Abstract
Background
Stiripentol is an antiseizure medication with multiple potential mechanisms of action, indicated as adjunctive therapy in people with Dravet syndrome, whose seizures are not adequately controlled with clobazam and valproate. However, there are scattered data on its efficacy in other epilepsy aetiologies and types. We previously reported our single-centre experience on the efficacy of adjunctive stiripentol treatment in a cohort of 132 patients with different types of refractory epilepsies.
Objective
We aimed to expand our analysis to a larger cohort of 196 patients with a long-term follow-up.
Methods
We retrospectively evaluated long-term efficacy, tolerability and predictors of treatment response in 196 patients with a long-term follow-up (range 0.5–232.8 months).
Results
After an initial median follow-up of 3 months after stiripentol introduction, we observed a responder rate of 53% including seizure freedom in 9%. At subsequent follow-ups at 12 and 24 months, responder rates were 29% and 22%, respectively. Aetiology was associated with sustained response over time, with Dravet syndrome being the aetiology with the highest responder rate (64%) at 48 months compared with syndromes with other genetic causes (13%) or unknown aetiology (38%). A higher responder rate over time was also observed in patients with generalised (44%) and combined focal and generalised epilepsies (28%) than in patients with focal epilepsies (20%). The highest relapse free-survival was observed when stiripentol was initiated at the youngest age (0–4 years) or in adulthood. The retention rate (i.e. proportion of patients who continued stiripentol with no change in either pharmacological or non-pharmacological therapy) was 53% at 12 months and 33% at 24 months.
Conclusions
Based on our findings, we suggest that stiripentol is an effective and well-tolerated therapeutic option not only in Dravet syndrome but also in other epilepsy syndromes with or without an established genetic aetiology. Response duration was influenced by age at stiripentol initiation across different aetiologies.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40801-022-00305-7.
Key Points
| Stiripentol can be effective not only in Dravet syndrome but also in other epilepsy syndromes. |
| The highest relapse-free survival was observed when stiripentol was initiated in early childhood or in adulthood. |
| Response duration was influenced by age at stiripentol initiation across different aetiologies. |
Introduction
Stiripentol (STP) is an antiseizure medication (ASM) with several possible mechanisms of action, including an increase of GABAergic inhibition [1], inhibition of lactate dehydrogenase [2], and blockage of sodium and calcium channels [3].
Stiripentol is approved in association with valproate and clobazam as an adjunctive treatment for Dravet syndrome (DS) in Europe, Canada, Japan, Argentina, Taiwan and USA in conjunction with clobazam, and in Australia in conjunction with valproate and benzodiazepines. It was the first drug for which a randomised controlled trial was conducted in DS [4]. Since then, one further controlled trial [5] and at least 15 open-label studies have been conducted, showing highly variable responder rates, from 48 to 63%, and seizure freedom rates between 10 and 40% [6, 7]. Stiripentol seems particularly effective in reducing the frequency of convulsive seizures and status epilepticus in DS [8], and this benefit is maintained in adulthood [9, 10]. The main adverse events, usually in combination with clobazam and/or valproate, include anorexia and weight loss, drowsiness, behavioural changes, neutropenia, insomnia, abdominal pain, ataxia, drooling and dystonia [4]. Adverse effects can be often reversed by adjusting the dose of STP and/or concomitant medications, although some may not (e.g. hyperammonemic encephalopathy) [11].
The use of STP in DS is well documented: STP is gradually increased up to a target dosage of 50 mg/kg/day in children and 20–30 mg/kg/day in adults, divided into two or three daily doses. Clobazam and valproate should be reduced concomitantly to STP introduction because of the STP metabolic inhibition of cytochrome P450 3A4 and cytochrome P450 2C19 [12].
We previously reported our single-centre experience on the efficacy of adjunctive STP treatment in a cohort of 132 children, adolescents and young adults with different types of refractory epilepsies including not only DS but also Lennox–Gastaut syndrome and focal and generalised epilepsies without encephalopathy [13]. Here, we expand our analysis to a larger cohort, and include an assessment of the long-term efficacy, tolerability and predictors of treatment response.
Methods
Patient Cohort and Treatment
Our study was approved by the Paediatric Ethics Committee of the Tuscany Region. Written informed consent was obtained by patients and/or their parents or legal guardians.
We reviewed the medical records of all patients followed at the Neuroscience Excellence Centre of Meyer Children’s Hospital of Florence between January 2007 and June 2020, consecutively treated with STP as add‐on therapy, irrespective of their type of drug‐resistant epilepsy. Data were extracted from the electronic health records. We have provided the data collection template in Table 1 of the Electronic Supplementary Material (ESM).
Table 1.
Kaplan–Meier estimates of the relapse-free survival in responders to treatment with stiripentol
| A | Responders, % (95% CI) | P value | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 months (n = 77) |
12 months (n = 56) |
24 months (n = 42) |
48 months (n = 22) |
60 months (n = 14) |
72 months (n = 12) |
96 months (n = 10) |
120 months (n = 3) |
|||
| 0.81 (0.72–0.87) |
0.68 (0.58–0.77) |
0.61 (0.50–0.71) |
0.45 (0.33–0.56) |
0.42 (0.30–0.54) |
0.42 (0.30–0.54) |
0.38 (0.25–0.51) |
0.34 (0.20–0.48) |
|||
