Abstract
Epilepsy is a common neurological disorder associated with alterations in cortical and subcortical brain networks. Despite a historical focus on gray matter regions involved in seizure generation and propagation, the role of white matter (WM) network disruption in epilepsy and its comorbidities has sparked recent attention. In this review, we describe patterns of WM alterations observed in focal and generalized epilepsy syndromes and highlight studies linking WM disruption to cognitive and psychiatric comorbidities, drug-resistance and poor surgical outcomes. Both tract-based and connectome-based approaches implicate the importance of extratemporal and temporo-limbic WM disconnection across a range of comorbidities, and an evolving literature reveals the utility of WM patterns for predicting outcomes following epilepsy surgery. We encourage new research employing advanced analytic techniques (e.g., machine learning) that will further shape our understanding of epilepsy as a network disorder and guide individualized treatment decisions. We also address the need for research that examines how neuromodulation and other treatments (e.g., laser ablation) impact WM networks, as well as research that leverages larger and more diverse samples, longitudinal designs, and improved MRI acquisitions. These steps will be critical to ensuring generalizability of current research and determining the extent to which neuroplasticity within WM networks can influence patient outcomes.
Keywords: epilepsy, diffusion tensor imaging, white matter, cognition, clinical outcomes
Epilepsy is defined by the presence of recurrent and unprovoked seizures and affects approximately 50 million people worldwide (Bell et al. 2014). Once considered predominantly a gray matter disease, epilepsy is now understood to affect white matter (WM) networks throughout the brain, typically characterized by loss of WM microstructure and disrupted network connectivity. These widespread alterations are observed in patients whose seizures originate in localized regions of the brain (i.e., focal epilepsy) as well as those whose seizures originate broadly and often bilaterally (i.e., generalized epilepsy). Although the origin(s) of WM injury in epilepsy are still debated, its consequences are now better appreciated, with converging studies demonstrating a contribution of WM disconnection to neurobehavioral comorbidities, measures of disease severity, and postsurgical outcomes.
In this review, we summarize new literature describing patterns of WM network alterations in adults with common focal and generalized epilepsy syndromes, including temporal lobe epilepsy (TLE), extratemporal focal epilepsy (ExE), and genetic generalized epilepsy (GGE). We focus our review on results obtained from diffusion-weighted imaging (dMRI) since dMRI has become the most widely used non-invasive method for interrogating WM microstructure and architecture in human neuroscience. We then provide evidence from dMRI research that WM alterations may underlie common cognitive and psychiatric comorbidities in epilepsy, as well as aid in the prediction of postoperative cognitive, seizure, and visual field outcomes. Finally, we address new data using advanced dMRI sequences and analytic procedures (e.g., machine learning), which may accelerate our understanding of the neurobiology of epilepsy and lead to enhanced predictions of patient-specific outcomes.
WM network abnormalities within and across epilepsy syndromes
The presence of WM abnormalities in epilepsy has long been observed, with earlier studies identifying WM hyperintensities, as well as global or regional WM volume loss in patients with different epilepsy syndromes. However, the extent of these abnormalities and their intrinsic patterns were not fully appreciated until the advent and application of dMRI tractography in the early 1990s (Yogarajah & Duncan 2007). In particular, dMRI has emerged as the method of choice for interrogating WM structure in epilepsy due to its ability to derive quantitative measures of individual fiber tract integrity and characterize the adverse effects of epilepsy on cortico-cortical disconnection, even in the absence of direct injury to the cortex.
However, epilepsy is not a single disorder. Instead, the epilepsies are a group of disorders that are unified by a common symptom (i.e., seizures) that can originate from almost anywhere in the brain. For this reason, WM regions, tracts, and networks affected by epilepsy do not follow one uniform pattern, but rather have some syndrome-specific features with abnormalities that are often most pronounced proximal to the seizure focus. Two recent, large-scale studies have well-characterized these patterns for the most common epilepsy syndromes (Slinger et al. 2016; Hatton et al. 2020), and therefore, each pattern is only briefly summarized below.
TLE:
TLE is the most common focal epilepsy syndrome in adults, and therefore, has received the most attention. In TLE, seizures most commonly arise from the hippocampus and other medial temporal lobe structures. For this reason, attention has focused on hippocampal efferent and afferent tracts, including the parahippocampal cingulum and fornix, as well as the uncinate fasciculus; Figure 1A. These tracts are among the most affected in patients who have gliosis and cell loss in the hippocampus (i.e., hippocampal sclerosis; HS), and in those with an early age of seizure onset and longer disease duration, with effects larger on the side ipsilateral to the seizure focus (Hatton et al. 2020). In addition, temporo-limbic tract alterations in TLE appear to follow a centrifugal pattern such that microstructural abnormalities increase along each tract as they approach the seizure focus (Concha et al. 2012). This pattern implies that WM alterations are likely intrinsic to the TLE syndrome, rather than general to epilepsy or secondary to treatment-related effects (e.g., anti-seizure medications; ASMs). However, other WM association tracts that course through the temporal lobe (e.g., inferior longitudinal fasciculus) and those distal to the seizure focus (e.g., corpus callosum, external capsule) also show marked WM changes bilaterally in TLE, providing evidence for broad network pathology in patients with focal epilepsy that could represent developmental (i.e., poor myelination of WM tracts) or iatrogenic (e.g., ASM) factors.
Figure 1. White matter tracts of interest and depiction of structural connectome.
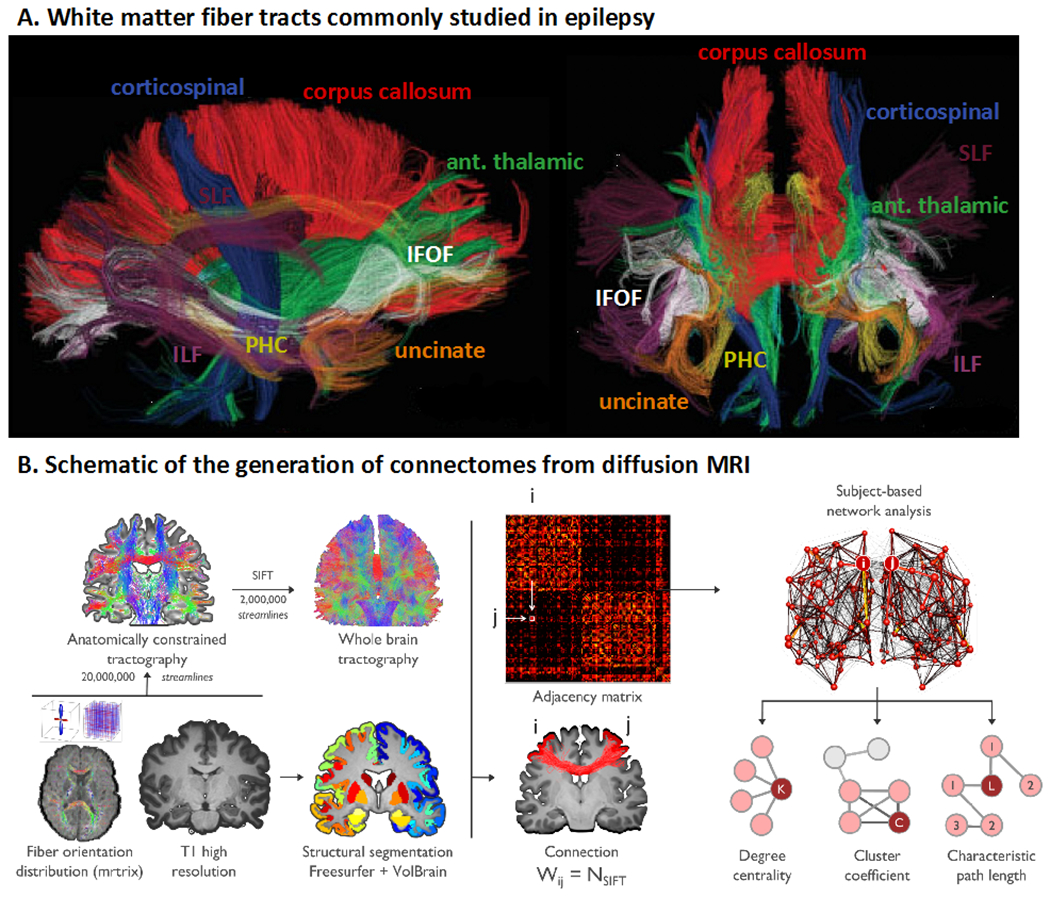
(A) DTI-derived fiber tracts that are commonly studied in relation to clinical and cognitive outcomes in epilepsy. ILF = inferior longitudinal fasciculus; PHC = parahippocampal cingulum; IFOF = inferior fronto-occipital fasciculus; uncinate = uncinate fasciculus; ant. thalamic = anterior thalamic radiations. Adapted from Hagler et al., 2009, with permission. (B) Schematic showing the construction of a diffusion MRI connectome. Preprocessed dMRI data are analyzed in an automatically parcellated anatomical space. Adjacency (i.e., connectivity) matrices are then generated by systematically assessing pairwise associations between pairs of all regions (with regions i and j given as an example). Connectivity matrices are equivalent to brain graphs, where brain regions correspond to nodes and structural connections correspond to edges. Connection weight (Wij) is defined as the number of fiber tract connections between two nodes (i and j). The final step (top right) includes graph theory analysis based on the adjacency matrix to extract brain network topological organization (i.e., degree centrality, cluster coefficient, characteristic path length). Adapted from Rodríguez-Cruces et al., 2020, with permission.
ExE:
Similarly, patients with ExE harbor a focal epilepsy syndrome with seizures originating from one or more extratemporal areas of the brain, typically in the frontal lobes. Although studies in ExE, such as frontal lobe epilepsy (FLE), are more scarce, decreases in fractional anisotropy (FA) and increases in mean diffusivity (MD) have been shown throughout the frontal lobe WM and frontostriatal fibers, with marked alterations along midline bundles and tracts, including the genu and body of the corpus callosum (Widjaja et al. 2014), anterior corona radiata, dorsal cingulum, and external capsule (Hatton et al. 2020). Associations between clinical variables and WM disruptions in ExE have been less consistent, with some studies demonstrating that an early age of onset and/or longer disease duration is associated with poorer WM network integrity (Wang et al. 2011; Lin et al. 2020) and others not finding associations (Hatton et al. 2020). The heterogeneity within ExE makes this syndrome challenging to study as a single group, and clinico-diffusion correlations more difficult to capture.
GGE:
GGE includes several related syndromes with generalized seizure onset, including juvenile myoclonic epilepsy (JME), where a predominant genetic contribution is suspected. Although patients with GGE do not have visible structural abnormalities on MRI, thalamocortical dysfunction is often present and accompanied by morphological alterations (Bernhardt et al. 2009; Whelan et al. 2018). Studies of WM disruption in GGE have suggested greatest alterations in fronto-midline fibers, including the genu and body of the corpus callosum, anterior corona radiata, external capsule (Hatton et al. 2020) and in thalamo-cortical pathways (Keller et al. 2011; Lee et al. 2014). In addition, alterations in pre-supplementary motor area to prefrontal connectivity patterns have been observed and appear unique to GGE syndromes (Vollmar et al. 2012). However, there is some evidence that WM alterations in GGE are less severe and widespread than those observed in focal epilepsy (Slinger et al. 2016).
Despite these syndrome-specific features, new results from the Enhancing NeuroImaging and Genetics through Meta-Analysis (ENIGMA)-Epilepsy working group have revealed striking similarities in WM compromise across these common epilepsy syndromes. In 1249 patients with TLE, FLE, and GGE compared to 1069 healthy controls, WM alterations were observed within 36 of 38 association, commissural and projection fibers (Hatton et al. 2020) - Figure 2. Across patient groups, reductions in FA and increases in MD were greatest in fronto-central WM, including the genu and body of the corpus callosum, dorsal cingulum and external capsule. Although the severity of these alterations varied across syndromes and was most pronounced in TLE with HS, bilateral alterations in many anterior midline fibers were uniform across groups. Although the underlying mechanism(s) that lead to this shared midline pathology are unknown, one possibility is that midline WM is more vulnerable to the direct impact of seizures (locally in GGE and FLE or from seizure propagation via the thalamus in TLE). Another possibility is that midline WM is more vulnerable to neurological or neuropsychiatric injury in general. In support of the latter, Hatton and colleagues observed very similar patterns of WM disruption between epilepsy and several neuropsychiatric disorders (e.g., bipolar, schizophrenia, depression), with the body and genu of the corpus callosum affected across all disorders. Indeed, several studies have demonstrated cross-disorder connectomic vulnerability, revealing that hub regions that are highly connected and potentially important for communication tend to be disproportionally affected by disease (van den Heuvel and Sporns 2013). Broad patterns of microstructural alterations shared across epilepsy syndromes were not previously appreciated due to a tendency of the field to segregate studies according to single epilepsy syndromes. Although some syndrome-specific findings were evident, shared patterns of WM injury could explain why cognitive and psychiatric co-morbidities can be quite similar in two patients with different syndromes, but heterogeneous within a syndrome. It is these clinico-diffusion associations that are the focus of this review.
Figure 2. White matter microstructural differences between all epilepsy syndromes compared to healthy controls.
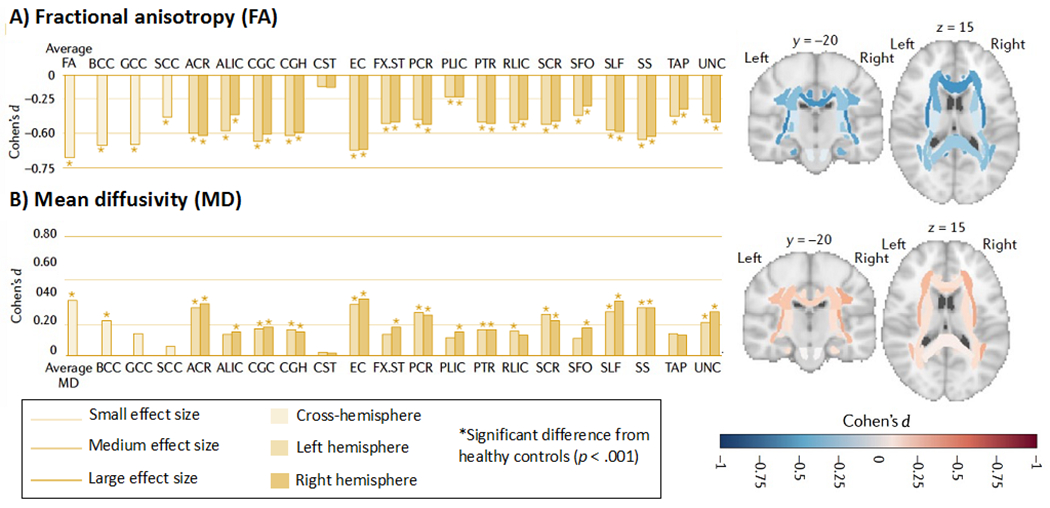
All values represent Cohen’s d effect size estimates for differences in (A) fractional anisotropy (FA) and (B) mean diffusivity (MD) between the epilepsy group and healthy controls. Positive effect sizes reflect diffusion values greater than controls; negative effect sizes represent values lower than controls; y and z values represent the slice number for the coronal and axial planes, respectively. Across all epilepsies, the greatest effects on FA were observed in the body of the corpus callosum (BCC) and genu of the corpus callosum (GCC), external capsule (EC), cingulum and corona radiata. Greatest effects on MD were observed in the EC, anterior corona radiata (ACR) and superior longitudinal fasciculus (SLF); ALIC, anterior limb, internal capsule; CGC, dorsal cingulum; CGH, parahippocampal cingulum; CST, corticospinal tract; FX- ST, fornix; PCR, posterior corona radiata; RLIC, rostral limb, internal capsule; SCC, splenium corpus callosum; SCR, superior corona radiata; SFO, superior frontal occipital fasciculus; SS, sagittal stratum; TAP, tapetum; UNC, uncinate fasciculus. These data are from the ENIGMA-Epilepsy working group (over 2,000 participants). Adapted from Hatton et al., 2020 with permission.
