | (56.3, 67.3) | 56.2 | (50.4, 62.0) | 56 | (50.2, 61.8) | 65.1 | (59.8, 70.4) | 66.7 | (61.5, 71.9) |
| Cardiopulmonary fitness | ||||||||||||||
| Resting systolic blood pressure*a | 50 | (44.1, 55.9) | 49.3 | (43.4, 55.2) | 56.6 | (50.8, 62.4) | 41.6 | (35.9, 47.3) | 42.9 | (37.2, 48.6) | 39 | (33.4, 44.6) | 34.6 | (29.3, 39.9) |
| Resting heart rate* | 26.2 | (21.7, 30.7) | 24.7 | (20.3, 29.1) | 31.6 | (26.5, 36.7) | 31.5 | (26.4, 36.6) | 31.9 | (26.8, 37.0) | 39 | (33.4, 44.6) | 43.2 | (37.5, 48.9) |
| Peak oxygen uptake | 21.4 | (17.5, 25.3) | 26 | (21.5, 30.5) | 25 | (20.6, 29.4) | 27 | (22.4, 31.6) | 13.2 | (10.5, 15.9) | 14.5 | (11.6, 17.4) | 13.6 | (10.8, 16.4) |
| Metabolism | ||||||||||||||
| Resting metabolic rate | 8.3 | (6.5, 10.1) | 9.6 | (7.6, 11.6) | 10.5 | (8.3, 12.7) | 2.2 | (1.7, 2.7) | 7.7 | (6.0, 9.4) | 9.6 | (7.6, 11.6) | 7.4 | (5.8, 9.0) |
| Flexibility | ||||||||||||||
| Sit and reach | 17.9 | (14.5, 21.3) | 13.7 | (10.9, 16.5) | 11.8 | (9.4, 14.2) | 12.4 | (9.9, 14.9) | 16.5 | (13.3, 19.7) | 25.3 | (20.9, 29.7) | 22.2 | (18.2, 26.2) |
| Dorsiflexion ROM | 13.1 | (10.4, 15.8) | 9.6 | (7.6, 11.6) | 11.8 | (9.4, 14.2) | 23.6 | (19.4, 27.8) | 15.4 | (12.3, 18.5) | 26.5 | (21.9, 31.1) | 19.8 | (16.1, 23.5) |
| Muscular strength | ||||||||||||||
| Hand grip strength | 53.6 | (47.8, 59.4) | 54.8 | (49.0, 60.6) | 44.7 | (38.9, 50.5) | 44.9 | (39.1, 50.7) | 45.1 | (39.3, 50.9) | 51.8 | (46.0, 57.6) | 42.5 | (36.8, 48.2) |
| Knee extension strength | 54.2 | (48.4, 60.0) | 65.3 | (60.0, 70.6) | 69.3 | (64.3, 74.3) | 70.5 | (65.6, 75.4) | 72.5 | (67.8, 77.2) | 82.9 | (79.6, 86.2) | 81.3 | (77.7, 84.9) |
| Balance | ||||||||||||||
| Sensory organization test | 20.2 | (16.4, 24.0) | 19.2 | (15.6, 22.8) | 25 | (20.6, 29.4) | 30.3 | (25.4, 35.2) | 34.1 | (28.8, 39.4) | 38.6 | (33.0, 44.2) | 34.6 | (29.3, 39.9) |
| Adaptive physical function | ||||||||||||||
| BOT-SF standardized score | 29.8 | (24.9, 34.7) | 27.4 | (22.7, 32.1) | 28.9 | (24.1, 33.7) | 33.7 | (28.5, 38.9) | 30.8 | (25.8, 35.8) | 32.5 | (27.4, 37.6) | 25.9 | (21.4, 30.4) |
Some participants were removed from the model due to missing some or all tests at each time point: Baseline, (n = 10) < 4 years old; 6-months, (n = 8) < 4 years old, (n = 13) no scheduled visit; 1 year (n = 6) < 4 years old, (n = 11) no scheduled visit, (n = 1) no show; 2 years, (n = 1) < 4 years old, (n = 4) no scheduled visit; 3 years, (n = 2) no scheduled visit, (n = 1) no show; 4 years, (n = 9) no scheduled visit, (n = 1) off study, (n = 1) COVID-19 cancellation; 5 years, (n = 6) no scheduled visit, (n = 5) COVID-19 cancellation, (n = 1) no show, (n = 1) off study
BOT-SF Bruininks-Oseretsky test of motor proficiency short form, CI Confidence interval, n Number, % cent, ROM Range of motion, SD Standard deviation
aFour participants were taking blood pressure medications at the time of assessment and were well controlled
*Above 1.5 SD
Table 4.
