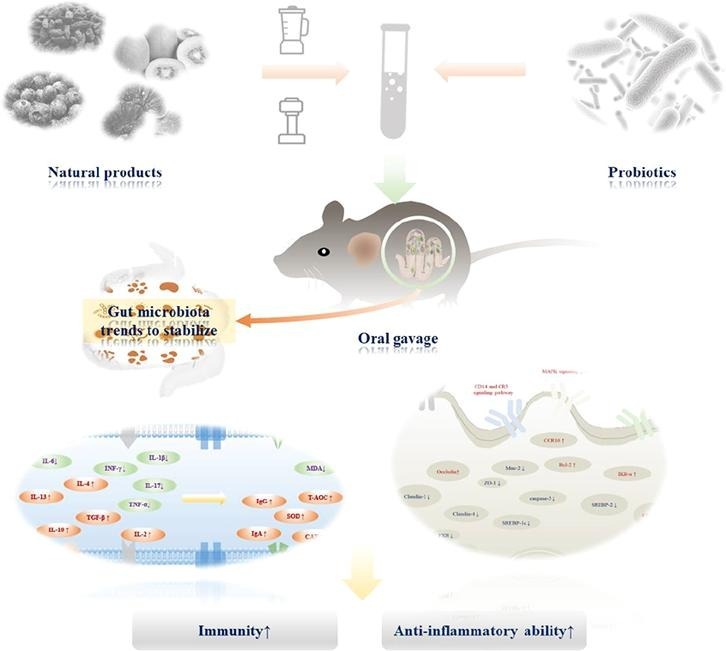
Graphical abstract
Keywords: Probiotics, Natural products, Active substances, Gut microbiota, Immunity, Anti-inflammatory activity
Abstract
Low immune function makes the body vulnerable to being invaded by external bacteria or viruses, causing influenza and inflammation of various organs, and this trend is shifting to the young and middle-aged group. It has been pointed out that natural products fermented by probiotic have benign changes about their active ingredients in some studies, and it have shown strong nutritional value in anti-oxidation, anti-aging, regulating lipid metabolism, anti-inflammatory and improving immunity. In recent years, the gut microbiota plays a key role and has been extensively studied in improving immunity and anti-inflammation activity. By linking the relationship between natural products fermented by probiotic, gut microbiota, immunity, and inflammation, this review presents the modulating effects of probiotics and their fermented natural products on the body, including immunity-enhancing and anti-inflammatory activities by modulating gut microbiota, and it is discussed that the current understanding of its molecular mechanisms. It may become a possible way to prevent COVID-19 through consuming natural products fermented by probiotic in our daily diet.
1. Introduction
The immune system is an important defense mechanism in the human body, which plays an extremely crucial role in preventing infectious diseases, inflammation, virus, cancer, cardiovascular and cerebrovascular diseases, et al. because it can identify and eliminate foreign invasion (Ahmed et al., 2022, Tischer et al., 2022). Before the 21st century, the weakening of immune system function and related symptoms have only been seen in immature children, the elderly, and people with related genetic defects. But now, this phenomenon is moving among the young and middle-aged population with the increasing pressure of people's life and work, bad living habits and lack of attention to health, it means that improving immunity is not only beneficial to enhance personal health, but also plays a positive role in promoting social progress and economic development. Patients with weakening of immune system function usually had symptoms such as weak constitution, easy to catch cold, malnutrition, and poor mental status caused by external virus infection (Schultze et al., 2021).
It is well known that most plants and animals in nature have nutritional value, which means that many active substances can be obtained from them (Mehany et al., 2021). Studies have found that it had a positive effect on the promotion of immunity through appropriate supplementation of active substances such as polyphenols, polysaccharides, polypeptides and vitamins, et al. in addition to strengthening exercise and forming good living habits (Liao et al., 2019, Sun et al., 2021). As an active microorganism that was mainly colonized in the human intestine and beneficial to the host, probiotics had positive effects in maintaining a benign balance of gut microbiota, promoting the absorption of active substances, and improving human physiological functions (Lof et al., 2022, Gu et al., 2022). The gut was not only an important digestive organ of the human body to digest food and provide energy for the human body (Guarner and Malagelada, 2003). At the same time, some studies have pointed out that there existed a large number of gut microbiota represented by Bacteroidetes and Firmicutes in the gut, and their benign homeostasis could improve body functions through fermenting active substances such as polysaccharides and dietary fibers that were difficult for the human body to absorb to regulate various signaling pathways (Xie et al., 2022, Xie et al., 2022, Fang et al., 2022). In the study of immune dysfunction caused by treating mice with cyclophosphamide, it was found that gavage polysaccharide could improve the gut microbiota of mice by up-regulating the relative abundance of Muribaculaceae, reducing the relative abundance of Lachnospiraceae, Helicobacteraceae, et al., then it could improve the content of metabolites represented by short-chain fatty acids to prevent infection of external viruses and improve the immunity of the body (Bai et al., 2022). In recent years, in the study of various biological models, researchers have found that the active substances from natural products fermented by probiotics reflected stronger functional activities in antiviral, anti-fatigue, anti-aging, anti-inflammatory, improving lipid metabolism and body immunity compared with the direct extraction of active substances from natural products (Jiao et al., 2018, Ofosu et al., 2022).
This review summarized the researches on natural products fermented by probiotics to improve immunity and antiviral activity with gut microbiota as the target through analyzing the interaction between natural products fermented by probiotics, gut microbiota, immune function and antiviral activity. The regulated signaling pathways and possible mechanisms were further sorted out and analyzed. It provided new ideas for the determination and in-depth exploration of the specific mechanism of natural products fermented by probiotics to improve gut microbiota, improve body immunity and antiviral activity, as well as the development of functional foods in the future, and may become a new means of preventing COVID-19 in addition to vaccines and drugs.
2. Research on active substances fermented by probiotics
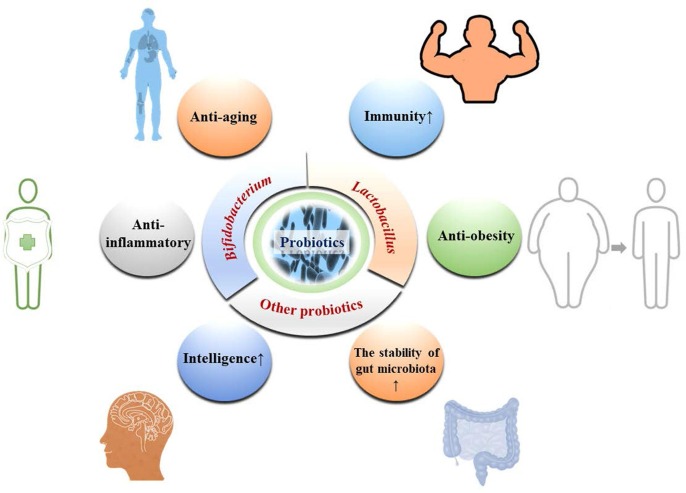
2.1. Probiotics
There existed a large number of microorganisms in the gut environment of the human body which were divided into three categories: probiotics, curative bacteria and opportunistic pathogenic bacteria according to whether they were beneficial to the human body (Rosen et al., 2017). Probiotics were defined as the ability of microorganisms to have a beneficial effect on host health when supplemented with a certain number of live microorganisms. It was determined by experts in the Food and Agriculture Organization of the United Nations and the World Health Organization in 2001 (Pineiro et al., 2007). This concept was developed primarily for strains whose members are Lactobacillus and Bifidobacterium available as probiotics in food (Pineiro et al., 2007). Obviously, the probiotics produced and applied in the food, biological, pharmaceutical and other industrial fields were rarely compared with the probiotics in gut of human (Fig. 1 ). On the one hand, most of the gut microbiota survived in anaerobic environment, which was not conducive to growth and reproduction in an aerobic environment (Margolles et al., 2021). On the other hand, different microorganisms might need to grow and reproduce in clusters and symbiotically, its activity could not be guaranteed when a single microorganism was isolated by modern science and technology, which was the main reason (Castellanos et al., 2020, Geva-Zatorsky et al., 2017). Probiotics have shown significant effects in promoting the digestion and absorption of nutrients, maintaining the homeostasis of gut microbiota, antivirus, inhibiting inflammation, and improving the body's antioxidant and immune capabilities, especially in people with low immunity, constipation, and people who were prone to inflammation (Manzoor et al., 2022, Marx et al., 2020, Su et al., 2020, Zepeda-Hernández et al., 2021). At the same time, the application of probiotics in fishery and breeding industry was also widely used to improve the reproductive ability and immunity of animals (Golder et al., 2022, Rohani et al., 2022). Adding probiotics based on Bacillus subtilis B10 to the feed, the blood urea nitrogen, IL-1β and serum alkalinity, phosphatase, lactate dehydrogenase and antioxidant enzyme activities of Pelodiscus sinensis were improved by up-regulating the expression of intestinal tight junction proteins and liver genes such as TLR8 and TLR5 (Xu et al., 2022). Colonization of Bacteroides ovatus in the mouse gut could produce acetate, propionic acid, butyric acid and other short-chain fatty acid metabolites compared with sterile controls, and generated glutamine by consuming tryptophan and glutamic acid, further improving the homeostasis of gut microbiota and increasing the concentration of neurotransmitters in the gut, which played a positive role in improving the immune activity of the body. (Horvath et al., 2022). In summary, probiotics had a very significant effect on the immune enhancement of the body, and have shown a strong application value in fishery and animal husbandry. However, the current research on probiotics and their improvement of body conditions was still insufficient. First, there are few probiotics used in human health research, although many types have been found. Secondly, there will also be a relationship of competition and mutual benefit between bacteria. Some studies have pointed out that mixing different types of probiotic strains has a better effect on the body than a single type of probiotics. Third, the in-depth mechanism research on the regulation of body immunity by probiotics still remains in the exploration of gene changes and protein expression, and there is no report on the deeper regulation mechanism. Therefore, it becomes the focus of future work to continuously discover and isolate new probiotic strains, and to explore and elucidate the effects of single type of probiotic and a mixture of different types of probiotics on the body.
Fig. 1.
A brief introduction to the main categories and nutritional activities of probiotics.
