Abstract
Osteoarthritis (OA) is a common chronic degenerative joint disease worldwide. The pathological features of OA are the erosion of articular cartilage, subchondral bone sclerosis, synovitis, and metabolic disorder. Its progression is characterized by aberrant expression of genes involved in inflammation, proliferation, and metabolism of chondrocytes. Effective therapeutic strategies are limited, as mechanisms underlying OA pathophysiology remain unclear. Significant research efforts are ongoing to elucidate the complex molecular mechanisms underlying OA focused on gene transcription. However, posttranscriptional alterations also play significant function in inflammation and metabolic changes related diseases. RNA binding proteins (RBPs) have been recognized as important regulators in posttranscriptional regulation. RBPs regulate RNA subcellular localization, stability, and translational efficiency by binding to their target mRNAs, thereby controlling their protein expression. However, their role in OA is less clear. Identifying RBPs in OA is of great importance to better understand OA pathophysiology and to figure out potential targets for OA treatment. Hence, in this manuscript, we summarize the recent knowledge on the role of dysregulated RBPs in OA and hope it will provide new insight for OA study and targeted treatment.
Keywords: RNA binding proteins, osteoarthritis, alternative splicing, mRNA stability, RNA metabolism
Introduction
Cell fate decisions including cellular identity, differentiation, morphology, and phenotype are directed by complex regulatory machinery (Schuschel et al., 2020). The regulatory mechanisms for precise patterns of gene expression range from epigenetic modifications in the transcript level, RNA modifications in posttranscriptional level to posttranslational modifications of the proteome (Zhang and Cao, 2019). The initiation of gene regulation is carried out by proteins that bind specific domain of DNA or RNA molecules. One class of such proteins is transcription factors (TFs), which regulated gene transcription by binding with DNA sequences (Neefjes et al., 2020). Another class of such proteins is RNA-binding proteins (RBPs), which primarily regulated RNA metabolism via binding to their target RNAs (Zhao et al., 2017). RBPs-mediated posttranscriptional regulation changes protein expression relatively quickly compared with TF-mediated transcription regulation. These precise regulation machineries contribute to the cellular homeostasis.
Posttranscriptional regulation is one important step of gene expression regulation (Lee et al., 2021). TFs regulated the produce of RNA transcript, and the maturation and fate of the mRNA was regulated by noncoding RNAs (e.g., miRNAs) and RBPs (Bansal and Arora, 2020; Kinoshita et al., 2021). RBPs are a series of proteins which could bind with double or single-stranded RNA and thereby demonstrate the function to influence the RNA fate by forming ribonucleoprotein (Glisovic et al., 2008; Bohnsack and Bohnsack, 2019). The main role of RBPs is RNA metabolism regulation, including mRNA stability (Shi et al., 2017), mRNA splicing (Rachez et al., 2021), translocation (Tolino et al., 2012), translation (Moore and von Lindern, 2018) as well as the corporation with noncoding RNAs (Ciafre and Galardi, 2013; Qin et al., 2020). The functions of RBPs are highly dependent on their structural features which is the RNA binding domains, and the most common domains are including RNA recognition motif (RRM), arginine-glycine-glycine (RGG) motif, the hnRNP-K homology domain (KH), cold-shock domain (CSD), double-stranded RBD (dsRBD), tyrosine rich domain and the zinc finger domain (ZF) (Maris et al., 2005; Dominguez et al., 2018; Qin et al., 2020; Dobrev et al., 2021).
Due to their important roles in gene expression regulation, RBPs are critical in human biology and development. Functional disruption of RBPs, thus, leads to lots of human diseases including cancers, metabolic-related diseases, inflammatory diseases, and so on. Osteoarthritis (OA) is a novel metabolic-related and inflammatory-related disease featured by changed cell morphology and the aberrant gene expression pattern (Martel-Pelletier et al., 2016). Chondrocytes are a single cell type in cartilage tissue that secrete type II collagen and are surrounded by a large number of extracellular matrix proteins. During the OA progression, the morphology and the expression of extracellular matrix proteins of chondrocytes were remarkedly changed (Liu et al., 2016; Aladal et al., 2022). Researchers reported that posttranscriptional alterations play a significant function in OA progression. For example, miR-877-5p alleviates chondrocyte dysfunction in OA models via repressing FOXM1 expression by binding with 3′-UTR of FOXM1 mRNA. Next, targeted METTL3 inhibition could alleviate the senescence of fibroblast-like synoviocytes (FLS) via posttranscriptional regulation of ATG7 and limit OA development in experimental animal models, providing a potential strategy for OA therapy (Chen et al., 2022).
In recent times, Yutaro Uchida and others summarized the emerging roles of RBPs in regulating inflammation diseases, such as rheumatoid arthritis (Uchida et al., 2019). Moreover, researchers reported that the concentrations of cold-inducible RNA-binding protein in synovial fluid are associated with severity in knee OA (Yu et al., 2017). In addition, in Wu’s article, they discussed the therapeutic potential and role of miRNA, lncRNA, and circRNA in OA, and they pointed that RBPs participated in this network (Wu et al., 2019). However, the biological significance of RBPs in OA remains unsystematically discussed. Emerging evidence indicated that lots of RBPs are dysregulated, which influences the development and progression of OA. Therefore, in our recent review, we will briefly summarize the current knowledge on the role of RBPs in OA.
RNA-binding proteins in regulating RNA metabolism
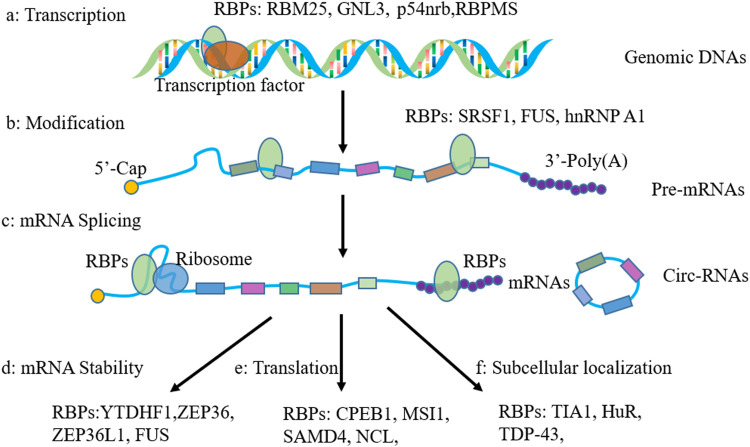
RNA metabolism consists of RNA synthesis, folding/unfolding, transport, modifications, processing, translocation as well as degradation. RBPs were reported, which demonstrate an important role in these processes. In this section, we will review the general role of RBPs in RNA metabolism (Figure 1).
FIGURE 1.
Functions of RNA-binding proteins in regulating gene expression. (A) regulation in gene transcription; (B) mRNA modifications; (C) function in RNA splicing; (D) Function in regulating RNA stability; (E) regulation of gene translation; (F) regulation of mRNA subcellular localization.
RNA synthesis
First, except traditional transcript factors regulated RNA synthesis, evidence suggests that some RBPs are also demonstrate function in regulating transcription. For example, RBM25 was an RBP involved in splicing regulation, and YY1 was a known RNA-dependent transcription factor. Researchers reported that RBM25 depletion attenuates all YY1-dependent chromatin binding, DNA looping, and transcription (Xiao et al., 2019). Moreover, IGFBP3 participated in the repair of double strand breaks via forming a nuclear complex with EGFR and DNA-PKCs, and then, it promoted the stability of tumor genome in response to DNA-destructive chemotherapy (Lin et al., 2014). Furthermore, researchers reported that nucleolar GTP binding protein 3, GNL3, participates in genomic regulation as RNA-binding protein and upregulates IL24 and PTN expression to promote the development of OA (Zhu et al., 2021).
Modifications
Second, RBPs also participated in pre-mRNA postprocessing, adding a poly (A) tail at the 3′ end of the mRNA or a cap at the 5′ end (Qin et al., 2020). The presence of methylated guanosyl cap and the poly (A) tail mediate the stability of mRNAs and protects them from exonuclease degradation. The length of poly (A) tail can be influenced by RBPs, and then, the stability, subcellular location, and translation efficiency of target mRNAs were regulated (Darnell, 2013). Researchers illustrated that cytosolic polyadenylation element-binding protein (CPEB1) extended poly (A) tails of lots cell cycle-related mRNAs and enhanced their translation efficiency (D'Ambrogio et al., 2013). Moreover, CPEB1 can directly target the 3′-UTR of SIRT1, control the poly (A) tail length and inhibit its translation, and suppress the stemness and resistance of liver cancer (Xu et al., 2018). In addition, RBPs is also involved in the m6A modification of mRNAs by regulating methyltransferase installation. Researchers reported that RBM15 and Zc3h13 associated with Mettl3, Mettl14, and WTAP to form methyltransferase complex (Wen et al., 2018).
