Abstract
Introduction: Photodynamic therapy (PDT) is now a widely used treatment modality in many fields of dentistry, including endodontics. The most common application of PDT in endodontics is to disinfect root canals. The purpose of this study was to present the experience of using PDT in root canal disinfection of three patients.
Case Presentation: Three patients referred to the Endodontics Department of Shahid Beheshti University of Medical Sciences were treated using 0.1 mg/mL Toluidine Blue (FotoSan® agent; CMS Dental, Denmark) irradiated with a light-emitting diode (LED) lamp (FotoSan®; CMS Dental, Denmark) with a mean wavelength of 630 nm and a mean power density of 3 W/cm2 over two 30-second periods.
Conclusion: PDT using LED lighting can be used in conjunction with conventional root canal treatment (RCT) to achieve great results.
Keywords: Photodynamic therapy, Disinfection, Endodontics, Root canal therapy
Introduction
Photons have a variety of therapeutic applications in medicine based on the reaction they cause. They can have photomechanical, photothermal, photobiostimulative, and photochemical effect, which occurs in photodynamic therapy (PDT).1,2
In 1900, PDT was discovered accidentally by a medical student named Oscar Raab while researching the toxicity of Acridine red dye on paramecia. He noticed that as the time of day and daylight changed, so did the toxicity of the dye. Five years later, von Tappeiner and Jesionek named this phenomenon “Photodynamic Phenomenon” after using it to treat skin cancer.3
The mechanism of PDT is based on a triad consisting of a non-toxic molecule known as a photosensitizer (PS), a light source (e.g., lasers, fluorescent lamps, LEDs), and molecular oxygen.3 The microorganisms first absorb the PS, and then it gets activated by light of a specific wavelength. The PS transfers the received energy to molecular oxygen and converts it into reactive species, causing microorganisms to die by affecting their membranes, proteins, and nucleic acids.4,5 The primary benefit of PDT is its low invasion and toxicity to the host site. Furthermore, there will be no need for systemic treatment because of its localized effect, and no side effects will occur.6
PDT has a variety of applications in different fields of dentistry, mainly in periodontics and endodontics.3 It is primarily used in the latter for root canal disinfection, which is the key variant determining the outcome of a root canal treatment (RCT). Due to irregularities in the anatomy of root canals, the traditional irrigation method using syringes may have limitations. As a result, novel techniques for activating irrigants for improved canal disinfection have been developed, including PDT.7This method has gained popularity in contemporary dentistry because of its several benefits, including high efficiency in reducing the bacterial load, reduced post-operative pain,8 and decreased size of periapical lesions.9 Moreover, it can increase the efficacy of chemo-mechanical root canal preparation in complex anatomies like c-shaped canals10 and retreatment of teeth with previous failed endodontic treatment.11
This study was aimed at sharing the experience of treating three different cases, including endodontic treatment of teeth in both anterior and posterior regions and retreatment, using PDT in conjunction with conventional RCT.
