Abstract
Background Intracranial hemorrhage (ICH) is one of the major devastating complications of anticoagulation. Matrix metalloproteinase (MMP) inhibition has been proposed as a novel pharmacological approach for ICH treatment.
Objectives We evaluated the effects of CM-352 (MMP-fibrinolysis inhibitor) in an experimental ICH model associated with oral anticoagulants as compared with clinically used prothrombin complex concentrate (PCC).
Methods ICH was induced by collagenase injection into the striatum of wild type (C57BL/6J) anticoagulated mice (warfarin or rivaroxaban) and Mmp10 −/− mice. Hematoma volume and neurological deficits were measured 24 hours later by diaminobenzidine staining and different behavioral tests. Circulating plasminogen activator inhibitor-1 (PAI-1) activity and interleukin-6 (IL-6) were measured in plasma samples and local inflammation was assessed by neutrophil infiltration. Finally, fibrinolytic effects of MMP-10 and rivaroxaban were evaluated by thromboelastometry and thrombin-activatable fibrinolysis inhibitor (TAFI) activation assays.
Results Only PCC reduced hemorrhage volume and improved functional outcome in warfarin-ICH, but both PCC and CM-352 treatments diminished hemorrhage volume (46%, p < 0.01 and 64%, p < 0.001, respectively) and ameliorated functional outcome in rivaroxaban-ICH. We further demonstrated that CM-352, but not PCC, decreased neutrophil infiltration in the hemorrhage area at 24 hours. The effect of CM-352 could be related to MMP-10 inhibition since Mmp10 −/− mice showed lower hemorrhage volume, better neurological score, reduced IL-6 levels and neutrophil infiltration, and increased PAI-1 after experimental ICH. Finally, we found that CM-352 reduced MMP-10 and rivaroxaban-related fibrinolytic effects in thromboelastometry and TAFI activation.
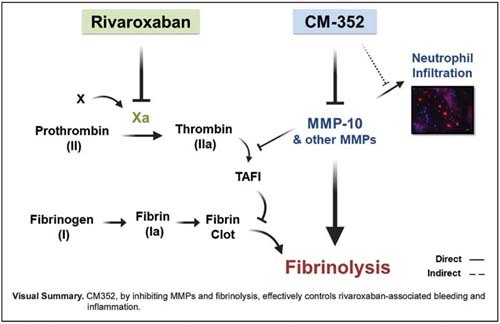
Conclusion CM-352 treatment, by diminishing MMPs and rivaroxaban-associated fibrinolytic effects, might be a novel antihemorrhagic strategy for rivaroxaban-associated ICH.
Keywords: anticoagulants, fibrinolysis, hemorrhagic, stroke, matrix metalloproteinases, thrombosis
Introduction
Almost 2 million people suffer from intracranial hemorrhage (ICH) worldwide every year with a 30-day mortality rate of around 50%; however, its overall incidence has not diminished during the past 30 years. 1
ICH is characterized by direct blood extravasation into the brain parenchyma, 2 thus, hematoma size and expansion are associated with poor outcome and neurological deterioration. 3 ICH is the most feared complication of oral anticoagulation since, accounting for nearly 15 to 20%, these patients present higher mortality rates and prolonged bleeding when compared with nonanticoagulated patients. 4
In ICH patients under vitamin-K antagonists (VKAs), clinical guidelines strongly recommend the use of prothrombin complex concentrate (PCC) as the first option for rapid anticoagulation reversal. 5 Direct oral anticoagulants (DOACs), which specifically inhibit thrombin or Xa, have decreased the risk of ICH. 6 Because of the safety of DOACs and the recent approval of their specific antidotes, 7 8 their use will further increase, multiplying the number of patients suffering from DOAC-ICH. 9 The delay of specific antidotes' approval 10 has led to the off-label use of PCC for the reversal of oral Xa inhibitors but without showing beneficial effects on hematoma expansion. 11 To date, no medical or surgical clinical trial has improved patient outcome after ICH, 12 therefore, remaining a challenging unsolved clinical and public health problem. 13
Matrix metalloproteinases (MMPs) are endogenous zinc-endopeptidases that play a relevant role in vascular remodeling, neuroinflammatory processes, and blood–brain barrier (BBB) disruption associated with the pathophysiology of ICH. 14 Besides, ICH patients presented increased MMP levels in the blood, cerebrospinal fluid, and perihematoma. 15 16 17
The potential of MMPs as pharmacological targets has not yet been fully identified. 18 Experimentally, MMP inhibition prevented hemorrhagic complications induced by tissue plasminogen activator (tPA), via protection of BBB tight junctions. 19 In addition, MMPs may play a role in thrombolysis, since the fibrinolytic system and MMPs cooperate in thrombus dissolution by directly targeting fibrin(ogen) or by collaborating with plasmin. 20 21 Specifically, our group described the fibrinolytic role of MMP-10 by preventing the activation of thrombin-activatable fibrinolysis inhibitor (TAFI) 22 in experimental models of stroke. 23 24 Based on these results, we developed a potent MMP inhibitor, CM-352, which inhibits MMP-10 and MMP-3 and fibrinolysis, 25 and effectively reduces bleeding, hematoma expansion, and functional impairment in different experimental models of hemorrhage with no signs of thrombotic side effects. 26 27
In this study, we aimed to explore the antihemorrhagic efficacy of CM-352 in a collagenase-induced ICH mouse model associated with oral anticoagulants (warfarin or rivaroxaban). We evaluated its antifibrinolytic and anti-inflammatory effects, compared with clinically used four-factor PCC.
Methods
Human Blood Samples
Citrated blood samples were obtained from healthy volunteers who provided informed consent in accordance with the Principles of Declaration of Helsinki on biomedical research involving human subjects.
Animals and Experimental Models
All animal experiments were performed in accordance with European Communities Council regulation (European Union) 2019/1010 for the care and use of laboratory animals and were approved by the Universidad de Navarra Animal Research Review Committee (Ref/092–15). Experiments were performed in 8 to 12 weeks old, 25 to 30 g weight, wild-type ( WT ) male C57BL/6J mice (Envigo, Barcelona, Spain), and MMP-10-deficient mice ( Mmp10 −/− , C57BL/6J ).
Mice were orally anticoagulated before the tail-bleeding model with warfarin (2 mg/kg, 24 hours) or rivaroxaban (3 mg/kg, 1 hour) and the collagenase-induced ICH model with warfarin (2 mg/kg, 24 hour) or rivaroxaban (10 mg/kg, 1 hour). Animals were randomly assigned to receive an intravenous bolus injection of saline, CM-352 (1 mg/kg) or four-factor PCC (100 UI/kg, Octaplex, Octapharma, Vienna, Austria).
Anticoagulation levels were measured 30 minutes before the experimental models in citrated blood samples obtained by submandibular vein puncture. Animals with international normalized ratio (INR) between 2.3 and 5 or plasma anti-Xa activity above 50% were subjected to the ICH model. Investigators were blinded to treatment groups. For further details, see the Supplementary Material and Supplementary Fig. S1(A, B) , available in the online version.
Neurological and Functional Evaluation
Behavioral assessments were performed in all mice before and 24 hours after the collagenase-induced ICH. Three different behavioral tests were performed: Bederson's, pole and coat-hanger test. See the Supplementary Material , for further details, available in the online version.
Sample Collection and Tissue Preparation
Animals for immunohistochemical analysis were euthanized 24 hours after collagenase-induced ICH using a CO 2 chamber and perfused with cold phosphate-buffered saline (PBS) and 4% paraformaldehyde (PAF; Sigma-Aldrich). Brains were removed, post-fixed in 4% PAF for 24 hours, frozen in isopentane, and stored until use at −80°C.