Percentage of participants with craniopharyngioma whose performance on physical fitness measures is 2.0 SD below (*or above) age- and sex-specific normative values during a 5-year follow-up period
| Baseline (n = 84) | 6-months (n = 73) | 1 year (n = 76) | 2 years (n = 89) | 3 years (n = 91) | 4 years (n = 83) | 5 years (n = 81) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | % | 95% CI | |
| Anthropometrics | ||||||||||||||
| Waist to height ratio* | 22.9 | (18.8, 27.0) | 34.7 | (29.4, 40.0) | 29.7 | (24.8, 34.6) | 27 | (22.4, 31.6) | 31.1 | (26.1, 36.1) | 33.7 | (28.5, 38.9) | 38.8 | (33.2, 44.4) |
| Body mass index* | 29.8 | (24.9, 34.7) | 37 | (31.5, 42.5) | 36.8 | (31.4, 42.2) | 30.3 | (25.4, 35.2) | 39.6 | (34.0, 45.2) | 39.8 | (34.2, 45.4) | 38.3 | (32.8, 43.8) |
| Cardiopulmonary fitness | ||||||||||||||
| Resting systolic blood pressure*a | 48.8 | (42.9, 54.7) | 46.6 | (40.8, 52.4) | 52.6 | (46.8, 58.4) | 38.2 | (32.7, 43.7) | 38.5 | (33.0, 44.0) | 32.9 | (27.7, 38.1) | 30.9 | (25.9, 35.9) |
| Resting heart rate* | 19 | (15.4, 22.6) | 20.5 | (16.7, 24.3) | 26.3 | (21.8, 30.8) | 24.7 | (20.3, 29.1) | 20.9 | (17.0, 24.8) | 32.9 | (27.7, 38.1) | 29.6 | (24.7, 34.5) |
| Peak oxygen uptake | 11.9 | (9.4, 14.4) | 17.8 | (14.4, 21.2) | 13.2 | (10.5, 15.9) | 18 | (14.5, 21.5) | 6.6 | (5.2, 8.0) | 9.6 | (7.6, 11.6) | 4.9 | (3.8, 6.0) |
| Metabolism | ||||||||||||||
| Resting metabolic rate | 3.6 | (2.8, 4.4) | 2.7 | (2.1, 3.3) | 5.3 | (4.1, 6.5) | 2.2 | (1.7, 2.7) | 6 | (4.7, 7.3) | 1.2 | (0.9, 1.5) | ||
| Flexibility | ||||||||||||||
| Sit and reach | 11.9 | (9.4, 14.4) | 6.8 | (5.3, 8.3) | 3.9 | (3.0, 4.8) | 5.6 | (4.4, 6.8) | 8.8 | (6.9, 10.7) | 12 | (9.5, 14.5) | 9.9 | (7.8, 12.0) |
| Dorsiflexion ROM | 7.1 | (5.6, 8.6) | 5.5 | (4.3, 6.7) | 6.6 | (5.2, 8.0) | 15.7 | (12.6, 18.8) | 8.8 | (6.9, 10.7) | 16.9 | (13.6, 20.2) | 12.3 | (9.8, 14.8) |
| Muscular strength | ||||||||||||||
| Hand grip strength | 40.5 | (34.9, 46.1) | 43.8 | (38.0, 49.6) | 35.5 | (30.1, 40.9) | 33.7 | (28.5, 38.9) | 28.6 | (23.8, 33.4) | 37.3 | (31.8, 42.8) | 37.5 | (32.0, 43.0) |
| Knee extension strength | 39.8 | (34.2, 45.4) | 52.8 | (47.0, 58.6) | 58.7 | (53.0, 64.4) | 52.3 | (46.5, 58.1) | 61.5 | (56.0, 67.0) | 73.2 | (68.6, 77.8) | 73.8 | (69.3, 78.3) |
| Balance | ||||||||||||||
| Sensory organization test | 14.3 | (11.4, 17.2) | 11 | (8.7, 13.3) | 19.7 | (16.0, 23.4) | 23.6 | (19.4, 27.8) | 26.4 | (21.8, 31.0) | 31.3 | (26.3, 36.3) | 25.9 | (21.4, 30.4) |
| Adaptive physical function | ||||||||||||||
| BOT-SF standardized score | 14.3 | (11.4, 17.2) | 12.3 | (9.8, 14.8) | 15.8 | (12.7, 18.9) | 13.5 | (10.8, 16.2) | 14.3 | (11.4, 17.2) | 18.1 | (14.6, 21.6) | 12.3 | (9.8, 14.8) |
Some participants were removed from the model due to missing some or all tests at each time point: Baseline, (n = 10) < 4 years old; 6-months, (n = 8) < 4 years old, (n = 13) no scheduled visit; 1 year (n = 6) < 4 years old, (n = 11) no scheduled visit, (n = 1) no show; 2 years, (n = 1) < 4 years old, (n = 4) no scheduled visit; 3 years, (n = 2) no scheduled visit, (n = 1) no show; 4 years, (n = 9) no scheduled visit, (n = 1) off study, (n = 1) COVID-19 cancellation; 5 years, (n = 6) no scheduled visit, (n = 5) COVID-19 cancellation, (n = 1) no show, (n = 1) off study
BOT-SF Bruininks-Oseretsky test of motor proficiency short form, CI Confidence interval, n Number, % cent, ROM Range of motion, SD Standard deviation
