Abstract
Objective
To synthesize recent empirical evidence for the prevention and management of falls and fear of falling in patients with Parkinson's disease (PD).
Data source
Database from PubMed, Cochrane Library, and EMBASE.
Study design
Systematic review.
Data collection
We searched the PubMed, Cochrane Library, and EMBASE databases for studies published from inception to February 27, 2021. Inclusion criteria were nonreview articles on prevention and management measures related to falls and fall prevention in Parkinson's disease patients.
Principal findings
We selected 45 articles and conducted in‐depth research and discussion. According to the causes of falls in PD patients, they were divided into five directions, namely physical status, pre‐existing conditions, environment, medical care, and cognition. In the cognitive domain, we focused on the fear of falling. On the above basis, we constructed a fall prevention model, which is a tertiary prevention health care network, based on The Johns Hopkins Fall Risk Assessment Tool to provide ideas for the prevention and management of falling and fear of falling in PD patients in clinical practice
Conclusions
Falls and fear of falls in patients with Parkinson's disease can be reduced by effective clinical prevention and management. Future studies are needed to explore the efficacy of treatment and prevention of falls and fear of falls.
Keywords: falls, fear of fall, management, Parkinson's disease, prevention, systematic review
1. INTRODUCTION
Parkinson's disease (PD), one of the most prevalent neurodegenerative diseases globally, causes heavy losses in social health and the economy unceasingly (GBD 2016 Parkinson's Disease Collaborators, 2018). Some experts forecasted that the number of PD patients would reach 9 million in 2030 (Dorsey & Bloem, 2018) and the neurodegenerative disease will be the dominating cause of related death by overriding cancer (Gammon, 2014). Fall is a common symptom of PD patients. Falls are often a symptom of PD, with two‐thirds of PD patients falling at least once annually (Latt et al., 2009; Paul et al., 2013), with >50% of them experiencing fall recurrence (Allen et al., 2013). Falls can increase the hospitalization rate and mortality rate (Hely et al., 1999; Soh et al., 2013; Temlett & Thompson, 2006), and PD patients with recurrent falls had decreased quality of life, even causing injury and disability (Michałowska et al., 2005; Pickering et al., 2007; Rahman et al., 2008; Soh et al., 2013). Moreover, falls may bring about fear of fall (FOF), which affects the movement of PD patients in turn and leads to the exacerbation of PD symptoms (Adkin et al., 2003; Chaudhuri et al., 2011; Rahman et al., 2011).
Some experts have suggested that assessing the joint effect of potential falls in PD patients may be useful for fall prediction (Gazibara et al., 2016a). Since our current understanding of risk factors for falls in PD patients remains poor, prevention and management strategies aiming to reduce fall occurrence have not been perfected, allowing for human and material resource loss. The combined effect of falls and FOF remains unknown. Further, ways to improve PD patients’ quality of life are arduous and lengthy.
This systematic review aims to summarize the latest evidence of falls and FOF in PD patients, outline the risk factors of falls and FOF, establish a new prevention and management model of falls and FOF, and provide ideas for clinical practice and prevention and management strategies of PD patients. It focuses on the physiological or psychological interventions in clinical practice to seek guidance and management strategies and to provide the scientific basis for future research.
2. METHODS
2.1. Literature search
Reports included within PubMed, Cochrane Library, and EMBASE databases published on and before February 27, 2021, were considered. The following search terms were used: fall/fear of falling, Parkinson's disease, and prevention/management, with no language limitations (Figure 1). No manual search approach was applied. The protocol of this systematic review was registered in PROSPERO (number 285709).
FIGURE 1.
Selected stratagem of searching literature
2.2. Study selection
Cross‐sectional, case‐control, prospective, and retrospective cohort studies of falls and FOF in PD were preliminarily screened. Secondary outcomes of interest included consequences of falling including injury, death, and material consumption. Reviews, case studies, and conference abstracts were excluded. Studies that summarized falls or made comparisons between falls and FOF were included. After identifying relevant articles, duplicate studies were removed. A detailed description of the search strategy and studies selected is illustrated in Figure 2. Titles and abstracts of the remaining articles were assessed. The remaining studies were further examined, with those containing information about falls, risk factors for falls, and prevention and management strategies selected. Full articles were examined to ensure relevant information was included. Two authors independently selected studies, with disagreements resolved via a discussion that included a third senior author (Figure 2).
FIGURE 2.
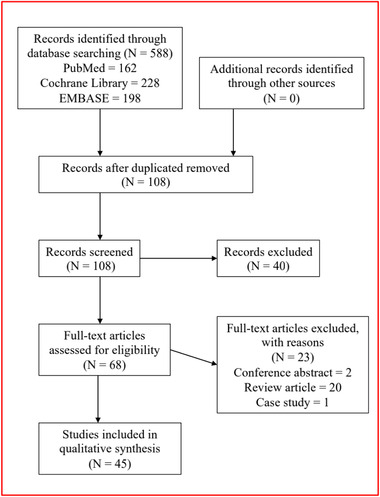
Methods of searching results and literature screening
2.3. Data extraction and quality assessment
We screened the full content to evaluate whether the information was potentially related. Two authors selected relevant studies independently, with disagreements resolved via discussion with a third senior author. The Newcastle–Ottawa Scale (NOS) was used to assess the quality of selected studies (Stang, 2010). Any disagreement between the two authors was also resolved by discussion with a third author. The NOS approach includes three domains (selection of study groups, comparability, and outcome assessment) to assess the quality of eligible studies. A study could be awarded up to one star for each item within the selection and outcome domains and up to two stars for comparability. If seven or more stars were awarded, we considered the study to be of high quality (Stang, 2010).
2.4. Data synthesis and analysis
Two broad outcome variables were considered, as follows: Risk factors for falls and FOF in PD patients (physical status, underlying health problems, environmental factors, medical care, and cognition function); and fall and FOF clinical practices (traditional measurement scales, fall prevention model, drug therapy, and surgery, and exercise). After selected studies were assessed, reasonable prevention‐management strategies for falls and FOF in PD were created.
3. RESULTS
3.1. Study selected characteristics
We obtained 162, 228, and 198 studies from the three databases, respectively. After duplicate removal, 108 studies remained. After all selection criteria were applied, 45 articles remained. Characteristics of included studies, and data regarding falls, FOF, fall‐ or FOF‐associated complications, prevention, and management are summarized in Table 1.
TABLE 1.
Summarized results of included studies
| Study, year, country, database used | Inclusion criteria | Study subjects | Outcome measures | NOS score a | Falls | Fear of falls | Complications of falls or fear of falls | Prevention and management |
|---|---|---|---|---|---|---|---|---|
| Gazibara T et al. 2014, Serbia, Neurology Clinic, Clinical Center of Serbia in Belgrade 2011–2012 | Age from 22 to 83 with PD. MMSE ≥ 24, walk independently for 10 m, stand for 90 s | 180 participants with PD, | Detailed interviews about falls information |
S*** C** O** |
Outside (57.2%), morning (53.9%), outside tripping OR: 7.90(3.21‐19.39), indoors lower extremity weakness β: 0.20(0.05‐0.72) and loss balance β: 0.19(0.05‐0.73) | NA | Soft‐tissue contusion (71.8%); fractures (12.7%) | Additional spatial visualization; using of cane; Particular prevention programs for PD at home and outside |
| Franzén E et al. 2016, Stockholm, Conradsson, Löfgren, Ståhle, Hagströmer, & Franzén, 2012 | PD; MMSE ≥ 24 | 89 patients with PD; Age from 61 to 87 | Structure questions, questionnaires and clinical assessments of falls; fear of falls |
S** C** O** |
Concerning about falling (48%) | Depression symptoms (β = 0.40) | NA | Focus on depressive symptoms, balance deficits, and mobility devices in rehabilitation programs of FOF |
| Paul SS et al. 2017, Australia, NSW Admitted Patients Data Collection 2005–2013 | PD and falls | 8487 fallers with PD | ICD10: S00‐T75 and T79 |
S*** C** O*** |
PD patients (2.5%); length of stay longer (M = 9d); indoor fall (44%) | NA | Fracture (35%); dementia (28.8%); comorbid (56.1%) | Early intervention to maintain mobility and reduce falls |
| Youn J et al. 2017, Korea, Movement Disorders Clinic at Samsung Medical Center, 2014–2015 | PD and falls | 45 participants with forward fallers, 17 with non‐forward fallers | forward PD fallers and non‐forward PD fallers |
S*** C** O*** |
Forward falls (72.6%) non‐forward falls (27.4%). Freeze of gait is frequency in forward falls | NA | NA | Prevention strategies focusing on postural instability; Using various scales can check balance problems in PD |
| Friedman SM et al. 2002, USA, Health Care Financing Administration | PD; MMSE ≥ 18 | 2,212 participants, aged from 65 to 84 | Falls and fear of falls |
S*** C** O*** |
Who with no FOF but falls at baseline were more likely to fear at follow‐up OR: 1.97(1.46‐2.64); cut back on activities OR: 2.51(1.52‐4.14) | Who with FOF were more likely to fall than who without fear OR: 2.22(1.65‐2.98) | Female, older age, worse GHQ (p < 0.05) | Identify high‐risk groups; White race, female, history of stroke, sedative use, FOF were predictors; Confirm FOF is useful assessment of risk |
| Balash Y et al. 2005, Israel, Clinic of the Movement Disorders Unit of the Tel Aviv Sourasky Medical Center 2002 | PD and falls | 350 non‐demented PD patients. | falls |
S*** C** O*** |
Advanced PD (p < 0.001); poor health (p = 0.002); duration of stance reduced (p < 0.001); Timed Up and Go time shorter in non‐falls (p < 0.001) | NA | Urinary incontinence OR: 1.95(1.17‐3.23) | Urinary incontinence can used for identify patients; Osteoporosis and treatment of osteopenia in elderly PD |
| Grimbergen YA et al. 2013, UK, database is not mentioned | PD and falls | 74 PD patients | Falls and fear of falls |
S*** C** O*** |
Balance confidence β = 0.28; Fall frequency β = 0.13 | FOF β = 0.34; FOF (R2 = 0.53) | NA | Management to improve quality of life at prevention of falls and assessment and treatment of FOF; Prevention strategies focusing on postural instability, cognitive and emotional domains |
| Allcock LM et al. 2009, UK, General Practitioners outside the community screening | PD and falls | 87 PD patients | Falls and fear of falls |
S*** C** O** |
Fall at least once (63%); falling twice or more (43.3%); accidental causes (38.9%); postural instability or dizziness (57.4%); | Continuity of attention reduced (p = 0.03) | Soft tissue injuries (81%) | Focus on cardiovascular and gait and balance orientated treatments and strategies to improve cognition |
| McKay JL et al. 2018, USA, community‐dwelling individuals 2011–2015 | PD and falls | 65 patients with PD and 73 normal | Falls |
S*** C** O** |
Falls (52%); impaired set shifting OR:1.29(1.03‐1.60); FOG (69%) | NA | NA | Set shifting may therefore be useful to include in fall risk assessments in older adults with and without PD |
| Gazibara T et al. 2016a, Serbia, Department of Movement Disorders, Neurology Clinic | PD and falls; walking for at least 10 m and standing for at least 90 s | 120 PD patients | Falls |
