Abstract
Background
Weight retention after pregnancy may contribute to obesity. It is known that diet and exercise are recommended components of any weight loss programme in the general population. However, strategies to achieve healthy body weight among postpartum women have not been adequately evaluated.
Objectives
The objectives of this review were to evaluate the effect of diet, exercise or both for weight reduction in women after childbirth, and to assess the impact of these interventions on maternal body composition, cardiorespiratory fitness, breastfeeding performance and other child and maternal outcomes.
Search methods
We searched the Cochrane Pregnancy and Childbirth Group's Trials Register (31 January 2012) and LILACS (31 January 2012). We scanned secondary references and contacted experts in the field. We updated the search of the Cochrane Pregnancy and Childbirth Group's Trials Register on 30 April 2013 and added the results to the awaiting classification section of the review.
Selection criteria
All published and unpublished randomised controlled trials (RCTs) and quasi‐randomised trials of diet or exercise or both, among women during the postpartum period.
Data collection and analysis
Both review authors independently assessed trial quality and extracted data. Results are presented using risk ratio (RR) for categorical data and mean difference (MD) for continuous data. Data were analysed with a fixed‐effect model. A random‐effects model was used in the presence of heterogeneity.
Main results
Fourteen trials were included, but only 12 trials involving 910 women contributed data to outcome analysis. Women who exercised did not lose significantly more weight than women in the usual care group (two trials; n = 53; MD ‐0.10 kg; 95% confidence interval (CI) ‐1.90 to 1.71). Women who took part in a diet (one trial; n = 45; MD ‐1.70 kg; 95% CI ‐2.08 to ‐1.32), or diet plus exercise programme (seven trials; n = 573; MD ‐1.93 kg; 95% CI ‐2.96 to ‐0.89; random‐effects, T² = 1.09, I² = 71%), lost significantly more weight than women in the usual care group. There was no difference in the magnitude of weight loss between diet alone and diet plus exercise group (one trial; n = 43; MD 0.30 kg; 95% CI ‐0.06 to 0.66). The interventions seemed not to affect breastfeeding performance adversely.
Authors' conclusions
Evidence from this review suggests that both diet and exercise together and diet alone help women to lose weight after childbirth. Nevertheless, it may be preferable to lose weight through a combination of diet and exercise as this improves maternal cardiorespiratory fitness and preserves fat‐free mass, while diet alone reduces fat‐free mass. This needs confirmation in large trials of high methodological quality. For women who are breastfeeding, more evidence is required to confirm whether diet or exercise, or both, is not detrimental for either mother or baby.
Plain language summary
Diet or exercise, or both, for weight reduction in women carrying excess weight after childbirth
Women naturally gain weight during pregnancy and many gradually lose it afterwards. Some women, though, find it difficult to lose the gained weight in the year or two following the birth of the baby and there is concern that this may be a health risk for them. The retention of weight gained during pregnancy may contribute to obesity, which can increase the risk of diabetes, heart disease and high blood pressure. It is suggested that women who return to their pre‐pregnancy weight by about six months have a lower risk of being overweight 10 years later. The review looked for randomised studies to assess the impact of dieting or exercise, or both, on women's weight loss in the months after giving birth. It paid particular attention to breastfeeding women to be sure that breastfeeding was not compromised. The review of trials found 14 studies, with 12 studies involving 910 women carrying excess weight after childbirth that contributed data for analysis. The findings suggest that diet combined with exercise or diet alone compared with usual care seemed to help with weight loss after giving birth. There is potential for these interventions to play a role in preventing future maternal obesity. There was not sufficient evidence to be sure that exercise or diet did not interfere with breastfeeding though it appeared not to in the included studies. It seems preferable to lose weight through a combination of dieting and exercise, compared to dieting alone, because exercise is thought to improve circulation and heart fitness, and to preserve lean body mass. Further research is needed.
Background
Description of the condition
Obesity related to childbearing
There is evidence suggesting that retention of weight gained during pregnancy contributes to female overweight and obesity (Gore 2003; Linne 2002; Linne 2003a; Rooney 2002). In women, being overweight or obese substantially raises the risk of serious diet‐related chronic disorders, including diabetes mellitus, heart disease and hypertension (Linne 2004; Manson 1990).
Postpartum weight retention
The weight retained after pregnancy is defined as the difference between postpartum and prepregnancy weight (IOM 1990). The Health Sciences Descriptor of Virtual Health Library states that postpartum or puerperium is "a period from delivery of the placenta until return of the reproductive organs to their normal non‐pregnant morphologic state. In humans, the puerperium generally lasts for six to eight weeks" (DeCs 2004). However, it is recommended to increase the definition of the postpartum period to one year, because many physiologic changes due to pregnancy remain up to one year after childbirth, such as the duration of breastfeeding (Mottola 2002).
Despite growing concern about weight‐related problems among postpartum women, neither a cut‐off point defining excess weight retention after childbirth, nor an ideal time to return to prepregnancy weight has been established in the literature. Linne et al carried out a study, which aimed to examine long‐term weight development after pregnancy in a 15‐year follow‐up study. The authors found that by six months postpartum, 56.3% of women who did not become overweight at 15‐year follow‐up had returned to within 1.5 kg of their prepregnancy weight, compared to 27.7% of whom became overweight. By one year, these figures had risen to 60.4% in the non‐overweight women and only 34.6% in the overweight group (Linne 2003b). Rooney and Schauberger reported that women who lost all pregnancy weight by six months postpartum, regardless of breastfeeding status, were only 2.4 kg heavier 10 years after childbirth, while women who retained postpartum weight were 8.3 kg heavier at 10‐year follow‐up. The authors argued that failure to lose pregnancy weight by six months postpartum is considered an important predictor of long‐term obesity. Although it seems beneficial that women return to pregestational weight by six months after childbirth, only 37% of women were able to lose the weight gained during pregnancy at this point (Rooney 2002). Studies estimated that, about one year after childbirth, women may retain 0.5 to 4.0 kg on average (AbuSabha 1998; Keppel 1993; Linne 2002; Linne 2003c; Ohlin 1990; Olson 2003). The average amount of weight retained as a result of pregnancy is relatively small; however, there is a subset of women that seems to be at greater risk of gaining significant amounts of weight with childbearing (Rossner 1992; Rossner 1995). In longitudinal studies, the proportion of women retaining 4.5 kg or more during postpartum ranges from 14% to 25% (Greene 1988; Olson 2003; Rossner 1995; Schauberger 1992). Women who retain a considerable amount of weight after delivery have a higher risk of doing so in subsequent gestations (Linne 2003c).
Postpartum weight retention might be determined by many factors, including low socio‐economic status, parity and high prepregnancy body mass index (BMI) (Crowell 1995; Schauberger 1992). However, excessive weight gain during pregnancy is the strongest predictor of postpartum weight retention. Various studies showed that the greater the gestational weight gain, the greater the postpartum weight retention (Gunderson 1999; Kac 2003; Linne 2003c; Rossner 1995). According to Olson et al, lower income women who gain more weight in pregnancy than the Institute of Medicine (IOM) recommends are at high risk for major gain with further childbearing (Olson 2003). Apart from that, the postpartum period might be related to an increase in food intake and a decrease in physical activity (Clark 1999; Sadurkis 1988; Symons Downs 2004). Consequently, it is considered a vulnerable period for gaining weight (Leermakers 1998). Thus, although gestational weight gain has a strong correlation with postpartum weight retention, gaining additional weight after delivery may also have a significant role in maternal obesity (Greene 1988).
Description of the intervention
Diet and exercise among breastfeeding women
Observational studies have demonstrated that long‐term and severe under‐nutrition was associated with milk volume reduction and lower nutrient concentration, whereas mild under‐nourishment had a weak correlation with change in milk volume and composition. These results suggest that when food intake is limited for a short period of time, maternal prolactin concentration level increases, which appears to ensure milk production (Coward 1984; Prentice 1994). However, the findings of dietary intervention studies are controversial. While some studies suggested that a calorie‐restricted diet had no impact on milk quantity and quality (Dusdieker 1994; McCrory 1999), other research reported that well‐nourished mothers who had consumed less than 1500 kcal/day experienced a decrease in milk volume and put the growth rate of their babies at risk (Strode 1986).
Likewise, the effect of exercise during postpartum in relation to lactation performance is still a contentious issue. Some trials, including exclusively breastfeeding mothers, indicated that exercise performed during postpartum had no adverse effect on lactation (Dewey 1994b; Lovelady 1995). Nevertheless, another study aimed to observe the infant acceptance of postexercise breast milk demonstrated a significant difference in acceptance of pre‐exercise and postexercise milk. Women had a significant increase in lactic acid levels in breast milk collected at 10 minutes and 30 minutes after the exercise period. The increase in lactic acid levels might affect milk palatability, making it have a sour taste that babies disliked. Furthermore, the lactic acid may have a degradative effect on milk immunoglobulin A concentration (Wallace 1992b), an important factor which confers protection against most infectious agents (Mestecky 1986).
Apart from the effect of postpartum weight loss programmes on lactation performance, it is important to examine the changes in maternal body composition imposed by different intervention strategies. It is desirable that women reduce the percentage of body fat and increase or preserve their lean mass during the intervention programme (Wood 2004). In order to identify which intervention optimises weight loss and fat reduction, while preserving or enhancing fat‐free mass, the results of some experimental studies should be pooled in a systematic manner.
How the intervention might work
Returning to prepregnancy weight
Although it is expected that breastfeeding women lose weight gradually, findings related to breastfeeding and postpartum weight loss are inconsistent (Crowell 1995; Schauberger 1992). Decline in physical activity and increase in caloric intake above the ordinary demand of lactation may explain why some breastfeeding women fail to return to prepregnancy weight. It is argued that the Recommended Dietary Allowance (RDA) for breastfeeding women is too high, and the need for increased calories for milk production may be offset by the reduction in physical activity and basal metabolic rate in breastfeeding women (Crowell 1995). Since behavioural change may also explain why some women fail to lose pregnancy‐related weight or gain additional weight, or both, in the first postpartum year (Olson 2003; Schauberger 1992), postpartum weight loss seems to be a critical issue for women who were overweight or obese before pregnancy. However, help strategies for returning to prepregnancy weight are also important for normal‐weight women who gained excessive weight during pregnancy.
Crowell highlights that a period of at least six months postpartum is necessary to facilitate weight loss with the purpose of helping women to return to prepregnancy weight without posing any risk to maternal and child health (Crowell 1995). Even though the IOM states that gradual weight loss during lactation (0.5 kg/week) appears safe for overweight women (IOM 1991), the best strategy in achieving postpartum weight reduction and the effect of high weight loss rate has not been critically evaluated.
It is known that diet and exercise impose energy deficit, therefore, they are recommended components of any weight loss programme in the general population (WHO 1998). Nonetheless, the effects of negative energy balance during the postpartum period, achieved by energy restriction intake, increased energy expenditure or the combination of both are still not fully understood. Since the growth rate of exclusively breastfed infants depends on the energy provided by maternal breast milk, it is paramount to assess the impact of diet and exercise on lactation performance (Wood 2004).
Why it is important to do this review
The diversity in magnitude of weight loss, body composition and effects on lactation performance found in the literature may be as a result of different study designs, selection criteria of control groups, sample sizes, type of participants and intervention strategies, duration of follow‐up, drop‐out rates and quality of weight measurements. Before the results of such studies can be applied in a clinical setting by healthcare professionals to determine an appropriated prescription of diet or exercise, or both, for postpartum women, these data must be selected using high‐quality criteria and summarised in an objective fashion.
Objectives
The primary objective of this review was to evaluate the effect of diet, exercise or both for weight reduction in women carrying excess weight after childbirth. Secondary objectives were to examine the impact of these interventions on maternal body composition; breastfeeding performance; cardiorespiratory fitness; infant weight gain and growth; and other child and maternal outcomes.
Methods
Criteria for considering studies for this review
Types of studies
We considered for inclusion randomised controlled trials and quasi‐randomised trials of diet or exercise or both, with a concurrent comparison group, in women during the postpartum period.
Types of participants
To be eligible, studies must have included women recruited to the intervention programme up to 24 months after childbirth. The participants were women who had given birth to a singleton healthy term infant; were aged at least 18 years; and were overweight or obese, or had gained excessive weight during pregnancy, or both. Normal‐weight women were eligible if, during pregnancy, they had gained weight above the IOM's recommendations or whose current weight had significantly exceeded their prepregnancy weight. Women who were underweight before pregnancy were not included. Participants were required to not be taking any medication that significantly interfered with body weight. There was no restriction in relation to maternal breastfeeding status.
Types of interventions
We considered interventions in postpartum women involving diet or exercise, or both.
The nutritional interventions included in this review were: (a) dietary advice intended to produce weight reduction delivered through group meetings, by telephone calls or by mail correspondence; (b) individualised dietary counselling; (c) prescription of a calorie‐restricted diet.
Exercise interventions included in this review were: (a) any type of exercise counselling that encouraged women to engage in regular recreational exercises (for example, walking, jogging, sports) in order to promote weight loss or improve physical fitness; (b) structured/individualised exercise programmes or interventions in which women participated in supervised exercise sessions.
We did not consider training programmes with exercise for preventing or treating pelvic or back pain and urinary incontinence. We included trials in which the stated objectives were not weight loss only if they involved one of the interventions mentioned above and assessed at least one relevant outcome measure.
There was no restriction concerning who delivered the interventions. Type, intensity, frequency, duration and timing (postpartum period at beginning and end) of the interventions varied between studies. Trial duration was defined according to the numbers of months over which each was conducted: short term (less than three months), medium term (from three to six months) and long term (longer than six months). Frequency, intensity, duration and timing of the intervention were extracted from the reports and described in the Characteristics of included studies table. We did not consider any type of intervention in combination with medication in this review.
Comparisons
Diet versus usual care;
exercise versus usual care;
diet plus exercise versus usual care;
diet versus exercise;
diet plus exercise versus exercise alone;
diet plus exercise versus diet alone.
Types of outcome measures
Primary outcomes
Change in body weight (kg), defined as body weight at the end minus body weight at the beginning of study (negative change implies postpartum weight loss);
percentage of women who returned to prepregnancy weight or lost weight retained after childbirth;
percentage of women who achieved healthy weight, according to WHO 1998 definitions (based on BMI classification) or weight loss of clinical significance (reduction of 5% of initial body weight).
Secondary outcomes
Change in percentage of body fat (%);
change in fat‐free mass (kg);
change in cardiorespiratory fitness (VO2 max, mL/kg/minute);
change in basal plasma prolactin concentration (µg/mL);
change in milk volume (g/day);
milk immunoglobulin (Ig) A concentration (µg/mL);
number of mothers who stop breastfeeding;
duration of breastfeeding in months (exclusive or predominant, according to WHO 1991 definitions);
percentage of partial or exclusive breastfeeding by the end of the intervention;
infant length gain (cm);
infant weight gain (g);
maternal morbidity (for example, anaemia, readmission to hospital);
adverse events (for example, exercise‐induced injuries, side effects of very low‐calorie diets);
maternal satisfaction with interventions;
compliance with interventions.
We gathered information on outcome measures related to milk volume, plasma prolactin concentration and infant length and weight gain only from trials which included exclusively lactating women.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Pregnancy and Childbirth Group’s Trials Register by contacting the Trials Search Co‐ordinator (31 January 2012). We updated this on 30 April 2013 and added the results to Studies awaiting classification.
The Cochrane Pregnancy and Childbirth Group’s Trials Register is maintained by the Trials Search Co‐ordinator and contains trials identified from:
monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL);
weekly searches of MEDLINE;
weekly searches of EMBASE;
handsearches of 30 journals and the proceedings of major conferences;
weekly current awareness alerts for a further 44 journals plus monthly BioMed Central email alerts.
Details of the search strategies for CENTRAL, MEDLINE and EMBASE, the list of handsearched journals and conference proceedings, and the list of journals reviewed via the current awareness service can be found in the ‘Specialized Register’ section within the editorial information about the Cochrane Pregnancy and Childbirth Group.
Trials identified through the searching activities described above are each assigned to a review topic (or topics). The Trials Search Co‐ordinator searches the register for each review using the topic list rather than keywords.
In addition, we searched LILACS (1983 to 31 January 2012) using the search strategy detailed in Appendix 1.
Searching other resources
We searched the citation lists of relevant publications, review articles and included studies. After the identification of studies, the primary author contacted some experts in the field via electronic mail. The list of potential included trials was sent to them. They were asked if they were aware of additional trials, published, unpublished or ongoing, that have been conducted in this area (postpartum weight loss).
We did not apply any language restrictions.
Data collection and analysis
For the methods used when assessing the trials identified in the previous version of this review, seeAppendix 2.
For this update we used the following methods when assessing the trials identified by the updated search.
Selection of studies
Two review authors independently assessed for inclusion all the potential studies identified as a result of the search strategy. Any disagreement was resolved through discussion.
Data extraction and management
A form to extract data was designed. For eligible studies, two review authors extracted the data using the agreed form. We resolved differences in data extraction by consensus, referring back to the original article. The data were entered into Review Manager software (RevMan 2011) and checked for accuracy.
When information regarding any of the above was unclear, we attempted to contact authors of the original reports to provide further details.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias for each study using the criteria outlined in theCochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). We resolved any disagreement by discussion.
(1) Random sequence generation (checking for possible selection bias)
We described for each included study the method used to generate the allocation sequence in sufficient detail to allow an assessment of whether it should produce comparable groups.
We assessed the method as:
low risk of bias (any truly random process, e.g. random number table; computer random number generator);
high risk of bias (any non‐random process, e.g. odd or even date of birth; hospital or clinic record number);
unclear risk of bias.
(2) Allocation concealment (checking for possible selection bias)
We described for each included study the method used to conceal allocation to interventions prior to assignment and assessed whether intervention allocation could have been foreseen in advance of, or during recruitment, or changed after assignment.
We assessed the methods as:
low risk of bias (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes);
high risk of bias (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);
unclear risk of bias.
(3) Blinding of outcome assessment (checking for possible detection bias)
Double blinding was impossible in these kinds of trials, as the participants knew which intervention they received. Therefore, we only considered blinding of outcome assessment. We described for each included study the methods used, if any, to blind outcome assessors from knowledge of which intervention a participant received. We assessed blinding separately for different outcomes or classes of outcomes.
We assessed methods used to blind outcome assessment as:
low, high or unclear risk of bias.
(4) Incomplete outcome data (checking for possible attrition bias due to the amount, nature and handling of incomplete outcome data)
We described for each included study, and for each outcome or class of outcomes, the completeness of data including attrition and exclusions from the analysis. We stated whether attrition and exclusions were reported and the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported, or was supplied by the trial authors, we re‐included missing data in the analyses which we undertook.
We assessed methods as:
low risk of bias (e.g. no missing outcome data; less than 20% of withdrawal or loss to follow‐up, missing outcome data balanced across groups);
high risk of bias (e.g. numbers or reasons for missing data imbalanced across groups; ‘as treated’ analysis done with substantial departure of intervention received from that assigned at randomisation);
unclear risk of bias.
