Abstract
Background:
The NCCN currently recommends several definitive radiotherapy options for men with unfavorable intermediate-risk (UIR) prostate cancer including external beam radiotherapy (EBRT) + androgen deprivation therapy (ADT) or EBRT + brachytherapy boost ± ADT. However, brachytherapy alone (BT) ± ADT is not well-defined and is currently not recommended for UIR disease. We hypothesized that men treated with BT ± ADT have comparable survival rates to men treated with EBRT ± ADT.
Methods:
31,783 men diagnosed between 2004–2015 with UIR prostate cancer were retrospectively reviewed from the National Cancer Database. Men were stratified into four groups: (i) EBRT (n=12,985), (ii) EBRT+ADT (n=12,960), (iii) BT (n=4,535), or (iv) BT+ADT (n=1,303). Inverse probability of treatment weighting (IPTW) was used to adjust for covariable imbalances and weight-adjusted multivariable analysis (MVA) using Cox regression modeling was used to compare overall survival (OS) hazard ratios.
Results:
Relative to EBRT alone, the following treatments were associated with improved OS: EBRT+ADT (Hazard Ratio (HR): 0.92 [95% Confidence Interval: 0.87–0.97], P=.002), BT alone (HR: 0.90 [0.83–0.98], P=.01), and BT+ADT (HR: 0.78 [0.69–0.88], P=.00006). In men who were not treated with ADT, brachytherapy correlated with improved OS relative to EBRT (HR: 0.92 [0.84–0.99]. P=.03). In men receiving ADT, brachytherapy correlated with improved OS relative to EBRT (HR: 0.84 [0.75–0.95]. P=.004). At 10 years follow-up, 56% and 63% of men receiving EBRT and brachytherapy were alive, respectively (P<.0001). IPTW was used to determine the average treatment effect of definitive brachytherapy. Relative to EBRT, definitive brachytherapy correlated with improved OS (HR: 0.90 [0.84–0.97], P=.009) on weight-adjusted MVA.
Conclusion:
Definitive brachytherapy was associated with improved OS compared to EBRT. The addition of ADT to both EBRT and definitive brachytherapy was associated with improved OS. These results suggest that definitive brachytherapy should be considered as an option for men with unfavorable intermediate-risk prostate cancer.
INTRODUCTION
Intermediate-risk prostate cancer represents the largest group of prostate cancers with considerable biologic and clinical heterogeneity, and is further subdivided into favorable and unfavorable intermediate-risk groups.1 Relative to favorable intermediate-risk disease, men with UIR disease have higher rates of biochemical recurrence, metastatic recurrence, and death from prostate cancer.2 The NCCN currently recommends three definitive treatment options for unfavorable intermediate-risk (UIR) prostate cancer including radical prostatectomy with or without pelvic lymph node dissection, external beam radiation therapy (EBRT) with 4–6 months of androgen deprivation therapy (ADT), or combination EBRT with a brachytherapy boost with or without ADT. The addition of ADT to EBRT is associated with improved biochemical control and is the considered standard of care,3,4 but the benefit of ADT in combination with brachytherapy is less well defined.5,6
Brachytherapy (BT) is an excellent treatment for patients with localized prostate cancer and is further classified into low-dose rate (LDR-BT) or high-dose rate (HDR-BT) brachytherapy. LDR-BT consists of delivering radiation at a rate of <2 Gray per hour via permanent implantation of sealed radioactive sources or seeds into the prostate, while HDR-BT is defined as radiation delivery at a rate >12 Gray per hour by temporarily implanting radioactive source into the prostate via catheters.7,8 In low-risk and favorable intermediate-risk prostate cancer, BT is an option for definitive therapy.1 In unfavorable intermediate- and high-risk prostate cancer, BT is often combined with external beam radiotherapy (EBRT) as a boost, which permits further dose escalation beyond doses that can be delivered routinely with EBRT. EBRT plus BT boost has demonstrated improved biochemical control over EBRT plus ADT alone in randomized clinical trials.9 However, there is limited data on the use of definitive BT in unfavorable intermediate-risk (UIR) prostate cancer, and the NCCN does not currently recommend definitive BT as an treatment option for men with UIR disease.1 Given that BT allows for significant dose-escalation relative to EBRT, we hypothesized that men with UIR prostate cancer in the National Cancer Database (NCDB) undergoing definitive BT ± ADT would have comparable overall survival (OS) to men receiving EBRT ± ADT.
