Abstract
Background
Otitis media is inflammation of the middle ear and is usually caused by infection. It affects people of all ages but is particularly common in young children. Around 164 million people worldwide have long‐term hearing loss caused by this condition, 90% of them in low‐income countries. As zinc supplements prevent pneumonia in disadvantaged children, we wanted to investigate whether zinc supplements could also prevent otitis media.
Objectives
To evaluate whether zinc supplements prevent otitis media in adults and children of different ages.
Search methods
We searched CENTRAL (2014, Issue 1), MEDLINE (1950 to February week 4, 2014) and EMBASE (1974 to March 2014).
Selection criteria
Randomised, placebo‐controlled trials of zinc supplements given at least once a week for at least a month for preventing otitis media.
Data collection and analysis
Two review authors independently assessed the eligibility and methodological quality of the included trials and extracted and analysed data. We summarised results using risk ratios (RRs) or rate ratios for dichotomous data and mean differences (MDs) for continuous data. We combined trial results where appropriate.
Main results
No new trials were identified for inclusion in this update. We identified 12 trials for inclusion, 10 of which contributed outcomes data. There were a total of 6820 participants. In trials of healthy children living in low‐income communities, two trials did not demonstrate a significant difference between the zinc‐supplemented and placebo groups in the numbers of participants experiencing an episode of definite otitis media during follow‐up (3191 participants); another trial showed a significantly lower incidence rate of otitis media in the zinc group (rate ratio 0.69, 95% confidence interval (CI) 0.61 to 0.79, n = 1621). A small trial of 39 infants undergoing treatment for severe malnutrition suggested a benefit of zinc for the mean number of episodes of otitis media (mean difference (MD) ‐1.12 episodes, 95% CI ‐2.21 to ‐0.03). Zinc supplements did not seem to cause any serious adverse events but a small minority of children were reported to have vomited shortly after ingestion of the supplements. The trial evidence included is generally of good quality, with a low risk of bias.
Authors' conclusions
Evidence on whether zinc supplementation can reduce the incidence of otitis media in healthy children under the age of five years living in low‐ and middle‐income countries is mixed. There is some evidence of benefit in children being treated for marasmus (severe malnutrition), but this is based on one small trial and should therefore be treated with caution.
Plain language summary
Zinc supplements for preventing middle ear infections
Background
Middle ear infections are common, especially among young children, usually causing earache and some temporary (occasionally permanent) hearing loss. Zinc is an essential micronutrient, which has a role in the optimal functioning of the immune system and resistance to infection. It must be consumed regularly as it cannot be stored in the body. Some people, especially children in low‐ and middle‐income countries, may not have adequate zinc intake from food alone. Researchers have examined the potential role of zinc supplements in preventing infective illnesses. Therefore we wanted to discover whether zinc supplements have any role in preventing middle ear infections.
Study characteristics
The review authors searched the medical literature for studies up to March 2014. We searched for trials which compared middle ear infections in people randomly selected to receive zinc supplements or who did not receive supplements. We found 10 eligible studies, all conducted amongst young children. The total number of participants was 6820. Nine trials were conducted in low‐ and middle‐income countries. Seven trials were conducted on healthy children. Participants included both males and females.
Results
The results of the trials provided no convincing evidence that zinc supplements reduce the occurrence of middle ear infections in healthy children. However, in one small study of severely malnourished children, those receiving zinc supplements had fewer middle ear infections. The only adverse effect was vomiting.
Quality of evidence
The trial evidence included is generally of good quality, with a low risk of bias. All the included trials included otitis media only as a secondary outcome. Therefore, there was a potential to miss trials which were less publicised or less well indexed within the electronic databases.
Background
This is the second update of the Cochrane review first published in 2010 (Abba 2010). The first update was published in 2012 (Gulani 2012).
Description of the condition
Otitis media (inflammation of the middle ear, usually caused by infection) affects people of all ages throughout the world, particularly young children under the age of three years, in whom it is very common. It causes acute pain for a day or so and temporary conductive deafness for longer. Usually it resolves quickly but it can lead to chronic conditions. The greatest burden is from chronic suppurative otitis media (discharge through a perforation in the ear drum), which affects between 65 to 328 million people worldwide; around 164 million people have hearing loss caused by this condition, 90% of them in low‐income countries (WHO 2004). Other complications of otitis media include meningitis and brain abscess, causing about 28,000 deaths worldwide, mostly in low‐income countries (WHO 2004).
Description of the intervention
Zinc is an essential micronutrient important for immune function and resistance to infection. It must be consumed regularly as it cannot be stored in the body. Mild to moderate zinc deficiency impairs immune function but has no obvious symptoms (Walker 2004). Zinc is found in a variety of foods, including most animal proteins and some nuts, beans and seeds. Absorption of zinc is inhibited by phytates, found in many cereals and legumes. Diarrhoea causes zinc loss from the body. Over 60% of children under the age of five years have zinc deficiency from inadequate diets in some low‐income countries (Caulfield 2004).
Zinc supplements are recommended by the World Health Organization (WHO)/United Nations International Children's Emergency Fund (UNICEF) as an adjunct in the treatment of diarrhoea (WHO/UNICEF 2004), based on an analysis of effectiveness and costs (Robberstad 2004). There have also been a number of successful trials using zinc supplements in the prevention and treatment of pneumonia and other respiratory infections (Bhutta 2004). A systematic review published in 1999 concluded that zinc supplements prevent pneumonia in economically disadvantaged children living in low‐ and middle‐income countries (ZICG 1999). Another systematic review concluded that zinc supplementation reduced the incidence of lower respiratory tract infections by approximately 15% (Brown 2009). Zinc supplementation in children is associated with a reduction in pneumonia mortality of 15% (Yakoob 2011).
How the intervention might work
Children who experience recurrent otitis media have lower zinc levels than healthy controls (Bondestam 1985). Zinc supplementation helps prevent and cure respiratory and diarrhoeal diseases and so it might have a similar effect against otitis media.
Why it is important to do this review
There are currently no other published reviews on zinc supplements for the prevention of otitis media, or hearing loss and other complications arising from otitis media. Although a preliminary search revealed no published zinc supplementation trials with otitis media as a primary outcome, some trials which assess zinc for the prevention of other acute respiratory infections also record otitis media as an outcome.
Objectives
To evaluate whether zinc supplements prevent otitis media in adults and children of different ages.
Methods
Criteria for considering studies for this review
Types of studies
Randomised controlled trials (RCTs).
Types of participants
People of all ages, male or female.
Types of interventions
Zinc salt supplements, at any dose, given at least once a week, for at least one month, versus placebo. We included trials giving other micronutrients in addition to zinc if the only difference between the two groups was zinc.
Types of outcome measures
Primary outcomes
Number of participants with at least one episode of definite acute otitis media (AOM) during follow‐up; diagnosed by clinical assessment of the ear.
Number of episodes of definite AOM per participant per year of follow‐up.
Number of days with definite otitis media (acute or chronic) per participant per year of follow‐up.
Secondary outcomes
Number of participants with at least one episode of probable AOM during follow‐up, indicated by ear pain, ear discharge or other indicator as specified by the trial author.
Number of episodes of probable AOM per participant per year of follow‐up.
Number of days with probable otitis media (acute or chronic) per participant per year of follow‐up.
Adverse events: any adverse events as reported by the trial authors.
Search methods for identification of studies
Electronic searches
For this update we searched the Cochrane Central Register of Controlled Trials (CENTRAL) (2014, Issue 1), which includes the Cochrane Acute Respiratory Infections Groups' Specialised Register, MEDLINE (February 2012 to February week 4, 2014) and EMBASE (February 2012 to March 2014). See Appendix 1 and Appendix 2 for details of the search strategy. For the 2012 review same search strategy was used. Details of earlier search strategies for the original review (Abba 2010) are in Appendix 3.
Searching other resources
We searched the trials registries, WHO ICTRP (http://www.who.int/ictrp) and the US National Institutes of Health Clinical Trials (http://www.clinicaltrials.gov) for completed and ongoing trials (latest search 16 May 2014).
We attempted to contact trial authors and other researchers in the field to identify additional studies that may be eligible for inclusion. We also contacted the WHO for unpublished and ongoing trials. We imposed no language or publication restrictions. We handsearched the references of all identified studies.
We designed the search strategy to identify all trials using zinc supplementation as an intervention, as we were aware that otitis media was likely to be only a secondary outcome in the majority of trials recording this outcome.
Data collection and analysis
Selection of studies
For the original review (Abba 2010), one previous review author (KA) screened the citations identified by the search strategy to exclude those which obviously did not refer to a study assessing zinc supplementation for the prevention of illness. Two review authors (KA, AG) independently screened the abstracts of the remaining citations for the 2010 review.
One review author (AG) independently screened the citations for the first review update (Gulani 2012) and this 2014 update. Where there was any doubt, we did not exclude citations at this stage. Two review authors (HS, AG) independently screened the abstracts for the updates. We obtained copies of the full‐text reports where abstracts were not available. Two review authors (HS, AG) obtained the full reports of potentially eligible studies and assessed them for inclusion in the review, using a pre‐designed eligibility form based on the inclusion criteria. We contacted trial authors for clarification in the event that it was unclear whether a trial was eligible for the review. We resolved any differences in opinion by discussion. We excluded studies that did not meet the criteria and, with the exception of those excluded on the basis of the citation alone, documented the reasons for exclusion.
Data extraction and management
Two review authors (KA, AG) independently extracted data using a tailored data extraction form for the original review (Abba 2010). Two review authors (HS, AG) independently extracted data for the updates. We extracted data on study design, participant characteristics, interventions and outcomes. We extracted the number of participants with the outcome, the total number randomised to each group and the total number analysed for dichotomous data. We planned to extract the arithmetic mean for each group and their standard deviations (SDs) for continuous data. If medians had been used, we planned also to extract ranges. We planned to extract geometric means if presented by the trial author because of skewed data. We resolved any discrepancies between the extracted data by discussion. We attempted to contact the trial authors for further details if data were unclear or not presented in the paper.
Assessment of risk of bias in included studies
Two review authors (KA, AG) independently assessed the risk of bias of the included trials using a proforma for the 2010 review. Two review authors (HS, AG) independently assessed the risk of bias for the 2012 update using the Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011). We resolved disagreements by discussion. We assessed the following risk of bias domains:
selection bias (random sequence generation and allocation concealment);
performance bias (blinding of participants and personnel);
detection bias (blinding of outcome assessment);
attrition bias (incomplete outcome data);
reporting bias (selective reporting);
other bias.
We expressed the judgement as 'low risk', 'high risk' or 'unclear risk' of bias.
We assessed whether, for the primary outcome of otitis media, the participants, care providers and investigators were blinded to which participants received zinc supplements, as described in Higgins 2011. For otitis media outcomes, we considered that incomplete outcomes data had been adequately addressed if 85% or more of the participants were included in the analysis, or if less than 85% were included but adequate steps were taken to ensure or demonstrate that this did not bias the results. We also examined the trial reports for any evidence of selective reporting of outcomes or any other issues that may bias the results. We reported the results of the assessment in 'Risk of bias' tables for each individual study. No new trials were included in this 2014 update.
Measures of treatment effect
We analysed the data using Review Manager 5.2 (RevMan 2012). We calculated the risk ratio (RR) and if appropriate we combined results from different trials for dichotomous data. We calculated mean differences (MDs) for continuous data. We presented all results with 95% confidence intervals (CI). We described data presented by the trial authors in other formats in the text.
Dealing with missing data
We contacted the trial authors with questions about missing data. We planned to conduct an intention‐to‐treat (ITT) analysis in studies where the drop‐out rate was significant. We only utilised the available data as the drop‐out rate was low in the included studies.
Assessment of heterogeneity
We planned to assess heterogeneity between the trials by examining the forest plot to check for overlapping CIs, using the Chi2 test for heterogeneity with a 10% level of significance and the I2 statistic, using a value of 50% to represent moderate levels of heterogeneity. We planned to use the random‐effects model if heterogeneity was detected and it remained clinically meaningful to combine the trials. We planned to explore heterogeneity using the following subgroups: type of supplement; length of supplementation and participant age (adults, children, children of different ages). However, there were insufficient trials with similar outcome measures to undertake these analyses.
Assessment of reporting biases
We planned to assess the likelihood of small study effects, such as publication bias, by examining the funnel plot for asymmetry. However, there were insufficient trials reporting on the same outcomes to do this.
Sensitivity analysis
Providing there were sufficient trials, we planned also to conduct a sensitivity analysis to investigate the robustness of the results, taking into account the quality components. However, there were insufficient trials to do this analysis.
Results
Description of studies
Results of the search
The updated and modified searches in 2014 yielded an additional 980 titles. After an initial scan, we identified seven citations that could not be excluded from the review on the basis of abstract alone. We retrieved full text of these citations. No new study was found for inclusion.
We retrieved 1311 records from searches of the electronic databases for the 2012 update. We identified seven trials that could not be excluded from the review on the basis of title alone. From the 2009 search we identified 5678 records. After an initial scan of the titles, we identified 144 citations that could not be excluded from the review on the basis of title alone. From this list, we identified 12 trials that met our inclusion criteria. Of the 12 included trials, two had extremely small sample sizes (21 and 49 participants respectively) and did not have any reported cases of otitis media in their participants, either in the intervention or control groups (Prasad 1999; Prasad 2007). These two trials are presented in the Characteristics of included studies table but as they have no relevant outcome data, they do not contribute to the conclusions.
Included studies
Location
Nine trials were conducted in low‐ and middle‐income countries, including Bangladesh (Brooks 2005), India (Bhandari 2002), South Africa (Bobat 2005), Burkina Faso (Muller 2001), Chile (Schlesinger 1992), Peru (Penny 2004), Ecuador (Wuehler 2008), Jamaica (Gardner 2005) and Indonesia (Lind 2004); and one trial was conducted in the USA (Heinig 2006). Two trials conducted in the USA were not included in the analysis because no cases of otitis media were identified (Prasad 1999; Prasad 2007).
Participants
All the trial participants included in the analysis were children aged under five years at the start of the trials. All but one (Bobat 2005) included only children under the age of three years. All trials included both males and females. One trial included only children who had HIV infection and were not receiving antiretroviral therapy (Bobat 2005) and one included only infants with marasmus (Schlesinger 1992), one enrolled only children with persistent diarrhoea (Penny 2004), while others included only healthy children or children from the general population. The two trials excluded from the analysis involved adults with sickle cell disease (Prasad 1999) and elderly adults attending a senior citizens' centre (Prasad 2007). The trials varied widely in size; one trial had fewer than 50 participants (Schlesinger 1992), two had more than 50 but fewer than 100 participants (Bobat 2005; Heinig 2006), two had more than 100 but fewer than 250 (Gardner 2005; Penny 2004), three had around 600 to 700 participants (Lind 2004; Muller 2001; Wuehler 2008) and the two largest trials had 1621 (Brooks 2005) and 2482 (Bhandari 2002) participants, respectively.
Interventions
Zinc supplements were provided in the form of zinc sulphate (Bobat 2005; Gardner 2005; Heinig 2006; Lind 2004; Muller 2001; Wuehler 2008), zinc gluconate (Bhandari 2002; Penny 2004; Prasad 2007), zinc acetate (Brooks 2005; Prasad 1999) and zinc chloride (Schlesinger 1992). Supplements were given for periods of 105 days (during nutritional rehabilitation) (Schlesinger 1992), four months (Bhandari 2002), six months (Bobat 2005; Gardner 2005; Heinig 2006; Lind 2004; Muller 2001; Penny 2004; Wuehler 2008) and 12 months (Brooks 2005). Zinc or placebo was given daily (or six days a week) in all trials, except one which gave supplements weekly (Brooks 2005). On average in most of the trials, the daily dose of elemental zinc was 10 mg (Bobat 2005; Brooks 2005; Gardner 2005; Lind 2004; Muller 2001). In one trial the daily dose of elemental zinc was 10 mg for infants and 20 mg for older children (Bhandari 2002). In one trial 20 mg of elemental zinc was given to all children 6 to 36 months of age (Penny 2004). In two trials involving adults, the dose of elemental zinc was 15 mg (Prasad 2007) and 50 mg (Prasad 1999). In one trial variable dosages of zinc (3 mg, 7 mg, 10 mg) were given in different arms (Wuehler 2008). In the trial involving marasmic infants the zinc dose in the supplemented group was on average 1.9 mg/kg/day (Schlesinger 1992). In one trial the zinc dose was as low as 5 mg (Heinig 2006).
