ABSTRACT.
Leishmaniasis is a protozoan disease caused by species of genus Leishmania. Immunosuppression increases the risk of severe clinical forms and impairs response to treatment. The expansion of the use of immunomodulatory drugs for different conditions has raised the number of these cases. In this report, we present a case of visceral leishmaniasis in a patient with multiple sclerosis (MS) under fingolimod treatment. He presented with the triad of fever, visceromegaly, and pancytopenia and was diagnosed by the presence of amastigotes in a bone marrow sample. Furthermore, we discuss the previous published cases of MS patients under different immunosuppressant therapies to highlight its risk in endemic areas and suggest a therapeutic approach.
INTRODUCTION
Leishmaniasis is a protozoan granulomatous disease endemic to South America, south Asia, Africa, and Mediterranean Basin. The disease is transmitted by phlebotomine sandflies and is caused by species of the genus Leishmania that are phagocytosed by macrophages and proliferate inside mononuclear phagocytic system cells. Clinical severity of the disease ranges from asymptomatic infection to disseminated forms of the infection with high morbidity and mortality depending on the characteristics of the parasite and the host response.1 In this regard, it is well-known that immunosuppression considerably increases not only the risk of severe clinical forms, but also predisposes to reactivation and impairs response to treatment.2
Most of the literature about leishmaniasis in immunosuppressed patients focused on persons with HIV infection,3 even so there is also a rising number of reviews and case reports of patients with rheumatological, gastrointestinal, and dermatological conditions requiring immunomodulatory drugs, including glucocorticosteroids (CS),4 cytostatic agents (i.e., methotrexate and purine analogues)5 and tumor necrosis factor (TNF)-α inhibitors6 as well as solid organ/hematologic transplant recipients.7
CASE PRESENTATION
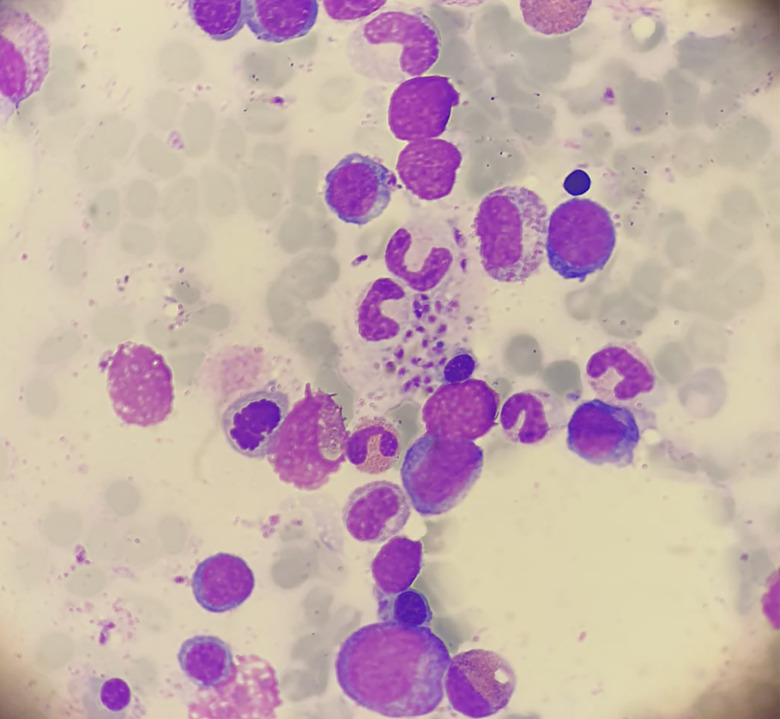
A 33-year-old man with relapsing remitting multiple sclerosis (MS) was admitted to our hospital because of fever. After diagnosis of MS in 2011, he had been initially treated with glatiramer acetate and then with interferon Beta-1a. Due to clinical and radiological activity, treatment was switched to fingolimod in 2013, achieving disease stability. He had lived in Barcelona (Spain) and had no history of recent travel over the past 10 years. He referred fever up to 40°C, chills, and abdominal pain localized in the right upper quadrant during the previous week. He did not have neurological symptoms. At admission, the patient had a temperature of 37.6°C, other vital signs were normal. Physical examination revealed tender hepatomegaly and splenomegaly with no adenopathy, jaundice, or additional findings. Laboratory test results included a hemoglobin concentration of 11.5 g/dL, leukocyte count of 1,260 cells/μL, platelet count of 59,000 cells/μL, 0.81% of reticulocytes, total bilirubin of 2.12 mg/dL, and elevated liver enzymes. A peripheral blood smear did not show immature cells or schistocytes. An abdominal computed tomography was performed, revealing an enlarged liver without focal lesions and homogeneous splenomegaly of 16 cm with no other remarkable findings. Given the suspected diagnosis of opportunistic infection, fingolimod was withdrawn. Blood cultures, serology for hepatotropic viruses, HIV, and blood cryptococcal antigen were negative. Real time polymerase chain reaction (PCR) for Leishmania spp. was performed in two blood samples and bone marrow biopsy and all of them were positive. In the bone marrow sample, Leishmania amastigotes could also be observed (Figure 1). A diagnosis of visceral leishmaniasis was established and the patient received treatment with liposomal amphotericin B at a dose of 4 mg/kg body weight once daily for 5 days followed by 5 weekly doses for a total dosage of 40 mg/kg. After treatment of the infection, the hemogram progressively recovered. As the patient presented negative anti-John Cunningham virus (JCV) antibody, MS treatment was restarted after 5 weeks by switching fingolimod to natalizumab neither leishmaniasis nor MS relapses after 6 months of follow-up.
