Abstract
Theoretical models of addiction suggest that alterations in addiction domains including incentive salience, negative emotionality, and executive control lead to relapse in alcohol use disorder (AUD). To determine whether the functional organization of neural networks underlying these domains predict subsequent relapse, we generated theoretically defined addiction networks. We collected resting functional magnetic resonance imaging data from 45 individuals with AUD during early abstinence (number of days abstinent M = 25.40, SD = 16.51) and calculated the degree of resting-state functional connectivity (RSFC) within these networks. Regression analyses determined whether the RSFC strength in domain-defined addiction networks measured during early abstinence predicted subsequent relapse (dichotomous or continuous relapse metrics). RSFC within each addiction network measured during early abstinence was significantly lower in those that relapsed (vs. abstained) and predicted subsequent time to relapse. Lower incentive salience RSFC during early abstinence increased the odds of relapsing. Neither RSFC in a control network nor clinical self-report measures predicted relapse. The association between low incentive salience RSFC and faster relapse highlights the need to design timely interventions that enhance RSFC in AUD individuals at risk of relapsing faster.
Keywords: addiction domains, incentive salience, relapse prediction, resting-state functional connectivity, time to relapse
Introduction
The path to recovery from alcohol use disorder (AUD) is often disrupted by high relapse rates (40–60%; McLellan et al. 2000; Maisto et al. 2018). Relapse in AUD is driven by maladaptive behaviors such as an exaggerated appetitive drive toward alcohol use, inability to regulate mood and stress, and inability to avoid and control alcohol consumption (Kwako et al. 2019). Theoretical models of addiction have categorized these maladaptive behaviors into three addiction domains: 1) reward and incentive salience, 2) negative emotionality, and 3) executive functioning (Koob and Volkow 2010, 2016; Kwako et al. 2018, 2019). These distinct domains are associated with underlying neural networks. Functional connectivity patterns within these neural networks have been found to play a role in perpetuating use and in repeated relapse (Koob and Volkow 2016; Zehra et al. 2018). These addiction domains and underlying neural substrates are described below with a focus on AUD.
Reward and Incentive Salience (IS) Domain
Under the IS domain, alcohol consumption is driven by its rewarding effects. Animal and human research suggest that the rewarding effects of substance use are mediated by dopamine signaling in the mesolimbic dopamine system (Vollstädt-Klein et al. 2010; Sanchez-Roige et al. 2014; Everitt and Robbins 2016). Initial consumption has been associated with exaggerated dopamine release in the nucleus accumbens, a dopaminergic hub within the ventral striatum, which sends signals to the motor cortex for continued goal-directed behavior toward substance use. After continued and repeated substance use, the dorsal regions of the dopaminergic system (i.e., caudate and putamen) are increasingly engaged as substance use shifts from goal-directed behavior to habitual behavior (Vollstädt-Klein et al. 2010; Murray et al. 2012; Everitt and Robbins 2016). Dysregulation in the ventral and dorsal striatum triggers neuroadaptations in the dopaminergic system, characterized by blunted dopaminergic responses (desensitization) to rewarding stimuli, including rewards derived from substance use (Koob and Le Moal 2008; George et al. 2012). Reward desensitization drives an organism to try to compensate for dopaminergic dysfunction by seeking incrementally larger amounts of alcohol (Leyton and Vezina 2014) or to relapse after periods of abstinence (Wang et al. 2012).
Negative Emotionality (NE) Domain
Under the NE domain, alcohol consumption is driven by attempts to avoid aversive experiences, such as negative affect or withdrawal symptoms and associated distress (Koob and Volkow 2016). Heightened stress (e.g., due to withdrawal or aversive experiences) has been found to be mediated by increased concentration of corticotropin-releasing factor in the extended amygdala (Volkow et al. 1997; Volkow et al. 2007; Koob 2010). Further, dopamine and k-opioid dysregulations in the striatum and habenula underlie the blunted reward sensitivity associated with negative affect (Matsumoto and Hikosaka 2007; Walker and Koob 2008; Wise 2008; Baker et al. 2016; Bazov et al. 2018). Mesolimbic dysregulation (e.g., in amygdala, striatum, and habenula) is involved in the manifestation of withdrawal symptoms including heightened stress and negative affect, which increases the vulnerability to relapse in an attempt to avoid these aversive experiences.
Executive Functioning (EF) Domain
Under the EF domain, alcohol consumption is posited to be driven by poor executive functioning, particularly the inability to stop habitual unwanted behaviors or to exert executive control over emotion dysregulations. Down-regulation of dopamine signaling described under the IS and NE domains above also extends to frontal regions, impairing top-down executive control (Goldstein and Volkow 2011; Tang et al. 2015; Volkow et al. 2019). Both reduced prefrontal dopaminergic signaling and reduced prefrontal glutamatergic signaling disrupt proper executive control over habit-induced craving, affecting decision making, cognitive flexibility, error monitoring, and emotion regulation needed to avoid return to substance use (Goldstein and Volkow 2011). The EF domain has been classified into two separate systems: the “EF-Go” and “EF-Stop” systems (Koob and Volkow 2016). Functional dysregulations in the EF-Go circuit, including the middle anterior cingulate cortex, inferior frontal cortex, insula, and medial prefrontal cortex, ostensibly mediate inability to control habit-induced craving driven by striatum (particularly caudate). Functional dysregulation in the EF-Stop circuit, including anterior cingulate cortex, dorsolateral prefrontal cortex, inferior frontal cortex, medial prefrontal cortex, orbitofrontal cortex, and amygdala, ostensibly mediate poor inhibitory control and poor control of affective responses in the EF-Stop system. Poor executive control in both the EF-Go and EF-Stop systems have been associated with higher vulnerability to relapse (Goldstein and Volkow 2011).
Resting State Functional Connectivity (RSFC) and Addiction Treatment Outcome
The literature cited above highlights the role of addiction domains and underlying neural substrates on the perpetuation of substance use and relapse. Our group contributed to this literature by reporting that lower resting-state functional connectivity (RSFC) of individual regions of interest (e.g., anterior cingulate and nucleus accumbens) and other key brain regions (e.g., dorsolateral prefrontal cortex and insula) are potential neural markers of relapse in AUD (Camchong et al. 2013; Camchong et al. 2014). While our previous studies have defined subsequent relapse as a dichotomous variable (Camchong et al. 2013; Camchong et al. 2014; Camchong et al. 2017), it is important to predict more detailed relapse metrics to better inform timely and detailed AUD interventions. The current paper extends previous findings in two main ways. First, we adopt an integrative and theory-driven framework in the identification of potential markers of relapse in AUD (Voon et al. 2020) through the examination of RSFC strength across regions within each of the aforementioned domain-defined addiction networks (i.e., IS, NE, EF-Go, and EF-Stop). This permits the ability to examine more nuanced neurobiological patterns specific to addiction beyond the information that can be derived using individual regions of interest. Second, we investigate whether RSFC within domain-defined addiction networks can predict detailed relapse metrics (e.g., time to relapse, number of drinks after relapse, and number of drinking days after relapse), beyond predicting a dichotomous variable of relapse.
The analysis of resting-state functional MRI (magnetic resonance imaging) data allows us to examine intrinsic functional organization (Biswal et al. 1997) of addiction networks in the absence of potentially confounding variables (e.g., variability in motivation or effort needed for task performance; Pacheco-Colón et al. 2018). While we recognize that the addiction domains described above overlap both neurally and chronologically throughout the addiction cycle (Kwako et al. 2018, 2019), the purpose of this paper is to investigate the “independent” role of each addiction neural network underlying each addiction domain. Moreover, if overlapping networks are combined into one regression model, there are potential multicollinearity issues that would yield misleading results (Allen 1997; Gujarati 2011). Therefore, RSFC within each addiction network defined above was examined as a separate variable of interest.
The current paper is the first to directly examine the relationship between the strength of RSFC within domain-defined addiction networks measured during early abstinence and subsequent detailed relapse metrics in AUD. First, we sought to investigate whether RSFC of domain-defined addiction networks during early abstinence was a marker of dichotomous (relapse vs. no relapse) or continuous (time to relapse, number of drinks after relapse, and number of drinking days after relapse) relapse metrics. Second, to investigate whether the relationship between relapse metrics and RSFC is unique to the domain-defined addiction networks (vs. generalized RSFC alterations), we examined RSFC of a visual network, a network that has not been explicitly identified as involved in the maintenance of addiction, as a control brain network. Based on the literature, we hypothesized that RSFC within each of the domain-defined addiction networks would be related to relapse, while the visual network would not be related to relapse in AUD. There was no a priori hypothesis favoring predictive ability of one domain-defined addiction network over another. Third, we examined whether clinical self-report measures (i.e., depression, anxiety, and craving) or history of past substance use predicted relapse metrics.
Materials and Methods
Participants
All participants were recruited 1–2 weeks after being admitted to a 28-day in-patient addiction treatment program in Minneapolis, MN, as part of a longitudinal study. The current paper analyzed neuroimaging data collected from individuals with AUD during early abstinence (mean number of days abstinent until MRI session = 25.40, SD = 16.51). All participants provided written informed consent and received monetary compensation for the time spent participating. The consent process and all procedures were reviewed and approved by the Institutional Review Board at the University of Minnesota.
