ABSTRACT.
Some patients with visceral leishmaniasis (VL), or kala-azar, suffer relapses and low quality of life despite adequate drug therapy, especially those co-infected with HIV. Occasionally, physicians indicate splenectomy, but the benefit of the procedure needs to be analyzed systematically. Therefore, a retrospective open cohort study was conducted in Teresina, Brazil. Inpatients from a reference hospital with relapsing VL who had a rescue splenectomy between 2012 and 2019 after the nationally recommended drug therapy failed were studied. The procedure’s risks and benefits were assessed in a limited-resource setting. The primary outcomes were surgical complications, complete blood count, CD4+ cell count, hospitalizations, survival time, and medical complications preceding death. Thirteen adult patients received medical and surgical indications of splenectomy (12 men and one woman). Eleven had HIV infection. Two had early and two had late complications. Four died, all of whom were infected with HIV. An additional HIV-coinfected patient, apart from the cohort, died just before surgery. The death rate after surgery was 13.3 overall and 22.1 per 100 person-years among HIV-infected patients (31% overall and 36%, respectively). The impressive rise of complete blood counts and reduction of blood transfusions and hospitalizations were observed among all patients. Also, a meaningful increase in CD4+ cells in HIV-infected patients was noted. Splenectomy may benefit patients with relapsing VL. However, before performing splenectomy, available combined drug therapy for VL should be tried.
INTRODUCTION
Visceral leishmaniasis (VL), or kala-azar, is a systemic infection caused by Leishmania infantum and L. donovani, transmitted by sandflies. It is an obligate parasite of mononuclear phagocytes, distributed mainly in the spleen, liver, and bone marrow, causing symptoms such as fever, hepatosplenomegaly, pancytopenia, and hemorrhage that may lead to death. The disease usually responds to a specific treatment. However, some patients may have a relapsing course, particularly those coinfected with HIV-1, an association described in various regions of the world.1,2 Both infections exert a detrimental synergistic effect on the cellular immune response by targeting similar cells.3 HIV-infected patients’ immune status is particularly favorable for Leishmania multiplication, increasing the risk of progression of an asymptomatic VL infection to active disease2,4,5 and mortality. In addition, VL accelerates the progression of HIV infection to AIDS and may induce the expression of latent viruses.2 Characteristically, coinfected patients under highly active antiretroviral therapy (HAART) with relapsing disease evolve with undetectable viral load and low CD4+ cell count.2,6
Several reports in the literature of patients with VL who are not responsive to the etiological treatment and secondary prophylaxis show they do have an improved outcome when submitted to splenectomy, albeit not performing as well when infected by HIV.6–11 The spleen behaves as a reservoir of Leishmania in humans, and some studies have shown a decrease in the action of drugs acting on parasites located in the spleen.6,9,12
The literature on splenectomy in patients with VL is limited to individual case reports6–11,13 and a Spanish series of three HIV-infected patients. In this series, the patients had a satisfactory response, as seen by the improvement in hematological and clinical parameters and the rapid rise of CD4+ cell count. However, splenectomy did not always cure L. infantum infection or prevent relapse.6 Finally, improvement in CD4+ cell count after splenectomy in patients with HIV but without VL was noted before the introduction of HAART.13
A significant proportion of patients with VL and coinfected with HIV remain for months and even years living with VL recurrences and undergo consecutive drug treatments. Faced with this bleak scenario where little can be done for these people, splenectomy was performed as rescue therapy in patients with drug-refractory VL, whether or not coinfected with HIV, who accepted the proposal of a surgical approach. However, knowledge of this long-term benefit of this procedure is scarce, and thus investigating this intervention and its consequences in this group of patients through the systematic collection of data is fundamental to assess its benefits. Therefore, an open retrospective cohort study of patients to whom splenectomy was indicated as salvage therapy was designed to evaluate the intervention’s long-term effect for patients with VL.
METHODS
This study analyzes a retrospective open cohort involving individuals with VL unresponsive to drug treatment, coinfected with HIV or not, followed at the Institute of Tropical Diseases “Natan Portella” (IDTNP), in Teresina, Brazil. The first patient included in the cohort was admitted for treating VL in May 2009. After three relapses, on May 2, 2012, he was underwent surgery. The last patient was splenectomized on April 8, 2018. Data collection ended on February 1, 2019. Splenectomy was indicated by both the attending infectious diseases specialist and the consulting surgeon as a rescue therapy after failure to respond to the Brazilian recommended treatment with pentavalent antimonial, liposomal amphotericin B, or both. To confirm the diagnosis of VL, the presence of typical signs and symptoms accompanied by at least one of the following laboratory tests was required: identification of the parasite in bone marrow by smear or culture in Novy-McNeal-Nicolle media and serology. Serological diagnosis of HIV infection was performed according to current national recommendations at the time of data collection. This consisted of a rapid immunochromatographic test (Rapid Check HIV 1 and 2, Alere, Livermore, CA). If reactive, another serological test was used as confirmation, usually another rapid test or an indirect immunofluorescence test (Oswaldo Cruz Foundation, Rio de Janeiro, Brazil), or an immunoblot test available on the market with the authorization by the Brazilian agency Anvisa, and according to the recommendations of the Ministry of Health at that time.
