ABSTRACT.
Chagas disease is a neglected parasitic infection and a major public health problem in the Americas. It remains underdiagnosed in the United States and internationally due to the lack of affordable testing and disparities in healthcare, particularly for those most at risk. We describe a proof-of-concept lateral flow immunoassay employing a recombinant Chagas multiantigen conjugated to gold nanoshells (AuNS) to detect circulating human anti-Chagas IgG antibodies. This is one of the first lateral flow immunoassays to capitalize on the larger surface area of AuNS compared with nanoparticles that can help amplify low-magnitude signals. Results were compared with 42 positive and negative Chagas serum samples, of which a subset of 27 samples was validated against an ELISA (Hemagen®). The sensitivity and specificity of our assay were 83% and 95%, respectively. These results suggest that an AuNS-based rapid testing for Chagas disease could facilitate in-field screening/diagnosis with a performance comparable to commercial methods.
INTRODUCTION
Infection with the protozoan parasite Trypanosoma cruzi, the cause of Chagas disease, affects approximately 6–7 million people worldwide and causes more than 10,000 deaths annually.1 Chagas disease is a vector-borne illness primarily transmitted by triatomine insects.2–4 Vectorial transmission occurs in endemic regions of the Americas where the triatomine insects maintain sylvatic, peridomestic, and domestic life cycles.5–7 If Chagas disease is left untreated at early stages, the infection passes into a silent chronic phase that can persist for decades or life. Chronic Chagas disease can manifest 10–30 years after exposure with irreversible cardiovascular, digestive, neurological, or mixed complications.4,7–10 Importantly, during the latent state, infected individuals can transmit the disease to others through nonvectorial pathways, which include blood transfusion,11,12 organ transplantation, and13,14 congenital.15,16
Ensuring early diagnosis of Chagas disease is essential for transmission control and clinical management.17 Unfortunately, current screening methods not only lack prioritization and inadequate coverage in both endemic and nonendemic settings, but they remain a major challenge.9,18–20 During the acute phase of the disease, blood parasitemia can be detected by direct microscopy methods or by culturing techniques. As the disease progresses, however, diagnosis is more difficult and relies on the detection of anti-T. cruzi antibodies, mostly IgG, through the standard serological methods such as indirect immunofluorescence assay (IFA), indirect hemagglutination assay (IHA), and ELISA.21 As Chagas tests often lead to inconclusive results, current guidelines recommend evaluating suspected cases through at least two diagnostic tests.20,22,23 In the case that both confirmatory tests are serological, the two tests should detect different antigens. Given that Chagas disease most often affects marginalized populations living in limited-resource settings, there is a need not only to improve standard diagnostic tools, but also to promote better diagnostic coverage through the development of reliable, affordable, and accurate point-of-care tests. While a diverse set of rapid diagnostic tools have been developed,24–26 only four tests have been cleared by the Food and Drug Administration (FDA) for diagnostic use, which include ORTHO T. cruzi ELISA Test System (Ortho Clinical Diagnostics, Raritan, NJ), Hemagen Chagas’ Kit ELISA (Hemagen Diagnostics, Inc., Columbia, MD), Wiener Chagatest Recombinante v. 3.0 ELISA (Wiener Laboratories, Rosario, Argentina), and InBios Chagas Detect Plus (CDP) Rapid Test (InBios International, Inc., Seattle, WA). Therefore, affordable, easy-to-use, minimal operator-dependent steps, and easily interpretable diagnostic tools are still needed for the better detection, management, and control of Chagas disease, in both endemic and nonendemic settings.