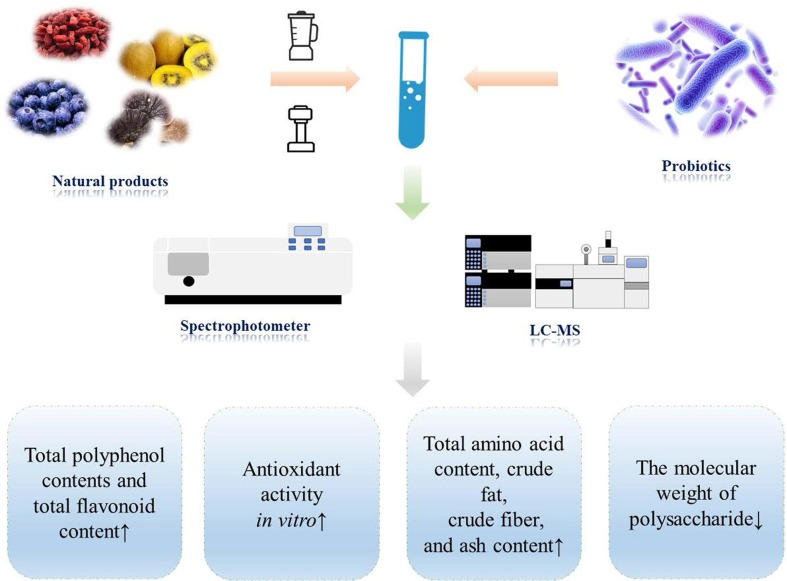
2.2. Active substances fermented by probiotics
Active substances extracted from natural products have shown significant biological activity in improving gut microbiota and regulating immunity (Chen et al., 2021, Annunziata et al., 2021) (Fig. 2 ) (Table 1 ). Polysaccharides were not easily digested in the human body, and were usually fermented in the gut to produce metabolites represented by short-chain fatty acids. At the same time, polysaccharides could promote the proliferation of probiotics in the intestinal tract, inhibited the growth of harmful bacteria, and regulated the homeostasis of the intestinal tract, which means that the gut microbiota and polysaccharides can interact to improve the immunity of the body, and jointly promote the healthy and sustainable development of the body (Chen et al., 2022). It was proposed that the nutritional properties of natural products fermented by probiotics played a better role than single natural products based on these researches. In the study of carrot polysaccharide fermented by L. plantarum NCU 116, it was found that the functional activity of carrot polysaccharide was enhanced after being fermented (Wan et al., 2021). Further exploration found that the molecular weight of carrot polysaccharide decreased after fermentation, and the links between repeating units were avoided, reducing the polydisperse size distribution (Wan et al., 2021). Qin et al. (2021) studied the polysaccharide composition and activity in vitro of Liubao tea before and after fermentation, it was found that the content of crude polysaccharide and each monosaccharide in Liubao tea increased after fermentation, the molecular weight decreased, and the anti-coagulation and binding ability with bile acids were significant increased, which means that the content of active substances in natural products increases after fermentation and is more easily absorbed and utilized by the human body (Qin et al., 2021). When mice were given bitter gourd juice fermented by L. plantarum NCU116, it was found that the concentration of short-chain fatty acids in mice increased, and the pH of colon contents decreased compared with the unfermented group, which means that the effect of bitter gourd juice fermented with probiotics is more significant in regulating body metabolism, improving immunity and antivirus activity (Gao et al., 2019). In conclusion, natural products fermented by probiotics had a significantly higher proportion of active substances extracted. The active substances were easier to be digested and absorbed by the body and played a positive regulating role compared with unfermented substances. However, there were still parts that need to be improved and studied in depth. For example, most of studies used a single species of bacteria in the research on natural products fermented by probiotic. At present, there were still few researches on the basic components, in vitro and in vivo nutrition of multi-bacteria composite fermentation active substances. At the same time, in the study of compound probiotic fermentation active substances, it was clearly expressed that there was a significant difference in biological activity compared with single bacterial species fermented and unfermented active substances, but the reasons and mechanisms for the synergistic effect of fermentation had not been thoroughly explored. It might become the main direction of the next research for researchers.
Fig. 2.
Changes in active substances obtained from natural products fermented by probiotic.
Table 1.
Changes in active substances before and after natural products fermented by probiotic.
| Natural products | Probiotic name or category | Changes in active substances | References |
|---|---|---|---|
| Grapefruit | Mixed lactic acid bacteria | Naringin, diosmin, gallic acid↑; Ferulic acid, vanillic acid↓ | (Tang et al., 2022) |
| Kumquat | Mixed lactic acid bacteria | Gallic acid↑; Ferulic acid, vanillic acid↓ | (Tang et al., 2022) |
| Navel orange | Mixed lactic acid bacteria | Diosmin, gallic acid↑; Hesperidin and ferulic acid, vanillic acid, neohesperidin↓ | (Tang et al., 2022) |
| Dioscorea opposita Thunb. | Saccharomyces boulardi | The molecular weight of polysaccharide↓ | (Shao et al., 2022) |
| Ipomoea batatas (L.) Lam | Saccharomyces boulardii | Total amino acid content, crude fat, crude fiber, ADF, NDF, and ash content↑ | (Campbell et al., 2017) |
| Red bayberry | Lactic acid bacteria, Acetic bacteria and Saccharomyces cerevisiae | Total polyphenol contents and total flavonoid content↑; Total anthocyanins content↓ | (Zhu et al., 2020) |
| Blueberry pomace |
L. rhamnosus GG and L. plantarum-1 |
Lactic acid, total phenols and flavonoids↑; Citric acid↓ | (Yan et al., 2019) |
| Brassica campestris L. | Active dry yeast | Riboflavin, nicotinic acid, nicotinamide, free amino acids, phenolic compounds, oligopeptides and fatty acids↑; Fructose, glucose↓ | (Yan et al., 2019) |
| Coffee | Lactobacillus spp. | Major volatiles, most active ingredients and antioxidant capacity were preserved | (Chan et al., 2020) |
| Mango |
Lactobacillus bulgaricus S1, Streptococcus thermophilus 6063, Lactobacillus plantarum Lp-115 |
solubledietary fiber, carotenoids, total phenols, and ascorbic acid↑ | (Wang et al., 2021) |
| Ganoderma lucidum | Lactobacillus acidophilus and Bifidobacterium breve | The main components of Ganoderma lucidum such as Ganoderma acid A have undergone structural changes | (Li et al., 2021) |
| Dendrobium officinal | Bacillus sp. DU-106 | Monosaccharide molecular weight, mannose↑ | (Tian et al., 2019) |
| Whole-grain lupin, quinoa and wheat |
Bifidobacterium animalis subsp. lactis DSM10140, B. longum subsp. longum DSM20097 and B. breve DSM20213 |
Total phenols↑ | (Ayyash et al., 2018) |
| Dimocarpus longan Lour. | Lactobacillus fermentum | Reducing sugar↑; The molecular weight↓ | (Huang et al., 2020) |
| Purple potato flour | Lactobacillus plantarum CGMCC 14177, L. plantarum CGMCC 15358 | Protein, ash, resistant starch and the first limiting amino acid↑; Total starch↓ | (Gong et al., 2022) |
| Anacardium occidentale |
Lactobacillus delbrueckii, Lactobacillus jhonsoni, Lactobacillus rhamnosus and Bifidobacterium longum |
The bio-accessibility index of total phenolics, flavonoids, antioxidant capacity ORAC↑ | (Santana et al., 2022) |
| Actinidia deliciosa |
Lactobacillus acidophilus 85, Lactobacillus helveticus 76 and Lactobacillus plantarum 90 |
Total phenolics and flavonoids↑ | (Wang et al., 2022) |
| Blueberry and blackberry juice | Lactobacillus plantarum and Streptococcus thermophilus | Syringic acid, ferulic acid, gallic acid and lactic acid↑; P-coumaric acid, protocatechuic acid, chlorogenic acid, critic acid and malic acid, cyannindin-3-glucoside and peonidin-3-glucoside↓ | (Wu et al., 2021, Wu et al., 2021) |
| Blueberry | Autochthonous lactic acid bacteria | Total phenolics, rutin, myricetin and gallic acid↑; Anthocyanin, paraben and caffeic acid↓ | (Li et al., 2021) |
| Mulberry | L. plantarum Lp-115 (ATCC SD5209), L. acidophilus La-14 (ATCC SD5212) and L. paracasei Lpc-37(ATCC SD5275) | Total anthocyanins, phenols and flavonoids↑ | (Kwaw et al., 2018) |
3. The relationship between fermented natural products, gut microbiota, immunity and anti-inflammatory activity
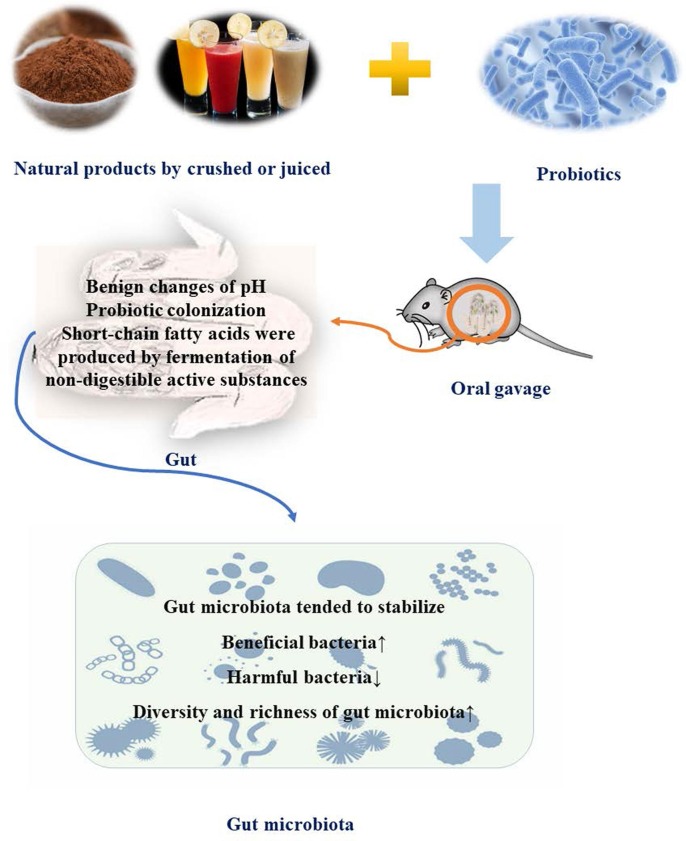
3.1. The relationship between fermented natural products and gut microbiota
Inflammation, the imbalance of gut microbiota, and various infectious diseases were more susceptible to illness in patients with immunocompromised, it means that improving the body's immunity plays a crucial role in resisting various pathogenic bacteria, as well as anti-inflammatory (Agarwala et al., 2022; Al-Rashidi, 2022, Greer et al., 2013, Kiely et al., 2022). Some studies have clarified that the gut was an important place for probiotic fermentation active substances to improve body functions, and the gut microbiota was an important target for its role (Chaikham and Rattanasena, 2017, Ma et al., 2021, Ma et al., 2021). The modulating effect of low-fat ice cream supplemented with Lactobacillus casei 01 and Lactobacillus acidophilus LA5 on colonic microbiota was investigated in a gut model in vitro and found that the content of Lactobacillus and Bifidobacterium increased in the colon while other harmful microorganisms such as Clostridium and Escherichia coli gradually decreased and produced beneficial microbial metabolites, while toxic ammonia levels decreased significantly in the study of Chaikham et al. (2017). Adding complex probiotics with Lactobacillus acidophilus, Enterococcus faecium and Bifidobacterium bifidium and dietary fiber to the feed significantly improved the lipase activity in the feed, the activity of lipase in the feed was significantly improved, and the levels of amylase, protease and alkaline phosphatase also increased significantly. At the same time, it was found that the levels of total autochthonous gut microbiota were significantly increased in vivo experimental study of fish, and skin mucous immune parameters were also significantly improved compared with the control group (Mirghaed et al., 2018). In the study of collagen peptide-jackfruit mixture fermented by lactic acid bacteria, Ma et al. found that the in vitro antioxidant capacity and lactic acid content of the fermented products were significantly increased. The fermentation products were found to significantly increase the immune organ index, reduce colon tissue damage, and stimulate the secretion of cytokines and immunoglobulins in immunosuppressed mice through reducing the relative abundance of pathogenic bacteria, increasing the relative abundance of beneficial bacteria, improving the composition of gut microbiota and increasing the content of short-chain fatty acids (Ma et al., 2021). Ginseng polysaccharide fermented by Lactobacillus has strong ameliorating effect on gut microbiota and immunity, diarrhea symptoms and gut inflammation in rats with antibiotic-associated diarrhea (Qu et al., 2021).
In conclusion, the active substances have improved antioxidant and other activities in vitro after probiotic fermentation. In the research of experiments in vivo on animal model by constructing various immune dysfunctional such as mice, fish, and rabbits, it was found that their immunity and related anti-inflammatory, and antiviral activities were enhanced after the natural products fermented by probiotics. Meanwhile, it was found that the stable gut microbiota of the biological model after treatment trended to be normal, the beneficial bacteria proliferated steadily, the pathogenic bacteria gradually decreased, and the intestinal environment was close to normal immune individuals of its gut microbiota. On the contrary, although various diseases were closely related to the decline of immune function, the different drugs and methods used in establishing animal models lead to different changes about gut microbiota after treatment with fermented products. The description of boosting immunity and improving related symptoms in those researches was not clear, which means that it is inconvenient to clarify that the gut microbiota is the target of fermented immunity to improve immunity.
