Abstract
Aims
Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVC) is characterized by ventricular arrhythmias (VAs) and sudden cardiac death (SCD). We aimed to develop a model for individualized prediction of incident VA/SCD in ARVC patients.
Methods and results
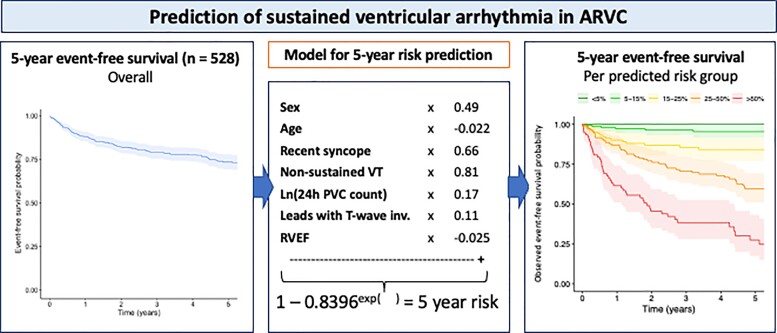
Five hundred and twenty-eight patients with a definite diagnosis and no history of sustained VAs/SCD at baseline, aged 38.2 ± 15.5 years, 44.7% male, were enrolled from five registries in North America and Europe. Over 4.83 (interquartile range 2.44–9.33) years of follow-up, 146 (27.7%) experienced sustained VA, defined as SCD, aborted SCD, sustained ventricular tachycardia, or appropriate implantable cardioverter-defibrillator (ICD) therapy. A prediction model estimating annual VA risk was developed using Cox regression with internal validation. Eight potential predictors were pre-specified: age, sex, cardiac syncope in the prior 6 months, non-sustained ventricular tachycardia, number of premature ventricular complexes in 24 h, number of leads with T-wave inversion, and right and left ventricular ejection fractions (LVEFs). All except LVEF were retained in the final model. The model accurately distinguished patients with and without events, with an optimism-corrected C-index of 0.77 [95% confidence interval (CI) 0.73–0.81] and minimal over-optimism [calibration slope of 0.93 (95% CI 0.92–0.95)]. By decision curve analysis, the clinical benefit of the model was superior to a current consensus-based ICD placement algorithm with a 20.3% reduction of ICD placements with the same proportion of protected patients (P < 0.001).
Conclusion
Using the largest cohort of patients with ARVC and no prior VA, a prediction model using readily available clinical parameters was devised to estimate VA risk and guide decisions regarding primary prevention ICDs (www.arvcrisk.com).
Keywords: Arrhythmogenic right ventricular cardiomyopathy, Implantable cardioverter-defibrillators, Sudden cardiac death, Ventricular arrhythmias
Introduction
Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVC) is an inherited cardiomyopathy characterized by progressive fibrofatty replacement of the myocardium which predisposes patients to ventricular arrhythmias (VAs) and sudden cardiac death (SCD). Once the diagnosis is established, a primary goal of management is prevention of SCD, for which implantable cardioverter-defibrillators (ICDs) are a common consideration. There is agreement that most ARVC patients with a prior history of sustained VA or resuscitated sudden cardiac arrest (SCA) benefit from secondary prevention ICDs.1 However, for the primary prevention population, there is no established risk stratification scheme.
Over the past two decades, multiple attempts have been made to identify factors associated with VA in this clinically challenging population.2–4 While these studies have significantly contributed to our understanding of clinical, demographic, and behavioural factors associated with arrhythmic risk, the relatively small patient populations provided insufficient statistical power to assess the independent predictive value of potentially correlated risk factors.5
To overcome this important limitation, we assembled a large cohort of patients with ARVC from five registries (Johns Hopkins, Dutch, Nordic, Swiss, and Canadian) without a history of sustained VAs at baseline. Our aim was (i) to develop a model for individualized prediction of incident sustained VA in patients with ARVC using readily available clinical variables; and (ii) to compare this new model to a current consensus-based ICD placement algorithm.
Methods
Study design
We conducted an observational, retrospective, longitudinal cohort study in accordance with the Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis (TRIPOD) statement.6
Study population
The study population was drawn from five ARVC registries encompassing 14 academic centres (Supplementary material online, Table S1) in six countries. Each registry is itself a longitudinal cohort study. From each registry, we included all patients who (i) were diagnosed with definite ARVC by the current 2010 Task Force Criteria (TFC)7 and (ii) had not experienced spontaneous sustained VA or SCA at diagnosis. The study conforms to the Helsinki declaration and was approved by local ethics and/or institutional review boards.
Study outcomes
The primary study outcome was the first sustained VA following diagnosis by the TFC. Sustained VA was defined as a composite of the occurrence of SCD, SCA, spontaneous sustained ventricular tachycardia (VT) (VT lasting ≥30 s at ≥100 b.p.m. or with haemodynamic compromise requiring cardioversion), ventricular fibrillation/flutter (VF), or appropriate ICD intervention. The first episode of a rapid sustained VA (defined as SCD, SCA, VF, or VT >250 b.p.m.), heart transplantation, cardiovascular mortality, and all-cause mortality were also recorded.
Predictors
Potential predictors were pre-specified based on clinical experience and current literature on arrhythmic risk stratification in ARVC including: (i) a recent systematic review and meta-analysis5 and (ii) the International Task Force Consensus (ITFC) Statement for Treatment of ARVD/C1 and a recent analysis of the performance of its risk stratification algorithm.8 Variables considered were sex, age, recent (<6 months) cardiac syncope, non-sustained VT (NSVT), number of premature ventricular complexes (PVCs) on 24-h Holter monitoring, extent of T-wave inversion (TWI) on anterior and inferior leads, right ventricular ejection fraction (RVEF), and left ventricular ejection fraction (LVEF). Each predictor variable was determined at the time of diagnosis, defined as 1 year before to 1 year after the date of diagnosis by TFC but always prior to occurrence of the primary outcome. Definitions of predictor variables are provided in Supplementary material online, Table S2. In Supplementary material online, Table S3, we describe the rationale for selecting each predictor, as well as the rationale for excluding other variables.