Alternative splicing
Third, RBPs regulated RNA splicing, another major pre-mRNA processing event. Some RBPs can interact with core proteins to form spliceosome complexes (Fredericks et al., 2015). The main types of aberrant splicing are constitutive splicing, including exon skipped, exon included, alternative five or three splice-sites, intron retention, and mutually exclusive exon, and they contribute to protein diversity and mRNA stability (Frankiw et al., 2019). For example, RNA-binding protein RBM10 was reported to inhibit the splicing of exon nine of NUMB mRNA (Collins et al., 2017). Feng et al. reported that SRSF1 was responsible for the mechanical stress-induced alternative splicing of cyclin D1 (Feng et al., 2021). RBFOX2 interacted with hnRNPC, hnRNPM, and SRSF1 to regulate splicing of a large number of transcripts implicated in cell differentiation and development (Zhou et al., 2021). In recent times, RBPs have also been reported to participate in the splicing and formation of circRNAs. Ni and others reported that FUS binds to the GUGGU sequences at nt 358 and 409 proximal to the back-splicing sites (exon 8) and one sequence at nt 499 proximal to the back-splicing sites (exon 10) of SLC7A2 to induce circSLC7A2 formation (Ni et al., 2021).
Subcellular localization
mRNA was generated in the nucleus and could be translocated into the cytoplasm. RBPs can recognize the cis motif or secondary structure of mRNA and mediate the localization of RNA to a specific subcellular compartment. The hnRNPs family member proteins are in charge of RNA transporting from the nucleus into the cytoplasm (Mofatteh and Bullock, 2017). More importantly, a recent study also revealed that RBPs mediated the translocation of mRNAs to stress granule (SGs), a ribonucleoprotein complex. Researchers reported that TIA-1 and HuR bind selectively to mRNAs with high AREs in their UTRs and recruit them to SGs (Bley et al., 2015). What’s more, the RBP TIA-1 binds p53 mRNA and mediates it with RNA particle localization. When the cell was damaged, p53 mRNA is released from the stress particles and binds to polysomes, resulting in the translation of p53 (Diaz-Munoz et al., 2017). Poly (A) binding protein (PABP), a cytoplasmic RNA-binding protein, regulated mRNA translation and stability by binding to the 3′ poly (A) tail. Researchers reported that PABP recruited localized mRNA and translation at the cell membrane (Bohm et al., 2018). In addition, recently studies reported that some RBPs showed significant function in regulating mRNA sorting to exosomes. Ann L Wozniak and others revealed that the RNA-binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation (Wozniak et al., 2020). Also, they reported that sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs (Villarroya-Beltri et al., 2013).
RNA stability
The eukaryotic mRNAs will be degradation. The cap structure at the 5′ end and poly (A) at the 3′ end play an important function in preventing mRNAs degradation (Hamilton et al., 2010). Researchers reported that almost 16% of transcripts 3′-UTR contain an AU-rich original, which is the main structure involved in reversing degradation of mRNAs (Gruber et al., 2011). Also, in this process, AU-rich original binding proteins promote the mRNA degradation or enhance their stability (Fallmann et al., 2016). Researchers reported that PTBP3 binds to the 3′-UTR of ZEB1 mRNA to prevent its degradation and promote EMT of breast cancer (Hou et al., 2018). Moreover, our group reported that CIRP binds to the 3′-UTR of CTNNB1 mRNA to promote its stability and the progression of nonsmall lung cancer (Liao et al., 2021). Furthermore, ZFC3H, RBP zinc-finger C3H1 domain-containing protein, play a crucial role in the regulating the degradation of nuclear RNAs via acting as an adaptor protein mediated the interaction between nuclear RNAs and the exonucleases complex (Silla et al., 2018). Next, the insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs) are a family of well-conserved mRNA-binding proteins consisting of two RRMs and four KH domains, and their cellular function is stabilization of bound mRNAs (Degrauwe et al., 2016). In addition, researchers reported that YTHDF1 mediated the stability of Bcl2 mRNA and inhibited the apoptosis and autophagy of chondrocytes in inflammation (He et al., 2022a).
Translation
The translation process is complex and exhibits an initiation stage, extension stage, and termination stage. Studies showed that RBPs eIF4F and PABP are involved in the translation activation. An internal ribosome entry site (IRES) is found in the 5′-UTR sequence of some mRNAs. Our group reported that CIRP binds with IRES sequence of CTNNB1 mRNA and promotes its translation (Liao et al., 2021). In addition to binding with the 5′-UTR of mRNAs to influence translation, some RBPs regulate the translation by binding 3′-UTR. For example, researchers reported that MSI1 binds to the 3′-UTR untranslated regions of mRNAs, interacts with the poly (A)–binding protein, and competes for eIF-4G. As a result, it disturbs the translation initiation (Kawahara et al., 2008). Further, MSI2 was reported promoted tumorigenesis by the inhibition of NUMB mRNA translation (Pereira et al., 2012). Moreover, Dab2 and ILEI mRNAs 3′-UTR contain TGF-β-activated translation (BAT) stem-loop elements, which can be recognized by hnRNPE1, and then, the translation was silenced (Chaudhury et al., 2010). Also, interestingly, hnRNP-K could enhance the translation of c-myc mRNA by binding to the internal ribosome entry site in the 5′-UTR (Evans et al., 2003), and it also could silence the translation of 15 lipoxygenase (LOX) mRNA by binding to its differentiation control element located in the 3′-UTR (Ostareck et al., 1997).
RNA-binding proteins in osteoarthritis
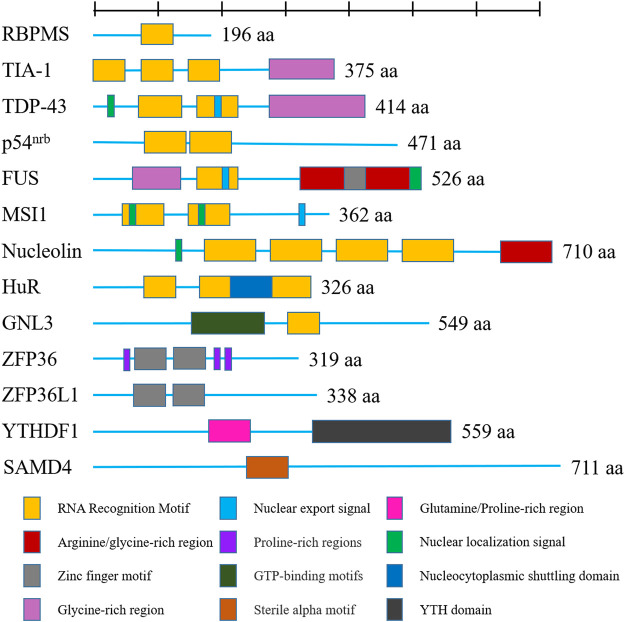
RBPs are increasingly revealed as crucial players in OA, as they have been proven to play an important function in the regulation of gene expression by influencing RNA metabolism. In the past decade, mounting studies illustrated that the role of RBPs in OA via their actions in RNA metabolism, including transcription, RNA alternative splicing, and mRNA stability. In our present review, we will discuss the function of RBPs in OA in the following aspects: 1) regulation in gene transcription, 2) function in RNA splicing, 3) function in regulating RNA stability, 4) RBPs in stress granules, 5) RBPs in regulating translation. The RBPs playing a major role in OA discussed in this review were shown in Figure 2.
FIGURE 2.
Shown are human RNA-binding proteins playing a major role in osteoarthritis as discussed in this review. Domains are color-coded and proteins are scaled to amino acid length. Different colors represent different functional domains.
RNA-binding proteins in regulating gene transcription in osteoarthritis
As we all know, the gene transcription regulation is traditionally known to be mediated by DNA-binding TFs. Recent studies indicate that some RBPs also demonstrate an important function in controlling gene transcription. Splicing factor proline and glutamine-rich protein, SFPQ, has been reported to cooperate with HDAC1 to suppress the CD40 transcription (Pan et al., 2019). Hata et al. illustrated that paraspeckle regulatory protein 54-kDa nuclear RNA-binding protein, p54nrb, associated with SOX9 to enhance the gene promoter activity of Col2a1 and promoted chondrocyte differentiation. Moreover, researchers reported that p54nrb could control the splicing and maturation of Col2a1 mRNA because its RRMs are highly homologous to the RRM domain of polypyrimidine tract-binding protein-associated splicing factor (Hata et al., 2008). Zhu and others reported that nucleolar GTP binding protein 3, GNL3, participates in genomic regulation as RNA-binding protein, and then, it upregulates IL24 and PTN expression to promote the development of OA (Zhu et al., 2021). Furthermore, RNA-binding protein with multiple splicing, RBPMS, has been shown to physically interact with Smads and enhance Smads’ transcriptional activity (Sun et al., 2006). Besides, Shanmugaapriya et al. reported that RBPMS expression was significantly decreased in aged and osteoarthritic cartilage, which suggested its potential role in the maintenance of normal articular cartilage (Shanm ugaapriya et al., 2016). Furthermore, Gu et al. reported that FUS might work together with Runx2 to mediate the transcription of Col10a1 and then regulate chondrocyte hypertrophic differentiation (Gu et al., 2014). In addition, DiGeorge syndrome critical region 8, DGCR8, a double-stranded RNA-binding protein, was reported to play a significant function in maintaining heterochromatin organization and attenuating senescence and OA (Deng et al., 2019).