Outcomes
Primary
Two trials reported on the number of children having at least one episode of otitis media during follow‐up, diagnosed by clinical assessment of the ear (Bhandari 2002; Muller 2001); one trial also reported on the number of children with more than one episode during follow‐up (Bhandari 2002).
Three trials reported on the number of episodes of diagnosed otitis media with ear suppuration per participant per year of follow‐up (Brooks 2005; Heinig 2006; Schlesinger 1992), although one trial presented these data only in graph form and the exact numbers could not be extracted (Heinig 2006).
No trials reported on the number of days with definite otitis media.
Two trials in adults reported individual infections and described the type of infections. None of the infections reported in these trials were otitis media (Prasad 1999; Prasad 2007).
Secondary
Two trials reported on the number of participants with at least one episode of probable otitis media, defined as ear discharge reported by the mother (Bhandari 2002; Gardner 2005), although one trial did not present any numerical data for this comparison (Gardner 2005).
One trial reported on the mean number of days with reported ear discharge (Bhandari 2002). Another reported on the total number of days with ear discharge for the entire population within each group (Lind 2004); these data are not presented in meta‐analysis because of the potential that they may be skewed by a small number of children with chronic otitis media. Another recorded mean proportion of days with earache or ear discharge and presented the conclusion but not the actual data (Wuehler 2008).
Adverse events
Three trials reported on vomiting following supplementation (Bhandari 2002; Lind 2004; Penny 2004) and one trial reported on withdrawals from the study due to regurgitation of the supplement syrup (Bobat 2005).
Other
One trial, involving only children with HIV infection attending an HIV clinic but not receiving antiretroviral treatment, reported on the number of clinic attendances and the number of attendances where otitis media was diagnosed.
Excluded studies
We excluded 134 studies and the reasons for their exclusion are documented in the Characteristics of excluded studies table. Seven new trials were excluded in this update (Basnet 2012; Kartasurya 2012; Sempertegui 2014; Shah 2012; Shah 2013; Trevor 2011; Wadhwa 2013).
Risk of bias in included studies
This section reports only on the included trials that contributed to data in the analysis. The overall risk of bias is presented graphically in Figure 1 and summarised in Figure 2.
1.
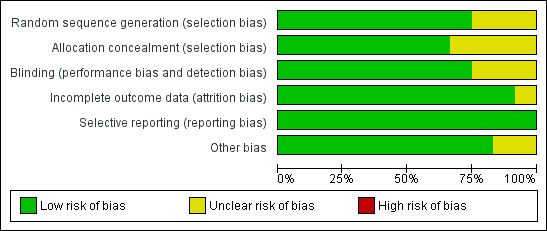
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
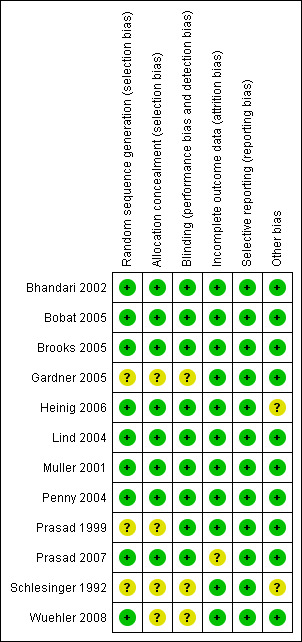
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Seven of the trials included in the analysis reported an adequate method of random sequence generation and of concealment of the allocation sequence. One trial reported adequate sequence generation but did not describe allocation concealment (Wuehler 2008). In the remaining two trials the methods of sequence generation and allocation sequences were not described (Gardner 2005; Schlesinger 1992).
Blinding
Seven of the trials included in the analysis reported that knowledge of the allocated interventions was adequately concealed during the trial (blinding of all relevant individuals involved in the trial); the remaining three trials did not provide details of blinding (Gardner 2005; Schlesinger 1992; Wuehler 2008), although Schlesinger 1992 and Wuehler 2008 were stated to be "double‐blind" and "double‐masked", respectively.
Incomplete outcome data
All 10 of the trials included in the analysis had either almost complete outcome data, or had adequately addressed any incomplete outcome data.
Selective reporting
None of the trials included in the analysis showed any evidence of selective reporting, although this was difficult to assess because the outcomes of interest were not the primary outcomes of any of the trials.
Other potential sources of bias
There was no clear evidence of any other bias in any of the trials included in the analysis, although one trial did not give clear information on how the trial sample was selected (Heinig 2006) and another did not provide sufficient methodological details to judge the risk of other bias (Schlesinger 1992).
Effects of interventions
Primary outcomes
1. Number of participants with at least one episode of definite acute otitis media (AOM) during follow‐up
In two trials conducted in community settings (Bhandari 2002; Muller 2001), there was no significant difference between zinc or placebo in the number of children with at least one episode of definite otitis media during follow‐up (3191 participants, two trials, Analysis 1.1).
1.1. Analysis.
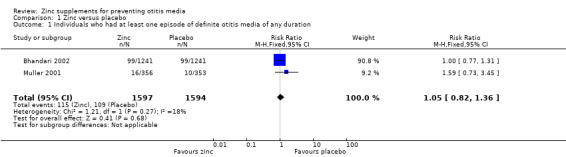
Comparison 1 Zinc versus placebo, Outcome 1 Individuals who had at least one episode of definite otitis media of any duration.
2. Number of episodes of definite AOM per participant per year of follow‐up
There was also no significant difference between zinc and placebo groups in the numbers experiencing more than one definite episode (2482 participants, one trial, Analysis 1.2) (Bhandari 2002).
1.2. Analysis.
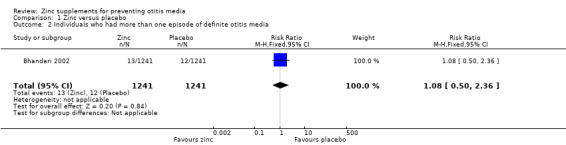
Comparison 1 Zinc versus placebo, Outcome 2 Individuals who had more than one episode of definite otitis media.
One community trial reported a lower incidence rate of diagnosed otitis media with zinc supplements compared with placebo (rate ratio (RR) 0.69, 95% confidence interval (CI) 0.61 to 0.79) (Analysis 1.4) (Brooks 2005). This trial included a younger age group than the two trials described above (60 days to six months, compared with six to 36 months and six to 31 months).
1.4. Analysis.
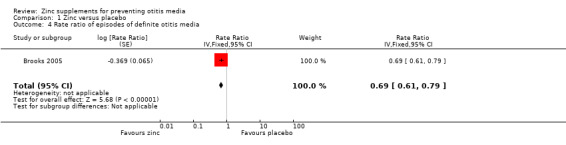
Comparison 1 Zinc versus placebo, Outcome 4 Rate ratio of episodes of definite otitis media.
In a small trial involving infants with marasmus (Schlesinger 1992), there was a significant benefit of zinc on mean number of episodes of otitis media (mean difference (MD) ‐1.12 episodes, 95% CI ‐2.21 to ‐0.03, 39 participants) (Analysis 1.3). Another trial, involving breast‐fed infants in the USA, presented data on episodes of diagnosed otitis media per 100 days at risk, in graph form only (Heinig 2006); although it was not possible to extract numerical data, it was apparent from the graph that there was no significant difference between the zinc and placebo groups, the rate in each group being approximately 0.6 episodes per 100 days at risk.
1.3. Analysis.
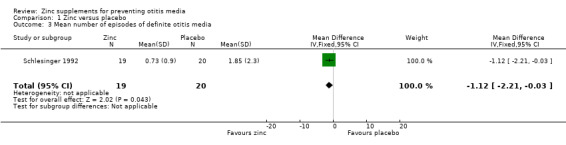
Comparison 1 Zinc versus placebo, Outcome 3 Mean number of episodes of definite otitis media.
In a trial involving 96 children with HIV infection and not receiving antiretroviral therapy (Bobat 2005), ear infection was diagnosed in 39 of 360 (10.8%) scheduled clinic visits and 46 of 407 (11.3%) total visits of children in the zinc group; while in the placebo group, ear infection was diagnosed in 52 of 370 (14.1%) scheduled visits and 65 of 447 (14.5%) total visits. The trial authors reported that this represented no significant differences between the groups.
3. Number of days with definite otitis media (acute or chronic) per participant per year of follow‐up
There were no trials reporting number of days with definite otitis media.
Secondary outcomes
1. Number of participants with at least one episode of probable AOM during follow‐up
In a trial within a community setting (Bhandari 2002), there was no significant difference between the zinc and placebo groups in the number of children who had at least one episode of probable otitis media of any duration (2482 participants, one trial) (Analysis 1.5).
1.5. Analysis.
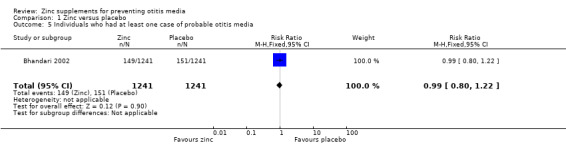
Comparison 1 Zinc versus placebo, Outcome 5 Individuals who had at least one case of probable otitis media.
2. Number of episodes of probable AOM per participant per year of follow‐up
In a community trial (Gardner 2005), there was no difference between the zinc‐supplemented and placebo groups in the median number of episodes of diagnosed ear infection (0.2 (range 0 to 5) compared with 0.0 (range 0 to 2)).
3. Number of days with probable otitis media (acute or chronic) per participant per year of follow‐up
Bhandari 2002 reported no significant difference between the zinc and placebo groups in mean days with ear discharge (3145 participants) (Analysis 1.6). In one community trial including infants from birth to 12 months (Lind 2004), the zinc‐supplemented group spent a total of 21 of 30,275 days of follow‐up with ear discharge, compared with 37 of 30,423 days in the placebo group (risk ratio (RR) 0.57, 95% CI 0.33 to 0.97, 340 participants) (Analysis 1.7). This appears to represent a significant risk reduction in the zinc‐supplemented group in the number of days spent with ear discharge. However, it is unclear from these data how many children were affected by ear discharge and whether the data were skewed by one or two individuals who may have developed chronic ear discharge.
1.6. Analysis.
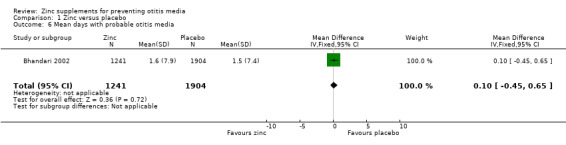
Comparison 1 Zinc versus placebo, Outcome 6 Mean days with probable otitis media.
1.7. Analysis.
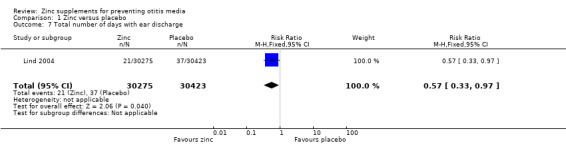
Comparison 1 Zinc versus placebo, Outcome 7 Total number of days with ear discharge.
In another community trial (Gardner 2005), there was no difference between the zinc‐supplemented and placebo groups in the median time with ear infection (0.0 (range 0 to 28) compared with 0.0 (range 0 to 15)).
A further community trial reported that the overall proportion of days spent with earache or ear discharge was less than 1% in the zinc and placebo groups and that there was no significant difference between the groups (Wuehler 2008).
4. Adverse events
One trial reported no difference between zinc and placebo in the proportion of doses given where vomiting followed within 15 minutes (0.6% in each group, 246 participants) (Penny 2004), while another reported more days of vomiting with zinc than placebo (MD 1.70 days, 95% CI 1.31 to 2.09, 2842 participants, one trial) (Analysis 1.8) (Bhandari 2002). One trial reported no significant difference between the zinc and placebo groups in the number of children who vomited after ingesting the supplement (100 participants) (Analysis 1.9) (Lind 2004). One reported that eight of 1421 children in the zinc group discontinued supplementation because of vomiting, compared with 0 of 1421 in the placebo group (Bhandari 2002). However, this difference was not significant (Analysis 1.10). One trial reported that 9.1% of the participants dropped out before the end of the study (Bobat 2005). The most common reason in both the zinc and placebo groups was a reaction to the taste of the syrup resulting in regurgitation; this was most common in the youngest participants. No trials reported any serious adverse events.
1.8. Analysis.
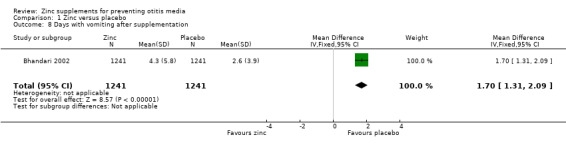
Comparison 1 Zinc versus placebo, Outcome 8 Days with vomiting after supplementation.
1.9. Analysis.
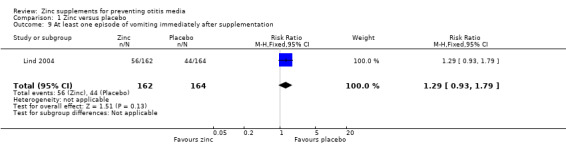
Comparison 1 Zinc versus placebo, Outcome 9 At least one episode of vomiting immediately after supplementation.
1.10. Analysis.
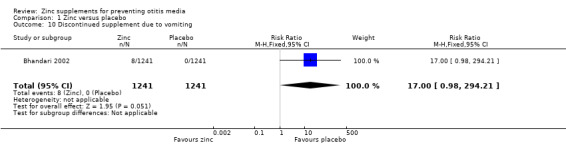
Comparison 1 Zinc versus placebo, Outcome 10 Discontinued supplement due to vomiting.
Discussion
Summary of main results
Six of the included trials showed no evidence of a difference between zinc supplements and placebo on otitis media incidence or prevalence in children. These included a small trial of children with HIV/AIDS, another small trial of breast‐fed infants in the USA and three larger trials conducted within poor communities in low‐ or middle‐income countries. One trial conducted within a community setting showed a possible benefit of zinc in infants from birth to 12 months but the findings were difficult to interpret. However, two trials appeared to demonstrate a definite significant benefit of zinc supplementation; one of these trials involved only infants with marasmus, the other involved healthy infants aged 60 days to 12 months living in a poor urban community. While the two trials including only infants under the age of 12 months appeared to show a beneficial effect of zinc and other trials also including the older age groups showed no beneficial effect, it is not possible to assess whether this effect is due to age differences as analyses stratified by age were not available for the other studies included in the review.
Overall completeness and applicability of evidence
All of the trials included in the analysis involved only young children under the age of five years and most included only children under the age of three years, the age group in which otitis media is most common. Nine of the 10 trials were conducted in low‐ and middle‐income countries, where zinc deficiency among young children is common and supplements most likely to be beneficial. Therefore these trials were applicable to real life situations where the use of supplements to prevent otitis media and other adverse health outcomes may be considered. In all the included trials, otitis media was a secondary outcome; the primary outcomes usually being more serious illnesses such as pneumonia, malaria, diarrhoea, lower respiratory tract infection or death. However, as data on otitis media were collected using the same methods as the primary outcomes, this should not have adversely affected the quality of the data collected. The main weakness of the data is that most trials, when presenting data, did not differentiate between otitis media and chronic suppurative otitis media, which may have more severe consequences, including permanent deafness.
