Abstract
The antagonistic pleiotropy hypothesis is a well-known evolutionary theory to explain the aging process. It proposes that while a particular gene may possess beneficial effects during development, it can exert deleterious properties in the aging process. The aryl hydrocarbon receptor (AhR) has a significant role during embryogenesis, but later in life, it promotes several age-related degenerative processes. For instance, AhR factor (i) controls the pluripotency of stem cells and the stemness of cancer stem cells, (ii) it enhances the differentiation of embryonal stem cells, especially AhR signaling modulates the differentiation of hematopoietic stem cells and progenitor cells, (iii) it also stimulates the differentiation of immunosuppressive Tregs, Bregs, and M2 macrophages, and finally, (iv) AhR signaling participates in the differentiation of many peripheral tissues. On the other hand, AhR signaling is involved in many processes promoting cellular senescence and pathological processes, e.g., osteoporosis, vascular dysfunction, and the age-related remodeling of the immune system. Moreover, it inhibits autophagy and aggravates extracellular matrix degeneration. AhR signaling also stimulates oxidative stress, promotes excessive sphingolipid synthesis, and disturbs energy metabolism by catabolizing NAD+ degradation. The antagonistic pleiotropy of AhR signaling is based on the complex and diverse connections with major signaling pathways in a context-dependent manner. The major regulatory steps include, (i) a specific ligand-dependent activation, (ii) modulation of both genetic and non-genetic responses, (iii) a competition and crosstalk with several transcription factors, such as ARNT, HIF-1α, E2F1, and NF-κB, and (iv) the epigenetic regulation of target genes with binding partners. Thus, not only mTOR signaling but also the AhR factor demonstrates antagonistic pleiotropy in the regulation of the aging process.
Keywords: Immunosuppression, Kynurenine, Lifespan, Longevity, RelB, Retrotransposon
Introduction
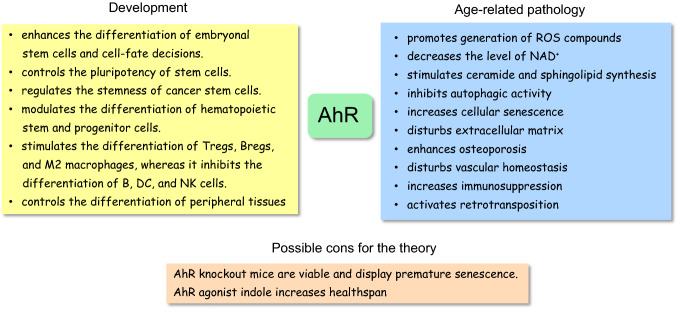
The aryl hydrocarbon receptor (AhR) is an evolutionarily conserved transcription factor which first appeared over 600 million years ago [1]. The AhR factor is a ligand-regulated transcription factor which is an ancient member of the basic-helix/loop/helix per Arnt-sim (bHLH/PAS) family [2]. Originally, the AhR factor was studied as an environmental sensor for many xenobiotics, such as 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) and polycyclic aromatic hydrocarbons (PAH). Currently, it is known that the AhR factor is not only involved in chemical defence, but it has also a crucial role in developmental biology and in the function of the immune system [3–5]. For instance, AhR signaling regulates the pluripotency of embryonic stem cells and affects their differentiation into diverse tissue types (Fig. 1). Moreover, Ahr signaling controls the differentiation of immune cells, especially enhancing the generation of immunosuppressive phenotypes (see below). On the other hand, with aging, AhR signaling increases cellular senescence and osteoporosis, inhibits autophagy, and disturbs vascular homeostasis (Fig. 1). Interestingly, the function of the AhR factor is a good example of antagonistic pleiotropy, i.e., a particular gene has crucial functions during development, but its activity evokes detrimental responses later in the life [6]. Here, I will shortly introduce the theory of antagonistic pleiotropy and then describe in detail many important functions of the AhR factor during embryogenesis and for comparison, examine its role as an enhancer of age-related degenerative processes.
Fig. 1.
AhR signaling represents an example of antagonistic pleiotropy in the regulation of the developmental and aging processes. Major beneficial developmental and harmful age-related properties have been listed for comparison. There are some cons for the theory which have been discussed in the text
AhR-driven antagonistic pleiotropy in development and aging process
The antagonistic pleiotropy hypothesis is a well-known evolutionary theory explaining the aging process, originally proposed by George Williams in 1957 [6, 7]. The antagonistic pleiotropy theory postulates that a particular gene regulates several functional traits which support the developmental process, but which exert detrimental effects during the aging process. The theory predicts that natural selection favors the vigor of youth over the frailty of old age. Those genes revealing characteristics of antagonistic pleiotropy enhance reproduction and growth at a young age, but these same genes have deleterious effects later in life promoting the aging process. The theoretical basis underpinning the antagonistic pleiotropy and its functional explanations in the aging process have been reviewed elsewhere in detail [6, 8]. The disposable soma theory, adapted from the antagonistic pleiotropy theory, suggests that organisms have a limited amount of resources which are allocated to reproduction and growth at the cost of repair processes during the aging process [9]. Subsequent studies on genetics, e.g., on the long-lived mutants, have provided novel insights into the evolutionary mechanisms controlling the aging process [8, 10]. For instance, the age-1 and daf-2 mutants in Caenorhabditis elegans as well as the chico and Inr mutants in Drosophila increased lifespan, but correspondingly, they exhibited a strongly reduced reproduction or sterility in C. elegans and Drosophila [8]. These genes are driving the insulin/insulin-like growth factor (IGF) receptor signaling pathway. In mammals, this pathway activates the mammalian target of rapamycin (mTOR) pathway which is known to display antagonistic pleiotropy in the regulation of the aging process [11, 12]. The insulin/IGF-1/TOR axis undertakes several crucial functions during reproduction and the growth of the organism, whereas the same pathway has many detrimental effects during the aging process. For instance, the inhibition of autophagy with aging increases the accumulation of garbage into aged cells [12]. Interestingly, there is clear indication that AhR signaling also inhibits autophagy, but it seems that it is mediated through the E2F1 signaling rather than the insulin/IGF1/mTOR signaling pathway (see below).
AhR stimulates developmental processes
Across the evolution from single-cell organisms to multicellular animals, AhR signaling has undertaken functions far beyond its role as an environmental sensor (Fig. 1). For instance, it is known that AhR signaling is important in many developmental processes and in the immune defence of the organism [5, 13, 14]. During embryogenesis, the expression of the AhR gene displays significant stage-specific alterations [14–16]. The AhR gene was strongly expressed in mouse fertilized eggs and up to the four-cell morula stage, but afterwards the expression level disappeared during the cleavage phase until it became evident again in the early blastocyst. After implantation, the expression of the AhR gene increased although it displayed cell type- and tissue-specific expression levels. Interestingly, a global decline in the expression of mouse AhR gene during the morula and early blastula phase coincided with a decrease in the DNA methylation state during increased proliferation [14]. Studies conducted in the AhR-knockout mice have revealed significant developmental perturbations in some tissues, especially in liver, spleen, and cardiac muscle, as well as some crucial impairments in their immune system [17–19]. Fernandez-Salguero et al. [20] reported that the AhR-null (AhR−/−) mice were viable and fertile, but had a 45% mortality and displayed clearly a sick phenotype by 13 months of age. Intriguingly, the constitutively active AhR gene (CA-AhR) expressed in transgenic mice also impaired the developmental processes of liver and kidney [21], disturbed neurogenesis [22], and increased the risk of cancers, e.g., in stomach [23]. It is clear that the AhR gene performs many homeostatic functions in the developmental processes that explain why its expression is stringently regulated.
Currently, there is convincing evidence that AhR factor has an important role in the regulation of pluripotency of embryonal stem (ES) cells since it promotes their differentiation into diverse cell lineages (Fig. 1). Ko et al. [24] demonstrated that several pluripotency factors, i.e., OCT4, NANOG, and SOX2, were able to inhibit the expression of AhR gene in mouse ES cells by binding to the silencer domain of the AhR gene. Several other investigators have also revealed that the AhR gene needs to be repressed so that ES cells can maintain their mitotic progression and pluripotency [16, 25]. For instance, OCT4 and NANOG factors displayed an overexpression in the mouse AhR−/− embryos inhibiting their differentiation process [16]. On the other hand, an increased activation of AhR factor inhibited the expression of OCT4 and NANOG and consequently promoted the differentiation of mouse ES cells [5, 14, 16]. Recently, Gonzalez-Rico et al. [26] demonstrated that AhR factor was able to bind to two Alu elements flanking the human NANOG gene thus assembling a chromatin loop which inhibited the expression of the NANOG gene. This AhR-driven retrotransposon-mediated chromatin modification inhibited the expression of human NANOG factor and subsequently induced the differentiation of human NTERA-2 cells. Moreover, Cheng et al. [27] demonstrated that the activation of AhR factor induced the binding of AhR factor to the promoter of human OCT4 gene, inhibited its expression, and consequently induced the differentiation of several cancer stem-like cells, thus reducing their tumorigenic potential. However, several studies have indicated that the expression of AhR maintained and enhanced the stemness of cancer stem cells. For instance, Stanford et al. [28] revealed that an increased expression of AhR factor augmented the development of human cells with cancer stem-like properties which enhanced tumorigenesis by increasing their migration and invasion. Yan et al. [29] also reported that the activation of AhR signaling in the radioresistant human epithelial cancer cells induced the expression of genes associated with the stem-like phenotype, e.g., ABCG2, c-MYC, and CXCR4 genes. They also revealed that nuclear IKKα protein was involved in the AhR-mediated regulation of stemness in human epithelial cancer cells.
AhR factor has a crucial role in the maintenance of hematopoietic stem cells (HSC) and progenitor cells and subsequently it is involved in their differentiation to a diverse set of immune cells in a cell-specific manner [4, 30, 31]. There is clear evidence that the activation of AhR factor is a negative regulator of hematopoiesis inhibiting excessive proliferation and thus it maintains homeostasis in the immune system [30, 32, 33]. Vaughan et al. [33] demonstrated that the inhibition of AhR signaling in mice through its knockout or the antagonist treatments induced a myeloid-biased increase in HSCs and progenitor cells. There also appeared an increase in the frequency of progenitors committed to pregranulocyte/premonocyte lineages. The role of AhR signaling has been elucidated in the differentiation of the progenitor cells into effector and regulatory immune cells. For instance, Li et al. [34] revealed that an increased AhR signaling impaired the human B cell lymphopoiesis from hematopoietic stem cells to early-B and pro-B cells. AhR signaling also disturbed the differentiation of human natural killer (NK) cells [35] and mouse dendritic cells (DC) [36]. Interestingly, Tousif et al. [37] demonstrated that AhR signaling promoted the differentiation of B cells into the regulatory B (Breg) cells in a mouse model of lung cancer. In addition, there is convincing evidence that an activation of AhR signaling induced the differentiation of mouse T cells into regulatory T (Treg) cells [38, 39] (Fig. 1). It seems that an increase in AhR signaling disturbs the function of effector immune cells, whereas it augments immunosuppressive phenotypes.
The developmental regulation of AhR signaling is not limited to the embryonal differentiation or the hematopoiesis, but it also affects the terminal differentiation and the growth of several tissues, e.g., neurogenesis, cardiovascular development, and osteogenesis [40–42]. In this respect, it seems that AhR signaling has both beneficial and detrimental effects in a context-dependent manner during development. It is also evident that different models, i.e., the knockout/antagonist or the overexpression/agonist treatments, generate non-physiological responses in long-term experiments. Neurogenesis has been frequently studied with the aim of clarifying the role of AhR signaling during development of the brain. Latchney et al. [40] reported that both the deletion and the activation of AhR signaling disturbed neurogenesis and impaired memory and cognition in growing mice. The 3-month-old AhR-deficient mice experienced an abnormal neuronal differentiation and a reduced cell survival. The AhR-null mice also revealed a reduced myelination of neurons [43]. On the other hand, Kimura et al. [22] reported that the long-term activation of AhR (CA-AhR) impaired the dendritic growth and the positioning of cortical mouse pyramidal neurons. Recently, Wei et al. [44] demonstrated that certain tryptophan-metabolizing microbes in the gut were able to enhance mouse hippocampal neurogenesis by promoting synaptic maturation and activity via AhR signaling. These studies indicate that AhR signaling is crucial for neurogenesis although the overexpression of AhR factor disturbs the programmed development of brain.
There is clear evidence that AhR signaling affects the development of peripheral tissues. For instance, studies on cardiovascular development have revealed that there is an abnormal morphogenesis induced by either AhR ablation or agonist treatments [41]. Disturbances involved alterations, e.g., in tissue structures, cellular metabolism, and cardiac functions leading to defects similar to those encountered in congenital heart disease (CHD). Pulignani et al. [45] reported that the presence of the genetic variant Arg554Lys in the AhR protein was associated with an increased risk of CHD. It is known that an AhR-deficiency can induce cardiac hypertrophy and increase arterial blood pressure in mice [46]. The activation of AhR signaling also regulates the development of bones and kidneys. Osteogenesis of the bones involves a balance between the activities of osteoblasts and osteoclasts during the developmental phase and this continues to ensure the maintenance of bone homeostasis later in the life. There is abundant evidence indicating that AhR signaling suppresses osteoblastogenesis, whereas it increases osteoclastogenesis during the development and remodeling of the bone [42, 47]. Izawa et al. [42] demonstrated that the overexpression of AhR signaling in mice enhanced osteoclastogenesis which promoted osteoporosis with aging. On the other hand, treatments with antagonists of AhR signaling, e.g., isopsoralen, stimulated the differentiation of osteoblasts and subsequently increased bone mineralization [48]. Falahatpisheh et al. [49] demonstrated that the expression of AhR was crucial for the development of mouse kidneys. They reported that AhR signaling regulated signaling via the Wilms’ tumor suppressor 1 (WT1) during nephrogenesis, especially affecting the development of kidney glomeruli. In conclusion, it seems that a constant activity of AhR signaling maintains developmental homeostasis in the tissues, and thus either a decline or an excessive activation of AhR signaling impairs embryogenesis.
AhR promotes the age-related degenerative processes
Several aging studies have revealed that AhR signaling is associated with many age-related degenerative processes [50, 51] (Fig. 1). It appears that some of the properties driven by AhR are crucial in the early growth of embryos, but subsequently become harmful during the aging process, e.g., the repression of autophagy enhances the growth of tissues during embryogenesis, but it disturbs cellular homeostasis with aging [52]. Moreover, apoptosis and cellular senescence are important tissue remodeling mechanisms during development, but the accumulation of senescent cells with aging enhances the aging process [53, 54]. The expression of matrix-degrading metalloproteinases (MMP), a function controlled by AhR signaling, has an important role in the development of tissues during embryogenesis, but with aging, an increased expression of MMPs has many harmful effects on tissue integrity [55]. The increased inflammatory microenvironment with aging enhances the activity of AhR signaling which might promote the age-related remodeling of the immune system (see below). It seems that certain properties of AhR factor are driving the growth of organism, but they have detrimental effects with aging.
It has been difficult to obtain direct evidence on the role of AhR activity in the aging process because both the depletion and overexpression of mammalian AhR factor disturb the physiological functions of AhR signaling causing pathological consequences and leading to premature aging. One reason might be the fact that the expression of the AhR gene is stringently regulated in a context-dependent manner. In addition, it is not only the expression level of AhR factor since a number of endogenous and exogenous ligands, either agonists or antagonists, regulate the context-dependent activity of AhR factor. However, the genetic models of Caenorhabditis elegans have been exploited in the lifespan studies on the mutants of the ahr-1 gene. For instance, Eckers et al. [56] demonstrated that the ahr-1 (ju 145) mutants displayed an increased mean lifespan as well as they revealed an improved motility and heat resistance. However, Sonowal et al. [57] reported that certain indoles, AhR agonists, from commensal bacteria extended the healthspan of many organisms, e.g., C. elegans, Drosophila, and mice, although indoles did not affect maximum lifespan (Fig. 1). On the other hand, the extrinsic activation of AhR factor in mouse skin promoted the aging process [50]. It is known that chronic UV radiation (UVR) can induce the AhR-mediated photoaging in the skin [58]. Next, I will examine the properties of AhR signaling which are able to generate the essential hallmarks of the aging process.
