Abstract
The archaeon Sulfolobus solfataricus grows optimally at 80°C and pH 2.5 to 3.5 on carbon sources such as yeast extracts, tryptone, and various sugars. Cells rapidly accumulate glucose. This transport activity involves a membrane-bound glucose-binding protein that interacts with its substrate with very high affinity (Kd of 0.43 μM) and retains high glucose affinity at very low pH values (as low as pH 0.6). The binding protein was extracted with detergent and purified to homogeneity as a 65-kDa glycoprotein. The gene coding for the binding protein was identified in the S. solfataricus P2 genome by means of the amino-terminal amino acid sequence of the purified protein. Sequence analysis suggests that the protein is anchored to the membrane via an amino-terminal transmembrane segment. Neighboring genes encode two membrane proteins and an ATP-binding subunit that are transcribed in the reverse direction, whereas a homologous gene cluster in Pyrococcus horikoshii OT3 was found to be organized in an operon. These data indicate that S. solfataricus utilizes a binding-protein-dependent ATP-binding cassette transporter for the uptake of glucose.
Glucose is one of the most important carbon sources for many living organisms. It is metabolized mostly via a mechanism that involves catabolite repression. Such a “glucose effect” has also been described for the extremely thermoacidophilic archaeon Sulfolobus solfataricus (9). The pathways of glucose metabolism in this archaeon have been well characterized (22). However, little is known about the mechanism by which glucose is taken up by S. solfataricus. In bacteria, glucose transport occurs often via the phosphoenolpyruvate-dependent sugar phosphotransferase transport system. In Archaea, a phosphotransferase system most probably is absent, since the available archaeal genome sequences do not give any indications of the presence of such systems (23). An alternative mechanism for uptake of glucose is ion symport. In bacteria, mostly H+/glucose symporters are found, whereas in eukaryotes, Na+/glucose symporters are more common. Most of the bacterial sugar symporters belong to a well-characterized family, the major facilitator superfamily (17). A third mechanism described for sugar uptake is via a binding-protein dependent ATP-binding cassette (ABC) transporter. A well-studied ABC transporter is the maltose transport system of Escherichia coli (2). It comprises a binding protein in the periplasm, two inner membrane components, and two identical domains, which catalyze the hydrolysis of ATP, the energy source for this transport system.
A binding-protein-dependent ABC transporter for trehalose and maltose has been described for the archaeon Thermococcus litoralis (10). This system is equipped with a binding protein that has an exceptionally high substrate-binding affinity (26). We have analyzed the mechanism of glucose transport in the extremely thermoacidophilic S. solfataricus. Our data indicate that glucose is taken up via a high-affinity-binding-protein-dependent ABC transporter. The binding protein is a secreted glycoprotein that is anchored to the cytoplasmic membrane by a membrane-spanning domain and that shows a very low pH optimum for glucose binding.
MATERIALS AND METHODS
Organisms and growth conditions.
S. solfataricus P1 was obtained from W. Zillig (Max-Planck-Institute for Biochemistry, Martinsried, Germany), and S. solfataricus P2 (DSM 1617) was obtained from the Deutsche Sammlung von Mikroorganismen und Zellkultur GmbH (Braunschweig, Germany). Cells were grown aerobically at 80°C in the mineral base of Allen, as modified by Brock et al. (3), supplemented with 0.1% yeast, 0.2% tryptone, and 0.5% sucrose or 0.5% glucose as the sole carbon source at pH 3.
Uptake experiments.
Cells grown in 50-ml cultures were harvested at an optical density at 600 nm of 0.5 to 0.8. The cells were washed twice in medium without carbon source, resuspended at 10 mg of protein/ml, and preincubated for 15 min at 60°C. Subsequently, 10 μl of this cell suspension was added to 90 μl of prewarmed medium without carbon source containing different concentrations of radiolabelled sugars (specific activities: [14C]glucose, 291 mCi/mmol; [3H]glucose, 13 Ci/mmol) (Amersham, ’s Hertogenbosch, The Netherlands). At different time intervals, the reaction was stopped by adding 2 ml of ice-cold 0.1 M LiCl, and the mixture was rapidly filtered through 0.45-μm-pore-size BA 85 nitrocellulose filters (Protran; Schleicher & Schuell, Dassel, Germany). Filters were washed with 2 ml of 0.1 M LiCl and dissolved and counted in 2 ml of scintillation fluid (Packard). For the determination of the kinetic parameters Km and Vmax of glucose uptake in cells, incubation was stopped after 10 s. Data were analyzed by the direct linear plot.
Preparation of membranes of S. solfataricus.
