Abstract
Background:
Conduct Disorder (CD) is a common syndrome with far-reaching effects. Risk factors for the development of CD span social, psychological, and biological domains. Researchers note that predictive models of CD are limited if the focus is on a single risk factor or, even, a single domain. Machine learning methods are optimized for the extraction of trends across multi-domain data but have yet to be implemented in predicting the development of CD.
Methods:
Social (e.g., family, income), psychological (e.g., psychiatric, neuropsychological), and biological (e.g., resting-state graph metrics) risk factors were measured using data from the baseline visit of the Adolescent Brain Cognitive DevelopmentSM Study when youth were 9–10-years-old (n = 2,368). Applying a feed-forward neural network machine learning method, risk factors were used to predict CD diagnoses two years later.
Results:
A model with factors that included social, psychological, and biological domains outperformed models representing factors within any single domain, predicting the presence of a CD diagnosis with 91.18% accuracy. Within each domain, certain factors stood out in terms of their relationship to CD (social: lower parental monitoring, more aggression in the household, lower income; psychological: greater ADHD and ODD symptoms, worse crystallized cognition and card sorting performance; biological: disruptions in the topology of subcortical and frontoparietal networks).
Conclusions:
The development of an accurate, sensitive, and specific predictive model of CD has the potential to aid in prevention and intervention efforts. Key risk factors for CD appear best characterized as reflecting unpredictable, impulsive, deprived, and emotional external and internal contexts.
Keywords: Conduct Disorder, machine learning, graph analysis, family, biopsychosocial
Conduct disorder (CD) is a psychiatric diagnosis present in approximately 3% of school-aged children and is characterized by aggressive, rule-breaking, destructive, and deceitful behaviors (1). Youth with CD are at increased risk for academic underachievement, family dysfunction, legal system involvement, substance misuse, emotional distress, suicidality, teen pregnancy, and a host of health problems. Consequently, CD is associated with a high individual, family, and societal burden, constituting a leading reason for referral to mental health services among youth and a main cause of disability, worldwide (2, 3).
An extensive body of research identifies risk factors for CD that span social, psychological, and biological domains. CD is one of the few childhood psychiatric disorders with a substantial environmental influence (4, 5). Parenting associated with harsh, coercive (e.g., corporal punishment, shouting, swearing and threatening) and inconsistent discipline are robust risk factors for CD, particularly for conduct problems that emerge in childhood (2, 5). Other environmental risk factors include low socioeconomic status and community violence. Low socioeconomic status is associated with an exponential increase in risk for persistent CD (6, 7). Additionally, witnessing or being the victim of community violence increases the odds of developing CD by approximately 2-fold and 4-fold, respectively (8). Individual psychological factors related to CD broadly reflect difficulties with behavioral and emotional regulatory capacities (9–11). For example, most studies show that attention-deficit/hyperactivity disorder (ADHD) and oppositional defiant disorder (ODD), two developmental disorder associated with impulsivity and negative affect, are psychiatric precursors to CD (12). In addition, compared to typically developing youth or youth with ADHD in the absence of CD, neurocognitive impairments in CD youth are sizeable, with some meta-analyses documenting overall medium sized effects (13). Finally, studies of neural structure, function, and connectivity show consistent atypicalities in youth with CD (2, 14–20). Specific patterns of reduced gray matter volume and structural connectivity, resting-state graph metrics of small-world properties, and higher variability in electroencephalogram signals uniquely characterize youth with CD (16–20). A great deal of knowledge has been accumulated about various risk factors for CD; however, often it is the presence of multiple risk factors, across domains, that most reliably predicts the development of CD.
In one study of 309 male youth that used longitudinal path models, CD diagnosis was best predicted by both caregiving characteristics (e.g., harshness) and youth psychological factors (e.g., intelligence, negative emotionality, information processing) (21). In another longitudinal study of 17,206 youth in the United Kingdom, the cumulative risk associated with low socioeconomic status and neurocognitive abilities demonstrated more explanatory power in predicting elevated levels of conduct problems than any single risk factor alone (22). This research suggests that predictive models of CD are limited if the focus is on a single risk factor or, even, a single domain (e.g., psychological only) (21, 23). However, previous studies examining multi-domain predictors of CD contain methodological limitations related to mass univariate testing and descriptive group-based analyses (24).
Machine learning methods increasingly are being used in psychiatry to address these methodological limitations. Such methods can be flexibly optimized for the extraction of trends across multiple pieces of information/domains. They also can generate individual predictions from multi-dimensional data, providing multivariate signatures that are valid at the single-subject level, and identify key features that could be used as markers to monitor the onset or continuity of syndrome illness (25–27). In research on CD, only a handful of studies have been conducted that implement machine learning methods (5, 16–19); but, they have been cross-sectional and only provide correlational evidence between a single domain of factors (e.g., brain or parenting) and CD. No research has used machine learning methods to examine the utility of risk factors across multiple domains for predicting the development of CD.
The present study applied machine learning methods to examine the prediction of CD using risk factors from the social, psychological, and biological domains. Data were from the Adolescent Brain Cognitive DevelopmentSM Study (ABCD Study®), a longitudinal multi-site study following the biopsychosocial development of over 10,000 youth starting at 9–10 years old (release 3.0; DOI 10.15154/1520591). We used a combined theory- and data-driven approach to identify relevant risk factors for inclusion in our model and explore the relative importance of those risk factors for predicting CD. We trained a feed-forward neural network (FNN) model1 (27, 28) to predict CD diagnosis when youth were 11–12-years-old using risk factors from the social, psychological, and biological domains, measured when youth were 9–10-years-old. FNN models are well-suited for analyzing linear and non-linear relationships between predictors and outcomes, identifying salient predictors, and suppressing non-relevant predictors (27, 29, 30). We first used a subsample to train the FNN model, and then we applied the trained model to data from an independent subsample. We also estimated the relative importance of each risk factor. We had no specific hypotheses about which risk factors would emerge as most important for discriminating between those with and without CD.
Methods and Materials
Participants
Participants were youth who completed the baseline session (at ages 9–10-years-old) and the two-year follow-up session (ages 11–12-years-old) of the multisite ABCD Study (see Supplement for more details). For the present analyses, participants were included if they: (a) had CD data available from their baseline session, (b) were not missing any data for key variables, and (c) had valid rs-fMRI data released from their baseline session that also passed the ABCD Study overall MRI quality checks (31). Further, given the large number of ABCD Study families with multiple children and/or twins that participated in the study, siblings were overrepresented in the sample (32). To help control for any family-related effects, only one, randomly selected, child per family was used in the current analyses, yielding a final sample of n = 2,368 (Table 1).
Table 1.
Demographics Summary
| Correlations | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | % of sample | Mean | Std. Dev. | Min | Max | 1 | 2a | 3b | |
| 1. | Age | 2368 | — | 9.53 | .50 | 8.00 | 11.00 | — | .01 | −.00 |
| 2. | sexa | 2368 | — | −.04* | ||||||
| Male | 1199 | 50.63 | ||||||||
| Female | 1169 | 49.37 | ||||||||
| 3. | Raceb | 2368 | — | |||||||
| White | 1385 | 58.50 | ||||||||
| Black | 227 | 9.59 | ||||||||
| Hispanic | 468 | 19.76 | ||||||||
| Asian | 55 | 2.32 | ||||||||
| Other | 233 | 9.83 | ||||||||
| 4. | Baseline CD | 2368 | ||||||||
| CD Diagnosis | 54 | 2.28 | ||||||||
| No CD Diagnosis | 2314 | 97.72 | ||||||||
| 5. | Two-Year CD | 2368 | ||||||||
| CD Diagnosis | 56 | 2.36 | ||||||||
| No CD Diagnosis | 2312 | 97.64 | ||||||||
Significant at alpha = .05 under the Spearman correlation test.