METHODS
Patient Cohort and Covariables
De-identified patients from 2004 and 2015 with histologically diagnosed adenocarcinoma of the prostate were identified in the NCDB. Patients were included that met criteria for UIR prostate cancer, defined as intermediate-risk disease and either: primary Gleason 4 disease, diffuse disease (≥ 50% biopsy cores positive), or 2 of 3 intermediate-risk factors (cT2b-T2c, PSA: 10 ≤ x ≤ 20, Gleason 3+4 disease). Patients with any of the following criteria were excluded: (i) nodal or metastatic disease; (ii) missing information on clinical T-stage, Gleason score, or PSA; (iii) moderate-to-severe comorbid disease burden, defined as a Charlson/Deyo comorbidity index (CDCI) scores >1, (iv) prior prostate surgery or pelvic radiation, (v) receipt of chemotherapy or immunotherapy, (vi) ADT status or ADT start date in relation to diagnosis was unknown, (vii) ADT was initiated >180 days after diagnosis, (viii) information of the cumulative EBRT radiation dose, number of fractions, or the numbers of days after diagnosed when radiotherapy was initiated was unknown; (ix) or radiation was initiated >180 days after diagnosis. Selection of the final patient cohort is summarized in Supplemental Figure 1. Radiotherapy treatment included: (i) EBRT or (ii) definitive BT. EBRT was delivered with conventional fractionation (≥ 72 Gy in 1.8–2.0 Gy per fraction). Patients receiving BT included LDR-BT and HDR-BT. As the allowed EBRT dose/fractionation in this study was consistent with NCCN guidelines, we wanted to make sure that the brachytherapy patients included were also treated in a manner consistent with NCCN guidelines. Therefore, we excluded single-fraction HDR patients as this treatment is not listed as an acceptable option per NCCN guidelines and was recently shown to be associated with worse biochemical control.1,10,11 Covariables were selected a priori and included age, race, ethnicity, year of diagnosis, CDCI score, insurance status, educational attainment within the patient’s area of residence (divided into quartiles on the basis of residents in the patient’s zip code who did not graduate high school), median income quartiles (divided into quartiles on the basis of residents in the patient’s zip code), treatment at an academic center, PSA at diagnosis, Gleason score, and clinical T-stage.
Endpoints and Statistical Analysis
OS was the primary endpoint. Chi square and Student t-tests were used to detect significant differences among categorical and continuous variables, respectively. OS was estimated using the Kaplan-Meier method and log-rank tests were used to compare treatment arms. Multivariable (MVA) OS hazard ratios were estimated using Cox regression analysis. The inverse probability of treatment weighting (IPTW) was used to adjust for covariable imbalance. The probability of receiving a treatment was estimated using a binomial logistic regression model to generate propensity scores that included: age, race, and ethnicity, CDCI score, PSA, clinical T-stage, Gleason score, year of diagnosis, treatment at an academic center, insurance status, educational attainment within the patient’s area of residence, and median household income within the patient’s area of residence. Unstabilized inverse propensity weights were generated, with truncation of the most extreme weights as previously described (α = 0.0001),10 and a pseudo-sample population in which measured baseline covariables were balanced among treatment groups was generated. Acceptable covariable balance among treatment groups was verified using the standardized mean difference, with a standardized mean difference less than 0.1 (10%) considered a negligible difference among treatment populations.12 The standardized mean difference between BT and EBRT treatment groups for each covariable following adjustment was as follows: age (0.03), race (0.009), Hispanic ethnicity (0.009), CDCI (0.003), treatment at an academic center (0.03), insurance status (0.06), educational attainment (0.01), median income (0.001), PSA at diagnosis (0.02), clinical T-stage (0.004), Gleason score (0.004), period of diagnosis (0.07), and ADT treatment (0.006). MVA was performed with weights applied to the time-dependent Cox proportional hazard model to compare the effects of BT versus EBRT alone. IPTW-weighted Kaplan-Meier curves were generated as the weighted product limit estimator. All analysis was performed in R software (Vienna, Austria). All tests were two-sided. P values < 0.05 were considered statistically significant.
RESULTS
The study cohort included 31,783 UIR prostate cancer patients treated with: (i) EBRT (n = 12,985), (ii) EBRT+ADT (n = 12,960), (iii) BT alone (n = 4,535), or (iv) BT+ADT (n = 1,303). Radiation dose and fractionation regimens for EBRT and BT are summarized in Supplemental Table 1. Among patients receiving BT, 3,559 patients (61%) received LDR-BT-BT, 432 patients (7%) receiving HDR-BT, and 1,847 (32%) received BT not otherwise specified. Baseline characteristics among men stratified by treatment with EBRT or BT are summarized in Table 1. The average age of men receiving EBRT versus BT was 70 and 68 years-old, respectively. EBRT patients had a slightly higher proportion of men with a CDCI score of 0 relative to the BT arms.
Table 1.
Baseline characteristics of patients treated with BT versus EBRT.