aFour participants were taking blood pressure medications at the time of assessment and were well controlled
*Above 2.0 SD
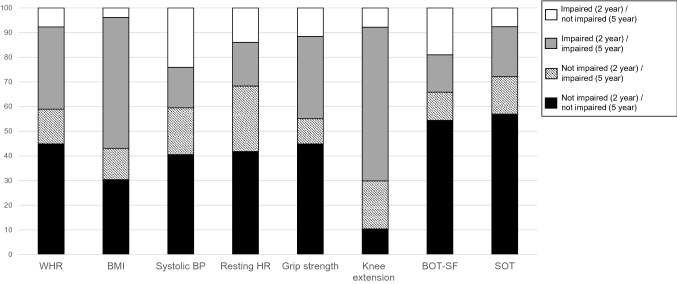
Figure 1 shows change in impairment status from the 2 to 5 years’ time point. Both BMI and knee extension strength had the largest proportion of participants who were impaired at both 2 and 5 years (51.9% and 62.8%, respectively). Resting HR had the highest proportion of participants who were not impaired at 2 years but became impaired at 5 years (26.3%). Systolic BP (23.8%) and performance on the BOT-SF (18.5%) had the highest proportions whose status changed from impaired at 2 years to not impaired at 5 years. Excluding the participants who started their treatment over 1 year after diagnosis did not substantially change the mean z-score, percent impaired, or percent who changed their impairment status (Online Resource Tables 2–4, and Online Resource Fig. 1). Additionally, excluding the participants who did not complete every time-point did not substantially change the mean z-score, percent impaired, or percent who changed their impairment status (Online Resource Tables 5–7, and Online Resource Fig. 2).
Fig. 1.
Percentage of participants with craniopharyngioma whose impairment status (below or above 1.5 standard deviations from normative values) changed, or whose impairment status stayed the same, from 2-year follow-up to 5-year follow-up. WHR Waist to height ratio, BMI body mass index, BP Blood pressure, HR Heart rate, BOT-SF Bruinink-Oseretsky test of motor proficiency test, SOT Sensory Organization Test. A total of 16 patients were excluded from the analysis due to missing the 2-year follow-up, the 5-year follow-up, or both
Discussion
The results of this study indicate that a majority of children with craniopharyngioma have BMI and fitness abnormalities at diagnosis that do not normalize by 5 years after PRT. The prevalence of impairment is highest for BMI and muscular strength and increases over time for both. Although the proportion with impaired cardiopulmonary fitness decreases over time, it is particularly concerning that more than one third of our cohort had elevated resting BP and HR 5 years after treatment. These data indicate that children with craniopharyngioma may benefit from interventions designed to improve BMI, strength, and resting indicators of cardiopulmonary fitness.
To our knowledge, this is the first study to report the results of a comprehensive physical fitness assessment in children with craniopharyngioma from treatment (baseline) through 5 years of follow-up. Although our study is consistent with others demonstrating children with craniopharyngioma have BMI impairments [29] and impaired cardiopulmonary fitness [3], our data describing that over 80% of this population have either impaired upper or lower body muscular strength 5 years after treatment is novel. Low grip strength among children in the general population is associated with future cardiovascular disease and metabolic risk [30]. In a longitudinal cohort of Swedish men (N = 1,078,685), those who had higher knee extension strength as adolescents had lower rates of cardiovascular disability as adults [31]. In addition, muscular strength deficits in children continue into adulthood [32], where they remain associated with cardiometabolic disease and physical disability [33].
