Abstract
Ethnopharmacological relevance
Shufeng Jiedu capsule (SFJDC) is a pure form of traditional Chinese medicine (TCM) that contains eight medicinal plants. Known for its anti-inflammatory and antipyretic effects, it is mostly used to treat upper respiratory tract infections and other infectious diseases, such as colds, pharyngitis, laryngitis, and tonsillitis. Both acute lung injury (ALI) and COVID-19 are closely related to lung damage, primarily manifesting as lung inflammation and epithelial cell damage. However, whether SFJDC can improve ALI and by what mechanism remain unclear. The purpose of this study was to explore whether SFJDC could be used as a prophylactic treatment for COVID-19 by improving acute lung injury.
Aim of the study
The purpose of this study was to determine whether SFJDC could protect against ALI caused by lipopolysaccharide (LPS), and we wanted to determine how SFJDC reduces inflammation and apoptosis pharmacologically and molecularly.
Materials and methods
Preadministering SFJDC at 0.1 g/kg, 0.3 g/kg, or 0.5 g/kg for one week was followed by 5 mg/kg LPS to induce ALI in mice. Observations included the study of lung histomorphology, tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6) secretion, as well as the ratio of lung wet/dry weights. In addition, RAW264.7 cells were treated for 24 h with 1 μg/mL LPS after being pretreated for 1 h with 0.5 mg/mL SFJDC. In the samples, we detected TNF-α, IL-1β, and IL-6. Cell apoptosis was detected by stimulating A549 cells for 24 h with RAW264.7 supernatant. Both in vitro and in vivo, the levels of A2A adenosine receptor (A2AAR), PKA, IκB, p-IκB, NF-κB P65 (P65), p–NF–κB P65 (p-P65), cleaved caspases-3 (Cc3), Bcl-2 associated X protein (Bax), and B-cell lymphoma-2 (Bcl-2) proteins were determined using Western blot analysis.
Results
Lung tissue morphology was improved as SFJDC decreased cytokine secretion, the ratio of lung wet/dry weights, and lung tissue secretion of proinflammatory cytokines. The expression of A2AAR was increased by SFJDC, and the phosphorylation of NF-κB was inhibited. TUNEL staining and flow cytometry showed that SFJDC inhibited apoptosis by reducing the expression of Cc3 and the ratio of Bax/Bcl-2.
Conclusions
According to the results of this study, SFJDC can reduce inflammation and inhibit apoptosis. A2AAR activation and regulation of NF-κB expression are thought to make SFJDC anti-inflammatory and anti-apoptotic. A wide range of active ingredients may result in an anti-inflammatory and antipyretic effect with SFJDC.
Keywords: Acute lung injury, Shufeng jiedu capsule, Lipopolysaccharide, Inflammation, Apoptosis, A2AAR/NF-κB pathway
Graphical abstract
Acronyms
- ALI
Acute lung injury
- ARDS
Acute respiratory distress syndrome
- A2AAR
A2A adenosine receptor
- Bax
Bcl-2 Associated X Protein
- Bcl-2
B-cell lymphoma-2
- Cc3
Cleaved-caspases-3
- CCK-8
Cell Counting Kit-8
- TCM
Traditional Chinese Medicine
- Dex
Dexamethasone
- IL-1β
Interleukin-1β
- IL-6
Interleukin-6
- LPS
Lipopolysaccharide
- P65
NF-κB P65
- p-P65
p–NF–κB P65
- SFJDC
Shufeng Jiedu Capsule
- TNF-α
Tumor necrosis factor-α
1. Introduction
Constantly changing COVID-19 strains around the globe not only a present threat to public health but also make it increasingly difficult to develop drugs and vaccines. In light of the growing understanding of COVID-19 infection, even though most cases are asymptomatic or mild, an increasing number of studies are being performed on the disease. In 10%–20% of COVID-19 cases, it can give rise to interstitial pneumonia and acute respiratory distress syndrome (ARDS) (Soy et al., 2020). In addition to fatigue, dry cough, and high fever, COVID-19 patients often suffer headaches, sore throats, and a loss of taste and smell (Wang et al., 2020). Relevant clinical studies have found that the pathological morphology of the lungs of COVID-19 patients/deceased patients can not be distinguished from those of acute lung injury (ALI) patients, showing increased lung tissue permeability, alveolar edema and alveolar collapse (Martin, 2022; Swenson and Swenson, 2021; Zarrilli et al., 2021a). In addition, in the lungs of patients with COVID-19 have been found hyperactive macrophages, along with a lot of proinflammatory cytokines such as tumor necrosis factor-α (TNF-α), interleukin-1β (IL-1β), and interleukin-6 (IL-6). Although the early COVID-19-induced pulmonary pathological state is characterized by atypical ALI, it gradually overlaps with typical ALI as proinflammatory cytokines continue to develop. (Habashi et al., 2021; Ragab et al., 2020; Zarrilli et al., 2021b). In addition, COVID-19 can also cause T-cell cell apoptosis, it can further cause the death of lung epithelial and endothelial cells if left unchecked (Ye et al., 2020). In severe cases, ARDS develops.
ALI also called ARDS, is another clinical term. In the case of ALI, the lung tissue may be damaged for various reasons, such as pneumonia, sepsis, acute pancreatitis, inhalation injury, etc (Mokra and Kosutova, 2015; Wan et al., 2018). Although medical advancements have made it easier to diagnose and treat acute respiratory infections, the incidence remains high. Data suggest that more than 10% of inpatients in ICUs have an acute respiratory infection or ARDS, and the overall death toll is between 35 and 40% (He et al., 2021; Mowery et al., 2020). Many cytokines and chemokines play an important role in the innate immune response as the first barrier against viral infection. However, the excessive production of cytokines and chemokines also leads to disordered immune defense, resulting in immune pathology (Channappanavar et al., 2016). Inflammation is an essential part of the immune response, since without it, the infection source cannot be addressed and the system cannot return to normal. However, some chronic infections cause excessive induction of cytokines and chemokines, resulting in cytokine storms that lead to an unbalanced inflammatory response (Channappanavar and Perlman, 2017; Kumar, 2020). According to recent research, excessive lung inflammation is thought to play a role in the development of ALI (Liu et al., 2020). In the pathogenesis of ALI/ARDS, multiple factors stimulate macrophage activation in the lung, activate associated inflammatory pathways such as the NF-κB signaling pathway, and release many proinflammatory cytokines, which promote the further deterioration of ALI (Fan and Fan, 2018; He et al., 2019; Hendrickson and Matthay, 2018). Inhibiting the release of inflammatory factors and alleviating the inflammatory response may be one of the effective ways to treat ALI.
In recent years, however, the role of adenosine receptors in lung injury has slowly come into view. Numerous studies have found that adenosine receptor can inhibit the further development of ALI by inhibiting inflammatory response, restoring vascular barrier, and improving lung histopathology (Gonzales et al., 2014; Hoegl et al., 2015). A2A adenosine receptor (A2AAR), as one of the four adenosine receptors, belongs to the G-protein conjugated superfamily with A1AR, A2BAR, and A3AR (Antonioli et al., 2013). It has been shown that a variety of immune cells express A2AAR, including macrophages, neutrophils, T cells, and natural killer cells, among others, and this has anti-inflammatory properties (Cai et al., 2018; Gessi et al., 2011; Haskó et al., 2008). Moreover, most of the role played by A2AAR activation can be mediated by cAMP (Shaikh and Cronstein, 2016; Sullivan et al., 2001), thus influencing the proinflammatory NF-κB pathway to regulate inflammation (Majumdar and Aggarwal, 2003). In recent years, A2AAR has been gradually found to play an anti-inflammatory role in several ALI models, such as LPS-induced model (He et al., 2013), ischemia-reperfusion model (Sharma et al., 2009), and lung transplantation model (Gazoni et al., 2008). As A2AAR has an anti-inflammatory effect, we were curious whether A2AAR can be involved in SFJDC-mediated regulation of ALI.