| Total | EBRT | BT | P | |
|---|---|---|---|---|
|
| ||||
| Total patients, n | 31,783 | 25,945 | 5,838 | |
| Age, Mean (SD) | 70 (7.5) | 70 (7.4) | 68 (7.7) | < 1 ×10−16 |
| Race | ||||
| White | 25,367 (80) | 20,612 (79) | 4755 (81) | 7.1 × 10−4 |
| Black | 5,563 (17) | 4,607 (18) | 956 (16) | |
| Other | 853 (3) | 726 (3) | 127 (3) | |
| Spanish or Hispanic Origin | ||||
| Non-Spanish, Non-Hispanic | 28,783 (90) | 23,452 (90) | 5,331 (91) | 0.03 |
| Spanish or Hispanic | 3,000 (10) | 2,493 (10) | 507 (9) | |
| Insurance | ||||
| Uninsured | 424 (1) | 363 (1) | 61 (1) | |
| Private Insurance | 8,536 (27) | 6,463 (25) | 2,073 (36) | < 2.2 × 10−16 |
| Medicare | 20,699 (65) | 17,317 (67) | 3,382 (58) | |
| Medicaid/Other Government | 1,666 (5) | 1,403 (5) | 263 (4) | |
| Unknown | 458 (2) | 399 (2) | 59 (1) | |
| Income Level | ||||
| < 38,000 | 6,163 (19) | 5,058 (20) | 1,105 (19) | |
| 38,000–47,999 | 7,562 (24) | 6,095 (23) | 1,467 (25) | 0.005 |
| 48,000–62,999 | 8,462 (27) | 6,875 (27) | 1,587 (27) | |
| > 63,000 | 9,455 (30) | 7808 (30) | 1,647 (28) | |
| Unknown | 141 (0) | 109 (0) | 32 (1) | |
| Education † | ||||
| < 7% | 7,460 (23) | 6,074 (24) | 1,386 (24) | |
| 7–12.9% | 10,680 (34) | 8,748 (34) | 1,932 (33) | 0.004 |
| 13–20.9% | 8,578 (27) | 6,920 (26) | 1,658 (29) | |
| ≥ 21% | 4,947 (16) | 4,112 (16) | 835 (14) | |
| Unknown | 118 (0) | 91 (0) | 27 (0) | |
| CDCI (Comorbidity) Score | ||||
| 0 | 27,511 (87) | 22,569 (87) | 4,942 (85) | 2.6 × 10−6 |
| 1 ‡ | 4,272 (13) | 3,376 (13) | 896 (15) | |
| Treatment at Academic Center | ||||
| No | 22,966 (72) | 18,794 (72) | 4,172 (71) | 0.13 |
| Yes | 8,817 (28) | 7,151 (28) | 1,666 (29) | |
| PSA, Mean (SD) | 8.3 (4.3) | 8.5 (4.3) | 7.5 (4.3) | < 1 ×10−16 |
| Gleason Score | ||||
| 3+3 | 1,503 (5) | 1,156 (4) | 347 (6) | 6.7 × 10−11 |
| 3+4 | 14,043 (44) | 11,334 (44) | 2709 (46) | |
| 4+3 | 16,237 (51) | 13,455 (52) | 2782 (48) | |
| Clinical T Stage | ||||
| ≤ cT2a | 22,736 (72) | 18,280 (71) | 4,456 (76) | < 2.2 × 10−16 |
| T2b-T2c | 8,032 (25) | 6,788 (26) | 1,244 (21) | |
| cT2, NOS | 1,015 (3) | 877 (3) | 138 (3) | |
| ADT | ||||
| No | 17,520 (55) | 12,985 (50) | 4,535 (78) | < 2.2 × 10−16 |
| Yes | 14,263 (45) | 12,960 (50) | 1,303 (22) | |
| Year of Diagnosis | ||||
| 2004–2007 | 8,235 (26) | 6,591 (25) | 1,644 (28) | 3.0 × 10−5 |
| 2008–2010 | 14,249 (45) | 11,671 (45) | 2,578 (44) | |
| 2011–2015 | 9,299 (29) | 7,683 (30) | 1,616 (28) | |
Summary statistics are represented as mean (standard deviation [SD]) for continuous variables and No. (%) for categorical variables.
Abbreviations: ADT, androgen deprivation therapy; BT, brachytherapy; CDCI, Charlson-Deyo Comorbidity Index; EBRT, external beam radiotherapy; PSA, prostate specific antigen.
Proportion of adults in the patient’s zip code who did not graduate from high school.
A CDCI score of 1 means that an individual has a history of one of the following: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, mild liver disease, or diabetes.
Unadjusted multivariate analysis of each treatment group is summarized in Table 2. Relative to patients treated with EBRT alone, improved OS was associated with EBRT+ADT (Hazard Ratio (HR): 0.92 [95% Confidence Interval (95% CI): 0.87–0.97], P=.002), BT alone (HR: 0.90 [0.83–0.98], P=.01), and BT+ADT (HR: 0.78 [0.69–0.88], P=.00006). BT was associated with better OS relative to EBRT (P<.0001, Figure 1A). In men treated in the absence of hormones, BT correlated with improved OS relative to EBRT (HR: 0.92 [0.84–0.99]. P=.03). At 10 years follow-up, 55% of men receiving EBRT and 60% of men receiving BT were alive (P<.0001, Figure 1B). In men treated with ADT, BT correlated with improved OS relative to EBRT (HR: 0.84 [0.75–0.95]. P=.004). At 10 years follow-up, 56% of men receiving EBRT and 63% of men receiving BT were alive (P=.0002, Figure 1C).
Table 2.