S** C** O** |
Indoors fall (61.0%); outdoors falls (68.3%); Slipping is strongly associated with outdoor falls Indoor falls were mostly preceded by postural instability, lower extremity weakness, vertigo | NA | Fractures (4.3%) about hip fracture and redial fracture. | Using of cane; Elevating feet when crossing obstacles more than perceiving; Assess joint effect of potential falls factors; Emphasizing on balance recovery and objects in environment |
| Hunter H et al. 2018, UK, ICICLE‐PD | PD and falls | 121 PD patients | Falls |
S** C* O** |
Fall diary to collect fall information | NA | Falls diary data reduced (n = 62) | Longitudinal use of falls diaries is feasible; Making personal monitoring |
| Hiorth YH 2014, Norway, Rogaland County, Western Norway | PD and falls | 211 PD patients | Falls |
S** C** O** |
Disease‐specific gait and axial impairments were the major risk factors for future falls in non‐fallers at baseline | NA | NA | specific education of patients and caregivers in using compensatory strategies; Early treatment strategies of PD are important |
| Gazibara T et al. 2017, Serbia, Department of Movement Disorders, Neurology Clinic 2011–2012 | PD and falls | 120 PD patients without falls in past 6 months | Falls |
S** C** O** |
Fall at least one times (35%); recurrent falls (54.7%); near‐falls (93.5%) | NA | NA | engaging in tailored physical exercise may have a favorable effect on occurrence of near‐fall episodes; Focusing on balance maintenance when experiencing freezing of gait could potentially be useful in reduction of near‐falls |
| Kiesmann M et al. 2020, France, EVAMAR‐AGEX | PD and falls | 79 PD patients | Falls within 6 months |
S** C** O** |
Traumatic (12%); single fallers (8%); zero fallers (34%) | NA | Hallucination (OR = 7.35); history of falls (OR = 11.78) | encouraging elder to maintain his or her cognitive abilities and physical activity focused on postural stability and posture |
| Dibble LE et al. 2006, USA, The University of Utah Rehabilitation and Wellness Clinic | PD and falls | 45 PD patients aged 39 to 90 | The individual unintentionally came to rest on the ground or other level |
S*** C** O** |
FRT (27.43 cm), BBS (50.20), DGI (19.92), TUG (11.67), CTUG (16.48) | NA | NA | Recode number of falls; More accurate predictive ability for falls in persons with PD |
| Hoskovcová M et al. 2015, Czech Republic |
PD and falls UK PD brain bank criteria Without a walking aid |
45 PD patients | Falls |
S** C** O** |
Fall one or more (60%) | NA | NA | Daylong monitoring of gait; Instrumented testing of gait in the OFF state |
| Moreno CM et al. 2015, Germany, database is not mentioned | PD and falls Hoehn & Yahr scale > 3 | 25 young PDs (12 fallers, 13 non‐fallers) | Falls |
S** C** O* |
Young PDs with an increased falling risk may benefit from leg‐extensors strengthening and stability training. | NA | NA | Focusing on leg‐extensors strengthening as well as on exercising the mechanisms; Developing appropriate exercise therapy |
| Gazibara T et al. 2016b, Serbia, the Department of Movement Disorders, Neurology Clinic, Clinical center of Serbia | PD and falls; walk independently for 10 m; statically stand for at least 90 s | 120 PD patients | Falls |
S** C** O*** |
Recurrent fallers (54.8%); outdoors falls in recurrent fallers (p = 0.017); slipping in single falls (36.8%); posture instability (33.0%); lower extremity weakness (p= 0.023) | NA |
Common: soft‐tissue contusion; few: radial fracture. |
Assess joint effect of potential falls factors; Enrolling in fall prevention programs; Guiding physical exercise is important |
| D'Cruz N et al. 2020, Belgium, the Movement Disorders Clinic of the University Hospital in Leuven | PD patients walk independently for at least 10 m | 60 PD patient without freezing of gait | Falls |
S** C** O** |
Conversion to FOG was predicted mainly by objective and clinical measures of motor dyscontrol | NA | NA | Focusing on motor dyscontrol apparent in repetitive gait and non‐gait tasks such as finger tapping, toe tapping and stepping in place; Screening FOG conversion risk |
| Ashburn A et al. 2008, UK, database is not mentioned | PD; independently mobile; Living at home; falls in 12 months | 142 PD patients | Falls |
S*** C** O*** |
Home falls (80%); other falls (12%); bedroom falls (30%); loss of balance (69%); setting off too quickly after standing (11%) | NA | 8 fallers fractures; 4 X‐ray; 3 need assistance. | Assisting individuals to deal with hazards cognitively and physically; Gait re‐education in PD must incorporate more than straight lines forward; Importance of cognitive; Falls diaries |
| Mark D et al. 2009, Australia, database is not mentioned |
PD; MMSE ≥ 24; independently walk; UK PD Brain Bank criteria |
130 PD patient | Falls |
S*** C** O** |
Falls at least once (45%); history of falls (P < 0.001); FOG (p= 0.004) | NA | Injurious falls (25%); 3 hip fractures, 1 radial fracture, and 1 tibial fracture | Focus on increased age, poor contrast sensitivity, slower cadence and TUG times, postural hypotension, bradykinesia, use of multiple medications, and PD‐specific factors. |
| Danielle PL et al. 2019, Brazil, the Movement Disorders Clinic in Fortaleza | PD patients; UK PD Brain Bank criteria | 218 PD patients | Falls |
S** C** O** |
Disease duration, modified HY stage, SE ADL score, LED, probable sarcopenia and positive SARC‐F (SARC‐F+) were associated with falls | NA | NA | Focus on sarcopenia in the older adults; Reduced quality of life; High‐protein diet and resistance exercise training. |
| Silvia DD Et Al. 2020, UK, V‐TIME Study | PD; walk for 5 m; on stable medication, self‐reported 2+ falls within 6 months | 282 fallers (109 older fallers, 19 MCI, 62 PD) | Falls |
S** C* O** |
PD falls 2 times for every 100k steps; FRA index more than other 2 groups (p= 0.043) | NA | NA | FRA index a preliminary but important step; V‐TIME intervention successfully reduced falls risks |
| Maria H et al. 2020, Sweden, Home and Health in People Ageing with PD (HHPD) | PD; mSADDE score; | 151 PD patients (mean = 68±8.8 y) | Falls |
S** C** O** |
Fall‐related activity avoidance (16% increased); concerns about falling (β = 0.589); | NA | NA | Activity avoidance can be a sound strategy in hazardous circumstances; Pain is a common symptom in PD adverse outcomes |
| Serene SP et al. 2014, Australia, private neurology clinics | PD ;age ≥ 40, MMSE ≥ 24; walk independently | 205 PD participants | Falls |
S*** C* O** |
Fall at least once (59%); freezing of gait (RR = 1.24); dyskinesia (RR = 1.14); stability (RR = 1.22); repeated sit to‐stand (RR = 1.19), fast walking speed (RR = 0.84); pull test (RR = 1.18) | NA | NA | Falls history probably represents a composite measure of individual risk factors; Impaired balance and cognition are important risk factors |
| Pattamon P et al. 2020, Thailand, Chulalongkorn Centre of Excellence for Parkinson's Disease | PD; H&Y stage 1–4; MMSE ≥ 21 | 305 PD patients | Having a history of at least one fall |
S** C* O** |
Faller (32%); recurrent fallers (19%); Model (sweep floor, reaching on tiptoes, walking in a crowded mall) |
NA | NA | Determining modifiable predictors of falling; Ranking high‐risk activities as the strongest predictors of falls/recurrent falls |
| Chayanin F et al. 2016, Japan, Chulalongkorn Centre of Excellence for Parkinson's Disease | PD; MMSE ≥ 24; | 184 PD patients and 52 normal people | Infrequent fallers; Frequent fallers |
S** C** O** |
HY stage higher (p < 0.001); ABC‐16 scores lower (p < 0.001); Walk on slippery sidewalks; Not holding rails on escalator; Bumped while walking in a crowd; Standing on chair to reach something | NA | NA | Activities that involved movement switching in the vertical orientation are significant predictors; Proper use of assisted devices |
| Tatjana G et al. 2017, Serbia, the Department of Movement Disorders, Neurology Clinic, Clinical Center of Serbia in Belgrade | PD; UK PD Brain Bank; MMSE ≥ 24; walk independently for 10 m and stand for 90 s | 120 PD patient | Falls and fear of falls |
S*** C** O** |
NA | FES scores higher (22.9%); Taking a bath or shower is the lowest level of confidence | NA | Installing hand rails, insert bathtub chairs and/or rubber mats; Enhancing confidence |
| Yaroslav W et al. 2010, Germany, Movement Disorders Outpatient Departments of University Hospitals 2003–2004 | PD; UK PD Brain Bank criteria | 100 PD patients | Health‐economic data |
S** C* O** |
Falling is related with total costs (p < 0.05) | NA | NA | Falls is additional factors increasing PD‐related costs; Evaluating the economic burden of PD |
| Taylor C et al. 2017, Canada, Ambulosono walking program | PD; step‐in‐place 5 m; Non‐dementia | 11 PD patients | H & Y; questionnaires |
S** C** O* |
Training effect (p= 0.047) | NA | NA | Easy and safe home‐based rehabilitation approach that may offer benefit to improving DT |
| Emma S et al. 2018, UK, Parkinson's UK (a UK charity) in Southampton | PD | 24 PD patients involved | Sensor data of trails |
S** C** O* |
Recalling repeated falls (21%); fall performances: cautious (19%), unstable (5%); Rise‐to‐Walk generated most near‐falls | NA | NA | Wearable sensors can detect subtle instability and might be a useful adjunct |
| Nader N et al. 2019, USA, database is not mentioned | PD | 18 participants with PD | Sensors of freezing of gaits. |
S** C* O** |
156 FOG in 18.4 min | NA | NA | Machine learning methods; Sensors detect FOG episodes, and the effects of cueing in ambient environments |
| Steve W P et al. 2016, UK, The North Tyneside Community Falls Prevention Service | PD; walk independently for 10 m | 35,288 PD participants with 60+ age. | Falls and fear of falls |
S*** C** O** |
NA | Fear of falls (n = 2,448) | NA | NTCFPS was highly effective in finding individuals with falls, syncope, and dizziness symptoms; Potential clinical and health economic effect |
| Tara M et al. 2015, New Zealand, New Zealand Brain Research Institute database | PD; NFOGQ (having FGO); MMSE ≥ 24 | 21 PD participants | Falls and fear of falls |
S** C** O*** |
Fall rate between 2 groups:1.22 (0.45‐3.26); adjusted 3.21(1.39‐9.38) | Worried about falling (17%); worried less about falling (39%); | NA | Capturing the difficulties experienced by patients in everyday life or their opinions on treatment acceptability and personal improvements |
| Daphne J et al. 2018, New Zealand, Leiden University Medical Center | UK PD Brain Bank clinical diagnostic criteria; age ≥ 18; stand unsupported for 20s+ | 30 PD patients and 30 controls | Tests of gait |
S** C** O** |
Walking speed, step length and stride length significant | NA | NA | It seems fair to conclude that the IWW is of added value in people with PD when assessing walking ability |
| Daniel SP et al. 2020, USA, database is not mentioned | PD; age ≥ 55; no other neurological disease and orthopedic injuries | 16 PD patients and 14 controls | Cognitive, neuromuscular, and protective stepping |
S** C** O** |
Muscle onset (p= 0.003), step length (p= 0.011), latency (P < 0.001), and step width (p= 0.001) | NA | NA | Part of population may prioritize cognition over gait, known as a “posture second” strategy; Single‐task protective stepping can be improved through practice in people with PD |
| Davide C et al. 2019, Italy, NEUROFALL group 2015–2016 | PD; walk 10 m independently | 113 patients evolved (32 PD) | Telephone contacts for falls information |
S** C** O** |