Relapses were diagnosed by clinical worsening with the presence of parasites in bone marrow. The attending physician determined the surgical indication of splenectomy in agreement with the consulting surgeon. The indications followed the principles summarized in Table 1, which included relapsing VL in the presence of drug prophylaxis with the Brazilian recommendation of liposomal amphotericin B biweekly.14–16
Table 1.
Principles followed by the attending physicians and the consulting surgeons for indicating splenectomy for relapsing visceral leishmaniasis at the Institute for Tropical Diseases “Natan Portella,” Teresina, Brazil, 2008–2019
| Patients with confirmed VL, coinfected or not with HIV. |
| Patients not responsive to available drugs* for VL in at least two cycles of drug therapy with an interval of 6 months. |
| Patients who adhered to secondary prophylaxis for VL, consisting of amphotericin B biweekly, as recommended by the Brazilian Ministry of Health. |
| HIV-infected patients, adherent to HAART. |
| Patients in clinical conditions to undergo the surgical procedure. |
| Patients who accepted surgery after being informed about the advantages and disadvantages; |
| Patients who have received vaccination against encapsulated germs at least 15 days before surgery. |
HAART = highly active anti-retroviral therapy; VL = visceral leishmaniasis.
Amphotericin B or pentavalent antimonials.
In addition to these principles, patients took a 7-day (patients without HIV infection) or 14-day (patients with HIV infection) course of liposomal amphotericin B immediately before or after the surgical procedure. Patients were followed up at IDTNP, where those infected with HIV regularly obtained HAART. Therefore, the only criterion was to have relapsing VL despite secondary prophylaxis. After surgery, patients were not assigned to prophylactic antiparasitic therapy.
The analyzed data were demographic and clinical variables before and after splenectomy (number of hospital admissions and red blood cell transfusions before and after surgery); variables of early surgical complications (hemorrhages, pancreatitis, surgical wound infection, and surgical site infection), and late postoperative complications (incisional hernia, intraabdominal collection, thrombosis, sepsis); laboratory variables before and after surgery (complete blood cell count [CBC], CD4+ cell count [BD FACScalibur, CD4, San Jose, CA], and HIV viral load [quantitative PCR was performed by using the Abbott m24sp Instrument, Abbott Laboratories. Abbot Park, IL]); surgical specimen data; survival from the day of surgery until February 1, 2019, or death. Immediate cause of death was determined through hospital records when death occurred in hospital or by family report if it occurred out of the hospital.
The surgical procedure was standard for endemic regions with limited resources. Surgical tweezers and wires with universal references were used. No unique material or orthosis or prosthesis were used. The laparotomy pathway was chosen due to the large spleen volume, which hinders laparoscopic surgery. For all splenectomies, two red blood cell concentrates were reserved. There was no need to secure an intensive care unit bed, as agreed by the hospital anesthesiologists. The surgeries were performed at the University Hospital of the Federal University of Piauí (HU-UFPI) and Hospital Getúlio Vargas (HGV), both in Teresina, Brazil.
Data analysis was performed using R software for Windows version 3.2.2 (https://www.r-project.org/) and Stata version 15.1 (Stata Corp., College Station, TX). To compare two independent means, when the data were normally distributed and homogeneous, the Student’s t test was used for independent samples. When the data were nonnormal and nonhomogeneous, the Mann-Whitney test was used. Student’s t test was used for paired samples to compare two means with normal distribution whenever indicated. When variables were nonnormally distributed, the Wilcoxon test was applied. The survival curve was estimated according to the Kaplan-Meier method.
The study was submitted to the Ethics Committee of the HU-UFPI, initially approved by the Research Project Evaluation Committee—CAPP (approval letter no. 09/17) and later by the ethics committee (opinion no. 2.428.145).
RESULTS
Demographics.
Thirteen patients were operated on, and therefore constituted the study retrospective cohort. The gender distribution was 12 male patients (92,3%) and one HIV-infected female (7.7%). Seven patients (58.3%) were under 40 years. The age distribution of HIV- and non-HIV-infected patients was similar (mean 40.5 years for the two without HIV infection and 41.8 years for those HIV-infected). Eleven (84.6%) patients were HIV-positive and two were HIV negative without any known immunosuppression condition (Table 2). Another patient with HIV not belonging to the cohort but treated during the cohort period, a 41-year-old man with a CD4+ cell count of 62 cells/μL and 84 cells/μL (measured 1 year and 6 months before death, respectively) and undetectable viral load, was assigned for splenectomy but died a few days earlier due to herpes virus encephalitis.