RNA-binding proteins in regulating mRNA splicing in osteoarthritis
In recent times, Katsoula et al. performed deep RNA sequencing and genome-wide differential splicing analysis of cartilage tissues of 124 OA patients, and they reported differential splicing for 209 genes, which consists of extracellular matrix proteins, proteoglycans, and integrin surface interactions terms (Katsoula et al., 2022). For example, periostin (POSTN) is a secretory matricellular matrix protein. Researchers reported that splicing isoforms one of POSTN were highly expressed only in chondrocytes and reported to possibly influence cell adhesion (Cai et al., 2019). These findings highlight the important role of gene alternative splicing in OA progression. FUS is an RNA-binding protein that regulates RNA alternative splicing, transcription, and RNA transportation. FUS demonstrates a Gly-rich domain, a RRM domain, two arginine/glycine-rich domain, and a ZF motif. Studies showed that FUS can bind to RNAs that contain enriched GUGGU or GUU motif (Lagier-Tourenne et al., 2012; Zhao et al., 2018). Researchers reported that FUS regulated the mRNA splicing. Ni and others illustrated that FUS binds to the GUGGU sequences at nt 358 and 409 proximal to the back-splicing sites (exon 8) and one sequence at nt 499 proximal to the back-splicing sites (exon 10) of SLC7A2 to promotes circSLC7A2 production. Also, the formed circSLC7A2 protects against OA via inhibiting miR-4498/TIMP3 axis (Ni et al., 2021). Moreover, Shen et al. also reported that FUS promoted the splicing of PDE4B pre-mRNA to produce the circPDE4B. Also, they found that circPDE4B was downregulated in OA, and it prevented articular cartilage degeneration and promoted repair through acting as a scaffold for RIC8A and MID1 (Shen et al., 2021). In addition, RBP QKI belongs to STAR family, and it is a splicing factor. Wu et al. reported that QKI promoted the formation of circPDE4D, which inhibited chondrocyte matrix degradation by regulating miR-103a-3p/FGF18 axis (Wu et al., 2021).
RNA-binding proteins in regulating mRNA stability in osteoarthritis
N6-methyladenosine (m6A) is the most prevalent modification in eukaryotic messenger RNAs (mRNAs). It was installed by “writers”: the m6A methyltransferases including METTL3/14, WTAP, and RBM15/15B, reverted by “erasers,” and the demethylases including FTO and ALKBH5 and recognized by its “readers,” including RBPs YTHDF1/2/3 and IGF2BPs (Huang et al., 2018; Chen et al., 2019). The readers were illustrated to play an important role in regulating the stability of m6A-bearing transcripts. For example, METTL3 facilitates tumorigenesis by enhancing c-Myc mRNA stability via YTHDF1-mediated m6A modification (Zhao et al., 2020). Researchers reported that YTHDF1 mediated the stability of Bcl2 mRNA and inhibited the apoptosis and autophagy of chondrocytes in inflammation (He et al., 2022a). Moreover, Chen et al. reported that METTL3 regulated m6A modification and mRNA stability of ATG7, and then, it affected autophagy to promote cellular senescence and OA progression (Chen et al., 2022). In addition, METTL3 expression was significantly upregulated in degenerative human endplate cartilage tissue. It mediated the methylation of SOX9 mRNA and disrupted its stability, thereby inhibiting the expression of collagen 2 (Xiao et al., 2022).
ZFP36 is an ancient RNA-binding protein that is also known as tristetraprolin (TTP) and TPA-inducible sequence 11 (TIS11). The ZFP36 family consists of ZFP36, ZFP36L1, ZFP36L2, and ZFP36L3. They share the similar structure, including two tandemly repeated ZF motifs (CCCH), which bind to AU-rich elements in the 3′-UTRs of target mRNAs to trigger mRNA destabilization and decay (Bermudez et al., 2011; Sanduja et al., 2011). Researchers reported that ZFP36 binds to AREs in the 3′-UTR of SOX-9 mRNA, acts as a suppressor of SOX-9, and then regulates anabolic and catabolic gene expression in chondrocytes (McDermott et al., 2016). Moreover, Son et al. found that RNA-binding protein, ZFP36L1, was increased in expression in OA chondrocyte. ZFP36L1 bound to the 3′-UTR of the HSPA1A mRNA, enhanced HSPA1A mRNA decay, and reduced the protein level of HSPA1A, which was reported to protect against OA pathogenesis by inhibiting chondrocyte apoptosis (Son et al., 2019).
FUS also has been reported to play function in maintain mRNA stability. For example, FUS binds GluA1 mRNA in the vicinity of the 3′ terminus and controls poly (A) tail maintenance, thus regulating stability (Udagawa et al., 2015). Next, Bai et al. found that FUS worked together with LncRNA-MM2P to bind with and stabilize SOX-9 mRNA and then promoted the chondrocyte-specific protein expression (Bai et al., 2020). HuR demonstrates three RRMs domain and increased the stability of target mRNAs by binding with the AU-rich elements (AREs) of them (Pabis et al., 2019). The 3′-UTR of COX-2 mRNA contains an ARE element, and Riina et al. reported that aurothiomalate inhibited HuR expression and then promoted the destabilization of COX-2 mRNA in human cartilage chondrocytes (Nieminen et al., 2008). In addition, Lv and others reported that the RNA-binding protein SND1 bound with HSPA5 at the 3′UTR, destabilized and suppressed its expression, and then promoted the degradation of GPX4 and ferroptosis in osteoarthritis chondrocytes (Lv et al., 2022).
RNA-binding proteins in stress granules in osteoarthritis
When cells undergo physiological stress, they will form SGs to respond adequately to stress and adapt to them. SGs are ribonucleoprotein complexes consisting of translationally stalled mRNAs and RBPs, such as TIA-1 and HuR (Wolozin and Ivanov, 2019). The protein structure of TIA-1 contains three RRMs domains and a C-terminal Gly-rich domain, which contribute to stress granule assembly (Zhao et al., 2018). HuR also demonstrates three RRMs domains and prevents the degradation and increase the stability of target mRNAs by binding with the AREs of them (Pabis et al., 2019). Researchers reported that they bind selectively to mRNAs with high AREs in their UTRs and recruit them to SGs (Bley et al., 2015). In IL-1β-treated in OA chondrocytes, TAI-1 and HuR work together to sequester COX-2 mRNAs, which are in SGs, and suppress its expression (Ansari and Haqqi, 2016). What’s more, Liu et al. reported that the expression of HuR was significantly increased in rheumatoid arthritis (RA) synovial tissue, and TNF-α could induce the nucleocytoplasmic shuttling of HuR (Liu et al., 2019). Another important RBP, TDP-43, plays an important role in splicing. It exhibits two RRMs, a nuclear localization signal (NLS), a nuclear export signal, and a Gly-rich domain. It is usually localized in the nucleus, while localized to SGs upon cellular stress (Khalfallah et al., 2018). Chang et al. reported that TDP-43 maintains chondrocyte homeostasis and prevents cartilage degradation in OA (Chang et al., 2021). Moreover, Huang and others revealed that TDP-43 increased RACK1 expression and then promoted SGs formation and inhibited inflammatory response in OA (Huang et al., 2017). Besides, another group also reported the function of TDP-43 in regulating SGs formation, RACK1 expression, inflammatory factors secretion by inhibiting the JNK, and p38 MAPK signaling pathway in OA (Huang et al., 2019).
RNA-binding proteins in regulating translation in osteoarthritis
CPEB1 (cytoplasmic polyadenylation element-binding protein 1) is an RNA-binding protein that binds the 3′-UTR-cytoplasmic polyadenylation element of its target mRNAs, mediates the extension or removal of poly (A) tail, and then regulates their translation (Udagawa et al., 2013). Researchers reported that CPEB1 was upregulated in articular cartilage from OA patients, and its expression level was correlated with disease severity (Li et al., 2019). MSI1 binds to the 3′-UTR untranslated regions of mRNAs, interacts with the poly (A)-binding protein, and competes for eIF-4G. As a result, it disturbs the translation initiation. Padial-Molina et al. revealed that MSI1 expressed in mesenchymal stromal cells, osteoblasts, and osteocytes, but not in chondrocytes, suggested its different function in the bone healing environment (Kawahara et al., 2008). Next, RNA-binding protein sterile alpha motif domain-containing protein 4 (SAMD4) is a special RNA-binding protein. It contains a sterile alpha motif, which directly binds RNA with stem-loop structures and led the translational repression of target mRNA (Baez and Boccaccio, 2005). Niu et al. found that Samd4-deficient mice displayed chondrocyte defects proved its crucial role in metabolic bone diseases (Niu et al., 2017). Nucleolin (NCL), a type of multifunctional nonribosomal protein, which exhibits four RRM-domains, is mainly found in the nucleolus and plays key roles in ribosome biogenesis. Also, it has been reported to bind with 5′-UTR of p53 mRNA or 3′-UTR of Bcl2 mRNA, respectively, to influence the translation or mRNA stabilization, respectively, (Takagi et al., 2005; Ishimaru et al., 2010). Deng et al. reported that NCL promotes articular chondrocyte proliferation via the MAPK/Erk1/2 pathway (Deng et al., 2020). In addition, Lee et al. reported that PUM1 interacted with the 3′-UTR of TLR4 to suppress its mRNA translation, and PUM1 overexpression protected the chondrocytes from inflammation-mediated disruption of the chondrogenic phenotype (Yoon et al., 2022).
