Abstract
Introduction
Dexmedetomidine (Dex) is suggested to be neuroprotective. However, influence of Dex on postoperative cognitive dysfunction (POCD) in the elderly remains unknown.
Methods
We performed a meta‐analysis of randomized controlled trials (RCTs) to evaluate the effect of Dex on POCD. Relevant studies were obtained by search of PubMed, Embase, and Cochrane's Library databases. A random‐effect model was used to pool the results.
Results
Fourteen RCTs including 1626 adults of 60 years or older who received surgery with general anesthesia were included. Because methodologically diverse scales were used for POCD, eight RCTs with POCD diagnosed with Mini‐Mental State Examination (MMSE) were included in the meta‐analysis, while the remaining six RCTs with POCD diagnosed with other scales were qualitative synthesized. Pooled results of RCTs with MMSE showed that Dex significantly reduced the incidence of POCD (risk ratio: 0.47, 95% confidence interval: 0.37–0.60, p < 0.001) with no significant heterogeneity (I 2 = 0%) or publication bias (p for Egger's regression test = 0.579). For the remaining six RCTs with POCD diagnosed with other scales, three of them showed that Dex was associated with a significantly lower incidence of POCD, while the other three RCTs did not show a significant difference.
Conclusions
Dex is associated with a reduced risk of POCD in elderly patients receiving surgeries with general anesthesia, and the results were mainly obtained in studies with POCD diagnosed with MMSE. Based on these findings, Dex may be considered as a preventative measure for POCD in elderly patients.
Keywords: dexmedetomidine, elderly, meta‐analysis, postoperative cognitive dysfunction, surgery
Graphical Abstract
A systematic review and meta‐analysis of 14 randomized controlled trials showed that dexmedetomidine is associated with a reduced risk of postoperative cognitive dysfunction in elderly patients receiving surgeries with general anesthesia, and the results were mainly obtained in studies with postoperative cognitive dysfunction diagnosed with MMSE. Based on these findings, Dex may be considered as a preventative measure for postoperative cognitive dysfunction in elderly patients.

1. INTRODUCTION
Postoperative cognitive dysfunction (POCD), which is defined as a decline in cognitive function after the surgery, is a common postoperative cognitive disorder in patients following surgeries with general anesthesia, particularly in the elderly population (Belrose & Noppens, 2019; Brodier & Cibelli, 2021; Urits et al., 2019). Previous studies showed that the incidence of POCD varied between 20% and 40% in people aged 60 years or older (Lin et al., 2020). Moreover, POCD has been related to prolonged hospitalization, impaired functional ability, and increased mortality of patients after surgeries (L. Gao et al., 2005; Ruggiero et al., 2017). Therefore, development of novel strategy to prevent the incidence of POCD is of great clinical significance, especially in the elderly population.
Dexmedetomidine (Dex) is a well‐applied perioperative medication for patients that received surgeries with general anesthesia (Abowali et al., 2021). Pharmacologically, Dex is a highly selective α2‐adrenoreceptor agonist, which exerts various clinical efficacies during perioperative periods, such as sedation, analgesia, anti‐anxiety, and diuresis (Keating, 2015; Lee, 2019). Besides, accumulating evidence showed that Dex may also confer neuroprotective effects (Jiang et al., 2017; Y. Wang et al., 2016). However, previous clinical studies evaluating the influence of Dex on POCD in elderly patients who received surgery showed inconsistent results (J. Chen et al., 2013; Ding et al., 2015; Y. Gao et al., 2020; Y. Li et al., 2015; Z. Li et al., 2021, 2020; Mansouri et al., 2019; Mohamed & Shaaban, 2014; Shi et al., 2020; K. Wang et al., 2015; Xu et al., 2017; Zhang et al., 2014; Zhao et al., 2020; M. Zhou et al., 2019). Some randomized controlled trials (RCTs) suggested that Dex may reduce POCD in the elderly population (Y. Li et al., 2015; Z. Li et al., 2021; Mohamed & Shaaban, 2014; Shi et al., 2020; Zhang et al., 2014; Zhao et al., 2020), while others did not (J. Chen et al., 2013; Ding et al., 2015; Y. Gao et al., 2020; Liu et al., 2020; Mansouri et al., 2019; K. Wang et al., 2015; Xu et al., 2017; M. Zhou et al., 2019). Although two early meta‐analyses (Man et al., 2015; C. Zhou et al., 2016) showed that Dex may be associated with preserved postoperative cognitive function, only RCTs published before 2015 were included. Moreover, the relatively small number of available RCTs prevented further analyses regarding the influences of study characteristics on the outcome (Man et al., 2015; C. Zhou et al., 2016). With the accumulated studies in recent years (Y. Gao et al., 2020; Z. Li et al., 2021; Liu et al., 2020; Mansouri et al., 2019; Shi et al., 2020; Xu et al., 2017; Zhao et al., 2020; M. Zhou et al., 2019), we performed an updated meta‐analysis to evaluate the influence of Dex on the incidence of POCD in the elderly population. Moreover, possible influences of characteristics, such as type of the surgery, regimen of anesthetics, using of Dex loading dose, instrument for POCD measuring, and so on, on the outcome were also studied.
2. METHODS
The PRISMA 2020 (Preferred Reporting Items for Systematic Reviews and Meta‐Analyses 2020) statement (Page, McKenzie, et al., 2021a; Page, Moher, et al., 2021b) and the Cochrane Handbook guidelines (Higgins et al., 2021) were followed during the designing and implementation of the study.
2.1. Search strategy
PubMed, Embase, and the Cochrane Library (Cochrane Center Register of Controlled Trials) databases were searched for relevant studies with a combined strategy of: (1) “dexmedetomidine”; (2) “cognition” OR “cognitive” OR “dementia” OR “cognit*” OR “deliri*” OR “mild cognitive impairment*” OR “mild‐cognitive impairment*” OR “neuropsycholo*” OR “POCD” OR “postoperative cognitive” OR “post‐operative cognitive” OR “MMSE” OR “mini‐mental state examination” OR “cerebral function” OR “neurocognit*” OR “encephalopath” OR “cognition” OR “cognitive” OR “delirium”; and (3) “random” OR “randomized” OR “randomised” OR “randomly” OR “allocated” OR “control” OR “placebo.” Only clinical studies were considered. The references of related reviews and original articles were also searched as a complementation. The final database search was conducted on June 20, 2021.