(5) Selective reporting (checking for reporting bias)
We described for each included study how we investigated the possibility of selective outcome reporting bias and what we found.
We assessed the methods as:
low risk of bias (where it is clear that all of the study’s prespecified outcomes and all expected outcomes of interest to the review have been reported);
high risk of bias (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not prespecified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported);
unclear risk of bias.
(6) Other bias (checking for bias due to problems not covered by (1) to (5) above)
We described for each included study any important concerns we have about other possible sources of bias, such as extreme baseline imbalance between groups, lack of information on source of funding and research protocol published a priori.
We assessed whether each study was free of other problems that could put it at risk of bias:
low risk of other bias;
high risk of other bias;
unclear whether there is risk of other bias.
(7) Overall risk of bias
We made explicit judgements about whether studies were at high risk of bias, according to the criteria given in the Cochrane Handbook (Higgins 2011). With reference to (1) to (6) above, we assessed the likely magnitude and direction of the bias and whether we considered it was likely to impact on the findings. We explored the impact of the level of bias through undertaking sensitivity analyses ‐ seeSensitivity analysis.
Measures of treatment effect
Dichotomous data
For dichotomous data, we presented results as summary risk ratio with 95% confidence intervals.
Continuous data
For continuous data, We used the mean difference if outcomes were measured in the same way across trials. If required, we planned to use the standardised mean difference to combine trials that measured the same outcome, but used different methods.
Unit of analysis issues
Cluster‐randomised trials
We planned to include cluster‐randomised trials in the analyses along with individually‐randomised trials. If we had identified cluster trials, we planned to adjust their sample sizes using the methods described in the Cochrane Handbook (Secions 16.3.4 or 16.3.6) using an estimate of the intra cluster correlation co‐efficient (ICC) derived from the trial (if possible), from a similar trial or from a study of a similar population. If we use ICCs from other sources, we planned to report this and conduct sensitivity analyses to investigate the effect of variation in the ICC. If we had identified both cluster‐randomised trials and individually‐randomised trials, we planned to synthesise the relevant information. We would consider it reasonable to combine the results from both if there was little heterogeneity between the study designs and the interaction between the effect of intervention and the choice of randomisation unit was considered to be unlikely.
We would also acknowledge heterogeneity in the randomisation unit and perform a sensitivity analysis to investigate the effects of the randomisation unit.
Dealing with missing data
For included studies, we noted levels of attrition. We planned to explore the impact of including studies with high levels of missing data in the overall assessment of treatment effect by using sensitivity analysis if such studies were identified.
For all outcomes, we carried out analyses, as far as possible, on an intention‐to‐treat basis, i.e. we attempted to include all participants randomised to each group in the analyses, and all participants were analysed in the group to which they were allocated, regardless of whether or not they received the allocated intervention. The denominator for each outcome in each trial was the number randomised minus any participants whose outcomes were known to be missing.
Assessment of heterogeneity
We assessed statistical heterogeneity in each meta‐analysis using the T², I² and Chi² statistics. We regarded heterogeneity as substantial if the I² was greater than 30% and either the T² was greater than zero, or there was a low P value (less than 0.10) in the Chi² test for heterogeneity.
Assessment of reporting biases
If there had been 10 or more studies in the meta‐analysis, we planned to investigate reporting biases (such as publication bias) using funnel plots for all primary outcomes. We planned to assess funnel plot asymmetry visually. If asymmetry was suggested by a visual assessment, we planned to perform exploratory analyses to investigate it.
Data synthesis
We carried out statistical analysis using the Review Manager software (RevMan 2011). We used fixed‐effect meta‐analysis for combining data where it was reasonable to assume that studies were estimating the same underlying treatment effect: i.e. where trials were examining the same intervention, and the trials’ populations and methods were judged sufficiently similar. If there was clinical heterogeneity sufficient to expect that the underlying treatment effects differ between trials, or if substantial statistical heterogeneity was detected, we used random‐effects meta‐analysis to produce an overall summary if an average treatment effect across trials was considered clinically meaningful. The random‐effects summary was treated as the average of the range of possible treatment effects and we discussed the clinical implications of treatment effects differing between trials. If the average treatment effect was not clinically meaningful, we did not combine the trials.
If we used random‐effects analyses, the results were presented as the average treatment effect with 95% confidence intervals, and the estimates of T² and I².
Subgroup analysis and investigation of heterogeneity
We investigated substantial heterogeneity using subgroup analyses and sensitivity analyses. We considered whether an overall summary was meaningful, and if it was, used a random‐effects analysis to produce it.
We carried out the following subgroup analyses, if sufficient data were available:
dietary advice versus prescription of caloric restriction;
exercise counselling versus structured/individualised exercise programme or supervised exercise sessions;
duration of intervention: short‐term and medium‐term versus long‐term.
The following outcomes will be used in subgroup analysis:
change in body weight;
percentage of women who returned to prepregnancy weight;
percentage of women who achieved healthy weight;
change in percentage of body fat.
We assessed subgroup differences by interaction tests available within RevMan (RevMan 2011).
Sensitivity analysis
If we identified substantial heterogeneity that was not explained by subgroup analyses, we investigated it using sensitivity analyses based on the 'Risk of bias' assessment. We planned to carry out sensitivity analyses to explore the effect of trial quality assessed by concealment of allocation, high attrition rates, or both, with poor quality studies being excluded from the analyses in order to assess whether this made any difference to the overall result.
Results
Description of studies
Results of the search
We found 24 reports of trials which qualified for inclusion in this review. Some papers reported results or description of the same trial. We considered reports by Dewey 1994b, Prentice 1994 and Lovelady 1995, which described the effects of aerobic exercise among women during lactation, as a single study. Likewise, we considered articles by Lovelady 2000, Lovelady 2001, Lovelady 2006 and Mukherjea 2000, which described the effect of energy restriction and exercise among breastfeeding women, as a single study. Furthermore, two or more reports describing the same study were found for Ferrara 2011 (two reports); Kearney 2006 (two reports); Krummel 2010 (two reports) and Ostbye 2009 (three reports). After accounting for duplicate reports of the same study, the review included a total of 14 trials. One article contributed information for three comparison groups: diet versus usual care; diet plus exercise versus usual care; diet plus exercise versus diet alone (McCrory 1999).
We were able to get outcome data for all trials except three. O'Toole et al stated that fat‐free mass was measured, but data were not available in the article (O'Toole 2003). Huang et al and Kearney et al reported postpartum weight retention (weight at the end of the intervention ‐ pre‐gestational weight) instead of postpartum weight loss (weight at the end of the intervention ‐ weight at the beginning of the intervention) (Huang 2011; Kearney 2006). Therefore, these trials did not contribute data to the statistical analysis.
Included studies
The trials were primarily conducted in the United States (Dewey 1994a; Ferrara 2011; Kearney 2006; Krummel 2010; Leermakers 1998; Lovelady 2000; Lovelady 2009; McCrory 1999; O'Toole 2003; Ostbye 2009); two were conducted in Australia (Armstrong 2003; Armstrong 2004); one in the UK (Craigie 2011) and one in Tawian (Huang 2011). Most trials were classified as short‐ and medium‐term studies, and five trials comprised long‐term interventions, ranging from six months to a one‐year long intervention programme (Ferrara 2011; Kearney 2006; Krummel 2010; O'Toole 2003; Ostbye 2009). Although the majority of trials involved a prescription of a calorie‐restricted diet, the trials by Leermakers 1998 and Krummel 2010 involved nutritional education. All trials involved aerobic exercise programmes; four trials were based on supervised exercise sessions (Armstrong 2003; Dewey 1994a; Lovelady 2000; Lovelady 2009); five focused on self‐monitored sessions (Craigie 2011; Krummel 2010; Leermakers 1998; McCrory 1999; O'Toole 2003) and two trials combined supervised exercise sessions with self‐monitored sessions (Armstrong 2004; Ostbye 2009).
The recruitment period ranged from three weeks to 24 months postpartum. Only two trials recruited women during pregnancy (Ferrara 2011; Huang 2011). Four trials included exclusively breastfeeding mothers (Dewey 1994a; Lovelady 2000; Lovelady 2009; McCrory 1999) and seven trials exclusively included women who were overweight/obese after childbirth or who gained excessive weight gain during pregnancy or had high postpartum weight retention (Craigie 2011; Kearney 2006; Leermakers 1998; Lovelady 2000; Lovelady 2009; O'Toole 2003; Ostbye 2009).
Nineteen reports from an updated search in April 2013 have been added to Characteristics of studies awaiting classification and will be assessed at the next update in December 2013.
Excluded studies
Of the 28 excluded reports, four articles were related to the same study by Fahrenwald 2004, and three articles were related to same study by Kinnunen 2007. These articles were considered as a single study, leaving the number of 23 excluded studies.
We found seven ongoing trial. Details for each trial can be found in the following tables: Characteristics of included studies; Characteristics of excluded studies and Characteristics of ongoing studies.
Risk of bias in included studies
Allocation
In nine out of 14 trials, the method of randomisation was adequate (Armstrong 2003; Armstrong 2004; Craigie 2011; Dewey 1994a; Ferrara 2011; Huang 2011; Lovelady 2000; McCrory 1999; Ostbye 2009). In the remaining five trials, it is stated that intervention was randomly assigned, but the method was not reported (Kearney 2006; Krummel 2010; Leermakers 1998; Lovelady 2009; O'Toole 2003). Allocation concealment was adequate in six trials (Armstrong 2003; Armstrong 2004; Dewey 1994a; Lovelady 2000; O'Toole 2003; Ostbye 2009). In the remaining eight trials the allocation process was unreported (Craigie 2011; Ferrara 2011; Huang 2011; Kearney 2006; Krummel 2010; Leermakers 1998; Lovelady 2009; McCrory 1999).
Blinding
Only two trials reported that outcome data were collected by investigators blinded to group allocation (Craigie 2011; Ferrara 2011).
Incomplete outcome data
Follow‐up attrition rates were less than 20% in six trials (Armstrong 2004; Dewey 1994a; Kearney 2006; Lovelady 2000; Lovelady 2009; McCrory 1999).
Selective reporting
All trials, except four, reported all relevant outcomes (Armstrong 2003; Armstrong 2004; Huang 2011; Kearney 2006).
Other potential sources of bias
Nine trials were free of other potential bias, such as extreme baseline imbalance between groups, lack of information on source of funding or research protocol published a priori (Craigie 2011; Dewey 1994a; Kearney 2006; Krummel 2010; Lovelady 2000; Lovelady 2009; McCrory 1999; O'Toole 2003; Ostbye 2009).
Overall, only two trials presented low risk of bias in five out of the six items investigated (Dewey 1994a; Lovelady 2000). Details for each trial can be found in the following figures: Figure 1; Figure 2.
1.
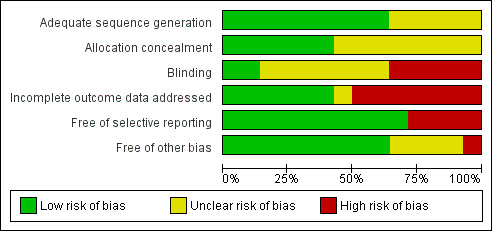
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
2.
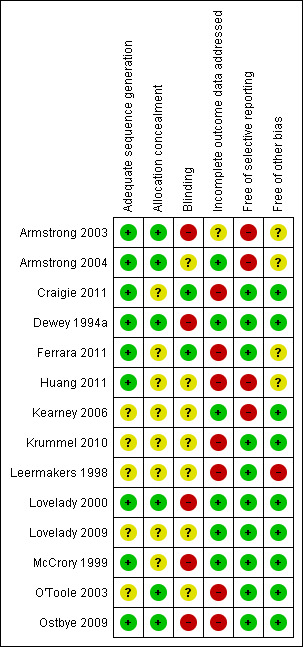
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Effects of interventions
In total, 14 trials were included, but only 12 trials involving 910 women contributed data to outcome analysis. All included studies were identified by the Cochrane Pregnancy and Childbirth Group's Trials Register and none of them were indexed within the LILACS database.
Initially, the results about heterogeneity assessment are presented, and then findings are shown in sequential order, starting with comparison one and the primary outcomes, followed by the secondary outcomes.
Heterogeneity
We used a fixed‐effect model to analyse these data. We found an I² value of 44% in Comparison 2 (exercise versus usual care) for change in percentage of body fat. However, the heterogeneity was not statistically significant (P > 0.1). Additionally, we found significant heterogeneity in two outcomes (change in body weight; change in percentage body fat) included in Comparison 3 (diet plus exercise versus usual care). The results of postpartum weight loss using a fixed‐effect model showed an I² value of 71% (Chi² = 20.98; df = 6; P < 0.01). When the data were analysed using a random‐effects model the mean difference (MD) changed from ‐1.53 kg (95% confidence interval (CI) ‐1.83 to ‐1.24) to average ‐1.93 (95% CI ‐2.96 to ‐0.89), random‐effects, T² = 1.09, I² = 71%, Analysis 3.1. Similarly, the results of change in percentage of body fat using a fixed‐effect model showed an I² value of 83% (Chi² = 17.22; df = 3; P < 0.001). The random‐effects model showed that MD changed from ‐1.69 kg (95% CI ‐2.20 to ‐1.17) to average ‐2.19 (95% CI ‐3.52 to ‐0.86); random‐effects, T² = 1.45, I² = 83%, Analysis 3.4.
3.1. Analysis.
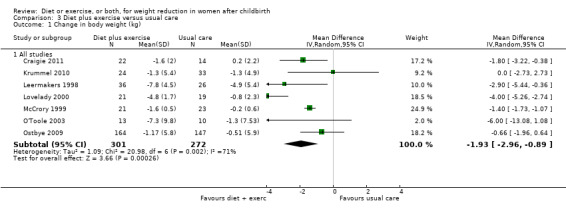
Comparison 3 Diet plus exercise versus usual care, Outcome 1 Change in body weight (kg).
3.4. Analysis.
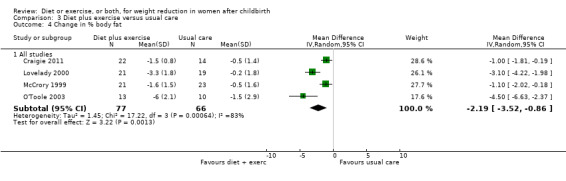
Comparison 3 Diet plus exercise versus usual care, Outcome 4 Change in % body fat.
For the primary outcome (change in body weight), for the comparison diet plus exercise versus usual care, we performed all prespecified subgroup analyses by type and duration of the intervention. Heterogeneity was eliminated when restricting the analysis to trials involving dietary advice (I² = 0%) compared to those involving caloric restriction (I² = 78%) Analysis 5.1. The borderline P value (P = 0.05) for the interaction test might indicate that the magnitude of the weight loss is higher in the trials involving caloric restriction (average MD ‐2.54 kg; 95% CI ‐3.92 to ‐1.17) compared to the dietary advice (average MD ‐0.63 kg; 95% CI ‐1.90 to 0.64) (see Comparisons 5), Analysis 5.1. Heterogeneity was significantly reduced when restricting the analysis to trials involving exercise counselling (I² = 20%) compared to those involving structured/individualised exercise programme or supervised exercise sessions (I² = 82%), Analysis 6.1. However, no significant subgroup differences in the intervention effect was observed (P = 0.26) (see Comparisons 6), Analysis 6.1. Heterogeneity was reduced when restricting the analysis to medium‐ and long‐term trials (I² = 27%) compared with short‐term trials (I² = 93%), Analysis 7.1. However, no significant subgroup difference in the intervention effect was observed (P = 0.39) (see Comparison 7), Analysis 7.1.
5.1. Analysis.
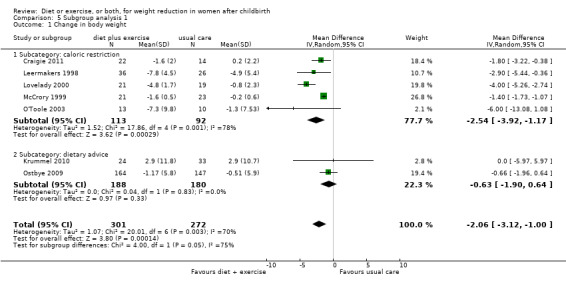
Comparison 5 Subgroup analysis 1, Outcome 1 Change in body weight.
6.1. Analysis.
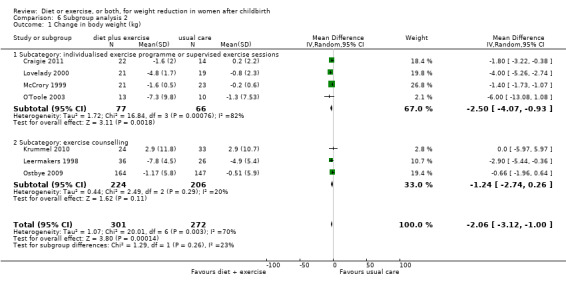
Comparison 6 Subgroup analysis 2, Outcome 1 Change in body weight (kg).
7.1. Analysis.
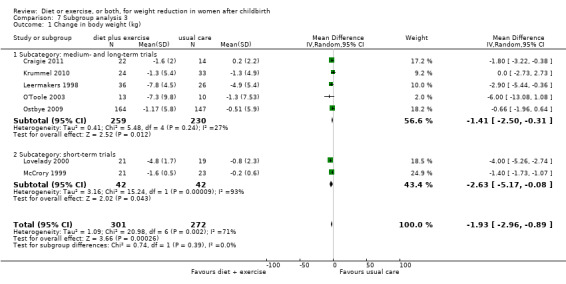
Comparison 7 Subgroup analysis 3, Outcome 1 Change in body weight (kg).
Again, for the comparison diet plus exercise versus usual care, for the secondary outcome (change in percentage of body fat) none of the prespecified subgroup analyses explained the heterogeneity (results not shown). Sensitivity analyses, excluding trials at high risk of bias did not explain the heterogeneity, (Analysis 8.1; Analysis 8.2). The only differences clinically between the trials were the length of the trial and the time of recruitment. Lovelady 2000 was a short‐term trial (10 weeks duration) and recruited women at early postpartum (four weeks postpartum). The other trials recruited women mostly in late postpartum.
8.1. Analysis.
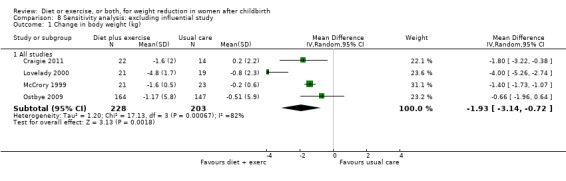
Comparison 8 Sensitivity analysis: excluding influential study, Outcome 1 Change in body weight (kg).
8.2. Analysis.
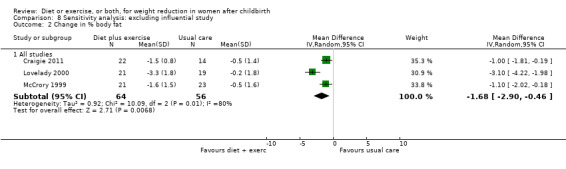
Comparison 8 Sensitivity analysis: excluding influential study, Outcome 2 Change in % body fat.