RNA-binding proteins in signaling pathway of osteoarthritis
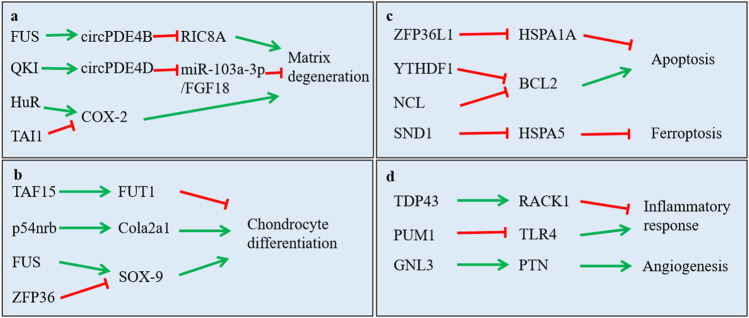
As we all know, OA acted as degenerative disease that was characterized by osteophyte formation, inflammatory response, angiogenesis and aberrant anabolic, and catabolic metabolism-induced cartilage destruction. Growing evidence indicated that RBPs participated in these pathways to effect OA progression, especially anabolic and catabolic metabolism. We summarized the function of RBPs on these pathways in Table 1 and Figure 3.
TABLE 1.
General overview of osteoarthritis-related RNA-binding proteins and their function.
| RBP | Target DNA or mRNA(s) | Function | Phenotype/Function | References |
|---|---|---|---|---|
| p54nrb | Col2a1 | Transcription | Enhance the gene promoter activity of Col2a1 and promoted chondrocyte differentiation | Hata et al. (2008) |
| GNL3 | IL24 and PTN | Genomic regulation | Induce articular osteocyte apoptosis and angiogenesis | Zhu et al. (2021) |
| RBPMS | Physically interact with Smads | Transcription | Form a counter-regulatory mechanism with TGF-β and IL-1β to maintenance the homeostasis of normal articular cartilage | (Sun et al., 2006) (Shanm ugaapriya et al., 2016) |
| FUS | Col10a1 promoter | Transcription | Mediate the transcription of Col10a1 and regulate chondrocyte hypertrophic differentiation | Gu et al. (2014) |
| DGCR8 | — | Maintaining heterochromatin organization | Alleviated hMSC aging and osteoarthritis | Deng et al. (2019) |
| FUS | SLC7A2 mRNA | Splicing | Mediate the formation of circSLC7A2 and inhibit the miR-4498/TIMP3 axis and inflammatory response | Ni et al. (2021) |
| FUS | PDE4B mRNA | Splicing | Mediate the formation of circPDE4B and regulates chondrocyte cell viability and extracellular matrix metabolism | Shen et al. (2021) |
| YTHDF1 | Bcl2 mRNA | Stability | Inhibited the apoptosis and autophagy of chondrocytes | He et al. (2022a) |
| METTL3 | Atg7 mRNA | Stability | Affected autophagy to promote cellular senescence | Chen et al. (2022) |
| METTL3 | Sox9 mRNA | Stability | Mediated the methylation and degradation of SOX9 mRNA, suppressed collagen 2 expression | Xiao et al. (2022) |
| ZFP36 | Sox9 mRNA | Stability | Increased sox9 expression and then regulating anabolic and catabolic gene expression | McDermott et al. (2016) |
| ZFP36L1 | HSPA1A mRNA | Stability | Enhanced HSPA1A mRNA decay, and inhibited chondrocyte apoptosis | Son et al. (2019) |
| FUS | Sox9 mRNA | Stability | Stabilized SOX-9 mRNA, and promoted the chondrocyte-specific protein expression | Bai et al. (2020) |
| HuR | Cox-2 mRNA | Stability | Stabilized cox-2 mRNA and OA progression | Nieminen et al. (2008) |
| TAI-1 | Cox-2 mRNA | Location | Sequester COX-2 mRNA to SGs and delayed its translation | Ansari and Haqqi, (2016) |
| TDP43 | RACK1 mRNA | Location | Promoted SGs formation and inhibited inflammatory response | (Huang et al., 2017; Chang et al., 2021) |
| CPEB1 | — | Translation | CPEB1 overexpression aggravated the catabolic effect of IL-1β on chondrocytes in vitro | Li et al. (2019) |
| SAMD4 | Mig6 | Translation | Samd4-deficient mice displayed chondrocyte defects | Niu et al. (2017) |
| Nucleolin | p53 mRNA | Translation | Promotes articular chondrocyte proliferation | (Takagi et al., 2005; Deng et al., 2020) |
FIGURE 3.
Function of RNA-binding proteins in regulating main signaling pathway in osteoarthritis. (A) regulation in extracellular matrix degradation of chondrocytes; (B) regulation in chondrocyte differentiation; (C) regulation in cell apoptosis, proliferation and ferroptosis; (D) regulation in inflammatory response and angiogenesis.
First, RBPs play important role in anabolic and catabolic metabolism of chondrocytes. Such as SOX-9 being a well-known important factor for chondrocyte differentiation, researchers reported that ZFP36 and FUS both could bind with SOX-9 mRNA to repress or increase it expression, respectively (McDermott et al., 2016) (Bai et al., 2020). They affected the anabolic and catabolic process of chondrocytes by the regulation of SOX-9 expression. Moreover, Chang et al. reported that TDP-43 alleviated cartilage catabolic metabolism and maintained chondrocyte homeostasis under oxidative stress through regulating SGs dynamics (Chang et al., 2021). Furthermore, Shen et al. reported that FUS promoted the formation of circPDE4B. circPDE4B downregulated the expression of catabolic genes including MMP3, MMP13, and ADAMTS4, while it upregulated the expression of anabolic genes, including SOX9 and COL2A1 (Shen et al., 2021). Wu’s group reported that QKI modulated the splicing of PDE4D to form circPDE4D, which protected against OA by inducing the expression of aggrecan and reducing the expression of catabolic enzymes (MMP3, MMP13, ADAMTS4, and ADAMTS5) (Wu et al., 2021).
Second, RBPs also regulated the cell senescence, autophagy, apoptosis, and inflammatory response of mesenchymal stem cells, chondrocytes, and synovial fibroblasts. Researchers reported that PUM1 binds with TLR4 mRNA and suppressed its expression, and then, it inhibited cellular aging of MSCs and OA progression (Yoon et al., 2022). Fu and others reported that TAF15 was upregulated in cartilage tissues from osteoarthritis patient. TAF15 worked with lncRNA XIST to stabilize FUT1 expression and inhibit chondrogenic differentiation of BMSCs (He et al., 2022b). Moreover, researchers reported that DGCR8 could reverse premature senescent phenotypes of hMSCs and prevent and osteoarthritis progression (Deng et al., 2019). Meulenbelt et al. revealed that IGFBP5 and IGFBP6 were involved in the cellular senescence of chondrocytes in osteoarthritis (Houtman et al., 2021). Also, Wang et al. found that YTHDF1 mediated the stability of Bcl2 mRNA and overexpressed BCL2 protein to bind with Beclin1 protein to inhibited autophagy (He et al., 2022a). Furthermore, researchers found that CPEB1 induced chondrocytes apoptosis and promoted joint fracture-induced osteoarthritis (Chen et al., 2020). ZFP36L1 inhibited chondrocytes apoptosis by binding to the mRNA of HSPA1A and increasing its expression (Son et al., 2019). Besides, researchers reported that HuR was related to the chondrocyte hypertrophic differentiation (Gu et al., 2014). In addition, researchers found that HuR expression was significantly elevated in RA FLS, and its expression demonstrates a positive relationship with the NLRP3 inflammasome activation (Liu et al., 2019). Liu et al. reported that the proliferation, migration, and invasion of FLS was related to the expression of PTBP1 (Liu et al., 2021). These results indicated the potential function of RBPs on inflammatory response of OA.
Discussion and future perspectives
Osteoarthritis is one of the most common joint diseases worldwide, with an incidence of about 28% in the world population over 60 years old. The pathological features of OA are the erosion of articular cartilage, subchondral bone sclerosis, synovitis, and metabolic disorder (Martel-Pelletier et al., 2016; Mandl, 2019). Although various studies showed that gender, age, and obesity are the main risks factors of OA, the pathophysiology of OA was not fully illustrated (Bijlsma et al., 2011; Glyn-Jones et al., 2015). An increasing number of studies showed that the cell morphology is changed, and the aberrant expression of genes involved in inflammation, proliferation, and metabolism are existed in the initiation and progression of OA (Charlier et al., 2019; Li et al., 2021). Significant research efforts are ongoing to elucidate the complex molecular mechanisms underlying OA focused on gene transcription (Fisch et al., 2018). For example, Sox9, an essential transcription factor for cartilage differentiation and function, regulated the transcription of miR-455 to repress HIF-2α expression and coordinately regulate cartilage homeostasis (Ito et al., 2021). Also, importantly, posttranscriptional alterations play significant function in inflammation and metabolic changes related diseases, including OA.
Emerging in vitro and in vivo research indicated that lots of RBPs are dysregulated in chondrocytes, synovial fibroblasts, and osteoblasts and results in significant effects on OA progress. RNA alternative splicing, RNA stability, transcription, and translation are the major types of RBPs-RNA interaction events contributing to OA development based on acknowledges to date. These studies highlight the potential possibility of targeting RBPs to restore the cellular homeostasis of chondrocytes and prevent the progression of OA. In recent times, RNA interference-based silencing, genetic ablation, or adenovirus-mediated overexpression of RBPs have been used to test their influences on OA progression. For example, Young-Ok Son reported that genetic ablation or silencing of Zfp36l1 significantly abrogates experimental OA in mice (Son et al., 2019). Furthermore, Samd4-deficient mice also display chondrocyte defects and indicated its important function in maintaining chondrocytes homeostasis (Niu et al., 2017). Moreover, Chang and others reported that intraarticular injection of recombinant TDP-43 could significantly prevent ECM degradation of cartilage and subchondral bone remodeling in vivo (Chang et al., 2021).
