Abstract
Background
This review is one of six looking at the primary medical management options for patients with chronic rhinosinusitis.
Chronic rhinosinusitis is common and is characterised by inflammation of the lining of the nose and paranasal sinuses leading to nasal blockage, rhinorrhoea, facial pressure/pain and loss of sense of smell. The condition can occur with or without nasal polyps. The use of topical (intranasal) corticosteroids has been widely advocated for the treatment of chronic rhinosinusitis given the belief that inflammation is a major component of this condition.
Objectives
To assess the effects of intranasal corticosteroids in people with chronic rhinosinusitis.
Search methods
The Cochrane ENT Information Specialist searched the Cochrane ENT Trials Register; Central Register of Controlled Trials (CENTRAL 2015, Issue 8); MEDLINE; EMBASE; ClinicalTrials.gov; ICTRP and additional sources for published and unpublished trials. The date of the search was 11 August 2015.
Selection criteria
Randomised controlled trials (RCTs) with a follow‐up period of at least three months comparing intranasal corticosteroids (e.g. beclomethasone dipropionate, triamcinolone acetonide, flunisolide, budesonide) against placebo or no treatment in patients with chronic rhinosinusitis.
Data collection and analysis
We used the standard methodological procedures expected by Cochrane. Our primary outcomes were disease‐specific health‐related quality of life (HRQL), patient‐reported disease severity and the commonest adverse event ‐ epistaxis. Secondary outcomes included general HRQL, endoscopic nasal polyp score, computerised tomography (CT) scan score and the adverse events of local irritation or other systemic adverse events. We used GRADE to assess the quality of the evidence for each outcome; this is indicated in italics.
Main results
We included 18 RCTs with a total of 2738 participants. Fourteen studies had participants with nasal polyps and four studies had participants without nasal polyps. Only one study was conducted in children.
Intranasal corticosteroids versus placebo or no intervention
Only one study (20 adult participants without polyps) measured our primary outcome disease‐specific HRQL using the Rhinosinusitis Outcome Measures‐31 (RSOM‐31). They reported no significant difference (numerical data not available) (very low quality evidence).
Our second primary outcome, disease severity , was measured using the Chronic Sinusitis Survey in a second study (134 participants without polyps), which found no important difference (mean difference (MD) 2.84, 95% confidence interval (CI) ‐5.02 to 10.70; scale 0 to 100). Another study (chronic rhinosinusitis with nasal polyps) reported an increased chance of improvement in the intranasal corticosteroids group (RR 2.78, 95% CI 1.76 to 4.40; 109 participants). The quality of the evidence was low.
Six studies provided data on at least two of the individualsymptoms used in the EPOS 2012 criteria to define chronic rhinosinusitis (nasal blockage, rhinorrhoea, loss of sense of smell and facial pain/pressure). When all four symptoms in the EPOS criteria were available on a scale of 0 to 3 (higher = more severe symptoms), the average MD in change from baseline was ‐0.26 (95% CI ‐0.37 to ‐0.15; 243 participants; two studies; low quality evidence). Although there were more studies and participants when only nasal blockage and rhinorrhoea were considered (MD ‐0.31, 95% CI ‐0.38 to ‐0.24; 1702 participants; six studies), the MD was almost identical to when loss of sense of smell was also considered (1345 participants, four studies; moderate quality evidence).
When considering the results for the individual symptoms, benefit was shown in the intranasal corticosteroids group. The effect size was larger for nasal blockage (MD ‐0.40, 95% CI ‐0.52 to ‐0.29; 1702 participants; six studies) than for rhinorrhoea (MD ‐0.25, 95% CI ‐0.33 to ‐0.17; 1702 participants; six studies) or loss of sense of smell (MD ‐0.19, 95% CI ‐0.28 to ‐0.11; 1345 participants; four studies). There was heterogeneity in the analysis for facial pain/pressure (MD ‐0.27, 95% CI ‐0.56 to 0.02; 243 participants; two studies). The quality of the evidence was moderate for nasal blockage, rhinorrhoea and loss of sense of smell, but low for facial pain/pressure.
There was an increased risk of epistaxis with intranasal corticosteroids (risk ratio (RR) 2.74, 95% CI 1.88 to 4.00; 2508 participants; 13 studies; high quality evidence).
Considering our secondary outcome, general HRQL, one study (134 participants without polyps) measured this using the SF‐36 and reported a statistically significant benefit only on the general health subscale. The quality of the evidence was very low.
It is unclear whether there is a difference in the risk of local irritation (RR 0.94, 95% CI 0.53 to 1.64; 2124 participants; 11 studies) (low quality evidence).
None of the studies treated or followed up patients long enough to provide meaningful data on the risk of osteoporosis or stunted growth (children).
Other comparisons
We identified no other studies that compared intranasal corticosteroids plus co‐intervention A versus placebo plus co‐intervention A.
Authors' conclusions
Most of the evidence available was from studies in patients with chronic rhinosinusitis with nasal polyps. There is little information about quality of life (very low quality evidence). For disease severity, there seems to be improvement for all symptoms (low quality evidence), a moderate‐sized benefit for nasal blockage and a small benefit for rhinorrhoea (moderate quality evidence). The risk of epistaxis is increased (high quality evidence), but these data included all levels of severity; small streaks of blood may not be a major concern for patients. It is unclear whether there is a difference in the risk of local irritation (low quality evidence).
Plain language summary
Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis
Review question
We reviewed the evidence for the benefits and harms of intranasal (in the nose) steroids given to people with chronic rhinosinusitis.
Background
Chronic rhinosinusitis is a common condition that is defined as inflammation of the nose and paranasal sinuses (a group of air‐filled spaces behind the nose, eyes and cheeks). Patients with chronic rhinosinusitis experience at least two or more of the following symptoms for at least 12 weeks: blocked nose, discharge from their nose or runny nose (rhinorrhoea), pain or pressure in their face and/or a reduced sense of smell (hyposmia). Some people will also have nasal polyps, which are grape‐like swellings of the normal nasal lining inside the nasal passage and sinuses. Topical (intranasal) corticosteroids are used with the aim of reducing inflammation in order to improve patient symptoms.
Study characteristics
We included 18 randomised controlled trials (RCTs) with a total of 2738 participants in this review. Most studies were relatively small, with as few as 9 or 10 patients per intervention arm. The largest study had 748 patients in total. Most were conducted in tertiary referral centres in northern Europe, the US and Canada. Fourteen studies only included participants with chronic rhinosinusitis with nasal polyps and four studies had participants without nasal polyps. Only one study was conducted in children. The studies looked at a range of types, doses and methods of administration (e.g. spray, drops) of intranasal corticosteroids.
Key results and quality of the evidence
One study (20 participants) reported no statistically significant difference in disease‐specific health‐related quality of life. Another measured general health‐related quality of life and reported a statistically significant benefit only on a subscale for general health. Both studies recruited participants with chronic rhinosinusitis without nasal polyps. The quality of the evidence was very low (we have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect).
Disease severity was measured in one study (chronic rhinosinusitis without nasal polyps, 134 participants), which found no important difference. Another study (chronic rhinosinusitis with nasal polyps) reported an increased chance of improvement in the intranasal corticosteroids group. The quality of the evidence was low (our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect).
When each type of symptom was measured separately (nasal blockage, rhinorrhoea, loss of sense of smell, facial pain/pressure), benefit was shown in the intranasal corticosteroids group. The quality of the evidence was moderate for nasal blockage, rhinorrhoea and loss of sense of smell, but low for facial pain/pressure (moderate quality evidence means we are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different).
There was an increased risk of nosebleeds (epistaxis) with intranasal corticosteroids (high quality evidence). However, it was unclear whether there was a difference in the risk of local (nose or throat) irritation (low quality evidence).
None of the studies treated or followed up patients long enough to provide meaningful data on the risk of osteoporosis (fragile bones) or stunted growth (in children).
Conclusions
Most of the evidence available was from studies in patients with chronic rhinosinusitis with nasal polyps. There is little information about quality of life and the quality of this evidence is very low. For disease severity, there seems to be improvement for all symptoms (low quality evidence), a moderate‐sized benefit for nasal blockage and a small benefit for rhinorrhoea (moderate quality evidence). The risk of nosebleeds is increased (high quality evidence), but this included all levels of severity; for some patients small streaks of blood may not be a major concern. It is unclear whether there is a difference in the risk of local irritation (low quality evidence).
Summary of findings
Summary of findings for the main comparison. Intranasal corticosteroids for people with chronic rhinosinusitis.
| Intranasal corticosteroids for people with chronic rhinosinusitis | ||||||
| Patient or population: chronic rhinosinusitis Setting: all Intervention: intranasal corticosteroids Comparison: placebo | ||||||
|
Outcomes № of participants (studies) |
Relative effect (95% CI) | Anticipated absolute effects* (95% CI) | Quality | What happens | ||
| With placebo | With intranasal corticosteroids | Difference | ||||
| Disease‐specific HRQL measured as median change from baseline (RSOM‐31) № of participants: 20 (1 RCT) |
— | Median 5 points lower | Median 62 points lower | — | ⊕⊝⊝⊝ VERY LOW 1,5 | Not enough information to conclude whether there is a difference |
| Disease severity ‐ measured as change from baseline using the Chronic Sinusitis Survey at 20 weeks № of participants: 134 (1 RCT) |
— | Chronic Sinusitis Survey score was 7.35 | — | MD 2.84 higher (5.02 lower to 10.7 higher) than placebo | ⊕⊕⊝⊝ LOW 1, 2 | There was no clinically important difference |
| Disease severity ‐ analysed as the proportion of patients who reported improvement on a global symptom score № of participants:109 (1 RCT) |
RR 2.78 (1.76 to 4.40) | Study population | ⊕⊕⊝⊝ LOW 1, 2 | There was an increased chance of improvement with intranasal corticosteroids | ||
| 273 per 1000 | 758 per 1000 (480 to 1000) |
485 more per 1000 (207 more to 927 more) |
||||
| Disease severity measured as average change from baseline at 12 to 20 weeks (range 0 to 3 points) for a combination of symptoms* | ||||||
№ of participants: 243 (2 RCTs) |
— | — | — | MD 0.26 lower (0.37 lower to 0.15 lower) than placebo | ⊕⊕⊝⊝ LOW 1, 2 | The improvement in the intranasal corticosteroids group was higher (moderate effect size). Lower score = less severe symptoms. |
№ of participants: 1345 (4 RCTs) |
— | — | — | MD 0.31 lower (0.38 lower to 0.23 lower) than placebo | ⊕⊕⊕⊝ MODERATE 2 | The improvement in the intranasal corticosteroids group was higher (moderate effect size) |
№ of participants: 1702 (6 RCTs) |
— | — | — | MD 0.31 lower (0.38 lower to 0.24 lower) than placebo | ⊕⊕⊕⊝ MODERATE 2 | The improvement in the intranasal corticosteroids group was higher (moderate effect size) |
| Disease severity measured as average change from baseline at 12 to 20 weeks (range 0 to 3 points) for individual symptoms* | ||||||
№ of participants: 1702 (6 RCTs) |
— | — | — | MD 0.4 lower (0.52 lower to 0.29 lower) than placebo | ⊕⊕⊕⊝ MODERATE 2 | The improvement in the intranasal corticosteroids group was higher (moderate effect size) |
№ of participants: 1702 (6 RCTs) |
— | — | — | MD 0.25 lower (0.33 lower to 0.17 lower) than placebo | ⊕⊕⊕⊝ MODERATE 2 | The improvement in the intranasal corticosteroids group was higher (small effect size) |
№ of participants: 1345 (4 RCTs) |
— | — | — | MD 0.19 lower (0.28 lower to 0.11 lower) than placebo | ⊕⊕⊕⊝ MODERATE 2 | The improvement in the intranasal corticosteroids group was higher (small effect size) |
№ of participants:243 (2 RCTs) |
— | — | — | MD 0.27 lower (0.56 lower to 0.02 higher) than placebo | ⊕⊕⊝⊝ LOW 1, 2, 6 | The improvement in the intranasal corticosteroids group was higher (moderate effect size) |
| Generic HRQL ‐ measured using SF‐36 № of participants: 134 (1 RCT) |
Statistical significance only for the general health sub‐scale. No statistically significant difference for the other sub‐scales. | ⊕⊝⊝⊝ VERY LOW 1, 5 | Unclear whether there is a difference in general HRQL | |||
| Adverse events ‐ epistaxis № of participants: 2508 (13 RCTs) |
RR 2.74 (1.88 to 4.00) | Study population | ⊕⊕⊕⊕ HIGH | The risk of epistaxis is higher in the intranasal corticosteroids group | ||
| 29 per 1000 | 79 per 1000 (54 to 115) |
50 more per 1000 (25 more to 86 more) |
||||
| Moderate | ||||||
| 39 per 1000 | 105 per 1000 (72 to 154) |
67 more per 1000 (34 more to 115 more) |
||||
| Adverse events ‐ local irritation № of participants: 2124 (11 RCTs) |
RR 0.94 (0.53 to 1.64) | Study population | ⊕⊕⊝⊝ LOW 3, 4 | It is uncertain whether there is an important difference between the intranasal corticosteroids and placebo groups in the risk of local irritation | ||
| 21 per 1000 | 20 per 1000 (11 to 34) |
1 fewer per 1000 (10 fewer to 13 more) |
||||
| Moderate | ||||||
| 26 per 1000 | 24 per 1000 (14 to 42) |
2 fewer per 1000 (12 fewer to 16 more) |
||||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; EPOS: European Position Paper on Rhinosinusitis and Nasal Polyps 2012; HRQL: health‐related quality of life; OR: odds ratio; RCT: randomised controlled trial; RR: risk ratio | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1Evidence only came from small trial(s). The overall sample size is very small. 2Outcome was not measured using a validated tool. 3Inconsistency in how outcome was reported across studies. The number of overall events is likely to be underestimated. 4Overall event rates likely to have been underestimated. The confidence interval is wide. 5Evidence only from one study, which had a high risk of reporting bias for this outcome. 6Unexplained heterogeneity observed for this outcome.
Background
Description of the condition
Chronic rhinosinusitis is defined as inflammation of the nose and paranasal sinuses characterised by two or more symptoms, one of which must be nasal blockage/obstruction/congestion or nasal discharge (anterior/posterior nasal drip). The other possible symptoms include facial pain/pressure, reduction or loss of sense of smell (in adults) or cough (in children). Symptoms must have continued for at least 12 weeks. In addition people must have either mucosal changes within the ostiomeatal complex and/or sinuses as evidenced by a computerised tomography (CT) scan and/or endoscopic signs of at least one of the following: nasal polyps, mucopurulent discharge primarily from middle meatus or oedema/mucosal obstruction primarily in the middle meatus (EPOS 2012).
Chronic rhinosinusitis represents a common source of ill health; 11% of UK adults reported chronic rhinosinusitis symptoms in a worldwide population study (Hastan 2011). Symptoms, including nasal obstruction, nasal discharge, facial pain, anosmia and sleep disturbance, have a major impact on quality of life, reportedly greater in several domains of the SF‐36 than angina or chronic respiratory disease (Gliklich 1995). Acute exacerbations, inadequate symptom control and respiratory disease exacerbation are common. Complications are rare, but may include visual impairment and intracranial infection.
Two major phenotypes of chronic rhinosinusitis have been identified based on the presence or absence of nasal polyps on examination. Nasal polyps are tumour‐like hyperplastic swellings of the nasal mucosa, most commonly originating from within the ostiomeatal complex (Larsen 2004). Chronic rhinosinusitis with nasal polyps (CRSwNP) is diagnosed when polyps are seen (on direct or endoscopic examination) bilaterally in the middle meatus. The acronym CRSsNP is used for the condition in which no polyps are present.
Although the aetiology of chronic rhinosinusitis is not fully understood, it may involve abnormalities in the host response to irritants, commensal and pathogenic organisms and allergens, obstruction of sinus drainage pathways, abnormalities of normal mucociliary function, loss of the normal mucosal barrier or infection. Two typical profiles may be observed with respect to inflammatory mediators; in eosinophilic chronic rhinosinusitis, which is typically associated with nasal polyps, high levels of eosinophils, immunoglobulin E (IgE) and interleukin (IL)‐5 may be found, while in neutrophilic chronic rhinosinusitis, more often associated with chronic rhinosinusitis without polyps, neutrophils predominate, with elevated interferon (IFN) gamma, IL‐8 and tumour necrosis factor (TNF) (EPOS 2012).
While treatment decisions should be made based on an understanding of the patient's chronic rhinosinusitis phenotype and likely aetiology, in practice treatment may be initiated without knowledge of the polyp status, particularly in primary care. This review (and most of its companion reviews) consider patients with and without polyps together in the initial evaluation of treatment effects. However, subgroup analyses explore potential differences between them.
The most commonly used interventions for chronic rhinosinusitis are used either topically (sprayed into the nose) or systemically (by mouth) and include steroids, antibiotics and saline.
Description of the intervention
Anti‐inflammatory therapy plays a significant role in the treatment of chronic rhinosinusitis. This includes corticosteroids and low‐dose macrolides. Topical corticosteroids are more widely used than oral steroids because treatment can be given for longer without significant adverse effects.
Intranasal corticosteroid therapy is often prescribed for patients with chronic rhinosinusitis, but with considerable variability in timing, frequency, dose, topical delivery method and the specific agent used (Benninger 2003; Spector 1998). The topical delivery method may affect the amount of steroid that comes into contact with the paranasal sinus mucosa (Grobler 2008; Harvey 2009). The simplest nasal delivery methods are drops, sprays, aerosols, nebulisers and atomisers. These contrast with methods involving direct sinus cannulation and nasal irrigation with squeeze bottles and neti pots, which are likely to provide better delivery to the sinuses, especially in the post‐sinus surgery setting (Grobler 2008; Harvey 2009; Thomas 2013).
Classes of topical corticosteroid include first‐generation intranasal steroids (beclomethasone dipropionate, triamcinolone acetonide, flunisolide and budesonide) and newer preparations (fluticasone propionate, mometasone furoate, ciclesonide and fluticasone furoate).
How the intervention might work
The use of topical (intranasal) corticosteroids has been widely advocated for the treatment of chronic rhinosinusitis given the belief that inflammation is a major component of this condition (Fokkens 2007; Hamilos 2000; McNally 1997). The mechanism of action is a combination of anti‐inflammatory effects (for example, reducing pro‐inflammatory, and increasing anti‐inflammatory, gene transcription and reducing airway inflammatory cell infiltration) and suppression of the production of pro‐inflammatory mediators, cell chemotactic factors and adhesion molecules (Mullol 2009). Several factors could affect the relative levels of effectiveness or harm from using intranasal corticosteroids. It has been suggested that the type of steroid, dose and method of delivery (which affects the bioavailability) may contribute to the relative effectiveness of the treatment. In addition, it is unclear whether patient characteristics will affect levels of effectiveness (i.e. whether they have polyps and whether they are adults or children). Another uncertainty is whether the duration of treatment is important. If heterogeneity in effects was indeed observed, we planned to explore these factors through subgroup analyses.
Why it is important to do this review
Intranasal corticosteroids are the mainstay and currently recommended treatment for chronic rhinosinusitis. This review incorporates an update of two previous Cochrane reviews (Kalish 2012; Snidvongs 2011). Unlike the previous reviews, this review focuses on the effects of intranasal corticosteroids when compared to no treatment or placebo, to establish their effectiveness in the treatment of chronic rhinosinusitis. The relative effects of different types, doses and methods of delivery are investigated in a separate review (Chong 2016a).
This review is one of a suite of reviews looking at management options for patients with chronic rhinosinusitis (Chong 2016a; Chong 2016b; Head 2016a; Head 2016b; Head 2016c), and we use the same outcome measures across the reviews. We have not included studies designed to evaluate interventions in the immediate peri‐surgical period, which are focused on assessing the impact of the intervention on the surgical procedure or on modifying the post‐surgical results (preventing relapse).
Objectives
To assess the effects of intranasal corticosteroids in people with chronic rhinosinusitis.
Methods
Criteria for considering studies for this review
Types of studies
We included studies with the following design characteristics:
randomised controlled trials, including cluster‐randomised trials and quasi‐randomised trials (cross‐over trials were only to be included if the data from the first phase were available); and
patients were followed up for at least two weeks.
We excluded studies with the following design characteristics:
randomised patients by side of nose (within‐patient controlled) because it is difficult to ensure that the effects of any of the interventions considered can be localised; or
perioperative studies, where the sole purpose of the study was to investigate the effect of intranasal corticosteroids on surgical outcome.
Types of participants
Patients with chronic rhinosinusitis, whether with or without polyps.
We excluded studies that included a majority of patients with:
cystic fibrosis;
allergic fungal sinusitis/eosinophilic fungal/mucinous rhinosinusitis;
aspirin‐exacerbated respiratory disease;
antrochoanal polyps (benign polyps originating from the mucosa of the maxillary sinus);
malignant polyps;
primary ciliary dyskinesia;
a history of surgery for nasal polyps within six weeks of entry to the study.
Types of interventions
All intranasal corticosteroids; this included nasal sprays and nasal drops.
First‐generation intranasal corticosteroids:
Beclomethasone dipropionate
Triamcinolone acetonide
Flunisolide
Budesonide
Second‐generation intranasal corticosteroids:
Ciclesonide
Fluticasone furoate
Fluticasone propionate
Mometasone furoate
Betamethasone sodium phospate
If other interventions were used, these should have been used in both treatment arms. Allowed co‐interventions included:
nasal saline irrigation;
antibiotics;
intermittent nasal decongestants.
The comparators were placebo or no intervention.
The main comparison pair was:
intranasal corticosteroids versus placebo or no intervention.
Other possible comparison pairs included:
intranasal corticosteroids plus co‐intervention A versus placebo plus co‐intervention A.
This review is part of a larger series of six reviews for the treatment of chronic rhinosinusitis.
Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis (this review).
Different types of intranasal steroids for chronic rhinosinusitis (Chong 2016a). This review compares different classes, doses and delivery methods of intranasal corticosteroids for chronic rhinosinusitis.
Short‐course oral steroids alone for chronic rhinosinusitis (Head 2016a). This review compares short‐course oral steroids alone with placebo or no intervention, or against other pharmacological interventions such as antibiotics or nasal saline irrigation.
Short‐course oral steroids as an adjunct therapy for chronic rhinosinusitis (Head 2016b). This review compares oral steroids where they have been used as add‐on therapy to other treatments for chronic rhinosinusitis (such as intranasal corticosteroids, antibiotics or saline solution).