AhR signaling generates diverse cellular stresses
Increased AhR signaling can induce many cellular stresses, such as oxidative stress, sphingolipid accumulation, and the depletion NAD+, all of which disturb cellular functions and enhance the aging process (Fig. 1). Interestingly, it is known that oxidative stress is a potent enhancer of the aging process, but it is an important mechanism during the embryonic development phase [59]. Particularly, redox regulation has a crucial role in the self-renewal and lineage commitment of stem cells [60, 61]. ROS compounds can control the activity of many key transcription factors associated with both the developmental and the aging processes, e.g., NF-κB, HIF-1α, and NRF2. ROS compounds regulate not only the development of stem cells, but also organismal aging in a concentration-dependent manner [62]. Low concentrations induce adaptive responses, whereas higher concentrations trigger pathological changes. There is convincing evidence that the activation of AhR signaling generates ROS compounds [63]. Certain target genes of AhR transcription factor are associated with the generation of ROS/oxidative stress, e.g., cytochrome P450 family 1 subfamily A member 1 (CYP1A1) and the p40phox component of NADPH oxidase [63, 64] (Fig. 2). CYP1A1, a member of cytochrome P450 family, is located in mitochondria and is involved in the production of superoxide in mitochondria [63]. The p40phox component has an important role in the ROS production by NADPH oxidase [64]. AhR signaling promotes many age-related processes via the generation of ROS compounds, e.g., inflammatory responses, cellular senescence, and remodeling of ECM (see below). As a pleiotropic factor, AhR can also activate the antioxidant pathways which have an important role in the maintenance of the cellular redox status [65]. In fact, the AhR-NRF2 signaling is the best characterized of the antioxidant pathways driven by AhR signaling (Fig. 2). It is known that ROS compounds can control both the developmental and the aging processes through an epigenetic regulation of the chromatin landscape [66].
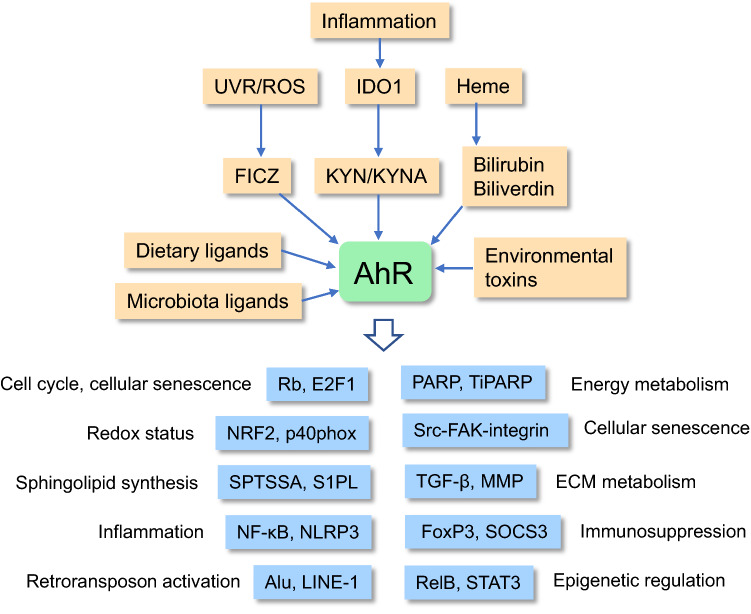
Fig. 2.
Age-related properties induced by the ligand-activated AhR signaling. A large variety of endogenous and exogenous ligands activate AhR signaling which promotes the aging process in a context-dependent manner. Certain dietary, environmental, and microbiota ligands can be antagonists for AhR activation (see text). E2F1 E2F transcription factor 1, FAK focal adhesion kinase, FICZ 6-formylindolo[3,2-b]carbazole, FoxP3 forkhead box P3, IDO1 indoleamine 2,3-dioxygenase, KYNA kynurenic acid, KYN kynurenine, MMP matrix metalloproteinase, NF-κB nuclear factor-κB, NLRP3 NOD- LRR- and pyrin domain-containing protein 3, NRF2 nuclear factor-erythroid factor 2-related factor 2, p40phox p40 component of NADPH oxidase, PARP poly(ADP-ribose) polymerase, Rb retinoblastoma, RelB RELB proto-oncogene, ROS reactive oxygen species, SOCS3 suppressor of cytokine signaling 3, SPTSSA serine palmitoyltransferase small subunit A, Src SRC proto-oncogene, STAT3 signal transducer and activator of transcription 3, TiPARP TCDD-inducible poly(ADP-ribose) polymerase, TGF-β transforming growth factor-β, UVR ultraviolet radiation
Sphingolipids are important membrane components, but they also have many signaling functions, e.g., they are involved in the regulation of cell proliferation, adhesion, autophagy, apoptosis, and many immune functions [67]. Sphingolipids have a crucial role in the developmental processes including brain development and stem cell differentiation [68, 69]. On the other hand, sphingolipids, especially ceramide, regulates cellular senescence and the aging process [70, 71]. Majumder et al. [72] utilized a genome-wide CRISPR/Cas9 screening to demonstrated that AhR factor increased the levels of several sphingolipids in mouse liver and lung. AhR factor induced the transcription of mouse serine palmitoyltransferase small subunit A (SPTSSA) gene. The SPT complex is the first and rate-limiting step in the synthesis of sphingolipids (Fig. 2). Moreover, Wang et al. [73] reported that AhR factor inhibited the expression of human sphingosine-1-phosphate lyase (S1PL) enzyme which controls the degradation of S1P to phosphoethanolamine. This means that the activation of AhR signaling is able not only to enhance the synthesis of sphingolipids, but also to inhibit their degradation. Ceramide and S1P are the most important signaling components of sphingolipids and they have many opposing functions, called the sphingolipid rheostat. For instance, ceramides induce cell-cycle arrest, cellular senescence, apoptosis, and neurodegeneration, whereas S1P has many beneficial properties, e.g., it controls lymphocyte differentiation and trafficking as well as it promotes neuroprotection [74, 75]. Different approaches have revealed that the level of ceramides increases with aging, thus probably promoting senescence and the aging process [70, 71]. Interestingly, Huang et al. [76] demonstrated that the inhibition of SPT reduced sphingolipid synthesis and significantly extended the lifespan of yeast. Given that AhR signaling stimulated the expression of SPT, this might accelerate the aging process of mammals. Currently, it is known that the AhR-induced activation of SPT is associated with the appearance of many age-related diseases, e.g., hepatic lipid accumulation in mice [77].
The maintenance of energy homeostasis is crucial for both developmental processes and healthspan extension. NAD+ is a coenzyme which not only controls energy status, but it also supplies the NAD+-consuming enzymes, e.g., poly(ADP-ribose)polymerases (PARP) and sirtuins (SIRT1-7) [78]. Interestingly, the activation of AhR factor can disturb energy balance by reducing the cellular level of NAD+. Ma [79] was the first investigator who demonstrated that TCDD induced the AhR-mediated expression of a novel PARP enzyme, called TiPARP (ARTD14/PARP7). Subsequently, MacPherson et al. [80] revealed that human TiPARP enzyme was a mono-ADP-ribosyltransferase which also inhibited the transactivation of the AhR gene. This shows a negative feedback loop in AhR signaling. The TiPARP enzyme has an important role in the developmental process since the TiPARP knockout mice experienced disturbances in the development of GABAergic neurons and the loss of TiPARP induced an abnormal layering of mouse cerebral cortex [81]. There is convincing evidence that the activation of AhR factor reduced the level of NAD+ under diverse experimental conditions [82]. It seems that AhR signaling decreases the level of NAD+ attributed to the activation of TiPARP, whereas concurrently it increases the mono-ADP-ribosylation of many proteins, thus affecting the maintenance of homeostasis. There is robust evidence that the cellular level of NAD+ declines in senescent cells and during aging in many tissues [83, 84]. Currently, the role of AhR signaling in these age-related alterations is not completely clear.
AhR signaling promotes cellular senescence and controls apoptosis
The accumulation of senescent cells into tissues is a major hallmark of the aging process [85]. Senescent cells undergo an irreversible arrest of cell-cycle which has been attributed to an activation of several tumor suppressors, such as p53, p16Ink4a, p21Cip1, p27Kip1, and retinoblastoma (Rb) protein [85, 86]. There is abundant evidence that the activation of AhR signaling by several agonists arrests the proliferation of different cell types and consequently triggers a senescent phenotype [87–89]. Interestingly, AhR signaling also inhibits the proliferation of stem cells, e.g., embryonal stem cells [25], hematopoietic stem cells [90], and bone marrow mesenchymal stem cells [89] (Fig. 2). It seems that there are several mechanisms through which AhR factor can repress the cell-cycle progression. For instance, AhR factor can increase the expression of p21Cip1 and p27Kip1 proteins and thus halt cell proliferation. Jackson et al. [91] demonstrated that the activation of AhR by TCDD induced the expression of p21Cip1 protein and consequently inhibited the regeneration of mouse liver. The p21Cip1 inhibited the G1-phase cyclin-dependent kinase 2 (CDK2) activity and induced a G0/G1 cell-cycle arrest. Accordingly, Kolluri et al. [92] reported that the activation of AhR factor induced the expression of p27Kip1 protein and suppressed the proliferation of developing mouse thymus and rat hepatoma cells. There are several studies indicating that the AhR protein can bind to the Rb protein and consequently repress the function of E2F transcription factors, thus inhibiting DNA synthesis and cell-cycle progression [93, 94] (Fig. 2). Marlowe et al. [94] revealed that the binding of AhR protein to E2F transcription factors displaced the p300 protein from the complex and subsequently inhibited the transcription of many E2F-regulated genes which control the S phase progression. It is known that the E2F1 transcription factor inhibits the Forkhead box O transcription factors (FoxO) and thus controls cellular senescence and organismal aging [95]. Moreover, the aging process is associated with a progressive decline in the numbers of stem cells [96]. Currently, the role of AhR signaling in the exhaustion of age-related stem cells needs to be clarified.
Programmed cell death, i.e., apoptosis, is a crucial architect of mammalian development [54]. Although AhR signaling has a fundamental role in embryogenesis, there are only a few reports on its effects on apoptosis during developmental processes. For instance, the Bax-dependent apoptosis driven by AhR signaling controlled the development of murine fetal ovarian germ cells [97]. However, there is a substantial literature examining the role of AhR signaling in apoptosis, both in pro-apoptotic and anti-apoptotic responses, later in the life. It seems that the activation of AhR signaling by environmental toxins, e.g., TCDD and polycyclic aromatic hydrocarbons (PAH), induces an apoptotic cell death in diverse cell types [98, 99]. There appears to be several mechanisms in the toxin-induced apoptosis although the ROS-induced mitochondrial dysfunction seems to be the major process. However, it is not known how AhR signaling is driving the endogenously-induced apoptosis or resistance to apoptosis. For instance, it has been demonstrated that C2-ceramides can trigger apoptosis in murine hepatomas [100]. On the other hand, there is robust evidence that AhR signaling can prevent apoptotic cell death in many tissues. As a proof of principle, Elizondo et al. [101] reported that embryonic fibroblasts obtained from the AhR-knockout mice were predisposed to apoptosis in cell culture. Moreover, when AhR signaling was activated by tumor promoters, this proved to be a potent inhibitor of apoptosis and an effective inducer of tumorigenesis [102]. For instance, Bekki et al. [103] demonstrated that AhR signaling activated by kynurenine (KYN) exposure enhanced apoptosis resistance in human breast cancer cells. Interestingly, there is clear evidence indicating that the resistance to apoptosis increases in both cellular senescence and the aging process [104, 105]. Marlowe et al. [106] demonstrated that the AhR protein formed a complex with the E2F1 factor and thus inhibited the E2F1-induced apoptosis in mouse hepatoma cells and human osteosarcoma cells. Currently, it needs to be clarified whether the resistance to the apoptosis associated with aging and cellular senescence is related to AhR signaling. It is clear that any decline in the extent of apoptosis with aging would prevent the elimination of unfit cells and thus disturb tissue homeostasis.
AhR signaling inhibits autophagic degradation
Autophagy is a catabolic process which involves the degradation of intracellular proteins and organelles via the lysosomal pathway. Mammalian target of rapamycin (mTOR), a major regulator of protein synthesis and cellular growth, is a potent inhibitor of autophagy. Schmeisser and Parker [12] have reviewed the potential role of mTOR-dependent autophagy as a model of antagonistic pleiotropy. A low level of autophagy enhances the growth of tissues during development, but with aging, it has detrimental effects on tissue homeostasis. There is robust evidence that autophagic degradation declines with aging and that there are disturbances in the function of the autophagy-lysosome pathway [52, 107]. Interestingly, there are observations indicating that AhR signaling is able to suppress autophagic activity. Kondrikov et al. [89] demonstrated that KYN, an endogenous agonist for AhR, inhibited autophagy via the activation of AhR signaling in the bone marrow mesenchymal stem cells (BMSC) obtained from aged mice. The physiological levels of KYN disrupted autophagic flux and inhibited macroautophagy induced by serum-starvation in mouse BMSCs. Kim et al. [108] reported that the activation of AhR signaling with TCDD and proinflammatory cytokines in human keratinocytes decreased the expression of several autophagy-related factors including Beclin 1, ATG5, and LC3 proteins. The production of LC3-positive autophagosomes was also down-regulated in activated keratinocytes. Surprisingly, the inhibition of autophagy with chloroquine increased the expression of AhR in the cytokine-stimulated keratinocytes. Yang and Chan [109] also observed that the inhibition of autophagy by chemical agents increased the level of the AhR protein in human HeLa cells. They revealed that the AhR protein was degraded through the p62/LC3-mediated selective autophagy in diverse human cell lines. Thus, the inhibition of autophagy, e.g., by mTOR signaling, enhances the AhR-related responses. Currently, the mechanism of the AhR-induced repression of autophagy still needs to be clarified. Polager et al. [110] demonstrated that the E2F1 factor stimulated autophagy by increasing the expression of several autophagy genes, e.g., ATG1, ATG5, LC3, and DRAM genes, in human osteosarcoma cells. This means that the E2F1 factor does not only arrest cell cycle and inhibit apoptosis, but it also stimulates autophagy in order to maintain homeostasis. As discussed earlier, AhR is a potent inhibitor of E2F1 signaling [93, 94, 106] and thus it could promote the age-related inhibition of autophagy. One might speculate that the AhR factor, as an effective growth regulator during development, can inhibit certain catabolic activities with aging although they are crucial for the maintenance of cellular integrity, i.e., autophagy and apoptosis. The inhibition of autophagy by AhR signaling is a good example of how antagonistic pleiotropy can drive the aging process.
AhR signaling aggravates extracellular matrix degeneration
The extracellular matrix (ECM) has a crucial role in both the developmental morphogenesis of tissues and in the maintenance of homeostasis during the aging process. Tissue development is dependent on a constant remodeling of ECM structures involving a dynamic balance between both synthetic and catabolic processes [111]. ECM provides the microenvironment for both cellular differentiation and tissue morphogenesis during embryogenesis. This developmental process is regulated by a diverse network of signaling mechanisms and the enzymes controlling the homeostasis of ECM. On the other hand, it is known that the aging process is associated with many degenerative activities in the ECM of many tissues, e.g., in the skin and cardiac muscle [112, 113]. For example, there is an increased expression and activity of many matrix metalloproteinases (MMP), an accumulation of collagen with an increased level of cross-linking, and an enhanced fragmentation of ECM components; these are all common hallmarks of age-related degeneration of ECM integrity. Moreover, the degeneration of ECM modifies immune responses thus exposing tissues to inflammation and many diseases [114]. Interestingly, AhR signaling has an important role in the ECM remodeling and the maintenance of its homeostasis [115, 116]. It is known that AhR signaling stimulates the expression and activity of different MMPs, e.g., MMP1 and MMP9, and thus enhances the degradation of ECM structures in a context dependent manner (Fig. 2). There is robust evidence indicating that the expression of many MMPs increases with aging in different tissues [113]. AhR signaling co-operates with several signaling pathways, such as TGF-β, NF-κB, and integrin pathways, in the regulation of cell adhesion and ECM metabolism [116, 117]. The TGF-β cytokine is a pleiotropic regulator of ECM remodeling, i.e., its signaling is stringently controlled in a cell-specific manner [117, 118]. However, AhR and TGF-β factors display mutually repressive signaling mechanisms in many conditions [117]. For instance, AhR signaling regulates the expression of the latency-associated protein 1 (LTBP-1) which inhibits the activation of latent TGF-β in the ECM. This regulation is under the epigenetic control and is thus a context-dependent process. It has been proposed that the AhR/LTBP-1/TGF-β axis has a crucial role in many pathological conditions [117]. Tomkiewicz et al. [119] demonstrated that the activation of AhR factor stimulated the Src kinase which subsequently activated focal adhesion kinase (FAK) and enhanced cell migration in human HepG2 cells. Moreover, TCDD exposure increased the expression of several integrin proteins, e.g., ITGβ1, ITGβ3, and ITGα1, in human HepG2 cells. Interestingly, Rapisarda et al. [120] revealed that an increased expression of ITGβ3 induced the senescence of several human cell types by activating TGF-β signaling. In this respect, the activation of AhR signaling enhances cell migration during the developmental phase as well as cell senescence and ECM degradation with aging.