Quality of the evidence
The trial evidence included is generally of good quality, with a low risk of bias. Seven of the nine trials included in the analysis reported adequate allocation concealment, seven reported blinding the participants and trial staff and all 10 reported a low rate of loss to follow‐up. The majority were carefully conducted community trials, with active mechanisms to promote adherence to the intervention and active case finding.
Potential biases in the review process
All the included trials, as expected, included otitis media only as a secondary outcome. There was therefore the potential to miss trials which were less publicised or less well indexed within the electronic databases. We tried to avoid this by conducting a wide search and assessing the relevance of each paper identified in that search carefully. However, only one author (KA for the 2010 review and AG for 2012 and 2014 update) was able to find the time to assess all titles identified in the search, so there is a small possibility that relevant trials may have been inappropriately excluded at this stage. There are no other obvious sources of potential bias.
Agreements and disagreements with other studies or reviews
We are unaware of any similar reviews covering this topic.
Authors' conclusions
Implications for practice.
Evidence on whether zinc supplementation can reduce the incidence of otitis media in healthy children under the age of five years living in low‐ and middle‐income countries is mixed. Three out of the five trials assessing this outcome demonstrated no significant effect, with point estimates close to no effect; another trial suggested a possible benefit of zinc but the findings were difficult to interpret and another trial appeared to demonstrate a significant benefit. The trial demonstrating a benefit of zinc included only children aged 60 days to 12 months. There were no trials in adults or older children but both otitis media and zinc deficiency are much less common in these groups.
There is no clear evidence on whether zinc supplements can reduce otitis media specifically in children with HIV/AIDS, as only one small study was conducted among this group and involved only children not receiving antiretroviral treatment.
There is some evidence that zinc supplements may reduce otitis media in infants being treated for marasmus (severe malnutrition). However, this conclusion is based only on one small trial, so must be viewed with caution.
Implications for research.
Although initial results do not seem promising, it is still unclear whether zinc supplements can prevent otitis media in poor community settings and, if so, which particular types of communities may benefit and which age groups.
Future trials assessing zinc supplements for the prevention of morbidity in childhood could incorporate otitis media as an outcome. Such trials should take care to collect and code data on otitis media outcomes accurately, including differentiating between different forms of otitis media, and to analyse and present the data in a manner that is appropriate and suitable for combining with other trial data in meta‐analyses.
Feedback
Zinc supplements for preventing otitis media, 2 March 2010
Summary
Feedback: This is a carefully conducted review but needs rebalancing for the general reader in my opinion with a few carefully placed tweaks.
1. Otitis media was a secondary outcome or collected incidentally in these trials of zinc in children. This is referred to at the end of the background but only briefly. The naive reader might believe that all these trials were done to examine zinc on otitis media. It should be made absolutely explicit that the question arose as the trials were being conducted and this analysis was simply to see if this was a secondary effect of giving zinc supplements. This is not mentioned in the narrative description of studies or in the results.
2. Otitis media is common world wide, but zinc supplements would only be considered in areas where marginal zinc deficiency is common. This again is implicit if you know the topic but not to the reader who doesn't know this. it would not be considered out of the context of low income rural areas in developing countries‐and probably would not be given for its effects on OM alone but because of its effects on life threatening ARI. SHOULD THE TITLE ACTUALLY SAY "IN developing countries" or "areas where zinc deficiency is common"?
Submitter agrees with default conflict of interest statement: I certify that I have no affiliations with or involvement in any organization or entity with a financial interest in the subject matter of my feedback.
Reply
1. We have tried to make it more explicit in the discussion.
2. We cannot change the title as it was predecided in the initial protocol to include studies from all over the world.
Contributors
Paul Garner
What's new
| Date | Event | Description |
|---|---|---|
| 10 March 2014 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 10 March 2014 | New search has been performed | Searches updated. No new trials were identified for inclusion. Seven new trials were excluded (Basnet 2012; Kartasurya 2012; Sempertegui 2014; Shah 2012; Shah 2013; Trevor 2011; Wadhwa 2013). |
History
Protocol first published: Issue 3, 2007 Review first published: Issue 2, 2010
| Date | Event | Description |
|---|---|---|
| 14 February 2012 | New search has been performed | Searches conducted. No new trials were included or excluded in this update. |
| 14 February 2012 | New citation required but conclusions have not changed | Our conclusions remain unchanged. |
| 7 September 2010 | Feedback has been incorporated | Feedback comment added to review. |
| 30 November 2008 | Amended | Converted to new review format. |
Acknowledgements
The authors wish to thank the following people for commenting on the draft protocol: Anne Lyddiatt, Renzo Mora, Betsy Blazek‐O'Neill, Rick Shoemaker and Abigail Fraser. We also wish to thank Paul Garner who provided advice and supervision for the protocol. We wish to thank Katharine Abba (KA) who wrote the protocol, selected the included studies, assessed their quality, undertook the analysis and drafted the 2010 review. We wish to thank the following people for commenting on the original draft review: Anne Lyddiatt, Renzo Mora, Abdullah Brooks, Mark Jones and Hans van der Wouden.
Appendices
Appendix 1. MEDLINE search strategy
We used the following search terms to search MEDLINE and CENTRAL. We combined the MEDLINE search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity‐ and precision‐maximising version (2008 revision); Ovid format (Lefebvre 2011). We modified the search for EMBASE (see Appendix 2).
1 Zinc/ (43649) 2 Zinc Sulfate/ (1282) 3 (zinc* or zn).tw,nm. (96313) 4 or/1‐3 (96313) 5 exp Otitis Media/ (19936) 6 otitis media.tw. (14174) 7 Respiratory Tract Infections/ (27922) 8 or/5‐7 (49675) 9 exp Dietary Supplements/ (28337) 10 8 and 9 (168) 11 4 or 10 (96453)
Appendix 2. EMBASE search strategy
#14. #10 AND #13 4,502 7 Jul 2011 #13. #11 OR #12 874,749 7 Jul 2011 #12. random*:ab,ti OR placebo*:ab,ti OR factorial*:ab,ti OR crossover*:ab,ti OR 'cross over':ab,ti OR 'cross‐over':ab,ti OR volunteer*:ab,ti OR assign*:ab,ti OR allocat*:ab,ti OR ((singl* OR doubl*) NEAR/1 blind*):ab,ti AND [embase]/lim 834,722 7 Jul 2011 #11. 'randomized controlled trial'/exp OR 'single blind procedure'/exp OR 'double blind procedure'/exp OR 'crossover procedure'/exp AND [embase]/lim 244,750 7 Jul 2011 #10. #3 OR #9 88,439 7 Jul 2011 #9. #4 AND #8 162 7 Jul 2011 #8. #5 OR #6 OR #7 46,899 7 Jul 2011 #7. 'respiratory tract infection'/de AND [embase]/lim 27,444 7 Jul 2011 #6. 'otitis media':ab,ti AND [embase]/lim 13,341 7 Jul 2011 #5. 'otitis media'/exp AND [embase]/lim 18,524 7 Jul 2011 #4. 'diet supplementation'/de AND [embase]/lim 37,692 7 Jul 2011 #3. #1 OR #2 88,312 7 Jul 2011 #2. zinc:ab,ti AND [embase]/lim 62,243 7 Jul 2011 #1. 'zinc'/de OR 'zinc sulfate'/de AND [embase]/lim 57,057 7 Jul 2011
Appendix 3. Previous search strategy
We used the following search terms to search MEDLINE and CENTRAL. We combined the MEDLINE search string with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE.
MEDLINE (Ovid)
exp Zinc/
zinc.tw.
exp Dietary Supplements/
1 or 2 or 3
EMBASE (Elsevier)
#1. 'zinc'/exp AND #2. zinc:ti,ab #3. 'diet supplementation'/de #4. #1 OR #2 OR #3 #5. random*:ti,ab OR placebo*:ti,ab,de OR 'double‐blind':ti,ab #6. #4 AND #5
Data and analyses
Comparison 1. Zinc versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Individuals who had at least one episode of definite otitis media of any duration | 2 | 3191 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.05 [0.82, 1.36] |
| 2 Individuals who had more than one episode of definite otitis media | 1 | 2482 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.08 [0.50, 2.36] |
| 3 Mean number of episodes of definite otitis media | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | ‐1.12 [‐2.21, ‐0.03] |
| 4 Rate ratio of episodes of definite otitis media | 1 | Rate Ratio (Fixed, 95% CI) | 0.69 [0.61, 0.79] | |
| 5 Individuals who had at least one case of probable otitis media | 1 | 2482 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.99 [0.80, 1.22] |
| 6 Mean days with probable otitis media | 1 | 3145 | Mean Difference (IV, Fixed, 95% CI) | 0.10 [‐0.45, 0.65] |
| 7 Total number of days with ear discharge | 1 | 60698 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.57 [0.33, 0.97] |
| 8 Days with vomiting after supplementation | 1 | 2482 | Mean Difference (IV, Fixed, 95% CI) | 1.70 [1.31, 2.09] |
| 9 At least one episode of vomiting immediately after supplementation | 1 | 326 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.29 [0.93, 1.79] |
| 10 Discontinued supplement due to vomiting | 1 | 2482 | Risk Ratio (M‐H, Fixed, 95% CI) | 17.0 [0.98, 294.21] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Bhandari 2002.
| Methods | RCT (simple randomisation in blocks of 8) Duration: February 1998 to September 2000 | |
| Participants | Number: 2482 enrolled Inclusion criteria: aged 6 to 30 months, male and female Exclusion criteria: consent refused, likely to move out of the study area within the next 4 months, needed urgent admission to hospital on the enrolment day, received a massive dose of vitamin A within last 2 months | |
| Interventions | Group1: zinc gluconate syrup taken daily for 4 months (contained 10 mg elemental zinc for infants and 20 mg for older children) Group 2: placebo syrup | |
| Outcomes |
Included in the review
In passive surveillance at clinics: acute suppurative OM defined as pus draining from ear for < 2 weeks duration plus otoscopic examination reveals redness, decreased mobility and/or bulging of ear drum. 2 episodes of OM separated by ≥ 6 weeks
Chronic suppurative OM defined as pus discharge from ear for > 2 weeks and/or recurrent ear discharge
In active surveillance: reported discharge from the ear, obtained during weekly surveillance visit by fieldworkers
Measures include children with at least 1 episode of clinically diagnosed OM during follow‐up, children with at least 1 episode of reported ear discharge during follow‐up and mean number of days with reported ear discharge at follow‐up Not included in the review Acute LRTIs Pneumonia Diarrhoea |
|
| Notes | Location: India Setting: community setting (urban slum) Source of funding: Indian Council for Medical Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomisation scheme was generated by a statistician at Statens Serum Institute using SAS software" Comment: done |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomisation scheme was generated by a person not otherwise involved with this study" Comment: done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The zinc and placebo syrups were similar in appearance, taste and packaging. Masking was maintained during analysis by coding the groups A and B" Comment: done |
| Incomplete outcome data (attrition bias) Otitis media | Low risk | 2226 of 2482 (89%) participants followed up for 4 months, a similar percentage in the placebo (91%) and zinc (88%) groups. Only 8 children (all in the zinc group) discontinued the trial because of vomiting |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Bobat 2005.
| Methods | RCT. Randomised in blocks of 8 in 3 age strata Duration: March 2003 to September 2004 | |
| Participants | Number: 96 enrolled Inclusion criteria: HIV‐1 infection, aged 6 to 60 months (age strata 6 to 23, 24 to 42 and 42 to 60 months), male or female, hospital outpatients, not receiving antiretroviral therapy. Children receiving a single dose of nevirapine to prevent transmission were included Exclusion criteria: none stated | |
| Interventions | Group 1: zinc sulphate tablets (10 mg elemental zinc) daily for 6 months Group 2: placebo | |
| Outcomes |
Included in the review
Presence of OM diagnosed by history and examination, including otoscopy, at routine and additional visits to the HIV clinic Not included in the review Watery diarrhoea Pneumonia URTI HIV‐1 viral load and CD4 + T lymphocyte % |
|
| Notes | Location: South Africa Setting: hospital outpatients Source of funding: Johns Hopkins Family Health and Child Survival Cooperative Agreement with the Office of Health, Infectious Diseases and Nutrition, Global Health Bureau, US Agency for International Development | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation lists were computer generated" Comment: done |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomisation lists were generated at the WHO" Comment: done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The investigators were unaware of the treatment allocation until follow‐up was completed" Comment: done |
| Incomplete outcome data (attrition bias) Otitis media | Low risk | 85 of 96 children (89%) completed the protocol. Of the 11 that did not complete the study, 2 dropped out and 9 died |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Brooks 2005.
| Methods | RCT. Random assignment with permuted blocks of variable length between 2 and 8 Duration: April 1999 to August 2000 for recruitment | |
| Participants | Number: 1655 enrolled, 1621 randomised Inclusion criteria: aged 60 days to 12 months, male or female Exclusion criteria: known or suspected tuberculosis, chronic respiratory or congenital heart disease, severe malnutrition requiring hospital admission | |
| Interventions | Group 1: zinc acetate 70 mg once weekly for 12 months Group 2: placebo identical in colour, odour and taste once weekly for 12 months | |
| Outcomes |
Included in the review
Mean number of episodes of suppurative OM defined as 'purulent ear discharge'. Suspected cases were found during once‐weekly home visits by trained research assistants using a standardised calendar questionnaire, then referred to the clinic for a definite diagnosis Not included in the review Episodes of diagnosed diarrhoea, URTI, reactive airways disease or bronchiolitis, pneumonia and severe pneumonia. Cases were found during once‐weekly home visits by trained research assistants using a standardised calendar questionnaire Death Height and weight Serum zinc and copper, blood haemoglobin and white blood cells |
|
| Notes | Location: Kamalapur, southeastern Dhaka, Bangladesh Setting: poor, urban community Source of funding: Johns Hopkins Family Health and Child Survival Cooperative Agreement with the US Agency for International Development, the Swiss Development Corporation and a co‐operative agreement between US Agency for International Development and core donors to the Centre for Health and Population Research | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignment... was done with permuted blocks of variable length between two and eight" Comment: probably done |
| Allocation concealment (selection bias) | Low risk | Quote: "ACME Laboratories, Ltd prepared, labelled and masked the identity of both preparations" Comment: done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The placebo was designed to be identical to the zinc syrup in colour, odour and taste" Comment: done |
| Incomplete outcome data (attrition bias) Otitis media | Low risk | Data were included in the analysis up the point of a child's withdrawal, even if they did not complete the observation period. Exit interviews were done for all drop‐outs, to determine any possible biases |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Gardner 2005.
| Methods | RCT. Stratified randomisation in 2 age groups Duration: not stated | |
| Participants | Number: 126 enrolled Inclusion criteria: age 9 to 30 months (stratified 9 to 18 months and 19 to 30 months), male or female, weight for age z‐score below ‐1.5 SDs of the National Centre for Health Statistics references, weight for age below ‐2 SDs of the National Centre for Health Statistics references Exclusion criteria: twins, children with physical or mental impairments that could affect development | |
| Interventions | Group 1: 10 mg elemental zinc as zinc sulphate were given daily, as a flavoured syrup, for 6 months Group 2: placebo with identical appearance, odour and taste The trial had a factorial design, with some children in the zinc and placebo groups also receiving psychosocial stimulation. All children in both zinc and placebo groups also received a brand of mixed micronutrients containing vitamins and iron | |
| Outcomes |
Included in the review
Number of episodes of illness and number of days ill. Pain or discharge from the ear recorded by a fieldworker visiting the families every 7th day Not included in the review Development using 4 sub‐scales of the Griffiths Mental Development Scales at enrolment and at 6 months Weight, length, weight‐for‐age z‐score, height‐for‐age z‐score, at enrolment and at 6 months Number of episodes of illness and number of days ill. Symptoms recorded by a fieldworker visiting the families every 7th day: apathy, anorexia, fever, coughing, nasal discharge, diarrhoea, vomiting, rapid or difficult breathing, any skin condition. Clinic visits for illness and hospital admissions were also recorded |
|
| Notes | Location: the parishes of Kingston, St Andrew and St Catherine, Jamaica Setting: nutrition clinics (outpatients) Source of funding: the Nestle Foundation, The Grace Kennedy Foundation (Jamaica). Dr Jeffrey Meeks and the Matalon and Methado families provided further financial assistance. Zinc supplement donated by Federated Pharmaceuticals Limited | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomly assigned to receive zinc supplement or placebo" Comment: probably done but methods not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | The participants were blinded using placebos but blinding of investigators is not described |
| Incomplete outcome data (attrition bias) Otitis media | Low risk | 114 of 126 participants (91%) completed the study and were included in the analysis. The trial authors report that the children who withdrew were not significantly different from those remaining in the study |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Heinig 2006.