A total of 66 participants were consented. From the 66 total subjects, 21 did not have available fMRI (functional magnetic resonance imaging) data after consenting for the following reasons: Seven left the treatment program and were no longer reachable before their neuroimaging session, four were found to be no longer eligible (one because of identified cognitive impairment, two because the identified “primary” substance use disorder diagnosis was not alcohol, but stimulant and heroin, and one because of identified unknown metal in their bodies), four voluntarily withdrew participation before the neuroimaging session, two were excluded from group analyses because their resting-state data did not meet our image quality threshold (see Resting-State Data Quality Assessment section), one because of technical issues during fMRI scan, and three were lost to follow-up after the neuroimaging session. As a result, complete data for the scope of this paper were available for 45 abstinent individuals with AUD (Table 1).
Table 1.
Demographics and history of alcohol use in individuals with AUD during early abstinence
| Treatment outcome at 4-month follow-up | ||||
|---|---|---|---|---|
| All AUD (n = 45) | ABS (n = 25) | REL (n = 20) | ABS versus REL | |
| Characteristic | Mean or n (SD or %) | Mean or n (SD or %) | Mean or n (SD or %) | T-test or χ2 (italics) |
| Age | 42.50 (9.41) | 43.92 (9.10) | 40.45 (9.83) | P = 0.227 |
| Education | 14.13 (2.27) | 14.00 (1.78) | 14.35 (2.83) | P = 0.615 |
| Female, n % | 18 (39.1%) | 6 (24%) | 12 (60%) | P = 0.014 |
| Employeda, n % | 17 (39.1%) | 12 (44.4%) | 5 (25%) | P = 0.209 |
| Age of AUD onset | 26.76 (9.84) | 25.16 (8.74) | 29.50 (10.49) | P = 0.137 |
| # of standard drinks: Past 6 months | 2585.98 (1955.41) | 2808.74 (1942.76) | 2171.64 (1911.44) | P = 0.385 |
| # of drinking days: Past 6 months | 100.98 (59.00) | 106.68 (58.43) | 91.10 (60.22) | P = 0.277 |
| # of days abstinent until the MRI session | 25.20 (16.38) | 26.12 (14.52) | 24.75 (18.91) | P = 0.785 |
Notes: AUD, alcohol use disorder; MRI, magnetic resonance imaging; SD, standard deviation; ABS, those that remained abstinent in the 4-month follow-up period; REL, those that relapsed during the 4-month follow-up period; χ2, Chi-Square; P, significance probability value.
aEmployed at the time of entering the addiction treatment program.
Inclusion and exclusion criteria can be found in the Supplementary Material A. Participants underwent random alcohol and drug tests in the treatment program. All subjects’ substance use history for the past 6 months before entering the treatment program was recorded using the Timeline Follow-Back (TLFB) (Sobell and Sobell 1992) and administered for alcohol and for each other substance used (excluding caffeine) (Table 1).
All participants completed clinical self-report measures that have been associated with relapse (Oliva et al. 2018; Stohs et al. 2019; Pareaud et al. 2021) (Table 2): the Beck Depression Inventory (BDI, Beck et al. 1988); the State Anxiety Inventory (STAI, Spielberger 1983); and the Penn Alcohol Craving Scale (PACS, Flannery et al. 1999). One participant did not complete self-report measures. Participants completed follow-up interviews 1 and 4 months after the neuroimaging session to query dichotomous and continuous relapse metrics.
Table 2.
Self-report measures by group
| Mean (standard deviation) | |||
|---|---|---|---|
| Self-report measure | ABS (n = 25) | REL (n = 20) | Independent samples t-tests |
| Beck Depression Inventory (BDI) | 21.92 (12.57) | 18.90 (11.28) | P = 0.41 |
| State Anxiety Inventory (STAI) | 40.61 (12.48) | 44.73 (11.47) | P = 0.29 |
| Penn Alcohol Craving Scale (PACS) | 21.21 (8.12) | 21.10 (6.46) | P = 0.96 |
Notes: ABS, those that remained abstinent in the 4-month follow-up period; REL, those that relapsed during the 4-month follow-up period; P, significance probability value.
Dichotomous Relapse Metrics: Group Definition
Participants were considered to be in the relapsing group (REL) if they reported consuming at least one drink during the 1-month or the 4-month follow-up period. Participants who had not consumed any alcohol and/or nonprescribed drug during the 1-month or the 4-month follow-up period were considered to be in the abstaining group (ABS).
Continuous Relapse Metrics
Detailed TLFB (Sobell and Sobell 1992) data were collected from the REL group, recording date of relapse, number of drinks, and number of drinking days after relapse during the 4-month follow-up period. Time to relapse to alcohol use was operationalized as the number of days until a participant’s self-reported first use of alcohol. The variable representing the number of drinks (standard drink, equivalent to 0.6 ounces of pure alcohol; Centers for Disease Control and Prevention [CDC], 2021) after relapse was operationalized as the number of drinks reported to have been consumed in the 4-month follow-up period. Number of days drinking was operationalized as the number of days reported to have consumed at least one drink during the 4-month follow-up period (Table 1).
Imaging Data Acquisition
MRI data were acquired from a 3T Siemens Prisma scanner at the University of Minnesota’s Center for Magnetic Resonance Research (CMRR). Acquisition parameters closely matching those created by the Human Connectome Project (HCP) (Glasser et al. 2013; Smith et al. 2013; Uğurbil et al. 2013). The images collected included: a T1-weighted MPRAGE image [TR = 2400 ms, TE = 2.24 ms, slices = 208, voxel size = 0.8 mm3], a T2-weighted SPACE image [TR = 3200 ms, TE = 564 ms, slices = 208, voxel size = 0.8 mm3], spin echo EPI time series images for the resting-state fMRI, and resting-state fMRI [TR = 800 ms, TE = 37 ms, slices = 72, volumes = 520, voxel size = 2.0 mm3, 7 min duration]. During the resting-state fMRI scan, the participant was asked to keep their eyes open, look directly at a fixation cross, to not think of anything in particular, and remain awake (confirmed by participant at the end of the MRI session).
Resting-State Data Quality Assessment
Prior to data processing, resting-state fMRI data quality was assessed using methods similar to those outlined in Power et al (Power et al. 2012). Framewise Displacement (FD) and DVARS (“D” refers to the temporal derivative of time courses, and “VARS” refers to root means square (RMS) variance over voxels) were calculated on the resting-state fMRI scans. FD is a measure of the head position change relative to the previous time point. DVARS is a measure of the RMS signal change from the previous time point, calculated over the brain mask (Power et al. 2012). Volumes with FD > 0.5 mm and/or DVARS > 0.008 were flagged as bad along with the previous volume and next two volumes. Resting scans with more than 30% flagged volumes were rejected from the study. Two participants were excluded from analyses because they failed these quality criteria.
Individual Anatomical MRI Data Processing
T1-weighted and T2-weighted images were processed using the HCP minimal preprocessing pipeline (Glasser et al. 2013). To preprocess the T1- and T2-weighted data, the following steps in the minimal preprocessing HCP pipeline were done: Aligned the T1-weighted and T2-weighted to each other; Registered T1 and T2 images to standard MNI space; Corrected for gradient inhomogeneities with gradient distortion correction; Projected images to surface space with FreeSurfer; Motion correction; Removal of nonbrain tissue; Gray and white matter segmentation; Intensity normalization; Extraction and inflation to the cortical surface; Output to CIFTI space by extracting NIFTI volumes containing subcortical structural and GIFTI surface files corresponding to the left and right cortical hemispheres, respectively (Glasser et al. 2013).
Individual FMRI Data Preprocessing
After anatomical processing, the following were completed in chronological order on resting-state fMRI data. To account for spin-history stabilization effects, the first eight volumes of each time series were removed. Then, the nonlinear distortions produced by the gradient were corrected using the HCP version of the gradunwarp package (https://github.com/Washington-University/gradunwarp). EPI (echo-planar imaging) realignment was then conducted to correct for motion by registering each volume to the single band reference image via FLIRT with 6 degrees of freedom. To correct for distortion in the phase encoding direction, a pair of opposing phase encoding spin echo EPI field maps was used to estimate a distortion field with the FSL tool “top-up” and was then applied to the EPI fMRI images with FLIRT. Following distortion correction, EPI to T1-weighted surface registration and subsequently native to MNI nonlinear registration were performed. Finally, the voxels of the MNI registered volumes were resampled onto cortical surfaces and extracted to CIFTI space.