To address the need for a field-friendly test for Chagas disease, we sought to determine the diagnostic potential of gold nanoshells (AuNS). Gold nanoshells are a class of plasmonic nanoparticles composed of a dielectric silica core that is surrounded by a thin gold shell.27,28 Their unique composition allows for versatile optical absorption and scattering properties, which are of great biomedical potential for enhanced therapeutic and diagnostic applications.28–34 In this article, we report the development of a lateral flow assay (LFA) that employs 150-nm diameter AuNS conjugated to a synthetic array of T. cruzi recombinant antigens. These recombinant molecules represent antigens found in the different morphological stages of T. cruzi and hence, might obviate the need for multiple serological tests that rely on single antigen or parasite lysates. This proof-of-concept design showed that our qualitative point-of-care test can obtain results with similar analytical performance to standard serological assays, but with minimum processing of sample and a 15-minute incubation/turnaround time.35
MATERIALS AND METHODS
Conjugation of Chagas chimeric polypeptide.
A 9.9-kDa synthetic chimeric polypeptide bearing four Chagas antigens (PEP-2, TcD, TcE, and SAPA) was purchased (Peptides International Inc., Louisville, KY, #CCH-001). The polypeptide was reconstituted with distilled water (Invitrogen, Waltham, MA, #10977015) and 25 µg were conjugated to 1 mL of BioReadyTM 150 nm carboxyl AuNS (NanoComposix, San Diego, CA, #GSXR150), as per the manufacturer’s instructions. This conjugation reaction typically yields 20–30 µg of protein per 1 mL gold at 20 optical density (OD). After the AuNS conjugation, serum-positive samples were used to determine the optimal OD of the AuNS-conjugated chimeric protein. A final solution of 5 OD conjugated AuNS was made by diluting the stock in conjugate diluent (0.1× phosphate-buffered saline [PBS], 0.5% bovine serum albumin [BSA], 0.5% Tween 20, and 0.05% sodium azide). A glass conjugate pad was soaked in this solution (Millipore Sigma, Burlington, MA, GFDX103000), which was dried at 37°C for 1 hour. The dried conjugate pad was stored with desiccant packs for at least 24 hours before use.
Preparation of the lateral flow immune strip.
The antibodies for test and control lines were obtained from Thermo Fisher Scientific (Waltham, MA) (#31142, RRID: AB_228405) and BioXCell (Lebanon, NH) (#RT0266, RRID AB_2687790), respectively. Standard procedures to determine the optimal dilutions of test and control antibodies were followed. Briefly, the concentrations of the conjugate were kept constant while assessing the diluted antibody pairs (diluted in 1× PBS). Spot assays were used to determine the optimal enrichment zones at 15 minutes postincubation. Strong signals were observed for the polyclonal rabbit antihuman IgG antibody and the ReadyTag anti-6-His when using 1.5 and 3 mg/mL, respectively. Therefore, a solution of these concentrations for each antibody was prepared in larger scale, and was dispensed onto a polystyrene-backed nitrocellulose membrane (GE Healthcare, Chicago, IL, FF170HP Plus) using an automatic dispenser. The membrane was dried at 37°C for 1 hour and stored with silica gel desiccant packs until further use.
The LFA test strip was composed of the pretreated conjugate pad, a sample pad (Millipore Burlington, MA, #CFSP203000), and an absorption pad (MDI, Ambala Cantonment, India, FR-1[0.6]). To assemble the LFA test strip, the LFA components were attached to the adhesive sites of the nitrocellulose membrane with an overlap of 0.2 cm. The assembled cards were cut with an automated cutter into 4 × 0.4 cm strips. The test strips were stored at room temperature sealed in a package with desiccant packs until use.
Serological analysis.
The performance of the Chagas AuNS test strips was evaluated using a total of 15 positive and 27 negative Chagas samples were purchased from Discovery Life Sciences (Los Osos, CA). These samples were previously characterized via an ORTHO® T. cruzi ELISA Test System, Abbott PRISM Chagas, or an unspecified FDA-cleared test ( Supplemental Tables S1 and S2).