| Age group, years | 0–4 | 0.83 (0.65–0.92) | 0.83 (0.65–0.92) | 0.79 (0.61–0.90) | 0.61 (0.41–0.77) | 0.61 (0.41–0.77) | 0.61 (0.41–0.77) | 0.51 (0.26–0.71) | 0.41 (0.16–0.65) | 0.0348 |
| 5–9 | 0.81 (0.60–0.92) | 0.73 (0.51–0.86) | 0.61 (0.38–0.78) | 0.34 (0.14–0.54) | 0.22 (0.05–0.47) | 0.22 (0.05–0.47) | 0.22 (0.52–0.47) | – | ||
| 10–18 | 0.78 (0.54–0.90) | 0.35 (0.15–0.56) | 0.29 (0.10–0.50) | 0.29 (0.10–0.50) | 0.29 (0.10–0.50) | 0.29 (0.10–0.50) | 0.28 (0.11–0.50) | 0.29 (0.10–0.50) | ||
| 19+ | 0.87 (0.56–0.96) | 0.79 (0.49–0.93) | 0.68 (0.34–0.87) | 0.57 (0.23–0.80) | 0.57 (0.23–0.80) | 0.57 (0.23–0.80) | 0.57 (0.23–0.80) | – | ||
| Epilepsy duration, months | ≤ 60 | 0.80 (0.67–0.89) | 0.78 (0.64–0.87) | 0.73 (0.58–0.84) | 0.52 (0.35–0.66) | 0.47 (0.29–0.63) | 0.47 (0.29–0.63) | 0.38 (0.20–0.58) | 0.31 (0.12–0.52) | 0.2978 |
| > 60 | 0.83 (0.69–0.91) | 0.58 (0.42–0.71) | 0.49 (0.32–0.63) | 0.38 (0.22–0.54) | 0.38 (0.22–0.54) | 0.38 (0.22–0.54) | 0.38 (0.23–0.54) | 0.38 (0.22–0.54) | ||
| Seizure type | Focal onset with/without impaired awareness | 0.73 (0.28–0.92) | 0.73 (0.28–0.92) | 0.73 (0.28–0.92) | 0.73 (0.28–0.92) | 0.73 (0.28–0.92) | 0.73 (0.28–0.92) | 0.73 (0.28–0.93) | – | 0.3827 |
| Focal to bilateral | 0.74 (0.45–0.89) | 0.74 (0.45–0.89) | 0.53 (0.22–0.77) | 0.53 (0.22–0.77) | 0.35 (0.07–0.66) | – | – | – | ||
| GTCS | 0.91 (0.75–0.97) | 0.68 (0.48–0.81) | 0.63 (0.43–0.78) | 0.59 (0.38–0.75) | 0.59 (0.38–0.75) | 0.59 (0.38–0.74) | 0.47 (0.21–0.69) | 0.35 (0.11–0.61) | 0.2572 | |
|
Other GS (non-convulsive) |
0.69 (0.40–0.86) | 0.62 (0.34–0.80) | 0.62 (0.34–0.80) | 0.31 (0.08–0.58) | 0.31 (0.08–0.58) | 0.31 (0.08–0.58) | 0.31 (0.08–0.58) | – | ||
| Epilepsy type | Focal | 0.74 (0.51–0.87) | 0.74 (0.51–0.87) | 0.60 (0.35–0.79) | 0.60 (0.35–0.79) | 0.48 (0.20–0.72) | 0.48 (0.20–0.72) | 0.48 (0.20–0.72) | – | 0.8090 |
| Generalised | 0.84 (0.70–0.92) | 0.66 (0.50–0.78) | 0.63 (0.47–0.75) | 0.50 (0.33–0.65) | 0.50 (0.33–0.65) | 0.49 (0.33–0.65) | 0.33 (0.14–0.54) | 0.33 (0.14–0.54) | ||
| Combined | 0.85 (0.64–0.94) | 0.73 (0.50–0.86) | 0.63 (0.40–0.79) | 0.33 (0.15–0.53) | 0.33 (0.15–0.53) | 0.33 (0.15–0.53) | 0.41 (0.22–0.60) | – | ||
| Aetiology | Dravet syndrome | 0.96 (0.74–0.99) | 0.91 (0.70–0.98) | 0.91 (0.70–0.98) | 0.60 (0.36–0.78) | 0.60 (0.36–0.78) | 0.60 (0.36–0.78) | 0.53 (0.28–0.73) | 0.45 (0.21–0.67) | 0.1567 |
| Other genetic | 0.71 (0.44–0.87) | 0.59 (0.33–0.78) | 0.44 (0.19–0.67) | 0.36 (0.13–0.60) | 0.36 (0.13–0.60) | 0.36 (0.13–0.60) | 0.36 (0.13–0.60) | – | ||
| Unknown | 0.78 (0.59–0.89) | 0.63 (0.43–0.78) | 0.50 (0.30–0.67) | 0.45 (0.26–0.63) | 0.45 (0.26–0.63) | 0.45 (0.26–0.63) | 0.45 (0.26–0.63) | – | ||
| Other | 0.80 (0.58–0.91) | 0.59 (0.36–0.77) | 0.59 (0.36–0.77) | 0.42 (0.18–0.65) | 0.28 (0.06–0.56) | – | – | – | ||
| ASMs in add-on therapy | ≤ 2 | 0.85 (0.74–0.92) | 0.68 (0.55–0.78) | 0.58 (0.45–0.70) | 0.41 (0.28–0.54) | 0.41 (0.28–0.54) | 0.41 (0.28–0.54) | 0.35 (0.20–0.51) | 0.35 (0.20–0.51) | 0.9285 |
| > 2 | 0.72 (0.51–0.85) | 0.72 (0.51–0.85) | 0.72 (0.51–0.85) | 0.56 (0.32–0.76) | 0.42 (0.15–0.68) | 0.42 (0.15–0.68) | 0.42 (0.14–0.68) | – | ||
ASMs antiseizure medications, CI confidence interval, GS generalised-onset seizures, GTCS generalised tonic‐clonic seizures
P values refer to the comparison among the categories of each clinical variable as measured by the log rank test
Stiripentol was administered according to the titration schedule included in the Summary of Product Characteristics. Monitoring of plasma concentrations of concomitant ASMs was performed, where indicated, on a clinical basis and, if necessary, dose adjustments were done, based on clinical and laboratory findings.
In this pragmatic and retrospective cohort study, follow‐up visits for this difficult‐to‐treat population were scheduled according to a tailored plan based on each individual’s clinical status, but never exceeded a 6‐month interval. Information was collected on age at the time of STP treatment, sex, age at seizure onset, aetiology, epilepsy syndrome, highest total daily dose and treatment duration of STP, type and dosage of concomitant ASMs, previous ASMs, seizure type and frequency before and during STP administration, adverse events and reasons for discontinuation. Seizures, epilepsy type and aetiology were classified according to the International League Against Epilepsy criteria [14]. Seizure frequency was recorded in a diary we customarily use in our epilepsy clinic and assessed at each follow‐up visit, every 3–6 months. Seizure frequency at baseline was defined as the monthly number of seizures in the 3 months preceding the initial STP administration. ‘Responders’ were defined as patients who achieved a 50% or higher reduction in baseline seizure frequency after the target dose was reached. ‘Non-responders’ were patients with a <50% reduction or no change or worsening in seizure frequency. Tolerability was assessed by recording the type and number of adverse events.
Statistical Analysis
Survival curves (relapse‐free survival) were used to assess the probability of remaining responders within the responder group. Retention rate (i.e. proportion of patients who continued STP with no change in the therapy regime either pharmacological or non-pharmacological) and failure‐free survival (time lag between STP initiation and STP failure) were calculated for the entire study population. In the retention rate analysis, failure of STP was defined as one or more of the following occurrences: withdrawal because of documented inefficacy at the maximum dosage, withdrawal because of adverse events, and addition of another ASM or of a non-pharmacological therapy (i.e. surgery, vagus nerve stimulation, ketogenic diet). We included patients with a follow-up shorter than 12 months (n = 48) to capture those with early relapse and describe our ‘real-world’ experience of the use of STP in the clinical setting.