Multivariable analysis showing overall survival hazard ratios associated with EBRT+ADT, BT, and BT+ADT relative to EBRT alone using unweighted Cox regression.
| HR [95% CI] | P | |
|---|---|---|
|
| ||
| Age | 1.05 [1.04–1.05] | < 2 × 10−16 |
| Race | ||
| White | 1.0 | - |
| Black | 1.01 [0.94–1.09] | 0.77 |
| Other | 0.66 [0.55–0.80] | 2.2 × 10−5 |
| Spanish or Hispanic Origin | ||
| Non-Spanish, Non-Hispanic | 1.0 | - |
| Spanish or Hispanic | 0.97 [0.89–1.05] | 0.40 |
| Insurance | ||
| Uninsured | 1.0 | - |
| Private Insurance | 0.99 [0.75–1.31] | 0.97 |
| Medicaid | 1.25 [0.95–1.64] | 0.12 |
| Medicare | 1.34 [0.99–1.83] | 0.053 |
| Unknown | 1.23 [0.88–1.72] | 0.22 |
| Income Level | ||
| < 38,000 | 1.0 | - |
| 38,000–47,999 | 0.90 [0.84–0.98] | 0.01 |
| 48,000–62,999 | 0.87 [0.80–0.94] | 0.008 |
| > 63,000 | 0.82 [0.75–0.91] | 7.1 × 10−5 |
| Education † | ||
| < 7% | 1.0 | - |
| 7–12.9% | 1.11 [1.03–1.19] | 0.007 |
| 13–20.9% | 1.12 [1.03–1.23] | 0.01 |
| ≥ 21% | 1.15 [1.03–1.28] | 0.012 |
| CDCI (Comorbidity) Index | ||
| 0 | 1.0 | - |
| 1 ‡ | 1.41 [1.32–1.51] | < 2 × 10−16 |
| Treatment at Academic Center | ||
| No | 1.0 | - |
| Yes | 0.95 [0.89–1.00] | 0.055 |
| PSA | 1.02 [1.01–1.03] | 5.6 × 10−14 |
| Gleason Score | ||
| 3+3 | 1.0 | - |
| 3+4 | 0.93 [0.82–1.04] | 0.21 |
| 4+3 | 0.96 [0.85–1.09] | 0.51 |
| Clinical T Stage | ||
| ≤ cT2a | 1.0 | - |
| T2b-T2c | 1.14 [1.07–1.20] | 1.7 × 10−5 |
| T2, not otherwise specified | 1.19 [1.04–1.35] | 0.010 |
| Year of Diagnosis | ||
| 2004–2007 | 1.0 | - |
| 2008–2010 | 1.04 [0.99–1.10] | 0.14 |
| 2011–2015 | 0.95 [0.85–1.06] | 0.34 |
| Treatment | ||
| EBRT | 1.0 | - |
| EBRT + ADT | 0.92 [0.87–0.97] | 0.002 |
| BT | 0.90 [0.83–0.98] | 0.01 |
| BT + ADT | 0.78 [0.69–0.88] | 5.7 × 10−5 |
Abbreviations: ADT, androgen deprivation therapy; BT, brachytherapy; CDCI, Charlson-Deyo Comorbidity Index; EBRT, external beam radiotherapy; HR, hazard ratio; PSA, prostate specific antigen; 95% CI, 95% confidence interval.
Proportion of adults in the patient’s zip code who did not graduate from high school.
A CDCI score of 1 corresponds to men with a history of one of the following: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, mild liver disease, or diabetes.
Figure 1:
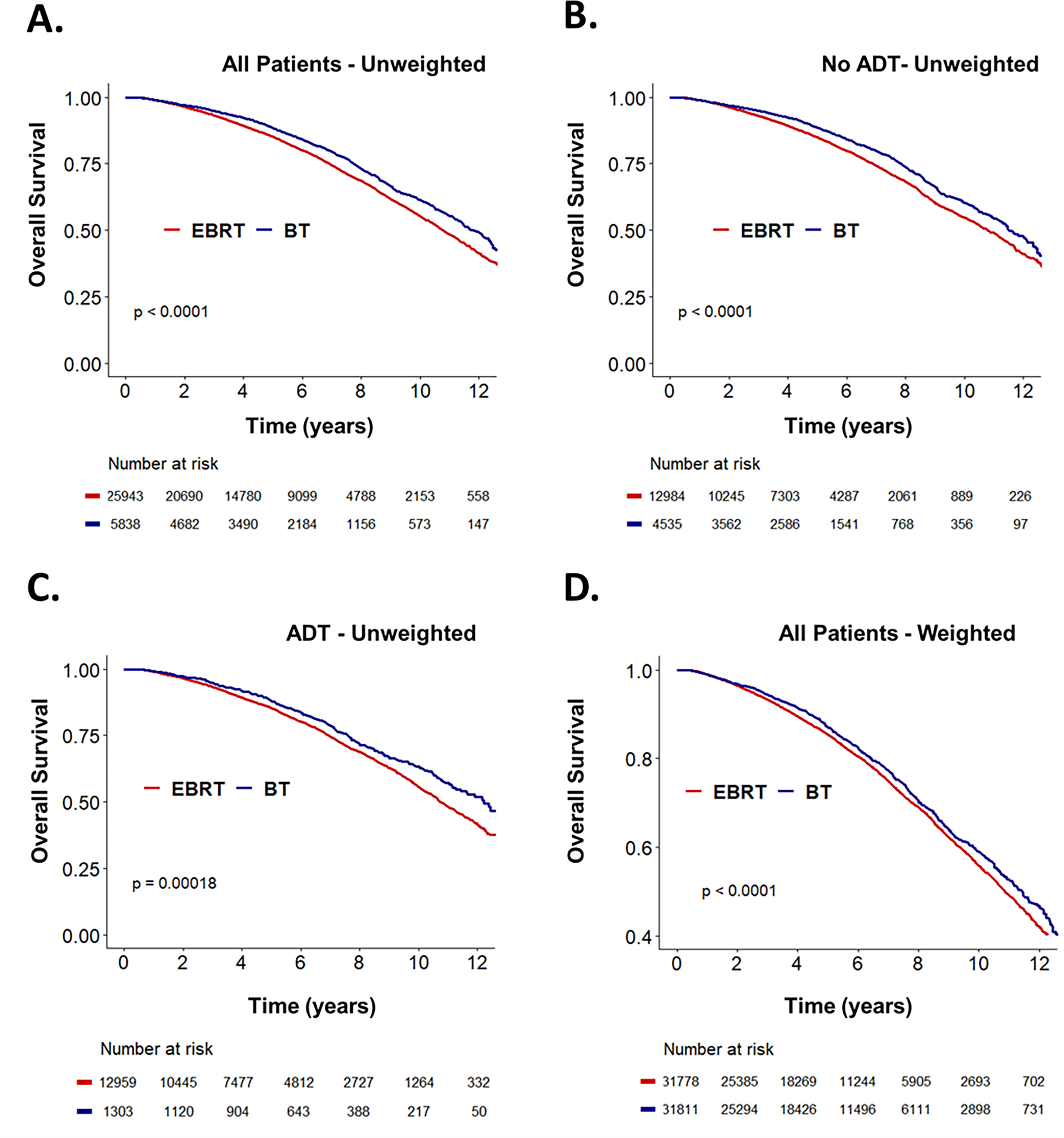
Unweighted Kaplan-Meier curves showing overall survival stratified by treatment with either definitive brachytherapy (BT) or external beam radiotherapy (EBRT) for (A) all men with unfavorable intermediate-risk (UIR) disease, (B) the subgroup of men treated without androgen-deprivation therapy (ADT), and (C) the subgroup of men treated with ADT. (D) Inverse probability of treatment weigting-adjusted Kaplan-Meier curves stratified by treatment with either BT or EBRT for all men with UIR disease.
Given the significant potential selection bias for EBRT versus BT, IPTW was used to balance covariables that influenced both treatment allocation and outcomes, and weight-adjusted Cox regression was used to determine the average treatment effect of definitive BT on groups with balanced cofounders. Relative to men treated with EBRT, definitive BT was associated with improved OS (HR: 0.91 [0.84–0.98, P=.009) on weight-adjusted MVA (Table 3). The addition of ADT was associated with a comparable improvement in OS (HR: 0.90 [0.83–0.97], P=.004). Advanced age (HR: 1.05 [1.05–1.06], P<2×10−16), higher CDCI score (1.35 [1.22–1.50], P=4.7×10−9), and higher PSA (HR: 1.03 [1.02–1.04], P=9.1×10−7) correlated with reduced OS; with higher clinical stage trending toward significantly reduced OS (HR: 1.10 [0.99–1.20], P=.08). Relative to men treated with EBRT, BT treatment was associated with significantly improved OS (P<.0001, Figure 1D). We next compared whether adding ADT improves survival in patents undergoing brachytherapy. Relative to BT alone, BT+ADT was associated with improved OS on propensity-weighted MVA (HR: 0.84 [0.73–0.96], P=.01) (Supplemental Table 2). Additional analysis was performed to address potential confounders. Since OS can be confounded by death from other causes, we restricted analysis to patients without any reported underlying comorbidities (CDCI score of 0), and propensity weighted MVA showed that both ADT (HR: 0.90 [0.83–0.97], P=.009) and brachytherapy (HR: 0.92 [0.85–0.99], P=.04) remained associated with significantly improved OS (Supplemental Table 3).
Table 3.
Average treatment effect of definitive BT versus EBRT using IPTW-adjusted multivariable cox regression analysis.