Animals for western blot analysis were euthanized under the same conditions, perfused with PBS, and brain tissue was frozen in liquid nitrogen, and stored at −80°C until posterior protein analysis.
Citrated blood samples were collected after euthanasia, by cardiac puncture. Samples were centrifuged first at 2,500 × g for 10 minutes, then at 13,000 × g for 2 minutes at 4°C, and finally stored at −80°C.
Histological, Immunohistochemical, and Protein Analysis
Frozen brains were cut into serial 20-μm thick coronal sections for histological (hemorrhage volume) and immunohistochemical analysis (neutrophil and neutrophil extracellular traps [NETs]). Additionally, interleukin-6 (IL-6) was analyzed in frozen brain homogenates by western blot as described in detail in the Supplementary Material , available in the online version.
Hemostatic and Inflammatory Parameters
To study the role of different hemostatic parameters, TAFI activation, MMP-10 and Xa activities were measured in purified systems. Additionally, to evaluate systemic inflammation and fibrinolysis 24 hours after the ICH experimental model, plasma levels of IL-6 and plasminogen activator inhibitor-1 (PAI-1) activity were measured by enzyme-linked-immunosorbent assay and chromogenic assays, respectively. See the Supplementary Material , available in the online version, for further details.
Thromboelastometry (ROTEM) with Adherent Endothelial Cells
Human endothelial cells (EC; Eahy926) were seeded onto microbeads to create transferable EC microcarriers. 28 Thromboelastometry (ROTEM) experiments were performed using human citrated blood samples and different therapeutic concentrations of tPA, rivaroxaban, CM-352, and MMP-10. Clotting time (CT) and lysis time (LT) were analyzed. See the Supplementary Material , available in the online version, for further details.
Statistical Analysis
Data are presented as mean ± standard deviation of the mean. Normality was assessed using the Kolmogorov–Smirnov test. Two independent samples were compared using the Mann–Whitney U two-tailed test and two related samples were compared using the Wilcoxon signed-rank test. The analysis for multiple observations was performed by the Kruskal–Wallis test according to the data distribution. Statistical significance was established as p < 0.05. The statistical analysis was performed with SPSS (SPSS version 15.0 for Windows).
Results
Anticoagulant Regimens
No differences between INR values of mice anticoagulated with warfarin included in the tail bleeding and collagenase-ICH experimental models ( Supplementary Fig. S2A , available in the online version) were found among the different treatments ( Supplementary Fig. S2B , available in the online version). Furthermore, we demonstrated that the INR values returned to normal 30 minutes after PCC administration (3.4 ± 0.75 vs. 0.86 ± 0.05, p < 0.05, Supplementary Fig. S2C , available in the online version).
A kinetic study was performed to determine the time-lapse of plasma Xa-inhibition by rivaroxaban ( Supplementary Fig. S3A , available in the online version). WT mice were only included if anti-Xa activity was 50% above the mean basal value (3.84 mIU) 30 minutes after rivaroxaban administration. Plasma Xa activity was similar among the studied groups ( Supplementary Fig. S3B , available in the online version). All mice under rivaroxaban anticoagulation were included in the tail-bleeding model.
CM-352 Effect on Mice Models of Experimental Hemorrhage
Considering that CM-352 efficiently reduced hemorrhage volume in the collagenase-induced ICH model with rats, 27 we first confirmed that CM-352 was effective in mice. As shown in Fig. 1(A, B) , CM-352 reduced the hematoma volume of mice 24 hours after collagenase-induced ICH when compared with saline (mm 3 : 5.78 ± 1.46 saline vs. 3.79 ± 1.93 CM-352, p < 0.05) and prevented hemorrhage-related functional decline ( p < 0.05 for saline, Fig. 1C ). These results support the efficacy of CM-352 reducing hematoma volume and neurological deficit in rodent models of ICH.
Fig. 1.
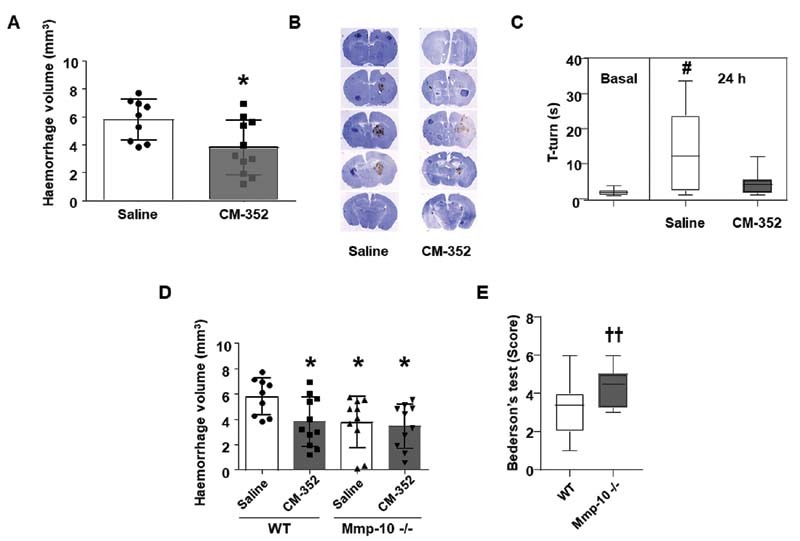
Nonanticoagulated mice 24 hours after experimental ICH. (A) Hemorrhage volume and (B) representative DAB staining images showing hemorrhage volume of wild-type ( WT ) mice treated with saline and CM-352 under no anticoagulation. (C) Functional evaluation (pole test). (D) Hemorrhage volume and (E) functional evaluation (Bederson's test) in WT- and MMP-10-deficient ( Mmp10 −/− ) mice. Treatment: CM-352 (1 mg/kg). Mean ± SD, * p < 0.05 vs. saline; # p < 0.05 vs. basal; †† p < 0.01 vs. WT , using the Kruskal–Wallis and Mann–Whitney U-test, n ≥ 9 /group. ICH, intracranial hemorrhage; SD, standard deviation.
To assess whether MMP-10 inhibition could be involved in the antihemorrhagic effect of CM-352, we performed the collagenase-ICH experimental model in MMP-10-deficient mice ( Mmp10 −/− ). As shown in Fig. 1D , Mmp10 −/− mice presented smaller hematoma volume compared with WT mice (mm 3 : 5.78 ± 1.46 WT vs. 3.77 ± 2.04 Mmp10 −/− , p < 0.05) that was not further reduced upon CM-352 administration. Finally, Mmp10 −/− mice showed an improved score in the Bederson's test when compared with WT 24 hours after ICH ( p < 0.01, Fig. 1E ), suggesting that the beneficial effects of CM-352 in ICH might be partially explained by MMP-10 inhibition.
Then, we assessed the anti-hemorrhagic efficacy of CM-352 and PCC in the tail-bleeding model associated with oral anticoagulants. Our results showed that CM-352 reduced the bleeding time as effectively as PCC in warfarin and rivaroxaban anticoagulated mice when compared with controls ( Supplementary Fig. S4A and B respectively, available in the online version).
Once we confirmed that both CM-352 and PCC efficiently controlled acute bleeding in mice under oral anticoagulation, we tested the antihemorrhagic effects of CM-352 and PCC in the model of collagenase-induced ICH associated with oral anticoagulants.