Nearly two-thirds of 5-year survivors of craniopharyngioma in this study had elevated BMI values. Previous data show that among 65 children who had hypothalamus sparing surgery, BMI values at 6 months (z-score = 1.56) and 12 months (z-score = 1.89) post-surgery were similar to our participants, especially those with craniotomies [29], well into the obese range. Another group reported that among 45 children treated for craniopharyngioma, mean BMI z-score 7 years after diagnosis was 2.10 ± 1.70 [13]. This is slightly higher than the mean among our participants with 2 years less of follow-up, but supports our finding of a weight gain trend during survivorship. Obese adolescents often become more obese with age [34], and have higher rates of cardiometabolic morbidity [35] and mortality [36] in adulthood. Data from otherwise healthy adolescents (n = 1782) show that those with obesity were 4.51 (95% CI 2.83–7.19) times more likely to have left ventricular hypertrophy, 2.24 (95% CI 1.46–3.45) times more likely to have elevated systolic BP, and 2.10 (95% CI 1.06–4.17) times more likely to have elevated diastolic BP than those without obesity [37]. Our cohort is already at risk for cardiovascular disability from muscular strength impairment; obesity could compound this risk. Approximately one third of the participants did not have impaired BMI at 24 or 60 months and less than 4 percent went from impaired to not impaired. Our participants who had craniotomies had higher BMI values than those who did not, so less invasive surgery may be one of many factors, including normal tissue-sparing PRT, in preventing obesity among some survivors 2 and 5 years after PRT.
In fact, these children already have early markers of future cardiovascular disease risk. A third had elevated systolic BP, and nearly half had elevated resting HR at 5 years of follow-up. Data from multiple studies (males, N sizes ranging from 2104 to 803,505) indicate that a one SD higher systolic BP in adolescence increases risk for ischemic stroke (Hazard Ratio [HR], 1.04; 95% CI 1.02–1.07) and hemorrhagic stroke (HR, 1.12; 95% CI 1.07–1.17) in adulthood [38], that both elevated systolic and diastolic BPs in adolescence increase risk for cardiovascular mortality in adulthood [39], and that elevated resting HR in adolescence is associated with heart failure, dilated cardiomyopathy [40], and cardiovascular and all-cause mortality [41]. Fortunately, interventions to address strength deficits improve markers of vascular flow in lean children [42], measures of glycemic control in children with insulin resistance [43], function in children with cerebral palsy, [44] frequency domain components of HR variability in children with cystic fibrosis [45], and both skeletal muscle mass and visceral adiposity in children and adolescents with obesity [46]. Interventions to promote weight loss such as diet, medications, and resistance and aerobic training, are also beneficial in pediatric populations [47].
Amongst our cohort, whose radiation exposure was proton based, both RMR and peak oxygen uptake improved during this 5-year study. These results differ from those presented for a small cohort (N = 8) of children with craniopharyngioma treated with traditional radiotherapy [48]. The RMR was significantly impaired for obese participants with craniopharyngioma compared to predicted values (mean kcals ± SD; 1541 ± 112.6 vs. 1809 ± 151.8, p = 0.01) [48]. This was a 17% decrease compared to obese controls who had no significant difference compared to predicted values (1647 ± 33.2 vs. 1652 ± 40.2, p = 0.8) [48]. Our cohort’s improved RMR could indicate that the normal tissue-sparing PRT may reduce hypothalamic dysfunction, which is postulated to be associated with low RMR in this population [49]. Additionally, our peak oxygen uptake data agree with a study that evaluated craniopharyngioma survivors treated with surgery alone where relative peak oxygen uptake 5.7 years post-surgery was 20–25% lower than among healthy controls [3]. Participants in our study, all of whom had PRT, had a mean z-score at 5 years of − 0.5 ± 0.9, 19.1% lower than expected compared to normative values. Although this comparative study has a small sample size who received traditional radiotherapy, it appears that in addition to sparing tissue responsible for controlling metabolism, PRT exposure does not adversely affect peak oxygen uptake beyond that conferred by surgical resection.