Cells were suspended in 20 mM Bis-Tris propane (pH 6.5) containing a small amount of DNase I and subsequently passed through a French pressure cell at 800 lb/in2. Unbroken cells were removed by low-speed centrifugation at 3,000 × g for 20 min at 4°C, and membranes were collected from the supernatant by centrifugation (100,000 × g for 45 min at 4°C). To remove peripheral membrane proteins, the pellet was resuspended in 20 mM Bis-Tris propane (pH 6.5)–6 M urea at a protein concentration of 5 mg/ml. After 30 min on ice, the suspension was centrifuged (100,000 × g for 45 min at 4°C) and the membrane pellet was resuspended in 20 mM Bis-Tris propane (pH 6.5) and stored in liquid nitrogen. Alternatively, membranes were extracted with 22 mM Na2CO3 for 40 min at 45°C (8).
Binding assays.
Binding of radiolabelled substrates to membranes or solubilized protein was assayed as described by Richarme and Kepes (18). Basically, 10 μl of membranes (10 mg/ml) or purified protein (0.3 mg/ml) was added to 90 μl of 50 mM glycine HCl (pH 2) and preincubated for 5 min at 60°C. [14C]glucose was added at various concentrations, and the suspension was incubated at 60°C. At various time intervals, the reaction was stopped by the addition of 2 ml of a chilled 70% saturated ammonium sulfate solution, and the mixture was filtered through 0.2-μm-pore size BA 83 nitrocellulose filters and washed once with 2 ml of the same solution. Filters were dissolved in 2 ml of scintillation fluid, and the radioactivity was counted. Binding data were analyzed by the method of Scatchard (20).
Purification of GBP from membranes of S. solfataricus.
Membranes of S. solfataricus were solubilized in a buffer containing 20 mM Bis-Tris propane (pH 6.5) and 2% Triton X-100. The suspension was incubated for 2 h at 37°C, and insoluble material was removed by centrifugation (100,000 × g for 45 min). The supernatant was diluted with 20 mM Bis-Tris propane (pH 6.5) containing 0.5 M NaCl to yield a final concentration of 0.05% Triton X-100. Subsequently, the material was applied to a concanavalin A (ConA)-Sepharose (Pharmacia, Roosendaal, The Netherlands) column equilibrated with buffer A (20 mM Bis-Tris propane [pH 6.5], 0.5 M NaCl, 0.05% Triton X-100). The column was washed with 5 volumes of the same buffer, and bound glycoproteins were eluted with a linear gradient of 0 to 200 mM α-methylmannopyranoside in buffer A. Fractions were assayed for glucose-binding activity as described above. α-Methylmannopyranoside did not interfere with glucose binding, since the glucose-binding assay was not influenced by the presence of a 100-fold excess of α-methylmannopyranoside. Active fractions were pooled, and NaCl was removed overnight by dialysis against 1,000 volumes of buffer B (20 mM Bis-Tris propane [pH 6.5], 0.05% Triton X-100). The dialysis buffer was replaced three times. The protein fraction was subsequently applied to a HR5/5 Mono Q column (Pharmacia, Uppsala, Sweden) preequilibrated with buffer B. Proteins were eluted with a linear gradient of 0 to 500 mM NaCl. The glucose-binding protein (GBP) eluted at 120 mM NaCl and was subsequently dialyzed against 1,000 volumes of 50 mM glycine HCl (pH 2)–0.05% Triton X-100 with 10% glycerol. Samples were routinely analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE).
Detection of binding activity in nondenaturating polyacrylamide gels.
Native PAGE was performed as described by Schaegger and von Jagow (21). The glucose-binding activity in these gels was determined as follows. A gel strip was incubated at 80°C for 20 min in 20 mM Bis-Tris propane (pH 6.5) that contained 1 μM [14C]glucose (specific activity, 291 mCi/mmol). The gel strip was washed for 10 min in water and fixed for 5 min in 50% (vol/vol) methanol–10% (vol/vol) acetic acid. After being washed with water for 5 min, the gel was dried and exposed to a high-sensitivity X-ray film (Kodak).
Cloning of the gene encoding the GBP.
Two degenerate primers (see Fig. 5A) were designed on the basis of the N-terminal amino acid sequence of the purified GBP and used in a PCR with genomic DNA isolated from S. solfataricus P1 and P2. The resulting PCR product of 80 bp was ligated in an pGEM-T-easy vector (Promega) and subsequently sequenced. The obtained sequences were used to screen the S. solfataricus P2 database (24a) and GenBank (6a).