From WM tracts to WM networks
Extrapolating from the study of specific WM tracts, a mounting literature has aggregated connectivity information across multiple regions to study macroscale brain network reorganization in epilepsy. These studies have utilized approaches from complex systems analysis such as graph theory, as a formalism to examine changes in WM network topology (Larivière et al. 2021; Tavakol et al. 2019). Such macroscopic analyses initially generate systematic representations of connectivity, so-called ‘connectomes’, based on WM tract properties between all pairs of cortical and subcortical regions - Figure 1B. The topology of the resulting connectomes can then be analyzed at a global scale (by studying network clustering that relates to local communication efficiency, or by studying path length that reflects global efficiency), by examining submodules within the networks through network decomposition techniques, or by studying network embedding of individual regions, with hub mapping being a prominent example of the latter. Complementing graph-theoretical network descriptions, complementary approaches from network neuroscience have emerged, including the use of network communication models that assess how a structurally-wired connectome can generate brain dynamics (Girardi-Schappo et al. 2021), or the study of spatial trends in network organization, also referred to as connectivity gradients (Huntenburg et al. 2018).
Similar to the study of WM tracts, the most robust connectomics literature has focused on TLE. These studies have described shifts in cortical as well as subcortical network topology in TLE at global, modular, and nodal scales. One of the earliest graph theoretical studies in TLE reported reductions in both global and local efficiency in a group of left TLE patients relative to controls, in addition to alterations in hub topography in TLE (Liu et al. 2014). These studies have been extended to assess the utility of connectome measures to predict postoperative seizure outcome (see “Postsurgical seizure outcomes” section and Table 3). Other studies provided connectome-level evidence for a broad association between degree of mesiotemporal pathology and WM alterations in TLE. In one study, the authors observed overall more marked network reorganization in patients with more severe HS (based on histopathology) compared to TLE patients with only subtle hippocampal pathology (Bernhardt et al. 2019) - Figure 3. Other recent investigations used connectome-informed dynamic communication models, underscoring that the alterations in the brain’s WM architecture may relate to delayed dynamic signal flow, and ultimately cognitive impairments across multiple domains in TLE (Girardi-Schappo et al. 2021).
Table 3.
Studies examining white matter associations with seizure lateralization, drug-resistance, postsurgical seizure outcomes, and postsurgical visual field deficits*
| Author & year | Clinical outcome | Epilepsy syndrome | N | Approach & ROI(s) | Findings |
|---|---|---|---|---|---|
| Ahmadi et al. [2009] | Seizure lateralization | TLE | 11 LTLE, 10 RTLE, 21 HC | Tract-based DTI (CG, PH, SLF, ILF, UF, fornix, ATR, IFOF) | FA of the UF and PH correctly lateralized 90% of all cases (100% of all patients without HS). |
| An et al. [2014] | Seizure lateralization | TLE | 17 LTLE, 15 RTLE, 34 HC | Whole-brain DTI | FA of the bilateral UF, right SLF, IFOF, ILF and right posterior corona radiata differentiated LTLE vs. RTLE with 91% accuracy. |
| Besson et al. [2014] | Seizure lateralization | TLE | 19 LTLE, 20 RTLE, 28 HC | Whole-brain DTI (SC) | Patients with LTLE had more severe connectivity reductions and a strongly lateralized fronto-temporal disconnection patterns compared to RTLE patients. |
| Concha et al. [2012] | Seizure lateralization | TLE | 30 TLE, 21 HC | Tract-based DTI (UF, ILF, AF) | MD of clusters along the ILF correctly lateralized 87% of patients (91% in patients with hippocampal atrophy, 71% of patients with normal hippocampal volume). |
| Kamiya et al. [2016] | Seizure lateralization | TLE | 29 LTLE, 15 RTLE, 14 HC | Whole-brain DTI + graph theory | FA of left PCG, cuneus, and bilateral hippocampi differentiated LTLE vs. RTLE with 73 to 86% accuracy, with an AUC of 0.82 to 0.91. |
| Nazem-Zadeh et al. [2014] | Seizure lateralization | TLE | 20 TLE, 23 HC | Tract-based DTI (PIC, COF, hippocampus) | Lower FA in the PIC & COF ipsilateral to side of seizure onset was observed in 10/10 MTS patients and 15/20 total TLE patients. Hippocampal MD, hippocampal volume, and hippocampal FLAIR signal lateralized 19/20 cases. |
| Nazem-Zadeh et al. [2016] | Seizure lateralization | TLE | 31 TLE, 23 HC | Tract-based DTI (CC, CG, fornix) | 11 FA measurements in the cingulate, callosal and forniceal subregions lateralized all TLE cases. |
| Pustina et al. [2015] | Seizure lateralization | TLE | 28 LTLE; 28 RTLE | Tract-based DTI (fornix, PHC, UF, ILF) | FA asymmetry of the ILF predicted seizure lateralization with 71% accuracy, although PET (glucose metabolism) asymmetry outperformed DTI. Metabolism was closely related with white matter in LTLE. |
| Labate et al. [2015] | Drug resistance | TLE | 48 benign MTLE, 38 refractory MTLE, 54 HC | DTI (temporal lobe WM) | Compared to benign MTLE patients, refractory MTLE patients had higher MD and lower FA in the ipsilateral temporal WM. Temporal lobe FA was the most accurate in discriminating refractory vs. benign MTLE patients, with an AUC of 74%. AUCs of temporal lobe MD, hippocampal volume, and HS were 64%, 65%, and 66%, respectively. |
| Labate et al. [2020] | Drug resistance | TLE | 39 stable MTLE, 16 refractory MTLE | Whole-brain DTI | Benign MTLE patients who became refractory (rMTLE) exhibited reduced WM volume in the AF, corticospinal tracts, L retrosplenial cingulum, and L ILF prior to the development of drug-resistance. Following progression to rMTLE, patients had decreased FA in the CC, SLF, and major bundles of the R hemisphere compared to patients who remained stable. |
| McKavanagh et al. [2021] | Drug resistance | GGE | 10 non-refractory GGE, 23 refractory GGE, 39 HC | Whole-brain DTI (SC) | Refractory patients had more significant FA network disruption, while non-refractory patients had reduced diffusion network alterations and demonstrated increased nodal volume. |
| Park et al. [2020] | Drug resistance | Focal epilepsy of unknown etiology | 64 ASM good responders, 20 ASM poor responders | Whole-brain DTI + graph theory | Assortativity coefficient (i.e., the tendency of nodes with similar properties to be more connected) was higher in ASM good responders than in ASM poor responders. |
| Bonilha et al. [2013] | Postsurgical seizure outcome (ATL) | TLE | 20 unilateral MTLE, 18 HC | Whole-brain DTI (SC) | Patients who did not become seizure-free following surgery had higher connectivity between the ipsilateral MTL & LTL, ipsilateral MTL & parietal lobe, and contralateral temporal pole & parietal lobe. |
| Bonilha et al. [2015] | Postsurgical seizure outcome (ATL) | TLE | 35 TLE | Whole brain DTI (SC) | Fewer abnormalities within a subnetwork consisting of the ipsilateral hippocampus, amygdala, thalamus, superior frontal region, lateral temporal gyri, insula, orbitofrontal cortex, cingulate, and lateral occipital gyrus was correlated with greater likelihood of post-ATL seizure freedom. WM alone was able to predict postsurgical seizure freedom with 90% specificity, and adding clinical data increased this to 94%. |
| Gleichgerrcht et al. [2018] | Postsurgical seizure outcome (ATL, laser ablation) | TLE | 50 unilateral TLE | Whole-brain DTI (SC) | SC was able to accurately classify seizure outcomes with a PPV (seizure freedom) = 88 ° 7% and NPV (seizure refractoriness) = 79 ± 8%, compared to the <50% accuracy of a model with clinical variables alone. |
| Gleichgerrcht et al. [2020] | Postsurgical seizure outcome (resection, laser ablation) | TLE | Training sample of 121 TLE; Validation sample of 47 TLE | Whole-brain DTI (SC) | Patients with abnormally integrated network nodes were less likely to achieve post-surgical seizure freedom (AUC = .88 based on regional betweenness centrality). This was higher than any other machine learning indices or gray matter volume. Nodes of bilateral parahippocampal gyri and superior temporal gyri contributed the most to the model. |
| Keller et al. [2017] | Postsurgical seizure outcome (amygdalohippocampectomy) | TLE | 43 TLE-HS, 44 HC | DTI (FF, PWMB, UF); automated fiber quantification | FA was lower in contralateral PWMB and ipsilateral FF in patients who became seizure-free compared to those who did not. PWMB classified outcomes with an AUC of 0.81 and ipsilateral FF classified outcomes with an AUC of 0.71. |
| Moreira da Silva et al. [2020] | Postsurgical seizure outcome (ATL) | TLE | 26 LTLE, 23 RTLE, 17 HC | Whole-brain DTI (SC + graph theory) | All TLE patients experienced decreased quantitative anisotropy (QA) in the ipsilateral UF and IFOF. LTLE patients with larger reductions in QA were more likely to be seizure-free postoperatively. Widespread increases in nodal betweenness centrality was not associated with seizure outcomes. |
| Morgan et al. [2017] | Postsurgical seizure outcome (ATL, laser ablation, amygdalohippocampectomy) | TLE | 7 LTLE, 15 RTLE, 35 HC | Whole-brain DTI (SC) | Patients with connectivity patterns similar to a previously proposed seizure propagation network had a more favorable postsurgical seizure outcome. The connectivity model was able to distinguish between patients with unfavorable outcomes from those who were seizure-free or had favorable outcomes with 100% accuracy, outperforming clinical and demographic variables. |
| Taylor et al. [2018] | Postsurgical seizure outcome | TLE | 53 TLE | Whole-brain DTI (SC + graph theory) | Epilepsy surgery reduced network efficiency by <10% in the majority of patients. 15 network connections (8 of which connected the ipsilateral temporal lobe) differentiated seizure-free from non seizure-free outcomes with 79% accuracy and 65% specificity. |
| Borius et al. [2014] | Postsurgical VFD (ATL) | TLE | 16 TLE, 13 HC | Tract-based DTI | Increased percentage of virtual fibers reconstructed by tractography and damaged by surgery was associated with greater visual impairment. A threshold of 5.5% of injured virtual fibers predicted a VFD with 71% sensitivity and 88% specificity. |
| David et al. [2021] | Postsurgical VFD (Amygdalohippocampectomy) | TLE | 28 TLE | Whole-brain DTI (SC) | Reduction of post-surgical FA in the ipsilateral optic radiation was associated with increased severity of VFD. Patients with VFD had increased postoperative local connectivity changes compared to patients without VFD. No preoperative WM correlates of VFD severity were observed. |
| Winston et al. [2012] | Postsurgical VFD (ATL) | TLE | 20 TLE | Tract-based DTI (optic radiations) | Patients who experienced postsurgical VFDs had resections that extended posterior to the anterior limit of Meyer’s loop with significant correlation between distance and degree of VFD. |
| Winston et al., [2014] | Postsurgical VFD (ATL) | TLE | 21 TLE with and 44 TLE without intraoperative dMRI | Tract-based DTI (optic radiations) | The VFDs in the contralateral superior quadrant were significantly lower with intraoperative dMRI guidance than without. Whereas 13% of the cohort without intraoperative dMRI failed to meet criteria for driving, all patients in the intraoperative dMRI cohort met this criteria. |
Note: we provide an update on postsurgical VFD studies not previously reviewed in Piper et al., 2014.
AF = arcuate fasciculus; ASM = anti-seizure medication; ATL = anterior temporal lobectomy; ATR = anterior thalamic radiations; AUC = area under the curve; CC = corpus callosum; CG = cingulate gyrus; COF = crus of fornix; CST = corticospinal tract; FF = fimbria-fornix; GGE = genetic generalized epilepsy; HC = healthy controls; HS = hippocampal sclerosis; IFOF = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; LTL = lateral temporal lobe; MTL = mesial temporal lobe; MTLE = mesial temporal lobe epilepsy; PC = participation coefficient; PH = parahippocampal gyrus; PHC = parahippocampal cingulum; PIC = posteroinferior cingulum; PWMB = parahippocampal white matter bundle; SC = structural connectome; SLF = superior longitudinal fasciculus; TLE = temporal lobe epilepsy; UF = uncinate fasciculus; VFD = visual field deficit; WMD = within-module degree
Figure 3. Group differences in network topology.
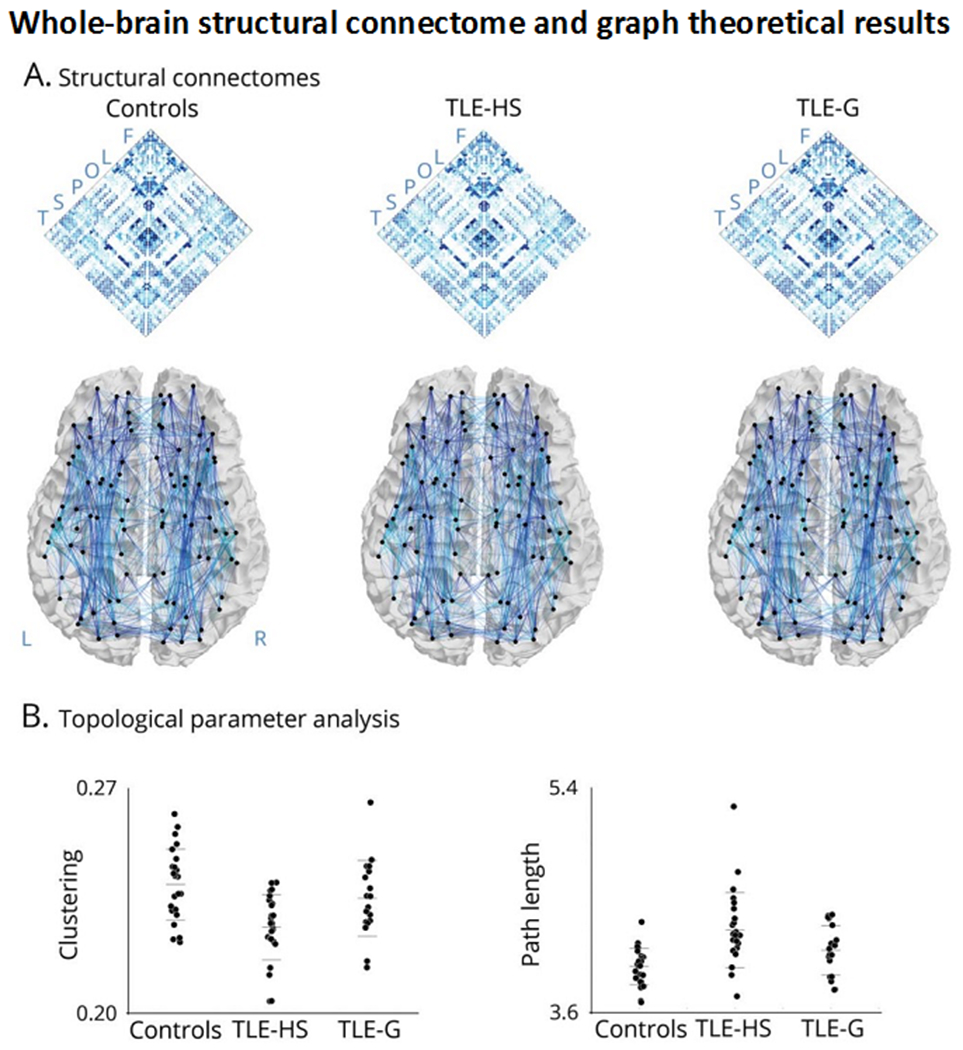
Panel A shows whole-brain structural connectomes in healthy controls, temporal lobe epilepsy (TLE) patients with hippocampal sclerosis (TLE-HS), and TLE patients with isolated gliosis (TLE-G). Maps were generated using diffusion tractography between all regions. Letters refer to regional groupings of the nodes (F = frontal; L = limbic; O = occipital; P = parietal; S = sub-cortical; T= temporal). Panel B depicts whole-brain graph theoretical results showing a markedly increased path length and decreased clustering coefficient in TLE-HS compared to controls and TLE-G, whereas those with TLE-G are only moderately affected compared to controls. Reproduced from Bernhardt et al., 2019, with permission.