The qualitative detection of anti-Chagas IgG was carried out by adding 1:1 serum to the running buffer (1× PBS, 1% Tween 20) ratio. For each serological LFA analysis, 50 µL of human serum were pipetted onto the sample pad that was followed by the addition of 50 µL of running buffer. The test strips were incubated at room temperature for 15 minutes, to allow for the anti-Chagas antibodies present in the serum samples to interact with the AuNS conjugate. The performance of the AuNS-LFA design was validated against a commercial Chagas testing kit, Hemagen Chagas’ Kit. Due to the limited volume of samples, validation experiments for the qualitative detection of anti-Chagas IgG were conducted using only a subset of 27 Chagas serum samples (12 positive and 15 negative). The sample assessment and interpretation of the Hemagen test were performed in accordance with the manufacturer’s manual.
RESULTS
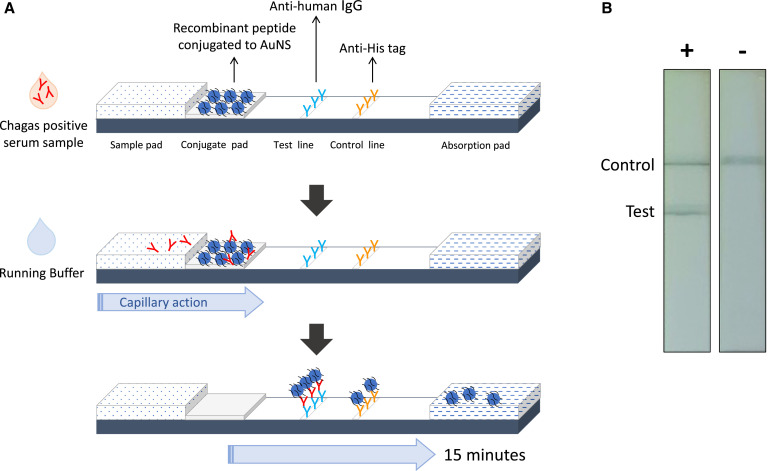
To improve the diagnosis of Chagas disease at chronic stages, we focused on the development of a qualitative AuNS-based diagnostic tool to detect anti-T. cruzi IgG antibodies in serum. Figure 1A illustrates the novel LFA format, where the immobilized AuNS were conjugated to a His-tagged polypeptide bearing four Chagas antigens. Upon the addition of a serum sample into the sample pad, putative anti-Chagas IgG antibodies present within the sample interact with pretreated AuNS to form a colorimetric complex that is immobilized by an antiheavy chain of human IgG at the test line, whereas unbound AuNS are captured by an anti-His antibody at the control line (Figure 1B). After a 15-minute incubation, the AuNS-LFA provided a readout that appears as a high-contrast blue-gray line when bound to the dispensed antibodies. Test results were recorded as positive, when a colored band was observed in both control and test lines, or negative when the assay displayed a single-colored band at the control line. If no band was observed at the control line, the test result was invalidated.
Figure 1.
Schematic representation of the gold nanoshells Chagas test strip. (A) Human serum specimens are introduced into the sample pad and, immediately after, a running buffer solution is added to facilitate the capillary action of the reagents across the membrane. Putative anti-Chagas IgG antibodies present within the sample are then able to interact with the pretreated gold nanoshells (AuNS) that are immobilized on the conjugate pad. The AuNS have been conjugated to a multi-antigen polypeptide bearing four Chagas antigens. As the sample migrates toward the absorption pad, the formed Chagas positive antibody–AuNS complexes are captured at the test line, whereas unbound AuNS particles are captured at the control line. (B) Visualization of the test and control lines after 15 minutes of incubation at room temperature. This figure appears in color at www.ajtmh.org.
A total of 42 previously characterized Chagas specimens were used to evaluate the qualitative patterns of the AuNS-LFA format (Table 1, Supplemental Figure S1). The AuNS-LFA accurately predicted positivity in Chagas samples 22 out of 27 times, 81.4%, with three invalidated tests. Twenty-seven Chagas-negative samples were evaluated against the AuNS-LFA test. The AuNS-LFA predicted the negative status of the samples 42 out of the 43 times, 97.7%.
Table 1.