In warfarin-anticoagulated mice, PCC was able to significantly reduce the hemorrhage at 24 hours when compared with saline (mm 3 : 6.35 ± 2.89 saline vs. 3.88 ± 1.46 PCC, p < 0.05, Fig. 2A ), while the effect of CM-352 did not reach statistical significance. Moreover, treatment with PCC also preserved the functional outcome in the 5-second score test, whereas CM-352 and saline treatments did not ( Fig. 2B ).
Fig. 2.
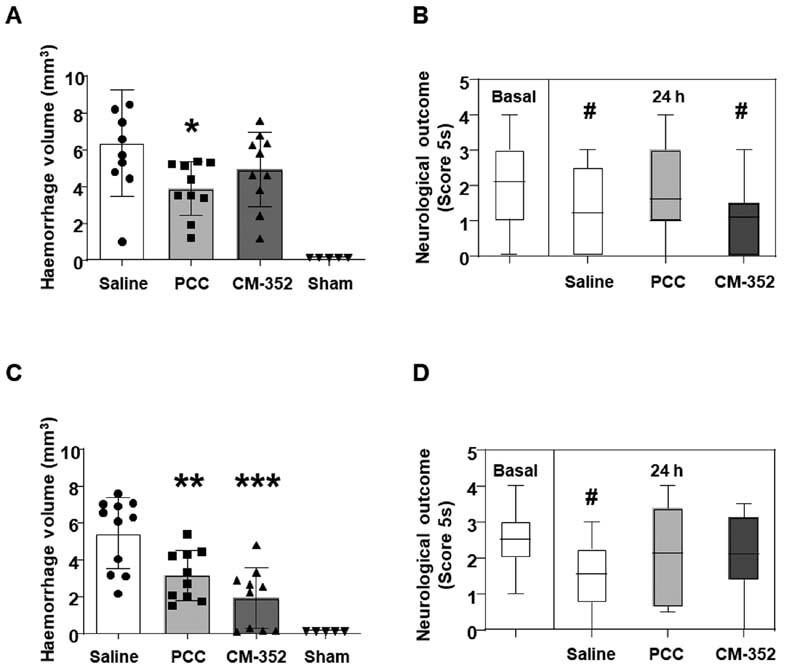
Anticoagulated mice 24 hours after experimental ICH. (A) Hemorrhage volume and (B) functional evaluation (coat hanger) in warfarin-anticoagulated mice. (C) Hemorrhage volume and (D) functional evaluation (coat hanger) in rivaroxaban-anticoagulated mice. Treatments: warfarin (2 mg/kg), rivaroxaban (10 mg/kg), PCC (100 UI/kg), and CM-352 (1 mg/kg). Mean ± SD, * p < 0.05, ** p < 0.01, and *** p < 0.001 vs. saline; # p < 0.05 vs. basal, using the Kruskal–Wallis and Mann–Whitney U-test, n ≥ 5 /group. ICH, intracranial hemorrhage; SD, standard deviation.
In mice under rivaroxaban anticoagulation, CM-352-treated animals achieved a beneficial response, with a 64% reduction of the hematoma volume when compared with saline 24 hours after ICH induction (mm 3 : 5.76 ± 1.68 saline vs. 2.11 ± 1.63 CM-352, p < 0.001). Likewise, PCC-treated mice showed a 46% reduction in hematoma volume when compared with saline (mm 3 : 5.76 ± 1.68 saline vs. 3.14 ± 1.65 PCC, p < 0.01, Fig. 2C ), with no further differences between CM-352 and PCC. Functional activity scores showed an improved 5-second score test in PCC and CM-352 groups when compared with the neurological deficits observed in the saline group ( p < 0.05 for saline, Fig. 2D ). No differences in the rest of the neurological scores were found (data not shown). As anticipated, sham-operated mice did not develop cerebral injury ( Fig. 2A, C ). Together, these results suggest that only PCC is effective in controlling the hematoma expansion and neurological function in warfarin-associated ICH, while both CM-352 and PCC are effective in rivaroxaban-associated ICH.
CM-352 Impact on MMP-10 and Xa in the Presence of Oral Anticoagulants
Enzymatic activity assays were performed to exclude an interaction of warfarin or rivaroxaban on the anti-MMP10 activity of CM-352, or that of CM-352 on the inhibitory effect of rivaroxaban on Xa. As shown in Supplementary Fig. S5A [available in the online version], neither rivaroxaban nor warfarin affects the anti-MMP10 activity of CM-352. Similarly, CM-352 did not alter the inhibitory effect of rivaroxaban on Xa ( Supplementary Fig. S5B , available in the online version).
CM-352 Restores Rivaroxaban and MMP-10 Fibrinolytic Effects in Vitro
Taking into account the potential fibrinolytic activity described for rivaroxaban, 29 we analyzed whether CM-352 could modulate it. Kinetics of clot formation and lysis (tPA-mediated) were analyzed by thromboelastometry in the presence of beads coated with endothelial cells to provide the system with cell membranes and thrombomodulin ( Fig. 3A ). As expected, CT increased dose-dependently in presence of rivaroxaban ( p < 0.05, Fig. 3B ). Furthermore, our results showed that rivaroxaban exhibited a fibrinolytic effect shortening the LT in a dose-dependent manner when compared with the control ( p < 0.01 for 460 nmol/L, Fig. 3C ). Interestingly, CM-352 blocked the fibrinolytic effect induced by rivaroxaban ( p < 0.01 and p < 0.05, for 1.8 and 3.7 μmol/L, Fig. 3E ) without changes in CT ( Fig. 3D ).
Fig. 3.
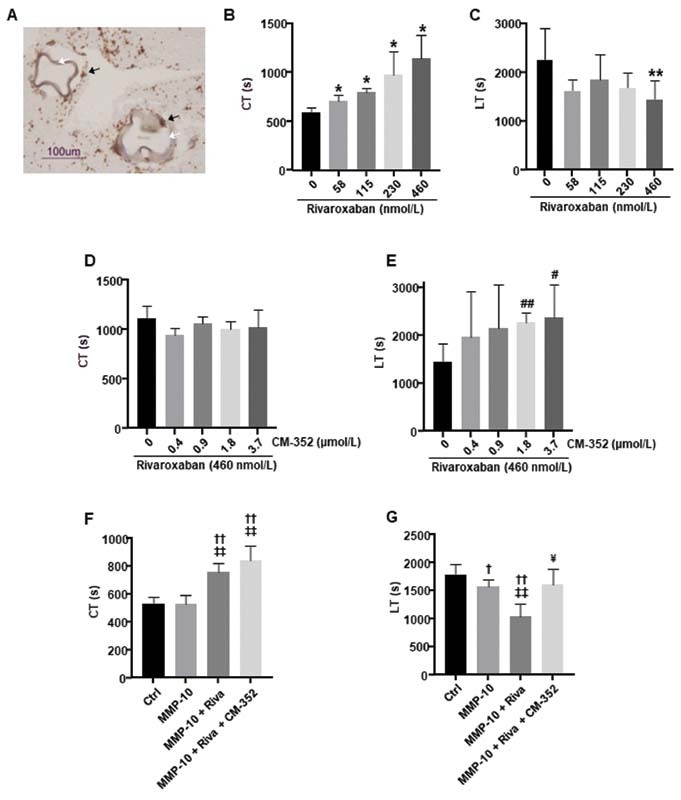
Thromboelastometric analysis with adherent EC microcarriers using human whole blood samples. (A) Representative image of CD31-positive cells ( brown, black arrows ) surrounding the surface of Cytodex 3 EC microcarriers ( white arrows ) in a blood clot obtained by ROTEM. Scale bar = 100 µm. (B) Clotting time (CT) and (C) lysis time (LT) in the presence of 58, 115, 230, or 460 nmol/L of rivaroxaban. (D) CT and (E) LT in the presence of 0.4, 0.9, 1.8, or 3.7 µmol/L of CM-352 and 460 nmol/L of rivaroxaban. (F) CT and (G) LT in the presence of MMP-10 (200 nmol/L), rivaroxaban (460 nmol/L), and CM-352 (1.8 µmol/L). CT and LT times expressed in seconds (s) are presented in the graphs. Mean ± SD, * p < 0.05 and ** p < 0.01 vs. 0 nmol/L; # p < 0.05 and ## p < 0.01 vs. 0 µmol/L; † p < 0.05 and †† p < 0.01 vs. Ctrl; ‡‡ p < 0.01 vs. MMP-10; ¥ p < 0.05 vs. MMP-10 + Riva, using Kruskal–Wallis and Mann–Whitney U-test, n ≥ 3/group. EC, endothelial cell; ROTEM, rotational thromboelastometry; SD, standard deviation; TAFI, thrombin-activatable fibrinolysis inhibitor.