| Methods | RCT (individual randomisation stratified by sex) Duration: November 1994 to August 1997 for recruitment and data collection | |
| Participants | Number: 85 enrolled and randomised Inclusion criteria: healthy male or female term infant weighing > 2500 g, healthy nonsmoking mother, mother planning to breast feed (without using formula milk on a daily basis) for ≥ 10 months and not to introduce complementary food before 4 months. Infants were recruited within 3 months of birth and included in the trial between the ages of 4 and 10 months Exclusion criteria: mother under 19 years old, chronic medical condition that may interfere with lactation, planning to leave the study area during the study period | |
| Interventions | Group 1: 5 mg elemental zinc (as zinc sulphate) in drops each day between the ages 4 and 10 months Group 2: placebo drops | |
| Outcomes |
Included in the review
Episodes of physician diagnosed OM per 100 child days at risk Not included in the review Length, weight, head circumference, midarm circumference, skinfold thickness at triceps, subscapular, flank and quadriceps at ages 4 and 10 months Plasma zinc, iron, copper, ferritin, immunoglobulin G2 and G4; and erythrocyte superoxide dismutase at ages 4 and 10 months Timing of introduction of complementary foods, nutrient intake from complementary foods Respiratory illness, diarrhoea, fever (without other symptoms), other illness Motor development measured using Alberta Infant Motor Scale |
|
| Notes | Location: USA Setting: community setting, recruitment through local paediatrician's offices Source of funding: US Department of Agriculture | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Random assignment to groups was done by using the Moses‐Oakford algorithm" Comment: done |
| Allocation concealment (selection bias) | Low risk | Quote: "An assistant who was not in contact with the study subjects labelled the bottles with 1 of 4 colours" Comment: done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Each mother‐infant pair was assigned to a colour group so that neither the primary investigator nor the mothers would know whether the infants received the zinc supplement" Comment: done |
| Incomplete outcome data (attrition bias) Otitis media | Low risk | 70 of 85 participants (82%) were included in the analysis. Of these, 3 were lost to follow‐up and 12 became ineligible because of consumption of formula milk. The analysis was undertaken both including and excluding the 12 ineligible participants and the findings were the same; therefore data are presented only for the 70 infants who completed the trial |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Not clear how participants were originally selected |
Lind 2004.
| Methods | RCT Duration: July 1997 to May 1999 | |
| Participants | Number: 680 randomised Inclusion criteria: healthy male or female singleton infants under 6 months of age Exclusion criteria: metabolic or neurological disorders; handicaps affecting development, feeding or activity; severe or protracted illness; haemoglobin < 90 g/L | |
| Interventions | Group 1: 10 mg iron as ferrous sulphate Group 2: 10 mg zinc as zinc sulphate Group 3: 10 mg iron plus 10 mg zinc Group 4: placebo All interventions given as a sweet‐tasting syrup, given daily for 6 months | |
| Outcomes |
Included in the review
Total number of days (for entire trial treatment groups) with ear discharge recorded by a fieldworker visiting families every 3rd day Not included in the review Weight, length and knee‐heel length, head circumference and mid arm circumference at 12 months of age Infant development, measured using the Bayley Scales of Infant Development, at 12 months of age Symptoms recorded by a fieldworker visiting the families every third day: fever (mothers own definition), coryza, cough, difficult or rapid breathing, diarrhoea or vomiting. Incidence of diarrhoeal disease and LRTIs during the 6 months of the trial were the primary outcomes Perceived side effects of the intervention |
|
| Notes | Location: Purworejo, Central Java, Indonesia Setting: community setting, in a health and demographic surveillance area. Authors reported a high prevalence of child stunting and micronutrient deficiencies Source of funding: the Swedish Agency for Research Cooperation with Developing Countries, the Swedish Medical Research Council, the Swedish Foundation for International Cooperation in Research and Education, the Swedish Medical Society, the Maud and Birger Gustavsson Foundation and Umea University Foundation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was planned and generated by an independent statistician and performed in blocks of 20" Comment: done |
| Allocation concealment (selection bias) | Low risk | Quote: "Randomization was planned and generated by an independent statistician".... "The pharmaceutical company marked the 4 different supplements with letter codes to which the researchers and participants were blinded" Comment: done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "Researchers and field staff were blinded to the information on group assignment, because this information was kept in safes at the administrative offices.. until after the intention‐to‐treat analyses" Comment: done |
| Incomplete outcome data (attrition bias) Otitis media | Low risk | 662 of 680 participants (97%) had complete morbidity data. Analysis showed no differences between those who completed the trial and those who did not |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Muller 2001.
| Methods | RCT (randomisation in blocks of 30) Duration: June 1999 to December 1999 | |
| Participants | Number: 709 enrolled and randomised Inclusion criteria: aged between 6 and 31 months, male or female, permanent residents in participating villages Exclusion criteria: serious underlying disease, absence for more than 14 consecutive days during the study period | |
| Interventions | Group 1: 12.5 mg zinc sulphate, in tablet form, daily (except Sundays) for 6 months Group 2: placebo identical in appearance and taste | |
| Outcomes |
Included in the review
Number of children with at least 1 episode of clinically diagnosed OM, at period cross‐sectional surveys Not included in the review Daily reports, for 6 months, from the parents on reported symptoms other than earache, visits to healthcare providers and any treatments received Incidence of malaria, detected by daily temperature monitoring and testing of children with a temperature of 37.5 °C or higher At baseline, 3 months and 6 months, cross‐sectional survey data including: personal characteristics and risk factors (age, sex, ethnicity, use of mosquito nets), clinical data (including diagnosed OM), anthropometric data (weight, height or length, mid arm circumference) and malaria parasite density from thick and thin blood films. Packed cell volumes and serum zinc concentrations were measured in a random sample of 100 |
|
| Notes | Location: 18 villages in rural northwestern Burkina Faso Setting: community setting, in rural area where malaria is common Source of funding: the World Health Organization and Deutsche Forschungsgemeinschaft | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Children were allocated zinc or placebo in blocks of 30 by computer generated random permutated codes" Comment: done |
| Allocation concealment (selection bias) | Low risk | Quote: "Children were allocated by computer generated random permuted codes (prepared by the World Health Organization)" Comment: done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The tablets were identical in appearance and taste" "The randomization code was broken after the database was closed" Comment: done |
| Incomplete outcome data (attrition bias) Otitis media | Low risk | 685 of 709 (97%) children were included in the analysis. Children absent from the trial for more than 14 consecutive days were excluded |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | Participants selected for the study randomly |
Penny 2004.
| Methods | RCT. Block randomisation, stratified by current breastfeeding status Duration: not stated | |
| Participants | Number: 246 enrolled Inclusion criteria: aged 6 to 36 months with diarrhoea for ≥ 14 days, male or female, intending to remain in the study area the next 6 months Exclusion criteria: none stated | |
| Interventions | Group 1: 20 mg zinc per day as zinc gluconate Group 2: 20 mg zinc per day as zinc gluconate plus a mixture of other micronutrients Group 3: placebo The 3 interventions were indistinguishable by taste and appearance. They were in the form of a dry powder with added sugar, flavouring and colouring and were administered by dissolving in boiled water in the participants' homes All interventions were given daily for 6 months after the episode of diarrhoea had ended | |
| Outcomes |
Included in the review
Incidence and prevalence of ear infection, assessed by fieldworkers who made daily visits to the children's home, asked the caregivers about symptoms and examined the child if new symptoms or worsening of existing symptoms was reported. (Awaiting data from author) Not included in the review Incidence and prevalence of various illnesses and symptoms, assessed by fieldworkers who made daily visits to the children's home, asked the caregivers about symptoms and examined the child if new symptoms or worsening of existing symptoms were reported. Illnesses reported on included diarrhoea, cough, lower respiratory infection, pneumonia, fever, anorexia Weight, length, mid‐upper arm circumference and skinfold thickness on biceps, triceps, suprailiac, subscapular. Measured at baseline, 15 days, then monthly Haemoglobin, haematocrit and plasma zinc measured at baseline, 15 days and 6 months |
|
| Notes | Location: Canto Grande, a shanty town on the outskirts of Lima, Peru Setting: community setting Source of funding: Thrasher Research Fund and the World Health Organization; additional funds were provided by the University of California Pacific Rim Programme | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "With the use of a computer‐generated, block randomization scheme..." Comment: done |
| Allocation concealment (selection bias) | Low risk | Quote: "Each study number had been linked previously to 1 of 9 letter codes, each of which indicated one of the three treatment groups" Comment: done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The identities of the codes were not available to the field staff or investigators until after the data had been cleaned and analyzed" Comment: done |
| Incomplete outcome data (attrition bias) Otitis media | Low risk | 6/246 (2%) children were withdrawn from the study by their parents. For the remaining children, information on morbidity was available for > 90% of days during the period of supplementation |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Prasad 1999.
| Methods | Randomised controlled trial Duration: 4 years | |
| Participants | Number: 21 enrolled
Inclusion criteria: adults (male or female) with sickle cell disease, sickle cell haemoglobin C, or sickle cell beta thalassaemia, who were attending a clinic and who were identified as zinc‐deficient using cellular zinc levels and immunological parameters Exclusion criteria: non‐ambulatory, receiving more than 6 transfusions per year, drug dependent, neurological or psychiatric deficits, taking immunosuppressive drugs, HIV positive, hepatitis B, already taking zinc supplements |
|
| Interventions | Group 1: no supplement for 1 year, placebo for 1 year, followed by 50 mg elemental zinc as zinc acetate daily for 2 years Group 2: 50 mg elemental zinc as zinc acetate daily for 3 years | |
| Outcomes | Infections, classified by type and diagnosed at the clinic | |
| Notes | Location: Detroit, USA Source of funding: FDA grant | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Mentioned "randomized groups" |
| Allocation concealment (selection bias) | Unclear risk | Not stated |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Acutely symptomatic, febrile patients were evaluated by an infectious disease consultant who was a blinded observer" |
| Incomplete outcome data (attrition bias) Otitis media | Low risk | No loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
Prasad 2007.
| Methods | RCT Duration: 12 months | |
| Participants | Number: 50 enrolled Inclusion criteria: elderly men and women attending a senior citizens' centre Exclusion criteria: life expectancy less than 8 months, progressive neoplastic disease, significant kidney disease, significant liver disease, people self supplementing with zinc, people unable to provide informed consent for participation |
|
| Interventions | Group 1: 1 capsule zinc gluconate (15 mg elemental zinc) every day for 12 months Group 2: placebo capsules every day in the same manner | |
| Outcomes | Any infection, classified by type of infection, diagnosed by a nurse practitioner | |
| Notes | Location: Detroit, USA Source of funding: NIH grant and Labcatal Laboratories, France | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Elderly subjects were randomly assigned in pairs to the zinc supplemented or the placebo group with the use of envelopes that each contained two smaller envelopes" Comment: done |
| Allocation concealment (selection bias) | Low risk | Quote: "Elderly subjects were randomly assigned in pairs to the zinc supplemented or the placebo group with the use of envelopes that each contained two smaller envelopes" Comment: done |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Quote: "The nurse practitioner was blinded to the treatment assignment" Quote: "The persons caring for the patient...were blinded to the assignment" |
| Incomplete outcome data (attrition bias) Otitis media | Unclear risk | 1 participant dropped out of the study on day 2. There was no other loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of any other bias |
Schlesinger 1992.
| Methods | RCT Duration: not stated | |
| Participants | Number: 39 enrolled Inclusion criteria: marasmic infants, male or female Exclusion criteria: none stated | |
| Interventions | Group 1: zinc fortified (zinc chloride 15 mg/L) nutritional recovery formula, based on full fat, powdered cow's milk Group 2: identical nutritional recovery formula but without the zinc Interventions were given daily for 105 days | |
| Outcomes |
Included in the review
Mean number of episodes of ear suppuration, detected by daily recording of signs and symptoms Not included in the review Haemoglobin, ferritin, plasma zinc and copper, anaemia and iron stores at admission, 30 days, 60 days and 105 days Height for age, weight for age and height for weight z‐scores at admission, 30 days, 60 days and 105 days Positive skin tests indicating immunocompetence at admission and 105 days Mean number of episodes, mean duration of days of each episode and mean percentage of infected days with illness for URTI, LRTI, acute diarrhoea, skin and mucous candidiasis, purulent conjunctivitis, within the 105 days duration. These were detected by daily recording of signs and symptoms |
|
| Notes | Location: Santiago, Chile Setting: nutritional recovery centre inpatients | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Not described |
| Allocation concealment (selection bias) | Unclear risk | No described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "controlled double blind design" Comment: probably done but methods not described |
| Incomplete outcome data (attrition bias) Otitis media | Low risk | 39 of 39 participants (100%) included in the analysis |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Unclear risk | Not enough information provided about the methods |
Wuehler 2008.