| HR [95% CI] | P | |
|---|---|---|
|
| ||
| Age | 1.05 [1.05–1.06] | < 2 × 10−16 |
| Race | ||
| White | 1.0 | - |
| Black | 1.03 [0.91–1.17] | 0.58 |
| Other | 0.69 [0.49–0.99] | 0.04 |
| Spanish or Hispanic Origin | ||
| Non-Spanish, Non-Hispanic | 1.0 | - |
| Spanish or Hispanic | 0.98 [0.86–1.11] | 0.74 |
| Insurance | ||
| Uninsured | 1.0 | - |
| Private Insurance | 1.11[0.77–1.61] | 0.57 |
| Medicare | 1.24 [0.86–1.79] | 0.25 |
| Medicaid/Other Government | 1.33 [0.88–1.99] | 0.17 |
| Unknown | 1.35 [0.84–2.10] | 0.23 |
| Income Level | ||
| < 38,000 | 1.0 | - |
| 38,000–47,999 | 0.92 [0.82–1.04] | 0.21 |
| 48,000–62,999 | 0.87 [0.76–0.99] | 0.04 |
| > 63,000 | 0.82 [0.71–0.96] | 0.01 |
| Education † | ||
| < 7% | 1.0 | - |
| 7–12.9% | 1.01 [0.89–1.14] | 0.86 |
| 13–20.9% | 1.01 [0.88–1.16] | 0.93 |
| ≥ 21% | 1.09 [0.92–1.29] | 0.30 |
| Treatment at Academic Center | ||
| No | 1.0 | - |
| Yes | 0.92 [0.84–1.01] | 0.08 |
| Charlson Comorbidity Index | ||
| 0 | 1.0 | - |
| 1 ‡ | 1.35 [1.22–1.50] | 4.7 × 10−9 |
| PSA | 1.03 [1.02–1.04] | 9.1 × 10−7 |
| Gleason Score | ||
| 3+3 | 1.0 | - |
| 3+4 | 0.90 [0.76–1.07] | 0.23 |
| 4+3 | 0.98 [0.81–1.18] | 0.80 |
| Clinical T Stage | ||
| ≤ T2a | 1.0 | - |
| T2b-T2c | 1.09 [0.99–1.20] | 0.08 |
| T2, NOS | 1.20 [0.92–1.56] | 0.17 |
| Year of Diagnosis | ||
| 2004–2007 | 1.0 | - |
| 2008–2010 | 1.02 [0.93–1.11] | 0.65 |
| 2011–2015 | 0.92 [0.77–1.10] | 0.34 |
| ADT | ||
| No | 1.0 | |
| Yes | 0.90 [0.83–0.97] | 0.004 |
| Treatment | ||
| EBRT | 1.0 | - |
| Brachytherapy | 0.91 [0.84–0.98] | 0.009 |
Abbreviations: ADT, androgen deprivation therapy; BT, brachytherapy; CDCI, Charlson-Deyo Comorbidity Index; EBRT, external beam radiotherapy; HR, hazard ratio; PSA, prostate specific antigen; 95% CI, 95% confidence interval.
Proportion of adults in the patient’s zip code who did not graduate from high school.
A CDCI score of 1 corresponds to men with a history of one of the following: myocardial infarction, congestive heart failure, peripheral vascular disease, cerebrovascular disease, dementia, chronic pulmonary disease, rheumatologic disease, peptic ulcer disease, mild liver disease, or diabetes.
DISCUSSION
In this study of men with unfavorable intermediate-risk prostate cancer, definitive brachytherapy was associated with a statistically significant improvement in OS compared to EBRT. A survival benefit was observed for BT versus EBRT alone and BT+ADT versus EBRT+ADT, after adjusting for measured confounders. We observed the following trends among the four treatment groups. Men treated with EBRT without ADT had higher-mortality rates relative to all other treatment groups. Relative to men treated with EBRT without ADT, men receiving BT without ADT or men receiving EBRT+ADT had better survival rates. The best outcomes were in men receiving BT+ADT. IPTW analysis showed that ADT and BT were independently associated with improved OS, suggesting that treatment with both may be associated with the best outcomes. To our knowledge, this is the first large retrospective study comparing single-modality EBRT to BT in men with UIR prostate cancer and this study adds to the existing literature on the comparative effectiveness of definitive BT for UIR disease.
EBRT is the principal radiation treatment modality used for definitive treatment of localized prostate cancer. In men with UIR prostate cancer, 30% of men will eventually develop recurrent disease, and EBRT treatment is often intensified by adding ADT and/or dose-escalating with a BT boost. Prior studies have overwhelmingly shown that adding 4 to 6 months of ADT to EBRT is associated with reduced biochemical recurrence, and have even shown a cancer-specific survival benefit 3,4,13 The ASCENDE-RT trial, which randomized men with UIR and high-risk disease to dose-escalated EBRT alone or EBRT plus a BT boost, showed a biochemical-free survival benefit in men receiving dose-escalation with BT boost.9 While this study used a mixed population of UIR and high-risk patients, a recent retrospective study showed that EBRT plus a BT boost improved biochemical recurrence-free survival in men with UIR disease.14 Consistent with these prior studies, the NCCN currently recommends that men with UIR disease are treated with either EBRT and short-term ADT or EBRT+BT boost ± ADT.1
While BT monotherapy is an accepted treatment option for men with favorable intermediate-risk disease in the NCCN guidelines, the NCCN does not currently recommend BT monotherapy for men with UIR disease.1 This recommendation is based on limited data. While there is ample data on the use of a BT boost in combination with EBRT for UIR disease, there is less data on definitive BT for this patient cohort, as the distinction between favorable intermediate- and unfavorable intermediate-risk disease is a relatively recent convention. Prior retrospective studies comparing treatment-related outcomes of men with favorable intermediate- and unfavorable intermediate-risk prostate cancer treated with BT monotherapy have arrived at different conclusions. In Berlin et al, outcomes following BT monotherapy were evaluated in 258 intermediate-risk patients, which failed to show a statistical difference in biochemical recurrence or distant metastases between favorable intermediate- and unfavorable intermediate-risk patients.15 In contrast, a recent retrospective analysis of 1,200 men with intermediate-risk prostate cancer showed that LDR-BT among UIR disease had a much higher risk of biochemical failure and distant metastasis compared to favorable intermediate-risk disease.16 Similar to patients treated with EBRT, this would suggest that men treated with BT may benefit from treatment escalation with short-term ADT or EBRT. However, studies addressing treatment escalation in patients receiving BT are sparse and limited to men with primarily favorable intermediate-risk disease, where they have shown no benefit to treatment escalation with ADT or EBRT.16–18 In contrast, our study shows that the addition of ADT to BT monotherapy is associated with a survival benefit.