AhR signaling provokes osteoporosis and vascular dysfunction
AhR signaling has an important role in the control of homeostasis in bones and vascular tissues. The balance between the activities of osteoblasts and osteoclasts regulates the mineralization and resorption of the bone [121]. There is abundant evidence that the activation of AhR signaling arrests the proliferation of human osteoblasts [122], whereas it stimulates the formation of osteoclasts thus increasing bone resorption and enhancing osteoporosis with aging [42, 47, 123]. Eisa et al. [47] reported that KYN, an agonist of AhR, induced the generation of osteoclasts from mouse macrophages via the activation of the receptor of nuclear factor κB ligand (RANKL) signaling. KYN treatment also enhanced the resorption of mouse bone. However, recent studies have revealed that the AhR-driven osteoclastogenesis in mouse macrophages is dependent on both the concentration of indoxyl sulfate, an endogenous AhR agonist, as well as the duration of treatment [124]. A short-term and low-dose exposure stimulated mouse osteoclast differentiation, whereas a long-term and high-dose treatment inhibited the differentiation of mouse macrophages into osteoclasts. Refaey et al. [125] reported that feeding of adult mice with KYN increased the serum level of RANKL, a marker for osteoclastic activity, as well as it induced osteoporotic changes in the bone microarchitecture. KYN supplementation also augmented the adiposity of bone marrow. It is known that the activity of indoleamine 2,3-dioxygenase 1 (IDO1) and consequently the serum levels of KYN significantly increase with aging [126, 127]. Accordingly, Chung et al. [128] reported that the aging process notably upregulated the markers of osteoclastogenesis in human bone marrow cells, such as the expression levels of RANK and c-fms genes. Currently, the mechanisms behind the pleiotropic responses of AhR signaling in the maintenance of bone homeostasis remain to be elucidated.
AhR signaling regulates not only vascular development, but also sprouting angiogenesis which can have either beneficial or detrimental effects in diverse diseases. It is known that different AhR agonists inhibit the proliferation, migration, and tube formation of human umbilical vein/artery endothelial cells (HUVEC/HUAEC) [129]. Ichihara et al. [130] demonstrated that a hindlimb ischemia induced a markedly increased angiogenesis in the AhR-knockout mice in comparison with that of their normal counterparts. The enhanced angiogenesis was associated with an increased expression of hypoxia-inducible factor-1α (HIF-1α) which stimulated the expression of vascular endothelial growth factor (VEGF) subsequently enhancing angiogenesis. Given that AhR and HIF-1α factors share the same nuclear transporter ARNT protein [131], it seems that a reduced level of AhR factors could well increase the nuclear transport of HIF-1α (see below). It is known that exposure to indoxyl sulfate was able to promote rat endothelial senescence [132] and enhance the disruption of rat blood–brain barrier [133] via an activation AhR signaling. Indoxyl sulfate, a uremic toxin produced from L-tryptophan, has been claimed to be associated with some serious vascular diseases, e.g., chronic kidney disease and cardiovascular disease [134]. Eckers et al. [56] demonstrated that the overexpression of AhR factor or its stimulation with agonists impaired the activation of endothelial nitric oxide synthase (eNOS) and the production of NO in human endothelial cells. eNOS has a crucial role in the maintenance of vascular homeostasis and it is evident that its reduced activation could lead to the development of vascular diseases [135].
AhR signaling induces the remodeling of immune system
The aging process is associated with a significant remodeling of both arms of the immune system, i.e., adaptive and innate immunity [136–138]. Common hallmarks of immunoaging include (i) an involution of the thymus, (ii) a chronic low-grade inflammation called inflammaging, (iii) increased immunosuppressive activity attempting to counteract inflammaging, and (iv) immunosenescence of effector immune cells reducing the functional activity of the immune system with aging. Interestingly, AhR signaling seems to be able to regulate these processes in a context-dependent manner. It is known that the activation of AhR signaling can promote an atrophy/involution of mouse thymus which disturbs the maturation of lymphocytes with aging thus enhancing immunosenescence [82, 139]. Laiosa et al. [139] revealed that the activation of AhR signaling within intrathymic lymphocyte progenitor cells arrested their proliferation and consequently induced the atrophy of mouse thymus. Diani-Moore et al. [82] reported that the stimulation of AhR signaling by TCDD activated poly (ADP-ribose) polymerase (TiPARP) which caused the loss of NAD+ and subsequently elicited thymic atrophy in mice. The exposure to a PARP inhibitor increased the level of NAD+ and prevented the atrophy of thymus. Age-related involution of thymus is an important contributor to inflammaging and immunosenescence [140].
There is clear evidence that AhR signaling can induce both pro-inflammatory and anti-inflammatory responses through different signaling pathways [4, 141, 142]. Commonly, many environmental pollutants, e.g., polycyclic aromatic hydrocarbons (PAH), are known to stimulate oxidative stress and induce inflammatory responses [143, 144]. The AhR-induced ROS production probably is the major mechanism underpinning the generation of inflammation since ROS are well-known inducers of inflammatory responses [145]. Vogel et al. [144] demonstrated that inflammatory stimuli also induced the expression of AhR factor in human dendritic cells. They revealed that the promoter of the human AhR gene contained three putative NF-κB binding sites; of these, one motif mediated the RelA/p50-induced transcription of the AhR gene which explains why an increased NF-κB signaling is associated with the stimulation of AhR signaling. Subsequently, AhR signaling is able to control the function of a large set of lymphoid cells generating either pro- or anti-inflammatory responses [4, 146]. Moreover, bilirubin and biliverdin, the catabolic metabolites of heme protein, are also agonists of the AhR factor [147] (Fig. 2). It is known that diverse stresses can increase the levels of these compounds which possess anti-inflammatory activity by inhibiting the activation of NF-κB and inflammasomes [148].
There is convincing evidence that AhR signaling is driving an anti-inflammatory regulation rather than pro-inflammatory responses. Huai et al. [149] demonstrated that the AhR factor suppressed the function of inflammasomes by inhibiting the transcription of the NLRP3 gene in mice. They revealed that AhR factor was able to bind to the xenobiotic response element (XRE) in the promoter of the NLRP3 gene and inhibited its transcription (Fig. 2). Inflammasomes are responsible for the activation of a diverse set of inflammatory responses driven by the innate immune system. AhR factor can also suppress inflammatory responses by inducing the expression of SOCS3 protein, a suppressor of cytokine signaling [150]. Wada et al. [150] revealed that the mouse and human promoter sequences of the SOCS3 gene contained a transcriptionally active XRE site through which AhR factor inhibited the transactivation of the SOCS3 gene in mouse liver and human HepG2 cells. They also reported that the activation of AhR signaling suppressed hepatic steatosis occurring when mice were fed a high fat diet. Moreover, the activation of AhR signaling stimulates the differentiation of immunosuppressive phenotypes of many immune cells, i.e., (i) induction of myeloid-derived suppressor cells (MDSC) [151], (ii) Tregs [38], (iii) Bregs [37], (iv) tolerogenic dendritic cells (tolDC) [152], and (v) M2 macrophages (Mreg) [153]. For instance, AhR signaling stimulated the expression of Forkhead box 3 (FoxP3) protein, a master regulator of Tregs [38] (Fig. 2). AhR factor can also induce immunosuppressive responses via the non-genomic Src-STAT3 pathway. Zhu et al. [154] reported that AhR factor promoted the expression of IL-10 through the Src-STAT3 pathway in mouse inflammatory macrophages.
It is known that a chronic state of inflammation, such as inflammaging, triggers a compensatory immunosuppression which prevents excessive inflammatory responses [155]. There is clear evidence that the aging process induces the activation of immunosuppressive network which most probably counteracts inflammaging [138]. Interestingly, it seems that AhR signaling has a key role in the induction of inflammation-induced immunosuppression. It is recognized that NF-κB signaling, a major inducer of inflammatory responses, is also able to transactivate the expression of AhR factor [144]. Moreover, it is known that inflammation stimulates the expression of the IDO1 enzyme which activates the KYN pathway thus producing KYN and kynurenic acid (KYNA), potent agonists for AhR [39, 127] (Fig. 2). Consequently, the activation of AhR signaling can promote the expression of IDO1 [156], i.e., a positive feedback loop between IDO1 and AhR signaling. Given that AhR signaling stimulates anti-inflammatory/immunosuppressive responses (see above), this provides a counteracting regulation commonly observed not only in inflammaging [157], but also in age-related inflammatory diseases. An increased immunosuppressive activity with aging inhibits the functional activity of the immune system evoking a state called immunosenescence [158]. Immunosenescence in conjunction with immunosuppression increases a risk for cancers, enhances sensitivity to infections, and exposes elderly people to many age-related diseases [159]. For instance, it is well-known phenomenon that vaccination efficiency and the efficacy of immunotherapies decline as the individual enters old age. These observations are indications that there is a remodeling of the immune system with aging. Immunosuppressive mechanisms, e.g., the secretion of anti-inflammatory cytokines and ROS/RNS compounds as well as the depletion of certain amino acids via catabolism also promote degenerative bystander effects in inflamed host tissues [160]. For instance, TGF-β signaling can induce fibrosis, cellular senescence, osteoporosis, muscle atrophy, and alterations in ECM [161]. It seems that acute inflammatory responses stimulate the expression of AhR factor which consequently enhances immunosuppression and ultimately promotes immunosenescence and immunoaging of the immune system.
AhR signaling is increased in age-related inflammatory diseases
Currently, there are many review articles describing the role of AhR signaling in inflammatory diseases, e.g., atherosclerosis, neurodegenerative diseases, rheumatoid arthritis, and chronic infections [162, 163]. Given that AhR signaling has a crucial role in cardiovascular physiology, disturbances in its function have been linked with several cardiovascular diseases, e.g., atherosclerosis and ischemic heart disease [164]. For instance, AhR signaling can enhance inflammatory responses by promoting oxidative stress and cellular senescence in the intimal layer of the blood vessel wall. Moreover, Vogel et al. [165] demonstrated that TCDD stimulated the differentiation of human U937 macrophages into atherogenic foam cells. The foam cells secreted inflammatory mediators and MMPs thus disturbing ECM structures and inducing chronic inflammation in blood vessel wall. However, Kim et al. [166] reported that AhR signaling inhibited the transition of smooth muscle cells to chondromyocytes in human atherosclerotic lesions. This implies that AhR signaling has both beneficial and harmful effects in atherosclerotic tissue. There seem to exist tissue-specific differences in the AhR-induced responses to ischemic insults. Seong et al. [167] revealed that the activation of AhR signaling with an endogenous agonist, 2-[1′H-indole-3-carbonyl]-thiazole-4 carboxylic acid methyl ester (ITE), improved the function of mouse cardiac muscle after myocardial infarction. In fact, ITE treatment increased the number of immunosuppressive FoxP3-positive Tregs and shifted the pro-inflammatory M1 macrophages towards the anti-inflammatory M2 phenotype. On the other hand, Cuartero et al. [168] demonstrated that the activation of KYN/AhR pathway enhanced the extent of acute brain damage after middle cerebral artery occlusion (MCAO) in mice. An ischemic insult increased the nuclear translocation of AhR factor to neurons in peri-infarct and core regions. They also reported that the KYN-induced increase in infarct volume was AhR-dependent since it did not appear in the AhR antagonist-treated or in the AhR-knockout mice. Chen et al. [169] reported that AhR immunoreactivity mainly increased in activated microglia and astrocytes after MCAO in mice. They also observed that the activation of AhR after an ischemic stroke increased astrogliosis and suppressed neurogenesis in adult mice. These studies indicate that the responses of AhR signaling are context-dependent in inflammatory states.
Ramos-Garcia et al. [170] demonstrated that the expression of AhR was increased with aging in the human post-mortem hippocampal samples. The increase was more evident in non-neuronal cells than neurons, especially the cytoplasm of astrocytes displayed a robust immunostaining. They also revealed that there was a strong increase in the expression level of AhR protein in the hippocampal samples of patients with Alzheimer’s disease (AD) as compared to that of healthy elderly people. Currently, the role of AhR signaling in the pathogenesis of AD is unknown. However, Duan et al. [171] demonstrated that the neurotoxicity of β-amyloid peptide was dependent on IDO1/KYN/AhR signaling in rat primary neurons. There is clear evidence that uremic toxins aggravate vascular inflammation in many chronic inflammatory diseases, e.g., in cardiovascular diseases [172]. Uremic toxins, e.g., indoxyl sulfate, derived from the gut microbiota, are potent agonists for AhR factor [173]. Chronic kidney disease (CKD) leads to the accumulation of uremic toxins in the circulation. It has been reported that indoxyl sulfate promotes the AhR-mediated production of ROS/RNS compounds which impair the redox balance of endothelial cells and induce vascular inflammation [174]. These toxins not only enhance inflammation in the kidney, but they accelerate vascular or even organismal aging throughout the body [175, 176]. It is known that the presence of CKD exposes the patient to many other chronic diseases, such as cardiovascular diseases [172] and Alzheimer’s disease [177]. Given that AhR signaling is a pleiotropic regulator in inflammatory states, it will provide a great challenge for drug discovery projects.
Potential molecular mechanisms associated with the AhR-promoted aging process
The AhR is a transcription factor which integrates many upstream signaling pathways to a multitude of downstream functions. Here, the principles of the regulation will be briefly described, mainly focusing on the mechanisms which appear to control the aging process. The signaling mechanisms and functions undertaken by the AhR factor have been described in detail elsewhere [3, 178, 179]. The AhR protein belongs to the family of bHLH/PAS domain factors possessing functional sites for ligand binding, dimerization, HSP90 interface, and DNA-binding [3]. A cytoplasmic complex of AhR contains the interaction with chaperones p23, XAP2, and HSP90 [180]. After ligand binding, AhR factor is translocated to the nucleus where it heterodimerizes with AhR nuclear translocator (ARNT) protein [181]. The AhR/ARNT complex binds to the specific dioxin/xenobiotic response element (DRE/XRE) and this can activate the transcription of multiple genes. However, AhR factor can also form complexes with other transcription factors, e.g., RelB, E2F1, and estrogen receptor (ER), and subsequently bind to their specific binding sites, i.e., not that of DRE/XRE. These non-canonical pathways indicate that AhR factor can affect gene expression which is not controlled by the AhR/ARNT complex [182]. The ARNT factor can form a complex with the AhR repressor (AhRR) protein which means that AhRR protein competes with AhR factor for binding to the ARNT factor. Given that the AhRR/ARNT complex lacks a transactivation domain, it indicates that the complex inhibits AhR signaling [183]. In addition to the genomic regulation, AhR factor is also able to affect signaling in a non-genomic manner. There is clear evidence that ligand binding to AhR factor in the cytoplasm can activate Src kinase which subsequently stimulates focal adhesion kinase (FAK) and promotes integrin clustering and cellular plasticity in human HepG2 cells [119] (Fig. 2). The AhR/Src signaling can also induce the Src/IDO1 and Src/STAT3 pathways which are inducers of many immunosuppressive properties [154].
Given that AhR factor is a ligand-dependent transcription factor, its ligands, either agonists or antagonists, have a key role in the regulation of its activity. The AhR factor was first characterized as a sensor for environmental toxins, such as TCDD and PAH compounds. In addition to xenobiotic ligands, it has been revealed that it can bind a number of endogenous ligands, such as many tryptophan metabolites as well as the metabolites of the arachidonic acid and heme pathways [178]. There exist significant differences in the specificity of ligands in the activation of AhR factor concerning ligand structures, tissues, and even animal species. Currently, it has been speculated that the most important ligands related to the aging process and age-related diseases could be metabolites of L-tryptophan degradation [179]. In the host cells, the activation of IDO1/IDO2 and tryptophan 2–3-dioxygenase (TDO) stimulates the kynurenine pathway generating kynurenine (KYN) and kynurenic acid (KYNA) which are potent activators of AhR factor [127, 179]. Interestingly, many inflammatory mediators stimulate the activation of IDO1 and the levels of KYN are significantly elevated with aging [126, 127]. Ultraviolet radiation (UVR) generates 6-formylindolo[3,2-b]carbazole (FICZ) from tryptophan and FICZ is a potential inducer of skin photoaging [58]. Gut microbiota are also an important producer of tryptophan metabolites, especially indole compounds, which can gain access to the circulation and subsequently activate AhR signaling in many tissues [173]. Moreover, Marinelli et al. [184] revealed that the butyrate produced by the microbiota was an activating ligand for the AhR factor. There is convincing evidence indicating that microbiome can have a crucial role in the aging process and especially in age-related diseases [185, 186]. Currently, it seems that the IDO1/KYN pathway and gut microbiome provide important endogenous ligands which can participate in the regulation of AhR signaling.