Table 2.
Demographic variables and HIV infection status of 13 patients, HIV infected or not, with relapsing visceral leishmaniasis who underwent splenectomy
| Variable | All, n (%) | HIV-infected, n (%) |
|---|---|---|
| Gender | ||
| Male | 12 (92) | 10 (91) |
| Female | 1 (8) | 1 (9) |
| Age group (years) | ||
| <40 | 7 (54) | 6 (55) |
| ≥40 | 6 (46) | 5 (45) |
| HIV infection | ||
| No | 2 (15) | 0 (0) |
| Yes | 11 (85) | 11 (100) |
Intervention.
Because the anti-Leishmania indication for patients with HIV coinfection and relapsing VL (coinfected or not) in Brazil is restricted to amphotericin B, all patients received at least two treatments in a minimum interval of 6 months. However, patient 1 (non-HIV infected) and patient 3 (HIV infected) received pentavalent antimonial as the initial treatment. Anti-HIV medication use at the time of splenectomy, number of years of VL diagnosis before surgery, and each VL treatment is shown in Supplemental Table 1. The first patient underwent surgery at HGV Hospital in May 2012, and the other 12 cases were operated on at HU-UFPI up to April 2018. Two patients were operated on twice: the only female patient, who was HIV infected, underwent a second surgery 18 months after the first to resect an accessory spleen. The sixth patient, also HIV infected, had to be reoperated on the same day of surgery due to intraabdominal bleeding. The length of postsurgical hospitalization ranged from 3 days to a maximum of 8 days, with an average of 5.8 days.
Complications.
Most patients (n = 11; 84.6%) progressed without early postoperative complications during the first 30 days after surgery. Complications experienced by the other two patients (15.4%) included intraabdominal hemorrhage, hematoma of the surgical wound, and diarrhea. Two patients (15.4%) experienced late surgical complications, which occur 30 or more days after surgery; both had post–kala-azar dermal leishmaniasis (PKDL), one in month 5 and the other at month 8 after surgery (Table 3).
Table 3.
Complications and outcomes of splenectomy in 13 patients with relapsing visceral leishmaniasis
| All patients | HIV-infected | |
|---|---|---|
| Complications and outcomes | n (%) | n (%) |
| Early surgical complications | ||
| No | 11 (85) | 9 (82) |
| Yes | 2 (15) | 2 (18) |
| Late surgical complications | ||
| No | 11 (85) | 9 (82) |
| Yes | 2 (15) | 2 (18) |
| Outcome | ||
| Survival during follow-up | 9 (69) | 7 (64) |
| Death | 4 (31) | 4 (36) |
Four patients died at 11, 244, 329, and 742 days after surgery; all were HIV infected. Symptoms before death and probable causes of death were as follows: foot wound followed by signs of infection and rapid progression to death in patient 2, almost 11 months after splenectomy. He was asymptomatic before the complication. Sepsis was considered the most likely cause of his death. Patient 4 died more than 2 years after the surgery. After a subsequent splenectomy, she had a difficult outcome after the excision of an accessory spleen, with rapid improvement followed by CD4+ cell-count drop. She progressed to PKDL that was unresponsive to treatment with liposomal amphotericin B and died with mental alteration and upper digestive hemorrhage; portal vein thrombosis is a likely cause of death. Patient 6 had a complicated course of 8 months postsurgery. He was a patient with early surgical complications and difficult surgical wound healing who died after dyspnea, interpreted as probable Pneumocystis jiroveci pneumonia. Patient 12 died 11 days after surgery and transfer to clinical care at IDTNP, with a progression compatible with hospital sepsis (Table 4). No complication occurred in non-HIV-infected patients.
Table 4.
Possible causes of the four deaths after splenectomy in 13 patients
| Patient no. | HIV infection | Lifetime after surgery (days) | Early complications | Late complications | Circumstances of death | Likely cause of death | Source of information |
|---|---|---|---|---|---|---|---|
| 2 | Yes | 329 | No | No | Foot wound followed by acute infection | Sepsis | Wife |
| 4 | Yes | 742 | No | PKDL | Behavioral changes followed by upper digestive bleeding | Portal vein thrombosis | Daughter |
| 6 | Yes | 244 | Intraabdominal hemorrhage | No | Respiratory distress | Pneumocystosis | Mother, hospital file |
| 12 | Yes | 11 | Hematoma at surgical wound, diarrhea | No | Sepsis | Postoperative infection | Hospital files |
Analytics.