A Kaplan–Meier survival analysis was conducted to calculate relapse-free survival in responders and the failure‐free survival in the whole population. A survival analysis was also performed in relation to the duration of epilepsy, seizure type, epilepsy type, aetiology and number of concomitant ASMs. Survival curves of two or more groups were compared using the log‐rank test.
A multi-variable Cox regression analysis was conducted. We built a model for the relapse rate in the responder cohort and a model for the failure rate (rate of discontinuation or addition of another ASM) in the whole cohort. We chose the variables to be included in the models based on their statistical significance (i.e. significant association with a relapse or discontinuation in the univariate analysis) or if they were considered clinically relevant regardless of the results observed in the univariate analysis. The following variables were included in the multivariable models: age group, epilepsy duration, epilepsy type, aetiology, concurrent treatment with clobazam and concurrent treatment with valproate.
A p-value of <0.05 was considered to be statistically significant. All analyses were performed with Stata version 11 software (Statacorp LLC, College Station, TX, USA).
Results
Clinical Data
Our study comprised 196 patients (88 were female, median age 8.3 years, age range 0.4–46.5 years). The majority were children (aged ≤ 18 years) [n = 171, 87%]. The median age at onset of epilepsy was 1.3 years (range 0–21 years), and median epilepsy duration was 4.6 years (range 0.2–43.6 years). Median time of the first clinical follow-up after STP introduction was 3.1 months (interquartile range 1.6–6.8 months), which was the time when STP response status was assessed. For responders, median time to achieve seizure control after STP introduction was 1.5 months (interquartile range 1.0–2.1 months). The epilepsy type was classified as generalised in 84 patients (43%), focal in 61 (31%) and combined in 51 (26%). Seizure types included focal to bilateral tonic-clonic seizures in 36 patients (59%), generalised-onset tonic-clonic seizures in 46 (55%), other generalised-onset seizures in 36 (45%) and focal-onset with and without impaired awareness in 25 (41%). The most prevalent aetiology was genetic, including DS (n = 39, 20%) and other genetic causes (n = 32, 16%), followed by unknown (n = 64, 33%), structural (n = 51, 26%), immune or infectious (n = 7, 4%) and metabolic (n = 3, 1.5). The list of other genetic aetiologies is provided in Table 2 of the ESM. Stiripentol was added to three or fewer ASMs in 140 patients (74%), whilst in a minority it was added to more than three ASMs (n = 49, 26%). Concomitant treatment in most patients included clobazam (n = 181, 92%) and valproate (n = 109, 56%).
Table 2.
Kaplan–Meier estimates of the probability of continuing stiripentol with no change in treatment schedule
| Responders, % (95% CI) | P value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 6 months (n = 139) | 12 months (n = 103) | 24 months (n = 64) | 48 months (n = 28) | 60 months (n = 18) | 72 months (n = 17) | 96 months (n = 14) | 120 months (n = 4) | |||
| 0.79 (0.72–0.84) | 0.64 (0.56–0.71) | 0.47 (0.39–0.54) | 0.31 (0.24–0.39) | 0.26 (0.18–0.34) | 0.26 (0.18–0.34) | 0.26 (0.18–0.34) | 0.13 (0.05–0.26) | – | ||
| Age group, years | 0–4 | 0.83 (0.72–0.90) | 0.70 (0.57–0.79) | 0.61 (0.47–0.72) | 0.45 (0.31–0.58) | 0.33 (0.19–0.47) | 0.33 (0.19–0.47) | 0.33 (0.19–0.47) | 0.15 (0.03–0.34) | 0.0898 |
| 5–9 | 0.82 (0.68–0.90) | 0.54 (0.38–0.67) | 0.38 (0.23–0.52) | 0.17 (0.06–0.33) | 0.17 (0.06–0.33) | 0.17 (0.06–0.33) | 0.17 (0.06–0.33) | – | ||
| 10–18 | 0.69 (0.53–0.80) | 0.64 (0.49–0.76) | 0.33 (0.19–0.47) | 0.26 (0.14–0.40) | 0.26 (0.14–0.40) | 0.26 (0.14–0.40) | 0.26 (0.14–0.40) | 0.26 (0.14–0.40) | ||