(1) Diet versus usual care
Primary outcomes
Only one trial, involving only exclusively breastfeeding women, contributed data for this comparison group. Women who followed a calorie‐restricted diet lost significantly more weight than women who received usual care (n = 45; MD ‐1.70 kg; 95% CI ‐2.08 to ‐1.32), Analysis 1.1. The other primary outcome measures were not assessed in the study.
1.1. Analysis.
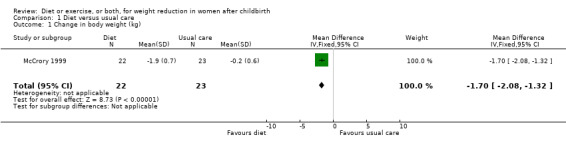
Comparison 1 Diet versus usual care, Outcome 1 Change in body weight (kg).
Secondary outcomes
Data were available for the following prespecified outcomes: change in percentage of body fat, fat‐free mass, basal plasma prolactin concentration and milk volume. Women allocated in the diet group lost significantly more fat‐free mass than women in the usual care (MD ‐0.90 kg; 95% CI ‐1.38 to ‐0.42), Analysis 1.3. There were not significant differences between the diet and control groups in relation to body fat (MD ‐0.40% body fat; 95% CI ‐1.15 to 0.35), Analysis 1.2; plasma prolactin concentration (MD 2.24 µg/mL; 95% CI ‐13.95 to 18.43), Analysis 1.4; and milk volume (MD ‐18.00 g/day; 95% CI ‐63.87 to 27.87), Analysis 1.5.
1.3. Analysis.
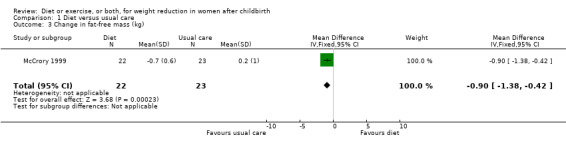
Comparison 1 Diet versus usual care, Outcome 3 Change in fat‐free mass (kg).
1.2. Analysis.
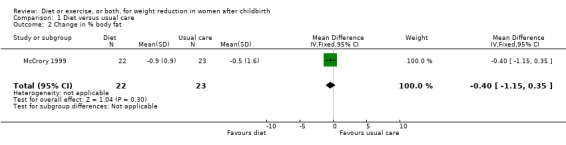
Comparison 1 Diet versus usual care, Outcome 2 Change in % body fat.
1.4. Analysis.
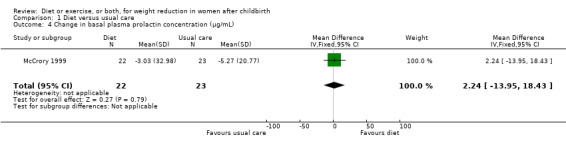
Comparison 1 Diet versus usual care, Outcome 4 Change in basal plasma prolactin concentration (µg/mL).
1.5. Analysis.
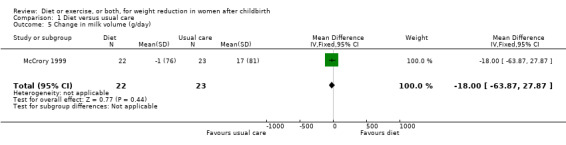
Comparison 1 Diet versus usual care, Outcome 5 Change in milk volume (g/day).
(2) Exercise versus usual care
Primary outcomes
Data were available for only one primary outcome, which showed that exercise was not significantly associated with postpartum weight loss among exclusively breastfeeding women, (two trials; n = 53; MD ‐0.10 kg; 95% CI ‐1.90 to 1.71), Analysis 2.1.
2.1. Analysis.
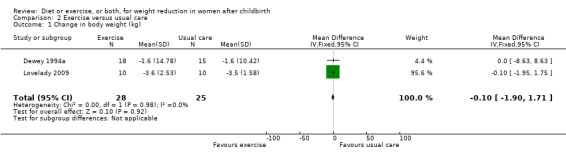
Comparison 2 Exercise versus usual care, Outcome 1 Change in body weight (kg).
Secondary outcomes
No significant differences were found between the exercise and usual care groups regarding change in percentage of body fat (two trials; n = 53; MD ‐2.51% body fat; 95% CI ‐7.80 to 2.78; random‐effects, Tau² = 6.47, I² = 44%), Analysis 2.2; plasma prolactin concentration (one trial; n = 33; MD ‐6.73 µg/mL; 95% CI ‐54.62 to 41.16), Analysis 2.5; milk volume (one trial; n = 33; MD 40.00 g/day; 95% CI ‐109.16 to 189.16), Analysis 2.6; and infant weight gain (two trials; n = 53; MD ‐124.52 g; 95% CI ‐576.60 to 327.57), Analysis 2.7. However, we found significant improvement in cardiorespiratory fitness (four trials n = 92; MD 6.73 mL/kg/minute; 95% CI 4.28 to 9.17), Analysis 2.4; and fat‐free mass (two trials; n = 53; MD 0.88 kg; 95% CI 0.06 to 1.69), Analysis 2.3, in the exercise group compared with the usual care group.
2.2. Analysis.
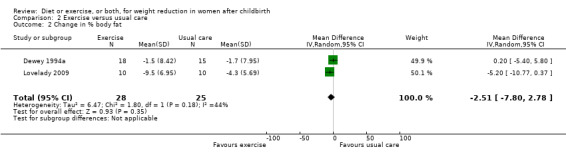
Comparison 2 Exercise versus usual care, Outcome 2 Change in % body fat.
2.5. Analysis.
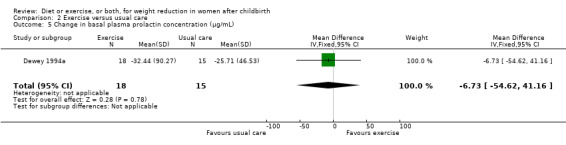
Comparison 2 Exercise versus usual care, Outcome 5 Change in basal plasma prolactin concentration (µg/mL).
2.6. Analysis.
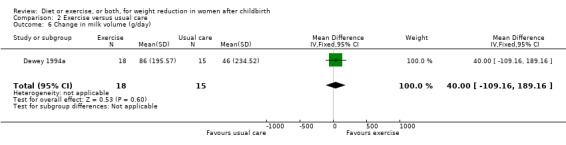
Comparison 2 Exercise versus usual care, Outcome 6 Change in milk volume (g/day).
2.7. Analysis.
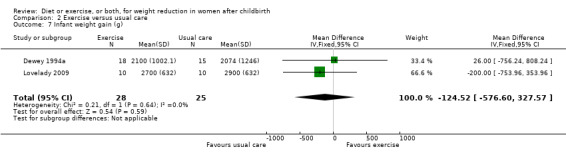
Comparison 2 Exercise versus usual care, Outcome 7 Infant weight gain (g).
2.4. Analysis.
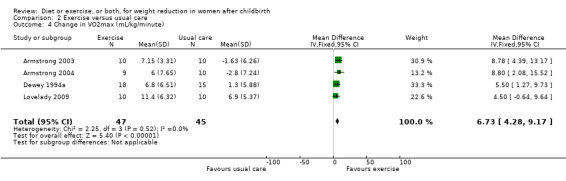
Comparison 2 Exercise versus usual care, Outcome 4 Change in VO2max (mL/kg/minute).
2.3. Analysis.
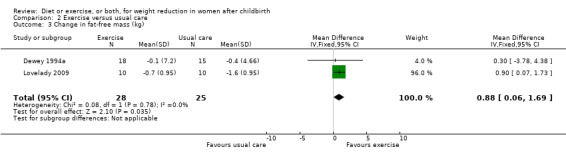
Comparison 2 Exercise versus usual care, Outcome 3 Change in fat‐free mass (kg).
(3) Diet plus exercise versus usual care
Primary outcomes
Diet combined with exercise was significantly associated with postpartum weight loss (seven trials; n = 573; MD ‐1.93 kg; 95% CI ‐2.96 to ‐0.89; random‐effects, T² = 1.09, I² = 71%), Analysis 3.1. Women who followed a dietary and exercise programme were significantly more likely to return to prepregnancy weight (three trials; n = 258; risk ratio (RR) 2.00; 95% CI 1.31 to 3.05), Analysis 3.2, and achieve healthy weight (three trials; n = 99; RR 4.41; 95% CI 1.38 to 14.13), Analysis 3.3, than women who received usual care.
3.2. Analysis.
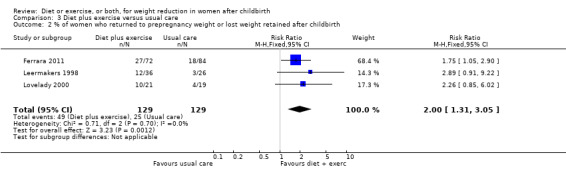
Comparison 3 Diet plus exercise versus usual care, Outcome 2 % of women who returned to prepregnancy weight or lost weight retained after childbirth.
3.3. Analysis.
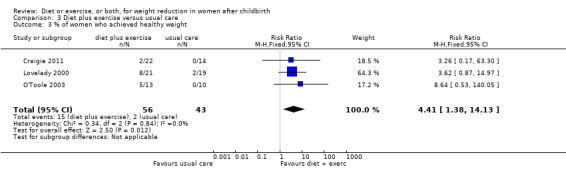
Comparison 3 Diet plus exercise versus usual care, Outcome 3 % of women who achieved healthy weight.
Secondary outcomes
Diet combined with exercise significantly reduced the percentage of body fat (four trials; n = 143; MD ‐2.19% body fat; 95% CI ‐3.52 to ‐0.86; random‐effects, T² = 1.45, I² = 83%), Analysis 3.4 and improved cardiorespiratory fitness (two trials; n = 63; MD 3.76 mL/kg/minute; 95% CI 1.46 to 6.07), Analysis 3.6, among postpartum women compared with usual care. No significant differences were found between the diet plus exercise and usual care groups regarding change in fat‐free mass (two trials; n = 84; MD ‐0.20 kg; 95% CI ‐0.67 to 0.27), Analysis 3.5; plasma prolactin concentration (one trial; n = 43; MD 3.40 µg/mL; 95% CI ‐6.77 to 13.57), Analysis 3.7; milk volume (one trial; n = 45; MD ‐33.00 g/day; 95% CI ‐81.25 to 15.25), Analysis 3.8; percentage of partial or exclusive breastfeeding (one trial; n = 161; RR 1.31; 95% CI 0.99 to 1.74), Analysis 3.9; infant length gain (one trial; n = 40; MD 0.50 cm; 95% CI ‐0.65 to 1.65), Analysis 3.10; and infant weight gain (one trial; n = 40; MD 64.00 g; 95% CI ‐271.87 to 399.87), Analysis 3.11.
3.6. Analysis.
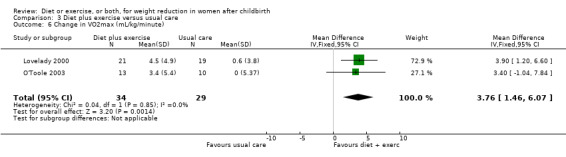
Comparison 3 Diet plus exercise versus usual care, Outcome 6 Change in VO2max (mL/kg/minute).
3.5. Analysis.
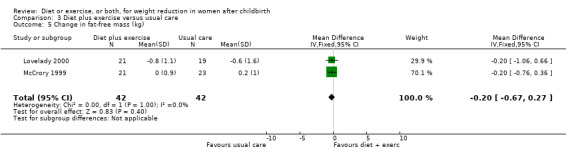
Comparison 3 Diet plus exercise versus usual care, Outcome 5 Change in fat‐free mass (kg).
3.7. Analysis.
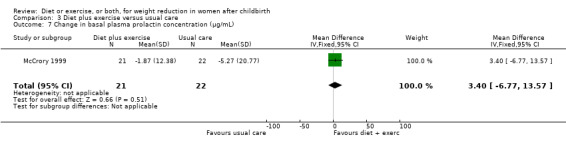
Comparison 3 Diet plus exercise versus usual care, Outcome 7 Change in basal plasma prolactin concentration (µg/mL).
3.8. Analysis.
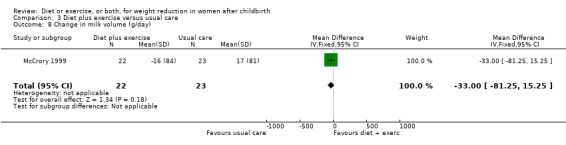
Comparison 3 Diet plus exercise versus usual care, Outcome 8 Change in milk volume (g/day).
3.9. Analysis.
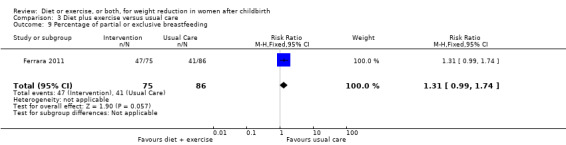
Comparison 3 Diet plus exercise versus usual care, Outcome 9 Percentage of partial or exclusive breastfeeding.
3.10. Analysis.
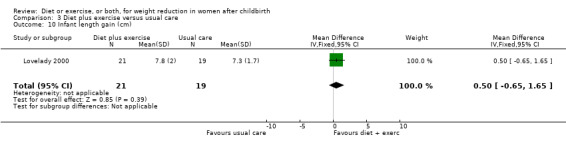
Comparison 3 Diet plus exercise versus usual care, Outcome 10 Infant length gain (cm).
3.11. Analysis.
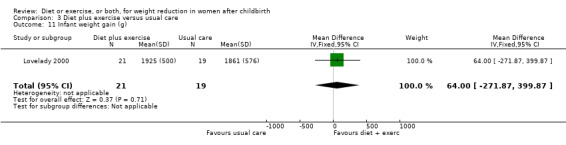
Comparison 3 Diet plus exercise versus usual care, Outcome 11 Infant weight gain (g).
(4) Diet versus exercise
No study reporting this comparison group was identified.
(5) Diet plus exercise versus exercise alone
No study reporting this comparison group was identified.
(6) Diet plus exercise versus diet alone
Primary outcomes
Only one trial, involving only exclusively breastfeeding women, contributed data for this comparison group. There was no significant difference in weight loss between the diet and diet plus exercise groups (n = 43; MD 0.30 kg; 95% CI ‐0.06 to 0.66), Analysis 4.1. The other primary outcome measures were not assessed.
4.1. Analysis.
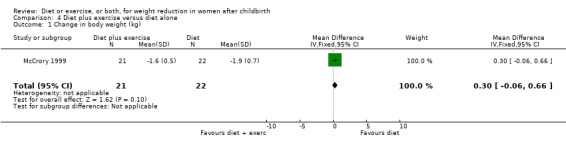
Comparison 4 Diet plus exercise versus diet alone, Outcome 1 Change in body weight (kg).
Secondary outcomes
Women allocated in the diet plus exercise group lost more body fat than women in the diet group (MD ‐0.70% body fat; 95% CI ‐1.44 to 0.04), Analysis 4.2. On the other hand, the diet group lost significantly more fat‐free mass than the diet plus exercise group (MD 0.70 kg; 95% CI 0.24 to 1.16), Analysis 4.3. Non‐significant results were observed regarding plasma prolactin concentration (MD 1.16 µg/mL; 95% CI ‐13.86 to 16.18), Analysis 4.4, and milk volume (MD ‐15.00 g/day; 95% ‐62.34 to 32.34), Analysis 4.5.
4.2. Analysis.
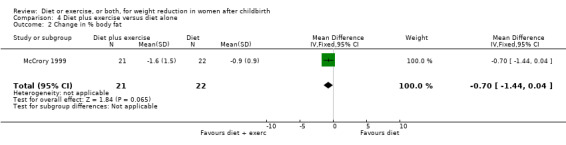
Comparison 4 Diet plus exercise versus diet alone, Outcome 2 Change in % body fat.
4.3. Analysis.
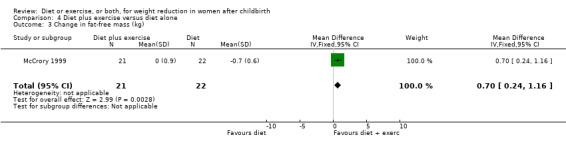
Comparison 4 Diet plus exercise versus diet alone, Outcome 3 Change in fat‐free mass (kg).
4.4. Analysis.
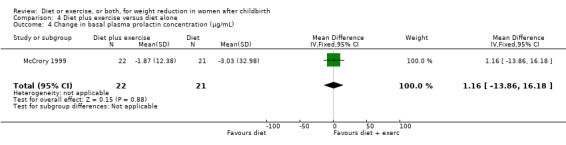
Comparison 4 Diet plus exercise versus diet alone, Outcome 4 Change in basal plasma prolactin concentration (µg/mL).
4.5. Analysis.
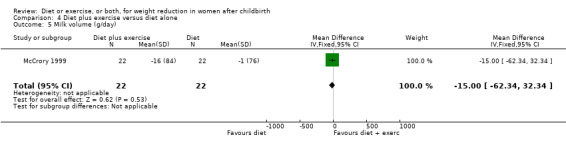
Comparison 4 Diet plus exercise versus diet alone, Outcome 5 Milk volume (g/day).
Discussion
Postpartum weight loss
The results suggest diet or diet plus exercise are effective strategies in reducing body weight. Exercise alone seems to have no or little effect on weight loss, body fatness and fat‐free mass, but significantly improved maternal cardiovascular fitness. These results about weight loss require confirmation because they are based primarily on two trials, including only 53 women (Dewey 1994a; Lovelady 2009). However, the effect of exercise programmes on cardiovascular fitness seems consistent across four trials (Armstrong 2003; Armstrong 2004; Dewey 1994b; Lovelady 2009). One possible reason for no difference on body weight between the exercise and usual care groups is that women who exercised could have increased their energy consumption. Thus, they did not reach the energy deficit required to impose weight loss. However, Dewey et al reported that the difference in energy intakes at baseline remained unaltered during the study period. The authors suggested that the mothers who exercised compensated their increased energy expenditure by reducing other daily activities (Dewey 1994b). On the other hand, Lovelady et al reported that both groups slightly decreased energy (kcal) intake over time; however, this was not significant between groups (Lovelady 2009).
In contrast to our finding, a meta‐analysis evaluating the effect of exercise, with or without dieting, on the body composition of overweight women found that aerobic exercise without dietary restriction among women caused a modest but significant weight loss (1.4 kg in 12 weeks), compared with sedentary controls. Similar to our results, the study showed little effect of aerobic exercise on fat‐free mass. The meta‐analysis demonstrated that resistance exercise had little effect on weight loss, but increased significantly fat‐free mass (Garrow 1995). We could not test this hypothesis because all of the included trials involved aerobic exercises.