2.2. Study selection
Studies that fulfilled the following criteria were included: (1) Articles published in English or Chinese; (2) designed as parallel‐group RCTs; (3) included elderly patients (60 years or older) scheduled for surgery with general anesthesia who were randomly allocated to a Dex treatment group or a control group with placebo or blank treatment; and (4) reported the incidence of POCD in the perioperative periods. The diagnostic criteria of POCD outcomes in the meta‐analysis were in accordance with that applied in the included studies. Reviews, studies with non‐elderly patients, preclinical studies, observational studies, and repeated reports were excluded.
2.3. Data extraction and quality assessment
Database search, data extraction, and quality evaluation were conducted by two independent authors. If disagreement occurred, it was resolved by discussion with the corresponding author. We extracted data regarding study information (first author, publication year, and study country), study design (blind or open‐label), patient information (number of participants, range of age, and sex), surgery type, perioperative anesthetics and anesthesia depth monitoring, regimens of Dex and control, and diagnostic strategy for POCD. Quality evaluation was achieved using the Cochrane's Risk of Bias Tool (Higgins et al., 2021) according to the following aspects: (1) Random sequence generation; (2) allocation concealment; (3) blinding of participants and personnel; (4) blinding of outcome assessors; (5) incomplete outcome data; (6) selective outcome reporting; and (7) other potential bias.
2.4. Statistical analysis
Incidence of POCD was separately evaluated via risk ratios (RRs) and their 95% confidence intervals (CIs) in this meta‐analysis. We used the Cochrane's Q test to detect the heterogeneity (Higgins et al., 2021). The I 2 statistic was also calculated, and an I 2 > 50% reflected significant heterogeneity. Pooled analyses were calculated using a random‐effect model because this method incorporates the influence of potential heterogeneity and retrieves a more generalized result (Higgins et al., 2021). Sensitivity analysis by excluding one study at a time was used to evaluate the influence of each study on the pooled results of the meta‐analysis (Higgins et al., 2021). Predefined subgroup analyses were used to evaluate the possible influences of study characteristics on the effect of Dex on POCD risk. Publication bias was evaluated by visual inspection of funnel plots, and the Egger's regression asymmetry test (Higgins et al., 2021). p values <0.05 were considered statistically significant. The RevMan (Version 5.1; Cochrane, Oxford, UK) and Stata software (Version 12.0; Stata, College Station, TX) were applied for statistical analyses.
3. RESULTS
3.1. Search results
The process of database search and study identification is shown in Figure 1. Briefly, 751 articles were obtained through the database search, and 622 were retrieved after exclusion of duplicated records. Among them, 573 articles were subsequently excluded based on titles and abstracts primarily because these studies were irrelevant to the aim of the meta‐analysis. Of the 49 articles that underwent full‐text review, 35 were further excluded for the reasons presented in Figure 1. Finally, 14 RCTs (J. Chen et al., 2013; Ding et al., 2015; Y. Gao et al., 2020; Y. Li et al., 2015; Z. Li et al., 2021; Liu et al., 2020; Mansouri et al., 2019; Mohamed & Shaaban, 2014; Shi et al., 2020; K. Wang et al., 2015; Xu et al., 2017; Zhang et al., 2014; Zhao et al., 2020; M. Zhou et al., 2019) were included.
FIGURE 1.
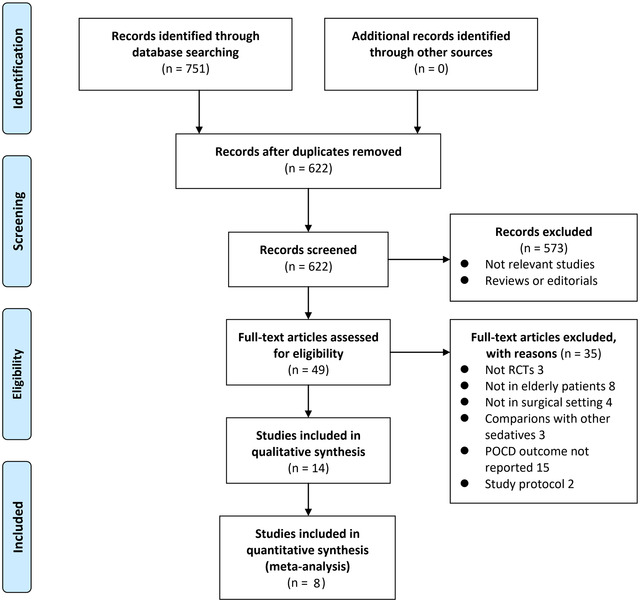
Flow chart of literature search
3.2. Study characteristics
Because methodologically diverse scales were used for POCD, eight RCTs with POCD diagnosed with the Mini‐Mental State Examination (MMSE) were included in the meta‐analysis (J. Chen et al., 2013; Y. Gao et al., 2020; Y. Li et al., 2015; Z. Li et al., 2021; Liu et al., 2020; Mansouri et al., 2019; Zhang et al., 2014; Zhao et al., 2020), while the remaining six RCTs with POCD diagnosed with other scales were qualitative synthesized (Ding et al., 2015; Mohamed & Shaaban, 2014; Shi et al., 2020; K. Wang et al., 2015; Xu et al., 2017; M. Zhou et al., 2019). Table 1 shows the characteristics of the included studies. Overall, 14 RCTs with 1626 elderly patients were included in the current meta‐analysis (J. Chen et al., 2013; Ding et al., 2015; Y. Gao et al., 2020; Y. Li et al., 2015; Z. Li et al., 2021; Liu et al., 2020; Mansouri et al., 2019; Mohamed & Shaaban, 2014; Shi et al., 2020; K. Wang et al., 2015; Xu et al., 2017; Zhang et al., 2014; Zhao et al., 2020; M. Zhou et al., 2019). Twelve of the studies were performed in China (J. Chen et