Spearman correlations were used to examine the effect of Sex (dichotomously-coded).
Spearman correlations were used to examine the effect of Race (dichotomously-coded, white vs. non-white).
Measures
Social
Perhaps the most studied risk factors in the social domain relate to disruptions in neighborhood and family functioning, therefore, we included predictors related to neighborhood safety, family income, and parenting practices (2, 33, 34).
Neighborhood.
The severity of neighborhood crime was assessed with the Neighborhood Safety/Crime Survey administered to participants’ parents. The question “My neighborhood is safe from crime” was answered on a Likert scale (1 “Strongly disagree”; 5 “Strongly agree”).
Family.
The Parental Monitoring Survey was completed by the youth and asked about parental awareness of location, interactions with peers/afterschool, and plans, as well as communication with parents about plans and activities. Higher scores indicated greater parental involvement (1 “Never”; 5 “Always”). The Family Environment Scale-Family Conflict evaluated parent-reported perceptions (0 “no”; 1 “Yes”) of whether family members fought, threw objects, criticized, hit, were in competition with one another, became openly angry, lost their temper, disagreed, and yelled. For most items on the scale, higher scores indicated more conflict, except for the items that asked about being openly angry, losing temper, disagreeing, and yelling where higher scores indicated lower occurrences of these events. The survey also asked about the frequency of family dinner in an average week (1 “Never”; 5 “Always”). Finally, family income was estimated using the total combined income for the 12-months preceding the assessment.
Psychological
Psychological risk factors included common diagnostic antecedents of CD, namely ADHD and ODD (4, 34), as well as, neurocognitive predictors given the well-replicated effects relating CD to deficits in executive functions, verbal abilities, and neurocognition broadly (9, 35).
Psychiatric Diagnoses.
CD, ADHD, and ODD diagnoses were determined using the Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime version (K-SADS-PL) (36). At the two-year follow-up assessment, 2.36% (n = 56) of the sample met for CD. Of those 56, 21 youth also presented with CD at baseline, therefore CD at baseline was included as a covariate (see Covariates below).
Neurocognitive.
The NIH Toolbox cognition battery is a battery of seven different neurocognitive tasks (37), including a: list sorting task assessing working memory, picture vocabulary task assessing language and verbal abilities, Flanker task assessing cognitive control and attention, dimensional change card sorting task assessing cognitive flexibility, pattern comparison task assessing visual processing speed, picture sequence task assessing episodic memory and visuospatial sequencing, and oral reading task assessing reading ability. Additionally, composite scores are calculated to measure fluid cognition (the capacity to process and integrate information, act, and solve novel problems) and crystallized cognition (the ability to utilize skills and knowledge acquired via prior learning) (38). Fully-corrected T-scores were used in the present analyses.
Biological
Global and local connectivity graph metrics across default, dorsal attention, frontoparietal, salience, subcortical, and ventral attention networks were included as biological, specifically neural, predictors. To maintain comparability with prior studies and manage computational complexity, we opted to use graph metrics as higher-order indicators of the widespread connectivity anomalies previous research has documented in youth with CD (14, 15, 39–42).
Graph Metrics.
For each participant, 15–20 min of resting state (rs)-fMRI data was acquired. Data acquisition occurred across 3–4 separate rs-fMRI sequences, each of which was 5-minutes in duration. During each rs-fMRI sequence, participants were instructed to stay still and gaze at a central fixation cross. Imaging parameters were harmonized across all 21 data collection sites and scanner models (31, 43). Graph metrics of rs-fMRI connectivity were calculated using the methods detailed elsewhere (14). Global graph metrics of interest included global clustering and global efficiency and node-level graph metrics of interest included between centrality (BC), efficiency, and degree for specific networks theoretically relevant to CD (i.e., default, dorsal attention, frontoparietal, salience, subcortical, and ventral attention networks; 14, 42, 44) (Table 2).
Table 2.
Descriptions of Graph Metrics
| Metric | Definition |
|---|---|
| Global Clustering | The fraction of nodes that form triangular connections (i.e., the fraction of nodes whose neighbors are also interconnected with each other) at the network level. High global clustering indicates greater functional segregation, efficient local connectivity, and robustness to disruption. |
| Global Efficiency | A metric related to the average inverse shortest path length across an entire graph. Graphs with high global efficiency allow information to travel through fewer connections to get from a node to any other node in the network, increasing the efficiency of neural communication. |
| Node-Level Betweenness Centrality (BC) | The number of shortest paths passing through a specific node in the global flow of information. Nodes with high BC have more information passing through them (i.e., are more central). Node-level BC measures were estimated for the default, dorsal attention, frontoparietal, salience, subcortical, and ventral attention networks. |
| Node-Level Degree | The number of connections between a specific node and other nodes in the network. Nodes with high degree have greater connections and may act as more of a hub in the global flow of information. Node-level degree measures were gathered for the default, dorsal attention, frontoparietal, salience, subcortical, and ventral attention networks. |
| Node-Level Efficiency | A metric related to the inverse shortest path length of a specific node within a smaller neighborhood. Nodes with high mean efficiency allow information to travel through fewer connections to get to other nodes in that neighborhood, increasing the efficiency of neural communication. Node-level efficiency measures were gathered for the default, dorsal attention, frontoparietal, salience, subcortical, and ventral attention networks. |
Covariates
Research collection site, sex (dichotomously coded, male vs. female), race (dichotomously coded, white vs. non-white), age, and baseline CD diagnosis were included as covariates in all models.
Analytic approach
We built a FNN model to classify the presence or absence of CD using the high-dimensional data (i.e., biopsychosocial risk factors) in the R nnet package (45). FNN architecture is defined by the number of layers within the network and the number of “neurons” within each layer. Information moves forward from the input neurons, through the hidden neurons, and to the output neurons. At each layer, the model learns complex patterns and relationships by conducting linear and non-linear transformations on the data. The architecture of our model consisted of 52 input neurons to represent all risk factors and covariates (Figure 1). To determine the number of hidden neurons, we ran an optimization loop that iterated through possible architectures with 2 to 26 neurons (the number of neurons in a layer should not be more than ½ the number of predictors). The model with 18 neurons had the highest test accuracy (46–48). As standard with binary classification models, one output neuron calculates the probability that a participant meets the criteria for two-year CD based on their input features.
Figure 1. Network Architecture.