The ARNT protein is not a specific binding partner for AhR and AhRR, but it can also bind some other transcription factors, e.g., hypoxia-inducible factor-1α (HIF-1α) [187]. This means that there exists a competition between the AhR and HIF-1α factors for the recruitment of the ARNT protein and subsequent DNA binding [131, 187, 188]. The ARNT factor has also been called HIF-β because it dimerizes with HIF-1α protein. There is robust evidence that hypoxia/HIF-1α suppressed the gene expression via AhR signaling, whereas the activation of AhR inhibited the HIF-1α-driven gene expression [187–189]. The conditional knockout of mouse Arnt gene prevented both the AhR and HIF-1α-driven induction of target genes [190]. It has been demonstrated that an increased expression of HIF-1α and hypoxia resistance are both associated with an extension of lifespan, e.g., in subterranean naked mole-rats [191]. Studies on C. elegans have also revealed that hypoxia and the consequent stabilization of HIF-1α protein can extend lifespan in the co-operation with other longevity factors [192]. Currently, it is not known whether an increased HIF-1α stabilization in hypoxic conditions decreases AhR signaling and thus could reduce the AhR-promoted degenerative processes.
There is clear evidence that AhR factor co-operates with the NF-κB signaling system [179]. The NF-κB pathway is a major regulator of immune responses, but it also controls apoptosis, cellular senescence, and even the aging process [193, 194]. Vogel et al. [195] demonstrated that AhR factor interacted with RelB component, a member of NF-κB family driving non-canonical signaling, and subsequently the complex became bound to the specific RelB/AhR responsive element (RelBAhRE) in the promoter of the IL-8 gene and increased the promoter activity in human macrophages. Interestingly, the RelB/AhR complex was able to bind to specific NF-κB sites as well as to the DRE/XRE sites. The RelB-induced DNA-binding did not require ARNT protein, but protein kinase A (PKA) signaling clearly activated the transcription. Consequently, Ishihara et al. [196] revealed that the RelB-enhanced expression of AhR-driven cytokine genes was dependent on which ligand was binding to the AhR factor. For instance, TCDD and FICZ promoted the RelB-dependent expression of CCL20, whereas indole-3-carbinol suppressed the expression of CCL20 in the LPS-stimulated mouse macrophages. The function of the RelB factor has been associated with many developmental processes in the immune system, especially the differentiation of dendritic cells [197]. It is known that RelB protein can modify the chromatin landscape and thus promote alterations in cell phenotypes. For instance, RelB protein can form a repressive complex with histone H3 lysine methyltransferase G9a in the IL-1β promoter of human THP-1 monocytes and thus induce tolerance to endotoxin [198]. Currently, it is not known whether the RelB/AhR complex can be associated with the RelB-dependent epigenetic regulation.
Currently, it is known that epigenetic regulation controls the expression of the AhR gene and subsequently AhR protein can modify, e.g., the activity of retrotransposons [13, 26]. Epigenetic mechanisms have a crucial role both in the early development and the aging process [199, 200]. There are several studies indicating that the promoter sequences of the human AhR gene contained DNA methylation sites which controlled the silencing of the gene [201]. Moreover, Ko et al. [24] demonstrated that the promoter of the mouse AhR gene contained clusters of binding sites for the pluripotency factors NANOG and OCT3/4 which mediated the silencing of the AhR gene through an association of polycomb group (PcG) proteins with the methylated histones. Accordingly, the differentiation of mouse embryonal stem cells increased the acetylation level of these histone sites that enhanced the transcription of the AhR gene. Garrison et al. [202] reported that exposure of human HepG2 and MCF7 cells to histone deacetylase (HDAC) inhibitors robustly increased the activity of the AhR promoter. These observations highlight that the transactivation of the AhR gene is under the epigenetic regulation through DNA and histone methylation. Interestingly, there are studies indicating that AhR factor is able to regulate the epigenetic state of target genes. Singh et al. [203] demonstrated that in mice, an experimental colitis decreased the presence of Tregs and increased the activation of Th17 cells. Interestingly, TCDD exposure promoted the differentiation of Tregs and inhibited the activity of Th17 cells. They also revealed that TCDD treatment significantly reduced the methylation of CpG sites in the promoter of the FoxP3 gene, whereas it increased the methylation level of the IL-17 promoter. These changes attenuated clinical and inflammatory markers of mouse colitis. Clinical studies have also revealed that the activation of the AhR-HDAC8 axis promoted the progress of human hepatocellular carcinoma [204]. AhR factor stimulated the expression of HDAC8 via the AhR-ARNT complex in human hepatoma cells. Wajda et al. [205] have reviewed the studies on the role of AhR factor in the epigenetic regulation of the immune system. Currently, it is clear that epigenetic mechanisms control the aging process both at the stem cell and organismal level [96, 200]. Epigenetic regulation is a tempting model to explain how antagonistic pleiotropy could control both the developmental and aging processes.
Several epigenome-wide association studies (EWAS) have demonstrated that smoking is associated with DNA hypomethylation at the intron 3 of the AhRR gene (cg05575921) in peripheral blood mononuclear cells (PBMC) [206, 207]. Dawes et al. [208] reported that increased cigarette consumption enhanced the hypomethylation of the intron in PBMC and saliva samples in a dose-dependent manner. Interestingly, there are several reports indicating that the alterations in the DNA methylation signatures of smokers significantly correlated with the markers of the epigenetic aging clock, e.g., with the GrimAge and DNAmPhenoAge biomarkers [209, 210]. These studies indicated that the hypomethylation of CpG site at the intron 3 of the AhRR gene accelerated the aging process although the mechanism is still unknown. Moreover, AhR factor is able to regulate the differentiation of embryonic stem cells via the control of retrotransposon Alu and LINE-1 sequences. Alu and LINE-1 transposons are normally silenced with heterochromatin structures, but there are studies indicating that many transposons are activated as the individual ages [211]. Wood et al. [212] demonstrated that the genetic suppression of transposable elements extended the lifespan in Drosophila. There are many observations that AhR factor regulates the activity of transposable elements, e.g., Alu and LINE-1 [26, 213] (Fig. 2). It is known that AhR ligands can reactivate LINE-1 retrotransposon in many human and mouse cell lines [213]. The retrotransposition of LINE-1 has been associated with the activation of immune system and thus it can induce both inflammation and autoimmunity [214]. There are observations that LINE-1 caused DNA damages and enhanced cellular senescence [215]. Given that retrotransposons have beneficial effects during embryogenesis, St. Laurent 3rd et al. [215] proposed that the activation of retrotransposition with aging represents an antagonistic pleiotropy in the regulation of the aging process.
Anti-aging therapeutic treatments suppress AhR signaling
Substantial research efforts have been exerted in the search for anti-aging therapeutics. It is known that metformin and rapamycin treatments are able to extend the healthspan and lifespan of mice [216, 217]. Interestingly, there is clear evidence that metformin and rapamycin can suppress AhR signaling in different models [218, 219]. It seems that autophagy is involved in these results since both metformin and rapamycin are potent inducers of autophagy and it is known that autophagy is an important regulator of the aging process [52, 107]. Interestingly, Yang and Chan [109] demonstrated that the AhR protein was degraded via selective autophagy in several human cell lines. Accordingly, chloroquine, an inhibitor of autophagy, significantly increased the protein level of AhR factor. Nguyen et al. [220] reported that the p23 co-chaperone of the AhR complex prevented the degradation of AhR factor. The levels of AhR and ARNT were also robustly reduced in mouse hepatoma cells with a p23 knockdown. Although the mechanism of p23 in the degradation of AhR factor is unknown, it seems that autophagy controls the protein level and the activity of AhR signaling. In addition to metformin and rapamycin, also a short-term nutrient deprivation was able to induce autophagy and down-regulate AhR factor in human HeLa cells [109]. It is widely accepted that the anti-aging response of caloric restriction is induced by an increase in cellular autophagy [221]. There is robust evidence that certain plant polyphenols are potent modulators of AhR factor, either as agonists or antagonists [222, 223]. Xue et al. [222] categorized different polyphenols according to their agonistic/antagonistic properties; the most potent agonists were chrysin, baicalein, and quercetin, whereas the most powerful antagonists were kaempferol, resveratrol, luteolin, and curcumin. Especially, resveratrol, a potential anti-aging compound, displays many anti-inflammatory and therapeutic effects in age-related degenerative diseases, e.g., cardiovascular diseases, cancers, osteoporosis, neurodegenerative diseases, and sarcopenia [224]. However, the role of AhR signaling as a potential target of anti-aging therapeutics needs to be clarified.
AhR knockout mice: Cons for antagonistic pleiotropy theory?
The AhR-null (AhR−/−) mice revealed crucial developmental defects in many tissues, especially in liver, spleen, and cardiovascular system [17, 18, 20]. Moreover, there appeared clear impairments in the immune system, e.g., proliferation of hematopoietic stem cells (HSC) was robustly increased which promoted premature exhaustion of HSCs and thus augmented myeloproliferative disorders [19]. The AhR-null mice displayed such pathological changes as cardiovascular lesions, hepatic fibrosis, and increased tumorigenesis [17, 20]. The mortality of the AhR-null mice was significantly increased both right after birth and later in life [17, 225]. All these alterations are consistent with the theory of antagonistic pleiotropy. Surprisingly, recent studies have revealed that lack of AhR factor is associated with premature aging process characterized by enhanced cellular senescence and increased serum levels of both pro-inflammatory and anti-inflammatory cytokines, such as IL-6, TNF-α, and IL-10 [225, 226]. Nacarino-Palma et al. [226] reported that AhR depletion significantly increased tumor incidence in mouse liver with aging. Moreover, they demonstrated that the deficiency of AhR factor robustly expanded the number of senescent cells in the liver preceding the aging process. For instance, there was a clear overexpression of senescence markers, such as senescence-associated β-galactosidase, p16INK4a, and p21CIP1. An increased cellular senescence was not only a liver-specific process since AhR-deficient embryonic and adult fibroblasts displayed increased cellular senescence in vitro cell cultures. These results indicate that lack of AhR factor accelerates premature aging process and tumorigenesis, robustly present already at the age of 12–15 months [225, 226]. Given that AhR signaling promotes cellular senescence with aging (see above), these observations on premature aging process in the AhR-null mice seem to be contradictory to the antagonistic pleiotropy theory (Fig. 1).
There is substantial evidence that natural killer (NK) cells and cytotoxic CD8+ T cells have a crucial role in the immune surveillance of senescent and tumor cells [227–229]. Sagiv et al. [227] revealed that NK cells targeted senescent human fibroblast and killed them by secreting perforin, a pore-forming cytolytic protein present in the granules of NK and CD8+ T cells. NK and CD8+ T cells also exploit the perforin/granzyme mechanism to induce apoptosis of tumor cells [230]. Consequently, Ovadya et al. [231] reported that there was a robust accumulation of senescent cells in the tissues of perforin-knockout mice which displayed a premature aging process. Interestingly, there is convincing evidence that AhR factor is required for the maintenance of liver-resident NK cells in mice [232]. Zhang et al. [232] demonstrated that there was a robust decline in the number of NK cells in the AhR-null mice. Moreover, AhR deficient mice could not mount an NK cell memory response to hapten challenges. Accordingly, Shin et al. [233] reported that the activation of AhR factor was required for the proper cytolytic activity of NK cells in tumor surveillance, i.e., NK cells from the AhR-null mice possessed an intrinsic defect in tumoricidal activity. These results imply that an impaired surveillance capacity of NK cells could cause an accumulation of senescent and tumor cells in the AhR-null mice. An enhanced cellular senescence of AhR-depleted fibroblasts in cell culture might be attributed to metabolic disturbances caused by the absence of the AhR-driven gene expression, both via genomic and non-genomic pathways. It seems that a lack of AhR factor disturbs normal development and leads to pathological processes rather than represents a real aging process.
Conclusions
The antagonistic pleiotropy theory is an old evolutionary explanation for the cellular senescence and the aging process. There are rather few genes revealing the clear-cut examples of the antagonistic pleiotropy. Blagosklonny [11] provided the evidence that mTOR was a good example of antagonistic pleiotropy, i.e., it drives developmental processes, its knockout has detrimental effects during embryogenesis, but it promotes many age-related degenerative processes later in life. Interestingly, similar properties have been associated with the expression of AhR factor although the mTOR and AhR factors represent very different types of regulation. The AhR factor is an ancient sensor for diverse environmental ligands, initially its role was thought to be confined to defending the organism from a range of chemical threats. Nowadays, it is recognized that it is also involved in early developmental processes, especially the differentiation of stem cells and immune cells. There is substantial evidence that with aging, AhR factor is involved in several processes promoting cellular senescence and age-related pathological conditions, such as osteoporosis, vascular dysfunction, and the remodeling of the immune system. Accordingly, there is evidence that an increase in AhR signaling occurs in many age-related diseases, such as atherosclerosis, cancers, and rheumatoid arthritis. This pleiotropy poses difficulties for targeting AhR for drug discovery purposes. For instance, many AhR agonists, e.g., phytochemicals and microbiota compounds, prevent inflammation but an increase in the level of AhR signaling can promote different age-related degenerative processes. Nonetheless, treatment with tapinarof, a natural AhR agonist, resolved skin inflammation in patients with psoriasis and atopic dermatitis [234]. Currently, there are also some drug discovery projects aimed to reveal novel antagonistic ligands to inhibit AhR signaling. Treatments with AhR antagonists have yielded promising results in rheumatoid arthritis [235] and in cancers, e.g., melanoma and glioma [236]. Some researchers have claimed that AhR factor is a survival and longevity factor based on the health problems and shorter lifespan of the knockout mice. However, it seems that the knockout technology, even a conditional knockout, is unable to confirm directly whether AhR factor is driving the aging process since as a pleiotropic factor AhR signaling is able to regulate different and sometimes opposing traits in the organism. For instance, there are observations that indoles, an agonist of AhR factor, can extend healthspan, but not maximum lifespan in worms, flies, and mice [57] (Fig. 1). However, indoles from commensal bacteria contains numerous compounds which can be processed to different metabolites with diverse agonist/antagonist activities. It is also known that there exist many species-specific differences in the responses of AhR signaling [237] but their role in antagonistic pleiotropy needs to be clarified.
Acknowledgements
The author thanks Dr. Ewen MacDonald for checking the language of the manuscript.
Funding
Open access funding provided by University of Eastern Finland (UEF) including Kuopio University Hospital. The author declares that no funds, grants, or other support were received during the preparation of this manuscript.
Availability of data and material
Not applicable.
Declarations
Conflict of interest
The author declares no conflict of interest.