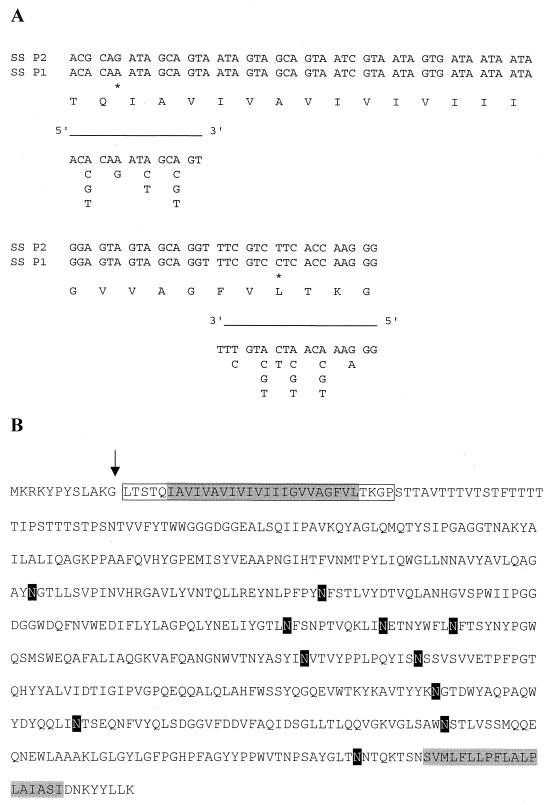
FIG. 5.
Amino acid sequence of the GBP. (A) Sequence of the amino terminus of the purified protein. Also indicated are the nucleotide sequences of the PCR primers used to clone the gene encoding GBP. An asterisk indicates differences in the nucleotide sequence of S. solfataricus P1 and P2. (B) Complete amino acid sequence of the binding protein derived from the nucleotide sequence found in the S. solfataricus genomic bank. The sequenced amino-terminal fragment is boxed, the positions of the putative transmembrane segments are shaded in grey, and putative glycosylation sites are shaded in black.
Other techniques.
For the amino-terminal sequence analysis, purified GBP was transferred to a polyvinylidene difluoride membrane. DNA and protein sequencing was performed by Eurosequence (Groningen, The Netherlands). Staining of glycoproteins in SDS-PAGE was performed as described by Wardi and Michos (25). Protein concentrations were determined with the DC kit (Bio-Rad, Veenendaal, The Netherlands).
RESULTS
Sugar transport in S. solfataricus cells.
S. solfataricus can grow on various sugars as sole carbon and energy source (7). Uptake of glucose, galactose, fructose, sucrose, and maltose occurs rapidly. Glucose is transported most rapidly and was therefore studied in greater detail. The rate of glucose uptake is highly dependent on the external pH (Fig. 1). The uptake rate is highest at pH 3.0, strongly decreased above pH 4.0, and is no longer detectable at pH 5.0 and above. At higher pH values, the ΔpH and the internal pool of ATP rapidly diminish (16), causing transient glucose uptake or no uptake at all. The apparent Km for glucose uptake was found to be 1.9 μM at pH 3.0 and 60°C, with a Vmax of 0.9 nmol min−1 (mg of protein)−1. A 10-fold excess of galactose and mannose significantly inhibited the uptake of glucose, while 2-deoxyglucose only marginally affected uptake (Fig. 2A). A 100-fold excess of 2-deoxyglucose decreased the glucose uptake only by 50%. Fructose, sucrose, and maltose had no effect on glucose uptake (data not shown). These data suggest that S. solfataricus cells take up glucose and most probably also galactose and mannose in an energy-dependent manner by the same high-affinity transport system.
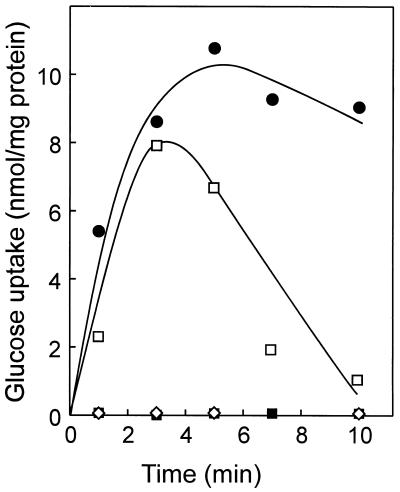
FIG. 1.
pH dependence of glucose uptake by S. solfataricus cells. Uptake assays were performed at 60°C and 1 μM [14C]glucose at pH 3 (●), pH 4 (□), pH 5 (■), and pH 6 (◊). The rapid decrease in radioactivity at pH 4 after 3 min was probably due to the metabolic degradation of glucose into rapidly diffusible products.
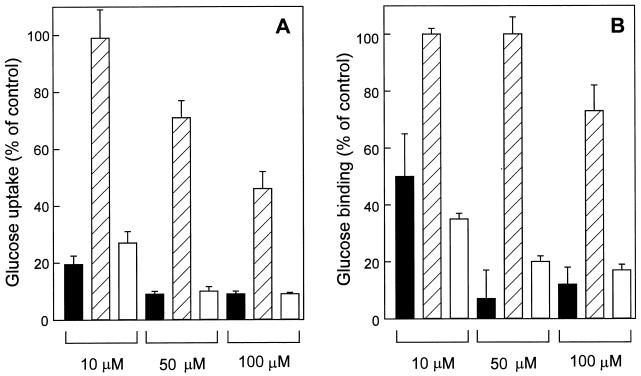
FIG. 2.