| 19+ | 0.79 (0.56–0.90) | 0.68 (0.44–0.83) | 0.58 (0.34–0.75) | 0.29 (0.07–0.56) | 0.29 (0.07–0.56) | 0.29 (0.07–0.56) | 0.29 (0.07–0.56) | – | ||
| Epilepsy duration, months | ≤ 60 | 0.78 (0.69–0.85) | 0.62 (0.51–0.71) | 0.49 (0.38–0.58) | 0.33 (0.23–0.43) | 0.25 (0.15–0.36) | 0.25 (0.15–0.36) | 0.24 (0.15–0.36) | 0.11 (0.03–0.26) | 0.8503 |
| > 60 | 0.78 (0.68–0.86) | 0.66 (0.54–0.75) | 0.45 (0.33–0.57) | 0.28 (0.16–0.41) | 0.28 (0.16–0.41) | 0.28 (0.16–0.41) | 0.28 (0.16–0.41) | 0.28 (0.16–0.41) | ||
| Seizure type | Focal | 0.84 (0.62–0.94) | 0.45 (0.23–0.64) | 0.2 (0.06–0.39) | 0.15 (0.04–0.33) | 0.15 (0.04–0.33) | 0.15 (0.04–0.33) | 0.15 (0.04–0.33) | – | 0.3603 |
| Focal to bilateral | 0.70 (0.50–0.81) | 0.51 (0.33–0.66) | 0.37 (0.20–0.53) | 0.24 (0.10–0.41) | 0.24 (0.10–0.41) | 0.24 (0.10–0.41) | – | – | ||
| GTCS | 0.84 (0.69–0.92) | 0.76 (0.59–0.86) | 0.55 (0.37–0.69) | 0.51 (0.34–0.66) | 0.46 (0.27–0.62) | 0.46 (0.27–0.62) | 0.46 (0.27–0.62) | 0.34 (0.13–0.57) | 0.2438 | |
| Other GS | 0.73 (0.55–0.84) | 0.66 (0.47–0.79) | 0.57 (0.38–0.72) | 0.31 (0.12–0.52) | 0.31 (0.12–0.52) | 0.31 (0.12–0.52) | 0.31 (0.12–0.52) | – | ||
| Epilepsy type | Focal | 0.76 (0.62–0.85) | 0.48 (0.34–0.61) | 0.30 (0.18–0.43) | 0.20 (0.1–0.32) | 0.20 (0.1–0.32) | 0.20 (0.1–0.32) | 0.20 (0.09–0.32) | – | 0.0311 |
| Generalised | 0.79 (0.68–0.86) | 0.71 (0.59–0.80) | 0.56 (0.43–0.67) | 0.44 (0.30–0.56) | 0.40 (0.26–0.54) | 0.40 (0.26–0.54) | 0.39 (0.26–0.56) | 0.24 (0.08–0.44) | ||
| Combined | 0.82 (0.69–0.90) | 0.71 (0.57–0.82) | 0.54 (0.39–0.67) | 0.28 (0.14–0.43) | 0.14 (0.04–0.30) | 0.14 (0.04–0.30) | 0.14 (0.04–0.30) | - | ||
| Aetiology | Dravet syndrome | 0.97 (0.83–0.99) | 0.92 (0.76–0.97) | 0.82 (0.64–0.92) | 0.64 (0.44–0.78) | 0.50 (0.29–0.67) | 0.50 (0.29–0.67) | 0.50 (0.29–0.67) | 0.19 (0.04–0.43) | 0.0001 |
| Other genetic | 0.78 (0.59–0.89) | 0.56 (0.36–0.72) | 0.40 (0.22–0.57) | 0.13 (0.03–0.32) | 0.13 (0.03–0.32) | 0.13 (0.03–0.32) | 0.13 (0.03–0.32) | – | ||
| Unknown | 0.74 (0.61–0.83) | 0.66 (0.52–0.76) | 0.46 (0.32–0.59) | 0.38 (0.24–0.52) | 0.30 (0.15–0.48) | 0.30 (0.15–0.48) | 0.30 (0.15–0.48) | – | ||
| Other | 0.72 (0.59–0.82) | 0.49 (0.35–0.62) | 0.28 (0.16–0.41) | 0.13 (0.05–0.26) | 0.13 (0.05–0.26) | 0.13 (0.05–0.26) | 0.13 (0.05–0.26) | 0.13 (0.05–0.26) | ||
| ASM in add-on therapy | ≤ 2 | 0.81 (0.73–0.87) | 0.65 (0.56–0.73) | 0.46 (0.37–0.54) | 0.27 (0.19–0.36) | 0.22 (0.14–0.31) | 0.22 (0.14–0.31) | 0.22 (0.14–0.31) | 0.17 (0.08–0.27) | 0.3662 |
| > 2 | 0.70 (0.54–0.81) | 0.61 (0.44–0.74) | 0.53 (0.35–0.68) | 0.48 (0.30–0.64) | 0.42 (0.23–0.58) | 0.42 (0.23–0.58) | 0.42 (0.23–0.58) | – | ||
ASMs antiseizure medications, CI confidence interval, GS generalised-onset seizures, GTCS generalised tonic‐clonic seizures
P-values refer to the comparison among the categories of each clinical variable as measured by the log rank test
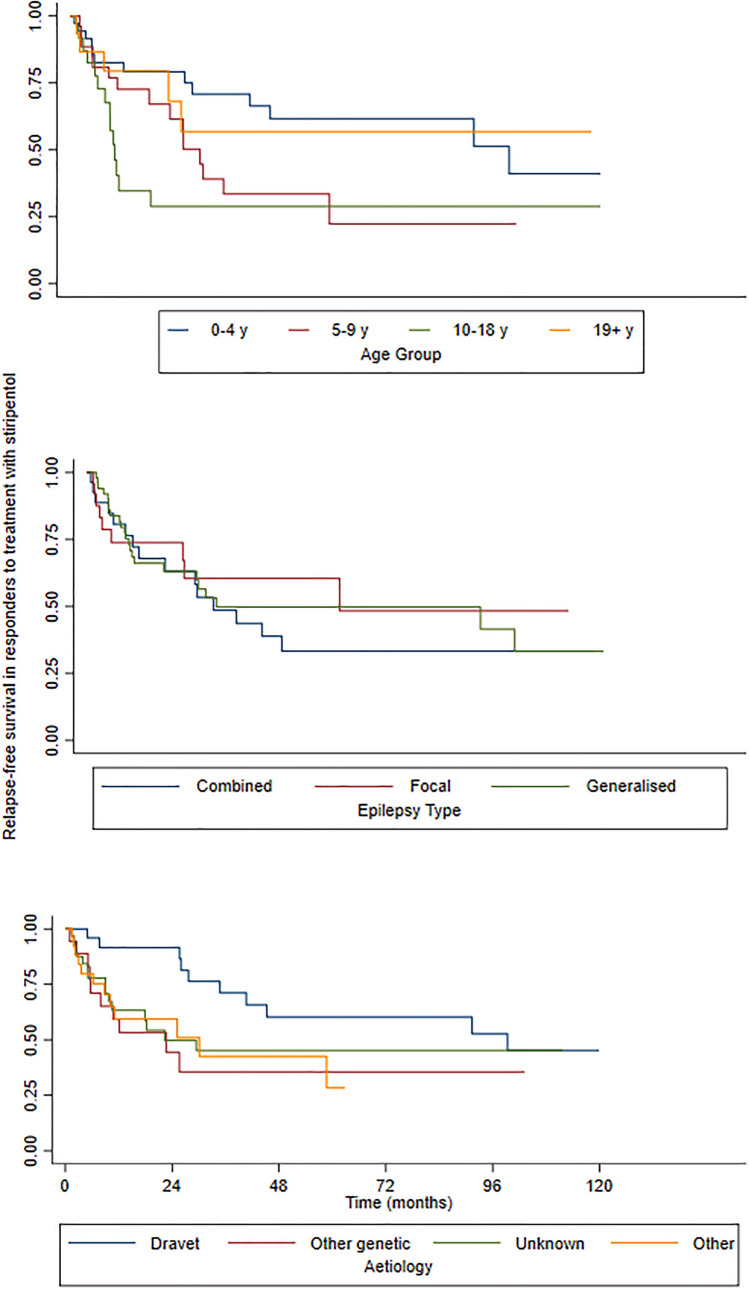
Median duration of follow-up during STP treatment was 12.2 months (range 0.5– 232.8). Patients who initially responded to STP were 103 (53%), with 18 (9%) who became seizure free. The highest response rate was observed for age 0–4 years and >18 years (54.5 and 64%, respectively), and in generalised (60.7%) and combined (52.9%) epilepsies. There was no significant difference between responders and non-responders across age groups (p = 0.507), median STP dosage (p = 0.71), aetiology (p = 0.176), epilepsy type (p = 0.063) and duration (p = 0.338), and the number of ASMs in add-on therapy (p = 0.204). Concomitant treatment with valproate was more frequent in responders (p = 0.001), whilst there was no difference in other concomitant ASMs between responders and non-responders. In line with our previous study [13], almost half of patients with focal to bilateral tonic-clonic seizures (47.2%) and about a third of patients with focal-onset seizures with or without impaired awareness (32%) responded to add-on STP.