Fall in the past 6 months; number of falls in the education group were evenly distributed; higher number of falls in subjects with higher level of participation | NA | NA | Education program improved ability to carry out activities and decreased participation restrictions without a concomitant increase of number of falls |
| Colleen GC et al. 2015; Australia, Sydney and regional and rural New South Wales | PD; age ≥ 40; walk independently; stable medication intake for 2 w; fall at least once in 1 y | 231 PD patients | Falls |
S*** C** O** |
Exercise group compared control group (IRR = 0.73, p= 0.18); 69% reduction in falls in the exercise group; No significant interaction effect between fall history or physical function on rate of falls | NA | NA | Minimally supervised exercise programs aimed at reducing falls in people with PD should be implemented early in the disease process |
| Jennifer L et al. 2012, Australia, clinics and rehabilitation centers in Melbourne | PD; be able to walk; MMSE ≥ 24 | 210 participants | Mobility, activity limitations, and quality of life. |
S** C** O** |
Recurrent falls (64%); after intervention: 19 person falls 3–9 times,7 falls 10+ times | NA | Arthritis (44%); cancer or heart disease (23%) | Increased confidence might increase activity or risk taking and result in further falls; Strategy training and strength training can be safely implemented in a community‐based sample of people with idiopathic PD |
| Juliana M F et al. 2019, Brazil, database is not mentioned | PD; clock test > 4 | 9 PD older | After the booklets and games, creating the codes |
S* C* O* |
NA | NA | NA | care reduce the emotional, social and physical overload; Physiotherapy and physical activity can improve motor symptoms |
| Changhong Y et al. 2020, Korea, database is not mentioned | PD; H&Y stage 1–3; MMSE ≥ 24 | 23 participants | Test scores |
S** C** O** |
Step test (ES = 0.341); TUG test (ES = 0.299); AP (ES = 0.293); ML (ES = 0.299); step length (ES = 0.332), step velocity (ES = 0.301), and toe‐clearance height (ES = 0.285) | NA | NA | Exercise program may improve their overall movement; Progressive resistance exercise program |
| Tatjana G et al. 2015, Serbia, Department of Movement Disorders, Neurology Clinic | PD; able to walk; stand for 90 s | 42 PD patients | Indoor falls and outdoor falls |
S** C** O** |
Indoor falls (61%); outdoor falls (68%); falls dominantly occurred in daytime; outdoors (tripping, slipping) | NA | NA | Causes of falls involved both extrinsic and intrinsic factors; Using of cane; Emphasizing on balance recovery and negotiation of objects in environment |
| Laura A et al. 2020, USA, database is not mentioned | PD; age ≥ 18; sMMSE ≥ 4 | 18 PD patients | Adverse events; FPMQ; FES‐I |
S** C** O** |
Falls (n = 10); fall decreased (elimination of recurrent fallers); 6 home falls; 4 community falls; FPMQ & FPSS had interaction between assessment time and practice | NA | NA | Learning about body awareness in yoga may have been more mindful of fall prevention; Yoga decreased the number of recurrent fallers |
| Natalie E et al. 2015, Australia, database is not mentioned | PD; MMSE ≥ 24; age ≥ 40; walk independently | 115 PD participants | Adherence information of exercise; FES‐I; SF‐6D |
S** C** O** |
Adherence (72%); bodily pain (86%); less bodily pain (more SF‐6D) | NA | NA | Effective treatment of pain could therefore improve adherence to exercise |
| Tatjana G et al. 2016, Serbia, the Department of Movement Disorders, Neurology Clinic, Clinical Center | PD; walk for 10 m; stand for 90 s | 300 PD patients | H&Y scores; UPDRS; FES; SADS; NFOG; HAMD; HAMA |
S** C** O** |
Recurrent fallers (19.2%); single fallers (45.2%); FES, SADS, NFOG, HAMD, HAMA positively correlated. | NA | NA | Frequent falls report less fear of falling as compared with infrequent fallers; Worse motor performance at baseline were more likely to experience recurrent falls |
| Kim C S et al. 2019, UK, National Health Service hospitals and clinics in UK | PD; Residuals; UK Brain Bank criteria; MMSE ≥ 24 | 474 PD participants | Falls; fractures; near falling; CST; GDS; FES; NFIG |
S** C** O** |
PDSAFE Near falling compared to control group (OR = 0.67) ;better balance (p = 0.026); better CST (p= 0.041); repeat falls (p = 0.111); rate of falling (p = 0.088) | NA | NA | Personalizing plan; Individual motivation; Integrating their training into everyday functional tasks |
| Emma L S et al. 2013, UK, geriatrician's clinic in UK | PD | 255 PD questionnaires | Falls information (where, what, why, how, then) |
S* C** O** |
Single fallers(n = 19), recurrent falls(n = 86), very frequent fallers (n = 31); unfamiliar building (38%); during walking (52%); tripping (24%) | NA | Hurt (40%); immediate healthcare (16%) | Teaching them how to get up alone; Hypervigilance; Taking care to avoid trips; Sensitivity of fatigue |
FOF = fear of falls; FOG = freezing of gait; PD = Parkinson's disease.
Scale domains: S selection of study groups, C comparability, O outcome assessment.
3.2. Classification of falls
Falls are common symptoms of PD. Fall events were defined as “some unexpected events that caused the person to unintentionally land on any lower surface such as an object, flood, or ground.” (Canning et al., 2015; Del Din et al., 2020; Foongsathaporn et al., 2016; Gazibara et al., 2016; Hoskovcová et al., 2015; Lima et al., 2020; Maki et al., 1994; Martin et al., 2015; Moreno Catalá et al., 2015; Parry et al., 2016; Paul et al., 2014) Some studies classified falls according to their frequency of occurrence, as follows: Never, few, or less; every month or year; and disabled. (Grimbergen et al., 2013) Several studies distinguished falls based on whether they occurred indoors or outdoors (Gazibara et al., 2014, 2016a, 2016b; Paul et al., 2017). Falls were also classified based on whether they occurred in a forward or non‐forward direction (Youn et al., 2017). Despite the fact that studies classified falls using different methods, all aimed to reduce falls or assess risk factors for falls.
The factors that may cause the fall of PD patients are summarized in Figure 3. We broke these risk factors down into five components: Physical status, pre‐existing conditions, environment, medical care, and cognition. These five parts complement each other and generally do not constitute falls in PD patients by a single factor. PD is a neurodegenerative disease, and its disease mechanism is likely to lead to falls. Therefore, we pay more attention to factors other than PD that may affect falls. Next, we will explain each of these five aspects.
FIGURE 3.
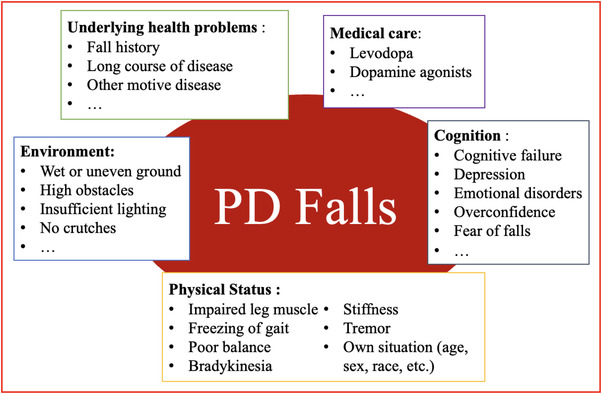
Schematic diagram of fall risk factors in patients with Parkinson's disease
3.2.1. Physical status
Conclusions in different studies were inconsistent. Several predicting factors concentrated on freezing of gait (FOG), postural instability, weakness of leg muscle, and cognitive failure (Allcock et al., 2009; Ashburn et al., 2001; Bloem et al., 2001; Duncan et al., 2012; Foreman et al., 2011; Kerr et al., 2010; Latt et al., 2009; Pickering et al., 2007; Robinson et al., 2005; Wood et al., 2002). The effect of prediction was not exactly the same such as in the FOG analysis (Kerr et al., 2010; Latt et al., 2009) or in the frequency of fall analysis (Mak & Pang, 2010; Thomas et al., 2010). In clinical practice, physiological indicators such as leg weakness, postural instability, and FOG may catch physicians’ eye. Compared with the past situations, these physiological indicators can be modifiable if conducting an effective intervention. Clinicians can assess the modifiable risk factors and calculate the absolute probability of falls in the future, seeking guidance for evaluating and intervening based on indicators of motion to improve PD patients' lives (Wallace & Johansen, 2018). However, due to insufficient sample size or potential defect of studies design, these indicators' effect cannot be accurately evaluated and measured. Possible factors, such as postural instability and impaired lower limb muscle function, improved the patient's quality of life, no matter how much they improve future falls (Gazibara et al., 2014, 2016a, 2016b). Of particular concern were impaired leg muscle (Kerr et al., 2010; Latt et al., 2009), FOG (Camicioli & Majumdar, 2010; Latt et al., 2009; Paul et al., 2014), poor balance (Latt et al., 2009; Paul et al., 2014), action inconvenience (Balash et al., 2005; Cole et al., 2010; Dibble et al., 2008; Foreman et al., 2011; Kerr et al., 2010; Koller et al., 1989; Latt et al., 2009; Latt et al., 2009; Lim et al., 2008; Mak & Pang, 2010; Mak & Pang, 2009; Matinolli et al., 2011; Plotnik et al., 2011; Robinson et al., 2005; Schaafsma et al., 2003; Smulders et al., 2012). In addition, some studies have shown that the fixed attributes of people, such as age, gender, and race, have nothing to do with falls (Allcock et al., 2009; Ashburn et al., 2001; Balash et al., 2005; Latt et al., 2009; Matinolli et al., 2007; Parashos et al., 2013; Paul et al., 2013; Pickering et al., 2007), but these are all studies with small samples, and a larger sample size is needed to prove the true internal connection.
3.2.2. Pre‐existing conditions
A pre‐existing poor condition is known as an underlying health problem. Age can be seen as a variable that describes health status, so old age can also be seen as a potential underlying disease. Some risk factors, include seriously injured (Allcock et al., 2009; Ashburn et al., 2001; Balash et al., 2005; Bloem et al., 2001; Camicioli & Majumdar, 2010; Contreras & Grandas, 2012; Latt et al., 2009; Lim et al., 2008; Matinolli et al., 2007; Parashos et al., 2013; Paul et al., 2013; Pickering et al., 2007), a longer course of underlying disease (Contreras & Grandas, 2012; Lim et al., 2008; Matinolli et al., 2007; Parashos et al., 2013; Paul et al., 2013; Wood et al., 2002), and, history of fall (Allcock et al., 2009; Ashburn et al., 2001; Bloem et al., 2001; Camicioli & Majumdar, 2010; Latt et al., 2009; Matinolli et al., 2011; Paul et al., 2013; Pickering et al., 2007; Wood et al., 2002), and they have been verified to be associated with falls in PD patients. For example, some researchers believe that the history of falls is a good predictor of future falls in PD patients in terms of underlying health problems (Pickering et al., 2007). Although factors related to underlying disease (including fixed factors such as age and sex) cannot be changed in the short term, they may be used to identify high‐risk groups requiring immediate preventive intervention. Further, treatment of underlying diseases may be prioritized, if appropriate.
3.2.3. Environment factors
The external physical environment is an important reason for PD patients to fall, such as wet or uneven ground, high obstacles, and insufficient lighting, causing majority of the tripping or slipping (Grimbergen et al., 2004; Hely et al., 2005; Olanow et al., 2009). An observational study of fall conditions included 300 PD participants. Of the 180 people who reported falling, the conditions associated with falling included the following characteristics: Outdoors, early morning, daytime, tripping, slipping, and unsteady posture (Gazibara et al., 2014). Preventive actions that can reduce the likelihood of a fall in a complex environment include using crutches, elevating feet higher when crossing obstacles, or using armrests or pads (Gazibara et al., 2017). These external environmental factors are noteworthy in the management of PD patients, and they are also one of the factors that can most reduce the occurrence of falls.