While studies have reported increased recurrence risk in men with UIR, very few studies have compared definitive BT with EBRT in the UIR cohort. In this large retrospective study, we show that BT achieved comparable survival outcomes as EBRT with ADT. The addition of ADT to BT monotherapy provided a further survival advantage over BT alone. Given that EBRT+ADT is currently recommended for treatment of UIR, our results provide additional data to support the use of definitive BT ± ADT for treatment of UIR disease since patients receiving BT monotherapy did as well or better than patients receiving EBRT+ADT. This is consistent with prior biological studies suggesting a benefit to dose-escalation with BT monotherapy in prostate cancer. Interestingly, 34% of men received prophylactic pelvic lymph node radiation in the EBRT arms, while no men received nodal RT in the BT monotherapy arms. Thus, men treated with BT monotherapy appeared to do better, despite the fact that 34% of men receiving EBRT were treated with larger radiation fields which could reduce the risk of nodal relapse.
There are several limitations to our study, including unmeasured confounders and selection biases inherent to retrospective studies. Information on ADT duration, BT dose, and BT dosimetry are not recorded in the NCDB. In addition, information on the type of BT (LDR-BT versus HDR-BT) and total fractions was missing for a proportion of patients. While the comorbidity index (CDCI) was equally balanced between groups, CDCI scores are a surrogate for performance status and not a true measure of overall performance. However, prior surgical studies have reported that adding ASA/ECOG performance scores to models already containing CDCI scores to address confounding yielded no improvements in risk adjustment models for comparative assessment of cancer outcomes.19 MVA and IPTW were used to adjust for measured confounders. However, these techniques cannot address unmeasured confounders (i.e, performance status, BT dose and plan quality, etc.), which may have influenced treatment decision or treatment outcomes. Lastly, our analysis is limited by the fact that NCDB does not code for prostate-specific mortality or recurrence, which represent better endpoints for assessing the relationship between treatment and the natural disease course.
In conclusion, this large retrospective analysis of more than 30,000 men demonstrates that definitive BT is associated with improved OS compared to EBRT in men with UIR prostate cancer. Given that EBRT+ADT is a recommended treatment option for men with UIR prostate cancer,1 and BT+ADT achieved better results than EBRT+ADT, our results provide evidence that argues in favor of incorporating definitive BT+ADT into the NCCN guidelines. These results should be considered hypothesis generating and need further validation in randomized clinical trials. NRG GU-010 is a soon-to-open randomized cooperative group trial for men with UIR that will allow definitive brachytherapy but does not randomize or stratify by EBRT vs. BT to answer this question directly. Definitive brachytherapy is already an appealing option for patients because it is a highly conformal treatment with the lowest integral dose to normal tissues and is associated with less erectile dysfunction,9,17 greater patient convenience relative to standard or moderately hypofractionated radiotherapy, and is more cost-effective compared to some EBRT dose/fractionation schemes.20 Our finding of similar or better efficacy for BT relative to EBRT further adds to the treatment’s appeal. While our results require validation in randomized trials, definitive BT represents a viable alternative to EBRT ± ADT for men with UIR disease.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by institutional funds from the Departments of Radiation Oncology and Siteman Cancer Center at Washington University/Barnes Jewish Hospital in St Louis.
Funding:
BCB was funded under an NCI Cancer Clinical Investigator Team Leadership Award (CCITLA) (P30 CA091842–20S2).
Footnotes
Disclosures: The authors have disclosed that they have not received any financial consideration from any person or organization to support the preparation, analysis, results, or discussion of this article.
REFERENCES
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology (NCCN Guidelines): Prostate Cancer (Version 2.2021). https://www.nccn.org/professionals/physician_gls/pdf/prostate.pdf. Accessed March 2, 2021.