3.2. The relationship between gut microbiota, immunity and anti-inflammatory activity
The human gut was parasitized by microorganisms from birth until death, and the gut microbiota would change with the growth environment, diet and living habits of people, it means that the health of the body will also be related to changes with gut microbiota (Ardissone et al., 2014). The gut environment was worse in people with diseases caused by a weakened immune system compared with normal people, it was mainly manifested in the lower proportion of beneficial bacteria and higher proportion of pathogenic bacteria in the gut tract of the diseased population. The diversity and stability of bacteria gut microbiota are poor although everyone in the world has a different gut microbiota (Al-Rashidi et al., 2022). Inflammatory bowel disease is a common disease in people with weakened immune systems. Compared with healthy people, people with ulcerative colitis showed greater changes in gut microbiota, mainly manifested as decreased abundance of Eubacterium rectale and Akkermansia, and the diversity of gut microbiota was decreased (Pittayanon et al., 2020). Hepatitis and cirrhosis as one of the common diseases of immunocompromised patients, it was found that alpha diversity indices including Simpson, Chao1, ACE and Shannon were increased in patients with compensated cirrhosis compared with patients with decompensated cirrhosis through the technology of 16S rRNA sequencing. At the same time, the proportion of beneficial bacteria including Bifidobacterium and Lactobacillus decreased, while the proportion of harmful bacteria such as Enterobacter increased in patients with liver cirrhosis through comparing patients with liver cirrhosis and normal population. And the abundance of intestinal harmful Streptococcus and Ruminococcus in patients with decompensated cirrhosis was higher than that in patients with compensated cirrhosis, meaning that the gut microbiota is severely damaged in patients with decompensated cirrhosis (Shu et al., 2022). In fact, the body's immune-related cancers are closely linked to the gut microbiota (Chen et al., 2021; Gori et al., 2019, Zhou et al., 2021). In the study of mice with colitis integrated tumor treated of AOM/DSS-induced, it was found that compared with the normal group, the diversity of intestinal flora was significantly reduced, and the structure of the gut was significantly changed. Meanwhile, the abundance of Bacteroidetes increased and the abundance of Firmicutes decreased at the phylum level. The relative abundance of Lactobacillus and Bifidobacterium was significantly lower than that of the normal group, while the relative abundance of Oscillibacter, Desulfovibrio, Alistipes, Lachnoclostridium, and Parasutterella increased (Guo et al., 2021).
The variation of gut microbiota for different diseases was mostly the ratio of Firmicutes and Bacteroidetes at the phylum level in the description of the correlation between gut microbiota and immunity, and richness and diversity of gut microbiota decreased. The changes of the same disease at the genus level were different in different studies, which might be due to the differences in the genetic of people in each region, food culture, living habits and other external factors, it means that we need to find out the specific bacteria that coexist between the same disease and the different bacteria between different diseases in different regions and populations, in order to explore, judge and treat related diseases in the later stage by improving the gut microbiota. And this may become the focus of future research in this aspect.
4. The possible mechanism of probiotic fermented natural products to improve immunity and anti-inflammatory by targeting gut microbiota
4.1. A brief introduction to the pathogenesis of immune dysfunction and inflammation
Inflammation response in the body was caused by factors such as genes, bad living habits, and external environment, which acted on the malignant changes of gut microbiota (Ye et al., 2021). This process involved the damage of the intestinal immune barrier, the activation of inflammatory factors, the weakening of the body's ability to recognize and eliminate external viruses and bacteria, and changed in a series of signaling factors and enzymes (Ge et al., 2021, Shamoon et al., 2019). The external environment caused the body's gut microbiota imbalance, which further triggered the damage of the intestinal immune barrier and triggered the expression of inflammatory factors and the malignant regulation of genes. It increased the level of inflammation in the body and the expression of enzymes was unbalanced, and finally affected various organs of the body, causing inflammation of organs such as colitis and pneumonia. Meanwhile, it was more susceptible to infectious diseases because of the degradation of the immune barrier, and the ability to resist external bacterial and viral aggression was weakened (Praveen et al., 2019, Tan and Nie, 2020, Zhu et al., 2021). It was detailed on the possible mechanisms of inflammation, changes in the gut microbiota, the intestinal immune barrier, and changes in specific signaling pathways and their factors in this section. The possible mechanism of natural products fermented by probiotic to modulate immune and anti-inflammatory functions by improving gut microbiota had also been speculated and elucidated.
4.2. Possible mechanism by which natural products fermented by probiotics improve gut microbiota
The mechanism of natural products fermented by probiotic to improve gut microbiota could be roughly analyzed from two aspects: the regulating effect of probiotics on gut microbiota and the regulating effect of fermentation products on gut microbiota. Natural products fermented by probiotics had lower molecular weight of active substances and were more easily absorbed by the body compared with direct intake of active substances, it means that the bioavailability of fermented active substances is greatly improved (Hu et al., 2022). Probiotics inhibited the reproduction of harmful bacteria, reduced the proportion of harmful bacteria, and promoted the healthy and stable development of gut microbiota when a large number of probiotics entered the intestinal tract, because some probiotics competed with harmful bacteria (Coyte and Rakoff-Nahoum, 2019, Müller et al., 2019, Patnode et al., 2019) (Fig. 3 ) (Table 2 ). When the active substances entered the intestinal tract, part of the digestible active substances were directly absorbed by the body organs, and the other part of the indigestible active substances were fermented by the beneficial bacteria of gut microbiota (Li et al., 2022a, Li et al., 2022b, Osbelt et al., 2021). On the one hand, pH of the intestinal environment changed during the fermentation process, which would be adjusted in the direction of suitable reproduction of beneficial bacteria, resulting in the death of harmful bacteria due to changes in the breeding environment. On the other hand, metabolites represented by short-chain fatty acids were produced in large quantities, which also inhibited the reproduction of harmful bacteria (Li et al., 2022c, Ma et al., 2021, Wei et al., 2022). In general, the active substances of probiotics fermented natural products were more effective in regulating gut microbiota than simply ingesting active substances through improving the pH value of the intestinal environment and producing metabolites, it inhibited the reproduction of harmful bacteria and proliferated beneficial bacteria.
Fig. 3.
Modulation of gut microbiota with active substances extracted from natural products fermented by probiotic.
Table 2.
Gut microbiota improvement after ingestion of probiotic-fermented natural products.
| Natural products | Active substances | Probiotic name or category | In vivo or in vitro | Model | Changes in gut microbiota | References |
|---|---|---|---|---|---|---|
| Ganoderma lucidum | Water extracts | Lactobacillus acidophilus and Bifidobacterium | In vivo | Mice with dexamethasone-induced immunosuppressed | The relative abundance of Lactobaccilus ↑; The relative abundance of Enterococcus↓ Lachnospiraceae_bacterium_DW17, Dorea_sp_5–2, rumen_bacterium_NK4A214 and Lachnospiraceae_bacterium_DW52 ↑ |
(Li et al., 2021) |
| Goji berry juice | polysaccharides, amino acids, phenolics and protein |
Lactobacillus plantarum, Lactobacillus reuteri and Streptococcus thermophilus |
In vivo | Mice with DSS-induced ulcerative colitis | The relative abundance of Bacteroidetes↑; The relative abundance of Firmicutes↓; The relative abundance of Muribaculaceae-unclassified↑; The relative abundance of Lachnospiraceae-NK4A136-group↓ | (Liu et al., 2021) |
| Fagopyrum esculentum | Gamma-aminobutyric acid, rutin, total polyphenols and total flavonoids | Bacillus sp. DU-106 and Lactobacillus plantarum | In vivo | Mice with high-fat diet-induced hyperlipidemia | The relative abundance of Bacteroides, Lactobacillus, and Blautia ↑; The ratio of Firmicutes to Bacteroidetes↓; Gut microbiota approached the normal group | (Yan et al., 2022) |
| Anethum graveolens essential oil | – | Lactobacillus acidophilus | In vitro | – | Escherichia coli O157↓ | (Mojaddar et al., 2021) |
| Black tartary buckwheat | Tyrosine, lysine, total flavonoids, total polyphenols, quercetin, and kaempferol | Bacillus sp. DU-106 | In vivo | Mice with high-fat diet-induced hyperlipidemia | The relative abundance of Lactobacillus, Faecalibaculum, and Allobaculum↑; The relative abundance of Romboutsia↓ | (Ren et al., 2021) |
| Apium graveolens L. | Total polyphenols, flavonoids, vitamin C | Mixed probiotics | In vivo | Mice with high-fat diet-induced hyperlipidemia | The ratio of Firmicutes/Bacteroidetes and the relative abundance of Lactobacillus, Ruminococcaceae_UCG-014, Faecalibaculum and Blautia↑; the relative abundance of Alloprevotella and Helicobacter↓ | (Zhao et al., 2021) |
| Blueberry pomace | Polyphenols | Lactobacillus casei | In vitro | – | Escherichia coli, Enterococcus Firmicutes and Bacteroidetes↓; Bifidobacterium, Ruminococcus, Lactobacillus, Akkermansia and butyrate-producing bacteria↑ | (Cheng et al., 2020) |
| Raspberry | Polyphenols | Lactobacillus casei | In vivo | Normal mice | the Firmicutes to Bacteroidetes ratio↓; Verrucomicrobia↑; the relative abundance of Blautia, Ruminiclostridium_9↓; the relative abundance of Lactobacillus↑ | (Wu et al., 2021) |
| Mulberry pomace | Phenolic compounds and dietary fibers | Lactobacillus plantarum | In vitro | – | Lactobacillus, Bifidobacterium, Ruminococcus, butyrateproducing bacteria and Akkermansia↑; Escherichia coli and Enterococcus↓ | (Tang et al., 2021) |
| Blueberry pomace | – | Lactobacillus casei | In vivo | Mice with high-fat diet-induced hyperlipidemia | The Firmicutes to Bacteroidetes ratio↓; Bifidobacterium, Lactobacillus and Akkermansia↑ | (Cheng et al., 2020) |
4.3. Possible mechanisms by which the gut microbiota modulates immune and anti-inflammatory activity
4.3.1. Gut microbiota improves immunity and exerts anti-inflammatory function by maintaining intestinal immune barrier function
The proliferation of probiotics about gut microbiota played an important role in enhancing immunity and improving inflammatory response. The proliferation of Lactobacillus of gut microbiota could maintain the barrier function of intestinal epithelial cells and enhance the protective immune response (Yan and Polk, 2020). At the same time, Lactobacillus rhamnosus GG of gut microbiota could derive the soluble protein p40. On the one hand, it could release the epidermal growth factor receptor in intestinal epithelial cells by stimulating the activity of a disintegrin and metalloproteinase 17 (Yan et al., 2013). On the other hand, p40 could induce ligand production by upregulating the proliferation of epidermal growth factor in intestinal epithelial cells, which would increase the production of IgA in the gut (Wang et al., 2017). The IgA in intestinal mucus interacts with immunogenic substances to prevent it from adhering to intestinal mucosal cells. In addition, IgA lost its adhesion ability by agglutinating external invading bacteria or blocking its flagella, it means that Lactobacillus rhamnosus GG of gut microbiota plays an important role in inhibiting cytokine-induced apoptosis and improving the protective function of the intestinal mucosal barrier and anti-inflammatory (Nagafusa and Sayama, 2020, Zhang and Zhang, 2018). In addition, short-chain fatty acids, including acetic acid and its salts, propionic acid and its salts, et al. played an important role in improving the immune system of the intestinal mucosa. They were metabolites produced by beneficial bacteria of gut microbiota fermenting active substances that were difficult to digest by the human body (Akhtar et al., 2021, Ding et al., 2018). As an important factor of immune tolerance, regulatory T cells played a key role in the regulation of immunity and anti-inflammation of short-chain fatty acids (Dupraz et al., 2021). It was found that native CD4+ T cells exposed to peripheral TGF-β cytokines produced IL-10-producing inducible regulatory T cells that were eventually converted into regulatory T cells through feeding mice with butyrate supplementation feed (Richards et al., 2016). However, short-chain fatty acids could be produced by the fermentation of beneficial bacteria of gut microbiota, which means that the homeostasis of gut microbiota can improve the immune mucosa of the intestinal epithelium by productizing the short-chain fatty acids to regulate regulatory T cells (Richards et al., 2016).