Data collection
Data were collected independently by each registry according to standard operating procedures (Supplementary material online, Table S4). Outcomes were adjudicated at each centre via review of electrocardiogram (ECG) tracings, ICD interrogation tracings, as well as medical and death records. ECGs were interpreted through an ECG core laboratory by two cardiologists–electrophysiologists (J.C.T. and R.T.) blinded to the rest of the data and outcomes. Genetic variants were adjudicated according to the American College of Medical Genetics and Genomics guidelines by consensus of specialists in cardiac genetics (B.M., J.D.H.J., J.P.v.T., and C.A.J.).9
Statistical analysis
Analyses were performed using SAS software version 9.4 (SAS Institute, Cary, NC, USA) and RStudio version 1.1.414 (Boston, MA, USA). Categorical variables were summarized as frequencies (%) and compared using the χ2 or the Fisher's exact tests, as appropriate. Continuous variables were presented as mean ± standard deviation or median [interquartile range (IQR)], and compared using the independent sample t-test or the Mann–Whitney U test. Follow-up duration was calculated from the date of diagnosis to the date of reaching the endpoint or censoring, which was defined as death from any other cause, heart transplantation, or the most recent follow-up visit at which the endpoint could be ascertained. The overall probability of survival free from sustained VA was estimated using the Kaplan–Meier method.
Missing data
Potential bias from missing data was evaluated by comparing characteristics of patients with one or more missing predictor variables to patients with complete data. Missing quantitative values for RVEF and LVEF were imputed manually when qualitative assessment was present (as detailed in Supplementary material online, Table S4). Other missing data were assumed to be missing at random and imputed using multiple imputation with chained equations.10 The multiple imputation model included all pre-specified predictors as well as proband status, QRS duration, right ventricular volume, ICD carrier status, together with the outcome, and a cumulative baseline hazard estimation.11 A total of 25 imputed datasets were generated, and the final inference estimations were combined using Rubin's rules.12 A complete case analysis and an analysis without manual imputation of RVEF and LVEF were conducted as sensitivity analyses.
Model development and validation
The association between the pre-specified predictors and the primary outcome was assessed using Cox regression. Proportional-hazard assumptions were verified as well as linearity of the association for continuous predictors. The final model was fitted using stepwise backward selection based on Akaike's Information Criterion.6 The discriminative performance of the model was measured using Harrell's C-statistic.
The model was validated using 200 bootstrap samples. The degree of optimism was estimated by the average calibration slope of the bootstrap samples.13 Agreement between predicted and observed outcomes was evaluated graphically using calibration plots that incorporated grouped Kaplan–Meier estimates and the continuous hazard regression function.14 Calibration analyses were repeated for patient subgroups including genotype and ICD status.
Model presentation
For an individual patient, the risk of sustained VA was calculated using the following equation:
where S0(t) is the baseline survival probability at time t (i.e. at 5 years), and LP (linear predictor) is the sum of the products of the predictors and associated coefficients for a given patient.
Clinical utility
To assess the implications of our model in clinical practice, we compared performance of our model to that of the consensus-based algorithm for ICD placement published in the ITFC Statement for Treatment of ARVC.1 First, we explored the clinical impact of potential thresholds for ICD implantation by evaluating the proportions of appropriate and inappropriate treatment at each of these thresholds. Second, we performed a decision curve analysis to evaluate the clinical benefit of our model. In this analysis, the clinical benefit was assessed by the ‘net benefit'; a weighted measure of the balance between appropriate and inappropriate ICD implantations.15 A value of 0 indicates no benefit, while higher values indicate greater benefit.
Results
Study population
The study population consisted of 528 patients with definite ARVC and no history of sustained VA or SCA at time of diagnosis. Almost half (n = 236, 44.7%) of the population was male with an average age at diagnosis of 38.2 ± 15.5 years. Probands (n = 263, 49.8%), the first affected individual in a family seeking medical attention for ARVC, and family members (n = 265, 50.2%) were equally represented. Two-thirds (n = 340, 64.4%) of patients had a pathogenic or likely pathogenic variant (e.g. mutation) in an ARVC-associated gene. Other clinical and demographic characteristics are summarized in Table 1. The study population had balanced representation from North America (n = 259, 49.1%) and Europe (n = 269, 50.9%). Characteristics of patients contributed by each registry are shown in Supplementary material online, Table S5.
Table 1.
Baseline clinical characteristics
| Overall | Patients without sustained VA | Patients with sustained VA | P-value | |
|---|---|---|---|---|
| Total | 528 (100.0) | 382 (72.3) | 146 (27.7) | |
| Demographics | ||||
| Male sex | 236 (44.7) | 155 (40.6) | 81 (55.5) | 0.003 |
| Age at diagnosis (years) | 38.16 ± 15.47 | 39.73 ± 15.84 | 34.05 ± 13.67 | <0.001 |
| Caucasian ethnicity (n = 498) | 485 (91.9) | 348 (91.1) | 137 (93.8) | 0.064 |
| Proband status | 263 (49.8) | 151 (39.5) | 112 (76.7) | <0.001 |
| Pathogenic mutation (n = 504) | 340 (64.4) | 248 (64.9) | 92 (63.0) | 0.599 |
| PKP2 | 258 (48.9) | 185 (48.4) | 73 (50.0) | 0.582 |
| DSP | 23 (4.4) | 18 (4.7) | 5 (3.4) | |
| DSG2 | 17 (3.2) | 15 (3.9) | 2 (1.4) | |
| PLN | 26 (4.9) | 19 (5.0) | 7 (4.8) | |
| Multiple mutations | 6 (1.1) | 4 (1.0) | 2 (1.4) | |
| Other | 10 (1.9) | 7 (1.8) | 3 (2.1) | |
| History | ||||
| Symptoms | 307 (58.1) | 190 (49.7) | 117 (80.1) | <0.001 |
| Cardiac syncope | 107 (20.3) | 59 (15.4) | 48 (32.9) | <0.001 |
| Recent cardiac syncope (n = 519) | 48 (9.1) | 23 (6.0) | 25 (17.1) | <0.001 |
| ECG/continuous ECG monitoring | ||||
| TWI in ≥3 precordial leads (n = 517) | 298 (56.4) | 193 (50.5) | 105 (71.9) | <0.001 |
| TWI in ≥2 inferior leads (n = 506) | 85 (16.1) | 53 (13.9) | 32 (21.9) | 0.021 |
| NSVT (n = 470) | 231 (43.8) | 145 (38.0) | 86 (58.9) | <0.001 |
| 24 h PVC count (n = 425) | 1007 (278–3731) | 833 (125–2768) | 2782 (992–5918) | <0.001 |
| Imaging | ||||
| RVEF (%) (n = 510)a | 43.80 ± 10.40 | 45.40 ± 9.55 | 39.33 ± 11.37 | <0.001 |
| LVEF (%) (n = 515) | 57.66 ± 8.42 | 58.16 ± 8.00 | 56.34 ± 9.34 | 0.029 |
| Treatment at baseline | ||||
| ICD | 218 (41.3) | 136 (35.6) | 82 (56.2) | <0.001 |
| Beta-blockers (n = 511) | 200 (37.9) | 142 (37.2) | 58 (39.7) | 0.343 |
| Anti-arrhythmic drugs (n = 510) | 82 (15.5) | 50 (13.1) | 32 (21.9) | 0.019 |
Variables are expressed as frequency (%), mean ± standard deviation, or median (IQR). Total number of patients for a given variable mentioned if missing data.