Figure 1.
Bone marrow aspiration: We observe intracellular and extracellular amastigotes of leishmania. This figure appears in color at www.ajtmh.org.
DISCUSSION
Multiple sclerosis treatment has changed considerably over the past 25 years with the advent of new disease-modifying therapies (DMTs).8 Disease-modifying therapies induce immunomodulation and depletion of T or B cells, providing not only higher efficacy but at the same time new clinical challenges, as an increased risk of infection. It has been suggested that Th1 cells and T central memory cells would play a particularly important role in the host defense against Leishmania spp.9 Among the waxing body of literature dealing with infectious risks in patients with MS under DMTs,10 there are only a few case reports that have addressed specifically the issue of leishmaniasis. After describing another case of visceral leishmaniasis in a patient under treatment with fingolimod, our case arises the question about the risk of developing severe clinical forms of leishmaniasis in patients treated with DMTs.
Sphingosine 1 phosphate receptor modulators, such as fingolimod, avoid migration of CCR7-positive T lymphocytes such as CD8 + naïve, central memory, and Th17 and Th17/Th1-helper cells out of the lymph nodes, but also impairing cellular immune response and TNF alpha production.9 Three cases of leishmaniasis have been reported in patients under treatment with fingolimod; the first by Artemiadis et al. presented as visceral leishmaniasis in a 37-year-old woman from Greece who was treated with fingolimod for 29 months. A blood quantitative polymerase chain reaction (qPCR) was positive for Leishmania infantum and the patient was treated with liposomal amphotericin B, with complete response to treatment.11 The second, reported by Hernández Clares et al. was a 32-year-old woman from Spain receiving fingolimod for 25 months presented an aggressive cutaneous leishmaniasis by Leishmania infantum in the form of granulomatous infiltration of the outer ear accompanied by painful cervical adenopathies. Intralesional treatment with amphotericin B was attempted unsuccessfully, requiring intravenous liposomal amphotericin B.12 The last one, reported by Williams et al. was a 61-year-old man from Australia receiving fingolimod for 6 years who presented a visceral leishmaniasis by Leishmania infantum probably acquired after a trip to southern Italy.13 In all cases, prolonged lymphopenia attributable to fingolimod was observed, which is presumably associated with an increased risk of infection progression. Here, we report another case of visceral leishmaniasis in a patient under fingolimod treatment of 7 years and grade III lymphopenia, supporting the hypothesis that fingolimod treatment would increase the risk of more aggressive forms of leishmaniasis.