Case Presentation
Three patients visited the Department of Endodontics at Shahid Beheshti Dental School in Tehran, Iran:
Case 1
The first patient was a 32-year-old woman, referred with severe pain and gingival swelling in the mandibular right molar region, close to the crowns of teeth #46 and #47. She stated that the pain had begun about four weeks before and that swelling had appeared after two weeks. In her medical history, there was nothing noteworthy to mention. The diagnostic radiography revealed that tooth #47 had received previous endodontic treatment and that there were a lucent lesion and a marginal gap between the class II amalgam restoration and the remaining structure. The unsuitable restoration and marginal gap were confirmed by intraoral examination. A sinus tract was also discovered in the buccal gingiva, in the area of swelling. The path of the tract was traced back to tooth #47. The probing depth in the buccal area of tooth #47 was 2 mm, with normal mobility. The periapical diagnosis was a chronic periapical abscess, and the remaining structure of the tooth was restorable. First, we informed the patient about the success rate and prognosis of PDT, as well as alternative treatment options in the event of a likely failure (e.g., apicoectomy, extraction, or others). Following that, a consent form was signed. To reduce the oral bacterial load, the patient was instructed to rinse her mouth with 0.2 % chlorhexidine (CHX) mouthwash (Najo Co., Tehran, Iran). After local anesthesia with 1.8 mL of lidocaine HCl 2% with epinephrine 1:80000 (Darupakhsh Pharmaceutical Mfg. Co., Tehran, Iran) using the inferior alveolar nerve (IAN) block technique, the old amalgam restoration and remaining caries were removed using carbide burs (Meisinger®, USA). A rubber dam was placed, and the obturation materials were removed from the coronal 1/3 of the root canals using gates Glidden (Dentsply®, Maillefer, Ballaigues, Switzerland) of size 3 at 300 rpm. To remove the remaining gutta-percha from the apical 2/3, 0.1 mL of chloroform (Merck®, Darmstadt, Germany) was applied for 2 minutes, followed by using ProTaper Universal Retreatment files (Dentsply®, Maillefer, Ballaigues, Switzerland). E-Connect Pro was the rotary motor used for canal preparation and determining working length (Eighteeth®, Jiangsu, China). Irrigation was done using 5 mL 5.25% NaOCl solution after each step. The root canal preparation was then modified using ProTaper Gold rotary files (Dentsply®, Maillefer, Ballaigues, Switzerland) through the crown-down technique. The finishing files used in mesial and distal root canals were F2 and F3 respectively. After the last irrigation step, sodium thiosulfate 5% (Merck, Darmstadt, Germany) was used to neutralize the adverse effects of NaOCl. Next, 0.5 mL of low viscosity 0.1 mg/mL Toluidine Blue (FotoSan® agent; CMS Dental, Denmark) was injected as a PS in each canal. With a #25 endodontic K-file circumferential movement, the PS was brushed to the root canal walls for 1 minute. The excessive unabsorbed PS was then removed using sterile paper cones (GAPA Dent Co, Tianjin City, China). The PS was irradiated with a Light-emitting diode (LED) lamp (FotoSan®; CMS Dental, Denmark) operating at a mean wavelength of 630 nm and a mean power density of 3 W/cm2 over two 30-second continuous periods. Safety precautions such as goggles for the patient and operator were considered. The endodontic tip of the device, with a diameter of 0.5 mm, was inserted into the canal until it reached the apical 1/3 of the working length and gradually moved out. This process was carried out for each canal. The canals were then irrigated to remove any remaining PS and then were dried with sterile paper cones (GAPA Dent Co, Tianjin City, China). Finally, the root canals were obturated laterally with gutta-percha (GAPA Dent Co, Tianjin City, China), and the crown got restored with a temporary restoration (Cavit; 3M ESPE®, St. Paul, MN, USA). The patient was referred to the department of operative dentistry for the final restoration. After 6 months in the follow-up session, no signs and symptoms were evident, and the periapical lesion was healing (Figure 1).
Figure 1.
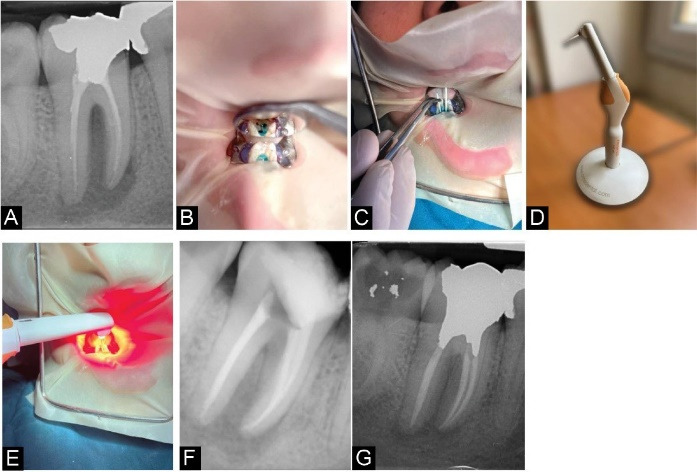
Case Report 1. (A) Diagnostic radiograph showing a periapical lesion related to tooth #47. (B) Root canals filled with FotoSan agent after removing the obturation material and old restoration. (C) Removing the excessive amount of the photosensitizer using paper cones. (D) FotoSan LED device. (E) Irradiation of the photosensitizer with an LED. (F) Post-operative radiograph. (G) The 6-month follow-up radiograph, showing the shrinkage in the lesion’s size.