There were significant and expressive increase in hemoglobin (from mean values of 8.1 g/dL before to 10.5 g/dL after splenectomy), hematocrit (from 27.8% to 33.2%), leukocyte count (2,152.3/µL to 11,105.6/µL), and platelets (from 124,346.2/µL to 303,638.5/µL) after surgery. The first CD4+ cell count after splenectomy increased 4-fold, and viral load remained undetectable in the pre- and postoperative periods. Six patients received red blood cell transfusions up to 10 days after the surgery. However, the mean number of red blood cell transfusions per patient was 5.2 and 5.7 before surgery, respectively, for all patients and for those who were HIV infected. From 10 days post-splenectomy, no patient received a blood transfusion up to the end of data collection. The number of hospitalizations was significantly lower after surgery, dropping from 0.39 to 0.05 per patient after the surgery among all patients and those with HIV (P = 0.001). The same pattern of CBC and relapses was observed when the HIV-infected patients were analyzed separately (Table 5). Four patients had the last creatinine dose before splenectomy above the cutoff level of 13.0 mg/L, and three had it at the first measurement after the surgery. The mean concentration was 13.0 mg/L before and 12.7 after (P = 0.839) splenectomy.
Table 5.
Laboratory data and clinical evolution after splenectomy of 13 patients with relapsing visceral leishmaniasis
| Laboratory data and clinical evolution | All patients | HIV-infected patients | ||||
|---|---|---|---|---|---|---|
| Presurgery, mean (95% CI) | Postsurgery, mean (95% CI) | P value | Presurgery, mean (95% CI) | Postsurgery, mean (95% CI) | P value | |
| Hemoglobin* | 8.1 g/dL (7.3–8.8) | 10.7 g/dL (9.2–12.2) | 0.002† | 8.0 g/dL (7.1–8.0) | 10.2 g/dL (8.7–11.7) | 0.01 |
| Hematocrit* | 25.8% (23.4–28.2) | 33.2% (29.1–37.2) | 0.001 | 25.5% (22.6–28.3) | 31.8% (27.7–36.0) | 0.002 |
| Leukocytes* | 2,152 cells/µL (1,090–3,820)‡ | 11,105 cells/µL (1,703.4–20,507.8)§ | 0.002‖ | 2,190 cells/µL (1,645–2,735)‡ | 11,770 cells/µL (393–23,147)‡ | 0.003§ |
| Platelets* | 124,346 platelets/µL (99,263–149,429) | 303,638.5 platelets/µL (181,258–426,019) | 0.005 | 126,473 platelets/µL (98,966–153,980) | 292,664 platelets/µL (145,045–440,283) | 0.01 |
| CD4+ count‡ | 69.7 cells/µL (53.1–86.3) | 278.1 cells/µL (155.5–400.7) | 0.003 | 69.7 cells/µL (53.1–86.3) | 278.1 cells/µL (155.5–400.7) | 0.003 |
| Viral load‡ | Below detection level | Below detection level | – | Below detection level | Below detection level | – |
| No. of RBC transfusions¶ | 5.2 (2.4–8.1) | None# | 0.002 | 5.7 (2.4–9.0) | None# | 0.003 |
| No. of per-patient hospital admissions | 0.39 (0.32–0.46) | 0.05 (0.00–0.09) | 0.001 | 0.45 (0.35–0.55) | 0.05 (0.00–0.13) | 0.001 |
CI = confidence interval; RBC = red blood cells; PKDL = post–kala-azar dermal leishmaniasis.
A few days before and after splenectomy (in-hospital).
Student’s t test.
Last before and after splenectomy (range 9–323 days before and 17–258 days after).
Median 2,200 cells/µL and 7,320 cells/µL, before and after splenectomy, respectively for all patents and 2,200 cells/µL and 7,450 cells/µL for HIV-infected.
Wilcoxon matched-pairs signed-rank test.
Excluded the transfusions performed immediately after the surgery.