Further, we tested the effects of CM-352 using rivaroxaban and MMP-10 as fibrinolytic agents. Rivaroxaban delayed the CT ( p < 0.01) independently of MMP-10 and CM-352 ( Fig. 3F ). The LT was reduced by MMP-10 alone and was further accelerated when combined with rivaroxaban ( p < 0.05 and p < 0.01, respectively), while CM-352 reverted their effect ( p < 0.05, Fig. 3G ). These results suggest that CM-352 restores rivaroxaban and MMP-10 fibrinolytic effects.
CM-352 Prevents MMP-10-Dependent TAFI Inactivation
Additionally, we assessed whether CM-352 could prevent MMP-10-induced TAFI inactivation. 22 As shown in Fig. 4 , MMP-10 alone or in combination with rivaroxaban reduced TAFI activation ( p < 0.05) that was restored by CM-352, also in the presence of rivaroxaban. These results suggest that the observed antifibrinolytic effects of CM-352 might depend on MMP-10 inhibition.
Fig. 4.
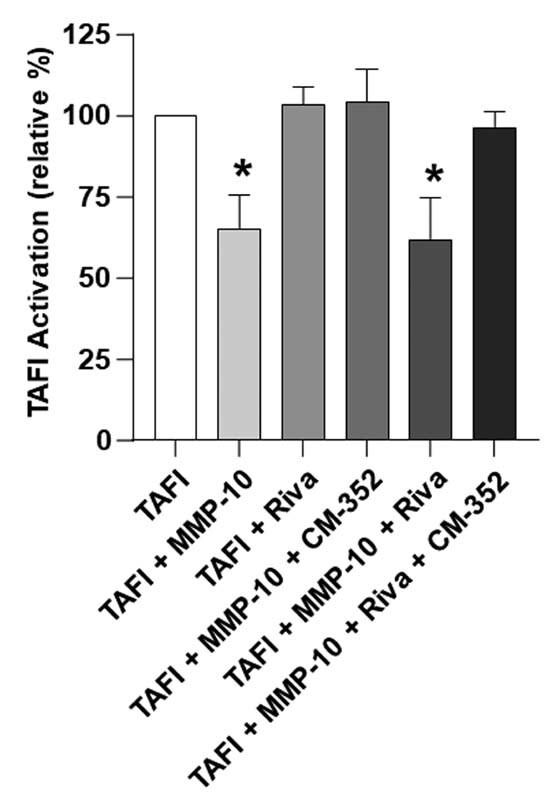
MMP-10-dependent TAFI activation. TAFI (30 nmol/L) activation measured in the presence of MMP-10 (4 nmol/L), rivaroxaban (4 nmol/L), and CM-352 (4 nmol/L). TAFI relative activation (%) is shown. Mean ± SD, * p < 0.05 vs. TAFI, using Kruskal–Wallis and Mann–Whitney U-test, n ≥ 3/group. SD, standard deviation; TAFI, thrombin-activatable fibrinolysis inhibitor.
MMP-10 Inhibition Contributes to Reducing Inflammation and Fibrinolysis after Experimental ICH
To assess whether PCC and CM-352 were able to modulate the systemic inflammatory status of the animals 24 hours after the ICH induction, we measured circulating levels of IL-6 in untreated, warfarin, and rivaroxaban-anticoagulated mice ( Fig. 5A–C ), finding no differences in IL-6 plasma levels after ICH in any of the assessed experimental condition. However, Mmp10 −/− animals showed decreased IL-6 expression in plasma (pg/mL: 24.70 ± 9.66 saline Mmp10 −/− and 22.85 ± 4.93 CM-352 Mmp10 −/− vs. 33.84 ± 9.06 saline WT , p < 0.05, Fig. 5A ) as well as in brain tissue ( Supplementary Fig. S6A , available in the online version) after ICH induction when compared with WT .
Fig. 5.
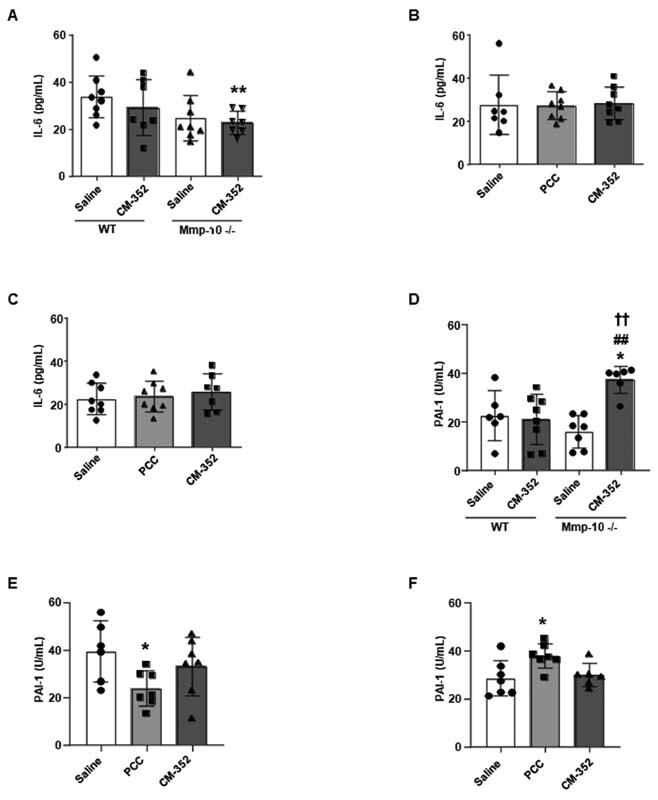
Systemic inflammation and fibrinolysis 24 hours after experimental ICH. Plasma IL-6 levels of (A) nonanticoagulated wild-type ( WT ) and MMP-10-deficient ( Mmp10 −/− ) animals, (B) warfarin, and (C) rivaroxaban anticoagulated mice. (D) PAI-1 activity in nonanticoagulated WT and MMP-10-deficient ( Mmp10 −/− ) animals, (E) warfarin, and (F) rivaroxaban anticoagulated mice. Mean ± SD, * p < 0.05 vs. saline WT ; ## p < 0.01 vs. CM-352 WT ; †† p < 0.01 vs. saline Mmp10 −/− ; using Kruskal–Wallis and Mann–Whitney U-test, n ≥ 6/group. ICH, intracranial hemorrhage; IL-6, interleukin-6; SD, standard deviation.