Case 2
The second patient was a 48-year-old woman. The chief complaint was a non-suppurative extra-oral fistula and a feeling that tooth #46 seemed taller than neighboring teeth. The fistula had been evident for the last two months. Her medical history did not draw attention, and she was not under any medication. The diagnostic x-ray showed a periapical lesion close to tooth #46. Then, the extra-oral fistula was traced with a gutta-percha size 45, and a second radiograph was taken, confirming the lesion originating from the mentioned tooth. Pocket depth of 3 mm was seen, but the mobility was normal. The responses to vitality tests were negative. It was diagnosed as pulp necrosis with a chronic abscess based on the evidence. Root canal therapy alongside PDT was selected as the treatment plan. The information about treatment options was provided, and the consent form was signed in the same way as in case 1. After using CHX mouthwash, local anesthesia was applied through the IAN block. Then, a diamond bur (Meisinger®, USA) was used to open an access cavity. The rubber dam was applied; root canal instrumentation was performed using ProTaper Gold rotary files (Dentsply, Maillefer, Ballaigues, Switzerland), and irrigation was performed as previously described. The crown-down preparation was completed with the F2 file in mesial and F3 in distal canals. The PDT procedure was carried out in the same manner as in case 1. The root canal was obturated with gutta-percha using the warm vertical technique (E and Q Master®, Meta Biomed Co., Ltd, Cheongju city, Chungbuk, Korea). The crown got restored with a temporary restoration (Cavit; 3M ESPE, St. Paul, MN, USA) covering a small piece of cotton roll. The permanent restoration was done in the restorative department. The 6-month follow-up session showed complete healing as the patient declined any symptoms, and clinical and radiographic views were normal (Figure 2).
Figure 2.
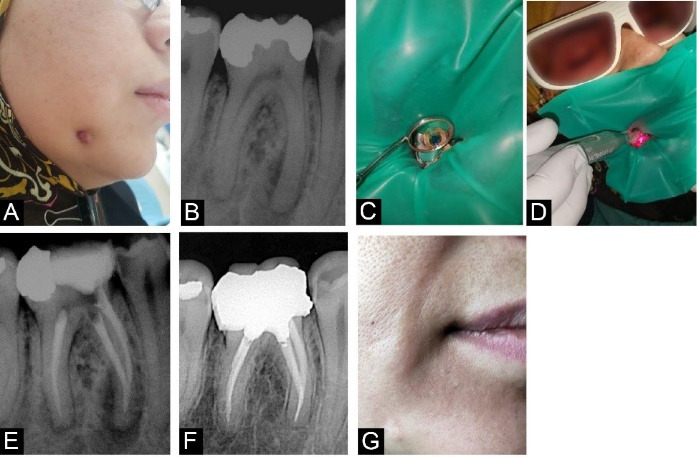
Case Report 2. (A) Extra-oral fistula (B) Diagnostic radiograph, showing a periapical lesion close to tooth #46 (C) Root canals filled with FotoSan agent (D) Irradiation of the photosensitizer with an LED (E) post-operative radiograph (F) 6-month follow-up radiograph (G) 6-month follow up extra-oral photograph.
Case 3
The third patient was a 27-year-old female who visited the dental school with the chief complaint of sensitivity to percussion in tooth #41, which had been noticeable for two weeks. She had no notable item in her medical history. The diagnostic radiograph revealed a periapical lesion close to the mentioned teeth. There was no periodontal pocket around the teeth in question, with physiologic mobility. The tooth was sensitive to percussion with mild sensitivity to gingival palpation. No response to the vitality tests was recorded. Based on clinical examination and radiographic view, the diagnosis was pulp necrosis accompanied by symptomatic apical periodontitis. Thus, we decided to do root canal therapy alongside PDT. As in previous cases, information about treatment options was provided, and the consent form was signed. Mouthwash rinse and local anesthesia using the infiltration technique were done. The access cavity was then prepared with a diamond bur (Meisinger®, USA). Following rubber dam isolation of tooth #41, canal instrumentation was performed using ProTaper Gold rotary files (Dentsply®, Maillefer, Ballaigues, Switzerland), and irrigation was performed as previously described. The last file used in the crown-down technique was F3, and the PDT procedure was carried out in the same manner as in Case 1. Following that, the root canal was obturated with gutta-percha (GAPA Dent Co, Tianjin City, China) using the warm vertical technique (E and Q Master®, Meta Biomed Co., Ltd, Cheongju city, Chungbuk, Korea). The access cavity was closed with a cotton role, and the crown was dressed with a temporary restoration (Cavit; 3M ESPE®, St. Paul, MN, USA). Finally, the patient was referred to the restorative department to receive permanent restoration. In the 6-month follow-up session, the patient reported no symptoms, and no clinical and radiographic signs were present, indicating complete healing (Figure 3).