Extensive Functional Processing
The structural and functional preprocessing pipeline was followed by the extensive functional pipeline. Because the rest fMRI analysis relied on temporal correlations, the primary focus of the extensive functional pipeline was to correct temporal artifacts. First, to linearly detrend the data, a high band pass filter cutoff of 2000s with a slow roll off (Smith et al. 2013) was applied. Second, the data were denoised using ICAFIX (Griffanti et al. 2014; Salimi-Khorshidi et al. 2014) with a customized FIX classifier using 15 randomly selected scanning sessions from the current data (trained by J. Camchong—ICAFIX Pipeline Documentation, see Supplementary Material B for methodology rationale) (https://github.com/Washington-University/HCPpipelines/wiki/Installation-and-Usage-Instructions#the-ica-fix-pipeline). “FIX” classified the spatial components as “signal” or “noise.” Components classified as noise were regressed out from the data (multiplied by the associated time series and subtracted from the original dataset) (Smith et al. 2013). Following the ICAFIX process, surface-based functional alignment was run on the ICAFIX denoised data with the tool MSMAll (Robinson et al. 2014). MSMAll employed myelin maps, resting-state fMRI network maps, and resting-state fMRI visuotopic maps to align a participant’s cortical data to a group template. Once complete, the MSMAll and ICAFIX fMRI concatenated data were dissociated resulting in the resting-state fMRI data that were used for subsequent analyses.
Individual Data Segmentation
Following the above steps, the resting-state fMRI individual data were parcellated into distinct and nonoverlapping regions using the cortical Schaefer 400 (i.e., 400 regions across the cortex) (Schaefer et al. 2018) and the subcortical Harvard-Oxford atlases (Desikan et al. 2006). Within each region, the individual grayordinates (Van Essen and Glasser 2016) were averaged together per time point to generate a parcellated time series of the resting-state fMRI data.
Calculating Functional Connectivity Matrices within Domain-Defined Addiction Networks
Regions corresponding to each domain-defined addiction network (Koob and Volkow 2016) were identified within the Schaefer 400 and the subcortical Harvard-Oxford atlases (Table 3 and Supplementary Material C). Although each addiction domain represents specific maladaptive behaviors in the addiction cycle (Koob and Volkow 2016), it is important to note that they overlap spatially (Table 3;Kwako et al. 2018) since some regions play a different role within different domains. Because the scope of this current paper is to determine whether the functional organization within separate domain-defined addiction networks is related to treatment outcome, the RSFC within each domain-defined addiction network was calculated and analyzed as a separate variable.
Table 3.
Bilateral brain regions within each addiction domain
| Regions in each addiction domain (Koob and Volkow 2016; Kwako et al. 2018) | ||||
|---|---|---|---|---|
| IS consumption driven to experience reward |
NE consumption driven to avoid withdrawal and negative emotions |
EF-Go consumption driven by habit-induced craving |
EF-Stop consumption driven by poor inhibitory and affective control |
|
| Subcortical | Amygdala | Amygdala | ||
| Caudate | Caudate | Caudate | ||
| Habenula | ||||
| Nucleus accumbens | Nucleus accumbens | Nucleus accumbens | ||
| Pallidum | Pallidum | Pallidum | ||
| Putamen | Putamen | Putamen | ||
| Cortical a | Motor cortexb | |||
| Anterior cingulate cortex | Anterior cingulate cortex | |||
| Dorsolateral prefrontal cortex | ||||
| Inferior frontal cortex | Inferior frontal cortex | |||
| Insulac | ||||
| Medial prefrontal cortex | Medial prefrontal cortex | |||
| Orbitopolar frontal cortex | ||||
Notes: IS, incentive salience; NE, negative emotionality; EF-Go, executive functioning go; EF-Stop, executive functioning stop.
aCorresponding Schaefer regions in Supplementary MaterialC.
bIncluding: Inferior and superior premotor, premotor, somatosensory, supplementary motor.
cIncluding: Anterior agranular insular complex, frontal opercular, insula granular, middle insula, posterior insula, posterior opercular. NOTE: To test the robustness of the composition of these domain-defined addiction networks, bootstrapping analyses were conducted and detailed in Supplementary MaterialH.
The time series within each of the regions was identified to be part of each domain-defined addiction network: The incentive salience (IS; Fig. 1), negative emotionality (NE; Fig. 2), and executive functioning “Go” (EF-Go; Fig. 3) and executive functioning “Stop” (EF-Stop; Fig. 4) networks (Koob and Volkow 2016; Kwako et al. 2019) were extracted. The time series of each region within each domain-defined addiction network was used to produce an NxN connectivity matrix representing pairwise temporal Pearson correlations. Each node in the matrix corresponded to an individual region and each edge corresponded to the temporal correlation of the paired nodes. The following additional steps were taken on the four generated connectivity matrices for each domain-defined addiction network. First, the diagonal of each of the matrices was removed as this represents the correlation of a region with itself. Second, the remaining Pearson correlation values were transformed to Z-scores (Fisher Z-transformation). Third, the Fisher Z-scores were vectorized and averaged to produce one measure of RSFC (temporal correlation) within each of the four domain-defined addiction networks. Finally, the temporal correlations were standardized across subjects to remove the impact of scaling when fitting group-level models.
Figure 1.
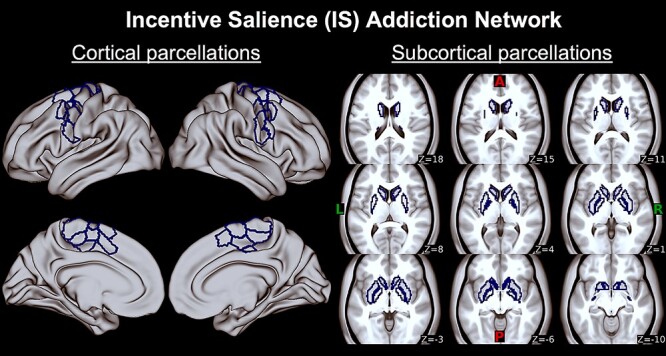
Incentive salience (IS) addiction network. First two columns are IS regions corresponding to cortical Schaefer 400 atlas parcels on bilateral motor cortex displayed on an MNI surface brain (see Table 3 and Supplementary Material C for list of regions). Last three columns are IS regions corresponding to subcortical Harvard-Oxford atlas parcels on bilateral caudate, nucleus accumbens, pallidum, and putamen on axial slices (z) displayed on an MNI average brain. A, anterior; P, posterior; L, left; R, right.
Figure 2.
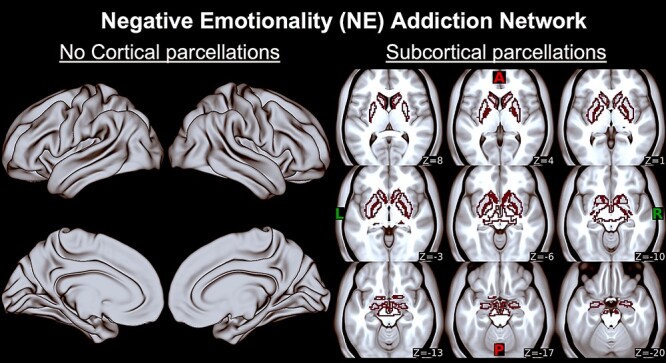
Negative emotionality (NE) addiction network. Last three columns are EF-Go regions corresponding to subcortical Harvard-Oxford atlas parcels on bilateral amygdala, caudate, habenula, nucleus accumbens, pallidum, and putamen on axial slices (z) displayed on an MNI average brain. There are no cortical parcels within this addiction network, so two first columns with the MNI surface brain are empty. A, anterior; P, posterior; L, left; R, right.
Figure 3.
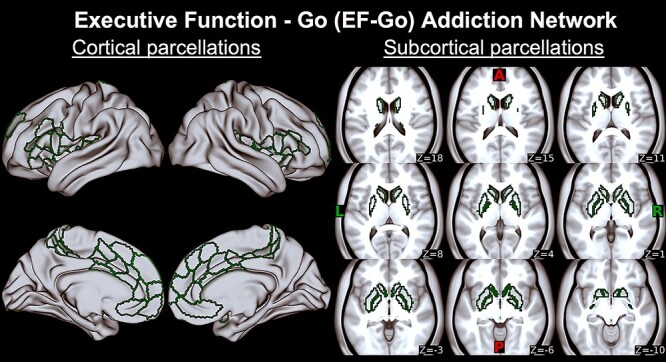
Executive function Go (EF-Go) addiction network. First two columns are EF-Go regions corresponding to cortical Schaefer 400 atlas parcels on bilateral anterior cingulate cortex, mid cingulate cortex, inferior frontal cortex, insula (anterior agranular insular complex, frontal opercular, insula granular, middle insula, posterior insula, and posterior opercular), and medial prefrontal cortex displayed on an MNI surface brain (Table 3 and Supplementary Material C include list of regions). Last three columns are EF-Go regions corresponding to subcortical Harvard-Oxford atlas parcels on bilateral caudate, nucleus accumbens, pallidum, and putamen on axial slices (z) displayed on an MNI average brain. A, anterior; P, posterior; L, left; R, right.
Figure 4.