DSG2, desmoglein-2; DSP, desmoplakin; ICD, implantable cardioverter-defibrillator; IQR, interquartile range; LVEF, left ventricular ejection fraction; NSVT, non-sustained ventricular tachycardia; PKP2, plakophilin-2; PLN, phospholamban; PVC, premature ventricular complex; RVEF, right ventricular ejection fraction; TWI, T-wave inversion; VA, ventricular arrhythmia.
RVEF estimation was based on quantitative measurement by CMR in 327 patients, by echocardiography in 160, by qualitative CMR assessment in 20, and by angiography in 3.
Overall, 390 (73.8%) patients had complete data for the pre-specified predictors. Missing data occurred for six of the eight predictors: recent cardiac syncope (n = 9, 1.7%), NSVT (n = 58, 11.0%), PVC count (n = 103, 19.5%), sum of TWI in anterior and inferior leads (n = 22, 4.2%), RVEF (n = 19, 3.6%), and LVEF (n = 13, 2.5%).
Outcomes
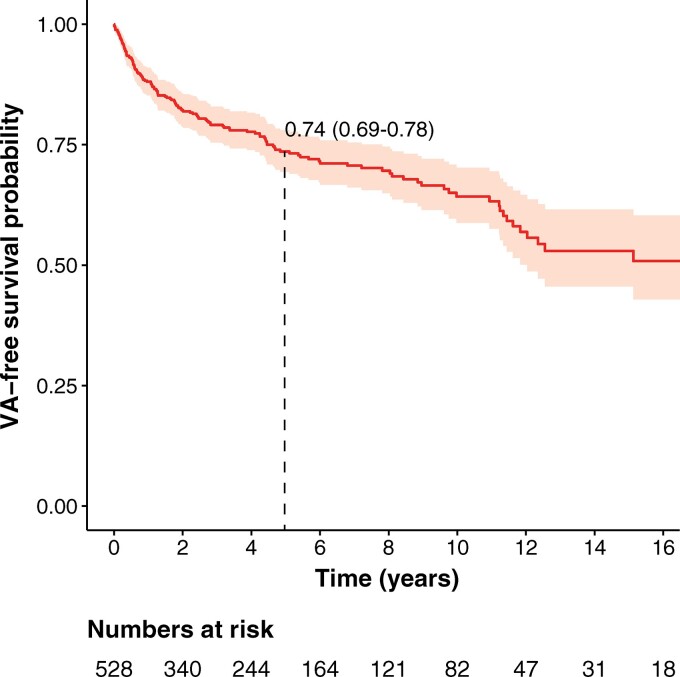
During a median follow-up of 4.83 years (IQR 2.44–9.33 years), 146 (27.7%) patients experienced the composite outcome, with a corresponding annual event rate of 5.6% [95% confidence interval (CI) 4.7–6.6]. Figure 1 shows the cumulative survival free from first sustained VA. As shown in the figure, events occurred throughout follow-up, with a cumulative event-free survival at 5 years of 73.6% (95% CI 69.4–78.0%). The most common first sustained VA was appropriate ICD therapy (n = 102, 70.0%), followed by spontaneous sustained VT (n = 35, 23.9%), SCA (n = 6, 4.1%), and SCD (n = 3, 2.0%). Rapid sustained VAs (VT with cycle length <240 ms, SCA, or SCD) were experienced by 53 (10.0%) patients during follow-up at an annual event rate of 1.7% (95% CI 1.3–2.2). At last follow-up, 18 (3.4%) patients had died and 14 (2.7%) had undergone heart transplantation.
Figure 1.
Cumulative survival free from sustained ventricular arrhythmia. Plotted is the cumulative event-free survival for any ventricular arrhythmia with 95% confidence intervals (shaded area). Dotted line represents cumulative 5-year survival.
Model development
Table 1 shows baseline characteristics of patients with and without sustained VA during follow-up. Table 2 summarizes development of the risk prediction model. As shown in these tables, each pre-specified predictor had a significant (P < 0.05) univariable linear or log-linear relationship with the primary outcome. All predictors were, therefore, fitted into a multivariable model, after which stepwise backward selection was performed leading to the removal of LVEF from the final model. As a sensitivity analysis, we repeated this process (i) for patients with complete data and (ii) without manual imputation for RVEF. As can be appreciated from Supplementary material online, Table S6, this resulted in inclusion of the same predictor variables (i.e. excluding LVEF) with only small changes to the coefficients in the resulting model.
Table 2.
Ventricular arrhythmia risk prediction model
| Univariable model | Multivariable (final model) | |||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Male sex | 1.74 (1.26–2.42) | <0.001 | 1.63 (1.17–2.29) | 0.005 |
| Age (per year increase) | 0.98 (0.97–0.99) | 0.001 | 0.98 (0.97–0.99) | <0.001 |
| Recent cardiac syncope | 2.57 (1.66–3.97) | <0.001 | 1.93 (1.20–3.11) | 0.007 |
| Prior NSVT | 3.15 (2.12–4.68) | <0.001 | 2.25 (1.47–3.44) | <0.001 |
| 24 h PVC count (ln)a | 1.32 (1.17–1.48) | <0.001 | 1.19 (1.05–1.34) | 0.013 |
| Leads with TWI anterior + inferior | 1.20 (1.12–1.29) | <0.001 | 1.12 (1.02–1.23) | 0.014 |
| RVEF (per % decrease) | 1.05 (1.03–1.06) | <0.001 | 1.03 (1.01–1.04) | 0.002 |
| LVEF (per % decrease) | 1.02 (1.01–1.04) | 0.011 | Not included in the final model | |
LVEF, left ventricular ejection fraction; NSVT, non-sustained ventricular tachycardia; PVC, premature ventricular complex; RVEF, right ventricular ejection fraction; TWI, T-wave inversion.