Glucose uptake by S. solfataricus cells (A) and binding of glucose to purified GBP (B) in the presence of competing substrates. Uptake and binding assays were performed at 60°C and 1 μM [14C]glucose in the presence of the indicated concentration of mannose (black bars), 2-deoxyglucose (hatched bars), and galactose (white bars). Cells were preincubated for 30 s with nonlabelled sugars in medium (pH 2.5) without carbon source, and uptake was stopped after 10 s. GBP was preincubated for 1 min with nonlabelled sugars in buffer (pH 2), and the binding reaction was stopped after 2 min.
Isolated membranes of S. solfataricus bind glucose with high affinity.
To further characterize glucose transport in S. solfataricus, membrane vesicles are the preferred model system for uptake studies, as they are devoid of substrate-metabolizing activities and since the energy supply for transport across the membranes of these vesicles can be well controlled. However, attempts to construct closed membrane vesicles from S. solfataricus have so far been unsuccessful, partly due to the difficulty in removing the membrane-anchored S-layer. Membranes derived by French press treatment of Sulfolobus cells formed vesicle-like structures, which were, however, leaky for protons and small ions and thus were unable to maintain a proton motive force. These membranes nevertheless showed a distinct glucose-binding activity, which was strongly pH dependent (Fig. 3A) with an optimum at pH 1.5. At this pH value, binding occurred most rapidly.
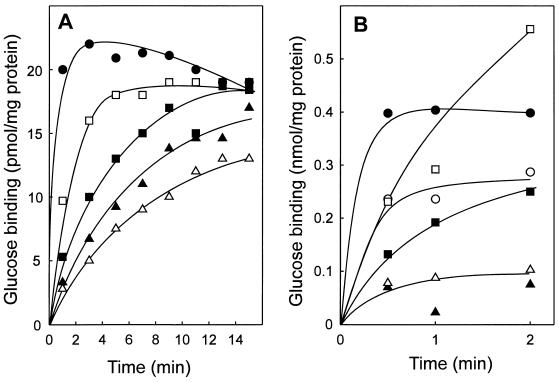
FIG. 3.
pH dependence of glucose binding to S. solfataricus membranes (A) and purified GBP (B). Binding assays were performed at 60°C in the presence of 1 μM [14C]glucose. The buffers used for the different pH values were 250 mM HCl (pH 0.6) (○ [only in panel B]), 50 mM HCl (pH 1.5) (●), 50 mM glycine HCl (pH 2 [□] and 3 [■], and 50 mM citric acid NaOH (pH 4 [▵] and 5 [▴]).
Glucose binding in membranes could not be inhibited by the addition of the ionophores valinomycin and nigericin, nor was it affected by the presence of the detergent Triton X-100. Moreover, in a total-membrane-protein extract obtained after detergent solubilization, the same pH dependence of glucose binding was observed as for the intact membranes (data not shown). Therefore, it is concluded that the S. solfataricus membranes harbor a glucose-binding activity.
Glucose binding is mediated by a membrane-bound 65-kDa glycoprotein.
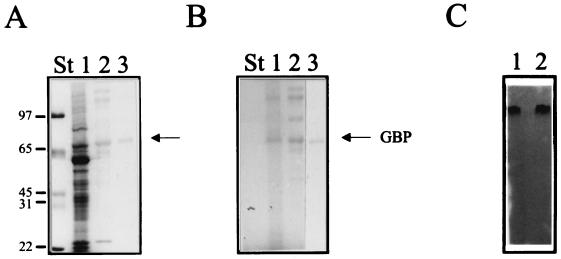
To identify the GBP, the protein was purified to homogeneity from S. solfataricus membranes by using the binding activity to monitor the purification. The activity appeared to be tightly associated with the membranes since it resists treatment of the membranes with chaotropic agents like urea or Na2CO3 (pH 10). Extraction of the protein from the membrane required a high concentration of the detergent Triton X-100 (2%). Initially, the protein was further purified by Mono Q fast protein liquid chromatography (FPLC) by using the glucose-binding assay to monitor the activity. Activity appeared to be related to a 65-kDa polypeptide in SDS-PAGE (Fig. 4A). A glycoprotein-specific stain indicated that this binding protein is glycosylated (Fig. 4B). This enabled a larger-scale purification by means of ConA-Sepharose affinity chromatography. ConA-Sepharose specifically binds the α-glucosyl and α-mannosyl side chains of glycoproteins. By increasing the concentration of methyl-α-d-mannopyranoside, five to seven glycoproteins could be eluted from the ConA-Sepharose column after loading with a total Triton X-100 extract of S. solfataricus membranes. The protein was subsequently purified to homogeneity by FPLC Mono Q. The purified 65-kDa protein retained the ability to bind [14C]glucose on a native PAGE gel (Fig. 4C). Moreover, the same activity-staining technique reveals the presence of a single band in the total-membrane extracts that corresponds to the purified GBP (Fig. 4C). The isolated GBP exhibited the same pH optimum curve as was found in membranes or solubilized membrane proteins (Fig. 3B). The optimal pH value was around 1.5, but significant binding activity could even be detected at a pH of 0.6 (Fig. 3B). The Kd for glucose binding was determined to be 430 nM at pH 2 and 60°C. The substrate specificity was tested by adding nonlabelled sugar to the binding-assay mixture. As with glucose transport activity in intact cells, binding of glucose to the purified binding protein was strongly inhibited by galactose and mannose but not by 2-deoxyglucose (Fig. 2B). We were unable to detect any significant level of 2-[3H]deoxyglucose binding to the membrane vesicles or purified binding protein (data not shown). Taken together, these data demonstrate that glucose binding by S. solfataricus cells is mediated by a membrane-bound glycoprotein with an apparent molecular mass of 65 kDa.