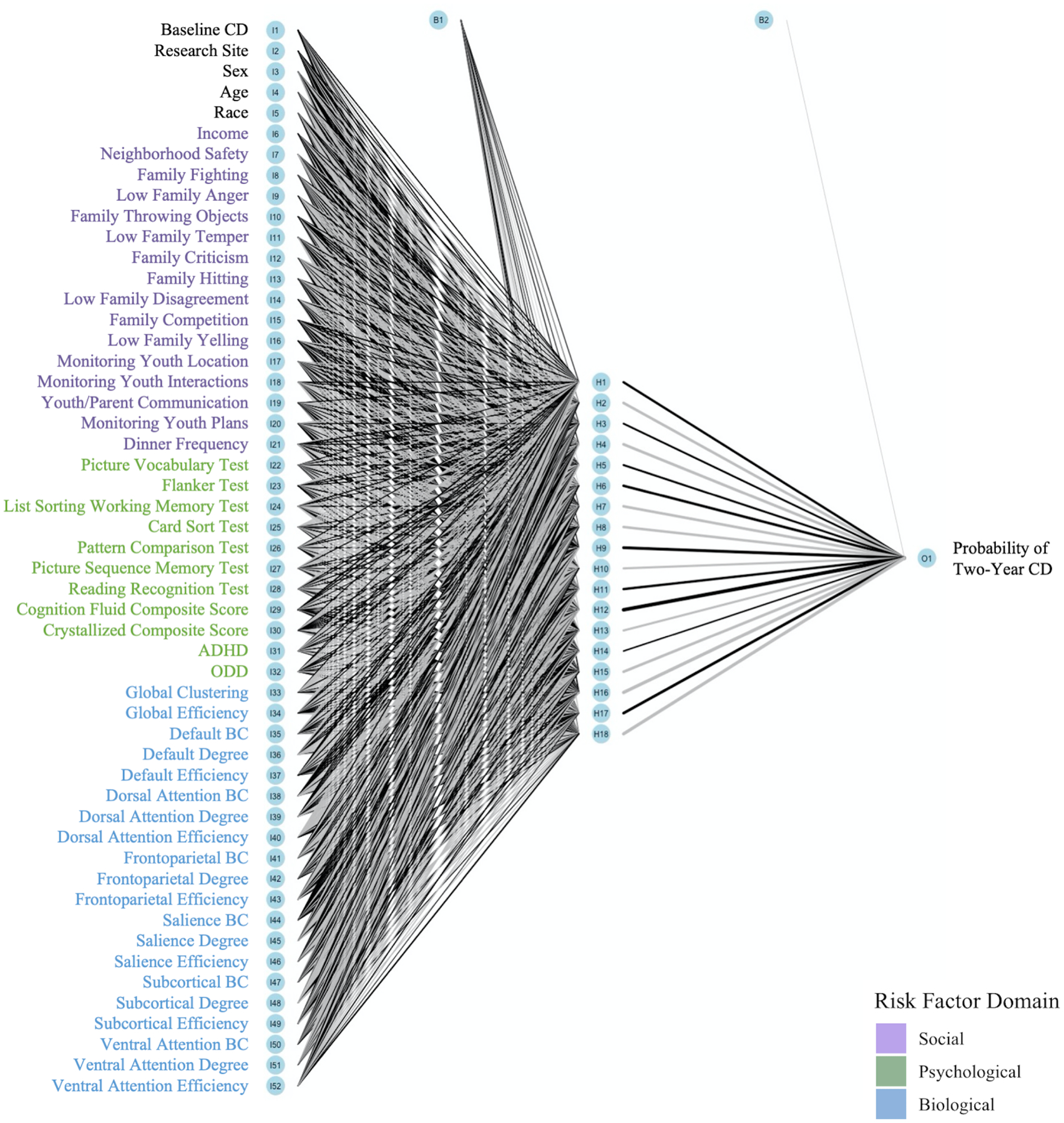
Note. The model consists of 52 input neurons, 18 hidden neurons, and one output neuron. Line thickness is proportional to the magnitude of each weight and bias term. Black lines indicate positive parameters and grey lines indicate negative parameters.
Classification models can encounter issues when datasets are unbalanced (i.e., contain one group that is much smaller than the other) (49). Given that only 2.36% of participants were diagnosed with CD at the two-year follow-up assessment, we used a smoothed bootstrapping method to undersample the larger class and oversample the smaller class until they were equal in size while still preserving the data distribution (50) using the ROSE package in R (51, 52). The ROSE algorithm estimates the conditional density underlying the two classes of the training set based on the probability distribution of randomly selected observations and the covariance matrix, allowing for the synthetic generation of observations from the minority class. Prior to bootstrapping (no over/under sampling), within the training set 1547 had no CD diagnosis and 39 participants had a CD diagnosis; after applying the smoothed bootstrapping to oversample for CD the synthetic data that enlarged the feature space for the minority class (i.e., CD diagnosis) resulted in 825 participants with no CD and 761 with CD.
In order to address the potential for overfitting (53), 67% of the dataset was randomly selected for model training and 33% for model testing. We adopted the standard approach of adding Gaussian noise to the training set to prevent the model from memorizing patterns or trends in the data (54). In addition, all models included a regularization decay term that penalized weight parameters for overfitting to the training set (55).
Each classifier was trained to predict whether an individual participant met the diagnostic criteria for CD in two years based on their risk factors at baseline. All predictors were standardized before training to improve model fitting. Risk factors were then transformed by a series of weight and bias terms and summed to calculate hidden layer neurons. These sums then passed through a non-linear activation function that activated neurons above a certain threshold and deactivated neurons below that threshold (see Supplement for details).
Neural networks are an advantageous method because they maximize prediction accuracy on unseen observations (24, 56). The holdout method is a standard validation technique that sets aside 33% of the data for testing the performance of the model (57). All predictions and performance measures were calculated on the out-of-sample testing set.
Results
Model Performance
A confusion matrix was examined to evaluate model efficacy by displaying correct and incorrect classifications. We compared the predictions against the known diagnostic status of participants at their two-year follow-up assessment (Table 3). The confusion matrix was used to calculate performance measures that normalize the number of true positive and true negative predictions by the sample size of the model, allowing for comparison of classification accuracy across different approaches. Accuracy, sensitivity, and specificity were examined to determine the precision of the model (see Supplement).
Table 3.
Confusion Matrix
| Predicted Class | |||
|---|---|---|---|
| No CD | CD | ||
| True Class | No CD | 357 (True Negative) | 44 (False Positive) |
| CD | 25 (False Negative) | 356 (True Positive) | |
The FNN model demonstrated an accuracy of 91.18% and an area under the curve of .9578, suggesting a strong balance between sensitivity and specificity, while maximizing overall prediction accuracy. The sensitivity of the model was 89.03%, suggesting that the model was effective at detecting CD among participants with CD. The model specificity was 93.44%, indicating that the network was best at determining the absence of CD among typically developing participants.
Additional analyses were conducted to test if the: (1) FNN model performed better than traditional inferential statistics (i.e., logistic regression), (2) selected biopsychosocial risk factors were more predictive of CD compared to other syndromes, and (3) biopsychosocial model for CD performed better than models only within risk factor domains (see Supplement for additional models). The biopsychosocial FNN model yielded higher predictive accuracy, sensitivity, and specificity for CD than all other models.
Feature Importance
Olden’s algorithm in R was used to identify the relative importance of risk factors on the classification task by deconstructing and assessing weighted connections associated with each predictor (58). Greater ADHD and ODD symptomatology, frontoparietal efficiency, and reports of family members throwing objects positively related to CD at the two-year follow-up. Conversely, lower crystallized cognitive ability, card sorting ability, subcortical efficiency, frontoparietal degree, income, and parental monitoring predicted CD at the two-year follow-up (Figure 2) (see Supplement for feature importance reliability). Sensitivity analyses, which illustrate how the probability of CD diagnosis and risk factors covary while holding other factors constant (at the minimum, maximum, and 20th, 40th, 60th, and 80th quartiles), were conducted using the Lek’s profile method in R. These analyses supported the feature importance designations (Figure 3).