Tolerability
Treatment-emergent adverse events were reported in 38 (19.4%) of 196 patients, including mainly neurological symptoms (n = 26, 13%), followed by gastrointestinal manifestations (n = 5, 2.5%), skin reactions (n = 2, 1%) and urinary symptoms (n = 1, 0.5%). No serious adverse events were observed.
Efficacy
After an initial response observed at the first follow-up in 103/196 (56%), the responder rate was 56/196 (29%) at 12 months and 42/196 (22%) at 24 months. The initial responder status was maintained in 56/103 (54%) at 12 months and in 42/103 (41%) at 24 months.
After performing a univariate association analysis, we found a significant difference between treatment response and concomitant treatment with valproate (69, 63.3% of responders, vs 40, 36.7% of non-responders), and generalised-onset tonic-clonic seizure type when compared with other generalised-onset seizures (non-convulsive). There was no other factor significantly associated with treatment response. The probability of remaining responders at different follow-up intervals is reported in Table 1.
Age at STP initiation emerged as a predictive factor of sustained response as a higher rate of response over time was observed in the 0–4 years age group and in the adult group (p = 0.035, Table 1). None of the other analysed factors significantly affected relapse-free survival.
The responder rate in people with focal-onset seizures with/without impaired awareness remained stable when compared with subgroups with other seizure types (Table 1). The relapse-free survival in subjects with DS was longer and more stable over time than that observed in the other aetiologies. No significant differences were observed in the relapse-free survival by the number of ASMs used in add-on therapy. The probability of remaining responders over time stratified by age, epilepsy type and aetiology is shown in Fig. 1.
Fig. 1.
Kaplan–Meier survival estimates of the relapse-free survival over time, stratified by age, epilepsy type and aetiology. y years
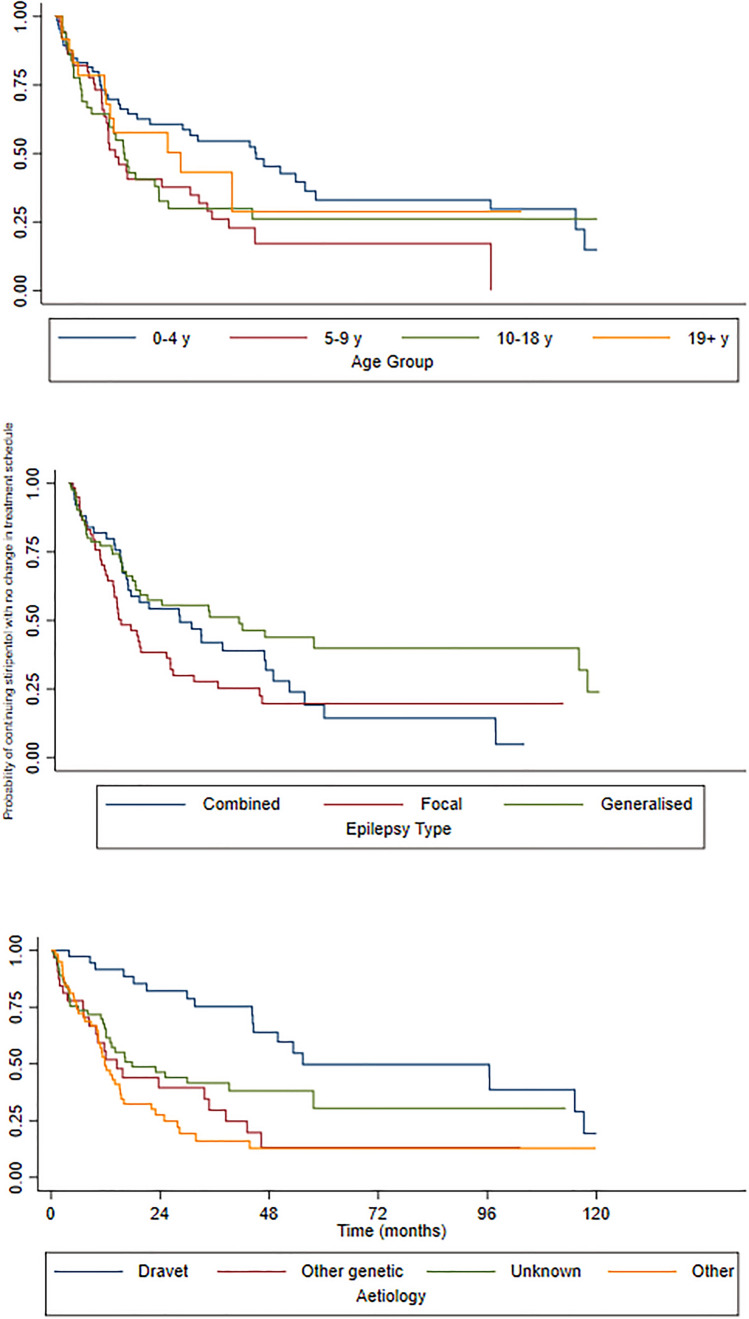
We also analysed the STP retention rate (i.e. probability of continuing STP with no change in the treatment schedule) as a proxy of efficacy and tolerability. All 196 subjects were included from STP initiation to the first treatment change, defined as STP withdrawal and/or introduction of any pharmacological or non-pharmacological treatment including surgery. The median time of STP retention was 20 months. Retention rates were 139/196 (71%) at 6 months, 103/196 (53%) at 12 months and 64/196 (33%) at 24 months. The probability of STP retention over time is reported in Table 2.
There was a difference in retention rates across the age groups, although not statistically significant: a greater retention of STP over time was observed in the age groups 0–4 years and adults (p = 0.089). Aetiology and epilepsy type were significantly associated with STP retention rate. At 60 and 72 months, 50% of subjects with DS remained taking STP and the probability of continuing STP with no change in the treatment schedule was higher than with other aetiologies (p = 0.0001). Subjects with generalised and combined types of epilepsies had a higher probability of continuing STP with no change in the treatment schedule than those with focal epilepsies (p = 0.0311). The probability of continuing STP over time stratified by age, epilepsy type and aetiology is shown in Fig. 2. We note that there is a greater difference in STP efficacy as measured by the retention rate among epilepsy types (highest for generalised epilepsies) and aetiologies (highest for DS), compared with STP efficacy measured by relapse-free survival where no statistical difference emerged among different epilepsy types or aetiologies. The multivariable Cox regression analysis confirmed aetiology to be a strong predictor of both relapse and retention rates: with DS as the reference category, the hazard ratio (HR) for relapse rate in the responder cohort was 3.6 (95% confidence interval [CI] 1.4–9.4, p = 0.009) for other genetic aetiologies (non-DS), 3.8 (95% CI 1.4–10.3, p = 0.008) for other aetiologies (i.e. structural, immune/infectious, metabolic) and 2.4 (95% CI 1.0–5.9, p = 0.044) for an unknown aetiology; the HR for retention rate in the overall cohort was 3.3 (95% CI 1.7–6.3, p < 0.001) for other genetic aetiologies (non-DS), 3.7 (95% CI 2.0–7.0, p < 0.001) for other aetiologies (i.e. structural, immune/infectious, metabolic) and 2.4 (95% CI 1.3–4.4, p = 0.005) for an unknown aetiology. The epilepsy type was associated with retention rate, with generalised epilepsy having a lower risk of STP discontinuation than other epilepsy types (HR 0.6, 95% CI 0.4–0.9; p = 0.033). There was also a trend towards significance of paediatric patients showing a higher risk of relapse than adults (HR 2.9 l, 95% CI 1.0–8.5; p = 0.051) (Table 3).