- 2.Zumsteg ZS, Spratt DE, Pei I, et al. A new risk classification system for therapeutic decision making with intermediate-risk prostate cancer patients undergoing dose-escalated external-beam radiation therapy. Eur Urol. 2013;64(6):895–902. [DOI] [PubMed] [Google Scholar]
- 3.D’Amico AV, Chen MH, Renshaw AA, Loffredo M, Kantoff PW. Androgen suppression and radiation vs radiation alone for prostate cancer: a randomized trial. JAMA. 2008;299(3):289–295. [DOI] [PubMed] [Google Scholar]
- 4.Jones CU, Hunt D, McGowan DG, et al. Radiotherapy and short-term androgen deprivation for localized prostate cancer. N Engl J Med. 2011;365(2):107–118. [DOI] [PubMed] [Google Scholar]
- 5.Pickles T, Morris WJ, Keyes M. High-intermediate prostate cancer treated with low-dose-rate brachytherapy with or without androgen deprivation therapy. Brachytherapy. 2017;16(6):1101–1105. [DOI] [PubMed] [Google Scholar]
- 6.Pickles T, Tyldesley S, Hamm J, Virani SA, Morris WJ, Keyes M. Brachytherapy for Intermediate-Risk Prostate Cancer, Androgen Deprivation, and the Risk of Death. Int J Radiat Oncol Biol Phys. 2018;100(1):45–52. [DOI] [PubMed] [Google Scholar]
- 7.Davis BJ, Horwitz EM, Lee WR, et al. American Brachytherapy Society consensus guidelines for transrectal ultrasound-guided permanent prostate brachytherapy. Brachytherapy. 2012;11(1):6–19. [DOI] [PubMed] [Google Scholar]
- 8.Yamada Y, Rogers L, Demanes DJ, et al. American Brachytherapy Society consensus guidelines for high-dose-rate prostate brachytherapy. Brachytherapy. 2012;11(1):20–32. [DOI] [PubMed] [Google Scholar]
- 9.Morris WJ, Tyldesley S, Rodda S, et al. Androgen Suppression Combined with Elective Nodal and Dose Escalated Radiation Therapy (the ASCENDE-RT Trial): An Analysis of Survival Endpoints for a Randomized Trial Comparing a Low-Dose-Rate Brachytherapy Boost to a Dose-Escalated External Beam Boost for High- and Intermediate-risk Prostate Cancer. Int J Radiat Oncol Biol Phys. 2017;98(2):275–285. [DOI] [PubMed] [Google Scholar]
- 10.Cole SR, Hernan MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Siddiqui ZA, Gustafson GS, Ye H, et al. Five-Year Outcomes of a Single-Institution Prospective Trial of 19-Gy Single-Fraction High-Dose-Rate Brachytherapy for Low- and Intermediate-Risk Prostate Cancer. Int J Radiat Oncol Biol Phys. 2019;104(5):1038–1044. [DOI] [PubMed] [Google Scholar]
- 12.Austin PC, Stuart EA. Moving towards best practice when using inverse probability of treatment weighting (IPTW) using the propensity score to estimate causal treatment effects in observational studies. Stat Med. 2015;34(28):3661–3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bolla M, Maingon P, Carrie C, et al. Short Androgen Suppression and Radiation Dose Escalation for Intermediate- and High-Risk Localized Prostate Cancer: Results of EORTC Trial 22991. J Clin Oncol. 2016;34(15):1748–1756. [DOI] [PubMed] [Google Scholar]
- 14.Abugharib AE, Dess RT, Soni PD, et al. External beam radiation therapy with or without low-dose-rate brachytherapy: Analysis of favorable and unfavorable intermediate-risk prostate cancer patients. Brachytherapy. 2017;16(4):782–789. [DOI] [PubMed] [Google Scholar]
- 15.Berlin A, Moraes FY, Sanmamed N, et al. International Multicenter Validation of an Intermediate Risk Subclassification of Prostate Cancer Managed with Radical Treatment without Hormone Therapy. J Urol. 2019;201(2):284–291. [DOI] [PubMed] [Google Scholar]
- 16.Tom MC, Reddy CA, Smile TD, et al. Validation of the NCCN prostate cancer favorable- and unfavorable-intermediate risk groups among men treated with I-125 low dose rate brachytherapy monotherapy. Brachytherapy. 2020;19(1):43–50. [DOI] [PubMed] [Google Scholar]
- 17.Bruner DW, Moughan J, Prestidge BR, et al. Patient Reported Outcomes of NRG Oncology/RTOG 0232: A Phase III Study Comparing Combined External Beam Radiation and Transperineal Interstitial Permanent Brachytherapy with Brachytherapy Alone in Intermediate Risk Prostate Cancer. Int J Radiat Oncol Biol Phys. 2018;102(3):S2–S3. [Google Scholar]
- 18.Keyes M, Merrick G, Frank SJ, Grimm P, Zelefsky MJ. American Brachytherapy Society Task Group Report: Use of androgen deprivation therapy with prostate brachytherapy-A systematic literature review. Brachytherapy. 2017;16(2):245–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dobbins TA, Badgery-Parker T, Currow DC, Young JM. Assessing measures of comorbidity and functional status for risk adjustment to compare hospital performance for colorectal cancer surgery: a retrospective data-linkage study. BMC Med Inform Decis Mak. 2015;15:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah C, Lanni TB Jr., Ghilezan MI, et al. Brachytherapy provides comparable outcomes and improved cost-effectiveness in the treatment of low/intermediate prostate cancer. Brachytherapy. 2012;11(6):441–445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