Both diet and diet combined with exercise were significantly associated with postpartum weight loss when compared to the usual care group. Women assigned to the combined intervention were significantly more likely to return to prepregnancy weight and achieve healthy weight, which may help to prevent women from becoming overweight or obese after childbearing. There was no difference in the magnitude of weight loss and change in percentage of body fat between the diet and diet plus exercise groups. However, the decrease in fat‐free mass was significantly higher in the diet group than in the diet plus exercise group. According to the preliminary results, it seems advisable to lose weight by a combination of dieting and exercise, rather than by dieting alone, because the former improves the cardiovascular fitness level of the mothers and preserves fat‐free mass. Diet alone, on the other hand, reduces maternal fat‐free mass. This finding corroborates other meta‐analyses, which found that exercise provides some conservation of fat‐free mass during weight loss by dieting (Ballor 1994; Garrow 1995). Although this review showed that change in body weight was statistically significant in the diet plus exercise group, the magnitude of postpartum weight loss was moderate (approximately 2 kg). Due to lack of information about maternal health outcomes related to excess body weight and the small number of studies included in the meta‐analysis, the clinical importance of the intervention programme remains unclear, particularly for women who were already overweight or obese before pregnancy. Since the data were mostly gathered in affluent countries, it is unknown if these findings can be applied to other populations.
It is important to note that there was considerable clinical heterogeneity between trials (in Comparison 3), probably because of differences in the type or length/period of the intervention and differences in the participants' characteristics. Statistical heterogeneity was also identified. Due to the small number of trials, all explanations for the observed heterogeneity remain highly speculative. Therefore, overall effects were calculated using a random‐effects model.
It was not possible to adequately assess the presence of publication bias via funnel plot due to the limited number of studies included in the preselected outcomes in all comparison groups (less than 10 trials).
Effect of interventions on breastfeeding performance
Results on breastfeeding performance were limited to trials that included exclusively breastfeeding women (four studies). The findings indicated that none of the interventions adversely affected milk volume and plasma prolactin concentration. Due to lack of data, we could only evaluate impact on infant length and weight gain among women who followed a diet plus exercise intervention. The results showed no significant difference in both outcomes. Milk Ig A concentration, number of women who stopped breastfeeding and breastfeeding duration were not assessed in any trial. Only one trial evaluated the percentage of partial or exclusive breastfeeding and found no adverse effect of the intervention (diet plus exercise) on this outcome (Ferrara 2011). However, there was a tendency of lower percentage or partial or exclusive breastfeeding in the intervention group compared with usual care group. Within these limits and those imposed by small sample sizes, the results seem reasonably consistent, showing that the interventions appear safe for breastfeeding women.
Authors' conclusions
Implications for practice.
Preliminary findings suggest that exercise alone improves cardiovascular fitness, but does not increase the rate of postpartum weight loss. Furthermore, diet combined with exercise or diet alone compared with usual care enhance weight loss during postpartum and play a role in preventing future maternal obesity. However, it may be preferable to lose weight through a combination of dieting and exercise to dieting alone, because the former improves maternal cardiovascular fitness level and preserves lean body mass. Diet or exercise, or both, appears safe for breastfeeding women. Unfortunately, the available data are insufficient to infer important risks or other potential benefits for the mother or infant. Methodological shortcomings of some trials, especially the small sample size, the small number of studies reviewed for each outcome, and the diversity in the nature, duration and frequency of the interventions argue caution in applying these encouraging results.
Implications for research.
Future trials will require much larger sample sizes to detect potential effects on milk volume, plasma prolactin concentration and infant length and weight gain. In addition, the studies should assess the potential impacts on milk Ig A concentration, number of women who stopped breastfeeding and breastfeeding duration. Other outcomes, such as maternal morbidity and adverse events should also be studied. In addition, it would be interesting to examine the impact of weight‐loss programmes on maternal self‐image and self‐esteem.
The suggestion that regular aerobic exercise may not affect weight loss and body composition also merits further study. Likewise, future trials should attempt to confirm the limited evidence suggesting that diet alone or diet plus exercise enhance postpartum weight loss. It is still not clear if diet plus exercise is an effective strategy in low‐income women, which suggests this as an area for future study. Future trials should ensure strict and concealed randomisation, intention‐to‐treat analysis, and adequate blinding of examiners. Finally, since adherence to weight‐loss programmes requires considerable effort, more information is necessary on women's satisfaction and compliance with such interventions. These outcomes should be evaluated in a systematic fashion.
Feedback
Whiting, July 2007
Summary
I feel the conclusions in the abstract could be worded more carefully. The first sentence says:
"Preliminary evidence from this review suggests that dieting and exercise together appear to be more effective than diet alone at helping women to lose weight after childbirth, because the former improves maternal cardiorespiratory fitness level and preserves fat‐free mass, while diet alone reduces fat‐free mass."
The results do not show that diet and exercise are more effective at "helping women to lose weight". The confidence intervals for weight‐loss from diet and weight‐loss from diet and exercise together in the results overlap comprehensively, i.e. they result in the same amount of weight‐loss. Also in the results it is stated (that one study showed) that "there was no difference in the magnitude of weight loss between the diet and diet plus exercise groups".
While I agree that diet plus exercise might be better for women's health than diet alone, I feel that this analysis does not suggest that it is so.
(Summary of feedback from David Whiting, July 2007)
Reply
I agree there is no clear difference in the magnitude of weight loss between diet, and diet plus exercise, compared with normal care. We accept that the wording of the conclusions in the abstract is incorrect and have amended this.
(Summary of response from Amanda R Amorim Adegboye, November 2007)
Contributors
Feedback: David Whiting
Reply: Amanda R Amorim Adegboye
What's new
| Date | Event | Description |
|---|---|---|
| 15 May 2012 | New citation required but conclusions have not changed | Review updated. Eight new trials included and incorporated into the review, but conclusions not changed. We updated the search of the Cochrane Pregnancy and Childbirth Group's Trials Register on 30 April 2013 and added the results to the awaiting classification section of the review, to be assessed at the next update in December 2013. |
| 31 January 2012 | New search has been performed | Search updated. |
History
Protocol first published: Issue 1, 2006 Review first published: Issue 3, 2007
| Date | Event | Description |
|---|---|---|
| 5 December 2011 | Amended | Search updated. Twenty‐three reports added to Studies awaiting classification (Kinnunen 2007a; Bastian 2010; Brouwer 2006a; Craigie 2011a; Cramp 2006a; Davenport 2011a; Ebbeling 2007a; Ferrara 2008; Ferrara 2011a; Fjeldsoe 2010a; Huang 2011a; Kearney 2005; Kearney 2006a; Keller 2011a; Krummel 2010a; Liu 2009a; Lovelady 2009a; Mohammad 2011a; Moreau 2007a; Norman 2010a; Ostbye 2008a; Ostbye 2009a; Stendell‐Hollis 2011a). |
| 1 August 2008 | Amended | Contact details updated |
| 4 February 2008 | Amended | Converted to new review format. |
| 4 February 2008 | Feedback has been incorporated | We have replied to the previously published feedback, as a result of which we have also edited the Abstract's Conclusions. |
| 23 April 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
As part of the prepublication editorial process, this review has been commented on by three peers (an editor and two referees who are external to the editorial team), one or more members of the Pregnancy and Childbirth Group's international panel of consumers and the Group's Statistical Adviser.
We thank A Adegboye for the constructive criticism and help with grammar and spelling.
We thank Paulo Lourenco for his help with this update (2013) and for his contribution as an author on previous versions of the review.
The National Institute for Health Research (NIHR) is the largest single funder of the Cochrane Pregnancy and Childbirth Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the NIHR, NHS or the Department of Health.
Appendices
Appendix 1. LILACS search strategy
LILACS (1983 to 31 January 2012)
((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal)))
AND Tw postpartum OR Tw post‐partum OR Tw puerperium OR Tw mother$ OR Tw postpartal OR Tw post‐partal OR Tw lactating women OR Tw nursing women OR Tw breastfeeding OR Tw breast‐feeding
AND Tw exercis$ OR (Tw physic$ activ$) OR Tw exert$ OR (Tw physic$ fit$) OR Tw sport$ OR Tw training OR (Tw physical education) OR Tw fat$ OR Tw energ$ OR Tw calori$ OR Tw carbohydrate$ OR diet OR Tw diet‐therapy OR Tw dietary‐carbohydrates OR Tw dietary‐fats
Appendix 2. Methods used to assess trials included in previous versions of this review
The following methods were used to assess Armstrong 2003; Dewey 1994a; Leermakers 1998; Lovelady 2000; McCrory 1999; O'Toole 2003; Armstrong 2004; Bopp 2005; Carey 1997; Duckman 1968; Fahrenwald 2004; Fly 1998; Gregory 1997; Koltyn 1997; Krummel 2004; Lovelady 2003; Ostbye 2003; Quinn 1999; Wallace 1991; Wallace 1992a; Wallace 1992b; Wright 2002.
Trial selection
Three independent authors (AR Amorim, PMC Lourenco and YM Linne) considered studies for inclusion. The selection process was divided into two stages. Initially, we scanned titles, abstracts and keywords of every article retrieved to determine whether each article met the predetermined eligibility criteria, such as: included postpartum women involved at least one of the selected interventions and assessed one or more relevant clinical outcomes. In the presence of doubt about article inclusion, the decision was taken at the next stage. In the second stage, we obtained the full text of the article to clarify doubts about eligibility criteria. The discrepancies in selecting studies were resolved by discussion. Details of excluded studies are available in the Characteristics of excluded studies table.
Data extraction
The three authors independently extracted information from the included studies and entered data into the Review Manager software (RevMan 2003). Data extraction forms, developed by the primary author were tested in a pilot study. When needed, we requested further information or data from trial authors. We resolved differences in data extraction by consensus, referring back to the original article.
Multiple publications
In order to identify instances of multiple publication, we extracted information about characteristics of the participants, type of intervention, time period and place of study from all papers. Additionally, the primary author contacted the trial authors to confirm if the articles reported results of the same study. They were asked if participants, type of intervention and time period of study were exactly the same. In the case of multiple publications, we considered the most complete articles, such as those including greater numbers of outcomes and more methodological information, as primary references.
Quality assessment
We assessed methodological quality of each included study according to the criteria described in the Cochrane Reviewers' Handbook (Alderson 2004). Methods used for generation of the randomisation sequence were described for each trial.
Quality scores for concealment of allocation: (A) adequate: assignment to groups was determined by central off‐site randomisation, sequentially‐numbered, sealed, opaque envelopes or other appropriate schemes and so could not be influenced by the investigators; (B) unclear; (C) inadequate: alternation, the use of case record numbers, dates of birth or day of the week, tossing a coin, and any procedure that is entirely transparent before allocation; (D) not used.
For completeness of follow‐up: (A) adequate: less than 20% of withdrawal or loss to follow‐up; (B) unclear; (C) inadequate: more than 20% of withdrawal or loss to follow‐up.
For blinding of outcome assessment: (A) adequate: the investigator who assessed the results did not know the allocated treatment; (B) unclear; (C) no blinding: the investigator knew the allocated treatment.
Double blinding was impossible in these kinds of trials, as the participants knew which intervention they received. Blinding of those assessing the results (single blinding) was, however, highlighted and we planned to consider it in a separate sensitivity analysis.
Based on these quality criteria, we subdivided studies into the following three broad categories: (A) low risk of bias: all quality criteria met; (B) moderate risk of bias: one or more of the quality criteria only partly met; (C) high risk of bias: one or more criteria not met.
The authors evaluated methodological quality of trials independently. We did not assess trials blindly, as we knew the names of trial authors and institutions, as well as the source of publication. Differences highlighted here were resolved through consultation with the other authors, and a judgment was made based on consensus. We did not exclude studies on the basis of a low‐quality score. Thus, this classification was used as the basis of a sensitivity analysis.
Data analysis
When data were available, sufficiently similar and of sufficient quality, we performed statistical analyses using the Review Manager software (RevMan 2003). For continuous outcomes, results were expressed as mean difference between the postintervention values, or the difference between baseline values and postintervention values. When all trials assessed the same outcome, but measured it in a variety of ways or in different scales, the standardised mean difference was used as a summary statistic. For dichotomous outcomes, results for each study were expressed as risk ratios. Both dichotomous and continuous outcomes were presented with 95% confidence intervals. When information was provided in the article, an intention‐to‐treat analysis was planned to be performed.
Assessment of heterogeneity
Firstly, we analysed all data with a fixed‐effect model. The I² statistic was applied to describe the proportion of total variation in study estimates that was due to heterogeneity. An I² of more than 50% was considered as notable heterogeneity. When we found high levels of heterogeneity, we performed subgroup and sensitivity analyses, excluding the trials most susceptible to bias. Whether pooling of results seemed appropriate, heterogeneity that was not explained by subgroup and sensitivity analyses was modelled using a random‐effects analysis, which assumes that the effect size varies across studies.
Subgroup analyses
These analyses aimed to assess whether particular groups of participants could obtain more benefit from an intervention than other groups could or evaluate if the treatment effect varied with different intervention characteristics.
Our prespecified subgroups were based on:
dietary advice versus prescription of caloric restriction;
exercise counselling (self‐supervised exercise) versus structured exercise programme (supervised exercise sessions);
duration of intervention: short‐term and medium‐term versus long‐term.
We did not conduct all subgroup analyses, due to insufficient data. We carried out only the analyses for postpartum weight loss in the comparison group of diet plus exercise versus usual care. We will include these analyses in future updates, once sufficient data are available. Only the primary outcomes listed above will be included in the subgroup analyses.
Sensitivity analyses
Sensitivity analyses aimed to assess robustness of results to allocation concealment, blinding of outcome assessors, losses to follow up and other study characteristics. We planned to perform these analyses in order to explore the influence of the following factors on effect size:
repeating the analysis, excluding unpublished studies;
repeating the analysis, taking account of study quality, as previously specified in quality assessment section. The results of high‐quality studies will be compared with those of poorer quality studies, where studies rated A for all quality criteria will be compared with those rated B or C;
repeating the analysis, excluding quasi‐randomised trials;
repeating the analysis, excluding any very large or long‐term trials to establish how much they dominate the result.
Our prespecified sensitivity analyses have not been completely conducted, due to the small number of studies included in the meta‐analysis. We repeated only the analysis excluding any very large or long‐term trials in the comparison group of diet plus exercise versus usual care. We will include the entire analysis in future updates, when sufficient data become available.
We also planned to use funnel plots and a simple graphical test to assess for evidence of bias (Egger 1997). However, the number of eligible studies was too few to allow adequate assessment.
Data and analyses
Comparison 1. Diet versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in body weight (kg) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐1.7 [‐2.08, ‐1.32] |
| 2 Change in % body fat | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐1.15, 0.35] |
| 3 Change in fat‐free mass (kg) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.38, ‐0.42] |
| 4 Change in basal plasma prolactin concentration (µg/mL) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | 2.24 [‐13.95, 18.43] |
| 5 Change in milk volume (g/day) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐18.0 [‐63.87, 27.87] |
Comparison 2. Exercise versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in body weight (kg) | 2 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.10 [‐1.90, 1.71] |
| 2 Change in % body fat | 2 | 53 | Mean Difference (IV, Random, 95% CI) | ‐2.51 [‐7.80, 2.78] |
| 3 Change in fat‐free mass (kg) | 2 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.88 [0.06, 1.69] |
| 4 Change in VO2max (mL/kg/minute) | 4 | 92 | Mean Difference (IV, Fixed, 95% CI) | 6.73 [4.28, 9.17] |
| 5 Change in basal plasma prolactin concentration (µg/mL) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | ‐6.73 [‐54.62, 41.16] |
| 6 Change in milk volume (g/day) | 1 | 33 | Mean Difference (IV, Fixed, 95% CI) | 40.0 [‐109.16, 189.16] |
| 7 Infant weight gain (g) | 2 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐124.52 [‐576.60, 327.57] |
Comparison 3. Diet plus exercise versus usual care.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in body weight (kg) | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All studies | 7 | 573 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐2.96, ‐0.89] |
| 2 % of women who returned to prepregnancy weight or lost weight retained after childbirth | 3 | 258 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.00 [1.31, 3.05] |
| 3 % of women who achieved healthy weight | 3 | 99 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.41 [1.38, 14.13] |
| 4 Change in % body fat | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 All studies | 4 | 143 | Mean Difference (IV, Random, 95% CI) | ‐2.19 [‐3.52, ‐0.86] |
| 5 Change in fat‐free mass (kg) | 2 | 84 | Mean Difference (IV, Fixed, 95% CI) | ‐0.20 [‐0.67, 0.27] |
| 6 Change in VO2max (mL/kg/minute) | 2 | 63 | Mean Difference (IV, Fixed, 95% CI) | 3.76 [1.46, 6.07] |
| 7 Change in basal plasma prolactin concentration (µg/mL) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 3.40 [‐6.77, 13.57] |
| 8 Change in milk volume (g/day) | 1 | 45 | Mean Difference (IV, Fixed, 95% CI) | ‐33.0 [‐81.25, 15.25] |
| 9 Percentage of partial or exclusive breastfeeding | 1 | 161 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.31 [0.99, 1.74] |
| 10 Infant length gain (cm) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.5 [‐0.65, 1.65] |
| 11 Infant weight gain (g) | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 64.0 [‐271.87, 399.87] |
Comparison 4. Diet plus exercise versus diet alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in body weight (kg) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.30 [‐0.06, 0.66] |
| 2 Change in % body fat | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | ‐0.70 [‐1.44, 0.04] |
| 3 Change in fat‐free mass (kg) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 0.7 [0.24, 1.16] |
| 4 Change in basal plasma prolactin concentration (µg/mL) | 1 | 43 | Mean Difference (IV, Fixed, 95% CI) | 1.16 [‐13.86, 16.18] |
| 5 Milk volume (g/day) | 1 | 44 | Mean Difference (IV, Fixed, 95% CI) | ‐15.0 [‐62.34, 32.34] |
Comparison 5. Subgroup analysis 1.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in body weight | 7 | 573 | Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐3.12, 1.00] |
| 1.1 Subcategory: caloric restriction | 5 | 205 | Mean Difference (IV, Random, 95% CI) | ‐2.54 [‐3.92, ‐1.17] |
| 1.2 Subcategory: dietary advice | 2 | 368 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐1.90, 0.64] |
Comparison 6. Subgroup analysis 2.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in body weight (kg) | 7 | 573 | Mean Difference (IV, Random, 95% CI) | ‐2.06 [‐3.12, 1.00] |
| 1.1 Subcategory: individualised exercise programme or supervised exercise sessions | 4 | 143 | Mean Difference (IV, Random, 95% CI) | ‐2.50 [‐4.07, ‐0.93] |
| 1.2 Subcategory: exercise counselling | 3 | 430 | Mean Difference (IV, Random, 95% CI) | ‐1.24 [‐2.74, 0.26] |
Comparison 7. Subgroup analysis 3.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in body weight (kg) | 7 | 573 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐2.96, ‐0.89] |
| 1.1 Subcategory: medium‐ and long‐term trials | 5 | 489 | Mean Difference (IV, Random, 95% CI) | ‐1.41 [‐2.50, ‐0.31] |
| 1.2 Subcategory: short‐term trials | 2 | 84 | Mean Difference (IV, Random, 95% CI) | ‐2.63 [‐5.17, ‐0.08] |
Comparison 8. Sensitivity analysis: excluding influential study.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Change in body weight (kg) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 1.1 All studies | 4 | 431 | Mean Difference (IV, Random, 95% CI) | ‐1.93 [‐3.14, ‐0.72] |
| 2 Change in % body fat | 3 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 2.1 All studies | 3 | 120 | Mean Difference (IV, Random, 95% CI) | ‐1.68 [‐2.90, ‐0.46] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Armstrong 2003.