3.2.4. Medical care
In terms of medications, long‐term and high‐dose levodopa use reduced complications associated with falls (Hauser et al., 2007; Holloway et al., 2004; Parkinson Study Group, 2000; Parkinson Study Group CALM Cohort Investigators, 2009; Rascol et al., 2000). Continuous carbidopa, levodopa enteral suspension, or continuous subcutaneous apomorphine injection reduced pain levels due to complications in PD (Antonini & Nitu, 2018; Katzenschlager et al., 2018; Olanow et al., 2014). In a previously published randomized controlled trial (RCT), effects of levodopa, a dopamine agonist, and a MAO‐B inhibitor were compared, revealing that levodopa had a better activity score than the other therapeutics considered (Gray et al., 2014).
3.2.5. Cognition function
Neurodegenerative symptoms of PD can lead to falling (Chaudhuri & Schapira, 2009; Chaudhuri et al., 2015, 2016). Cognitive function is particularly important in affecting falls, and good cognition can play a role in preventing falls (Chaudhuri et al., 2013). There are some factors that can be prevented and managed such as depression (Aarsland et al., 2017; Ashburn et al., 2001; Balash et al., 2005; Contreras & Grandas, 2012; Matinolli et al., 2007; Robinson et al., 2005; Wood et al., 2002), cognitive impairment (Camicioli & Majumdar, 2010; Chaudhuri et al., 2006; Ffytche et al., 2017; Latt et al., 2009; Paul et al., 2014), overconfidence (Davidsdottir et al., 2008; Gullett et al., 2013; Halperin et al., 2020; Halperin et al., 2020; Lin et al., 2014; Yakubovich et al., 2020), and FOF (Ashburn et al., 2001; Cole et al., 2010; Contreras & Grandas, 2012; Lim et al., 2008; Mak & Pang, 2009; Mak & Pang, 2009; Matinolli et al., 2007; Rahman et al., 2011; Robinson et al., 2005). These factors are grouped under cognitive classification in Figure 3. Of the most important are the effects of overconfidence and fear of falling on falls. People with PD show visual dependence because visual‐motor cues are disordered when they combine with the brain's vestibule (Bertolini et al., 2015; Halperin et al., 2020; Yakubovich et al., 2020). Perceptual overconfidence is evident, not only in vision but also in other senses such as smell (Almeida & Lebold, 2010; Azulay et al., 1999; Azulay et al., 2002; Bronstein et al., 1990; Cooke et al., 1978; Cowie et al., 2010). Visual overconfidence is associated with gait and balance and can predict the risk of falls (Curtze et al., 2016; Mak & Pang, 2009). A controlled study of 20 PD patients and 21 healthy people found that both groups had a high level of confidence in the correct target, but PD patients were more confident for the wrong reasons compared to normal people (Halperin et al., 2020).
Most falls can have minor or severe consequences, including physical and psychological injuries. One of the most important psychological harms, as opposed to overconfidence, is FOF, which is also a cognitive disorder (Adkin et al., 2003; Rahman et al., 2011). FOF has been defined as “a continuous concern about falling that contributed to individual refraining from activities” (Tinetti & Powell, 1993). The etiology and clinical symptoms of FOF show a big difference and it needs joint methods to measure. Some experts used questionnaires to estimate FOF such as the question “In general, are you afraid of falling over?” (Yardley & Smith, 2002). FOF was used to be called “basiphobia,” which is a specific type of phobia, and it manifests itself as a severe fear of standing or walking (Bhala et al., 1982; Gai et al., 2009). Although falling has been illustrated to cause FOF, FOF can also cause falls in reverse. FOF may induce falls through the change of gaits or restriction of movement (Grimbergen et al., 2013; Mak & Pang, 2009; Peto et al., 1995). Reduced activity may also lead to a reduction in the number of falls, but not the probability of falls. This suggests that FOF may indicate a decline in function due to reduced activity, leading to an increased risk of falling (Mak & Pang, 2010).
A study of the elderly in the community suggested that FOF was a dynamic process, in which the fearful stage alternated with the non‐fearful stage (Oh‐Park et al., 2011). Parkinson's disease, as a neurodegenerative disease, presents a more complex FOF than the common elderly person. Compared with healthy elderly individuals, PD patients show a higher tendency for emotional disorders (Hadjistavropoulos et al., 2011; Trollor et al., 2006), and about one‐third of PD patients suffered from anxiety disorders (Broen et al., 2016). FOF was an essential factor affecting the quality of life of PD patients (Brozova et al., 2009; Grimbergen et al., 2013). Several studies have shown that the degree of fear in recurrent fallers and frequent fallers was higher than in single fallers and low‐frequency fallers (Mak & Pang, 2010; Rahman et al., 2011; Thomas et al., 2010). A more individualized treatment approach in PD patients with FOF will bring more healthy and economic benefits to patients (Winter et al., 2010).
3.3. Clinical practices of falls and FOF in PD patients
3.3.1. Traditional measurement scales and fall prevention model
Some traditional scales are used to predict falls and FOF, mainly including the following three scales: Consequences of falling (COF) (Yardley & Smith, 2002), falls efficacy scale (FES) (Tinetti et al., 1990), and survey of activities and fear of falling in the elderly (SAFFE) (Lachman et al., 1998; Yardley & Smith, 2002). COF rated 12 questions that described falling, with higher scores indicating greater fear of falling. FES is a self‐efficacy rating of 10 activities of daily living without falling, with higher scores indicating less confidence or a high fear of falling. And Saffe is an improved survey of activity and fear of falling in the elderly, where higher scores are associated with greater avoidance of activity. Besides, Beck Depression Inventory (BDI) (Beck et al., 1961), Beck Anxiety Inventory (BAI) (Beck et al., 1988), and assessment of quality of life scale (QOLS) (Peto et al., 1995) are measurements of mood, and can be auxiliary diagnosis methods of FOF. The scales mentioned earlier are given in Table 2.
TABLE 2.
The detail information for 5 scales of fear of falls
| Name of scales | Description | Number of questions | Score range | Scaling of score |
|---|---|---|---|---|
| Consequences of Falling (CoF) | Evaluating falls and how concerned you are about falls | 12 | 12–48 | Higher = Greater concern about falls |
| Falls Efficacy Scale (FES) | Assessing non‐hazardous activities of daily life with non‐falls | 10 | 10–100 | Higher = High fear of falls |
| Survey of Activities and Fear of Falling in the Elderly (SAFFE) | Assessing elderly people about fear of falls in daily activities | 17 | 0–34 | Higher = Higher avoidance of activities |
| Beck Depression Inventory (BDI) | Assessing depression levels | 21 | 0–63 | Higher = Greatest depression |
| Beck Anxiety Inventory (BAI) | Assessing anxiety levels | 21 | 0–63 | Higher = Greatest anxiety |
| Quality of life scale (QOLS) | Assessing quality of life | 15 | 15–105 | Higher = Greater QoL |
Recently, a prospective study developed a new scale, which is an instrument to evaluate FOF in PD (Terroba‐Chambi et al., 2019). A 2‐stage and 1‐year follow‐up design validated the new scale as a self‐assessed tool for PD patients. The new scale named “Fear of Falls Scale” (FFS) contained 24 questions about daily life and clinical experience (10 questions used to evaluate the severity of motor balance ability, 10 questions used to evaluate FOF of the severity in the motor task, and 4 open questions used to supply physical activity information). It illustrated that FFS, which has simple and short duration characters, is a useful instrument to assess FOF in clinics.
We used The Johns Hopkins Fall Risk Assessment Tool (JHFRAT) to create the fall prevention model (details in Figure 4) to evaluate patients to determine what kind of management and treatment they receive. JHFRAT is a scale developed by Johns Hopkins University in 2003 (Poe et al., 2005). It scores patients on a scale of 0–28 based on age, history of falls, bowel movements, medication, equipment use, mobility, and cognition. Based on their scores, the JHFRAT classified patients into low‐ (0‐5 points), moderate‐ (6‐13 points), and high‐risk (>13 points) groups. JHFRAT has been shown to be a good predictor of fall risk in many studies and has been recognized by scholars worldwide (Ariza‐Zafra et al., 2020; Hur et al., 2017; Klinkenberg & Potter, 2017; Luo et al., 2020; Poe et al., 2018). We applied the risk group score to tertiary prevention in public health. In primary prevention, for a patient with a score of 0–5, we recommend a combination of medication (if necessary) and physical activity to prevent falls. In secondary prevention, we recommend that patients adhere to medication in addition to their primary prevention‐management strategy in order to improve exercise status and mental health and reduce the risk of falls and FOF. In tertiary prevention, due to the severity of the patient's condition and many complications, relevant surgical treatment should be carried out as soon as possible if the patient meets the surgical indications.
FIGURE 4.
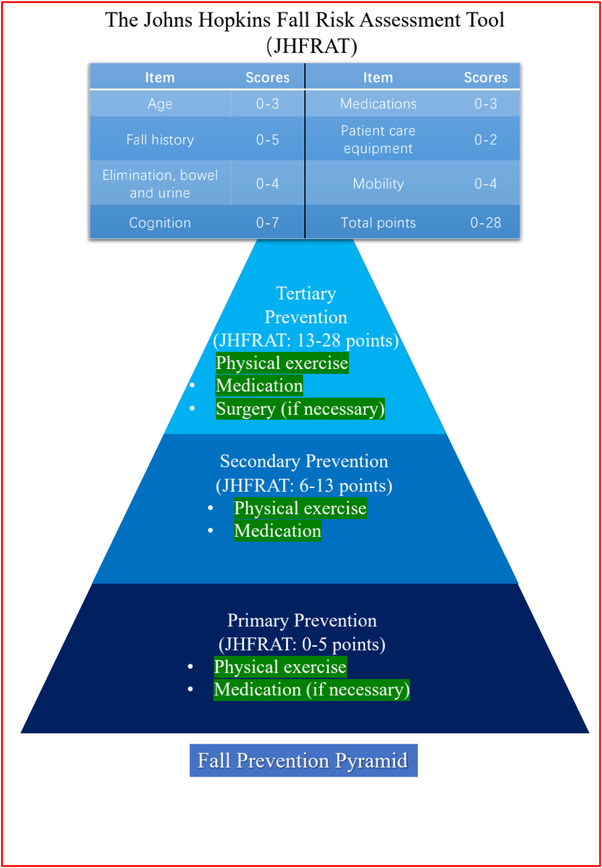
Detailed rules of The Johns Hopkins Fall Risk Assessment Tool scores and Falls Prevention Pyramid
3.3.2. Drug therapy and surgery methods
Drug therapy is the most common clinical treatment for patients with PD. Clinically, physicians are more likely to observe complications related to motion. Complications related to motion, such as bradykinesia, stiffness, and tremor (Postuma et al., 2015), can be treated with medication and can improve the patient's motor function and prevent falls. Dyskinesia, represented by a decrease in the velocity and amplitude of repeated movements, and stiffness, represented by an increase in auxiliary joint resistance, can be treated with levodopa (Chou et al., 2018; Hauser et al., 2000; Jankovic, 2005). Several randomized controlled trials have shown that levodopa or dopamine agonists are effective in treating motor symptoms in patients with early PD (Hauser et al., 2007; Holloway et al., 2004; Parkinson Study Group, 2000; Parkinson Study Group CALM Cohort Investigators, 2009; Rascol et al., 2000). Since the pathological feature of PD patients is insufficient secretion of dopamine, some studies have confirmed that continuous infusion of dopamine can reduce motor complications in advanced PD patients (Antonini & Nitu, 2018; Katzenschlager et al., 2018; Olanow et al., 2014). DAs currently in use include ropinirole and pramipexole, and many RCTs have been evaluated (Lieberman et al., 1998; Lieberman et al., 1997; Möller et al., 2005; Pahwa et al., 2007; Rascol et al., 1996; Schapira et al., 2011). In terms of clinical management, early PD drugs are recommended for treatment daily with 25–100 mg treatment of carbidopa‐levodopa immediate release to relieve motor symptoms and prevent falls (Freitas et al., 2016; Grosset, 2009, 2010). If the patient fluctuates during activity, the frequency of dosing may need to be increased. Adjuvant drugs can be MAO‐B inhibitors, mixed selective MAO‐B inhibitors, or ion channel inhibitors (Aradi & Hauser, 2020).