Saline irrigation for chronic rhinosinusitis (Chong 2016b). This review compares nasal saline irrigation for chronic rhinosinusitis with both placebo/no intervention and with intranasal corticosteroids or antibiotics.
Systemic and topical antibiotics for chronic rhinosinusitis (Head 2016c). This review compares both topical and systemic antibiotics with placebo/no treatment, two different antibiotics with each other and antibiotics with intranasal corticosteroids.
Types of outcome measures
We analysed the following outcomes in the review, but we did not use them as a basis for including or excluding studies.
Primary outcomes
Health‐related quality of life, using disease‐specific health‐related quality of life scores, such as the Sino‐Nasal Outcome Test‐22 (SNOT‐22), Rhinosinusitis Outcome Measures‐31 (RSOM‐31) and SNOT‐20.
Disease severity, as measured by patient‐reported symptom score (such as the Chronic Sinusitis Survey (CSS) questionnaire and visual analogue scales).
Significant adverse effect: epistaxis.
Secondary outcomes
Health‐related quality of life, using generic quality of life scores, such as the SF‐36, EQ‐5D and other well‐validated instruments.
Other local adverse effects: local irritation (including oral thrush, sore throat and other local nasal irritation such as dryness, itchiness etc.).
-
Other systemic adverse effects:
in children ‐ stunted growth (minimum time point: six months of treatment and follow‐up);
in adults – osteoporosis.
Nasal endoscopic score (depending on population, either nasal polyps size score or endoscopy score, e.g. Lund‐Mackay/Lund‐Kennedy).
Computerised tomography (CT) scan score (e.g. Lund‐Mackay).
Outcomes were measured at three to six months, six to 12 months and more than 12 months. For adverse events, we analysed data from the longest time periods.
Search methods for identification of studies
The Cochrane ENT Information Specialist conducted systematic searches for randomised controlled trials and controlled clinical trials. There were no language, publication year or publication status restrictions. The date of the search was 11 August 2015.
Electronic searches
The Information Specialist searched:
the Cochrane Register of Studies ENT Trials Register (searched 11 August 2015);
the Cochrane Central Register of Controlled Trials (CENTRAL 2015, Issue 7);
-
Ovid MEDLINE (1946 to July week 5 2015);
Ovid MEDLINE (In‐Process & Other Non‐Indexed Citations) (searched 11 August 2015);
PubMed (as a top up to searches in Ovid MEDLINE) (searched 11 August 2015);
Ovid EMBASE (1974 to 2015 week 32);
ClinicalTrials.gov, www.clinicaltrials.gov (search via the Cochrane Register of Studies) (searched 11 August 2015);
World Health Organization (WHO) International Clinical Trials Registry Platform (ICTRP) (searched 11 August 2015);
Google Scholar (searched 11 August 2015).
The Information Specialist modelled subject strategies for databases on the search strategy designed for CENTRAL. Where appropriate, they were combined with subject strategy adaptations of the highly sensitive search strategy designed by Cochrane for identifying randomised controlled trials and controlled clinical trials (as described in the Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0, Box 6.4.b. (Handbook 2011). Search strategies for major databases including CENTRAL are provided in Appendix 1.
Searching other resources
We scanned the reference lists of identified publications for additional trials and contacted trial authors where necessary. In addition, the Information Specialist searched PubMed, The Cochrane Library and Google to retrieve existing systematic reviews relevant to this systematic review, so that we could scan their reference lists for additional trials.
Data collection and analysis
Selection of studies
At least two review authors independently screened all titles and abstracts of the studies obtained from the database searches to identify potentially relevant studies. At least two review authors evaluated the full text of each potentially relevant study to determine if it met the inclusion and exclusion criteria for this review.
We resolved any differences by discussion and consensus, with the involvement of a third author for clinical and/methodological input where necessary.
Data extraction and management
Two review authors independently extracted data from each study using a standardised data collection form (see Appendix 2). Whenever a study had more than one publication, we retrieved all publications to ensure complete extraction of data. Where there were discrepancies in the data extracted by different review authors, we checked these against the original reports and resolved differences by discussion and consensus, with the involvement of a third author or a methodologist where appropriate. We contacted the original study authors for clarification or for missing data whenever possible. If differences were found between publications of a study, we contacted the original authors for clarification. We used data from the main paper(s) if no further information was found.
We included key characteristics of the studies, such as study design, setting, sample size, population and how outcomes were defined or collected in the studies. In addition, we also collected baseline information on prognostic factors or effect modifiers. For this review, this included:
presence or absence of nasal polyps;
baseline polyp score (where appropriate);
whether the patient has had previous sinus surgery.
For the outcomes of interest to the review, we extracted the findings of the studies on an available case analysis basis; i.e. we included data from all patients available at the time points based on the treatment randomised whenever possible, irrespective of compliance or whether patients had received the treatment as planned.
In addition to extracting pre‐specified information about study characteristics and aspects of methodology relevant to risk of bias, we extracted the following summary statistics for each trial and each outcome:
For continuous data: the mean values, standard deviations and number of patients for each treatment group. Where endpoint data were not available, we extracted the values for change from baseline. We analysed data from measurement scales such as SNOT‐22 and EQ‐5D as continuous data.
For binary data: the numbers of participants experiencing an event and the number of patients assessed at the time point.
For ordinal scale data: if the data appeared to be approximately normally distributed or if the analysis that the investigators performed suggested parametric tests were appropriate, then we treated the outcome measures as continuous data. Alternatively, if data were available, we planned to convert into binary data.
We prespecified the time points of interest for the outcomes in this review. While studies may have reported data at multiple time points, we only extracted the longest available data within the time points of interest. For example, for 'short' follow‐up periods, our time point was defined as 'three to six months' post‐randomisation. If a study reported data at three, four and six months, we only extracted and analysed the data for the six‐month follow‐up.
Assessment of risk of bias in included studies
Two review authors independently assessed the risk of bias of each included study. We followed the guidance in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011) and we used the Cochrane 'Risk of bias' tool. With this tool we assessed the risk of bias as 'low', 'high' or 'unclear' for each of the following six domains:
sequence generation;
allocation concealment;
blinding of participants, personnel and outcome assessment;
incomplete outcome data;
selective reporting;
other sources of bias.
Measures of treatment effect
We summarised the effects of dichotomous outcomes (e.g. proportion of patients with symptom resolution) as risk ratios (RR) with CIs. For the key outcomes that we presented in the 'Summary of findings' table, we also expressed the results as absolute numbers based on the pooled results and compared to the assumed risk. We also planned to calculate the number needed to treat to benefit (NNTB) using the pooled results. The assumed baseline risk is typically either (a) the median of the risks of the control groups in the included studies, this being used to represent a 'medium risk population' or, alternatively, (b) the average risk of the control groups in the included studies is used as the 'study population' (Handbook 2011). If a large number of studies were available, and where appropriate, we also planned to present additional data based on the assumed baseline risk in (c) a low‐risk population and (d) a high‐risk population.
For continuous outcomes, we expressed treatment effects as a mean difference (MD) with standard deviation (SD) or as standardised mean difference (SMD) if different scales had been used to measure the same outcome. We provided a clinical interpretation of the SMD values.
Unit of analysis issues
This review did not use data from phase II of cross‐over studies or from studies where the patient was not the unit of randomisation, i.e. studies where the side (right versus left) was randomised.
If we had found cluster‐randomised trials, we would have analysed these according to the methods in section 16.3.3 of the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011).
Dealing with missing data
We tried to contact study authors via email whenever the outcome of interest was not reported, if the methods of the study suggested that the outcome had been measured. We did the same if not all data required for meta‐analysis had been reported, unless the missing data were standard deviations. If standard deviation data were not available, we approximated these using the standard estimation methods from P values, standard errors or 95% CIs if these are reported as detailed in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011). If it was impossible to estimate these, we contacted the study authors.
Apart from imputations for missing standard deviations, we conducted no other imputations. However, we carried out calculations relating to disease severity (measured by patient‐reported symptom scores) as most of the data measured individual symptoms rather than using validated instruments (see 'Imputing total symptom scores' below). We extracted and analysed data for all outcomes using the available case analysis method.
Imputing total symptom scores
Where a paper did not present information for the total disease severity in terms of patient‐reported symptom scores but did present data for the results of individual symptoms, we used the symptoms covering the important domains of the EPOS chronic rhinosinusitis diagnosis criteria (EPOS 2012) to calculate a total symptom score. The EPOS 2012 criteria for chronic rhinosinusitis require at least two symptoms. One of the symptoms must be either nasal blockage or nasal discharge; other symptoms can include facial pressure/pain, loss of sense of smell (for adults) or cough (for children). Where mean final values or changes from baseline were presented in the paper for the individual symptoms we summed these to calculate a 'total symptom score'. We calculated standard deviations for the total symptom score as if the symptoms were independent, random variables that were normally distributed. We acknowledge that there is likely to be a degree of correlation between the individual symptoms, however we used this process because the magnitude of correlation between the individual symptoms is not currently well understood (no evidence found). If the correlation is high, the summation of variables as discrete variables is likely to give a conservative estimate of the total variance of the summed final score. If the correlation is low, this method of calculation will underestimate the standard deviation of the total score. However, the average patient‐reported symptom scores have a correlation coefficient of about 0.5; if this is also applicable to chronic rhinosinusitis symptoms, the method used should have minimal impact (Balk 2012). As this method of calculation does not take into account weighting of different symptoms (no evidence found), we downgraded all the disease severity outcomes for lack of use of validated scales whenever this occurred.
Assessment of heterogeneity
We assessed clinical heterogeneity (which may be present even in the absence of statistical heterogeneity) by examining the included trials for potential differences between studies in the types of participants recruited, interventions or controls used and the outcomes measured.
We assessed statistical heterogeneity by visually inspecting the forest plots and by considering the Chi² test (with a significance level set at P value < 0.10) and the I² statistic, which calculates the percentage of variability that is due to heterogeneity rather than chance, with I² values over 50% suggesting substantial heterogeneity (Handbook 2011).
Assessment of reporting biases
We assessed reporting bias as between‐study publication bias and within‐study outcome reporting bias.
Outcome reporting bias (within‐study reporting bias)
We assessed within‐study reporting bias by comparing the outcomes reported in the published report against the study protocol, whenever this could be obtained. If the protocol was not available, we compared the outcomes reported to those listed in the methods section. If results are mentioned but not reported adequately in a way that allows analysis (e.g. the report only mentions whether the results were statistically significant or not), bias in a meta‐analysis is likely to occur. We sought further information from the study authors. If no further information could be found, we noted this as being a 'high' risk of bias. Quite often there was insufficient information to judge the risk of bias; we noted this as an 'unclear' risk of bias (Handbook 2011).
Publication bias (between‐study reporting bias)
We planned to assess funnel plots if sufficient trials (more than 10) were available for an outcome. If we had observed asymmetry of the funnel plot, we would have conducted more formal investigation using the methods proposed by Egger 1997.
Data synthesis
We conducted all meta‐analyses using Review Manager 5.3 (RevMan 2014). For dichotomous data, we planned to analyse treatment differences as a risk ratio (RR) calculated using the Mantel‐Haenszel methods. We analysed time‐to‐event data using the generic inverse variance method.
For continuous outcomes, if all the data were from the same scale, we planned to pool mean values obtained at follow‐up with change outcomes and report this as a MD. However, if the SMD had to be used as an effect measure, we did not plan to pool change and endpoint data.
When statistical heterogeneity is low, random‐effects versus fixed‐effect methods yield trivial differences in treatment effects. However, when statistical heterogeneity is high, the random‐effects method provides a more conservative estimate of the difference.
Subgroup analysis and investigation of heterogeneity
We planned to conduct some subgroup analyses regardless of whether statistical heterogeneity was observed, as these are widely suspected to be potential effect modifiers. For this review, this included:
phenotype of patients: whether patients have chronic rhinosinusitis without nasal polyps, chronic rhinosinusitis with nasal polyps, a mixed group or the status of polyps is not known or not reported. We planned to undertake the subgroup analysis because although there appears to be a considerable overlap between the two forms of chronic rhinosinusitis with regards to inflammatory profile, clinical presentation and effect of treatment (Cho 2012; DeMarcantonio 2011; Ebbens 2010; Fokkens 2007; Ragab 2004; Ragab 2010; van Drunen 2009), there is some evidence pointing to differences in the respective inflammatory profiles (Kern 2008; Keswani 2012; Tan 2011; Tomassen 2011; Zhang 2008; Zhang 2009), and potentially even differences in treatment outcome (Ebbens 2011).
We planned to present the main analyses of this review according to the subgroups of phenotypes of chronic rhinosinusitis in forest plots and all other subgroup analysis results in tables. When studies had a mixed group of patients, we analysed the study as one of the subgroups (rather than as a mixed group) if more than 80% of patients belonged to one category. For example, if 81% of patients had chronic rhinosinusitis without nasal polyps, we analysed the study as that subgroup.
In addition to polyps status, we also planned to conduct the following subgroup analyses in the presence of statistical heterogeneity:
patient age (children versus adults);
dose;
duration of treatment;
method of delivery.
Sensitivity analysis
We planned to carry out sensitivity analyses to determine whether the findings were robust to the decisions made in the course of identifying, screening and analysing the trials. We planned to conduct sensitivity analysis for the following factors, whenever possible:
impact of model chosen: fixed‐effect versus random‐effects model;
risk of bias of included studies: excluding studies with high risk of bias (we defined these as studies that had a high risk of allocation concealment bias and a high risk of attrition bias (overall loss to follow‐up of 20%, differential follow‐up observed);
how outcomes were measured: we planned to investigate the impact of including data where the validity of the measurement is unclear.
If any of these investigations found a difference in the size of the effect or heterogeneity, we would mention this in the Effects of interventions section.
GRADE and 'Summary of findings' table
We used the GRADE approach to rate the overall quality of evidence for each outcome using the GDT tool (http://www.guidelinedevelopment.org/) for the main comparison pairs listed in the Types of interventions section. The quality of evidence reflects the extent to which we are confident that an estimate of effect is correct and we applied this in the interpretation of results. There are four possible ratings: 'high', 'moderate', 'low' and 'very low'. A rating of 'high' quality evidence implies that we are confident in our estimate of effect and that further research is very unlikely to change our confidence in the estimate of effect. A rating of 'very low' quality implies that any estimate of effect obtained is very uncertain.
The GRADE approach rates evidence from RCTs that do not have serious limitations as high quality. However, several factors can lead to the downgrading of the evidence to moderate, low or very low. The degree of downgrading is determined by the seriousness of these factors:
study limitations (risk of bias);
inconsistency;
indirectness of evidence;
imprecision;
publication bias.
The 'Summary of findings' table presents only the seven top priority outcomes (disease‐specific health‐related quality of life, disease severity score, adverse effects and generic quality of life score). We did not include the outcomes of endoscopic score and CT scan score in the 'Summary of findings' table.
Results
Description of studies
Results of the search
The searches retrieved a total of 2470 references after removal of duplicates. We identified one additional reference from other sources. We screened the titles and abstracts and subsequently removed 2297 references. We assessed 87 full texts for eligibility. We excluded 45 studies (55 references), with reasons. We included 18 studies (24 references). We identified three ongoing studies and there are five studies awaiting assessment because we cannot locate the full‐text papers.
A flow chart of study retrieval and selection is provided in Figure 1.
1.
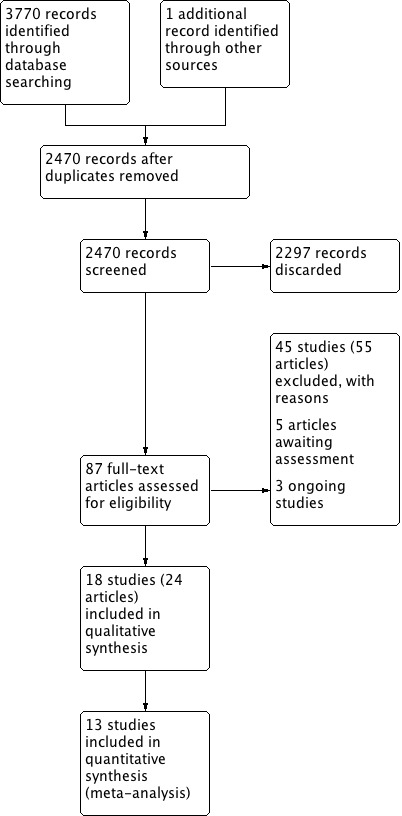
Process for sifting search results and selecting studies for inclusion.
Included studies
Design
All studies included studies were double‐blinded randomised controlled trials. All but three studies (Holmberg 1997; Lang 1983; Lund 2004) had a treatment and follow‐up duration of between 12 to 16 weeks. Holmberg 1997 and Lund 2004 had a treatment duration of 20 and 26 weeks respectively.
Some of these studies had multiple treatment arms: four compared different doses of intranasal corticosteroids against placebo (Chur 2013; Penttilla 2000; Small 2005; Stjarne 2006), whereas Johansen 1993 compared two different types of delivery methods (nasal spray versus nasal drops) against placebo.
Setting
Most studies were published more than 10 years ago (Aukema 2005; Holmberg 1997; Holopainen 1982; Johansen 1993; Keith 2000; Lang 1983; Lund 1998; Lund 2004; Parikh 2001; Penttilla 2000; Small 2005). Most of these were conducted in tertiary referral centres in northern Europe, the US and Canada. Chur 2013, Lund 2004, Small 2005 and Stjarne 2006 included other centres around the world, while Zhou 2015 was conducted in China.
Population and sample size
One study recruited 127 children aged 6 to 17 (Chur 2013); the rest were conducted in adults. Most studies were relatively small, with as few as 9 or 10 patients per intervention arm. The largest study was Zhou 2015, with 748 patients in total.
The populations included in the studies with regards to phenotypes of chronic rhinosinusitis were:
with polyps: Aukema 2005; Chur 2013; Holmberg 1997; Johansen 1993; Keith 2000; Lang 1983; Lund 1998; Penttilla 2000; Small 2005; Stjarne 2006; Stjarne 2006a; Vlckova 2009; Holopainen 1982; Zhou 2015;
without polyps: Hansen 2010; Lund 2004; Mosges 2011; Parikh 2001.
Intervention and comparison
The following types of intranasal corticosteroids were used:
Fluticasone propionate: 400 µg per day given as nasal drops in Aukema 2005, Keith 2000 and Penttilla 2000, nasal spray in Holmberg 1997, Lund 1998 and Parikh 2001, and as a 800 µg/day dose using a breadth actuated inhaler in Hansen 2010 and Vlckova 2009. Penttilla 2000 also had a higher‐dose treatment arm of 800 µg per day.
Beclomethasone propionate: 400 µg per day delivered as nasal sprays in Holmberg 1997 and Lund 1998. Lang 1983 used nasal drops at 800 µg per day.
Mometasone furoate nasal spray, given as 200 µg once daily in Small 2005, Stjarne 2006 and Stjarne 2006a. 400 µg per day was used in Mosges 2011, Zhou 2015 and in the higher‐dose arm of Stjarne 2006. Chur 2013 used either 100 µg a day for children aged 6 to 11 or 200 µg per day for children aged 12 to 17. It also had a group using a higher dose, doubling the doses to 200 µg and 400 µg per day respectively.
Budesonide nasal spray was used in Johansen 1993, Holopainen 1982 (400 µg per day) and Lund 2004 (128 µg per day).
Outcomes
Health‐related quality of life, using disease‐specific health‐related quality of life scores
One study used the Rhinosinusitis Outcome Measure (RSOM‐31) (Hansen 2010). Stjarne 2006a reported that scores for quality of life were recorded at every study visit using an investigator‐administered scale with the following items: "nose breathing", "experience of smell and taste", "interference with daily activities caused by nasal symptoms" and "sleep disturbance". It is unlikely that this is a validated quality of life instrument.
Disease severity, as measured by patient‐reported symptom score
All studies but one (Lang 1983) measured disease severity as reported by patients, but only Lund 2004 used a validated scale (Chronic Sinusitis Survey ‐ CSS). However, Lund 2004 reported that at the time of the study, a validated version of the Hungarian questionnaire was not available (the study was conducted in 19 centres, six of which were in Hungary).
Most studies presented the results (partially) in graphs as median or mean values. The method of measurement was reported in several ways and differed in the choice of scale, type/combination of symptoms measured, timing of measures and scoring/analysis method (see Appendix 3).
Due to these variations, it was unclear whether the total scores between studies were comparable with each other and we had to make modifications in order to standardise scores before meta‐analysis could be conducted (see Methods and Potential biases in the review process).
Significant adverse effect: epistaxis
All but five studies reported the number of patients with epistaxis and these could be meta‐analysed (Holmberg 1997; Johansen 1993; Lang 1983; Lund 1998; Parikh 2001). Holmberg 1997 and Johansen 1993 did not provide enough information about the types of adverse effects experienced and how many patients experienced them, whereas the other three studies were very small (fewer than 15 patients per treatment arm) and it was unclear whether epistaxis was not experienced by any patients or not reported.
The studies included nosebleeds of all severities, including mild.
Health‐related quality of life, using generic quality of life scores, such as the SF‐36, EQ‐5D and other well‐validated instruments
Lund 2004 used the SF‐36, but only reported for which subscale a statistically significant difference was found.
Other local adverse effects: local irritation (including oral thrush, sore throat and other local nasal irritation such as dryness, itchiness etc.)
A variety of types of possible local irritation symptoms were reported in the studies, ranging from "itchiness" to "nasal burns" and "nasopharyngeal pain/discomfort". Some studies used broader classifications whereas others used more specific description. For example, Vlckova 2009 reported an unusual combination of "adverse events", listing "rhinalgia" and "nasal discomfort" separately alongside headache; they also reported "rhinitis" as an adverse event alongside "sneezing" and "rhinorrhoea".
Other systemic adverse effects (in children ‐ stunted growth, in adults ‐ osteoporosis)
Only one study followed up patients in the longer term (Lang 1983), and this adverse effect was not reported or relevant in the other studies.
Nasal endoscopic score (either nasal polyps size score or endoscopy score, e.g. Lund‐Kennedy)
Lund 1998 and Stjarne 2006a did not report any endoscopy results.
Studies that recruited patients with nasal polyps mostly used a 0‐ to 3‐point scale. In most studies, this was the Lildholdt scale, but the definitions were not reported clearly in the other studies. Whenever studies reported these as mean values at endpoint or change from baseline, they summed the scores from both sides of the nose (0 to 6 range). However, no definitions were given in the studies that reported the results as proportions of people who had improved.
One study used a 0 to 4 scale (Holmberg 1997), after some modification to the Lildholdt scale. The investigators in Aukema 2005 scored the volume of polyps on a 0 to 10 cm visual analogue scale after conducting endoscopy.