Figure 2. Feature Importance.
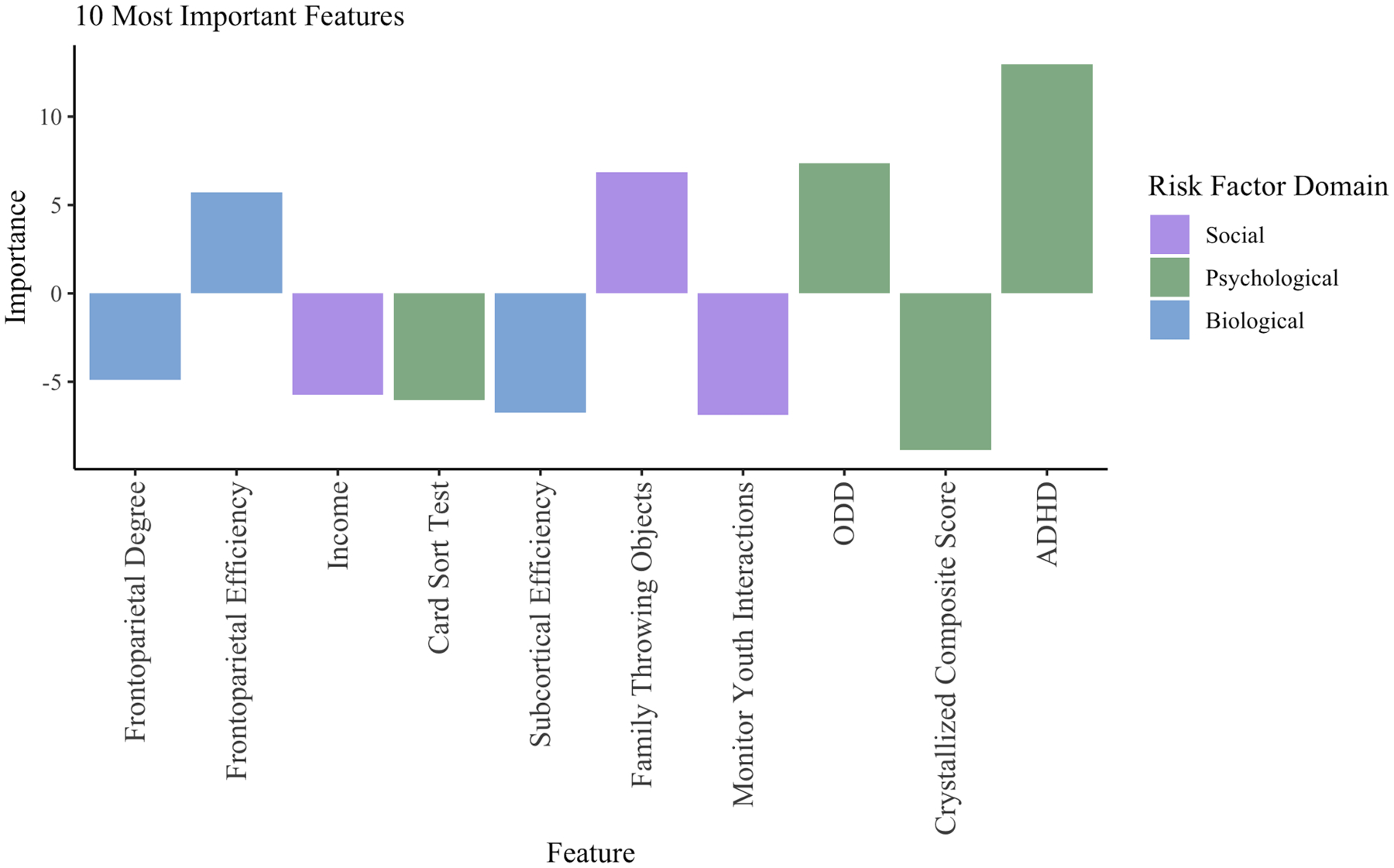
Note. Features are ordered from left to right by increasing absolute value of importance. The importance values assigned to each variable are in units that are based directly on the summed product of the connection weights. The 10 most important features in the model, excluding covariates, are highlighted. Bars in the positive direction represent a positive association with a CD diagnosis, whereas bars in the negative direction represent a negative association with a CD diagnosis.
Figure 3. Sensitivity Plots.
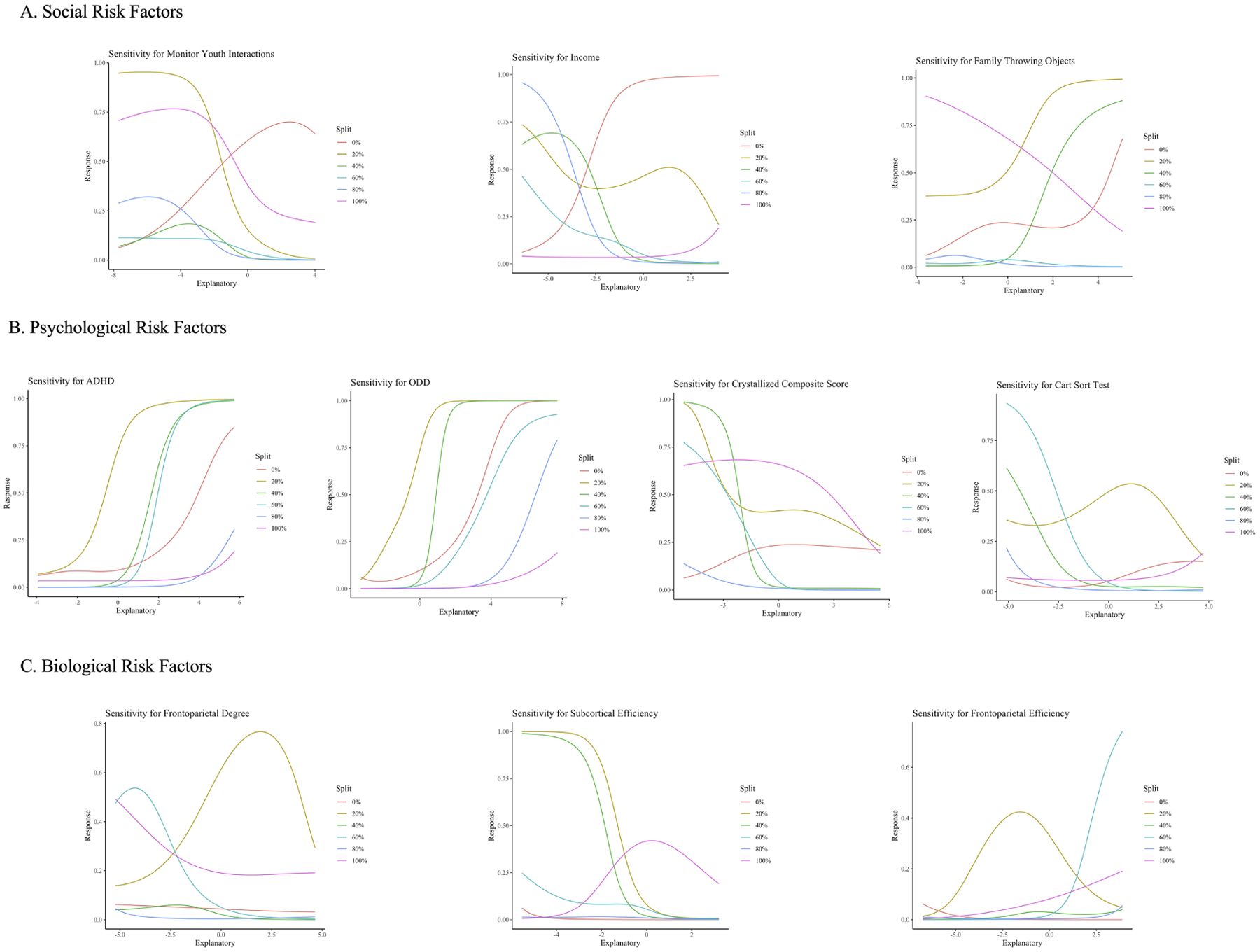
Note. The sensitivity plots display the relationship between model predictions and the risk factors. The explanatory variable denotes risk factor values, and the response variable denotes the probability that a participant is diagnosed with CD at the two-year follow-up assessment. Panel A presents sensitivity plots for the social risk factors, Panel B presents sensitivity plots for psychological risk factors, and Panel C presents sensitivity plots for biological risk factors. The risk factors of family members throwing objects, ADHD and ODD symptomatology, and frontoparietal efficiency positively correlate with the likelihood of developing CD, while the risk factors of income, parental monitoring, crystallized cognition, card sorting ability, subcortical efficiency, and frontoparietal degree negatively correlate with the likelihood of developing CD.