Glucocorticosteroids, among other mechanisms, reduce T-lymphocytes and derived cytokines, which are the main host defense against intracellular microorganisms like Leishmania, which may promote the development of visceral leishmaniasis, as well as chronicity and relapses in these patients. Murine models have demonstrated an association between continued exposition to CS and an increase in amastigote burden in the spleen. In different series of cases and case reports of leishmaniasis in CS-treated patients, diagnostic delay, clinically severe disease, and partial response to treatment were observed and likely attributable to the steroid therapy.4,14
Azathioprine, although barely used nowadays, is a purine analogue that blocks de novo synthesis of purines and induces apoptosis of T-lymphocytes. It has been reported to increase the risk of developing bacterial, viral, fungal, and protozoal infections. Although no cases of leishmaniasis have been published in MS patients on azathioprine treatment, two cases have been described, one with Crohn Disease and the other with midline granulomatosis.5,15
Alemtuzumab, an anti-CD52 monoclonal antibody, is thought to decrease the number of circulating B and T lymphocytes and their subsequent repopulation, although the exact mechanism of action is still unknown.16 Infections have been described in patients with alemtuzumab, mainly involving the respiratory and urinary tracts.10 Even though leishmaniasis has not been reported in patients with MS, one case was published in Italy in a patient with chronic lymphocytic leukaemia.17
Rituximab is an anti-CD20 that produces B cell depletion through various mechanisms. Some cases of visceral leishmaniasis have been described in patients with lymphoproliferative disorders under treatment with rituximab and there is evidence that its activity is not restricted to B lymphocytes, since it also may reduce the activity of both peripheral and tissue-resident T lymphocytes.18 However, in patients, rituximab is often used in combination with other drugs and we must also consider the contribution of the underlying disease to the total immunosuppression.19 In relation to other anti-CD20 antibodies, neither ocrelizumab nor ofatumumab have been related to leishmaniasis. However, its limited use compared with rituximab should be considered.
No visceral leishmaniasis cases have been reported for patients under treatment with glatiramer acetate, natalizumab, teriflunomide, and dimethyl fumarate or desoxiadenosine analogs as cladribine and mitoxantrone although they also modify cellular immunity. For interferon beta not only has not been observed an increased risk of specific infections, but also a possible protective effect against Leishmania have been described depending on the dosing and treatment protocol used.20,21
Liposomal amphotericine B has proved to have the best safety profile and compelling evidence of its efficacy in immunosuppressed patients.6 One of the major concerns is the reintroduction of immunosuppression. Taking into account the available information, it seems reasonable to choose alternative DMTs with lower risk of recurrence such as natalizumab ensuring close clinical follow-up and blood qPCR. All in all, the proportion of MS individuals treated with fingolimod that develop aggressive forms of leishmaniasis remains low, hence it does not appear reasonable to completely avoid its administration. Nevertheless, the case we report, on the same line as the previously described, highlights that treatment with fingolimod, should be done carefully in areas endemic for Leishmania spp.
CONCLUSION
In patients from endemic areas under treatment with any of the mentioned DMTs presenting fever, cytopenia, or organomegaly it is worth considering leishmaniasis to avoid delayed diagnosis. Early withdrawal of the DMTs and a rapid confirmation of the diagnosis, so that systemic treatment can be started in the shortest time possible are critical for the management of such cases.
REFERENCES
- 1. Burza S, Croft SL, Boelaert M, 2018. Leishmaniasis. Lancet 392: 951–970. [DOI] [PubMed] [Google Scholar]
- 2. Pagliano P, Ascione T, Di Flumeri G, Boccia G, De Caro F, 2016. Visceral leishmaniasis in immunocompromised: diagnostic and therapeutic approach and evaluation of the recently released IDSA guidelines. Infez Med 24: 265–271. [PubMed] [Google Scholar]
- 3. Fernández-Guerrero ML, Robles P, Rivas P, Mójer F, Muñíz G, de Górgolas M, 2004. Visceral leishmaniasis in immunocompromised patients with and without AIDS: a comparison of clinical features and prognosis. Acta Trop 90: 11–16. [DOI] [PubMed] [Google Scholar]