Studies that included patients without polyps used the Lund‐Kennedy scale (Hansen 2010; Mosges 2011; Parikh 2001). It was unclear whether Lund 2004 used endoscopy scores as an outcome ‐ this was not reported.
Computerised tomography (CT) scan score (e.g. Lund‐Mackay)
Only one study reported a CT scan score (Aukema 2005). The Lund‐Mackay score was used (0 to 24 points, higher score = more severe).
Excluded studies
We excluded 55 papers (45 studies) after reviewing the full text. Further details of the reasons for exclusion can be found in the Characteristics of excluded studies table.
We excluded 23 studies due to the population, most of these (19 studies) because all patients received surgery at the start of the trial and the intranasal steroids were used to try to prevent recurrence of polyps (Bross‐Soriano 2004; Cassano 1996; Dijkstra 2004; Drettner 1982; el Naggar 1995; Gulati 2001; Hartwig 1988; Jorissen 2009; Jurkiewicz 2004; Kang 2008; Karlsson 1982; Malmberg 1988; Passali 2003; Rotenberg 2011; Rowe‐Jones 2005; Slifirski 2009; Stjarne 2009; Vento 2012; Virolainen 1980). Other reasons for excluding studies based on the population were related to patients not meeting the current criteria for chronic rhinosinusitis (ALA 2015), chronic allergic or bacterial sinusitis where less than 50% of patients had chronic disease (Cuenant 1986), aspirin‐induced asthma and chronic eosinophilic rhinitis (Mastalerz 1997), and recurrent or chronic maxillary sinusitis (Qvarnberg 1992). We excluded three studies because the studies were designed to look at the impact of intranasal corticosteroids on outcomes when given perioperatively: all patients underwent surgery during the period of the trial (Albu 2010; Ehnhage 2009; Saunders 1999). Two studies compared steroids with placebo or no treatment but we excluded them as they used a catheter or tube to administer steroids directly to the participant's sinuses (Furukido 2005; Lavigne 2002). One study was a clinical trial that appeared to meet the inclusion criteria but the clinical trials registry website stated that the trial had been terminated early (Optinose 2012). The reason for early termination is not provided.
We excluded the remaining 16 papers as they did not meet the minimum requirements for the duration of treatment and follow‐up. Ten of these studies had a follow‐up time of one month or less (Chalton 1985; Johansson 2002; Kapucu 2012; Keith 1995; Lildholdt 1995; Mygind 1975; Ruhno 1990; Taub 1968; Toft 1982; Wang 2015), whereas the remaining six treated and followed up patients for between six and eight weeks (Filiaci 2000; Jankowski 2001; Jankowski 2009; Man 2013; Meltzer 1993; Tos 1998).
Ongoing studies
We identified three relevant ongoing studies, all of which are in adults with chronic rhinosinusitis with nasal polyps (NCT01622569; NCT01624662; NCT01013701). Two of these are large, multicentre trials each with a planned population of over 300 patients (NCT01622569; NCT01624662). These two trials will make the same comparisons, comparing three different doses of fluticasone proportionate (400 µg bid, 200 µg bid and 100 µg bid) with placebo. All of the arms will use a novel bi‐directional device. The studies were completed in October 2015 but no study data were available at the time of writing this review. The other trial compares fluticasone furoate with placebo for 16 weeks (NCT01013701). The trial information was registered in 2009 and the ClinicalTrials.gov website reports that the recruitment status of the trial is unknown as the information has not been recently validated. We attempted to contact the investigators but we did not receive a response.
Risk of bias in included studies
We included 18 studies in this review. Nine of these had low risk of bias for both selection and blinding (Keith 2000; Lund 2004; Mosges 2011; Parikh 2001; Penttilla 2000; Small 2005; Stjarne 2006; Stjarne 2006a; Zhou 2015). Lang 1983 was only available as an abstract and therefore there was insufficient information to judge the risk of bias for most domains. We did most of the ratings based solely on the study report(s), as the trials were not registered and no protocols were available.
See Figure 2 for the 'Risk of bias' graph (our judgements about each risk of bias item presented as percentages across all included studies) and Figure 3 for the 'Risk of bias' summary (our judgements about each risk of bias item for each included study).
2.
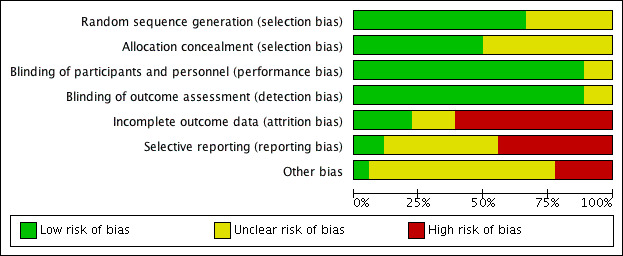
'Risk of bias' graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
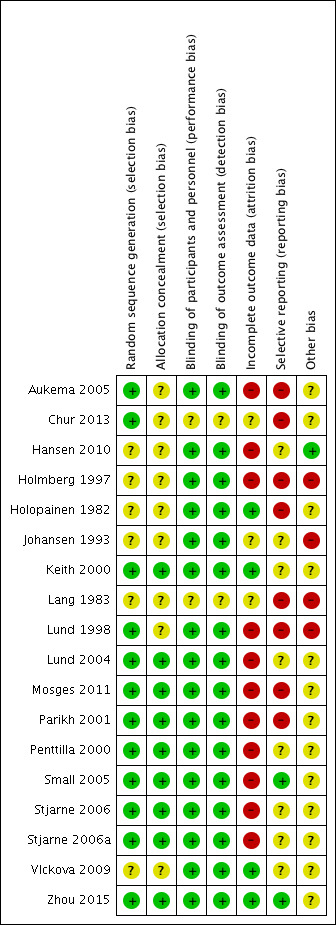
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Sequence generation
Six of the studies only stated that the trials were randomised but did not provide further information about how sequence generation was conducted. We therefore judged them to be at an unclear risk of bias (Hansen 2010; Holmberg 1997; Holopainen 1982; Johansen 1993; Lang 1983; Vlckova 2009).
Another two studies also did not provide details of their randomisation procedures, but we judged this to be a low risk of bias because these studies were conducted quite recently as multinational trials and therefore should have used adequate methodology to ensure adequate sequence generation (Penttilla 2000; Small 2005). The rest of the studies either specified that the randomisation was conducted by another unit supporting clinical trials (the pharmacy for Parikh 2001) or provided a clear description of how computerised sequence generation was used.
Allocation concealment
We also rated nine studies as low risk of bias for allocation concealment (Keith 2000; Lund 2004; Mosges 2011; Parikh 2001; Penttilla 2000; Small 2005; Stjarne 2006; Stjarne 2006a; Zhou 2015). As for sequence generation, we considered the large multinational RCTs to have adequate methodology although they did not provide specific information about the allocation concealment method (Penttilla 2000; Small 2005; Stjarne 2006a). An exception to this is Chur 2013, which we rated as unclear risk of bias for allocation concealment. Despite the adequate methods used to generate a random sequence, blocked randomisation was used and the effectiveness of the blinding was unclear in the absence of a 'double‐dummy' design in this multi‐arm trial.
We rated the other studies as unclear risk of bias due to lack of information.
Blinding
The ratings for the risk of performance bias versus detection bias were closely related for this review. Most of the outcomes were assessed by the patients and the overall risks of bias were low when both participants and investigators were adequately blinded. We did not find information to suggest that the clinicians could have obtained extra information from blood tests etc. to allow them to 'guess' which treatment the patients were allocated. We considered the majority (16) of the studies to be at low risk of bias for blinding. We considered two studies to be at unclear risk: Chur 2013 did not use a double‐dummy design to mask the regimens and there was no information in Lang 1983.
Incomplete outcome data
We considered only four trials to have a low risk of attrition bias: Holopainen 1982; Keith 2000; Vlckova 2009 and Zhou 2015.
Eleven studies had a high risk of attrition bias due to unbalanced drop‐out rates between the placebo and treatment groups, with higher drop‐out rates in the placebo groups (Aukema 2005; Hansen 2010; Penttilla 2000, Small 2005; Stjarne 2006; Stjarne 2006a), an overall high rate of drop‐outs (Lund 2004), or a combination of these factors (Holmberg 1997; Lund 1998; Parikh 2001). In Mosges 2011, only 10% of patients did not complete the full study, but it was unclear why the study only included 75% of the sample in intention‐to‐treat (ITT) analysis due to "major protocol violation" and 61% in the per protocol population due to "minor protocol violation".
The risk of attrition bias was unclear for three of the included studies (Chur 2013; Johansen 1993; Lang 1983). These studies did not provide enough information to adequately judge the risk. For example, Johansen 1993 reported that 5/91 (5.5%) participants did not complete the study. There was no information on how many were randomised to each group in Johansen 1993 and so it is difficult to determine whether this could have affected the results.
Selective reporting
Many of the study reports presented effectiveness outcomes only in graphs and only provided limited, selective information, for example P values or mean values only when statistical significance was noted.
We considered only two studies to have low risk of bias, with all expected outcomes reported (Small 2005; Zhou 2015).
We considered the risk of selective reporting to be high in eight studies (Aukema 2005; Chur 2013; Holmberg 1997; Holopainen 1982; Lang 1983; Lund 1998; Mosges 2011; Parikh 2001). These studies either presented outcomes that were not pre‐specified in the methods section or downplayed and provided insufficient information about prespecified outcomes. Others used an unclear or arbitrary method to combine data or report some of the outcomes (or both).
The primary endpoint in Chur 2013 was "safety" (cortisol levels) and despite presenting the mean change values for effectiveness outcomes, they did not provide any P values or standard deviations. The study's rationale for collecting the data and not fully reporting them was: "No statistical analysis of efficacy end points was pre‐specified in the study protocol, and only descriptive efficacy statistics were collected." We observed that these values (mean changes) were similar between groups and unlikely to be statistically significant and so poor reporting due to lack of beneficial effects cannot be ruled out.
We considered the remaining eight studies to be at unclear risk. There was not enough information in the methods and/or protocol and we found it difficult to judge whether there was a risk of reporting bias.
Other potential sources of bias
Use of validated outcome measures
The lack of use of validated outcome measures is a major bias concern in this review. If an instrument is insensitive to measuring differences, this biases towards a finding of 'no difference' in the studies and also in this review.
None of the included studies mentioned using validated outcome measures, for either of the primary effectiveness outcomes (disease‐specific health‐related quality of life and disease severity/symptom scores).
Almost all studies attempted to measure change in symptom scores as measured by patients, but none reported validation of the instruments being used. Most studies used a 0 to 3 scale in the "diaries", but used different methods to calculate the result (period of time, combination of scores). There is no evidence that a 0‐ to 3‐point scale, especially when used as a single scale, has the sensitivity to distinguish between groups of patients who improved versus those who did not (discriminant validity) or whether the different method of scoring was valid.
The scales used to measure nasal polyps were generally well described. However, it is again unclear whether a 0‐ to 3‐point scale has the discriminant validity to detect a difference in the small trials seen.
Effects of interventions
See: Table 1
Where the range of scales and values for minimal important differences were unclear, we used the standardised mean difference (SMD) as a guide to estimate the effect sizes. As suggested in the Cochrane Handbook for Systematic Reviews of Interventions (Handbook 2011), we used standard rules of thumb in the interpretation of effect sizes (SMD, or Cohen's effect size of < 0.41 = small, 0.40 to 0.70 = moderate, > 0.70 = large) (Cohen 1988). Established scales such as the SF‐12 may have other rules of thumb to estimate the minimal important difference (MID = 0.5 SMD) and we use those to guide our interpretation whenever available (Jaeschke 1989; Revicki 2008).
Although we had planned to present data for patients with our without polyps in subgroups as a visual comparison, this was not necessary for the effectiveness outcomes because no more than one study contributed to the analysis, with the exception of polyps score data.
Intranasal corticosteroids compared to placebo or no intervention
Three of the 18 studies did not contribute any data for meta‐analysis (Holmberg 1997; Johansen 1993; Parikh 2001). In our protocol, we had specified that results from studies of participants with chronic rhinosinusitis with nasal polyps and without nasal polyps would be presented as subgroups in the forest plots (Chong 2015). However, this was only possible for the outcomes of epistaxis and local irritation. Of the four studies in patients with chronic rhinosinusitis without nasal polyps (Hansen 2010; Lund 2004; Mosges 2011; Parikh 2001), only Lund 2004 contributed data for the effectiveness outcomes (disease severity).
Health‐related quality of life, using disease‐specific health‐related quality of life scores
Hansen 2010 (chronic rhinosinusitis without nasal polyps) was the only study reporting the used of a disease‐specific health‐related quality of life questionnaire, the Rhinosinusitis Outcome Measures‐31 (RSOM‐31). The median change from baseline (median 178 points) was ‐62 points for the intranasal corticosteroids group (n = 10) and ‐5 points for the placebo group (n = 10, from a median baseline score of 187 points). The difference was not statistically significant (Mann‐Whitney U test).
Disease severity, as measured by patient‐reported symptom score (such as the Chronic Sinusitis Survey (CSS) questionnaire and visual analogue scales)
Chronic Sinusitis Survey
Lund 2004 (chronic rhinosinusitis without nasal polyps) reported that at the time of the study, a validated version of the Hungarian questionnaire was not available (the study was conducted in 19 centres, six of which were in Hungary). Only the mean change and 95% confidence interval (CI) for each group was reported, without a sample size. We imputed the sample size based on the number of patients available at the end of the study and it seems that all available patients had filled in the CSS. The mean difference (MD) was 2.84 points (95% CI ‐5.02 to 10.70; 134 participants) between groups (range 0 to 100, higher values = better) (Analysis 1.1). The magnitude of the difference is insignificant.
1.1. Analysis.
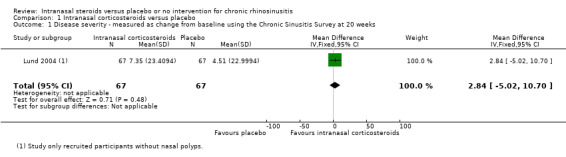
Comparison 1 Intranasal corticosteroids versus placebo, Outcome 1 Disease severity ‐ measured as change from baseline using the Chronic Sinusitis Survey at 20 weeks.
Global rating scale
Vlckova 2009 used a five‐point global rating scale (very much improved, improved, same, worse or very much worse). At 12 weeks, 76% of patients in the treatment group were "improved" or "very much improved" compared to 27% of patients in the placebo group (risk ratio (RR) 2.78, 95% CI 1.76 to 4.40; 109 participants; one study) (Analysis 1.2).
1.2. Analysis.
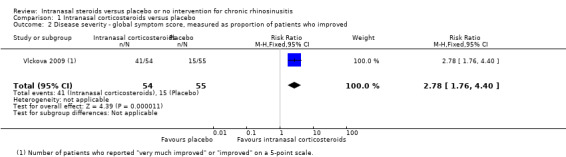
Comparison 1 Intranasal corticosteroids versus placebo, Outcome 2 Disease severity ‐ global symptom score, measured as proportion of patients who improved.
Combined symptom scores
Six studies provided information on mean and standard deviation values, or had enough information in their graphs for us to estimate these values for various symptom scores (Aukema 2005; Lund 2004; Small 2005; Stjarne 2006; Vlckova 2009; Zhou 2015). Of these, only Vlckova 2009 and Lund 2004 (chronic rhinosinusitis without nasal polyps) reported an overall combined score for all four groups of symptoms covered by EPOS 2012 (nasal blockage, nasal discomfort (facial and sinus pain/pressure) and rhinitis symptoms (nasal secretion, itching, irritation and sneezing), and loss of sense of smell). Parikh 2001 also included these symptoms in their diary card and symptom scores, but it is less clear how these were calculated. Although the means and standard deviations were reported for this study, we did not include it in the meta‐analysis because the values were obviously skewed (mean of 29.4 (standard deviation (SD) 37) for intranasal corticosteroids group (n = 9), mean of 16.9 (SD 48.5) (n = 13) for placebo group, P value = 0.39 using Mann Whitney U test).
Therefore we only included six studies in our analysis. We report the average values when all four types of symptoms mentioned in EPOS were measured versus when only three types (loss of sense of smell, nasal blockage and nasal discharge) and two types of symptoms (nasal blockage and nasal discharge) were measured. All studies used 0‐ to 3‐point scales in their diaries, except for Aukema 2005, which used a 0 to 100 visual analogue scale (VAS) measured during follow‐up. All studies reported change from baseline, except for Aukema 2005, which reported the mean difference at the end of the study. To allow for ease of interpretation, we converted these 0 to 100 VAS scores into 0 to 3 by a division of 33.333. All studies used the 'usual dose' of intranasal steroids, except for Vlckova 2009, which only used a higher dose of fluticasone propionate (800 µg/day), delivered in two divided doses. Two studies, Small 2005 and Stjarne 2006, had two treatment arms using mometasone furoate nasal spray with low (200 µg/day) and high (400 µg/day) doses. Only one study included patients without nasal polyps (Lund 2004). All studies were conducted in adults.
The pooled results are as follows:
Combined symptom score for four EPOS domains, average score: MD ‐0.26 (95% CI ‐0.37 to ‐0.15; 243 participants; two studies; I2 = 46%), scale range: 0 to 3, lower = better, indicating less severe symptoms in the intranasal corticosteroids group (Analysis 1.3).
Combined symptom score for three EPOS domains (nasal blockage, rhinorrhoea and loss of sense of smell), average score: MD ‐0.31 (95% CI ‐0.38 to ‐0.23; 1345 participants; four studies; I2 = 0%), scale range: 0 to 3, lower = better (Analysis 1.3).
Combined symptom score for two EPOS domains (only nasal blockage and rhinorrhoea), average score: MD ‐0.31 (95% CI ‐0.38 to ‐0.24; 1702 participants; six studies; I2 = 0%), scale range: 0 to 3, lower = better (Analysis 1.3).
1.3. Analysis.
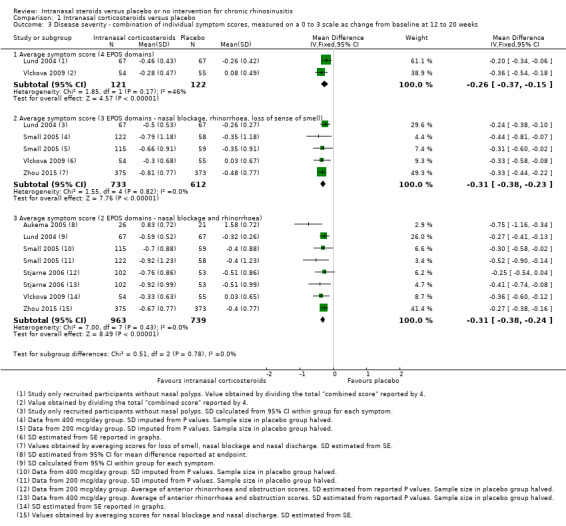
Comparison 1 Intranasal corticosteroids versus placebo, Outcome 3 Disease severity ‐ combination of individual symptom scores, measured on a 0 to 3 scale as change from baseline at 12 to 20 weeks.
The observed mean differences correspond to a moderate effect size (the SMD was about 0.4 for the two and three domain average symptom scores and 0.6 for the four domain average symptom score). The quality of the evidence is low for the four domain scores and moderate for the three and two domains scores (facial pain/pressure not considered), with the main concerns being the use of non‐validated symptom scores and a high risk of reporting bias (many studies did not publish the results in detail and this could be linked to a lack of observed efficacy).
Individual symptom scores
Nasal blockage: MD ‐0.40 (95% CI ‐0.52 to ‐0.29; 1702 participants; six studies; I2 = 47%) (Analysis 1.4).
Rhinorrhoea: MD ‐0.25 (95% CI ‐0.33 to ‐0.17; 1702 participants; six studies; I2 = 6%) (Analysis 1.4).
Loss of sense of smell: MD ‐0.19 (95% CI ‐0.28 to ‐0.11; 1345 participants; four studies; I2 = 0%) (Analysis 1.4).
Facial pain/pressure: MD ‐0.27 (95% CI ‐0.56 to 0.02; 243 participants; two studies; I2 = 78%). Of these two studies, Lund 2004 included patients with chronic rhinosinusitis without nasal polyps, whereas Vlckova 2009 included patients with chronic rhinosinusitis with nasal polyps. Due to the differences in type, dose and delivery method (fluticasone propionate 800 µg per day using breadth actuated inhaler versus budesonide 128 µg per day as a nasal spray), the source of heterogeneity was unclear.
1.4. Analysis.
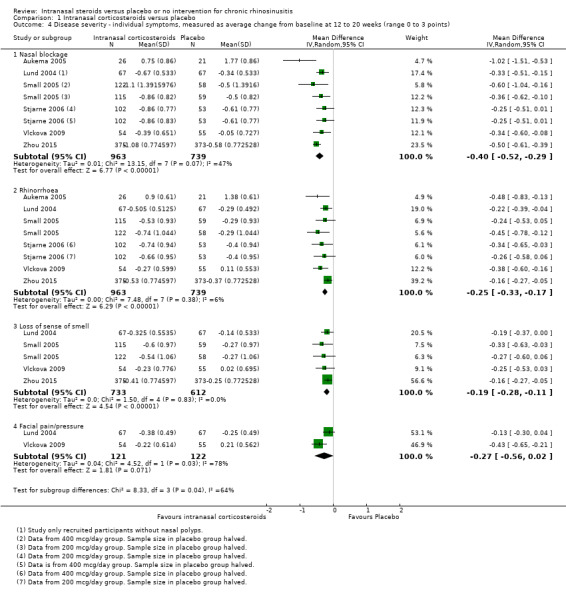
Comparison 1 Intranasal corticosteroids versus placebo, Outcome 4 Disease severity ‐ individual symptoms, measured as average change from baseline at 12 to 20 weeks (range 0 to 3 points).
We used a random‐effects model to conduct the analysis; if a fixed‐effect model is used the statistical significance of the pooled MD for facial pain is ‐0.24 (95% CI ‐0.37 to ‐0.11). The quality of the evidence is moderate for nasal blockage, rhinorrhoea and loss of sense of smell, but low for facial pain/pressure.
Significant adverse effect: epistaxis
The risk of epistaxis was higher in the intranasal corticosteroids group (RR 2.74, 95% CI 1.88 to 4.00; 2508 participants; 13 studies; I2 = 0%) (Analysis 1.5). The quality of the evidence is high.