FIG. 4.
Purification of the GBP from S. solfataricus membranes. (A and B) Coomassie brilliant blue (A) and glycoprotein (B) staining of SDS-PAGE gels of Triton X-100-solubilized membrane proteins (lane 1), the glycoprotein fraction eluting from the ConA-Sepharose column (lane 2), and the purified binding protein after FPLC Mono Q (lane 3). The positions of the molecular mass standards are indicated. (C) [14C]glucose staining of a native PAGE gel of purified GBP (lane 1) and solubilized membrane proteins (lane 2).
Genetic characterization of the GBP.
To identify the gene coding for the GBP, the amino-terminal amino acid sequence of the purified protein was determined. A stretch of 31 amino acid residues could unequivocally be determined (Fig. 5A), and this sequence was used to design two degenerate primers to amplify part of the gene by PCR. The primers allowed the PCR amplification of an 80-bp DNA fragment when either S. solfataricus P1 or P2 chromosomal DNA was used as the template. The translated nucleotide sequence of the PCR product corresponded to the short amino-terminal sequence of the purified GBP (Fig. 5) and allowed the identification of the complete open reading frame (ORF) (accession code c42_036) in the genomic database of S. solfataricus P2 (Fig. 5B). This gene codes for a protein of 61 kDa. The size difference with respect to the purified protein (∼65 kDa) is most probably due to the glycosylation of the mature protein. The protein contains 11 possible glycosylation sites (Fig. 5B), while hydropathy analysis revealed strong hydrophobic regions at the amino and carboxyl termini of the protein. Both may form a transmembrane segment that anchors the protein to the cytoplasmic membrane.
The amino-terminal amino acid sequence of the purified GBP completely matched the predicted sequence from the DNA database, except that the first 12 amino acids were lacking in the purified protein. One possibility is that the protein is truncated as a result of a proteolytic degradation. The other possibility is that the protein is processed after synthesis and before transport over the cytoplasmic membrane (1).
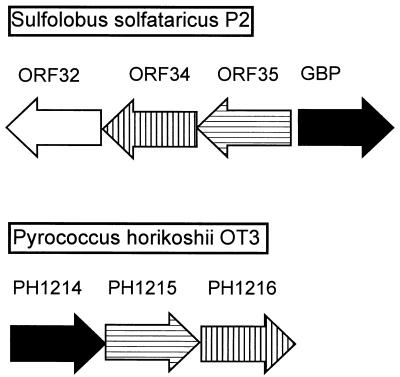
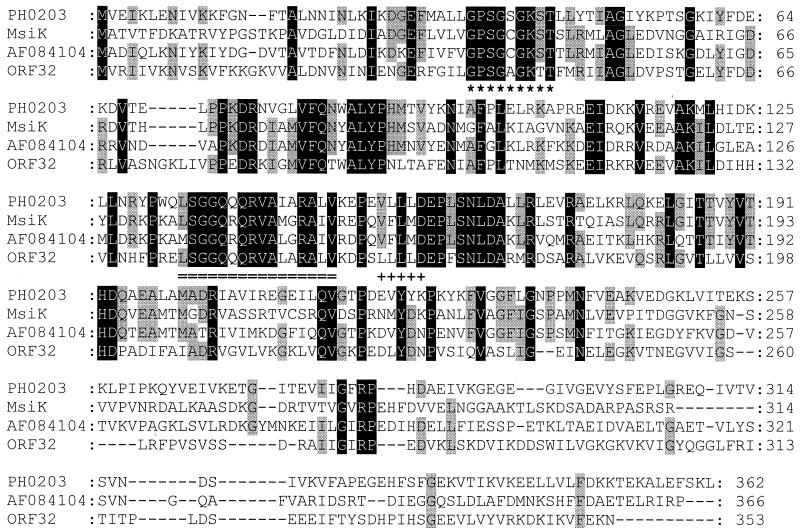
Data bank searches revealed that the GBP has 24% (40% similarity) and 19% (30% similarity) identical residues to the products of the P. horikoshii PH1214 (accession no. G3915507) and PH1039 (accession no. G3915490) genes, respectively. It is 15% identical (29% similar) to the product of the bxlE gene of Streptomyces lividans (accession no. G3941369), which corresponds to a putative sugar-binding protein. Downstream of PH1214 of P. horikoshii, two other ORFs are located in the same transcription direction, PH1215 (accession no. G3915506) and PH1216 (accession no. G3915506) (Fig. 6). These two ORFs code for integral membrane proteins that are homologous to sugar permeases bearing the inner membrane component signature typical of binding-protein-dependent transport systems. Further analysis of the DNA sequence surrounding the GBP gene of S. solfataricus revealed three upstream genes, i.e., ORF32, ORF34, and ORF35, that are transcribed in the reverse direction (Fig. 6). The ORF34 and ORF35 products show homology to binding-protein-dependent sugar permeases, while the ORF32 product is similar to several ATP-binding proteins (Fig. 7). Furthermore, ORF34 and PH1215 (28% identity, 51% similarity), and ORF35 and PH1216 (24% identity, 54% similarity) are homologous (Fig. 6). This genetic organization suggests that the GBP is a subunit of an ABC transporter.