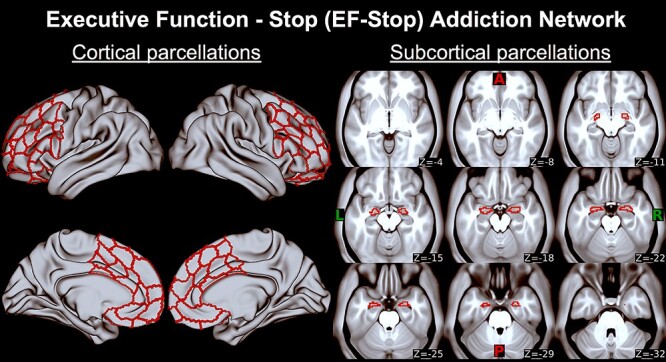
Executive function Stop (EF-Stop) addiction network. First two columns are EF-Stop regions corresponding to cortical Schaefer 400 atlas parcels on bilateral anterior cingulate cortex, dorsolateral prefrontal cortex (BA 6, 8, 9, 46), inferior frontal cortex, medial prefrontal cortex, and orbitopolar frontal cortex (BA 10, 47) displayed on an MNI surface brain (Table 3 and Supplementary Material C include list of regions). Last three columns are EF-Stop regions corresponding to subcortical Harvard-Oxford atlas parcels on bilateral amygdala on axial slices (z) displayed on an MNI average brain. A, anterior; P, posterior; L, left; R, right.
RSFC in Control Networks
To determine whether findings are specific to domain-defined addiction networks versus whole-brain RSFC alteration, the RSFC within Schaefer regions corresponding to primary visual network was examined (Supplementary MaterialD).
Group-Level Analyses
First, to determine whether there were significant demographic differences on dichotomous relapse metrics (ABS vs. REL defined at 1-month and 4-month follow-up periods), independent samples t-tests with bootstrapping (2000 bootstrapping samples; Konietschke and Pauly 2014) were conducted on age, years of education, clinical self-report measures (i.e., BDI, STAI, and PACS), and substance use history (age of onset of alcohol dependence, number of drinks in the past 6 months, number of days drinking in the past six months, and number of days abstinent until MRI data collection day). To determine whether there were sex (as a biological variable) differences between groups (REL vs. ABS), Pearson Chi-square tests were conducted (Table 1). Supplementary Material E includes analyses examining motion as a covariate.
Second, to determine whether the strength of RSFC within domain-defined addiction networks measured during early abstinence predicted relapse as a dichotomous variable (REL vs. ABS defined at 1-month and 4-month follow-up periods), a binary logistic regression with each domain-defined addiction network as a separate independent variable and adjusting for sex was conducted. To determine whether there were significant group differences in RSFC strength within domain-defined addiction networks, analysis of variance with bootstrapping (2000 samples; Konietschke and Pauly 2014) adjusting for sex was conducted for each of the domain-defined addiction networks.
Third, to determine whether the strength of RSFC within the domain-defined addiction networks measured during early abstinence predicted continuous relapse metrics (i.e., time to relapse, number of drinks after relapse, and number of drinking days after relapse—as dependent variables), linear regressions were conducted.
Finally, to determine whether clinical self-report measures (BDI, STAI, and PACS) predicted dichotomous or continuous relapse metrics (i.e., time to relapse, number of drinks after relapse, and number of drinking days after relapse), binary logistic and linear regressions were conducted respectively.
Results
Dichotomous Group Definition
Eleven out of forty-five participants (23.9%) relapsed at the 1-month follow-up (number of days to relapse M = 9.60, SD = 6.92). Twenty of the forty-five participants (44.4%) relapsed in the 4-month follow-up period with an average time to relapse of 52.20 days (SD = 48.57, n = 20). From the 20 individuals that relapsed during the 4-month follow-up period, 94.7% relapsed to alcohol only and 5.3% relapsed to a combination of alcohol and drugs. Fifteen (of twenty) REL reported 213.63 average number of drinks (SD = 252.86) and 19.93 average number of drinking days (SD = 18.20) after relapse in the 4-month follow-up period. While the remaining 5 (of 20) in the REL group reported to have relapsed over the phone, they were not available to complete the in-person visit in which detailed TLFB information after relapse (number of drinks consumed and number of drinking days) would be collected.
Demographic and Self-Report Differences between Subsequent ABS and REL
Because Chi-Square test revealed significant sex differences, with a higher proportion of females in the REL group at the 4-month follow-up than the ABS group (X2 (1, N = 45) = 6.00, P = 0.014) (Table 1), Cox proportional hazards analysis was conducted to model sex differences in survival (relapse) rate. Results suggested that being female increased the odds (OR, odds ratio) of relapse during the 4-month period [B = 0.959, SE = 0.459, Wald χ2 = 4.355, P = 0.037, OR = 1.06–6.42] (Supplementary Material G). Subsequent group analyses adjust for sex.
Self-Report Measures Measured during Early Abstinence—Comparison between Subsequent REL and ABS
Independent samples t-tests showed no significant difference between REL and ABS groups in number of days of abstinence before the MRI scan session (Table 1), past substance use (number of drinks in the past 6 months, number of days drinking in the past 6 months, and age of onset of alcohol use disorder) (Table 1), clinical self-report measures (BDI, STAI, and PACS, Table 2), current and lifetime substance use disorder (Table 4), or psychiatric diagnoses (Table 5). Results remained nonsignificant after adjusting for sex.
Table 4.
Counts of lifetime and current substance use disorder for all participants with alcohol use disorder
| Lifetime diagnosis count | Current diagnoses count | |||||
|---|---|---|---|---|---|---|
| Substance | ABS (n = 25) | REL (n = 20) | χ2 Sig. | ABS (n = 25) | REL (n = 20) | χ2 Sig. |
| Marihuana | 11 | 4 | P = 0.09 | 2 | 1 | P = 0.70 |
| Cocaine | 6 | 2 | P = 0.20 | 0 | 0 | — |
| Methamphetamine | 2 | 1 | P = 0.67 | 0 | 0 | — |
| Opioids | 3 | 0 | P = 0.11 | 0 | 0 | — |
| Hallucinogens | 0 | 1 | P = 0.66 | 0 | 0 | — |
| Nicotine | 14 | 11 | P = 0.94 | 12 | 10 | P = 0.50 |
Notes: ABS, those that remained abstinent in the 4-month follow-up period; REL, those that relapsed during the 4-month follow-up period; χ2, Chi-Square; P, significance (Sig.) probability value. Analyses correcting for comorbid substance use showed that having a lifetime or current comorbid substance use did not have an effect on predicting subsequent dichotomous or continuous treatment outcomes.
Table 5.
Counts of lifetime and current psychiatric diagnoses
| Lifetime diagnosis count (%) | Currenta diagnoses count (%) | |||||
|---|---|---|---|---|---|---|
| Psychiatric diagnosesb | ABS (n = 25) | REL (n = 20) | χ2 Sig. | ABS (n = 25) | REL (n = 20) | χ2 Sig. |
| MDD | 12 (48.0%) | 6 (30.0%) | P = 0.22 | 11 (44.0%) | 6 (30.0%) | P = 0.34 |
| GAD | 10 (40.0%) | 8 (40.0%) | P = 1.0 | 9 (36.0%) | 6 (30.0%) | P = 0.67 |
| PTSD | 6 (24.0%) | 7 (35.0%) | P = 0.42 | 6 (24.0%) | 6 (30.0%) | P = 0.65 |
| Social phobia | 4 (16.0%) | 5 (25.0%) | P = 0.45 | 4 (16.0%) | 5 (25.0%) | P = 0.45 |
| PD | 4 (16.0%) | 4 (20.0%) | P = 0.73 | 0 | 2 (10.0%) | P = 0.11 |
| Agoraphobia | 1 (4.0%) | 1 (5.0%) | P = 0.87 | 0 | 1 (5.0%) | P = 0.26 |
| ADHD | 1 (4.0%) | 2 (10.0%) | P = 0.42 | 1 (4.0%) | 1 (5.0%) | P = 0.87 |
Notes: ABS, those that remained abstinent in the 4-month follow-up period; REL, those that relapsed during the 4-month follow-up period; χ2, Chi-Square; MDD, major depressive disorder; GAD, generalized anxiety disorder; PTSD, posttraumatic stress disorder; PD, panic disorder; ADHD, attention deficit hyperactivity; P, significance (Sig.) probability value.
aParticipants with current diagnoses were clinically stable.
bNo lifetime or current diagnoses for the following disorders in the current sample: dysthymia, hypomania, bipolar disorder (without psychosis episodes), obsessive compulsive disorder, antisocial personality disorder, conduct disorder.
Predicting Dichotomous Relapse Metrics Based on RSFC Measured during Early Abstinence
For all the results reported hereafter, the following concepts apply. First, when referring to any RSFC metric, it should be noted that all RSFC metrics reported here refer to one MRI session collected during early abstinence for all participants, while they were in the addiction treatment program. Second, when referring to group differences, REL versus ABS, it should be noted that group membership was defined at subsequent follow-up time points 1 and 4 months later.
Predicting Dichotomous Relapse Defined at the 1-Month Follow-Up Using RSFC Measured during Early Abstinence
Binary logistic regression conducted with group membership (REL vs. ABS) defined at the 1-month follow-up period indicated that higher IS RSFC during early abstinence decreases the odds of relapse in the subsequent month, after adjusting for sex (OR = 0.32, P = 0.037, 95% CI: 0.11–0.93). RSFC within the other addiction networks showed a statistical trend predicting 1-month relapse, after adjusting for sex: 1) NE RSFC (OR = 0.47, P = 0.08, 95% CI: 0.20–1.09), 2) EF-Go RSFC (OR = 0.36, P = 0.06, 95% CI: 0.13–1.04), and 3) EF-Stop RSFC (OR = 0.39, P = 0.06, 95% CI: 0.14–1.05).