| Methods | Intervention was randomly assigned. The procedure was based on a 4‐block randomised sequence (information not published). Allocation using sealed opaque envelopes. | |
| Participants | 20 women who had a child between the ages of 6 weeks and 12 months and were experiencing depressive symptomatology. | |
| Interventions | Intervention: social support and aerobic exercise. The exercise programme consisted of supervised pram‐walking group sessions 3 times per week for 30‐40 minutes at an intensity of 60% to 75% of age‐predicted heart rate for 12 weeks. Control: the control group was not involved in the multi‐intervention programme. Trial duration: medium‐term. |
|
| Outcomes | VO2 max and adherence to intervention. Other outcomes not considered in this review: postpartum depression and social support. |
|
| Notes | Data suggested good follow‐up (no drop outs) and no differences between groups at baseline. A total of 36 exercise sessions were offered and the mean number of sessions attended was 23.7 (66% of adherence). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | The procedure was based on a 4‐block randomised sequence. |
| Allocation concealment | Low risk | Allocation using sealed opaque envelopes (information not published). |
| Blinding All outcomes | High risk | The investigator who assessed the results knew the allocated treatment (information not published). |
| Incomplete outcome data addressed All outcomes | Unclear risk | Data suggested good follow‐up (no drop outs) but information is not clearly described in the report. |
| Free of selective reporting | High risk | Some outcomes of relevance were not described (e.g. weight loss). |
| Free of other bias | Unclear risk | Source of funding not mentioned. No mention of any research protocol published a priori. The characteristics of participants were not significantly different between groups at baseline. |
Armstrong 2004.
| Methods | Randomised controlled trial. The procedure of randomisation was based upon a 4‐block, randomised sequence. Sealed envelopes were opened in a sequential manner. Each envelope contained a code (A or B) assigning the woman to either the exercise or social support group. It was stressed that the process was random and that the investigator had no control over who was selected into which group. | |
| Participants | 19 women between 6 weeks and 18 months postpartum with an Edinburgh Postnatal Depression score of ≥ 12 at the screening phase and without a medical condition that would prevent regular aerobic exercise. | |
| Interventions | Intervention: 12‐week pram‐walking exercise programme. Women were encouraged to attend 2 pram‐walking sessions (Mondays and Wednesdays) at 09.30 hours on flat walking paths at an area on the Gold Coast. They were required to do the third session needed to improve cardiovascular endurance independently. Muscle stretches were done before and after the exercise and heart rate was recorded at the end of the session. Participants walked for approximately 40 min each session and it was essential that the participants walked at a moderate intensity (60% to 75% of age‐predicted heart rate). Control: this group received social support. Women met once per week on Tuesdays from 09.30 hours to 11.00 hours at a room within the local community centre. No specific topics were discussed. Instead, the women could talk openly about any issues that were of concern or interest to them. Trial duration: medium‐term. |
|
| Outcomes | VO2 max and adherence to intervention. Other outcomes not considered in this review: postpartum depression and social support. |
|
| Notes | Data suggested good follow‐up (no drop outs) and no differences between groups at baseline. The overall attendance was 75% for the pram‐walking group and 73% for the social support intervention group. There was a common pattern for both groups in relation to attendance. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Randomisation was based upon a 4‐block, randomised sequence. |
| Allocation concealment | Low risk | Sealed envelopes. |
| Blinding All outcomes | Unclear risk | No detail provided. |
| Incomplete outcome data addressed All outcomes | Low risk | Data suggested good follow‐up (no drop outs) but information is not clearly described in the report. |
| Free of selective reporting | High risk | Some outcomes of relevance were not described (e.g. weight loss). |
| Free of other bias | Unclear risk | Source of funding not mentioned. No mention of any research protocol published a priori. The characteristics of participants were not significantly different between groups at baseline. |
Craigie 2011.
| Methods | Computer‐based randomisation, using a 1:1 random sampling procedure. | |
| Participants | 52 women who were not pregnant, 6‐18 months postpartum with a BMI > 25 kg/m2 living in areas of deprivation within Tayside, UK. | |
| Interventions | Intervention: the 12‐week intervention were allocated a trained lifestyle counsellor who delivered the intervention by 3 face‐to‐face consultations at monthly intervals and 3 structured telephone calls between consultation to identify progress towards goals and challenges. A personalised dietary prescription of estimated energy requirements minus 500 kcal was calculated with verbal and written guidance on food groups, frequency of consumption and portion size. Personalised physical activity goals were also set towards achieving 150 minutes of moderate to vigorous activity per week. Participants were provided with 4‐week walking plans, a pedometer and a weight logbook for self‐monitoring. Control: the group received usual care and 1‐off consultation with a lifestyle counsellor after follow‐up assessment. All participants received a weight loss booklet. Trial duration: medium‐term. |
|
| Outcomes | Postpartum weight loss, percentage of women who had weight loss of clinical significance, change in percentage of body fat, and feasibility and acceptability of the intervention. Other outcomes not considered in this review: change in waist circumference, BMI and minutes of moderate‐vigorous physical activity per day. |
|
| Notes | 65 women met the inclusion criteria and were appointed for a baseline visit but 11 women subsequently declined to participate and 2 were excluded due to low BMI. In total 52 women enrolled in the study. Loss to follow‐up was 31% (24% and 39% for intervention and control group respectively) including 3 participants who became pregnant during the study and were excluded. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Randomisation was computer‐based. |
| Allocation concealment | Unclear risk | No detail provided. |
| Blinding All outcomes | Low risk | Assessments were performed, primarily within a hospital setting but on occasions within the participants’ home, by a research assistant blinded to randomisation allocation. |
| Incomplete outcome data addressed All outcomes | High risk | 31% of loss to follow‐up. |
| Free of selective reporting | Low risk | Outcomes of relevance described. |
| Free of other bias | Low risk | Supported by the Medical Research Council (Ref GO701771) and NHS Research Scotland (NRS) through NHS Tayside. No mention of any research protocol published a priori. The characteristics of participants were not significantly different between groups at baseline. |
Dewey 1994a.
| Methods | Randomisation using a random‐number table. Allocation using sealed, opaque envelopes (information not published). | |
| Participants | 33 sedentary, non‐smoking women, without chronic disease, whose infants were being exclusively breastfed. | |
| Interventions | Intervention: 45 minutes of supervised aerobic exercise session at an intensity of 60% to 70% of maximal heart rate reserve, 5 times per week for 12 weeks, beginning at 6‐8 weeks' postpartum. Control: no regular aerobic exercise during the same time period. Trial duration: medium‐term. | |
| Outcomes | Postpartum weight loss, body fat, fat‐free mass, VO2 max, milk volume, infant weight gain and plasma prolactin concentration. Other outcomes not considered in this review: energy expenditure and energy intake. |
|
| Notes | A total of 38 women enrolled in the study and 5 women did not complete the study (4 in the control group). These women had similar characteristics to those who remained, however, their infants had significantly lower birth weights. There was a higher proportion of female infants in the exercise group (65%) than in control (46%). All women were able to exclusively breastfeed their infants during the study period. Research assistants visited the homes at each exercise session to assure compliance. Data concerning fat free mass were extracted from Lovelady 1995. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Randomisation using a random‐number table. |
| Allocation concealment | Low risk | Allocation using sealed, opaque envelopes (information not published). |
| Blinding All outcomes | High risk | The investigator who assessed the results knew the allocated treatment (information not published). |
| Incomplete outcome data addressed All outcomes | Low risk | 13% of loss to follow‐up. |
| Free of selective reporting | Low risk | Outcomes of relevance described. |
| Free of other bias | Low risk | Supported by a grant (HD 24112) from the National Institutes of Health. Study guided by a research protocol and previous validation studies. The characteristics of participants were not significantly different between groups at baseline. |
Ferrara 2011.
| Methods | Computer‐based randomisation. | |
| Participants | 197 English‐speaking women with gestational diabetes mellitus, aged 18 years or older without high‐risk pregnancy (i.e., drug or alcohol abuse, chronic health problems, or pregnancy complications). | |
| Interventions | Intervention: Intervention was initiated during pregnancy and continued until 12 months postpartum. Intervention consisted of advice on diet, exercise and breastfeeding. 2 trained dietitians delivered the intervention. The prenatal phase consisted of 1 in‐person session and 2 individual telephone counselling contacts. During the postpartum phase women were asked to reach their weight goal during the first 12‐months postpartum and were given a handbook that contained written materials organized in 16 sessions. There was a core curriculum of 8 sessions with up to 8 additional sessions offered to those
who desired more contact. The sessions were conducted over the telephone except for the first and the last, which were conducted in‐person. Women were encouraged to perform 150 min of moderate or harder physical activity per week and to consume 25% or less of total calories from fat per day. Control: women received usual care and printed educational materials that included publicly available information on gestational diabetes mellitus. In the postpartum period, they received 2 newsletters focusing on issues related to infant safety and general health. Trial duration: long‐term. |
|
| Outcomes | Percentage of women who returned to prepregnancy weight if it was normal, or achieved a 5% reduction from prepregnancy weight if overweight at 6 weeks, 7 and 12 months postpartum, percentage of partial or exclusive breastfeeding at 6 weeks and 7 months postpartum, satisfaction and compliance with intervention Data on weight at 12 months postpartum were preferably used in the analysis. Other outcomes not considered in this review: change in percent of calories from dietary fat and change in moderate or vigorous physical activity (min/ wk) at 6 weeks and 7 months postpartum. |
|
| Notes | Small differences in baseline characteristics were observed between women in the intervention and usual care conditions regarding education and 1‐h glucose value from the diagnostic 100 g oral glucose tolerance test (lower values in the intervention group). In total, 197 women enrolled in the study. Participant retention at 12 months postpartum was 75% in the intervention group and 83% in the control group. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Randomisation was computer‐based. |
| Allocation concealment | Unclear risk | No details provided. |
| Blinding All outcomes | Low risk | Data were collected by research assistants who were unaware of the condition assignment. |
| Incomplete outcome data addressed All outcomes | High risk | 21% of loss to follow‐up. |
| Free of selective reporting | Low risk | Outcomes of relevance described. |
| Free of other bias | Unclear risk | Supported by a grant (R18‐DK067334) from the National Institute of Diabetes and Digestive and Kidney Diseases and a grant from the Kaiser Garfield Foundation. No mention of any research protocol published a priori. The characteristics of participants were slightly different between groups at baseline. |
Huang 2011.
| Methods | Randomised controlled trial. Using a randomised table, the researcher assigned pregnant women to the control group or to 1 of the 2 intervention groups. | |
| Participants | 128 women aged18 years or older, without cognitive impairment or psychiatric illness, able to speak and read Chinese, not participating in another study, and planning to give birth at the study site. All participants were recruited during pregnancy. | |
| Interventions | Intervention: intervention began 24–48 hours after birth and extended to 6 months postpartum. The intervention was delivered at bedside in the obstetric units and during regularly scheduled clinic visits by a nurse. The nurse discussed with each participant how to design an individualised dietary and physical activity education plan based on the participant’s baseline information. The plan consisted of 1 primary counselling session, 1 brochure and 2 booster sessions at 6 weeks postpartum and 3 months postpartum. Control: usual care plus participation in face‐to‐face discussions in the health education room with nurse educators about individual concerns, e.g. sexual life during pregnancy, preparation for breastfeeding, birth and first signs of labour. Trial duration: medium‐term. |
|
| Outcomes | Weight retention at 6 months postpartum. Other outcomes not considered in this review: health‐promoting behaviour, self‐efficacy, body image and social support. |
|
| Notes | The study did not contribute data for the statistical analysis. The study aimed at examining the effect of individual counselling about diet and physical activity among child‐bearing women during 2 periods: from pregnancy through to 6 months postpartum, and from birth through to 6 months postpartum. Only the second arm (intervention initiated during postpartum) was considered in this review. In total, 240 women were randomised and 51 women (16 in the control ; 16 in the postpartum intervention and 16 in the pregnancy intervention) who dropped out were not statistically different in age, parity, employment, education or BMI. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Randomisation using a random‐number table. |
| Allocation concealment | Unclear risk | No detail provided. |
| Blinding All outcomes | Unclear risk | No detail provided. |
| Incomplete outcome data addressed All outcomes | High risk | 20% of loss to follow‐up (including data for the intervention groups considered in this review). |
| Free of selective reporting | High risk | Some outcomes of relevance were not described (e.g. weight loss). |
| Free of other bias | Unclear risk | Supported by a grant from the NationalScienceCouncil,Taiwan (NSC 93‐2314‐B‐182‐079). No mention of any research protocol published a priori. The characteristics of participants were not significantly different between groups at baseline. |
Kearney 2006.
| Methods | Randomised controlled trial, no detail provided. | |
| Participants | 21 English‐speaking women, aged 21 years or older with a pregnancy weight gain of at least 30 lb (14 kg) who had delivered healthy singleton infants. | |
| Interventions | Intervention: a nurse‐delivered motivational intervention in enhancing weight loss between 2 and 8 months postpartum. Structured diet and exercise program was not provided. Women were motivated to use information and programs for lifestyle change already available to them. Women were offered reimbursement of $50 for program costs if they enrolled in a commercial weight loss program. The intervention began at 2 months postpartum and continued monthly contact with both groups over the next 6 months, for a total of 3 home visits (at 2, 5, and 8 months) and 4 phone calls (at 3, 4, 6, and 7 months). Control: the control group received friendly support but no structured counselling. Trial duration: long‐term. |
|
| Outcomes | Body weight, BMI and weight retention at 8 months postpartum. | |
| Notes | The study did not contribute data for the statistical analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Randomisation stated, but method not reported. |
| Allocation concealment | Unclear risk | No detail provided. |
| Blinding All outcomes | Unclear risk | No detail provided. |
| Incomplete outcome data addressed All outcomes | Low risk | Only 1 woman was lost to follow‐up at 3 months postpartum. Despite the group size imbalance (control = 14 women, intervention = 7 women), non‐parametric tests showed no statistically significant difference in demographics, BMI, smoking, breastfeeding, exercise, and work hours. |
| Free of selective reporting | High risk | Some outcomes of relevance were not described (e.g. weight loss). |
| Free of other bias | Low risk | Supported by a Research Incentive Grant awarded by Boston College. No mention of any research protocol published a priori. The characteristics of participants were not significantly different between groups at baseline. |
Krummel 2010.
| Methods | Randomised controlled trial, no detail provided. | |
| Participants | 151 postpartum women (up to 2 years), over the age of 18 years, not underweight, and enrolled in WIC in the participating counties. | |
| Interventions | Intervention: were enrolled in a facilitated discussion group (10 sessions) and received monthly personalised feedback on self‐monitoring records for nutrition and physical activity behaviours during 12 months. Topics included in the facilitated discussion group: lifestyle change, portion estimation, finding the fat, meeting dietary needs with the Food Guide Pyramid, activity adoption and maintenance, progressive relaxation and deep breathing for stress management, supportive environments, emotional eating, social support, and maintaining behaviour change. They also received the newsletters and counselling session. The intervention was delivered by a team formed by nutritionists, exercise physiologists, psychologist, and health educator. Control group: called self‐guided group received usual care in addition to 1 counselling session with a dietitian and monthly newsletters. Trial duration: long‐term. |
|
| Outcomes | Postpartum weight loss. Other outcomes not considered in this review: changes in waist circumference, dietary intake (calorie, fat, and fibre), steps (pedometer), perceived stress and depression. |
|
| Notes | At enrolment, 73 women were randomised to the control group and 78 to the intervention group. After 12 months follow‐up, only 33 women and 24 remained in the control and intervention group, respectively. Comparing women who stayed active versus those who dropped out, the active women were more likely to be educated, have a lower BMI, and be in the control group. The attendance level was low. The average number of discussions attended was 4 (out of 10) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Randomisation stated, but method not reported. |
| Allocation concealment | Unclear risk | No detail provided. |
| Blinding All outcomes | Unclear risk | No detail provided. |
| Incomplete outcome data addressed All outcomes | High risk | 62% of loss to follow‐up. |
| Free of selective reporting | Low risk | Outcomes of relevance described. |
| Free of other bias | Low risk | Supported by the NIH, NICHD, R01,D39102 grant to DK. To guide intervention development, 8 focus groups (n = 38 women) of women, who were WIC participants but not eligible for the study, were held prior to the intervention. The characteristics of participants were not significantly different between groups at baseline for the entire population (n = 151). However, participants and drop outs were slightly different. |
Leermakers 1998.
| Methods | Randomisation stated, but method not reported. | |
| Participants | 62 women who had given birth in the past 3‐12 months and whose weight exceeded their prepregnancy weight by at least 6.8 kg. Women who were breastfeeding their infant were excluded from the study. | |
| Interventions | Intervention: 2 group sessions held at the beginning of intervention and at month 2. Women were instructed in the group sessions to follow a diet of 1000‐1500 kcal per day, begin an aerobic programme and self‐monitor. Correspondence material consisted of 16 lessons focused on low‐fat and low‐caloric eating habits and increasing physical activity, delivered over 6 months. Participants were instructed to begin an aerobic exercise program, consisting primarily of walking, and to gradually increase the frequency and duration of their walking until they reached 2 miles per day on at least 5 days per week. Telephone contacts were made weekly or biweekly, depending on participants' requests during 6‐month intervention period. Control: the control group did not receive any treatment, but participants were given an informational brochure about healthy eating and exercise. Trial duration: medium‐term. | |
| Outcomes | Postpartum weight loss, percentage of women who returned to prepregnancy weight and adherence to intervention. Other outcomes not considered in this review: energy expenditure, energy intake, dietary fat intake. |
|
| Notes | A total of 90 women enrolled in the study and 28 women dropped out (11 in the intervention group and 17 in the control). The drop outs were significantly heavier at baseline and retained significantly more weight after pregnancy than completers. The intervention group was significantly older and had a greater percentage of married women, compared to control group. Women returned 10.1 self‐monitoring records (40.4% of adherence) and 7.6 homework assignments (50.7% of adherence). They received an average of 10.3 telephone contacts during the 6‐month programme. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Randomisation stated, but method not reported. |
| Allocation concealment | Unclear risk | No detail provided. |
| Blinding All outcomes | Unclear risk | No detail provided. |
| Incomplete outcome data addressed All outcomes | High risk | 31% of loss to follow‐up. Using an intent‐to‐treat approach, missing data were imputed to post‐treatment weight data by assuming that women who did not complete the post‐treatment assessment had no weight change from their pre‐treatment weight. |
| Free of selective reporting | Low risk | Outcomes of relevance described. |
| Free of other bias | High risk | Supported by a Pilot Feasibility Grant from the Obesity Nutrition Research Center (DK46204). No mention of any research protocol published a priori. The intervention group was significantly older and had a greater percentage of married women, compared to control group. |
Lovelady 2000.