FIG. 6.
Organization of the genomic region around the GBP of S. solfataricus and its homolog, PH1214, of P. horikoshii OT3. Homologous genes are indicated by the same shading pattern. ORF34, ORF35, PH1215, and PH1216 code for putative membrane proteins, and ORF32 encodes an ATP-binding protein belonging to the ABC superfamily.
FIG. 7.
Alignment of the ORF32 product of the ABC operon of S. solfataricus with other ATP-binding proteins (PH0203 of P. horikoshii, MsiK of Streptomyces lividans, and AF084104 of Bacillus firmus). The ABC transporter family signature is doubly underlined, and the Walker A (∗) and Walker B (+) motifs of the nucleotide-binding site are indicated. Residues which are conserved in all four proteins are shaded in black, and residues found in only three of the four aligned proteins are shaded in grey.
DISCUSSION
In this study we have investigated the mechanism of glucose transport in Sulfolobus solfataricus. Transport of glucose is mediated by a high-affinity-binding-protein-dependent system that is specific for glucose, galactose, and mannose. We were unable to detect any 2-deoxyglucose binding to the membrane vesicles and to the purified binding protein. Moreover, 2-deoxyglucose is a very poor inhibitor of glucose binding and transport. This implies that a hydroxyl group at the C-2 position of the sugar is critical for binding of the substrate but that there is no discrimination between C-2 and C-4 epimers of glucose. Studies by Cusdin et al. (4) suggest that 2-deoxyglucose is transported in a different strain of S. solfataricus (DSM 1616) by a glucose-galactose-mannose transporter, albeit with a 15-fold-lower affinity than glucose. In our study, the difference in affinity between 2-deoxyglucose and glucose appears to be even larger. The exact reason for this discrepancy is not clear but could relate to differences in the strains used. Nevertheless, both studies show that 2-deoxyglucose is a poor substrate for this transporter.
A transporter with affinity for glucose and galactose has previously been identified in Brucella abortus (5). This system presumably catalyzes a sugar/H+ symport reaction. Energetically, such a mechanism would also be favourable for S. solfataricus, since it maintains a very large ΔpH across its membrane (16). Uptake of glucose by S. solfataricus could possibly be mediated by a binding protein that is associated with a secondary transport system (12, 24), but the gene encoding the GBP is located adjacent to genes encoding two integral membrane proteins and one ATPase subunit that are typical of ABC transporters. Since these three genes are transcribed in the reverse direction relative to the binding protein, a direct link is not immediately obvious. However, homologues of these genes in the genome of P. horikoshii OT3 (14) are contained in a single operon-like structure. Moreover, the two integral membrane proteins, the ORF34 and ORF35 products, are also homologous to many other sugar permeases belonging to an ABC transporter. We therefore conclude that the GBP is a subunit of an ABC transport system. The binding-protein-dependent maltose/trehalose transporters of Thermococcus litoralis (10) and S. shibatae (27) and the glucose transporter of S. solfataricus exhibit very high affinities for their sugar substrates, i.e., in the submicromolar range. The high affinity of the binding protein allows these archaeal cells to utilize carbon sources efficiently in substrate-poor environments such as the hydrothermal vents in the deep sea or the hot sulfuric pools.
We noted that a short amino-terminal sequence of 12 amino acids is not present in the purified GBP although it is predicted on the basis of the nucleotide sequence. Similar observations have been made for the flagellin proteins in methanogenes (13). This suggests that these proteins may use a similar mechanism of processing and possibly even secretion (1).