Predicting Dichotomous Relapse Defined at the 4-Month Follow-Up Using RSFC Measured during Early Abstinence
Binary logistic regression conducted with group membership (REL vs. ABS) defined at the 4-month follow-up period indicated that RSFC within any of the considered networks was not associated with 4-month relapse status, after adjusting for sex: 1) IS RSFC (OR = 0.93, P = 0.83, 95% CI: 0.47–1.82), 2) NE RSFC (OR = 1.31, P = 0.43, 95% CI: 0.67–2.55), 3) EF-Go RSFC (OR = 0.92, P = 0.82, 95% CI: 0.47–1.81), and 4) EF-Stop RSFC (OR = 0.87, P = 0.70, 95% CI: 0.44–1.74).
RSFC during Early Abstinence Is Significantly Different between AUD That Relapsed versus Abstained in the Subsequent Month
Independent samples t-tests revealed significantly lower RSFC within the IS, EF-Go, and EF-Stop addiction networks during early abstinence between subsequent REL versus ABS (Fig. 5). After adjusting for sex, IS RSFC group differences (subsequent REL vs. ABS) during early abstinence remained significant (P = 0.027). There was a trend in RSFC group differences in the other addiction networks after adjusting for sex (NE P = 0.07; EF-Go P = 0.05; EF-Stop P = 0.06).
Figure 5.
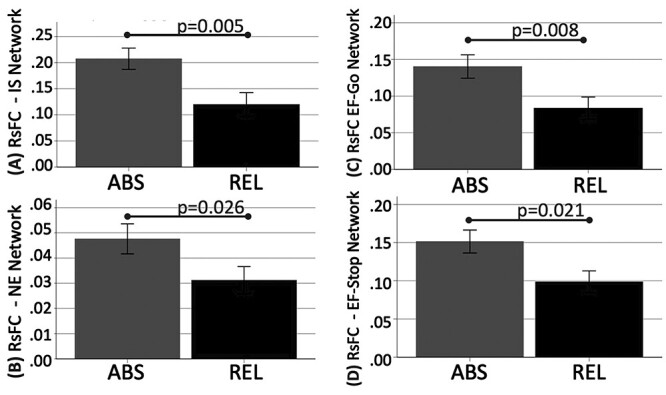
Lower resting-state functional connectivity (RSFC) in subsequent relapsers (REL, red bars) versus abstainers (ABS, blue bars) defined at the 1-month follow-up within the (A) incentive salience (IS; 95% Confidence Interval: 0.033–0.146), (B) negative emotionality (NE; 95% Confidence Interval: 0.03–0.15), (C) executive function Go (EF-Go; 95% Confidence Interval: 0.017–0.102), and (D) executive function Stop (EF-Stop; 95% Confidence Interval: 0.016–0.095) networks. After adjusting for sex as a biological variable, only the IS RSFC difference remained significant.
RSFC during Early Abstinence Was Not Different between AUD That Relapsed versus Abstained in the 4-Month Follow-Up
Independent samples t-tests revealed no significant RSFC differences between groups defined at the 4-month follow-up when not adjusting for sex (IS P = 0.437; NE P = 0.372; EF-Go P = 0.533; EF-Stop P = 0.365) or after adjusting for sex (IS P = 0.839; NE P = 0.442; EF-Go P = 0.824; EF-Stop P = 0.713).
RSFC of Primary Visual (Control) Network during Early Abstinence Was Not Different between AUD That Relapsed versus Abstained during Follow-Up
Independent samples t-tests revealed no significant RSFC differences within the primary visual network (Supplementary Material D) between REL and ABS groups defined at 1-month follow-up when not adjusting for sex (P = 0.571) and after adjusting for sex (P = 0.660). No significant RSFC differences were found when groups were defined at the 4-month follow-up when not adjusting for sex (P = 0.415) and after adjusting for sex (P = 0.311).
Predicting Continuous Relapse Metrics during Follow-Up Based on RSFC Measured during Early Abstinence
Time to relapse (number of days to first drink since MRI scan) during the 4-month follow-up period could be significantly predicted based on the strength of RSFC within the IS, NE, EF-Go, and EF-Stop addiction domain networks during early abstinence (number of days to relapse increased as strength in RSFC increased). These analyses were significant before and after adjusting for sex.
For the IS network, a significant regression equation was found (ANOVA: F(1,18) = 7.553, P = 0.013), with an R2 of 0.296 (Fig. 6A). RSFC within the IS network measured during early abstinence significantly predicted time to relapse during the follow-up period (Coefficient: t = 2.748, P = 0.013, β = 0.544). The squared semipartial coefficient (sr2) of 0.544 (sr2 estimated how much variance in days to relapse was uniquely predictable based on the strength of RSFC within the IS network) indicated that 54.4% of the variance in time to relapse was uniquely accounted for by the IS network RSFC strength.
Figure 6.
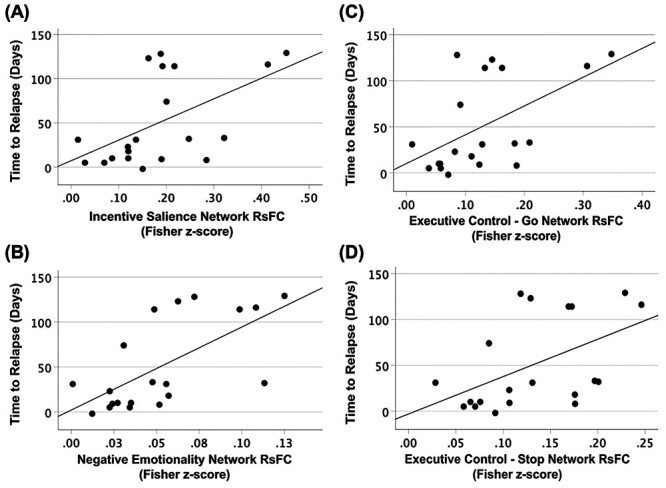
Linear regression scatter plots showing significant association between time to relapse (measured in days) and resting-state functional connectivity (RSFC) in the (A) incentive salience (t = 2.70, P = 0.015, β = 0.537), (B) negative emotionality (t = 3.63, P = 0.002, β = 0.650), (C) executive control—Go (t = 2.719, P = 0.014, β = 0.540), and (D) executive control—Stop (t = 2.451, P = 0.025, β = 0.500) addiction networks.
For the NE network, a significant regression equation was found (ANOVA: F(1,18) = 11.397, P = 0.003), with an R2 of 0.388 (Fig. 6B). RSFC within the NE network measured during early abstinence significantly predicted time to relapse during the follow-up period (Coefficient: t = 3.376, P = 0.003, β = 0.623). The squared semipartial coefficient (sr2) of 0.623 indicated that 62.3% of the variance in time to relapse was uniquely accounted for by the IS network RSFC strength.
For the EF-Go network, a significant regression equation was found (ANOVA: F(1,18) = 7.030, P = 0.016), with an R2 of 0.281 (Fig. 6C). RSFC within the EF-Go network measured during early abstinence significantly predicted time to relapse during the follow-up period (Coefficient: t = 2.651, P = 0.016, β = 0.530). The squared semipartial coefficient (sr2) of 0.530 indicated that 53.0% of the variance in time to relapse was uniquely accounted for by the IS network RSFC strength.
For the EF-Stop network, a significant regression equation was found (ANOVA: F(1,18) = 5.717, P = 0.028), with an R2 of 0.241 (Fig. 6D). RSFC within the EF-Stop network measured during early abstinence significantly predicted time to relapse during the follow-up period (Coefficient: t = 2.391, P = 0.028, β = 0.491). The squared semipartial coefficient (sr2) of 0.491 indicated that 49.1% of the variance in time to relapse was uniquely accounted for by the IS network RSFC strength.
For the primary visual cortex (control) network, the regression equation was not significant (ANOVA: F(1,18) = 2.732, P = 0.116), with an R2 of 0.132. Results remained nonsignificant after adjusting for sex (F(2,17) = 1.304, P = 0.297). That is, RSFC of primary visual cortex measured during early abstinence did not predict time to relapse during the follow-up period.
Number of drinks or the number of drinking days after relapse during the 1-month or 4-month follow-up period was not predicted by RSFC measured during early abstinence within the addiction or visual cortex networks (P > 0.05). Results remained nonsignificant after adjusting for sex.
No Relapse Prediction Using Self-Report Measures (BDI, STAI, and PACS) Measured during Early Abstinence
Predicting REL versus ABS at 1-Month Follow-Up
Binary logistic regression conducted with group membership defined at the 1-month follow-up period indicated that self-report measures measured during early abstinence were not associated with 1-month relapse, after adjusting for sex.
Predicting REL versus ABS at 4-Month Follow-Up
Binary logistic regression conducted with group membership defined at the 4-month follow-up period indicated that self-report measures measured during early abstinence were not associated with 4-month relapse, after adjusting for sex.
Predicting Continuous Relapse Metrics
Time to relapse, number of drinks after relapse, or number of drinking days after relapse was not predicted with any of the self-report measures measured during early abstinence.