| Methods | RCT Duration: November 2001 to April 2005 | |
| Participants | Number: 631 enrolled Inclusion criteria: children 12 to 29 months old, male or female, length for age z‐score < ‐1.3 for children 12 to 20 months old, < ‐1.5 for children 21 to 29 months old (stunted), assessed by comparison with WHO/NCHS international reference data. All received iron supplements for 1 month before the start of the trial Exclusion criteria: anaemia assessed by haemoglobin < 10.5 g/dL, adjusted for altitude, chronic disease or congenital defect that restricts normal growth | |
| Interventions | Group 1: placebo
Group 2: 3 mg zinc per day
Group 3: 7 mg zinc per day
Group 4: 10 mg zinc per day
Group 5: 10 mg zinc plus 0.5 mg copper per day Zinc was given as zinc sulphate and copper given as copper sulphate. All supplements were given for 6 months |
|
| Outcomes |
Included in the review
Earache or discharge from the ear during each day, recorded during home visits 3 to 5 times per week using a systematic, symptom‐based questionnaire and observation of the child Not included in the review Child's general health status, appetite, number and consistency of stools, symptoms of cough, fever, nasal discharge, vomiting Length and weight at 0, 3 and 6 months Zinc, iron and copper status as measured in blood samples |
|
| Notes | Location: Ecuador, various communities Source of funding: US Department of Agriculture, US Agency for International Development Micronutrient Program, the United Nations Children Fund, Bristol‐Meyers/ Squibb Freedom to Discover grant and Grupo Farma del Ecuador | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The randomization lists... were generated... by using a fixed block randomization procedure" Decision: done |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Quote: "double‐masked" Decision: unclear, probably done |
| Incomplete outcome data (attrition bias) Otitis media | Low risk | 30 days or more of morbidly data were obtained for 590 of 631 participants (94%) |
| Selective reporting (reporting bias) | Low risk | No evidence of selective reporting |
| Other bias | Low risk | No evidence of other bias |
C: celsius FDA: Food and Drug Administration (US) g/dL: grams/decilitre g/L: grams/litre LRTI: lower respiratory tract infection mg: milligram mg/L: milligrams/litre OM: otitis media RCT: randomised controlled trial SD: standard deviation URTI: upper respiratory tract infection WHO/NCHS: World Health Organization/National Center for Health Statistics
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Abdulhamid 2008 | No relevant outcomes |
| Al‐Sonboli 2003 | No relevant outcomes |
| Alam 2011 | No relevant outcomes |
| Arsenault 2008 | No relevant outcomes |
| Bacqui 2002 | No relevant outcomes |
| Bacqui 2003 | No relevant outcomes |
| Bansal 2011 | No relevant outcomes |
| Basnet 2012 | No relevant outcomes |
| Bates 1993 | No relevant outcomes |
| Behrens 1990 | No relevant outcomes |
| Berger 2006 | No relevant outcomes |
| Bhandari 2007 | No relevant outcomes |
| Bhutta 1999 | No relevant outcomes |
| Bogden 1988 | No relevant outcomes |
| Bogden 1990 | No relevant outcomes |
| Boran 2006 | No relevant outcomes |
| Brooks 2004 | No relevant outcomes |
| Brooks 2005b | No relevant outcomes |
| Brown 2007 | No relevant outcomes |
| Carcamo 2006 | No relevant outcomes |
| Castillo‐Duran 1987 | No relevant outcomes |
| Castillo‐Duran 1995 | No relevant outcomes |
| Castillo‐Duran 2001 | No relevant outcomes |
| Cavan 1993 | No relevant outcomes |
| Chandyo 2010 | No relevant outcomes |
| Chang 2006 | No relevant outcomes |
| Coles 2008 | No relevant outcomes |
| Czerwinski 1974 | No relevant outcomes |
| Dehbozorgi 2007 | No relevant outcomes |
| Dijkhuizen 2001 | No relevant outcomes |
| Dijkhuizen 2008 | No relevant outcomes |
| Doherty 1998 | No relevant outcomes |
| Duchateau 1981 | Not a randomised controlled trial |
| Dutta 2000 | No relevant outcomes |
| Eggert 1982 | No relevant outcomes |
| Ertekin 2003 | No relevant outcomes |
| Fahmida 2007 | No relevant outcomes |
| Fajolu 2008 | No relevant outcomes |
| Faruque 1999 | No relevant outcomes |
| Fawzi 2005 | No relevant outcomes |
| Fischer Walker 2007 | No relevant outcomes (contacted trial author for clarification) |
| Fischer Walker 2009 | No relevant outcomes |
| Floersheim 1980 | Not a randomised controlled trial |
| Fortes 1998 | No relevant outcomes |
| Friel 1993 | No relevant outcomes |
| Friis 1997 | No relevant outcomes |
| Giroden 1999 | Intervention is not zinc alone (zinc plus selenium sulphide) |
| Golden 1978 | Intervention is not oral zinc (topical zinc cream) |
| Goransson 1978 | No relevant outcomes |
| Grazioso 1993 | No relevant outcomes |
| Gupta 1995 | No relevant outcomes |
| Gupta 2003 | No relevant outcomes |
| Hambridge 1993 | No relevant outcomes |
| Hemalatha 1993 | No relevant outcomes |
| Hettiarachchi 2008 | No relevant outcomes |
| Hodkinson 2007 | No relevant outcomes |
| Intorre 2008 | No relevant outcomes |
| Izadi 2010 | No relevant outcomes |
| Johnson 2007 | No relevant outcomes |
| Jonsson 1996 | No relevant outcomes |
| Kartasurya 2012 | No relevant outcomes |
| Khanum 1988 | No relevant outcomes |
| Khatun 2001 | No relevant outcomes |
| Kilic 1998 | No relevant outcomes |
| Kurogol 2007 | No relevant outcomes |
| Labadie 1986 | No relevant outcomes |
| Lin 2008 | No relevant outcomes |
| Linday 2004 | Not a randomised controlled trial |
| Lira 1998 | No relevant outcomes |
| Lockitch 1989 | No relevant outcomes |
| Long 2006 | No relevant outcomes |
| Luabeya 2007 | No relevant outcome |
| Mahomed 1989 | No relevant outcomes |
| Makonnen 2003 | No relevant outcomes |
| Marchisio 2010 | Intervention is not zinc alone (propolis and zinc suspension) |
| Mocchegiani 1995 | No relevant outcomes |
| Mocchegiani 1999 | Not a randomised controlled trial |
| Muller 2003 | No relevant outcomes |
| Munguia 2003 | No relevant outcomes |
| Naheed 2009 | Zinc supplements given for less than 1 month |
| Ninh 1996 | No relevant outcome |
| Osendarp 2002 | No relevant outcomes |
| Osendarp 2007 | No relevant outcomes |
| Rahman 2002 | No relevant outcomes |
| Rawer 1987 | No relevant outcomes |
| Richard 2006 | Not a relevant article |
| Rivera 1998 | No relevant outcomes |
| Rosado 1997 | No relevant outcomes |
| Roy 1997 | No relevant outcomes |
| Roy 1999 | Supplements given for a period shorter than 1 month |
| Roy 2007 | Intervention not zinc alone (zinc plus multivitamins) |
| Roy 2008a | Supplements given for a period shorter than 1 month |
| Roy 2008b | No relevant outcomes |
| Ruel 1997 | No relevant outcomes (checked with author) |
| Ruz 1997 | No relevant outcomes |
| Sachdev 1990 | No relevant outcomes |
| Safai‐Kutti 1990 | Not a randomised controlled trial |
| Safai‐Kutti 1991 | No relevant outcomes |
| Samman 1987 | No relevant outcomes |
| Sancho Martinez 2007 | Not a randomised controlled trial (review article) |
| Sazawal 1995 | No relevant outcomes |
| Sazawal 1996 | No relevant outcomes |
| Sazawal 2001 | No relevant outcomes |
| Sazawal 2007 | No relevant outcomes |
| Sempertegui 2014 | No relevant outcomes |
| Shah 2012 | No relevant outcomes |
| Shah 2013 | No relevant outcomes |
| Shankar 2000 | No relevant outcomes |
| Silva 2006 | No relevant outcomes |
| Simmer 1988 | Not a randomised controlled trial |
| Simmer 1991 | No relevant outcomes |
| Singh 1994 | No relevant outcomes |
| Smith 1999 | No relevant outcomes |
| Sur 2003 | No relevant outcomes |
| Thu 1999 | Intervention not zinc alone (multiple micronutrients) |
| Tielsch 2007 | No relevant outcomes |
| Trevor 2011 | Review article. Not a randomised trial |
| Udomkesmalee 1992 | No relevant outcomes |
| Valery 2005 | No relevant outcomes |
| Van Horn 2003 | Intervention not zinc (promotion of diet low in saturated fat) |
| Vasudevan 1997 | No relevant outcomes |
| Veverka 2009 | No relevant outcomes |
| Wadhwa 2013 | No relevant outcomes |
| Walravens 1976 | No relevant outcomes |
| Walravens 1989 | No relevant outcomes |
| Walravens 1992 | No relevant outcomes |
| Wasantwisut 2006 | No relevant outcomes |
| Wazewska‐Czyzewska 1978 | No relevant outcomes |
| Weismann 1978 | No relevant outcomes |
| Weismann 1979 | No relevant outcomes |
| Wieringa 2007 | No relevant outcomes |
| Yang 2002 | No relevant outcomes |
| Zeba 2008 | Intervention not zinc alone (zinc plus vitamin A) |
| Zemel 2002 | No relevant outcomes |
Differences between protocol and review
There was a change in authorship between the protocol and the review. Paul Garner contributed to the protocol but did not participate in the review process. Katharine Abba contributed to the original review (Abba 2010). Anjana Gulani and Harshpal Singh Sachdev contributed to the original review, the first update (Gulani 2012) and this 2014 update, but were not involved in the protocol.
Contributions of authors
Harshpal Singh Sachdev (HS) and Anjana Gulani (AG) selected the included studies, assessed their quality, undertook the analysis and drafted the 2014 update. HS provided advice and comments on the findings of the updated review and contributed to the writing.
Sources of support
Internal sources
Sitaram Bhartia Institute of Science and Research, India.
External sources
No sources of support supplied
Declarations of interest
Harshpal Singh Sachdev: none known. Anjana Gulani: none known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Bhandari 2002 {published and unpublished data}
- Bhandari N, Bahl R, Taneja S, Strand T, Molbak K, Ulvik RJ, et al. Effect of routine zinc supplementation on pneumonia in children aged 6 months to 3 years: randomised controlled trial in an urban slum. BMJ 2002;324(7350):1358‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhandari N, Bhal T, Taneja S, Strand T, Molbak K, Ulvik RJ, et al. Substantial reduction in severe diarrhoea morbidity by daily zinc supplementation in young north Indian children. Pediatrics 2002;109(6):86‐91. [DOI] [PubMed] [Google Scholar]
Bobat 2005 {published data only}
- Bobat R, Coovadia H, Stephen C, Naidoo KL, McKerrow N, Black RE, et al. Safety and efficacy of zinc supplementation in children with HIV‐1 infection in South Africa: a randomised double‐blind placebo‐controlled trial. Lancet 2005;366:1862‐7. [DOI] [PubMed] [Google Scholar]
Brooks 2005 {published data only}
- Brooks WA, Santosham M, Naheed A, Goswami D, Wahed MA, Diener‐West M, et al. Effect of weekly zinc supplements on incidence of pneumonia and diarrhoea in children younger than 2 years in an urban, low‐income population in Bangladesh: randomised controlled trial. Lancet 2005;366(9490):999‐1004. [DOI] [PubMed] [Google Scholar]
Gardner 2005 {published data only}
- Gardner JM, Powell CA, Baker‐Henningham H, Walker SP, Cole TJ, Grantham‐McGregor SM. Zinc supplementation and psychosocial stimulation: effects on the development of undernourished Jamaican children. American Journal of Clinical Nutrition 2005;82(2):399‐405. [DOI] [PubMed] [Google Scholar]
Heinig 2006 {published data only}
- Heinig MJ, Brown KH, Lonnerdal B, Dewey KG. Zinc supplementation does not affect growth, morbidity, or motor‐development of US term breastfed infants at 4‐10 months of age. American Journal of Clinical Nutrition 2006;84(3):594‐601. [DOI] [PubMed] [Google Scholar]
Lind 2004 {published data only}
- Lind T, Lonnerdal B, Stenlund H, Gamayanti IL, Ismail, Seswandhana T, et al. A community‐based randomised controlled trial of iron and zinc supplementation in Indonesian infants: effects of growth and development. American Journal of Clinical Nutrition 2004;80(3):729‐36. [DOI] [PubMed] [Google Scholar]
- Lind T, Lonnerdal B, Stenlund H, Ismail D, Seswandhana R, Ekstrom EC, et al. A community‐based randomized controlled trial of iron and zinc supplementation in Indonesian Infants: interactions between iron and zinc. American Journal of Clinical Nutrition 2003;77(4):883‐90. [DOI] [PubMed] [Google Scholar]
Muller 2001 {published data only}
- Muller O, Becher H, Zweeden AB, Ye Y, Diallo DA, Konate AT, et al. Effect of zinc supplementation on malaria and other causes of morbidity in west African children: randomised double blind placebo controlled trial. BMJ 2001;322(7302):1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
Penny 2004 {published data only}
- Penny ME, Marin RM, Duran A, Peerson JM, Lanata CF, Lonnerdal B, et al. Randomized controlled trial of the effect of daily supplementation with zinc or multiple micronutrients on morbidity, growth, and micronutrient status of young Peruvian children. American Journal of Clinical Nutrition 79;3:457‐65. [DOI] [PubMed] [Google Scholar]
Prasad 1999 {published data only}
- Prasad AS, Beck FWJ, Kaplan J, Pranatharthi H, Chandrasekar JO, Fitzgerald JT, et al. Effect of zinc supplementation on incidence of infections and hospital admissions in sickle cell disease. American Journal of Hematology 1999;61:194‐202. [DOI] [PubMed] [Google Scholar]
Prasad 2007 {published data only}
- Prasad AS, Beck FW, Bao B, Fitzgerald JT, Snell DC, Steinberg JD, et al. Zinc supplementation decreases incidence of infections and hospital admissions in sickle cell disease (SCD). American Journal of Hematology 1999;61(3):194‐202. [DOI] [PubMed] [Google Scholar]
Schlesinger 1992 {published data only}
- Schlesinger L, Arevalo M, Arredondo AS, Diaz M, Lonnerdal B, Stekel A. Effect of a zinc‐fortified formula on immunocompetence and growth of malnourished infants. American Journal of Clinical Nutrition 1992;56(3):491‐8. [DOI] [PubMed] [Google Scholar]
Wuehler 2008 {published data only}
- Wuehler SE, Sempertegui F, Brown KH. Dose‐response trial of prophylactic zinc supplements, with or without copper, in young Ecuadorian children at risk of zinc deficiency. American Journal of Clinical Nutrition 87;3:723‐33. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Abdulhamid 2008 {published data only}
- Abdulhamid I, Beck FWJ, Miliard S, Chen X, Prasad A. Effect of zinc supplementation on respiratory infections in children with cystic fibrosis. Pediatric Pulmonology 2008;43(3):281‐7. [DOI] [PubMed] [Google Scholar]
Alam 2011 {published data only}
- Alam DS, Yunus M, Arifeen S, Chowdury HR, Larson CP, Sack DA, et al. Zinc treatment for 5 or 10 days is equally efficacious in preventing diarrhea in the subsequent 3 months among Bangladeshi children. Journal of Nutrition 2011;141:312‐5. [DOI] [PubMed] [Google Scholar]
Al‐Sonboli 2003 {published data only}
- Al‐Sonboli N, Gurgel RQ, Shenkin A, Hart CA, Cuevas LE. Zinc supplementation in Brazilian children with acute diarrhoea. Annals of Tropical Paediatrics 2003;23(1):3‐8. [DOI] [PubMed] [Google Scholar]