al., 2013; Ding et al., 2015; Y. Gao et al., 2020; Y. Li et al., 2015; Z. Li et al., 2021; Liu et al., 2020; Shi et al., 2020; K. Wang et al., 2015; Xu et al., 2017; Zhang et al., 2014; Zhao et al., 2020; M. Zhou et al., 2019), and the other two were performed in Egypt and Iran (Mansouri et al., 2019; Mohamed & Shaaban, 2014), respectively. All of these studies were double‐blinded RCTs except for two studies, which were single‐blinded (Y. Gao et al., 2020; Zhang et al., 2014). For most of the included studies, non‐cardiac surgeries were performed (J. Chen et al., 2013; Ding et al., 2015; Y. Li et al., 2015; Z. Li et al., 2021; Liu et al., 2020; Mansouri et al., 2019; Mohamed & Shaaban, 2014; Shi et al., 2020; K. Wang et al., 2015; Xu et al., 2017; Zhang et al., 2014; Zhao et al., 2020), while for two studies, cardiac surgeries were performed (Y. Gao et al., 2020; M. Zhou et al., 2019). For three studies (Ding et al., 2015; Mohamed & Shaaban, 2014; M. Zhou et al., 2019), sevoflurane was used during anesthesia with intravenous anesthetics such as propofol, remifentanil, or sufentanil. A loading dose and subsequent continuously intravenous administration of Dex was applied in most of the included studies except for two studies (Shi et al., 2020; K. Wang et al., 2015), in which a loading dose of Dex was not applied. Dex was administered during surgical procedure in all of the included studies, while in two studies, Dex was also administered in intensive care unit (ICU) after the surgery (K. Wang et al., 2015; Zhao et al., 2020). Placebo of normal saline was applied in all the included studies. As for the evaluation strategy of POCD, the MMSE was performed in eight studies (J. Chen et al., 2013; Y. Gao et al., 2020; Y. Li et al., 2015; Z. Li et al., 2021; Liu et al., 2020; Mansouri et al., 2019; Zhang et al., 2014; Zhao et al., 2020), while in the other studies, instruments such as the Montreal Cognitive Assessment (MoCA), Stroop color test, and Chinese Neurocognitive Scale were used (Ding et al., 2015; Mohamed & Shaaban, 2014; Shi et al., 2020; K. Wang et al., 2015; Xu et al., 2017; M. Zhou et al., 2019). Patients with POCD were identified within 24 h after surgery in all of the included studies.
TABLE 1.
Characteristics of the included RCTs
| Study | Country | Design | Surgical procedure | No. of patients | Age range (years) | Male (%) | Anesthesia regimen | Anesthesia depth monitoring | Dex regimens | Loading dose of Dex | Timing and duration of Dex | Control | Diagnosis of outcomes |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| POCD diagnosed with MMSE | |||||||||||||
| Chen 2013 | China | R, DB, PC | Laparoscopic cholecystectomy | 122 | 60∼75 | 52.5 | Propofol and remifentanil | NR | 1 ug/kg for 10 min after induction, and 0.4 ug/kg/h until the end of surgery | Yes | During surgery | NS | Postoperative MMSE < 27 |
| Zhang 2014 | China | R, SB, PC | Laparoscopic surgery for colorectal cancer | 80 | 65∼85 | 58.8 | Propofol and remifentanil | Nareotrend Index 45∼55 | 0.5 ug/kg for 15 min before induction, and 0.2, 0.5, or 0.8 ug/kg/h until the end of surgery | Yes | During surgery | NS | Postoperative MMSE decline ≥ 2 |
| Li 2015 | China | R, DB, PC | Laparoscopic cholecystectomy | 100 | 60∼75 | 54 | Propofol and remifentanil | BIS 40∼60 | 1 ug/kg for 10 min after induction, and 0.4 ug/kg/h until the end of surgery | Yes | During surgery | NS | A postoperative decrease of minimally 1 SD in MMSE |
| Mansouri 2019 | Iran | R, DB, PC | Cataract surgery | 100 | ≥ 65 | 44 | Nitrous oxide | NR | 1 ug/kg for 10 min at induction | Yes | During surgery | NS | Postoperative MMSE < 26 |
| Gao 2020 | China | R, SB, PC | Minimally invasive CABG | 60 | 65∼75 | 48.3 | Propofol and sufentanil | BIS 40∼60 | 1 ug/kg for 15 min after induction, and 0.3–0.5 ug/kg/h until the end of surgery | Yes | During surgery | NS | Postoperative decline of MMSE |
| Zhao 2020 | China | R, DB, PC | Non‐cardiac surgery | 416 | ≥ 65 | 56.3 | Propofol and remifentanil | BIS 40∼60 | 1 ug/kg for 15 min before induction, and 100, 200, or 400 ug in patient‐controlled intravenous analgesia | Yes | During surgery and in ICU | NS | Postoperative MMSE decline ≥ 2 |
| Liu 2020 | China | R, DB, PC | Colorectal cancer radical resection | 48 | ≥ 65 | 58.3 | Propofol and remifentanil | BIS 40∼55 | 0.5 μg/kg for 15 min at induction and 0.6 μg/kg/h from induction to the end of surgery | Yes | During surgery | NS | Postoperative MMSE decline ≥ 2 |
| Li 2021 | China | R, DB, PC | Spine surgery | 120 | 65∼90 | 56.7 | Propofol and remifentanil | BIS 40∼60 | 0.3 ug/kg for 10 min before induction, and 0.2, 0.5, and 0.8 ug/kg/h until the end of surgery | Yes | During surgery | NS | A decrease of minimally 1 SD in 2 or more postoperative neurocognitive tests by MMSE |