Exploratory correlation analyses revealed that anxiety and craving levels were associated with RSFC in the NE and EF networks (see Supplementary MaterialF).
Discussion
This paper is innovative because it combined two novel approaches to investigate whether RSFC measured during early abstinence in AUD can predict treatment outcomes. First, we used an integrative and theory-driven framework to determine whether RSFC within neural networks known to underlie addiction domains (Koob and Volkow 2016; Kwako et al. 2018, 2019) could predict relapse in AUD enrolled in an addiction treatment program. Second, analyses included both dichotomous and continuous relapse metrics collected during a 4-month follow-up period. These novel approaches provided evidence that degree of RSFC within domain-defined addiction networks collected during early abstinence in AUD can 1) predict relapse (REL vs. ABS) within 1 month and 2) predict time to relapse in the subsequent 4 months. The ability to predict time to relapse is particularly important to inform timely interventions targeted to those at risk of relapse, especially earlier relapse.
RSFC Strength and Sex Predicted Relapse as a Dichotomous Variable (REL vs. ABS)
The binary logistic prediction models suggested that those with lower IS RSFC were more likely to relapse in the 1-month follow-up period (with a similar trend found in the NE, EF-Go, and EF-Stop) (Table 7). The finding that reduced RSFC within the IS network predicts relapse as a dichotomous variable is in line with reports using seed-based and graph theory analysis. First, our previous RSFC paper found that lower RSFC of the nucleus accumbens seed (part of the IS network) and cortical and subcortical regions (including putamen) in a whole-brain analysis predicted relapse (Camchong et al. 2013). This effect was not found when examining the RSFC of the visual cortex as a seed (Camchong et al. 2013). Second, a recent paper using seed-based connectivity methodology reported similar findings of lower RSFC of nucleus accumbens and medial prefrontal cortex measured during early abstinence as predictors of subsequent relapse during a 6-month follow-up period (Yang et al. 2021). Finally, a recent paper using graph theory methodology reported that those who subsequently relapse show lower interconnectedness of caudate and thalamus with the default mode network, and higher interconnectedness of these regions in other networks (including a salience network) when compared with abstainers and healthy controls (Muller and Meyerhoff 2021). Current findings complement and extend the above literature (Camchong et al. 2013; Muller and Meyerhoff 2021; Yang et al. 2021) by reporting that lower RSFC within a network underlying the incentive salience domain (Koob and Volkow 2016; Kwako et al. 2018, 2019) predicts subsequent relapse. While the ability to predict subsequent relapse (REL vs. ABS) during the 1-month follow-up period was highest with the IS network (with statistical significance), data also showed a trend in the other domain-defined addiction networks.
Table 7.
Statistics showing binary logistic regression results when predicting dichotomous group membership (relapsers vs. abstainers) during the 1-month and 4-month follow-up period
| Odds ratio of relapsing During 1-month follow-up period |
Odds ratio of relapsing During 4-month follow-up period |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Predictors | B | SE (B) | Wald χ2 | P | OR, 95% CI | B | SE (B) | Wald χ2 | P | OR, 95% CI |
| IS | −1.13 | 0.54 | 4.36 | 0.04 | 0.32, 0.11–0.93 | −0.07 | 0.34 | 0.05 | 0.83 | 0.93, 0.47–1.82 |
| Sex | 0.24 | 0.73 | 0.10 | 0.75 | 1.27, 0.30–5.34 | 1.66 | 0.68 | 5.93 | 0.01 | 5.26, 1.38–20.0 |
| NE | −0.76 | 0.43 | 3.08 | 0.08 | 0.47, 0.20–1.09 | 0.27 | 0.34 | 0.62 | 0.43 | 1.31, 0.67–2.55 |
| Sex | 0.70 | 0.72 | 0.95 | 0.33 | 2.01, 0.49–8.19 | 1.68 | 0.67 | 6.24 | 0.01 | 5.37, 1.44–20.1 |
| EF-Go | −1.01 | 0.54 | 3.54 | 0.06 | 0.36, 0.13–1.04 | −0.08 | 0.34 | 0.05 | 0.82 | 0.92, 0.47–1.81 |
| Sex | 0.43 | 0.72 | 0.37 | 0.54 | 1.54, 0.38–6.31 | 1.67 | 0.67 | 6.14 | 0.01 | 5.31, 1.42–19.9 |
| EF-Stop | −0.94 | 0.51 | 3.46 | 0.06 | 0.39, 0.14–1.05 | −0/14 | 0.35 | 0.15 | 0.70 | 0.87, 0.44–1.74 |
| Sex | 0.39 | 0.71 | 0.30 | 0.59 | 1.48, 0.36–5.99 | 1.64 | 0.68 | 5.89 | 0.02 | 5.17, 1.37–19.5 |
Notes: Underlined P-values are significant at P < 0.05. IS, resting-state functional connectivity in the incentive salience network; NE, resting-state functional connectivity in the negative emotionality network; EF-Go, resting-state functional connectivity in the executive functioning-Go network; EF-Stop, resting-state functional connectivity in the executive functioning Stop network; B, unstandardized regression Beta weight; SE (B), standard error of the Beta weight; Wald χ2, Chi-Square statistic for the individual predictor variable; P, significance probability value; OR odds ratio; 95% CI for OR, 95% confidence interval for the odds ratio.
Sex as a Biological Variable Predicted Dichotomous Relapse
Within our sample, females were more likely to relapse in the 4-month follow-up period (Supplementary Material G). The literature regarding the association between sex and relapse outcomes, however, is inconsistent. While there are preclinical and clinical reports consistent with our findings showing a significantly higher proportion of females in the relapse group (Rubonis et al. 1994; Kippin et al. 2005; Becker et al. 2017), there are also reports that males are more likely to relapse than females (Walitzer and Dearing 2006; Agosti 2013). This inconsistency may be associated with the variability of factors surrounding relapse that may be also related to sex differences such as mental health (e.g., PTSD and negative affect), hormonal differences, or neurochemical differences (Giacometti and Barker 2020). Within our sample, females reported more severe symptoms of depression, anxiety, and craving than males, although the difference was a statistical trend (P = 0.055, P = 0.072, P = 0.064, respectively). While sex predicted relapse as a dichotomous variable, it did not predict continuous variables of relapse: time to relapse, number of drinks, or number of days drinking. Future research focusing on sex as a biological variable with larger samples is needed.
RSFC Strength within Domain-Defined Addiction Networks Predicted Time to Relapse
Time to relapse as a treatment outcome in AUD is crucial information for timely clinical or neuromodulation interventions. This is the first paper that reports the ability to predict time to relapse based on resting fMRI within domain-defined addiction networks in AUD. The association between lower RSFC of domain-defined addiction networks during early abstinence and subsequent shorter time to relapse reported in this paper may indicate that RSFC of networks associated with incentive salience, negative emotionality, and executive functioning during early abstinence is key to recovery in AUD. Although the ultimate goal is to prevent relapse outright, it is important to identify the markers of early relapse risk in order to stage interventions to those most vulnerable appropriately. It should be noted that while we found sex differences between groups (those that relapsed were more likely to be female), the effect of predicting time to relapse with RSFC of domain-defined addiction networks remained significant after adjusting for sex. While RSFC within each addiction network predicted time to relapse, RSFC within the control network (visual network) did not predict relapse metrics, suggesting that the predictive value was specific to RSFC within domain-defined addiction networks (Koob and Volkow 2016; Kwako et al. 2018, 2019).
Time to relapse in AUD has been previously predicted with other neuroimaging metrics (i.e., task-evoked fMRI and gray matter volume). For example, when presented with neutral stimuli, lower connectivity between anterior cingulate and mid cingulate cortex in recovering individuals with AUD can predict longer time to relapse in alcohol use disorder (Zakiniaeiz et al. 2017). Current results add to the literature by showing that RSFC strength across regions within domain-defined addiction networks measured during early abstinence can predict time to relapse over a 4-month follow-up period in individuals with AUD.
Clinical Self-Report Measures Did Not Predict Relapse Metrics
The lack of group difference (ABS vs. REL) as well as the inability to predict relapse metrics suggests that clinical symptomatology (depression, anxiety, and craving; Table 2) reported by AUD individuals during early abstinence were not reliable predictors of treatment outcome within our sample. Moreover, the count of clinical diagnoses (current or lifetime; Table 5) was not significantly different between groups and was not associated with relapse metrics in our sample.
Current findings are inconsistent with previous papers reporting time to relapse prediction from self-report measures such as craving in cocaine use disorder (Paliwal et al. 2008) or depression in AUD (Greenfield et al. 1998). Sample differences across studies may contribute to the discrepancy between the current and previous findings. The lack of association between clinical self-report measures and relapse metrics could be because in the current sample the degree of clinical severity reported during early abstinence was moderate (Spielberger 1983; Beck et al. 1988; Emons et al. 2019). Participants in the current sample were undergoing group and individual counseling in the addiction treatment program and were receiving psychotropic medication (Table 6) to treat clinical symptoms, suggesting their symptomatology was stable. It should be noted that a high proportion of AUD participants in the current sample had comorbid clinical diagnoses (e.g., PTSD, MDD, and GAD). While the proportion of these diagnoses between subsequent abstainers and relapsers was comparable, their presence needs to be further explored in larger studies addressing clinical diagnoses comorbidities with AUD.