# Ten days after surgery.
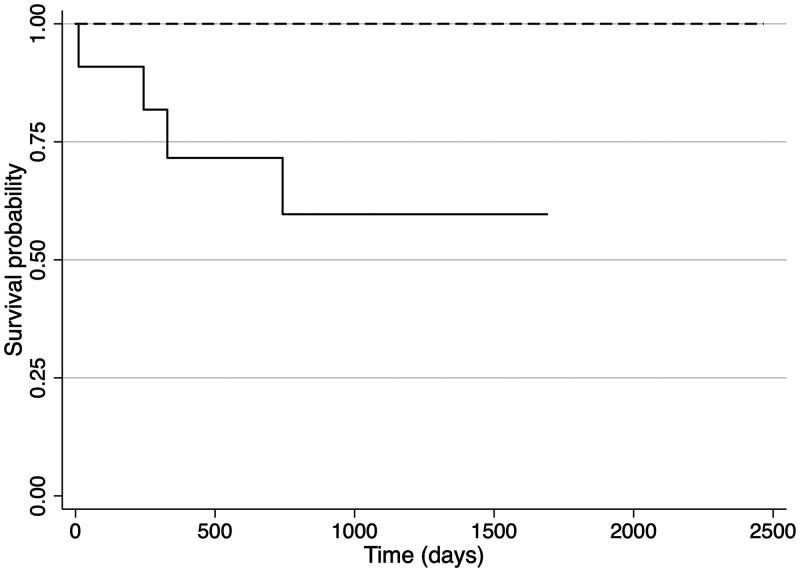
Overall mortality was 4 of 13, or (30.7%) and among HIV-infected patients, it was 36.3%. The death rate was 13.3 and 22.1 per 100 person-years after the surgery, respectively, for all and for the HIV-infected patients. One death occurred 11 days after surgery, with the probability of early survival (30 days) of 0.92 (92.3%). The total time at risk was 10.984 days, and the mean survival time was 844.9 days (2.3 years) with a median of 785 days (2.2 years), with a minimum of 11 days and a maximum of 2,466 days (6.8 years). For the two non-HIV-infected patients, the mean survival time was 1,871.5 days (5.1 years). However, for the HIV-infected, the mean survival time was 658.3 days (1.8 years). The survival analysis is shown in Figure 1. The Supplemental Table 2 summarizes the dataset.
Figure 1.
Kaplan-Meier survival curve after splenectomy of patients with relapsing visceral leishmaniasis. Dashed line: patients without HIV; solid line: patients with HIV.
DISCUSSION
This cohort of patients who underwent splenectomy with the intent to cure relapsing VL, which was not responding to the Brazilian recommendations for treatment, demonstrated that the two patients without HIV infection or any other known immunosuppression had a long-term safe outcome. However, the 11 patients infected with HIV responded differently. Although the viral load was undetected, all had low CD4+ count, indicating severe immunosuppression and AIDS.17 Two patients developed PKDL, and four died. Unfortunately, a cohort of similar patients who did not undergo surgery was not available, and therefore a measure of its effect was not possible. However, except for one patient with early death, all, including those with late death, had improved quality of life with reduced hospitalizations and CBC and increased CD4+ count.
As with all surgical procedures, splenectomy is not free of complications, mainly when performed in patients with chronic comorbidities. Early severe or late complications such as fulminant post-splenectomy infection (FPSI), thrombotic events, subphrenic abscess, and surgical wound complications have been described.18–23 However, the number of early and late surgical complications observed in this study was surprisingly small, given the patients’ immunosuppression status as measured by the CD4+ cell counts.
PKDL, the most frequent late complication of splenectomy in this study, is a condition in which Leishmania parasites persist on the skin after apparently successful treatment of VL, probably due to incomplete immune response.24 It is rare in infections caused by L. infantum but common in L. donovani infection in the Indian subcontinent and East Africa.24,25 One of the patients described here had a slow but progressive and complete regression of his skin lesions. However, the other remained with them up to death, showing that in splenectomized patients, PKDL can be harder to treat.26 Individuals with PKDL usually have no general symptom, and most of the time, lesions cause only aesthetic problems. However, if left untreated, the infectious lesions can last from years to decades.24,25 This study shows that removing the spleen somehow facilitates cutaneous parasitism and deserves further investigation.
Patients with refractory VL, coinfected or not with HIV, have a typical pattern of undergoing multiple hospitalizations. Relapses are much more common among HIV-coinfected patients.27 Therefore, this study’s most objective way to evaluate patients’ response to splenectomy was through the number of new hospitalizations. The number in the postsurgical period dropped significantly and occurred only among patients who died. Of course, deaths censored the number of postsurgical blood transfusions and hospital admissions. Nonetheless, because the deaths seem to have been somehow related to the procedure itself, it appears reasonable to imagine that the causes of the previous hospital post-splenectomy admissions are risk factors for later death. In the other series of splenectomy in coinfected patients, the mean number of hospitalizations was also reduced after surgery.6 Patients with different types of immunosuppression, not infected with HIV, can develop relapsing VL,28 but neither of the two patients without HIV from this cohort had a known cause of immunosuppression. Some sort of unknown parasitic or host genetic factor may have played a role in their therapeutic failure because, several years after the splenectomy, both remain healthy.