On the other hand, small molecule inhibitors targeting RBPs are being explored. These RBPs-targeting molecules were well used for the treatment of cancers and other diseases. For instance, CMLD-2 and MS-444 are two HuR inhibitors, and their application for colorectal and colon cancer treatment was in the preclinical stage (Wu et al., 2015; Lang et al., 2017). Moreover, Resveratrol was reported an inhibitor of RBFox2, and the clinical trials of it to treat melanoma are now in phase II (Choi et al., 2019). The bioactive plant diterpene oridonin was a direct inhibitor of NCL and has been shown as a special function for using in the treatment of cancer, inflammation, and viral infection (Vasaturo et al., 2018). Further, importantly, researchers reported that aurothiomalate could reduce the expression of HuR and then inhibited the COX-2 expression in chondrocytes and prevented the progression of OA (Nieminen et al., 2008). I-BET151was reported to target and repress the activity of IGF2BP3, and studies showed that I-BET151 suppresses the expression of inflammatory genes and matrix-degrading enzymes in RA synovial fibroblasts (Klein et al., 2016), in addition to suppressing the IL-1β- and TNF-α-induced expression and activity of several matrix-degrading enzymes in human chondrocytes (Dai et al., 2018). Further studies are needed to elucidate the effects and safety of accurate RBPs molecular inhibitor for the OA treatment.
Conclusion
In summary, this review underlines the function and influence of RBPs in OA and thus the importance of understanding their interaction networks. However, the regulatory network of RBPs in OA is still complex and not completely understood. Future studies will further contribute to this knowledge and will hopefully enable the discovery of novel potential targeted therapies.
Author contributions
QY, ZD, WX, and JX participated in the literature review. JY and JH designed the figures. QY, JH, and WS1 written the draft of the review. WS1, ZD, and WS2 polished the manuscript. QY and ZD contribute equally to this work. All authors read and approved the final manuscript.
Funding
This work was supported by funds from the National Natural Sciences Foundation of China (No.82003126), Shenzhen Science and Technology Projects (No. JSGG20191129094218565), (No. JCYJ20190807102601647), (No. JCYJ20210324103604013), Scientific Research Foundation of Southwest Medical University (No. 2021ZKMS009), Luzhou Science and Technology Program (No. 2021-JYJ-71).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations
AREs, AU-rich elements; BAT, TGF-β-activated translation; CPEB1, cytosolic polyadenylation element-binding protein; eIF4, eukaryotic translation initiation factor 4; GNL3, nucleolar GTP binding protein three; HuR, human antigen R; IGFBP3, insulin-like growth factor binding protein three; IRES, internal ribosome entry site; lncRNA, long noncoding RNA; MSI1, Musashi1; ncRNAs, noncoding RNAs; NCL, nucleolin; OA, osteoarthritis; PABP, poly (A) binding protein; RBPs, RNA binding proteins; RBM, RNA-binding motif; RRMs, RNA recognition elements; RGG, arginine/glycine-rich region; SR, serine/arginine; SGs, stress granules; SFPQ, splicing factor proline and glutamine-rich protein; TEAD, TEA domain transcriptional; 3′-UTR, 3′-untranslated regions; ZEB1, zinc-finger E-box binding homeobox one; ZFP36, zinc-finger protein homolog 36; Znf, zinc-finger motif
References
- Aladal M., You W., Huang R., Huang J., Deng Z., Duan L., et al. (2022). Insights into the implementation of Fibronectin 1 in the cartilage tissue engineering. Biomed. Pharmacother. 148, 112782. 10.1016/j.biopha.2022.112782 [DOI] [PubMed] [Google Scholar]
- Ansari M. Y., Haqqi T. M. (2016). Interleukin-1β induced stress granules sequester COX-2 mRNA and regulates its stability and translation in human OA chondrocytes. Sci. Rep. 6, 27611. 10.1038/srep27611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baez M. V., Boccaccio G. L. (2005). Mammalian Smaug is a translational repressor that forms cytoplasmic foci similar to stress granules. J. Biol. Chem. 280 (52), 43131–43140. 10.1074/jbc.M508374200 [DOI] [PubMed] [Google Scholar]
- Bai J., Zhang Y., Zheng X., Huang M., Cheng W., Shan H., et al. (2020). LncRNA MM2P-induced, exosome-mediated transfer of Sox9 from monocyte-derived cells modulates primary chondrocytes. Cell Death Dis. 11 (9), 763. 10.1038/s41419-020-02945-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bansal P., Arora M. (2020). RNA binding proteins and non-coding RNA's in cardiovascular diseases. Adv. Exp. Med. Biol. 1229, 105–118. 10.1007/978-981-15-1671-9_5 [DOI] [PubMed] [Google Scholar]
- Bermudez O., Jouandin P., Rottier J., Bourcier C., Pages G., Gimond C. (2011). Post-transcriptional regulation of the DUSP6/MKP-3 phosphatase by MEK/ERK signaling and hypoxia. J. Cell. Physiol. 226 (1), 276–284. 10.1002/jcp.22339 [DOI] [PubMed] [Google Scholar]
- Bijlsma J. W., Berenbaum F., Lafeber F. P. (2011). Osteoarthritis: An update with relevance for clinical practice. Lancet 377 (9783), 2115–2126. 10.1016/S0140-6736(11)60243-2 [DOI] [PubMed] [Google Scholar]
- Bley N., Lederer M., Pfalz B., Reinke C., Fuchs T., GlaB M., et al. (2015). Stress granules are dispensable for mRNA stabilization during cellular stress. Nucleic Acids Res. 43 (4), e26. 10.1093/nar/gku1275 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohm B. B., Fehrl Y., Janczi T., Schneider N., Burkhardt H. (2018). Cell adhesion-induced transient interaction of ADAM15 with poly(A) binding protein at the cell membrane colocalizes with mRNA translation. PLoS One 13 (9), e0203847. 10.1371/journal.pone.0203847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnsack K. E., Bohnsack M. T. (2019). RNA-binding proteins chaperone ribonucleoprotein complex assembly to solve the RNA-folding problem. Cell 179 (6), 1248–1250. 10.1016/j.cell.2019.11.011 [DOI] [PubMed] [Google Scholar]
- Cai L., Brophy R. H., Tycksen E. D., Duan X., Nunley R. M., Rai M. F. (2019). Distinct expression pattern of periostin splice variants in chondrocytes and ligament progenitor cells. FASEB J. 33 (7), 8386–8405. 10.1096/fj.201802281R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang L., Liu A., Xu J., Xu X., Dai J., Wu R., et al. (2021). TDP-43 maintains chondrocyte homeostasis and alleviates cartilage degradation in osteoarthritis. Osteoarthr. Cartil. 29 (7), 1036–1047. 10.1016/j.joca.2021.03.015 [DOI] [PubMed] [Google Scholar]
- Charlier E., Deroyer C., Ciregia F., Malaise O., Neuville S., Plener Z., et al. (2019). Chondrocyte dedifferentiation and osteoarthritis (OA). Biochem. Pharmacol. 165, 49–65. 10.1016/j.bcp.2019.02.036 [DOI] [PubMed] [Google Scholar]
- Chaudhury A., Hussey G. S., Ray P. S., Jin G., Fox P. L., Howe P. H. (2010). TGF-beta-mediated phosphorylation of hnRNP E1 induces EMT via transcript-selective translational induction of Dab2 and ILEI. Nat. Cell Biol. 12 (3), 286–293. 10.1038/ncb2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen R., Ye B., Xie H., Huang Y., Wu Z., Wu H., et al. (2020). miR-129-3p alleviates chondrocyte apoptosis in knee joint fracture-induced osteoarthritis through CPEB1. J. Orthop. Surg. Res. 15 (1), 552. 10.1186/s13018-020-02070-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X., Gong W., Shao X., Shi T., Zhang L., Dong J., et al. (2022). METTL3-mediated m(6)A modification of ATG7 regulates autophagy-GATA4 axis to promote cellular senescence and osteoarthritis progression. Ann. Rheum. Dis. 81 (1), 87–99. 10.1136/annrheumdis-2021-221091 [DOI] [PubMed] [Google Scholar]
- Chen X. Y., Zhang J., Zhu J. S. (2019). The role of m(6)A RNA methylation in human cancer. Mol. Cancer 18 (1), 103. 10.1186/s12943-019-1033-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S., Sa M., Cho N., Kim K. K., Park S. H. (2019). Rbfox2 dissociation from stress granules suppresses cancer progression. Exp. Mol. Med. 51 (4), 1–12. 10.1038/s12276-019-0246-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciafre S. A., Galardi S. (2013). microRNAs and RNA-binding proteins: A complex network of interactions and reciprocal regulations in cancer. RNA Biol. 10 (6), 935–942. 10.4161/rna.24641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins K. M., Kainov Y. A., Christodolou E., Ray D., Morris Q., Hughes T., et al. (2017). An RRM-ZnF RNA recognition module targets RBM10 to exonic sequences to promote exon exclusion. Nucleic Acids Res. 45 (11), 6761–6774. 10.1093/nar/gkx225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Ambrogio A., Nagaoka K., Richter J. D. (2013). Translational control of cell growth and malignancy by the CPEBs. Nat. Rev. Cancer 13 (4), 283–290. 10.1038/nrc3485 [DOI] [PubMed] [Google Scholar]