Testing of commercially purchased blood or serum specimens confirmed Chagas-positive by an FDA-cleared test
| Assay | Positive | Negative | Invalid | Total |
|---|---|---|---|---|
| FDA cleared test | 15 | 27 | 0 | 42 |
| Gold nanoshells | 22* | 42†‡ | 3 | 67 |
FDA = Food and Drug Administration.
All-positive specimens (15) were tested twice with strips made from different nitrocellulose cards. With the first card, 12 out of 15 specimens agreed with FDA-cleared test results. In the second card, 10 out of 12 were found positive and 3 tests were invalidated because the control line was not observed.
All but 11 specimens were tested twice with strips made from different cards.
Denotes the total of samples that tested negative out of 43, with only 1 false-positive sample.
The performance of the AuNS-LFA was compared against Hemagen Chagas’ Kit, which is a commercially available ELISA for the qualitative assessment of anti-Chagas IgG antibodies. A total of 27 Chagas serum specimens, 12 positives and 15 negatives, were concurrently evaluated to provide an estimate of the assay performance (Table 2, Supplemental Tables S3 and S4). The Hemagen test showed a sensitivity and specificity of 91% and 100%, respectively. The AuNS-LFA yielded 83% sensitivity and 95% specificity values. The calculated positive predictive value of the AuNS-LFA was 95%.
Table 2.
Performance of AuNS-LFA to detect anti-Chagas IgG antibodies when compared with a commercially available testing kit
| Gold nanoshells | True positive | False positive | Total |
|---|---|---|---|
| Positive | 19* | 1 | 20 |
| Negative | 4 | 20 | 24 |
AuNS = gold nanoshells; LFA = lateral flow assay.
Only 11 out of the 12 positive specimens that were assessed for IgG using Hamagen® were considered due to incongruencies with FDA-cleared test results. These samples were evaluated twice with strips made from different nitrocellulose cards. One result obtained with strips from the second card was invalidated because the control line was not observed.
DISCUSSION
Due to the wide genetic and antigenic diversity of T. cruzi, no single test has proven optimal for the assessment of Chagas disease.9,18–20 To avoid inconclusive test results, the concomitant assessment of samples through at least two different immunological assays is recommended.20,22,23 But even when these recommendations are met, high heterogeneity in the performance of standard tests can result in a misclassified or inconclusive Chagas diagnosis.35–37 One known contributor to test performance variability is the implementation of different T. cruzi antigens and their respective antigenic preparations. Studies comparing antigen preparations have observed a higher consensus among positive sera and lower cross-reactivity when using chimeric recombinant T. cruzi peptides.38–40 Based on their potential for better assay performance, several research groups have looked into employing different synthetic protein constructs to improve the assessment of Chagas disease.24,25 Our study also sought to assess the integration of a chimeric recombinant polypeptide bearing four previously characterized Chagas antigens,41–44 but using a novel LFA design and a new class of plasmonic nanoparticle.
We developed a proof-of-concept LFA that employs 150 nm AuNS conjugated to a recombinant Chagas multiantigen for the qualitative detection of anti-Chagas IgG antibodies. When evaluating 42 previously characterized positive and negative Chagas samples, the AuNS-LFA showed a positive predictive value of 81.4% and a negative predictive value of 97.7%. We used a subset of the samples to validate our assay against a commercial FDA-cleared ELISA assay, which has been previously documented to have a sensitivity and specificity ranging between 87.7–100% and 99.68–100%, respectively.21,45 During our validation experiments, the Hemagen test showed a sensitivity of 91% and specificity of 100%. This performance was higher than the AuNS-LFA test, which demonstrated a sensitivity and specificity of 83% and 95%, respectively, and an overall accuracy of 95% in detecting circulating anti-T. cruzi IgG antibodies. Other studies21,35,46 that have compared the performance of the same four FDA-cleared assays to detect chronic Chagas have shown results consistent with AuNS-LFA. This further confirms the large heterogeneity that exists among tests and the need for better rapid, sensitive, and reliable diagnostic tools. Our results, here, highlight the potential utility of a rapid test such as AuNS-LNF to identify the exposure to T. cruzi, particularly in resource-limited settings, where access to intensive testing tools and trained personnel is limited.