This study is not without limitations. First, due to the rarity of craniopharyngioma, our study’s sample size was relatively small (n = 94), so we were unable to perform multivariable analyses on how treatment impacted the physical function status of our participants. It is important for future studies to examine which clinical and treatment parameters impact the function of children with craniopharyngioma. Second, our study did not collect lifestyle covariates such as physical activity or diet information. As it stands, it is unclear what impacts physical function the most, treatment or lifestyle. Future studies should consider how the interaction between lifestyle and treatment impact physical function of children with craniopharyngioma. Third, there was some missing outcome data (ranging from 4 to 22% depending on timepoint), which may have biased our results. Missing data were most often due to a child not being able to perform a task, thus estimates of impairment prevalence are likely low. All tests were attempted with every participant, even the very young, but there were times when a test was not appropriate due to age. Alternatively, because most of the measured outcomes were effort based, there may be some misclassification of impairment status. Also, it can be difficult to interpret BMI and physical performance changes during puberty. This could lead to misclassification of the outcomes.
Our findings are the first to document fitness from treatment through 5 years of survival in children with craniopharyngioma. The high proportion and rising trajectory of poor strength, obesity and elevated resting HR and BP over 5-years of follow-up, particularly for patients who had craniotomies, likely increases risk for future cardiovascular disease in these children. Multifaceted interventions, including a combination of pharmaceutical (weight loss medications), behavioral (diet, exercise), and caregiver education are needed to address these impairments.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank Amy Sketch of the Department of Epidemiology and Cancer Control at St. Jude Children’s Research Hospital for assistance with data abstraction and BOT-SF scoring. We would also like to thank Tracie Gatewood of the Department of Epidemiology and Cancer Control at St. Jude Children’s Research Hospital for help in finalizing for manuscript submission.
Author contributions
All authors contributed to the study conception and design. Acquisition of data, interpretation of data, drafting the article, and final approval was performed by Robyn Partin and Matthew Wogksch. Analysis of data, revising the article, and final approval was performed by Robyn Partin. Interpretation of data, revising the article, and final approval was performed by Jason Halford and Heather Conklin. Conception and design, revising the article, and final approval was performed by Daniel Indelicato. All authors read and approved the final manuscript.
Funding
This work was supported by the American Lebanese Syrian Associated Charities (ALSAC) and the St. Jude Cancer Center Support [CORE] Grant [P30 CA 21765 (Roberts)].
Data availability
Data are available upon request from the corresponding author.
Declarations
Competing interest
The authors have no relevant financial or non-financial interests to disclose.
Ethics approval
The study protocol was approved by the St. Jude Children Research Hospital Institutional Review Board.
Consent to participate
Written informed consent was obtained from all participants.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Craniopharyngioma—childhood: statistics (2021). http://www.cancer.net/cancer-types/craniopharyngioma-childhood/statistics. Accessed June 16 2021
- 2.Crom DB. Metabolic abnormalities in an adult survivor of pediatric craniopharyngioma. Oncology. 2008;22:43–46. [PubMed] [Google Scholar]
- 3.Piguel X, Abraham P, Bouhours-Nouet N, Gatelais F, Dufresne S, Rouleau S, Coutant R. Impaired aerobic exercise adaptation in children and adolescents with craniopharyngioma is associated with hypothalamic involvement. Eur J Endocrinol. 2012;166:215–222. doi: 10.1530/EJE-11-0742. [DOI] [PubMed] [Google Scholar]