| POCD diagnosed with MoCA | |||||||||||||
| Xu 2017 | China | R, DB, PC | Laparoscopic ovarian cystectomy | 96 | 63∼85 | 0 | Propofol and sufentanil | BIS 45∼55 | 0.8 ug/kg/h for 10 min before induction, and 0.5 ug/kg/h until the end of surgery | Yes | During surgery | NS | Postoperative MoCA < 27 or postoperative MoCA decline ≥ 2 |
| Zhou 2019 | China | R, DB, PC | Cardiac surgery | 76 | 60∼80 | 46.1 | Sevoflurane, propofol and sufentanil | BIS 40∼60 | 0.4 ug/kg/h until the end of surgery | No | During surgery | NS | A decrease of minimally 1 SD in 2 or more postoperative neurocognitive tests by MoCA |
| POCD diagnosed with the Chinese Neurocognitive Scale | |||||||||||||
| Wang 2015 | China | R, DB, PC | Spine surgery | 152 | ≥ 60 | 50.7 | Propofol and remifentanil | BIS 50∼60 | 3 ug/kg in patient‐controlled intravenous analgesia | No | During surgery and in ICU | NS | A decrease of minimally 1 SD in 2 or more postoperative neurocognitive tests by Chinese Neurocognitive Scale |
| Ding 2015 | China | R, DB, PC | Robot‐assisted laparoscopic radical prostatectomy | 100 | 65∼80 | 100 | Sevoflurane, propofol and remifentanil | BIS 40∼60 | 0.8 ug/kg/h for 10 min before induction, and 0.3 ug/kg/h until the end of surgery | Yes | During surgery | NS | A decrease of minimally 1 SD in 2 or more postoperative neurocognitive tests by Chinese Neurocognitive Scale |
| POCD diagnosed with other scales | |||||||||||||
| Mohamed 2014 | Egypt | R, DB, PC | Abdominal surgery | 50 | ≥ 60 | 90 | Sevoflurane, fentanyl | NR | 1 ug/kg/h for 10 min before induction, and 0.4 ug/kg/h until the end of surgery | Yes | During surgery | NS | A decrease of minimally 1 SD in 2 or more postoperative neurocognitive tests by Stroop color test |
| Shi 2020 | China | R, DB, PC | Thoracoscopic lobectomy | 106 | ≥ 65 | 100 | Propofol, remifentanil and cisatracurium | BIS 45∼60 | 0.5 μg/kg/h from induction to the end of surgery | No | During surgery | NS | A decrease of minimally 1 SD in 2 or more postoperative neurocognitive tests by a comprehensive test scale of four domains |
Abbreviations: RCT, randomized controlled trials; DB, double blind; SB, single blind; PC, placebo controlled; CABG, coronary artery bypass grafting; NR, not reported; BIS, Bispectral index; Dex, Dexmedetomidine; ICU, intensive care unit; NS, normal saline; MMSE, Mini‐Mental State Examination; SD, standard deviation; MoCA, Montreal Cognitive Assessment; POCD, postoperative cognitive dysfunction.
3.3. Data quality
Table 2 shows the details of study quality evaluation. All of these studies were double‐blinded RCTs except for two studies, which were single‐blinded (Y. Gao et al., 2020; Zhang et al., 2014). Methods of random sequence generation were reported in nine RCTs (Ding et al., 2015; Y. Li et al., 2015; Z. Li et al., 2021; Liu et al., 2020; Mohamed & Shaaban, 2014; Shi et al., 2020; K. Wang et al., 2015; Zhao et al., 2020; M. Zhou et al., 2019), and information of allocation concealment was reported in five RCTs (J. Chen et al., 2013; Z. Li et al., 2021; Liu et al., 2020; Mohamed & Shaaban, 2014; M. Zhou et al., 2019). Incomplete outcome data, selective reporting, and other sources of biases were judged to be of low risks in all of the included studies.
TABLE 2.
Details of quality evaluation of the included RCTs via the Cochrane's Risk of Bias Tool
| Study | Random sequence generation | Allocation concealment | Blinding of participants | Blinding of outcome assessment | Incomplete outcome data addressed | Selective reporting | Other sources of bias |
|---|---|---|---|---|---|---|---|
| POCD diagnosed with MMSE | |||||||
| Chen 2013 | Unclear | Low | Low | Low | Low | Low | Low |
| Zhang 2014 | Unclear | Unclear | Low | Unclear | Low | Low | Low |
| Li 2015 | Low | Unclear | Low | Low | Low | Low | Low |
| Mansouri 2019 | Unclear | Unclear | Low | Low | Low | Low | Low |
| Gao 2020 | Unclear | Unclear | Low | Unclear | Low | Low | Low |
| Zhao 2020 | Low | Unclear | Low | Low | Low | Low | Low |
| Liu 2020 | Low | Low | Low | Low | Low | Low | Low |
| Li 2021 | Low | Low | Low | Low | Low | Low | Low |
| POCD diagnosed with MoCA | |||||||
| Xu 2017 | Unclear | Unclear | Low | Low | Low | Low | Low |
| Zhou 2019 | Low | Low | Low | Low | Low | Low | Low |
| POCD diagnosed with the Chinese Neurocognitive Scale | |||||||
| Wang 2015 | Low | Unclear | Low | Low | Low | Low | Low |
| Ding 2015 | Low | Unclear | Low | Low | Low | Low | Low |
| POCD diagnosed with other scales | |||||||
| Mohamed 2014 | Low | Low | Low | Low | Low | Low | Low |
| Shi 2020 | Low | Unclear | Low | Low | Low | Low | Low |
Abbreviations: RCTs, randomized controlled trials; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; POCD, postoperative cognitive dysfunction.