To compare the diagnostic utility of AuNS with standard gold nanoparticles (AuNP), we conjugated the Chagas chimeric polypeptide to AuNP and evaluated its performance in a spot assay (data not shown). Initial assessment of the LFA design using AuNP yielded very faint or no signals at either the test or control lines and thus, precluded a direct comparison between AuNP- and AuNS-LFA strips. We suspect that the observed differences in the signal are due to incompatibilities with the conjugation and/or LFA design. Noteworthy is that most LFA formats conjugate polyclonal or monoclonal antihuman antibodies to their nanoparticles and dispense protein antigens in the nitrocellulose membrane. In contrast, we conjugated the chimeric protein to the AuNS and use an antihuman antibody as a test line. A major advantage of this changed configuration was that less chimeric protein was required to obtain a signal readout of results. Another advantage of using a tagged chimeric polypeptide was that it allowed us to use an anti-tag antibody as the control line. To our knowledge, the implementation of a tagged protein as a key component of the sandwich LFA format is unique to our AuNS-LFA design. This is a practice that not only reduced the time and effort in finding antibody pairs but it also mitigated the cost of assay materials. The cost of a single prototype AuNS-LFA card was $14.75 (US dollars). Each card can make up to 75 test strips (4 cm × 0.4 cm), which translates into $0.20 cost for materials per single Chagas evaluation. Given that the estimated rapid diagnostic test for Chagas disease costs $4–$7 per single determination,47 AuNS-LFA is an affordable alternative for Chagas surveillance.
Although additional studies are still warranted to fully understand the clinical utility of AuNS-LFA, this data suggest that such platforms may provide a sensitive and specific alternative to standard immunological assays. This technology, intended for the rapid and accurate diagnosis of Chagas disease, carries many potential advantages relative to standard serological methods. First, LFA is a rapid diagnostic method that is affordable, utilizes minimum input of sample, has a quick turnover of results, and is easy to perform and interpret with minimum training. Second, the unique optical properties of AuNS allow for stronger extinction per particle.29,30 This translates into higher sensitivity in an LFA format when compared with the ubiquitous 40 nm colloidal gold nanospheres.27,31 Third, the use of a synthetic recombinant polypeptide bearing four Chagas antigens not only offers higher sensitivity compared with single antigen or parasite lysate-based tests, but it might also obliterate the need to use multiple single antigen tests to confirm Chagas disease. Last, AuNS-LFA represents a significant cost-reduction compared with traditional methods. Taken together, these characteristics are of great value to oversee the serodiagnosis of Chagas disease in both high- and low-risk populations; but with the highest impact in limited-recourse settings, where the disease predominantly afflicts marginalized populations with limited or no healthcare access.
Limitations of the study.
Assay validation was limited by the small sample size of seropositive samples. Nevertheless, preliminary data justifies additional studies of point-of-care platforms employing AuNS to further understand their clinical utility and in-field performance.
Supplemental Material
ACKNOWLEDGMENTS
We appreciate and acknowledge the technical support for the serum sample analysis received from Vicky Simon, manager of the Human Nutritional Chemistry Service Laboratory in the Division of Nutritional Sciences, and Balaji Srinivasan for the feedback during the assay development and writing of the manuscript.
Note: Supplemental tables and figure appear at www.ajtmh.org.
REFERENCES
- 1. WHO , 2020. Chagas Disease (Also Known as American Trypanosomiasis). Geneva, Switzerland: World Health Organization. Available at: https://www.who.int/news-room/fact-sheets/detail/chagas-disease-(american-trypanosomiasis). Accessed November 18, 2020.