Additionally, we evaluated plasma PAI-1 activity 24 hours after ICH as a marker of systemic inflammatory and hemostasis status. In mice under no oral anticoagulation, we found no differences in PAI-1 activity 24 hours after ICH except for an increment observed only in CM-352-treated Mmp10 −/− animals ( p < 0.05 vs. saline WT; p < 0.01 vs. CM-352 WT and p < 0.01 vs. saline Mmp-10 −/− , Fig. 5D ). In warfarin-anticoagulated mice, we observed that PCC-treated mice depicted lower PAI-1, but, on the other hand, in rivaroxaban-anticoagulated mice, PCC-treated mice showed higher PAI-1 activity when compared with controls after ICH ( p < 0.05 vs. saline, Fig. 5E, F , respectively). We observed no changes in PAI-1 activity after CM-352 treatment in any of our anticoagulant experimental groups after ICH.
We also analyzed the effect of PCC and CM-352 on local inflammation by examining neutrophil infiltration in the hemorrhage area at 24 hours. Neutrophil infiltration into brain tissue after ICH was similar in untreated or warfarin-anticoagulated mice, regardless of the treatment (saline, CM-352, or PCC, Fig. 6A, B ). However, in mice anticoagulated with rivaroxaban, we found that only CM-352 reduced neutrophil infiltration when compared with controls (neutrophils/µm 2 : 93.30 ± 35.78 saline vs. 42.51 ± 27.50 CM-352, p < 0.05, Fig. 6C ). Additionally, in this group of mice, we observed that CM-352 diminished the density of NETs in the hemorrhage area as compared with controls (NETs/µm 2 : 37.39 ± 21.10 saline vs. 11.23 ± 6.79 CM-352, p < 0.05, Supplementary Fig. S6B, C , available in the online version).
Fig. 6.
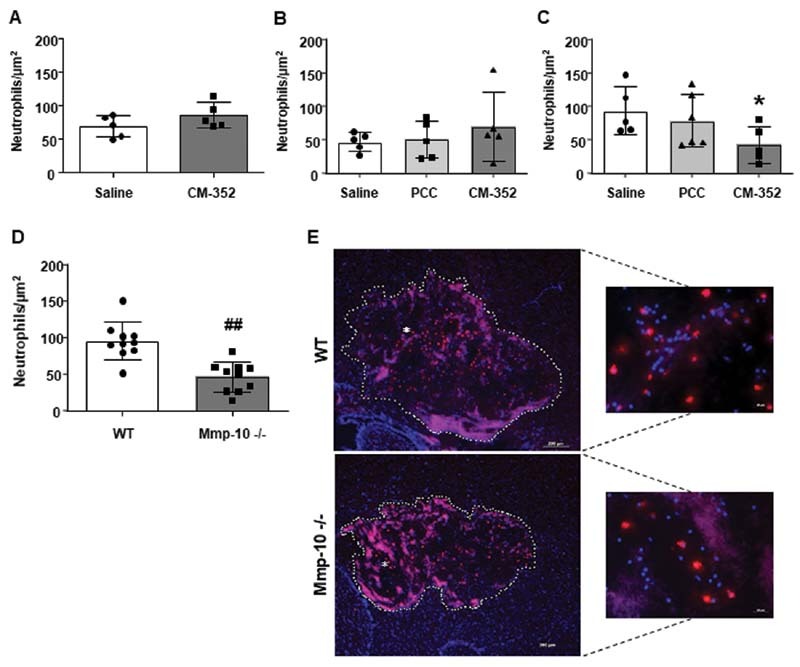
Local inflammation 24 hours after experimental ICH. Neutrophil infiltration in the hemorrhage area of (A) nonanticoagulated, (B) warfarin, and (C) rivaroxaban anticoagulated mice. (D) Neutrophil infiltration in the hemorrhage area of wild-type ( WT ) and MMP-10-deficient ( Mmp10 −/− ) animals. (E) Representative immunofluorescence images showing neutrophils ( red ) and DAPI ( blue ) in the hemorrhage area ( white dots ) of WT and Mmp10 −/− mice. Scale = 200 µm. Magnification images of selected areas (*). Scale = 20 µm. Mean ± SD, * p < 0.05 vs. saline and ## p < 0.01 vs. WT using Kruskal–Wallis and Mann–Whitney U-test, n ≥ 4/group. ICH, intracranial hemorrhage; SD, standard deviation.
These results suggest that CM-352 treatment might diminish local inflammation after experimental ICH associated with rivaroxaban anticoagulation. Moreover, we found that neutrophil infiltration in Mmp10 −/− animals was also decreased when compared with WT (neutrophils/µm 2 : 77.69 ± 18.92 WT vs. 46.41 ± 20.36 Mmp10 −/− , p < 0.01, Fig. 6D, E ).
Altogether, our results suggest MMP-10 inhibition could help to reduce inflammation and control hemostasis after experimental ICH.
Discussion
Here we reported that CM-352 effectively reduced hematoma volume and functional impairment in rivaroxaban-associated ICH. Furthermore, CM-352 prevented rivaroxaban and MMP-10-related fibrinolytic effects. Additionally, we reported that: (1) CM-352 and PCC effectively controlled experimental bleeding under oral anticoagulation with warfarin or rivaroxaban; (2) PCC reduced hematoma volume and functional decline in experimental ICH associated with warfarin or rivaroxaban; and (3) MMP-10-deficient animals showed smaller hematoma and better neurological function, suggesting that inhibition of MMP-10 by CM-352 could be related to the beneficial effects of CM-352 after experimental ICH.
MMP inhibition has been hypothesized as a promising strategy for the treatment of ICH. 30 In fact, our group previously demonstrated the effectiveness and safety of CM-352 after experimental collagenase-induced ICH in rats, and discarded any effect of CM-352 on collagenase activity. 27 In line with these results, the current study shows that CM-352 successfully reduces brain hemorrhage and prevents neurological decline in collagenase-induced ICH in mice. Particularly, we showed that MMP-10-deficient animals consistently displayed reduced hematoma volume and improved neurological function after experimental ICH while CM-352 did not present any additional advantage on these parameters. Taken together, these data indicate that the beneficial effects of CM-352 may partially depend on MMP-10 inhibition.
VKAs are widely prescribed effective anticoagulants for the prevention and treatment of thrombotic events, but the VKA-related major bleeding complications (rates between 10 and 16%) must be taken into consideration. The risk of warfarin-associated ICH may reach 1 to 2% per year, and this risk increases up to 4.2% in older patients. 31 In this context, CM-352 treatment does not control ICH or improve motor activity in our experimental conditions. This result could be due to a lower efficacy of CM-352 on MMP inhibition or fibrinolysis, although our data indicate that MMP-10 inhibition or PAI-1 activity is not modified by CM-352 in the presence of warfarin.