Connectome analyses of WM organization in other epilepsy syndromes are less frequently reported. In GGE, a recent study showed bi-hemispheric alterations in several connectivity parameters compared to controls, and demonstrated an association between network architecture and drug response in patients (McKavanagh et al. 2021). These findings are complemented by a connectome-informed machine learning study in JME, showing that structural connectome and conventional dMRI measures can discriminate between patients and controls with more than 80% accuracy (Lee et al. 2021). Finally, a recent study applied computational modelling to structural and functional connectome data in both GGE and TLE patients, and identified increases in subcortical drive contributing to cortical dynamics in GGE, while TLE patients presented with reduced subcortical drive and imbalanced excitation-inhibition of cortical microcircuits, potentially suggesting an important differentiation between focal and generalized epilepsy syndromes at macro- and microscales (Weng et al. 2020).
WM associations with cognitive and psychiatric co-morbidities
WM associations with cognition in epilepsy
WM integrity is critical for the integration of cortico-cortical networks that support cognition. However, only recently has compromise to specific WM tracts and networks been linked to domain-specific cognitive impairments in epilepsy (for reviews see Allone et al. 2017; Leyden et al. 2015). The majority of work has focused on cognitive impairment in TLE, but new data addressing how WM injury disrupts cognition in FLE and JME are now emerging. A review of TLE studies between 2005 and 2014 is provided in Leyden and colleagues (2015). We provide an update on the state of the field, focusing on studies from 2015 to 2021 for TLE, and studies of other epilepsy syndromes not previously reviewed (Table 1).
Table 1a.
Studies examining white matter associations with cognition
| Author & year | Epilepsy syndrome | N | Cognitive domain(s) examined | Approach & ROI(s) | Findings |
|---|---|---|---|---|---|
| Chang et al. [2019] | TLE | 46 TLE; 33 HC | Verbal memory | Tract-based DTI (SWM of left medial temporal/posterior cingulate & left lateral temporal regions) | Lower FA and higher MD within the SWM of the left medial temporal/posterior cingulate regions explained the most variance in verbal memory scores compared to other imaging measures. |
| Balachandra et al. [2020] | TLE | 81 TLE | Verbal memory | Tract-based DTI (fornix, PHC, UF, & IFOF) and SC of a temporal sub-network | A combination of SC and hippocampal volume yielded the highest accuracy (81%) for classification of memory impaired versus non-impaired TLEs (90% sensitivity and 67% specificity). SC alone outperformed clinical-variables only and hippocampal volume. |
| Chang et al. [2017] | TLE | 14 LTLE; 26 HC | Language | Tract-based DTI (AF & IFOF) | Right-lateralized FA of the AF was associated with right-lateralized language activation on fMRI. |
| Munsell et al. [2019] | TLE | 24 LTLE (left-hemisphere language dominant) | Language | Whole-brain DTI (SC) | Identified a distributed bilateral white matter network of regions (anterior and medial temporal and frontal lobes) associated with naming performance. The model explained 60% of variance in naming performance. |
| Kaestner et al [2020] | TLE | 82 TLE | Language | Tract-based DTI (AF, IFOF, ILF, UF) and SC of a temporal lobe sub-network | The SC model yielded greater AUC (0.73) and classification accuracy for language impaired vs. non-impaired groups (79%) compared to a tract-based model (AUC = 0.54). SC revealed a WM network contributing to language impairment that was widely distributed, bilateral, and mainly lateral temporal. |
| Kaestner et al [2019] | TLE | 85 TLE; 47 HC | Language | Tract-based DTI (AF, ILF) | The language-impaired TLE group had lower FA in the left ILF and left AF compared to HC, but did not differ from the non-language impaired group. |
| Reyes et al. [2019] | TLE | 70 TLE; 46 HC | Language; verbal memory | Tract-based (AF, UF, ILF, & PHC) and whole-brain DTI | The language and memory impaired group and the memory impaired group showed distinct patterns of WM abnormalities relative to HC. Language and memory impaired group had widespread SWM abnormalities and altered global network topology. Language and memory impaired group also had lower FA of the bilateral AF and ILF and the left UF relative to HC. The language-only impaired group had poorer perisylvian network structure relative to the other groups. |
| O’Muircheartaigh et al. [2011] | JME | 28 JME; 24 HC | Language; verbal and visual memory; executive function | Whole-brain DTI | Lower supplementary motor area FA was associated with poorer naming and verbal expression. Lower posterior cingulate cortex FA was associated with poorer set shifting/cognitive flexibility. |
| Lin et al. [2020] | TLE and FLE | 22 FLE; 22 TLE with focal cortical dysplasia; 22 HC | Chinese version of MoCA) | DTI (graph theory) | Higher local efficiency was associated with better MoCA scores in TLE. |
| Liu et al. [2016] | TLE | 10 LTLE; 16 RTLE; 20 HC | MMSE and the Attention Network Test (ANT) | DTI (selected regions based on abnormal functional connectivity) | FA of the commissural fibers connecting the bilateral parahippocampal gyri were smaller in RTLE than in HC. Lower FA of these fibers was associated with poorer performance on a measure of alertness (ANT) in RTLE. |
| Diao et al. [2015] | TLE | 14 LTLE; 15 HC | Executive function; attention | Tract-based DTI (UF) | In LTLE and HC, lower FA of the left UF was associated with poorer executive function/working memory. |
| Kim et al. [2012] | JME | 25 patients with JME; 30 HC | Executive function; attention | Whole-brain DTI | JME performed worse than HC on most executive function tests. However, FA was not significantly correlated with any of the tests. |
| Reyes et al. [2018] | TLE | 32 TLE (16 LTLE); 24 HC | Executive function (set-shifting) | Restriction spectrum imaging (RSI) of inferior and superior fronto-striatal tracts | Lower neurite density of the bilateral inferior fronto-striatal tracts was associated with poorer performance on verbal set-shifting/response inhibition in TLE, which was driven by LTLE. |
| Vaessen et al [2012] | TLE and FLE | 39 patients with frontal or temporal seizure focus; 23 HC | IQ | Whole brain DTI and WM volume; graph theory | Patients with severe cognitive impairment had lower clustering and higher path length compared to HC and patients with little to no cognitive impairment. No differences found in WM volume. Lower IQ was associated with lower clustering and higher path length. |
| Knake et al. [2017] | JME | 20 JME: 20 HC | Verbal & visual memory; executive function; attention; fluency; personality | Whole-brain DTI | No associations found between DTI and neuropsychological measures. Microstructural changes in the cingulum were related to the personality trait agreeableness. |
| Mirò et al. [2015] | TLE | 21 TLE (14 LTLE; 7 bilateral MTS); 15 HC | Verbal & visual memory; perceptual reasoning; language; processing speed | Tract-based DTI and WM volume | TLE patients with bilateral MTS had lower FA and WM volume in temporal and extra-temporal tracts when compared to left TLE and HC but no significant associations were found with neuropsychological scores. |
| Dinkelacker et al. [2015] | TLE with MTS | 22 RLTE (18 with cognitive data); 22 LTLE (18 with cognitive data); 24 HC | Verbal & visual memory; executive function; spatial orientation; IQ | Tract-based DTI (hippocampus as the seed and thalamus as target region) | Higher hippocampal-thalamic connectivity (i.e. higher number of fiber counts) was associated with worse executive function in TLE (primarily in the Trail Making Test); the same relationship held in LTLE and RLTE separately (though uncorrected for multiple comparisons). |
| Rodríguez-Cruces et al. [2018] | TLE | 16 LTLE (7 with MTS); 10 RTLE (8 with MTS); 23 HC | Verbal & visual memory; processing speed; working memory; perceptual reasoning; verbal comprehension | Whole-brain DTI | In LTLE lower FA of the left anterior corona radiata right, superior corona radiata and left external capsule was associated with poorer working memory. Lower FA of the left external capsule was associated with poorer processing speed. Cluster analysis revealed three cognitive profiles (normal, mainly memory-impaired and domain-general impairment) associated with the degree and spread of WM abnormalities. |
| Rodríguez-Cruces et al. [2020] | TLE | 34 TLE; 24 HC | Verbal & visual memory; processing speed; working memory; perceptual reasoning; verbal comprehension | Whole-brain DTI (SC) | Less efficient WM connectome organization was observed in patients with more cognitive impairment. Network topology characterized cognitive performance better than measures of morphometry (e.g. cortical thickness). |
AF = arcuate fasciculus; FA = fractional anisotropy; FLE = frontal lobe epilepsy; HC = healthy controls; HS = hippocampal sclerosis; IFOF = inferior frontal occipital fasciculus; ILF = inferior longitudinal fasciculus; JME = juvenile myoclonic epilepsy; LTLE = left TLE; MD = mean diffusivity; MTS = mesial temporal sclerosis; PHC = parahippocampal cingulum; ROI = region of interest; SC = structural connectome; SWM = superficial white matter; TLE = temporal lobe epilepsy; UF = uncinate fasciculus
Memory
It is well established that the hippocampus and its projections are critical to learning and memory. However, an emerging literature has characterized how broader WM network disruption contributes to memory impairments in epilepsy (Table 1a). Damage to temporo-limbic association tracts, including the uncinate fasciculus, inferior longitudinal fasciculus, parahippocampal cingulum, and inferior fronto-occipital fasciculus is most commonly associated with impairments in verbal learning and memory in TLE (for review see Leyden et al. 2015). A few studies have also examined the superficial WM (SWM) or U-shaped WM fibers directly beneath the cortex that are important for maintaining short-range cortico-cortical connectivity. These studies have revealed that microstructural loss within the left entorhinal, broader medial temporal, and posterior cingulate SWM also contributes to verbal memory impairments in TLE, and may explain more of the variance in memory performances than functional oscillations or cortical thinning in adjacent cortex (Chang et al. 2019). In particular, the entorhinal WM contains major afferent connections from the entorhinal cortex to CA3 and the dentate gyrus of the hippocampus via the perforant path and angular bundle. These WM tracts are known to be important for episodic memory encoding (e.g., pattern separation) and likely disrupt a critical memory circuit in TLE.
Leveraging network models of WM connectivity, Balachandra, Kaestner and colleagues. 2020 found that a structural connectome of a temporal sub-network (i.e., temporal to extratemporal connections) was able to classify TLE patients as verbal memory-impaired vs non memory-impaired with 81% accuracy. The connectome’s strong performance may reflect its ability to identify temporo-limbic and association tracts commonly implicated in memory, in conjunction with short-range connections connecting adjacent temporal lobe cortex - Figure 4.
Figure 4. Structural connectome predicts verbal memory in temporal lobe epilepsy.
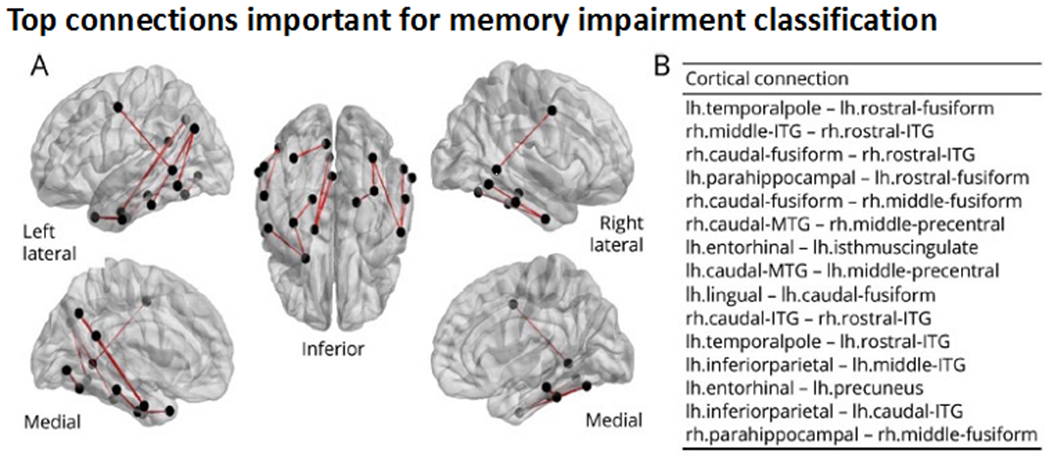
Comprehensive white matter neuronal network mapping (i.e., the structural connectome) was able to predict verbal memory impairment in TLE and highlighted the importance of short-range temporal-temporal connections to memory. Panel A shows a glass brain visualization of the top 15 connections important for classification of patients as memory-impaired versus unimpaired. Panel B shows names of top 15 most important connections ordered by most important (top) to least important (bottom). ITG = inferior temporal gyrus; lh = left hemisphere; MTG = middle temporal gyrus; rh = right hemisphere. Reproduced from Balachandra, Kaestner et al., 2020, with permission.
Associations between WM and visual memory are scarce, with only two studies reporting that damage to the right uncinate fasciculus (Diehl et al. 2008) and right parahippocampal gyrus WM (Yogarajah et al. 2008) is associated with visual memory impairment in TLE.
Pre-to-postoperative associations with memory.
Anterior temporal lobectomy (ATL) is the most common surgical procedure performed for treatment of drug-resistant TLE. However, ATL involves the removal of the anterior hippocampus, amygdala, lateral temporal cortex and sub-adjacent WM, leading to a high risk for postsurgical memory decline in many patients (Sherman et al. 2011). Only two studies have examined WM associations with postoperative memory decline (Table 1b). One study highlighted the importance of a fronto-temporal tract transected during surgery (i.e., uncinate fasciculus) and the integrity of WM beneath the entorhinal cortex to memory decline following ATL. A second study did not find associations between WM integrity in the ipsilateral temporal lobe (i.e., fornix) and memory decline (Elliott et al. 2018). However, the surgical sample in the second study was small and the surgeries were heterogeneous, limiting interpretability of the results. Thus, while some data support the importance of the uncinate fasciculus to postoperative memory outcomes, there are not enough data to draw reliable conclusions.