- Dai J., Zhou S., Ge Q., Qin J., Chen D., Xu Z., et al. (2018). Bi-directional regulation of cartilage metabolism by inhibiting BET proteins-analysis of the effect of I-BET151 on human chondrocytes and murine joints. J. Orthop. Surg. Res. 13 (1), 118. 10.1186/s13018-018-0797-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell J. E., Jr. (2013). Reflections on the history of pre-mRNA processing and highlights of current knowledge: A unified picture. RNA 19 (4), 443–460. 10.1261/rna.038596.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Degrauwe N., Suva M. L., Janiszewska M., Riggi N., Stamenkovic I. (2016). IMPs: An RNA-binding protein family that provides a link between stem cell maintenance in normal development and cancer. Genes Dev. 30 (22), 2459–2474. 10.1101/gad.287540.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng L., Ren R., Liu Z., Song M., Li J., Wu Z., et al. (2019). Stabilizing heterochromatin by DGCR8 alleviates senescence and osteoarthritis. Nat. Commun. 10 (1), 3329. 10.1038/s41467-019-10831-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng Q., Yu X., Deng S., Ye H., Zhang Y., Han W., et al. (2020). Midkine promotes articular chondrocyte proliferation through the MK-LRP1-nucleolin signaling pathway. Cell. Signal. 65, 109423. 10.1016/j.cellsig.2019.109423 [DOI] [PubMed] [Google Scholar]
- Diaz-Munoz M. D., Kiselev V. Y., Le Novere N., Curk T., Ule J., Turner M. (2017). Tia1 dependent regulation of mRNA subcellular location and translation controls p53 expression in B cells. Nat. Commun. 8 (1), 530. 10.1038/s41467-017-00454-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobrev N., Ahmed Y. L., Sivadas A., Soni K., Fischer T., Sinning I. (2021). The zinc-finger protein Red1 orchestrates MTREC submodules and binds the Mtl1 helicase arch domain. Nat. Commun. 12 (1), 3456. 10.1038/s41467-021-23565-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez D., Freese P., Alexis M. S., Su A., Hochman M., Palden T., et al. (2018). Sequence, structure, and context preferences of human RNA binding proteins. Mol. Cell 70 (5), 854–867. 10.1016/j.molcel.2018.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. R., Mitchell S. A., Spriggs K. A., Ostrowski J., Bomsztyk K., Ostarek D., et al. (2003). Members of the poly (rC) binding protein family stimulate the activity of the c-myc internal ribosome entry segment in vitro and in vivo . Oncogene 22 (39), 8012–8020. 10.1038/sj.onc.1206645 [DOI] [PubMed] [Google Scholar]
- Fallmann J., Sedlyarov V., Tanzer A., Kovarik P., Hofacker I. L. (2016). AREsite2: An enhanced database for the comprehensive investigation of AU/GU/U-rich elements. Nucleic Acids Res. 44 (D1), D90–D95. 10.1093/nar/gkv1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Xu X., Fan X., Yi Q., Tang L. (2021). BAF57/SMARCE1 interacting with splicing factor SRSF1 regulates mechanical stress-induced alternative splicing of cyclin D1. Genes (Basel) 12 (2), 306. 10.3390/genes12020306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisch K. M., Gamini R., Alvarez-Garcia O., Akagi R., SaitoM., Muramatsu Y., et al. (2018). Identification of transcription factors responsible for dysregulated networks in human osteoarthritis cartilage by global gene expression analysis. Osteoarthr. Cartil. 26 (11), 1531–1538. 10.1016/j.joca.2018.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankiw L., Baltimore D., Li G. (2019). Alternative mRNA splicing in cancer immunotherapy. Nat. Rev. Immunol. 19 (11), 675–687. 10.1038/s41577-019-0195-7 [DOI] [PubMed] [Google Scholar]
- Fredericks A. M., Cygan K. J., Brown B. A., Fairbrother W. G. (2015). RNA-binding proteins: Splicing factors and disease. Biomolecules 5 (2), 893–909. 10.3390/biom5020893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glisovic T., Bachorik J. L., Yong J., Dreyfuss G. (2008). RNA-binding proteins and post-transcriptional gene regulation. FEBS Lett. 582 (14), 1977–1986. 10.1016/j.febslet.2008.03.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glyn-Jones S., Palmer A. J. R., Agricola R., Price A. J., Vincent T. L., Weinans H., et al. (2015). Osteoarthritis. Lancet 386 (9991), 376–387. 10.1016/S0140-6736(14)60802-3 [DOI] [PubMed] [Google Scholar]
- Gruber A. R., Fallmann J., Kratochvill F., Kovarik P., Hofacker I. L. (2011). AREsite: A database for the comprehensive investigation of AU-rich elements. Nucleic Acids Res. 39 (Database issue), D66–D69. 10.1093/nar/gkq990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu J., Lu Y., LiF., Qiao L., Wang Q., LiN., et al. (2014). Identification and characterization of the novel Col10a1 regulatory mechanism during chondrocyte hypertrophic differentiation. Cell Death Dis. 5, e1469. 10.1038/cddis.2014.444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton T., Novotny M., Pavicic P. J., Herjan T., Hartupee J., Sun D., et al. (2010). Diversity in post-transcriptional control of neutrophil chemoattractant cytokine gene expression. Cytokine 52 (1-2), 116–122. 10.1016/j.cyto.2010.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hata K., Nishimura R., Muramatsu S., Matsuda A., Matsubara T., Amano K., et al. (2008). Paraspeckle protein p54nrb links Sox9-mediated transcription with RNA processing during chondrogenesis in mice. J. Clin. Invest. 118 (9), 3098–3108. 10.1172/JCI31373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He J. Y., Cheng M., Ye J. L., Peng C. H., Chen J., Luo B., et al. (2022). YY1-induced lncRNA XIST inhibits cartilage differentiation of BMSCs by binding with TAF15 to stabilizing FUT1 expression. Regen. Ther. 20, 41–50. 10.1016/j.reth.2022.02.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y., Wang W., Xu X., Yang B., Yu X., Wu Y., et al. (2022). Mettl3 inhibits the apoptosis and autophagy of chondrocytes in inflammation through mediating Bcl2 stability via Ythdf1-mediated m(6)A modification. Bone 154, 116182. 10.1016/j.bone.2021.116182 [DOI] [PubMed] [Google Scholar]
- Hou P., Li L., Chen F., Chen Y., Liu H., Li J., et al. (2018). PTBP3-Mediated regulation of ZEB1 mRNA stability promotes epithelial-mesenchymal transition in breast cancer. Cancer Res. 78 (2), 387–398. 10.1158/0008-5472.CAN-17-0883 [DOI] [PubMed] [Google Scholar]
- Houtman E., Tuerlings M., Riechelman J., Suchiman E. H. E. D., van der Wal R. J. P., Nelissen R. G. H. H., et al. (2021). Elucidating mechano-pathology of osteoarthritis: Transcriptome-wide differences in mechanically stressed aged human cartilage explants. Arthritis Res. Ther. 23 (1), 215. 10.1186/s13075-021-02595-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Weng H., Sun W., Qin X., Shi H., Wu H., et al. (2018). Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 20 (3), 285–295. 10.1038/s41556-018-0045-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang H., Zhang Z. F., Qin F. W., Tang W., Liu D. H., Wu P. Y., et al. (2019). The mechanism of TDP-43 gene expression on inflammatory factors and the JNK and p38 MAPK signalling pathways in ischaemic hypoxic stress dependence. Int. Wound J. 16 (3), 724–729. 10.1111/iwj.13087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Y., Huang Q., Su H., Mai X., Feng E., Cao Z., et al. (2017). TAR DNA-binding protein 43 inhibits inflammatory response and protects chondrocyte function by modulating RACK1 expression in osteoarthritis. Biomed. Pharmacother. 85, 362–371. 10.1016/j.biopha.2016.11.037 [DOI] [PubMed] [Google Scholar]