Figure 3.
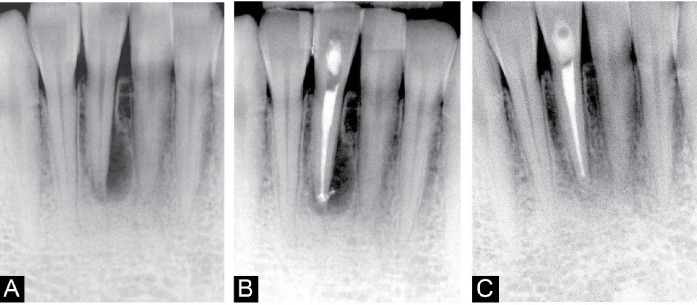
Case Report 3. (A) Diagnostic radiograph showing a periapical lesion related to tooth #41. (B) Post-operative radiograph. (C) 6-month follow-up radiograph, showing the shrinkage in lesion’s size
Discussion
Efficacy of Photodynamic Therapy
The success of an endodontic treatment directly depends on a number of factors, including adequate root canal disinfection.7 Endodontic files and irrigant solutions are used to perform the traditional cleaning and shaping of a root canal. Several studies, however, warn that due to the complexity of anatomy, biofilm growth, and other factors, complete elimination of intracanal microorganisms may not be possible using current techniques. As a result, because achieving direct contact of the irrigant with all microorganisms is nearly impossible, adjunctive methods such as PDT may be beneficial.5,12 PDT provides advantages such as immediate effect, selectivity, reaching complex anatomical areas, and a lower risk of bacteremia.12 Several studies have shown that this procedure can provide maximum root canal disinfection; for instance, Chrepa et al conducted a systematic review of studies on bacterial load reduction after PDT. The disinfection through PDT was found to reduce the bacterial load by 91.3% to 100%.13 Pourhajibagher and Bahador concluded in another review that integrating PDT with conventional chemo-mechanical root canal debridement would contribute to an increased reduction in the microbial load.14 In line with the research mentioned earlier, Vendramini et al stated that PDT in adjunction with conventional RCT would decrease intracanal bacterial biofilms.5 Besides, some studies disputed the superiority of PDT over other disinfection methods. Hecker et al demonstrated that the disinfection attained via PDT was less effective than NaOCl.15 Gergova et al proposed the use of NaOCl and CHX as the most effective disinfection method,16 while Bago et al reported that it is the combination of PDT and NaOCl that would achieve the greatest reduction in the number of bacteria.17
PDT and Periapical Tissues
Conejero et al concluded in a retrospective study that combining PDT with conventional RCT may shorten the healing period of periapical lesions while also reducing the need for calcium hydroxide therapy and additional treatment sessions.18 Silva et al, on the other hand, demonstrated that calcium hydroxide dressing could stimulate angiogenesis and bone formation more intensely than PDT, resulting in better apical periodontitis repair.19 Lopes et al recognized PDT as an effective adjunct method to endodontic treatment, resulting in satisfactory regression of periapical lesions.9 In the case of complex anomalies like dens invaginatus, Figueiredo et al showed that PDT might help the clinician in the nonsurgical management of the involved tooth even in the presence of a periapical lesion.20
PDT and Post-operative Pain
In multiple studies, PDT has been shown to significantly reduce pain following endodontic treatment.8,21 Although, in studies conducted by Barciela et al22 and Yoshinari et al,23 no significant difference was evident between experienced post-operative pain of patients who received additional PDT and those who did not.