At present, there is no satisfactory drug therapy program for the clinical treatment of exercise complications. There are many individual differences in these strategies for alleviating PD symptoms. There are surgical methods of deep brain stimulation targeting at globus pallidus and subthalamic nucleus (Follett et al., 2010; Odekerken et al., 2013). Relevant RCTs show that deep brain stimulation of the subthalamic nucleus can improve motor symptoms and prevent falls (Lhommée et al., 2018). But on the other hand, subthalamic nucleus deep brain stimulation (STN‐DBS) surgery does not have an active effect on non‐motor symptoms (Amami et al., 2015; Gratwicke et al., 2018). A cohort study showed that 6 years after STN‐DBS surgery, dopamine addiction and impulse control disorders decreased, but non‐operative mental fluctuations decreased, and apathy increased (Abbes et al., 2018). In other words, surgical treatment can only improve patients' movement status and improve their physical quality to reduce falls. Moreover, these studies have not assessed the effect of mental state on falls, making it difficult to rule out a confounding effect on falls. While there has been some progress with surgery, there are still many unsolved problems in the prevention of falls, and the side effects of drugs are huge.
3.3.3. Physical exercise
Although levodopa has shown promising results in the treatment of PD patients, there are serious limitations to long‐term levodopa therapy. In addition to traditional medical therapy, exercise or physical therapy is more helpful to improve the patient's motion and non‐motion status, and has benefits in increasing confidence, preventing falls, and improving quality of life (Cruise et al., 2011; Mak et al., 2017; Reynolds et al., 2016). Exercise is defined as any physical activity resulting from the expenditure of energy to contract skeletal muscles. In a large cohort, moderate‐to‐heavy exercise participants were found to have a lower risk of PD (LaHue et al., 2016), with even about two‐thirds of risk decreasing in men (Corcos et al., 2012; Mak et al., 2017). Some studies have found that physical exercise can slow down the onset and progression of PD (Cheng et al., 2016; Mak et al., 2017). Several large RCTs also found that exercise can improve symptoms of cognitive decline and bradykinesia and can effectively prevent the occurrence of falls (Corcos et al., 2012; Mak et al., 2017). Some studies can also prove that exercise intervention can alleviate the non‐motor symptoms of PD, which can relieve the mental stress of PD patients, enhance their self‐confidence, and reduce FOF (Aarsland et al., 2005; Cruise et al., 2011). Drugs do not perform well in improving the mood of PD patients, and some drugs may produce many side effects in the treatment of PD patients (Reynolds et al., 2016). This highlights the huge two‐sided benefits of exercise compared to drugs (Reynolds et al., 2016; Sacheli et al., 2018). Progressive exercise intervention for patients can restore partly behavioral ability in the physical function and prevent falls. An RCT involving 130 PD patients who were assigned treadmill‐based training therapy and physical training via biosensors showed a 55% reduction in falls within 6 months (Mirelman et al., 2016). Another study on treadmills showed that treadmills improved baroreflex sensitivity, significantly improved blood pressure, and reduced some hypotensive falls (Ganesan et al., 2014). A large study reported that low‐intensity treadmill exercise in PD patients can be as effective as medication, suggesting that aerobic exercise may improve cardiopulmonary function (Schenkman et al., 2018). Not only that, but other forms of exercise, such as Taijiquan, also have the effects of improving the movement status of patients (Song et al., 2017).
4. DISCUSSION
To our knowledge, this is the first review of clinical interventions and management of falls and FOF and the first presentation of a novel fall prevention theory. We focus on summarizing the existing association between falls and FOF in PD patients and attempt to summarize clinically feasible prevention and management schemes. The etiology of PD is complex, and the mechanism of this degenerative disease still needs to be studied, but this does not conflict with using existing research findings to find ways to prevent falls and the fear of falling. Because fall is a kind of subjectively unwilling but physically irresistible behavior, and the duration of falls is short, the complications of falls are extremely harmful, so it is particularly important to find a way to prevent falls. FOF is likely to occur in PD patients with or without previous falls. On the one hand, this may be the result of the neurodegenerative lesion, and on the other hand, it may be the side effect caused by PD patients' subjective perception of a different gait from the previous gait (Maki et al., 1994). FOF may lead to falls through gait changes or active restriction. If left uncontrolled, falls and FOF will interact with each other, leading to a vicious circle, and patient's quality of life will suffer unprecedented impacts. We summarized the predictive methods and influencing factors of fall and FOF, as well as the relevant methods of clinical prevention and management, which all consistently indicate that the control and management of PD are urgent.
According to our conclusion, the fall prevention model regards physical exercise as a necessary part of each stage, not only because of its good efficacy and its strong effect on improving patients' physical fitness and reducing falls, but also because it is a controllable and individualized treatment method. In primary prevention, we recommend that moderate‐intensity exercise and rehabilitation activities be used as the primary means of preventing falls and FOF, and, if necessary, take medications such as levodopa as recommended by the clinician. With the increase of JHFRAT score, we recommend that the symptoms of PD should be treated primarily, and that medication and low‐intensity exercise should be used to assist in preventing falls. Exercises are mainly low‐risk and low‐intensity exercises such as walking and tai chi. In tertiary prevention, exercise is listed as a non‐essential management item. Exercise as much as possible under the premise that patients can accept, do not cause injury and have protection, and stimulate motor nerve and cell metabolism can also reduce the risk of falling due to muscle atrophy to a certain extent (Allcock et al., 2009; Ashburn et al., 2001; Bloem et al., 2001; Duncan et al., 2012; Foreman et al., 2011; Kerr et al., 2010; Robinson et al., 2005; Wood et al., 2002).
We also looked at a state of near‐fall that could be reduced by physical exercise. A near‐fall is a transient state before a fall, which leads to two outcomes, one that leads directly to the fall, and the other that occurs shortly before the fall called “a fall initiated but arrested by support from a wall, railing, other person and so on” (Gray & Hildebrand, 2000). Near‐falls are common in people with PD. Someone will often fall if he or she loses balance and have nothing to cling to (Maidan et al., 2014). In a prospective cohort study of 120 PD patients, the association between near‐falls and falls was explored by questionnaire collection and exercise scale scores. FES was also used to assess the extent of FOF (Gazibara et al., 2017). The results showed that there was a significant association between non‐falls and falls or near‐falls in the scale scores, but no statistical association between falls and near‐falls was found. This suggests that near‐falls should be listed as a complication of PD along with falls. Medical costs for fall‐related consequences also increase as falls have more serious consequences (Pressley et al., 2003). It illustrated that taking some physical measures to reduce near‐falls or prevent falls in the state of near‐falls will bring great benefits (Gazibara et al., 2017). A multicenter randomized controlled trial also reported the risk of falls and near‐falls. After excluding the nonconforming population, 474 PD patients were randomly assigned to the experimental group for exercise and strategic intervention (PDSAFE), and for a period of 3 months for random monitoring of falls and economic evaluation (Ashburn et al., 2019). PDSAFE is a home‐based training program under the guidance of physiotherapists that includes postural control training, gait freeze reduction training, and learning feedback (Hulbert et al., 2019). This study found that the intervention significantly reduced the severity of falls and near‐falls, and the decline increased in PD patients with cognitive impairment.
The collection of fall information plays an important role in the prevention of falls. The use of retrospective self‐report may cause recall bias and may underestimate the frequency of falls (Hauer et al., 2006; Lamb et al., 2005). It is very important during intervention and management of fall prevention to correctly identify people at high risk of falls (Allen et al., 2013). A cohort study used diary data to explore its feasibility, and found that despite the high rate of loss of follow‐up, the characteristics of the people lost to follow‐up were similar to the baseline characteristics of the people who kept a diary, and the diary data was also feasible (Hunter et al., 2018). In addition, postural monitoring of patients with Parkinson's disease is crucial for the prediction and prevention of falls. With the continuous development of science and technology, more and more high‐tech equipment is being applied to the field of disease monitoring and prevention. Wearable devices, such as watches and wristbands, can already correctly identify the types of activities that occur in everyday life (Pärkkä et al., 2006). Different from traditional data collection methods, sensor devices can capture the existence of micro‐data and can use machine learning to develop relevant algorithms, which can not only understand the subtle changes in patients' falls but also objectively monitor PD symptoms and daily changes in a remote place and home (Arora et al., 2015; Lakshminarayana et al., 2014; Weiss et al., 2010). There is already a large study using wearable sensors to collect data on falls in Parkinson's patients (Silva de Lima et al., 2016). If PD patients can be screened out early and primary prevention can be carried out in time for people with suspected PD, the number of PD patients can be fundamentally reduced. For example, a large recent case‐control study with 274 participants used liquid chromatography‐mass spectrometry (LCMS) to separate and detect the presence of lipids and small molecules in the cortex of patients with PD to identify serum biomarkers of PD (Sinclair et al., 2021). The results showed that ceramides, triacylglycerol, and fatty acyl classes in PD patients decreased, while glycosphingolipid and fatty acyl metabolites increased, which is helpful for the development of skin test paper for PD patients, and has an important role in the field of public health.
Finally, the causes of falls and FOF in PD patients are complex, and epidemiological studies should be combined with basic studies to avoid various confusion caused by inaccurate data. The quaility of life of PD patients can only be improved with the participation of the whole society. Our study did not (and it was difficult to) quantify the effectiveness of prevention and management strategies in reducing falls and fear of falls, and the included literature lacked homogeneity in inclusion and exclusion criteria. We hope that there will be more research on the prevention and treatment of FOF in PD patients when they fall in the future. Although there is no data analysis in this review to support the accuracy and feasibility of the fall prevention model, the fall prevention model is established based on the existing studies and the latest theoretical basis, which can still be used as a reference measure for the prevention and management of falls in clinical practice and FOF in PD patients
5. CONCLUSION
Falls and FOF in PD patients can be reduced by effective clinical prevention and management. Although PD patients have a high rate of falls, FOF is common. More research is needed to explore the treatment and prevention of falls and FOF. The key is to identify these PD patients who are at high risk for falls or FOF, identify and reduce the occurrence of falls and FOF through monitoring, medication, physical exercise, and other means, in order to detect and reduce the occurrence of motor and non‐motor complications. The fall prevention model established by us expands the treatment methods of clinicians for PD patients and adopts a comprehensive prevention approach to reduce the incidence of falls and FOF. PD patients would benefit from this integrated prevention and management approach.
CONFLICT OF INTEREST
The authors declare no potential conflicts of interest.
AUTHOR CONTRIBUTIONS
Wen‐Yi Liu, Tao‐Hsin Tung, Chencheng Zhang, and Leiyu Shi conducted the study and drafted the manuscript. Wen‐Yi Liu and Tao‐Hsin Tung participated in the design of the study and performed data synthesis. Chencheng Zhang and Leiyu Shi conceived the study and participated in its design and coordination. All of the authors read and approved the final manuscript.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.2690.