In conclusion, gut microbiota improved immunity and anti-inflammatory by maintaining intestinal immune barrier function and improving intestinal epithelial tissue cells. On the one hand, beneficial bacteria dissolved soluble protein p40 to release epidermal growth factor receptor 17 and inhibited cytokine-induced apoptosis. On the other hand, the short-chain fatty acids produced by beneficial bacteria fermenting the indigestible active substances could improve the intestinal epithelial mucosa by regulating regulatory T cells, and finally achieved the improvement of immunity and anti-inflammatory activity. Of course, the mechanism of gut microbiota improving immunity and anti-inflammatory by maintaining intestinal immune barrier function was not only the above expression because its mechanism was very complex and huge, which means that clarifying its mechanism of action has become the focus of future in-depth research.
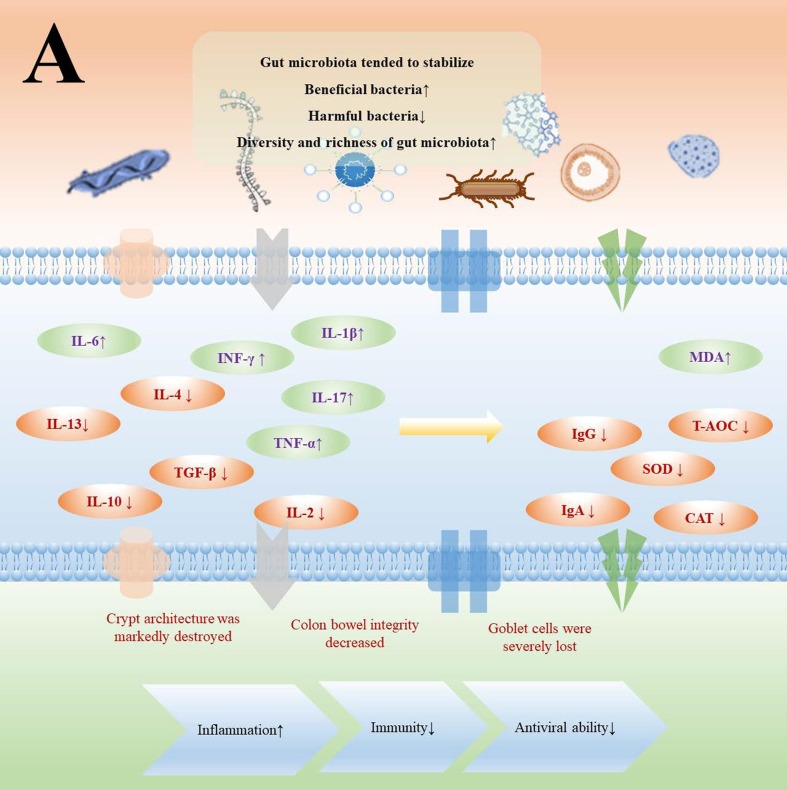
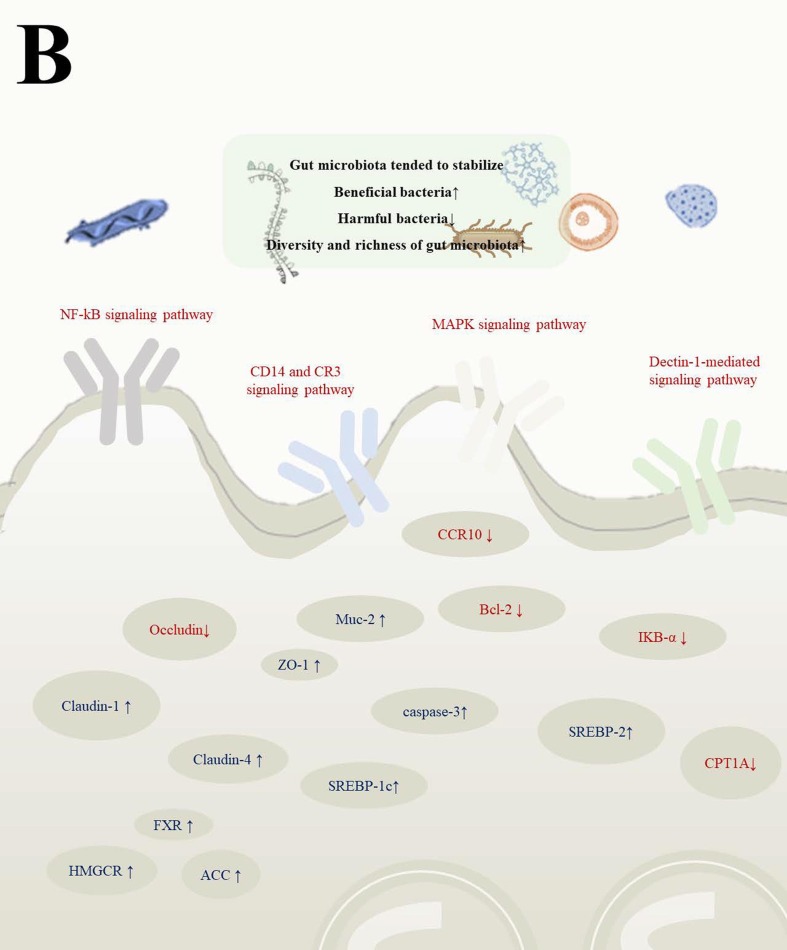
4.3.2. Gut microbiota regulates signaling pathway and its factors to improve immunity and anti-inflammatory
IL-6, TNF and IL-1β were pro-inflammatory cytokines, while cell permeability proteins IL-4, IL-10, IL-13 and TGF were anti-inflammatory cytokines, which played an important role in regulating inflammatory responses (Liao et al., 2022) (Fig. 4 A). And the changes of these inflammatory factors were regulated by signaling pathways including: NF-kB signaling pathway and MAPK signaling pathway in TLR2/4 signaling pathway, Arachidonic acid dependent pathway, CD14 and CR3 signaling pathway, Dectin-1-mediated signaling pathway and others (Li et al., 2022c, Fitzgerald and Kagan, 2020) (Fig. 4B). The improvement of gut microbiota could inhibit the expression of inflammatory factors to improve immunity and anti-inflammatory activities by regulating gene expression (Aggeletopoulou et al., 2019) (Table 3 ). Different microorganisms had different regulatory mechanisms on the expression of various inflammatory factors, and this process was closely related to gene expression. Probiotic-fermented fish diet significantly reduced serum LPS levels, gut inflammation scores, and MDA levels in zebrafish through increasing the relative abundance of Firmicutes and Actinobacteria and reducing the relative abundance of Proteobacteria. At the same time, the gene expression of SVCV-2d was significantly decreased in the spleen, and the gene expression of IFNφ1, IFNφ2, IFNφ3 and MxC was significantly increased, which means that the improvement of gut microbiota can achieve antiviral and anti-inflammatory effects by regulating gene expression (Xie et al., 2022). Porphyromonadaceae, Helicobacter, Parasutterella, Parabacteroides, Oscillibacter and Lachnospiraceae of gut microbiota played key roles in the inflammatory response, and the gene expression of COX-2, MCP-1, NLPR3 was down-regulated, and ZO-1, FFAR2, FFAR3 gene expression was up-regulated along with benign changes of gut microbiota in mice. Finally, the expression of pro-inflammatory factors such as TNF-α, IL-1β, and IFN-γ was inhibited (Peng et al., 2019). Interestingly, the fermentation of carrageenan fructose could increase the proportion of Prevotella, Bifidobacterium, Lactobacillius and Prevotellaceae of gut microbiota, inhibited the reproduction of Bacteroides and Parabacteroides, it promoted secretion of IL-1β, TNF-α, SIgA and mucin 2, which means that there exists a part of the fermented active substances that have a negative regulatory effect on the body and promote the inflammatory response (Sun et al., 2019). In fact, gut microbiota modulated inflammatory factors, improved inflammation and immunity by regulating related signaling pathways and their factors (Xie et al., 2022, Peng et al., 2019). In general, the benign regulation of gut microbiota such as proliferation of beneficial bacteria, increased diversity and abundance of microbiota led to the down-regulation of pro-inflammatory cytokines such as IL-6, TNF and IL-1β and up-regulation of anti-inflammatory cytokines such as IL-4, IL-10, IL-13, TGF. This process was the effect of the regulation of related signaling pathways, such as insulin signaling pathway, MAPK signaling pathway. Finally, anti-inflammatory activity and improved immunity were manifested through benign improvements in the gut environment and cytokines. At the same time, a small number of active substances fermented by probiotics did not show good organism regulation, which means that it is still necessary to delve into the specific reasons (Sun et al., 2019).
Fig. 4.
Interrelation and mechanism of gut microbiota with inflammation and immunity (A: Imbalance of gut microbiota led to decreased immunity and antiviral ability by affecting the expression of inflammatory factors. B: Gene expression in various diseases caused by imbalance of gut microbiota in mice).
Table 3.
Immunity and inflammation changes with gut microbiota by regulating signaling factors.