Discussion
The present study selected biopsychosocial risk factors that were identified in prior work and applied a machine learning model that was able to predict CD two years after the measurement of risk factors. It did so with high accuracy, specificity, and sensitivity. The biopsychosocial model also outperformed models examining risk factors within single domains. Further, the methods used in the present study allowed for a more nuanced identification of key factors and, importantly, it ranked them in terms of how well they predicted CD. Collectively, these risk factors are reflective of unpredictable, impulsive, deprived, and emotional external and internal contexts.
Key Risk Factors
In the social domain, family-level risk factors are related consistently to the development of CD (4, 34). In the present study, low parental monitoring, throwing objects, and low family income emerged as particularly important predictors. Parental monitoring of youth activities, plans, and peers commonly is viewed as essential for attenuating a variety of risky adolescent behaviors, including substance use, sexual activity, illegal behaviors, and association with delinquent peers (12). Further, it is positively associated with other beneficial parenting practices, such as warmth, cohesion, and involvement (59). Thus, it is unsurprising that low parental monitoring emerged as an important factor among CD sequelae. Additionally, harsh and aggressive parenting behaviors predict youth conduct problems (60). For instance, throwing objects in the household, although not specifically focused on in previous work, appears in parental quality inventories and reflects harsh and emotional parenting behaviors that are well-documented in relation to the onset and maintenance of CD. Youth who observe their family members throwing objects may learn that using aggressive behavior is a legitimate way to express feelings or achieve goals. Finally, the negative association between income and CD reinforces prior studies that find strong connections between low family socioeconomic status and CD development (61). Adversities often associated with low socioeconomic status and familial dysfunction may damage psychological functioning and contribute to the etiology of CD.
Among the strongest predictors of CD were those within the psychological domain. The influence of ADHD and ODD on the development of CD has been detailed in cross-sectional and longitudinal studies (61–63). ADHD, ODD, and CD often are conceptualized as externalizing disorders that share latent characteristics and premorbid genetic and environmental risk factors (64–66) (see Supplement). Further, both the genetic and environmental factors associated with externalizing disorders implicate several neuropsychological factors that contribute to changes in cognition.
Two neuropsychological factors of importance for predicting CD are lower crystallized cognition and card sorting performance. Crystallized cognition is believed to be dependent on past learning and acculturation, and is heavily influenced by education and cultural exposure, particularly during childhood (38). Thus, this component of cognition might be best interpreted alongside the social risk factors discussed above; specifically, low socioeconomic resources, lack of parental supervision, and home environments with displays of aggression may hinder the opportunities and experiences necessary to build verbal knowledge and skills. Furthermore, the observed deficits in card sorting are notable given previous research indicating that youth with CD struggle, in particular, with flexibly adjusting their behavior to new rules and information (67–70). These psychiatric and neurocognitive vulnerabilities highlight the early neurodevelopmental differences that characterize youth with CD.
Lastly, in the biological domain, evidence of disruptions in the topology of subcortical and frontoparietal networks is consistent with well-established theories and empirical evidence that anomalous functioning and connectivity between subcortical and cortical regions hinder affective and cognitive functioning in youth with CD (2, 14, 71–73). Low subcortical efficiency appears especially relevant for the etiopathogenesis of CD. A topographical aberration in cortical-subcortical communication that disrupts neural information processing could impact detecting, reacting to, and remembering salient or affective information. Disrupted cortical-subcortical communication may contribute to the propensity for some youth to engage in behaviors that put themselves at risk and violate the rights of others.
Similarly, the ranking of frontoparietal efficiency and degree as important biological risk factors reinforces findings that youth with CD often experience disruptions in networks related to inhibition, flexibility, and executive functioning (15, 39–42). While the positive feature importance of frontoparietal efficiency suggests that CD is associated with faster and/or less costly neural communication between the frontoparietal network and other networks in its immediate “neighborhood” (i.e., other networks closely connected to the frontoparietal network), the negative feature importance of frontoparietal degree indicates that this network shares fewer direct connections with other networks. The co-occurrence of high efficiency and low degree suggests that a frontoparietal network that is functionally segregated from the core of other networks contributes to the risk for CD. Excessive functional segregation may inhibit flexible communication and information integration between more distal nodes or networks, contributing to the neural and behavioral deficits observed among children with CD.
Limitations and Future Directions
Several key limitations should be noted. First, feature importance quantifies the relative influence of predictors on CD classification based on other predictors in the model and cannot measure their absolute influence. Furthermore, the training of each classifier incorporates inherent variation as samples are randomly separated into training and testing sets, and model parameters are randomly initialized before their optimization. As a result, performance measures and feature importance scores may vary for each training of the same model (25). Second, since we used curated data from the ABCD Study, we were limited to conducting the graph analysis at a network level. As a result, we were unable to take a more fine-grained approach to look at how node-level differences in specific regions of the cortex or subcortical structures (e.g., the amygdala) may represent biological risk factors. Finally, consistent with previous national community samples (74), the prevalence of CD is low in the current sample, particularly in comparison to samples with legal system-involved youth. The present study provides a proof-of-concept approach to diagnostic predictive modeling for CD; however, replication of the current findings and refinement of the model is needed.