As stated in recent guidelines, PCC is recommended to decrease mortality and normalize the INR in ICH occurring under the use of VKAs. 32 Clinical and experimental data showed that PCC improved hematoma expansion and outcome after warfarin-associated ICH. 33 34 In line with these results, we observed that PCC reduced bleeding time as well as hematoma volume and functional decline after experimental warfarin-associated ICH. These data might be explained by INR normalization after PCC treatment and the subsequent reduction of fibrinolytic activity, which in turn could decrease PAI-1 activity. Some studies already reported four-factor PCC treatment for acute warfarin reversal in adult patients 35 ; however, high doses of PCC might led to overcorrection of thrombin generation and increase the risk of thrombotic complications. 35 36
PCC is also recommended (off-label) for the treatment of DOAC-associated ICH, when specific reversal antidotes are not available. 37 A recent multicenter clinical study in factor Xa inhibitor-related ICH patients demonstrated that PCC achieved excellent hemostasis with very low thrombotic events (4%). 38 Similarly, a recent meta-analysis in patients with DOAC-associated severe bleeding reported that thromboembolism rates were higher in patients treated with andexanet (specific-Xa antidote) compared with those treated with PCC (10.7 vs. 4.3%). 10 Nevertheless, PCC still lacks the clinical efficacy required to improve patients' outcome, thus, further studies are needed to answer this clinical need. 11 Yet, experimentally Zhou et al described that PCC treatment prevented hematoma expansion in a murine model of rivaroxaban-associated ICH. 39 Our results confirm and extend these data showing an increase in PAI-1 activity that could be explained by an increased endogenous thrombin generation as already described in previous studies evaluating PCC treatment to reverse the anticoagulant effect of direct factor Xa inhibitors. 40 However, absence of the prothrombin time (PT) restoration or incomplete correction of thrombin generation has also been described, 40 so there remains significant uncertainty regarding the efficacy and potential harms associated with these agents.
Notably, we showed for the first time that CM-352 is as effective as PCC-reducing hematoma and preventing functional impairment after rivaroxaban-associated ICH, suggesting that CM-352 can be a promising therapeutic approach in anticoagulant-associated ICH. Recently, some reports have described that Xa-DOACs, aside from their anticoagulant effects, enhance fibrinolysis by increasing urokinase plasminogen activator, 41 suggesting that these patients might specially benefit from antifibrinolytic compounds to prevent hemorrhage. In addition, the fibrinolytic activity of rivaroxaban has also been related to the reduction of TAFI activation since decreasing thrombin generation would reduce resistance to fibrinolysis. 29 Nevertheless, this effect might be dual since reduction of thrombin generation could also reduce fibrinolysis by lowering tPA release from endothelium. 42 Moreover, in experimental studies, the role of rivaroxaban as a cofactor of tPA inducing the fibrinolytic activity of FXa has been also described. 43 Therefore, we performed thromboelastometry experiments to assess the fibrinolytic effect of rivaroxaban and its modulation by CM-352. Interestingly, we found that rivaroxaban exhibited a fibrinolytic effect in blood of healthy volunteers, which was restored by CM-352 without altering coagulation. Our group previously described the profibrinolytic properties of MMP-10, 23 24 therefore, additional thromboelastometry experiments were performed in the presence of MMP-10. We showed that the profibrinolytic effect of MMP-10 was enhanced by rivaroxaban and blocked by CM-352, suggesting that CM-352 reverses the fibrinolytic activity of MMP-10 and rivaroxaban.
Like rivaroxaban, MMP-10 displays its fibrinolytic mechanism by cleaving TAFI and so preventing its activation. 22 Our data suggest that the antifibrinolytic effects of CM-352 might be mainly related to MMP activity inhibition, since CM-352 (1) did not affect rivaroxaban inactivation of FXa neither its anticoagulant activity, and (2) it was able to preserve TAFI activation in the presence of MMP-10 independently of rivaroxaban. Nevertheless, CM-352 might participate in other rivaroxaban-enhanced fibrinolysis mechanisms. Therefore, although CM-352 did not alter rivaroxaban inhibition of FXa, it may change the fibrinolytic effect of rivaroxaban-treated FXa.
Inflammatory cells and molecules localized in the lesion site and peripheral areas have been associated with secondary damage after ICH. 16 We have evaluated systemic and local inflammatory status by measuring, plasma, and brain levels of IL-6, as well neutrophil infiltration and NET formation in the hemorrhage area after experimental ICH.
Systemically, our results show that CM-352 has no apparent effect on inflammation regardless of the anticoagulant treatment. However, MMP-10-deficient animals treated with CM-352 present reduced levels of IL-6 in plasma and increased PAI-1 activity. Previous studies reported that IL-6 regulates PAI-1 expression 42 and additionally that PAI-1 might be related to a reduction in MMP activity, 44 45 suggesting that MMP-10 inhibition might contribute to control systemic inflammation and fibrinolysis and to the subsequent protective effect observed in the ICH experimental model.
Locally, in line with hematoma volume reduction and neurological outcome improvement, CM-352 diminishes neutrophil infiltration and NET formation in rivaroxaban-associated ICH. Additionally, MMP-10-deficient animals present lower neutrophil infiltration and IL-6 after ICH. Likewise, experimental ICH studies have described that MMP-9- and -12-deficient mice exhibited diminished neutrophil infiltration in the lesion area associated with less brain damage. 46 47 Altogether, our data suggest that the beneficial effects of MMP-10 inhibition in ICH might be partially related to reduced systemic and local inflammation.
We are aware of the limitations of our study and extrapolation of the results should be applied with caution. Age and sex are nonmodifiable risk factors for ICH, and in this study, we used adult male animals, therefore, further experimental studies should address gender differences and the effect of aging when assessing its therapeutic potential for ICH. We have not examined the anti-Xa activity levels in mice anticoagulated with rivaroxaban and subjected to the tail-bleeding model. However, all the control animals bled for 30 minutes assuring effective anticoagulation. In addition, we have found that the beneficial effects of CM-352 might be related, at least in part, to MMP-10 inhibition, although CM-352 is a pan-MMP inhibitor that also inhibits MMP-2, MMP-9, and MMP-12 activity in the nanomolar range, thus the reported benefits could also involve the modulation of other MMPs. 26 27 Hence, further experiments using other MMP-deficient animals should be performed to establish a cause–effect relationship.
Conclusion
CM-352 and PCC effectively control oral anticoagulant-associated acute bleeding. In anticoagulant-associated ICH, only PCC reduces hemorrhage and improves functional outcome in warfarin-anticoagulated mice, while both PCC and CM-352 prevent hematoma expansion and functional impairment in mice anticoagulated with rivaroxaban. The mechanism behind the effect of CM-352 in experimental ICH might depend on MMP-10 inhibition and its antifibrinolytic and anti-inflammatory effects. Additionally, CM-352 prevents rivaroxaban and MMP-10-related fibrinolytic effects in thromboelastometry, as well as in TAFI activation. Therefore, CM-352 has the potential to provide a paradigm shift for rivaroxaban-associated ICH.
Acknowledgments
We thank Agustina Salicio, Lara Montori, and Miriam Belzunce (Laboratory of Atherothrombosis, CIMA Universidad de Navarra, Instituto de Investigación Sanitaria de Navarra, IdisNA, Pamplona, Spain) for the help with the experimental work.
Funding Statement
Funding This work was supported by ISCIII (PI15/01807 and PI19/00065”), co-funded by ERDF, “A way to make Europe”, the Spanish Society of Thrombosis and Haemostasis (SETH), the Navarra Government (02/2015), and the Virto Group (Navarra, Spain).
Conflict of Interest M.N.-O.: recipient of a PhD scholarship from the Asociación de Amigos de la Universidad de Navarra (ADA). J.Oy., J.A.R, J.A.P., and J.Or. were involved in a granted patent family (EP 12382285) that was licensed to Hemostatics Pharmaceuticals S.L. The other authors declare no significant conflict of interest.
Author Contributions
M.N.O. participated in the design of the project, experimental work, statistical analysis, and wrote, reviewed, and edited the manuscript; J.M.E. participated in the analysis of data, and edited and reviewed the manuscript; C.R. participated in experimental work, data analysis, and reviewed the manuscript; J.A.R., B.Z., J.H., J.O., J.A.P., and R.M. participated in the design of the project and reviewed the manuscript; A.P.L., and R.L. have provided intellectual content and reviewed the manuscript; and J.O. was in charge of project design, supervised the work and wrote, edited, and reviewed the manuscript.