- 2. Gourbiere S, Dorn P, Tripet F, Dumonteil E, 2012. Genetics and evolution of triatomines: from phylogeny to vector control. Heredity 108: 190–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Angheben A, Boix L, Buonfrate D, Gobbi F, Bisoffi Z, Pupella S, Gandini G, Aprili G, 2015. Chagas disease and transfusion medicine: a perspective from non-endemic countries. Blood Transfus 13: 540–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Echeverria LE, Morillo CA, 2019. American trypanosomiasis (Chagas disease). Infect Dis Clin North Am 33: 119–134. [DOI] [PubMed] [Google Scholar]
- 5. Garcia MN. et al. , 2016. One health interactions of Chagas disease vectors, canid hosts, and human residents along the Texas-Mexico border. PLoS Negl Trop Dis 10: e0005074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lynn MK, Bossak BH, Sandifer PA, Watson A, Nolan MS, 2020. Contemporary autochthonous human Chagas disease in the USA. Acta Trop 205: 105361. [DOI] [PubMed] [Google Scholar]
- 7. Stanaway JD, Roth G, 2015. The burden of Chagas disease: estimates and challenges. Glob Heart 10: 139–144. [DOI] [PubMed] [Google Scholar]
- 8. Garcia MN. et al. , 2015. Development of chagas cardiac manifestations among Texas blood donors. Am J Cardiol 115: 113–117. [DOI] [PubMed] [Google Scholar]
- 9. Perez-Molina JA, Molina I, 2018. Chagas disease. Lancet 391: 82–94. [DOI] [PubMed] [Google Scholar]
- 10. Nolan M, Aguilar D, Misra A, Gunter S, Erickson T, Gorchakov R, Rivera H, Montgomery S, Murray K, 2021. Trypanosoma cruzi in nonischemic cardiomyopathy patients, Houston, Texas, USA. Emerg Infect Dis J 27: 1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Blumental S, Lambermont M, Heijmans C, Rodenbach MP, El Kenz H, Sondag D, Bottieau E, Truyens C, 2015. First documented transmission of Trypanosoma cruzi infection through blood transfusion in a child with sickle-cell disease in Belgium. PLoS Negl Trop Dis 9: e0003986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ries J, Komarek A, Gottschalk J, Brand B, Amsler L, Jutzi M, Frey BM, 2016. A case of possible Chagas transmission by blood transfusion in Switzerland. Transfus Med Hemother 43: 415–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cura CI, Lattes R, Nagel C, Gimenez MJ, Blanes M, Calabuig E, Iranzo A, Barcan LA, Anders M, Schijman AG, 2013. Early molecular diagnosis of acute Chagas disease after transplantation with organs from Trypanosoma cruzi-infected donors. Am J Transplant 13: 3253–3261. [DOI] [PubMed] [Google Scholar]
- 14. Salvador F. et al. , 2018. Prevalence of Chagas disease among solid organ-transplanted patients in a nonendemic country. Am J Trop Med Hyg 98: 742–746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Jackson Y, Myers C, Diana A, Marti HP, Wolff H, Chappuis F, Loutan L, Gervaix A, 2009. Congenital transmission of Chagas disease in Latin American immigrants in Switzerland. Emerg Infect Dis 15: 601–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Barona-Vilar C. et al. , 2012. Prevalence of Trypanosoma cruzi infection in pregnant Latin American women and congenital transmission rate in a non-endemic area: the experience of the Valencian Health Programme (Spain). Epidemiol Infect 140: 1896–1903. [DOI] [PubMed] [Google Scholar]
- 17. Forsyth CJ, Stigler Granados P, Pacheco GJ, Betancourt JA, Meymandi SK, 2019. Current gaps and needs for increasing access to healthcare for people with Chagas disease in the USA. Curr Trop Med Rep 6: 13–22. [Google Scholar]
- 18. Dumonteil E, Herrera C, 2017. Ten years of Chagas disease research: looking back to achievements, looking ahead to challenges. PLoS Negl Trop Dis 11: e0005422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Moure Z, Sulleiro E, Iniesta L, Guillen C, Molina I, Alcover MM, Riera C, Pumarola T, Fisa R, 2018. The challenge of discordant serology in Chagas disease: the role of two confirmatory techniques in inconclusive cases. Acta Trop 185: 144–148. [DOI] [PubMed] [Google Scholar]
- 20. Edwards MS, Stimpert KK, Montgomery SP, 2017. Addressing the challenges of Chagas disease: an emerging health concern in the United States. Infect Dis Clin Pract 25: 118–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hochberg NS, Wheelock A, Hamer DH, Marcus R, Nolan MS, Meymandi S, Gilman RH, 2021. Chagas disease in the United States: a perspective on diagnostic testing limitations and next steps. Am J Trop Med Hyg 104: 800–804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. CDC , 2019. DPDx - Laboratory Identification of Parasites of Public Health Concern - American Trypanosomiasis. Available at: https://www.cdc.gov/dpdx/trypanosomiasisAmerican/index.html. Accessed May 26, 2021.