- Ishimaru D., Zuraw L., Ramalingam S., Sengupta T. K., Bandyopadhyay S., Reuben A., et al. (2010). Mechanism of regulation of bcl-2 mRNA by nucleolin and A+U-rich element-binding factor 1 (AUF1). J. Biol. Chem. 285 (35), 27182–27191. 10.1074/jbc.M109.098830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito Y., Matsuzaki T., Ayabe F., Mokuda S., Kurimoto R., Matsushima T., et al. (2021). Both microRNA-455-5p and -3p repress hypoxia-inducible factor-2α expression and coordinately regulate cartilage homeostasis. Nat. Commun. 12 (1), 4148. 10.1038/s41467-021-24460-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsoula G., Steinberg J., Tuerlings M., de Almeida R. C., Southam L., Swift D., et al. (2022). A molecular map of long non-coding RNA expression, isoform switching and alternative splicing in osteoarthritis. Hum. Mol. Genet. 1, ddac017. 10.1093/hmg/ddac017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawahara H., Imai T., Imataka H., Tsujimoto M., Matsumoto K., Okano H. (2008). Neural RNA-binding protein Musashi1 inhibits translation initiation by competing with eIF4G for PABP. J. Cell Biol. 181 (4), 639–653. 10.1083/jcb.200708004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khalfallah Y., Kuta R., Grasmuck C., Prat A., Durham H. D., Vande Velde C. (2018). TDP-43 regulation of stress granule dynamics in neurodegenerative disease-relevant cell types. Sci. Rep. 8 (1), 7551. 10.1038/s41598-018-25767-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita C., Kubota N., Aoyama K. (2021). Interplay of RNA-binding proteins and microRNAs in neurodegenerative diseases. Int. J. Mol. Sci. 22 (10), 5292. 10.3390/ijms22105292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein K., Kabala P. A., Grabiec A. M., Gay R. E., Kolling C., Lin L. L., et al. (2016). The bromodomain protein inhibitor I-BET151 suppresses expression of inflammatory genes and matrix degrading enzymes in rheumatoid arthritis synovial fibroblasts. Ann. Rheum. Dis. 75 (2), 422–429. 10.1136/annrheumdis-2014-205809 [DOI] [PubMed] [Google Scholar]
- Lagier-Tourenne C., Polymenidou M., Hutt K. R., Vu A. Q., Baughn M., Huelga S. C., et al. (2012). Divergent roles of ALS-linked proteins FUS/TLS and TDP-43 intersect in processing long pre-mRNAs. Nat. Neurosci. 15 (11), 1488–1497. 10.1038/nn.3230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang M., Berry D., Passecker K., Mesteri I., Bhuju S., Ebner F., et al. (2017). HuR small-molecule inhibitor elicits differential effects in adenomatosis polyposis and colorectal carcinogenesis. Cancer Res. 77 (9), 2424–2438. 10.1158/0008-5472.CAN-15-1726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J. W., Chun Y. L., Kim A. Y., Lloyd L. T., Ko S., Yoon J. H., et al. (2021). Accumulation of mitochondrial RPPH1 RNA is associated with cellular senescence. Int. J. Mol. Sci. 22 (2), E782. 10.3390/ijms22020782 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L., Lan J., Ye Y., Yang B., Yang X., Cai Z. (2019). CPEB1 expression correlates with severity of posttraumatic ankle osteoarthritis and aggravates catabolic effect of IL-1β on chondrocytes. Inflammation 42 (2), 628–636. 10.1007/s10753-018-0920-6 [DOI] [PubMed] [Google Scholar]
- Li Z., Huang Z., Zhang H., Lu J., Tian Y., Wei Y., et al. (2021). P2X7 receptor induces pyroptotic inflammation and cartilage degradation in osteoarthritis via NF-κB/NLRP3 crosstalk. Oxid. Med. Cell. Longev. 2021, 8868361. 10.1155/2021/8868361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y., Feng J., Sun W., Wu C., Li J., Jing T., et al. (2021). CIRP promotes the progression of non-small cell lung cancer through activation of Wnt/β-catenin signaling via CTNNB1. J. Exp. Clin. Cancer Res. 40 (1), 275. 10.1186/s13046-021-02080-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin M. Z., Marzec K. A., Martin J. L., Baxter R. C. (2014). The role of insulin-like growth factor binding protein-3 in the breast cancer cell response to DNA-damaging agents. Oncogene 33 (1), 85–96. 10.1038/onc.2012.538 [DOI] [PubMed] [Google Scholar]
- Liu F., Feng X. X., Zhu S. L., Lin L., Huang H. Y., Zhang B. Y., et al. (2021). Long non-coding RNA SNHG1 regulates rheumatoid synovial invasion and proliferation by interaction with PTBP1. Int. Immunopharmacol. 90, 107182. 10.1016/j.intimp.2020.107182 [DOI] [PubMed] [Google Scholar]
- Liu Q., Hu X., Zhang X., Duan X., Yang P., Zhao F., et al. (2016). Effects of mechanical stress on chondrocyte phenotype and chondrocyte extracellular matrix expression. Sci. Rep. 6, 37268. 10.1038/srep37268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Wei W., Wang Y., Wan C., Bai Y., Sun X., et al. (2019). TNF-α/calreticulin dual signaling induced NLRP3 inflammasome activation associated with HuR nucleocytoplasmic shuttling in rheumatoid arthritis. Inflamm. Res. 68 (7), 597–611. 10.1007/s00011-019-01244-w [DOI] [PubMed] [Google Scholar]
- Lv M., Cai Y., Hou W., Peng K., Xu K., Lu C., et al. (2022). The RNA-binding protein SND1 promotes the degradation of GPX4 by destabilizing the HSPA5 mRNA and suppressing HSPA5 expression, promoting ferroptosis in osteoarthritis chondrocytes. Inflamm. Res. 71 (4), 461–472. 10.1007/s00011-022-01547-5 [DOI] [PubMed] [Google Scholar]
- Mandl L. A. (2019). Osteoarthritis year in review 2018: Clinical. Osteoarthr. Cartil. 27 (3), 359–364. 10.1016/j.joca.2018.11.001 [DOI] [PubMed] [Google Scholar]
- Maris C., Dominguez C., Allain F. H. (2005). The RNA recognition motif, a plastic RNA-binding platform to regulate post-transcriptional gene expression. FEBS J. 272 (9), 2118–2131. 10.1111/j.1742-4658.2005.04653.x [DOI] [PubMed] [Google Scholar]
- Martel-Pelletier J., Barr A. J., Cicuttini F. M., Conaghan P. G., Cooper C., Goldring M. B., et al. (2016). Osteoarthritis. Nat. Rev. Dis. Prim. 2, 16072. 10.1038/nrdp.2016.72 [DOI] [PubMed] [Google Scholar]
- McDermott B. T., ElliS S., Bou-Gharios G., Clegg P. D., Tew S. R. (2016). RNA binding proteins regulate anabolic and catabolic gene expression in chondrocytes. Osteoarthr. Cartil. 24 (7), 1263–1273. 10.1016/j.joca.2016.01.988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mofatteh M., Bullock S. L. (2017). SnapShot: Subcellular mRNA localization. Cell 169 (1), 178. 10.1016/j.cell.2017.03.004 [DOI] [PubMed] [Google Scholar]
- Moore K. S., von Lindern M. (2018). RNA binding proteins and regulation of mRNA translation in erythropoiesis. Front. Physiol. 9, 910. 10.3389/fphys.2018.00910 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neefjes M., van Caam A. P. M., van der Kraan P. M. (2020). Transcription factors in cartilage homeostasis and osteoarthritis. Biol. (Basel) 9 (9), E290. 10.3390/biology9090290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ni W., Jiang C., Wu Y., Zhang H., Wang L., Yik J. H. N., et al. (2021). CircSLC7A2 protects against osteoarthritis through inhibition of the miR-4498/TIMP3 axis. Cell Prolif. 54 (6), e13047. 10.1111/cpr.13047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieminen R., Vuolteenaho K., Riutta A., Kankaanranta H., van der Kraan P. M., Moilanen T., et al. (2008). Aurothiomalate inhibits COX-2 expression in chondrocytes and in human cartilage possibly through its effects on COX-2 mRNA stability. Eur. J. Pharmacol. 587 (1-3), 309–316. 10.1016/j.ejphar.2008.03.016 [DOI] [PubMed] [Google Scholar]
- Niu N., Xiang J. F., Yang Q., Wang L., Wei Z., Chen L. L., et al. (2017). RNA-binding protein SAMD4 regulates skeleton development through translational inhibition of Mig6 expression. Cell Discov. 3, 16050. 10.1038/celldisc.2016.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostareck D. H., OstAreck-Lederer A., WilMM., Thiele B. J., MannM., Hentze M. W. (1997). mRNA silencing in erythroid differentiation: HnRNP K and hnRNP E1 regulate 15-lipoxygenase translation from the 3' end. Cell 89 (4), 597–606. 10.1016/s0092-8674(00)80241-x [DOI] [PubMed] [Google Scholar]
- Pabis M., Popowicz G. M., Stehle R., Fernandez-Ramos D., ASami S., Warner L., et al. (2019). HuR biological function involves RRM3-mediated dimerization and RNA binding by all three RRMs. Nucleic Acids Res. 47 (2), 1011–1029. 10.1093/nar/gky1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y. Y., Yang J. X., Mao W., Wang X. X. (2019). RNA-binding protein SFPQ cooperates with HDAC1 to suppress CD40 transcription in pulmonary adventitial fibroblasts. Cell Biol. Int. 44, 166–176. 10.1002/cbin.11216 [DOI] [PubMed] [Google Scholar]
- Pereira J. K., Traina F., Machado-Neto J. A., Duarte A. d. S. S., Lopes M. R., Saad S. T. O., et al. (2012). Distinct expression profiles of MSI2 and NUMB genes in myelodysplastic syndromes and acute myeloid leukemia patients. Leuk. Res. 36 (10), 1300–1303. 10.1016/j.leukres.2012.06.010 [DOI] [PubMed] [Google Scholar]