| Model | Changes in gut microbiota | Changes in immunity and inflammation | Changes in signaling pathways and its factors | References |
|---|---|---|---|---|
| Mice with dexamethasone-induced immunosuppressed | The relative abundance of Lactobaccilus ↓; The relative abundance of Enterococcus↑ Lachnospiraceae_bacterium_DW17, Dorea_sp_5–2, rumen_bacterium_NK4A214 and Lachnospiraceae_bacterium_DW52 ↓ |
Colonic crypt architecture was markedly destroyed with more histological inflammation; Weight, spleen index, IL-17, TNF-α↑; Lipopolysaccharide↑ | Occludin↑ | (Li et al., 2021) |
| Mice with DSS-induced ulcerative colitis | The relative abundance of Bacteroidetes↓; The relative abundance of Firmicutes ↑; The relative abundance of Muribaculaceae-unclassified↓; The relative abundance of Lachnospiraceae-NK4A136-group↑ | Crypts were damage, goblet cells were severely lost and colon bowel integrity decreased; T-SOD, IL-4 and IL-10↓; MPO, GSH, NO, TNF-α, IL-6, IL-1β and IFN-γ↑ | The gene expression of ZO-1, claudin-1↓ | (Liu et al., 2021) |
| Mice with high-fat diet-induced hyperlipidemia | The relative abundance of Bacteroides, Lactobacillus, and Blautia ↓; The ratio of Firmicutes to Bacteroidetes↑ | The level of SOD, CAT and GSH-Px↓; The level of MDA, TNF-α, IL-1β and IL-6↑ | The gene expression of CPT1A and PPARα↓; The gene expression of SREBP-1c, ACC, HMGCR, LXR, SREBP-2↑ | (Yan et al., 2022, Yan et al., 2019) |
| Mice with high-fat diet-induced hyperlipidemia | The relative abundance of Lactobacillus, Faecalibaculum, and Allobaculum↓; The relative abundance of Romboutsia↑ | – | The gene expression of FXR and SREBP1↑; The gene expression of PPARα↓ | (Ren et al., 2021) |
| Normal mice | The Firmicutes to Bacteroidetes ratio↓; Verrucomicrobia↑; the relative abundance of Blautia, Ruminiclostridium_9↓; the relative abundance of Lactobacillus↑ | – | The gene expression of ZO-1, Claudin-1, Claudin-4, Ocdudin, E-cadherin and Muc-2↑ | (Wu et al., 2021) |
| Mice with high-fat diet-induced hyperlipidemia | The Firmicutes to Bacteroidetes ratio↑; Bifidobacterium, Lactobacillus and Akkermansia↓ | The level of sIgA↑; TGF-β↓ | The gene expression of CCL28 and CCR10↓ | (Cheng et al., 2020) |
| Cyclophosphamide (CTX)-induced mice | Diversity and richness of gut microbiota↓; the relative abundance of Bacteroidetes↓; the relative richness of Firmicutes↑; the relative abundance of Erysipelatoclostridum ↑ |
The level of TNF-α, IL-2, IL-6, INF-γ, Ig-A, Ig-G↓ | The gene expression of TLR4, MyD88, p65and NF-κB↓ | (Liu et al., 2021b, Liu et al., 2021a) |
| Mice with high-fat diet-induced hyperlipidemia | Akkermansia and Lachnospiraceae was specific bacteria | TNF-α, IL-6, IL-10 and IL-1β in small intestine and brain↑ | The protein expression of IL-1β, TNF-α in small intestine and brain↑ | (Li et al., 2021c, Li et al., 2021a, Li et al., 2021b) |
| Mice with D-galactose–induced aging | The proportion of Bacteroidetesand↓; the proportions of Firmicutes and Verrucomicrobia↑ | The level of SOD, CAT in serum and liver↓; The level of MDA in serum and liver↑; IL-1β↑ | The protein expression of Bax, NF-KB and caspase-3↑; The protein expression of IKB-α and Bcl-2↓ | (Chen et al.,2022) |
| Mice with LPS-induced inflammation | The abundance of Bacteroidetes and Proteobacteria↓; The abundance of Actinobacteria and Firmicutes↑; The abundance of Lactobacillius, Alistipes, Odoribacter and Ruminoccaceae↓; The abundance of Bacteroides and Staphylococcus↑ | The level of IL-1β, IL-6, TNF-α, IL-10↑; The level of SOD and T-AOC↓; The level of MDA↑ | – | (Zhang et al., 2020) |
5. Enlightenment of natural products fermented by probiotic for the auxiliary prevention of COVID-19 through modulating gut microbiota to improve immunity and inflammatory response.
5.1. A brief introduction of COVID-19
The world first patient with COVID-19, the coronavirus caused by the spread of SARS-CoV-2, appeared on November 17, 2019 (The Lancet, 2020). And the virus had infected hundreds of millions of people and killed more than 600,000 people. In fact, SARS-CoV-2 was more likely to infect the elderly, children, and people with weakened immune systems. And there was no vaccine or drug that could cure it because of its extremely fast mutation rate. Its essence was the inflammation caused by viral infection in the lungs, and the inflammation of the body was closely related to the deterioration of gut microbiota (Dhar and Mohanty, 2020). There existed an inextricable link between gut microbiota disturbance, low immunity, inflammatory and COVID-19. The gut microbiota of immunocompromised patients was significantly worse than that of normal individuals. At the same time, the decline in immunity led to a weakening of the ability to resist external virus aggression. The decline in immunity caused to a weakening of the ability to resist external virus aggression, resulting in lung inflammation of the organism caused by SARS-CoV-2 virus infection. Therefore, in this part, we mainly introduced the changes of gut microbiota of patients with COVID-19, in order to expect that natural products fermented by probiotics could prevent the COVID-19 in adjunct vaccines and drugs by improving gut microbiota to adjust the immunity and anti-inflammatory effects.
5.2. The gut microbiota and inflammatory factors of patients with COVID-19
Some studies have pointed out that the gut microbiota of patients with COVID-19 continued to change during hospitalization, and the inflammation of the body promoted the severity of COVID-19 (Mizutani et al., 2022). The abundance of Blautia and Ruminococcus increased of gut microbiota of patients with colitis, and the severity and lethality increased when they had COVID-19 (Cai et al., 2021). Compared with healthy controls or patients with seasonal influenza, patients with COVID-19 had significantly lower gut microbiota diversity and higher levels of IL-18 in stool samples (Tao et al., 2020). Opportunistic pathogens such as Enterococcus faecalis and Saccharomyces cerevisiae were enriched in the gut microbiota. In febrile COVID patients, Bacteroides fragilis and Eubacterium ramulus were reduced, which induced that the lymphocytes, CD3+ T cells, CD4+ T cells were significantly decreased, and AST, LDH, CRP, IL-6, IL-10 were significantly increased (Zhou et al., 2021). And three months after the recovery of patients with COVID-19, their gut microbiota could not recover (Tian et al., 2021).
In conclusion, patients with COVID-19 have significant differences in gut microbiota and inflammatory indicators compared with normal people. However, the treatment and prevention of diseases should not only consider vaccines or drugs, daily diet and physical protection are also very important. In our above discussion, it was found that the natural products after fermentation of probiotics could regulate the gut microbiota through increasing the proportion of beneficial bacteria and reducing the proportion of harmful bacteria to improve the diversity and richness of gut microbiota to achieve the purpose of improving immunity and reducing the level of inflammatory factors, which means that ingestion of natural products fermented by probiotics in daily life provides possible ideas and methods to assist prevention of SARS-CoV-2 invasion.
6. Conclusions and prospects
Active substances obtained from natural products fermented by probiotics showed extremely high nutritional value in regulating immunity and anti-inflammatory, and gut microbiota has become an important research hotspot. The gut microbiota was regulated by the action of active substances, which further affects the intestinal mucosal immune system and inhibits the expression of inflammatory factors, thereby improving the body's immunity and anti-inflammatory activity. Meanwhile, it should be focused on in-depth exploration of its underlying mechanisms and signaling pathways.
As an important target for active substances to regulate the body's health, the gut microbiota is gradually accepted by medicine by looking for characteristic bacteria as a means of judging and determining whether one has a certain disease. At the same time, the improvement of the gut microbiota by active substances opens up new possibilities for the treatment of complementary diseases. As the largest public health problem in the world in recent years, the prevention of COVID-19 by ingesting natural products fermented by probiotics in daily meals has become a new idea.
Ethics statement
This is a review article and the articles covered have passed the ethics statement.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was financially supported by the Key Research and Development Program of Jiangxi Province (20192ACB60008) and the Science and Technology Planning Project of Fujian Province (2021L3007).
Data availability
No data was used for the research described in the article.
References
- Agarwala R., Maria I.J., Dewan P., Rahman M.M., Hosen Z., Adnan M. Exploring the impact of daily food habit and modification of lifestyle for boosting immunity against COVID-19. Heliyon. 2022;8(2):e8983. doi: 10.1016/j.heliyon.2022.e08983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aggeletopoulou I., Konstantakis C., Assimakopoulos S.F., Triantos C. The role of the gut microbiota in the treatment of inflammatory bowel diseases. Microbial Pathogenesis. 2019;137 doi: 10.1016/j.micpath.2019.103774. [DOI] [PubMed] [Google Scholar]
- Ahmed M.M., Wang A.C., Elos M., Chial H.J., Sillau S., Solano D.A., Coughlan C., Aghili L., Anton P., Markham N., Adame V., Gardiner K.J., Boyd T.D., Potter H. The innate immune system stimulating cytokine GM-CSF improves learning/memory and interneuron and astrocyte brain pathology in Dp16 down syndrome mice and improves learning/memory in wild-type mice. Neurobiology of Disease. 2022;105694 doi: 10.1016/j.nbd.2022.105694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar M., Chen Y., Ma Z., Zhang X., Shi D., Khan J.A., Liu H. Gut microbiota-derived short chain fatty acids are potential mediators in gut inflammation. Animal Nutrition. 2021 doi: 10.1016/j.aninu.2021.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Rashidi H.E. Gut microbiota and immunity relevance in eubiosis and dysbiosis. Saudi Journal of Biological Sciences. 2022;29(3):1628–1643. doi: 10.1016/j.sjbs.2021.10.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annunziata G., Sureda A., Orhan I.E., Battino M., Arnone A., Jiménez-García M., Capó X., Cabot J., Sanadgol N., Giampieri F., Tenore G.C., Kashani H.R.K., Silva A.S., Habtemariam S., Nabavi S.F., Nabavi S.M. The neuroprotective effects of polyphenols, their role in innate immunity and the interplay with the microbiota. Neuroscience & Biobehavioral Reviews. 2021;128:437–453. doi: 10.1016/j.neubiorev.2021.07.004. [DOI] [PubMed] [Google Scholar]