Traditional Chinese medicine (TCM) has recently drawn international attention and made great contributions to the fight against COVID-19. Chinese medicine compounds are composed of a variety of Chinese herbal medicines because they contain a variety of active ingredients that can affect different targets and pathways and play a synergistic role to achieve prevention and treatment. Shufeng Jiedu Capsule (SFJDC), as a pure TCM preparation, is composed of eight medicinal plants (Xu et al., 2022). As shown in Table 1 . Due to its anti-inflammatory and antipyretic effects, it is often used for upper respiratory tract infections (Xia et al., 2021). Additionally, since 2009, SFJDC has played a significant role in treating atypical pneumonia and influenza A (H1N1), which is the first-line TCM for epidemic diseases after being accredited by the National Health Commission (Lu, 2020; Xia et al., 2021). SFJDC has also been reported to relieve inflammation by activating NrF2 antioxidants and regulating the MAPK/NF-κB signaling pathway (Liao et al., 2021; Tao et al., 2014). Our aim was to investigate whether SFJDC has any beneficial effect on lipopolysaccharide (LPS) -induced lung inflammation in mice to further intervene in the early stage of COVID-19.
Table 1.
An explicit list of SFJDC ingredients.
| Ingredients | Medicinal parts and sources | Chinese name |
|---|---|---|
| Polygonum cuspidatum | The root and rhizome of Polygonum cuspidatum Siebold & Zucc. (WCSP) | Hu-Zhang |
| Forsythia suspensa | The fruit of Forsythia suspensa (Thunb.) Vahl. (WCSP) | Lian-Qiao |
| Radix isatidis | The root of Isatis indigotica Fortune ex Lindl. (WCSP) | Ban-Lan-Gen |
| Bupleurum chinense | The root of Bupleurum chinense DC. (WCSP) | Chai-Hu |
| Patrinia scabiosaefolia | As a whole of Patrinia scabiosifolia Link. (TRO) | Bai-Jiang-Cao |
| Verbena officinalis | The ground part of Verbena officinalis L. (WCSP) | Ma-Bian-Cao |
| Rhizoma phragmitis | The rhizome of Phragmites communis Trin. (WCSP) | Lu-Gen |
| Liquorice | The root and rhizome of Glycyrrhiza uralensis Fisch. (ILDIS) | Gan-Cao |
Note: All the medicinal materials in the formula have been certified (http://www.theplantlist.org).
2. Materials and methods
2.1. Reagents and antibodies
SFJDC was provided by Anhui Jiren Pharmaceutical Co., Ltd. (Batch number:3200509, Bozhou, Anhui). Dexamethasone (Dex) injection (H37021969, 5 mg/mL) was produced by Chenxin Pharmaceutical Co., Ltd. (Shandong Lukang). LPS was purchased from Sigma Biological Company (NO: L2880-10 mg, Shanghai, China). Reagents ZM241385 and CGS21680 were purchased from GLPBIO Biological Company (Shanghai, China). IκB, p-IκB, P65, p-P65, and Cc3 antibodies were purchased from Shenyang Wanlei Biological Company. Bax, Bcl-2, and PKA antibodies were obtained from Affinity Biological Company (Jiangsu, China). The antibody against the A2A receptor was available from Bioss Biotech (Beijing, China). β-actin antibody was obtained from Zhongshan Jinqiao Biotechnology Co., Ltd (Beijing, China). The cell counting kit was obtained from Bioss (Beijing, China). ELISA kits (TNF-α, IL-6, and IL-1β) were obtained from Suzhou Calvin Biotechnology Co., Ltd. An Annexin V-FITC/PI Apoptosis Kit was purchased from Beibo Biological Co., Ltd (Shanghai, China).
2.2. Animal models
We accepted mice (C57/BL 6J, 20–22 g, male, 7 ± 1 weeks old) from the Animal Experiment Center at Anhui Medical University. Before the animal model was established, all the mice were housed for a week and were given randomly selected fodder and water. In six groups, the mice were randomly distributed into the following groups: control group, LPS group (5 mg/kg), SFJDC 0.1 g/kg, SFJDC 0.3 g/kg, SFJDC 0.5 g/kg, and Dex group (5 mg/kg), with 6 mice in each group. The drug group was intragastrically injected for 7 consecutive days before modeling, and the remaining groups were intragastrically injected with an equal amount of normal saline. Three hours after the last administration, mice in each group were intraperitoneally injected with LPS solution (5 mg/kg, 0.5 mg LPS dissolved in 1 mL sterile double steamed water) of 0.2 mL (Sun et al., 2020; Wang et al., 2021), except for the control group. Mice were killed 12 h later. Blood and lung tissue were collected for subsequent experiments.
All experimental animals used in this study were handled according to guidelines published by the National Institutes of Health (NIH Publication 85-23, 1985 revision) and approved by the Ethics Committee of Anhui Medical University (Approval number: LLSC20190527/190301).
2.3. Cell culture and coculture
RAW264.7 cells are mouse mononuclear macrophages and are one of the most commonly used cells to study the inflammatory response. We obtained RAW264.7 cells from the Shanghai Branch of the Chinese Academy of Sciences. The School of Pharmacy, Anhui Medical University, provided A549 cells. RAW264.7 and A549 cells were cultured in the ratio of DMEM (HyClone, USA) to fetal bovine serum (9:1) in a 37 °C constant temperature incubator with 5.0% CO2. After pretreatment with 500 mg/mL SFJDC for 1 h, the cells were incubated with LPS (1 μg/mL) for 24 h in the LPS + SFJDC group. Meanwhile, the model group was incubated with LPS (1 μg/mL) for 24 h. Then, A549 cells were stimulated for 24 h with RAW264.7-cell supernatant, and their apoptosis indices were determined.
2.4. Immunofluorescence staining
We divided RAW264.7 cells into three groups: normal, model, and SFJDC. Then, 4% paraformaldehyde was used to immobilize RAW264.7 cells for 30 min, and 3% BSA was used to block them for 30 min. For the purpose of comparing protein expression between groups, the groups of cells were incubated in the bottom of the glass dish with A2AAR (1:200) and P65 (1:200) at 4 °C for 12 h. After that, they were incubated for 1 h in the dark with fluorescent secondary antibody. To complete the procedure, the nuclei were stained for 15 min with an anti-fluorescence quenching agent with DAPI. Laser scanning confocal microscopy was used to capture the images (Carl Zeiss, Germany).
2.5. Histopathological and TUNEL staining
A total of 24 h of formalin was applied to fresh lungs before paraffin embedding of 4 μm sections was performed. To estimate tissue changes and cell apoptosis, HE and TUNEL staining were performed. The images were then observed using an Austrian 3DHISTECH pathological section scanner.
2.6. ELISA
Serum was collected after holding whole blood for 2 h and centrifugation at 3000 RPM for 20 min. Mouse serum and the supernatant of RAW264.7-cell culture were tested to determine the content of TNF-α, IL-6, and IL-1β according to the ELISA kit requirements. With a microporous plate analyzer (Mithras LB 940, Germany), the absorbance at 450 nm was measured.
2.7. Determination of cAMP concentration
Frozen lung tissue was pretreated with cold PBS. Then the tissue was broken into homogenates and centrifuged at 3000 RPM for 10 min. We analyzed the cAMP level in the supernatant in accordance with the instructions provided by the manufacturer (Wuhan Ilerite Biotechnology Co., Ltd.). We also quantified the intracellular cAMP levels following the manufacturer's instructions.
2.8. Cell viability screening
Cell viability was determined using the Cell Counting Kit-8 (CCK-8) as directed by the specification. A multifunction microporous plate analyzer was used to measure absorbance at 450 nm (Mithras LB 940, Germany).
2.9. Western blot
In RAW264.7 cells, A549 cells, and lung tissues, proteins were exposed using RIPA buffer (EpiZyme, Shanghai, China). The protein concentration was detected with a BCA protein detection kit (Beyotime, Shanghai, China). Proteins were isolated from samples using SDS–PAGE. Protein-free rapid blocking buffer (EpiZyme, Shanghai, China) was used to block the PVDF membrane for 15 min upon transfer of the isolated protein into the PVDF membrane (Bio-Rad, CA, USA). The diluted primary antibody was reacted with PVDF membranes overnight at 4 °C. Next, the PVDF membrane was incubated at room temperature for 1 h with the corresponding secondary antibody (anti-rabbit or anti-mouse). Specific experimental conditions are shown in Supplementary Table 1. Finally, proteins on PVDF membranes were imaged using an ultrasensitive ECL kit (GlpBio, USA) and protein expression was quantified using ImageJ.