Arsenault 2008 {published data only}
- Arsenault JE, Lopez de Romana D, Penny ME, Loan MD, Brown KH. Additional zinc delivered in a liquid supplement, but not in a fortified porridge, increased fat‐free mass accrual among young Peruvian children with mild‐to‐moderate stunting. Journal of Nutrition 2008;138:108‐14. [DOI] [PubMed] [Google Scholar]
Bacqui 2002 {published data only}
- Bacqui AH, Black RE, Arifeen S, Yunus M, Chakraorty J, Ahmed S, et al. Effect of zinc supplementation started in diarrhoea on morbidity and mortality in Bangladeshi children: community randomised trial. BMJ 2002;325(7372):1059‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bacqui 2003 {published data only}
- Bacqui AH, Zaman K, Persson LA, Arifeen S, Yunus M, Begum N, et al. Simultaneous weekly supplementation of iron and zinc is associated with lower morbidity due to diarrhoea and acute lower respiratory infection in Bangladeshi infants. Journal of Nutrition 2003;133(12):4150‐7. [DOI] [PubMed] [Google Scholar]
Bansal 2011 {published data only}
- Bansal A, Parmar VR, Basu S, Kaur J, Jain S, Saha A, et al. Zinc supplementation in severe acute lower respiratory tract infection in children: a triple‐blind randomized placebo controlled trial. Indian Journal of Pediatrics 2011;78:33‐7. [DOI] [PubMed] [Google Scholar]
Basnet 2012 {published data only}
- Basnet S, Shrestha PS, Sharma A, Mathisen M, Prasai R, Bhandari N, et al. A randomized controlled trial of zinc as adjuvant therapy for severe pneumonia in young children. Pediatrics 2012;129:701‐8. [DOI] [PubMed] [Google Scholar]
Bates 1993 {published data only}
- Bates CJ, Evans PH, Dardenne M, Prentice A, Lunn PG, Northrop‐Clewes CA, et al. A trial of zinc supplementation in young rural Gambian children. British Journal of Nutrition 1993;69(1):243‐55. [DOI] [PubMed] [Google Scholar]
Behrens 1990 {published data only}
- Behrens RH, Tomkins AM, Roy SK. Zinc supplementation during diarrhoea, a fortification against malnutrition?. Lancet 1990;336(8712):442‐3. [DOI] [PubMed] [Google Scholar]
Berger 2006 {published data only}
- Berger J, Ninh NX, Khan NC, Nhien NV, Lien DK, Trung NQ, et al. Efficacy of combined iron and zinc supplementation on micronutrient status and growth in Vietnamese infants. European Journal of Clinical Nutrition 2006;4:443‐54. [DOI] [PubMed] [Google Scholar]
Bhandari 2007 {published data only}
- Bhandari N, Taneja S, Mazumder S, Bahl R, Fontaine O, Bhan MK, et al. Adding zinc to supplemental iron and folic acid does not affect mortality and severe morbidity in young children. Journal of Nutrition 2007;137(1):112‐7. [DOI] [PubMed] [Google Scholar]
Bhutta 1999 {published data only}
- Bhutta ZA, Nizami SQ, Isani Z. Zinc supplementation in malnourished children with persistent diarrhoea in Pakistan. Pediatrics 1999;103(4):e42. [DOI] [PubMed] [Google Scholar]
Bogden 1988 {published data only}
- Bogden JD, Oleske JM, Lavenhar MA, Munves EM, Kemp FW, Bruening KS, et al. Zinc and immunocompetence in elderly people: effects of zinc supplementation for 3 months. American Journal of Clinical Nutrition 1988;48(3):655‐63. [DOI] [PubMed] [Google Scholar]
Bogden 1990 {published data only}
- Bogden JD, Oleske JM, Lavenhar MA, Munves EM, Kemp FW, Bruening KS, et al. Effects of one‐year supplementation with zinc and other micronutrients on cellular immunity in the elderly. Journal of the American College of Nutrition 1990;9(3):214‐25. [DOI] [PubMed] [Google Scholar]
Boran 2006 {published data only}
- Boran P, Tokuc G, Vagas E, Oktem S, Gokduman MK. Impact of zinc supplementation in children with acute diarrhoea in Turkey. Archives of Disease in Childhood 2006;91(4):296‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Brooks 2004 {published data only}
- Brooks WA, Yunus M, Santosham M, Wahed MA, Nahar K, Yeasmin S, et al. Zinc for severe pneumonia in very young children: a double‐blind placebo‐controlled trial. Lancet 2004;363(9422):1683‐8. [DOI] [PubMed] [Google Scholar]
Brooks 2005b {published data only}
- Brooks WA, Santosham M, Roy SK, Faruque AS, Wahed MA, Nahar K, et al. Efficacy of zinc in young infants with acute watery diarrhoea. American Journal of Clinical Nutrition 2005;82(3):605‐10. [DOI] [PubMed] [Google Scholar]
Brown 2007 {published data only}
- Brown KH, Romana DL, Arsenault JE, Peerson JM, Penny ME. Comparison of the effects of zinc delivered in a fortified food or a liquid supplement on the growth, morbidity, and plasma zinc concentrations of young Peruvian children. American Journal of Clinical Nutrition 2007;85(2):538‐47. [DOI] [PubMed] [Google Scholar]
Carcamo 2006 {published data only}
- Carcamo C, Hooton T, Weiss NS, Gilman R, Wener MH, Chavez V, et al. Randomized controlled trial of zinc supplementation for persistent diarrhoea in adults with HIV‐1 infection. Journal of Acquired Immune Deficiency Syndromes 2006;43(2):197‐201. [DOI] [PubMed] [Google Scholar]
Castillo‐Duran 1987 {published data only}
- Castillo‐Duran C, Heresi G, Fisberg M, Uauy R. Controlled trial of zinc supplementation during recovery from malnutrition; effects on growth and immune function. American Journal of Clinical Nutrition 1987;45(3):602‐8. [DOI] [PubMed] [Google Scholar]
Castillo‐Duran 1995 {published data only}
- Castillo‐Duran C, Rodriguez A, Venegas G, Alvarez P, Icaza G. Zinc supplementation and growth of infants born small for gestational age. Journal of Pediatrics 1995;127(2):206‐11. [DOI] [PubMed] [Google Scholar]
Castillo‐Duran 2001 {published data only}
- Castillo‐Duran C, Perales CG, Hertrampf ED, Marin VB, Rivera FA, Icaza G. Effect of zinc supplementation on development and growth of Chilean infants. Journal of Pediatrics 2001;138(2):229‐35. [DOI] [PubMed] [Google Scholar]
Cavan 1993 {published data only}
- Cavan KR, Gibson RS, Grazioso CF, Isalgue Am, Ruz M, Solomus NW. Growth and body composition of periurban Guatemalan children in relation to zinc status: a longitudinal intervention trial. American Journal of Clinical Nutrition 1993;57(3):344‐52. [DOI] [PubMed] [Google Scholar]
Chandyo 2010 {published data only}
- Chandyo RK, Shrestha PS, Valentiner‐Branth P, Mathisen M, Basnet S, Ulak M, et al. Two weeks of zinc administration to Nepalese children with pneumonia does not reduce the incidence of pneumonia or diarrhea during the next six months. Journal of Nutrition 2010;140:1677‐82. [DOI] [PubMed] [Google Scholar]
Chang 2006 {published data only}
- Chang AB, Torzillo PJ, Boyce NC, White AV, Stewart PM, Wheaton GR, et al. Zinc and vitamin A supplementation in Indigenous Australian children hospitalised with lower respiratory tract infection: a randomised controlled trial. Medical Journal of Australia 2006;184(3):107‐12. [DOI] [PubMed] [Google Scholar]
Coles 2008 {published data only}
- Coles CL, Sherchand JB, Khatry SK, Katz J, LeClerq SC, Mullany LC, et al. Zinc modifies the association between nasopharyngeal Streptococcus pneumoniae carriage and risk of acute lower respiratory infection among young children in rural Nepal. Journal of Nutrition 2008;138(12):2462‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Czerwinski 1974 {published data only}
- Czerwinski AW, Clark ML, Serafetinides AE, Perrier C, Huber Q. Safety and efficacy of zinc sulphate in geriatric patients. Clinical Pharmacology and Therapeutics 1974;15(4):436‐7. [DOI] [PubMed] [Google Scholar]
Dehbozorgi 2007 {published data only}
- Dehbozorgi P, Mohseni P, Mazloom Z. The influence of zinc sulfate supplementation on the growth of school age children in villages around Shiraz 2002,2003. Journal of Medical Sciences 2007;7(4):690‐3. [Google Scholar]
Dijkhuizen 2001 {published data only}
- Dijkhuizen MA, Wieringa FT, West CE, Martuti S, Muhilal S. Effects of iron and zinc supplementation in Indonesian infants on micronutrient status and growth. Journal of Nutrition 2001;131(11):286‐5. [DOI] [PubMed] [Google Scholar]
Dijkhuizen 2008 {published data only}
- Dijkhuizen MA, Winichagoon P, Wieringa FT, Wasantwisut E, Utomo B, Ninh NX, et al. Zinc supplementation improved length growth only in anemic infants in a multi‐country trial of iron and zinc supplementation in South‐East Asia. Journal of Nutrition 2008;138(10):1969‐75. [DOI] [PubMed] [Google Scholar]
Doherty 1998 {published data only}
- Doherty CP, Sarkar MA, Shakur MS, Ling SC, Elton RA, Cutting WA. Zinc and rehabilitation from severe protein‐energy malnutrition: higher‐dose regimens are associated with increased mortality. American Journal of Clinical Nutrition 1998;68(3):742‐8. [DOI] [PubMed] [Google Scholar]
Duchateau 1981 {published data only}
- Duchateau J, Delepesse G, Vrijens R, Collett H. Beneficial effects of oral zinc supplementation on the immune response of old people. American Journal of Medicine 1981;70(5):1001‐4. [DOI] [PubMed] [Google Scholar]
Dutta 2000 {published data only}
- Dutta P, Mitra U, Datta A, Niyogi SK, Dutta S, Manna B, et al. Impact of zinc supplementation in malnourished children with acute watery diarrhoea. Journal of Tropical Paediatrics 2000;46(5):259‐63. [DOI] [PubMed] [Google Scholar]
Eggert 1982 {published data only}
- Eggert JV, Siegler RL, Edomkesmalee E. Zinc supplementation in chronic renal failure. International Journal of Pediatric Nephrology 1982;3(1):21‐4. [PubMed] [Google Scholar]
Ertekin 2003 {published data only}
- Ertekin MV, Uslu H, Karslioglu I, Ozbek E, Osbek A. Effect of oral zinc supplementation on agents of oropharyngeal infections in patients receiving radiotherapy for head and neck cancer. Journal of International Medical Research 2003;31(4):253‐66. [DOI] [PubMed] [Google Scholar]
Fahmida 2007 {published data only}
- Fahmida U, Rumawas JS, Utomo B, Patmonodewo S, Schultink W. Zinc‐iron, but not zinc‐alone, supplementation increased linear growth of stunted infants with low haemoglobin. Asia Pacific Journal of Clinical Nutrition 2007;16(2):301‐9. [PubMed] [Google Scholar]
Fajolu 2008 {published data only}
- Fajolu IB, Amokpae A, Oduwole AO, Silva BO, Abidoye RO, Renner JK. Zinc supplementation in children with acute diarrhoea. Nigerian Quarterly Journal of Hospital Medicine 2008;18(2):101‐3. [DOI] [PubMed] [Google Scholar]
Faruque 1999 {published data only}
- Faruque AS, Mahalanabis D, Haque SS, Fuchs GJ, Habte D. Double‐blind, randomized controlled trial of zinc or vitamin A supplementation in young children with acute diarrhoea. Acta Paediatrica 1999;88(2):154‐60. [DOI] [PubMed] [Google Scholar]
Fawzi 2005 {published data only}
- Fawzi WW, Villamore E, Msamanga GI, Antelman G, Aboud S, Urassa W, et al. Trial of zinc supplements in relation to pregnancy outcomes, hematologic indicators, and T cell counts among HIV‐1 infected women in Tanzania. American Journal of Clinical Nutrition 2005;81(1):161‐7. [DOI] [PubMed] [Google Scholar]
Fischer Walker 2007 {published data only}
- Fischer Walker CL, Bhutta ZA, Bhandari N, Teka T, Shahid F, Taneja S, et al. Zinc during and in convalescence from diarrhoea has no demonstrable effect on subsequent morbidity and anthropometric status among infants < 6 months of age. American Journal of Clinical Nutrition 85;3:887‐94. [DOI] [PubMed] [Google Scholar]
- Fischer Walker CL, Black RE, Bacqui AH. Does age affect the response to zinc therapy for diarrhoea in Bangladeshi infants?. Journal of Health, Population and Nutrition 2008;26(1):105‐9. [PMC free article] [PubMed] [Google Scholar]
Fischer Walker 2009 {published data only}
- Fischer Walker CL, Baqui AH, Ahmed S, Zaman K, Arifeen S, Begum N, et al. Low‐dose weekly supplementation of iron and/or zinc does not affect growth among Bangladeshi infants. European Journal of Clinical Nutrition 2009;63(1):87‐92. [DOI] [PubMed] [Google Scholar]
Floersheim 1980 {published data only}
- Floersheim GL, Boesch JH. The effect of oral zinc sulphate on human immunologic reactivity [Wirkung von oralem Zinksulfat auf die immunologische Reactivitat beim Menschen]. Schweizerische Medizinische Wochenschrift 1980;110(26):998‐1006. [PubMed] [Google Scholar]
Fortes 1998 {published data only}
- Fortes C, Forastiere F, Agabiti N, Fano V, Pacifici R, Virgili F, et al. The effects of zinc and vitamin A supplementation on immune response in an older population. Journal of the American Geriatrics Society 1998;46(1):19‐26. [DOI] [PubMed] [Google Scholar]
Friel 1993 {published data only}
- Friel JK, Andrews WL, Matthew JD, Long DR, Cornel AM, Cox M, et al. Zinc supplementation in very‐low‐birth‐weight infants. Journal of Pediatric Gastroenterology and Nutrition 1993;17(1):97‐104. [DOI] [PubMed] [Google Scholar]
Friis 1997 {published data only}
- Friis H, Ndhlovu P, Mduluza T, Kaondera K, Sandstrom B, Michaelson KF, et al. The impact of zinc supplementation on Schistosoma mansoni reinfection rate and intensities: a randomised, controlled trial among rural Zimbabwean schoolchildren. European Journal of Clinical Nutrition 1997;51(1):33‐7. [DOI] [PubMed] [Google Scholar]
- Friis H, Ndhlovu P, Mduluza T, Kaondera K, Sandstrom B, Michaelson KF, et al. The impact of zinc supplementation on growth and body composition: a randomised controlled trial among rural Zimbabwean school children. European Journal of Clinical Nutrition 1997;51(1):38‐45. [DOI] [PubMed] [Google Scholar]
Giroden 1999 {published data only}
- Giroden F, Blanche D, Mongret AL, Lombart M, Brunet‐Lecompte P, Arnaud L, et al. Effects of a two‐year supplementation with low doses of antioxidant vitamins and/or minerals in elderly subjects on levels of nutrients and antioxidant defense parameters. Journal of the American College of Nutrition 1997;16(4):357‐65. [DOI] [PubMed] [Google Scholar]
- Giroden F, Galan P, Monget AL, Boutron‐Ruault MC, Brunet‐Lecomte P, Preziosi P, et al. Impact of trace elements and vitamin supplementation on immunity and infections in institutionalized elderly patients: a randomized controlled trial. MIN.VIT.AOX, geriatric network. Archives of Internal Medicine 1999;159(7):748‐54. [DOI] [PubMed] [Google Scholar]
- Giroden F, Lombard M, Galan P, Brunet Lecomte P, Monget AL, Arnaud L, et al. Effect of micronutrient supplementation on infection in institutionalised elderly subjects: a controlled trial. Annals of Nutrition and Metabolism 1997;41(2):98‐107. [DOI] [PubMed] [Google Scholar]
Golden 1978 {published data only}
- Golden MH, Harland PS, Golden BE, Jackson AA. Zinc and immunocompetence in protein‐energy malnutrition. Lancet 1978;1(8076):1226‐8. [DOI] [PubMed] [Google Scholar]
Goransson 1978 {published data only}
- Goransson K, Liden S, Odsell L. Oral zinc in acne vulgaris: a clinical and methodological study. Acta Dermato‐Venereologica 1978;58(5):443‐8. [PubMed] [Google Scholar]