3.4. Meta‐analysis for the studies of POCD evaluated using the MMSE
Pooled results of eight RCTs (J. Chen et al., 2013; Y. Gao et al., 2020; Y. Li et al., 2015; Z. Li et al., 2021; Liu et al., 2020; Mansouri et al., 2019; Zhang et al., 2014; Zhao et al., 2020) with POCD evaluated using the MMSE showed that Dex significantly reduced the incidence of POCD (RR: 0.47, 95% CI: 0.37–0.60, p < 0.001) in elderly patients with no significant heterogeneity (p for Cochrane's Q test = 0.98, I2 = 0%; Figure 2). All of these eight RCTs did not include sevoflurane in the anesthesia regimen, and Dex was all administered with a loading dose. Sensitivity analysis by excluding one RCT at a time showed consistent results (RR: 0.46 to 0.49, p values all < 0.05). Subgroup analysis showed consistent results in single‐ and double‐blinded studies (p for subgroup analysis = 0.46, Table 3). Specifically, sensitivity analyses limited to studies from China (RR: 0.47, 95% CI: 0.360.60, p < 0.001), patients with non‐cardiac surgery (RR: 0.47, 95% CI: 0.37–0.61, p < 0.001), and studies with Dex use only within the surgery process (RR: 0.47, 95% CI: 0.37–0.59, p < 0.001; Table 3) showed consistent results.
FIGURE 2.

Forest plots for the meta‐analysis of effect of Dex on the risk of POCD in elderly population in studies of POCD diagnosed with the MMSE
TABLE 3.
Results of subgroup and sensitivity analyses for the meta‐analysis of Dex on POCD evaluated by MMSE
| Study characteristics | Datasets number | RR (95% CI) | I2 | P for subgroup effect | P for subgroup difference |
|---|---|---|---|---|---|
| Design | |||||
| Double blind | 6 | 0.48 [0.37, 0.62] | 0% | < 0.001 | |
| Single blind | 2 | 0.36 [0.18, 0.74] | 0% | 0.005 | 0.46 |
| Only Chinese studies | 7 | 0.47 [0.36, 0.60] | 0% | < 0.001 | |
| Only non‐cardiac surgeries | 7 | 0.47 [0.37, 0.61] | 0% | < 0.001 | |
| Only Dex used in surgery | 7 | 0.47 [0.37, 0.59] | 0% | < 0.001 |
Abbreviations: RR, risk ratio; CI, confidence interval; Dex, dexmedetomidine.
3.5. Qualitative synthesis for the studies of POCD evaluated using other instruments
Results of the remaining six RCTs with POCD diagnosed with instruments other than MMSE are summarized in Table 4. Two Chinese studies used MoCA as the instruments for the detection of POCD in elderly patients after surgeries (Xu et al., 2017; M. Zhou et al., 2019). Although the incidence of POCD seemed lower in patients receiving Dex as compared to those of the control group, the differences were not statistically significant (Xu et al., 2017; M. Zhou et al., 2019). For another two studies of POCD diagnosed with the Chinese Neurocognitive Scale, one study showed that Dex significantly reduced the incidence of POCD as compared to control (K. Wang et al., 2015), while the other study failed to show a significant difference of POCD incidence between patients allocated to the Dex and control groups (Ding et al., 2015). The remaining two studies, using Stroop color test and the comprehensive test scale of four domains for the diagnosis of POCD, respectively, showed that Dex was effective in reducing the incidence of POCD in elderly patients after surgeries (Mohamed & Shaaban, 2014; Shi et al., 2020).
TABLE 4.
Results of studies with POCD diagnosed with scales other than MMSE
| Study | Diagnosis scale for POCD | Incidence of POCD in Dex group | Incidence of POCD in control group | P for difference of POCD incidence |
|---|---|---|---|---|
| Xu 2017 | MoCA | 0% (0/48) | 4.2% (2/48) | 0.29 |
| Zhou 2019 | MoCA | 15.8% (6/38) | 31.6% (12/38) | 0.12 |
| Wang 2015 | Chinese Neurocognitive Scale | 8.0% (6/75) | 19.5% (15/77) | 0.04 |
| Ding 2015 | Chinese Neurocognitive Scale | 22% (11/50) | 34% (17/50) | 0.19 |
| Mohamed 2014 | Stroop color test | 13.3% (8/60) | 35% (7/20) | < 0.001 |
| Shi 2020 | A comprehensive test scale of four domains | 13.2% (7/53) | 35.8% (19/53) | 0.01 |
Abbreviations: RR, risk ratio; CI, confidence interval; Dex, dexmedetomidine; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; POCD, postoperative cognitive dysfunction.
3.6. Publication bias
The funnel plots for the meta‐analysis of POCD were symmetrical, suggesting low risk of publication bias (Figure 3). Egger's regression test also showed low risk of publication bias (p for Egger's regression test = 0.579).
FIGURE 3.

Funnel plots for the publication bias within the meta‐analysis of effect of Dex on the risk of POCD in elderly population in studies of POCD diagnosed with the MMSE
4. DISCUSSION
In this study, by pooling the results of available RCTs, the results of the meta‐analysis showed that compared to placebo, Dex significantly reduced the incidence of POCD as evaluated by MMSE in the elderly population following surgery with general anesthesia. Besides, results of subgroup analyses showed that the potential preventative efficacy of Dex on POCD in elderly population was consistent in single‐blind and double‐blind studies. Sensitivity analyses showed consistent results in studies from China, in patients with noncardiac surgeries, and in studies with Dex administered within the surgeries only. For the remaining six studies with POCD evaluated by instruments other than MMSE, three of them showed Dex could significantly reduce the risk of POCD, while the other three showed a nonsignificantly reduced incidence of POCD following Dex. Taken together, these findings indicated that Dex is associated with a reduced risk of POCD in elderly patients receiving surgeries with general anesthesia, and the results were mainly obtained in studies with POCD diagnosed with MMSE. Based on these findings, Dex may be considered as a preventative measure for POCD in these patients.