PVC count had a log-linear relationship.
The following formula allows for the calculation of the 5-year risk of sustained VA:
where LP = 0.488*sex − 0.022*age + 0.657*history of recent cardiac syncope + 0.811*history of NSVT + 0.170*ln(24 h PVC count) + 0.113*Sum of anterior and inferior leads with TWI − 0.025*RVEF.
Supplementary material online, Table S7 provides the probability of survival (S0(t)) at 1, 2, 3, and 4 years to facilitate calculating risk for shorter time durations.
Supplementary material online, Table S8 illustrates the use of this risk calculator in 3 patients from our cohort. An online application to calculate risk for an individual patient is available at: www.arvcrisk.com.
Model validation
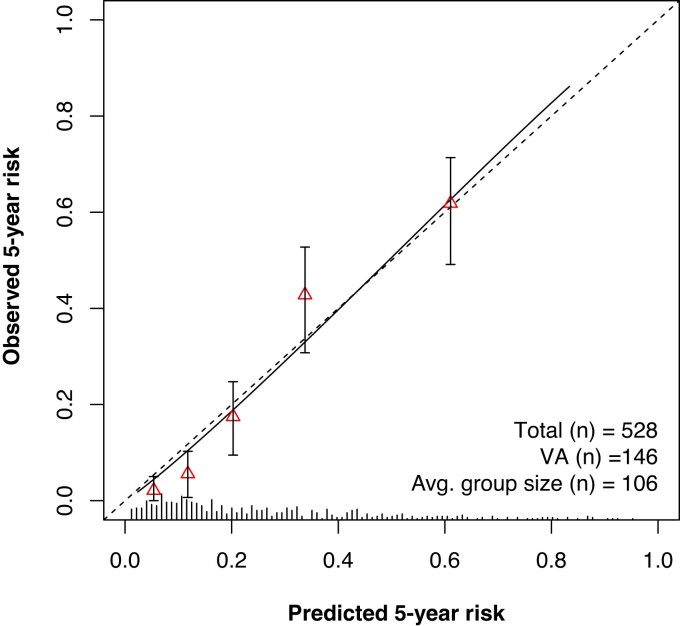
The optimism-corrected C-statistic of the predictive model was 0.77 (95% CI 0.73–0.81). Internal validation with bootstrapping revealed a calibration slope of 0.93 (95% CI 0.92–0.95), reflecting a small degree of over-optimism. Figure 2 presents a graphical representation of calibration, showing good overall agreement between the predicted and observed 5-year risk. Calibration plots showing similarly good agreement for shorter follow-up durations can be found in Supplementary material online, Figure S1. Additional calibration plots for patients stratified by ICD carrier status and genotype are presented in Supplementary material online, Figure S2. As can be appreciated from this figure, predicted and observed 5-year risk remained concordant in these patient subgroups.
Figure 2.
Calibration plot showing the agreement between predicted (x axis) and observed (y axis) 5-year risk of the primary outcome. Triangles represent binned Kaplan–Meier estimates with 95% confidence intervals for quintiles of predicted risk. Straight line is the continuous calibration hazard regression. Dotted line represents perfect calibration. Spike histogram on the x axis reflects the number of patients with a predicted risk corresponding to the x axis value. VA, ventricular arrhythmia.
Clinical utility
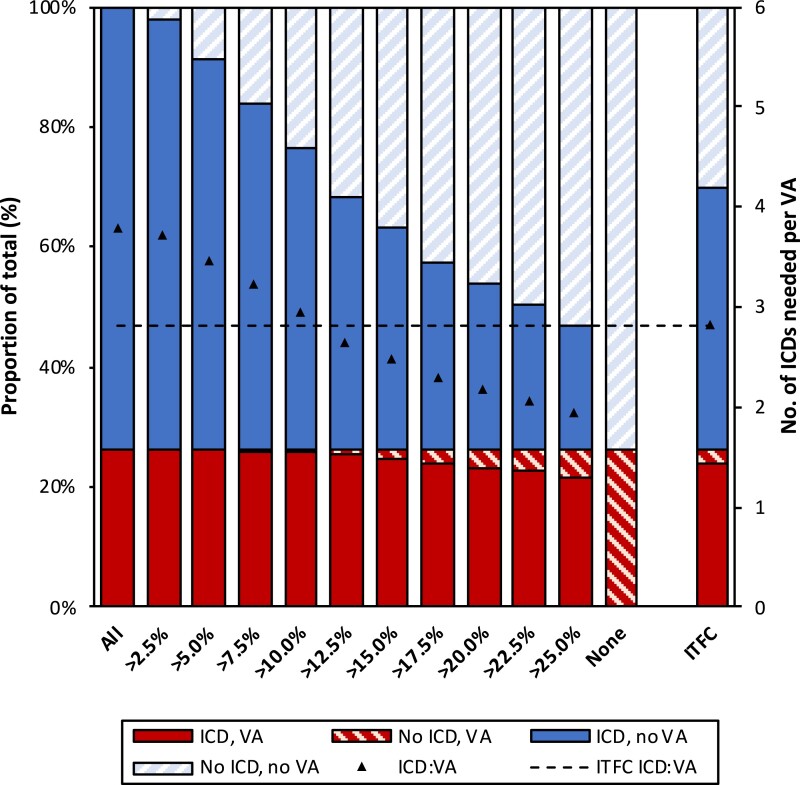
To assess the implications of our model in clinical practice, we explored the impact of potential 5-year VA risk thresholds for ICD implantation in our model vs. the ITFC consensus algorithm (i.e. ICD implantation in those with an ITFC Class I/IIa indication).1 This is laid out in Figure 3. As can be appreciated from the two last columns of Supplementary material online, Table S9, applying the ITFC algorithm would have resulted in treating 355 (67.2%) patients and protecting 25 (89.9%) of those who subsequently developed VA. In comparison, to provide the same level of protection (89.9%), our model would result in the implantation of 283 (53.6%) ICDs, thereby reducing the total number of ICD implants by 20.3% [(355–283)/355] (P < 0.001).