Liu, W.‐Y. , Tung, T.‐H. , Zhang, C. , & Shi, L. (2022). Systematic review for the prevention and management of falls and fear of falling in patients with Parkinson's disease. Brain and Behavior, 12, e2690. 10.1002/brb3.2690
Contributor Information
Wen‐Yi Liu, Email: 66886908@qq.com.
Leiyu Shi, Email: lshi2@jhu.edu.
DATA AVAILABILITY STATEMENT
All data underlying the findings are within the paper.
REFERENCES
- Aarsland, D. (2017). Cognitive decline in Parkinson disease. Nature reviews Neurology, 13(4), 217–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aarsland, D. , Zaccai, J. , & Brayne, C. (2005). A systematic review of prevalence studies of dementia in Parkinson's disease. Movement Disorders, 20(10), 1255–1263. [DOI] [PubMed] [Google Scholar]
- Abbes, M. (2018). Subthalamic stimulation and neuropsychiatric symptoms in Parkinson's disease: Results from a long‐term follow‐up cohort study. Journal of Neurology, Neurosurgery, and Psychiatry, 89(8), 836–843. [DOI] [PubMed] [Google Scholar]
- Adkin, A. L. , Frank, J. S. , & Jog, M. S. (2003). Fear of falling and postural control in Parkinson's disease. Movement Disorders, 18(5), 496–502. [DOI] [PubMed] [Google Scholar]
- Allcock, L. M. (2009). Impaired attention predicts falling in Parkinson's disease. Parkinsonism & Related Disorders, 15(2), 110–115. [DOI] [PubMed] [Google Scholar]
- Allen, N. E. , Schwarzel, A. K. , & Canning, C. G. (2013). Recurrent falls in Parkinson's disease: A systematic review. Parkinson's Disease, 2013, 906274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida, Q. J. , & Lebold, C. A. (2010). Freezing of gait in Parkinson's disease: A perceptual cause for a motor impairment? Journal of Neurology, Neurosurgery, and Psychiatry, 81(5), 513–518. [DOI] [PubMed] [Google Scholar]
- Amami, P. (2015). Impulse control behaviours in patients with Parkinson's disease after subthalamic deep brain stimulation: De novo cases and 3‐year follow‐up. Journal of Neurology, Neurosurgery, and Psychiatry, 86(5), 562–564. [DOI] [PubMed] [Google Scholar]
- Antonini, A. , & Nitu, B. (2018). Apomorphine and levodopa infusion for motor fluctuations and dyskinesia in advanced Parkinson disease. Journal of Neural Transmission (Vienna), 125(8), 1131–1135. [DOI] [PubMed] [Google Scholar]
- Aradi, S. D. , & Hauser, R. A. (2020). Medical management and prevention of motor complications in Parkinson's disease. Neurotherapeutics, 17(4), 1339–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariza‐Zafra, F. J. (2020). Cross‐cultural adaptation and validation of the Spanish version of the Johns Hopkins Fall Risk Assessment Tool. Disability and Rehabilitation, 1–8. [DOI] [PubMed] [Google Scholar]
- Arora, S. (2015). Detecting and monitoring the symptoms of Parkinson's disease using smartphones: A pilot study. Parkinsonism & Related Disorders, 21(6), 650–653. [DOI] [PubMed] [Google Scholar]
- Ashburn, A. (2001). A community‐dwelling sample of people with Parkinson's disease: Characteristics of fallers and non‐fallers. Age and Ageing, 30(1), 47–52. [DOI] [PubMed] [Google Scholar]
- Ashburn, A. (2001). Predicting fallers in a community‐based sample of people with Parkinson's disease. Gerontology, 47(5), 277–281. [DOI] [PubMed] [Google Scholar]
- Ashburn, A. (2019). Exercise‐ and strategy‐based physiotherapy‐delivered intervention for preventing repeat falls in people with Parkinson's: The PDSAFE RCT. Health Technology Assessment (Winchester, England), 23(36), 1–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azulay, J. P. (1999). Visual control of locomotion in Parkinson's disease. Brain, 122(Pt 1), 111–120. [DOI] [PubMed] [Google Scholar]
- Azulay, J. P. (2002). Increased visual dependence in Parkinson's disease. Perceptual and Motor Skills, 95(3 Pt 2), 1106–1114. [DOI] [PubMed] [Google Scholar]
- Balash, Y. (2005). Falls in outpatients with Parkinson's disease: Frequency, impact and identifying factors. Journal of Neurology, 252(11), 1310–1315. [DOI] [PubMed] [Google Scholar]
- Beck, A. T. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4, 561–571. [DOI] [PubMed] [Google Scholar]
- Beck, A. T. (1988). An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology, 56(6), 893–897. [DOI] [PubMed] [Google Scholar]
- Bertolini, G. (2015). Impaired tilt perception in Parkinson's disease: A central vestibular integration failure. PLoS One, 10(4), e0124253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhala, R. P. , O'Donnell, J. , & Thoppil, E. (1982). Ptophobia: Phobic fear of falling and its clinical management. Physical Therapy, 62(2), 187–190. [DOI] [PubMed] [Google Scholar]
- Bloem, B. R. (2001). Prospective assessment of falls in Parkinson's disease. Journal of Neurology, 248(11), 950–958. [DOI] [PubMed] [Google Scholar]
- Broen, M. P. (2016). Prevalence of anxiety in Parkinson's disease: A systematic review and meta‐analysis. Movement Disorders, 31(8), 1125–1133. [DOI] [PubMed] [Google Scholar]
- Bronstein, A. M. (1990). Visual control of balance in cerebellar and parkinsonian syndromes. Brain, 113(Pt 3), 767–779. [DOI] [PubMed] [Google Scholar]
- Brozova, H. (2009). Fear of falling has greater influence than other aspects of gait disorders on quality of life in patients with Parkinson's disease. Neuro Endocrinology Letters, 30(4), 453–457. [PubMed] [Google Scholar]
- Camicioli, R. , & Majumdar, S. R. (2010). Relationship between mild cognitive impairment and falls in older people with and without Parkinson's disease: 1‐Year prospective cohort study. Gait & Posture, 32(1), 87–91. [DOI] [PubMed] [Google Scholar]
- Canning, C. G. (2015). Exercise for falls prevention in Parkinson disease: A randomized controlled trial. Neurology, 84(3), 304–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri, K. R. (2011). Parkinson's disease: The non‐motor issues. Parkinsonism & Related Disorders, 17(10), 717–723. [DOI] [PubMed] [Google Scholar]
- Chaudhuri, K. R. (2015). The burden of non‐motor symptoms in Parkinson's disease using a self‐completed non‐motor questionnaire: A simple grading system. Parkinsonism & Related Disorders, 21(3), 287–291. [DOI] [PubMed] [Google Scholar]
- Chaudhuri, K. R. , Healy, D. G. , & Schapira, A. H. (2006). Non‐motor symptoms of Parkinson's disease: Diagnosis and management. Lancet Neurology, 5(3), 235–245. [DOI] [PubMed] [Google Scholar]
- Chaudhuri, K. R. , Rizos, A. , & Sethi, K. D. (2013). Motor and nonmotor complications in Parkinson's disease: An argument for continuous drug delivery? Journal of Neural Transmission, 120(9), 1305–1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhuri, K. R. , & Sauerbier, A. (2016). Unravelling the nonmotor mysteries of Parkinson disease. Nature Reviews Neurology, 12(1), 10–11. [DOI] [PubMed] [Google Scholar]
- Chaudhuri, K. R. , & Schapira, A. H. (2009). Non‐motor symptoms of Parkinson's disease: Dopaminergic pathophysiology and treatment. Lancet Neurology, 8(5), 464–474. [DOI] [PubMed] [Google Scholar]
- Cheng, F. Y. (2016). Positive effects of specific exercise and novel turning‐based treadmill training on turning performance in individuals with Parkinson's disease: A randomized controlled trial. Science Reports, 6, 33242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou, K. L. (2018). The spectrum of “off” in Parkinson's disease: What have we learned over 40 years? Parkinsonism & Related Disorders, 51, 9–16. [DOI] [PubMed] [Google Scholar]
- Cole, M. H. (2010). Falls in Parkinson's disease: Kinematic evidence for impaired head and trunk control. Movement Disorders, 25(14), 2369–2378. [DOI] [PubMed] [Google Scholar]
- Contreras, A. , & Grandas, F. (2012). Risk of falls in Parkinson's disease: A cross‐sectional study of 160 patients. Parkinson's Disease, 2012, 362572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke, J. D. , Brown, J. D. , & Brooks, V. B. (1978). Increased dependence on visual information for movement control in patients with Parkinson's disease. Canadian Journal of Neurological Sciences, 5(4), 413–415. [DOI] [PubMed] [Google Scholar]
- Corcos, D. M. , Comella, C. L. , & Goetz, C. G. (2012). Tai chi for patients with Parkinson's disease. New England Journal of Medicine, 366(18), 1737–1738. [DOI] [PubMed] [Google Scholar]
- Cowie, D. (2010). Insights into the neural control of locomotion from walking through doorways in Parkinson's disease. Neuropsychologia, 48(9), 2750–2757. [DOI] [PubMed] [Google Scholar]
- Cruise, K. E. (2011). Exercise and Parkinson's: Benefits for cognition and quality of life. Acta Neurologica Scandinavica, 123(1), 13–19. [DOI] [PubMed] [Google Scholar]
- Curtze, C. (2016). Objective gait and balance impairments relate to balance confidence and perceived mobility in people with Parkinson disease. Physical Therapy, 96(11), 1734–1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidsdottir, S. (2008). Impact of optic flow perception and egocentric coordinates on veering in Parkinson's disease. Brain, 131(Pt 11), 2882–2893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Din, S. (2020). Falls risk in relation to activity exposure in high‐risk older adults. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 75(6), 1198–1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibble, L. E. (2008). Diagnosis of fall risk in Parkinson disease: An analysis of individual and collective clinical balance test interpretation. Physical Therapy, 88(3), 323–332. [DOI] [PubMed] [Google Scholar]
- Dorsey, E. R. , & Bloem, B. R. (2018). The Parkinson pandemic—A call to action. JAMA Neurology, 75(1), 9–10. [DOI] [PubMed] [Google Scholar]
- Duncan, R. P. (2012). Accuracy of fall prediction in Parkinson disease: Six‐month and 12‐month prospective analyses. Parkinson's Disease, 2012, 237673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ffytche, D. H. (2017). The psychosis spectrum in Parkinson disease. Nature Reviews Neurology, 13(2), 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follett, K. A. (2010). Pallidal versus subthalamic deep‐brain stimulation for Parkinson's disease. New England Journal of Medicine, 362(22), 2077–2091. [DOI] [PubMed] [Google Scholar]
- Foongsathaporn, C. (2016). What daily activities increase the risk of falling in Parkinson patients? An analysis of the utility of the ABC‐16 scale. Journal of the Neurological Sciences, 364, 183–367. [DOI] [PubMed] [Google Scholar]
- Foreman, K. B. (2011). Testing balance and fall risk in persons with Parkinson disease, an argument for ecologically valid testing. Parkinsonism & Related Disorders, 17(3), 166–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freitas, M. E. , Ruiz‐Lopez, M. , & Fox, S. H. (2016). Novel levodopa formulations for Parkinson's disease. CNS Drugs, 30(11), 1079–1095. [DOI] [PubMed] [Google Scholar]
- Gai, J. , Gomes, L. , & Jansen De Cárdenas, C. (2009). Ptophobia: The fear of falling in elderly people. Acta Medica Portuguesa, 22(1), 83–88. [PubMed] [Google Scholar]
- Gammon, K. (2014). Neurodegenerative disease: Brain windfall. Nature, 515(7526), 299–300. [DOI] [PubMed] [Google Scholar]