2.10. Flow cytometry and apoptosis
The rate of apoptosis in A549 cells was determined using the Annexin V-FITC/PI apoptosis kit (Best Bio, China). Following a 1:10 dilution of binding solution, the collected cells were centrifuged and resuspended in 400 μl binding solution. Incubation was conducted in darkness in a 4 °C temperature range for 15 min after adding 5 μl of V-FITC staining solution to the cell suspension. PI staining solution was added, and the cells were incubated for 5 min on ice in the dark before flow cytometry (Beckman Coulter, USA).
2.11. RT-qPCR analysis
RAW264.7 cells, A549 cells and lung tissue were all treated with TRIzol reagent (Accurate Biology, Hunan, China) for total RNA extraction. After the extracted RNA was quantified, the RNA was reverse transcribed using the TAKARA procedure and the Evo M-MLV Mix Kit (Accurate Biology, Hunan, China). By using a SYBR Green Premix qPCR kit (Accurate Biology, Hunan, China) and cDNA as templates, the mouse gene was amplified by qPCR. See Supplementary Table 2 for primer sequences. The reference gene GAPDH was used internally in this experiment.
2.12. Statistical analysis
All data are presented as the mean ± SEM (GraphPad Prism 8.0), and statistical significance was determined by a T test and one-way ANOVA. Data were analyzed as follows: #*P ≤ 0.05, ##**P ≤ 0.01, and ###***P ≤ 0.001.
3. Results
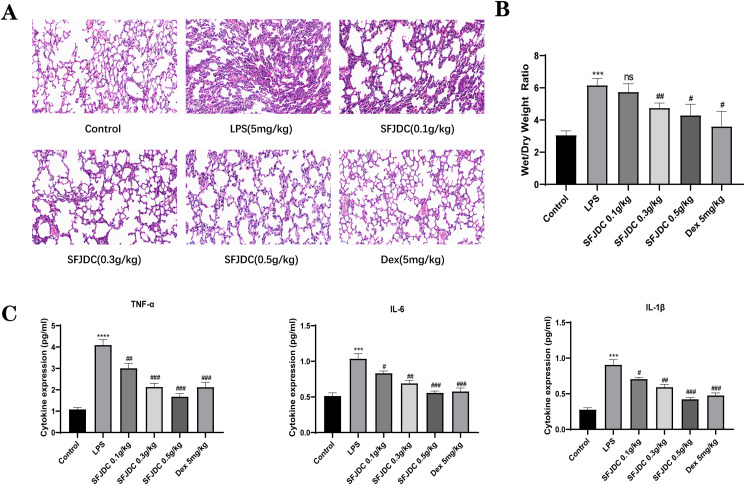
3.1. SFJDC attenuates LPS-induced lung injury
Serum and lung tissues from mice were examined by ELISA kits, HE staining, and wet/dry lung specific gravity methods to determine the role of SFJDC in LPS-induced lung damage. We first observed the morphological changes in lung tissues by comparing the HE-stained pathological sections (Fig. 1 A). The lung tissues of control mice did not display any obvious morphological changes. LPS-induced mice showed alveolar collapse, thickening of alveolar walls, and inflammatory cell infiltration compared with their control counterparts. After SFJDC pretreatment, lung injury induced by LPS gradually resolved and followed a dose-dependent trend. Moreover, LPS increased the ratio of wet/dry lung weight in mice, but the ratio slowly decreased with increasing SFJDC dose, according to the wet/dry lung weight analysis (Fig. 1B). As shown in Fig. 1C, we used ELISA kits to detect representative inflammatory factors. Following LPS stimulation, the levels of TNF-α, IL-1β, and IL-6 increased significantly, and these levels decreased dose-dependently after pretreatment with SFJDC. According to these results, SFJDC can reduce LPS-induced lung injury.
Fig. 1.
SFJDC can relieve mouse lung injury caused by LPS inhalation. (A) Representative lung tissue sections stained with HE (original multiples: 200 × , scale = 50 μm). (B) Lung tissue wet/dry weight ratio. (C) TNF-α, IL-1β, and IL-6 concentrations were determined with ELISA in mouse serum. Compared to the control group, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Compared to the LPS group, #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001. Each set of data was based on six replicates.
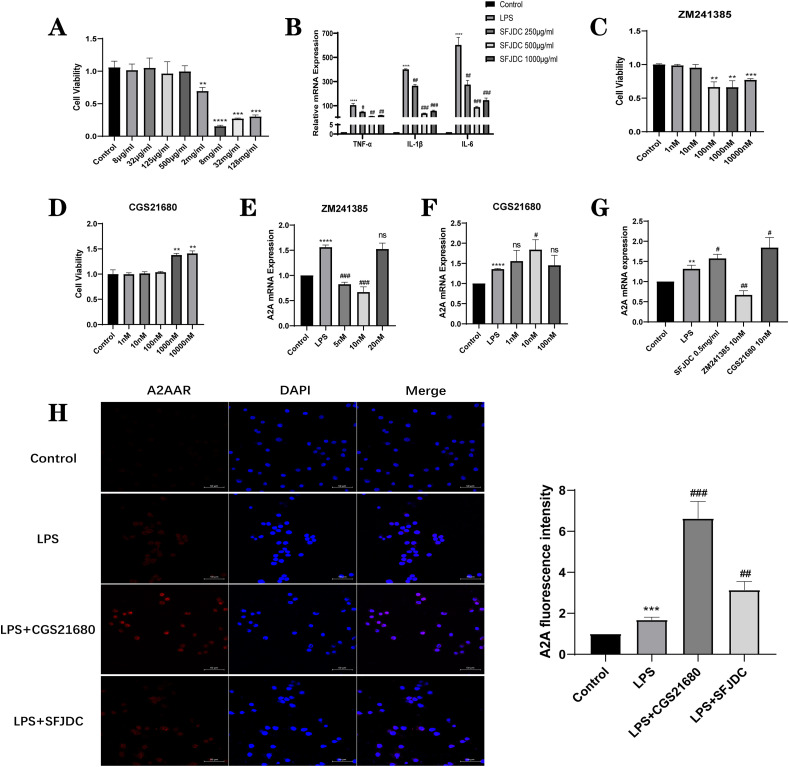
3.2. SFJDC activates A2AAR to reduce RAW264.7-cell inflammation
To study the effect of SFJDC on A2AAR in RAW264.7 cells, we used RT–qPCR and immunofluorescence staining. Previous studies have shown that RAW264.7 cells can be the subject of an in vitro inflammatory model by providing 1 μg/mL LPS for 24 h (Zhang et al., 2020). Meanwhile, CCK-8 was used to identify the optimal concentration of SFJDC for RAW264.7 cells by detecting the differences in cell viability between different concentrations (Fig. 2 A). At 2 mg/mL, SFJDC showed significant effects on cell viability. Therefore, SFJDC concentrations of 0.25 mg/mL, 0.5 mg/mL and 1.0 mg/mL were selected to detect representative inflammatory factors (Fig. 2B). At the mRNA level, we found that 0.5 mg/mL SFJDC significantly decreased TNF-α, IL-1β, and IL-6 expression. Therefore, 0.5 mg/mL SFJDC can be used as the optimal concentration in the RAW264.7-cell inflammation model.
Fig. 2.
SFJDC can excite A2AAR. (A) RAW264.7 cells using CCK-8. (B) SFJDC stimulated TNF-α, IL-1β, and IL-6 mRNA levels in the supernatant of RAW264.7 cells at 250 μg/mL, 500 μg/mL and 1000 μg/mL (C) and (D) RAW264.7 cells using CCK-8. (E), (F) and (G) A2AAR mRNA expression in RAW264.7 cells. (H) Representative A2AAR immunofluorescence image (scale = 50 μm) and A2AAR fluorescence intensity calculation. Compared to the control group, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Compared to the LPS group, #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001. Each set of data was based on three replicates.