Table 6.
Current medications by group (abstainers vs. relapsers)
| Current count (%) | |||
|---|---|---|---|
| Type of medication | ABS (n = 25) | REL (n = 20) | χ2 Sig. |
| Antidepressant | 8 (32.0%) | 7 (35.0%) | P = 0.83 |
| Anxiolytic | 9 (36.0%) | 6 (30.0%) | P = 0.67 |
| Blood pressure stabilizer | 6 (24.0%) | 4 (20.0%) | P = 0.75 |
| Cholesterol | 2 (8.0%) | 0 (0.0%) | P = 0.20 |
| Craving | 0 (0.0%) | 5 (25.0%) | P = 0.008* |
| Diabetes | 7 (28.0%) | 2 (10.0%) | P = 0.13 |
| Sleep | 10 (40.0%) | 6 (30.0%) | P = 0.49 |
Notes: ABS, those that remained abstinent in the 4-month follow-up period; REL, those that relapsed during the 4-month follow-up period; χ2, Chi-Square; P, significance (Sig.) probability value. The finding of a significantly higher proportion of individuals taking craving medication (four using naltrexone, one using acamprosate) in the REL group versus the ABS group needs to be further examined in larger scale studies.
* P < 0.05.
Based on the above discussion, we believe that within the scope of the current data, the functional organization of domain-defined addiction networks measured during early abstinence offers a more objective and predictive measure of relapse vulnerability than self-report clinical measures within the current AUD sample. Future research with a wider range of self-reported measures needs to be conducted to further explore these measures as predictors of relapse metrics (i.e., time to relapse and drinking severity after relapse).
Considerations
The following issues cannot be determined within the scope of this paper. First, we present cross-sectional RSFC data. We cannot determine whether RSFC of abstainers and relapsers differed before the baseline MRI session or whether the differences resulted from diverging neuroplastic alterations during early abstinence. Second, the prediction of long-term length of abstinence is not possible with our 4-month follow-up period. That is, those that were categorized as being in the ABS group could have subsequently relapsed outside of the observed follow-up period. Third, we investigated whether AUD outcomes could be predicted using RSFC within networks selected based on theoretical models of addiction (Koob and Volkow 2016; Kwako et al. 2018, 2019). A direct methodological comparison between our approach and traditional individual ROI analyses is beyond the scope of this paper. However, in the future, it will be important to examine whether RSFC within these theoretically defined networks predict addiction outcomes more strongly than RSFC networks defined with a single ROI. Fourth, the association between clinical symptomatology and RSFC within domain-defined addiction networks and relapse metrics needs to be further examined in individuals with AUD with more severe symptomatology. Finally, while RSFC within domain-defined addiction networks predicted time to relapse, it did not predict the severity of drinking after relapse in the 4-month follow-up period. Future longitudinal studies examining a longer treatment outcome trajectory and exploring data-driven network definition are warranted.
Conclusions
Current findings suggest that reduced RSFC within neural networks underlying incentive salience, negative emotionality, and executive control can predict subsequent relapse in AUD. This paper provided unique and crucial evidence showing that among individuals with AUD in an addiction treatment program, relapse and time to relapse can be predicted based on RSFC of domain-defined addiction networks. These predictions were not found when using an RSFC control network (primary visual) or self-report measures as predictors. The association between low RSFC in addiction networks and shorter time to relapse presents a biomarker of vulnerability to relapse and highlights the importance of allocating resources to design timely interventions dedicated to modulating RSFC of domain-defined addiction networks in individuals with AUD at risk of relapsing faster.
Supplementary Material
Contributor Information
J Camchong, Department of Psychiatry and Behavioral Sciences, University of Minnesota, MN 55454, USA.
A F Haynos, Department of Psychiatry and Behavioral Sciences, University of Minnesota, MN 55454, USA.
T Hendrickson, University of Minnesota Informatics Institute, University of Minnesota, Minneapolis, MN 55455, USA.
M B Fiecas, Division of Biostatistics, School of Public Health, University of Minnesota, Minneapolis, MN 55455, USA.
C S Gilmore, Geriatric Research, Education, and Clinical Center (GRECC), Minneapolis VA Health Care System, Minneapolis, MN 55417, USA.
B A Mueller, Department of Psychiatry and Behavioral Sciences, University of Minnesota, MN 55454, USA.
M G Kushner, Department of Psychiatry and Behavioral Sciences, University of Minnesota, MN 55454, USA.
K O Lim, Department of Psychiatry and Behavioral Sciences, University of Minnesota, MN 55454, USA; Geriatric Research, Education, and Clinical Center (GRECC), Minneapolis VA Health Care System, Minneapolis, MN 55417, USA.
Funding
National Institute of Health (K01AA026349, to M.F. and J.C.; UL1TR002494 to J.C.; K23MH112867 to A.F.H.; R34AA025761, R01AA029077 to M.K.; UG3DA048508, R01DA038984, MH116987 to K.O.L.; P41EB027061, P30NS076408, S10OD017974-01 to CMRR), the Westlake Wells Foundation (to J.C.), Klarman Family Foundation (to A.F.H.), and Hilda and Preston Davis Foundation (to A.F.H.).
Notes
The opinions and assertions expressed herein are those of the authors and do not necessarily reflect the official policy or position of the National Institute of Health, Westlake Wells Foundation, Klarman Family Foundation, or Hilda and Preston Davis Foundation. Conflict of Interest: All authors have declared that there are no competing or potential conflicts of interest.
References
- Agosti V. 2013. Predictors of alcohol dependence relapse during recurrence of major depression. J Addict Dis. 32(1):79–84. [DOI] [PubMed] [Google Scholar]
- Allen MP, editor. 1997. The problem of multicollinearity. In: Understanding regression analysis. Boston, MA: Springer US, pp. 176–180. [Google Scholar]
- Baker PM, Jhou T, Li B, Matsumoto M, Mizumori SJY, Stephenson-Jones M, Vicentic A. 2016. The lateral Habenula circuitry: reward processing and cognitive control. J Neurosci. 36(45):11482–11488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazov I, Sarkisyan D, Kononenko O, Watanabe H, Yakovleva T, Hansson AC, Sommer WH, Spanagel R, Bakalkin G. 2018. Dynorphin and κ-opioid receptor dysregulation in the dopaminergic reward system of human alcoholics. Mol Neurobiol. 55(8):7049–7061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck AT, Steer RA, Carbin MG. 1988. Psychometric properties of the Beck depression inventory: twenty-five years of evaluation. Clin Psychol Rev. 8(1):77–100. [Google Scholar]
- Becker JB, McClellan ML, Reed BG. 2017. Sex differences, gender and addiction. J Neurosci Res. 95(1–2):136–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswal BB, Van Kylen J, Hyde JS. 1997. Simultaneous assessment of flow and BOLD signals in resting-state functional connectivity maps. NMR Biomed. 10(4–5):165–170. [DOI] [PubMed] [Google Scholar]
- Camchong J, Lim KO, Kumra S. 2017. Adverse effects of cannabis on adolescent brain development: a longitudinal study. Cereb Cortex. 27(3):1922–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, MacDonald AW III, Mueller BA, Nelson B, Specker S, Slaymaker V, Lim KO. 2014. Changes in resting functional connectivity during abstinence in stimulant use disorder: a preliminary comparison of relapsers and abstainers. Drug Alcohol Depend. 139(June):145–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camchong J, Stenger A, Fein G. 2013. Resting-state synchrony during early alcohol abstinence can predict subsequent relapse. Cereb Cortex. 23(9):2086–2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essen DC, Glasser DC. 2016. The human connectome project: progress and prospects. Cerebrum. 2016:1–16. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5198757/. [Google Scholar]
- Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BTet al. 2006. An automated Labeling system for subdividing the human cerebral cortex on MRI scans into Gyral based regions of interest. Neuroimage. 31(3):968–980. [DOI] [PubMed] [Google Scholar]
- Emons WH, Habibović M, Pedersen SS. 2019. Prevalence of anxiety in patients with an implantable cardioverter defibrillator: measurement equivalence of the HADS-A and the STAI-S. Qual Life Res. 28(11):3107–3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Robbins TW. 2016. Drug addiction: updating actions to habits to compulsions ten years on. Annu Rev Psychol. 67(1):23–50. [DOI] [PubMed] [Google Scholar]
- Flannery BA, Volpicelli JR, Pettinati HM. 1999. Psychometric properties of the Penn alcohol craving scale. Alcohol Clin Exp Res. 23(8):1289–1295. [PubMed] [Google Scholar]
- George O, Le Moal M, Koob GF. 2012. Allostasis and addiction: role of the dopamine and corticotropin-releasing factor systems. Physiol Behav. 106(1):58–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giacometti LL, Barker JM. 2020. Sex differences in the glutamate system: implications for addiction. Neurosci Biobehav Rev. 113(June):157–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glasser MF, Sotiropoulos SN, Wilson JA, Coalson TS, Fischl B, Andersson JL, Xu J, Jbabdi S, Webster M, Polimeni JRet al. 2013. The minimal preprocessing pipelines for the human connectome project. Neuroimage. 80(October):105–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein RZ, Volkow ND. 2011. Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci. 12(11):652–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfield SF, Weiss RD, Muenz LR, Vagge LM, Kelly JF, Bello LR, Michael J. 1998. The effect of depression on return to drinking: a prospective study. Arch Gen Psychiatry. 55(3):259–265. [DOI] [PubMed] [Google Scholar]