- Ardissone A., Cruz D.M., Davis-Richardson A., Rechcigl K.T., Li N. Meconium microbiome analysis identifies bacteria correlated with premature birth. PLoS ONE. 2014;9(3):e90784. doi: 10.1371/journal.pone.0090784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayyash M., Johnson S.K., Liu S., Al-Mheiri A., Abushelaibi A. Cytotoxicity, antihypertensive, antidiabetic and antioxidant activities of solid-state fermented lupin, quinoa and wheat by Bifidobacterium species: In-vitro investigations. LWT. 2018;95:295–302. doi: 10.1016/j.lwt.2018.04.099. [DOI] [Google Scholar]
- Bai Y., Zeng Z., Xie Z., Chen G., Chen D., Sun Y., Zeng X., Liu Z. Effects of polysaccharides from Fuzhuan brick tea on immune function and gut microbiota of cyclophosphamide-treated mice. The Journal of Nutritional Biochemistry. 2022;101 doi: 10.1016/j.jnutbio.2022.108947. [DOI] [PubMed] [Google Scholar]
- Cai C., Zhang X., Liu Y., Shen E., Feng Z., Guo C., Han Y., Ouyang Y., Shen H. Gut microbiota imbalance in colorectal cancer patients, the risk factor of COVID-19 mortality. Gut Pathogens. 2021;13(1) doi: 10.1186/s13099-021-00466-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C., Nanjundaswamy A.K., Njiti V., Xia Q., Chukwuma F. Value-added probiotic development by high-solid fermentation of sweet potato with Saccharomyces boulardii. Food Science & Nutrition. 2017;5(3):633–638. doi: 10.1002/fsn3.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellanos N., Diez G.G., Antunez-Almagro C., Bailen M., Bressa C., Gonzalez Soltero R., Perez M., Larrosa M. A critical mutualism - competition interplay underlies the loss of microbial diversity in sedentary lifestyle. Frontiers in Microbiology. 2020;10 doi: 10.3389/fmicb.2019.03142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaikham P., Rattanasena P. Combined effects of low-fat ice cream supplemented with probiotics on colon microfloral communities and their metabolites during fermentation in a human gut reactor. Food Bioscience. 2017;17:35–41. doi: 10.1016/j.fbio.2016.12.005. [DOI] [Google Scholar]
- Chen G., Zeng Z., Xie M., Peng Y., Zhou W., Xu W., Sun Y., Zeng X., Liu Z. Fermentation characteristics and probiotic activity of a purified fraction of polysaccharides from Fuzhuan brick tea. Food Science and Human Wellness. 2022;11(3):727–737. doi: 10.1016/j.fshw.2021.12.030. [DOI] [Google Scholar]
- Chan M.Z.A., Toh M., Liu S. Growth, survival, and metabolic activities of probiotic Lactobacillus spp. in fermented coffee brews supplemented with glucose and inactivated yeast derivatives. Food Research International. 2020;137 doi: 10.1016/j.foodres.2020.109746. [DOI] [PubMed] [Google Scholar]
- Chen H., Dong L., Chen X., Ding C., Hao M., Peng X., Zhang Y., Zhu H., Liu W. Anti-aging effect of phlorizin on D-galactose–induced aging in mice through antioxidant and anti-inflammatory activity, prevention of apoptosis, and regulation of the gut microbiota. Experimental Gerontology. 2022;163 doi: 10.1016/j.exger.2022.111769. [DOI] [PubMed] [Google Scholar]
- Chen X., Cai B., Wang J., Sheng Z., Yang H., Wang D., Chen J., Ning Q. Mulberry leaf-derived polysaccharide modulates the immune response and gut microbiota composition in immunosuppressed mice. Journal of Functional Foods. 2021;83 doi: 10.1016/j.jff.2021.104545. [DOI] [Google Scholar]
- Chen Y., Liu B., Wei Y., Kuang D. Influence of gut and intratumoral microbiota on the immune microenvironment and anti-cancer therapy. Pharmacological Research. 2021;174 doi: 10.1016/j.phrs.2021.105966. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Tang S., Huang Y., Liang F., Fang Y., Pan S., Wu T., Xu X. Lactobacillus casei-fermented blueberry pomace augments sIgA production in high-fat diet mice by improving intestinal microbiota. Food & Function. 2020;11(7):6552–6564. doi: 10.1039/D0FO01119C. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Wu T., Chu X., Tang S., Cao W., Liang F., Fang Y., Pan S., Xu X. Fermented blueberry pomace with antioxidant properties improves fecal microbiota community structure and short chain fatty acids production in an in vitro mode. LWT. 2020;125 doi: 10.1016/j.lwt.2020.109260. [DOI] [Google Scholar]
- Coyte K.Z., Rakoff-Nahoum S. Understanding competition and cooperation within the mammalian gut microbiome. Current Biology. 2019;29(11):R538–R544. doi: 10.1016/j.cub.2019.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar D., Mohanty A. Gut microbiota and Covid-19- possible link and implications. Virus Research. 2020;285 doi: 10.1016/j.virusres.2020.198018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding X.M., Li D.D., Bai S.P., Wang J.P., Zeng Q.F., Su Z.W., Xuan Y., Zhang K.Y. Effect of dietary xylooligosaccharides on intestinal characteristics, gut microbiota, cecal short-chain fatty acids, and plasma immune parameters of laying hens. Poultry Science. 2018;97(3):874–881. doi: 10.3382/ps/pex372. [DOI] [PubMed] [Google Scholar]
- Dupraz L., Magniez A., Rolhion N., Richard M.L., Da Costa G., Touch S., Mayeur C., Planchais J., Agus A., Danne C., Michaudel C., Spatz M., Trottein F., Langella P., Sokol H., Michel M. Gut microbiota-derived short-chain fatty acids regulate IL-17 production by mouse and human intestinal γδ T cells. Cell Reports. 2021;36(1) doi: 10.1016/j.celrep.2021.109332. [DOI] [PubMed] [Google Scholar]
- Fang S., Wang T., Li Y., Xue H., Zou J., Cai J., Shi R., Wu J., Ma Y. Gardenia jasminoides Ellis polysaccharide ameliorates cholestatic liver injury by alleviating gut microbiota dysbiosis and inhibiting the TLR4/NF-κB signaling pathway. International Journal of Biological Macromolecules. 2022;205:23–36. doi: 10.1016/j.ijbiomac.2022.02.056. [DOI] [PubMed] [Google Scholar]
- Fitzgerald K.A., Kagan J.C. Toll-like receptors and the control of immunity. Cell. 2020;180(6):1044–1066. doi: 10.1016/j.cell.2020.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao H., Wen J., Hu J., Nie Q., Chen H., Xiong T., Nie S., Xie M. Fermented Momordica charantia L. juice modulates hyperglycemia, lipid profile, and gut microbiota in type 2 diabetic rats. Food Research International. 2019;121:367–378. doi: 10.1016/j.foodres.2019.03.055. [DOI] [PubMed] [Google Scholar]
- Ge T., Yao X., Zhao H., Yang W., Zou X., Peng F., Li B., Cui R. Gut microbiota and neuropsychiatric disorders: Implications for neuroendocrine-immune regulation. Pharmacological Research. 2021;173 doi: 10.1016/j.phrs.2021.105909. [DOI] [PubMed] [Google Scholar]
- Geva-Zatorsky, N., Sefik, E., Kua, L., Pasman, L., Tan, T. G., Ortiz-Lopez, A., Yanortsang, T. B., Yang, L., Jupp, R., Mathis, D., Benoist, C., & Kasper, D. L. (2017). Mining the human gut microbiota for immunomodulatory organisms. Cell 168(5), 928. http://doi.org/10.1016/j.cell.2017.01.022. [DOI] [PMC free article] [PubMed]
- Golder H.M., Séon Simon A.A., Santigosa E., de Ondarza M., Lean I.J. Effects of probiotic interventions on production efficiency, survival rate, and immune responses of shrimp: A meta-analysis and meta-regression. Aquaculture. 2022;552 doi: 10.1016/j.aquaculture.2022.737973. [DOI] [Google Scholar]
- Gong S., Yu Y., Li W., Wu J., Wang Z. Effects of amylolytic Lactobacillus fermentation on the nutritional quality and digestibility of purple potato flour. Journal of Food Composition and Analysis. 2022;107 doi: 10.1016/j.jfca.2021.104363. [DOI] [Google Scholar]
- Gori S., Inno A., Belluomini L., Bocus P., Bisoffi Z., Russo A., Arcaro G. Gut microbiota and cancer: How gut microbiota modulates activity, efficacy and toxicity of antitumoral therapy. Critical Reviews in Oncology/Hematology. 2019;143:139–147. doi: 10.1016/j.critrevonc.2019.09.003. [DOI] [PubMed] [Google Scholar]
- Greer R.L., Morgun A., Shulzhenko N. Bridging immunity and lipid metabolism by gut microbiota. Journal of Allergy and Clinical Immunology. 2013;132(2):253–262. doi: 10.1016/j.jaci.2013.06.025. [DOI] [PubMed] [Google Scholar]
- Gu Y., Li X., Chen H., Sun Y., Yang L., Ma Y., Yong Chan E.C. Antidiabetic effects of multi-species probiotic and its fermented milk in mice via restoring gut microbiota and intestinal barrier. Food Bioscience. 2022;47 doi: 10.1016/j.fbio.2022.101619. [DOI] [Google Scholar]
- Guarner F., Malagelada J. Gut flora in health and disease. The Lancet. 2003;361(9356):512–519. doi: 10.1016/S0140-6736(03)12489-0. [DOI] [PubMed] [Google Scholar]
- Guo C., Guo D., Fang L., Sang T., Wu J., Guo C., Wang Y., Wang Y., Chen C., Chen J., Chen R., Wang X. Ganoderma lucidum polysaccharide modulates gut microbiota and immune cell function to inhibit inflammation and tumorigenesis in colon. Carbohydrate Polymers. 2021;267 doi: 10.1016/j.carbpol.2021.118231. [DOI] [PubMed] [Google Scholar]
- Horvath T.D., Ihekweazu F.D., Haidacher S.J., Ruan W., Engevik K.A., Fultz R., Hoch K.M., Luna R.A., Oezguen N., Spinler J.K., Haag A.M., Versalovic J., Engevik M.A. Bacteroides ovatus colonization influences the abundance of intestinal short chain fatty acids and neurotransmitters. IScience. 2022;104158 doi: 10.1016/j.isci.2022.104158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu X., Zeng J., Shen F., Xia X., Tian X., Wu Z. Citrus pomace fermentation with autochthonous probiotics improves its nutrient composition and antioxidant activities. LWT. 2022;157 doi: 10.1016/j.lwt.2022.113076. [DOI] [Google Scholar]
- Huang F., Hong R., Yi Y., Bai Y., Dong L., Jia X., Zhang R., Wang G., Zhang M., Wu J. In vitro digestion and human gut microbiota fermentation of longan pulp polysaccharides as affected by Lactobacillus fermentum fermentation. International Journal of Biological Macromolecules. 2020;147:363–368. doi: 10.1016/j.ijbiomac.2020.01.059. [DOI] [PubMed] [Google Scholar]
- Jiao Y., Kuang H., Hu J., Chen Q. Structural characterization and anti-hypoxia activities of polysaccharides from the sporocarp, fermentation broth and cultured mycelium of Agaricus bitorquis (Quél.) Sacc. Chaidam in mice. Journal of Functional Foods. 2018;51:75–85. doi: 10.1016/j.jff.2018.10.017. [DOI] [Google Scholar]
- Kiely M., Lord B., Ambs S. Immune response and inflammation in cancer health disparities. Trends in Cancer. 2022;8(4):316–327. doi: 10.1016/j.trecan.2021.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwaw E., Ma Y., Tchabo W., Apaliya M.T., Wu M., Sackey A.S., Xiao L., Tahir H.E. Effect of lactobacillus strains on phenolic profile, color attributes and antioxidant activities of lactic-acid-fermented mulberry juice. Food Chemistry. 2018;250:148–154. doi: 10.1016/j.foodchem.2018.01.009. [DOI] [PubMed] [Google Scholar]
- Li H., Liu S., Liu Y., Li W., Niu A., Ren P., Liu Y., Jiang C., Inam M., Guan L. Effects of in vitro digestion and fermentation of Nostoc commune Vauch. polysaccharides on properties and gut microbiota. Carbohydrate Polymers. 2022;281 doi: 10.1016/j.carbpol.2021.119055. [DOI] [PubMed] [Google Scholar]
- Li M., Li P., Tang R., Lu H. Resveratrol and its derivates improve inflammatory bowel disease by targeting gut microbiota and inflammatory signaling pathways. Food Science and Human Wellness. 2022;11(1):22–31. doi: 10.1016/j.fshw.2021.07.003. [DOI] [Google Scholar]