Moreover, we selected the specific inhibitor ZM241385 and agonist CGS21680 that target A2AAR, and their effects on the viability of RAW264.7 cells were detected by CCK-8 (Fig. 2C and D). Meanwhile, at the mRNA level (Fig. 2E and F), ZM241385 and CGS21680 at 10 nM significantly inhibited and excited the expression of A2AAR compared with other concentrations.
Subsequently, the expression of A2AAR was detected at the mRNA levels of 0.5 mg/mL SFJDC and 10 nM ZM241385 and CGS21680 (Fig. 2G). Based on the results, SFJDC was able to modulate the expression of A2AAR in comparison with LPS and ZM241385. However, it was lower than that in the CGS21680 agonist group. We also detected the expression of A2AAR by immunofluorescence staining (Fig. 2H) and found that both SFJDC and the agonist CGS21680 could upregulate the expression of the A2AAR, so we hypothesized that SFJDC had the same effect as the agonist CGS21680. According to the above results, we speculate that SFJDC may reduce RAW264.7-cell inflammation by activating the A2AAR.
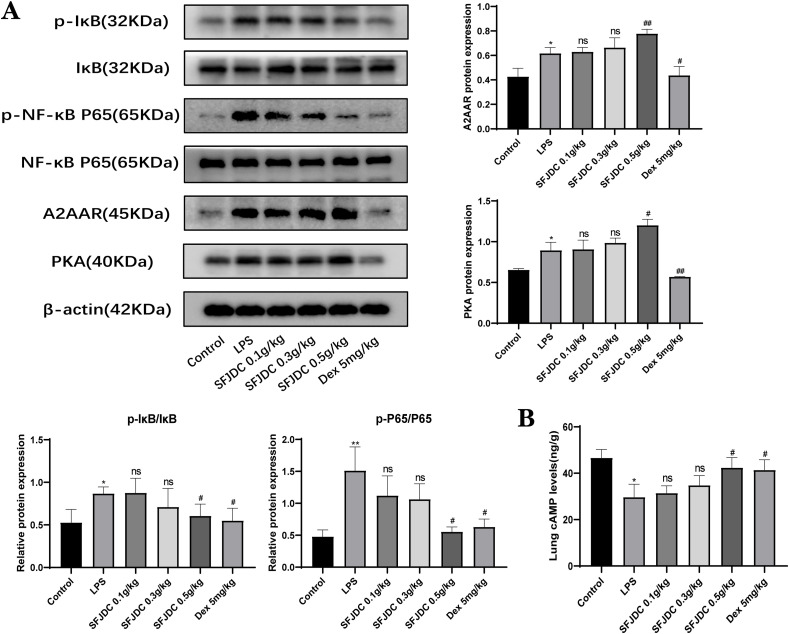
3.3. SFJDC inhibits the alveolar macrophage inflammatory response by regulating A2A/cAMP/NF-κB signaling
According to studies, cAMP and NF-κB signaling pathways have been seen as mechanisms by which A2AAR inhibits inflammation. Western blot analysis revealed the presence of A2AAR, PKA, IκB, p-IκB, P65 and p-P65 expression (Fig. 3 A). In the LPS group, A2AAR and PKA were found to be expressed at slightly higher levels than in the control group. Meanwhile, different doses of SFJDC can upregulate the expression of A2AAR and PKA. Compared to the control group, the ratios of p-IκB/IκB and p-P65/P65 gradually returned to normal after SFJDC treatment. However, the levels of the proteins IκB and P65 did not change significantly. According to our results, cAMP levels changed significantly in the animal model of lung injury caused by LPS, but then recovered to a normal, controllable range with gradual dose increases in SFJDC (Fig. 3B). Based on our results, SFJDC may promote cAMP production and reduce inflammation by activating A2AAR to modulate NF-κB signaling pathways.
Fig. 3.
SFJDC affects A2AAR/NF-κB signaling. (A) Western blots were used to detect the levels of A2AAR, PKA, IκB, p-IκB, P65, and p-P65 in lung tissues. (B) cAMP content in lung tissue homogenate. Compared to the control group, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Compared to the LPS group, #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001. Each set of data was based on six replicates.
3.4. SFJDC attenuates LPS-induced inflammation in RAW264.7 cells through A2A/cAMP/NF-κB signaling
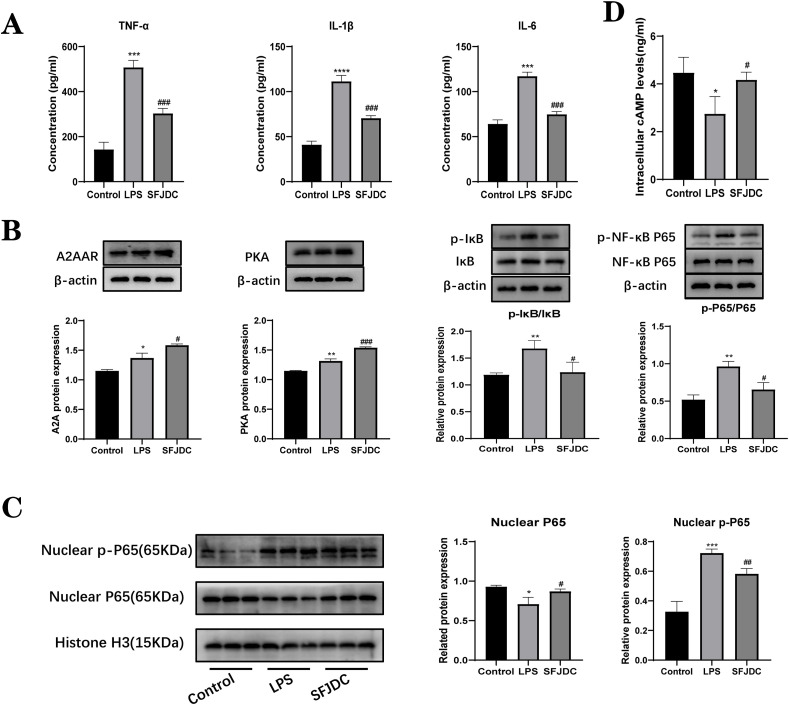
To further verify the function of SFJDC in macrophages, RAW264.7 cells were used for in vitro experiments. We measured the levels of inflammatory factors in RAW264.7 supernatants in the control, LPS, and SFJDC groups by ELISA (Fig. 4 A). In the LPS group, there was a clear difference between the levels of TNF-α, IL-1β, and IL-6 compared to those in the control group. Following pretreatment with SFJDC, TNF-α, IL-1β, and IL-6 levels significantly decreased. Subsequently, the protein expression levels of A2AAR, PKA, IκB, p-IκB, P65 and p-P65 were further detected by Western blotting (Fig. 4B). According to the results, the expression of A2AAR and PKA was slightly upregulated in the LPS group, and the expression of A2AAR and PKA was also upregulated in the SFJDC group, and the trend was consistent with that in lung tissue. After SFJDC treatment, the p-IκB/IκB and p-P65/P65 ratios in the LPS group also showed a decreasing and increasing trend, returning to normal levels. Meanwhile, IκB and P65 proteins did not exhibit significant changes. For this reason, we performed Western blot analysis with nuclear proteins (Fig. 4C), revealing that P65 protein expression was drastically reduced in the LPS group, while the expression of p-P65 increased significantly after entering the nucleus, which was significantly reversed by SFJDC. We also detected the cAMP level in RAW264.7 cells and found that the cAMP level in the LPS group changed significantly compared with that in the control group, and returned to the normal level after SFJDC pretreatment (Fig. 4D). Accordingly, it was found that SFJDC regulates the expression of A2AAR, promotes cAMP production, and inhibits P65 phosphorylation to reduce the release of inflammatory factors and decrease inflammatory responses in RAW264.7 cells.
Fig. 4.
Effects of SFJDC on RAW264.7 cells. (A) TNF-α, IL-1β, and IL-6 concentrations were determined with ELISA in RAW264.7 cells. (B) Protein levels of A2AAR, PKA, IκB, p-IκB, P65 and p-P65. (C) Nucleoprotein levels of P65 and p-P65 in RAW264.7 cells. (D) cAMP levels in RAW264.7 cells were detected by ELISA kit. Compared to the control group, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Compared to the LPS group, #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001. Each set of data was based on three replicates.