Figure 3.
Outcomes of patients associated with model-based implantable cardioverter-defibrillator implantation thresholds. The implications of implanting an implantable cardioverter-defibrillator in all (left bar) or none (second-to-right bar) of the patients are shown, as well as the implications of treating all patients as per International Task Force Consensus Statement (far right bar). The rest of the bars show the impact of using different implantable cardioverter-defibrillator placement thresholds based on the risk calculated by our model. Each bar represents the complete cohort (n = 528) and colour coding represents the proportion of patients experiencing sustained ventricular arrhythmia (red) or absence thereof (blue) as well as the placement (solid colours) vs. the non-placement (striped colours) of an implantable cardioverter-defibrillator. The black triangles represent the number of implantable cardioverter-defibrillators needed to protect one patient developing ventricular arrhythmia, with a horizontal dotted line for the reference value (i.e. treatment as per International Task Force Consensus Statement). Left y axis denotes proportion of patients (corresponding to the colour coding); right y axis denotes the number of implantable cardioverter-defibrillators needed to protect one patient (corresponding to the black triangles). ICD, implantable cardioverter-defibrillator; ICD:VA, ratio of implantable cardioverter-defibrillator placements required to protect one patient developing ventricular arrhythmia; ITFC, International Task Force Consensus Statement.
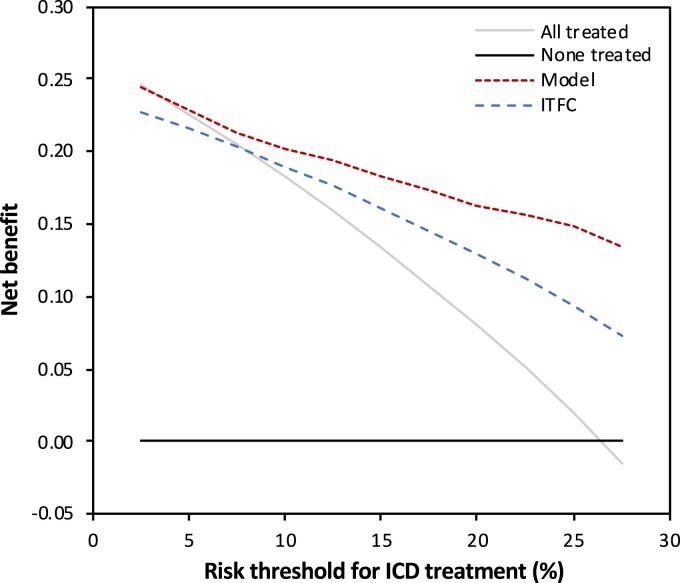
We subsequently compared the clinical performance of our model to the ITFC algorithm using decision curve analysis. As shown in Figure 4, our proposed model was associated with the highest net benefit within the entire range of potential treatment thresholds for ICD placement. This suggests superiority in clinical practice regardless of implantation threshold.
Figure 4.
Decision curve analysis comparing the clinical utility of our model (red dotted line) to the International Task Force Consensus Statement algorithm (blue dotted line). The clinical utility of both treatment strategies is compared by plotting the net benefit (y axis) for a range of potential implantable cardioverter-defibrillator placement thresholds based on the 5-year risk of VA (x axis). Our model showed the highest net benefit for all potential thresholds (ranging from 2.5% to 27.5%). This indicates that our model would result in the highest weighted balance of appropriate vs. inappropriate implantable cardioverter-defibrillator placements, regardless of the clinically preferred risk threshold. ICD, implantable cardioverter-defibrillator; ITFC, International Task Force Consensus Statement.
Discussion
Main findings
We developed and internally validated the first prediction model to generate individualized risk estimates for sustained VA in patients with ARVC. This model accurately distinguished patients who had incident sustained VA during follow-up from those who did not using seven non-invasive parameters that are readily available to the clinician. Predicted and observed risks were concordant both in the overall population and in key patient subgroups. In addition, the model compared favourably to a clinically available treatment algorithm, suggesting greater utility in everyday clinical practice.
Prior studies
This work builds upon numerous efforts in the ARVC community directed towards optimization of arrhythmic risk stratification. Indeed, selection of pre-specified predictors was based on a meta-analysis that included 45 studies examining the association of clinical and demographic characteristics with VAs.5 Despite this wealth of data, a lack of systematically analysed results complicates their translation to clinical care. Interpretation of the results of prior studies has been significantly hampered by limited sample sizes and heterogeneous study populations.5 In addition, none of the prior studies were designed to derive a prediction model that can be applied to clinical care. The 2015 ITFC Statement for Treatment of ARVC was a major step forward in consolidating the literature and proposing an algorithm for ICD placement.1 Nevertheless, the ITFC recommendations were based on expert opinion and provided only risk strata with a crude estimate of risk. Opportunities for improvement were subsequently raised.8 Therefore, to the present day, there is no uniformly accepted risk stratification algorithm for ARVC.
Model development and validation
In order to be widely applicable, a risk stratification algorithm should be derived from a broad population, simple, and easy to use. As such, we assembled the largest cohort to date of ARVC patients from multinational transatlantic registries, and measured seven easily available clinical parameters. Our model showed good discrimination between those with vs. without sustained VA (as indicated by the C-statistic), and good agreement between observed and predicted sustained VA risk (as determined by the calibration plots). In addition, sensitivity analyses revealed that the relationships between predictors and outcome were comparable in key patient subgroups.
The need for accurate ventricular arrhythmia risk prediction in arrhythmogenic right ventricular dysplasia/cardiomyopathy
Our study quantifies the high rate of VA events in ARVC patients without a pre-existing history of sustained VA (5.6% per year). While this event rate is significantly higher than for other types of non-ischaemic cardiomyopathies, it is comparable to previous studies in primary prevention ARVC populations, which reported annual event rates of 2–10%.2,16 Faced with this high event rate, many clinicians would agree that the majority of patients with definite ARVC benefit from ICD placement. However, ICD placement has significant drawbacks in this usually young and active population, including a considerable risk of complications and inappropriate interventions.17 Appropriate patient selection is thus of paramount importance.