| Methods | Women were randomly assigned using a random‐number table, after stratification according to the sex of their infants. Once the random sequence was generated, each participant's number and their group assignment was written down and placed in an envelope and sealed (information not published). | |
| Participants | 40 healthy, sedentary, non‐smoking and exclusively breastfeeding women, who were overweight at 4 weeks postpartum and had delivered a full‐term infant weighing at least 2500 g and had not delivered by caesarean section. | |
| Interventions | Intervention: restriction of 500 kcal from the average of reported daily energy intake and estimated energy requirements. 45 minutes of supervised aerobic exercise 4 times per week at an intensity of 65% to 80% of maximal heart rate reserve for 10 weeks, beginning at 4 weeks postpartum. Control: usual dietary intake and not exercise more than once per week for 10 weeks. All women were given a multivitamin supplement containing at least 50% of the recommended dietary allowances for lactating women. Trial duration: short‐term. |
|
| Outcomes | Postpartum weight loss, percentage of women who achieved a BMI below 25, percentage of women who were within 1 kg of their prepregnancy weight, body fat, fat‐free mass, VO2max, infant weight gain and infant length gain. Other outcomes not considered in this review: Skin‐fold thickness and energy intake. |
|
| Notes | A total of 48 women enrolled in the study and 8 women dropped out of the study (6 in the intervention group and 2 in the control). The drop outs were significantly heavier before pregnancy; tended to have higher BMI and heavier infants at birth and lower level of cardiovascular fitness compared to women who completed the study. Research assistants visited the homes at each exercise session to assure compliance. All participants, but 1 were able to exercise 4 days per week. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Randomisation using a random‐number table. |
| Allocation concealment | Low risk | Allocation using sealed, opaque envelopes (information not published). |
| Blinding All outcomes | High risk | The investigator who assessed the results knew the allocated treatment (information not published). |
| Incomplete outcome data addressed All outcomes | Low risk | 16.7% of loss to follow‐up. |
| Free of selective reporting | Low risk | Outcomes of relevance described. |
| Free of other bias | Low risk | Supported by grants from the National Institutes of Health (HD 34222) and the North Carolina Agricultural Research Service. No mention of any research protocol published a priori. The characteristics of participants were not significantly different between groups at baseline. |
Lovelady 2009.
| Methods | Randomised controlled trial. The randomisation was stratified by parity because loss of bone density during lactation may be different between primiparous and multiparous women. | |
| Participants | 20 healthy (free from chronic disease), non‐smoking, sedentary, exclusively breastfeeding women with a BMI of 25–30 kg/m2 at 3 weeks postpartum. | |
| Interventions | Intervention: 16‐week home based exercise program that focused on increasing core strength of the body and aerobic exercise 3 times per week. Research assistants travelled to the home 3 days/ week to train mothers in the exercise program and to ensure exercise compliance during the study. Control: women were instructed not to perform resistance exercise or aerobic exercise. They were allowed to walk their babies in strollers at a casual pace (not faster than 2 mph). They were offered the exercise program after they completed the baseline and end point measurements. Women in both groups were instructed not to restrict their calorie intake and were given multivitamin supplement without minerals. Trial duration: medium‐term. |
|
| Outcomes | The primary outcome was bone mineral density. However, postpartum weight loss, fat mass, lean body mass, cardiorespiratory fitness (VO2max) and infant weight gain were also considered. | |
| Notes | In total, 24 women were recruited and completed baseline measurements. 4 women (1 in the control and 3 in the exercise group) did not complete the study because they were not able to exclusively breastfeed their infants throughout the 16‐week period. There were no significant differences in their baseline characteristics compared with the women who completed the study. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Randomisation stated, but method not reported. |
| Allocation concealment | Unclear risk | No detail provided. |
| Blinding All outcomes | Unclear risk | No detail provided. |
| Incomplete outcome data addressed All outcomes | Low risk | 16.7% of loss to follow‐up. |
| Free of selective reporting | Low risk | Outcomes of relevance described. |
| Free of other bias | Low risk | Supported by a grant from the North Carolina Agricultural Research Service. No mention of any research protocol published a priori. The characteristics of participants were not significantly different between groups at baseline. |
McCrory 1999.
| Methods | Random assignment of participants was computer‐based using Moses‐Oakford algorithm with variables block size. | |
| Participants | 67 non‐smoking, exclusively breastfeeding women, who had no chronic illnesses, were not taking medication regularly and had delivered a single healthy, term infant. Participants were randomised at 8‐16 weeks postpartum. | |
| Interventions | Intervention I: diet group ‐ 35% of energy deficit for 11 days. Intervention II: diet plus exercise group ‐ 35% of net energy deficit for 11 days (60% by dietary restriction and 40% by additional exercise). Women in this group performed aerobic exercises during 86 minutes per session at an intensity of 50% to 70% of maximal heart rate on 9 of the 11 days. Exercise sessions were self‐supervised. Control: no energy restriction and exercise. Trial duration: short‐term. |
|
| Outcomes | Postpartum weight loss, body fat, fat‐free mass, milk volume and plasma prolactin concentration. Other outcomes not considered in this review: milk energy output and milk energy density. |
|
| Notes | Of the 68 participants, 1 withdrew after assignment to the diet plus exercise group, but before the intervention began. Of the remaining 67 participants, 1 in the diet plus exercise group did not continue with the intervention after day 8. Data for the latter participant were included in the analysis up to the time that she stopped participating in the intervention. Data suggested good compliance with the intervention. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Randomisation was computer‐based. |
| Allocation concealment | Unclear risk | No detail provided. |
| Blinding All outcomes | High risk | The investigator who assessed the results knew the allocated treatment (information not published). |
| Incomplete outcome data addressed All outcomes | Low risk | 3% of loss to follow‐up. |
| Free of selective reporting | Low risk | Outcomes of relevance described. |
| Free of other bias | Low risk | Supported by NIH grant (HD 24112). No mention of any research protocol published a priori. But the study seems to be guided by a previous short‐term intervention study in lactating women. The characteristics of participants were not significantly different between groups at baseline. |
O'Toole 2003.
| Methods | Interventions were randomly assigned, but method not reported. Allocation using blinded drawing of labels containing group assignment. | |
| Participants | 23 postpartum women, who were overweight prior to pregnancy, had gained more than 15 kg during pregnancy and were more than 5 kg heavier than prepregnancy at the time of enrolment. Participants were randomised between 6 weeks and 6 months postpartum. | |
| Interventions | Intervention I: structured diet and physical activity group, which included individualised diet prescriptions derived from baseline measurements, daily food and activity diaries and healthy cooking demonstration.
A specific, individualised activity plan consisting of moderate intensity activity guided by heart rate was developed for each participant. The intervention also included educational group sessions held once a week for 12 weeks, biweekly for the following 2 months, and monthly up to 1 year postpartum. Intervention II: self‐directed group based on general advice about diet and exercise. This group participated in a single 1‐hour educational session about healthy diet and exercise practices. Participants were given some brochures about nutrition and a food guide pyramid. Trial duration: long‐term. |
|
| Outcomes | Postpartum weight loss, percentage of women who achieved a BMI below 25, body fat, fat‐free mass (values not available) and VO2 max. Other outcomes not considered in this review: energy intake and energy expenditure in physical activity (kcal/week) |
|
| Notes | 40 women enrolled in the study, but 29 remained at 12 weeks postpartum (73% of retention) and 23 remained up to 1 year postpartum (58% of retention). There were no differences between those who finished the study and those who dropped out. Data on fat‐free mass were not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Unclear risk | Randomisation stated, but method not reported. |
| Allocation concealment | Low risk | Allocation using blinded drawing of labels containing group assignment. |
| Blinding All outcomes | Unclear risk | No detail provided. |
| Incomplete outcome data addressed All outcomes | High risk | 42% of lost to follow‐up (up to 1 year postpartum). |
| Free of selective reporting | Low risk | Outcomes of relevance described. |
| Free of other bias | Low risk | Study supported by the American Heart Association Heartland Affiliate, award 0051330Z. No mention of any research protocol published a priori. The characteristics of participants were not significantly different between groups at baseline. |
Ostbye 2009.
| Methods | Participants were randomised 1:1 to the intervention or control group (stratified by black versus other and primiparous versus multiparous) using block randomisation. | |
| Participants | 450 overweight or obese women, aged 18 years or older, enrolled at 6 weeks postpartum. | |
| Interventions | Intervention: 8 healthy‐eating classes, 10 physical activity classes, and 6 telephone‐counselling sessions over 9 months. Emphasis was placed on reducing total caloric intake through a decrease in calorie‐dense foods and an increase in fruit and vegetable consumption, and on increasing physical activity to the recommended 30 minutes a day, 5 times a week. Every 6 weeks, women received 1 of 6 counselling sessions from a trained counsellor, lasting about 20 minutes each. These sessions were delivered primarily over the phone, but occasionally in person. Control: women in the control group received biweekly newsletters with general tips for postpartum mothers. Trial duration: long‐term. |
|
| Outcomes | Postpartum weight loss at 12 months postpartum. Other outcomes not considered in this review: energy intake, calories from fat, intake of certain foods, self‐reported physical activity and television time. |
|
| Notes | 70% of participants completed the follow‐up measures. At the follow‐up assessment, 24 women were pregnant again, and 5 had delivered a second baby; these 29 women were excluded from all analyses. 9% of weights recorded at the follow‐up assessment were self‐reported. In the intervention group, participants attended a mean of 3.8 classes and completed a mean of 3.3 counselling calls. Ten women completed no classes or calls. Those who took part in the classes were more likely to be older, white, and married, and have more education and higher income than those who did not participate. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation | Low risk | Randomisation in block. |
| Allocation concealment | Low risk | No detail provided. |
| Blinding All outcomes | High risk | 9% of weights recorded at the follow‐up assessment were self‐reported. |
| Incomplete outcome data addressed All outcomes | High risk | 30% of loss to follow‐up. Imbalanced data on weight change between groups. |
| Free of selective reporting | Low risk | Outcomes of relevance described. |
| Free of other bias | Low risk | This study was funded through the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK; R01DK064986). Study guided by a research protocol published a priori. The characteristics of participants were not significantly different between groups at baseline. |
BMI: body mass index WIC: the Special Supplemental Food Program for Women, Infants and Children
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Bopp 2005 | 1. Non‐clinical trial. The participants were grouped according to their exercise habits into exercise or sedentary group. The experimental part of the study consisted of returning, of a sub sample of exercise group, to the laboratory 2 additional times for rest and exercise sessions. 2. The experimental part of the study did not intend to create a caloric deficit for weight control, improve cardiorespiratory fitness or encourage women to increase their physical activity level. |
| Carey 1997 | 1. The intervention did not intend to create a caloric deficit for weight control, improve cardiorespiratory fitness or encourage women to increase their physical activity level. The intervention consisted of only 4 laboratory visits to perform exercise at 100%, 50% and 70% of VO2max and non‐exercise control session to determine if breast milk composition changed following exercise conducted at different intensities. 2. The study did not involve sedentary women as a control group. Every woman served as both an exercising volunteer and a non‐exercising control during the rest session. |
| Cramp 2006 | 1. The trial did not assess any outcome of interest. The intervention target was to improve physical activity adherence. |
| Davenport 2011 | 1. Use of historical control group. |
| Duckman 1968 | 1. Intervention for postpartum weight control involved medication. |
| Ebbeling 2007 | 1.The trial did not assess any outcome of interest. The study describes the conceptualisation and development of a theory‐based healthful eating and physical activity Intervention for postpartum women who are low income. No data are presented. |
| Fahrenwald 2004 | 1. Inclusion of individuals younger than 18 years of age. |
| Fjeldsoe 2010 | 1. The trial did not assess any outcome of interest. The primary outcome of this trial was moderate‐vigorous physical activity and our secondary outcomes included the targeted psychosocial constructs of the intervention. |
| Fly 1998 | 1. The intervention did not intend to create a caloric deficit for weight control, improve cardiorespiratory fitness or encourage women to increase their physical activity level. The intervention consisted of 2 laboratory visits for a maximal graded exercise test and resting control period. 2. The study did not involve sedentary women as a control group. Every woman served as both an exercising volunteer and a non‐exercising control during the rest session on different days. |
| Gregory 1997 | 1. The intervention did not intend to create a caloric deficit for weight control, improve cardiorespiratory fitness or encourage women to increase their physical activity level. 2. The study did not involve sedentary women as a control group. Every woman served as both an exercising volunteer and a non‐exercising control on different days. |
| Kinnunen 2007 | 1. Cluster‐controlled trial, but participating clinics were not randomly selected. 2. The primary aim of the main study was to prevent gestational diabetes. The intervention was initiated during pregnancy and not continued after delivery. |
| Koltyn 1997 | 1. The trial did not assess any outcome of interest. |
| Liu 2009 | 1.The trial did not assess any outcome of interest. |
| Lovelady 2003 | 1. Non‐clinical trial. The participants were grouped according to their exercise habits into exercise or sedentary group. The experimental part of the study consisted of returning, of a sub sample of exercise group, to the laboratory 2 additional times for rest and exercise sessions. 2. The experimental part of the study did not intend to create a caloric deficit for weight control, improve cardiorespiratory fitness or encourage women to increase their physical activity level. |
| Mohammad 2011 | 1. The intervention did not intent to create a caloric deficit for weight control, improve cardiorespiratory fitness or encourage women to increase their physical activity level or adopt healthier lifestyle. The intervention consisted in testing the effect of an isocaloric, isonitrogenous galactose drink on rates of lipolysis and fat oxidation during 3 days. 2. Randomised, cross‐over, single‐blinded design. |
| Moreau 2007 | 1. Intervention did not involve diet and/or exercise. The intervention involved administration of a nutraceutical compound (multivitamins soft‐capsules with Omega 3 and 6 fatty acids) for postpartum women. |
| Norman 2010 | 1.The trial did not assess any outcome of interest. The trial focused on maternal well‐being and risk of postnatal depression only. |
| Ostbye 2003 | 1. Non‐intervention study. The purpose of this study was to better understand the attitudes and preferences for weight loss among postpartum women. 2. The study refers to a planned trial. It is stated in the article that an intervention study is being designed; however, no more information was provided. |
| Quinn 1999 | 1. The comparison groups (high carbohydrate diet plus exercise versus moderate carbohydrate diet plus exercise) are not included in this review. 2. Dietary intervention involved no change in energy intake or dietary advice for weight reduction. 3. Exercise intervention did not intend to create a caloric deficit for weight control, improve cardiorespiratory fitness or encourage women to increase their physical activity level. The exercise programme consisted of 4 laboratory visits: 1 for maximal graded exercise test, 2 exercise sessions at different intensities and 1 rest session. |
| Wallace 1991 | 1. The intervention did not intent to create a caloric deficit for weight control, improve cardiorespiratory fitness or encourage women to increase their physical activity level. The intervention consisted of a maximal graded exercise test. 2. The study did not involve sedentary women as a control group. Every woman was assigned to an exercise test. The study compared data from pre‐exercise rest, exercise test and postexercise period. 3. Inclusion of women who had delivery over 12 months. |
| Wallace 1992a | 1. The intervention did not intend to create a caloric deficit for weight control, improve cardiorespiratory fitness or encourage women to increase their physical activity level. The intervention consisted of a maximal graded exercise test. The women were randomly assigned to group E which nursed prior to maximal exercise test and group F which did not nurse. 2. The study did not involve sedentary women as a control group. Every woman was assigned to an exercise test. |
| Wallace 1992b | 1. The intervention did not intend to create a caloric deficit for weight control, improve cardiorespiratory fitness or encourage women to increase their physical activity level. The intervention consisted of a maximal graded exercise test to assess the infant acceptance of postexercise breast milk. 2. The study did not involve sedentary women as a control group. Every woman was assigned to an exercise test. |
| Wright 2002 | 1. The intervention did not intend to create a caloric deficit for weight control, improve cardiorespiratory fitness or encourage women to increase their physical activity level. The intervention consisted of 4 laboratory visits: 1 for instructions, 2 for performing a maximal intensity and moderate exercise test, respectively and 1 rest session. 2. The study did not involve sedentary women as a control group. Every woman served as both an exercising volunteer and a non‐exercising control during the rest period on different days. |
Characteristics of ongoing studies [ordered by study ID]
Keller 2011.
| Trial name or title | Madres para la Salud (Mothers for Health) |
| Methods | Participants will be randomly assigned to the intervention or control group, using Random Allocation Software. The total number of participants is entered into the software, and is computed for 2 groups. Randomisation occurs after the baseline data collection. |
| Participants | Inclusion: habitually sedentary Latinas who are between the ages of 18 and 35, at least 6‐weeks but less than 6 months post childbirth, and physically able to participate in moderate intensity walking. Exclusion: participation in regular, strenuous physical activity exceeding 150 min of moderate physical activity weekly, severe musculoskeletal or cardiorespiratory problems that would preclude physical activity, currently pregnant or planning on becoming pregnant within the next 12 months, current use of antidepressants, infectious illness, acute or chronic systemic inflammation, BMI < 25 or BMI > 35, or regularly taking high doses of oral steroid medication, and women with osteoporosis at baseline. |
| Interventions | Intervention: 12 weekly walking sessions and support interventions with Promotoras. Control: standard care plus health newsletters and follow‐up phone calls. |
| Outcomes | Weight loss and body composition. |
| Starting date | Not stated. |
| Contact information | Colleen Keller. College of Nursing and Health Innovation, Arizona State University, Phoenix, AZ, United States. |
| Notes | The study has been completed, but results regarding weight loss have not been analysed yet. |
Peterson 2002.
| Trial name or title | Enhanced Expanded Food and Nutrition Education Program (EFNEP). |
| Methods | No details provided. |
| Participants | 700 postpartum women from 2 urban areas who are WIC eligible. |
| Interventions | Participants are randomised to the usual WIC care or Enhanced EFNEP intervention arm. The usual WIC care consists of nutrition education and breastfeeding consultation at the first postpartum and follow‐up visits up to 12 months from delivery. The Enhanced EFNEP intervention consists of usual WIC care plus a sustained, multi‐component intervention including home visits, group classes and monthly telephone counselling in the first 12 months postpartum and after 6 months of maintenance. The purpose of the study is to test the efficacy of an educational model in improving diet, activity and weight loss among new mothers. |
| Outcomes | BMI, fat mass and body fat distribution. |
| Starting date | Not stated. |
| Contact information | Peterson KE, Departments of Maternal and Child Health, and Nutrition, Harvard School of Public Health, Boston, MA ‐ USA. |
| Notes |
Phelan 2010.
| Trial name or title | Fit Moms ‐ an Internet‐based Postpartum Weight Loss Program (FM). |
| Methods | Randomised controlled trial. |
| Participants | Inclusion: age 18‐35 years; delivery within 6‐52 weeks (up to 6 months postpartum), exceed prepregnancy weight by at least 6.8 kg (15 pounds); current BMI > 22; English speaking; has computer with Internet access; literacy of at least 5th grade reading level. Exclusion: pregnant or planning to become pregnant; relocating in the next year; serious medical problem (i.e. heart disease, cancer, renal disease and diabetes), for which physician supervision of diet and exercise prescription is needed. |
| Interventions | Intervention: enhanced WIC weight loss program. Participants randomised into this condition will receive standard WIC care, but will also receive weight loss classes provided through the Internet. Topics will cover behavioural weight loss topics, based off the protocols of the Look AHEAD program. Control: standard WIC care. Participants randomised to this group will receive standard WIC care and an information packet surround healthy eating and activity topics. |
| Outcomes | Feasibility and effectiveness (weight loss) of protocol for WIC counsellors reinforcing adherence to web‐based program. |
| Starting date | June 2010. |
| Contact information | Dr. Suzanne Phelan, California Polytechnic State University, USA. |
| Notes | The study has been completed but results have not been published yet. |
Phelan 2011.