- 23. Pane S. et al. , 2018. Serological evaluation for Chagas disease in migrants from Latin American countries resident in Rome, Italy. BMC Infect Dis 18: 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Silva ED. et al. , 2020. Development of a new lateral flow assay based on IBMP-8.1 and IBMP-8.4 chimeric antigens to diagnose Chagas disease. BioMed Res Int 2020: 1803515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Luquetti AO. et al. , 2003. Chagas’ disease diagnosis: a multicentric evaluation of Chagas Stat-Pak, a rapid immunochromatographic assay with recombinant proteins of Trypanosoma cruzi. Diagn Microbiol Infect Dis 46: 265–271. [DOI] [PubMed] [Google Scholar]
- 26. Ponce C. et al. , 2005. Validation of a rapid and reliable test for diagnosis of Chagas’ disease by detection of Trypanosoma cruzi-specific antibodies in blood of donors and patients in Central America. J Clin Microbiol 43: 5065–5068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.nanoComposix, 2017. Increased Sensitivity of Lateral Flow Assays Using Gold Nanoshells. Available at: https://cdn.shopify.com/s/files/1/0257/8237/files/Nanoshells_for_Increased_Sensitivty_in_Lateral_Flow_-_nanoComposix_-_v1.1.pdf?13398085647345355955. Accessed May 20, 2021.
- 28. Oldenburg SJ, Averitt RD, Westcott SL, Halas NJ, 1998. Nanoengineering of optical resonances. Chem Phys Lett 288: 243–247. [Google Scholar]
- 29. Hirsch LR, Gobin AM, Lowery AR, Tam F, Drezek RA, Halas NJ, West JL, 2006. Metal nanoshells. Ann Biomed Eng 34: 15–22. [DOI] [PubMed] [Google Scholar]
- 30. Averitt RD, Westcott SL, Halas NJ, 1999. Linear optical properties of gold nanoshells. J Opt Soc Am B 16: 1824–1832. [Google Scholar]
- 31. Gonzalez-Moa MJ, Van Dorst B, Lagatie O, Verheyen A, Stuyver L, Biamonte MA, 2018. Proof-of-concept rapid diagnostic test for onchocerciasis: exploring peptide biomarkers and the use of gold nanoshells as reporter nanoparticles. ACS Infect Dis 4: 912–917. [DOI] [PubMed] [Google Scholar]
- 32. Hirsch LR, Jackson JB, Lee A, Halas NJ, West JL, 2003. A whole blood immunoassay using gold nanoshells. Anal Chem 75: 2377–2381. [DOI] [PubMed] [Google Scholar]
- 33. Rastinehad AR. et al. , 2019. Gold nanoshell-localized photothermal ablation of prostate tumors in a clinical pilot device study. Proc Natl Acad Sci USA 116: 18590–18596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Sershen SR, Westcott SL, Halas NJ, West JL, 2000. Temperature-sensitive polymer-nanoshell composites for photothermally modulated drug delivery. J Biomed Mater Res 51: 293–298. [DOI] [PubMed] [Google Scholar]
- 35. Afonso AM, Ebell MH, Tarleton RL, 2012. A systematic review of high quality diagnostic tests for Chagas disease. PLoS Negl Trop Dis 6: e1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Santos FL, de Souza WV, Barros Mda S, Nakazawa M, Krieger MA, Gomes Yde M, 2016. Chronic Chagas disease diagnosis: a comparative performance of commercial enzyme immunoassay tests. Am J Trop Med Hyg 94: 1034–1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Verani JR. et al. , 2009. Geographic variation in the sensitivity of recombinant antigen-based rapid tests for chronic Trypanosoma cruzi infection. Am J Trop Med Hyg 80: 410–415. [PubMed] [Google Scholar]