PDT in Endodontic Retreatment
Besides initial endodontic treatment, PDT can be used in the retreatment of teeth with failed previous RCT. Asnaashari et al demonstrated that using adjunct PDT to eliminate intraradicular microbiota in teeth with filled canals and apical periodontitis could improve the efficacy of conventional RCT.11 According to Tavares et al, combining PDT with nonsurgical retreatment can attain sufficient microbial reduction.24
Different Photosensitizers Used in PDT
As previously stated, PDT requires PS, a light source, and oxygen. In the presented cases, FotoSan agent, a low viscosity 0.1 mg/mL toluidine blue ortho (TBO), was chosen from various PS options. According to Gambarini et al, the FotoSan agent, whether light-activated or not, has comparable cytotoxicity to other irrigants, such as 17% EDTA and 2% CHX. As a result, it can be used for disinfection with the same precautions that other irrigating solutions require.25 According to Bago et al, using TBO as PS would result in a 99.99% reduction in the bacterial load.17 Pourhajibagher et al investigated the efficacy of various PSs against Enterococcus faecalis. It was determined that the antimicrobial effect of PDT using 0.1 mg/mL TBO was greater than that of 0.1 mg/mL methylene blue (MB).26
Different Light Properties Used in PDT
Along with traditional root canal therapy, lasers and LEDs can be used in root canal disinfection. However, LEDs are less harmful due to lower thermal productivity and less tissue injury.27 Furthermore, LEDs use less energy, need less space, and are more economical.28 Unlike lasers, the light from an LED diverges as it moves away from the source, resulting in lower power than desired. Nonetheless, this can be improved using an intracanal fiber.29 Light can be applied for various time durations, but Yildirim et al believe that 1-minute irradiation is sufficient for an antimicrobial effect against E. faecalis.30 Nunes et al found that increased irradiation time resulted in a greater antimicrobial effect; however, this enhancement was not statistically significant.31
Moradi Eslami et al demonstrated that activating 1mg/mL TBO with 630 nm FotoSan LED light reduces the thickness of the intracanal biofilm. Besides that, PDT is preferred for disinfection over triple antibiotic paste because it is a single-session procedure with no microbial resistance. Furthermore, because of the broader spectrum, choosing LEDs over lasers as the light source can increase feasibility, safety, accessibility, and efficacy.32 Er Karaoğlu et al compared the disinfection efficiency of three different PSs activated by a 630-nm FotoSan LED (313 mol MB, 327 mol TBO, and 6 mol TM-ZnPc). It was concluded that all three activated PSs significantly reduced the bacterial load following root canal chemo-mechanical preparation.33 Borba et al reported that E. faecalis reduction following PDT with a high power LED is 60% greater than that following PDT with low power LED, similar to the one we used.34 Rios et al investigated the efficacy of PDT against E. faecalis using a FotoSan agent irradiated with an LED lamp after conventional irrigation with 6% NaOCl. It was determined that this modality is significantly more efficient than NaOCl or PDT alone. As a result, PDT via an LED lamp and TBO as PS can be used in addition to conventional RCT.27
Based on the findings of previous studies, we decided to use the combination of LED and TBO in three different patients for more efficient root canal disinfection. However, there are numerous ways to apply PDT, each with its own set of options for selecting a light source, PS, and so on. Based on the current literature, using a laser to activate MB or TBO is the more common method for PDT, alongside conventional RCT. The exposure time, energy, and output power should all be in sync to achieve the desired irradiation dose. Nonetheless, more research appears to be required to develop PDT unit protocols that can simultaneously achieve the highest efficiency and safety.
Conclusion
Among the various methods of root canal disinfection, PDT in conjunction with conventional root canal therapy can assist us in achieving an intracanal environment with the lowest microorganism load. Based on the results of three reported patients who were treated using PDT, it was concluded that activating low viscosity 0.1 mg/mL Toluidine Blue (FotoSan® agent) with a 635-nm LED (FotoSan®; CMS Dental, Denmark) with a mean power density of 3 W/cm2 during two 30-second periods can be an efficient option in root canal disinfection.