1.5. Analysis.
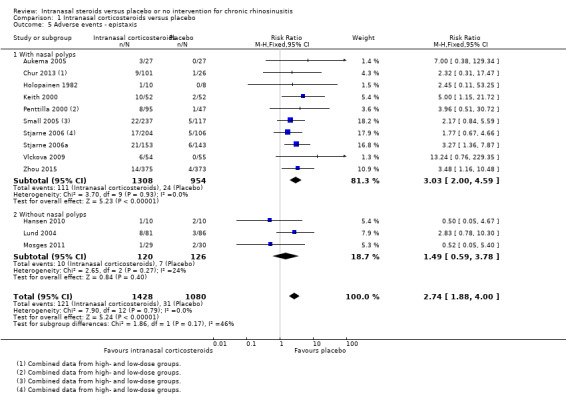
Comparison 1 Intranasal corticosteroids versus placebo, Outcome 5 Adverse events ‐ epistaxis.
Health‐related quality of life, using generic quality of life scores, such as the SF‐36, EQ‐5D and other well‐validated instruments
Lund 2004 used the SF‐36, but only stated that there was a statistically significant improvement in the general health subscale in patients on intranasal corticosteroids compared to placebo. Apart from stating that no other significant differences were observed, no other details were reported.
Other local adverse effects: local irritation (including oral thrush, sore throat and other local nasal irritation such as dryness, itchiness etc.)
It is unclear whether there is an important difference in the risk of local irritation between participants taking intranasal corticosteroids or placebo (RR 0.94, 95% CI 0.53 to 1.64; 2124 participants; 11 studies; I2 = 0%) (Analysis 1.6). The quality of the evidence is low (we are uncertain about this estimate), because the reporting of local irritation effects varied a lot between studies. This was sometimes finely split into many types of local irritation and we could only use the numbers for the most commonly reported types of irritation to avoid double counting in this review. The actual event rate for all types of local irritation is higher than reported in this analysis.
1.6. Analysis.
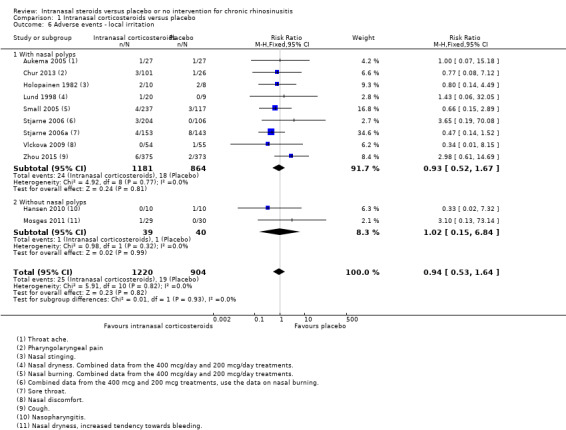
Comparison 1 Intranasal corticosteroids versus placebo, Outcome 6 Adverse events ‐ local irritation.
Other systemic adverse effects (in children ‐ stunted growth, in adults ‐ osteoporosis)
None of the studies treated or followed up patients for long enough to report these adverse events.
Nasal endoscopic score (depending on population, either nasal polyps size score or endoscopy score, e.g. Lund‐Mackay/Lund‐Kennedy)
Three studies reported polyps score results as the mean change from baseline on a 0‐ to 6‐point scale. Five studies reported this as the proportion of patients who had an improvement. Vlckova 2009 reported both the mean improvement and the proportion of patients with an improvement. Aukema 2005 measured polyps size on a 0 to 10 cm visual analogue scale and seems to have reported the values at the end of the study. We did not include this in the analysis as it was unclear what the scale was and whether it was valid. The MD was ‐24.70 (95% CI ‐48.00 to ‐1.40, n = 47) and we observed heterogeneity when it was combined with the other studies.
Chronic rhinosinusitis with nasal polyps ‐ reduction in polyps size
The MD in polyps score was ‐0.58 (95% CI ‐0.90 to ‐0.26; 1417 participants; four studies; I2 = 83%) indicating less severity for the intranasal corticosteroids group (Analysis 1.7). All reported the sum of polyps score from both sides of the nose (range 0 to 6). One study, Vlckova 2009, had an effect that was larger than the other studies, with a mean difference of ‐1.21 (95% CI ‐1.56 to ‐0.86) points between treatment arms in the reduction of polyps size score. When this study is removed, the heterogeneity is resolved and the observed effect size is smaller (MD ‐0.35, 95% CI ‐0.47 to ‐0.24; 1308 participants; three studies; I2 = 0%).
1.7. Analysis.
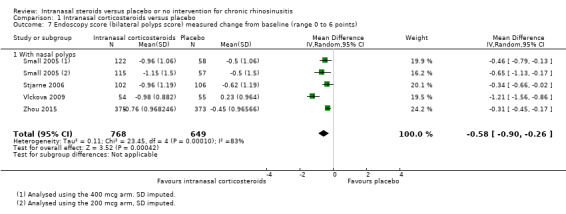
Comparison 1 Intranasal corticosteroids versus placebo, Outcome 7 Endoscopy score (bilateral polyps score) measured change from baseline (range 0 to 6 points).
Five studies reported the proportion of participants who had an improvement in their polyps scores. The chance of an improvement was higher in patients on intranasal corticosteroids (RR 1.77, 95% CI 1.06 to 2.95; 676 participants; five studies; I2 = 66%) (Analysis 1.8). The observed heterogeneity was resolved when we removed a study with an outlier effect (Vlckova 2009), but the RR became slightly smaller (RR 1.46, 95% CI 1.12 to 1.90; 567 participants; four studies; I2 = 0%).
1.8. Analysis.
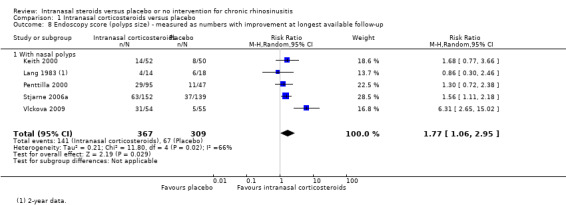
Comparison 1 Intranasal corticosteroids versus placebo, Outcome 8 Endoscopy score (polyps size) ‐ measured as numbers with improvement at longest available follow‐up.
Using a method recommended in the Cochrane Handbook for Systematic Reviews of Interventions, we converted the continuous outcome data into proportions and analysed them together using the generic inverse variance method. For Vlckova 2009, which reported both the mean difference and proportions, we used the values reported for proportions to avoid double counting and to minimise imputations. The overall pooled odds ratio (OR) was 2.07 (95% CI 1.48 to 2.91; 1984 participants; eight studies) (Analysis 1.9). The observed heterogeneity was quite substantial (> 50%). We observed that Vlckova 2009 seemed to have a larger effect than the other studies. If this study is removed from the analysis, the OR is slightly lower (OR 1.71, 95% CI 1.43 to 2.04; 1875 participants; seven studies) and heterogeneity is no longer observed.
1.9. Analysis.
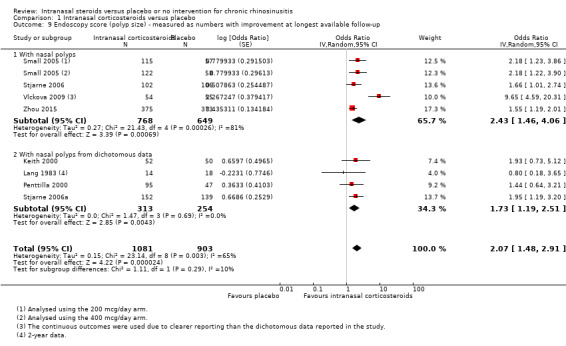
Comparison 1 Intranasal corticosteroids versus placebo, Outcome 9 Endoscopy score (polyp size) ‐ measured as numbers with improvement at longest available follow‐up.
Chronic rhinosinusitis without nasal polyps ‐ endoscopy score
The studies that included patients without polyps, such as Hansen 2010, Mosges 2011 and Parikh 2001, used a modified Lund‐Kennedy score. However, the results were either only partially reported or could not be meta‐analysed due to highly skewed distribution (very small sample sizes). Lund 2004 did not report the endoscopy score as an outcome and it was unclear whether this was measured.
Parikh 2001 reported the percentage of change and standard deviation. This was ‐22.3% (SD 61.8) (n = 9) for the intranasal corticosteroids group and +19.9% (SD 58.3) (n = 13). This was not statistically significant (Mann Whitney U test). Mosges 2011 showed the total endoscopy score for redness, oedema and discharge on a chart. There were no statistically significant differences between groups. Similarly, Hansen 2010 reported no statistically significant differences between groups for different aspects of the assessment, except for oedema.
Computerised tomography (CT) scan score (e.g. Lund‐Mackay)
Only one study reported the CT scan score (Aukema 2005). The MD was ‐4.82 (95% CI ‐7.27 to ‐2.37; 47 participants; one study; I2 = 0%) (Analysis 1.10). The Lund‐Mackay score was used (0 to 24 points, higher score = more severe).
1.10. Analysis.
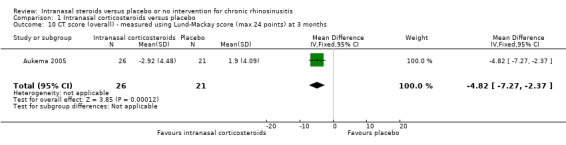
Comparison 1 Intranasal corticosteroids versus placebo, Outcome 10 CT score (overall) ‐ measured using Lund‐Mackay score (max 24 points) at 3 months.
Discussion
Summary of main results
This review includes a total of 18 studies. However, many studies were very small and did not measure and/or report data in a way that allowed for meta‐analysis.
Only one very small study (n = 20) reported disease‐specific health‐related quality of life, using the Rhinosinusitis Outcome Measures‐31 (RSOM‐31). Lund 2004 described using the SF‐36, but reported that only the general health subscale showed a statistically significant difference between groups.
We found that intranasal corticosteroids improved patient symptom scores, when these were measured as a combined score, with scores for individual items or as a global score (non‐validated scales). Apart from rhinorrhoea and loss of sense of smell, which had small effect sizes when measured as individual items, the effect sizes observed using other parameters corresponded to moderate effect sizes. However, because these data could only be obtained from a few studies (many studies did not report enough detail when the results were not 'significant') there is a risk of reporting bias.
Epistaxis was probably the most consistently reported outcome, with 13 out of 18 studies reporting this. The risk was increased in patients using intranasal corticosteroids (risk ratio (RR) 2.74, 95% confidence interval (CI) 1.88 to 4.00; 2508 participants; 13 studies). However, local irritation was very inconsistently reported, with studies using various definitions to report the data. The RR obtained was 0.94 (95% CI 0.53 to 1.64; 2124 participants; 11 studies; I2 = 0%) and it is unclear whether there is an important increase in risk. None of the studies treated or followed up patients for long enough to provide meaningful data on osteoporosis risk or risk of stunted growth.
There seemed to be an increased odds of polyp improvement in people who had chronic rhinosinusitis with nasal polyps. However, the results for endoscopy score were not well reported in studies that included participants with chronic rhinosinusitis without polyps.
Overall completeness and applicability of evidence
A good range of types and doses of intranasal corticosteroids are included in this review. However, the main body of evidence is in patients with chronic rhinosinusitis with polyps. Studies in chronic rhinosinusitis without polyps tended to be smaller, had poorer reporting and did not contribute enough information to the meta‐analyses to allow us to evaluate whether there are differences in effectiveness between these subgroups. Only one of these studies was conducted in children (up to 18 years old) and therefore it is again unclear whether the evidence is applicable to children. Most studies were conducted for three to four months, with the exception of three studies that were conducted for 20 weeks (Aukema 2005), 26 weeks (Lund 2004), and two years (Lang 1983), respectively. Therefore, it is unclear whether the observed effectiveness is maintained if intranasal corticosteroids are used over longer periods and whether these benefits can be maintained after patients stop treatment.
Most of the studies did not make any reference to whether saline irrigation could be used. However, Vlckova 2009 specified that patients could not use nasal saline. This study showed larger effect sizes than the other studies and unlike most studies, where participants in both the intervention and placebo groups showed some improvement over time, the participants in the placebo group in this study got worse with time. It is unclear whether this is a random observation or due to the patient population, the high doses used or the exclusion of normal saline.
Quality of the evidence
The studies included in this review were relatively well conducted, with most using good methodology for selection of patients and blinding.
However, we had serious concerns about how the effectiveness outcomes were measured, analysed and reported. The validity of the tools used to measure outcomes is a major concern. Most studies did not use validated tools to measure and score symptom severity scales and this reduces our confidence in the estimates of effect. Moreover, there is also a possibility of reporting bias, as studies only tended to report enough detail to allow for meta‐analysis when they found a statistical significant result. These reasons significantly reduced our confidence in the estimates of effect sizes and we rated the quality of the evidence as moderate for disease severity outcomes. The exception was those outcomes involving facial pain/pressure, but there was some unexplained heterogeneity and there were fewer data. For quality of life, the quality of the evidence is very low.
The reporting methodology for 'local irritation' was also a concern and along with the imprecision observed we considered the evidence for this outcome to be of low quality.
However, we considered the evidence for the risk of epistaxis to be of high quality; this is an outcome that appears to have been collected and reported consistently across studies.
Potential biases in the review process
There were two major challenges to meta‐analysis in the review of effectiveness outcomes: 1) the lack of use of validated tools and variations in how outcomes were measured and reported and 2) the outcomes were often not fully reported.
Lack of use of validated tools and variations in how outcomes were measured and reported
The lack of use of validated instruments to measure patient‐important outcomes, such as the impact on quality of life and disease severity, is probably the single most important issue that hampers our ability to meta‐analyse results or to compare results between studies. Studies used a variety of scales, timings and analysis methods to measure different combinations of symptoms. Some studies may have measured one group of symptoms (e.g. nasal discharge) as several separate items (anterior rhinorrhoea, post‐nasal drip), but not measured other types of symptoms at all (e.g. loss of sense of smell and facial pain/pressure). In the absence of evidence for both disease‐specific health‐related quality of life and disease severity measured with validated tools (only one study respectively, Hansen 2010 and Lund 2004, reported these), some 'standardisation' of these measures had to be conducted in order to allow the results to be pooled. We took the decision to combine the scores for individual symptoms to create a total symptoms score. The methods we used to do this are described in the methods section (Dealing with missing data). The symptoms we included were based on the EPOS 2012 diagnostic criteria, but this score was not a validated measure and the correlation between symptoms was not accounted for in the results.
In addition to making an assumption of no correlation between symptoms, we also had to make other decisions to standardise the data:
Most studies only specified that diaries were completed in the morning, whereas others were completed in the morning and evening and may, or may not, have reported the results separately. Where studies presented both morning and evening scores, we only used the morning score values in this review, to allow for standardisation across studies. We avoided taking an average of morning and evening data and estimating the standard deviations, since these should be taken as paired data and this may further overestimate the size of the standard deviation (see below for considerations when imputing standard deviations).
Some studies only measured the outcomes at the endpoint. We had to assume that the difference between changes and endpoints would produce a similar mean difference between groups and therefore could be pooled. We made this assumption for Aukema 2005.
Aukema 2005 used a 10 cm visual analogue scale. Since this was only one small study, we had to assume that the scales were linear and could be converted to a 0‐ to 3‐point scale; we tested this in a sensitivity analysis. Although the effect size observed for this study was larger, it did not have an impact on the results.
Fortunately, most of the studies had measured symptom severity using diaries with a 0‐ to 3‐point scale and we could use the mean differences to look at the size of the effect. However, what is a minimal important difference (MID) for a 0‐ to 3‐point scale is not known and to assist interpretation we still had to look at the standardised mean difference (SMD) and used Cohen's effect size as a rule of thumb. Bearing in mind that we did factor in correlations (which will result in smaller standard deviations), this means that the effect sizes could be larger than estimated. Nevertheless, we still found moderate to large effect sizes in disease severity scores. In fact, a moderate effect size was found in Lund 2004 using the individual or combined scores, even though the study did not find a clinically important difference using the Chronic Sinusitis Survey (CSS), a validated tool.
Local irritation is another outcome where there were many variations in reporting, in terms of categorisation and descriptions used. Where studies reported more than one type of local irritation (e.g. nasal burning and nasal irritation were both reported), we took the data for the outcome with the higher total event rate. If rates were the same for both outcomes, we chose the one terminology that was closest to the description of general irritation (e.g. nasal irritation would be used in this example), with the review author blinded to the data.
Outcomes were often not fully reported
Many of the data for the included studies were presented in the papers in graphs or charts. Where this was the case, we extracted the data from the paper using an online program (http://arohatgi.info/WebPlotDigitizer/app/). There will inevitably be a degree of error in using these data, both from inaccuracies during the printing process and the process used to collect the data. Where P values were reported as less than a certain threshold (e.g. P value < 0.05), calculations were based on P value = 0.05. This is a conservative estimate for standard deviation values and we were conscious of the need to minimise imputations as much as possible. For example, Small 2005 had two arms (a high‐dose and a low‐dose group) and only reported the P values compared to placebo for many outcomes. Rather than trying to estimate what these P values may be when 'combined' and risk inflating the standard deviation further, we entered this study twice as a high‐dose and low‐dose group, and halved the sample size in the placebo group to avoid double‐counting.
Agreements and disagreements with other studies or reviews
This review aimed to answer the clinical question of whether intranasal corticosteroids are effective in patients who have chronic rhinosinusitis. It is one of a series of reviews looking at the (relative) effectiveness and safety of different medical interventions for chronic rhinosinusitis.
Although the intranasal steroids included in this review comprised different types of molecules, doses, regimens and delivery methods, we observed no statistical heterogeneity that suggested that these factors could result in different levels of effectiveness and adverse events such as epistaxis and local irritation. This observation about the impact of variations in the types and doses of intranasal corticosteroids is consistent with our companion review, which looks at different types and doses of intranasal corticosteroids (Chong 2016a). Chong 2016a also did not find any important differences between high and low doses and there was very little evidence to draw conclusions about the other aspects. However, Chong 2016a did find it possible that the risk of adverse effects is increased when higher doses are used, whereas in this review we did not observe any heterogeneity.
This review only compared intranasal steroids against placebo. We have compared short‐course oral steroids against intranasal corticosteroids in Head 2016a and Head 2016b; antibiotics against intranasal corticosteroids in Head 2016c; and nasal saline against intranasal corticosteroids in Chong 2016b. When compared to oral corticosteroids, patients who received oral corticosteroids instead of intranasal corticosteroids seemed to benefit more in terms of reduced disease severity and polyps size for short follow‐up periods (two to three weeks), but it was uncertain whether the benefit persisted (difference minimises by three months) (Head 2016a). The antibiotics review only found one very small study comparing antibiotics (12 weeks of 250 mg clarithromycin) against 200 µg of mometasone furoate spray and did not find an important different between groups for overall disease severity, but found the endoscopy score to be slightly better in the antibiotics group (Head 2016c). The saline review only found one very small study comparing intranasal corticosteroids versus nebulised saline; intranasal corticosteroids were much more effective than nebulised saline (Chong 2016b). However, most of the evidence for intranasal corticosteroids compared against other interventions is of very low quality (we have very little confidence in the effects estimated ‐ the evidence is inconclusive).
Unlike previous reviews of intranasal steroids, which focused on either patients with polyps (Kalish 2012) or without polyps (Snidvongs 2011), this review includes all types of chronic rhinosinusitis patients. However, we limited inclusion to studies that had followed up patients for at least three months and we excluded patients who had just undergone surgery. The impact of intranasal steroids in reducing recurrence in patients who have just had sinus surgery is not the clinical question addressed by this review.
Authors' conclusions
Implications for practice.
Patients with chronic rhinosinusitis with nasal polyps showed an improvement in symptoms on intranasal corticosteroids. However, data are lacking for patients without polyps and it is unclear whether they derive a similar level of benefit.
The risk of adverse effects such as epistaxis and local irritation is increased in people taking intranasal corticosteroids, although the severity of the epistaxis is unknown, as is whether patients discontinue usage as a result. If epistaxis is limited to streaks of blood in the mucus it may be tolerated by the patient and be safe to continue treatment. However, it may be a factor that affects compliance. Ensuring good technique in usage of spray by patients may help to reduce these effects, especially where spraying of the septum is avoided. Different intranasal corticosteroid delivery nozzles may also have a bearing on this.
Implications for research.
As of August 2015, most studies of intranasal steroids have been conducted in participants with chronic rhinosinusitis with nasal polyps. The evidence we found suggests that chronic rhinosinusitis patients show an improvement in symptoms with intranasal corticosteroids. Recent international trials using the Optinose device (Navigate trials I and II) have been completed and they include different doses within their protocols and a comparison with placebo. Further information will therefore be forthcoming once these results are published (NCT01622569; NCT01624662).
Future research should recruit patients with chronic rhinosinusitis diagnosed using the EPOS 2012 criteria and include both patients with and without nasal polyps (stratified randomisation by subgroup). Intranasal corticosteroids should be compared against placebo and this should be considered against a background of nasal irrigation, including in the placebo arm. The intervention and follow‐up should be carried out for at least three or six months, since intranasal corticosteroids are used as a long‐term treatment for a chronic condition.
A key area of weakness across all of the included studies was the absence of both disease‐specific and generic health‐related quality of life tools as outcome measures. It is recommended that any future research uses primary outcome measures that are relevant to patients and any disease‐specific instruments used should be validated in people with chronic rhinosinusitis. Many studies chose to use polyp scores as their primary outcome measure yet the correlation between endoscopic results and patient symptoms is unclear. The methods for defining and recording adverse events should be considered at the protocol stage and adverse events recorded should include epistaxis and local irritation; longer‐term effects such as osteoporosis should also be considered.
This review is one of a suite of reviews of medical treatments for chronic rhinosinusitis, each of which features its own research recommendations. Across all reviews, key features of future research are as follows:
Trials should be adequately powered and imbalances in prognostic factors (for example, prior sinus surgery) must be accounted for in the statistical analysis.
Study participants should be diagnosed with chronic rhinosinusitis using the EPOS 2012 criteria and should primarily be recruited based on their symptoms. Different patient phenotypes (that is, those with and without nasal polyps) should be recognised and trials should use stratified randomisation within these subgroups or focus on one or other of the phenotypes.
Studies should focus on outcomes that are important to patients and use validated instruments to measure these. Validated chronic rhinosinusitis‐specific health‐related quality of life questionnaires exist, for example the Sino‐Nasal Outcome Test‐22 (SNOT‐22). Patients may find dichotomised outcomes easiest to interpret; for example the percentage of patients achieving a minimal clinically important difference (MCID) or improvement for that outcome. Such MCIDs or cut‐off points should be included in the study protocol and clearly outlined in the methods section.