After splenectomy, the mean length of hospital stay was consistent with the average patients submitted to splenectomy by other causes. For example, a study with 90 elective splenectomies for five years in Canada showed that, in the absence of complications, patients remain in the hospital from four to six days after open splenectomy.29 Another study comparing the length of hospital stay between the open and laparoscopic pathways in patients with idiopathic thrombocytopenic purpura showed an average of nine days of postoperative hospitalization for the laparoscopic approach and 11 days for the open surgery.30
Few studies in the literature have evaluated the mortality and survival of coinfected patients submitted to splenectomy. This study describes the procedure in patients with two severe diseases of protracted and chronic evolution in a poor-resources setting; the natural evolution to death would be hypothetically higher than each one without the other. In a study published in 2017, Sousa Gomes et al. studied 760 cases of leishmania-HIV coinfected patients between 2001 and 2010 with a mortality rate of 25% and three times higher among coinfected patients in comparison to patients with VL only.27 Despite the more extended observation period in the study mentioned above that led to more significant mortality, this study's population consisted of people with more severe disease, with pancytopenia, very low CD4+ cell count, and successive red blood cells transfusions and hospital admissions. The most feared post-splenectomy complication is FPSI,2,5,31–33 but it is now infrequent due to vaccination against encapsulated germs.22 The risk of development of FPSI depends on the surgical indication for splenectomy.31 Styrt et al. estimated the risk of fatal occurrence of FPSI in 1:800 to 1:1000 adults.32 In most of their cases, the lethal outcome happened in the first 48 hours, despite the use of broad-spectrum antibiotics and intensive care.34–36 The second-to-last patient operated on in this cohort had a fulminant evolution in a few hours. It must be reminded that, indeed, sepsis is strongly associated with AIDS.37 The other three deaths occurred lately. Thus, it cannot be determined whether splenectomy contributed to their death38 or whether the deaths occurred due to the irreversible progression of immune failure resulting from HIV infection before the surgical procedure. Their low CD4+ count suggests that they already had a high chance of death without splenectomy, as it occurred with the patient with herpetic encephalitis. On the other hand, among the immunocompetent patients, the approach was curative.
Regarding the CBC and CD4+ cell count laboratory values before and after surgery, all results were significantly higher in the postsurgical period, independent of HIV infection status. Interestingly, viral load remained undetectable between pre- and postsurgical periods. A similar outcome was found by Troya et al. in 2007 in their three patients.6 According to them, splenectomy has three advantages: eliminating many parasites that were not affected by anti-Leishmania drugs, avoiding severe cytopenia due to inflammatory hypersplenism, and leading to increased CD4+ cells and improved survival.6 Similarly, Alon and Chowers, in a case described in 2012 of coinfected patients, reported that the patient had normalized blood and CD4+ count profile after surgery.11 The improvement in CBC and CD4+ count profiles reduced the number of hospitalizations because the main reasons for hospital readmission are anemia requiring red cells transfusion or opportunistic infections in patients with low CD4+ cell count.39 It is worth noting that of nine surviving patients, none were readmitted after splenectomy.
This study shows that splenectomy can benefit immunocompetent patients with relapsing VL and those coinfected with HIV who are not responsive to either the treatments for VL or HAART. The approach improved the quality of life by reducing the number of blood transfusions, hospital readmissions and opportunistic infections, and the effects of inflammatory hypersplenism by improving the CBC and increasing the CD4+ cell count, albeit unable to improve already established renal failure. Unfortunately, the established drug-related kidney damage was not reversed by the procedure. HIV-infected patients did not perform as well given that all four deaths happened in this group. On the other hand, it is unclear whether the effect of splenectomy on HIV infection was independent of the response to infection by L. infantum.13 Additionally, splenectomy indications for these patients should be thoroughly and carefully evaluated and are indicated only as a rescue approach when specific drug therapeutic possibilities have been exhausted. Recent findings suggest that a combination of anti-Leishmania therapy with locally available drugs should be tried first.40,41 Furthermore, the decision to perform splenectomy should only be made after patients’ informed consent.
The study has several limitations. First, drug therapy before the decision for splenectomy was not exhausted because the Brazilian official recommendation for secondary prevention of VL relapses included only amphotericin B; miltefosine and paromomycin are not currently available in Brazil. Moreover, combination therapy had not been introduced in Brazil during the study period and was not prescribed by the assistant physicians. Finally, albeit the highest ever published in this population, the number of patients was small and included only two without HIV coinfection, making it difficult to draw more robust conclusions about introducing the procedure into the routine medical practice.
Finally, evaluation of both the attending physician and surgeon is required for completion of the following criteria: confirmation of VL refractory to anti-Leishmania therapeutic and best prophylactic drug treatments; multiple hospital readmissions, either for VL relapses or opportunistic infections in patients with concomitant HIV; and patients must be in clinical condition to undergo the surgical procedure. As the last comment, the occurrence of low CD4+ cell count simultaneously to undetectable viral load is a secret hidden by relapsing Leishmania/HIV infection that should be unveiled in future more in-depth analyses of the excised spleens.