| Trial name or title | Prevention of Postpartum Weight Retention in Low Income WIC Women. |
| Methods | Randomised controlled trial. |
| Participants | Inclusion: age 18‐40 years; delivery within 6‐24 weeks (up to 6 months postpartum), exceed prepregnancy weight by at least 6.8 kg (15 pounds); current BMI > 22; English speaking; has computer with Internet access; literacy of at least 5th grade reading level. Exclusion: pregnant or planning to become pregnant; relocating in the next year; serious medical problem (i.e. heart disease, cancer, renal disease and diabetes), for which physician supervision of diet and exercise prescription is needed. |
| Interventions | Intervention: this group will be allowed access to an online weight loss program supplemented by monthly group meetings. The program is designed to help low income women lose weight through lifestyle intervention. Control: the control group will received Standard Care as provided through WIC. |
| Outcomes | Women randomised to the weight loss group will be assessed at study entry, 6 months, and 12 months. Weight is the primary outcome. |
| Starting date | July 2011. |
| Contact information | Peterson KE, Departments of Maternal and Child Health, and Nutrition, Harvard School of Public Health, Boston, MA ‐ USA. |
| Notes | July 2015 (estimated primary completion date for data collection). |
Redman 2011.
| Trial name or title | Postpartum Weight Loss and Exercise (PRIDE). |
| Methods | Randomised controlled trial. |
| Participants | 50 postpartum women ≥ 18 years to 45 years (inclusive) of age who experienced GDM during index pregnancy. |
| Interventions | Intervention I: face‐to‐face group: participants randomised to the face‐to‐face intervention will attend motivational meetings held once per week in Phase I and biweekly in Phase II. Behavioural sessions will be led by a trained interventionist and will take place at Pennington Biomedical Research Center. Intervention II: Telehealth group: participants randomised to the Telehealth intervention will receive behavioural counselling through Trestletree, phone system. Control: women will be provided a pedometer and written material on a healthy lifestyle. |
| Outcomes | Incidence of glucose abnormalities (impaired fasting glucose, impaired glucose tolerance, type 2 diabetes) and health outcomes (changes in body weight, body fat, waist circumference and blood lipids) in women with a history of gestational diabetes, 12 months postpartum. |
| Starting date | February 2011. |
| Contact information | Leanne M. Redman, Pennington Biomedical Research Center. |
| Notes | February 2012 (estimated date for final data collection date for primary outcome measure). |
Stendell‐Hollis 2011.
| Trial name or title | Not stated. |
| Methods | Randomised controlled trial. Randomisation was performed using a table of random numbers. |
| Participants | Inclusion: lactating women living in the greater Tucson, AZ area who were between 18 and 40 years of age and in general good health with no diagnosis or history of diabetes, liver or kidney disease, or cancer (other than non‐melanoma skin cancer). Primipara or multipara women were eligible if their infants were between the ages of 2 weeks and 6 months and met the following criteria: breastfeed for a minimum of 3 times per day for at least 6 additional months; use a non‐soy based formula if planning to supplement; refrain from oestrogen‐containing contraceptives; avoid use of all vitamins/supplements for the duration of the study with the exception of the study provided prenatal vitamins. Exclusion: use of tobacco products or having a family history of food allergies. |
| Interventions | Intervention: Mediterranean‐style diet rich in walnuts. Control: USDA's MyPyramid diet for pregnancy and breastfeeding. All participants were provided nutrition education, lifestyle counselling, and support to adopt and adhere to the assigned study diet via 1 on‐1 diet education with a Registered Dietitian at the baseline, 2 week, and 2‐month clinic visits; written materials as well as telephone consultations with a registered dietitian bi‐monthly for the first 2 months on study and then once during the third month of the study. Participants in both groups were instructed to consume the study provided prenatal vitamin daily. |
| Outcomes | Anthropometric measurements (BMI, % body fat, waist circumference, hip circumference and waist to hip ratio). |
| Starting date | No detail provided. |
| Contact information | Nicole R. Stendell‐Hollis. Nutritional Sciences Department, University of Arizona, 1177 E. 4th St., Tucson, AZ, 85721, USA. nhollis@email.arizona.edu |
| Notes | The study has been completed but results regarding postpartum reduction in anthropometric measurements (main outcome) have not been published yet. |
Winkvist 2011.
| Trial name or title | Short‐ and Long‐Term Effects of Physical Activity and Dietary Restriction Postpartum (LEVA). |
| Methods | Randomised controlled trial. |
| Participants | 68 women with prepregnancy BMI 25 ‐ 34.9 at 10 weeks postpartum. |
| Interventions | Intervention I: Diet 12‐week diet modification intervention by dietician. Intervention II: Exercise 12‐week physical exercise modification intervention by physical therapist Intervention III: Diet and Exercise 12‐week diet and exercise behavioral modification by dietician and physical therapist. Control: standard procedure. |
| Outcomes | Weight loss, body composition, cardiovascular fitness, blood lipids, insulin levels and inflammation markers. |
| Starting date | May 2007 (Starting date). August 2010 (final data collection date for primary outcome measures). |
| Contact information | Anna Winkvist, Professor, The University of Gothenburg. |
| Notes | The study has been completed but main findings regarding postpartum weight loss have not been published yet. |
BMI: body mass index GDM: gestational diabetes mellitus WIC: the Special Supplemental Food Program for Women, Infants and Children
Differences between protocol and review
In the protocol the inclusion criteria was restricted to women recruited up to 12 months postpartum. In the review update, we extended the recruitment period to 24 months postpartum. In the review update, we also included one additional outcome related to breastfeeding performance (percentage of partial or exclusive breastfeeding by the end of the intervention). In the protocol, only the duration of breastfeeding in months was considered.
Contributions of authors
Amanda R Amorim Adegboye developed the protocol and the review and was responsible for revising the drafts in response to editorial comments. Yvonne Linne commented on the drafts and participated in the data extraction and quality assessment of the selected studies.
Sources of support
Internal sources
No sources of support supplied
External sources
Brazilian Foundation (CAPES), Brazil.
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Armstrong 2003 {published data only}
- Armstrong K, Edwards H. The effects of exercise and social support on mothers reporting depressive symptoms: a pilot randomized controlled trial. International Journal of Mental Health Nursing 2003;12:130‐8. [DOI] [PubMed] [Google Scholar]
Armstrong 2004 {published data only}
- Armstrong K, Edwards H. The effectiveness of a pram‐walking exercise programme in reducing depressive symptomatology for postnatal women. International Journal of Nursing Practice 2004;10(4):177‐94. [DOI] [PubMed] [Google Scholar]
Craigie 2011 {published data only}
- Craigie AM, Macleod M, Barton KL, Treweek S, Anderson AS. Supporting postpartum weight loss in women living in deprived communities: Design implications for a randomised control trial. European Journal of Clinical Nutrition 2011;65(8):952‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Dewey 1994a {published and unpublished data}
- Dewey KG, Lovelady CA, Nommsen‐Rivers LA, McCrory MA, Lonnerdal B. A randomized study of the effects of aerobic exercise by lactating women on breast‐milk volume and composition. New England Journal of Medicine 1994;330(7):449‐53. [DOI] [PubMed] [Google Scholar]
- Lovelady CA, Nommsen‐Rivers LA, McCrory MA, Dewey KG. Effects of exercise on plasma lipids and metabolism of lactating women. Medicine & Science in Sports & Exercise 1995;27(1):22‐8. [PubMed] [Google Scholar]
- Prentice A. Should lactating women exercise?. Nutrition Reviews 1994;52(10):358‐60. [DOI] [PubMed] [Google Scholar]
Ferrara 2011 {published data only}
- Ferrara A. Diet, exercise and breastfeeding intervention program for women with gestational diabetes (DEBI Trial). ClinicalTrials.gov (http://clinicaltrials.gov/) (accessed 20 February 2008) 2008.
- Ferrara A, Hedderson MM, Albright CL, Ehrlich SF, Quesenberry CP Jr, Peng T, et al. A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care 2011;34(7):1519‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Huang 2011 {published data only}
- Huang TT, Yeh CY, Tsai YC. A diet and physical activity intervention for preventing weight retention among Taiwanese childbearing women: a randomised controlled trial. Midwifery 2011;27(2):257‐64. [DOI] [PubMed] [Google Scholar]
Kearney 2006 {published data only}
- Kearney M. Influences on postpartum weight retention: pilot study. Virginia Henderson International Nursing Library (www.nursinglibrary.org) (accessed 10 January 2007) 2005.
- Kearney MH, Simonelli MC. Intervention fidelity: lessons learned from an unsuccessful pilot study. Applied Nursing Research 2006;19(3):163‐6. [DOI] [PubMed] [Google Scholar]
Krummel 2010 {published data only}
- Krummel D, Semmens E, MacBride AM, Fisher B. Lessons learned from the mothers' overweight management study in 4 West Virginia WIC Offices. Journal of Nutrition Education and Behavior 2010;42(3 Suppl):S52‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krummel DA, Semmens E, Boury J, Gordon PM, Larkin KT. Stages of change for weight management in postpartum women. Journal of the American Dietetic Association 2004;104(7):1102‐8. [DOI] [PubMed] [Google Scholar]
Leermakers 1998 {published data only}
- Leermakers EA, Anglin K, Wing RR. Reducing postpartum weight retention through a correspondence intervention. International Journal of Obesity and Related Metabolic Disorders 1998;22(11):1103‐9. [DOI] [PubMed] [Google Scholar]
Lovelady 2000 {published and unpublished data}
- Lovelady CA, Garner KE, Moreno KL, Williams JP. The effect of weight loss in overweight, lactating women on the growth of their infants. New England Journal of Medicine 2000;342(7):449‐53. [DOI] [PubMed] [Google Scholar]
- Lovelady CA, Stephenson KG, Kuppler KM, Williams JP. The effects of dieting on food and nutrient intake of lactating women. Journal of the American Dietetic Association 2006;106(6):908‐12. [DOI] [PubMed] [Google Scholar]
- Lovelady CA, Williams JP, Garner KE, Moreno KL, Taylor Ml, Leklem JE. Effect of energy restriction and exercise on vitamin B‐6 status of women during lactation. Medicine & Science in Sports & Exercise 2001;33(4):512‐8. [DOI] [PubMed] [Google Scholar]
- Mukherjea R, Moser‐Veillon P, Lovelady C. Effect of exercise and energy restriction on leptin during lactation. Advances in Experimental Medicine and Biology 2000;478:417‐8. [DOI] [PubMed] [Google Scholar]
Lovelady 2009 {published data only}
- Lovelady CA, Bopp MJ, Colleran HL, Mackie HK, Wideman L. Effect of exercise training on loss of bone mineral density during lactation. Medicine & Science in Sports & Exercise 2009;41(10):1902‐7. [DOI] [PubMed] [Google Scholar]
McCrory 1999 {published and unpublished data}
- McCrory MA, Nommsen‐Rivers LA, Mole PA, Lonnerdal B, Dewey KG. Randomized trial of the short‐term effects of dieting compared with dieting plus aerobic exercise on lactation performance. American Journal of Clinical Nutrition 1999;69(5):959‐67. [DOI] [PubMed] [Google Scholar]
O'Toole 2003 {published data only}
- O'Toole ML, Sawicki MA, Artal R. Structured diet and physical activity prevent postpartum weight retention. Journal of Women's Health 2003;12(10):991‐8. [DOI] [PubMed] [Google Scholar]
Ostbye 2009 {published data only}
- Bastian LA, Pathiraja VC, Krause K, Namenek Brouwer RJ, Swamy GK, Lovelady CA, et al. Multiparity is associated with high motivation to change diet among overweight and obese postpartum women. Womens Health Issues 2010;20(2):133‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostbye T, Krause KM, Brouwer RJ, Lovelady CA, Morey MC, Bastian LA, et al. Active Mothers Postpartum (AMP): rationale, design, and baseline characteristics. Journal of Women's Health 2008;17(10):1567‐75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostbye T, Krause KM, Lovelady CA, Morey MC, Bastian LA, Peterson BL, et al. Active Mothers Postpartum: a randomized controlled weight‐loss intervention trial. American Journal of Preventive Medicine 2009;37(3):173‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
Bopp 2005 {published data only}
- Bopp M, Lovelady C, Hunter C, Kinsella T. Maternal diet and exercise: effects on long‐chain polyunsaturated fatty acid concentrations in breast milk. Journal of the American Dietetic Association 2005;105(7):1098‐103. [DOI] [PubMed] [Google Scholar]
Carey 1997 {published data only}
- Carey GB, Quinn TJ, Goodwin SE. Breast milk composition after exercise of different intensities. Journal of Human Lactation 1997;13(2):115‐20. [DOI] [PubMed] [Google Scholar]
Cramp 2006 {published data only}
- Cramp AG, Brawley LR. Moms in motion: a group‐mediated cognitive‐behavioral physical activity intervention. International Journal of Behavioral Nutrition and Physical Activity 2006;3:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
Davenport 2011 {published data only}
- Davenport MH, Giroux I, Sopper MM, Mottola MF. Postpartum exercise regardless of intensity improves chronic disease risk factors. Medicine and Science in Sports and Exercise 2011;43(6):951‐8. [DOI] [PubMed] [Google Scholar]
Duckman 1968 {published data only}
- Duckman S, Chen W, Weir JH. Double‐blind evaluation of chlorphentermine hydrochloride vs placebo in postpartum weight control. Current Therapeutic Research 1968;10:619‐25. [PubMed] [Google Scholar]
Ebbeling 2007 {published data only}
- Ebbeling CB, Pearson MN, Sorensen G, Levine RA, Hebert JR, Salkeld JA, et al. Conceptualization and development of a theory‐based healthful eating and physical activity intervention for postpartum women who are low income. Health Promotion Practice 2007;8(1):50‐9. [DOI] [PubMed] [Google Scholar]
Fahrenwald 2004 {published data only}
- Fahrenwald NL, Atwood JR, Johnson DR. Mediator analysis of Moms on the move. Western Journal of Nursing Research 2005;27(3):271‐91. [DOI] [PubMed] [Google Scholar]
- Fahrenwald NL, Atwood JR, Walker SN, Johnson DR, Berg K. A randomized pilot test of "Moms on the Move": a physical activity intervention for WIC mothers. Annals of Behavioral Medicine 2004;27(2):82‐90. [DOI] [PubMed] [Google Scholar]
- Fahrenwald NL, Sharma M. Development and expert evaluation of "Moms on the Move," a physical activity intervention for WIC mothers. Public Health Nursing 2002;19(6):423‐39. [DOI] [PubMed] [Google Scholar]
- Fahrenwald NL, Walker SN. Application of the Transtheoretical Model of behavior change to the physical activity behavior of WIC mothers. Public Health Nursing 2003;20(4):307‐17. [DOI] [PubMed] [Google Scholar]
Fjeldsoe 2010 {published data only}
- Fjeldsoe BS, Miller YD, Marshall AL. MobileMums: a randomized controlled trial of an SMS‐based physical activity intervention. Annals of Behavioral Medicine 2010;39(2):101‐11. [DOI] [PubMed] [Google Scholar]
Fly 1998 {published data only}
- Fly AD, Uhlin KL, Wallace JP. Major mineral concentrations in human milk do not change after maximal exercise testing. American Journal of Clinical Nutrition 1998;68(2):345‐9. [DOI] [PubMed] [Google Scholar]
Gregory 1997 {published data only}
- Gregory RL, Wallace JP, Gfell LE, Marks J, King BA. Effect of exercise on milk immunoglobulin A. Medicine & Science in Sports & Exercise 1997;29(12):1596‐601. [DOI] [PubMed] [Google Scholar]
Kinnunen 2007 {published data only}
- Aittasalo M, Pasanen M, Fogelholm M, Kinnunen TI, Ojala K, Luoto R. Physical activity counseling in maternity and child health care ‐ a controlled trial. BMC Women's Health 2008;8:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen TI, Aittasalo M, Koponen P, Ojala K, Mansikkamäki K, Weiderpass E, et al. Feasibility of a controlled trial aiming to prevent excessive pregnancy‐related weight gain in primary health care. BMC Pregnancy and Childbirth 2008;8:37‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen TI, Pasanen M, Aittasalo M, Fogelholm M, Weiderpass E, Luoto R. Reducing postpartum weight retention‐‐a pilot trial in primary health care. Nutrition Journal 2007;6:21‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
Koltyn 1997 {published data only}
- Koltyn KF, Schultes SS. Psychological effects of an aerobic exercise session and a rest session following pregnancy. Journal of Sports Medicine and Physical Fitness 1997;37(4):287‐91. [PubMed] [Google Scholar]
Liu 2009 {published data only}
- Liu N, Mao L, Sun X, Liu L, Yao P, Chen B. The effect of health and nutrition education intervention on women's postpartum beliefs and practices: a randomized controlled trial. BMC Public Health 2009;9:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lovelady 2003 {published data only}
- Lovelady CA, Hunter CP, Geigerman CM. Effect of exercise on immunologic factors in breast milk. Pediatrics 2003;111(2):148‐52. [DOI] [PubMed] [Google Scholar]
Mohammad 2011 {published data only}
- Mohammad MA, Sunehag AL, Rodriguez LA, Haymond MW. Galactose promotes fat mobilization in obese lactating and nonlactating women. American Journal of Clinical Nutrition 2011;93(2):374‐81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Moreau 2007 {published data only}
- Moreau M, Chadoutaud B, Msika P. An innovative nutraceutical compound for postpartum localized overweight or for postpartum body remodeling. Journal of the American Academy of Dermatology 2007;56(2):AB89, Abstract no: P1019. [Google Scholar]
Norman 2010 {published data only}
- Norman E, Sherburn M, Osborne RH, Galea MP. An exercise and education program improves well‐being of new mothers: a randomized controlled trial. Physical Therapy 2010;90(3):348‐55. [DOI] [PubMed] [Google Scholar]
Ostbye 2003 {published data only}