- Ganesan, M. (2014). Treadmill gait training improves baroreflex sensitivity in Parkinson's disease. Clinical Autonomic Research, 24(3), 111–118. [DOI] [PubMed] [Google Scholar]
- Gazibara, T. (2014). Circumstances of falls and fall‐related injuries among patients with Parkinson's disease in an outpatient setting. Geriatric Nursing (New York, N.Y.), 35(5), 364–369. [DOI] [PubMed] [Google Scholar]
- Gazibara, T. (2016a). Recurrent falls in Parkinson's disease after one year of follow‐up: A nested case‐control study. Archives of Gerontology and Geriatrics, 65, 17–24. [DOI] [PubMed] [Google Scholar]
- Gazibara, T. (2016b). Indoor and outdoor falls in persons with Parkinson's disease after 1 year follow‐up study: Differences and consequences. Neurol Sci, 37(4), 597–602. [DOI] [PubMed] [Google Scholar]
- Gazibara, T. (2017). Near‐falls in people with Parkinson's disease: Circumstances, contributing factors and association with falling. Clinical Neurology and Neurosurgery, 161, 51–55. [DOI] [PubMed] [Google Scholar]
- GBD 2016 Parkinson's Disease Collaborators . (2018). Global, regional, and national burden of Parkinson's disease, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurology, 17(11), 939–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratwicke, J. (2018). Bilateral deep brain stimulation of the nucleus basalis of meynert for Parkinson disease dementia: A randomized clinical trial. JAMA Neurology, 75(2), 169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray, P. , & Hildebrand, K. (2000). Fall risk factors in Parkinson's disease. Journal of Neuroscience Nursing, 32(4), 222–228. [DOI] [PubMed] [Google Scholar]
- Gray, R. (2014). Long‐term effectiveness of dopamine agonists and monoamine oxidase B inhibitors compared with levodopa as initial treatment for Parkinson's disease (PD MED): A large, open‐label, pragmatic randomised trial. Lancet, 384(9949), 1196–1205. [DOI] [PubMed] [Google Scholar]
- Grimbergen, Y. A. (2013). Impact of falls and fear of falling on health‐related quality of life in patients with Parkinson's disease. Journal of Parkinsons Diseae, 3(3), 409–413. [DOI] [PubMed] [Google Scholar]
- Grimbergen, Y. A. , Munneke, M. , & Bloem, B. R. (2004). Falls in Parkinson's disease. Current Opinion in Neurology, 17(4), 405–415. [DOI] [PubMed] [Google Scholar]
- Grosset, D. (2009). Adherence to antiparkinson medication in a multicenter European study. Movement Disorders, 24(6), 826–832. [DOI] [PubMed] [Google Scholar]
- Grosset, D. (2010). Therapy adherence issues in Parkinson's disease. Journal of the Neurological Sciences, 289(1–2), 115–118. [DOI] [PubMed] [Google Scholar]
- Gullett, J. M. (2013). Reliability of three Benton Judgment of Line Orientation short forms in idiopathic Parkinson's disease. The Clinical Neuropsychologist, 27(7), 1167–1178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjistavropoulos, T. , Delbaere, K. , & Fitzgerald, T. D. (2011). Reconceptualizing the role of fear of falling and balance confidence in fall risk. Journal of Aging and Health, 23(1), 3–23. [DOI] [PubMed] [Google Scholar]
- Halperin, O. (2020). Overconfidence in visual perception in Parkinson's disease. European Journal of Neuroscience. [DOI] [PubMed]
- Halperin, O. (2020). Self‐motion perception in Parkinson's disease. European Journal of Neuroscience. [DOI] [PubMed]
- Hauer, K. (2006). Systematic review of definitions and methods of measuring falls in randomised controlled fall prevention trials. Age and Ageing, 35(1), 5–10. [DOI] [PubMed] [Google Scholar]
- Hauser, R. A. (2000). A home diary to assess functional status in patients with Parkinson's disease with motor fluctuations and dyskinesia. Clinical Neuropharmacology, 23(2), 75–81. [DOI] [PubMed] [Google Scholar]
- Hauser, R. A. (2007). Ten‐year follow‐up of Parkinson's disease patients randomized to initial therapy with ropinirole or levodopa. Movement Disorders, 22(16), 2409–2417. [DOI] [PubMed] [Google Scholar]
- Hely, M. A. (1999). The Sydney multicentre study of Parkinson's disease: Progression and mortality at 10 years. Journal of Neurology, Neurosurgery, and Psychiatry, 67(3), 300–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hely, M. A. (2005). Sydney multicenter study of Parkinson's disease: Non‐L‐dopa‐responsive problems dominate at 15 years. Movement Disorders, 20(2), 190–199. [DOI] [PubMed] [Google Scholar]
- Holloway, R. G. (2004). Pramipexole vs levodopa as initial treatment for Parkinson disease: A 4‐year randomized controlled trial. Archives of Neurology, 61(7), 1044–53. [DOI] [PubMed] [Google Scholar]
- Hoskovcová, M. (2015). Predicting falls in Parkinson disease: What is the value of instrumented testing in OFF medication state? PLoS One, 10(10), e0139849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hulbert, S. (2019). Staying safe”—A narrative review of falls prevention in people with Parkinson's—PDSAFE. Disability and Rehabilitation, 41(21), 2596–2605. [DOI] [PubMed] [Google Scholar]
- Hunter, H. (2018). Longitudinal falls data in Parkinson's disease: Feasibility of fall diaries and effect of attrition. Disability and Rehabilitation, 40(19), 2236–2241. [DOI] [PubMed] [Google Scholar]
- Hur, E. Y. (2017). Longitudinal evaluation of Johns Hopkins fall risk assessment tool and nurses' experience. Journal of Nursing Care Quality, 32(3), 242–251. [DOI] [PubMed] [Google Scholar]
- Jankovic, J. (2005). Motor fluctuations and dyskinesias in Parkinson's disease: Clinical manifestations. Movement Disorders, 20(Suppl 11), S11–S16. [DOI] [PubMed] [Google Scholar]
- Katzenschlager, R. (2018). Apomorphine subcutaneous infusion in patients with Parkinson's disease with persistent motor fluctuations (TOLEDO): A multicentre, double‐blind, randomised, placebo‐controlled trial. Lancet Neurology, 17(9), 749–759. [DOI] [PubMed] [Google Scholar]
- Kerr, G. K. (2010). Predictors of future falls in Parkinson disease. Neurology, 75(2), 116–124. [DOI] [PubMed] [Google Scholar]
- Klinkenberg, W. D. , & Potter, P. (2017). Validity of the Johns Hopkins Fall Risk Assessment Tool for Predicting Falls on Inpatient Medicine Services. Journal of Nursing Care Quality, 32(2), 108–113. [DOI] [PubMed] [Google Scholar]
- Koller, W. C. (1989). Falls and Parkinson's disease. Clinical Neuropharmacology, 12(2), 98–105. [DOI] [PubMed] [Google Scholar]
- Lachman, M. E. (1998). Fear of falling and activity restriction: The survey of activities and fear of falling in the elderly (SAFE). Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 53(1), P43–P50. [DOI] [PubMed] [Google Scholar]
- LaHue, S. C. , Comella, C. L. , & Tanner, C. M. (2016). The best medicine? The influence of physical activity and inactivity on Parkinson's disease. Movement Disorders, 31(10), 1444–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshminarayana, R. (2014). August 15. Smartphone‐ and internet‐assisted self‐management and adherence tools to manage Parkinson's disease (SMART‐PD): Study protocol for a randomised controlled trial (v7). Trials, 15, 374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, S. E. (2005). Development of a common outcome data set for fall injury prevention trials: The Prevention of Falls Network Europe consensus. Journal of the American Geriatrics Society, 53(9), 1618–1622. [DOI] [PubMed] [Google Scholar]
- Latt, M. D. (2009). Acceleration patterns of the head and pelvis during gait in older people with Parkinson's disease: A comparison of fallers and nonfallers. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences, 64(6), 700–706. [DOI] [PubMed] [Google Scholar]
- Latt, M. D. (2009). Clinical and physiological assessments for elucidating falls risk in Parkinson's disease. Movement Disorders, 24(9), 1280–1289. [DOI] [PubMed] [Google Scholar]
- Lhommée, E. (2018). Behavioural outcomes of subthalamic stimulation and medical therapy versus medical therapy alone for Parkinson's disease with early motor complications (EARLYSTIM trial): Secondary analysis of an open‐label randomised trial. Lancet Neurology, 17(3), 223–231. [DOI] [PubMed] [Google Scholar]
- Lieberman, A. (1998). A multicenter trial of ropinirole as adjunct treatment for Parkinson's disease. Neurology, 51(4), 1057–1062. [DOI] [PubMed] [Google Scholar]
- Lieberman, A. , Ranhosky, A. , & Korts, D. (1997). Clinical evaluation of pramipexole in advanced Parkinson's disease: Results of a double‐blind, placebo‐controlled, parallel‐group study. Neurology, 49(1), 162–168. [DOI] [PubMed] [Google Scholar]
- Lim, I. (2008). Identifying fallers with Parkinson's disease using home‐based tests: Who is at risk? Movement Disorders, 23(16), 2411–2415. [DOI] [PubMed] [Google Scholar]
- Lima, D. P. (2020). Clinical correlates of sarcopenia and falls in Parkinson's disease. PLoS One, 15(3), e0227238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, C. C. (2014). Effects of Parkinson's disease on optic flow perception for heading direction during navigation. Experimental Brain Research, 232(4), 1343–1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, S. , Kalman, M. , & Haines, P. (2020). Evaluating a fall risk assessment tool in an emergency department. Journal for Healthcare Quality, 42(4), 205–214. [DOI] [PubMed] [Google Scholar]
- Maidan, I. (2014). Introducing a new definition of a near fall: Intra‐rater and inter‐rater reliability. Gait & Posture, 39(1), 645–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mak, M. K. (2017). Long‐term effects of exercise and physical therapy in people with Parkinson disease. Nature Reviews Neurology, 13(11), 689–703. [DOI] [PubMed] [Google Scholar]
- Mak, M. K. , & Pang, M. Y. (2009). Fear of falling is independently associated with recurrent falls in patients with Parkinson's disease: A 1‐year prospective study. Journal of Neurology, 256(10), 1689–1695. [DOI] [PubMed] [Google Scholar]
- Mak, M. K. , & Pang, M. Y. (2009). Balance confidence and functional mobility are independently associated with falls in people with Parkinson's disease. Journal of Neurology, 256(5), 742–9. [DOI] [PubMed] [Google Scholar]
- Mak, M. K. , & Pang, M. Y. (2010). Parkinsonian single fallers versus recurrent fallers: Different fall characteristics and clinical features. Journal of Neurology, 257(9), 1543–1551. [DOI] [PubMed] [Google Scholar]
- Maki, B. E. , Holliday, P. J. , & Topper, A. K. (1994). A prospective study of postural balance and risk of falling in an ambulatory and independent elderly population. Journal of Gerontology, 49(2), M72–M84. [DOI] [PubMed] [Google Scholar]
- Martin, T. (2015). A randomized controlled feasibility trial of a specific cueing program for falls management in persons with Parkinson disease and freezing of gait. Journal of Neurologic Physical Therapy, 39(3), 179–184. [DOI] [PubMed] [Google Scholar]
- Matinolli, M. (2007). Postural sway and falls in Parkinson's disease: A regression approach. Movement Disorders, 22(13), 1927–1935. [DOI] [PubMed] [Google Scholar]