3.5. SFJDC inhibited LPS-induced apoptosis of lung epithelial cells
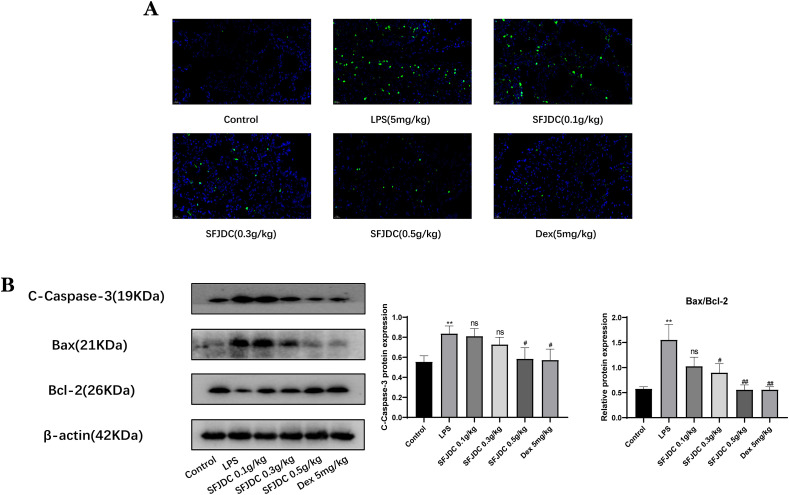
Apoptosis plays a crucial role in various diseases and body homeostasis. As an important lung barrier, apoptosis of lung epithelial cells triggers or promotes the evolution of lung diseases (Chambers et al., 2018; Li et al., 2018). We used TUNEL staining (Fig. 5 A) to study whether SFJDC influenced the apoptosis of lung epithelial cells following LPS exposure. Based on these results, SFJDC significantly decreased lung epithelial cell death and inhibited apoptosis in a concentration-dependent manner. Additionally, Western blot analysis of the apoptotic proteins Cc3, Bax and Bcl-2 confirmed their expression in the cells (Fig. 5B). A higher Bax/Bcl-2 ratio and increased Cc3 expression were found following LPS stimulation, which was reversed after SFJDC treatment. Based on these results, we concluded that SFJDC can inhibit LPS-induced apoptosis in lung tissues.
Fig. 5.
SFJDC inhibited apoptosis in lung tissue. (A) Apoptosis was detected in lung tissues using a TUNEL kit (scale = 20 μm). (B) Cc3, Bax, and Bcl-2 protein levels. Compared to the control group, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Compared to the LPS group, #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001. Each set of data was based on six replicates.
3.6. Effect of SFJDC on apoptosis of A549 cells
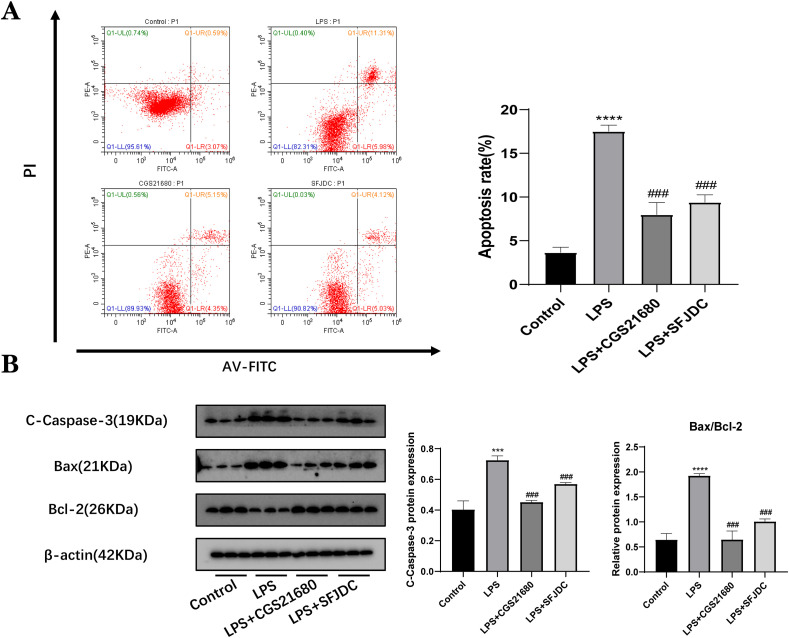
Lung epithelial cells are closely associated with inflammation. Inflammatory factors are known to influence apoptosis in lung epithelial cells. At the same time, numerous studies have revealed that the release of proinflammatory mediators can activate the apoptosis signaling pathway in lung epithelial cells. The aim of this study was to determine whether SFJDC could affect lung epithelial cell apoptosis via regulation of the inflammatory response of macrophages. To establish the coculture model, the supernatant from RAW264.7 cells was collected and A549 cells were cultured for 24 h. A549 cells were significantly more prone to apoptosis when cocultured with LPS-stimulated RAW264.7 cells than when cocultured with a control group. The A549 cells were cultured in the supernatants of the CGS21680 group and the SFJDC group, and flow cytometry confirmed that both A2AAR-mediated inflammation and SFJDC-mediated inflammation could reduce apoptosis (Fig. 6 A).
Fig. 6.
SFJDC and CGS21680 inhibited the apoptosis rate of A549 cells. (A) Flow cytometry measured the apoptotic rate of A549 cells. (B) Protein levels of Cc-3, Bax, and Bcl-2. Compared to the control group, *P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001. Compared to the LPS group, #P ≤ 0.05, ##P ≤ 0.01, ###P ≤ 0.001. Each set of data was based on three replicates.
Apoptosis-related proteins were also detected for further investigation. Western blot results showed that Cc3 and the Bax/Bcl-2 ratio of A549 cells cultured in the A2AAR agonist CGS21680 group and SFJDC group were decreased compared with the LPS group (Fig. 6B). Based on the above results, we concluded that apoptosis of lung epithelial cells is closely related to inflammation and that SFJDC can inhibit apoptosis of lung epithelial cells by reducing the inflammatory response.
4. Discussion
Acute lung injury can be caused by a variety of factors, and diffuse inflammation and tissue damage are the main characteristics (Gouda and Bhandary, 2019; Zhao et al., 2020). It is often manifested by severe damage to the vascular barrier and the expression of a large number of proinflammatory cytokines (Hoegl et al., 2015). This was consistent with LPS-induced ALI (Li et al., 2020; Qian et al., 2019). SFJDC, as a TCM compound preparation, has been proven to have a variety of active components and targets, which can exert anti-inflammatory, antibacterial, antiviral and other immunomodulatory effects through synergistic effects (Xia et al., 2021; Xu et al., 2022). According to our experiment, SFJDC could improve LPS-induced ALI.
In recent years, due to the discovery and mining of an increasing number of signaling pathways, the connections between signaling pathways have become more complex, and the biological effects have become increasingly diverse. The purine energy signaling pathway has received extensive attention during this period, as has its involvement in many diseases (Velázquez-Miranda et al., 2019; Zhao et al., 2022). A2AAR plays a key role in inhibiting the systemic inflammatory response and tissue-specific negative feedback regulation mechanisms (Eckle et al., 2009; Ohta and Sitkovsky, 2001). It has been reported that A2AAR knockout mice showed a decrease in overall lung function, and A2AAR stimulation significantly reduced neutrophil infiltration in lung tissue and the expression of related proinflammatory cytokines in LPS-induced ALI (He et al., 2013). Jörg Reutershan et al. also demonstrated that mice lacking A2AAR exhibited more severe microvascular permeability in the LPS-induced ALI model compared with wild-type mice. At the same time, the injection of A2AAR agonist not only reduced the permeability of pulmonary microvessels, but also inhibited the secretion of proinflammatory cytokines by macrophages (Reutershan et al., 2007). Joyce N Gonzales et al. found an increase in pulmonary vascular permeability induced by LPS, which was also closely related to excessive cells apoptosis, but the effect of LPS was prevented after activation of A2AAR (Gonzales et al., 2014). Moreover, A2AAR's positive relationship with cAMP is another key factor in its function. Activation of A2AAR is accompanied by an increase in cAMP level, which further inhibits P65 translocation to the nucleus. (Link et al., 2000; Sands et al., 2004; Shaikh and Cronstein, 2016). These are consistent with our results. Therefore, SFJDC plays an anti-inflammatory and anti-apoptosis role in ALI may be related to stimulating A2AAR expression and inhibiting NF-κB phosphorylation.