Though the biopsychosocial model was a more precise classifier of CD compared to models within individual domains, further research should ascertain which risk factors represent the most reliable predictors of CD. This is especially true for factors in the biological domain. On the one hand, incorporating brain imaging in models outperformed some clinician-rated measures (e.g., neuropsychiatric scores) and added incremental value when combined with various sociodemographic predictors (see psychological and biological models in the Supplement and see 75). On the other hand, questions remain about the feasibility of brain imaging in daily practice and the reliability of neural measurement particularly during development (76). Currently, there is insufficient work to designate the neural risk factors that characterize CD. However, the growth of consortium-based research provides an opportunity for large-scale validation of neural markers for CD (77), and the hope to develop generalizable models. Furthermore, if reliable brain-based markers are identified, it might be possible to generate empirically derived proxy measures that meaningfully capture the underlying construct (78). While it seems that brain-based metrics capture important variance related to CD risk, which metrics and in what context these metrics most robustly relate to CD remains an open question.
Conclusions
The present study is a step towards developing an evidence-based approach to early intervention for CD. We used a combination of theory and data-driven methods to identify key risk factors for CD and achieved a high level of accuracy in prediction. These findings advance a literature on biopsychosocial models of CD and reinforce the importance of moving toward multidomain screening in school, medical, and psychiatric settings. In these settings, asking youth about their family situations and having youth complete brief assessments of psychiatric and neuropsychological functioning is feasible. While direct measurement of brain topology is not practical, there is promise in developing proxies for the latent constructs, which could open new doors for tailored screenings where resources are limited. Additionally, the approach employed in this study, which allows for single-subject prediction, would have great utility in legal settings. CD is overrepresented within the legal system (79) and the ability to develop accurate predictive models would be an improvement over current approaches routinely utilized in the legal system that have limited predictive value. Information gleaned from the approach used in the present study could better identify which interventions, based on person-specific biopsychosocial factors, might be effective, thereby preventing youth from becoming ensnared in the legal system (80). Further, the specification of neural factors predictive of CD may motivate clinical trials of targeted neuromodulatory interventions. CD is a complex disorder and advances in methodology, such as machine-learning, allow us greater precision in the identification of risk factors, both in terms of their relative prominence in the etiology as well as maintenance of CD. These same methods also provide guidance for employing interventions that can mitigate the harm and distress caused by CD.
Supplementary Material
Acknowledgments.
Data used in the preparation of this article were obtained from the Adolescent Brain Cognitive DevelopmentSM (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive (NDA). This is a multisite, longitudinal study designed to recruit more than 10,000 children age 9–10 and follow them over 10 years into early adulthood. The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/consortium_members/. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in the analysis or writing of this report. This manuscript reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators. The ABCD data repository grows and changes over time. The ABCD data used in this report came from 10.15154/1520591. DOIs can be found at https://nda.nih.gov/study.html?id=1042.
Funding Statement.
The ABCD Study® is supported by the National Institutes of Health and additional federal partners under award numbers U01DA041048, U01DA050989, U01DA051016, U01DA041022, U01DA051018, U01DA051037, U01DA050987, U01DA041174, U01DA041106, U01DA041117, U01DA041028, U01DA041134, U01DA050988, U01DA051039, U01DA041156, U01DA041025, U01DA041120, U01DA051038, U01DA041148, U01DA041093, U01DA041089, U24DA041123, U24DA041147.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest. All authors report no biomedical financial interests or potential conflicts of interest.
Despite the name, neural networks are not exclusive to neurobiological variables. The algorithm adopts this title because its nodes or units of information processing are loosely modeled on the neurons in a biological brain. Neural networks can process both neural and non-neural data.
References
- 1.American Psychiatric Association (2013): Diagnostic and statistical manual of mental disorders (DSM-5). Washington, D. C.: American Psychiatric Publishing. [Google Scholar]
- 2.Fairchild G, Hawes DJ, Frick PJ, Copeland WE, Odgers CL, Franke B, et al. (2019): Conduct disorder. Nature Reviews Disease Primers. 5:43. [DOI] [PubMed] [Google Scholar]
- 3.Rivenbark JG, Odgers CL, Caspi A, Harrington H, Hogan S, Houts RM, et al. (2018): The high societal costs of childhood conduct problems: evidence from administrative records up to age 38 in a longitudinal birth cohort. Journal of Child Psychology and Psychiatry. 59:703–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loeber R, Burke JD, Pardini DA (2009): Development and etiology of disruptive and delinquent behavior. Annual Review of Clinical Psychology. 5:291–310. [DOI] [PubMed] [Google Scholar]
- 5.Pauli R, Tino P, Rogers JC, Baker R, Clanton R, Birch P, et al. (2020): Positive and negative parenting in conduct disorder with high versus low levels of callous–unemotional traits. Development and psychopathology. 1–12. [DOI] [PubMed] [Google Scholar]
- 6.Piotrowska PJ, Stride CB, Croft SE, Rowe R (2015): Socioeconomic status and antisocial behaviour among children and adolescents: A systematic review and meta-analysis. Clinical psychology review. 35:47–55. [DOI] [PubMed] [Google Scholar]
- 7.Moore AA, Silberg JL, Roberson-Nay R, Mezuk B (2017): Life course persistent and adolescence limited conduct disorder in a nationally representative US sample: prevalence, predictors, and outcomes. Social psychiatry and psychiatric epidemiology. 52:435–443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greger HK, Myhre AK, Lydersen S, Jozefiak T (2015): Previous maltreatment and present mental health in a high-risk adolescent population. Child Abuse & Neglect. 45:122–134. [DOI] [PubMed] [Google Scholar]
- 9.Ogilvie JM, Stewart AL, Chan RCK, Shum DHK (2011): Neuropsychological measures of executive function and antisocial behavior: A meta-analysis. Criminology. 49:1063–1107. [Google Scholar]
- 10.Azeredo A, Moreira D, Barbosa F (2018): ADHD, CD, and ODD: Systematic review of genetic and environmental risk factors. Research in developmental disabilities. 82:10–19. [DOI] [PubMed] [Google Scholar]
- 11.Kim-Cohen J, Arseneault L, Caspi A, Tomás MP, Taylor A, Moffitt TE (2005): Validity of DSM-IV conduct disorder in 4½–5-year-old children: A longitudinal epidemiological study. American Journal of Psychiatry. 162:1108–1117. [DOI] [PubMed] [Google Scholar]
- 12.Murray J, Farrington DP (2010): Risk factors for conduct disorder and delinquency: key findings from longitudinal studies. The Canadian Journal of Psychiatry. 55:633–642. [DOI] [PubMed] [Google Scholar]
- 13.Morgan AB, Lilienfeld SO (2000): A meta-analytic review of the relation between antisocial behavior and neuropsychological measures of executive function. Clinical Psychology Review. 20:113–136. [DOI] [PubMed] [Google Scholar]