Trials and other high‐quality studies should use consistent outcomes and adhere to reporting guidelines, such as CONSORT, so that results can be compared across future trials. The development of a standardised set of outcomes, or core outcome set, for chronic rhinosinusitis, agreed by researchers, clinicians and patients, will facilitate this process.
Acknowledgements
This project is one of a suite of reviews on the medical treatment of chronic rhinosinusitis, funded by the National Institute for Health Research (award reference 14/174/03).
This project was also supported by the National Institute for Health Research, via Cochrane Infrastructure, Cochrane Programme Grant or Cochrane Incentive funding to Cochrane ENT. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
We would like to express our thanks to the external peer reviewer, Professor Wytske Fokkens, the consumer referee Joan Blakley and the Cochrane ENT editors for their detailed and insightful comments, which helped to strengthen this review. Thank you also to acting Co‐ordinating Editor, Professor Richard Harvey, for his oversight of this publication.
The authors are grateful for the assistance provided by Jenny Bellorini and Samantha Faulkner, with editorial support and searching for studies.
Appendices
Appendix 1. Search strategies
| CENTRAL | Ovid MEDLINE |
| #1 MeSH descriptor: [Sinusitis] explode all trees #2 MeSH descriptor: [Rhinitis] this term only #3 MeSH descriptor: [Rhinitis, Atrophic] this term only #4 MeSH descriptor: [Rhinitis, Vasomotor] this term only #5 MeSH descriptor: [Paranasal Sinus Diseases] this term only #6 MeSH descriptor: [Paranasal Sinuses] explode all trees #7 rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis #8 kartagener* near syndrome* #9 inflamm* near sinus* #10 (maxilla* or frontal*) near sinus* #11 #1 or #2 or #3 or #4 or #5 or #6 or #7 or #8 or #9 or #10 #12 MeSH descriptor: [Chronic Disease] explode all trees #13 MeSH descriptor: [Recurrence] explode all trees #14 chronic or persis* or recurrent* #15 #12 or #13 or #14 #16 #11 and #15 #17 CRSsNP #18 (sinusitis or rhinitis) near (chronic or persis* or recurrent*) #19 #16 or #17 or #18 #20 MeSH descriptor: [Nasal Polyps] explode all trees #21 MeSH descriptor: [Nose] explode all trees #22 MeSH descriptor: [Nose Diseases] explode all trees #23 #21 or #22 #24 MeSH descriptor: [Polyps] explode all trees #25 #23 and #24 #26 (nose or nasal or rhino* or rhinitis or sinus* or sinonasal) near (papilloma* or polyp*) #27 rhinopolyp* or CRSwNP #28 #19 or #20 or #25 or #26 or #27 #29 MeSH descriptor: [Steroids] explode all trees #30 MeSH descriptor: [Adrenal Cortex Hormones] explode all trees #31 MeSH descriptor: [Glucocorticoids] explode all trees #32 MeSH descriptor: [Anti‐Inflammatory Agents] explode all trees #33 MeSH descriptor: [Anti‐Inflammatory Agents, Non‐Steroidal] explode all trees #34 #32 not #33 #35 steroid* or glucocorticoid* or corticosteroid* or glucosteroid* or cyclocosteroid* #36 beclomethasone or beclometasone or beclamet or beclocort or becotide #37 betamethasone or betadexamethasone or flubenisolone or celeston* or cellestoderm or betnelan or oradexon #38 dexamethasoneor dexameth or dexone or dexametasone or decadron or dexasone or hexadecadron or hexadrol or methylfluorprednisolone or millicorten #39 flunisolide or fluticasone or hydrocortisone or cortisol or cortifair or cortril or hyrocortone or cortef or epicortisol or efcortesol or Cortisone #40 methylprednisolone or medrol or metripred or urbason #41 mometasone or prednisolone or precortisyl or deltacortril or deltastab or prednesol or deltasone or prednisone or cortan or liquid next pred or meticorten #42 paramethasone or triamcinolone or aristocort or volon or atolone or kenacort or orasone or panasol or prednicen #43 corticoid* or betamethason* or betamethasone or hydrocortison* or celesto* or dexamethason* or hexadecadrol or budesonid* or horacort or pulmicort or rhinocort or methylfluorprednisolone or flunisolid* or nasalide or fluticason* or flonase or flounce or mometason* or nasonex or triamclinolon* or nasacort or tri next nasal or aristocort or Ciclesonide #44 #29 or #30 or #31 or #34 or #35 or #36 or #37 or #38 or #39 or #40 or #41 or #42 or #43 #45 #28 and #44 |
1 exp Sinusitis/ 2 paranasal sinus diseases/ or rhinitis/ or rhinitis, atrophic/ or rhinitis, vasomotor/ 3 exp Paranasal Sinuses/ 4 (rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis).ab,ti. 5 (kartagener* adj3 syndrome*).ab,ti. 6 (inflamm* adj5 sinus*).ab,ti. 7 ((maxilla* or frontal*) adj3 sinus*).ab,ti. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp chronic disease/ 10 exp Recurrence/ 11 (chronic or persis* or recurrent*).ab,ti. 12 9 or 10 or 11 13 8 and 12 14 CRSsNP.ab,ti. 15 ((sinusitis or rhinitis) adj3 (chronic or persis* or recurrent*)).ab,ti. 16 13 or 14 or 15 17 exp Nasal Polyps/ 18 exp Nose/ or exp Nose Diseases/ 19 exp Polyps/ 20 18 and 19 21 ((nose or nasal or rhino* or rhinitis or sinus* or sinonasal) adj3 (papilloma* or polyp*)).ab,ti. 22 (rhinopolyp* or CRSwNP).ab,ti. 23 16 or 17 or 20 or 21 or 22 24 exp Steroids/ 25 exp Adrenal Cortex Hormones/ 26 exp Glucocorticoids/ 27 exp Anti‐Inflammatory Agents/ 28 exp Anti‐Inflammatory Agents, Non‐Steroidal/ 29 27 not 28 30 (steroid* or glucocorticoid* or corticosteroid* or glucosteroid* or cyclocosteroid* orbeclomethasone or beclometasone or beclamet or beclocort or becotide or betamethasone or betadexamethasone or flubenisolone or celeston* or cellestoderm or betnelan or oradexon or dexamethasone or dexameth or dexone or dexametasone or decadron or dexasone or hexadecadron or hexadrol or methylfluorprednisolone or millicorten or flunisolide or fluticasone or hydrocortisone or cortisol or cortifair or cortril or hyrocortone or cortef or epicortisol or efcortesol or Cortisone or methylprednisolone or medrol or metripred or urbason or mometasone or prednisolone or precortisyl or deltacortril or deltastab or prednesol or deltasone or prednisone or cortan or liquid next pred or meticorten or paramethasone or triamcinolone or aristocort or volon or atolone or kenacort or orasone or panasol or prednicen).ab,ti. 31 (corticoid* or betamethason* or betamethasone or hydrocortison* or celesto* or dexamethason* or hexadecadrol or budesonid* or horacort or pulmicort or rhinocort or methylfluorprednisolone or flunisolid* or nasalide or fluticason* or flonase or flounce or mometason* or nasonex or triamclinolon* or nasacort or (tri adj3 nasal) or aristocort or Ciclesonide).ab,ti. 32 24 or 25 or 26 or 29 or 30 or 31 33 23 and 32 |
| Ovid Embase | Trial registries (via CRS) |
| 1 exp sinusitis/ or paranasal sinus disease/ 2 atrophic rhinitis/ or chronic rhinitis/ or rhinosinusitis/ or vasomotor rhinitis/ 3 exp paranasal sinus/ 4 (rhinosinusitis or nasosinusitis or pansinusitis or ethmoiditis or sphenoiditis).tw. 5 (kartagener* adj3 syndrome*).tw. 6 (inflamm* adj5 sinus*).tw. 7 ((maxilla* or frontal*) adj3 sinus*).tw. 8 1 or 2 or 3 or 4 or 5 or 6 or 7 9 exp chronic disease/ 10 exp recurrent disease/ 11 (chronic or persis* or recurrent*).tw. 12 9 or 10 or 11 13 8 and 12 14 CRSsNP.tw. 15 ((sinusitis or rhinitis) adj3 (chronic or persis* or recurrent*)).tw. 16 13 or 14 or 15 17 exp nose polyp/ 18 exp nose disease/ or exp nose/ 19 exp polyp/ 20 18 and 19 21 ((nose or nasal or rhino* or rhinitis or sinus* or sinonasal) adj3 (papilloma* or polyp*)).tw. 22 (rhinopolyp* or CRSwNP).tw. 23 16 or 20 or 21 or 22 24 exp *corticosteroid/ 25 exp steroid/ 26 exp antiinflammatory agent/ 27 exp nonsteroid antiinflammatory agent/ 28 26 not 27 29 (steroid* or glucocorticoid* or corticosteroid* or glucosteroid* or cyclocosteroid* or beclomethasone or beclometasone or beclamet or beclocort or becotide or betamethasone or betadexamethasone or flubenisolone or celeston* or cellestoderm or betnelan or oradexon or dexamethasone or dexameth or dexone or dexametasone or decadron or dexasone or hexadecadron or hexadrol or methylfluorprednisolone or millicorten or flunisolide or fluticasone or hydrocortisone or cortisol or cortifair or cortril or hyrocortone or cortef or epicortisol or efcortesol or Cortisone or methylprednisolone or medrol or metripred or urbason or mometasone or prednisolone or precortisyl or deltacortril or deltastab or prednesol or deltasone or prednisone or cortan or liquid next pred or meticorten or paramethasone or triamcinolone or aristocort or volon or atolone or kenacort or orasone or panasol or prednicen).tw. 30 24 or 28 or 29 31 23 and 30 |
ClinicalTrials.gov Condition: rhinitis OR sinusitis OR rhinosinusitis OR (nose AND polyp*) OR (nasal AND polyp*) OR CRSsNP OR CRSwNP OR CRS ICTRP Title: rhinitis OR sinusitis OR rhinosinusitis OR CRSsNP OR CRSwNP OR CR OR All: (nose AND polyp*) OR (nasal AND polyp*) NB These searches were run from 1 March 2015 to 11 August 2015, when these terms were last searched to populate the Cochrane ENT trials register in CRS |
Appendix 2. Data extraction form
| REF ID: | Study title: |
| Date of extraction: | Extracted by: |
| General comments/notes (internal for discussion): |
| Flow chart of trial | ||
| Group A (Intervention) | Group B (Comparison) | |
| No. of people screened | ||
| No. of participants randomised ‐ all | ||
| No. randomised to each group | ||
| No. receiving treatment as allocated | ||
| No. not receiving treatment as allocated ‐ Reason 1 ‐ Reason 2 |
||
| No. dropped out (no follow‐up data for any outcome available) |
||
| No. excluded from analysis1 (for all outcomes) ‐ Reason 1 ‐ Reason 2 |
||
| 1This should be the people who received the treatment and were therefore not considered 'drop‐outs' but were excluded from all analyses (e.g. because the data could not be interpreted or the outcome was not recorded for some reason). | ||
| Information to go into 'Characteristics of included studies' table | |
| Methods | X arm, double/single/non‐blinded, [multicentre] parallel‐group/cross‐over/cluster‐RCT, with x duration of treatment and x duration of follow‐up |
| Participants |
Location: country, no of sites etc. Setting of recruitment and treatment: Sample size:
Participant (baseline) characteristics:
Inclusion criteria:[state diagnostic criteria used for CRS, polyps score if available] Exclusion criteria: |
| Interventions |
Intervention (n = x): drug name, method of administration, dose per day/frequency of administration, duration of treatment Comparator group (n = y): Use of additional interventions (common to both treatment arms): |
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
| Funding sources | 'No information provided'/'None declared'/State source of funding |
| Declarations of interest | 'No information provided'/'None declared'/State conflict |
| Notes | |
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Quote: "…" Comment: |
|
| Allocation concealment (selection bias) | Quote: "…" Comment: |
|
| Blinding of participants and personnel (performance bias) | Quote: "…" Comment: |
|
| Blinding of outcome assessment (detection bias) | Quote: "…" Comment: |
|
| Incomplete outcome data (attrition bias) | Quote: "…" Comment: |
|
| Selective reporting (reporting bias) | Quote: "…" Comment: |
|
| Other bias (see section 8.15) Insensitive/non‐validated instrument? |
Quote: "…" Comment: |
|
| Other bias (see section 8.15) | Quote: "…" Comment: |
| Findings of study: continuous outcomes | |||||||
| Results (continuous data table) | |||||||
| Outcome | Group A | Group B | Other summary stats/Notes | ||||
| Mean | SD | N | Mean | SD | N | Mean difference (95% CI), P values etc. | |
| Disease‐specific HRQL (instrument name/range) Time point: |
|||||||
| Generic HRQL (instrument name/range) Time point: |
|||||||
| Symptom score (overall) (instrument name/range) Time point: |
|||||||
|
Added total ‐ if scores reported separately for each symptom (range) Time point: |
|||||||
| Nasal blockage/obstruction/congestion (instrument name/range) |
|||||||
| Nasal discharge (instrument name/range) |
|||||||
| Facial pain/pressure (instrument name/range) |
|||||||
| Smell (reduction) (instrument name/range) |
|||||||
| Headache (instrument name/range) |
|||||||
| Cough (in children) (instrument name/range) |
|||||||
| Polyp size (instrument name/range) |
|||||||
| CT score (instrument name/range) |
|||||||
| Comments: | |||||||
| Results (dichotomous data table) | ||||||
| Outcome | Applicable review/intervention | Group A | Group B | Other summary stats/notes | ||
| No. of people with events | No. of people analysed | No. of people with events | No. of people analysed | P values, RR (95% CI), OR (95% CI) | ||
| Epistaxis/nose bleed | INCS Saline irrigation |
|||||
| Local irritation (sore throat, oral thrush, discomfort) | INCS Saline irrigation |
|||||
| Osteoporosis (minimum 6 months) | INCS | |||||
| Stunted growth (children, minimum 6 months) | INCS | Can also be measured as average height | ||||
| Mood disturbances | OCS | |||||
| Gastrointestinal disturbances (diarrhoea, nausea, vomiting, stomach irritation) |
OCS Antibiotics |
|||||
| Insomnia | OCS | |||||
| Osteoporosis (minimum 6 months) | INCS OCS |
|||||
| Discomfort | Saline irrigation | |||||
| Skin irritation | Antibiotics | |||||
| Anaphylaxis or other serious allergic reactions such as Stevens‐Johnson | Antibiotics | |||||
| Comments: | ||||||
Appendix 3. Methods of measurement
The method of measurement in the included studies was reported in several ways:
Choice of scale: A variety of scales and choice of symptoms were used across the studies. A 10 cm visual analogue scale (VAS) was used in Aukema 2005 and Hansen 2010. Holmberg 1997 and Lund 1998 used a five‐point scale (0 to 4), and most studies used a four‐point scale (range 0 to 3) to measure the severity of each type of symptom. The most commonly used definition was 0 = none, 1 = mild, 2 = moderate, 3 = severe, with some variation between studies. Parikh 2001 used both a 10 cm VAS and also a diary card (scale unclear).
Type/combination of symptoms measured: Of the four possible domains mentioned in EPOS 2012 for adults (nasal blockage/obstruction, nasal discharge, loss of sense of smell and facial pressure/pain), the most consistently measured and reported type of symptom was nasal blockage/obstruction. "Nasal discharge" symptoms were also often measured but there were a variety of ways of measuring this. Some studies reported "rhinitis symptoms" (it was unclear whether this was from a single item or a combination), whereas others may only have measured this as a single symptom (e.g. rhinorrhoea), or as a combination of slightly different symptoms ("anterior nasal discharge", "posterior drip", "purulent nasal discharge"). Loss of sense of smell was less commonly measured. Facial pain/pressure was only measured in six studies (Aukema 2005; Lund 1998; Lund 2004; Mosges 2011; Parikh 2001; Vlckova 2009). However, "headache" was reported as an "adverse effect" in certain studies. Only six of the included studies attempted to assess all of the four symptoms used to define CRS in EPOS 2012 (nasal blockage/obstruction/congestion, nasal discharge (anterior/posterior nasal drip), loss of sense of smell and facial pain/pressure (adults)/cough (children)) (Aukema 2005; Lund 1998; Lund 2004; Mosges 2011; Parikh 2001; Vlckova 2009). Facial pain/pressure was not measured by most studies.
-
Timing of measures:
Whether scores were filled every day:Johansen 1993 only required diaries to be filled once a week. Holopainen 1982 only required patients to fill their "daily diary" for two weeks before each visit. Aukema 2005 seemed to only require patients to fill the VAS at every visit. Parikh 2001 only required patients to fill in a 10 cm VAS "symptom score" at the beginning and end of the study and a diary card "once every week".
Morning versus morning and evening: Some studies clearly stated that they required patients to fill in diary cards twice a day (morning and evening) (Keith 2000; Hansen 2010; Penttilla 2000; Stjarne 2006a; Vlckova 2009), whereas others only required scores in the morning (Small 2005; Stjarne 2006; Zhou 2015). This information was not reported in some studies (Lund 1998), and seemed to require a mixture of different timings depending on the type of symptoms in Holmberg 1997. Keith 2000, Penttilla 2000 and Vlckova 2009 presented the data for the morning and evening scores separately. Stjarne 2006a did not report the scores apart from stating that these were statistically significant different compared placebo, whereas Hansen 2010 only provided an overall score. Where studies presented both morning and evening scores, we only used the morning score values in this review, to allow for standardisation across studies.
Period of time taken into account:Lund 1998, Small 2005; Vlckova 2009 and Zhou 2015 clearly stated that it was a four‐week or monthly period. Mosges 2011 used the area under the curve for a 16‐week period, while Holopainen 1982 seemed to average the daily diary scores, which patients had to fill in "daily for a fortnight before every check up". It can only be assumed that Stjarne 2006 and Stjarne 2006a also used four‐week averages. There was no information for Chur 2013, Hansen 2010 and Lund 1998.
-
Scoring/analysis method:
Overall score versus individual symptom score: Some studies used the overall score for all the symptoms measured. Others seemed to combine several types of symptoms (e.g. "rhinitis symptoms" can be established by a combination of several individual questions (anterior rhinorrhoea, postnasal drip, nasal blockage), or by a single question). The rationale for weighting types of symptoms in their scoring and reporting was not discussed or provided at all.
Weighting of symptoms in scores: Apart from Aukema 2005, which clearly stated that facial pain was "counted twice because this symptom was considered most bothering", none of the studies provided any information on how scores were weighted/calculated and the rationale for their analysis.
Method of reporting: Most studies used the mean scores (or median for smaller studies) for individual symptom scores with or without the total symptom scores. However, other studies reported this as proportions of people above a certain threshold of change from baseline, or above/below a certain threshold at the "endpoint". Penttilla 2000 reported the proportion of patients who had symptoms scores < 2 points (0‐ to 3‐point scale). Stjarne 2006a used the proportion of patients who had an improvement of >= 1 point compared to baseline. Three studies used different cut‐offs to present these as the percentage of patients with scores above a certain value (Holmberg 1997; Keith 2000; Lund 1998): either different trial periods (Keith 2000; Lund 1998), or as percentage of "blocks of treatment period" with certain thresholds (Holmberg 1997). These choices of cut‐offs or treatment period were not specified in the methods sections of the study report.