Another unusual aspect of the GBP is its extreme acid resistance. The protein exhibits a very low pH optimum, i.e., around pH 1.5, which is comparable to that of pepsin in the stomach (6). In this respect, GBP differs from two other extracellular enzymes of Sulfolobus that have been analyzed, i.e., α-glucosidase (19) and an esterase (11), which exhibit pH optima of 4.5 and 6, respectively. The purified GBP in detergent solution appeared somewhat more acid susceptible than the membrane-bound enzyme did (compare Fig. 3A and B).
In gram-negative bacteria, binding proteins exist in a soluble form in the periplasm. S. solfataricus lacks an outer membrane and is instead surrounded by an S-layer. This hexagonal-paracrystalline-proteinaceous structure contains large pores of 4 to 5 nm (15) to allow contact with the external medium. It is thought that this structure serves as a molecular sieve, but it is not known if it can act as a barrier for proteins that are present in the space between the cytoplasmic membrane and the S-layer. The transmembrane segment(s) of GBP most probably serves as an anchor to the membrane, like the lipid moiety that retains binding proteins at the cytoplasmic membrane of gram-positive bacteria. Although the exact membrane topology of the protein is not yet known, it is most likely that the major part of the protein is located outside where it is glycosylated and where it can perform its function as a binding protein. Future experiments will address the membrane topology of this protein.
ACKNOWLEDGMENTS
We thank Carola Fuchs and Sigi Peters, Technical University Hamburg, Harburg, Germany, for the large-scale growth of S. solfataricus.
This work was supported by a TMR grant from the European Commission (ERBFMBIC971980) and a grant from the Deutsche Akademische Austauschdienst (D/97/18761).
REFERENCES
- 1.Albers S-V, Konings W N, Driessen A J M. A unique short signal sequence in membrane anchored proteins of Archaea. Mol Microbiol. 1999;30:1595–1596. doi: 10.1046/j.1365-2958.1999.01286.x. [DOI] [PubMed] [Google Scholar]
- 2.Boos W, Shuman H. Maltose/maltodextrin system of Escherichia coli: transport, metabolism, and regulation. Microbiol Mol Biol Rev. 1998;62:204–229. doi: 10.1128/mmbr.62.1.204-229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brock T D, Brock K M, Belly R T, Weiss R L. Sulfolobus: a new genus of sulfur-oxidizing bacteria living at low pH and high temperature. Arch Mikrobiol. 1972;84:54–68. doi: 10.1007/BF00408082. [DOI] [PubMed] [Google Scholar]
- 4.Cusdin F S, Robinson M J, Holman G D, Hough D W, Danson M J. Characterisation of glucose transport in the hyperthermophilic archaeon Sulfolobus solfataricus. FEBS Lett. 1996;387:193–195. doi: 10.1016/0014-5793(96)00497-8. [DOI] [PubMed] [Google Scholar]
- 5.Essenberg R C, Candler C, Nida S K. Brucella abortus strain 2308 putative glucose and galactose transporter gene: cloning and characterization. Microbiology. 1997;143:1549–1555. doi: 10.1099/00221287-143-5-1549. [DOI] [PubMed] [Google Scholar]
- 6.Fox P F, Whitaker J R. Isolation and characterization of sheep pepsin. Biochem J. 1977;161:389–398. doi: 10.1042/bj1610389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6a.GenBank database. 1 June 1999, revision date. Sequences. [Online.] http://www.ncbi.nlm.nih.gov/Entrez/nucleotide.html. [3 June 1999, last date accessed.]