To our knowledge, two previous meta‐analyses (Man et al., 2015; C. Zhou et al., 2016) published in 2015 and 2016, respectively, have devalued the influences of Dex on postoperative cognitive function. One study including RCTs published before 2015 showed that perioperative dexmedetomidine treatment is associated with significantly better neurocognitive function postoperatively in comparison with both saline controls and comparator anesthetics (Man et al., 2015). However, this study focused on the changes of MMSE scores after surgery, rather than the incidence of POCD, and included patients with a wide range of ages rather than the elderly population (Man et al., 2015). The other meta‐analysis combined the results of RCTs published until 2015 and showed that dexmedetomidine reduced the incidence of POCD in elderly patients after general anesthesia (C. Zhou et al., 2016). However, results of studies comparing Dex with placebo and other active sedatives were both combined, which made it difficult to interpret the results. Moreover, neither of the meta‐analyses included subgroup analyses to evaluate whether the results were affected by differences of study characteristics. Since considerable RCTs have been published after these meta‐analyses (Man et al., 2015; C. Zhou et al., 2016), we performed an updated analysis to systematically evaluate the influence of Dex on the incidence of POCD in elderly patients after surgery with general anesthesia. Our study has several strengths compared to the previous ones. Firstly, we adopted rigorous literature search, as well as strict inclusion and exclusion criteria to focus on the comparison between Dex and placebo in elderly patients after surgery. Secondly, an up‐to‐date literature search was performed and the numbers of studies and patients included in this meta‐analysis is much larger than the previous ones. Finally, considering the potential clinical heterogeneity which may result due to the difference in instruments used for the diagnosis of POCD, we quantitatively evaluated the influence of Dex on POCD in studies using the MMSE in a meta‐analysis, and qualitatively synthesized the results of the studies using other instruments for the diagnosis of POCD. Overall, we found that perioperative use of Dex is associated with a significantly reduced risk of POCD in elderly patients after general anesthesia. Sensitivity analysis confirmed the robustness of the finding, which was not primarily driven by either of the included studies. Subgroup analyses also showed that the possible preventative efficacy of Dex for POCD was not significantly affected by difference of study design. Taken together, based on these findings, Dex should be recommended as a potential preventative strategy for POCD in elderly patients.
Pathologically, neuroinflammation induced by surgery has been considered as one of the primary mechanisms underlying the pathogenesis of POCD (Luo et al., 2019; Umholtz & Nader, 2017). Accumulating evidence from animal studies showed that Dex could alleviate neuroinflammation induced by surgery or lipopolysaccharide via regulation of systematic inflammatory cytokines including interleukin 1β, tumor necrosis factor‐α, and NF‐κB (N. Chen et al., 2019), inhibiting the expressions of Toll‐like receptor 4 (Yamanaka et al., 2017; X. Y. Zhou et al., 2020), and through α2 adrenoceptor‐mediated anti‐inflammatory pathways (R. Li et al., 2020). However, a recent clinical study showed that Dex preserved postoperative cognitive function in elderly patients who received total knee arthroplasty without significant modulation on peripheral inflammation (Mei et al., 2020), suggesting mechanisms besides anti‐inflammation are also involved. Future studies are warranted to determine the mechanisms underlying the benefits of Dex on postoperative cognitive function in the elderly.
This study also has limitations. Firstly, as previously indicated, instruments for neurocognitive testing and diagnostic criteria for POCD varied among the included studies, and the difference in the definition of POCD may affect the results of the meta‐analysis. In view of the emerged consensus regimens for neurocognitive testing and diagnostic criteria for POCD, such as the Recommendations for the Nomenclature of Cognitive Change associated with Anaesthesia and Surgery (2018) (Evered et al., 2018), studies evaluating the possible preventative strategies for POCD diagnosed with standardized criteria are needed. Furthermore, most of the studies were performed in Chinese population, and all of the studies were performed in the developing countries. Studies from developed countries are needed to validate the consistency of the findings. Accordingly, role of Dex on POCD in elderly population with other ethnicities remains to be evaluated. In addition, we did not have access to the individual patient data. Accordingly, potential influences of patient or study characteristics on the outcomes of the meta‐analysis were unable to be evaluated, such as the sex, comorbidities, and concurrent medications of the patients. Finally, studies are needed to determine whether the differences in the regimens and doses of Dex could affect the possible preventative efficacy of Dex on POCD in the elderly.
5. CONCLUSION
In conclusion, results of this meta‐analysis showed that Dex is associated with a reduced risk of POCD in elderly patients receiving surgeries with general anesthesia, and the results were mainly obtained in studies with POCD diagnosed with MMSE. These findings support that Dex should considered as a preventative measure for POCD in this population.
CONFLICT OF INTEREST
The authors declare that they have no conflicts of interest.
AUTHOR CONTRIBUTIONS
HY, GC and BL contributed to the conception and design of the study. HY and HK performed literature search, study identification, quality evaluation, and data extraction. HY, HK, and JF performed the statistical analysis. HY, GC, and BL wrote the first draft of the manuscript. All authors contributed to manuscript revision, and read and approved the submitted version.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/brb3.2665
Yu, H. , Kang, H. , Fan, J. , Cao, Ge. , & Liu, B. (2022). Influence of dexmedetomidine on postoperative cognitive dysfunction in the elderly: A meta‐analysis of randomized controlled trials. Brain and Behavior, 12, e2665. 10.1002/brb3.2665
Contributor Information
Ge Cao, Email: caoge_5233@21cn.com.
Bin Liu, Email: liubinwch_01@tom.com.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.