- 14.Tillem S, Conley MI, Baskin-Sommers A (2021): Conduct disorder symptomatology is associated with an altered functional connectome in a large national youth sample. Development and psychopathology. 1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu F-M, Zhou J-S, Zhang J, Xiang Y-T, Zhang J, Liu Q, et al. (2015): Functional connectivity estimated from resting-state fMRI reveals selective alterations in male adolescents with pure conduct disorder. PloS one. 10:e0145668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang J, Cao W, Wang M, Wang N, Yao S, Huang B (2019): Multivoxel pattern analysis of structural MRI in children and adolescents with conduct disorder. Brain imaging and behavior. 13:1273–1280. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J, Li X, Li Y, Wang M, Huang B, Yao S, et al. (2020): Three dimensional convolutional neural network-based classification of conduct disorder with structural MRI. Brain imaging and behavior. 14:2333–2340. [DOI] [PubMed] [Google Scholar]
- 18.Zhang J, Liu W, Zhang J, Wu Q, Gao Y, Jiang Y, et al. (2018): Distinguishing adolescents with conduct disorder from typically developing youngsters based on pattern classification of brain structural MRI. Frontiers in Human Neuroscience. 12:152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang J, Liu Y, Luo R, Du Z, Lu F, Yuan Z, et al. (2020): Classification of pure conduct disorder from healthy controls based on indices of brain networks during resting state. Medical & Biological Engineering & Computing. 58:2071–2082. [DOI] [PubMed] [Google Scholar]
- 20.Tor HT, Ooi CP, Lim-Ashworth NS, Wei JKE, Jahmunah V, Oh SL, et al. (2021): Automated detection of conduct disorder and attention deficit hyperactivity disorder using decomposition and nonlinear techniques with EEG signals. Computer Methods and Programs in Biomedicine. 200:105941. [DOI] [PubMed] [Google Scholar]
- 21.Trentacosta CJ, Hyde LW, Goodlett BD, Shaw DS (2013): Longitudinal prediction of disruptive behavior disorders in adolescent males from multiple risk domains. Child Psychiatry & Human Development. 44:561–572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gutman LM, Joshi H, Schoon I (2019): Developmental trajectories of conduct problems and cumulative risk from early childhood to adolescence. Journal of youth and adolescence. 48:181–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frick PJ, Dickens C (2006): Current perspectives on conduct disorder. Current psychiatry reports. 8:59–72. [DOI] [PubMed] [Google Scholar]
- 24.Dwyer DB, Falkai P, Koutsouleris N (2018): Machine learning approaches for clinical psychology and psychiatry. Annual review of clinical psychology. 14:91–118. [DOI] [PubMed] [Google Scholar]
- 25.Nielsen AN, Barch DM, Petersen SE, Schlaggar BL, Greene DJ (2020): Machine learning with neuroimaging: Evaluating its applications in psychiatry. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. 5:791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yarkoni T, Westfall J (2017): Choosing prediction over explanation in psychology: Lessons from machine learning. Perspectives on Psychological Science. 12:1100–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shafiei SB, Lone Z, Elsayed AS, Hussein AA, Guru KA (2020): Identifying mental health status using deep neural network trained by visual metrics. Translational psychiatry. 10:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan MI, Mitchell TM (2015): Machine learning: Trends, perspectives, and prospects. Science. 349:255–260. [DOI] [PubMed] [Google Scholar]
- 29.LeCun Y, Bengio Y, Hinton G (2015): Deep learning. nature. 521:436–444. [DOI] [PubMed] [Google Scholar]
- 30.Zhang Z, Beck MW, Winkler DA, Huang B, Sibanda W, Goyal H (2018): Opening the black box of neural networks: methods for interpreting neural network models in clinical applications. Annals of translational medicine. 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hagler DJ Jr, Hatton S, Cornejo MD, Makowski C, Fair DA, Dick AS, et al. (2019): Image processing and analysis methods for the Adolescent Brain Cognitive Development Study. Neuroimage. 202:116091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Iacono WG, Heath AC, Hewitt JK, Neale MC, Banich MT, Luciana MM, et al. (2018): The utility of twins in developmental cognitive neuroscience research: How twins strengthen the ABCD research design. Developmental cognitive neuroscience. 32:30–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazdin AE (1995): Conduct disorders in childhood and adolescence. Sage. [Google Scholar]
- 34.Loeber R, Burke JD, Lahey BB, Winters A, Zera M (2000): Oppositional Defiant and Conduct Disorder: A Review of the Past 10 Years, Part I. Journal of the American Academy of Child & Adolescent Psychiatry. 39:1468–1484. [DOI] [PubMed] [Google Scholar]
- 35.Moffitt TE (1993): Life-course-persistent and adolescence-limited antisocial behavior: A developmental taxonomy. Psychological review. 100:674–701. [PubMed] [Google Scholar]
- 36.Kaufman J, Birmaher B, Axelson D, Perepletchikova F, Brent D, Ryan N (2013): Kiddie Schedule for Affective Disorders and Schizophrenia Present and Lifetime Version 2013 (K-SADS-PL). Pittsburgh, PA: Western Psychiatric Institute and Yale University. [Google Scholar]
- 37.Gershon RC, Slotkin J, Manly JJ, Blitz DL, Beaumont JL, Schnipke D, et al. (2013): IV. NIH Toolbox Cognition Battery (CB): measuring language (vocabulary comprehension and reading decoding). Monographs of the Society for Research in Child Development. 78:49–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Akshoomoff N, Beaumont JL, Bauer PJ, Dikmen SS, Gershon RC, Mungas D, et al. (2013): VIII. NIH Toolbox Cognition Battery (CB): composite scores of crystallized, fluid, and overall cognition. Monographs of the Society for Research in Child Development. 78:119–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aghajani M, Klapwijk ET, van der Wee NJ, Veer IM, Rombouts SA, Boon AE, et al. (2016): Disorganized Amygdala Networks in Conduct-Disordered Juvenile Offenders With Callous-Unemotional Traits. Biol Psychiatry. [DOI] [PubMed] [Google Scholar]
- 40.Cohn MD, Pape LE, Schmaal L, van den Brink W, van Wingen G, Vermeiren RR, et al. (2015): Differential relations between juvenile psychopathic traits and resting state network connectivity. Hum Brain Mapp. 36:2396–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Finger EC, Marsh AA, Blair KS, Reid ME, Sims C, Ng P, et al. (2011): Disrupted Reinforcement Signaling in the Orbitofrontal Cortex and Caudate in Youths With Conduct Disorder or Oppositional Defiant Disorder and a High Level of Psychopathic Traits. American Journal of Psychiatry. 168:152–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou J, Yao N, Fairchild G, Cao X, Zhang Y, Xiang YT, et al. (2016): Disrupted default mode network connectivity in male adolescents with conduct disorder. Brain Imaging Behav. 10:995–1003. [DOI] [PubMed] [Google Scholar]
- 43.Casey B, Cannonier T, Conley MI, Cohen AO, Barch DM, Heitzeg MM, et al. (2018): The adolescent brain cognitive development (ABCD) study: imaging acquisition across 21 sites. Developmental cognitive neuroscience. 32:43–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Passamonti L, Fairchild G, Fornito A, Goodyer IM, Nimmo-Smith I, Hagan CC, et al. (2012): Abnormal anatomical connectivity between the amygdala and orbitofrontal cortex in conduct disorder. PloS one. 7:e48789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ripley B, Venables W, Ripley MB (2016): Package ‘nnet’. R package version. 7:700. [Google Scholar]
- 46.Sheela KG, Deepa SN (2013): Review on methods to fix number of hidden neurons in neural networks. Mathematical Problems in Engineering. 2013. [Google Scholar]