- 4.Conklin HM, Ness KK, Ashford JM, Scoggins MA, Ogg RJ, Han Y, Li Y, Bradley JA, Boop FA, Merchant TE. Cognitive performance, aerobic fitness, motor proficiency, and brain function among children newly diagnosed with craniopharyngioma. J Int Neuropsychol Soc. 2019;25:413–425. doi: 10.1017/S1355617718001170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lustig RH, Post SR, Srivannaboon K, Rose SR, Danish RK, Burghen GA, Xiong X, Wu S, Merchant TE. Risk factors for the development of obesity in children surviving brain tumors. J Clin Endocrinol Metab. 2003;88:611–616. doi: 10.1210/jc.2002-021180. [DOI] [PubMed] [Google Scholar]
- 6.Roemmler-Zehrer J, Geigenberger V, Stormann S, Ising M, Pfister H, Sievers C, Stalla GK, Schopohl J. Specific behaviour, mood and personality traits may contribute to obesity in patients with craniopharyngioma. Clin Endocrinol. 2015;82:106–114. doi: 10.1111/cen.12523. [DOI] [PubMed] [Google Scholar]
- 7.Chemaitilly W, Li Z, Huang S, Ness KK, Clark KL, Green DM, Barnes N, Armstrong GT, Krasin MJ, Srivastava DK, Pui CH, Merchant TE, Kun LE, Gajjar A, Hudson MM, Robison LL, Sklar CA. Anterior hypopituitarism in adult survivors of childhood cancers treated with cranial radiotherapy: a report from the St. Jude lifetime cohort study. J Clin Oncol. 2015;33:492–500. doi: 10.1200/JCO.2014.56.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ness KK, Baker KS, Dengel DR, Youngren N, Sibley S, Mertens AC, Gurney JG. BMI, muscle strength deficits and mobility limitations in adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2007;49:975–981. doi: 10.1002/pbc.21091. [DOI] [PubMed] [Google Scholar]
- 9.Umer A, Kelley GA, Cottrell LE, Giacobbi P, Jr, Innes KE, Lilly CL. Childhood obesity and adult cardiovascular disease risk factors: a systematic review with meta-analysis. BMC Public Health. 2017;17:683. doi: 10.1186/s12889-017-4691-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Auyeung TW, Kwok T, Lee J, Leung PC, Leung J, Woo J. Functional decline in cognitive impairment–the relationship between physical and cognitive function. Neuroepidemiology. 2008;31:167–173. doi: 10.1159/000154929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fjalldal S, Holmer H, Rylander L, Elfving M, Ekman B, Osterberg K, Erfurth EM. Hypothalamic involvement predicts cognitive performance and psychosocial health in long-term survivors of childhood craniopharyngioma. J Clin Endocrinol Metab. 2013;98:3253–3262. doi: 10.1210/jc.2013-2000. [DOI] [PubMed] [Google Scholar]
- 12.Crom DB, Smith D, Xiong Z, Onar A, Hudson MM, Merchant TE, Morris EB. Health status in long-term survivors of pediatric craniopharyngiomas. J Neurosci Nurs. 2010;42:323–328. doi: 10.1097/jnn.0b013e3181f8a59d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vinchon M, Weill J, Delestret I, Dhellemmes P. Craniopharyngioma and hypothalamic obesity in children. Childs Nerv Syst. 2009;25:347–352. doi: 10.1007/s00381-008-0754-x. [DOI] [PubMed] [Google Scholar]
- 14.Kiehna EN, Merchant TE. Radiation therapy for pediatric craniopharyngioma. Neurosurg Focus. 2010;28:E10. doi: 10.3171/2010.1.FOCUS09297. [DOI] [PubMed] [Google Scholar]
- 15.Merchant TE, Hua CH, Shukla H, Ying X, Nill S, Oelfke U. Proton versus photon radiotherapy for common pediatric brain tumors: comparison of models of dose characteristics and their relationship to cognitive function. Pediatr Blood Cancer. 2008;51:110–117. doi: 10.1002/pbc.21530. [DOI] [PubMed] [Google Scholar]
- 16.Roth CL, Hunneman DH, Gebhardt U, Stoffel-Wagner B, Reinehr T, Muller HL. Reduced sympathetic metabolites in urine of obese patients with craniopharyngioma. Pediatr Res. 2007;61:496–501. doi: 10.1203/pdr.0b013e3180332cd6. [DOI] [PubMed] [Google Scholar]
- 17.Fournier-Goodnight AS, Ashford JM, Merchant TE, Boop FA, Indelicato DJ, Wang L, Zhang H, Conklin HM. Neurocognitive functioning in pediatric craniopharyngioma: performance before treatment with proton therapy. J Neurooncol. 2017;134:97–105. doi: 10.1007/s11060-017-2492-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Mei Z, Curtin LR, Roche AF, Johnson CL. CDC growth charts: United States. Adv Data. 2000;314:1–27. [PubMed] [Google Scholar]
- 19.Thompson WR, Gordon NR, Pescatello LS. ACSM’s guidelines for exercise testing and prescription. 8. Philadelphia: Lippincott Williams & Wilkins; 2010. [Google Scholar]