REFERENCES
- Abowali, H. A. , Paganini, M. , Enten, G. , Elbadawi, A. , & Camporesi, E. M. (2021). Critical review and meta‐analysis of postoperative sedation after adult cardiac surgery: Dexmedetomidine versus propofol. Journal of Cardiothoracic and Vascular Anesthesia, 35(4), 1134–1142. 10.1053/j.jvca.2020.10.022 [DOI] [PubMed] [Google Scholar]
- Belrose, J. C. , & Noppens, R. R. (2019). Anesthesiology and cognitive impairment: A narrative review of current clinical literature. BMC Anesthesiol, 19(1), 241. 10.1186/s12871-019-0903-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodier, E. A. , & Cibelli, M. (2021). Postoperative cognitive dysfunction in clinical practice. BJA Educ, 21(2), 75–82. 10.1016/j.bjae.2020.10.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J. , Yan, J. , & Han, X. (2013). Dexmedetomidine may benefit cognitive function after laparoscopic cholecystectomy in elderly patients. Exp Ther Med, 5(2), 489–494. 10.3892/etm.2012.811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, N. , Chen, X. , Xie, J. , Wu, C. , & Qian, J. (2019). Dexmedetomidine protects aged rats from postoperative cognitive dysfunction by alleviating hippocampal inflammation. Mol Med Rep, 20(3), 2119–2126. 10.3892/mmr.2019.10438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding, L. , Zhang, H. , Mi, W. , He, Y. , Zhang, X. , Ma, X. , & Li, H. (2015). Effects of dexmedetomidine on recovery period of anesthesia and postoperative cognitive function after robot‐assisted laparoscopicradical prostatectomy in the elderly people. Zhong Nan Da Xue Xue Bao Yi Xue Ban, 40(2), 129–135. 10.11817/j.issn.1672-7347.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Evered, L. , Silbert, B. , Knopman, D. S. , Scott, D. A. , DeKosky, S. T. , Rasmussen, L. S. , & Eckenhoff, R. G. (2018). Recommendations for the nomenclature of cognitive change associated with anaesthesia and surgery‐2018. Anesthesiology, 129(5), 872–879. 10.1097/ALN.0000000000002334 [DOI] [PubMed] [Google Scholar]
- Gao, L. , Taha, R. , Gauvin, D. , Othmen, L. B. , Wang, Y. , & Blaise, G. (2005). Postoperative cognitive dysfunction after cardiac surgery. Chest, 128(5), 3664–3670. 10.1378/chest.128.5.3664 [DOI] [PubMed] [Google Scholar]
- Gao, Y. , Zhu, X. , Huang, L. , Teng, J. , & Li, F. (2020). Effects of dexmedetomidine on cerebral oxygen saturation and postoperative cognitive function in elderly patients undergoing minimally invasive coronary artery bypass surgery. Clinical Hemorheology and Microcirculation, 74(4), 383–389. 10.3233/CH-190590 [DOI] [PubMed] [Google Scholar]
- Higgins, J. , Thomas, J. , Chandler, J. , Cumpston, M. , Li, T. , Page, M. , & Welch, V. (2021). Cochrane handbook for systematic reviews of interventions version 6.2. The Cochrane Collaboration, www.training.cochrane.org/handbook [Google Scholar]
- Jiang, L. , Hu, M. , Lu, Y. , Cao, Y. , Chang, Y. , & Dai, Z. (2017). The protective effects of dexmedetomidine on ischemic brain injury: A meta‐analysis. Journal of Clinical Anesthesia, 40, 25–32. 10.1016/j.jclinane.2017.04.003 [DOI] [PubMed] [Google Scholar]
- Keating, G. M. (2015). Dexmedetomidine: A review of its use for sedation in the intensive care setting. Drugs, 75(10), 1119–1130. 10.1007/s40265-015-0419-5 [DOI] [PubMed] [Google Scholar]
- Lee, S. (2019). Dexmedetomidine: Present and future directions. Korean J Anesthesiol, 72(4), 323–330. 10.4097/kja.19259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, R. , Lai, I. K. , Pan, J. Z. , Zhang, P. , & Maze, M. (2020). Dexmedetomidine exerts an anti‐inflammatory effect via alpha2 adrenoceptors to prevent lipopolysaccharide‐induced cognitive decline in Mice. Anesthesiology, 133(2), 393–407. 10.1097/ALN.0000000000003390 [DOI] [PubMed] [Google Scholar]
- Li, Y. , He, R. , Chen, S. , & Qu, Y. (2015). Effect of dexmedetomidine on early postoperative cognitive dysfunction and peri‐operative inflammation in elderly patients undergoing laparoscopic cholecystectomy. Exp Ther Med, 10(5), 1635–1642. 10.3892/etm.2015.2726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, Z. , Li, H. , Yao, S. , Cheng, M. , & Chen, J. (2021). Effects of dexmedetomidine doses on postoperative cognitive dysfunction and serum beta‐ amyloid and cytokine levels in elderly patients after spine surgery: A randomized controlled trial. Nan Fang Yi Ke Da Xue Xue Bao, 41(4), 600–606. 10.12122/j.issn.1673-4254.2021.04.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin, X. , Chen, Y. , Zhang, P. , Chen, G. , Zhou, Y. , & Yu, X. (2020). The potential mechanism of postoperative cognitive dysfunction in older people. Experimental Gerontology, 130, 110791. 10.1016/j.exger.2019.110791 [DOI] [PubMed] [Google Scholar]
- Liu, Y. , Zhu, X. , He, Z. , Sun, Z. , Wu, X. , & Zhong, J. (2020). Protective effect of dexmedetomidine infusion combined with epidural blockade on postoperative complications after surgery: A prospective randomized controlled clinical trial. Journal of International Medical Research, 48(6), 10.1177/0300060520930168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo, A. , Yan, J. , Tang, X. , Zhao, Y. , Zhou, B. , & Li, S. (2019). Postoperative cognitive dysfunction in the aged: The collision of neuroinflammaging with perioperative neuroinflammation. Inflammopharmacology, 27(1), 27–37. 10.1007/s10787-018-00559-0 [DOI] [PubMed] [Google Scholar]
- Man, Y. , Guo, Z. , Cao, J. , & Mi, W. (2015). Efficacy of perioperative dexmedetomidine in postoperative neurocognitive function: A meta‐analysis. Clinical and Experimental Pharmacology & Physiology, 42(8), 837–842. 10.1111/1440-1681.12432 [DOI] [PubMed] [Google Scholar]