Consent for publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hahn ME, Karchner SI, Merson RR. Diversity as opportunity: insights from 600 million years of AHR evolution. Curr Opin Toxicol. 2017;2:58–71. doi: 10.1016/j.cotox.2017.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nebert DW. Aryl hydrocarbon receptor (AHR): "pioneer member" of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of "sensors" of foreign and endogenous signals. Prog Lipid Res. 2017;67:38–57. doi: 10.1016/j.plipres.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stockinger B, Di Meglio P, Gialitakis M, Duarte JH. The aryl hydrocarbon receptor: multitasking in the immune system. Annu Rev Immunol. 2014;32:403–432. doi: 10.1146/annurev-immunol-032713-120245. [DOI] [PubMed] [Google Scholar]
- 4.Gutierrez-Vazquez C, Quintana FJ. Regulation of the immune response by the aryl hydrocarbon receptor. Immunity. 2018;48:19–33. doi: 10.1016/j.immuni.2017.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zablon HA, Ko CI, Puga A. Converging roles of the aryl hydrocarbon receptor in early embryonic development, maintenance of stemness, and tissue repair. Toxicol Sci. 2021;182:1–9. doi: 10.1093/toxsci/kfab050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gaillard JM, Lemaitre JF. The Williams' legacy: a critical reappraisal of his nine predictions about the evolution of senescence. Evolution. 2017;71:2768–2785. doi: 10.1111/evo.13379. [DOI] [PubMed] [Google Scholar]
- 7.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution. 1957;11:398–411. doi: 10.2307/2406060. [DOI] [Google Scholar]
- 8.Austad SN, Hoffman JM. Is antagonistic pleiotropy ubiquitous in aging biology? Evol Med Public Health. 2018;2018:287–294. doi: 10.1093/emph/eoy033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kirkwood TB. Evolution of ageing. Nature. 1977;270:301–304. doi: 10.1038/270301a0. [DOI] [PubMed] [Google Scholar]
- 10.Gems D. The hyperfunction theory: an emerging paradigm for the biology of aging. Ageing Res Rev. 2022;74:101557. doi: 10.1016/j.arr.2021.101557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blagosklonny MV. Revisiting the antagonistic pleiotropy theory of aging: TOR-driven program and quasi-program. Cell Cycle. 2010;9:3151–3156. doi: 10.4161/cc.9.16.13120. [DOI] [PubMed] [Google Scholar]
- 12.Schmeisser K, Parker JA. Pleiotropic effects of mTOR and autophagy during development and aging. Front Cell Dev Biol. 2019;7:192. doi: 10.3389/fcell.2019.00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mulero-Navarro S, Fernandez-Salguero PM. New trends in aryl hydrocarbon receptor biology. Front Cell Dev Biol. 2016;4:45. doi: 10.3389/fcell.2016.00045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ko CI, Puga A. Does the aryl hydrocarbon receptor regulate pluripotency? Curr Opin Toxicol. 2017;2:1–7. doi: 10.1016/j.cotox.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abbott BD, Birnbaum LS, Perdew GH. Developmental expression of two members of a new class of transcription factors: I. Expression of aryl hydrocarbon receptor in the C57BL/6N mouse embryo. Dev Dyn. 1995;204:133–143. doi: 10.1002/aja.1002040204. [DOI] [PubMed] [Google Scholar]
- 16.Nacarino-Palma A, Gonzalez-Rico FJ, Rejano-Gordillo CM, Ordiales-Talavero A, Merino JM, Fernandez-Salguero PM. The aryl hydrocarbon receptor promotes differentiation during mouse preimplantational embryo development. Stem Cell Reports. 2021;16:2351–2363. doi: 10.1016/j.stemcr.2021.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fernandez-Salguero P, Pineau T, Hilbert DM, McPhail T, Lee SS, Kimura S, Nebert DW, Rudikoff S, Ward JM, Gonzalez FJ. Immune system impairment and hepatic fibrosis in mice lacking the dioxin-binding Ah receptor. Science. 1995;268:722–726. doi: 10.1126/science.7732381. [DOI] [PubMed] [Google Scholar]
- 18.Schmidt JV, Su GH, Reddy JK, Simon MC, Bradfield CA. Characterization of a murine Ahr null allele: involvement of the Ah receptor in hepatic growth and development. Proc Natl Acad Sci U S A. 1996;93:6731–6736. doi: 10.1073/pnas.93.13.6731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Singh KP, Bennett JA, Casado FL, Walrath JL, Welle SL, Gasiewicz TA. Loss of aryl hydrocarbon receptor promotes gene changes associated with premature hematopoietic stem cell exhaustion and development of a myeloproliferative disorder in aging mice. Stem Cells Dev. 2014;23:95–106. doi: 10.1089/scd.2013.0346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fernandez-Salguero P, Ward JM, Sundberg JP, Gonzalez FJ. Lesions of aryl-hydrocarbon receptor-deficient mice. Vet Pathol. 1997;34:605–614. doi: 10.1177/030098589703400609. [DOI] [PubMed] [Google Scholar]
- 21.Brunnberg S, Andersson P, Lindstam M, Paulson I, Poellinger L, Hanberg A. The constitutively active Ah receptor (CA-Ahr) mouse as a potential model for dioxin exposure—effects in vital organs. Toxicology. 2006;224:191–201. doi: 10.1016/j.tox.2006.04.045. [DOI] [PubMed] [Google Scholar]
- 22.Kimura E, Kubo KI, Endo T, Ling W, Nakajima K, Kakeyama M, Tohyama C. Impaired dendritic growth and positioning of cortical pyramidal neurons by activation of aryl hydrocarbon receptor signaling in the developing mouse. PLoS ONE. 2017;12:e0183497. doi: 10.1371/journal.pone.0183497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Andersson P, McGuire J, Rubio C, Gradin K, Whitelaw ML, Pettersson S, Hanberg A, Poellinger L. A constitutively active dioxin/aryl hydrocarbon receptor induces stomach tumors. Proc Natl Acad Sci U S A. 2002;99:9990–9995. doi: 10.1073/pnas.152706299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko CI, Wang Q, Fan Y, Xia Y, Puga A. Pluripotency factors and polycomb group proteins repress aryl hydrocarbon receptor expression in murine embryonic stem cells. Stem Cell Res. 2014;12:296–308. doi: 10.1016/j.scr.2013.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ko CI, Fan Y, de Gannes M, Wang Q, Xia Y, Puga A. Repression of the aryl hydrocarbon receptor is required to maintain mitotic progression and prevent loss of pluripotency of embryonic stem cells. Stem Cells. 2016;34:2825–2839. doi: 10.1002/stem.2456. [DOI] [PubMed] [Google Scholar]
- 26.Gonzalez-Rico FJ, Vicente-Garcia C, Fernandez A, Munoz-Santos D, Montoliu L, Morales-Hernandez A, Merino JM, Roman AC, Fernandez-Salguero PM. Alu retrotransposons modulate Nanog expression through dynamic changes in regional chromatin conformation via aryl hydrocarbon receptor. Epigenetics Chromatin. 2020;13:15. doi: 10.1186/s13072-020-00336-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cheng J, Li W, Kang B, Zhou Y, Song J, Dan S, Yang Y, Zhang X, Li J, Yin S, Cao H, Yao H, Zhu C, Yi W, Zhao Q, Xu X, Zheng M, Zheng S, Li L, Shen B, Wang YJ. Tryptophan derivatives regulate the transcription of Oct4 in stem-like cancer cells. Nat Commun. 2015;6:7209. doi: 10.1038/ncomms8209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanford EA, Wang Z, Novikov O, Mulas F, Landesman-Bollag E, Monti S, Smith BW, Seldin DC, Murphy GJ, Sherr DH. The role of the aryl hydrocarbon receptor in the development of cells with the molecular and functional characteristics of cancer stem-like cells. BMC Biol. 2016;14:20. doi: 10.1186/s12915-016-0240-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yan B, Liu S, Shi Y, Liu N, Chen L, Wang X, Xiao D, Liu X, Mao C, Jiang Y, Lai W, Xin X, Tang CE, Luo D, Tan T, Jia J, Liu Y, Yang R, Huang J, Zhou H, Cheng Y, Cao Y, Yu W, Muegge K, Tao Y. Activation of AhR with nuclear IKKα regulates cancer stem-like properties in the occurrence of radioresistance. Cell Death Dis. 2018;9:490. doi: 10.1038/s41419-018-0542-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Angelos MG, Kaufman DS. Advances in the role of the aryl hydrocarbon receptor to regulate early hematopoietic development. Curr Opin Hematol. 2018;25:273–278. doi: 10.1097/MOH.0000000000000432. [DOI] [PubMed] [Google Scholar]
- 31.Trikha P, Lee DA. The role of AhR in transcriptional regulation of immune cell development and function. Biochim Biophys Acta Rev Cancer. 2020;1873:188335. doi: 10.1016/j.bbcan.2019.188335. [DOI] [PubMed] [Google Scholar]
- 32.Boitano AE, Wang J, Romeo R, Bouchez LC, Parker AE, Sutton SE, Walker JR, Flaveny CA, Perdew GH, Denison MS, Schultz PG, Cooke MP. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science. 2010;329:1345–1348. doi: 10.1126/science.1191536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vaughan KL, Franchini AM, Kern HG, Lawrence BP. The aryl hydrocarbon receptor modulates murine hematopoietic stem cell homeostasis and influences lineage-biased stem and progenitor cells. Stem Cells Dev. 2021;30:970–980. doi: 10.1089/scd.2021.0096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Bhattacharya S, Zhou J, Phadnis-Moghe AS, Crawford RB, Kaminski NE. Aryl hydrocarbon receptor activation suppresses EBF1 and PAX5 and impairs human B lymphopoiesis. J Immunol. 2017;199:3504–3515. doi: 10.4049/jimmunol.1700289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Scoville SD, Nalin AP, Chen L, Chen L, Zhang MH, McConnell K, Beceiro Casas S, Ernst G, Traboulsi AA, Hashi N, Williams M, Zhang X, Hughes T, Mishra A, Benson DM, Saultz JN, Yu J, Freud AG, Caligiuri MA, Mundy-Bosse BL. Human AML activates the aryl hydrocarbon receptor pathway to impair NK cell development and function. Blood. 2018;132:1792–1804. doi: 10.1182/blood-2018-03-838474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bankoti J, Rase B, Simones T, Shepherd DM. Functional and phenotypic effects of AhR activation in inflammatory dendritic cells. Toxicol Appl Pharmacol. 2010;246:18–28. doi: 10.1016/j.taap.2010.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tousif S, Wang Y, Jackson J, Hough KP, Strenkowski JG, Athar M, Thannickal VJ, McCusker RH, Ponnazhagan S, Deshane JS. Indoleamine 2, 3-dioxygenase promotes aryl hydrocarbon receptor-dependent differentiation of regulatory B cells in lung cancer. Front Immunol. 2021;12:747780. doi: 10.3389/fimmu.2021.747780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Quintana FJ, Basso AS, Iglesias AH, Korn T, Farez MF, Bettelli E, Caccamo M, Oukka M, Weiner HL. Control of T(reg) and T(H)17 cell differentiation by the aryl hydrocarbon receptor. Nature. 2008;453:65–71. doi: 10.1038/nature06880. [DOI] [PubMed] [Google Scholar]
- 39.Mezrich JD, Fechner JH, Zhang X, Johnson BP, Burlingham WJ, Bradfield CA. An interaction between kynurenine and the aryl hydrocarbon receptor can generate regulatory T cells. J Immunol. 2010;185:3190–3198. doi: 10.4049/jimmunol.0903670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Latchney SE, Hein AM, O'Banion MK, DiCicco-Bloom E, Opanashuk LA. Deletion or activation of the aryl hydrocarbon receptor alters adult hippocampal neurogenesis and contextual fear memory. J Neurochem. 2013;125:430–445. doi: 10.1111/jnc.12130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carreira VS, Fan Y, Wang Q, Zhang X, Kurita H, Ko CI, Naticchioni M, Jiang M, Koch S, Medvedovic M, Xia Y, Rubinstein J, Puga A. Ah receptor signaling controls the expression of cardiac development and homeostasis genes. Toxicol Sci. 2015;147:425–435. doi: 10.1093/toxsci/kfv138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Izawa T, Arakaki R, Mori H, Tsunematsu T, Kudo Y, Tanaka E, Ishimaru N. The nuclear receptor AhR controls bone homeostasis by regulating osteoclast differentiation via the RANK/c-Fos signaling axis. J Immunol. 2016;197:4639–4650. doi: 10.4049/jimmunol.1600822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shackleford G, Sampathkumar NK, Hichor M, Weill L, Meffre D, Juricek L, Laurendeau I, Chevallier A, Ortonne N, Larousserie F, Herbin M, Bieche I, Coumoul X, Beraneck M, Baulieu EE, Charbonnier F, Pasmant E, Massaad C. Involvement of aryl hydrocarbon receptor in myelination and in human nerve sheath tumorigenesis. Proc Natl Acad Sci U S A. 2018;115:E1319–E1328. doi: 10.1073/pnas.1715999115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wei GZ, Martin KA, Xing PY, Agrawal R, Whiley L, Wood TK, Hejndorf S, Ng YZ, Low JZY, Rossant J, Nechanitzky R, Holmes E, Nicholson JK, Tan EK, Matthews PM, Pettersson S. Tryptophan-metabolizing gut microbes regulate adult neurogenesis via the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2021;118:e2021091118. doi: 10.1073/pnas.2021091118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pulignani S, Borghini A, Vecoli C, Foffa I, Ait-Ali L, Andreassi MG. A functional aryl hydrocarbon receptor genetic variant, alone and in combination with parental exposure, is a risk factor for congenital heart disease. Cardiovasc Toxicol. 2018;18:261–267. doi: 10.1007/s12012-017-9436-9. [DOI] [PubMed] [Google Scholar]
- 46.Lund AK, Goens MB, Kanagy NL, Walker MK. Cardiac hypertrophy in aryl hydrocarbon receptor null mice is correlated with elevated angiotensin II, endothelin-1, and mean arterial blood pressure. Toxicol Appl Pharmacol. 2003;193:177–187. doi: 10.1016/j.taap.2003.08.008. [DOI] [PubMed] [Google Scholar]
- 47.Eisa NH, Reddy SV, Elmansi AM, Kondrikova G, Kondrikov D, Shi XM, Novince CM, Hamrick MW, McGee-Lawrence ME, Isales CM, Fulzele S, Hill WD. Kynurenine promotes RANKL-induced osteoclastogenesis in vitro by activating the aryl hydrocarbon receptor pathway. Int J Mol Sci. 2020;21:7931. doi: 10.3390/ijms21217931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ge L, Cui Y, Cheng K, Han J. Isopsoralen enhanced osteogenesis by targeting AhR/ERα. Molecules. 2018;23:2600. doi: 10.3390/molecules23102600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Falahatpisheh MH, Nanez A, Ramos KS. AHR regulates WT1 genetic programming during murine nephrogenesis. Mol Med. 2011;17:1275–1284. doi: 10.2119/molmed.2011.00125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vogeley C, Esser C, Tüting T, Krutmann J, Haarmann-Stemmann T. Role of the aryl hydrocarbon receptor in environmentally induced skin aging and skin carcinogenesis. Int J Mol Sci. 2019;20:6005. doi: 10.3390/ijms20236005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brinkmann V, Ale-Agha N, Haendeler J, Ventura N. The aryl hydrocarbon receptor (AhR) in the aging process: another puzzling role for this highly conserved transcription factor. Front Physiol. 2020;10:1561. doi: 10.3389/fphys.2019.01561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cuervo AM. Autophagy and aging: keeping that old broom working. Trends Genet. 2008;24:604–612. doi: 10.1016/j.tig.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sacco A, Belloni L, Latella L. From development to aging: the path to cellular senescence. Antioxid Redox Signal. 2021;34:294–307. doi: 10.1089/ars.2020.8071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wanner E, Thoppil H, Riabowol K. Senescence and apoptosis: Architects of mammalian development. Front Cell Dev Biol. 2021;8:620089. doi: 10.3389/fcell.2020.620089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zitka O, Kukacka J, Krizkova S, Huska D, Adam V, Masarik M, Prusa R, Kizek R. Matrix metalloproteinases. Curr Med Chem. 2010;17:3751–3768. doi: 10.2174/092986710793213724. [DOI] [PubMed] [Google Scholar]
- 56.Eckers A, Jakob S, Heiss C, Haarmann-Stemmann T, Goy C, Brinkmann V, Cortese-Krott MM, Sansone R, Esser C, Ale-Agha N, Altschmied J, Ventura N, Haendeler J. The aryl hydrocarbon receptor promotes aging phenotypes across species. Sci Rep. 2016;6:19618. doi: 10.1038/srep19618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sonowal R, Swimm A, Sahoo A, Luo L, Matsunaga Y, Wu Z, Bhingarde JA, Ejzak EA, Ranawade A, Qadota H, Powell DN, Capaldo CT, Flacker JM, Jones RM, Benian GM, Kalman D. Indoles from commensal bacteria extend healthspan. Proc Natl Acad Sci U S A. 2017;114:E7506–E7515. doi: 10.1073/pnas.1706464114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Murai M, Tsuji G, Hashimoto-Hachiya A, Kawakami Y, Furue M, Mitoma C. An endogenous tryptophan photo-product, FICZ, is potentially involved in photo-aging by reducing TGF-β-regulated collagen homeostasis. J Dermatol Sci. 2018;89:19–26. doi: 10.1016/j.jdermsci.2017.10.002. [DOI] [PubMed] [Google Scholar]