What is known about this topic?
Intracranial hemorrhage (ICH) is the most feared complication of oral anticoagulation.
Matrix metalloproteinase (MMP) inhibition has been proposed as a novel pharmacological approach for ICH treatment.
What does this paper add?
Prothrombin concentrate complex (PCC) and CM-352 are effective treatments reducing hemorrhage volume and functional decline in rivaroxaban-associated ICH, while only PCC is effective in warfarin-associated ICH mouse model.
The effect of CM-352 could be related to MMP-10 inhibition, since Mmp10 −/− mice showed lower hemorrhage volume and inflammation, and better neurological score after experimental ICH.
CM-352 prevents and attenuates MMP-10 and rivaroxaban-related fibrinolytic effects.
Supplementary Material
References
- 1.VISTA-ICH Collaboration ; ICH Growth Individual Patient Data Meta-analysis Collaborators . Al-Shahi Salman R, Frantzias J, Lee R J. Absolute risk and predictors of the growth of acute spontaneous intracerebral haemorrhage: a systematic review and meta-analysis of individual patient data. Lancet Neurol. 2018;17(10):885–894. doi: 10.1016/S1474-4422(18)30253-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nadeau C A, Dietrich K, Wilkinson C M. Prolonged blood-brain barrier injury occurs after experimental intracerebral hemorrhage and is not acutely associated with additional bleeding. Transl Stroke Res. 2019;10(03):287–297. doi: 10.1007/s12975-018-0636-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Haller J T, Wiss A L, May C C, Jones G M, Smetana K S. Acute management of hypertension following intracerebral hemorrhage. Crit Care Nurs Q. 2019;42(02):129–147. doi: 10.1097/CNQ.0000000000000247. [DOI] [PubMed] [Google Scholar]
- 4.Kuramatsu J B, Huttner H B. Management of oral anticoagulation after intracerebral hemorrhage. Int J Stroke. 2019;14(03):238–246. doi: 10.1177/1747493019828555. [DOI] [PubMed] [Google Scholar]
- 5.Veltkamp R, Purrucker J. Management of spontaneous intracerebral hemorrhage. Curr Neurol Neurosci Rep. 2017;17(10):80. doi: 10.1007/s11910-017-0783-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ruff C T, Giugliano R P, Braunwald E.Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials Lancet 2014383(9921):955–962. [DOI] [PubMed] [Google Scholar]
- 7.ANNEXA-4 Investigators . Connolly S J, Crowther M, Eikelboom J W. Full study report of andexanet alfa for bleeding associated with factor Xa inhibitors. N Engl J Med. 2019;380(14):1326–1335. doi: 10.1056/NEJMoa1814051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pollack C V, Jr, Reilly P A, van Ryn J. Idarucizumab for dabigatran reversal - full cohort analysis. N Engl J Med. 2017;377(05):431–441. doi: 10.1056/NEJMoa1707278. [DOI] [PubMed] [Google Scholar]
- 9.Ruff C T, Giugliano R P, Antman E M. Management of bleeding with non-vitamin K antagonist oral anticoagulants in the era of specific reversal agents. Circulation. 2016;134(03):248–261. doi: 10.1161/CIRCULATIONAHA.116.021831. [DOI] [PubMed] [Google Scholar]
- 10.Gómez-Outes A, Alcubilla P, Calvo-Rojas G. Meta-analysis of reversal agents for severe bleeding associated with direct oral anticoagulants. J Am Coll Cardiol. 2021;77(24):2987–3001. doi: 10.1016/j.jacc.2021.04.061. [DOI] [PubMed] [Google Scholar]
- 11.RETRACE II (German-Wide Multicenter Analysis of Oral Anticoagulation-Associated Intracerebral Hemorrhage II) Investigators . Gerner S T, Kuramatsu J B, Sembill J A. Association of prothrombin complex concentrate administration and hematoma enlargement in non-vitamin K antagonist oral anticoagulant-related intracerebral hemorrhage. Ann Neurol. 2018;83(01):186–196. doi: 10.1002/ana.25134. [DOI] [PubMed] [Google Scholar]
- 12.Kim J Y, Bae H J. Spontaneous intracerebral hemorrhage: management. J Stroke. 2017;19(01):28–39. doi: 10.5853/jos.2016.01935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hemorrhagic Stroke Academia Industry (HEADS) Roundtable Participants . Selim M. Unmet needs and challenges in clinical research of intracerebral hemorrhage. Stroke. 2018;49(05):1299–1307. doi: 10.1161/STROKEAHA.117.019541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dang B, Duan X, Wang Z, He W, Chen G. A therapeutic target of cerebral hemorrhagic stroke: matrix metalloproteinase- 9. Curr Drug Targets. 2017;18(12):1358–1366. doi: 10.2174/1389450118666170427151657. [DOI] [PubMed] [Google Scholar]
- 15.Castellazzi M, Tamborino C, De Santis G. Timing of serum active MMP-9 and MMP-2 levels in acute and subacute phases after spontaneous intracerebral hemorrhage. Acta Neurochir Suppl (Wien) 2010;106:137–140. doi: 10.1007/978-3-211-98811-4_24. [DOI] [PubMed] [Google Scholar]
- 16.Florczak-Rzepka M, Grond-Ginsbach C, Montaner J, Steiner T. Matrix metalloproteinases in human spontaneous intracerebral hemorrhage: an update. Cerebrovasc Dis. 2012;34(04):249–262. doi: 10.1159/000341686. [DOI] [PubMed] [Google Scholar]
- 17.Wang H X, Yang Q D, Liu B Q. TIMP-1 polymorphisms in a Chinese Han population with intracerebral hemorrhage. Int J Neurosci. 2014;124(01):61–67. doi: 10.3109/00207454.2013.823604. [DOI] [PubMed] [Google Scholar]
- 18.Montaner J, Ramiro L, Simats A. Matrix metalloproteinases and ADAMs in stroke. Cell Mol Life Sci. 2019;76(16):3117–3140. doi: 10.1007/s00018-019-03175-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishiro K, Ishiguro M, Suzuki Y, Tsuruma K, Shimazawa M, Hara H. A broad-spectrum matrix metalloproteinase inhibitor prevents hemorrhagic complications induced by tissue plasminogen activator in mice. Neuroscience. 2012;205:39–48. doi: 10.1016/j.neuroscience.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 20.Lijnen H R, Juhan-Vague I. The fibrinolytic system and obesity. Thromb Haemost. 2002;88(05):882. [PubMed] [Google Scholar]
- 21.Mühl D, Ghosh S, Uzuelli J A, Lantos J, Tanus-Santos J E. Increases in circulating matrix metalloproteinase-9 levels following fibrinolysis for acute pulmonary embolism. Thromb Res. 2010;125(06):549–553. doi: 10.1016/j.thromres.2010.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Orbe J, Barrenetxe J, Rodriguez J A. Matrix metalloproteinase-10 effectively reduces infarct size in experimental stroke by enhancing fibrinolysis via a thrombin-activatable fibrinolysis inhibitor-mediated mechanism. Circulation. 2011;124(25):2909–2919. doi: 10.1161/CIRCULATIONAHA.111.047100. [DOI] [PubMed] [Google Scholar]
- 23.Roncal C, Martinez de Lizarrondo S, Salicio A. New thrombolytic strategy providing neuroprotection in experimental ischemic stroke: MMP10 alone or in combination with tissue-type plasminogen activator. Cardiovasc Res. 2017;113(10):1219–1229. doi: 10.1093/cvr/cvx069. [DOI] [PubMed] [Google Scholar]