- Griffanti L, Salimi-Khorshidi G, Beckmann CF, Auerbach EJ, Douaud G, Sexton CE, Zsoldos E, Ebmeier KP, Filippini N, Mackay CEet al. 2014. ICA-based artefact removal and accelerated fMRI acquisition for improved resting state network imaging. Neuroimage. 95(July):232–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gujarati DN. 2011. Econometrics by example. Vol 1. New York: Palgrave Macmillan. [Google Scholar]
- Kippin TE, Fuchs RA, Mehta RH, Case JM, Parker MP, Bimonte-Nelson HA, See RE. 2005. Potentiation of cocaine-primed reinstatement of drug seeking in female rats during Estrus. Psychopharmacology (Berl). 182(2):245–252. [DOI] [PubMed] [Google Scholar]
- Konietschke F, Pauly M. 2014. Bootstrapping and permuting paired t-test type statistics. Stat Comput. 24(3):283–296. [Google Scholar]
- Koob GF. 2010. The role of CRF and CRF-related peptides in the dark side of addiction. Brain Res. 1314(February):3–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. 2008. Addiction and the brain antireward system. Annu Rev Psychol. 59(1):29–53. [DOI] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. 2010. Neurocircuitry of addiction. Neuropsychopharmacology. 35(1):217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. 2016. Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiat. 3(8):760–773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Bickel WK, Goldman D. 2018. Addiction biomarkers: dimensional approaches to understanding addiction. Trends Mol Med. 24(2):121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwako LE, Schwandt ML, Ramchandani VA, Diazgranados N, Koob GF, Volkow ND, Blanco C, Goldman D. 2019. Neurofunctional domains derived from deep behavioral phenotyping in alcohol use disorder. Am J Psychiatry. 176(9):744–753. 10.1176/appi.ajp.2018.18030357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M, Vezina P. 2014. Dopamine ups and downs in vulnerability to addictions: a neurodevelopmental model. Trends Pharmacol Sci. 35(6):268–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maisto SA, Hallgren KA, Roos CR, Witkiewitz K. 2018. Course of remission from and relapse to heavy drinking following outpatient treatment of alcohol use disorder. Drug Alcohol Depend. 187(June):319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto M, Hikosaka O. 2007. Lateral Habenula as a source of negative reward signals in dopamine neurons. Nature. 447(7148):1111–1115. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Lewis DC, O’Brien CP, Kleber HD. 2000. Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA. 284(13):1689–1695. [DOI] [PubMed] [Google Scholar]
- Muller AM, Meyerhoff DJ. 2021. Maladaptive brain organization at 1 month into abstinence as an indicator for future relapse in patients with alcohol use disorder. Eur J Neurosci. 53(8):2923–2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray JE, Belin D, Everitt BJ. 2012. Double dissociation of the dorsomedial and dorsolateral striatal control over the acquisition and performance of cocaine seeking. Neuropsychopharmacology. 37(11):2456–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliva F, Nibbio G, Vizzuso P, Sodano AJ, Ostacoli L, Carletto S, Picci RL. 2018. Gender differences in anxiety and depression before and after alcohol detoxification: anxiety and depression as gender-related predictors of relapse. Eur Addict Res. 24(4):163–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacheco-Colón I, Limia JM, Gonzalez R. 2018. Nonacute effects of cannabis use on motivation and reward sensitivity in humans: a systematic review. Psychol Addict Behav. 32(5):497–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliwal P, Hyman SM, Sinha R. 2008. Craving predicts time to cocaine relapse: further validation of the now and brief versions of the cocaine craving questionnaire. Drug Alcohol Depend. 93(3):252–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pareaud M, Girard M, Nubukpo P. 2021. Factors for maintaining abstinence at 2 and 6 months after alcohol withdrawal. J Psychiatr Pract. 27(1):2–13. [DOI] [PubMed] [Google Scholar]
- Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE. 2012. Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage. 59(3):2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EC, Jbabdi S, Glasser MF, Andersson J, Burgess GC, Harms MP, Smith SM, Van Essen DC, Jenkinson M. 2014. MSM: a new flexible framework for multimodal surface matching. Neuroimage. 100(October):414–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubonis AV, Colby SM, Monti PM, Rohsenow DJ, Gulliver SB, Sirota AD. 1994. Alcohol cue reactivity and mood induction in male and female alcoholics. J Stud Alcohol. 55(4):487–494. [DOI] [PubMed] [Google Scholar]
- Salimi-Khorshidi G, Douaud G, Beckmann CF, Glasser MF, Griffanti L, Smith SM. 2014. Automatic denoising of functional MRI data: combining independent component analysis and hierarchical fusion of classifiers. Neuroimage. 90(April):449–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Roige S, Baro V, Trick L, Peña-Oliver Y, Stephens DN, Duka T. 2014. Exaggerated waiting impulsivity associated with human binge drinking, and high alcohol consumption in mice. Neuropsychopharmacology. 39(13):2919–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaefer A, Kong R, Gordon EM, Laumann TO, Zuo X-N, Holmes AJ, Eickhoff SB, Thomas Yeo BT. 2018. Local-global parcellation of the human cerebral cortex from intrinsic functional connectivity MRI. Cereb Cortex. 28(9):3095–3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Beckmann CF, Andersson J, Auerbach EJ, Bijsterbosch J, Douaud G, Duff E, Feinberg DA, Griffanti L, Harms MPet al. 2013. Resting-state fMRI in the human connectome project. Neuroimage. 80(October):144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. 1992. Timeline follow-back. In: Measuring alcohol consumption. Totowa, New Jersey: Humana Press, pp. 41–72. [Google Scholar]
- Spielberger CD 1983. Manual for the State-Trait Anxiety Inventory (STAI). Palo Alto, CA: Consulting Psychologists Press.
- Stohs ME, Schneekloth TD, Geske JR, Biernacka JM, Karpyak VM. 2019. Alcohol craving predicts relapse after residential addiction treatment. Alcohol Alcohol. 54(2):167–172. [DOI] [PubMed] [Google Scholar]
- Tang Y-Y, Posner MI, Rothbart MK, Volkow ND. 2015. Circuitry of self-control and its role in reducing addiction. Trends Cogn Sci. 19(8):439–444. [DOI] [PubMed] [Google Scholar]
- Uğurbil K, Xu J, Auerbach EJ, Moeller S, Vu AT, Duarte-Carvajalino JM, Lenglet C, Wu X, Schmitter S, van de Moortele PFet al. 2013. Pushing spatial and temporal resolution for functional and diffusion MRI in the human connectome project. Neuroimage. 80(October):80–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. 1997. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 386(6627):830–833. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Michaelides M, Baler R. 2019. The neuroscience of drug reward and addiction. Physiol Rev. 99(4):2115–2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. 2007. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 27(46):12700–12706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollstädt-Klein S, Wichert S, Rabinstein J, Bühler M, Klein O, Ende G, Hermann D, Mann K. 2010. Initial, habitual and compulsive alcohol use is characterized by a shift of cue processing from ventral to dorsal striatum. Addiction. 105(10):1741–1749. [DOI] [PubMed] [Google Scholar]
- Voon V, Grodin E, Mandali A, Morris L, Doñamayor N, Weidacker K, Kwako L, Goldman D, Koob GF, Momenan R. 2020. Addictions NeuroImaging assessment (ANIA): towards an integrative framework for alcohol use disorder. Neurosci Biobehav Rev. 113(June):492–506. [DOI] [PubMed] [Google Scholar]
- Walitzer KS, Dearing RL. 2006. Gender differences in alcohol and substance use relapse. Clin Psychol Rev. 26(2):128–148. [DOI] [PubMed] [Google Scholar]
- Walker BM, Koob GF. 2008. Pharmacological evidence for a motivational role of κ-opioid systems in ethanol dependence. Neuropsychopharmacology. 33(3):643–652. 10.1038/sj.npp.1301438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, Wong CT, Hoffman W, Jayne M, Alia-Klein Net al. 2012. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 17(9):918–925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. 2008. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 14(2–3):169–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X, Meng Y-J, Tao Y-J, Deng R-H, Wang H-Y, Li X-J, Wei W, Hua Y, Wang Q, Deng Wet al. 2021. Functional connectivity of nucleus accumbens and medial prefrontal cortex with other brain regions during early-abstinence is associated with alcohol dependence and relapse: a resting-functional magnetic resonance imaging study. Front Psychiat. 12(January):609458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zakiniaeiz Y, Scheinost D, Seo D, Sinha R, Constable RT. 2017. Cingulate cortex functional connectivity predicts future relapse in alcohol dependent individuals. NeuroImage Clin. 13:181–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zehra A, Burns J, Liu CK, Manza P, Wiers CE, Volkow ND, Wang G-J. 2018. Cannabis addiction and the brain: a review. J Neuroimmune Pharmacol. 13(4):438–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