- 7.Grogan D W. Phenotypic characterization of the archaebacterial genus Sulfolobus: comparison of five wild-type strains. J Bacteriol. 1989;171:6710–6719. doi: 10.1128/jb.171.12.6710-6719.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Grogan D W. Organization and interactions of cell envelope proteins of the extreme thermoacidophile Sulfolobus acidocaldarius. Can J Microbiol. 1996;42:1163–1171. [Google Scholar]
- 9.Haseltine C, Rolfsmeier M, Blum P. The glucose effect and regulation of α-amylase synthesis in the hyperthermophilic archaeon Sulfolobus solfataricus. J Bacteriol. 1996;178:945–950. doi: 10.1128/jb.178.4.945-950.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Horlacher R, Xavier K B, Santos H, DiRuggiero J, Kossmann M, Boos W. Archaeal binding protein-dependent ABC transporter: molecular and biochemical analysis of the trehalose/maltose transport system of the hyperthermophilic archaeon Thermococcus litoralis. J Bacteriol. 1998;180:680–689. doi: 10.1128/jb.180.3.680-689.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huddleston S, Yallop C A, Charalambous B M. The identification and partial characterization of a novel inducible extracellular thermostable esterase from the archaeon Sulfolobus shibatae. Biochem Biophys Res Commun. 1995;216:495–500. doi: 10.1006/bbrc.1995.2650. [DOI] [PubMed] [Google Scholar]
- 12.Jacobs M H, Van der Heide T, Driessen A J M, Konings W N. Glutamate transport in Rhodobacter sphaeroides is mediated by a novel binding protein-dependent secondary transport system. Proc Natl Acad Sci USA. 1996;93:12786–12790. doi: 10.1073/pnas.93.23.12786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kalmokoff M L, Jarrell K F. Cloning and sequencing of a multigene family encoding the flagellins of Methanococcus voltae. J Bacteriol. 1991;173:7113–7125. doi: 10.1128/jb.173.22.7113-7125.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kawarabayasi Y, Sawada M, Horikawa H, Haikawa Y, Hino Y, Yamamoto S, Sekine M, Baba S, Kosugi H, Hosoyama A, Nagai Y, Sakai M, Ogura K, Otsuka R, Nakazawa H, Takamiya M, Ohfuku Y, Funahashi T, Tanaka T, Kudoh Y, Yamazaki J, Kushida N, Oguchi A, Aoki K, Kikuchi H. Complete sequence and gene organization of the genome of a hyper-thermophilic archaebacterium, Pyrococcus horikoshii OT3. DNA Res. 1998;5:55–76. doi: 10.1093/dnares/5.2.55. [DOI] [PubMed] [Google Scholar]
- 15.Koenig H. Archaeobacterial cell envelopes. Can J Microbiol. 1988;34:395–406. [Google Scholar]
- 16.Moll R, Schäfer G. Chemiosmotic H+ cycling across the plasma membrane of the thermoacidophilic archaebacterium Sulfolobus acidocaldarius. FEBS Lett. 1988;232:359–363. [Google Scholar]
- 17.Pao S S, Paulsen I T, Saier M H., Jr Major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–34. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Richarme G, Kepes A. Study of binding protein-ligand interaction by ammonium sulphate assisted adsorption on cellulose ester filters. Biochim Biophys Acta. 1983;742:16–24. doi: 10.1016/0167-4838(83)90353-9. [DOI] [PubMed] [Google Scholar]
- 19.Rolfsmeier M, Haseltine C, Bini E, Clark A, Blum P. Molecular characterization of the α-glucosidase gene (malA) from the hyperthermophilic archaeon Sulfolobus solfataricus. J Bacteriol. 1998;180:1287–1295. doi: 10.1128/jb.180.5.1287-1295.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scatchard G. The attraction of proteins for small molecules and ions. Ann NY Acad Sci. 1949;51:660–672. [Google Scholar]
- 21.Schaegger H, von Jagow G. Blue native electrophoresis for isolation of membrane protein complexes in enzymatically active form. Anal Biochem. 1991;199:223–231. doi: 10.1016/0003-2697(91)90094-a. [DOI] [PubMed] [Google Scholar]
- 22.Schoenheit P, Schaefer T. Metabolism of hyperthermophiles. World J Microbiol Biotechnol. 1995;11:26–57. doi: 10.1007/BF00339135. [DOI] [PubMed] [Google Scholar]
- 23.Sensen C W, Charlebois R L, Chow C, Clausen I G, Curtis B, Doolittle W F, Duguet M, Garrett R A, Gaasterland T, Erauso G, Heikamp de Jong I, Jeffries A C, Kozera C, Medina N, De Moors A, van der Oost J, Phan H, Ragan M A, Schenk M E, She Q, Singh R K, Tolstrup N. Completing the sequence of the Sulfolobus solfataricus P2 genome. Extremophiles. 1998;2:305–312. doi: 10.1007/s007920050073. [DOI] [PubMed] [Google Scholar]
- 24.Shaw J G, Hamblin M J, Kelly D J. Purification, characterization and nucleotide sequence of the periplasmic C4-dicarboxylate-binding protein (DctP) from Rhodobacter capsulatus. Mol Microbiol. 1991;5:3055–3062. doi: 10.1111/j.1365-2958.1991.tb01865.x. [DOI] [PubMed] [Google Scholar]
- 24a.Sulfolobus sequence database. 22 May 1999, revision date. Sequences. [Online.] http//niji.imb.nrc.ca/sulfolobus/. [3 June 1999, last date accessed.]
- 25.Wardi A H, Michos G A. Alcian blue staining of glycoproteins in acrylamide disc electrophoresis. Anal Biochem. 1972;49:607–609. doi: 10.1016/0003-2697(72)90472-1. [DOI] [PubMed] [Google Scholar]
- 26.Xavier K B, Martins L O, Peist R, Kossmann M, Boos W, Santos H. High-affinity maltose/trehalose transport system in the hyperthermophilic archaeon Thermococcus litoralis. J Bacteriol. 1996;178:4773–4777. doi: 10.1128/jb.178.16.4773-4777.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yallop C A, Charalambous B M. Nutrient utilization and transport in the thermoacidophilic archaeon Sulfolobus shibatae. Microbiology. 1996;142:3373–3380. [Google Scholar]