Conflict of Interests
The Authors declare that there is no conflict of interest.
Ethical Considerations
All three patients signed consent forms describing the success rate and prognosis of the treatment, as well as alternative treatment options in the event of a likely failure.
Please cite this article as follows: Shahbazi S, Esmaeili S, Feli M, Asnaashari M. Photodynamic therapy in root canal disinfection: a case series and mini-review. J Lasers Med Sci. 2022;13:e19. doi:10.34172/jlms.2022.19.
References
- 1.Lubart R, Eichler M, Lavi R, Friedman H, Shainberg A. Low-energy laser irradiation promotes cellular redox activity. Photomed Laser Surg. 2005;23(1):3–9. doi: 10.1089/pho.2005.23.3. [DOI] [PubMed] [Google Scholar]
- 2.Jacques SL. Laser-Tissue Interactions: Photochemical, Photothermal, and Photomechanical. Surg Clin North Am. 1992;72(3):531–558. doi: 10.1016/S0039-6109(16)45731-2. [DOI] [PubMed] [Google Scholar]
- 3.Cieplik F, Deng D, Crielaard W, Buchalla W, Hellwig E, Al-Ahmad A. et al. Antimicrobial photodynamic therapy - what we know and what we don’t. Crit Rev Microbiol. 2018;44(5):571–589. doi: 10.1080/1040841X.2018.1467876. [DOI] [PubMed] [Google Scholar]
- 4.Diogo P, F Faustino MA, P M S Neves MG, Palma PJ, P Baptista I, Gonçalves T. et al. An Insight into Advanced Approaches for Photosensitizer Optimization in Endodontics-A Critical Review. J Funct Biomater. 2019;10(4):44. doi: 10.3390/jfb10040044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vendramini Y, Salles A, Portella FF, Brew MC, Steier L, de Figueiredo JAP. et al. Antimicrobial effect of photodynamic therapy on intracanal biofilm: A systematic review of in vitro studies. Photodiagnosis Photodyn Ther. 2020;32:102025. doi: 10.1016/j.pdpdt.2020.102025. [DOI] [PubMed] [Google Scholar]
- 6. Abdel-Kader MH. Photodynamic Therapy: From Theory to Application. Springer; 2014.
- 7.Do QL, Gaudin A. The Efficiency of the Er: YAG Laser and PhotonInduced Photoacoustic Streaming (PIPS) as an Activation Method in Endodontic Irrigation: A Literature Review. J Lasers Med Sci. 2020;11(3):316–334. doi: 10.34172/jlms.2020.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coelho MS, Vilas-Boas L, Tawil PZ. The effects of photodynamic therapy on postoperative pain in teeth with necrotic pulps. Photodiagnosis Photodyn Ther. 2019;27:396–401. doi: 10.1016/j.pdpdt.2019.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Lopes CS, de Azevedo Moreira S, Nícoli GA, Ramirez I, Viola NV. Endodontical treatment of periapical tooth injury with photodynamic therapy: Case report. Photodiagnosis Photodyn Ther. 2019;28:253–255. doi: 10.1016/j.pdpdt.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Mustafa M, Almnea R, Ajmal M, Alamri HM, Abdulwahed A, Divakar DD. Efficacy of root canal treatment in c-shaped canals with adjunctive photodynamic therapy using micro-CT. Photodiagnosis Photodyn Ther. 2021;34:102257. doi: 10.1016/j.pdpdt.2021.102257. [DOI] [PubMed] [Google Scholar]
- 11.Asnaashari M, Homayuni H, Paymanpour P. The Antibacterial Effect of Additional Photodynamic Therapy in Failed Endodontically Treated Teeth: A Pilot Study. J Lasers Med Sci. 2016;7(4):238–242. doi: 10.15171/jlms.2016.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mehrvarzfar P, Saghiri MA, Asatourian A, Fekrazad R, Karamifar K, Eslami G. et al. Additive effect of a diode laser on the antibacterial activity of 25% NaOCl, 2% CHX and MTAD against Enterococcus faecalis contaminating root canals: an in vitro study. J Oral Sci. 2011;53(3):355–60. doi: 10.2334/josnusd.53.355. [DOI] [PubMed] [Google Scholar]