Clinical utility
The greatest clinical utility of our model lies in the accurate individualized quantification of arrhythmic risk (Take home figure). By treating VA risk as a continuum instead of dividing patients into high-, intermediate-, and low-risk strata, we provide prognostic information that can aid clinical decision-making for prophylactic ICD placement. Importantly in this high-risk population, our model can help the clinician identify those who would fare well without an ICD. Of note, our study does not aim to prescribe ICD placement for a given patient. Instead, we seek to provide the clinician and patient with the necessary data to facilitate well-informed shared clinical decision-making.
Take home figure.
Prediction of sustained ventricular arrhythmia in arrhythmogenic right ventricular dysplasia/cardiomyopathy. ARVC, arrhythmogenic right ventricular dysplasia/cardiomyopathy; inv., inversion; PVC, premature ventricular complex; RVEF, right ventricular ejection fraction; VT, ventricular tachycardia.
While the acceptable risk threshold is undefined, our model performed better than the current consensus-based algorithm at any risk threshold. Importantly, our model results in a 20.3% reduction of ICD placement compared to the ITFC consensus algorithm, while protecting as many patients with VA events. Therefore, we believe that the model has the potential to set the standard for everyday clinical decision-making for primary prevention ICDs in patients with ARVC. To facilitate this, we have made our model available online as a ‘risk calculator’ on www.arvcrisk.com. Such a tool has had considerable clinical utility for arrhythmic risk prediction in hypertrophic cardiomyopathy.18,19 It is important to recognize that ARVC is a progressive condition. Thus, patients should be periodically re-stratified with right ventricular function assessment, ECG, and heart rhythm monitoring every 1–2 years as suggested in a recent expert consensus document.1 We provided risk estimates to facilitate shorter-term prediction and calibration plots establishing concordance between predicted and observed events over these shorter timeframes (Supplementary material online, Table S7 and Figure S1).
Limitations and future directions
Our study population was drawn from academic centres across Northern Europe and North America. Consistent with this, patients were predominantly Caucasian and pathogenic variants were primarily identified in PKP2. Results should consequently be extrapolated with caution to patients of other ethnic background or genotypes. Our ascertainment from tertiary care settings may have created a referral bias that could lead to overestimation of VA risk in a community-derived population. These limitations highlight the importance of external validation studies that include patients from community settings and with a diversity of ethnic backgrounds and genotypes. As in similar studies, we used a surrogate composite endpoint that included appropriate ICD therapy to infer risk of SCD. While most clinicians agree that ICD-treated VA represents a severe event, ICD therapies are an imperfect substitute for SCD.20 To address these limitations, we stratified the population by prophylactic ICD placement and demonstrated the model performed similarly well (Supplementary material online, Figure S2). There is certainly room for further iterations of the model by including other predictors. One of them is inducibility on programmed ventricular stimulation (PVS). The characteristics of the 214 (40.7%) patients who underwent PVS in our cohort with regards to the presence or absence of inducibility is presented in Supplementary material online, Table S10.
Conclusion
Based on the largest cohort to date of ARVC patients with no sustained VA history at diagnosis, we present a new prediction model to generate individualized estimates of the risk of incident VA. This model, based on readily available clinical parameters, performs better than the current consensus guideline and has the potential to set the standard for prophylactic ICD placement in ARVC.
Supplementary Material
Acknowledgements
The authors thank Rob Roudijk, MD and Freyja van Lint, MD for data collection. They thank the ARVC patients and families who have made this work possible.
Funding
This work was supported by the Canadian Heart Rhythm Society George Mines Traveling Fellowship to J.C.-T.; the Montreal Heart Institute Foundation ‘Bal du Coeur’ bursary to J.C.-T.; by a grant from the Fondation Leducq’ [grant number 16 CVD 02] to H.C.; the Dutch Heart Foundation [grant numbers 2015T058 to A.S.J.M.t.R.; CVON2015-12 eDETECT, 2012-10 PREDICT, CVON PREDICT Young Talent Program to A.S.J.M.t.R.]; the Netherlands Organisation for Scientific Research [040.11.586 to CAJ]; the Netherlands Heart Institute [project 06901]; the Swiss National Science Foundation [320030_160327]; the UMC Utrecht 2017 Alexandre Suerman Stipend to M.B.; and the UMC Utrecht Fellowship Clinical Research Talent to A.S.J.M.t.R. This project has received support from the European Union's Horizon 2020 research and innovation program under the ERA-NET Co-fund action no. 680969 (ERA-CVD DETECTIN-HF). The Johns Hopkins ARVD Program is supported by the Dr Francis P. Chiaramonte Private Foundation, the Leyla Erkan Family Fund for ARVD Research, the Dr Satish, Rupal, and Robin Shah ARVD Fund at Johns Hopkins, the Bogle Foundation, the Healing Hearts Foundation, the Campanella family, the Patrick J. Harrison Family, the Peter French Memorial Foundation, and the Wilmerding Endowments. The Zurich ARVC Program is supported by grants from the Georg und Bertha Schwyzer-Winiker Foundation, the Baugarten Foundation, and the Swiss Heart Foundation. The Johns Hopkins ARVD Program and the Zurich ARVC Program are also supported by a joint grant from the Leonie-Wild Foundation. Drs R.T. and M.T. are supported by the Marvin and Philippa Carsley Chair of Medicine. F.W.A. is supported by UCL Hospitals NIHR Biomedical Research Centre.
Contributor Information
Julia Cadrin-Tourigny, Division of Cardiology, Department of Medicine, Johns Hopkins Hospital, Carnegie 568D, 600 N. Wolfe St. Baltimore, MD, USA; Cardiovascular Genetics Center, Montreal Heart Institute, Université de Montréal, 5000 Bélanger St, Montréal, Canada.
Laurens P Bosman, Netherlands Heart Institute, 3501 DG, Utrecht, The Netherlands; Department of Cardiology, University Medical Center Utrecht, University of Utrecht, Heidelberglaan 100, CX Utrecht, The Netherlands.
Anna Nozza, Montreal Health Innovations Coordinating Center, Université de Montréal, 4100 Molson St, Suite 400, Montréal, Canada.
Weijia Wang, Division of Cardiology, Department of Medicine, Johns Hopkins Hospital, Carnegie 568D, 600 N. Wolfe St. Baltimore, MD, USA.
Rafik Tadros, Cardiovascular Genetics Center, Montreal Heart Institute, Université de Montréal, 5000 Bélanger St, Montréal, Canada.