Table 1b.
Studies examining white matter associations with postoperative change in cognition
| Author & year | Epilepsy syndrome & surgical intervention | N | Cognitive domain(s) examined | Approach & ROI(s) | Findings |
|---|---|---|---|---|---|
| Powell et al. [2008] | TLE (ATL) | 7 patients who underwent dominant ATL (6 LTLE; 1 RTLE) | Language (naming) | Whole-brain DTI and fMRI | Greater preoperative FA lateralization of tracts to the language-dominant hemisphere was associated with better naming score preoperatively, but with greater decline postoperatively. |
| Osipowitz et al. [2016] | TLE (ATL) | 15 LTLE with dominant hemisphere surgery; 20 HC | Language (fluency) | Pre- and postoperative tract-based DTI using deviation scores (i.e., change score = pre-surgical FA relative to HC minus postsurgical FA relative to HC) with seed regions determined by fMRI | Patients with poor postsurgical fluency outcomes showed greater deviation of FA (i.e., less similar to HC). Pre-to-post change on DTI, resting-state fMRI and task-based fMRI classified 87% of patients, explaining 52% of the variance. DTI contributed the most in terms of predictive power. Baseline DTI showed a trend in predicting change in fluency. |
| Pustina et al. [2014] | TLE (ATL) | 42 TLE (12 LTLE; 12 RTLE) 12 HC | Language (fluency) | Pre- and postoperative whole-brain DTI | In LTLE, better preoperative letter fluency was associated with higher FA in 3 clusters in which FA increased after surgery (left superior corona radiata, right SLF, and right UF). However, better postoperative letter and semantic fluency were associated with higher FA of the right SLF only. |
| Yogarajah et al. [2010] | TLE (ATL) | 26 LTLE; 20 RTLE | Language (fluency, naming) | Pre- and postoperative whole-brain DTI | Higher preoperative and postoperative FA and parallel diffusivity of the ipsilateral external capsule, posterior limb of the internal capsule, and corona radiata were associated with higher postoperative language scores. Patients with the greatest postsurgical increase in parallel diffusivity showed the smallest decline in verbal fluency. |
| Stasenko et al. [in press] | TLE (ATL) | 42 TLE (19 LTLE; 23 RTLE) | Verbal & visual memory | Preoperative tract-based DTI (UF, ILF) and SWM (entorhinal, parahippocampal) | In LTLE, greater left-lateralized FA asymmetry of the UF and entorhinal cortex pre-operatively was associated with greater postsurgical decline in prose memory. Left-lateralized FA asymmetry of the entorhinal cortex predicted greater decline in prose memory, even after controlling for hippocampal volume. |
| Elliott et al. 2018 | TLE (ATL, amygdalohippocampectomy) | 25 TLE (17 with cognitive data) and 12 HC | Verbal & visual memory; verbal comprehension, perceptual reasoning, working memory | Pre- and postoperative tract-based DTI (hippocampus, fornix, mammillary bodies) | No significant associations observed between pre- to postsurgical changes in FA or MD of the contralateral hippocampus and memory change. No significant correlation between cognitive change and postoperative change in mammillary body or fornix volume. |
ATL = anterior temporal lobectomy; FA = fractional anisotropy; HC = healthy controls; ILF = inferior longitudinal fasciculus; LTLE = left TLE; MD = mean diffusivity; RTLE = right TLE; SLF = superior longitudinal fasciculus; SWM = superficial white matter; TLE = temporal lobe epilepsy; UF = uncinate fasciculus
Language
Language impairments in TLE have frequently been associated with disruption to both perisylvian (i.e., arcuate fasciculus) and extra-sylvian (e.g., inferior longitudinal fasciculus) WM fibers. Left hemisphere fibers along the dorsal stream are important for mapping auditory sounds to articulatory (motor) representations (e.g., arcuate fasciculus), whereas fibers in the ventral stream are typically implicated in mapping auditory speech sounds to meaning–i.e., lexical semantic processing (e.g., inferior longitudinal fasciculus and inferior fronto-occipital fasciculus). Although these left hemisphere fronto-temporal tracts are implicated in language performance both in healthy individuals and TLE, right hemisphere fibers also correlate with language performance in TLE, including the right arcuate fasciculus, inferior fronto-occipital fasciculus, superior longitudinal fasciculus, and uncinate fasciculus (McDonald et al. 2008; Pustina et al. 2014). This suggests 1) right hemisphere contributions to language and/or 2) potential reorganization of language to the right hemisphere in some patients with a left-sided seizure focus. In support of the importance of right hemisphere networks to language, Kaestner and colleagues (2020) demonstrated that using a structural connectome and machine learning (XGBoost), a broad, bilateral pattern of WM abnormalities contributed to naming and fluency impairments in TLE. Although lateral temporal connections between superior temporal gyrus and pars opercularis were the most important features (i.e., fibers from the arcuate fasciculus), other widely distributed and interhemispheric connections also emerged. Similarly, Munsell and colleagues (2019) identified a distributed, bilateral WM network of regions that predicted naming performance in left TLE patients who were all left-hemisphere dominant for language, suggesting that right hemisphere WM contributions to language were not solely secondary to language reorganization.
Neuroplasticity of language networks.
A remarkable characteristic of the human brain is its ability to reorganize in response to injury. With language, this is most frequently observed as an interhemispheric shift, making asymmetry of WM tracts a popular method for probing language reorganization in epilepsy (Ellmore et al. 2010). For most healthy individuals, the left hemisphere is dominant for language. However, patients with left TLE in particular show reduced left-lateralization of language networks (i.e., a more symmetrical or right-lateralized representation) on fMRI and in WM integrity measured with dMRI. However, reduced asymmetry in perisylvian WM integrity sometimes but not always corresponds to reduced asymmetry in language activations on fMRI (e.g., Chang et al. 2017; Powell et al. 2007; but see Rodrigo et al. 2008). The mixed findings highlight the complexity of language reorganization in left TLE, and our need to better understand re-organization of WM language networks and how it relates to functional reorganization and language performance.
Pre-to-postoperative associations with language.
Only a few studies examined the association between WM integrity and postoperative language outcomes. Powell and colleagues (2008) found that greater preoperative asymmetry of fronto-temporal WM to the language-dominant hemisphere was associated with greater naming decline post-surgery, suggesting that direct surgical disruption to (presumably healthy) temporal lobe WM leads to decline in naming. Another study demonstrated that patients with pre-to-postsurgical decline in fluency had FA microstructure that looked less like that of controls (i.e., more abnormal), with abnormal WM profiles explaining more variance in language outcomes than language activation on fMRI (Osipowicz et al. 2016).
Other research has demonstrated associations between postoperative verbal fluency and higher FA of the right superior longitudinal fasciculus (Pustina et al. 2014)–a finding that may reflect a compensatory interhemispheric shift in language networks to the contralateral hemisphere. However, there is also evidence that greater pre-to-postsurgical increases in parallel diffusivity in the ipsilateral ventromedial temporal lobe are associated with better postoperative language scores in left TLE (Yogarajah et al. 2010). Taken together, the extant literature suggests that better language outcomes following ATL depend on both inter- and intra-hemispheric shifts in WM integrity in key dorsal and ventral language tracts. Although surgery incurs a risk of language decline, there appears to be potential for microstructural and functional reorganization in both ipsilateral and contralateral hemispheres that may help to mitigate language decline.
Executive function
Executive dysfunction is observed in a third to half of patients with TLE and has a higher prevalence in JME and FLE. However, unlike for language and memory, there is less consistent evidence linking specific WM tracts/regions to executive dysfunction in epilepsy. In adults, working memory impairments have been associated with damage to the superior longitudinal fasciculus, cingulum, and temporal lobe WM (Winston et al. 2013) as well as the uncinate fasciculus (Diao et al. 2015). In addition, poorer performance on set-shifting and response inhibition–two components of executive function–has been associated with lower neurite density of the bilateral inferior fronto-striatal tracts (Reyes et al. 2018). However, in another TLE study, poorer set-shifting performance was associated with heightened hippocampal-thalamic connectivity, interpreted to reflect a pathological increase of WM connectivity leading to less efficient executive function (Dinkelacker et al. 2015). These mixed results are unsurprising given that executive function is not a unitary construct, with different studies measuring different aspects of executive function. Interestingly, no study has examined the relationship between pre-to-postsurgical changes in executive function and WM connectivity. This would be a fruitful area for exploration as there is some evidence for postsurgical improvement of executive function (Sherman et al. 2011), and separately, normalization of fronto-temporal FA (e.g., Pustina et al. 2014).
Cognitive Phenotypes
The majority of studies have focused on cognitive domains in isolation as well as specific tracts, guided by a-priori hypotheses regarding structure-behavior relationships. However, recent studies have moved toward an examination of cognitive phenotypes, or patterns of cognitive impairment, and examined how these phenotypes map onto whole-brain microstructural pathology (Reyes et al. 2019; Rodríguez-Cruces et al. 2018; Rodríguez-Cruces et al. 2020) - Figure 5. These studies have identified three to four distinct cognitive phenotypes in TLE that have unique patterns of deep and superficial WM network abnormalities, some of which correspond to previously reported a-priori tracts. Most interesting is the observation that patients with a cognitively intact profile do not differ from healthy controls in WM network pathology, lending further validation to the biological relevance of these phenotypes and the importance of WM integrity to cognition.
Figure 5. Multi-domain cognitive phenotyping and whole-brain white matter connectome.
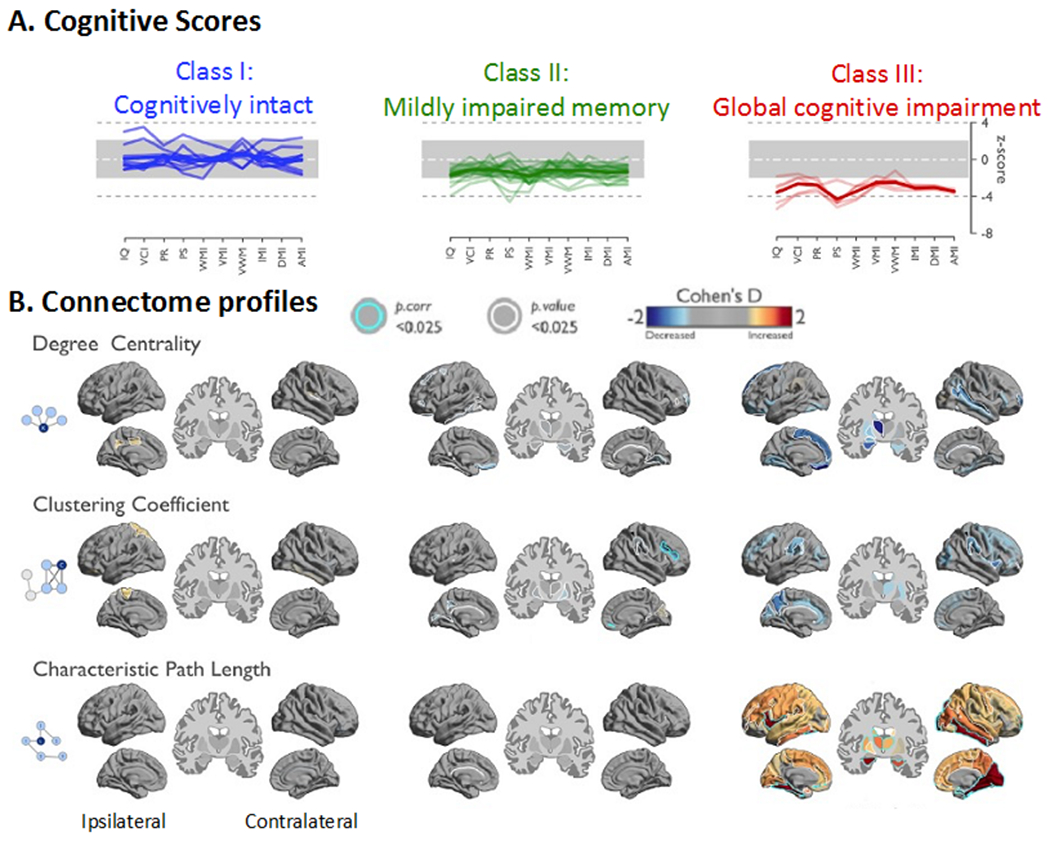
Patients with less efficient WM network organization showed more pronounced cognitive difficulties. WM connectome metrics were more closely associated with cognitive function than cortical thickness. (A) Hierarchical clustering of cognitive profiles converged on three cognitive classes in the temporal lobe epilepsy cohort: Patients in Class 1 had cognitive scores within normal range, those in Class 2 showed mild impairment in memory-specific domains, and Class 3 displayed pronounced impairment across all domains, with prominent reduction of processing speed. (B) Gradual network organization abnormalities were observed across Classes with most marked changes in Class 3, intermediate differences in Class 2, and only subtle changes in Class 1. Class 2 showed decreased clustering in the contralateral suborbital sulcus and inferior frontal sulcus. At a connectome-wide level, Class 3 showed the most marked increases of characteristic path length, while Classes 1 and 2 were rather normal. In Class 3, path length increases were most marked in the lateral and medial temporal lobes in both hemispheres, the ipsilateral frontal and the contralateral occipital lobe. Modified from Rodríguez-Cruces et al., 2020, with permission.
Associations with psychiatric comorbidities
Depression and anxiety
Depression affects approximately one out of four patients with epilepsy. Although once thought to reflect a reaction to psychosocial stressors associated with epilepsy, research now supports a bidirectional relationship between TLE and depression (Kanner et al. 2012), with WM abnormalities as one potential contributor. In a recent systematic review of the neuroimaging correlates of depression in epilepsy (Elkommos and Mula 2021), three studies examined WM microstructure. Two studies reported that WM abnormalities in fronto-temporo-limbic regions were associated with increased depressive symptoms in TLE (Kemmotsu et al. 2014; Kavanaugh et al. 2017). However, a third study did not find a significant difference between TLE with depression and anxiety compared to TLE alone in a post-hoc analysis (Stretton et al. 2015) - Table 2. In sum, there is some evidence that fronto-limbic network dysfunction may underlie a bidirectional link between TLE and depression, and this may influence which patients present with depression. However, prospective, longitudinal studies are needed that directly compare TLE with depression to TLE without depression and track whether the evolution of WM changes corresponds to the evolution or severity of depressive symptoms. Future investigations of these associations in other epilepsy syndromes is important.
Table 2.