- Li S., Liang T., Zhang Y., Huang K., Yang S., Lv H., Chen Y., Zhang C., Guan X. Vitexin alleviates high-fat diet induced brain oxidative stress and inflammation via anti-oxidant, anti-inflammatory and gut microbiota modulating properties. Free Radical Biology and Medicine. 2021;171:332–344. doi: 10.1016/j.freeradbiomed.2021.05.028. [DOI] [PubMed] [Google Scholar]
- Li S., Tao Y., Li D., Wen G., Zhou J., Manickam S., Han Y., Chai W.S. Fermentation of blueberry juices using autochthonous lactic acid bacteria isolated from fruit environment: Fermentation characteristics and evolution of phenolic profiles. Chemosphere. 2021;276 doi: 10.1016/j.chemosphere.2021.130090. [DOI] [PubMed] [Google Scholar]
- Li Y., Liu H., Qi H., Tang W., Zhang C., Liu Z., Liu Y., Wei X., Kong Z., Jia S., Du B., Yuan J., Wang C., Li M. Probiotic fermentation of Ganoderma lucidum fruiting body extracts promoted its immunostimulatory activity in mice with dexamethasone-induced immunosuppression. Biomedicine & Pharmacotherapy. 2021;141 doi: 10.1016/j.biopha.2021.111909. [DOI] [PubMed] [Google Scholar]
- Li Y., Xia D., Chen J., Zhang X., Wang H., Huang L., Shen J., Wang S., Feng Y., He D., Wang J., Ye H., Zhu Y., Yang L., Wang W. Dietary fibers with different viscosity regulate lipid metabolism via ampk pathway: Roles of gut microbiota and short-chain fatty acid. Poultry Science. 2022;101(4) doi: 10.1016/j.psj.2022.101742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao X., Hu S., Wang B., Qin H., Zhao J., He Z., Chen X., Liu Y., Qu P., Sun C., Zhang S. Dietary supplementation with polypeptides improved growth performance, antibacterial immune and intestinal microbiota structure of Litopenaeus vannamei. Fish & Shellfish Immunology. 2019;92:480–488. doi: 10.1016/j.fsi.2019.06.033. [DOI] [PubMed] [Google Scholar]
- Liao H., Ran R., Da C., Wang Z., Zhou K., Zhang H. Ski regulates the inflammatory response of reactive astrocytes induced by oxygen glucose deprivation/reoxygenation (OGD/R) through the NF-κB pathway. Neuroscience. 2022 doi: 10.1016/j.neuroscience.2022.02.015. [DOI] [PubMed] [Google Scholar]
- Liu F., Zhang L., Feng X., Ibrahim S.A., Huang W., Liu Y. Immunomodulatory activity of carboxymethyl pachymaran on immunosuppressed mice induced by cyclophosphamide. Molecules. 2021;26(19):5733. doi: 10.3390/molecules26195733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Fang H., Liu H., Cheng H., Pan L., Hu M., Li X. Goji berry juice fermented by probiotics attenuates dextran sodium sulfate-induced ulcerative colitis in mice. Journal of Functional Foods. 2021;83 doi: 10.1016/j.jff.2021.104491. [DOI] [Google Scholar]
- Lof J., Smits K., Melotte V., Kuil L.E. The health effect of probiotics on high-fat diet-induced cognitive impairment, depression and anxiety: A cross-species systematic review. Neuroscience & Biobehavioral Reviews. 2022;104634 doi: 10.1016/j.neubiorev.2022.104634. [DOI] [PubMed] [Google Scholar]
- Ma J., Piao X., Mahfuz S., Long S., Wang J. The interaction among gut microbes, the intestinal barrier and short chain fatty acids. Animal Nutrition. 2021 doi: 10.1016/j.aninu.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma T., Li C., Zhao F., Cao J., Zhang X., Shen X. Effects of co-fermented collagen peptide-jackfruit juice on the immune response and gut microbiota in immunosuppressed mice. Food Chemistry. 2021;365 doi: 10.1016/j.foodchem.2021.130487. [DOI] [PubMed] [Google Scholar]
- Manzoor S., Wani S.M., Ahmad Mir S., Rizwan D. Role of probiotics and prebiotics in mitigation of different diseases. Nutrition. 2022;96 doi: 10.1016/j.nut.2022.111602. [DOI] [PubMed] [Google Scholar]
- Margolles A., Ruiz L. Methods for Isolation and Recovery of Bifidobacteria. Methods in molecular biology (Clifton. N.J.) 2021;2278:1–12. doi: 10.1007/978-1-0716-1274-3_1. [DOI] [PubMed] [Google Scholar]
- Marx, W., Scholey, A., Firth, J., D Cunha, N. M., Lane, M., Hockey, M., Ashton, M. M., Cryan, J. F., O Neil, A., Naumovski, N., Berk, M., Dean, O. M., & Jacka, F. (2020). Prebiotics, probiotics, fermented foods and cognitive outcomes: A meta-analysis of randomized controlled trials. Neuroscience & Biobehavioral Reviews 118, 472-484. https://doi.org/10.1016/j.neubiorev.2020.07.036. [DOI] [PubMed]
- Mehany T., Khalifa I., Barakat H., Althwab S.A., Alharbi Y.M., El-Sohaimy S. Polyphenols as promising biologically active substances for preventing SARS-CoV-2: A review with research evidence and underlying mechanisms. Food Bioscience. 2021;40 doi: 10.1016/j.fbio.2021.100891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirghaed A.T., Yarahmadi P., Hosseinifar S.H., Tahmasebi D., Gheisvandi N., Ghaedi A. The effects singular or combined administration of fermentable fiber and probiotic on mucosal immune parameters, digestive enzyme activity, gut microbiota and growth performance of Caspian white fish (Rutilus frisii kutum) fingerlings. Fish & Shellfish Immunology. 2018;77:194–199. doi: 10.1016/j.fsi.2018.02.007. [DOI] [PubMed] [Google Scholar]
- Mizutani T., Ishizaka A., Koga M., Ikeuchi K., Saito M., Adachi E., Yamayoshi S., Iwatsuki-Horimoto K., Yasuhara A., Kiyono H., Matano T., Suzuki Y., Tsutsumi T., Kawaoka Y., Yotsuyanagi H., Perez D.R. Correlation analysis between gut microbiota alterations and the cytokine response in patients with coronavirus disease during hospitalization. Microbiology Spectrum. 2022:e1621–e1689. doi: 10.1128/spectrum.01689-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mojaddar Langroodi A., Mehdizadeh T., Majidi L., Neyriz-Naghadehi M. Lactobacillus acidophilus and Anethum graveolens essential oil in Iranian cheese against Escherichia coli O157:H7. Flavour and Fragrance Journal. 2021;36(2):190–196. https://doi.org/10.1002/ffj.3629. [Google Scholar]
- Müller J., Spriewald S., Stecher B., Stadler E., Fuchs T.M. Evolutionary stability of salmonella competition with the gut microbiota: How the environment fosters heterogeneity in exploitative and interference competition. Journal of Molecular Biology. 2019;431(23):4732–4748. doi: 10.1016/j.jmb.2019.06.027. [DOI] [PubMed] [Google Scholar]
- Nagafusa H., Sayama K. Age-related chemokine alterations affect IgA secretion and gut immunity in female mice. Biogerontology. 2020;21(5):609–618. doi: 10.1007/s10522-020-09877-9. [DOI] [PubMed] [Google Scholar]
- Ofosu F.K., Elahi F., Daliri E.B., Han S., Oh D. Impact of thermal treatment and fermentation by lactic acid bacteria on sorghum metabolite changes, their antioxidant and antidiabetic activities. Food Bioscience. 2022;45 doi: 10.1016/j.fbio.2021.101502. [DOI] [Google Scholar]
- Osbelt L., Wende M., Almási É., Derksen E., Muthukumarasamy U., Lesker T.R., Galvez E.J.C., Pils M.C., Schalk E., Chhatwal P., Färber J., Neumann-Schaal M., Fischer T., Schlüter D., Strowig T. Klebsiella oxytoca causes colonization resistance against multidrug-resistant K. pneumoniae in the gut via cooperative carbohydrate competition. Cell Host & Microbe. 2021;29(11):1663–1679. doi: 10.1016/j.chom.2021.09.003. [DOI] [PubMed] [Google Scholar]
- Patnode M.L., Beller Z.W., Han N.D., Cheng J., Peters S.L., Terrapon N., Henrissat B., Le Gall S., Saulnier L., Hayashi D.K., Meynier A., Vinoy S., Giannone R.J., Hettich R.L., Gordon J.I. Interspecies competition impacts targeted manipulation of human gut bacteria by fiber-derived glycans. Cell. 2019;179(1):59–73. doi: 10.1016/j.cell.2019.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng Y., Yan Y., Wan P., Chen D., Ding Y., Ran L., Mi J., Lu L., Zhang Z., Li X., Zeng X., Cao Y. Gut microbiota modulation and anti-inflammatory properties of anthocyanins from the fruits of Lycium ruthenicum Murray in dextran sodium sulfate-induced colitis in mice. Free Radical Biology and Medicine. 2019;136:96–108. doi: 10.1016/j.freeradbiomed.2019.04.005. [DOI] [PubMed] [Google Scholar]
- Pineiro M., Stanton C. Probiotic bacteria: Legislative framework - Requirements to evidence basis. Journal of Nutrition. 2007;137(3):850S–853S. doi: 10.1093/jn/137.3.850S. [DOI] [PubMed] [Google Scholar]
- Pittayanon R., Lau J.T., Leontiadis G.I., Tse F., Yuan Y., Surette M., Moayyedi P. Differences in gut microbiota in patients with vs without inflammatory bowel diseases: A systematic review. Gastroenterology. 2020;158(4):930–946. doi: 10.1053/j.gastro.2019.11.294. [DOI] [PubMed] [Google Scholar]
- Praveen M.A., Parvathy K.R.K., Balasubramanian P., Jayabalan R. An overview of extraction and purification techniques of seaweed dietary fibers for immunomodulation on gut microbiota. Trends in Food Science & Technology. 2019;92:46–64. doi: 10.1016/j.tifs.2019.08.011. [DOI] [Google Scholar]
- Qin H., Huang L., Teng J., Wei B., Xia N., Ye Y. Purification, characterization, and bioactivity of Liupao tea polysaccharides before and after fermentation. Food Chemistry. 2021;353 doi: 10.1016/j.foodchem.2021.129419. [DOI] [PubMed] [Google Scholar]
- Qu Q., Yang F., Zhao C., Liu X., Yang P., Li Z., Han L., Shi X. Effects of fermented ginseng on the gut microbiota and immunity of rats with antibiotic-associated diarrhea. Journal of Ethnopharmacology. 2021;267 doi: 10.1016/j.jep.2020.113594. [DOI] [PubMed] [Google Scholar]
- Ren Y., Wu S., Xia Y., Huang J., Ye J., Xuan Z., Li P., Du B. Probiotic-fermented black tartary buckwheat alleviates hyperlipidemia and gut microbiota dysbiosis in rats fed with a high-fat diet. Food & Function. 2021;12(13):6045–6057. doi: 10.1039/D1FO00892G. [DOI] [PubMed] [Google Scholar]
- Richards J.L., Yap Y.A., Mcleod K.H., Mackay C.R., Mariño E. Dietary metabolites and the gut microbiota: An alternative approach to control inflammatory and autoimmune diseases. Clinical & Translational Immunology. 2016;5(5):e82. doi: 10.1038/cti.2016.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohani M.F., Islam S.M., Hossain M.K., Ferdous Z., Siddik M.A., Nuruzzaman M., Padeniya U., Brown C., Shahjahan M. Probiotics, prebiotics and synbiotics improved the functionality of aquafeed: Upgrading growth, reproduction, immunity and disease resistance in fish. Fish & Shellfish Immunology. 2022;120:569–589. doi: 10.1016/j.fsi.2021.12.037. [DOI] [PubMed] [Google Scholar]
- Rosen C.E., Palm N.W. Functional classification of the gut microbiota: The key to cracking the microbiota composition code. BioEssays. 2017;39(12) doi: 10.1002/bies.201700032. [DOI] [PubMed] [Google Scholar]
- Santana Andrade J.K., Chagas Barros R.G., Gualberto N.C., Santos De Oliveira C., Shanmugam S., Narain N. Influence of in vitro gastrointestinal digestion and probiotic fermentation on the bioaccessibility of gallic acid and on the antioxidant potential of Brazilian fruit residues. LWT. 2022;153 doi: 10.1016/j.lwt.2021.112436. [DOI] [Google Scholar]