Data and analyses
Comparison 1. Intranasal corticosteroids versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Disease severity ‐ measured as change from baseline using the Chronic Sinusitis Survey at 20 weeks | 1 | 134 | Mean Difference (IV, Fixed, 95% CI) | 2.84 [‐5.02, 10.70] |
| 2 Disease severity ‐ global symptom score, measured as proportion of patients who improved | 1 | 109 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.78 [1.76, 4.40] |
| 3 Disease severity ‐ combination of individual symptom scores, measured on a 0 to 3 scale as change from baseline at 12 to 20 weeks | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Average symptom score (4 EPOS domains) | 2 | 243 | Mean Difference (IV, Fixed, 95% CI) | ‐0.26 [‐0.37, ‐0.15] |
| 3.2 Average symptom score (3 EPOS domains ‐ nasal blockage, rhinorrhoea, loss of sense of smell) | 4 | 1345 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.38, ‐0.23] |
| 3.3 Average symptom score (2 EPOS domains ‐ nasal blockage and rhinorrhoea) | 6 | 1702 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐0.38, ‐0.24] |
| 4 Disease severity ‐ individual symptoms, measured as average change from baseline at 12 to 20 weeks (range 0 to 3 points) | 6 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Nasal blockage | 6 | 1702 | Mean Difference (IV, Random, 95% CI) | ‐0.40 [‐0.52, ‐0.29] |
| 4.2 Rhinorrhoea | 6 | 1702 | Mean Difference (IV, Random, 95% CI) | ‐0.25 [‐0.33, ‐0.17] |
| 4.3 Loss of sense of smell | 4 | 1345 | Mean Difference (IV, Random, 95% CI) | ‐0.19 [‐0.28, ‐0.11] |
| 4.4 Facial pain/pressure | 2 | 243 | Mean Difference (IV, Random, 95% CI) | ‐0.27 [‐0.56, 0.02] |
| 5 Adverse events ‐ epistaxis | 13 | 2508 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.74 [1.88, 4.00] |
| 5.1 With nasal polyps | 10 | 2262 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.03 [2.00, 4.59] |
| 5.2 Without nasal polyps | 3 | 246 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.49 [0.59, 3.78] |
| 6 Adverse events ‐ local irritation | 11 | 2124 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.94 [0.53, 1.64] |
| 6.1 With nasal polyps | 9 | 2045 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.52, 1.67] |
| 6.2 Without nasal polyps | 2 | 79 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.02 [0.15, 6.84] |
| 7 Endoscopy score (bilateral polyps score) measured change from baseline (range 0 to 6 points) | 4 | 1417 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.90, ‐0.26] |
| 7.1 With nasal polyps | 4 | 1417 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.90, ‐0.26] |
| 8 Endoscopy score (polyps size) ‐ measured as numbers with improvement at longest available follow‐up | 5 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.06, 2.95] |
| 8.1 With nasal polyps | 5 | 676 | Risk Ratio (M‐H, Random, 95% CI) | 1.77 [1.06, 2.95] |
| 9 Endoscopy score (polyp size) ‐ measured as numbers with improvement at longest available follow‐up | 8 | 1984 | Odds Ratio (Random, 95% CI) | 2.07 [1.48, 2.91] |
| 9.1 With nasal polyps | 4 | 1417 | Odds Ratio (Random, 95% CI) | 2.43 [1.46, 4.06] |
| 9.2 With nasal polyps from dichotomous data | 4 | 567 | Odds Ratio (Random, 95% CI) | 1.73 [1.19, 2.51] |
| 10 CT score (overall) ‐ measured using Lund‐Mackay score (max 24 points) at 3 months | 1 | 47 | Mean Difference (IV, Fixed, 95% CI) | ‐4.82 [‐7.27, ‐2.37] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Aukema 2005.
| Methods | Double‐blinded, parallel‐group RCT, with 12 weeks duration of treatment and follow‐up | |
| Participants |
Location: Netherlands, single side Setting of recruitment and treatment: patients awaiting FESS at a university hospital Sample size:
Participant (baseline) characteristics:
Inclusion criteria: Patient on waiting list for FESS (recent CT score of ≥ 3 on one side, prior treatment with corticosteroid spray ≥ 3 months, total VAS score of ≥ 200 mm on 6 scales (range of 0 to 100 mm)) "...investigator had observed and approved the administration method (of the single dose nasal drops)" during the run‐in period |
|
| Interventions |
Intervention (n = 27): Nasules (fluticasone propionate single dose nasal drops, administered once daily at night) Comparator group (n = 27): placebo Patients were instructed to lie on their back with their head hanging down in a vertical position on the edge of a bed while administering the drops Use of additional interventions (common to both treatment arms): not described |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes: 1. Disease severity as measured by VAS of 0 to 100 mm for 6 symptoms (nasal blockage, rhinorrhoea, facial pain, mucus in throat, loss of sense of smell and headache) 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Other adverse effects: local irritation 4. Endoscopy score (polyp volume) using a VAS 5. CT scan score measured using Lund‐Mackay score (max 24 points) Other outcomes reported by the study:
|
|
| Notes | Study had a 2‐week run‐in period; patient's method of application was assessed before randomisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Medications were numbered by means of computerized randomization and were assigned in numeric order." Comment: likely to be adequately generated |
| Allocation concealment (selection bias) | Unclear risk | Quote: "All investigations were carried out by one investigator" Comment: no specific description provided. Sequence generation adequate; likely to depend on whether medications and packaging looked identical. Baseline risks potentially different. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "All investigations were carried out by one investigator...Double‐blind randomization to FPNDs or placebo took place after the investigator had observed and approved the administration method … Randomisation codes were not disclosed until a year after all the patients had finished the study" Comment: likely to be adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "All investigations were carried out by one investigator... …Randomisation codes were not disclosed until a year after all the patients had finished the study" Comment: likely to be adequate |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "At the end of the study, 6 patients of the placebo group and 1 of the FPND group had dropped out. Of the placebo group, 5 patients dropped out because they had a lot of complaints and did not want to complete the study. Three were prematurely scheduled for FESS (2 after 6 weeks and 1 after 2 weeks of treatment). Two patients resumed intranasal corticosteroids (after 4 and 8 weeks of treatment). One patient received oral steroids from her pulmonologist because she did not tolerate inhaled steroids (after 8 weeks of treatment) and was scheduled for FESS as well. The one dropout in the FPND group failed to return for the last visit, despite repeated requests. At the second‐to‐last visit, he was not much improved compared with inclusion, and he finally underwent surgery." Comment: it is unclear whether those patients considered "dropped out" were included in the analysis of the results for symptom score. The drop‐out was not balanced and was very likely to be related to lack of efficacy. |
| Selective reporting (reporting bias) | High risk | Comment: methods section did not specify which and how outcomes would be reported. Numerical data for 3 symptom scores (facial pain, loss of sense of smell and headache) was not shown. The total score on the VAS across 6 symptoms was not reported in the results (although it was used as a criterion to determine eligibility for surgery). Methods states that "Mann Whitney test was used to test a difference in CT score between treatment groups at the end of study", and "Wilcoxon signed rank test to test changes in CT score from baseline in either treatment group" but results reports mean difference and SD |
| Other bias | Unclear risk | Quote: "VAS of 0‐100 mm", "Lund Mackay" Comment: standard scales used for measuring outcomes. Inadequate information on the baseline characteristics to judge. There were slightly more current smokers, atopic patients and patients who had had surgery in the placebo group. Baseline data for outcomes not reported, e.g. "…the CT scores in the FPND patients were better at the start of the study" (page 1022) |
Chur 2013.
| Methods | 4 arm, "double blind", international, multicentre, parallel‐group RCT, with 4 months' duration of treatment and follow‐up | |
| Participants |
Location: 9 countries: Colombia, Guatemala, Honduras, Panama, Peru, Russia, South Africa, Ukraine, United States. No. of sites not presented. Setting of recruitment and treatment: not stated Sample size: 6 to 11 years Number randomised (6 to 11 years): 18 in intervention 1, 18 in intervention 2, 10 in comparison Number completed (6 to 11 years): no information 12 to 17 years Number randomised (12 to 17 years): 32 in intervention 1, 33 in intervention 2, 16 in comparison Number completed (12 to 17 years): no information Participant (baseline) characteristics: 6 to 11 years Age: twice daily group 9.6, once daily group 9.7, PL group 12.7 Gender M/F: twice daily group 5/13, once daily group 8/10, PL group 12/14 Main diagnosis: nasal polyps Polyps status: 100% with polyps Previous sinus surgery status: no information Other important effect modifiers: o Asthma: twice daily group 1, once daily group 3, PL group 6 o Eosinophilic: twice daily group 3, once daily group 5, PL group 9 12 to 17 years Age: twice daily group 14.4, once daily group 14.4, PL group 12.7 Gender M/F: twice daily group 15/18, once daily group 14/18, PL group 12/14 Main diagnosis: bilateral nasal polyps Polyps status: 100% with polyps Previous sinus surgery status: no information Other important effect modifiers: o Asthma: twice daily group 4, once daily group 9, PL group 6 o Eosinophilic: twice daily group 3, once daily group 9, PL group 9 Inclusion criteria: children aged 6 to 17 years with nasal polyposis Exclusion criteria: children younger than 6 years. Antrochoanal polyps, cystic fibrosis, acute rhinosinusitis, rhinitis medicamentosa, dyskinetic ciliary syndromes and aspirin allergy. Participants with asthma who received inhaled corticosteroids were required to be on no more than a moderate dosage regimen as defined by the 2005 Global Initiative for Asthma Guidelines (GINA) for 1 month before screening and to remain on it throughout the study (16); other forms of corticosteroids were prohibited. |
|
| Interventions |
6 to 11 years Intervention 1 (n = 18): mometasone furoate nasal spray, 100 µg once per day for 4 months Intervention 2 (n = 18): mometasone furoate nasal spray, 100 µg twice per day for 4 months Comparator group (n = 9): placebo once or twice daily (combined), for 4 months 12 to 17 years Intervention 1 (n = 26): mometasone furoate nasal spray, 200 µg once per day for 4 months Intervention 2 (n = 32): mometasone furoate nasal spray, 200 µg twice per day for 4 months Comparator group (n = 16): placebo once or twice daily (combined) for 4 months Use of additional interventions (common to both treatment arms): inhaled corticosteroids for patients with asthma (up to the equivalent of moderate dosage regimen according to GINA 2005) |
|
| Outcomes |
Outcomes of interest in the review: All outcomes were measured at 4 months Primary outcomes 1. Disease severity, measured as participant‐rated signs/symptoms including nasal congestion/obstruction, anterior rhinorrhoea/postnasal drip and loss of sense of smell; rated daily by participants on a 4‐point scale 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Other adverse effects: local irritation (including oral thrush, sore throat) 4. Polyps size; no details on scores used Other outcomes reported by the study: (Primary outcome) Effects on hypothalamic‐pituitary‐adrenal (HPA) axis function (24‐hour urinary free cortisol change from baseline and 24‐hour urinary free cortisol corrected for creatinine/adverse events Investigator‐evaluated polyp size (on a 4‐point scale) Investigator assessment of overall therapeutic response (on a 5‐point scale ranging from 0 (complete relief) to 4 (no relief) |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Subjects were randomly assigned to one of four treatment groups in a 4:4:1:1 ratio... stratified by age" Comment: pg 34, col 1, para 4 |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about allocation concealment provided |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Quote: "received MFNS 200 mcg once daily, MFNS 200 mcg twice daily, placebo once daily, or placebo twice daily" Comment: the abstract mentioned "double‐blind" and a placebo was used. However, instead of using a double‐dummy design, where all participants received the medication twice daily (with a placebo given for those who had once daily treatment), groups either had medication once or twice daily. Therefore, there was no blinding of participants in terms of knowing whether they were on the once daily or twice daily regimen. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: as above Comment: most of the outcomes are patient‐reported and therefore blinding of outcome assessment is affected |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no information about loss to follow‐up or exclusion. However, only 119/127 randomised patients (93%) were included in their primary endpoint analysis. There were more exclusions/drop‐outs from the 100 μg group compared with the higher‐dose group (6 (12%) versus 1) but no reasons were provided. Adverse effects and symptoms were reported based on 127 participants. It is unclear whether there were any imputations. |
| Selective reporting (reporting bias) | High risk | Quote: "No statistical analysis of efficacy end points was pre‐specified, in the study protocol, and only descriptive efficacy statistics were collected." Comment: we identified the protocol (NCT00378378) and the purpose was "to evaluate the safety and efficacy of Nasonex® (Mometasone Furoate Nasal Spray(MFNS)) in the treatment of nasal polyps in pediatric subjects between the ages of 6 and less than 18 years old. Safety will be the primary focus of this study." The study only reported the change from baseline in points and percentages but not the standard deviations and P values. The values from the treatment groups were very similar to the placebo group for some outcomes (e.g. for rhinorrhoea ‐43% for once daily versus ‐42%) and poor reporting due to lack of beneficial effects cannot be ruled out. Results for the "young (6‐11 years)" group and the "older (12‐17 years)" group were pooled together for the adverse events results and compared against the results separated by age group. This does not appear to be prespecified in the methods section. |
| Other bias | Unclear risk | Comment: there is no information regarding the validation of the symptom score |
Hansen 2010.
| Methods | 2‐arm, double‐blind, placebo‐controlled, parallel‐group RCT with 12 weeks of treatment and 14 weeks of follow‐up | |
| Participants |
Location: Netherlands, single site Setting of recruitment and treatment: ENT clinic in the Netherlands (Academic Medical Centre, Amsterdam) Sample size: Number randomised: 10 in intervention, 10 in comparison Number completed: 9 in intervention, 7 in comparison Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (n = 10): fluticasone propionate, administered using a breath actuated inhaler (Optinose) 400 µg twice daily (800 µg total daily dose), duration of treatment Comparator group (n = 10): matching placebo, administered twice daily Use of additional interventions (common to both treatment arms): Participants using saline rinses were permitted to continue to do so; 5 in the placebo group and 7 in the treatment group continued using nasal saline irrigation twice daily during the study Loratadine 10 mg tablets provided as rescue medication If a participant experienced a severe acute nasal blockage the investigator could authorise the use of a short course of oxymetazoline drops or spray for a maximum of 7 consecutive days and a maximum total of 10 days during the treatment period. Oxymetazoline was not to be used within 24 hours of a scheduled study visit. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes: 1. Health‐related quality of life, disease‐specific using RSOM‐31. A reduction in the average total symptom impact score > 1 is considered clinically relevant. 2. Disease severity symptom score, measured using a. A 10 cm VAS (not troublesome to most troublesome imaginable) "How troublesome are your symptoms of rhinosinusitis?" b. A diary (0 to 3 scale): 0 (none), 1 (mild – symptoms present but not troublesome), 2 (moderate – symptoms frequently troublesome but not interfering with daily activity or night‐time sleep) or 3 (symptoms troublesome and interfering with daily activity or night‐time sleep) to record nasal blockage, nasal discomfort and rhinitis symptoms. Participants also recorded sense of smell: 0 (normal), 1 (slightly impaired), 2 (moderately impaired) or 3 (absent). 3. Significant adverse effect: epistaxis Secondary outcomes: 4. Endoscopy (polyps size or overall score) using the Lund‐Mackay score Other outcomes reported by the study:
|
|
| Notes | Study had a 14‐ to 16‐day treatment‐free run‐in period at the beginning | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…subjects who met the eligibility criteria were randomized 1:1 …" Comment: no information on randomisation method provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information on how to maintain allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "…The Opt‐FP and placebo devices were identical in appearance. The spray pump in the Opt‐FP contained … Placebo matched FP exactly, except for the active ingredient... To deliver a dose of FP 400 μg bd. or placebo, subjects made two administrations per nostril, morning and evening. " Comment: matched placebo – identical‐looking and same formulation except for not including the active ingredient. Patients using the placebo administered it exactly the same number of times as the treatment group. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: as above Comment: key outcomes are patient‐reported – should remain well blinded until end of study |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "…The majority of subjects (9 Opt‐FP, 7 PBO) completed the study." Comment: the drop‐out rate is high (20%) and unbalanced (10% in active group and 30% in placebo group) enough to affect the findings of this small study (results were presented as medians). All withdrawals were related to adverse effects or worsening of symptoms. |
| Selective reporting (reporting bias) | Unclear risk | Comment: unclear why study reported "combined nasal symptoms" (rhinitis and blockage) only, whereas other symptoms (discomfort and smell) were reported separately. This was not prespecified. Reporting of some outcomes was confusing or incomplete. The results for the for "symptoms of rhinosinusitis" as measured on a 10 cm VAS were reported as having reduced 13 points in the treatment group. It was not clear if this was a mistake or whether it was 13%. For endoscopic evaluation, the study only showed data for the oedema score, which was statically significant, but not the nasal discharge score, which was not statistically significant. |
| Other bias | Low risk | Comment: instruments for the main patient‐reported symptoms were validated |
Holmberg 1997.
| Methods | 3‐arm, double‐blind, parallel‐group RCT, with a 26‐week duration of treatment and 2 additional weeks of follow‐up | |
| Participants |
Location: Sweden Setting of recruitment and treatment: outpatient clinics; single‐centre Sample size: Number randomised: 19 in FP group, 18 in BDP group, 18 in PL group Number completed: 15 in FP group, 16 in BDP group, 11 in PL group Participant (baseline) characteristics: Age mean (range): FP group: 54 (27 to 74); BDP group: 49 (26 to 68); PL group: 47 (21 to 71) Gender (M/F): FP group: 15/4; BDP group: 13/5; PL group: 14/4 Main diagnosis: bilateral polyposis with a polyp score of 1 or 2 Polyps status: 100% with polyps Previous sinus surgery status: 100% had a history of at least 1 polypectomy within the previous 5 years Other important effect modifiers: o Positive skin prick test (%): FP group: 3 (16%); BDP group: 6 (33%); PL group: 5/18 (27%) Inclusion criteria: bilateral polyposis with a polyp score of 1 or 2 Exclusion criteria: nasal polyposis with a score of 3 or 4 (or 0); concurrent nasal infection; an inability to cease treatment with systemic, inhaled or intranasal steroids or sodium cromoglycate on visit 1; had used antihistamines in the 48 hours prior to visit 1; had a contraindication to steroids or had any serious or unstable concurrent disease |
|
| Interventions |
FP group (n = 19): fluticasone propionate, aqueous nasal spray, 2 actuations of 50 μg each to each nostril morning and evening (400 μg/day) for 26 weeks BDP group (n = 18): beclomethasone dipropionate, aqueous nasal spray, 2 actuations of 50 μg each to each nostril morning and evening (400 μg/day) for 26 weeks PL group (n = 18): placebo, actuations to each nostril morning and evening, containing the same vehicle as the intervention solutions including benzalkonium chloride as a preservative, for 26 weeks Use of additional interventions (common to all treatment arms): A 4‐week run‐in period during which there was no treatment for polyposis except for rescue loratadine, which could be used by the patients. All patients were supplied with rescue loratadine tablets to use as relief medication, 10 mg loratadine once daily. Any use of rescue medication was documented on the patients' daily record cards. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes 1.Disease severity, measured by daily records of all nasal symptoms including: nasal blockage; sense of smell; sneezing and rhinorrhoea using a 4‐point rating system (0 = no symptoms; 1 = mild symptoms; 2= moderate symptoms; 4 = severe symptoms) 2. Physician assessment of symptoms. No details were provided on how these were measured. Measured at 26 weeks. 3. Significant adverse effect: epistaxis Secondary outcomes: 4. Polyp size by endoscopy (0‐ to 4‐point scale) Other outcomes reported by the study: 5. Polyp score 6. Peak nasal inspiratory flow 7. Physician's assessment of change in symptoms |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "randomized" Comment: pg 271, col 1, para 3 No further details provided |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided in the paper |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "placebo: 2 actuations to each nostril morning and evening containing the same vehicle, as the fluticasone and beclomethasone solutions including benzalkonium chloride as a preservative. The placebo solution was therefore identical to the active treatments but did not contain any active drug." Comment: pg 271, col 1, last para |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no further information. Should also be low if there is adequate blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 13/54 patients (24%) did not complete trial; 4/19 in fluticasone, 2/18 in beclomethasone, 7/18 (39%) in placebo group. Uneven drop‐out numbers (very high in placebo group). |
| Selective reporting (reporting bias) | High risk | Quote: "The primary efficacy endpoint was the physician's assessments of symptoms and polyp score on all clinic visits" Comment: the methods section described assessment of polyps and patient‐reported symptom scores. However, "physician assessment of outcomes and polyps score" were reported as primary outcomes in the results section. The results focused on "physician assessment of symptoms" and barely mentioned the results for the polyps (only "significant" for visit 5 on beclomethasone, not for fluticasone). In addition, there were some outcomes that seemed to have arbitrary, non‐predefined cut‐off points (% of days with symptom score < 2 in results). The denominator for the reported symptom scores outcome measures is not identified. |
| Other bias | High risk | Comment: primary outcome of physician assessment of outcomes was not well described in the paper with little information on the criteria used or any validation/inter‐rater reliability |
Holopainen 1982.
| Methods | 2‐arm, double‐blind, parallel‐group RCT, with a 16‐week duration of treatment and follow‐up | |
| Participants |
Location: Sweden, no information on number of sites Setting of recruitment and treatment: unclear Sample size: Number randomised: 10 in intervention, 9 in comparison Number completed: 10 in intervention, 8 in comparison Participant (baseline) characteristics: Age mean (range): group A: 43.5 (26 to 60); group B: 40 (18 to 62) Gender (M/F): group A: 6/4; group B: 4/5 Main diagnosis: perennial, intrinsic nasal symptoms associated with small nasal polyps Polyps status: 100% with polyps Previous sinus surgery status: unclear (there is a comment, "When necessary the number of polyps was reduced by surgical measures so that the test solution could easily be administered", but no further details are given) Other important effect modifiers: none provided Inclusion criteria: no further details available Exclusion criteria: none stated |
|
| Interventions |
Intervention (n = 10): budesonide nasal spray 400 μg daily, 2 puffs into each nostril, twice a day, for 16 weeks Comparator group (n = 9): placebo nasal spray (same solvent as intervention but without the active ingredient), 2 puffs into each nostril, twice a day, for 16 weeks Use of additional interventions (common to both treatment arms): all patients underwent a wash‐out period of 2 weeks before the study |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes 1. Disease severity, measured by patient‐reported symptom score cards recording nasal blocking, running, sneezing, itching and side effects according to a 0 to 3 scale daily for 2 weeks prior to check‐up. Last check‐up at 16 weeks. 2. Disease severity, measured by physician rhinoscopy to assess mucosal congestion and nasal discharge 3. Significant adverse effect: epistaxis Secondary outcomes: 4. Size of polyps (on a 0 to 3 scale) Other outcomes reported by the study: Saccharin test for measuring mucociliary activity Nasal smear for evaluating epithelial changes Biopsy of the nasal polyps Plasma cortisol determination Peak nasal inspiratory flow |
|
| Notes | Although the paper states "When necessary the number of polyps was reduced by surgical measures so that the test solution could be easily administered", there was no report of this having been carried out. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomly assigned…" Comment: no further information |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The placebo was identical with the active spray but without budesonide… the other a correspondent dose of only the solvent" Comment: identical‐looking and solvent used |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: low risk, since blinding is adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient with severe nasal blocking and obstructing polyps had to withdraw from the trial after 12 weeks of placebo treatment." This patient was not reported in the safety outcomes. Comment: only 1 drop‐out (5%). Unlikely to have an important impact on outcomes. |
| Selective reporting (reporting bias) | High risk | Comment: methods section reports that nasal discharge would be physician‐assessed by rhinoscopy (pg 222, bullet point 1). No results are reported for this outcome. |
| Other bias | Unclear risk | Comment: no information regarding the validation of the disease severity measures. Only limited information provided in the study about baseline characteristics, pre‐randomisation procedures etc. |
Johansen 1993.
| Methods | 3‐arm, double‐blind, multicentre, parallel‐group RCT, with 3 months duration of treatment and follow‐up | |
| Participants |
Location: 4 sites in Denmark, 1 site in Sweden Setting of recruitment and treatment: unclear Sample size: Number randomised: 91 (numbers allocated to each group unknown) Number completed: 86 (numbers allocated to each group unknown) Participant (baseline) characteristics:
Inclusion criteria: clinical diagnosis of eosinophilic nasal polyposis with polyp scores of 2 or less on each side. Eosinophilic polyposis was confirmed by nasal smear and/or biopsy. Exclusion criteria: Polyps surgically removed within 2 months Neutrophilic polyposis Systemic or topical nasal corticosteroid therapy within 2 months |
|
| Interventions |
Group A (n = unknown): budesonide aqua (Rhinocort Aqua), 50 µg in each nostril x 2, twice daily (400 µg/day), 3 months Group B (n = unknown): budesonide aerosol (Rhinocort Aerosol), 50 µg in each nostril x 2, twice daily (400 µg/day), 3 months Group C (n = unknown): placebo (aqua or aerosol), unclear dose, 3 months Use of additional interventions (common to all treatment arms): no information was provided about additional interventions |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes 1. Disease severity, measured weekly by patients. Symptoms included were nasal obstruction, sneezing and nasal secretions, recorded for each nasal cavity (scale 0 to 3). Change in sense of smell was recorded at clinical visits using a 0 to 3 scale 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Other adverse effects: local irritation (including oral thrush, sore throat) 4. Polyps size (assessed using a 0 to 3 scale – definitions provided) Other outcomes reported by the study:
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "...randomised…" Comment: mentioned in abstract but no further mention |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information provided |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The patients were treated with either budesonide aqua (Rhinocort Aqua) or budesonide aerosol (Rhinocort Aerosol), 50 mcg x 2 in each nostril, twice daily = 400 mcg/day or placebo aqua or aerosol." Comment: there should be adequate blinding for treatment versus placebo, but not for different forms of treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no further information |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "Five patients withdrew from the study…" Comment: low (5%) drop‐out rate. No reasons given for withdrawals. Patients who withdrew were not included in any of the outcomes (including safety outcomes). |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes reported in the methods are mentioned in the results section, but numerical information for the results is not provided |
| Other bias | High risk | Comment: no comment on the validation of outcome measurements The paper does not provide clear background characteristics for each group. The number randomised to each group was not provided. |
Keith 2000.