- 20.Mathiowetz V, Weber K, Volland G, Kashman N. Reliability and validity of grip and pinch strength evaluations. J Hand Surg Am. 1984;9:222–226. doi: 10.1016/s0363-5023(84)80146-x. [DOI] [PubMed] [Google Scholar]
- 21.Harbo T, Brincks J, Andersen H. Maximal isokinetic and isometric muscle strength of major muscle groups related to age, body mass, height, and sex in 178 healthy subjects. Eur J Appl Physiol. 2012;112:267–275. doi: 10.1007/s00421-011-1975-3. [DOI] [PubMed] [Google Scholar]
- 22.Ford-Smith CD, Wyman JF, Elswick RK, Jr, Fernandez T, Newton RA. Test-retest reliability of the sensory organization test in noninstitutionalized older adults. Arch Phys Med Rehabil. 1995;76:77–81. doi: 10.1016/s0003-9993(95)80047-6. [DOI] [PubMed] [Google Scholar]
- 23.Bruininks RHBB. The Bruininks-Oseretsky test of motor proficiency. 2. Circle Pines: AGS Publishing; 2005. [Google Scholar]
- 24.Griffiths A, Toovey R, Morgan PE, Spittle AJ. Psychometric properties of gross motor assessment tools for children: a systematic review. BMJ Open. 2018;8:e021734. doi: 10.1136/bmjopen-2018-021734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weir JB. New methods for calculating metabolic rate with special reference to protein metabolism. J Physiol. 1949;109:1–9. doi: 10.1113/jphysiol.1949.sp004363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cordingley D, Girardin R, Reimer K, Ritchie L, Leiter J, Russell K, Ellis MJ. Graded aerobic treadmill testing in pediatric sports-related concussion: safety, clinical use, and patient outcomes. J Neurosurg Pediatr. 2016;25:693–702. doi: 10.3171/2016.5.PEDS16139. [DOI] [PubMed] [Google Scholar]
- 27.Gahche J, Fakhouri T, Carroll DD, Burt VL, Wang CY, Fulton JE. Cardiorespiratory fitness levels among U.S. youth aged 12–15 years: United States, 1999–2004 and 2012. NCHS Data Brief. 2014;153:1–8. [PubMed] [Google Scholar]
- 28.Gahche JJ, Kit BK, Fulton JE, Carroll DD, Rowland T. Normative values for cardiorespiratory fitness testing among us children aged 6–11 years. Pediatr Exerc Sci. 2017;29:177–185. doi: 10.1123/pes.2016-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Elowe-Gruau E, Beltrand J, Brauner R, Pinto G, Samara-Boustani D, Thalassinos C, Busiah K, Laborde K, Boddaert N, Zerah M, Alapetite C, Grill J, Touraine P, Sainte-Rose C, Polak M, Puget S. Childhood craniopharyngioma: hypothalamus-sparing surgery decreases the risk of obesity. J Clin Endocrinol Metab. 2013;98:2376–2382. doi: 10.1210/jc.2012-3928. [DOI] [PubMed] [Google Scholar]
- 30.Castro-Pinero J, Perez-Bey A, Cuenca-Garcia M, Cabanas-Sanchez V, Gomez-Martinez S, Veiga OL, Marcos A, Ruiz JR, Up, Group DS Muscle fitness cut points for early assessment of cardiovascular risk in children and adolescents. J Pediatr. 2019;206:134–141.e133. doi: 10.1016/j.jpeds.2018.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Henriksson H, Henriksson P, Tynelius P, Ekstedt M, Berglind D, Labayen I, Ruiz JR, Lavie CJ, Ortega FB. Cardiorespiratory fitness, muscular strength, and obesity in adolescence and later chronic disability due to cardiovascular disease: a cohort study of 1 million men. Eur Heart J. 2020;41:1503–1510. doi: 10.1093/eurheartj/ehz774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fraser BJ, Schmidt MD, Huynh QL, Dwyer T, Venn AJ, Magnussen CG. Tracking of muscular strength and power from youth to young adulthood: longitudinal findings from the childhood determinants of adult health study. J Sci Med Sport. 2017;20:927–931. doi: 10.1016/j.jsams.2017.03.021. [DOI] [PubMed] [Google Scholar]
- 33.Peterson MD, Duchowny K, Meng Q, Wang Y, Chen X, Zhao Y. Low normalized grip strength is a biomarker for cardiometabolic disease and physical disabilities among U.S. and Chinese adults. J Gerontol A Biol Sci Med Sci. 2017;72:1525–1531. doi: 10.1093/gerona/glx031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.The NS, Suchindran C, North KE, Popkin BM, Gordon-Larsen P. Association of adolescent obesity with risk of severe obesity in adulthood. JAMA. 2010;304:2042–2047. doi: 10.1001/jama.2010.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reilly JJ, Kelly J. Long-term impact of overweight and obesity in childhood and adolescence on morbidity and premature mortality in adulthood: systematic review. Int J Obes. 2011;35:891–898. doi: 10.1038/ijo.2010.222. [DOI] [PubMed] [Google Scholar]