- 24.Navarro-Oviedo M, Roncal C, Salicio A. MMP10 promotes efficient thrombolysis after ischemic stroke in mice with induced diabetes. Transl Stroke Res. 2019;10(04):389–401. doi: 10.1007/s12975-018-0652-9. [DOI] [PubMed] [Google Scholar]
- 25.Orbe J, Sánchez-Arias J A, Rabal O. Design, synthesis, and biological evaluation of novel matrix metalloproteinase inhibitors as potent antihemorrhagic agents: from hit identification to an optimized lead. J Med Chem. 2015;58(05):2465–2488. doi: 10.1021/jm501940y. [DOI] [PubMed] [Google Scholar]
- 26.Orbe J, Rodríguez J A, Sánchez-Arias J A. Discovery and safety profiling of a potent preclinical candidate, (4-[4-[[(3R)-3-(hydroxycarbamoyl)-8-azaspiro[4.5]decan-3-yl]sulfonyl]phenoxy]-N-methylbenzamide) (CM-352), for the prevention and treatment of hemorrhage. J Med Chem. 2015;58(07):2941–2957. doi: 10.1021/jm501939z. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez J A, Sobrino T, López-Arias E. CM352 reduces brain damage and improves functional recovery in a rat model of intracerebral hemorrhage. J Am Heart Assoc. 2017;6(06):e006042. doi: 10.1161/JAHA.117.006042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zipperle J, Schlimp C J, Holnthoner W. A novel coagulation assay incorporating adherent endothelial cells in thromboelastometry. Thromb Haemost. 2013;109(05):869–877. doi: 10.1160/TH12-10-0767. [DOI] [PubMed] [Google Scholar]
- 29.Schultz N H, Holme P A, Henriksson C E. The influence of rivaroxaban on markers of fibrinolysis and endothelial cell activation/injury in patients with venous thrombosis. Thromb Res. 2019;177:154–156. doi: 10.1016/j.thromres.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 30.Navarro-Oviedo M, Muñoz-Arrondo R, Zandio B. Circulating TIMP-1 is associated with hematoma volume in patients with spontaneous intracranial hemorrhage. Sci Rep. 2020;10(01):10329. doi: 10.1038/s41598-020-67250-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammadi K, Yaribash S, Sani M A, Talasaz A H. Efficacy and safety of the fixed-dose versus variable-dose of 4-PCC for vitamin K antagonist reversal: a comprehensive systematic review and meta-analysis. Cardiovasc Drugs Ther. 2021 doi: 10.1007/s10557-021-07192-0. [DOI] [PubMed] [Google Scholar]
- 32.Christensen H, Cordonnier C, Kõrv J. European Stroke Organisation guideline on reversal of oral anticoagulants in acute intracerebral haemorrhage. Eur Stroke J. 2019;4(04):294–306. doi: 10.1177/2396987319849763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Illanes S, Zhou W, Schwarting S, Heiland S, Veltkamp R. Comparative effectiveness of hemostatic therapy in experimental warfarin-associated intracerebral hemorrhage. Stroke. 2011;42(01):191–195. doi: 10.1161/STROKEAHA.110.593541. [DOI] [PubMed] [Google Scholar]
- 34.Yasaka M, Brainsky A, Toyoda K. Prothrombin complex concentrate for vitamin K antagonist-associated intracranial hemorrhage: global evidence and the Japanese perspective. Circ J. 2017;81(11):1564–1573. doi: 10.1253/circj.CJ-17-0428. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka K A, Mazzeffi M, Durila M. Role of prothrombin complex concentrate in perioperative coagulation therapy. J Intensive Care. 2014;2(01):60. doi: 10.1186/s40560-014-0060-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grottke O, Braunschweig T, Spronk H MH. Increasing concentrations of prothrombin complex concentrate induce disseminated intravascular coagulation in a pig model of coagulopathy with blunt liver injury. Blood. 2011;118(07):1943–1951. doi: 10.1182/blood-2011-03-343046. [DOI] [PubMed] [Google Scholar]
- 37.Kuramatsu J B, Sembill J A, Huttner H B. Reversal of oral anticoagulation in patients with acute intracerebral hemorrhage. Crit Care. 2019;23(01):206. doi: 10.1186/s13054-019-2492-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neurocritical Care Society (NCS) Pharmacy Study Group . Panos N G, Cook A M, John S, Jones G M. Factor Xa inhibitor-related intracranial hemorrhage: results from a multicenter, observational cohort receiving prothrombin complex concentrates. Circulation. 2020;141(21):1681–1689. doi: 10.1161/CIRCULATIONAHA.120.045769. [DOI] [PubMed] [Google Scholar]
- 39.Zhou W, Zorn M, Nawroth P. Hemostatic therapy in experimental intracerebral hemorrhage associated with rivaroxaban. Stroke. 2013;44(03):771–778. doi: 10.1161/STROKEAHA.112.675231. [DOI] [PubMed] [Google Scholar]
- 40.Shaw J R, Siegal D M. Pharmacological reversal of the direct oral anticoagulants-a comprehensive review of the literature. Res Pract Thromb Haemost. 2018;2(02):251–265. doi: 10.1002/rth2.12089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Álvarez E, Paradela-Dobarro B, Raposeiras-Roubín S, González-Juanatey J R. Protective, repairing and fibrinolytic effects of rivaroxaban on vascular endothelium. Br J Clin Pharmacol. 2018;84(02):280–291. doi: 10.1111/bcp.13440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kang S, Kishimoto T. Interplay between interleukin-6 signaling and the vascular endothelium in cytokine storms. Exp Mol Med. 2021;53(07):1116–1123. doi: 10.1038/s12276-021-00649-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carter R LR, Talbot K, Hur W S. Rivaroxaban and apixaban induce clotting factor Xa fibrinolytic activity. J Thromb Haemost. 2018;16(11):2276–2288. doi: 10.1111/jth.14281. [DOI] [PubMed] [Google Scholar]
- 44.Tai S H, Chen H Y, Lee E J. Melatonin inhibits postischemic matrix metalloproteinase-9 (MMP-9) activation via dual modulation of plasminogen/plasmin system and endogenous MMP inhibitor in mice subjected to transient focal cerebral ischemia. J Pineal Res. 2010;49(04):332–341. doi: 10.1111/j.1600-079X.2010.00797.x. [DOI] [PubMed] [Google Scholar]
- 45.Quemener C, Gabison E E, Naïmi B. Extracellular matrix metalloproteinase inducer up-regulates the urokinase-type plasminogen activator system promoting tumor cell invasion. Cancer Res. 2007;67(01):9–15. doi: 10.1158/0008-5472.CAN-06-2448. [DOI] [PubMed] [Google Scholar]
- 46.Xue M, Hollenberg M D, Yong V W. Combination of thrombin and matrix metalloproteinase-9 exacerbates neurotoxicity in cell culture and intracerebral hemorrhage in mice. J Neurosci. 2006;26(40):10281–10291. doi: 10.1523/JNEUROSCI.2806-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wells J EA, Biernaskie J, Szymanska A, Larsen P H, Yong V W, Corbett D. Matrix metalloproteinase (MMP)-12 expression has a negative impact on sensorimotor function following intracerebral haemorrhage in mice. Eur J Neurosci. 2005;21(01):187–196. doi: 10.1111/j.1460-9568.2004.03829.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