Studies examining white matter associations with psychiatric outcomes
| Author & year | Epilepsy Syndrome | N | Psychiatric Comorbidity | Approach & ROI(s) | Findings |
|---|---|---|---|---|---|
| Kavanaugh et al. [2017] | TLE (most non-lesional) | 11 TLE without depression; 9 TLE with depression; 11 depression without TLE | Depression | Tract-based DTI in fronto-temporo-limbic tracts (CC, cingulum, fornix, SLF & UF) and less related tracts (corticospinal, pontine crossing fibers, cerebral peduncles) | Higher depressive symptoms in TLE were associated with lower FA in non fronto-temporo-limbic regions and marginally associated with lower FA in fronto-temporo-limbic regions. Associations most notable in those with childhood onset epilepsy. The relationship between FA and depressive symptoms in the depression without TLE group was not significant. |
| Kemmotsu et al. [2014] | TLE | 11 LTLE; 10 RTLE; 20 HC | Depression | Tract-based DTI (UF, amygdala, hippocampus) | In LTLE greater depressive symptoms were associated with lower FA of the bilateral UF and higher MD of the ipsilateral hippocampus. In RTLE greater depressive symptoms were associated with lower FA of the contralateral UF. However, fronto-limbic functional connectivity was a stronger predictor of depression than WM connectivity. |
| Stretton et al. [2015] | TLE | 17 TLE with depression and/or anxiety; 31 TLE without depression/anxiety; 30 HC | Depression/anxiety | Whole-brain DTI | No significant differences in WM integrity between groups. |
| Flügel et al. [2006] | TLE | 18 TLE with interictal psychosis; 20 TLE without- psychosis | Psychosis | Tract-based DTI (middle frontal and middle temporal gyri) | TLE with interictal psychosis had lower FA in fronto-temporal regions and higher MD in bilateral frontal regions compared to TLE-only. Lower FA was associated with worse visuospatial working memory. |
| Sone et al. [2020] | TLE | 20 TLE with interictal schizophrenia-like psychosis; 29 TLE without psychosis; 42 HC | Psychosis | Tract-based DTI and graph theory | TLE showed altered temporal and extra-temporal FA and MD compared to HC, which were more severe and widespread in TLE with psychosis. Differences between TLE with psychosis and TLE-only were found in the anterior thalamic radiation, IFOF & ILF. Global and local efficiency and increased path length were reduced in TLE compared to HC, with more severe changes in TLE with psychosis. Effect of psychosis found mainly in left limbic and prefrontal areas. |
CC = corpus callosum; DTI = diffusion tensor imaging; FA = fractional anisotropy; HC = healthy controls; IFOF = inferior fronto-occipital fasciculus; ILF = inferior longitudinal fasciculus; LTLE = left TLE; MD = mean diffusivity; RTLE = right TLE; SLF = superior longitudinal fasciculus; TLE = temporal lobe epilepsy; UF = uncinate fasciculus
Interictal psychosis
There is a prevailing view that a strong link exists between TLE and psychosis, and that damage to gray and WM may give rise to psychosis in epilepsy. A systematic review reported an almost eight-fold increased risk of psychosis in epilepsy relative to the general population, with an even higher risk in TLE (Clancy et al. 2014). Psychosis in epilepsy is classified as ictal or postictal if it is closely linked to seizure occurrence. Conversely, interictal psychosis is not temporally related to seizures and may not necessarily resolve in between seizure episodes. A recent study found differences between TLE with vs. without interictal psychosis in several temporo-limbic tracts (inferior fronto-occipital fasciculus, inferior longitudinal fasciculus) and the anterior thalamic radiations (Sone et al. 2020). In the same study, a graph theory analysis found that TLE with psychosis had a greater reduction in global and local efficiency compared to controls, with the effect of psychosis primarily in left limbic and prefrontal areas. A previous tract-based study reported lower FA in bilateral fronto-temporal regions and higher MD in bilateral temporal regions in TLE with psychosis compared to TLE alone (Flügel et al. 2006). Thus, psychosis in epilepsy may be associated with a distributed pattern of temporo-limbic pathology, not restricted to the mesial temporal lobe.
WM associations with seizure laterality, drug-resistance and postsurgical outcomes
Seizure onset laterality
Identifying the side of seizure onset is a crucial step in the presurgical evaluation of a patient with epilepsy. This presents a challenge for many patients with TLE whose seizures may not clearly lateralize on scalp-EEG, or for whom subtle epileptogenic lesions are not visible on conventional MRI. For this reason, dMRI has been proposed as a clinical decision support tool that could be used to map the underlying seizure networks and increase confidence in seizure laterality.
A number of studies have examined the utility of using dMRI to identify the side of seizure onset (e.g., Ahmadi et al. 2009; Concha et al. 2012; An et al. 2014; Nazem-Zadeh et al. 2014; Nazem-Zadeh et al. 2016) - Table 3. These studies have obtained accuracies from 71-91% for discriminating patients with right from left TLE using fronto-temporal WM tracts alone and reflect the tendency for patients with unilateral TLE to have greater WM tract damage on the side ipsilateral to the seizure focus and proximal to the seizure onset zone.
Beyond tract-based studies, Besson and colleagues (2014) used a structural connectome approach to demonstrate differences between left and right TLE, with more severe alterations in left TLE, who showed a strongly lateralized fronto-temporal disconnection pattern. Using graph theory, Kamiya and colleagues (2016) found decreased local efficiency in the left posterior cingulate gyrus, left cuneus, and bilateral hippocampus in left TLE. In contrast, only the right hippocampus showed altered network properties in right TLE. In this study, a support vector machine correctly classified between 73-86% of patients as having left versus right seizure onset. Taken together, preliminary evidence suggests that both tract-based and structural connectome measures of network pathology aid in lateralization of the seizure focus in TLE, with moderate to high classification accuracy across studies. With further refinements in machine learning algorithms and larger samples, dMRI may serve as clinically useful for augmenting pre-surgical seizure lateralization.
Drug-resistance
Only 60% of patients with epilepsy respond to the first two ASMs and less than 4% respond to further ASM trials. The remaining 30-40% are defined as “drug-resistant” and present a considerable treatment challenge. Labate and colleagues (2015) found that patients with drug-resistant mesial TLE had more severely reduced temporal lobe FA compared to patients with benign mesial TLE, irrespective of the presence of HS. In fact, temporal lobe FA was able to differentiate between refractory vs. benign TLE with an AUC of .74. In a follow-up study, patients whose mild mesial TLE eventually evolved into refractory mesial TLE had distinct microstructural alterations in the corticospinal tracts, superior longitudinal fasciculus, left cingulum, and left inferior longitudinal fasciculus prior to the development of drug-resistance (Labate et al. 2020). These data suggest that greater WM pathology both within and beyond the temporal lobe may predispose patients to develop drug-resistant seizures. Identifying these patterns at the onset of epilepsy could help to guide treatment decisions early, including identifying patients who are not likely to gain seizure control from ASMs and who should be considered for surgery or other non-pharmacologic treatments.
Postsurgical seizure outcomes
TLE and other focal epilepsies represent a spectrum of disorders with a wide range of postsurgical seizure outcomes (i.e., seizure-free versus not), even in patients with similar preoperative clinical features (Coan and Cendes, 2013). Keller and colleagues (2017) found that patients with TLE who had greater preoperative pathology in the ipsilateral dorsal fornix and contralateral parahippocampal WM were more likely to have poor seizure outcomes relative to those with less pathology. Furthermore, pathological changes in the ipsilateral fornix and uncinate were beyond the margins of the resection in patients with poor seizure outcomes, suggesting that insufficient disconnection of the temporal lobe epileptogenic network may lead to persisting seizures. Gleichgerrcht and colleagues (2020) quantified whether brain regions were situated on efficient communication pathways in the whole-brain network (i.e., regions with high betweenness centrality) to map patient-specific reorganization in structural hubs. Combining these measures with supervised machine learning, that study found that nodes most strongly associated with seizure freedom included the bilateral parahippocampal and superior temporal gyri - Figure 6. Bonilha and colleagues (2015) used a structural connectome model to predict post-ATL seizure outcomes in TLE with a positive predictive value (seizure freedom) of 88% and a negative predictive value (seizure refractoriness) of 79%. Network connections that contributed the highest accuracy were located not only in the ipsilateral temporal and extratemporal regions, but also in the contralateral hemisphere - Figure 6. These data suggest that broad WM network abnormalities both ipsilateral and contralateral to the seizure focus may increase risk for poor seizure outcomes, implying incomplete resection of the epileptogenic network as detected by dMRI.
Figure 6. Structural connectome and network topography as biomarkers for estimating post-surgical outcomes in patients with TLE.
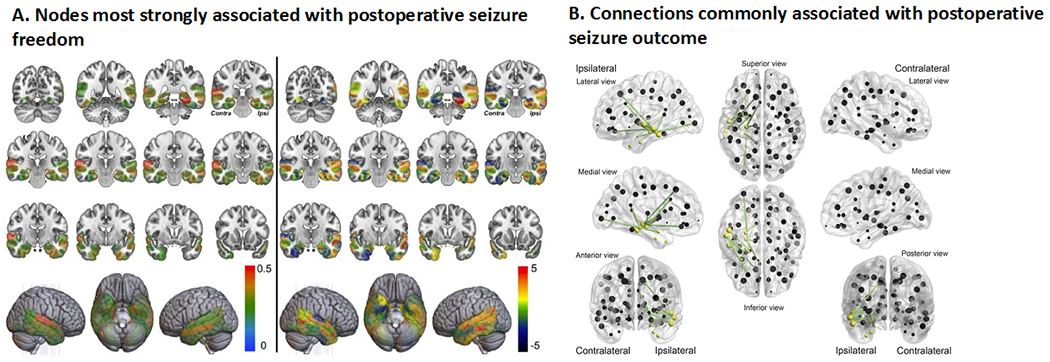
(A) Network integration in the medial and lateral temporal regions was related to post-surgical seizure outcomes, such that patients with abnormally integrated network nodes were less likely to achieve seizure freedom. The left panel of A illustrates feature importance for classification for a model of betweenness centrality (BC)—the degree to which other regions rely on a particular node for efficient (i.e., shortest amount of steps needed) flow of information. A higher BC indicates a more highly integrated region within the network. The ipsilateral parahippocampus, contralateral superior temporal gyrus, and bilateral entorhinal regions showed the highest importance. The right panel demonstrates group differences in BC between seizure-free and non-seizure free patients. Positive t-values indicate higher values in the non-seizure free group. Areas with stronger red color correspond to the most important (left panel) and most significantly different between the groups (right panel). Ipsi = ipsilateral (represents the side ipsilateral to the seizure onset). Reproduced from Gleichgerrcht et al., 2020, with permission. (B) In green are structural connectome links that were repeatedly chosen by the cross-validation model to have the highest ability to predict post-surgical seizure freedom. Yellow spheres represent the 8 cortical regions of interest defined as pertaining to the temporal region. Patients who exhibited greater weights among these links were less likely to become seizure-free after surgery. Reproduced from Bonilha et al., 2015, with permission.
Postsurgical visual field deficits
In the temporal lobe, the optic radiations project from the lateral geniculate of the thalamus, anteriorly and laterally over the temporal horn of the lateral ventricles before coursing posteriorly toward the occipital pole. During ATL, the anterior portion of the ventral visual pathway (i.e., Meyer’s loop) is removed, producing a visual field defect (VFD) [typically an incomplete (medial sector) quadrantanopia] in a majority of patients. VFDs can preclude patients from driving in some countries and states, significantly impacting their quality of life and independence (Gilliam et al. 1997). Converging evidence has demonstrated that the extent of degeneration along or transection of temporo-occipital fiber tracts predicts the severity of postoperative visual field defects following ATL (Chen et al. 2009; Powell et al. 2005; Taoka et al. 2005; Wieshmann et al. 1999; for review see Piper et al. 2014) - Table 3 and Figure 7. In fact, Winston and colleagues (2014) found that no patient failed to meet visual criteria for driving as a result of ATL resection when visualizing the optic radiations with tractography in comparison to 13% of controls who did not undergo tractography. As a result of these promising findings, preoperative dMRI has been proposed as a viable method for minimizing risk for VFDs. However, the optic radiations disperse broadly in the temporal lobe and can prove difficult to track, with differences in data acquisition and tractography algorithms leading to limited reproducibility of these fibers. Therefore, advanced dMRI models and tractography approaches are needed to augment the ability of dMRI tractography to minimize VFD associated with ATL.
Figure 7. Tractography of optic radiations decreases risk for post-surgical visual field deficits.
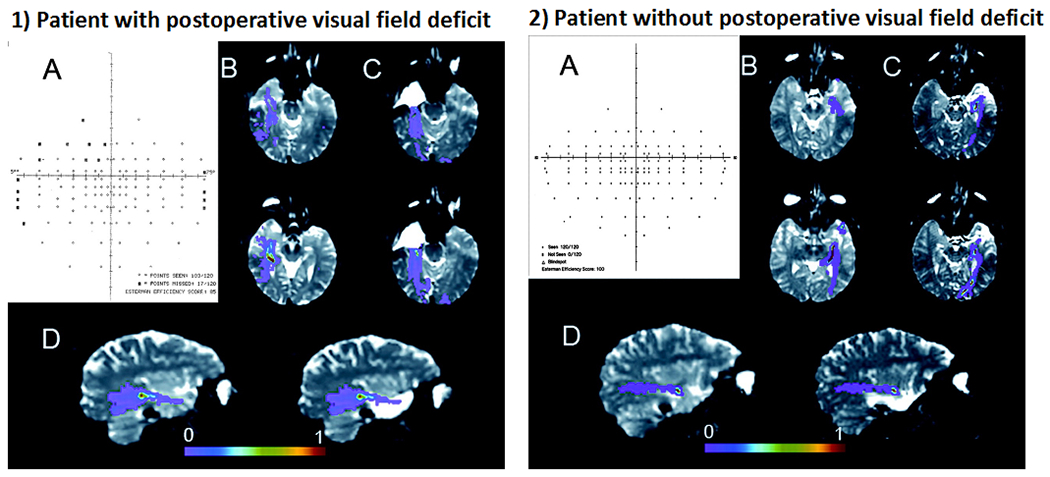
(1) Patient 1 demonstrated a postoperative visual field deficit (VFD) after anterior temporal lobectomy, manifesting as a superior quadrantanopia (A). This patient experienced a surgical disruption of the anterior segment of the Meyer’s loop (C). The preoperative right optic radiation tracts overlap with the resected anterior temporal lobe (D). (2) In contrast, Patient 2 did not experience a postoperative VFD (A). The anterior border of the Meyer’s loop remained intact (C), and the tracts do not overlap with the resected anterior temporal lobe (D). Thus, preoperative DTI tractography allows for identification of those patients at greatest risk of VFDs. The color bar represents a measure of connection probability to the starting point. Reproduced from Powell et al., 2005, with permission.
Advanced Diffusion Techniques
Although WM microstructural abnormalities are commonly observed in epilepsy using conventional dMRI and have been validated against histopathological measures of WM pathology (Concha et al. 2010), the full extent of neuropathological alterations in epilepsy requires more sensitive measures of microstructural properties. In particular, it is increasingly appreciated that FA and MD are non-specific measures of cerebral microstructure that are influenced by a number of tissue-related factors. In addition to axonal loss and demyelination, decreases in FA obtained from the basic tensor model may reflect the presence of crossing fibers or increases in extracellular diffusion due to edema or inflammation. Given the role that inflammation may play in the pathogenesis of some forms of epilepsy, a better understanding of the neurobiology behind decreased FA could help guide treatments in patients with different epilepsy syndromes.
Advancements in dMRI data acquisition (i.e., scanning parameters) and post-processing techniques have enabled more sensitive and/or specific measures of cerebral pathology in epilepsy. Studies in diffusion kurtosis imaging (DKI), a statistical method that uses multiple diffusion weightings (i.e., b-values) to probe non-Gaussian diffusion and estimate diffusion heterogeneity in tissue, have found that kurtosis measures reveal a broader and more robust pattern of microstructural abnormalities in TLE compared to conventional DTI (Bonilha et al. 2015; Lee et al. 2014). This may reflect a greater sensitivity of DKI to multiple pathologic factors including cell loss, inflammation, and axonal and dendritic reorganization.