Aditya Bhonsale, Division of Cardiology, Department of Medicine, Johns Hopkins Hospital, Carnegie 568D, 600 N. Wolfe St. Baltimore, MD, USA.
Mimount Bourfiss, Department of Cardiology, University Medical Center Utrecht, University of Utrecht, Heidelberglaan 100, CX Utrecht, The Netherlands.
Annik Fortier, Montreal Health Innovations Coordinating Center, Université de Montréal, 4100 Molson St, Suite 400, Montréal, Canada.
Øyvind H Lie, Department of Cardiology, Center for Cardiological Innovation, Oslo University Hospital, Postboks 4950 Nydalen, Oslo, Norway; University of Oslo, Postboks 1171, Blindern Oslo, Norway.
Ardan M Saguner, Department of Cardiology, University Heart Center Zurich, Raemistrasse 100, Zurich, Switzerland.
Anneli Svensson, Department of Cardiology, University Hosptial of Linköping, S-581 85 Linköping, Sweden.
Antoine Andorin, Cardiovascular Genetics Center, Montreal Heart Institute, Université de Montréal, 5000 Bélanger St, Montréal, Canada.
Crystal Tichnell, Division of Cardiology, Department of Medicine, Johns Hopkins Hospital, Carnegie 568D, 600 N. Wolfe St. Baltimore, MD, USA.
Brittney Murray, Division of Cardiology, Department of Medicine, Johns Hopkins Hospital, Carnegie 568D, 600 N. Wolfe St. Baltimore, MD, USA.
Katja Zeppenfeld, Department of Cardiology, Leiden University Medical Center, Albinusdreef 2, ZA Leiden, The Netherlands.
Maarten P van den Berg, Department of Cardiology, University Medical Center Groningen, University of Groningen, Groningen, The Netherlands.
Folkert W Asselbergs, Netherlands Heart Institute, 3501 DG, Utrecht, The Netherlands; Department of Cardiology, University Medical Center Utrecht, University of Utrecht, Heidelberglaan 100, CX Utrecht, The Netherlands; Faculty of Population Health Sciences, Institute of Cardiovascular Science, Institute of Health Informatics, University College London, 69-75 Chenies Mews, London, UK.
Arthur A M Wilde, Department of Clinical and Experimental Cardiology, Amsterdam UMC, University of Amsterdam, Heart Center, Meibergdreef 9, AZ, Amsterdam, The Netherlands.
Andrew D Krahn, Division of Cardiology, Department of Medicine, University of British Columbia 211 - 1033 Davie Street, Vancouver, BC, Canada.
Mario Talajic, Cardiovascular Genetics Center, Montreal Heart Institute, Université de Montréal, 5000 Bélanger St, Montréal, Canada.
Lena Rivard, Cardiovascular Genetics Center, Montreal Heart Institute, Université de Montréal, 5000 Bélanger St, Montréal, Canada.
Stephen Chelko, Division of Cardiology, Department of Medicine, Johns Hopkins Hospital, Carnegie 568D, 600 N. Wolfe St. Baltimore, MD, USA.
Stefan L Zimmerman, The Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins Hospital, 600 N. Wolfe St., Baltimore, MD, USA.
Ihab R Kamel, The Russell H. Morgan Department of Radiology and Radiological Science, Johns Hopkins Hospital, 600 N. Wolfe St., Baltimore, MD, USA.
Jane E Crosson, Division of Cardiology, Department of Medicine, Johns Hopkins Hospital, Carnegie 568D, 600 N. Wolfe St. Baltimore, MD, USA.
Daniel P Judge, Division of Cardiology, Department of Medicine, Johns Hopkins Hospital, Carnegie 568D, 600 N. Wolfe St. Baltimore, MD, USA.
Sing Chien Yap, Department of Cardiology, Thoraxcenter, Erasmus Medical Center, Dr. Molewaterplein 40, GD, Rotterdam, The Netherlands.
Jeroen F van der Heijden, Department of Cardiology, University Medical Center Utrecht, University of Utrecht, Heidelberglaan 100, CX Utrecht, The Netherlands.
Harikrishna Tandri, Division of Cardiology, Department of Medicine, Johns Hopkins Hospital, Carnegie 568D, 600 N. Wolfe St. Baltimore, MD, USA.
Jan D H Jongbloed, Department of Genetics, University of Groningen, University Medical Center Groningen, Hanzeplein 1, Groningen, The Netherlands.
Marie Claude Guertin, Montreal Health Innovations Coordinating Center, Université de Montréal, 4100 Molson St, Suite 400, Montréal, Canada.
J Peter van Tintelen, Netherlands Heart Institute, 3501 DG, Utrecht, The Netherlands; Department of Clinical Genetics, Academic Medical Center, University of Amsterdam, Meibergdreef 9, DD Amsterdam, The Netherlands.
Pyotr G Platonov, Department of Cardiology, Clinical Sciences, Lund University Hosptial, Lund, Sweden.
Firat Duru, Department of Cardiology, University Heart Center Zurich, Raemistrasse 100, Zurich, Switzerland.
Kristina H Haugaa, Department of Cardiology, Center for Cardiological Innovation, Oslo University Hospital, Postboks 4950 Nydalen, Oslo, Norway; University of Oslo, Postboks 1171, Blindern Oslo, Norway.
Paul Khairy, Cardiovascular Genetics Center, Montreal Heart Institute, Université de Montréal, 5000 Bélanger St, Montréal, Canada.
Richard N W Hauer, Netherlands Heart Institute, 3501 DG, Utrecht, The Netherlands; Department of Cardiology, University Medical Center Utrecht, University of Utrecht, Heidelberglaan 100, CX Utrecht, The Netherlands.
Hugh Calkins, Division of Cardiology, Department of Medicine, Johns Hopkins Hospital, Carnegie 568D, 600 N. Wolfe St. Baltimore, MD, USA.
Anneline S J M te Riele, Netherlands Heart Institute, 3501 DG, Utrecht, The Netherlands; Department of Cardiology, University Medical Center Utrecht, University of Utrecht, Heidelberglaan 100, CX Utrecht, The Netherlands.
Cynthia A James, Division of Cardiology, Department of Medicine, Johns Hopkins Hospital, Carnegie 568D, 600 N. Wolfe St. Baltimore, MD, USA.