- 47.Huang G-B, Babri HA (1998): Upper bounds on the number of hidden neurons in feedforward networks with arbitrary bounded nonlinear activation functions. IEEE transactions on neural networks. 9:224–229. [DOI] [PubMed] [Google Scholar]
- 48.Vujicic T, Matijevic T, Ljucovic J, Balota A, Sevarac Z (2016): Comparative analysis of methods for determining number of hidden neurons in artificial neural network. Central european conference on information and intelligent systems: Faculty of Organization and Informatics Varazdin, pp 219. [Google Scholar]
- 49.Qiao X, Liu Y (2009): Adaptive weighted learning for unbalanced multicategory classification. Biometrics. 65:159–168. [DOI] [PubMed] [Google Scholar]
- 50.Chawla NV, Bowyer KW, Hall LO, Kegelmeyer WP (2002): SMOTE: synthetic minority over-sampling technique. Journal of artificial intelligence research. 16:321–357. [Google Scholar]
- 51.Lunardon N, Menardi G, Torelli N (2014): ROSE: A Package for Binary Imbalanced Learning. R journal. 6. [Google Scholar]
- 52.Menardi G, Torelli N (2014): Training and assessing classification rules with imbalanced data. Data mining and knowledge discovery. 28:92–122. [Google Scholar]
- 53.Whelan R, Garavan H (2014): When optimism hurts: inflated predictions in psychiatric neuroimaging. Biological psychiatry. 75:746–748. [DOI] [PubMed] [Google Scholar]
- 54.Bishop CM (1995): Training with noise is equivalent to Tikhonov regularization. Neural computation. 7:108–116. [Google Scholar]
- 55.Ying X (2019): An overview of overfitting and its solutions. Journal of Physics: Conference Series: IOP Publishing, pp 022022. [Google Scholar]
- 56.Belloni A, Chernozhukov V, Hansen C (2014): High-dimensional methods and inference on structural and treatment effects. Journal of Economic Perspectives. 28:29–50. [Google Scholar]
- 57.Kim J-H (2009): Estimating classification error rate: Repeated cross-validation, repeated hold-out and bootstrap. Computational statistics & data analysis. 53:3735–3745. [Google Scholar]
- 58.Olden JD, Joy MK, Death RG (2004): An accurate comparison of methods for quantifying variable importance in artificial neural networks using simulated data. Ecological modelling. 178:389–397. [Google Scholar]
- 59.Racz SJ, McMahon RJ (2011): The relationship between parental knowledge and monitoring and child and adolescent conduct problems: A 10-year update. Clinical child and family psychology review. 14:377–398. [DOI] [PubMed] [Google Scholar]
- 60.Hoeve M, Dubas JS, Eichelsheim VI, Van der Laan PH, Smeenk W, Gerris JR (2009): The relationship between parenting and delinquency: A meta-analysis. Journal of abnormal child psychology. 37:749–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Loeber R, Green SM, Keenan K, Lahey BB (1995): Which boys will fare worse? Early predictors of the onset of conduct disorder in a six-year longitudinal study. Journal of the American Academy of Child & Adolescent Psychiatry. 34:499–509. [PubMed] [Google Scholar]
- 62.Van Lier PA, Der Ende Jv, Koot HM, Verhulst FC (2007): Which better predicts conduct problems? The relationship of trajectories of conduct problems with ODD and ADHD symptoms from childhood into adolescence. Journal of Child Psychology and Psychiatry. 48:601–608. [DOI] [PubMed] [Google Scholar]
- 63.Biederman J, Petty C, Dolan C, Hughes S, Mick E, Monuteaux M, et al. (2008): The long-term longitudinal course of oppositional defiant disorder and conduct disorder in ADHD boys: findings from a controlled 10-year prospective longitudinal follow-up study. Psychological medicine. 38:1027–1036. [DOI] [PubMed] [Google Scholar]
- 64.Tuvblad C, Zheng M, Raine A, Baker LA (2009): A common genetic factor explains the covariation among ADHD ODD and CD symptoms in 9–10 year old boys and girls. Journal of abnormal child psychology. 37:153–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Witkiewitz K, King K, McMahon RJ, Wu J, Luk J, Bierman KL, et al. (2013): Evidence for a multi-dimensional latent structural model of externalizing disorders. Journal of abnormal child psychology. 41:223–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lahey BB, Zald DH, Hakes JK, Krueger RF, Rathouz PJ (2014): Patterns of heterotypic continuity associated with the cross-sectional correlational structure of prevalent mental disorders in adults. JAMA psychiatry. 71:989–996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Blair RJ (2004): The roles of orbital frontal cortex in the modulation of antisocial behavior. Brain and Cognition. 55:198–208. [DOI] [PubMed] [Google Scholar]
- 68.Fairchild G, van Goozen SH, Stollery SJ, Aitken MR, Savage J, Moore SC, et al. (2009): Decision making and executive function in male adolescents with early-onset or adolescence-onset conduct disorder and control subjects. Biological psychiatry. 66:162–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Moffitt TE (1993): The neuropsychology of conduct disorder. Development and psychopathology. 5:135–151. [Google Scholar]
- 70.Matthys W, Vanderschuren LJ, Schutter DJ, Lochman JE (2012): Impaired neurocognitive functions affect social learning processes in oppositional defiant disorder and conduct disorder: Implications for interventions. Clinical child and family psychology review. 15:234–246. [DOI] [PubMed] [Google Scholar]
- 71.Fairchild G, Passamonti L, Hurford G, Hagan CC, von dem Hagen EA, van Goozen SH, et al. (2011): Brain structure abnormalities in early-onset and adolescent-onset conduct disorder. American Journal of Psychiatry. 168:624–633. [DOI] [PubMed] [Google Scholar]
- 72.Noordermeer SD, Luman M, Oosterlaan J (2016): A systematic review and meta-analysis of neuroimaging in oppositional defiant disorder (ODD) and conduct disorder (CD) taking attention-deficit hyperactivity disorder (ADHD) into account. Neuropsychology review. 26:44–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Blair RJR, Zhang R (2020): Recent neuro-imaging findings with respect to conduct disorder, callous-unemotional traits and psychopathy. Current opinion in psychiatry. 33:45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Merikangas KR, He J-P, Burstein M, Swanson SA, Avenevoli S, Cui L, et al. (2010): Lifetime prevalence of mental disorders in U.S. adolescents: results from the National Comorbidity Survey Replication--Adolescent Supplement (NCS-A). Journal of the American Academy of Child and Adolescent Psychiatry. 49:980–989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dadi K, Varoquaux G, Houenou J, Bzdok D, Thirion B, Engemann D (2021): Population modeling with machine learning can enhance measures of mental health. GigaScience. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kennedy JT, Harms MP, Korucuoglu O, Astafiev SV, Barch DM, Thompson WK, et al. (2021): Reliability and stability challenges in ABCD task fMRI data. bioRxiv. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Thompson PM, Jahanshad N, Ching CR, Salminen LE, Thomopoulos SI, Bright J, et al. (2020): ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Translational psychiatry. 10:1–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Brazil IA, van Dongen JDM, Maes JHR, Mars RB, Baskin-Sommers A (2016): Classification and treatment of antisocial individuals: From behavior to biocognition. Neuroscience & Biobehavioral Reviews. 91:259–277. [DOI] [PubMed] [Google Scholar]
- 79.Teplin LA, Abram KM, McClelland GM, Mericle AA, Dulcan MK, Washburn JJ (2006): Psychiatric Disorders of Youth in Detention. Juvenile Justice Bulletin. Office of Juvenile Justice and Delinquency Prevention. [Google Scholar]
- 80.Baskin-Sommers A, Chang S-A, Estrada S, Chan L (2021): Toward Targeted Interventions: Examining the Science Behind Interventions for Youth Who Offend. Annual Review of Criminology. 5. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