- Ostbye T, McBride C, Demark‐Wahnefried W, Bastian L, Morey M, Krause KM, et al. Interest in healthy diet and physical activity interventions peripartum among female partners of active duty military. Military Medicine 2003;168(4):320‐5. [PubMed] [Google Scholar]
Quinn 1999 {published data only}
- Quinn TJ, Carey GB. Does exercise intensity or diet influence lactic acid accumulation in breast milk?. Medicine & Science in Sports & Exercise 1999;31(1):105‐10. [DOI] [PubMed] [Google Scholar]
Wallace 1991 {published data only}
- Wallace JP, Rabin J. The concentration of lactic acid in breast milk following maximal exercise. International Journal of Sports Medicine 1991;12(3):328‐31. [DOI] [PubMed] [Google Scholar]
Wallace 1992a {published data only}
- Wallace JP, Ernsthausen K, Inbar G. The influence of the fullness of milk in the breasts on the concentration of lactic acid in postexercise breast milk. International Journal of Sports Medicine 1992;13(5):395‐8. [DOI] [PubMed] [Google Scholar]
Wallace 1992b {published data only}
- Wallace JP, Inbar G, Ernsthausen K. Infant acceptance of postexercise breast milk. Pediatrics 1992;89:1245‐7. [PubMed] [Google Scholar]
Wright 2002 {published data only}
- Wright KS, Quinn TJ, Carey GB. Infant acceptance of breast milk after maternal exercise. Pediatrics 2002;109:585‐9. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Albright 2012 {published data only}
- Albright CL, Steffen AD, Novotny R, Nigg CR, Wilkens LR, Saiki K, et al. Baseline results from Hawaii's Na Mikimiki Project: a physical activity intervention tailored to multiethnic postpartum women. Women & Health 2012;52(3):265‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bao 2010 {published data only}
- Bao W, Ma A, Mao L, Lai J, Xiao M, Sun G, et al. Diet and lifestyle interventions in postpartum women in China: study design and rationale of a multicenter randomized controlled trial. BMC Public Health 2010;10:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Bertz 2012 {published data only}
- Bertz F, Brekke HK, Ellegard L, Rasmussen KM, Wennergren M, Winkvist A. Diet and exercise weight‐loss trial in lactating overweight and obese women. American Journal of Clinical Nutrition 2012;96(4):698‐705. [DOI] [PubMed] [Google Scholar]
Boothe 2011 {published data only}
- Boothe AS, Brouwer RJ, Carter‐Edwards L, Ostbye T. Unmet social support for healthy behaviors among overweight and obese postpartum women: results from the Active Mothers Postpartum Study. Journal of Women's Health (2002) 2011;20(11):1677‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Colleran 2011 {published data only}
- Colleran HL, Sorvillo A, Cooney P, Wideman L, Lovelady CA. Effects of energy restriction and exercise on bone mineral density and hormones in overweight lactating women. FASEB Journal 2011;25:211.6. [Google Scholar]
Colleran 2012 {published data only}
- Colleran HL, Lovelady CA. Use of MyPyramid Menu Planner for Moms in a weight‐loss intervention during lactation. Journal of the Academy of Nutrition & Dietetics 2012;112(4):553‐8. [DOI] [PubMed] [Google Scholar]
Currie 2013 {published data only}
- Currie M, Reay R, Thompson J, Raphael B, Ellwood D. Physical exercise and postnatal emotional wellbeing: A randomised controlled trial (RCT) comparing two exercise and support programs. Journal of Paediatrics and Child Health 2013;49 Suppl 2:15. [Google Scholar]
French 2012 {published data only}
- French GM, Nicholson L, Skybo T, Klein EG, Schwirian PM, Murray‐Johnson L, et al. An evaluation of mother‐centered anticipatory guidance to reduce obesogenic infant feeding behaviors. Pediatrics 2012;130(3):e507‐17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Gaston 2011 {published data only}
- Gaston A, Gammage L. The effectiveness of a health‐based message on pregnant women's intentions to exercise postpartum. Journal of Reproductive & Infant Psychology 2011;29(2):162‐9. [Google Scholar]
Gilinsky 2012 {published data only}
- Gilinsky AS, Hughes AR, McInnes RJ. More Active Mums in Stirling (MAMMiS): a physical activity intervention for postnatal women. Study protocol for a randomized controlled trial. Trials 2012;13:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ladefoged 2012 {published data only}
- Ladefoged ML, Bor P, Svendlund R, Sondergaard N, Hojgaard A. The effect of introducing regular exercise during pregnancy and postpartum in physically inactive women. Acta Obstetricia et Gynecologica Scandinavica 2012;39(Suppl 159):102‐3. [Google Scholar]
Maturi 2011 {published data only}
- Maturi MS, Afshary P, Abedi P. Effect of physical activity intervention based on a pedometer on physical activity level and anthropometric measures after childbirth: A randomized controlled trial. BMC Pregnancy and Childbirth 2011;11:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Mohammad 2009 {published data only}
- Mohammad MA, Sunehag AL, Haymond MW. Effect of dietary macronutrient composition under moderate hypocaloric intake on maternal adaptation during lactation. American Journal of Clinical Nutrition 2009;89(6):1821‐7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Stendell‐Hollis 2011a {published data only}
- Stendell‐Hollis N, Thompson P, Winzerling J, Daines M, Thomson C. Adherence to a Mediterranean‐style diet and change in inflammatory biomarkers and fatty acid profiles in lactating women. FASEB Journal 2011;25:589.4. [Google Scholar]
Stendell‐Hollis 2013 {published data only}
- Stendell‐Hollis NR, Thompson PA, West JL, Wertheim BC, Thomson CA. A comparison of mediterranean‐style and mypyramid diets on weight loss and inflammatory biomarkers in postpartum breastfeeding women. Journal of Women's Health 2013;22(1):48‐57. [DOI] [PMC free article] [PubMed] [Google Scholar]
Surkan 2012 {published data only}
- Surkan PJ, Gottlieb BR, McCormick MC, Hunt A, Peterson KE. Impact of a health promotion intervention on maternal depressive symptoms at 15 months postpartum. Maternal & Child Health Journal 2012;16(1):139‐48. [DOI] [PubMed] [Google Scholar]
Walker 2012 {published data only}
- Walker LO, Sterling BS, Latimer L, Kim SH, Garcia AA, Fowles ER. Ethnic‐Specific Weight‐Loss Interventions for Low‐Income Postpartum Women: Findings and Lessons. Western Journal of Nursing Research 2012;34(5):654‐76. [DOI] [PubMed] [Google Scholar]
Watson 2005 {published data only}
- Watson N, Milat AJ, Thomas M, Currie J. The feasibility and effectiveness of pram walking groups for postpartum women in western Sydney. Health Promotion Journal of Australia 2005;16(2):93‐9. [DOI] [PubMed] [Google Scholar]
Wiltheiss 2013 {published data only}
- Wiltheiss GA, Lovelady CA, West DG, Brouwer RJ, Krause KM, Ostbye T. Diet quality and weight change among overweight and obese postpartum women enrolled in a behavioral intervention program. Journal of the Academy of Nutrition & Dietetics 2013;113(1):54‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to ongoing studies
Keller 2011 {published data only}
- Keller C, Records K, Ainsworth B, Belyea M, Permana P, Coonrod D, et al. Madres para la Salud: design of a theory‐based intervention for postpartum Latinas. Contemporary Clinical Trials 2011;32(3):418‐27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller C, Records K, Coe K, Ainsworth B, López SV, Nagle‐Williams A, et al. Promotoras' roles in integrative validity and treatment fidelity efforts in randomized controlled trials. Family and Community Health 2012;35(2):120‐9. [DOI] [PubMed] [Google Scholar]
Peterson 2002 {published data only}
- Peterson KE, Sorensen G, Pearson M, Hebert JR, Gottlieb BR, McCormick MC. Design of an intervention addressing multiple levels of influence on dietary and activity patterns of low‐income, postpartum women. Health Education Research 2002;17(5):531‐40. [DOI] [PubMed] [Google Scholar]
Phelan 2010 {published data only}
- Phelan S. Fit Moms‐ an internet‐based postpartum weight loss program (FM). http://clinicaltrials.gov/ct2/show/NCT01096888 (accessed 23 February 2011).
Phelan 2011 {published data only (unpublished sought but not used)}
- Phelan S. Prevention of postpartum weight retention in low income WIC women. http://clinicaltrials.gov/ct2/show/NCT01408147 (accessed 23 February 2012) 2011.
Redman 2011 {published data only}
- Redman L. Early postpartum treatment of gestational diabetes with weight loss and exercise. http://clinicaltrials.gov/ct2/show/NCT01296516 (accessed 23 February 2011).
Stendell‐Hollis 2011 {published data only}
- Stendell‐Hollis NR, Laudermilk MJ, West JL, Thompson PA, Thomson CA. Recruitment of lactating women into a randomized dietary intervention: Successful strategies and factors promoting enrollment and retention. Contemporary Clinical Trials 2011;32(4):505‐11. [DOI] [PubMed] [Google Scholar]
Winkvist 2011 {published data only}
- Winkvist A. Short‐ and long term effects of physical activity and dietary restriction postpartum (LEVA). http://clinicaltrials.gov/ct2/show/NCT01343238 (accessed 23 February 2012).
Additional references
AbuSabha 1998
- AbuSabha R, Greene G. Body weight, body composition, and energy intake changes in breastfeeding mothers. Journal of Human Lactation 1998;14(2):119‐24. [DOI] [PubMed] [Google Scholar]
Alderson 2004
- Alderson P, Green S, Higgins JPT, editors. Cochrane Reviews' Handbook 4.2.2 [updated March 2004]. http://www.cochrane.org/resources/handbook/hbook.htm (accessed 26 September 2004).
Ballor 1994
- Ballor DL, Poehlman ET. Exercise‐training enhances fat‐free mass preservation during diet‐induced weight loss: a meta‐analytical finding. International Journal of Obesity and Related Metabolic Disorders 1994;18(1):35‐40. [PubMed] [Google Scholar]
Clark 1999
- Clark M, Ogden J. The impact of pregnancy on eating behaviour and aspects of weight concern. International Journal of Obesity and Related Metabolic Disorders 1999;23(1):18‐24. [DOI] [PubMed] [Google Scholar]
Coward 1984
- Coward WA, Paul AA, Prentice AM. The impact of malnutrition on human lactation: observations from community studies. Federation Proceedings 1984;43(9):2432‐7. [PubMed] [Google Scholar]
Crowell 1995
- Crowell DT. Weight change in postpartum period. A review of the literature. Journal of Nurse‐Midwifery 1995;40(5):418‐23. [DOI] [PubMed] [Google Scholar]
DeCs 2004
- Health Sciences Descriptors (DeCS). Virtual Health Library (VHL). http://decs.bvs.br/ (accessed 11 August 2004).
Dewey 1994b
- Dewey KG, Lovelady CA, Nommsen‐Rivers LA, McCrory MA, Lonnerdal B. A randomized study of the effects of aerobic exercise by lactating women on breast‐milk volume and composition. New England Journal of Medicine 1994;330(7):449‐53. [DOI] [PubMed] [Google Scholar]
Dusdieker 1994
- Dusdieker LB, Hemingway DL, Stumbo PJ. Is milk production impaired by dieting during lactation?. American Journal of Clinical Nutrition 1994;59(4):833‐40. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ 1997;315(7109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Garrow 1995
- Garrow JS, Summerbell CD. Meta‐analysis: effect of exercise, with or without dieting, on the body composition of overweight subjects. European Journal of Clinical Nutrition 1995;49(1):1‐10. [PubMed] [Google Scholar]
Gore 2003
- Gore SA, Brown DM, West DS. The role of postpartum weight retention in obesity among women: a review of the evidence. Annals of Behavioral Medicine 2003;26(2):149‐59. [DOI] [PubMed] [Google Scholar]
Greene 1988
- Greene GW, Smiciklas‐Wright H, Scholl TO, Karp RJ. Postpartum weight change: how much of the weight gained in pregnancy will be lost after delivery?. Obstetrics & Gynecology 1988;71(5):701‐7. [PubMed] [Google Scholar]
Gunderson 1999
- Gunderson EP, Abrams B. Epidemiology of gestational weight gain and body weight changes after pregnancy. Epidemiologic Reviews 1999;21(2):261‐75. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S, editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
IOM 1990
- Institute of Medicine. Nutrition During Pregnancy. Washington, DC: National Academy Press, 1990. [Google Scholar]
IOM 1991
- Institute of Medicine. Nutrition During Lactation. Washington, DC: National Academy Press, 1991. [Google Scholar]
Kac 2003
- Kac G, D'Aquino Benicio MH, Valente JG, Velasquez‐Melendez G. Postpartum weight retention among women in Rio de Janeiro: a follow‐up study. Reports in Public Health 2003;19(1 Suppl):149S‐161S. [DOI] [PubMed] [Google Scholar]
Keppel 1993
- Keppel KG, Taffel SM. Pregnancy‐related weight gain and retention: implications of the 1990 Institute of Medicine Guidelines. American Journal of Public Health 1993;83(8):1100‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Krummel 2004
- Krummel DA, Semmens E, Boury J, Gordon PM, Larkin KT. Stages of change for weight management in postpartum women. Journal of the American Dietetic Association 2004;104(7):1102‐8. [DOI] [PubMed] [Google Scholar]
Linne 2002
- Linne Y, Barkeling B, Rossner S. Long‐term weight development after pregnancy. Obesity Reviews 2002;3(2):75‐83. [DOI] [PubMed] [Google Scholar]
Linne 2003a
- Linne Y, Rossner S. Easy to remain overweight after pregnancy. Lakartidningen 2003;100(49):4091‐5. [PubMed] [Google Scholar]
Linne 2003b
- Linne Y, Dye L, Barkeling B, Rossner S. Weight development over time in parous women‐‐the SPAWN study‐‐15 years follow‐up. International Journal of Obesity and Related Metabolic Disorder 2003;27:1516‐22. [DOI] [PubMed] [Google Scholar]
Linne 2003c
- Linne Y, Rossner S. Interrelationship between weight development and weight retention in subsequent pregnancies: the SPAWN study. Acta Obstetricia et Gynecologica Scandinavica 2003;82(4):318‐25. [DOI] [PubMed] [Google Scholar]
Linne 2004
- Linne Y. Effects of obesity on women's reproduction and complications during pregnancy. Obesity Reviews 2004;5(3):137‐43. [DOI] [PubMed] [Google Scholar]
Lovelady 1995
- Lovelady CA, Nommsen‐Rivers LA, McCrory MA, Dewey KG. Effects of exercise on plasma lipids and metabolism of lactating women. Medicine & Science in Sports & Exercise 1995;27(1):22‐8. [PubMed] [Google Scholar]
Lovelady 2001
- Lovelady CA, Williams JP, Garner KE, Moreno KL, Taylor Ml, Leklem JE. Effect of energy restriction and exercise on vitamin B‐6 status of women during lactation. Medicine & Science in Sports & Exercise 2001;33(4):512‐8. [DOI] [PubMed] [Google Scholar]
Lovelady 2006
- Lovelady CA, Stephenson KG, Kuppler KM, Williams JP. The effects of dieting on food and nutrient intake of lactating women. Journal of the American Dietetic Association 2006;106(6):908‐12. [DOI] [PubMed] [Google Scholar]
Manson 1990
- Manson JE. A prospective study of obesity and risk of coronary heart disease in women. New England Journal of Medicine 1990;322(13):882‐9. [DOI] [PubMed] [Google Scholar]
Mestecky 1986
- Mestecky J, Russell MW, Jackson S, Brown TA. The human IgA system: a reassessment. Clinical Immunology and Immunopathology 1986;40(1):105‐14. [DOI] [PubMed] [Google Scholar]
Mottola 2002
- Mottola MF. Exercise in the postpartum period: practical applications. Current Sports Medicine Reports 2002;1(6):362‐8. [DOI] [PubMed] [Google Scholar]
Mukherjea 2000
- Mukherjea R, Moser‐Veillon P, Lovelady C. Effect of exercise and energy restriction on leptin during lactation. Advances in Experimental Medicine and Biology 2000;478:417‐8. [DOI] [PubMed] [Google Scholar]
Ohlin 1990
- Ohlin A, Rossner S. Maternal body weight development after pregnancy. International Journal of Obesity 1990;14(2):159‐73. [PubMed] [Google Scholar]
Olson 2003
- Olson CM, Strawderman MS, Hinton PS, Pearson TA. Gestational weight gain and postpartum behaviors associated with weight change from early pregnancy to 1 y postpartum. International Journal of Obesity and Related Metabolic Disorders 2003;27(1):117‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Prentice 1994
- Prentice AM, Goldberg GR, Prentice A. Body mass index and lactation performance. European Journal of Clinical Nutrition 1994;48(3 Suppl):78S‐86S. [PubMed] [Google Scholar]
RevMan 2003 [Computer program]
- The Cochrane Collaboration. Review Manager (RevMan). Version 4.2 for Windows. Oxford, England: The Cochrane Collaboration, 2003.
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Rooney 2002
- Rooney BL, Schauberger CW. Excess pregnancy weight gain and long‐term obesity: one decade later. Obstetrics & Gynecology 2002;100(2):245‐52. [DOI] [PubMed] [Google Scholar]
Rossner 1992
- Rossner S. Pregnancy, weight cycling and weight gain in obesity. International Journal of Obesity and Related Metabolic Disorders 1992;16(2):145‐7. [PubMed] [Google Scholar]
Rossner 1995
- Rossner S, Ohlin A. Pregnancy as a risk factor for obesity: lessons from the Stockholm Pregnancy and Weight Development Study. Obesity Research 1995;3(2 Suppl):267S‐275S. [DOI] [PubMed] [Google Scholar]
Sadurkis 1988
- Sadurkis A, Kabir N, Wager J, Forsum E. Energy metabolism, body composition and milk production in healthy Swedish women during lactation. American Journal of Clinical Nutrition 1988;48(1):44‐9. [DOI] [PubMed] [Google Scholar]
Schauberger 1992
- Schauberger CW, Rooney BL, Brimer LM. Factors that influence weight loss in the puerperium. Obstetrics & Gynecology 1992;79(3):424‐9. [DOI] [PubMed] [Google Scholar]
Strode 1986
- Strode MA, Dewey KG, Lonnerdal B. Effects of short‐term caloric restriction on lactational performance of well‐nourished women. Acta Paediatrica Scandinavica 1986;75(2):222‐9. [DOI] [PubMed] [Google Scholar]
Symons Downs 2004
- Symons Downs D, Hausenblas HA. Women's exercise beliefs and behaviors during their pregnancy and postpartum. American College of Nurse‐Midwives 2004;49(2):138‐44. [DOI] [PubMed] [Google Scholar]
WHO 1991
- World Health Organization. Indicators for assessing breastfeeding practices. Report of an informal meeting. Geneva: WHO, 1991. [Google Scholar]
WHO 1998
- World Health Organization. Obesity: preventing and managing the global epidemic. Report of a WHO Consultation on Obesity. Geneva: WHO, 1998. [PubMed] [Google Scholar]
Wood 2004
- Wood S, Hildebrandt LA. Use of low‐carbohydrate diets during lactation. Implications for mothers and infants. Topics in Clinical Nutrition 2004;19(4):286‐96. [Google Scholar]
References to other published versions of this review
Amorim Adegboye 2007
- Amorim Adegboye AR, Linne YM, Lourenco PMC. Diet or exercise, or both, for weight reduction in women after childbirth. Cochrane Database of Systematic Reviews 2007, Issue 3. [DOI: 10.1002/14651858.CD005627.pub2] [DOI] [PubMed] [Google Scholar]