- 36.Engeland A, Bjorge T, Tverdal A, Sogaard AJ. Obesity in adolescence and adulthood and the risk of adult mortality. Epidemiology. 2004;15:79–85. doi: 10.1097/01.ede.0000100148.40711.59. [DOI] [PubMed] [Google Scholar]
- 37.Movahed MR, Bates S, Strootman D, Sattur S. Obesity in adolescence is associated with left ventricular hypertrophy and hypertension. Echocardiography. 2011;28:150–153. doi: 10.1111/j.1540-8175.2010.01289.x. [DOI] [PubMed] [Google Scholar]
- 38.Hogstrom G, Nordstrom A, Eriksson M, Nordstrom P. Risk factors assessed in adolescence and the later risk of stroke in men: a 33-year follow-up study. Cerebrovasc Dis. 2015;39:63–71. doi: 10.1159/000369960. [DOI] [PubMed] [Google Scholar]
- 39.Sundstrom J, Neovius M, Tynelius P, Rasmussen F. Association of blood pressure in late adolescence with subsequent mortality: cohort study of Swedish male conscripts. BMJ. 2011;342:d643. doi: 10.1136/bmj.d643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lindgren M, Robertson J, Adiels M, Schaufelberger M, Aberg M, Toren K, Waern M, Aberg ND, Rosengren A. Elevated resting heart rate in adolescent men and risk of heart failure and cardiomyopathy. ESC Heart Fail. 2020;7:1178–1185. doi: 10.1002/ehf2.12726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oh B, Gill TM, Shah I, Hughes AD, Deanfield JE, Kuh D, Hardy R. Association between resting heart rate across the life course and all-cause mortality: longitudinal findings from the medical research council (MRC) national survey of health and development (NSHD) J Epidemiol Community Health. 2014;68:883–889. doi: 10.1136/jech-2014-203940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu CC, McManus AM, So HK, Chook P, Au CT, Li AM, Kam JT, So RC, Lam CW, Chan IH, Sung RY. Effects of resistance training on cardiovascular health in non-obese active adolescents. World J Clin Pediatr. 2016;5:293–300. doi: 10.5409/wjcp.v5.i3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alvarez C, Ramirez-Campillo R, Ramirez-Velez R, Martinez C, Castro-Sepulveda M, Alonso-Martinez A, Izquierdo M. Metabolic effects of resistance or high-intensity interval training among glycemic control-nonresponsive children with insulin resistance. Int J Obes. 2018;42:79–87. doi: 10.1038/ijo.2017.177. [DOI] [PubMed] [Google Scholar]
- 44.Merino-Andres J, Garcia de Mateos-Lopez A, Damiano DL, Sanchez-Sierra A. Effect of muscle strength training in children and adolescents with spastic cerebral palsy: a systematic review and meta-analysis. Clin Rehabil. 2022;36:4–14. doi: 10.1177/02692155211040199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Estevez-Gonzalez AJ, Donadio MVF, Cobo-Vicente F, Fernandez-Luna A, Sanz-Santiago V, Villa Asensi JR, Iturriaga Ramirez T, Fernandez-Del-Valle M, Diez-Vega I, Larumbe-Zabala E, Perez-Ruiz M. Effects of a short-term resistance-training program on heart rate variability in children with cystic fibrosis-a randomized controlled trial. Front Physiol. 2021;12:652029. doi: 10.3389/fphys.2021.652029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lee S, Libman I, Hughan KS, Kuk JL, Barinas-Mitchell E, Chung H, Arslanian S. Effects of exercise modality on BMI and cardiovascular disease risk factors in adolescents with obesity: a randomized clinical trial. Appl Physiol Nutr Metab. 2020;45:1377–1386. doi: 10.1139/apnm-2019-0993. [DOI] [PubMed] [Google Scholar]
- 47.Ho M, Garnett SP, Baur LA, Burrows T, Stewart L, Neve M, Collins C. Impact of dietary and exercise interventions on weight change and metabolic outcomes in obese children and adolescents: a systematic review and meta-analysis of randomized trials. JAMA Pediatr. 2013;167:759–768. doi: 10.1001/jamapediatrics.2013.1453. [DOI] [PubMed] [Google Scholar]
- 48.Kim RJ, Shah R, Tershakovec AM, Zemel BS, Sutton LN, Grimberg A, Moshang T. Energy expenditure in obesity associated with craniopharyngioma. Childs Nerv Syst. 2010;26:913–917. doi: 10.1007/s00381-009-1078-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Muller HL. The diagnosis and treatment of craniopharyngioma. Neuroendocrinology. 2020;110:753–766. doi: 10.1159/000504512. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon request from the corresponding author.