- Qin H., Ni H., Liu Y., Yuan Y., Xi T., Li X., et al. (2020). RNA-binding proteins in tumor progression. J. Hematol. Oncol. 13 (1), 90. 10.1186/s13045-020-00927-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rachez C., Legendre R., Costallat M., Varet H., Yi J., Kornobis E., et al. (2021). HP1γ binding pre-mRNA intronic repeats modulates RNA splicing decisions. EMBO Rep. 22 (9), e52320. 10.15252/embr.202052320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanduja S., Blanco F. F., Dixon D. A. (2011). The roles of TTP and BRF proteins in regulated mRNA decay. Wiley Interdiscip. Rev. RNA 2 (1), 42–57. 10.1002/wrna.28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuschel K., Helwig M., Huttelmaier S., Heckl D., Klusmann J. H., Hoell J. I. (2020). RNA-binding proteins in acute leukemias. Int. J. Mol. Sci. 21 (10), E3409. 10.3390/ijms21103409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shanmugaapriya S., vAn CAAm A., de Kroon L., Vitters E. L., Walgreen B., van Beuningen H., et al. (2016). Expression of TGF-β signaling regulator RBPMS (RNA-Binding protein with multiple splicing) is regulated by IL-1β and TGF-β superfamily members, and decreased in aged and osteoarthritic cartilage. Cartilage 7 (4), 333–345. 10.1177/1947603515623991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S., Yang Y., Shen P., Ma J., Fang B., Wang Q., et al. (2021). circPDE4B prevents articular cartilage degeneration and promotes repair by acting as a scaffold for RIC8A and MID1. Ann. Rheum. Dis. 80 (9), 1209–1219. 10.1136/annrheumdis-2021-219969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi H., Wang X., Lu Z., Zhao B. S., Ma H., Hsu P. J., et al. (2017). YTHDF3 facilitates translation and decay of N(6)-methyladenosine-modified RNA. Cell Res. 27 (3), 315–328. 10.1038/cr.2017.15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silla T., Karadoulama E., Makosa D., Lubas M., Jensen T. H. (2018). The RNA exosome adaptor ZFC3H1 functionally competes with nuclear export activity to retain target transcripts. Cell Rep. 23 (7), 2199–2210. 10.1016/j.celrep.2018.04.061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son Y. O., Kim H. E., Choi W. S., Chun C. H., Chun J. S. (2019). RNA-binding protein ZFP36L1 regulates osteoarthritis by modulating members of the heat shock protein 70 family. Nat. Commun. 10 (1), 77. 10.1038/s41467-018-08035-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Ding L., Zhang H., Han J., Yang X., Yan J., et al. (2006). Potentiation of Smad-mediated transcriptional activation by the RNA-binding protein RBPMS. Nucleic Acids Res. 34 (21), 6314–6326. 10.1093/nar/gkl914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi M., Absalon M. J., McLure K. G., Kastan M. B. (2005). Regulation of p53 translation and induction after DNA damage by ribosomal protein L26 and nucleolin. Cell 123 (1), 49–63. 10.1016/j.cell.2005.07.034 [DOI] [PubMed] [Google Scholar]
- Tolino M., Kohrmann M., Kiebler M. A. (2012). RNA-binding proteins involved in RNA localization and their implications in neuronal diseases. Eur. J. Neurosci. 35 (12), 1818–1836. 10.1111/j.1460-9568.2012.08160.x [DOI] [PubMed] [Google Scholar]
- Uchida Y., Chiba T., Kurimoto R., Asahara H. (2019). Post-transcriptional regulation of inflammation by RNA-binding proteins via cis-elements of mRNAs. J. Biochem. 166 (5), 375–382. 10.1093/jb/mvz067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa T., Farny N. G., Jakovcevski M., Kaphzan H., Alarcon J. M., Anilkumar S., et al. (2013). Genetic and acute CPEB1 depletion ameliorate fragile X pathophysiology. Nat. Med. 19 (11), 1473–1477. 10.1038/nm.3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udagawa T., Fujioka Y., Tanaka M., Honda D., Yokoi S., Riku Y., et al. (2015). FUS regulates AMPA receptor function and FTLD/ALS-associated behaviour via GluA1 mRNA stabilization. Nat. Commun. 6, 7098. 10.1038/ncomms8098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasaturo M., Cotugno R., Fiengo L., Vinegoni C., Dal Piaz F., De Tommasi N. (2018). The anti-tumor diterpene oridonin is a direct inhibitor of Nucleolin in cancer cells. Sci. Rep. 8 (1), 16735. 10.1038/s41598-018-35088-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroya-Beltri C., Gutierrez-Vazquez C., Sanchez-Cabo F., Perez-Hernandez D., Vazquez J., Martin-Cofreces N., et al. (2013). Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 4, 2980. 10.1038/ncomms3980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen J., Lv R., Ma H., Shen H., He C., Wang J., et al. (2018). Zc3h13 regulates nuclear RNA m(6)A methylation and mouse embryonic stem cell self-renewal. Mol. Cell 69 (6), 1028–1038. 10.1016/j.molcel.2018.02.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolozin B., Ivanov P. (2019). Stress granules and neurodegeneration. Nat. Rev. Neurosci. 20 (11), 649–666. 10.1038/s41583-019-0222-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozniak A. L., Adams A., King K. E., Dunn W., Christenson L. K., Hung W. T., et al. (2020). The RNA binding protein FMR1 controls selective exosomal miRNA cargo loading during inflammation. J. Cell Biol. 219 (10), e201912074. 10.1083/jcb.201912074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu X., Lan L., Wilson D. M., Marquez R. T., Tsao W. C., Gao P., et al. (2015). Identification and validation of novel small molecule disruptors of HuR-mRNA interaction. ACS Chem. Biol. 10 (6), 1476–1484. 10.1021/cb500851u [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Hong Z., Xu W., Chen J., Wang Q., Chen J., et al. (2021). Circular RNA circPDE4D protects against osteoarthritis by binding to miR-103a-3p and regulating FGF18. Mol. Ther. 29 (1), 308–323. 10.1016/j.ymthe.2020.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Lu X., Shen B., Zeng Y. (2019). The therapeutic potential and role of miRNA, lncRNA, and circRNA in osteoarthritis. Curr. Gene Ther. 19 (4), 255–263. 10.2174/1566523219666190716092203 [DOI] [PubMed] [Google Scholar]
- Xiao L., Hu B., Ding B., Zhao Q., Liu C., Oner F. C., et al. (2022). N(6)-methyladenosine RNA methyltransferase like 3 inhibits extracellular matrix synthesis of endplate chondrocytes by downregulating sex-determining region Y-Box transcription factor 9 expression under tension. Osteoarthr. Cartil. 30 (4), 613–625. 10.1016/j.joca.2022.01.002 [DOI] [PubMed] [Google Scholar]
- Xiao R., Chen J. Y., Liang Z., Luo D., Chen G., Lu Z. J., et al. (2019). Pervasive chromatin-RNA binding protein interactions enable RNA-based regulation of transcription. Cell 178 (1), 107–121. 10.1016/j.cell.2019.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu M., Fang S., Song J., Chen M., Zhang Q., Weng Q., et al. (2018). CPEB1 mediates hepatocellular carcinoma cancer stemness and chemoresistance. Cell Death Dis. 9 (10), 957. 10.1038/s41419-018-0974-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon D. S., Byeon E., Kim D. H., Lee Y., Choi H., Park H. G., et al. (2022). TLR4 downregulation by the RNA-binding protein PUM1 alleviates cellular aging and osteoarthritis. Cell Death Differ. 1, 1. 10.1038/s41418-021-00925-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L., Li Q. H., Deng F., Yu Z. W., Luo X. Z., Sun J. L. (2017). Synovial fluid concentrations of cold-inducible RNA-binding protein are associated with severity in knee osteoarthritis. Clin. Chim. Acta. 464, 44–49. 10.1016/j.cca.2016.11.016 [DOI] [PubMed] [Google Scholar]
- Zhang Q., Cao X. (2019). Epigenetic regulation of the innate immune response to infection. Nat. Rev. Immunol. 19 (7), 417–432. 10.1038/s41577-019-0151-6 [DOI] [PubMed] [Google Scholar]
- Zhao B. S., Roundtree I. A., He C. (2017). Post-transcriptional gene regulation by mRNA modifications. Nat. Rev. Mol. Cell Biol. 18 (1), 31–42. 10.1038/nrm.2016.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M., Kim J. R., van Bruggen R., Park J. (2018). RNA-binding proteins in amyotrophic lateral sclerosis. Mol. Cells 41 (9), 818–829. 10.14348/molcells.2018.0243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao W., Cui Y., Liu L., Ma X., Qi X., Wang Y., et al. (2020). METTL3 facilitates oral squamous cell carcinoma tumorigenesis by enhancing c-myc stability via YTHDF1-mediated m(6)A modification. Mol. Ther. Nucleic Acids 20, 1–12. 10.1016/j.omtn.2020.01.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Couture S., Scott M. S., Abou Elela S. (2021). RBFOX2 alters splicing outcome in distinct binding modes with multiple protein partners. Nucleic Acids Res. 49 (14), 8370–8383. 10.1093/nar/gkab595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z., Xie J., Manandhar U., Yao X., Bian Y., Zhang B. (2021). RNA binding protein GNL3 up-regulates IL24 and PTN to promote the development of osteoarthritis. Life Sci. 267, 118926. 10.1016/j.lfs.2020.118926 [DOI] [PubMed] [Google Scholar]