Supplemental Material
ACKNOWLEDGMENTS
We are grateful for the contributions of the doctors, nurses, and clerks, especially José Mendes, from Tropical Diseases Institute “Natan Portella,” who helped coordinate all the datasets.
Note: Supplemental tables appear at www.ajtmh.org.
REFERENCES
- 1. Desjeux P Alvar J , 2003. Leishmania/HIV co-infections: epidemiology in Europe. Ann Trop Med Parasitol 97: 3–15. [DOI] [PubMed] [Google Scholar]
- 2. Alvar J et al. 2008. The relationship between leishmaniasis and AIDS: the second 10 years. Clin Microbiol Rev 21: 334–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Olivier M Badaró R Medrano FJ Moreno J , 2003. The pathogenesis of Leishmania/HIV co-infection: cellular and immunological mechanisms. Ann Trop Med Parasitol 97: 79–98. [DOI] [PubMed] [Google Scholar]
- 4. Wolday D Akuffo H Demissie A Britton S , 1999. Role of Leishmania donovani and its lipophosphoglycan in CD4+ T-cell activation-induced human immunodeficiency virus replication. Infect Immun 67: 5258–5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. van Griensven J Carrillo E López-Vélez R Lynen L Moreno J , 2014. Leishmaniasis in immunosuppressed individuals. Clin Microbiol Infect 20: 286–299. [DOI] [PubMed] [Google Scholar]
- 6. Troya J Casquero A Muñiz G Fern’ndez-Guerrero ML Górgolas M , 2006. The role of splenectomy in HIV-infected patients with relapsing visceral leishmaniasis. Parasitology 134: 621–624. [DOI] [PubMed] [Google Scholar]
- 7. Bryan P , 1952. Splenectomy in a case of kala-azar; comments on a case. Rev Esp Pediatr 8: 95–99. [PubMed] [Google Scholar]
- 8. Das A Sen Gupta PC , 1950. Relapse of kala-azar after splenectomy. Lancet 256: 681–683. [DOI] [PubMed] [Google Scholar]
- 9. Lyngdoh E Jain SC Barua P , 1971. Splenectomy in treatment of drug resistant kala-azar. J Indian Med Assoc 57: 458–461. [PubMed] [Google Scholar]
- 10. Dutra RA Dutra LF Reis M de O Lambert RC , 2012. Splenectomy in a patient with treatment-resistant visceral leishmaniasis: a case report. Rev Soc Bras Med Trop 45: 130–131. [DOI] [PubMed] [Google Scholar]
- 11. Alon D Chowers M , 2012. Successful therapeutic splenectomy in an HIV patient with relapsing visceral leishmaniasis. Int J STD AIDS 23: 289–290. [DOI] [PubMed] [Google Scholar]
- 12. Berman J , 2003. Current treatment approaches to leishmaniasis. Curr Opin Infect Dis 16: 397–401. [DOI] [PubMed] [Google Scholar]
- 13. Tsoukas CM , 1998. Effect of splenectomy on slowing human immunodeficiency virus disease progression. Arch Surg 133: 25–31. [DOI] [PubMed] [Google Scholar]
- 14. Brasil Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica , 2011. . Manual de Recomendações Para Diagnóstico, Tratamento e Acompanhamento de Pacientes Com a Coinfecção Leishmania-HIV. Normas e Manuais Técnicos.
- 15. Brasil Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Das Doenças Transmissívei , 2015. . Manual of Recommendations for Diagnosis, Treatment and Follow-up of Patients with Leishmaniasis–HIV Coinfection.
- 16. Brasil Ministério da Saúde, Secretaria de Vigilância em Saúde, Departamento de Vigilância Epidemiológica, 2014. . Manual de Vigilância e Controle da Leishmaniose Visceral.