In addition, diffusion spectrum imaging (DSI), a high-angular diffusion imaging (HARDI) technique, has been combined with the neurite orientation dispersion and density imaging (NODDI) model, a multicompartment diffusion model, to estimate structural connectivity and network properties in TLE (Lemkaddem et al. 2014). Restriction spectrum imaging (RSI) is another multicompartment (multi b-value) model well-positioned to evaluate whether decreases in FA are better explained by decreased axonal/neurite density, crossing fibers, and/or increases in extracellular diffusion (e.g., cerebrospinal fluid–filled spaces; inflammation), all within a clinically-feasible (4-6 min) time frame. This method has demonstrated that measures of WM pathology obtained with RSI are greater in magnitude, more lateralized to the epileptogenic hemisphere, and broader than those obtained with conventional DTI (Loi et al. 2016) - Figure 8.
Figure 8. Restriction spectrum imaging (RSI) provides a more robust measure of white matter injury in TLE relative to DTI.
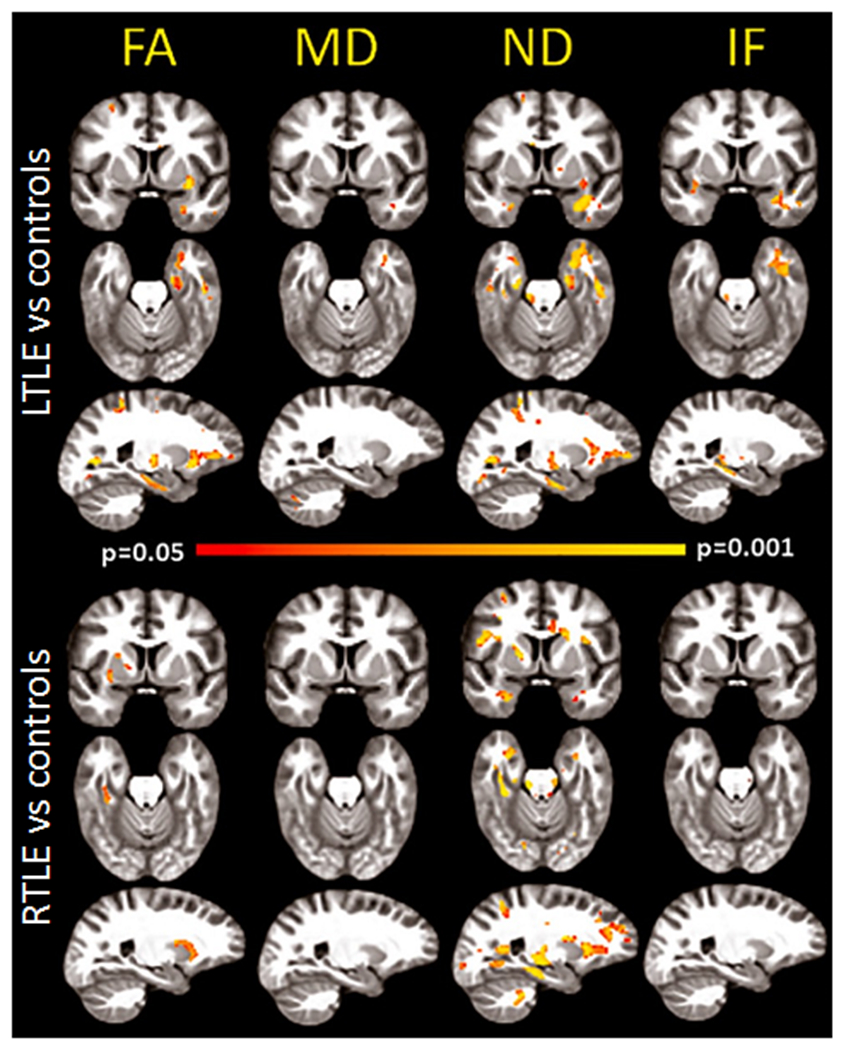
Voxel-based analysis of group comparisons between patients with right TLE (RTLE) and left TLE (LTLE) and age-matched controls. Areas of red-yellow represent decreased fractional anisotropy (FA) and neurite density (ND), and increased mean diffusivity (MD) and isotropic free (IF) water diffusion in patients compared to controls. Compared to FA, ND maps revealed a broader and more robust pattern of decreases of white matter integrity in TLE, with strong lateralization to the left hemisphere in LTLE, and to the right hemisphere in RTLE. Decreases were noted primarily in the anterior temporal lobe, with additional decreases in the inferior prefrontal white matter. Thus, neurite density using RSI may provide a more specific measure of WM pathology than standard DTI, distinguishing regions primarily affected by axonal/myelin loss from those where crossing fibers and increases extracellular water also play a role. Adapted from Loi et al., 2016, with permission.
Recent advancements in scanner hardware, such as stronger gradients and multiband acceleration methods, greatly reduce the practical difficulties of scanning with very high b-values (i.e., b=4000 or 5000) and a large number of diffusion directions. This allows for improved measurements, further improving the quality of tractography, as well as further separating intra-axonal from extracellular signals in epilepsy (Bryant et al. 2021). These studies suggest that advanced diffusion techniques may provide more sensitive measures of network pathology in TLE, greatly increase the specificity of connectome imaging, and further the identification of epilepsy-specific network abnormalities.
Summary and Future Directions
Over the past several decades, dMRI has greatly advanced our understanding of WM network disruption within and across epilepsy syndromes and reveals the critical role of WM disconnection in cognitive, psychiatric, and clinical outcomes in epilepsy. In particular, there is clear evidence demonstrating a link between memory and language impairments in epilepsy and disruption to bilateral medial temporal and fronto-temporal WM, respectively. Associations with executive dysfunction are less clear, but may be secondary to injury within fronto-temporal (i.e., uncinate fasciculus) and fronto-striatal pathways. Similarly, psychiatric co-morbidities are more likely to emerge in patients with distributed temporo-limbic WM pathology. With respect to clinical outcomes, there is strong evidence that WM patterns can facilitate lateralization of the seizure focus in TLE, and that the presence of extra-temporal pathology increases risk for drug-resistance as well as poor seizure outcomes following ATL. Finally, studies using dMRI tractography have demonstrated that visualization of the optic radiations can lead to improved visual field outcomes following surgery.
Despite these advances, future work is needed to replicate these findings in larger samples, expand to epilepsy syndromes beyond TLE, increase generalizability of findings by including more diverse populations, and utilize advanced analytical techniques. For instance, machine learning is well-suited for WM analyses in epilepsy for two main reasons: 1) Predictive models generated by training data can be tested in external samples and thus permit the evaluation of the generalizability of the results and 2) Machine learning allows for abridging complex data into variables that can be identified as relevant or discarded as non-crucial, as well as reducing data into fewer dimensions. Conventional machine learning approaches such as support vector machine and random-forest, among others, have been applied to WM in epilepsy and are excellent strategies for the identification of complex patterns and out-of-sample testing. Moreover, feed-forward neural networks or convolutional neural networks (CNN) are also well-suited to abridge and test information, with CNN being particularly relevant for 2D or 3D image-pattern detections, which can be derived from connectome-based matrices.
In addition, many unanswered questions remain regarding the origin and evolution of WM disruption in epilepsy, including: Does WM disruption lead to the development of seizures or do recurrent seizures result in progressive WM damage? What is the functional relevance and temporal course of FA changes (i.e., does increased FA or connectivity early in disease represent pathologically enhanced signal flow that occurs prior to white matter degradation?). How do WM networks reorganize after surgery and what is the time course of reorganization? Does the trajectory of WM recovery or re-organization correspond with cognitive or psychiatric improvements? And, how do patterns of reorganization within WM networks relate to functional reorganization? In addition, it is challenging to study direct associations between any single clinical seizure variable (e.g., age of seizure onset, seizure duration, drug resistance) and WM injury given the high interdependence of these variables. Many of these questions can be addressed with longitudinal studies of patients with new onset epilepsy and at multiple time points following surgery. Recent studies have developed nomograms, or easy-to-use risk stratification models that allow clinicians to estimate the probability of cognitive, emotional or seizure outcomes in adults considering epilepsy surgery (e.g., Jehi et al. 2015; Busch et al. 2018; Doherty et al. 2021). These studies have included clinical and demographic variables (e.g., side of seizure onset, education, cognitive score) as predictors of decline. Given new data suggesting that dMRI may add to the prediction of cognitive and seizure outcomes, future nomograms may benefit from the addition of markers of WM microstructure. In addition, no studies have used baseline WM integrity to risk-stratify patients with regard to cognitive or seizure outcomes following new surgical interventions that mostly spare collateral WM (e.g., laser ablation). Such studies may provide a more definitive answer as to the importance of WM integrity to a range of postsurgical outcomes.
As the field moves towards an understanding of epilepsy as a network disorder, there is an increased usage of neurostimulation to treat refractory epilepsy. The Responsive Neurostimulation System (RNS) delivers responsive stimulation to halt seizures, and also provides long-term neuromodulation. Similarly, deep brain stimulation (DBS) of thalamic nuclei and vagus nerve stimulation (VNS) of the peripheral part of the cranial nerve are neuromodulatory treatments for seizures that could impact WM connectivity. With respect to VNS, increased volume of WM microstructure in the vagus afferent network has been associated with increased treatment efficacy (Mithani et al. 2019). Further research examining WM changes following each of these neuromodulation treatments could improve patient selection and increase our understanding of WM neuroplasticity in epilepsy.
Lastly, there is a need to understand the impact of racial and ethnic health disparities on integrity of WM networks in epilepsy. Literature outside of epilepsy suggests a strong link between health disparities and brain and cognitive health. For instance, poorer WM integrity has been associated with fewer years of schooling, lower household income (Gianaros et al. 2013), and lower socioeconomic status (Shaked et al. 2019), which may lead to an increased risk for age-related and disease-related cognitive decline. However, minimal research exists on the additive impact of epilepsy and social determinants of health on WM integrity. Deeper phenotyping of our patients and efforts aimed at increasing the sociocultural, ethnic and racial characterization of our samples would enhance the generalizability of these findings and lead to a more enriched understanding of the causes and consequences of WM injury in epilepsy.
Box 1. Definitions and key terms.
Diffusion-weighted MRI (dMRI): A form of magnetic resonance imaging that generates images that utilize the diffusion patterns of water molecules, which allows for the detection of microstructural details of normal or abnormal anatomy of a given region in vivo and non-invasively. An extension of dMRI called diffusion tensor imaging (DTI) tractography is used widely for reconstructing white matter tracts in the brain.
Fractional anisotropy (FA): The most common summary measure of microstructural white matter integrity used in DTI studies that assesses the degree of anisotropy of water molecules, from which alterations in axonal diameter, fiber density, and myelination of white matter can be inferred. It ranges from 0 (i.e., isotropic movement of water molecules equally restricted in all directions) to 1 (i.e., anisotropic movement of water molecules, for example, in fiber bundles in which a diffusion occurs only along one axis and is fully restricted in all other directions). Although reduced FA is typically conceptualized as reflecting reduced myelin content, some findings suggest that increases in WM connectivity can reflect pathological wiring, although the origin of this is not well understood.
Mean diffusivity (MD): Another measure of microstructural integrity of white matter defined as an inverse measure of the membrane density that is more sensitive to cellularity, edema, and necrosis. Describes the rotationally invariant magnitude of water diffusion within tissue and can be affected by any disease process that affects the restriction of the barriers to the motion of water.
Radial diffusivity (RD): Reflects diffusivity perpendicular to axonal fibers and is influenced by changes in the axonal diameters or density. RD is thought to be more strongly related to myelin abnormalities (i.e., demyelination).
Structural connectome (SC): A comprehensive and individualized white matter analysis approach that examines a map of brain network connectivity. This requires measuring the strength of region-to-region connections within an individual. Connectome-based models have the potential to provide more fine-grained information about patterns of abnormal cortico-cortical connectivity underlying cognitive impairments than long-range tractography methods.
Graph theory: A mathematical framework that allows for the quantitative modeling and analysis of the topological properties of complex interconnected systems, that has made a considerable impact on understanding brain connectivity. Such an approach has been pivotal in a shift toward understanding temporal lobe epilepsy as a network disorder.
Hippocampal sclerosis (HS): A lesion characterized by cell loss and gliosis in the hippocampal formation usually seen as atrophy, increased signal, and loss of internal architecture of the hippocampus on MRI.
Parahippocampal cingulum (PHC): The inferior segment of the cingulum (a white matter tract projecting from the cingulate gyrus to the entorhinal cortex), which has been implicated in episodic memory. The PHC runs along the ventral aspect of the hippocampus.
Fornix: A C-shaped white matter bundle that serves as the main output tract of the hippocampus and plays a role in transmitting information from the hippocampus to the mammillary bodies and to the anterior nuclei of the thalamus. The fornix is believed to play an important role in cognition and episodic memory.
Uncinate fasciculus (UF): A curved relatively short fiber that connects the prefrontal and anterior temporal regions. Although its exact function is not understood, it has been associated with episodic and working memory, as well as with language (mainly semantic processing) and socio-emotional processing.
Inferior longitudinal fasciculus (ILF): A long range associative white matter tract that connects basal-temporal areas with the anterior temporal lobe, which is relayed to frontal and lateral temporal-parietal regions through additional white matter connections. The ILF serves as the ventral visual stream important for visual recognition (e.g., objects, faces, places) as well as an ‘indirect’ route for language. The ILF is thought to support multiple cognitive functions including object and face recognition, lexical and semantic processing, and emotion processing.
Inferior fronto-occipital fasciculus (IFOF): The IFOF, is a ‘direct’ language route as part of the ventral stream, proposed to connect the occipital cortex to the anterior temporal and inferior frontal cortices. The IFOF is thought to play a role in semantic processing via direct connections of basal-temporal areas with frontal and temporal-parietal cortex, with stimulation leading to disrupted semantic processing (i.e., semantic paraphasias or errors in speech that are related to an object’s meaning).
Arcuate fasciculus (AF): An association tract that connects the temporal and inferior parietal cortices to the frontal cortex, and specifically connects the inferior frontal gyrus (i.e., Broca’s area) and the superior temporal gyrus (i.e, Wernicke’s area). The AF is considered as part of the dorsal pathway for language, and is implicated in several language functions (e.g., syntax, repetition, phonological processing, and prosody).
Superficial white matter (SWM): A thin layer of white matter just underneath the cortex, comprised of short u-shaped association fibers that provide cortico-cortical connections between adjacent gyri, and represent most of the brain’s white matter connections. SWM is thought to play a role in brain maturation and neuroplasticity. Despite its importance in white matter connectivity, the application of SWM to neurological disease in humans (e.g., epilepsy, autism, Alzheimer’s disease) has been only recently applied.
Perforant path: The major input to the hippocampus that provides connections from the entorhinal cortex to hippocampal subfields including the dentate gyrus, CA1 and CA3, and the subiculum. This path has a major role in memory retention and retrieval.
Anterior temporal lobectomy (ATL): The most common resective surgery for medication-resistant temporal lobe epilepsy introduced in the 1950s. ATL achieves seizure freedom in 60-80% of patients and requires removal of the anterior portion of the inferior and middle temporal gyri, uncus, a portion of the amygdala, and the anterior 2-3 cm of the hippocampus and adjacent parahippocampal gyrus.
Perisylvian: Regions of the brain responsible for language found around the lateral sulcus (i.e., Sylvan fissure) of the left hemisphere that include deep white matter tracts that connect fronto-temporo-parietal regions.