- 59.Dennery PA. Effects of oxidative stress on embryonic development. Birth Defects Res C Embryo Today. 2007;81:155–162. doi: 10.1002/bdrc.20098. [DOI] [PubMed] [Google Scholar]
- 60.Bigarella CL, Liang R, Ghaffari S. Stem cells and the impact of ROS signaling. Development. 2014;141:4206–4218. doi: 10.1242/dev.107086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tan DQ, Suda T. Reactive oxygen species and mitochondrial homeostasis as regulators of stem cell fate and function. Antioxid Redox Signal. 2018;29:149–168. doi: 10.1089/ars.2017.7273. [DOI] [PubMed] [Google Scholar]
- 62.Ristow M, Schmeisser K. Mitohormesis: promoting health and lifespan by increased levels of reactive oxygen species (ROS) Dose Response. 2014;12:288–341. doi: 10.2203/dose-response.13-035.Ristow. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dalton TP, Puga A, Shertzer HG. Induction of cellular oxidative stress by aryl hydrocarbon receptor activation. Chem Biol Interact. 2002;141:77–95. doi: 10.1016/s0009-2797(02)00067-4. [DOI] [PubMed] [Google Scholar]
- 64.Wada T, Sunaga H, Ohkawara R, Shimba S. Aryl hydrocarbon receptor modulates NADPH oxidase activity via direct transcriptional regulation of p40phox expression. Mol Pharmacol. 2013;83:1133–1140. doi: 10.1124/mol.112.083303. [DOI] [PubMed] [Google Scholar]
- 65.Dietrich C. Antioxidant functions of the aryl hydrocarbon receptor. Stem Cells Int. 2016;2016:7943495. doi: 10.1155/2016/7943495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kietzmann T, Petry A, Shvetsova A, Gerhold JM, Görlach A. The epigenetic landscape related to reactive oxygen species formation in the cardiovascular system. Br J Pharmacol. 2017;174:1533–1554. doi: 10.1111/bph.13792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hannun YA, Obeid LM. Sphingolipids and their metabolism in physiology and disease. Nat Rev Mol Cell Biol. 2018;19:175–191. doi: 10.1038/nrm.2017.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hirabayashi Y, Furuya S. Roles of l-serine and sphingolipid synthesis in brain development and neuronal survival. Prog Lipid Res. 2008;47:188–203. doi: 10.1016/j.plipres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 69.Bieberich E. Ceramide in stem cell differentiation and embryo development: novel functions of a topological cell-signaling lipid and the concept of ceramide compartments. J Lipids. 2011;2011:610306. doi: 10.1155/2011/610306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Trayssac M, Hannun YA, Obeid LM. Role of sphingolipids in senescence: implication in aging and age-related diseases. J Clin Invest. 2018;128:2702–2712. doi: 10.1172/JCI97949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Li S, Kim HE. Implications of sphingolipids on aging and age-related diseases. Front Aging. 2022 doi: 10.3389/fragi.2021.797320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Majumder S, Kono M, Lee YT, Byrnes C, Li C, Tuymetova G, Proia RL. A genome-wide CRISPR/Cas9 screen reveals that the aryl hydrocarbon receptor stimulates sphingolipid levels. J Biol Chem. 2020;295:4341–4349. doi: 10.1074/jbc.AC119.011170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wang HC, Wong TH, Wang LT, Su HH, Yu HY, Wu AH, Lin YC, Chen HL, Suen JL, Hsu SH, Chen LC, Zhou Y, Huang SK. Aryl hydrocarbon receptor signaling promotes ORMDL3-dependent generation of sphingosine-1-phosphate by inhibiting sphingosine-1-phosphate lyase. Cell Mol Immunol. 2019;16:783–790. doi: 10.1038/s41423-018-0022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Aoki M, Aoki H, Ramanathan R, Hait NC, Takabe K. Sphingosine-1-phosphate signaling in immune cells and inflammation: roles and therapeutic potential. Mediat Inflamm. 2016;2016:8606878. doi: 10.1155/2016/8606878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Czubowicz K, Jesko H, Wencel P, Lukiw WJ, Strosznajder RP. The role of ceramide and sphingosine-1-phosphate in Alzheimer's disease and other neurodegenerative disorders. Mol Neurobiol. 2019;56:5436–5455. doi: 10.1007/s12035-018-1448-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Huang X, Liu J, Dickson RC. Down-regulating sphingolipid synthesis increases yeast lifespan. PLoS Genet. 2012;8:e1002493. doi: 10.1371/journal.pgen.1002493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Liu JR, Miao H, Deng DQ, Vaziri ND, Li P, Zhao YY. Gut microbiota-derived tryptophan metabolism mediates renal fibrosis by aryl hydrocarbon receptor signaling activation. Cell Mol Life Sci. 2021;78:909–922. doi: 10.1007/s00018-020-03645-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Canto C, Menzies KJ, Auwerx J. NAD+ metabolism and the control of energy homeostasis: a balancing act between mitochondria and the nucleus. Cell Metab. 2015;22:31–53. doi: 10.1016/j.cmet.2015.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ma Q. Induction and superinduction of 2,3,7,8-tetrachlorodibenzo-rho-dioxin-inducible poly(ADP-ribose) polymerase: role of the aryl hydrocarbon receptor/aryl hydrocarbon receptor nuclear translocator transcription activation domains and a labile transcription repressor. Arch Biochem Biophys. 2002;404:309–316. doi: 10.1016/s0003-9861(02)00339-9. [DOI] [PubMed] [Google Scholar]
- 80.MacPherson L, Tamblyn L, Rajendra S, Bralha F, McPherson JP, Matthews J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin poly(ADP-ribose) polymerase (TiPARP, ARTD14) is a mono-ADP-ribosyltransferase and repressor of aryl hydrocarbon receptor transactivation. Nucleic Acids Res. 2013;41:1604–1621. doi: 10.1093/nar/gks1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Grimaldi G, Vagaska B, Ievglevskyi O, Kondratskaya E, Glover JC, Matthews J. Loss of tiparp results in aberrant layering of the cerebral cortex. eNeuro. 2019 doi: 10.1523/eneuro.0239-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Diani-Moore S, Shoots J, Singh R, Zuk JB, Rifkind AB. NAD+ loss, a new player in AhR biology: prevention of thymus atrophy and hepatosteatosis by NAD+ repletion. Sci Rep. 2017;7:2268. doi: 10.1038/s41598-017-02332-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Braidy N, Guillemin GJ, Mansour H, Chan-Ling T, Poljak A, Grant R. Age related changes in NAD+ metabolism oxidative stress and Sirt1 activity in Wistar rats. PLoS ONE. 2011;6:e19194. doi: 10.1371/journal.pone.0019194. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 84.Chini CCS, Tarrago MG, Chini EN. NAD and the aging process: role in life, death and everything in between. Mol Cell Endocrinol. 2017;455:62–74. doi: 10.1016/j.mce.2016.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lopez-Otin C, Blasco MA, Partridge L, Serrano M, Kroemer G. The hallmarks of aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Puga A, Xia Y, Elferink C. Role of the aryl hydrocarbon receptor in cell cycle regulation. Chem Biol Interact. 2002;141:117–130. doi: 10.1016/s0009-2797(02)00069-8. [DOI] [PubMed] [Google Scholar]
- 87.Koizumi M, Tatebe J, Watanabe I, Yamazaki J, Ikeda T, Morita T. Aryl hydrocarbon receptor mediates indoxyl sulfate-induced cellular senescence in human umbilical vein endothelial cells. J Atheroscler Thromb. 2014;21:904–916. doi: 10.5551/jat.23663. [DOI] [PubMed] [Google Scholar]
- 88.Wan C, Liu J, Nie X, Zhao J, Zhou S, Duan Z, Tang C, Liang L, Xu G. 2, 3, 7, 8-Tetrachlorodibenzo-P-dioxin (TCDD) induces premature senescence in human and rodent neuronal cells via ROS-dependent mechanisms. PLoS ONE. 2014;9:e89811. doi: 10.1371/journal.pone.0089811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kondrikov D, Elmansi A, Bragg RT, Mobley T, Barrett T, Eisa N, Kondrikova G, Schoeinlein P, Aguilar-Perez A, Shi XM, Fulzele S, Lawrence MM, Hamrick M, Isales C, Hill W. Kynurenine inhibits autophagy and promotes senescence in aged bone marrow mesenchymal stem cells through the aryl hydrocarbon receptor pathway. Exp Gerontol. 2020;130:110805. doi: 10.1016/j.exger.2019.110805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gasiewicz TA, Singh KP, Bennett JA. The Ah receptor in stem cell cycling, regulation, and quiescence. Ann N Y Acad Sci. 2014;1310:44–50. doi: 10.1111/nyas.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jackson DP, Li H, Mitchell KA, Joshi AD, Elferink CJ. Ah receptor-mediated suppression of liver regeneration through NC-XRE-driven p21Cip1 expression. Mol Pharmacol. 2014;85:533–541. doi: 10.1124/mol.113.089730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kolluri SK, Weiss C, Koff A, Göttlicher M. p27(Kip1) induction and inhibition of proliferation by the intracellular Ah receptor in developing thymus and hepatoma cells. Genes Dev. 1999;13:1742–1753. doi: 10.1101/gad.13.13.1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Puga A, Barnes SJ, Dalton TP, Cy C, Knudsen ES, Maier MA. Aromatic hydrocarbon receptor interaction with the retinoblastoma protein potentiates repression of E2F-dependent transcription and cell cycle arrest. J Biol Chem. 2000;275:2943–2950. doi: 10.1074/jbc.275.4.2943. [DOI] [PubMed] [Google Scholar]
- 94.Marlowe JL, Knudsen ES, Schwemberger S, Puga A. The aryl hydrocarbon receptor displaces p300 from E2F-dependent promoters and represses S phase-specific gene expression. J Biol Chem. 2004;279:29013–29022. doi: 10.1074/jbc.M404315200. [DOI] [PubMed] [Google Scholar]
- 95.Xie Q, Peng S, Tao L, Ruan H, Yang Y, Li TM, Adams U, Meng S, Bi X, Dong MQ, Yuan Z. E2F transcription factor 1 regulates cellular and organismal senescence by inhibiting Forkhead box O transcription factors. J Biol Chem. 2014;289:34205–34213. doi: 10.1074/jbc.M114.587170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ermolaeva M, Neri F, Ori A, Rudolph KL. Cellular and epigenetic drivers of stem cell ageing. Nat Rev Mol Cell Biol. 2018;19:594–610. doi: 10.1038/s41580-018-0020-3. [DOI] [PubMed] [Google Scholar]
- 97.Matikainen TM, Moriyama T, Morita Y, Perez GI, Korsmeyer SJ, Sherr DH, Tilly JL. Ligand activation of the aromatic hydrocarbon receptor transcription factor drives Bax-dependent apoptosis in developing fetal ovarian germ cells. Endocrinology. 2002;143:615–620. doi: 10.1210/endo.143.2.8624. [DOI] [PubMed] [Google Scholar]
- 98.Lutz CT, Browne G, Petzold CR. Methylcholanthrene causes increased thymocyte apoptosis. Toxicology. 1998;128:151–167. doi: 10.1016/s0300-483x(98)00043-2. [DOI] [PubMed] [Google Scholar]
- 99.Svobodova J, Prochazkova J, Kabatkova M, Krkoska M, Smerdova L, Libalova H, Topinka J, Klema J, Kozubik A, Machala M, Vondracek J. 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) disrupts control of cell proliferation and apoptosis in a human model of adult liver progenitors. Toxicol Sci. 2019;172:368–384. doi: 10.1093/toxsci/kfz202. [DOI] [PubMed] [Google Scholar]
- 100.Reiners JJ, Jr, Clift RE. Aryl hydrocarbon receptor regulation of ceramide-induced apoptosis in murine hepatoma 1c1c7 cells. A function independent of aryl hydrocarbon receptor nuclear translocator. J Biol Chem. 1999;274:2502–2510. doi: 10.1074/jbc.274.4.2502. [DOI] [PubMed] [Google Scholar]
- 101.Elizondo G, Fernandez-Salguero P, Sheikh MS, Kim GY, Fornace AJ, Lee KS, Gonzalez FJ. Altered cell cycle control at the G(2)/M phases in aryl hydrocarbon receptor-null embryo fibroblast. Mol Pharmacol. 2000;57:1056–1063. [PubMed] [Google Scholar]
- 102.Schrenk D, Schmitz HJ, Bohnenberger S, Wagner B, Wörner W. Tumor promoters as inhibitors of apoptosis in rat hepatocytes. Toxicol Lett. 2004;149:43–50. doi: 10.1016/j.toxlet.2003.12.019. [DOI] [PubMed] [Google Scholar]
- 103.Bekki K, Vogel H, Li W, Ito T, Sweeney C, Haarmann-Stemmann T, Matsumura F, Vogel CF. The aryl hydrocarbon receptor (AhR) mediates resistance to apoptosis induced in breast cancer cells. Pestic Biochem Physiol. 2015;120:5–13. doi: 10.1016/j.pestbp.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Salminen A, Ojala J, Kaarniranta K. Apoptosis and aging: increased resistance to apoptosis enhances the aging process. Cell Mol Life Sci. 2011;68:1021–1031. doi: 10.1007/s00018-010-0597-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Sanders YY, Liu H, Zhang X, Hecker L, Bernard K, Desai L, Liu G, Thannickal VJ. Histone modifications in senescence-associated resistance to apoptosis by oxidative stress. Redox Biol. 2013;1:8–16. doi: 10.1016/j.redox.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Marlowe JL, Fan Y, Chang X, Peng L, Knudsen ES, Xia Y, Puga A. The aryl hydrocarbon receptor binds to E2F1 and inhibits E2F1-induced apoptosis. Mol Biol Cell. 2008;19:3263–3271. doi: 10.1091/mbc.e08-04-0359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Salminen A, Kaarniranta K. Regulation of the aging process by autophagy. Trends Mol Med. 2009;15:217–224. doi: 10.1016/j.molmed.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 108.Kim HR, Kang SY, Kim HO, Park CW, Chung BY. Role of aryl hydrocarbon receptor activation and autophagy in psoriasis-related inflammation. Int J Mol Sci. 2020;21:2195. doi: 10.3390/ijms21062195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Yang Y, Chan WK. Selective autophagy maintains the aryl hydrocarbon receptor levels in HeLa cells: a mechanism that is dependent on the p23 co-chaperone. Int J Mol Sci. 2020;21:3449. doi: 10.3390/ijms21103449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Polager S, Ofir M, Ginsberg D. E2F1 regulates autophagy and the transcription of autophagy genes. Oncogene. 2008;27:4860–4864. doi: 10.1038/onc.2008.117. [DOI] [PubMed] [Google Scholar]
- 111.Lu P, Takai K, Weaver VM, Werb Z. Extracellular matrix degradation and remodeling in development and disease. Cold Spring Harb Perspect Biol. 2011;3:a005058. doi: 10.1101/cshperspect.a005058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Quan T, Fisher GJ. Role of age-associated alterations of the dermal extracellular matrix microenvironment in human skin aging: a mini-review. Gerontology. 2015;61:427–434. doi: 10.1159/000371708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Freitas-Rodriguez S, Folgueras AR, Lopez-Otin C. The role of matrix metalloproteinases in aging: tissue remodeling and beyond. Biochim Biophys Acta Mol Cell Res. 2017;1864:2015–2025. doi: 10.1016/j.bbamcr.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 114.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10:712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 115.Hillegass JM, Murphy KA, Villano CM, White LA. The impact of aryl hydrocarbon receptor signaling on matrix metabolism: implications for development and disease. Biol Chem. 2006;387:1159–1173. doi: 10.1515/BC.2006.144. [DOI] [PubMed] [Google Scholar]
- 116.Kung T, Murphy KA, White LA. The aryl hydrocarbon receptor (AhR) pathway as a regulatory pathway for cell adhesion and matrix metabolism. Biochem Pharmacol. 2009;77:536–546. doi: 10.1016/j.bcp.2008.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Gomez-Duran A, Carvajal-Gonzalez JM, Mulero-Navarro S, Santiago-Josefat B, Puga A, Fernandez-Salguero PM. Fitting a xenobiotic receptor into cell homeostasis: how the dioxin receptor interacts with TGFβ signaling. Biochem Pharmacol. 2009;77:700–712. doi: 10.1016/j.bcp.2008.08.032. [DOI] [PubMed] [Google Scholar]
- 118.Meng XM, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nat Rev Nephrol. 2016;12:325–338. doi: 10.1038/nrneph.2016.48. [DOI] [PubMed] [Google Scholar]
- 119.Tomkiewicz C, Herry L, Bui LC, Metayer C, Bourdeloux M, Barouki R, Coumoul X. The aryl hydrocarbon receptor regulates focal adhesion sites through a non-genomic FAK/Src pathway. Oncogene. 2013;32:1811–1820. doi: 10.1038/onc.2012.197. [DOI] [PubMed] [Google Scholar]