Fig. 2.
Kaplan–Meier survival estimates of the probability of continuing stiripentol over time, stratified by age, epilepsy type and aetiology. y years
Table 3.
Cox regression analysis with relapse after initial response [i.e. a ≥50% reduction in seizure frequency] (a) and stiripentol discontinuation or addition of another ASM (b) as outcome variables
| Variables | Hazard ratio (CI 95%) | P value | ||||
|---|---|---|---|---|---|---|
| (a) Relapse after a ≥50% reduction in seizure frequency | ||||||
| Age | Adult | 1.0 | ||||
| Paediatric | 3.6 (1.2–11.2) | 0.024 | ||||
| Epilepsy duration, months | ≤ 60 | 1.0 | ||||
| > 60 | 1.5 (0.8–2.8) | 0.259 | ||||
| Aetiology | Genetic (DS) | 1.0 | ||||
| Other genetic | 3.9 (1.4–10.6) | 0.009 | ||||
| Unknown | 2.7 (1.1–6.7) | 0.035 | ||||
| Other | 4.5 (1.6–12.6) | 0.005 | ||||
| Epilepsy type | Combined | 1.0 | ||||
| Focal | 0.4 (0.1–1.0) | 0.045 | ||||
| Generalised | 0.6 (0.3–1.4) | 0.248 | ||||
| Concomitant ASMs | CLB | 3.7 (0.9–16.4) | 0.080 | |||
| VPA | 0.8 (0.4–1.6) | 0.469 | ||||
| (b) Stiripentol discontinuation or addition of another ASM | ||||||
| Age | Adult | 1.0 | ||||
| Paediatric | 1.6 (0.8–3.3) | 0.184 | ||||
| Epilepsy duration, months | ≤ 60 | 1.0 | ||||
| > 60 | 0.98 (0.6–1.5) | 0.929 | ||||
| Aetiology | Genetic (DS) | 1.0 | ||||
| Other genetic | 2.8 (1.4–5.6) | 0.003 | ||||
| Unknown | 2.40 (1.1–3.8) | 0.032 | ||||
| Other | 3.0 (1.5–6.0) | 0.001 | ||||
| Epilepsy type | Combined | 1.0 | ||||
| Focal | 0.8 (0.5–1.2) | 0.273 | ||||
| Generalised | 0.6 (0.4–1) | 0.037 | ||||
| Concomitant ASMs | CLB | 0.8 (0.4–1.6) | 0.467 | |||
| VPA | 0.7 (0.45–1.1) | 0.467 | ||||
ASMs antiseizure medications, CI confidence interval, CLB clobazam, DS Dravet syndrome, VPA sodium valproate
Discussion
Our cohort of 196 patients with DS and non-DS patients with severe epilepsies treated with STP with a long-term follow-up, the largest until now published, shows a responder rate of 53% and seizure freedom in 9% after an initial median follow-up of 3 months following STP introduction. The responder rate dropped at 29% at 12 months and 22% at 24 months. Despite the reduction in the responder rate over time, retention of STP maintained higher rates with 53% at 12 months and 33% at 24 months.
The presented results are in line with the majority of previous studies published on STP use in DS [4, 5, 8–10, 13, 15–20] and other epilepsy syndromes [13, 16, 21–23]. Previous publications reported a wide range for responder rates, defined as a >50% seizure reduction, from 23% [9] to 84% [16] for all seizure types in DS. Responder rates for generalised tonic-clonic seizures vary from 8% [9] to 49% [17] in DS. The wide reported range of efficacy is likely owing to the variability in study design, epilepsy syndrome, age span, and sample sizes, from small retrospective cohorts of adult patients with DS [9] to larger prospective controlled trials including various epilepsy syndromes and younger patients [16]. In this study, we included DS and other, less severe, epilepsy syndromes, and found that both epilepsy type and aetiology are associated with a sustained response over time, especially in the first 48 months, with generalised (44%) and combined focal and generalised epilepsies (28%) showing a higher responder rate than focal (20%) epilepsies, and DS being the aetiology with the highest responder rate (64%) among the other syndromes with a genetic or unknown aetiology (13–38%) (Table 2). There was no difference in median STP dosage between responders and non-responders, providing no evidence for a dose–effect relationship. In this series, we did not find a significant difference in the responder rate or in relapse-free survival between different seizure types; therefore, we could not confirm our previous finding of a higher responder rate in focal epilepsy without bilateral tonic-clonic seizures [13]. However, we noted that the probability of remaining responders at 60 months was 73% in patients with focal-onset seizures with or without impaired awareness and 35% in those with focal to bilateral tonic-clonic seizures (Table 1).
We did not find different degrees of STP efficacy across the various age groups. However, we observed a significant difference in the relapse-free survival with the highest probability of remaining responders when STP was initiated in the youngest age group (0–4 years) or in adulthood. Factors potentially explaining these differences include non-adherence in older children and adolescents and the development of tolerance to long-term comedication with clobazam. Unfortunately, we could not measure the effect of either variable in this study. Data from a historical French monocentric cohort showed that the efficacy of STP can be maintained over a very long term, and the epilepsy outcome was less severe when its use was initiated before adolescence [10]. Epilepsy in DS is often rapidly severe from the onset, with prolonged fever-related seizures and episodes of status epilepticus; therefore, early initiation of STP might be more effective in reducing seizure severity and status epilepticus. However, the initiation of STP in adulthood was also associated with a sustained higher response over time than initiation in age 5–18 years. The long-term efficacy of STP when initiated in adulthood in any epilepsy syndromes is a novel finding, as previous evidence of later initiation was only reported in two small cohorts with DS [9] and focal epilepsies [22], with evidence of lower responder rates. The evidence for efficacy of STP when initiated in adulthood has important implications also for regulatory reasons, as for example in the UK this drug does not have a marketing authorisation for initiation in patients with DS >18 years of age. Hence, at present for people with DS above this age, it may be only initiated off-label, outside the terms of its licence.