Grazioso 1993 {published data only}
- Grazioso CF, Islague M, Ramirez I, Ruz M, Solomons NW. The effect of zinc supplementation on parasitic reinfection of Guatemalan schoolchildren. American Journal of Clinical Nutrition 1993;57(5):673‐8. [DOI] [PubMed] [Google Scholar]
Gupta 1995 {published data only}
- Gupta VL, Chaubey BS. Efficacy of zinc therapy in prevention of crisis in sickle cell anaemia: a double blind, randomised controlled clinical trial. Journal of the Association of Physicians of India 1995;43(7):467‐70. [PubMed] [Google Scholar]
Gupta 2003 {published data only}
- Gupta DN, Mondal SK, Ghosh S, Rajendran K, Sur D, Manna B. Impact of zinc supplementation on diarrhoeal morbidity in rural children in West Bengal, India. Acta Paediatrica 2003;92(5):531‐6. [PubMed] [Google Scholar]
- Gupta DN, Rajendran K, Mondal SK, Ghosh S, Bhattacharya SK. Operational feasibility of implementing community‐based zinc supplementation: impact on childhood diarrhoeal morbidity. Pediatric Infectious Disease Journal 2007;26(4):306‐10. [DOI] [PubMed] [Google Scholar]
Hambridge 1993 {published data only}
- Hambridge KM, Chavez MN, Brown RM, Walravens PA. Zinc nutritional status of young middle‐income children and effects of consuming zinc‐fortified breakfast cereals. American Journal of Clinical Nutrition 1979;32(12):2532‐8. [DOI] [PubMed] [Google Scholar]
Hemalatha 1993 {published data only}
- Hemalatha P, Bhaskaram P, Khan MM. Role of zinc supplementation in the rehabilitation of severely malnourished children. European Journal of Clinical Nutrition 1993;47(6):395‐9. [PubMed] [Google Scholar]
Hettiarachchi 2008 {published data only}
- Hettiarachchi M, Liyanage C, Wickremasinghe R, Hilmers DC, Abrams SA. The efficacy of micronutrient supplementation in reducing the prevalence anaemia and deficiencies of zinc and iron among adolescents in Sri Lanka. European Journal of Clinical Nutrition 2008;62(7):856‐65. [DOI] [PubMed] [Google Scholar]
Hodkinson 2007 {published data only}
- Hodkinson CF, Kelly M, Alexander HD, Bradbury I, Robson PJ, Bonham MP, et al. The effect of zinc supplementation on the immune status of healthy older individuals aged 55‐70 years: the ZENITH Study. Journals of Gerontology. Series A, Biological Sciences and Medical Sciences 2007;62(6):598‐608. [DOI] [PubMed] [Google Scholar]
Intorre 2008 {published data only}
- Intorre F, Polito A, Andriollo‐Sanchez M, Azzini E, Raguzzini A, Toti E, et al. Effect of zinc supplementation on vitamin status of middle‐aged and older European adults: the ZENITH study. European Journal of Clinical Nutrition 2008;62(10):1215‐23. [DOI] [PubMed] [Google Scholar]
Izadi 2010 {published data only}
- Izadi P, Yarmohammadi M, Kholdi N, Izadi B, Anari S, Sedehi M. Effect of oral supplementation of zinc on treatment of otitis media with effusion. Medical Journal of the Islamic Republic of Iran 2010;24:126‐32. [Google Scholar]
Johnson 2007 {published data only}
- Johnson AR, Munoz A, Gottlieb JL, Jarrard DF. High dose zinc increases hospital admissions due to genitourinary complications. Journal of Urology 2007;177(2):639‐43. [DOI] [PubMed] [Google Scholar]
Jonsson 1996 {published data only}
- Jonsson B, Hauge B, Larsen MF, Hald F. Zinc supplementation during pregnancy: a double‐blind randomised controlled trial. Acta Obstetrica et Gynecologica Scandinavica 1996;75(8):725‐9. [DOI] [PubMed] [Google Scholar]
Kartasurya 2012 {published data only}
- Kartasurya MI, Ahmed F, Subagio HW, Rahfiludin MZ, Marks GC. Zinc combined with vitamin A reduces upper respiratory tract infection morbidity in a randomised trial in preschool children in Indonesia. British Journal of Nutrition 2012;108:2251‐60. [DOI] [PubMed] [Google Scholar]
Khanum 1988 {published data only}
- Khanum S, Alam AN, Anwar I, Akbar Ali M, Rahaman M. Effects of zinc supplementation on the dietary intake and weight gain of Bangladeshi children recovering from protein‐energy malnutrition. European Journal of Clinical Nutrition 1988;42(8):709‐14. [PubMed] [Google Scholar]
Khatun 2001 {published data only}
- Khatun UH, Malek MA, Black RE, Sarkar NR, Wahed MA, Fuchs G, et al. A randomized controlled trial of zinc, vitamin A or both in undernourished children with persistent diarrhea in Bangladesh. Acta Paediatrica 2001;90(4):376‐80. [PubMed] [Google Scholar]
Kilic 1998 {published data only}
- Kilic I, Ozalp I, Coskun T, Tokatli A, Emre S, Saldamli I, et al. The effect of zinc‐supplemented bread consumption on school children with asymptomatic zinc deficiency. Journal of Pediatric Gastroenterology and Nutrition 1998;26(2):167‐71. [DOI] [PubMed] [Google Scholar]
Kurogol 2007 {published data only}
- Kurogol Z, Bayram N, Atik T. Effects of zinc sulfate on common cold in children: randomized, double blind study. Pediatrics International 2007;49(6):842‐7. [DOI] [PubMed] [Google Scholar]
Labadie 1986 {published data only}
- Labadie H, Verneau A, Trinchet JC, Beaugrand M. Does oral zinc improve the cellular immunity of patients with alcoholic cirrhosis? [L'apport oral de zinc ameliore‐t‐il l'immunite cellulaire des malades atteints de cirrhose alcoolique?]. Gastoenterogie Clinique et Biologique 1986;10(12):799‐803. [PubMed] [Google Scholar]
Lin 2008 {published data only}
- Lin LC, Que J, Lin KL, Leung HW, Lu CL, Chang CH. Effects of zinc supplementation on clinical outcomes in patients receiving radiotherapy for head and neck cancers: a double‐blinded randomized study. International Journal of Radiation Oncology, Biology, Physics 2008;70(2):368‐73. [DOI] [PubMed] [Google Scholar]
Linday 2004 {published data only}
- Linday LA, Dolitsky JN, Shindledecker RD. Nutritional supplements as adjunctive therapy for children with chronic/recurrent sinusitis: pilot research. International Journal of Pediatric Otorhinolaryngology 2004;68(6):785‐93. [DOI] [PubMed] [Google Scholar]
Lira 1998 {published data only}
- Lira PI, Ashworth A, Morris SS. Effect of zinc supplementation on the morbidity, immune function, and growth of low‐birth‐weight, full‐term infants in northeast Brazil. American Journal of Clinical Nutrition 1998;68(Suppl 2):418‐24. [DOI] [PubMed] [Google Scholar]
Lockitch 1989 {published data only}
- Lockitch G, Puterman M, Godolphin W, Sheps S, Tingel AJ, Quigley G. Infection and immunity in Down syndrome: a trial of long‐term oral doses of zinc. Journal of Pediatrics 1989;114(5):781‐7. [DOI] [PubMed] [Google Scholar]
Long 2006 {published data only}
- Long KZ, Montoya Y, Hertzmark E, Santos JI, Rosado JL. A double‐blind, randomized, clinical trial of the effect of vitamin A and zinc supplementation on diarrhoeal disease and respiratory tract infections in children in Mexico City, Mexico. American Journal of Clinical Nutrition 2006;83(3):693‐700. [DOI] [PubMed] [Google Scholar]
Luabeya 2007 {published data only}
- Luabeya KA, Mpontshane N, Mackay M, Ward H, Elson I, Chagan M, et al. Zinc or multiple micronutrient supplementation to reduce diarrhea and respiratory disease in South African children: a randomized controlled trial. PloS One 2007;2:e541. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mahomed 1989 {published data only}
- Mahomed K, James DK, Golding J, McCabe R. Zinc supplementation during pregnancy: a double‐blind randomised controlled trial. BMJ 1989;299(6703):826‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Makonnen 2003 {published data only}
- Makonnen B, Venter A, Joubert G. A randomised controlled study of the impact of dietary zinc supplementation in the management of children with protein‐energy malnutrition in Lesotho. II: Special investigation. Journal of Tropical Pediatrics 2003;49(6):353‐60. [DOI] [PubMed] [Google Scholar]
- Makonnen B, Venter A, Joubert GA. A randomized controlled study of the impact of dietary zinc supplementation in the management of children with protein‐energy malnutrition in Lesotho. I: Mortality and morbidity. Journal of Tropical Pediatrics 2003;49(6):340‐52. [DOI] [PubMed] [Google Scholar]
Marchisio 2010 {published data only}
- Marchisio P, Esposito S, Bianchini S, Desantis C, Galeone C, Nazzari E, et al. Effectiveness of a propolis and zinc solution in preventing acute otitis media in children with a history of recurrent acute otitis media. International Journal of Immunopathology & Pharmacology 2010;23:567‐75. [DOI] [PubMed] [Google Scholar]
Mocchegiani 1995 {published data only}
- Mocchegiani E, Veccia S, Ancarani F, Scalise G, Fabris N. Benefits of oral zinc supplementation as an adjunct to zidovudine (AZT) therapy against opportunistic infections in AIDS. International Journal of Immunopharmacology 1995;17(9):719‐27. [DOI] [PubMed] [Google Scholar]
Mocchegiani 1999 {published data only}
- Mocchegiani E, Muzzioli M, Gaeti R, Veccia S, Viticchi C, Scalise G. Contribution of zinc to reduce CD4+ risk factor for 'severe' infection relapse in aging: parallelism with HIV. International Journal of Immunopharmacology 1999;21(4):271‐81. [DOI] [PubMed] [Google Scholar]
Muller 2003 {published data only}
- Muller O, Garenne M, Reitmaier P, Zweeden AB, Kouyate B, Becher H. Effect of zinc supplementation on growth in West African children: a randomized double‐blind placebo‐controlled trial in rural Burkina Faso. International Journal of Epidemiology 2003;32(6):1098‐102. [DOI] [PubMed] [Google Scholar]
Munguia 2003 {published data only}
- Munguia C, Paniagua R, Avili‐Diaz M, Nava‐Hernandez J, Rodrigues E, Ventura Mde J, et. Effects of zinc supplements on the nutritional status of patients undergoing continuous ambulatory peritoneal dialysis [Efect de suplementos de cinc sobre el estado nutricio de pacients en dialisis peritoneal continua ambulatoria]. Revista de Investigacion Clinica; Organo del Hospital de Enfermedades de la Nutricion 2003;55(5):519‐27. [PubMed] [Google Scholar]
Naheed 2009 {published data only}
- Naheed A, Walker Fischer CL, Mondal D, Ahmed S, Arifeen SE, Yunus M, et al. Zinc therapy for diarrhoea improves growth among Bangladeshi infants 6 to 11 months of age. Journal of Pediatric Gastroenterology and Nutrition 2009;48(1):89‐93. [DOI] [PubMed] [Google Scholar]
Ninh 1996 {published data only}
- Ninh NX, Thissen JP, Collette L, Gerard G, Khoi HH, Ketelslegers JM. Zinc supplementation increases growth and circulating insulin‐like growth factor 1 (IGF‐1) in growth‐retarded Vietnamese infants. American Journal of Clinical Nutrition 1996;63(4):514‐9. [DOI] [PubMed] [Google Scholar]
Osendarp 2002 {published data only}
- Osendarp SJ, Santosham M, Black RE, Wahed MA, Raaij JM, Fuchs GJ. Effect of zinc supplementation between 1 and 6 months of life on growth and morbidity of Bangladeshi infants in urban slums. American Journal of Clinical Nutrition 2002;76(6):1401‐8. [DOI] [PubMed] [Google Scholar]
Osendarp 2007 {published data only}
- Osendarp SJM, Prabhakar H, Fuchs GJ, Raaij JMA, Mahmud H, Tofail F, et al. Immunization with the heptavalent pneumococcal conjugate vaccine in Bangladeshi infants and effects of zinc supplementation. Vaccine 2007;25(17):3347‐54. [DOI] [PubMed] [Google Scholar]
Rahman 2002 {published data only}
- Rahman MM, Tofail F, Wahed MA, Fuchs GJ, Bacqui AH, Alvarez JO. Short‐term supplementation with zinc and vitamin A has no significant effect of the growth of undernourished Bangladeshi children. American Journal of Clinical Nutrition 2002;75(1):87‐91. [DOI] [PubMed] [Google Scholar]
- Rahman MM, Vermund SH, Wahed MA, Fuchs GJ, Bacqui AH, Alvarez JO. Simultaneous zinc and vitamin A supplementation in Bangladeshi children: a randomised double blind controlled trial. BMJ 2001;323(7308):314‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Rawer 1987 {published data only}
- Rawer P, Willems WR, Breidenbach T, Guttmann W, Pabst W, Schutterle G. Seroconversion rate, hepatitis B vaccination, hemodialysis, and zinc supplementation. Kidney International. Supplement 1987;22:149‐52. [PubMed] [Google Scholar]
Richard 2006 {published data only}
- Richard SA, Zavaleta N, Caulfield LE, Black RE, Witzig RS, Shankar AH. Zinc and iron supplementation for malaria, diarrhea, and respiratory infections in children in the Peruvian Amazon. American Journal of Tropical Medicine and Hygiene 2006;75(1):126‐32. [DOI] [PubMed] [Google Scholar]
Rivera 1998 {published data only}
- Rivera JA, Ruel MT, Santizo MC, Lonnerdal B, Brown KH. Zinc supplementation improves the growth of stunted rural Guatemalan infants. Journal of Nutrition 1998;128(3):556‐62. [DOI] [PubMed] [Google Scholar]
Rosado 1997 {published data only}
- Rosado JL, Lopez P, Munoz E, Martinez H, Allen LH. Zinc supplementation reduced morbidity, but neither zinc nor iron supplementation affected growth or body composition of Mexican preschoolers. American Journal of Clinical Nutrition 1997;65(1):13‐9. [DOI] [PubMed] [Google Scholar]
Roy 1997 {published data only}
- Roy SK, Tomkins AM, Akramuzzaman SM, Behrens RH, Haider R, Mahalanabis D, et al. Randomised controlled trial of zinc supplementation in malnourished Bangladeshi children with acute diarrhoea. Archives of Disease in Childhood 1997;77(3):196‐200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roy 1999 {published data only}
- Roy SK, Tomkins AM, Haider R, Behren RH, Akramuzzan SM, Mahalanabis D, et al. Impact of zinc supplementation on subsequent growth and morbidity in Bangladeshi children with acute diarrhoea. European Journal of Clinical Nutrition 1999;53(7):529‐34. [DOI] [PubMed] [Google Scholar]
Roy 2007 {published data only}
- Roy SK, Tomkins AM, Akramuzzaman SM, Chakroborty B, Ara G, Biswas R, et al. Impact of zinc supplementation on subsequent morbidity and growth in Bangladeshi children with persistent diarrhoea. Journal of Health, Population and Nutrition 2007;25(1):67‐74. [PMC free article] [PubMed] [Google Scholar]
Roy 2008a {published data only}
- Roy SK, Hossain MJ, Khatun W, Chakraborty B, Chowdhury A, Begum A, et al. Zinc supplementation in children with cholera in Bangladesh: randomised controlled trial. BMJ 2008;336(7638):266‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Roy 2008b {published data only}
- Roy SK, Raqib R, Khatun W, Azim T, Chowdhury R, Fuchs GJ, et al. Zinc supplementation in the management of shigellosis in malnourished children in Bangladesh. European Journal of Clinical Nutrition 2008;62(7):849‐55. [DOI] [PubMed] [Google Scholar]
Ruel 1997 {published data only}
- Ruel MT, Rivera JA, Santizo MC, Lonnerdal B, Brown KH. Impact of zinc supplementation on morbidity from diarrhoea and respiratory infections among rural Guatemalan children. Pediatrics 1997;99(6):808‐13. [DOI] [PubMed] [Google Scholar]
Ruz 1997 {published data only}