- 4. Pittalis S, Nicastri E, Spinazzola F, Ghirga P, De Marco M, Paglia MG, Narciso P, 2006. Leishmania infantum leishmaniasis in corticosteroid–treated patients. BMC Infect Dis 6: 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Valdés Delgado T, Cordero Ruiz P, Bellido Muñoz F, 2017. Visceral leishmaniasis infection in a patient with Crohn’s disease treated with azathioprine. J Crohn’s Colitis 11: 1282–1283. [DOI] [PubMed] [Google Scholar]
- 6. Bosch-Nicolau P, Ubals M, Salvador F, Sánchez-Montalvá A, Aparicio G, Erra A, Martinez de Salazar P, Sulleiro E, Molina I, 2019. Leishmaniasis and tumor necrosis factor alpha antagonists in the Mediterranean basin. A switch in clinical expression. PLoS Negl Trop Dis 13: e0007708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clemente WT, Mourão PHO, Lopez-Medrano F, Schwartz BS, García-Donoso C, Torre-Cisneros J, 2018. Visceral and cutaneous leishmaniasis recommendations for solid organ transplant recipients and donors. Transplantation 102 (2S Suppl 2): S8–S15. [DOI] [PubMed] [Google Scholar]
- 8. Tintore M, Vidal-Jordana A, Sastre-Garriga J, 2019. Treatment of multiple sclerosis—success from bench to bedside. Nat Rev Neurol 15: 53–58. [DOI] [PubMed] [Google Scholar]
- 9. Groves A, Kihara Y, Chun J, 2013. Fingolimod: direct CNS effects of sphingosine 1-phosphate (S1P) receptor modulation and implications in multiple sclerosis therapy. J Neurol Sci 328: 9–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Winkelmann A, Loebermann M, Reisinger EC, Hartung HP, Zettl UK, 2016. Disease-modifying therapies and infectious risks in multiple sclerosis. Nat Rev Neurol 12: 217–233. [DOI] [PubMed] [Google Scholar]
- 11. Artemiadis AK, Nikolaou G, Kolokythopoulos D, Tegos N, Terentiou A, Triantafyllou N, Papanastasiou I, 2015. Visceral leishmaniasis infection in a fingolimod-treated multiple sclerosis patient. Mult Scler 21: 795–796. [DOI] [PubMed] [Google Scholar]
- 12. Hernández Clares R, Sánchez Pedreño P, García Vazquez E, Carreón Guarnizo E, Meca Lallana JE, 2018. Aggressive cutaneous leishmaniasis in a patient with multiple sclerosis treated with fingolimod. Leishmaniasis cutánea agresiva en paciente con esclerosis múltiple tratada con fingolimod. Neurologia 33: 348–349 (Engl Ed). [DOI] [PubMed] [Google Scholar]
- 13. Williams E, Isles NS, Seemann T, Kilpatrick T, Grigg A, Leroi M, Howden BP, Kwong JC, 2020. Case report: confirmation by metagenomic sequencing of visceral leishmaniasis in an immunosuppressed returned traveler. Am J Trop Med Hyg 103: 1930–1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Motta AC, Arruda D, Souza CS, Foss NT, 2003. Disseminated mucocutaneous leishmaniasis resulting from chronic use of corticosteroid. Int J Dermatol 42: 703–706. [DOI] [PubMed] [Google Scholar]
- 15. Tejura N, Kim E, Dever LL, Chew D, 2019. Case report: mucocutaneous leishmaniasis masquerading as idiopathic midline granulomatous disease. Am J Trop Med Hyg 101: 1107–1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Informe de posicionamiento terapéutico de alemtuzumab (Lemtrada®) , 2015. Available at: http://www.aemps.gob.es/medicamentosUsoHumano/informesPublicos/docs/IPT-alemtuzumab-lemtrada.pdf. Accessed June 4, 2021.
- 17. Pitini V, Cascio A, Arrigo C, Altavilla G, 2012. Visceral leishmaniasis after alemtuzumab in a patient with chronic lymphocytic leukaemia. Br J Haematol 156: 1. [DOI] [PubMed] [Google Scholar]
- 18. Casabianca A, Marchetti M, Zallio F, Feyles E, Concialdi E, Ferroglio E, Biglino A, 2011. Seronegative visceral leishmaniasis with relapsing and fatal course following rituximab treatment. Infection 39: 375–378. [DOI] [PubMed] [Google Scholar]
- 19. Los-Arcos I, Aguilar-Company J, Ruiz-Camps I, 2020. Risk of infection associated with new therapies for lymphoproliferative syndromes. Riesgo de infección asociada a nuevas terapias para el tratamiento de los síndromes linfoproliferativos. Med Clin (Barc) 154: 101–107. [DOI] [PubMed] [Google Scholar]
- 20. Mattner J, Wandersee-Steinhäuser A, Pahl A, Röllinghoff M, Majeau GR, Hochman PS, Bogdan C, 2004. Protection against progressive leishmaniasis by IFN-beta. J Immunol 172: 7574–7582. [DOI] [PubMed] [Google Scholar]
- 21. Kumar R. et al. , 2020. Type I interferons suppress anti-parasitic immunity and can be targeted to improve treatment of visceral leishmaniasis. Cell Rep 30: 2512–2525.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]