- Schultze J.L., Aschenbrenner A.C. COVID-19 and the human innate immune system. Cell. 2021;184(7):1671–1692. doi: 10.1016/j.cell.2021.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamoon M., Martin N.M., O'Brien C.L. Recent advances in gut Microbiota mediated therapeutic targets in inflammatory bowel diseases: Emerging modalities for future pharmacological implications. Pharmacological Research. 2019;148 doi: 10.1016/j.phrs.2019.104344. [DOI] [PubMed] [Google Scholar]
- Shao Y., Kang Q., Zhu J., Zhao C., Hao L., Huang J., Lu J., Jia S., Yi J. Antioxidant properties and digestion behaviors of polysaccharides from Chinese yam fermented by Saccharomyces boulardii. LWT. 2022;154 doi: 10.1016/j.lwt.2021.112752. [DOI] [Google Scholar]
- Shu W., Shanjian C., Jinpiao L., Qishui O. Gut microbiota dysbiosis in patients with hepatitis B virus-related cirrhosis. Annals of Hepatology. 2022;27(2) doi: 10.1016/j.aohep.2022.100676. [DOI] [PubMed] [Google Scholar]
- Su G.L., Ko C.W., Bercik P., Falck-Ytter Y., Sultan S., Weizman A.V., Morgan R.L. AGA clinical practice guidelines on the role of probiotics in the management of gastrointestinal disorders. Gastroenterology. 2020;159(2):697–705. doi: 10.1053/j.gastro.2020.05.059. [DOI] [PubMed] [Google Scholar]
- Sun Y., Cui X., Duan M., Ai C., Song S., Chen X. In vitro fermentation of κ-carrageenan oligosaccharides by human gut microbiota and its inflammatory effect on HT29 cells. Journal of Functional Foods. 2019;59:80–91. doi: 10.1016/j.jff.2019.05.036. [DOI] [Google Scholar]
- Sun Y., Zhang Z., Cheng L., Zhang X., Liu Y., Zhang R., Weng P., Wu Z. Polysaccharides confer benefits in immune regulation and multiple sclerosis by interacting with gut microbiota. Food Research International. 2021;149 doi: 10.1016/j.foodres.2021.110675. [DOI] [PubMed] [Google Scholar]
- Tan H., Nie S. Deciphering diet-gut microbiota-host interplay: Investigations of pectin. Trends in Food Science & Technology. 2020;106:171–181. doi: 10.1016/j.tifs.2020.10.010. [DOI] [Google Scholar]
- Tang R., Yu H., Qi M., Yuan X., Ruan Z., Hu C., Xiao M., Xue Y., Yao Y., Liu Q. Biotransformation of citrus fruits phenolic profiles by mixed probiotics in vitro anaerobic fermentation. LWT. 2022;160 doi: 10.1016/j.lwt.2022.113087. [DOI] [Google Scholar]
- Tang S.X., Cheng Y.X., Wu T., Hu F.T., Pan S.Y., Xu X.Y. Effect of Lactobacillus plantarum-fermented mulberry pomace on antioxidant properties and fecal microbial community. Lwt-Food Science and Technology. 2021;147 doi: 10.1016/j.lwt.2021.111651. [DOI] [Google Scholar]
- Tao W., Zhang G., Wang X., Guo M., Zeng W., Xu Z., Cao D., Pan A., Wang Y., Zhang K., Ma X., Chen Z., Jin T., Liu L., Weng J., Zhu S. Analysis of the intestinal microbiota in COVID-19 patients and its correlation with the inflammatory factor IL-18. Medicine in Microecology. 2020;5 doi: 10.1016/j.medmic.2020.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancet T. Emerging understandings of 2019-nCoV. The Lancet. 2020;395(10221):311. doi: 10.1016/S0140-6736(20)30186-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Sun K., Meng T., Ye Z., Guo S., Li Z., Xiong C., Yin Y., Li H., Zhou L. Gut microbiota may not be fully restored in recovered COVID-19 patients after 3-month recovery. Frontiers in Nutrition. 2021;8 doi: 10.3389/fnut.2021.638825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian W., Dai L., Lu S., Luo Z., Qiu Z., Li J., Li P., Du B. Effect of Bacillus sp. DU-106 fermentation on Dendrobium officinale polysaccharide: Structure and immunoregulatory activities. International Journal of Biological Macromolecules. 2019;135 doi: 10.1016/j.ijbiomac.2019.05.203. [DOI] [PubMed] [Google Scholar]
- Tischer C., Kirjavainen P., Matterne U., Tempes J., Willeke K., Keil T., Apfelbacher C., Täubel M. Interplay between natural environment, human microbiota and immune system: A scoping review of interventions and future perspectives towards allergy prevention. Science of the Total Environment. 2022;821 doi: 10.1016/j.scitotenv.2022.153422. [DOI] [PubMed] [Google Scholar]
- Wan Y., Hong T., Shi H., Yin J., Koev T., Nie S., Gilbert R.G., Xie M. Probiotic fermentation modifies the structures of pectic polysaccharides from carrot pulp. Carbohydrate Polymers. 2021;251 doi: 10.1016/j.carbpol.2020.117116. [DOI] [PubMed] [Google Scholar]
- Wang J., Xie B., Sun Z. Quality parameters and bioactive compound bioaccessibility changes in probiotics fermented mango juice using ultraviolet-assisted ultrasonic pre-treatment during cold storage. LWT. 2021;137 doi: 10.1016/j.lwt.2020.110438. [DOI] [Google Scholar]
- Wang Y., Liu L., Moore D.J., Shen X., Peek R.M., Acra S.A., Li H., Ren X., Polk D.B., Yan F. An LGG-derived protein promotes IgA production through upregulation of APRIL expression in intestinal epithelial cells. Mucosal Immunology. 2017;10(2):373–384. doi: 10.1038/mi.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Feng Y., Yang N., Jiang T., Xu H., Lei H. Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: Bioactive phenolics, antioxidant activities and flavor volatiles. Food Chemistry. 2022;373 doi: 10.1016/j.foodchem.2021.131455. [DOI] [PubMed] [Google Scholar]
- Wei D., Ma P., Fan Q., Yu H., Peng Y., Li X. Yanning Syrup ameliorates the lipopolysaccharide-induced inflammation: Adjusting the gut microbiota, short-chain fatty acids, and the CD4+ T cell balance. Journal of Ethnopharmacology. 2022;283 doi: 10.1016/j.jep.2021.114729. [DOI] [PubMed] [Google Scholar]
- Wu, T., Chu, X., Cheng, Y., Tang, S., Zogona, D., Pan, S., & Xu, X. (2021). Modulation of Gut Microbiota by Lactobacillus casei Fermented Raspberry Juice In Vitro and In Vivo Foods (10, pp.). [DOI] [PMC free article] [PubMed]
- Wu Y., Li S., Tao Y., Li D., Han Y., Show P.L., Wen G., Zhou J. Fermentation of blueberry and blackberry juices using Lactobacillus plantarum, Streptococcus thermophilus and Bifidobacterium bifidum: Growth of probiotics, metabolism of phenolics, antioxidant capacity in vitro and sensory evaluation. Food Chemistry. 2021;348 doi: 10.1016/j.foodchem.2021.129083. [DOI] [PubMed] [Google Scholar]
- Xie H., Fang J., Farag M.A., Li Z., Sun P., Shao P. Dendrobium officinale leaf polysaccharides regulation of immune response and gut microbiota composition in cyclophosphamide-treated mice. Food Chemistry: X. 2022;13 doi: 10.1016/j.fochx.2022.100235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie M., Xie Y., Li Y., Zhou W., Zhang Z., Yang Y., Olsen R.E., Ringø E., Ran C., Zhou Z. Stabilized fermentation product of Cetobacterium somerae improves gut and liver health and antiviral immunity of zebrafish. Fish & Shellfish Immunology. 2022;120:56–66. doi: 10.1016/j.fsi.2021.11.017. [DOI] [PubMed] [Google Scholar]
- Xu S., Wang Q., Wang F., Li X., Wang B., Zhou Y., Zou P., Tang L., Yu D., Li W. Improved immune function of Chinese soft-shelled turtles (Pelodiscus sinensis) through oral probiotics via the TLR signaling pathway. Aquaculture. 2022;555 doi: 10.1016/j.aquaculture.2022.738126. [DOI] [Google Scholar]
- Yan F., Polk D. Probiotics and probiotic-derived functional factors—mechanistic insights into applications for intestinal homeostasis. Frontiers in Immunology. 2020;11 doi: 10.3389/fimmu.2020.01428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan F., Liu L., Dempsey P.J., Tsai Y., Raines E.W., Wilson C.L., Cao H., Cao Z., Liu L., Polk D.B. A Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate epidermal growth factor receptor*. Journal of Biological Chemistry. 2013;288(42):30742–30751. doi: 10.1074/jbc.M113.492397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J., Xue Q., Chen W., Wang K., Peng D., Jiang J., Li P., Du B. Probiotic-fermented rice buckwheat alleviates high-fat diet-induced hyperlipidemia in mice by suppressing lipid accumulation and modulating gut microbiota. Food Research International. 2022;155 doi: 10.1016/j.foodres.2022.111125. [DOI] [PubMed] [Google Scholar]
- Yan S., Li Q., Xue X., Wang K., Zhao L., Wu L. Analysis of improved nutritional composition of bee pollen (Brassica campestris L.) after different fermentation treatments. International Journal of Food Science & Technology. 2019;54(6):2169–2181. doi: 10.1111/ijfs.14124. [DOI] [Google Scholar]
- Yan Y., Zhang F., Chai Z., Liu M., Battino M., Meng X. Mixed fermentation of blueberry pomace with L. rhamnosus GG and L. plantarum-1: Enhance the active ingredient, antioxidant activity and health-promoting benefits. Food and Chemical Toxicology. 2019;131 doi: 10.1016/j.fct.2019.05.049. [DOI] [PubMed] [Google Scholar]
- Ye Z., Xu Y., Liu Y. Influences of dietary oils and fats, and the accompanied minor content of components on the gut microbiota and gut inflammation: A review. Trends in Food Science & Technology. 2021;113:255–276. doi: 10.1016/j.tifs.2021.05.001. [DOI] [Google Scholar]
- Zepeda-Hernández A., Garcia-Amezquita L.E., Requena T., García-Cayuela T. Probiotics, prebiotics, and synbiotics added to dairy products: Uses and applications to manage type 2 diabetes. Food Research International. 2021;142 doi: 10.1016/j.foodres.2021.110208. [DOI] [PubMed] [Google Scholar]
- Zhang X., Zhang N., Kan J., Sun R., Tang S., Wang Z., Chen M., Liu J., Jin C. Anti-inflammatory activity of alkali-soluble polysaccharides from Arctium lappa L. and its effect on gut microbiota of mice with inflammation. International Journal of Biological Macromolecules. 2020;154:773–787. doi: 10.1016/j.ijbiomac.2020.03.111. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Zhang H. Insights into the role of mucosal immunity in IgA nephropathy. Clinical Journal of the American Society of Nephrology. 2018;13(10):1584–1586. doi: 10.2215/CJN.04370418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao D., Cao J., Jin H., Shan Y., Fang J., Liu F. Beneficial impacts of fermented celery (Apium graveolens L.) juice on obesity prevention and gut microbiota modulation in high-fat diet fed mice. Food & Function. 2021;12(19):9151–9164. doi: 10.1039/D1FO00560J. [DOI] [PubMed] [Google Scholar]
- Zhou C., Zhou Y., Fang J. Gut microbiota in cancer immune response and immunotherapy. Trends in Cancer. 2021;7(7):647–660. doi: 10.1016/j.trecan.2021.01.010. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Shi X., Fu W., Xiang F., He X., Yang B., Wang X., Ma W. Gut microbiota dysbiosis correlates with abnormal immune response in moderate COVID-19 patients with fever. JOURNAL OF INFLAMMATION RESEARCH. 2021;14:2619–2631. doi: 10.2147/JIR.S311518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y., Jiang J., Yue Y., Feng Z., Chen J., Ye X. Influence of mixed probiotics on the the bioactive composition, antioxidant activity and appearance of fermented red bayberry pomace. LWT. 2020;133 doi: 10.1016/j.lwt.2020.110076. [DOI] [Google Scholar]
- Zhu Y., Mei Q., Fu Y., Zeng Y. Alteration of gut microbiota in acute pancreatitis and associated therapeutic strategies. Biomedicine & Pharmacotherapy. 2021;141 doi: 10.1016/j.biopha.2021.111850. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data was used for the research described in the article.