References
- 1. Corrado D, Wichter T, Link MS, Hauer R, Marchlinski F, Anastasakis A, Bauce B, Basso C, Brunckhorst C, Tsatsopoulou A, Tandri H, Paul M, Schmied C, Pelliccia A, Duru F, Protonotarios N, Estes NA 3rd, McKenna WJ, Thiene G, Marcus FI, Calkins H.. Treatment of arrhythmogenic right ventricular cardiomyopathy/dysplasia: an international task force consensus statement. Eur Heart J 2015;36:3227–3237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bhonsale A, James CA, Tichnell C, Murray B, Gagarin D, Philips B, Dalal D, Tedford R, Russell SD, Abraham T, Tandri H, Judge DP, Calkins H.. Incidence and predictors of implantable cardioverter-defibrillator therapy in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy undergoing implantable cardioverter-defibrillator implantation for primary prevention. J Am Coll Cardiol 2011;58:1485–1496. [DOI] [PubMed] [Google Scholar]
- 3. Corrado D, Calkins H, Link MS, Leoni L, Favale S, Bevilacqua M, Basso C, Ward D, Boriani G, Ricci R, Piccini JP, Dalal D, Santini M, Buja G, Iliceto S, Estes NA 3rd, Wichter T, McKenna WJ, Thiene G, Marcus FI.. Prophylactic implantable defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and no prior ventricular fibrillation or sustained ventricular tachycardia. Circulation 2010;122:1144–1152. [DOI] [PubMed] [Google Scholar]
- 4. Schuler PK, Haegeli LM, Saguner AM, Wolber T, Tanner FC, Jenni R, Corti N, Luscher TF, Brunckhorst C, Duru F.. Predictors of appropriate ICD therapy in patients with arrhythmogenic right ventricular cardiomyopathy: long term experience of a tertiary care center. PLoS One 2012;7:e39584.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Bosman LP, Sammani A, James CA, Cadrin-Tourigny J, Calkins H, van Tintelen JP, Hauer RNW, Asselbergs FW, Te Riele A. Predicting arrhythmic risk in arrhythmogenic right ventricular cardiomyopathy: a systematic review and meta-analysis. Heart Rhythm 2018;15:1097–1107. [DOI] [PubMed] [Google Scholar]
- 6. Moons KG, Altman DG, Reitsma JB, Ioannidis JP, Macaskill P, Steyerberg EW, Vickers AJ, Ransohoff DF, Collins GS.. Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 2015;162:W1–W73. [DOI] [PubMed] [Google Scholar]
- 7. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W.. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J 2010;31:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Orgeron GM, Te Riele A, Tichnell C, Wang W, Murray B, Bhonsale A, Judge DP, Kamel IR, Zimmerman SL, Tandri H, Calkins H, James CA.. Performance of the 2015 International Task Force Consensus Statement Risk Stratification Algorithm for Implantable Cardioverter-Defibrillator Placement in Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy. Circ Arrhythm Electrophysiol 2018;11:e005593.. [DOI] [PubMed] [Google Scholar]
- 9. Richards S, Aziz N, Bale S, Bick D, Das S, Gastier-Foster J, Grody WW, Hegde M, Lyon E, Spector E, Voelkerding K, Rehm HL; ACMG Laboratory Quality Assurance Committee. Standards and guidelines for the interpretation of sequence variants: a joint consensus recommendation of the American College of Medical Genetics and Genomics and the Association for Molecular Pathology. Genet Med 2015;17:405–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. van Buuren S, Boshuizen HC, Knook DL.. Multiple imputation of missing blood pressure covariates in survival analysis. Stat Med 1999;18:681–694. [DOI] [PubMed] [Google Scholar]
- 11. White IR, Royston P, Wood AM.. Multiple imputation using chained equations: issues and guidance for practice. Stat Med 2011;30:377–399. [DOI] [PubMed] [Google Scholar]
- 12. Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York, USA: John Wiley and Sons; 1987. [Google Scholar]
- 13. Steyerberg EW. SpringerLink (Service en Ligne). Clinical Prediction Models a Practical Approach to Development, Validation, and Updating. New York (NY: ): Springer; 2009. 1 texte électronique p. [Google Scholar]
- 14. Steyerberg EW, Vickers AJ, Cook NR, Gerds T, Gonen M, Obuchowski N, Pencina MJ, Kattan MW.. Assessing the performance of prediction models: a framework for traditional and novel measures. Epidemiology 2010;21:128–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Vickers AJ, Van Calster B, Steyerberg EW.. Net benefit approaches to the evaluation of prediction models, molecular markers, and diagnostic tests. BMJ 2016;352:i6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Folino AF, Buja G, Bauce B, Thiene G, Dalla Volta S, Nava A.. Heart rate variability in arrhythmogenic right ventricular cardiomyopathy correlation with clinical and prognostic features. Pacing Clin Electrophysiol 2002;25:1285–1292. [DOI] [PubMed] [Google Scholar]
- 17. Olde Nordkamp LR, Postema PG, Knops RE, van Dijk N, Limpens J, Wilde AA, de Groot JR.. Implantable cardioverter-defibrillator harm in young patients with inherited arrhythmia syndromes: a systematic review and meta-analysis of inappropriate shocks and complications. Heart Rhythm 2016;13:443–454. [DOI] [PubMed] [Google Scholar]
- 18. O'Mahony C, Jichi F, Pavlou M, Monserrat L, Anastasakis A, Rapezzi C, Biagini E, Gimeno JR, Limongelli G, McKenna WJ, Omar RZ, Elliott PM; Hypertrophic Cardiomyopathy Outcomes I. A novel clinical risk prediction model for sudden cardiac death in hypertrophic cardiomyopathy (HCM risk-SCD). Eur Heart J 2014;35:2010–2020. [DOI] [PubMed] [Google Scholar]
- 19. Authors/Task Force Members, Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H.. 2014 ESC Guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 20. Ellenbogen KA, Levine JH, Berger RD, Daubert JP, Winters SL, Greenstein E, Shalaby A, Schaechter A, Subacius H, Kadish A; Defibrillators in Non-Ischemic Cardiomyopathy Treatment Evaluation Investigators. Are implantable cardioverter defibrillator shocks a surrogate for sudden cardiac death in patients with nonischemic cardiomyopathy? Circulation 2006;113:776–782. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.