The retention rate of STP was significantly associated with the aetiology and epilepsy type. The highest retention rate over time was observed in DS and in generalised and combined focal and generalised epilepsy types. The retention rate provides a real-life measure of the balance between tolerance and efficacy, and in our cohort seemed more informative than the relapse-free survival for which no significant predictive factors emerged. Whilst there are extensive data on the efficacy and tolerability of STP in DS, there are much fewer data in other aetiologies and other epilepsy types. We found a high retention rate for the first 12 months also in other genetic epilepsies (non-DS) (Table 2), which suggests that STP may be an effective and well-tolerated option in other rare genetic epileptic encephalopathies where epilepsy is usually very difficult to treat, and precision treatment options are still lagging. However, we could not identify any common clinical or genetic features among responders or non-responders in patients with genetic epilepsies other than DS; we found instead a wide range of mutations in different genes (Table 2 of the ESM), a finding suggesting multiple pathophysiological mechanisms for epileptogenesis.
The role of aetiology in the prediction of drug response was confirmed in the multivariable Cox regression analysis (Table 3), suggesting a potential therapeutic effect targeted to the underlying aetiological mechanisms. Although a definite mechanism of action for STP has yet to be elucidated, several possible mechanisms have been postulated, including an increase of GABAergic inhibition [1, 24], inhibition of lactate dehydrogenase [2], and blockage of sodium and calcium channels [3]. There is also contrasting evidence on whether STP could be effective also without adjunctive clobazam [25]. In our cohort, there was no significant difference between responders (51.9%) and non-responders (48.1%) in patients who had clobazam in add-on therapy (92.4%), whilst we observed a higher prevalence of responders (63.3% vs 36.7%) in patients who had concomitant treatment with valproate (55.6%). No other comedications had a significantly different prevalence between responders vs non-responders but we cannot rely on a lack of statistical significance to exclude the role of a drug–drug interaction in the clinical effect of STP [26]. We also found additional evidence of efficacy of STP in the treatment of patients with focal epilepsies, a population for which no robust conclusive data on the use of this medication is available yet [27].
We observed a low prevalence of side effects in our cohort, with the most prevalent being neurological symptoms (13%), and gastroenterological manifestations (2.5%). Because of the retrospective design of this study, we could not conduct a systematic assessment of side effects, which are therefore likely to be under-reported. Previous studies showed a wide range of prevalence of neurological adverse effects, with somnolence/drowsiness/lethargy being the most common (6–79%), followed by appetite and weight loss (3–67%) [15, 18]. Tolerability often improved after reducing the dose of STP or other co-medications [10].
Study Limitations
Because of the retrospective nature of the study, serum concentrations of STP and concomitant ASMs were not systematically available for all patients and during all follow-up visits. Stiripentol exerts an inhibitory effect on hepatic cytochrome P450, therefore raising the serum concentrations of phenobarbital, phenytoin, carbamazepine, clobazam, valproate, diazepam, ethosuximide and tiagabine. This factor may well contribute to the response observed in our series, but its impact could not be measured in our study.
Conclusions
We conclude that STP can be an effective and well-tolerated therapeutic option in a wide spectrum of epilepsy types. In addition to its known efficacy in DS, it proved effective in other epilepsies with genetic aetiologies, and other generalised or focal epilepsy syndromes without established genetic aetiologies, with sustained response over time. Its approved use on DS relies on evidence gathered in controlled trials but not on a specific mechanism of action on epileptogenesis in this syndrome. It is therefore not unexpected that it can be effective in other epilepsy syndromes as well. Stiripentol seems more effective when initiated at an early age but there is also high relapse-free survival when initiated in adulthood. This is particularly important as increasingly more people with difficult-to-treat epilepsies receive a genetic diagnosis in adulthood [28]. Areas for future research may include prospective trials of STP in specific epilepsy syndromes or aetiologies and pharmacokinetic studies to more widely assess interactions with other ASMs.
Supplementary Information
Below is the link to the electronic supplementary material.
Authors’ Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by SB, VD, AB, ML and SM. The first draft of the manuscript was written by SB and all authors commented on previous versions of the manuscript. RG critically revised the manuscript and supervised the study. All authors read and approved the final manuscript.
Declarations
Funding
No funding was received for the preparation of this article.
Conflicts of Interest/Competing Interests
Renzo Guerrini has acted as an investigator for studies with Zogenix, Biocodex, UCB, Angelini and Eisai Inc. He has been a speaker and on advisory boards for Zogenix, Biocodex, Novartis, Biomarin and GW Pharma, outside the submitted work. Simona Balestrini reports personal fees from Biocodex, Eisai and UCB Pharma, outside the submitted work. Viola Doccini received a travel grant from Biocodex, partly related to the submitted work. Alessandra Boncristiano, Matteo Lenge and Salvatore De Masi, do not have any relevant competing interests.
Ethics Approval
Approval was obtained from the Paediatric Ethics Committee of the Tuscany Region. The procedures used in this study adhere to the tenets of the Declaration of Helsinki.
Consent to Participate
Written informed consent was obtained from all individual participants included in the study or their parents or legal guardians, as appropriate.
Consent for Publication
Not applicable.
Availability of Data and Material
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Code Availability
Not applicable.