- 13.Chrepa V, Kotsakis GA, Pagonis TC, Hargreaves KM. The effect of photodynamic therapy in root canal disinfection: a systematic review. J Endod. 2014;40(7):891–8. doi: 10.1016/j.joen.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 14.Pourhajibagher M, Bahador A. Adjunctive antimicrobial photodynamic therapy to conventional chemo-mechanical debridement of infected root canal systems: A systematic review and meta-analysis. Photodiagnosis Photodyn Ther. 2019;26:19–26. doi: 10.1016/j.pdpdt.2019.02.009. [DOI] [PubMed] [Google Scholar]
- 15.Hecker S, Hiller K-A, Galler KM, Erb S, Mader T, Schmalz G. Establishment of an optimized ex vivo system for artificial root canal infection evaluated by use of sodium hypochlorite and the photodynamic therapy. Int Endod J. 2013;46(5):449–457. doi: 10.1111/iej.12010. [DOI] [PubMed] [Google Scholar]
- 16.Gergova RT, Gueorgieva T, Dencheva-Garova MS, Krasteva-Panova AZ, Kalchinov V, Mitov I. et al. Antimicrobial activity of different disinfection methods against biofilms in root canals. J Investig Clin Dent. 2016;7(3):254–62. doi: 10.1111/jicd.12147. [DOI] [PubMed] [Google Scholar]
- 17.Bago I, Plečko V, Gabrić Pandurić D, Schauperl Z, Baraba A, Anić I. Antimicrobial efficacy of a high-power diode laser, photo-activated disinfection, conventional and sonic activated irrigation during root canal treatment. Int Endod J. 2013;46(4):339–347. doi: 10.1111/j.1365-2591.2012.02120.x. [DOI] [PubMed] [Google Scholar]
- 18.Conejero MJ, Almenar A, Forner L, Sanz JL, Llena C. Retrospective clinical evaluation of root canal treatment with or without photodynamic therapy for necrotic teeth and teeth subjected to retreatment. J Oral Sci. 2021;63(2):163–166. doi: 10.2334/josnusd.20-0429. [DOI] [PubMed] [Google Scholar]
- 19.Silva LABD, Lopes ZMS, Sá RC, Novaes Júnior AB, Romualdo PC, Lucisano MP. et al. Comparison of apical periodontitis repair in endodontic treatment with calcium hydroxide-dressing and aPDT. Braz Oral Res. 2019;33:e092. doi: 10.1590/1807-3107bor-2019.vol33.0092. [DOI] [PubMed] [Google Scholar]
- 20.Figueiredo VC, de Figueiredo NF, Tang S, Braga T, Amaral RR. The use of antimicrobial photodynamic therapy in the successful management of a nonsurgical complex type II Dens Invaginatus. Photodiagnosis Photodyn Ther. 2021;36:102540. doi: 10.1016/j.pdpdt.2021.102540. [DOI] [PubMed] [Google Scholar]
- 21.Alves-Silva EG, Arruda-Vasconcelos R, Louzada LM, de-Jesus-Soares A, Ferraz CCR, Almeida JFA. et al. The effect of photodynamic therapy on postoperative pain in teeth with primary endodontic infection. Photodiagnosis Photodyn Ther. 2022;37:102700. doi: 10.1016/j.pdpdt.2021.102700. [DOI] [PubMed] [Google Scholar]
- 22.Barciela B, da Silva Limoeiro AG, Bueno CE, Fernandes SL, Mandarini DR, Boer NC. et al. In vivo evaluation of painful symptomatology after endodontic treatment with or without the use of photodynamic therapy. J Conserv Dent. 2019;22(4):332–335. doi: 10.4103/JCD.JCD_39_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yoshinari F, Pereira K, Beraldo D, Silva J, Zafalon E, Silva P. Influence of Photodynamic Therapy in the Control of Postoperative Pain in Endodontic Treatment: A Cross-Sectional Randomized Clinical Trial. Pesqui Bras Odontopediatria Clin Integr. 2019;19:1–8. doi: 10.4034/PBOCI.2019.191.43. [DOI] [Google Scholar]