Executive function: A broad category of higher-level cognitive abilities including working memory, set-shifting, and inhibition.
Interictal psychosis: Psychosis that occurs in approximately 6% of individuals with epilepsy, with the onset not during or immediately following a seizure. Symptoms of interictal psychosis in epilepsy overlap with symptoms in schizophrenia, such as paranoid delusions and hallucinations.
Drug-resistant epilepsy: When a person has failed to become seizure-free with adequate trials of two antiseizure medications.
Quadrantanopia: A loss of vision in one quarter of the visual field. A homonymous superior quadrantanopia, which presents as a loss of vision in the same upper quadrant in both eyes, is common in patients who undergo ATL due to damage to the inferior optic radiations of the temporal lobe (i.e., Meyer’s loop). Individuals can compensate for the vision loss by tilting their head to bring the affected visual field into view.
Wallerian degeneration: An active process of injury-induced degeneration of the distal end of an axon after neuronal loss or death. Seizure-induced damage from abnormal neural firing and hyperexcitability may cause secondary white matter degeneration along the seizure propagation pathway. Using DTI, early axonal breakdown has been attributed to reduced parallel diffusivity, whereas later myelin degradation is attributed to elevated perpendicular diffusivity.
Acknowledgments
C.R.M was supported by NINDS R01NS120976, R01NS124585, R01122827, and R21NS107739. A.S. was supported by a Ruth L. Kirschtein Postdoctoral Individual National Research Service Award from NINDS (F32 NS119285-01A1). We thank Erik Kaestner, Donatello Arienzo, and Adam Schadler for helpful comments and discussion.
Footnotes
Declaration of Conflicting Interests: The Authors declare that there is no conflict of interest.
References
- Ahmadi ME, Hagler DJ Jr, McDonald CR, Tecoma ES, Iragui VJ, Dale AM, Halgren E. 2009. Side Matters: Diffusion Tensor Imaging Tractography in Left and Right Temporal Lobe Epilepsy. AJNR Am J Neuroradiol. 30(9):1740. doi: 10.3174/ajnr.A1650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allone C, Lo Buono V, Corallo F, Pisani LR, Pollicino P, Bramanti P, Marino S. 2017. Neuroimaging and cognitive functions in temporal lobe epilepsy: A review of the literature. J Neurol Sci. 381:7–15. doi: 10.1016/j.jns.2017.08.007. [DOI] [PubMed] [Google Scholar]
- An J, Fang P, Wang W, Liu Z, Hu D, Qiu S. 2014. Decreased white matter integrity in mesial temporal lobe epilepsy: a machine learning approach. Neuroreport. 25(10):788–794. doi: 10.1097/WNR.0000000000000178. [DOI] [PubMed] [Google Scholar]
- Balachandra AR, Kaestner E, Bahrami N, Reyes A, Lalani S, Macari AC, Paul BM, Bonilha L, McDonald CR. 2020. Clinical utility of structural connectomics in predicting memory in temporal lobe epilepsy. Neurology. 94(23):e2424–e2435. doi: 10.1212/WNL.0000000000009457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell GS, Neligan A, Sander JW. 2014. An unknown quantity—The worldwide prevalence of epilepsy. Epilepsia. 55(7):958–962. doi: 10.1111/epi.12605. [DOI] [PubMed] [Google Scholar]
- Bernhardt BC, Fadaie F, Liu M, Caldairou B, Gu S, Jefferies E, Smallwood J, Bassett DS, Bernasconi A, Bernasconi N. 2019. Temporal lobe epilepsy: Hippocampal pathology modulates connectome topology and controllability. Neurology. 92(19):e2209–e2220. doi: 10.1212/WNL.0000000000007447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Rozen DA, Worsley KJ, Evans AC, Bernasconi N, Bernasconi A. 2009. Thalamo-cortical network pathology in idiopathic generalized epilepsy: insights from MRI-based morphometric correlation analysis. NeuroImage. 46(2):373–381. doi: 10.1016/j.neuroimage.2009.01.055. [DOI] [PubMed] [Google Scholar]
- Besson P, Dinkelacker V, Valabregue R, Thivard L, Leclerc X, Baulac M, Sammler D, Colliot O, Lehéricy S, Samson S, et al. 2014. Structural connectivity differences in left and right temporal lobe epilepsy. NeuroImage. 100:135–144. doi: 10.1016/j.neuroimage.2014.04.071. [DOI] [PubMed] [Google Scholar]
- Bonilha Leonardo, Jensen JH, Baker N, Breedlove J, Nesland T, Lin JJ, Drane DL, Saindane AM, Binder JR, Kuzniecky RI. 2015. The brain connectome as a personalized biomarker of seizure outcomes after temporal lobectomy. Neurology. 84(18):1846–1853. doi: 10.1212/WNL.0000000000001548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Lee C-Y, Jensen JH, Tabesh A, Spampinato MV, Edwards JC, Breedlove J, Helpern JA. 2015. Altered microstructure in temporal lobe epilepsy: a diffusional kurtosis imaging study. AJNR Am J Neuroradiol. 36(4):719–724. doi: 10.3174/ajnr.A4185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant L, McKinnon ET, Taylor JA, Jensen JH, Bonilha L, de Bezenac C, Kreilkamp BAK, Adan G, Wieshmann UC, Biswas S, et al. 2021. Fiber ball white matter modeling in focal epilepsy. Hum Brain Mapp. 42(8):2490–2507. doi: 10.1002/hbm.25382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busch RM, Hogue O, Kattan MW, Hamberger M, Drane DL, Hermann B, Kim M, Ferguson L, Bingaman W, Gonzalez-Martinez J, et al. 2018. Nomograms to predict naming decline after temporal lobe surgery in adults with epilepsy. Neurology. 91(23):e2144–e2152. doi: 10.1212/WNL.0000000000006629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y-HA, Kemmotsu N, Leyden KM, Kucukboyaci NE, Iragui VJ, Tecoma ES, Kansal L, Norman MA, Compton R, Ehrlich TJ, et al. 2017. Multimodal imaging of language reorganization in patients with left temporal lobe epilepsy. Brain Lang. 170:82–92. doi: 10.1016/j.bandl.2017.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang Y-HA, Marshall A, Bahrami N, Mathur K, Javadi SS, Reyes A, Hegde M, Shih JJ, Paul BM, Hagler DJ Jr, et al. 2019. Differential sensitivity of structural, diffusion, and resting-state functional MRI for detecting brain alterations and verbal memory impairment in temporal lobe epilepsy. Epilepsia. 60(5):935–947. doi: 10.1111/epi.14736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Weigel D, Ganslandt O, Buchfelder M, Nimsky C. 2009. Prediction of visual field deficits by diffusion tensor imaging in temporal lobe epilepsy surgery. NeuroImage. 45(2):286–297. doi: 10.1016/j.neuroimage.2008.11.038. [DOI] [PubMed] [Google Scholar]
- Clancy MJ, Clarke MC, Connor DJ, Cannon M, Cotter DR. 2014. The prevalence of psychosis in epilepsy; a systematic review and meta-analysis. BMC Psychiatry. 14:75. doi: 10.1186/1471-244X-14-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coan AC, Cendes F. 2013. Understanding the spectrum of temporal lobe epilepsy: contributions for the development of individualized therapies. Expert Rev Neurother. 13(12):1383–1394. doi: 10.1586/14737175.2013.857604. [DOI] [PubMed] [Google Scholar]
- Concha L, Kim H, Bernasconi A, Bernhardt BC, Bernasconi N. 2012. Spatial patterns of water diffusion along white matter tracts in temporal lobe epilepsy. Neurology. 79(5):455–462. doi: 10.1212/WNL.0b013e31826170b6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Concha L, Livy DJ, Beaulieu C, Wheatley BM, Gross DW. 2010. In Vivo Diffusion Tensor Imaging and Histopathology of the Fimbria-Fornix in Temporal Lobe Epilepsy. J Neurosci. 30(3):996. doi: 10.1523/JNEUROSCI.1619-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diao L, Yu H, Zheng J, Chen Z, Huang D, Yu L. 2015. Abnormalities of the uncinate fasciculus correlate with executive dysfunction in patients with left temporal lobe epilepsy. Magn Reson Imaging. 33(5):544–550. doi: 10.1016/j.mri.2015.02.011. [DOI] [PubMed] [Google Scholar]
- Diehl B, Busch RM, Duncan JS, Piao Z, Tkach J, Lüders HO. 2008. Abnormalities in diffusion tensor imaging of the uncinate fasciculus relate to reduced memory in temporal lobe epilepsy. Epilepsia. 49(8):1409–1418. doi: 10.1111/j.1528-1167.2008.01596.x. [DOI] [PubMed] [Google Scholar]
- Dinkelacker V, Valabregue R, Thivard L, Lehéricy S, Baulac M, Samson S, Dupont S. 2015. Hippocampal-thalamic wiring in medial temporal lobe epilepsy: Enhanced connectivity per hippocampal voxel. Epilepsia. 56(8):1217–1226. doi: 10.1111/epi.13051. [DOI] [PubMed] [Google Scholar]
- Doherty C, Nowacki AS, Pat McAndrews M, McDonald CR, Reyes A, Kim MS, Hamberger M, Najm I, Bingaman W, Jehi L, et al. 2021. Predicting mood decline following temporal lobe epilepsy surgery in adults. Epilepsia. 62(2):450–459. doi: 10.1111/epi.16800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elkommos S, Mula M. 2021. A systematic review of neuroimaging studies of depression in adults with epilepsy. Epilepsy Behav EB. 115:107695. doi: 10.1016/j.yebeh.2020.107695. [DOI] [PubMed] [Google Scholar]
- Elliott CA, Gross DW, Wheatley BM, Beaulieu C, Sankar T. 2018. Longitudinal hippocampal and extra-hippocampal microstructural and macrostructural changes following temporal lobe epilepsy surgery. Epilepsy Res. 140:128–137. doi: 10.1016/j.eplepsyres.2018.01.008. [DOI] [PubMed] [Google Scholar]
- Ellmore TM, Beauchamp MS, Breier JI, Slater JD, Kalamangalam GP, O’Neill TJ, Disano MA, Tandon N. 2010. Temporal lobe white matter asymmetry and language laterality in epilepsy patients. NeuroImage. 49(3):2033–2044. doi: 10.1016/j.neuroimage.2009.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flügel D, Cercignani M, Symms MR, O’Toole A, Thompson PJ, Koepp MJ, Foong J. 2006. Diffusion tensor imaging findings and their correlation with neuropsychological deficits in patients with temporal lobe epilepsy and interictal psychosis. Epilepsia. 47(5):941–944. doi: 10.1111/j.1528-1167.2006.00527.x. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Marsland AL, Sheu LK, Erickson KI, Verstynen TD. 2013. Inflammatory pathways link socioeconomic inequalities to white matter architecture. Cereb Cortex N Y N 1991. 23(9):2058–2071. doi: 10.1093/cercor/bhs191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilliam F, Kuzniecky R, Faught E, Black L, Carpenter G, Schrodt R. 1997. Patient-validated content of epilepsy-specific quality-of-life measurement. Epilepsia. 38(2):233–236. doi: 10.1111/j.1528-1157.1997.tb01102.x. [DOI] [PubMed] [Google Scholar]
- Girardi-Schappo M, Fadaie F, Lee HM, Caldairou B, Sziklas V, Crane J, Bernhardt BC, Bernasconi A, Bernasconi N. 2021. Altered communication dynamics reflect cognitive deficits in temporal lobe epilepsy. Epilepsia. 62(4):1022–1033. doi: 10.1111/epi.16864. [DOI] [PubMed] [Google Scholar]
- Gleichgerrcht E, Keller SS, Drane DL, Munsell BC, Davis KA, Kaestner E, Weber B, Krantz S, Vandergrift WA, Edwards JC, et al. 2020. Temporal lobe epilepsy surgical outcomes can be inferred based on structural connectome hubs: a machine learning study. Ann Neurol. 88(5):970–983. doi: 10.1002/ana.25888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton SN, Huynh KH, Bonilha L, Abela E, Alhusaini S, Altmann A, Alvim MKM, Balachandra AR, Bartolini E, Bender B, et al. 2020. White matter abnormalities across different epilepsy syndromes in adults: an ENIGMA-Epilepsy study. Brain J Neurol. 143(8):2454–2473. doi: 10.1093/brain/awaa200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel MP, Sporns O. 2013. Network hubs in the human brain. Trends Cogn Sci. 17(12):683–696. doi: 10.1016/j.tics.2013.09.012. [DOI] [PubMed] [Google Scholar]
- Huntenburg JM, Bazin P-L, Margulies DS. 2018. Large-Scale Gradients in Human Cortical Organization. Trends Cogn Sci. 22(1):21–31. doi: 10.1016/j.tics.2017.11.002. [DOI] [PubMed] [Google Scholar]
- Jehi L, Yardi R, Chagin K, Tassi L, Russo GL, Worrell G, Hu W, Cendes F, Morita M, Bartolomei F, et al. 2015. Development and validation of nomograms to provide individualised predictions of seizure outcomes after epilepsy surgery: a retrospective analysis. Lancet Neurol. 14(3):283–290. doi: 10.1016/S1474-4422(14)70325-4. [DOI] [PubMed] [Google Scholar]
- Kaestner E, Balachandra AR, Bahrami N, Reyes A, Lalani SJ, Macari AC, Voets NL, Drane DL, Paul BM, Bonilha L, et al. 2020. The white matter connectome as an individualized biomarker of language impairment in temporal lobe epilepsy. NeuroImage Clin. 25:102125. doi: 10.1016/j.nicl.2019.102125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya K, Amemiya S, Suzuki Y, Kunii N, Kawai K, Mori H, Kunimatsu A, Saito N, Aoki S, Ohtomo K. 2016. Machine Learning of DTI Structural Brain Connectomes for Lateralization of Temporal Lobe Epilepsy. Magn Reson Med Sci MRMS Off J Jpn Soc Magn Reson Med. 15(1):121–129. doi: 10.2463/mrms.2015-0027. [DOI] [PubMed] [Google Scholar]
- Kanner AM, Schachter SC, Barry JJ, Hesdorffer DC, Hersdorffer DC, Mula M, Trimble M, Hermann B, Ettinger AE, Dunn D, et al. 2012. Depression and epilepsy: epidemiologic and neurobiologic perspectives that may explain their high comorbid occurrence. Epilepsy Behav EB. 24(2):156–168. doi: 10.1016/j.yebeh.2012.01.007. [DOI] [PubMed] [Google Scholar]
- Kavanaugh B, Correia S, Jones J, Blum A, LaFrance WC, Davis JD. 2017. White matter integrity correlates with depressive symptomatology in temporal lobe epilepsy. Epilepsy Behav EB. 77:99–105. doi: 10.1016/j.yebeh.2017.07.035. [DOI] [PubMed] [Google Scholar]
- Keller SS, Ahrens T, Mohammadi S, Möddel G, Kugel H, Ringelstein EB, Deppe M. 2011. Microstructural and volumetric abnormalities of the putamen in juvenile myoclonic epilepsy. Epilepsia. 52(9):1715–1724. doi: 10.1111/j.1528-1167.2011.03117.x. [DOI] [PubMed] [Google Scholar]