Inflammation is an important defense mechanism caused by injury, infection and stimulation (Leitch et al., 2008; Nathan, 2002). When inflammation is excessive, however, it can trigger an inflammatory cascade that can lead to lung injury (Gouda and Bhandary, 2019; Lee et al., 2018). Under the induction of LPS, this phenomenon was significantly amplified, the level of proinflammatory cytokines were significantly increased compared with the control group, and the inflammatory infiltration of lung tissue increased significantly as well, which was consistent with our results. However, after SFJDC pretreatment, these phenomena were significantly reversed and gradually returned to the normal level. NF-κB, a key inflammatory protein, also returns to homeostasis under the action of SFJDC. A2AAR and cAMP showed the same trend. At the same time, a high level of proinflammatory cytokines induces apoptosis of lung epithelial cells, destroys the integrity of the epithelial barrier and seriously damages normal gas exchange in the body, which is a major contributing factor to ALI (Ju et al., 2018; Li et al., 2019; Xie et al., 2018). Under LPS stimulation, macrophages were overactivated and secreted a large number of proinflammatory cytokines, which induced apoptosis of lung epithelial cells, which was verified by our flow cytometry assay and TUNEL assay. Apoptosis is closely related to the expression of Bax, Bcl-2, Cc3 and other molecules (Lindsay et al., 2011; Zhao et al., 2016). In our study, SFJDC was found to effectively inhibit LPS-induced apoptosis, downregulate the expression of Bax and Cc3, and upregulate the expression of Bcl-2 both in cells and animals. Based on the above results, We conclude that SFJDC can regulate inflammation and apoptosis through A2AAR, thereby inhibiting LPS-induced ALI.
During early studies of COVID-19, it was found that there was a high overlap between patients with COVID-19 and those with ALI in terms of onset time, medical imaging, and pathology. The onset time for COVID-19 patients is 8–12 days, and for ALI patients it is about 7 days. Both of them were characterized by tissue pneumonia, telangiectasia, endothelial injury, and mild to moderate pulmonary hypertension (Zarrilli et al., 2021a; Swenson and Swenson, 2021). Most importantly, the inflammatory response is a major factor driving the further development of COVID-19, which is highly consistent with ALI (Gustine and Jones, 2021; D'Agnillo et al., 2021). However, there are still limitations in this study. Unfortunately, our study did not explore clinical aspects, and the research conclusions could not be directly connected with clinical practice. Meanwhile, SFJDC's complexity raises the need for a deeper understanding of the components that play a role. The purpose of this study was to explore whether SFJDC could be used as a prophylactic treatment for COVID-19 by improving acute lung injury.
5. Conclusion
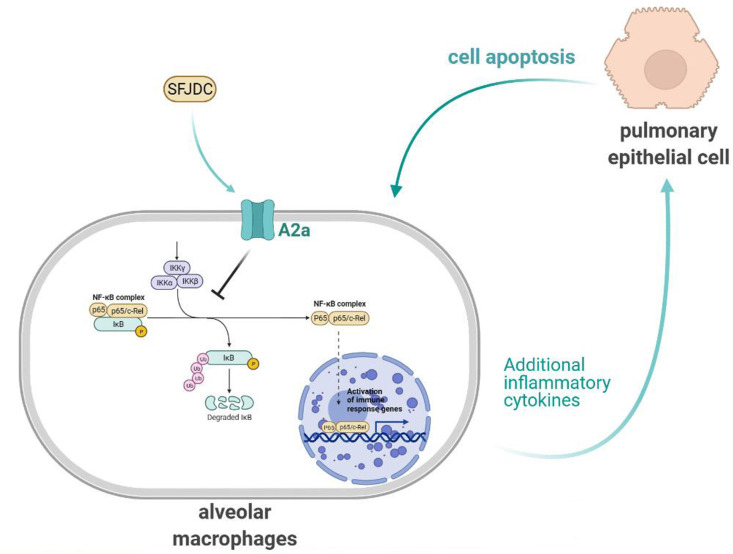
We conducted this research to probe the role and mechanism of SFJDC in ALI induced by LPS (Fig. 7 ). In animal and cell studies, we found that SFJDC may attenuate ALI by inhibiting the inflammatory response and cells apoptosis. The mechanism of SFJDC may be related to upregulation of A2AAR, increase of cAMP level and inhibition of phosphorylation of NF-κB. Although there are still some limitations to our study, SFJDC can inhibit the inflammatory response and apoptosis to reduce ALI, indicating that SFJDC could play a vital role in preventing and treating ALI. These findings provide valuable insights for the prevention and treatment of ALI by SFJDC, and also contribute to relevant rationalization for guiding clinical use of COVID-19.
Fig. 7.
Mechanism imaginary diagram.
CRediT authorship contribution statement
Junnan Cai: Conducted the research, Writing – original draft, prepared. Yu-lian Wang: Formal analysis, Writing – review & editing. Xiao-dong Sheng: prepared. Lei Zhang: Formal analysis. Xiongwen Lv: Conducted the research, Writing – original draft, All authors approved the final version of the manuscript.
Declaration of interest
We certify that we have no business or personal ties to any improper organizations or individuals, and that we have no interest of any kind in any products, services or companies that could affect or comment on the manuscript.
Acknowledgment
This study was supported by the collaborative Innovation Project of Universities in Anhui Province in 2020 (Item no. GXXT-2020-017).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jep.2022.115661.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Data availability
Data will be made available on request.