- 17. Li R Duffee D Gbadamosi-Akindele MF , 2021. CD4 Count. In: StatPearls. Treasure Island, FL: StatPearls Publishing. [PubMed]
- 18. Pizzuto J Ambriz R , 1984. Therapeutic experience on 934 adults with idiopathic thrombocytopenic purpura: multicentric trial of the Cooperative Latin American Group on Hemostasis and Thrombosis. Blood 64: 1179–1183. [PubMed] [Google Scholar]
- 19. Katkhouda N Hurwitz MB Rivera RT Chandra M Waldrep DJ Gugenheim J Mouiel J , 1998. Laparoscopic splenectomy. Ann Surg 228: 568–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Lozano-Salazar RR Herrera MF Vargas-Vorácková F López-Karpovitch X , 1998. Laparoscopic versus open splenectomy for immune thrombocytopenic purpura. Am J Surg 176: 366–369. [DOI] [PubMed] [Google Scholar]
- 21. Rosen M Brody F Walsh RM Tarnoff M Malm J Ponsky J , 2002. Outcome of laparoscopic splenectomy based on hematologic indication. Surg Endosc 16: 272–279. [DOI] [PubMed] [Google Scholar]
- 22. Sarangi J Coleby M Trivella M Reilly S , 1997. Prevention of post splenectomy sepsis: a population based approach. J Public Health (Oxf) 19: 208–212. [DOI] [PubMed] [Google Scholar]
- 23. Crary SE, Buchanan GR, 2009. Vascular complications after splenectomy for hematologic disorders. Blood 114: 2861–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zijlstra EE , 2016. The immunology of post-kala-azar dermal leishmaniasis (PKDL). Parasit Vectors 9: 464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Singh N Ramesh V Arora VK Bhatia A Kubba A Ramam M , 1998. Nodular post-kala-azar dermal leishmaniasis: a distinct histopathological entity. J Cutan Pathol 25: 95–99. [DOI] [PubMed] [Google Scholar]
- 26. Reinaldo LGC Araújo RJC Jr. Diniz TM de Deus Moura R Costa DL Eulálio KD Costa CHN , 2020. Recurrent kala-azar: report of two cured cases after total splenectomy. Rev Inst Med Trop Sao Paulo 62: e31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Leite de Sousa-Gomes M Romero GAS Werneck GL , 2017. Visceral leishmaniasis and HIV/AIDS in Brazil: are we aware enough? PLoS Negl Trop Dis 11: e0005772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Akuffo H Costa C van Griensven J Burza S Moreno J Herrero M , 2018. New insights into leishmaniasis in the immunosuppressed. PLoS Negl Trop Dis 12: e0006375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Feldman LS Demyttenaere SV Polyhronopoulos GN Fried GM , 2008. Refining the selection criteria for laparoscopic versus open splenectomy for splenomegaly. J Laparoendosc Adv Surg Tech A 18: 13–19. [DOI] [PubMed] [Google Scholar]
- 30. Qu Y Xu J Jiao C Cheng Z Ren S , 2014. Long-term outcomes of laparoscopic splenectomy versus open splenectomy for idiopathic thrombocytopenic purpura. Int Surg 99: 286–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Singer DB , 1973. Postsplenectomy sepsis. Perspect Pediatr Pathol 1: 285–311. [PubMed] [Google Scholar]
- 32. Styrt BA , 1996. Risks of infection and protective strategies for the asplenic patient. Infect Dis Clin Pract 5: 94–100. [Google Scholar]
- 33. Hansen K Singer DB , 2001. Asplenic-hyposplenic overwhelming sepsis: postsplenectomy sepsis revisited. Pediatr Dev Pathol 4: 105–121. [DOI] [PubMed] [Google Scholar]
- 34. Lynch AM Kapila R , 1996. Overwhelming postsplenectomy infection. Infect Dis Clin North Am 10: 693–707. [DOI] [PubMed] [Google Scholar]
- 35. Cunha BA , 1998. Infections in nonleukopenic compromised hosts (diabetes mellitus, SLE, steroids, and asplenia) in critical care. Crit Care Clin 14: 263–282. [DOI] [PubMed] [Google Scholar]
- 36. Marques RG Petroianu A , 2003. Infecção fulminante pós-esplenectomia. Arq Gastroenterol 40: 47–54. [DOI] [PubMed] [Google Scholar]
- 37. Droz N Hsia Y Ellis S Dramowski A Sharland M Basmaci R , 2019. Bacterial pathogens and resistance causing community acquired paediatric bloodstream infections in low- and middle-income countries: a systematic review and meta-analysis. Antimicrob Resist Infect Control 8: 207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Long B Koyfman A Gottlieb M , 2021. Complications in the adult asplenic patient: a review for the emergency clinician. Am J Emerg Med 44: 452–457 [DOI] [PubMed] [Google Scholar]
- 39. Lima IP Müller MC Holanda TA Harhay M Costa CHN Costa DL , 2013. Human immunodeficiency virus/Leishmania infantum in the first foci of urban American visceral leishmaniasis: clinical presentation from 1994 to 2010. Rev Soc Bras Med Trop 46: 156–160. [DOI] [PubMed] [Google Scholar]
- 40. Lindoso JAL Moreira CHV Cunha MA Queiroz IT , 2018. Visceral leishmaniasis and HIV coinfection: current perspectives. HIV AIDS (Auckl) 10: 193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vechi HT de Sousa ASV da Cunha MA Shaw JJ Luz KG , 2020. Case report: combination therapy with liposomal amphotericin B, N-methyl neglumine antimoniate, and pentamidine isethionate for disseminated visceral leishmaniasis in a splenectomized adult patient. Am J Trop Med Hyg 102: 268–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.