- Keller SS, Glenn GR, Weber B, Kreilkamp BAK, Jensen JH, Helpern JA, Wagner J, Barker GJ, Richardson MP, Bonilha L. 2017. Preoperative automated fibre quantification predicts postoperative seizure outcome in temporal lobe epilepsy. Brain J Neurol. 140(1):68–82. doi: 10.1093/brain/aww280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemmotsu N, Kucukboyaci NE, Leyden KM, Cheng CE, Girard HM, Iragui VJ, Tecoma ES, McDonald CR. 2014. Frontolimbic brain networks predict depressive symptoms in temporal lobe epilepsy. Epilepsy Res. 108(9):1554–1563. doi: 10.1016/j.eplepsyres.2014.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labate A, Caligiuri ME, Fortunato F, Ferlazzo E, Aguglia U, Gambardella A. 2020. Late drug-resistance in mild MTLE: Can it be influenced by preexisting white matter alterations? Epilepsia. 61(5):924–934. doi: 10.1111/epi.16503. [DOI] [PubMed] [Google Scholar]
- Labate A, Cherubini A, Tripepi G, Mumoli L, Ferlazzo E, Aguglia U, Quattrone A, Gambardella A. 2015. White matter abnormalities differentiate severe from benign temporal lobe epilepsy. Epilepsia. 56(7):1109–1116. doi: 10.1111/epi.13027. [DOI] [PubMed] [Google Scholar]
- Larivière S, Bernasconi A, Bernasconi N, Bernhardt BC. 2021. Connectome biomarkers of drug-resistant epilepsy. Epilepsia. 62(1):6–24. doi: 10.1111/epi.16753. [DOI] [PubMed] [Google Scholar]
- Lee C-Y, Tabesh A, Spampinato MV, Helpern JA, Jensen JH, Bonilha L. 2014. Diffusional kurtosis imaging reveals a distinctive pattern of microstructural alternations in idiopathic generalized epilepsy. Acta Neurol Scand. 130(3):148–155. doi: 10.1111/ane.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DA, Ko J, Kim HC, Shin KJ, Park BS, Kim IH, Park JH, Park S, Park KM. 2021. Identifying juvenile myoclonic epilepsy via diffusion tensor imaging using machine learning analysis. J Clin Neurosci Off J Neurosurg Soc Australas. 91:327–333. doi: 10.1016/j.jocn.2021.07.035. [DOI] [PubMed] [Google Scholar]
- Lemkaddem A, Daducci A, Kunz N, Lazeyras F, Seeck M, Thiran J-P, Vulliémoz S. 2014. Connectivity and tissue microstructural alterations in right and left temporal lobe epilepsy revealed by diffusion spectrum imaging. NeuroImage Clin. 5:349–358. doi: 10.1016/j.nicl.2014.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyden KM, Kucukboyaci NE, Puckett OK, Lee D, Loi RQ, Paul B, McDonald CR. 2015. What does diffusion tensor imaging (DTI) tell us about cognitive networks in temporal lobe epilepsy? Quant Imaging Med Surg. 5(2):247–263. doi: 10.3978/j.issn.2223-4292.2015.02.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H, Leng X, Qin C, Wang W, Zhang C, Qiu S. 2020. Altered White Matter Structural Network in Frontal and Temporal Lobe Epilepsy: A Graph-Theoretical Study. Front Neurol. 11:561. doi: 10.3389/fneur.2020.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Chen Z, Beaulieu C, Gross DW. 2014. Disrupted anatomic white matter network in left mesial temporal lobe epilepsy. Epilepsia. 55(5):674–682. doi: 10.1111/epi.12581. [DOI] [PubMed] [Google Scholar]
- Loi RQ, Leyden KM, Balachandra A, Uttarwar V, Hagler DJ, Paul BM, Dale AM, White NS, McDonald CR. 2016. Restriction spectrum imaging reveals decreased neurite density in patients with temporal lobe epilepsy. Epilepsia. 57(11):1897–1906. doi: 10.1111/epi.13570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald CR, Ahmadi ME, Hagler DJ, Tecoma ES, Iragui VJ, Gharapetian L, Dale AM, Halgren E. 2008. Diffusion tensor imaging correlates of memory and language impairments in temporal lobe epilepsy. Neurology. 71(23):1869–1876. doi: 10.1212/01.wnl.0000327824.05348.3b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKavanagh A, Kreilkamp BAK, Chen Y, Denby C, Bracewell M, Das K, De Bezenac C, Marson AG, Taylor PN, Keller SS. 2021. Sep 28. Altered Structural Brain Networks in Refractory and Nonrefractory Idiopathic Generalized Epilepsy. Brain Connect. doi: 10.1089/brain.2021.0035. [DOI] [PubMed] [Google Scholar]
- Munsell BC, Wu G, Fridriksson J, Thayer K, Mofrad N, Desisto N, Shen D, Bonilha L. 2019. Relationship between neuronal network architecture and naming performance in temporal lobe epilepsy: A connectome based approach using machine learning. Brain Lang. 193:45–57. doi: 10.1016/j.bandl.2017.08.006. [DOI] [PubMed] [Google Scholar]
- Nazem-Zadeh M-R, Elisevich K, Air EL, Schwalb JM, Divine G, Kaur M, Wasade VS, Mahmoudi F, Shokri S, Bagher-Ebadian H, et al. 2016. DTI-based response-driven modeling of mTLE laterality. NeuroImage Clin. 11:694–706. doi: 10.1016/j.nicl.2015.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nazem-Zadeh M-R, Schwalb JM, Elisevich KV, Bagher-Ebadian H, Hamidian H, Akhondi-Asl A-R, Jafari-Khouzani K, Soltanian-Zadeh H. 2014. Lateralization of temporal lobe epilepsy using a novel uncertainty analysis of MR diffusion in hippocampus, cingulum, and fornix, and hippocampal volume and FLAIR intensity. J Neurol Sci. 342(1–2):152–161. doi: 10.1016/j.jns.2014.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osipowicz K, Sperling MR, Sharan AD, Tracy JI. 2016. Functional MRI, resting state fMRI, and DTI for predicting verbal fluency outcome following resective surgery for temporal lobe epilepsy. J Neurosurg. 124(4):929–937. doi: 10.3171/2014.9.JNS131422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper RJ, Yoong MM, Kandasamy J, Chin RF. 2014. Application of diffusion tensor imaging and tractography of the optic radiation in anterior temporal lobe resection for epilepsy: a systematic review. Clin Neurol Neurosurg. 124:59–65. doi: 10.1016/j.clineuro.2014.06.013. [DOI] [PubMed] [Google Scholar]
- Powell HWR, Parker GJM, Alexander DC, Symms MR, Boulby PA, Barker GJ, Thompson PJ, Koepp MJ, Duncan JS. 2008. Imaging language pathways predicts postoperative naming deficits. J Neurol Neurosurg Psychiatry. 79(3):327–330. doi: 10.1136/jnnp.2007.126078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell HWR, Parker GJM, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott C a. M, Barker GJ, Koepp MJ, Duncan JS. 2005. MR tractography predicts visual field defects following temporal lobe resection. Neurology. 65(4):596–599. doi: 10.1212/01.wnl.0000172858.20354.73. [DOI] [PubMed] [Google Scholar]
- Powell HWR, Parker GJM, Alexander DC, Symms MR, Boulby PA, Wheeler-Kingshott CAM, Barker GJ, Koepp MJ, Duncan JS. 2007. Abnormalities of language networks in temporal lobe epilepsy. NeuroImage. 36(1):209–221. doi: 10.1016/j.neuroimage.2007.02.028. [DOI] [PubMed] [Google Scholar]
- Pustina D, Doucet G, Evans J, Sharan A, Sperling M, Skidmore C, Tracy J. 2014. Distinct Types of White Matter Changes Are Observed after Anterior Temporal Lobectomy in Epilepsy. PLOS ONE. 9(8):e104211. doi: 10.1371/journal.pone.0104211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Kaestner E, Bahrami N, Balachandra A, Hegde M, Paul BM, Hermann B, McDonald CR. 2019. Cognitive phenotypes in temporal lobe epilepsy are associated with distinct patterns of white matter network abnormalities. Neurology. 92(17):e1957–e1968. doi: 10.1212/WNL.0000000000007370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A, Uttarwar VS, Chang Y-HA, Balachandra AR, Pung CJ, Hagler DJ, Paul BM, McDonald CR. 2018. Decreased neurite density within frontostriatal networks is associated with executive dysfunction in temporal lobe epilepsy. Epilepsy Behav EB. 78:187–193. doi: 10.1016/j.yebeh.2017.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodrigo S, Oppenheim C, Chassoux F, Hodel J, de Vanssay A, Baudoin-Chial S, Devaux B, Meder J-F. 2008. Language lateralization in temporal lobe epilepsy using functional MRI and probabilistic tractography. Epilepsia. 49(8):1367–1376. doi: 10.1111/j.1528-1167.2008.01607.x. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Cruces R, Bernhardt BC, Concha L. 2020. Multidimensional associations between cognition and connectome organization in temporal lobe epilepsy. NeuroImage. 213:116706. doi: 10.1016/j.neuroimage.2020.116706. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Cruces R, Velázquez-Pérez L, Rodríguez-Leyva I, Velasco AL, Trejo-Martínez D, Barragán-Campos HM, Camacho-Téllez V, Concha L. 2018. Association of white matter diffusion characteristics and cognitive deficits in temporal lobe epilepsy. Epilepsy Behav EB. 79:138–145. doi: 10.1016/j.yebeh.2017.11.040. [DOI] [PubMed] [Google Scholar]
- Shaked D, Leibel DK, Katzel LI, Davatzikos C, Gullapalli RP, Seliger SL, Erus G, Evans MK, Zonderman AB, Waldstein SR. 2019. Disparities in Diffuse Cortical White Matter Integrity Between Socioeconomic Groups. Front Hum Neurosci. 13:198. doi: 10.3389/fnhum.2019.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman EMS, Wiebe S, Fay-McClymont TB, Tellez-Zenteno J, Metcalfe A, Hernandez-Ronquillo L, Hader WJ, Jetté N. 2011. Neuropsychological outcomes after epilepsy surgery: systematic review and pooled estimates. Epilepsia. 52(5):857–869. doi: 10.1111/j.1528-1167.2011.03022.x. [DOI] [PubMed] [Google Scholar]
- Slinger G, Sinke MRT, Braun KPJ, Otte WM. 2016. White matter abnormalities at a regional and voxel level in focal and generalized epilepsy: A systematic review and meta-analysis. NeuroImage Clin. 12:902. doi: 10.1016/j.nicl.2016.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sone D, Sato N, Shigemoto Y, Kimura Y, Maikusa N, Ota M, Foong J, Koepp M, Matsuda H. 2020. Disrupted White Matter Integrity and Structural Brain Networks in Temporal Lobe Epilepsy With and Without Interictal Psychosis. Front Neurol. 11:556569. doi: 10.3389/fneur.2020.556569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stretton J, Pope RA, Winston GP, Sidhu MK, Symms M, Duncan JS, Koepp M, Thompson PJ, Foong J. 2015. Temporal lobe epilepsy and affective disorders: the role of the subgenual anterior cingulate cortex. J Neurol Neurosurg Psychiatry. 86(2):144–151. doi: 10.1136/jnnp-2013-306966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taoka T, Sakamoto M, Iwasaki S, Nakagawa H, Fukusumi A, Hirohashi S, Taoka K, Kichikawa K, Hoshida T, Sakaki T. 2005. Diffusion tensor imaging in cases with visual field defect after anterior temporal lobectomy. AJNR Am J Neuroradiol. 26(4):797–803. [PMC free article] [PubMed] [Google Scholar]
- Tavakol S, Royer J, Lowe AJ, Bonilha L, Tracy JI, Jackson GD, Duncan JS, Bernasconi A, Bernasconi N, Bernhardt BC. 2019. Neuroimaging and connectomics of drug-resistant epilepsy at multiple scales: From focal lesions to macroscale networks. Epilepsia. 60(4):593–604. doi: 10.1111/epi.14688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vollmar C, O’Muircheartaigh J, Symms MR, Barker GJ, Thompson P, Kumari V, Stretton J, Duncan JS, Richardson MP, Koepp MJ. 2012. Altered microstructural connectivity in juvenile myoclonic epilepsy: the missing link. Neurology. 78(20):1555–1559. doi: 10.1212/WNL.0b013e3182563b44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W-H, Liou H-H, Chen C-C, Chiu M-J, Chen T-F, Cheng T-W, Hua M-S. 2011. Neuropsychological performance and seizure-related risk factors in patients with temporal lobe epilepsy: A retrospective cross-sectional study. Epilepsy Behav. 22(4):728–734. doi: 10.1016/j.yebeh.2011.08.038. [DOI] [PubMed] [Google Scholar]
- Weng Y, Larivière S, Caciagli L, Vos de Wael R, Rodríguez-Cruces R, Royer J, Xu Q, Bernasconi N, Bernasconi A, Thomas Yeo BT, et al. 2020. Macroscale and microcircuit dissociation of focal and generalized human epilepsies. Commun Biol. 3(1):1–11. doi: 10.1038/s42003-020-0958-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whelan CD, Altmann A, Botía JA, Jahanshad N, Hibar DP, Absil J, Alhusaini S, Alvim MKM, Auvinen P, Bartolini E, et al. 2018. Structural brain abnormalities in the common epilepsies assessed in a worldwide ENIGMA study. Brain J Neurol. 141(2):391–408. doi: 10.1093/brain/awx341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widjaja E, Kis A, Go C, Snead OC, Smith ML. 2014. Bilateral white matter abnormality in children with frontal lobe epilepsy. Epilepsy Res. 108(2):289–294. doi: 10.1016/j.eplepsyres.2013.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieshmann UC, Symms MR, Clark CA, Lemieux L, Franconi F, Parker GJ, Barker GJ, Shorvon SD. 1999. Wallerian degeneration in the optic radiation after temporal lobectomy demonstrated in vivo with diffusion tensor imaging. Epilepsia. 40(8):1155–1158. doi: 10.1111/j.1528-1157.1999.tb00834.x. [DOI] [PubMed] [Google Scholar]
- Winston GP, Daga P, White MJ, Micallef C, Miserocchi A, Mancini L, Modat M, Stretton J, Sidhu MK, Symms MR, et al. 2014. Preventing visual field deficits from neurosurgery. Neurology. 83(7):604–611. doi: 10.1212/WNL.0000000000000685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winston GP, Stretton J, Sidhu MK, Symms MR, Thompson PJ, Duncan JS. 2013. Structural correlates of impaired working memory in hippocampal sclerosis. Epilepsia. 54(7):1143–1153. doi: 10.1111/epi.12193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogarajah M, Focke NK, Bonelli SB, Thompson P, Vollmar C, McEvoy AW, Alexander DC, Symms MR, Koepp MJ, Duncan JS. 2010. The structural plasticity of white matter networks following anterior temporal lobe resection. Brain J Neurol. 133(Pt 8):2348–2364. doi: 10.1093/brain/awq175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogarajah M, Powell HWR, Parker GJM, Alexander DC, Thompson PJ, Symms MR, Boulby P, Wheeler-Kingshott CA, Barker GJ, Koepp MJ, et al. 2008. Tractography of the parahippocampal gyrus and material specific memory impairment in unilateral temporal lobe epilepsy. NeuroImage. 40(4):1755–1764. doi: 10.1016/j.neuroimage.2007.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]