References
- Antonioli L., Blandizzi C., Pacher P., Haskó G. Immunity, inflammation and cancer: a leading role for adenosine. Nat. Rev. Cancer. 2013;13:842–857. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- Cai Y., Li H., Liu M., Pei Y., Zheng J., Zhou J., Luo X., Huang W., Ma L., Yang Q., Guo S., Xiao X., Li Q., Zeng T., Meng F., Francis H., Glaser S., Chen L., Huo Y., Alpini G., Wu C. Disruption of adenosine 2A receptor exacerbates NAFLD through increasing inflammatory responses and SREBP1c activity. Hepatology. 2018;68:48–61. doi: 10.1002/hep.29777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers E., Rounds S., Lu Q. Pulmonary endothelial cell apoptosis in emphysema and acute lung injury. Adv. Anat. Embryol. Cell Biol. 2018;228:63–86. doi: 10.1007/978-3-319-68483-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Fehr A.R., Vijay R., Mack M., Zhao J., Meyerholz D.K., Perlman S. Dysregulated type I interferon and inflammatory monocyte-macrophage responses cause lethal pneumonia in SARS-CoV-infected mice. Cell Host Microbe. 2016;19:181–193. doi: 10.1016/j.chom.2016.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Channappanavar R., Perlman S. Pathogenic human coronavirus infections: causes and consequences of cytokine storm and immunopathology. Semin. Immunopathol. 2017;39:529–539. doi: 10.1007/s00281-017-0629-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Agnillo F., Walters K.A., Xiao Y., Sheng Z.M., Scherler K., Park J., Gygli S., Rosas L.A., Sadtler K., Kalish H., Blatti C.R., Zhu R., Gatzke L., Bushell C., Memoli M.J., O'Day S.J., Fischer T.D., Hammond T.C., Lee R.C., Cash J.C., Powers M.E., O'Keefe G.E., Butnor K.J., Rapkiewicz A.V., Travis W.D., Layne S.P., Kash J.C., Taubenberger J.K. Lung epithelial and endothelial damage, loss of tissue repair, inhibition of fibrinolysis, and cellular senescence in fatal COVID-19. Sci. Transl. Med. 2021;13:j7790. doi: 10.1126/scitranslmed.abj7790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckle T., Koeppen M., Eltzschig H.K. Role of extracellular adenosine in acute lung injury. Physiology. 2009;24:298–306. doi: 10.1152/physiol.00022.2009. [DOI] [PubMed] [Google Scholar]
- Fan E., Fan J. Regulation of alveolar macrophage death in acute lung inflammation. Respir. Res. 2018;19:50. doi: 10.1186/s12931-018-0756-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazoni L.M., Laubach V.E., Mulloy D.P., Bellizzi A., Unger E.B., Linden J., Ellman P.I., Lisle T.C., Kron I.L. Additive protection against lung ischemia-reperfusion injury by adenosine A2A receptor activation before procurement and during reperfusion. J. Thorac. Cardiovasc. Surg. 2008;135:156–165. doi: 10.1016/j.jtcvs.2007.08.041. [DOI] [PubMed] [Google Scholar]
- Gessi S., Merighi S., Varani K., Borea P.A. Adenosine receptors in health and disease. Adv. Pharmacol. 2011;61:41–75. doi: 10.1016/B978-0-12-385526-8.00002-3. [DOI] [PubMed] [Google Scholar]
- Gonzales J.N., Gorshkov B., Varn M.N., Zemskova M.A., Zemskov E.A., Sridhar S., Lucas R., Verin A.D. Protective effect of adenosine receptors against lipopolysaccharide-induced acute lung injury. Am. J. Physiol. Lung Cell Mol. Physiol. 2014;306:L497–L507. doi: 10.1152/ajplung.00086.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouda M.M., Bhandary Y.P. Acute lung injury: IL-17A-Mediated inflammatory pathway and its regulation by curcumin. Inflammation. 2019;42:1160–1169. doi: 10.1007/s10753-019-01010-4. [DOI] [PubMed] [Google Scholar]
- Gustine J.N., Jones D. Immunopathology of hyperinflammation in COVID-19. Am. J. Pathol. 2021;191:4–17. doi: 10.1016/j.ajpath.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habashi N.M., Camporota L., Gatto L.A., Nieman G. Functional pathophysiology of SARS-CoV-2-induced acute lung injury and clinical implications. J. Appl. Physiol. 2021;130:877–891. doi: 10.1152/japplphysiol.00742.2020. 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haskó G., Linden J., Cronstein B., Pacher P. Adenosine receptors: therapeutic aspects for inflammatory and immune diseases. Nat. Rev. Drug Discov. 2008;7:759–770. doi: 10.1038/nrd2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He M., Shi W., Yu M., Li X., Xu J., Zhu J., Jin L., Xie W., Kong H. Nicorandil attenuates LPS-induced acute lung injury by pulmonary endothelial cell protection via NF-κB and MAPK pathways. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/4957646. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He X., Hu J.L., Li J., Zhao L., Zhang Y., Zeng Y.J., Dai S.S., He F.T. A feedback loop in PPARγ-adenosine A2A receptor signaling inhibits inflammation and attenuates lung damages in a mouse model of LPS-induced acute lung injury. Cell. Signal. 2013;25:1913–1923. doi: 10.1016/j.cellsig.2013.05.024. [DOI] [PubMed] [Google Scholar]
- He Y.Q., Zhou C.C., Yu L.Y., Wang L., Deng J.L., Tao Y.L., Zhang F., Chen W.S. Natural product derived phytochemicals in managing acute lung injury by multiple mechanisms. Pharmacol. Res. 2021;163 doi: 10.1016/j.phrs.2020.105224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrickson C.M., Matthay M.A. Endothelial biomarkers in human sepsis: pathogenesis and prognosis for ARDS. Pulm. Circ. 2018;8 doi: 10.1177/2045894018769876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoegl S., Brodsky K.S., Blackburn M.R., Karmouty-Quintana H., Zwissler B., Eltzschig H.K. Alveolar epithelial A2B adenosine receptors in pulmonary protection during acute lung injury. J. Immunol. 2015;195:1815–1824. doi: 10.4049/jimmunol.1401957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju M., Liu B., He H., Gu Z., Liu Y., Su Y., Zhu D., Cang J., Luo Z. MicroRNA-27a alleviates LPS-induced acute lung injury in mice via inhibiting inflammation and apoptosis through modulating TLR4/MyD88/NF-κB pathway. Cell Cycle. 2018;17:2001–2018. doi: 10.1080/15384101.2018.1509635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar V. Pulmonary innate immune response determines the outcome of inflammation during pneumonia and sepsis-associated acute lung injury. Front. Immunol. 2020;11:1722. doi: 10.3389/fimmu.2020.01722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H., Abston E., Zhang D., Rai A., Jin Y. Extracellular vesicle: an emerging mediator of intercellular crosstalk in lung inflammation and injury. Front. Immunol. 2018;9:924. doi: 10.3389/fimmu.2018.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitch A.E., Duffin R., Haslett C., Rossi A.G. Relevance of granulocyte apoptosis to resolution of inflammation at the respiratory mucosa. Mucosal Immunol. 2008;1:350–363. doi: 10.1038/mi.2008.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Chen Q., He X., Alam A., Ning J., Yi B., Lu K., Gu J. Dexmedetomidine attenuates lung apoptosis induced by renal ischemia-reperfusion injury through α(2)AR/PI3K/Akt pathway. J. Transl. Med. 2018;16:78. doi: 10.1186/s12967-018-1455-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T., Wu Y.N., Wang H., Ma J.Y., Zhai S.S., Duan J. Dapk1 improves inflammation, oxidative stress and autophagy in LPS-induced acute lung injury via p38MAPK/NF-κB signaling pathway. Mol. Immunol. 2020;120:13–22. doi: 10.1016/j.molimm.2020.01.014. [DOI] [PubMed] [Google Scholar]
- Li X., Jamal M., Guo P., Jin Z., Zheng F., Song X., Zhan J., Wu H. Irisin alleviates pulmonary epithelial barrier dysfunction in sepsis-induced acute lung injury via activation of AMPK/SIRT1 pathways. Biomed. Pharmacother. 2019;118 doi: 10.1016/j.biopha.2019.109363. [DOI] [PubMed] [Google Scholar]
- Liao Q., Chen W., Tong Z., Xue M., Gu T., Yuan Y., Song Z., Tao Z. Shufeng Jiedu capsules protect rats against LPS-induced acute lung injury via activating NRF2-associated antioxidant pathway. Histol. Histopathol. 2021;36:317–324. doi: 10.14670/HH-18-293. [DOI] [PubMed] [Google Scholar]
- Lindsay J., Esposti M.D., Gilmore A.P. Bcl-2 proteins and mitochondria--specificity in membrane targeting for death. Biochim. Biophys. Acta. 2011;1813:532–539. doi: 10.1016/j.bbamcr.2010.10.017. [DOI] [PubMed] [Google Scholar]
- Link A.A., Kino T., Worth J.A., McGuire J.L., Crane M.L., Chrousos G.P., Wilder R.L., Elenkov I.J. Ligand-activation of the adenosine A2a receptors inhibits IL-12 production by human monocytes. J. Immunol. 2000;164:436–442. doi: 10.4049/jimmunol.164.1.436. [DOI] [PubMed] [Google Scholar]