- 24.Tavares WLF, Oliveira RR, Ferreira MVL, Sobrinho APR, Braga T, Amaral RR. The use of antimicrobial photodynamic therapy in the successful management of an invasive cervical resorption class 4: A case report with five years follow-up. Photodiagnosis Photodyn Ther. 2021;33:102126. doi: 10.1016/j.pdpdt.2020.102126. [DOI] [PubMed] [Google Scholar]
- 25.Gambarini G, Plotino G, Grande NM, Nocca G, Lupi A, Giardina B. et al. In vitro evaluation of the cytotoxicity of FotoSanTM light-activated disinfection on human fibroblasts. Med Sci Monit. 2011;17(3):MT21–5. doi: 10.12659/msm.881435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pourhajibagher M, Kazemian H, Chiniforush N, Hosseini N, Pourakbari B, Azizollahi A. et al. Exploring different photosensitizers to optimize elimination of planktonic and biofilm forms of Enterococcus faecalis from infected root canal during antimicrobial photodynamic therapy. Photodiagnosis Photodyn Ther. 2018;24:206–211. doi: 10.1016/j.pdpdt.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Rios A, He J, Glickman GN, Spears R, Schneiderman ED, Honeyman AL. Evaluation of photodynamic therapy using a light-emitting diode lamp against Enterococcus faecalis in extracted human teeth. J Endod. 2011;37(6):856–9. doi: 10.1016/j.joen.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 28.Rosario M, Decastro IC, Campos PF. Applicability of antimicrobial photodynamictherapy in dentistry. Arch Oral Sci Res. 2012;2(2):88–93. [Google Scholar]
- 29.Oda DF, Duarte MAH, Andrade FB, Moriyama LT, Bagnato VS, de Moraes IG. Antimicrobial action of photodynamic therapy in root canals using LED curing light, curcumin and carbopol gel. Int Endod J. 2019;52(7):1010–1019. doi: 10.1111/iej.13092. [DOI] [PubMed] [Google Scholar]
- 30.Yildirim C, Karaarslan ES, Ozsevik S, Zer Y, Sari T, Usumez A. Antimicrobial efficiency of photodynamic therapy with different irradiation durations. Eur J Dent. 2013;7(4):469–473. doi: 10.4103/1305-7456.120677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nunes MR, Mello I, Franco GC, de Medeiros JM, Dos Santos SS, Habitante SM. et al. Effectiveness of photodynamic therapy against Enterococcus faecalis, with and without the use of an intracanal optical fiber: an in vitro study. Photomed Laser Surg. 2011;29(12):803–8. doi: 10.1089/pho.2011.2995. [DOI] [PubMed] [Google Scholar]
- 32.Moradi Eslami L, Vatanpour M, Aminzadeh N, Mehrvarzfar P, Taheri S. The comparison of intracanal medicaments, diode laser and photodynamic therapy on removing the biofilm of Enterococcus faecalis and Candida albicans in the root canal system (ex-vivo study) Photodiagnosis Photodyn Ther. 2019;26:157–161. doi: 10.1016/j.pdpdt.2019.01.033. [DOI] [PubMed] [Google Scholar]
- 33.Er Karaoğlu G, Uğur Ydın Z, Erdönmez D, Göl C, Durmuş M. Efficacy of antimicrobial photodynamic therapy administered using methylene blue, toluidine blue and tetra 2-mercaptopyridine substituted zinc phthalocyanine in root canals contaminated with Enterococcusaecalis. Photodiagnosis Photodyn Ther. 2020;32:102038. doi: 10.1016/j.pdpdt.2020.102038. [DOI] [PubMed] [Google Scholar]
- 34.Borba ASM, da Silva Pereira SM, Borba MCM, Paschoal MAB, de Jesus Tavarez RR, de Castro Rizzi C. et al. Photodynamic therapy with high-power LED mediated by erythrosine eliminates Enterococcus faecalis in planktonic forms. Photodiagnosis Photodyn Ther. 2017;19:348–351. doi: 10.1016/j.pdpdt.2017.07.007. [DOI] [PubMed] [Google Scholar]