References
- 1.Chiron C. Stiripentol. Neurotherapeutics. 2007;4:123–125. doi: 10.1016/j.nurt.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sada N, Lee S, Katsu T, Otsuki T, Inoue T. Targeting LDH enzymes with a stiripentol analog to treat epilepsy. Science. 2015;347:1362–1367. doi: 10.1126/science.aaa1299. [DOI] [PubMed] [Google Scholar]
- 3.Verleye M, Buttigieg D, Steinschneider R. Neuroprotective activity of stiripentol with a possible involvement of voltage-dependent calcium and sodium channels: neuroprotective effect of stiripentol. J Neurosci Res. 2016;94:179–189. doi: 10.1002/jnr.23688. [DOI] [PubMed] [Google Scholar]
- 4.Chiron C, Marchand MC, Tran A, Rey E, d’Athis P, Vincent J, et al. Stiripentol in severe myoclonic epilepsy in infancy: a randomised placebo-controlled syndrome-dedicated trial. STICLO Study Group. Lancet. 2000;356:1638–1642. doi: 10.1016/S0140-6736(00)03157-3. [DOI] [PubMed] [Google Scholar]
- 5.Guerrini R, Tonnelier S, d’Athis P, Rey E, Vincent J, Pons G. Stiripentol in severe myoclonic epilepsy in infancy (SMEI): a placebo-controlled Italian trial. Epilepsia. 2002;43:155. doi: 10.1046/j.1528-1157.2002.13802.x. [DOI] [Google Scholar]
- 6.Cross JH, Caraballo RH, Nabbout R, Vigevano F, Guerrini R, Lagae L. Dravet syndrome: treatment options and management of prolonged seizures. Epilepsia. 2019;60(Suppl. 3):S39–48. doi: 10.1111/epi.16334. [DOI] [PubMed] [Google Scholar]
- 7.Frampton JE. Stiripentol: a review in Dravet syndrome. Drugs. 2019;79:1785–1796. doi: 10.1007/s40265-019-01204-y. [DOI] [PubMed] [Google Scholar]
- 8.Wirrell EC, Laux L, Franz DN, Sullivan J, Saneto RP, Morse RP, et al. Stiripentol in Dravet syndrome: results of a retrospective U.S. study. Epilepsia. 2013;54:1595–604. [DOI] [PubMed]
- 9.Balestrini S, Sisodiya SM. Audit of use of stiripentol in adults with Dravet syndrome. Acta Neurol Scand. 2017;135:73–79. doi: 10.1111/ane.12611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiron C, Helias M, Kaminska A, Laroche C, de Toffol B, Dulac O, et al. Do children with Dravet syndrome continue to benefit from stiripentol for long through adulthood? Epilepsia. 2018;59:1705–1717. doi: 10.1111/epi.14536. [DOI] [PubMed] [Google Scholar]
- 11.Zulfiqar Ali Q, Marques P, Selvarajah A, Tabarestani S, Sadoway T, Andrade DM. Starting stiripentol in adults with Dravet syndrome? Watch for ammonia and carnitine. Epilepsia. 2020;61:2435–2441. doi: 10.1111/epi.16684. [DOI] [PubMed] [Google Scholar]
- 12.Diacomit: summary of product characteristics. https://www.medicines.org.uk/emc/product/10300/smpc. Accessed 10 May 2022.
- 13.Rosati A, Boncristiano A, Doccini V, Pugi A, Pisano T, Lenge M, et al. Long-term efficacy of add-on stiripentol treatment in children, adolescents, and young adults with refractory epilepsies: a single center prospective observational study. Epilepsia. 2019;60:2255–2262. doi: 10.1111/epi.16363. [DOI] [PubMed] [Google Scholar]
- 14.Scheffer IE, Berkovic S, Capovilla G, Connolly MB, French J, Guilhoto L, et al. ILAE classification of the epilepsies: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:512–521. doi: 10.1111/epi.13709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Inoue Y, Ohtsuka Y. Long-term safety and efficacy of stiripentol for the treatment of Dravet syndrome: a multicenter, open-label study in Japan. Epilepsy Res. 2015;113:90–97. doi: 10.1016/j.eplepsyres.2015.03.012. [DOI] [PubMed] [Google Scholar]
- 16.Perez J, Chiron C, Musial C, Rey E, Blehaut H, d’Athis P, et al. Stiripentol: efficacy and tolerability in children with epilepsy. Epilepsia. 1999;40:1618–1626. doi: 10.1111/j.1528-1157.1999.tb02048.x. [DOI] [PubMed] [Google Scholar]
- 17.Myers KA, Lightfoot P, Patil SG, Cross JH, Scheffer IE. Stiripentol efficacy and safety in Dravet syndrome: a 12-year observational study. Dev Med Child Neurol. 2018;60:574–578. doi: 10.1111/dmcn.13704. [DOI] [PubMed] [Google Scholar]
- 18.Cho MJ, Kwon SS, Ko A, Lee S-T, Lee YM, Kim HD, et al. Efficacy of stiripentol in Dravet syndrome with or without SCN1A mutations. J Clin Neurol. 2018;14:22. doi: 10.3988/jcn.2018.14.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamada M, Suzuki K, Matsui D, Inoue Y, Ohtsuka Y. Long-term safety and effectiveness of stiripentol in patients with Dravet syndrome: interim report of a post-marketing surveillance study in Japan. Epilepsy Res. 2021;170:106535. doi: 10.1016/j.eplepsyres.2020.106535. [DOI] [PubMed] [Google Scholar]
- 20.Yıldız EP, Ozkan MU, Uzunhan TA, Bektaş G, Tatlı B, Aydınlı N, et al. Efficacy of stiripentol and the clinical outcome in Dravet syndrome. J Child Neurol. 2019;34:33–37. doi: 10.1177/0883073818811538. [DOI] [PubMed] [Google Scholar]
- 21.Chiron C, Tonnelier S, Rey E, Brunet M-L, Tran A, d’Athis P, et al. Stiripentol in childhood partial epilepsy: randomized placebo-controlled trial with enrichment and withdrawal design. J Child Neurol. 2006;21:496–502. doi: 10.1177/08830738060210062101. [DOI] [PubMed] [Google Scholar]
- 22.Habermehl L, Mross PM, Krause K, Immisch I, Chiru D, Zahnert F, et al. Stiripentol in the treatment of adults with focal epilepsy: a retrospective analysis. Seizure. 2021;88:7–11. doi: 10.1016/j.seizure.2021.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Farwell JR, Anderson GD, Kerr BM, Tor JA, Levy RH. Stiripentol in atypical absence seizures in children: an open trial. Epilepsia. 1993;34:305–311. doi: 10.1111/j.1528-1157.1993.tb02416.x. [DOI] [PubMed] [Google Scholar]
- 24.Quilichini PP, Chiron C, Ben-Ari Y, Gozlan H. Stiripentol, a putative antiepileptic drug, enhances the duration of opening of GABAA-receptor channels. Epilepsia. 2006;47:704–716. doi: 10.1111/j.1528-1167.2006.00497.x. [DOI] [PubMed] [Google Scholar]
- 25.Warner TA, Smith NK, Kang J-Q. The therapeutic effect of stiripentol in Gabrg2 mice associated with epileptic encephalopathy. Epilepsy Res. 2019;154:8–12. doi: 10.1016/j.eplepsyres.2019.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nickels KC, Wirrell EC. Stiripentol in the management of epilepsy. CNS Drugs. 2017;31:405–416. doi: 10.1007/s40263-017-0432-1. [DOI] [PubMed] [Google Scholar]
- 27.Brigo F, Igwe SC, Bragazzi NL. Stiripentol add-on therapy for drug-resistant focal epilepsy. Cochrane Database Syst Rev. 2020;5(5):CD009887. doi: 10.1002/14651858.CD009887.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Silvennoinen K, Martins Custodio H, Balestrini S, Rugg-Gunn F, England Research Consortium G, Sisodiya SM. Complex epilepsy: it’s all in the history. Pract Neurol. 2020. 10.1136/practneurol-2020-002522 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.