- Mansouri, N. , Nasrollahi, K. , & Shetabi, H. (2019). Prevention of cognitive dysfunction after cataract surgery with intravenous administration of midazolam and dexmedetomidine in elderly patients undergoing cataract surgery. Adv Biomed Res, 8(6), 10.4103/abr.abr_190_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mei, B. , Xu, G. , Han, W. , Lu, X. , Liu, R. , Cheng, X. , & Zhang, Y. (2020). The benefit of dexmedetomidine on postoperative cognitive function is unrelated to the modulation on peripheral inflammation: A single‐center, prospective, randomized study. Clinical Journal of Pain, 36(2), 88–95. 10.1097/AJP.0000000000000779 [DOI] [PubMed] [Google Scholar]
- Mohamed, S. , & Shaaban, A. R. (2014). The effect of dexmedetomidine on the incidence of postoperative cognitive dysfunction in elderly patients after prolonged abdominal surgery. Egyptian Journal of Anaesthesia, 30(4), 331–338. 10.1016/j.egja.2014.03.007 [DOI] [Google Scholar]
- Page, M. J. , McKenzie, J. E. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , & Moher, D. (2021a). The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. Bmj, 372, n71. 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Page, M. J. , Moher, D. , Bossuyt, P. M. , Boutron, I. , Hoffmann, T. C. , Mulrow, C. D. , & McKenzie, J. E. (2021b). PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ, 372, n160. 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero, C. , Bonamassa, L. , Pelini, L. , Prioletta, I. , Cianferotti, L. , Metozzi, A. , & Brandi, M. L. (2017). Early post‐surgical cognitive dysfunction is a risk factor for mortality among hip fracture hospitalized older persons. Osteoporosis International, 28(2), 667–675. 10.1007/s00198-016-3784-3 [DOI] [PubMed] [Google Scholar]
- Shi, H. , Du, X. , Wu, F. , Hu, Y. , Xv, Z. , & Mi, W. (2020). Dexmedetomidine improves early postoperative neurocognitive disorder in elderly male patients undergoing thoracoscopic lobectomy. Exp Ther Med, 20(4), 3868–3877. 10.3892/etm.2020.9113 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Umholtz, M. , & Nader, N. D. (2017). Anesthetic immunomodulation of the neuroinflammation in postoperative cognitive dysfunction. Immunological Investigations, 46(8), 805–815. 10.1080/08820139.2017.1373898 [DOI] [PubMed] [Google Scholar]
- Urits, I. , Orhurhu, V. , Jones, M. , Hoyt, D. , Seats, A. , & Viswanath, O. (2019). Current perspectives on postoperative cognitive dysfunction in the ageing population. Turk J Anaesthesiol Reanim, 47(6), 439–447. 10.5152/TJAR.2019.75299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, K. , Li, C. , Shi, J. , & Wei, H. (2015). Effects of patient‐controlled intravenous analgesia with dexmedetomidine and sufentanil on postoperative cognition in elderly patients after spine surgery. Zhonghua Yi Xue Za Zhi, 95(30), 2437–2441. [PubMed] [Google Scholar]
- Wang, Y. , Han, R. , & Zuo, Z. (2016). Dexmedetomidine‐induced neuroprotection: Is it translational? Transl Perioper Pain Med, 1(4), 15–19. [PMC free article] [PubMed] [Google Scholar]
- Xu, H. Y. , Fu, G. H. , & Wu, G. S. (2017). Effect of dexmedetomidine‐induced anesthesia on the postoperative cognitive function of elder patients after laparoscopic ovarian cystectomy. Saudi J Biol Sci, 24(8), 1771–1775. 10.1016/j.sjbs.2017.11.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka, D. , Kawano, T. , Nishigaki, A. , Aoyama, B. , Tateiwa, H. , Shigematsu‐Locatelli, M. , & Yokoyama, M. (2017). Preventive effects of dexmedetomidine on the development of cognitive dysfunction following systemic inflammation in aged rats. Journal of Anesthesia, 31(1), 25–35. 10.1007/s00540-016-2264-4 [DOI] [PubMed] [Google Scholar]
- Zhang, Y. , Xing, Z. , Xu, Y. , & Xu, S. (2014). Effects of different doses of dexmedetomidine on cognitive dysfunction in elderly patients early after laparoscopic surgery for colorectal cancer. Nan Fang Yi Ke Da Xue Xue Bao, 34(5), 743–746. [PubMed] [Google Scholar]
- Zhao, W. , Hu, Y. , Chen, H. , Wang, X. , Wang, L. , Wang, Y. , & Han, F. (2020). The effect and optimal dosage of dexmedetomidine plus sufentanil for postoperative analgesia in. elderly patients with postoperative delirium and early postoperative cognitive dysfunction: A single‐center, prospective, randomized, double‐blind, controlled trial. Front Neurosci, 14, 549516. 10.3389/fnins.2020.549516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, C. , Zhu, Y. , Liu, Z. , & Ruan, L. (2016). Effect of dexmedetomidine on postoperative cognitive dysfunction in elderly patients after general anaesthesia: A meta‐analysis. Journal of International Medical Research, 44(6), 1182–1190. 10.1177/0300060516671623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, M. , Lyu, Y. , Zhu, Y. , Jiang, T. , Wu, C. , Yang, J. , & Wang, L. (2019). Effect of ulinastatin combined with dexmedetomidine on postoperative cognitive dysfunction in patients who underwent cardiac surgery. Front Neurol, 10, 1293. 10.3389/fneur.2019.01293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, X. Y. , Liu, J. , Xu, Z. P. , Fu, Q. , Wang, P. Q. , Wang, J. H. , & Zhang, H. (2020). Dexmedetomidine ameliorates postoperative cognitive dysfunction by inhibiting Toll‐like receptor 4 signaling in aged mice. The Kaohsiung Journal of Medical Sciences, 36(9), 721–731. 10.1002/kjm2.12234 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this article. Further enquiries can be directed to the corresponding author.