| Methods | 2‐arm, double‐blind, multicentre, parallel‐group RCT, with 12‐week duration of blinded treatment and 12‐week duration of open treatment, with the active intervention followed by a final assessment 2 weeks after treatment had completed | |
| Participants |
Location: 11 sites in Canada and Finland Setting of recruitment and treatment: outpatient clinics Sample size: Number randomised: 52 in intervention, 52 in comparison Number completed: 51 in intervention, 47 in comparison Participant (baseline) characteristics: Age (mean ± SD): FP group: 49 ± 12; PL group: 47 ± 13 Gender (M/F): FP group: 38/14; PL group: 35/17 Main diagnosis: small or medium bilateral nasal polyposis Polyps status: 100% with polyps Previous sinus surgery status (n (%)): FP group: 38 (73%); PL group: 34 (65%) Other important effect modifiers: o 22% were atopic and allergic to one or more allergens o 2 patients in the study were reportedly aspirin‐sensitive (no information on which groups) o 52% in FP group and 44% in PL group had a polyposis history of > 10 years Inclusion criteria: outpatients aged 16 years and over with bilateral nasal polyposis. Polyps were graded by clinical assessment during rhinoscopic examination. Patients with a severity score of 1 (small) or 2 (medium) were included. Exclusion criteria: exclusion criteria included: Large (grade 3) polyps, indicating severe nasal obstruction Surgical treatment for nasal polyps during the last 3 months Cystic fibrosis Purulent nasal infection Allergic rhinitis Any disease likely to interfere with the study parameters or which gave evidence of any serious or unstable concurrent disease or psychological disorder Hypersensitivity or contraindication to steroids Currently receiving inhaled corticosteroids or those who had received depot or oral steroids during the previous 3 months Unable to cease treatment with intranasal steroids, or inhaled or intranasal sodium cromoglycate, at the screening visit. Astemizole during the last 6 weeks or other antihistamines within the last 48 hours, or received any other research medication during the previous month. Pregnant, lactating or, in the investigator's opinion, were not taking adequate contraceptive measures to avoid becoming pregnant during the study Had not correctly completed the daily diary card during the run‐in period |
|
| Interventions |
Intervention (n = 52): fluticasone propionate (unpreserved), nasal drops using head down and forwards position, 400 μg divided between both nostrils, once daily in the morning for 12 weeks Comparator group (n = 52): placebo nasal drops using head down and forwards position, to both nostrils once daily in the morning for 12 weeks Use of additional interventions (common to both treatment arms): loratadine tablets were provided as rescue medication for the relief of troublesome symptoms of rhinitis, to be used as needed, at a maximum dose of 10 mg once daily. No other medication was allowed for polyposis or rhinitis. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes 1. Disease severity, patient‐reported through daily diaries (nasal blockage, nasal discomfort and rhinitis symptoms) measured on a 4‐point scale (0 = none, 1 = mild, 2 = moderate, 3 = severe) at 12 weeks. Sense of smell was recorded as 0 (normal), 1 (slightly impaired), 2 (moderately impaired) or 3 (absent). 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Other adverse effects: local irritation (including oral thrush, sore throat) 4. Polyp size (0 to 3 score): 0 (no polyps), 1 (mild polyposis) = small polyps not reaching the upper edge of the inferior turbinate and causing only slight obstruction, 2 (moderate polyposis) = medium polyps reaching between the upper and lower edge of the inferior turbinate and causing troublesome obstruction, 3 (severe polyposis) = large polyps reaching below the lower edge of the inferior turbinate and causing almost/total obstruction Other outcomes reported by the study:
|
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "… computer randomized number …" Comment: proper sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: "… Each investigator was given a block of treatments, precoded with computer randomized numbers, which were assigned in ascending numerical order as patients presented." Comment: allocation concealment likely to be well maintained despite blocked randomisation; adequate blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "… FPND (400 mg unit dose preservative‐free suspension) and placebo solution were supplied in identical opaque nasal drop containers, in a foil pack …" Comment: adequate blinding |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: most outcomes are patient‐reported therefore blinding was likely to be well maintained |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 6/104 (5.8%) patients did not complete the study, 2 of these patients required polypectomy and so the last available outcome measures were reported for the efficacy outcomes. All patients were included in the safety analysis. |
| Selective reporting (reporting bias) | Unclear risk | Comment: results are presented in a different format to those presented in the methods section. For example the methods provides scales for each symptom but the results presents the percentage of time the value was below a certain value on the scale. It was unclear if this change was pre‐specified in the protocol or whether the study authors decided once the results had been processed. |
| Other bias | Unclear risk | Comment: no information is available regarding the validation of the scales used |
Lang 1983.
| Methods | 2‐arm, double‐blind, parallel‐group RCT, with a 2‐year duration of treatment and follow‐up | |
| Participants |
Location: unclear Setting of recruitment and treatment: unclear Sample size: Number randomised: 14 in intervention, 18 in comparison Number completed: unclear number of participants completed Participant (baseline) characteristics: Age (mean): 42 Gender (M/F): 17/15 Main diagnosis: clinical simple nasal polyps Polyps status: 100% with polyps Previous sinus surgery status: no details Other important effect modifiers: no details Inclusion criteria: no details Exclusion criteria: no details |
|
| Interventions |
Intervention (n = 14): beclomethasone dipropionate 400 μg twice daily for 2 years. No information on method of administration other than "Beconase topically in the nose" Comparator group (n = 18): placebo nasal insufflation twice daily for 2 years Use of additional interventions (common to both treatment arms): none mentioned |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes 1. Disease severity, measured by subjective assessment of nasal obstruction, sneezing and nasal discharge. No details of scale used. Assessment made every 4 weeks for 2 years. Secondary outcomes: 2. Size of nasal polyps – reported as the number of patients with "resolution" |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Support for judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "… randomly allocated …" Comment: no mention of method of randomisation |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Comment: no information provided |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Quote: "This allocation was kept blind from both patient and investigator." Comment: mentions a "placebo" was used but there was no information about precautions taken to make the products as similar as possible |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Quote: "This allocation was kept blind from both patient and investigator." Comment: method not specified |
| Selective reporting (reporting bias) | High risk | No information was provided about losses to follow‐up. Unlikely to have had no losses during a 2‐year study. |
| Other bias | High risk | Comment: abstract only. No information provided about adverse events. Very limited information provided. |
Lund 1998.
| Methods | Double‐blind, parallel‐group RCT, with 12 weeks of treatment | |
| Participants |
Location: UK Setting of recruitment and treatment: tertiary referral centre (Royal National ENT Hospital London) Sample size: Number randomised: 10 in fluticasone propionate, 10 in beclomethasone dipropionate, 9 in placebo Number completed: unclear, likely to be all Participant (baseline) characteristics: Age (mean, range): 52 (32 to 71, 46 (22 to 67) and 50 (27 to 69) in fluticasone propionate, beclomethasone dipropionate and placebo arms Gender (M/F): 7/3, 9/1 and 7/2 in fluticasone propionate, beclomethasone dipropionate and placebo arms Main diagnosis: "severe polyposis" Polyps status: all had polyps, median total polyps score of 4 (both nostrils) using Lund‐Mackay CT score Previous sinus surgery status: 66% had surgery (7/10 in fluticasone propionate and beclomethasone dipropionate arms, 5/9 in placebo) 59% had condition for more than 10 years All had allergy Inclusion criteria: Older than 16 years with a diagnosis of bilateral nasal polyposis requiring surgical intervention, meeting one or more of the following criteria: • a total polyp score of 4 or higher plus a CT scan score > 12; • a total polyp score of 3 or higher, a nasal blockage score of 2 or higher, plus a CT scan score >12; and • a total polyp score of 2 or higher, a nasal blockage score of 2 or higher, a CT scan > than 12, plus an UPSIT score > 32 Exclusion criteria: • Concurrent purulent nasal infection • A requirement for more than 1000 μg beclomethasone (or equivalent) per day for the treatment of asthma • An inability to cease treatment with parenteral and intranasal corticosteroids or cromolyn sodium (sodium cromoglycate) at visit 1, used astemizole in the 6 weeks before the study or other antihistamines in the 48 hours before visit 1, or a contraindication to corticosteroid medications |
|
| Interventions |
Intervention 1 (n = 10): fluticasone propionate aqueous nasal spray 400 µg per day, 2 actuations into each nostrils morning and night Intervention 2 (n = 10): beclomethasone dipropionate aqueous nasal spray 400 µg per day, 2 actuations into each nostrils morning and night Comparator (n = 9): placebo 2 sprays into each nostril twice a day Use of additional interventions (common to both treatment arms): terfenadine 60 mg as rescue medicine |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes:
Secondary outcomes:
Other outcomes reported by the study:
|
|
| Notes | Study had a 4‐week run‐in period 34 patients met criteria, 5 withdrew before randomisation (1 AE, 1 require polypectomy, 1 lack of efficacy, 2 did not return) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly allocated, using a computer‐generated random code and a block size of 6, to receive 1 of 3 treatments" |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Patients were randomly allocated, using a computer‐generated random code and a block size of 6, to receive 1 of 3 treatments" Comment: method not specified; blocked randomisation but adequate blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The placebo was identical to the active formulations with the active ingredient omitted and was indistinguishable from the active treatments, which were themselves identical in appearance, taste, and smell." Comment: there was a 4‐week pre‐treatment period where all patients were exposed to the placebo, but blinding should still be adequate |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: the same investigator did all the clinical assessments at all visits, but an identical placebo was used |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "last value carried forward technique" was used. Drop‐outs not balanced: 3/10 in fluticasone, 0/10 in beclomethasone and 4/9 in placebo. |
| Selective reporting (reporting bias) | High risk | Comment: patient‐reported symptoms (using diaries) were collected, but it was not specified how these were planned to be reported. Study only reported percentage of patients with 100% of days without nasal blockage, and the median % of days without nasal symptoms (different criteria). Other outcomes not reported at all. There was also a higher percentage of patients in the fluticasone group (70%) compared to 33% and 30% in the beclomethasone and placebo groups, but details were not reported. Only stated that 1 of the adverse events in the fluticasone group (throat irritation) was "predictable". |
| Other bias | High risk | Quote: "overall rhinitis symptoms (sneezing, rhinorrhoea, nasal itching)" Comment: symptoms scores (by patients and clinicians) were used but no mention of validation. Some items seem to be single symptom (e.g. nasal blockage), but others seems to encompass a few things (e.g. "overall rhinitis symptoms") Quote: "There was evidence, particularly from the acoustic rhinometric and PNIF data, that the patients randomly allocated to receive BDANS had milder symptoms than those randomly allocated to receive FPANS or placebo, even though all patients had been listed for surgical treatment on an equal basis before the study." Comment: baseline symptoms and other assessment scores were not reported. Unable to judge for other aspects. |
Lund 2004.
| Methods | Double‐blind, multicentre, parallel‐group RCT, with 20 weeks of treatment | |
| Participants |
Location: multicentre (19), UK (7), Hungary (6), South Africa (6) Setting of recruitment and treatment: all were ENT specialists, except 1 (in South Africa) Sample size: Number randomised: 81 in intervention, 86 in comparison Number completed: 67 in intervention, 67 in comparison Participant (baseline) characteristics: Age (mean): group A: 38; group B: 43 Gender (M/F): group A: 35/46; group B: 41/45 Main diagnosis: patients aged 18 years or over with chronic rhinosinusitis Polyps status: 0% with polyps Previous sinus surgery status: not provided Inclusion criteria: No nasal polyposis ≥ 18 years, with ≥ 12 weeks with at least 2 major symptoms Patients with a symptom score of ≥ 2 on a 4‐point scale for at least 1 of the symptoms for ≥ 4 out of 7 days during the last 7 days of the run‐in period Exclusion criteria: Sinonasal surgery within the previous 12 months |
|
| Interventions |
Intervention (n = 81): budesonide aqueous nasal spray, 128 μg (64 μg in each nostril twice daily), for 20 weeks Comparator group (n = 86): placebo nasal spray, for 20 weeks Use of additional interventions (common to both treatment arms): During the first 2 weeks of the run‐in period, all patients received co‐amoxiclav 250/125 mg three times daily, or 500 mg erythromycin twice daily The same antibiotics could be given for 2 weeks as needed to treat exacerbations (defined as episodes of worsening symptoms requiring a course of antibiotic therapy) |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes 1. Disease severity, measured by Chronic Sinusitis Survey (CSS) questionnaire at baseline and 20‐week time point (note: English‐speaking participants only as the survey was not validated in Hungarian) and patient‐reported scores for individual symptoms 2. Disease severity, measured by patient‐reported symptoms (facial pain, pressure or headache; facial congestion, nasal obstruction or blockage; nasal discharge; impairment of sense of smell). A combined symptom score (sum of the scores for the 4 domains of the symptoms above). 3. Significant adverse effect: epistaxis Secondary outcomes: 4. Health‐related quality of life, measured with the SF‐36 at baseline and 20‐week time point 5. Other adverse effects: local irritation (including oral thrush, sore throat) Other outcomes reported by the study:
|
|
| Notes | "Financial support" from AstraZeneca | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomisation was performed in balanced blocks of 4 by means of a computer program at the Department of Biostatistics…" Comment: pg 59, col 1, para 2 |
| Allocation concealment (selection bias) | Low risk | Quote: "Patients were allocated a treatment number in consecutive order and randomisation was performed in balanced blocks of 4 by means of a computer program…" "The treatment codes were known only to the persons responsible for packaging, who were not involved in the study in any other way" Comment: pg 59, col 1, para 2 |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "BANS and placebo aqueous sprays were identical in appearance and were both administered via the same vehicle." "The treatment codes were known only to the persons responsible for packaging, who were not involved in the study in any other way" Comment: pg 59, col 1, para 2, para 3 |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: "The treatment codes were known only to the persons responsible for packaging, who were not involved in the study in any other way " Comment: pg 59, col 1, para 2, para 3 |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 14/81 (17.3%) in intervention arm and 19/86 (22.1%) in comparison arm did not complete the study (overall rate 20%). No reasons for dropping out were provided. |
| Selective reporting (reporting bias) | Unclear risk | Comment: the methods section outlines the individual symptoms assessed, but the results are also presented as a "combined score" for which no information is available as to how it was calculated. Additionally, although all outcomes presented in the methods section are mentioned in the outcomes, this is not always in great detail, for example the results for the SF‐36 were presented as "There was a significant improvement in the general health sub‐scale of the SF‐36 questionnaire in the BANS treated group compared with placebo, but no other significant differences were observed." |
| Other bias | Unclear risk | Comment: no information about the validation of the combined symptom score. There is some information about validation of the chronic sinusitis score. 2 subgroups were reported (those with CT evidence of opacification and allergic versus non‐allergic patients). Not all outcomes are presented for these subgroups. There were pre‐randomisation procedures but these excluded patients with less symptomatic disease. |
Mosges 2011.
| Methods | 2‐arm, double‐blind, multicentre, parallel‐group RCT, with a 16‐week duration of treatment and follow‐up | |
| Participants |
Location: Germany, 9 sites Setting of recruitment and treatment: ear, nose and throat departments in university hospitals or ENT specialists' practices Sample size: Number randomised: 30 in intervention, 30 in comparison Number completed: 29 in intervention, 30 in comparison Participant (baseline) characteristics: Age: group A: 40 (19 to 63); group B: 44 (22 to 64) Gender (M/F): group A: 10/19; group B: 17/13 Main diagnosis: chronic rhinosinusitis (symptoms for a period longer than 8 weeks, or more than 4 episodes of a minimum length of 10 days each, over a 1‐year period; Lund score ≤ 10) Polyps status: 0% with polyps (exclusion criteria) Previous sinus surgery status: Discussion states "… only around 10% underwent preceding surgical treatment." However, surgery within 6 months and extensive surgery were exclusion criteria. Other important effect modifiers: Discussion states: "Allergy was present in only around 1 in 3 patients, who were distributed evenly between both treatment groups.". No other details provided. Inclusion criteria: Patients aged 18 to 65 years Clinical diagnosis of chronic sinusitis (a total symptom score of at least 5) confirmed at baseline by a coronal CT scan not older than 6 months. The scan was evaluated using the Lund scale. Exclusion criteria: Nasal polyps visible on endoscopic examination at baseline Patients with pansinusitis, Lund score > 10 Undergone nasal surgery within 6 months prior to study enrolment Patients who had undergone sinus surgery with opening of the lateral nasal wall at any time Inhalant or intranasal steroid therapy within 2 weeks prior to screening; systemic steroid therapy within 8 weeks prior to screening; antihistamine use within 12 hours to 14 days prior to screening depending on medication; regular use of decongestants within 24 hours to 3 days depending on medication; acute sinusitis or concurrent acute nasal infection, or upper respiratory tract infection, ongoing or within 2 weeks prior to screening |
|
| Interventions |
Intervention (n = 29): mometasone furoate nasal spray, 200 μg twice daily (morning and evening) to the lateral nasal wall (not to the septum), in the 'vertex to floor' position, over 16 weeks Comparator group (n = 30): placebo nasal spray, twice daily (morning and evening) to the lateral nasal wall (not to the septum), in the 'vertex to floor' position, over 16 weeks Use of additional interventions (common to both treatment arms): no information |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes 1. Total symptom score (TSS): sum of the 5 individual symptom score values (rhinorrhoea, postnasal drip, nasal obstruction, facial pain or pressure, and headache). Each symptom score was assessed on a 4‐point scale (0 = no symptoms; 1 = mild; 2 = moderate; 3 = severe) 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Endoscopy score (endoscopic evaluation at every visit) Other outcomes reported by the study: 4. Patient evaluation of therapeutic response 5. Compliance of medication used (measured by weighing bottles) 6. Other adverse events |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All qualified patients were randomized, according to a computer‐generated code in a 1:1 ratio…" |
| Allocation concealment (selection bias) | Low risk | Quote: "all patients who met the protocol requirements were assigned a randomization number that corresponded to the treatment unit they were given… The randomization schedule for blinding of treatments was maintained by the sponsor" Comment: no description of allocation concealment but should be low risk, as there is adequate sequence generation and double‐blinding |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The bottles of MFNS or placebo for the 16‐week treatment period had identical labels. The randomization schedule for blinding of treatments was maintained by the sponsor, and was disclosed only after study completion and database closure." Comment: sealed envelopes containing individual patient allocations were provided as a safety measure but whether any of these were opened is not stated; it is not likely to be enough to affect to affect blinding since only 6 (10%) withdrew |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: as above |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: only 6 patients dropped out from the study. However, the study excluded patients with "major protocol violation" (not defined) resulting in an "ITT" population of only 75% and an even lower per protocol population (39% excluded) |
| Selective reporting (reporting bias) | High risk | The following outcomes are stated in the methods section but not reported in paper: "patient compliance, Rhinosinusitis Disability Index, Work Productivity and Activity Impairment questionnaires" Comment: pg 242, col 2, para 1 |
| Other bias | Unclear risk | Comment: there is no mention of the validation of the "total symptom score" measure |
Parikh 2001.
| Methods | 2‐arm, double‐blind, parallel‐group RCT, with a 16‐week duration of treatment and follow‐up | |
| Participants |
Location: UK, single site Setting of recruitment and treatment: tertiary ENT clinic Sample size: Number randomised: 14 in intervention, 15 in comparison Number completed: 9 in intervention, 13 in comparison Participant (baseline) characteristics: Age: FP: 45.1 ± 10.7; PL: 48 ± 20 Gender (M/F): FP: 2/7; PL: 7/6 Main diagnosis: chronic rhinosinusitis Polyps status: FP: 2/9; PL: 2/13 Previous sinus surgery status, mean ± SD: FP: 3 ± 6.2; PL: 2.8 ± 3.4 Previous courses of steroids: no information Other important effect modifiers o Skin prick test positive: FP: 7/9; PL: 8/11 o Asthma: FP: 2/9; PL: 3/13 Inclusion criteria: More than 3 months history of recurrent discoloured rhinorrhoea (> 2 weeks per episode), accompanied by more than 2 of the following symptoms: nasal obstruction, headache, facial pain, fever Endoscopy or CT scan evidence of CRS Exclusion criteria: Acute exacerbation in the previous 2 weeks, on oral or depot corticosteroids in the previous 3 weeks, on INCS in the previous 2 weeks Other severe concurrent illness |
|
| Interventions |
Intervention (n = 9): fluticasone propionate nasal spray, 400 µg per day, administered twice daily Comparator group (n = 13): placebo, administered twice daily Use of additional interventions (common to both treatment arms): not described |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes: 1. Disease severity symptom score – measured using a 10 cm VAS for blockage, sense of smell, sneezing, discharge from the front of nose, discharge from the back of nose, headache, facial pain. Also used diary cards. 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Endoscopy: Lund‐Kennedy score 4. Local irritation – itchiness of the nose, throat and ear Other outcomes reported by the study: Middle meatus swabs Acoustic rhinometry Blood tests |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…randomisation code was generated and maintained by personnel in the pharmacy. The investigators were not involved in the process of randomisation" Comment: adequate randomisation |
| Allocation concealment (selection bias) | Low risk | Comment: no specific information; should be adequate since the investigators were not involved in randomisation and blinding was adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Placebo spray had benzalkonium chloride in the same concentration as fluticasone propionate, and other had rose scent to mask any differences in smell. The study medications were prepared and supplied by Glaxo…" Comment: identical‐looking, with the same composition and smell masking |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: as above, since most outcomes were patient‐reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 5/14 (36%) in treatment group and 2/15 (13%) in placebo group dropped out. The percentage is high and unbalanced. Most did not turn up for follow‐up. |
| Selective reporting (reporting bias) | High risk | Adverse effects such as epistaxis and local irrigation were mentioned as measured by one of the VAS, but were not reported separately. Unclear which symptoms were added up into the overall score for "symptom score" or "diary card score". |
| Other bias | Unclear risk | Comment: study used a 10 cm VAS for symptom scores. Although not formally validated, it should provide adequate discriminant validity for each item. Unclear which symptoms were added up into the overall score. |
Penttilla 2000.