- Matinolli, M. (2011). Recurrent falls and mortality in Parkinson's disease: A prospective two‐year follow‐up study. Acta Neurologica Scandinavica, 123(3), 193–200. [DOI] [PubMed] [Google Scholar]
- Michałowska, M. (2005). Falls in Parkinson's disease: Causes and impact on patients' quality of life. Functional Neurology, 20(4), 163–168. [PubMed] [Google Scholar]
- Mirelman, A. (2016). Addition of a non‐immersive virtual reality component to treadmill training to reduce fall risk in older adults (V‐TIME): A randomised controlled trial. Lancet, 388(10050), 1170–1182. [DOI] [PubMed] [Google Scholar]
- Möller, J. C. (2005). Long‐term efficacy and safety of pramipexole in advanced Parkinson's disease: Results from a European multicenter trial. Movement Disorders, 20(5), 602–610. [DOI] [PubMed] [Google Scholar]
- Moreno Catalá, M. , Woitalla, D. , & Arampatzis, A. (2015). Recovery performance and factors that classify young fallers and non‐fallers in Parkinson's disease. Human Movement Science, 41, 136–146. [DOI] [PubMed] [Google Scholar]
- Odekerken, V. J. (2013). Subthalamic nucleus versus globus pallidus bilateral deep brain stimulation for advanced Parkinson's disease (NSTAPS study): A randomised controlled trial. Lancet Neurology, 12(1), 37–44. [DOI] [PubMed] [Google Scholar]
- Oh‐Park, M. (2011). Transient versus persistent fear of falling in community‐dwelling older adults: Incidence and risk factors. Journal of the American Geriatrics Society, 59(7), 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow, C. W. (2014). Continuous intrajejunal infusion of levodopa‐carbidopa intestinal gel for patients with advanced Parkinson's disease: A randomised, controlled, double‐blind, double‐dummy study. Lancet Neurology, 13(2), 141–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanow, C. W. , Stern, M. B. , & Sethi, K. (2009). The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology, 72(21 Suppl 4), S1–136. [DOI] [PubMed] [Google Scholar]
- Pahwa, R. (2007). Ropinirole 24‐hour prolonged release: Randomized, controlled study in advanced Parkinson disease. Neurology, 68(14), 1108–1115. [DOI] [PubMed] [Google Scholar]
- Parashos, S. A. (2013). Falls in Parkinson disease: Analysis of a large cross‐sectional cohort. Journal of Parkinson's Disease, 3(4), 515–522. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group CALM Cohort Investigators . (2009). Long‐term effect of initiating pramipexole vs levodopa in early Parkinson disease. Archives of Neurology, 66(5), 563–570. [DOI] [PubMed] [Google Scholar]
- Parkinson Study Group . (2000). Pramipexole vs levodopa as initial treatment for Parkinson disease: A randomized controlled trial. JAMA, 284(15), 1931–1938. [DOI] [PubMed] [Google Scholar]
- Pärkkä, J. (2006). Activity classification using realistic data from wearable sensors. IEEE Transactions on Information Technology in Biomedicine, 10(1), 119–28. [DOI] [PubMed] [Google Scholar]
- Parry, S. W. (2016). A novel approach to proactive primary care‐based case finding and multidisciplinary management of falls, syncope, and dizziness in a one‐stop service: Preliminary results. Journal of the American Geriatrics Society, 64(11), 2368–2373. [DOI] [PubMed] [Google Scholar]
- Paul, S. S. (2013). Three simple clinical tests to accurately predict falls in people with Parkinson's disease. Movement Disorders, 28(5), 655–662. [DOI] [PubMed] [Google Scholar]
- Paul, S. S. (2014). The relative contribution of physical and cognitive fall risk factors in people with Parkinson's disease: A large prospective cohort study. Neurorehabilitation and Neural Repair, 28(3), 282–290. [DOI] [PubMed] [Google Scholar]
- Paul, S. S. (2017). Fall‐related hospitalization in people with Parkinson's disease. European Journal of Neurology, 24(3), 523–529. [DOI] [PubMed] [Google Scholar]
- Peto, V. (1995). The development and validation of a short measure of functioning and well being for individuals with Parkinson's disease. Quality of Life Research, 4(3), 241–248. [DOI] [PubMed] [Google Scholar]
- Pickering, R. M. (2007). A meta‐analysis of six prospective studies of falling in Parkinson's disease. Movement Disorders, 22(13), 1892–1900. [DOI] [PubMed] [Google Scholar]
- Plotnik, M. (2011). Postural instability and fall risk in Parkinson's disease: Impaired dual tasking, pacing, and bilateral coordination of gait during the “ON” medication state. Experimental Brain Research, 210(3–4), 529–538. [DOI] [PubMed] [Google Scholar]
- Poe, S. S. (2005). An evidence‐based approach to fall risk assessment, prevention, and management: Lessons learned. Journal of Nursing Care Quality, 20(2), 107–16. , quiz 117–118. [DOI] [PubMed] [Google Scholar]
- Poe, S. S. (2018). The Johns Hopkins Fall Risk Assessment Tool: A study of reliability and validity. Journal of Nursing Care Quality, 33(1), 10–19. [DOI] [PubMed] [Google Scholar]
- Postuma, R. B. (2015). MDS clinical diagnostic criteria for Parkinson's disease. Movement Disorders, 30(12), 1591–1601. [DOI] [PubMed] [Google Scholar]
- Pressley, J. C. (2003). The impact of comorbid disease and injuries on resource use and expenditures in parkinsonism. Neurology, 60(1), 87–93. [DOI] [PubMed] [Google Scholar]
- Rahman, S. (2008). Quality of life in Parkinson's disease: The relative importance of the symptoms. Movement Disorders, 23(10), 1428–1434. [DOI] [PubMed] [Google Scholar]
- Rahman, S. (2011). On the nature of fear of falling in Parkinson's disease. Behavioural Neurology, 24(3), 219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rascol, O. (1996). Ropinirole in the treatment of levodopa‐induced motor fluctuations in patients with Parkinson's disease. Clinical Neuropharmacology, 19(3), 234–245. [DOI] [PubMed] [Google Scholar]
- Rascol, O. (2000). A five‐year study of the incidence of dyskinesia in patients with early Parkinson's disease who were treated with ropinirole or levodopa. New England Journal of Medicine, 342(20), 1484–1491. [DOI] [PubMed] [Google Scholar]
- Reynolds, G. O. (2016). The therapeutic potential of exercise to improve mood, cognition, and sleep in Parkinson's disease. Movement Disorders, 31(1), 23–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, K. (2005). Falling risk factors in Parkinson's disease. Neurorehabilitation, 20(3), 169–182. [PubMed] [Google Scholar]
- Sacheli, M. A. (2018). Habitual exercisers versus sedentary subjects with Parkinson's disease: Multimodal PET and fMRI study. Movement Disorders, 33(12), 1945–1950. [DOI] [PubMed] [Google Scholar]
- Schaafsma, J. D. (2003). Gait dynamics in Parkinson's disease: Relationship to Parkinsonian features, falls and response to levodopa. Journal of the Neurological Sciences, 212(1‐2), 47–53. [DOI] [PubMed] [Google Scholar]
- Schapira, A. H. (2011). Extended‐release pramipexole in advanced Parkinson disease: A randomized controlled trial. Neurology, 77(8), 767–774. [DOI] [PubMed] [Google Scholar]
- Schenkman, M. (2018). Effect of high‐intensity treadmill exercise on motor symptoms in patients with de novo Parkinson disease: A phase 2 randomized clinical trial. JAMA Neurology, 75(2), 219–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva de Lima, A. L. (2016). Large‐scale wearable sensor deployment in Parkinson's patients: The Parkinson@Home Study Protocol. JMIR Research Protocols, 5(3), e172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair, E. (2021). Metabolomics of sebum reveals lipid dysregulation in Parkinson's disease. Nature Communication, 12(1), 1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smulders, K. (2012). Assessment of dual tasking has no clinical value for fall prediction in Parkinson's disease. Journal of Neurology, 259(9), 1840–1847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soh, S. E. (2013). Determinants of health‐related quality of life in people with Parkinson's disease: A path analysis. Quality of Life Research, 22(7), 1543–1553. [DOI] [PubMed] [Google Scholar]
- Song, R. (2017). The impact of Tai Chi and Qigong mind‐body exercises on motor and non‐motor function and quality of life in Parkinson's disease: A systematic review and meta‐analysis. Parkinsonism & Related Disorders, 41, 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stang, A. (2010). Critical evaluation of the Newcastle‐Ottawa scale for the assessment of the quality of nonrandomized studies in meta‐analyses. European Journal of Epidemiology, 25(9), 603–605. [DOI] [PubMed] [Google Scholar]
- Temlett, J. A. , & Thompson, P. D. (2006). Reasons for admission to hospital for Parkinson's disease. Internal Medicine Journal, 36(8), 524–526. [DOI] [PubMed] [Google Scholar]
- Terroba‐Chambi, C. (2019). Design and validation of a new instrument to assess fear of falling in Parkinson's disease. Movement Disorders, 34(10), 1496–1504. [DOI] [PubMed] [Google Scholar]
- Thomas, A. A. (2010). Falls and the falls efficacy scale in Parkinson's disease. Journal of Neurology, 257(7), 1124–1128. [DOI] [PubMed] [Google Scholar]
- Tinetti, M. E. , & Powell, L. (1993). Fear of falling and low self‐efficacy: A case of dependence in elderly persons. Journal of Gerontology, 48, Spec No, 35–38. [DOI] [PubMed] [Google Scholar]
- Tinetti, M. E. , Richman, D. , & Powell, L. (1990). Falls efficacy as a measure of fear of falling. Journal of Gerontology, 45(6), P239–P243. [DOI] [PubMed] [Google Scholar]
- Trollor, J. (2006). Prevalence of mental disorders in the elderly: The Australian National Mental Health and Well‐being Survey. Acta Neuropsychiatry, 18(6), 271–272. [DOI] [PubMed] [Google Scholar]
- Wallace, E. , & Johansen, M. E. (2018). Clinical prediction rules: Challenges, barriers, and promise. Annals of Family Medicine, 16(5), 390–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss, A. (2010). Can an accelerometer enhance the utility of the Timed Up & Go Test when evaluating patients with Parkinson's disease? Medical Engineering & Physics, 32(2), 119–125. [DOI] [PubMed] [Google Scholar]
- Winter, Y. (2010). Costs of Parkinson's disease in Eastern Europe: A Czech cohort study. Parkinsonism & Related Disorders, 16(1), 51–56. [DOI] [PubMed] [Google Scholar]
- Wood, B. H. (2002). Incidence and prediction of falls in Parkinson's disease: A prospective multidisciplinary study. Journal of Neurology, Neurosurgery, and Psychiatry, 72(6), 721–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubovich, S. (2020). Visual self‐motion cues are impaired yet overweighted during visual‐vestibular integration in Parkinson's disease. Brain Communications, 2(1), fcaa035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yardley, L. , & Smith, H. (2002). A prospective study of the relationship between feared consequences of falling and avoidance of activity in community‐living older people. The Gerontologist, 42(1), 17–23. [DOI] [PubMed] [Google Scholar]
- Youn, J. (2017). Falling direction can predict the mechanism of recurrent falls in advanced Parkinson's Disease. Science Reports, 7(1), 3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data underlying the findings are within the paper.