- 120.Rapisarda V, Borghesan M, Miguela V, Encheva V, Snijders AP, Lujambio A, O'Loghlen A. Integrin β3 regulates cellular senescence by activating the TGF-β pathway. Cell Rep. 2017;18:2480–2493. doi: 10.1016/j.celrep.2017.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Al-Bari AA, Al Mamun A. Current advances in regulation of bone homeostasis. FASEB Bioadv. 2020;2:668–679. doi: 10.1096/fba.2020-00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Watson ATD, Nordberg RC, Loboa EG, Kullman SW. Evidence for aryl hydrocarbon receptor-mediated inhibition of osteoblast differentiation in human mesenchymal stem cells. Toxicol Sci. 2019;167:145–156. doi: 10.1093/toxsci/kfy225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Park R, Madhavaram S, Ji JD. The role of aryl-hydrocarbon receptor (AhR) in osteoclast differentiation and function. Cells. 2020;9:2294. doi: 10.3390/cells9102294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Liu WC, Shyu JF, Lim PS, Fang TC, Lu CL, Zheng CM, Hou YC, Wu CC, Lin YF, Lu KC. Concentration and duration of indoxyl sulfate exposure affects osteoclastogenesis by regulating NFATc1 via aryl hydrocarbon receptor. Int J Mol Sci. 2020;21:3486. doi: 10.3390/ijms21103486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Refaey ME, McGee-Lawrence ME, Fulzele S, Kennedy EJ, Bollag WB, Elsalanty M, Zhong Q, Ding KH, Bendzunas NG, Shi XM, Xu J, Hill WD, Johnson MH, Hunter M, Pierce JL, Yu K, Hamrick MW, Isales CM. Kynurenine, a tryptophan metabolite that accumulates with age, induces bone loss. J Bone Miner Res. 2017;32:2182–2193. doi: 10.1002/jbmr.3224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pertovaara M, Raitala A, Lehtimäki T, Karhunen PJ, Oja SS, Jylhä M, Hervonen A, Hurme M. Indoleamine 2,3-dioxygenase activity in nonagenarians is markedly increased and predicts mortality. Mech Ageing Dev. 2006;127:497–499. doi: 10.1016/j.mad.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 127.Salminen A. Role of indoleamine 2,3-dioxygenase 1 (IDO1) and kynurenine pathway in the regulation of the aging process. Ageing Res Rev. 2022;75:101573. doi: 10.1016/j.arr.2022.101573. [DOI] [PubMed] [Google Scholar]
- 128.Chung PL, Zhou S, Eslami B, Shen L, LeBoff MS, Glowacki J. Effect of age on regulation of human osteoclast differentiation. J Cell Biochem. 2014;115:1412–1419. doi: 10.1002/jcb.24792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Li Y, Wang K, Zou QY, Jiang YZ, Zhou C, Zheng J. ITE suppresses angiogenic responses in human artery and vein endothelial cells: differential roles of AhR. Reprod Toxicol. 2017;74:181–188. doi: 10.1016/j.reprotox.2017.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ichihara S, Yamada Y, Ichihara G, Nakajima T, Li P, Kondo T, Gonzalez FJ, Murohara T. A role for the aryl hydrocarbon receptor in regulation of ischemia-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2007;27:1297–1304. doi: 10.1161/atvbaha.106.138701. [DOI] [PubMed] [Google Scholar]
- 131.Gradin K, McGuire J, Wenger RH, Kvietikova I, Fhitelaw ML, Toftgard R, Tora L, Gassmann M, Poellinger L. Functional interference between hypoxia and dioxin signal transduction pathways: competition for recruitment of the Arnt transcription factor. Mol Cell Biol. 1996;16:5221–5231. doi: 10.1128/MCB.16.10.5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Niwa T, Shimizu H. Indoxyl sulfate induces nephrovascular senescence. J Ren Nutr. 2012;22:102–106. doi: 10.1053/j.jrn.2011.10.032. [DOI] [PubMed] [Google Scholar]
- 133.Bobot M, Thomas L, Moyon A, Fernandez S, McKay N, Balasse L, Garrigue P, Brige P, Chopinet S, Poitevin S, Cerini C, Brunet P, Dignat-George F, Burtey S, Guillet B, Hache G. Uremic toxic Blood-Brain Barrier disruption mediated by AhR activation leads to cognitive impairment during experimental renal dysfunction. J Am Soc Nephrol. 2020;31:1509–1521. doi: 10.1681/ASN.2019070728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Leong SC, Sirich TL. Indoxyl sulfate—review of toxicity and therapeutic strategies. Toxins (Basel) 2016;8:358. doi: 10.3390/toxins8120358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Heiss C, Rodriguez-Mateos A, Kelm M. Central role of eNOS in the maintenance of endothelial homeostasis. Antioxid Redox Signal. 2015;22:1230–1242. doi: 10.1089/ars.2014.6158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Pawelec G, Solana R, Remarque E, Mariani E. Impact of aging on innate immunity. J Leukoc Biol. 1998;64:703–712. doi: 10.1002/jlb.64.6.703. [DOI] [PubMed] [Google Scholar]
- 137.Frasca D, Blomberg BB. Inflammaging decreases adaptive and innate immune responses in mice and humans. Biogerontology. 2016;17:7–19. doi: 10.1007/s10522-015-9578-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Salminen A. Activation of immunosuppressive network in the aging process. Ageing Res Rev. 2020;57:100998. doi: 10.1016/j.arr.2019.100998. [DOI] [PubMed] [Google Scholar]
- 139.Laiosa MD, Wyman A, Murante FG, Fiore NC, Staples JE, Gasiewicz TA, Silverstone AE. Cell proliferation arrest within intrathymic lymphocyte progenitor cells causes thymic atrophy mediated by the aryl hydrocarbon receptor. J Immunol. 2003;171:4582–4591. doi: 10.4049/jimmunol.171.9.4582. [DOI] [PubMed] [Google Scholar]
- 140.Thomas R, Wang W, Su DM. Contributions of age-related thymic involution to immunosenescence and inflammaging. Immun Ageing. 2020;17:2. doi: 10.1186/s12979-020-0173-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee YH, Lin CH, Hsu PC, Sun YY, Huang YJ, Zhuo JH, Wang CY, Gan YL, Hung CC, Kuan CY, Shie FS. Aryl hydrocarbon receptor mediates both proinflammatory and anti-inflammatory effects in lipopolysaccharide-activated microglia. Glia. 2015;63:1138–1154. doi: 10.1002/glia.22805. [DOI] [PubMed] [Google Scholar]
- 142.Bock KW. Human AHR functions in vascular tissue: pro- and anti-inflammatory responses of AHR agonists in atherosclerosis. Biochem Pharmacol. 2019;159:116–120. doi: 10.1016/j.bcp.2018.11.021. [DOI] [PubMed] [Google Scholar]
- 143.Brinchmann BC, Skuland T, Rambol MH, Szoke K, Brinchmann JE, Gutleb AC, Moschini E, Kubatova A, Kukowski K, Le Ferrec E, Lagadic-Gossmann D, Schwarze PE, Lag M, Refsnes M, Övrevik J, Holme JA. Lipophilic components of diesel exhaust particles induce pro-inflammatory responses in human endothelial cells through AhR dependent pathway(s) Part Fibre Toxicol. 2018;15:21. doi: 10.1186/s12989-018-0257-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Vogel CF, Van Winkle LS, Esser C, Haarmann-Stemmann T. The aryl hydrocarbon receptor as a target of environmental stressors - Implications for pollution mediated stress and inflammatory responses. Redox Biol. 2020;34:101530. doi: 10.1016/j.redox.2020.101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Forrester SJ, Kikuchi DS, Hernandes MS, Xu Q, Griendling KK. Reactive oxygen species in metabolic and inflammatory signaling. Circ Res. 2018;122:877–902. doi: 10.1161/CIRCRESAHA.117.311401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wang H, Wei Y, Yu D. Control of lymphocyte homeostasis and effector function by the aryl hydrocarbon receptor. Int Immunopharmacol. 2015;28:818–824. doi: 10.1016/j.intimp.2015.03.046. [DOI] [PubMed] [Google Scholar]
- 147.Phelan D, Winter GM, Rogers WJ, Lam JC, Denison MS. Activation of the Ah receptor signal transduction pathway by bilirubin and biliverdin. Arch Biochem Biophys. 1998;357:155–163. doi: 10.1006/abbi.1998.0814. [DOI] [PubMed] [Google Scholar]
- 148.Li Y, Huang B, Ye T, Wang Y, Xia D, Qian J. Physiological concentrations of bilirubin control inflammatory response by inhibiting NF-κB and inflammasome activation. Int Immunopharmacol. 2020;84:106520. doi: 10.1016/j.intimp.2020.106520. [DOI] [PubMed] [Google Scholar]
- 149.Huai W, Zhao R, Song H, Zhao J, Zhang L, Zhang L, Gao C, Han L, Zhao W. Aryl hydrocarbon receptor negatively regulates NLRP3 inflammasome activity by inhibiting NLRP3 transcription. Nat Commun. 2014;5:4738. doi: 10.1038/ncomms5738. [DOI] [PubMed] [Google Scholar]
- 150.Wada T, Sunaga H, Miyata K, Shirasaki H, Uchiyama Y, Shimba S. Aryl hydrocarbon receptor plays protective roles against high fat diet (HFD)-induced hepatic steatosis and the subsequent lipotoxicity via direct transcriptional regulation of Socs3 gene expression. J Biol Chem. 2016;291:7004–7016. doi: 10.1074/jbc.M115.693655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Neamah WH, Singh NP, Alghetaa H, Abdulla OA, Chatterjee S, Busbee PB, Nagarkatti M, Nagarkatti P. AhR activation leads to massive mobilization of myeloid-derived suppressor cells with immunosuppressive activity through regulation of CXCR2 and microRNA miR-150-5p and miR-543-3p that target anti-inflammatory genes. J Immunol. 2019;203:1830–1844. doi: 10.4049/jimmunol.1900291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Barroso A, Mahler JV, Fonseca-Castro PH, Quintana FJ. Therapeutic induction of tolerogenic dendritic cells via aryl hydrocarbon receptor signaling. Curr Opin Immunol. 2021;70:33–39. doi: 10.1016/j.coi.2021.02.003. [DOI] [PubMed] [Google Scholar]
- 153.Yang X, Liu H, Ye T, Duan C, Lv P, Wu X, Liu J, Jiang K, Lu H, Yang H, Xia D, Peng E, Chen Z, Tang K, Ye Z. AhR activation attenuates calcium oxalate nephrocalcinosis by diminishing M1 macrophage polarization and promoting M2 macrophage polarization. Theranostics. 2020;10:12011–12025. doi: 10.7150/thno.51144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Zhu J, Luo L, Tian L, Yin S, Ma X, Cheng S, Tang W, Yu J, Ma W, Zhou X, Fan X, Yang X, Yan J, Xu X, Lv C, Liang H. Aryl hydrocarbon receptor promotes IL-10 expression in inflammatory macrophages through Src-STAT3 signaling pathway. Front Immunol. 2018;9:2033. doi: 10.3389/fimmu.2018.02033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mira JC, Brakenridge SC, Moldawer LL, Moore FA. Persistent inflammation, immunosuppression and catabolism syndrome. Crit Care Clin. 2017;33:245–258. doi: 10.1016/j.ccc.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Vogel CF, Goth SR, Dong B, Pessah IN, Matsumura F. Aryl hydrocarbon receptor signaling mediates expression of indoleamine 2,3-dioxygenase. Biochem Biophys Res Commun. 2008;375(3):331–335. doi: 10.1016/j.bbrc.2008.07.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128(1):92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
- 158.Salminen A. Immunosuppressive network promotes immunosenescence associated with aging and chronic inflammatory conditions. J Mol Med (Berl) 2021;99:1553–1569. doi: 10.1007/s00109-021-02123-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Barbe-Tuana F, Funchal G, Schmitz CRR, Maurmann RM, Bauer ME. The interplay between immunosenescence and age-related diseases. Semin Immunopathol. 2020;42:545–557. doi: 10.1007/s00281-020-00806-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Salminen A. Increased immunosuppression impairs tissue homeostasis with aging and age-related diseases. J Mol Med (Berl) 2021;99:1–20. doi: 10.1007/s00109-020-01988-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tominaga K, Suzuki HI. TGF-β signaling in cellular senescence and aging-related pathology. Int J Mol Sci. 2019;20:5002. doi: 10.3390/ijms20205002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Neavin DR, Liu D, Ray B, Weinshilboum RM. The role of the aryl hydrocarbon receptor (AHR) in immune and inflammatory diseases. Int J Mol Sci. 2018;19:3851. doi: 10.3390/ijms19123851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cannon AS, Nagarkatti PS, Nagarkatti M. Targeting AhR as a novel therapeutic modality against inflammatory diseases. Int J Mol Sci. 2021;23:288. doi: 10.3390/ijms23010288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yi T, Wang J, Zhu K, Tang Y, Huang S, Shui X, Ding Y, Chen C, Lei W. Aryl hydrocarbon receptor: a new player of pathogenesis and therapy in cardiovascular diseases. Biomed Res Int. 2018;2018:6058784. doi: 10.1155/2018/6058784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Vogel CF, Sciullo E, Matsumura F. Activation of inflammatory mediators and potential role of ah-receptor ligands in foam cell formation. Cardiovasc Toxicol. 2004;4:363–373. doi: 10.1385/ct:4:4:363. [DOI] [PubMed] [Google Scholar]
- 166.Kim JB, Zhao Q, Nguyen T, Pjanic M, Cheng P, Wirka R, Travisano S, Nagao M, Kundu R, Quertermous T. Environment-sensing aryl hydrocarbon receptor inhibits the chondrogenic fate of modulated smooth muscle cells in atherosclerotic lesions. Circulation. 2020;142:575–590. doi: 10.1161/circulationaha.120.045981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Seong E, Lee JH, Lim S, Park EH, Kim E, Kim CW, Lee E, Oh GC, Choo EH, Hwang BH, Kim CJ, Ihm SH, Youn HJ, Chung WS, Chang K. Activation of aryl hydrocarbon receptor by ITE improves cardiac function in mice after myocardial infarction. J Am Heart Assoc. 2021;10:e020502. doi: 10.1161/JAHA.120.020502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cuartero MI, Ballesteros I, de la Parra J, Harkin AL, Abautret-Daly A, Sherwin E, Fernandez-Salguero P, Corbi AL, Lizasoain I, Moro MA. L-kynurenine/aryl hydrocarbon receptor pathway mediates brain damage after experimental stroke. Circulation. 2014;130:2040–2051. doi: 10.1161/circulationaha.114.011394. [DOI] [PubMed] [Google Scholar]
- 169.Chen WC, Chang LH, Huang SS, Huang YJ, Chih CL, Kuo HC, Lee YH, Lee IH. Aryl hydrocarbon receptor modulates stroke-induced astrogliosis and neurogenesis in the adult mouse brain. J Neuroinflamm. 2019;16:187. doi: 10.1186/s12974-019-1572-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ramos-Garcia NA, Orozco-Ibarra M, Estudillo E, Elizondo G, Gomez Apo E, Chavez Macias LG, Sosa-Ortiz AL, Torres-Ramos MA. Aryl hydrocarbon receptor in post-mortem hippocampus and in serum from young, elder, and Alzheimer's patients. Int J Mol Sci. 2020;21:1983. doi: 10.3390/ijms21061983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Duan Z, Zhang S, Liang H, Xing Z, Guo L, Shi L, Du L, Kuang C, Takikawa O, Yang Q. Amyloid β neurotoxicity is IDO1-Kyn-AhR dependent and blocked by IDO1 inhibitor. Signal Transduct Target Ther. 2020;5:96. doi: 10.1038/s41392-020-0188-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Moradi H, Sica DA, Kalantar-Zadeh K. Cardiovascular burden associated with uremic toxins in patients with chronic kidney disease. Am J Nephrol. 2013;38:136–148. doi: 10.1159/000351758. [DOI] [PubMed] [Google Scholar]
- 173.Schroeder JC, Dinatale BC, Murray IA, Flaveny CA, Liu Q, Laurenzana EM, Lin JM, Strom SC, Omiecinski CJ, Amin S, Perdew GH. The uremic toxin 3-indoxyl sulfate is a potent endogenous agonist for the human aryl hydrocarbon receptor. Biochemistry. 2010;49:393–400. doi: 10.1021/bi901786x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Lu CL, Zheng CM, Lu KC, Liao MT, Wu KL, Ma MC. Indoxyl-sulfate-induced redox imbalance in chronic kidney disease. Antioxidants (Basel) 2021;10:936. doi: 10.3390/antiox10060936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.White WE, Yaqoob MM, Harwood SM. Aging and uremia: is there cellular and molecular crossover? World J Nephrol. 2015;4:19–30. doi: 10.5527/wjn.v4.i1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kyriakidis NC, Cobo G, Dai L, Lindholm B, Stenvinkel P. Role of uremic toxins in early vascular ageing and calcification. Toxins (Basel) 2021;13:26. doi: 10.3390/toxins13010026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Shi Y, Liu Z, Shen Y, Zhu H. A novel perspective linkage between kidney function and Alzheimer's disease. Front Cell Neurosci. 2018;12:384. doi: 10.3389/fncel.2018.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Shinde R, McGaha TL. The aryl hydrocarbon receptor: connecting immunity to the microenvironment. Trends Immunol. 2018;39:1005–1020. doi: 10.1016/j.it.2018.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Gargaro M, Scalisi G, Manni G, Mondanelli G, Grohmann U, Fallarino F. The landscape of AhR regulators and coregulators to fine-tune AhR functions. Int J Mol Sci. 2021;22:757. doi: 10.3390/ijms22020757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Kudo I, Hosaka M, Haga A, Tsuji N, Nagata Y, Okada H, Fukuda K, Kakizaki Y, Okamoto T, Grave E, Itoh H. The regulation mechanisms of AhR by molecular chaperone complex. J Biochem. 2018;163:223–232. doi: 10.1093/jb/mvx074. [DOI] [PubMed] [Google Scholar]