- Ruz M, Castillo‐Duran C, Lara X, Codoceo J, Rebolledo A, Atalah E. A 14‐month zinc‐supplementation trial in apparently healthy Chilean preschool children. American Journal of Clinical Nutrition 1997;66(6):1406‐13. [DOI] [PubMed] [Google Scholar]
Sachdev 1990 {published data only}
- Sachdev HP, Mittal NK, Yadav HS. Oral zinc supplementation in persistent diarrhoea in infants. Annals of Tropical Paediatrics 1990;10(1):63‐9. [DOI] [PubMed] [Google Scholar]
Safai‐Kutti 1990 {published data only}
- Safai‐Kutti S. Oral zinc supplementation in anorexia nervosa. Acta Psychiatrica Scandinavica Supplementum 1990;36:114‐7. [PubMed] [Google Scholar]
Safai‐Kutti 1991 {published data only}
- Safai‐Kutti S, Selin E, Larsson S, Jagenburg R, Denfors I, Sten G, et al. Zinc therapy in children with cystic fibrosis. Beitrage Zur Infusionstherapie (Contributions to Infusion Therapy) 1991;27:104‐14. [PubMed] [Google Scholar]
Samman 1987 {published data only}
- Samman S, Roberts DC. The effect of zinc supplements on plasma zinc and copper levels and the reported symptoms in healthy volunteers. Medical Journal of Australia 1987;146(5):246‐9. [DOI] [PubMed] [Google Scholar]
Sancho Martinez 2007 {published data only}
- Sancho Martinez A, Saenz De Pipaon Marcos M, Quero Jimenez J. Effects of zinc supplementation in the first year of life. Revista Espanola de Pediatria 2007;63(6):464‐82. [Google Scholar]
Sazawal 1995 {published data only}
- Sazawal S, Black RE, Bhan MK, Bhandari N, Sinha A, Jalla S. Zinc supplementation in young children with acute diarrhoea in India. New England Journal of Medicine 1995;333(13):839‐44. [DOI] [PubMed] [Google Scholar]
Sazawal 1996 {published data only}
- Sazawal S, Black RE, Bhan MK, Jalla A, Sinha A, Bhandari N. Efficacy of zinc supplementation in reducing the incidence and prevalence of acute diarrhea: a community‐based, double‐blind, controlled trial. American Journal of Clinical Nutrition 1997;66(2):413‐8. [DOI] [PubMed] [Google Scholar]
- Sazawal S, Black RE, Bhan MK, Jalla S, Bhandari N, Sinha A, et al. Zinc supplementation reduces the incidence of persistent diarrhea and dysentery among low socioeconomic children in India. Journal of Nutrition 1996;126(2):443‐8. [DOI] [PubMed] [Google Scholar]
- Sazawal S, Black RE, Jalla S, Mazumder S, Sinha A, Bhan MK. Zinc supplementation reduces the incidence of acute lower respiratory infections in infants and pre‐school children: a double‐blind, controlled trial. Pediatrics 1998;102(1 Pt 1):1‐5. [DOI] [PubMed] [Google Scholar]
- Sazawal S, Jalla S, Mazumder S, Sinha A, Black RE, Bhan MK. Effect of zinc supplementation on cell‐mediated immunity and lymphocyte subsets in preschool children. Indian Pediatrics 1997;34(7):589‐97. [PubMed] [Google Scholar]
Sazawal 2001 {published data only}
- Sazawal S, Black RE, Menon VP, Dinghra P, Caulfield LE, Dhingra U, et al. Zinc supplementation in infants born small for gestational age reduces mortality: a prospective, randomized, controlled trial. Pediatrics 2001;108(6):1280‐6. [DOI] [PubMed] [Google Scholar]
Sazawal 2007 {published data only}
- Sazawal S, Black RE, Ramsan M, Chwaya HM, Dutta A, Dhingra U, et al. Effect of zinc supplementation on mortality in children aged 1‐48 months; a community‐based randomised placebo‐controlled trial. Lancet 2007;369(9565):927‐34. [DOI] [PubMed] [Google Scholar]
Sempertegui 2014 {published data only}
- Sempertegui F, Estrella B, Rodriguez Gomez D, Cabezas M, Salgado G, Sabin LL, et al. Zinc as an adjunct to the treatment of severe pneumonia in Ecuadorian children: a randomized controlled trial. American Journal of Clinical Nutrition 2014;99:497‐505. [DOI] [PubMed] [Google Scholar]
Shah 2012 {published data only}
- Shah GS, Dutta AK, Shah D, Mishra OP. Role of zinc in severe pneumonia: a randomized double bind placebo controlled study. Italian Journal of Pediatrics 2012;38:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
Shah 2013 {published data only}
- Shah UH, Abu‐Shaheen AK, Malik MA, Alam S, Riaz M, Al‐Tannir MA. The efficacy of zinc supplementation in young children with acute lower respiratory infections: a randomized double‐blind controlled trial. Clinical Nutrition 2013;32:193‐9. [DOI] [PubMed] [Google Scholar]
Shankar 2000 {published data only}
- Shankar AH, Genton B, Baisor M, Paino J, Tamja S, Adiguma T, et al. The influence of zinc supplementation on morbidity due to Plasmodium falciparum: a randomised trial in preschool children in Papua New Guinea. American Journal of Tropical Medicine and Hygiene 2000;62(6):663‐9. [DOI] [PubMed] [Google Scholar]
Silva 2006 {published data only}
- Silva AP, Vitolo MR, Zara LF, Castro CF. Effects of zinc supplementation on 1 to 5 year old children. Jornal de Pediatria 2006;82(3):227‐31. [DOI] [PubMed] [Google Scholar]
Simmer 1988 {published data only}
- Simmer K, Khanum S, Carlsson L, Thompson RP. Nutritional rehabilitation in Bangladesh: the importance of zinc. American Journal of Clinical Nutrition 1988;47(6):1036‐40. [DOI] [PubMed] [Google Scholar]
Simmer 1991 {published data only}
- Simmer K, Lort‐Phillips L, James C, Thompson RP. A double‐blind trial of zinc supplementation in pregnancy. European Journal of Clinical Nutrition 1991;45(3):139‐44. [PubMed] [Google Scholar]
Singh 1994 {published data only}
- Singh A, Failla ML, Deuster PA. Exercise‐induced changes in immune function: effects of zinc supplementation. Journal of Applied Physiology 1994;76(6):2298‐303. [DOI] [PubMed] [Google Scholar]
Smith 1999 {published data only}
- Smith JC, Makdani D, Hegar A, Rao D, Douglas LW. Vitamin A and zinc supplementation of preschool children. Journal of the American College of Nutrition 1999;18(3):213‐22. [DOI] [PubMed] [Google Scholar]
Sur 2003 {published data only}
- Sur D, Gupta DN, Mondal SK, Ghosh S, Manna B, Rajendran K, et al. Impact of zinc supplementation on diarrhoeal morbidity and growth pattern of low birth weight infants in Kolkata, India: a randomized, double‐blind, placebo‐controlled, community‐based study. Pediatrics 2003;112(6 Pt 1):1327‐32. [DOI] [PubMed] [Google Scholar]
Thu 1999 {published data only}
- Thu BD, Schultink W, Dillon D, Gross R, Leswara ND, Khoi HH. Effect of daily and weekly micronutrient supplementation on micronutrient deficiencies and growth in young Vietnamese children. American Journal of Clinical Nutrition 1999;69(1):80‐6. [DOI] [PubMed] [Google Scholar]
Tielsch 2007 {published data only}
- Tielsch JM, Khatry SK, Stoltzfus RJ, Katz J, LeClerq SC, Adhikari R, et al. Effect of daily zinc supplementation on child mortality in southern Nepal: a community‐based, cluster‐randomised, placebo‐controlled trial. Lancet 2007;370(9594):1230‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Trevor 2011 {published data only}
- Trevor D. Zinc sulphate for treatment and prevention of diarrhoea and other conditions in children in Papua New Guinea. Papua New Guinea Medical Journal 2011;54:17‐22. [PubMed] [Google Scholar]
Udomkesmalee 1992 {published data only}
- Udomkesmalee E, Dhanamitta S, Sirisinha S, Charokiatkul S, Tuntipopipat S, Banjong O, et al. Effect of vitamin A and zinc supplementation on the nutriture of children in Northeast Thailand. American Journal of Clinical Nutrition 1992;56(1):50‐7. [DOI] [PubMed] [Google Scholar]
Valery 2005 {published data only}
- Valery PC, Torzillo PJ, Boyce NC, White AV, Stewart PA, Wheaton GP, et al. Zinc and vitamin A supplementation in Australian Indigenous children with acute diarrhoea: a randomised controlled trial. Medical Journal of Australia 2005;182(10):530‐5. [DOI] [PubMed] [Google Scholar]
Van Horn 2003 {published data only}
- Horn L, Obarzanek E, Barton BA, Stevens BJ, Kwiterovich PO, Lasser NL, et al. A summary of results of the Dietary Intervention Study in Children (DISC): lessons learned. Progress in Cardiovascular Nursing 2003;18(1):28‐41. [DOI] [PubMed] [Google Scholar]
Vasudevan 1997 {published data only}
- Vasudevan A, Shendurnikar N, Kotecha PV. Zinc supplementation in severe malnutrition. Indian Pediatrics 1997;34(3):236‐8. [PubMed] [Google Scholar]
Veverka 2009 {published data only}
- Veverka DV, Wilson C, Martinez MA, Wenger R, Tamosuinas A. Use of zinc supplements to reduce upper respiratory infections in United States Air Force Academy cadets. Complementary Therapies in Clinical Practice 2009;15:91‐5. [DOI] [PubMed] [Google Scholar]
Wadhwa 2013 {published data only}
- Wadhwa N, Chandran A, Aneja S, Lodha R, Kabra S, Chaturvedi M, et al. Efficacy of zinc given as an adjunct in the treatment of severe and very severe pneumonia in hospitalized children 2‐24 mo of age: a randomized, double‐blind, placebo‐controlled trial. American Journal of Clinical Nutrition 2013;97:1387‐94. [DOI] [PubMed] [Google Scholar]
Walravens 1976 {published data only}
- Walravens PA, Hambridge KM. Growth of infants fed a zinc supplemented formula. American Journal of Clinical Nutrition 1976;29(10):1114‐21. [DOI] [PubMed] [Google Scholar]
Walravens 1989 {published data only}
- Walravens PA, Hambridge KM, Koepfer DM. Zinc supplementation in infants with a nutritional pattern of failure to thrive: a double‐blind, controlled study. Pediatrics 1989;83(4):532‐8. [PubMed] [Google Scholar]
Walravens 1992 {published data only}
- Walravens PA, Chakar A, Mokni R, Denise J, Lemonnier D. Zinc supplements in breastfed infants. Lancet 1992;340(8821):683‐5. [DOI] [PubMed] [Google Scholar]
Wasantwisut 2006 {published data only}
- Wasantwisut E, Winichagoon P, Chitchumroonchokchai C, Yamborisut U, Boonpraderm A, Pongcharoen T, et al. Iron and zinc supplementation improved iron and zinc status, but not physical growth, of apparently healthy, breastfed infants in rural communities of northeast Thailand. Journal of Nutrition 2006;136(9):2405‐11. [DOI] [PubMed] [Google Scholar]
Wazewska‐Czyzewska 1978 {published data only}
- Wazewska‐Czyzewska M, Wesierska‐Gadek J, Legutko L. Immunostimulatory effect of zinc in patients with acute lymphoblastic leukaemia. Folia Haematologica 1978;105(6):727‐32. [PubMed] [Google Scholar]
Weismann 1978 {published data only}
- Weismann K, Wanscher B, Krakauer R. Oral zinc therapy in geriatric patients with selected skin manifestations and low plasma zinc level. Acta Dermato‐Venereologica 1978;58(2):157‐61. [PubMed] [Google Scholar]
Weismann 1979 {published data only}
- Weismann K, Christensen E, Dreyer V. Zinc supplementation in alcoholic cirrhosis. A double‐blind clinical trial. Acta Medica Scandinavica 1979;205(5):361‐6. [DOI] [PubMed] [Google Scholar]
Wieringa 2007 {published data only}
- Wieringa FT, Berger J, Dijkhuizen MA, Hidayat A, Ninh NX, Utomo B, et al. Combined iron and zinc supplementation in infants improved iron and zinc status, but interactions reduced efficacy in a multicountry trial in southeast Asia. Journal of Nutrition 2007;137(2):466‐71. [DOI] [PubMed] [Google Scholar]
Yang 2002 {published data only}
- Yang YX, Han JH, Shao XP, He M, Bian LH, Wang Z, et al. Effect of micronutrient supplementation in preschool children in China. Biomedical and Environmental Sciences 2002;15(3):196‐202. [PubMed] [Google Scholar]
Zeba 2008 {published data only}
- Zeba AN, Sorgho H, Rouamba N, Zongo I, Rouamba J, Guiguemde RT, et al. Major reduction in malaria morbidity with combined vitamin A and zinc supplementation in young children in Burkina Faso: a randomized double blind trial. Nutrition Journal 2008;7:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Zemel 2002 {published data only}
- Zemel BS, Kawchak DA, Fung EB, Ohene‐Frempong K, Stallings VA. Effect of zinc supplementation on growth and body composition in children with sickle‐cell disease. American Journal of Clinical Nutrition 2002;75(2):300‐7. [DOI] [PubMed] [Google Scholar]
Additional references
Bhutta 2004
- Bhutta ZA. The role of zinc in child health in developing countries: taking the science where it matters. Indian Pediatrics 2004;41:429‐33. [PubMed] [Google Scholar]
Bondestam 1985
- Bondestam M, Foucard T, Gebre‐Mehdin M. Subclinical trace element deficiency in children with undue susceptibility to infections. Acta Paediatrica Scandinavica 1985;74(4):515‐20. [DOI] [PubMed] [Google Scholar]
Brown 2009
- Brown KH, Peerson JM, Baker SK, Hess SY. Preventive zinc supplementation among infants, preschoolers, and older prepubertal children. Food and Nutrition Bulletin 2009;30(Suppl 1):1‐40. [DOI] [PubMed] [Google Scholar]
Caulfield 2004
- Caulfield L, Black RE. Chapter 5: Zinc deficiency. In: Ezzata M, Lopez AD, Rodger A, Murray CJL editor(s). Comparative Quantification of Health Risks: Global and Regional Burden of Disease Attributable to Selected Major Risk Factors. Geneva: World Health Organization, 2004:262‐3. [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Robberstad 2004
- Robberstad B, Strand T, Black RE, Sommerfelt H. Cost‐effectiveness of zinc as adjunct therapy for acute childhood diarrhoea in developing countries. Bulletin of the World Health Organization 2004;82(7):523‐31. [PMC free article] [PubMed] [Google Scholar]
Walker 2004
- Walker CF, Black RE. Zinc and risk for infectious diseases. Annual Review of Nutrition 2004;24:255‐75. [DOI] [PubMed] [Google Scholar]
WHO 2004
- World Health Organization. Chapter 1: Global burden of disease due to chronic suppurative otitis media: disease, deafness, deaths and DALYs. Chronic Suppurative Otitis Media: Burden of Illness and Management Options. Geneva: World Health Organization, 2004. [Google Scholar]
WHO/UNICEF 2004
- WHO/UNICEF. Clinical management of acute diarrhoea: WHO/UNICEF joint statement. www.who.int/maternal_child_adolescent/documents/who_fch_cah_04_7/en/index.html (accessed 23 February 2012) 2004.
Yakoob 2011
- Yakoob MY, Theodoratou E, Jameen A, Imdad A, Eiseley TP, Ferguson J, et al. Preventive zinc supplementation in developing countries: impact on mortality and morbidity due to diarrhea, pneumonia and malaria. BMC Public Health 2011;11(Suppl 3):23. [DOI] [PMC free article] [PubMed] [Google Scholar]
ZICG 1999
- Zinc Investigators' Collaborative Group. Prevention of diarrhea and pneumonia by zinc supplementation in children in developing countries: pooled analysis of randomized controlled trials. Journal of Paediatrics 1999;135:689‐97. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Abba 2010
- Abba K, Gulani A, Sachdev HS. Zinc supplements for preventing otitis media. Cochrane Database of Systematic Reviews 2010, Issue 2. [DOI: 10.1002/14651858.CD006639.pub2] [DOI] [PubMed] [Google Scholar]
Gulani 2012
- Gulani A, Sachdev HS. Zinc supplements for preventing otitis media. Cochrane Database of Systematic Reviews 2012, Issue 4. [DOI: 10.1002/14651858.CD006639.pub3] [DOI] [PubMed] [Google Scholar]