- Liu Y., Xiang D., Zhang H., Yao H., Wang Y. Hypoxia-inducible factor-1: a potential target to treat acute lung injury. Oxid. Med. Cell. Longev. 2020 doi: 10.1155/2020/8871476. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu H. Drug treatment options for the 2019-new coronavirus (2019-nCoV) Biosci Trends. 2020;14:69–71. doi: 10.5582/bst.2020.01020. [DOI] [PubMed] [Google Scholar]
- Majumdar S., Aggarwal B.B. Adenosine suppresses activation of nuclear factor-kappaB selectively induced by tumor necrosis factor in different cell types. Oncogene. 2003;22:1206–1218. doi: 10.1038/sj.onc.1206184. [DOI] [PubMed] [Google Scholar]
- Martin T.R. Lung injury and repair in coronavirus disease 2019-related acute lung injury. Am. J. Pathol. 2022;192:406–409. doi: 10.1016/j.ajpath.2022.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mokra D., Kosutova P. Biomarkers in acute lung injury. Respir. Physiol. Neurobiol. 2015;209:52–58. doi: 10.1016/j.resp.2014.10.006. [DOI] [PubMed] [Google Scholar]
- Mowery N.T., Terzian W.T.H., Nelson A.C. Acute lung injury. Curr. Probl. Surg. 2020;57 doi: 10.1016/j.cpsurg.2020.100777. [DOI] [PubMed] [Google Scholar]
- Nathan C. Points of control in inflammation. Nature. 2002;420:846–852. doi: 10.1038/nature01320. [DOI] [PubMed] [Google Scholar]
- Ohta A., Sitkovsky M. Role of G-protein-coupled adenosine receptors in downregulation of inflammation and protection from tissue damage. Nature. 2001;414:916–920. doi: 10.1038/414916a. [DOI] [PubMed] [Google Scholar]
- Qian J., Chen X., Shu S., Zhang W., Fang B., Chen X., Zhao Y., Liu Z., Liang G. Design and synthesis novel di-carbonyl analogs of curcumin (DACs) act as potent anti-inflammatory agents against LPS-induced acute lung injury (ALI) Eur. J. Med. Chem. 2019;167:414–425. doi: 10.1016/j.ejmech.2019.02.042. [DOI] [PubMed] [Google Scholar]
- Ragab D., Salah E.H., Taeimah M., Khattab R., Salem R. The COVID-19 cytokine storm; what we know so far. Front. Immunol. 2020;11:1446. doi: 10.3389/fimmu.2020.01446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reutershan J., Cagnina R.E., Chang D., Linden J., Ley K. Therapeutic anti-inflammatory effects of myeloid cell adenosine receptor A2a stimulation in lipopolysaccharide-induced lung injury. J. Immunol. 2007;179:1254–1263. doi: 10.4049/jimmunol.179.2.1254. [DOI] [PubMed] [Google Scholar]
- Sands W.A., Martin A.F., Strong E.W., Palmer T.M. Specific inhibition of nuclear factor-kappaB-dependent inflammatory responses by cell type-specific mechanisms upon A2A adenosine receptor gene transfer. Mol. Pharmacol. 2004;66:1147–1159. doi: 10.1124/mol.104.001107. [DOI] [PubMed] [Google Scholar]
- Shaikh G., Cronstein B. Signaling pathways involving adenosine A2A and A2B receptors in wound healing and fibrosis. Purinergic Signal. 2016;12:191–197. doi: 10.1007/s11302-016-9498-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma A.K., Linden J., Kron I.L., Laubach V.E. Protection from pulmonary ischemia-reperfusion injury by adenosine A2A receptor activation. Respir. Res. 2009;10:58. doi: 10.1186/1465-9921-10-58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soy M., Keser G., Atagündüz P., Tabak F., Atagündüz I., Kayhan S. Cytokine storm in COVID-19: pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020;39:2085–2094. doi: 10.1007/s10067-020-05190-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan G.W., Rieger J.M., Scheld W.M., Macdonald T.L., Linden J. Cyclic AMP-dependent inhibition of human neutrophil oxidative activity by substituted 2-propynylcyclohexyl adenosine A(2A) receptor agonists. Br. J. Pharmacol. 2001;132:1017–1026. doi: 10.1038/sj.bjp.0703893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X., Cheng H., Liu B., Du Y., Dong J., Huang J. Icariin reduces LPS-induced acute lung injury in mice undergoing bilateral adrenalectomy by regulating GRα. Eur. J. Pharmacol. 2020;876 doi: 10.1016/j.ejphar.2020.173032. [DOI] [PubMed] [Google Scholar]
- Swenson K.E., Swenson E.R. Pathophysiology of acute respiratory distress syndrome and COVID-19 lung injury. Crit. Care Clin. 2021;37:749–776. doi: 10.1016/j.ccc.2021.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Z., Gao J., Zhang G., Xue M., Yang W., Tong C., Yuan Y. Shufeng Jiedu Capsule protect against acute lung injury by suppressing the MAPK/NF-κB pathway. Biosci Trends. 2014;8:45–51. doi: 10.5582/bst.8.45. [DOI] [PubMed] [Google Scholar]
- Velázquez-Miranda E., Díaz-Muñoz M., Vázquez-Cuevas F.G. Purinergic signaling in hepatic disease. Purinergic Signal. 2019;15:477–489. doi: 10.1007/s11302-019-09680-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan L., Meng D., Wang H., Wan S., Jiang S., Huang S., Wei L., Yu P. Preventive and therapeutic effects of thymol in a lipopolysaccharide-induced acute lung injury mice model. Inflammation. 2018;41:183–192. doi: 10.1007/s10753-017-0676-4. [DOI] [PubMed] [Google Scholar]
- Wang J., Jiang M., Chen X., Montaner L.J. Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J. Leukoc. Biol. 2020;108:17–41. doi: 10.1002/JLB.3COVR0520-272R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J., Cai X., Ma R., Lei D., Pan X., Wang F. Anti-inflammatory effects of sweroside on LPS-induced ALI in mice via activating SIRT1. Inflammation. 2021;44:1961–1968. doi: 10.1007/s10753-021-01473-4. [DOI] [PubMed] [Google Scholar]
- Xia L., Shi Y., Su J., Friedemann T., Tao Z., Lu Y., Ling Y., Lv Y., Zhao R., Geng Z., Cui X., Lu H., Schröder S. Shufeng Jiedu, a promising herbal therapy for moderate COVID-19:Antiviral and anti-inflammatory properties, pathways of bioactive compounds, and a clinical real-world pragmatic study. Phytomedicine. 2021;85 doi: 10.1016/j.phymed.2020.153390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie W., Lu Q., Wang K., Lu J., Gu X., Zhu D., Liu F., Guo Z. miR-34b-5p inhibition attenuates lung inflammation and apoptosis in an LPS-induced acute lung injury mouse model by targeting progranulin. J. Cell. Physiol. 2018;233:6615–6631. doi: 10.1002/jcp.26274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y., Yang L., Wang L., Chen F. Potential therapeutic effect of Shufeng Jiedu capsule and its major herbs on coronavirus disease 2019 (COVID-19): a review. Drug Discov Ther. 2022;15:289–299. doi: 10.5582/ddt.2021.01099. [DOI] [PubMed] [Google Scholar]
- Ye Q., Wang B., Mao J. The pathogenesis and treatment of the 'Cytokine Storm' in COVID-19. J. Infect. 2020;80:607–613. doi: 10.1016/j.jinf.2020.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrilli G., Angerilli V., Businello G., Sbaraglia M., Traverso G., Fortarezza F., Rizzo S., De Gaspari M., Basso C., Calabrese F., Dei T.A., Fassan M. The immunopathological and histological landscape of COVID-19-mediated lung injury. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarrilli G., Angerilli V., Businello G., Sbaraglia M., Traverso G., Fortarezza F., Rizzo S., De Gaspari M., Basso C., Calabrese F., Dei T.A., Fassan M. The immunopathological and histological landscape of COVID-19-mediated lung injury. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms22020974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Lang W., Wang S., Li B., Li G., Shi Q. Echinacea polysaccharide alleviates LPS-induced lung injury via inhibiting inflammation, apoptosis and activation of the TLR4/NF-κB signal pathway. Int. Immunopharm. 2020;88 doi: 10.1016/j.intimp.2020.106974. [DOI] [PubMed] [Google Scholar]
- Zhao H., Liu Z., Shen H., Jin S., Zhang S. Glycyrrhizic acid pretreatment prevents sepsis-induced acute kidney injury via suppressing inflammation, apoptosis and oxidative stress. Eur. J. Pharmacol. 2016;781:92–99. doi: 10.1016/j.ejphar.2016.04.006. [DOI] [PubMed] [Google Scholar]
- Zhao J., Zhang G., Cui W., Tian B. [Progress of neutrophil extracellular traps in airway inflammation of acute lung injury/acute respiratory distress syndrome: review] Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi. 2020;36:664–670. [PubMed] [Google Scholar]
- Zhao N., Xia G., Cai J., Li Z., Lv X.W. Adenosine receptor A2B mediates alcoholic hepatitis by regulating cAMP levels and the NF-KB pathway. Toxicol. Lett. 2022;359:84–95. doi: 10.1016/j.toxlet.2022.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.