- 181.Swanson HI. DNA binding and protein interactions of the AHR/ARNT heterodimer that facilitate gene activation. Chem Biol Interact. 2002;141:63–76. doi: 10.1016/s0009-2797(02)00066-2. [DOI] [PubMed] [Google Scholar]
- 182.Wright EJ, De Castro KP, Joshi AD, Elferink CJ. Canonical and non-canonical aryl hydrocarbon receptor signaling pathways. Curr Opin Toxicol. 2017;2:87–92. doi: 10.1016/j.cotox.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vogel CF, Haarmann-Stemmann T. The aryl hydrocarbon receptor repressor—more than a simple feedback inhibitor of AhR signaling: clues for its role in inflammation and cancer. Curr Opin Toxicol. 2017;2:109–119. doi: 10.1016/j.cotox.2017.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Marinelli L, Martin-Gallausiaux C, Bourhis JM, Beguet-Crespel F, Blottiere HM, Lapaque N. Identification of the novel role of butyrate as AhR ligand in human intestinal epithelial cells. Sci Rep. 2019;9:643. doi: 10.1038/s41598-018-37019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim M, Benayoun BA. The microbiome: an emerging key player in aging and longevity. Transl Med Aging. 2020;4:103–116. doi: 10.1016/j.tma.2020.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Ragonnaud E, Biragyn A. Gut microbiota as the key controllers of healthy aging of elderly people. Immun Ageing. 2021;18:2. doi: 10.1186/s12979-020-00213-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chan WK, Yao G, Gu YZ, Bradfield CA. Cross-talk between the aryl hydrocarbon receptor and hypoxia inducible factor signaling pathways. Demonstration of competition and compensation. J Biol Chem. 1999;274:12115–12123. doi: 10.1074/jbc.274.17.12115. [DOI] [PubMed] [Google Scholar]
- 188.Vorrink SU, Domann FE. Regulatory crosstalk and interference between the xenobiotic and hypoxia sensing pathways at the AhR-ARNT-HIF1α signaling node. Chem Biol Interact. 2014;218:82–88. doi: 10.1016/j.cbi.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Asai H, Hirata J, Hirano A, Hirai K, Seki S, Watanabe-Akanuma M. Activation of aryl hydrocarbon receptor mediates suppression of hypoxia-inducible factor-dependent erythropoietin expression by indoxyl sulfate. Am J Physiol Cell Physiol. 2016;310:C142–C150. doi: 10.1152/ajpcell.00172.2015. [DOI] [PubMed] [Google Scholar]
- 190.Tomita S, Sinal CJ, Yim SH, Gonzalez FJ. Conditional disruption of the aryl hydrocarbon receptor nuclear translocator (Arnt) gene leads to loss of target gene induction by the aryl hydrocarbon receptor and hypoxia-inducible factor 1α. Mol Endocrinol. 2000;14:1674–1681. doi: 10.1210/mend.14.10.0533. [DOI] [PubMed] [Google Scholar]
- 191.Buffenstein R. The naked mole-rat: a new long-living model for human aging research. J Gerontol A Biol Sci Med Sci. 2005;60:1369–1377. doi: 10.1093/gerona/60.11.1369. [DOI] [PubMed] [Google Scholar]
- 192.Leiser SF, Fletcher M, Begun A, Kaeberlein M. Life-span extension from hypoxia in Caenorhabditis elegans requires both HIF-1 and DAF-16 and is antagonized by SKN-1. J Gerontol A Biol Sci Med Sci. 2013;68:1135–1144. doi: 10.1093/gerona/glt016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Baeuerle PA, Henkel T. Function and activation of NF-κB in the immune system. Annu Rev Immunol. 1994;12:141–179. doi: 10.1146/annurev.iy.12.040194.001041. [DOI] [PubMed] [Google Scholar]
- 194.Salminen A, Huuskonen J, Ojala J, Kauppinen A, Kaarniranta K, Suuronen T. Activation of innate immunity system during aging: NF-κB signaling is the molecular culprit of inflammaging. Ageing Res Rev. 2008;7:83–105. doi: 10.1016/j.arr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- 195.Vogel CF, Sciullo E, Li W, Wong P, Lazennec G, Matsumura F. RelB, a new partner of aryl hydrocarbon receptor-mediated transcription. Mol Endocrinol. 2007;21:2941–2955. doi: 10.1210/me.2007-0211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Ishihara Y, Kado SY, Hoeper C, Harel S, Vogel CFA. Role of NF-κB RelB in aryl hydrocarbon receptor-mediated ligand specific effects. Int J Mol Sci. 2019;20:2652. doi: 10.3390/ijms20112652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Vogel CF, Wu D, Goth SR, Baek J, Lollies A, Domhardt R, Grindel A, Pessah IN. Aryl hydrocarbon receptor signaling regulates NF-κB RelB activation during dendritic-cell differentiation. Immunol Cell Biol. 2013;91:568–575. doi: 10.1038/icb.2013.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Chen X, El Gazzar M, Yoza BK, McCall CE. The NF-κB factor RelB and histone H3 lysine methyltransferase G9a directly interact to generate epigenetic silencing in endotoxin tolerance. J Biol Chem. 2009;284:27857–27865. doi: 10.1074/jbc.M109.000950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Lim CY, Knowles BB, Solter D, Messerschmidt DM. Epigenetic control of early mouse development. Curr Top Dev Biol. 2016;120:311–360. doi: 10.1016/bs.ctdb.2016.05.002. [DOI] [PubMed] [Google Scholar]
- 200.Horvath S, Raj K. DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat Rev Genet. 2018;19:371–384. doi: 10.1038/s41576-018-0004-3. [DOI] [PubMed] [Google Scholar]
- 201.Mulero-Navarro S, Carvajal-Gonzalez JM, Herranz M, Ballestar E, Fraga MF, Ropero S, Esteller M, Fernandez-Salguero PM. The dioxin receptor is silenced by promoter hypermethylation in human acute lymphoblastic leukemia through inhibition of Sp1 binding. Carcinogenesis. 2006;27:1099–1104. doi: 10.1093/carcin/bgi344. [DOI] [PubMed] [Google Scholar]
- 202.Garrison PM, Rogers JM, Brackney WR, Denison MS. Effects of histone deacetylase inhibitors on the Ah receptor gene promoter. Arch Biochem Biophys. 2000;374:161–171. doi: 10.1006/abbi.1999.1620. [DOI] [PubMed] [Google Scholar]
- 203.Singh NP, Singh UP, Singh B, Price RL, Nagarkatti M, Nagarkatti PS. Activation of aryl hydrocarbon receptor (AhR) leads to reciprocal epigenetic regulation of FoxP3 and IL-17 expression and amelioration of experimental colitis. PLoS ONE. 2011;6:e23522. doi: 10.1371/journal.pone.0023522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Wang LT, Chiou SS, Chai CY, Hsi E, Wang SN, Huang SK, Hsu SH. Aryl hydrocarbon receptor regulates histone deacetylase 8 expression to repress tumor suppressive activity in hepatocellular carcinoma. Oncotarget. 2017;8:7489–7501. doi: 10.18632/oncotarget.9841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Wajda A, Lapczuk-Romanska J, Paradowska-Gorycka A. Epigenetic regulations of AhR in the aspect of immunomodulation. Int J Mol Sci. 2020;21:6404. doi: 10.3390/ijms21176404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Philibert RA, Beach SR, Brody GH. Demethylation of the aryl hydrocarbon receptor repressor as a biomarker for nascent smokers. Epigenetics. 2012;7:1331–1338. doi: 10.4161/epi.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Bojesen SE, Timpson N, Relton C, Davey Smith G, Nordestgaard BG. AHRR (cg05575921) hypomethylation marks smoking behaviour, morbidity and mortality. Thorax. 2017;72:646–653. doi: 10.1136/thoraxjnl-2016-208789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Dawes K, Andersen A, Reimer R, Mills JA, Hoffman E, Long JD, Miller S, Philibert R. The relationship of smoking to cg05575921 methylation in blood and saliva DNA samples from several studies. Sci Rep. 2021;11:21627. doi: 10.1038/s41598-021-01088-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Yang Y, Gao X, Just AC, Colicino E, Wang C, Coull BA, Hou L, Zheng Y, Vokonas P, Schwartz J, Baccarelli AA. Smoking-related DNA methylation is associated with DNA methylation phenotypic age acceleration: the veterans affairs normative aging study. Int J Environ Res Public Health. 2019;16:2356. doi: 10.3390/ijerph16132356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Cardenas A, Ecker S, Fadadu RP, Huen K, Orozco A, McEwen LM, Engelbrecht HR, Gladish N, Kobor MS, Rosero-Bixby L, Dow WH, Rehkopf DH. Epigenome-wide association study and epigenetic age acceleration associated with cigarette smoking among Costa Rican adults. Sci Rep. 2022;12:4277. doi: 10.1038/s41598-022-08160-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Gorbunova V, Seluanov A, Mita P, McKerrow W, Fenyö D, Boeke JD, Linker SB, Gage FH, Kreiling JA, Petrashen AP, Woodham TA, Taylor JR, Helfand SL, Sedivy JM. The role of retrotransposable elements in ageing and age-associated diseases. Nature. 2021;596:43–53. doi: 10.1038/s41586-021-03542-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Wood JG, Jones BC, Jiang N, Chang C, Hosier S, Wickremesinghe P, Garcia M, Hartnett DA, Burhenn L, Neretti N, Helfand SL. Chromatin-modifying genetic interventions suppress age-associated transposable element activation and extend life span in Drosophila. Proc Natl Acad Sci U S A. 2016;113:11277–11282. doi: 10.1073/pnas.1604621113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Teneng I, Stribinskis V, Ramos KS. Context-specific regulation of LINE-1. Genes Cells. 2007;12:1101–1110. doi: 10.1111/j.1365-2443.2007.01117.x. [DOI] [PubMed] [Google Scholar]
- 214.Zhang X, Zhang R, Yu J. New understanding of the relevant role of LINE-1 retrotransposition in human disease and immune modulation. Front Cell Dev Biol. 2020;8:657. doi: 10.3389/fcell.2020.00657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.St-Laurent G, 3rd, Hammell N, McCaffrey TA. A LINE-1 component to human aging: do LINE elements exact a longevity cost for evolutionary advantage? Mech Ageing Dev. 2010;131:299–305. doi: 10.1016/j.mad.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Anisimov VN, Zabezhinski MA, Popovich IG, Piskunova TS, Semenchenko AV, Tyndyk ML, Yurova MN, Rosenfeld SV, Blagosklonny MV. Rapamycin increases lifespan and inhibits spontaneous tumorigenesis in inbred female mice. Cell Cycle. 2011;10:4230–4236. doi: 10.4161/cc.10.24.18486. [DOI] [PubMed] [Google Scholar]
- 217.Martin-Montalvo A, Mercken EM, Mitchell SJ, Palacios HH, Mote PL, Scheibye-Knudsen M, Gomes AP, Ward TM, Minor RK, Blouin MJ, Schwab M, Pollak M, Zhang Y, Yu Y, Becker KG, Bohr VA, Ingram DK, Sinclair DA, Wolf NS, Spindler SR, Bernier M, de Cabo R. Metformin improves healthspan and lifespan in mice. Nat Commun. 2013;4:2192. doi: 10.1038/ncomms3192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Do MT, Kim HG, Tran TT, Khanal T, Choi JH, Chung YC, Jeong TC, Jeong HG. Metformin suppresses CYP1A1 and CYP1B1 expression in breast cancer cells by down-regulating aryl hydrocarbon receptor expression. Toxicol Appl Pharmacol. 2014;280:138–148. doi: 10.1016/j.taap.2014.07.021. [DOI] [PubMed] [Google Scholar]
- 219.Kim HR, Kim JC, Kang SY, Kim HO, Park CW, Chung BY. Rapamycin alleviates 2,3,7,8-tetrachlorodibenzo-p-dioxin-induced aggravated dermatitis in mice with imiquimod-induced psoriasis-like dermatitis by inducing autophagy. Int J Mol Sci. 2021;22:3968. doi: 10.3390/ijms22083968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Nguyen PM, Wang D, Wang Y, Li Y, Uchizono JA, Chan WK. p23 co-chaperone protects the aryl hydrocarbon receptor from degradation in mouse and human cell lines. Biochem Pharmacol. 2012;84:838–850. doi: 10.1016/j.bcp.2012.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Chung KW, Chung HY. The effects of calorie restriction on autophagy: role on aging intervention. Nutrients. 2019;11:2923. doi: 10.3390/nu11122923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Xue Z, Li D, Yu W, Zhang Q, Hou X, He Y, Kou X. Mechanisms and therapeutic prospects of polyphenols as modulators of the aryl hydrocarbon receptor. Food Funct. 2017;8:1414–1437. doi: 10.1039/c6fo01810f. [DOI] [PubMed] [Google Scholar]
- 223.Goya-Jorge E, Jorge Rodriguez ME, Veitia MS, Giner RM. Plant occurring flavonoids as modulators of the aryl hydrocarbon receptor. Molecules. 2021;26:2315. doi: 10.3390/molecules26082315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zhou DD, Luo M, Huang SY, Saimaiti A, Shang A, Gan RY, Li HB. Effects and mechanisms of resveratrol on aging and age-related diseases. Oxid Med Cell Longev. 2021;2021:9932218. doi: 10.1155/2021/9932218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bravo-Ferrer I, Cuartero MI, Medina V, Ahedo-Quero D, Peña-Martínez C, Perez-Ruiz A, Fernandez-Valle ME, Hernandez-Sanchez C, Fernandez-Salguero PM, Lizasoain I, Moro MA. Lack of the aryl hydrocarbon receptor accelerates aging in mice. FASEB J. 2019;33:12644–12654. doi: 10.1096/fj.201901333R. [DOI] [PubMed] [Google Scholar]
- 226.Nacarino-Palma A, Rico-Leo EM, Campisi J, Ramanathan A, Gonzalez-Rico FJ, Rejano-Gordillo CM, Ordiales-Talavero A, Merino JM, Fernandez-Salguero PM. Aryl hydrocarbon receptor blocks aging-induced senescence in the liver and fibroblast cells. Aging (Albany NY) 2022;14:4281–4304. doi: 10.18632/aging.204103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sagiv A, Biran A, Yon M, Simon J, Lowe SW, Krizhanovsky V. Granule exocytosis mediates immune surveillance of senescent cells. Oncogene. 2013;32:1971–1977. doi: 10.1038/onc.2012.206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Pereira BI, Devine OP, Vukmanovic-Stejic M, Chambers ES, Subramanian P, Patel N, Virasami A, Sebire NJ, Kinsler V, Valdovinos A, LeSaux CJ, Passos JF, Antoniou A, Rustin MHA, Campisi J, Akbar AN. Senescent cells evade immune clearance via HLA-E-mediated NK and CD8+ T cell inhibition. Nat Commun. 2019;10:2387. doi: 10.1038/s41467-019-10335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Meza Guzman LG, Keating N, Nicholson SE. Natural killer cells: tumor surveillance and signaling. Cancers (Basel) 2020;12:952. doi: 10.3390/cancers12040952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Cullen SP, Brunet M, Martin SJ. Granzymes in cancer and immunity. Cell Death Differ. 2010;17:616–623. doi: 10.1038/cdd.2009.206. [DOI] [PubMed] [Google Scholar]
- 231.Ovadya Y, Landsberger T, Leins H, Vadai E, Gal H, Biran A, Yosef R, Sagiv A, Agrawal A, Shapira A, Windheim J, Tsoory M, Schirmbeck R, Amit I, Geiger H, Krizhanovsky V. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat Commun. 2018;9:5435. doi: 10.1038/s41467-018-07825-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Zhang LH, Shin JH, Haggadone MD, Sunwoo JB. The aryl hydrocarbon receptor is required for the maintenance of liver-resident natural killer cells. J Exp Med. 2016;213:2249–2257. doi: 10.1084/jem.20151998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Shin JH, Zhang L, Murillo-Sauca O, Kim J, Kohrt HE, Bui JD, Sunwoo JB. Modulation of natural killer cell antitumor activity by the aryl hydrocarbon receptor. Proc Natl Acad Sci U S A. 2013;110:12391–12396. doi: 10.1073/pnas.1302856110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Smith SH, Jayawickreme C, Rickard DJ, Nicodeme E, Bui T, Simmons C, Coquery CM, Neil J, Pryor WM, Mayhew D, Rajpal DK, Creech K, Furst S, Lee J, Wu D, Rastinejad F, Willson TM, Viviani F, Morris DC, Moore JT, Cote-Sierra J. Tapinarof is a natural AhR agonist that resolves skin inflammation in mice and humans. J Invest Dermatol. 2017;137:2110–2119. doi: 10.1016/j.jid.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 235.Nguyen NT, Nakahama T, Nguyen CH, Tran TT, Le VS, Chu HH, Kishimoto T. Aryl hydrocarbon receptor antagonism and its role in rheumatoid arthritis. J Exp Pharmacol. 2015;7:29–35. doi: 10.2147/JEP.S63549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Leclerc D, Staats Pires AC, Guillemin GJ, Gilot D. Detrimental activation of AhR pathway in cancer: an overview of therapeutic strategies. Curr Opin Immunol. 2021;70:15–26. doi: 10.1016/j.coi.2020.12.003. [DOI] [PubMed] [Google Scholar]
- 237.Xu X, Zhang X, Yuan Y, Zhao Y, Fares HM, Yang M, Wen Q, Taha R, Sun L. Species-specific differences in aryl hydrocarbon receptor responses: how and why? Int J Mol Sci. 2021;22:13293. doi: 10.3390/ijms222413293. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.