- 38. Santos FLN, Celedon PAF, Zanchin NIT, Brasil TdAC, Foti L, Souza WVd, Silva ED, Gomes YdM, Krieger MA, 2016. Performance assessment of four chimeric Trypanosoma cruzi antigens based on antigen-antibody detection for diagnosis of chronic Chagas disease. PLoS One 11: e0161100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Camussone C, Gonzalez V, Belluzo MS, Pujato N, Ribone ME, Lagier CM, Marcipar IS, 2009. Comparison of recombinant Trypanosoma cruzi peptide mixtures versus multiepitope chimeric proteins as sensitizing antigens for immunodiagnosis. Clin Vaccine Immunol 16: 899–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. da Silveira JF, Umezawa ES, Luquetti AO, 2001. Chagas disease: recombinant Trypanosoma cruzi antigens for serological diagnosis. Trends Parasitol 17: 286–291. [DOI] [PubMed] [Google Scholar]
- 41. Houghton RL, Benson DR, Reynolds L, McNeill P, Sleath P, Lodes M, Skeiky YA, Badaro R, Krettli AU, Reed SG, 2000. Multiepitope synthetic peptide and recombinant protein for the detection of antibodies to Trypanosoma cruzi in patients with treated or untreated Chagas’ disease. J Infect Dis 181: 325–330. [DOI] [PubMed] [Google Scholar]
- 42. Houghton RL, Benson DR, Reynolds LD, McNeill PD, Sleath PR, Lodes MJ, Skeiky YA, Leiby DA, Badaro R, Reed SG, 1999. A multi-epitope synthetic peptide and recombinant protein for the detection of antibodies to Trypanosoma cruzi in radioimmunoprecipitation-confirmed and consensus-positive sera. J Infect Dis 179: 1226–1234. [DOI] [PubMed] [Google Scholar]
- 43. Granjon E, Dichtel-Danjoy ML, Saba E, Sabino E, Campos de Oliveira L, Zrein M, 2016. Development of a novel multiplex immunoassay multi-cruzi for the serological confirmation of Chagas disease. PLoS Negl Trop Dis 10: e0004596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hernandez P, Heimann M, Riera C, Solano M, Santalla J, Luquetti AO, Beck E, 2010. Highly effective serodiagnosis for Chagas’ disease. Clin Vaccine Immunol 17: 1598–1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kelly EA, Bulman CA, Gunderson EL, Irish AM, Townsend RL, Sakanari JA, Stramer SL, Bern C, Whitman JD, 2021. Comparative performance of latest-generation and FDA-cleared serology tests for the diagnosis of Chagas disease. J Clin Microbiol 59: e00158–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Whitman JD, Bulman CA, Gunderson EL, Irish AM, Townsend RL, Stramer SL, Sakanari JA, Bern C, 2019. Chagas disease serological test performance in U.S. blood donor specimens. J Clin Microbiol 57: e01217–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Eguez KE, Alonso-Padilla J, Teran C, Chipana Z, Garcia W, Torrico F, Gascon J, Lozano-Beltran DF, Pinazo MJ, 2017. Rapid diagnostic tests duo as alternative to conventional serological assays for conclusive Chagas disease diagnosis. PLoS Negl Trop Dis 11: e0005501. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.