| Methods | 3‐arm, single‐blind, international, multicentre, parallel‐group RCT, with a 12‐week duration of treatment | |
| Participants |
Location: 12 centres in Denmark (3 centres), Finland (1 centre) and Sweden (1 centre) Setting of recruitment and treatment: outpatient clinics Sample size: Number randomised: 47 in 400 µg FPND twice daily, 48 in 400 µg FPND once daily, 47 in placebo Number completed: 45 in 400 µg FPND twice daily, 47 in 400 µg FPND once daily, 41 in placebo Participant (baseline) characteristics: Age: mean 51, range 22 to 83 Gender: M/F; 107/35 (%M: 75.4%) Main diagnosis: nasal polyposis Polyps status: 100% with polyps Previous sinus surgery status: 72% previous polypectomy (not within 3 months of trial) Inclusion criteria: at least 16 years old, bilateral mild or moderate nasal polyposis Exclusion criteria: severe polyposis (large polyps reaching below the lower edge of the inferior turbinate, causing total obstruction), concurrent purulent nasal infection, unable to cease treatment with intranasal steroids or sodium cromoglycate during run‐in period. Also excluded: people currently receiving inhaled corticosteroids or who had received depot or oral steroids within previous 3 months, patients who had received astemizole in the 6 weeks prior to the first clinic visit, patients who had undergone nasal polyp surgery in the previous 3 months, patients with hypersensitivity or contraindication to steroids, patients with allergic rhinitis or any other disease likely to interfere with outcomes, patients who were pregnant, lactating or likely to become pregnant during the study period. |
|
| Interventions |
Intervention A (n = 47): fluticasone propionate nasal drops (FPND), 400 µg twice daily for 12 weeks Intervention B (n = 48): fluticasone propionate nasal drops (FPND), 400 µg once daily for 12 weeks plus placebo drops once daily for 12 weeks Comparator group C (n = 47): placebo nasal drops twice daily for 12 weeks Process: contents were divided between both nostrils (200 µg per nostril) in the head down and forward position Use of additional interventions (common to both treatment arms): all patients underwent a 2‐week run‐in period during which they ceased all medication for polyposis except loratadine tables for relief of troublesome symptoms (10 mg daily maximum) Initial visit: physical and oropharyngeal examinations and details of clinical history Initial and 12‐week visit: blood and urine samples |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes 1. Disease severity, measured by assessing nasal blockage (0 to 3 scale) and overall rhinitis symptoms including sneezing, rhinorrhoea and nasal itching (0 to 3 scale) and sense of smell (0 to 3 scale) at 12 weeks after treatment 2. Nasal blockage, overall rhinitis 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Other adverse effects: local irritation 5. Polyps size Other outcomes reported by the study: peak nasal inspiratory flow (PNIF), olfactory function, rescue medication usage and adverse events |
|
| Notes | — | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…double blind randomised treatment...", Figure 1, pg 95 Comment: no further information provided, but this is an "international, multicentre" study in 12 centres across 3 countries with regional monitors. Should have adequate sequence generation procedures. |
| Allocation concealment (selection bias) | Low risk | Quote: "…double blind randomised treatment...", Figure 1, pg 95 Comment: no further information provided. As above, allocation concealment should be adequate. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "active and placebo nasal drops were provided in identical single‐dose containers …" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no further information provided. Should be adequate with use of adequate double‐blinding. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Sixteen patients were withdrawn during the randomized treatment phase, the majority due to lack of efficacy (five placebo, one FP 400 mg o.d., two FP 800 mg b.i.d.) or adverse events (five placebo, one FP 400 mg o.d., two FP 400 mg b.i.d.). One patient in the placebo group withdrew due to requirement for polypectomy. Two patients withdrew during the open phase, one requiring a polypectomy, the other for unspecified reasons", pg 97, col 2. Comment: 16/142 (11.3%) withdrew: 10/47 placebo, 4/47 400 µg twice daily and 2/48 400 µg once daily did not completed the study. All of these patients were included as the ITT population with imputation. Unbalanced drop‐out rates (21% in placebo) versus 8.5% and 4% in the twice and once daily active treatment groups. |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcome measures in the methods were discussed in the results section. However the diary card data were interpreted by using different cut‐off points, which do not appear to be pre‐specified in the methods section. Significant results were reported but not those that were not significant. |
| Other bias | Unclear risk | Comment: no mention of validation of symptom criteria used for the primary outcomes |
Small 2005.
| Methods | 3‐arm, double‐blind, multicentre, parallel‐group RCT, with 4‐month duration of treatment and follow‐up | |
| Participants |
Location: 44 medical centres "worldwide" Setting: no information Sample size: Number randomised: 122 in 400 µg, 115 in 200 µg, 117 in placebo group respectively Number completed: 109 in 400 µg, 101 in 200 µg, 95 in placebo group respectively Participant (baseline) characteristics:
Inclusion criteria: ≥ 18 years with an endoscopically confirmed diagnosis of bilateral nasal polyps (at least 1 on a scale of 0 to 3) and clinically significant nasal congestion/obstruction (average morning score 2 or higher on as scale of 0 to 3 for each of the last 7 days of the 14‐day run‐in period) If had asthma, had a documented FEV1 ≥ 80% of the predicted value within the 6 months before screening and no asthma exacerbations within 30 days before screening. Those treated with inhaled corticosteroids were required to be on a moderate, stable regimen of beclomethasone dipropionate ≤ 800 mg/d or equivalent for 1 month before screening and to remain on a stable regimen throughout the study period. Exclusion criteria: Seasonal allergic rhinitis within the past 2 years Sinus or nasal surgery within the previous 6 months or 3 nasal surgeries (or any surgical procedure preventing an accurate grading of polyps) Presumed fibrotic nasal polyposis, or complete or near complete nasal obstruction Nasal septal deviation requiring corrective surgery Nasal septal perforation Acute sinusitis, nasal infection or upper respiratory tract infection at screening or in the 2 weeks before screening; ongoing rhinitis medicamentosa Churg‐Strauss syndrome Dyskinetic ciliary syndromes Cystic fibrosis Glaucoma or a history of posterior subcapsular cataracts; allergies to corticosteroids or aspirin, or any other clinically significant disease that would interfere with the evaluation of therapy |
|
| Interventions |
400 µg group (n = 122): mometasone furoate nasal spray 200 μg twice daily (morning and evening) for 4 months 200 µg group (n = 115): mometasone furoate nasal spray 200 μg once daily (morning, matching placebo used in the evening) for 4 months Placebo group (n = 117): placebo nasal spray twice daily (morning and evening) for 4 months Use of additional interventions (common to both treatment arms): Acetaminophen (paracetamol) was encouraged for analgesic purposes; NSAIDs limited to 5 consecutive days if alternative analgesia was required. Antibiotics were administered for bacterial infections at the discretion of the principal investigator. Concomitant medications that would interfere with study evaluations were not permitted, including nasal sodium cromolyn; nasal atropine or ipratropium bromide; corticosteroids (except oral inhaled corticosteroids for asthma or mild‐strength or mid‐strength topical corticosteroids for dermatologic purposes); antihistamines; decongestants; topical, oral or ocular anti‐inflammatory drugs; or topical nasal or oral antifungal agents. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes 1. Disease severity, patient evaluation of symptoms (congestion/obstruction, loss of sense of smell, anterior rhinorrhoea and postnasal drip) measured daily on a diary card on a 4‐point scale (0 = none, 3 = severe) 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Other adverse effects: local irritation Other outcomes reported by the study: Polyps grade; bilateral score and proportion of patients demonstrating an improvement at endpoint Therapeutic response (rated by investigator) Peak nasal inspiratory flow Treatment compliance Number of withdrawals due to AE and events occurring in more than 2% of participants in any group |
|
| Notes | Supported by a grant from the Schering‐Plough Research Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…randomised in a 1:1:1 ratio to 3 treatment arms…" Comment: no further information. However, this is a large multinational RCT and should have adequate resources to conduct proper sequence generation. |
| Allocation concealment (selection bias) | Low risk | Comment: no information provided. However, this is a multinational trial with adequate blinding and should have adequate sequence generation and allocation concealment procedures. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind, double dummy"; "…matching placebo nasal spray …" Comment: pg 1276, col 1, para 1 and 2. "Matching placebo spray" mentioned and those on the 200 µg/day regimen were also given placebo nasal spray for the evening. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no information. Likely to remain well blinded until end of study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: 305/354 patients (86%) patients "completed 4‐month treatment period" Comment: higher % of patients not completing in the placebo group 22/117 (19%); compared to the twice daily or once daily groups 13/122 (11%) and 14/114 (12%), respectively. Study mentioned analyses based on "all randomised subjects" using the "ITT principle" and endpoint was "defined as the last non‐missing reading for the subject" for bilateral polyps score; however, unlikely all were analysed as numbers do not tally exactly with the "meta‐analysis subsequently reported" |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes reported in the methods section were reported in the results section |
| Other bias | Unclear risk | Comment: no information about the validation of outcome measures |
Stjarne 2006.
| Methods | 3‐arm, double‐blind, multicentre, parallel‐group RCT, with a 4‐month duration of treatment and follow‐up | |
| Participants |
Location: 24 centres in 17 countries worldwide Setting: study conducted from 25 June 2001 to 20 January 2003 Sample size: Number randomised: 102 in 400 µg, 102 in 200 µg, 106 in placebo group, respectively Number completed: 93 in 400 µg, 94 in 200 µg, 87 in placebo group, respectively Participant (baseline) characteristics: Main diagnosis: bilateral nasal polyps and clinically significant congestion/obstruction Age (mean): 400 µg: 47.6; 200 µg: 47.2; placebo: 50.9 Gender (%M/%F): 400 µg: 62/38; 200 µg: 70/30; placebo: 65/35 Polyps status: 100% with polyps Previous sinus surgery status: not more than 3 times or within past 6 months Other important effect modifiers: o Asthma history (%): 400 µg: 19; 200 µg: 15; placebo: 17 o Perennial allergic rhinitis history (%): 400 µg: 18; 200 µg: 14; placebo: 22 Inclusion criteria: ≥ 18 years with an endoscopically confirmed diagnosis of bilateral nasal and clinically significant nasal congestion/obstruction (average morning score 2 or higher on as scale of 0 to 3 for each of the last 7 days of the 14‐day run‐in period). If had asthma, had a documented FEV1 ≥ 80% of the predicted value within the 6 months before screening and no asthma exacerbations within 30 days before screening. Those treated with inhaled corticosteroids were required to be on a moderate, stable regimen of beclomethasone dipropionate ≤ 800 mg/d or equivalent for 1 month before screening and to remain on a stable regimen throughout the study period. Exclusion criteria: Seasonal allergic rhinitis within the past 2 years Sinus or nasal surgery within the previous 6 months or 3 nasal surgeries (or any surgical procedure preventing an accurate grading of polyps) Presumed fibrotic nasal polyposis, or complete or near complete nasal obstruction Nasal septal deviation requiring corrective surgery or nasal septal perforation Acute sinusitis, nasal infection or upper respiratory tract infection at screening or in the 2 weeks before screening Ongoing rhinitis medicamentosa Churg‐Strauss syndrome Dyskinetic ciliary syndromes Cystic fibrosis Glaucoma or a history of posterior subcapsular cataracts Allergies to corticosteroids or aspirin, or any other clinically significant disease that would interfere with the evaluation of therapy |
|
| Interventions |
400 µg group (n = 102): mometasone furoate nasal spray 200 μg twice daily (morning and evening) for 4 months 200 µg group (n = 102): mometasone furoate nasal spray 200 μg once daily (morning, matching placebo used in the evening) for 4 months Placebo group (n = 106): placebo nasal spray twice daily (morning and evening) for 4 months Use of additional interventions (common to both treatment arms): Acetaminophen (paracetamol) was encouraged for analgesic purposes; NSAIDs limited to 5 consecutive days if alternative analgesia was required. Antibiotics were administered for bacterial infections at the discretion of the principal investigator. Concomitant medications that would interfere with study evaluations were not permitted, including nasal sodium cromolyn; nasal atropine or ipratropium bromide; corticosteroids (except oral inhaled corticosteroids for asthma or mild‐strength or mid‐strength topical corticosteroids for dermatologic purposes); antihistamines; decongestants; topical, oral or ocular anti‐inflammatory drugs; or topical nasal or oral antifungal agents. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes 1. Disease severity, patient evaluation of symptoms (congestion/obstruction, loss of sense of smell, anterior rhinorrhoea and postnasal drip) measured daily on a diary card on a 4‐point scale (0 = none, 3 = severe) 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Other adverse effects: local irritation 4. Polyps grade; bilateral score and proportion of patients demonstrating an improvement at endpoint Other outcomes reported by the study: Therapeutic response (rated by investigator) Peak nasal inspiratory flow Treatment compliance Number of withdrawals due to AE and events occurring in more than 2% of participants in any group |
|
| Notes | Supported by a grant from the Schering‐Plough Research Institute | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed in blocks of 3 using random numbers generated by SAS function UNIFORM (SAS Institute, Cary, NC) with seed based on clock time. Randomization was stratified by the presence or absence of concurrent asthma." Comment: computerised randomisation |
| Allocation concealment (selection bias) | Low risk | Comment: although randomisation was blocked, blinding should be adequate |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "double blind"; "…matching placebo nasal spray …" Comment: "Matching placebo spray" mentioned; dosing regimen the same across all groups |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: no information. Likely to remain well blinded until end of study. |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "More than 85% of subjects completed the 4‐month treatment period, with more than twice as many placebo recipients as active drug recipients discontinuing during the treatment phase (18% vs 8%)." Comment: drop‐out rates not balanced |
| Selective reporting (reporting bias) | Unclear risk | Comment: although all outcomes mentioned in the methods were reported, these were mostly not in sufficient detail (e.g. only P values) |
| Other bias | Unclear risk | Comment: no information about the validation of outcome measures |
Stjarne 2006a.
| Methods | Double‐blind, multicentre, parallel‐group RCT, with a 4‐month duration of treatment and follow‐up | |
| Participants |
Location: 12 centres in Denmark, Finland, Norway and Sweden Setting: outpatient clinics Sample size: Number randomised: 153 in 200 µg, 145 in placebo group, respectively (298) Number completed: 134 in 200 µg, 101 in placebo group, respectively (235) Participant (baseline) characteristics: Main diagnosis: bilateral nasal polyps and clinically significant congestion/obstruction Age (mean): 53 (range 20 to 86) Gender (%M/%F): 200 µg group: 74.5/25.5; placebo group: 71.7/28.3 Polyps status: 100% with polyps Previous sinus surgery status: > 2 surgeries, 25.5% in 200 µg group 26.2% in placebo group Other important effect modifiers: Inclusion criteria: Age ≥ 18 years; a diagnosis of bilateral nasal polyps and clinically significant nasal congestion. Nasal congestion was defined as significant when the symptom score was ≥ 2 (on a scale of 0 to 3) for ≥ 4 days per week during the month before screening, at screening and at the baseline visit. Exclusion criteria: Nasal polyp surgery within the 6 months before screening; unhealed nasal surgery or trauma; polyp size of 3 (on a scale of 0 to 3); the presence of polyps that could interfere with nasal spray application; significant nasal structural abnormalities; ongoing concurrent nasal infections; glaucoma with narrow anterior chamber angle of the eye; rhinitis medicamentosa; or hereditary mucociliary dysfunction |
|
| Interventions |
200 µg group (n = 153): mometasone furoate nasal spray 200 μg once daily (morning, matching placebo used in the evening) for 16 weeks Placebo group (n = 145): placebo nasal spray twice daily (morning and evening) for 16 weeks Concomitant medications that would interfere with study evaluations were not permitted, including nasal sodium cromolyn; nasal atropine or ipratropium bromide; corticosteroids (except oral inhaled corticosteroids for asthma or mild‐strength or mid‐strength topical corticosteroids for dermatologic purposes); antihistamines; decongestants; topical, oral or ocular anti‐inflammatory drugs; or topical nasal or oral antifungal agents |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes 1. Disease severity – participants with improvement in score 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Other adverse effects: local irritation Other outcomes reported by the study: Investigator‐evaluated nasal congestion, sense of smell and rhinorrhoea PNIF Treatment response score Olfactory threshold |
|
| Notes | Participants who met the eligibility criteria at the screening visit (visit 1) underwent a 2‐ to 4‐week no treatment run‐in period. Criteria to remain in study/numbers subsequently excluded not reported. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "…randomized at the baseline visit (day 0, visit 2) according to a computer‐generated code…" Comment: adequate randomisation |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization schedule for the blinded treatments was maintained by the sponsor and only disclosed after the study was completed and the database closed." Comment: adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The placebo aqueous nasal spray was formulated to match MFNS exactly, except for the active ingredient." Comment: matching placebo |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: as above As key outcomes were patient‐reported and there was adequate blinding, it is likely there was adequate blinding for outcome assessment |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "Of the 298 subjects randomized to treatment, 235 (78.9%) completed the study. Premature withdrawals were more common in the placebo group than in the MFNS group (30.3% vs 12.4% of subjects, respectively)." Comment: drop‐out rates not balanced across groups |
| Selective reporting (reporting bias) | Unclear risk | Comment: all outcomes mentioned in the methods section were reported in the results section, although these were mostly not in sufficient detail. |
| Other bias | Unclear risk | Quote: "Approximately 10% of subjects were considered to be noncompliant with the dosing regimen (defined as missing study medication doses for ≥7 consecutive days up to a maximum of 10 days, using rescue medication for ≥10 days during treatment, or using prohibited concomitant medications)." Comment: there was a 2 to 4‐week run‐in period – criteria to remain in study not reported. There was no information about the validation of instruments used to measure symptoms. |
Vlckova 2009.
| Methods | Double‐blind, placebo‐controlled, multicentre, parallel‐group RCT, with a 12‐week duration of treatment and a 14‐week duration of follow‐up | |
| Participants |
Location: Czech Republic, 5 sites Setting of recruitment and treatment: 5 otorhinolaryngology hospital clinics Sample size: Number randomised: 54 in intervention, 55 in comparison Number completed: 54 in intervention, 52 in comparison Participant (baseline) characteristics: Age, mean (range): FP: 48.9 (18 to 65), PL: 47.0 (23 to 63) Gender (M/F): FP: 74/26; PL: 62/38 Main diagnosis: mild to moderate bilateral nasal polyposis Polyps status: all; about 50% in each arm had mild, 50% had moderate grade polyps Previous sinus surgery status: never had surgery FP: 23 (43%), PL: 15 (27%); had at least 4 surgeries FP: 4 (7%), PL: 7 (13%) Previous courses of steroids: no information Other important effect modifiers, if applicable (e.g. aspirin sensitivity, comorbidities of asthma): asthma: FP: 17%; PL: 18% Inclusion criteria: Age 18 to 65 years, a diagnosis of bilateral nasal polyposis graded as mild or moderate Verified airflow through both nostrils, an ability to close the soft palate and the ability to trigger the breath‐actuation mechanism of a device in accordance with the instructions for use Exclusion criteria: Large polyps (grade 3) Nasal polyp surgery during the 3 months before screening Cystic fibrosis, a purulent nasal infection, allergic rhinitis or other disease likely to interfere with the study parameters Depot or oral steroids during the previous 3 months Cleft palate Concomitant medications that would interfere with study evaluations were not permitted |
|
| Interventions |
Intervention (n = 54): fluticasone propionate 800 µg/day delivered with breadth actuated inhaler twice a day Comparator group (n = 55): placebo, twice a day Use of additional interventions (common to both treatment arms): Saline rinsing and devices that dilate the nostrils were prohibited Loratadine 10 mg tablets were provided as rescue medication for the relief of troublesome acute allergic symptoms. If a participant experienced a severe acute nasal blockage, the investigator could authorise the use of a short course of oxymetazoline drops or spray for a maximum of 7 consecutive days and a total maximum of 10 days during the treatment period. Oxymetazoline was not to be used within 24 hours of a scheduled study visit. |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes: 1. Disease severity symptom score, using both a global rating scale (very much improved; improved; same; worse or very much worse). Also used a diary with the following scoring system: 0 (none), 1 (mild – symptoms present but not troublesome), 2 (moderate – symptoms frequently troublesome but not interfering with daily activity or night time sleep) or 3 (symptoms troublesome and interfering with daily activity or night‐time sleep) for nasal blockage, nasal discomfort (facial and sinus pain and pressure) and rhinitis (nasal secretion, itching, irritation and sneezing). Sense of smell was rated as follows: 0 (normal), 1 (slightly impaired), 2 (moderately impaired) or 3 (absent). 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Endoscopy: polyp size was graded for each nostril using the Lildholdt scale. Some authors classify polyps causing total obstruction as grade 4. The score was presented for each nostril, the worst affected nostril and the summed score for both nostrils. 4. Adverse event: local irritation 5. CT scan Other outcomes reported by the study:
|
|
| Notes | Study had a 14‐ to 16‐day treatment‐free run‐in Compliance was high with 98.92% of administrations made in the Opt‐FP treatment group and 99.05% made in the placebo group |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "…randomized in a 1:1 ratio" Comment: no further description of randomisation methods |
| Allocation concealment (selection bias) | Unclear risk | Comment: no information about allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "…Opt‐FP and placebo breath‐actuated bi‐directional delivery devices were identical in appearance…. The placebo aqueous nasal spray was formulated to match FP exactly, except for the active ingredient... To deliver a dose of FP 400 μg b.i.d. or matching placebo, the subjects made two administrations to each nostril in the morning and the evening..." Comment: identical‐looking devices and same frequency of administration. Same formulation, except the omission of the active ingredient. Should be able to maintain good blinding. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Quote: as above Comment: most are patient‐reported outcomes therefore blinding should be adequate |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "A total of 106 subjects (97%) completed the study. Three subjects withdrew, all in the placebo group (one due to worsening of polyps, two withdrew consent)." Comment: low drop‐out rate |
| Selective reporting (reporting bias) | Unclear risk | Comment: no protocol was available. Most outcomes are reported in graphs. Global improvement score dichotomised when reported (not pre‐specified). |
| Other bias | Unclear risk | Comment: no mention of validation of questionnaires |
Zhou 2015.
| Methods | 2‐arm, double‐blind, multicentre, parallel‐group RCT, with a 16‐week duration of treatment and follow‐up | |
| Participants |
Location: China, 28 sites Setting of recruitment and treatment: not clear Sample size: 748 Number randomised: 375 in intervention, 373 in comparison Number completed: 350 in intervention, 336 in comparison Participant (baseline) characteristics:
Inclusion criteria:
Exclusion criteria:
|
|
| Interventions |
Intervention (n = 375): mometasone nasal spray, 400 µg per day (200 µg twice daily), 16 weeks Comparator group (n = 373): matching placebo nasal spray, twice daily for 16 weeks Use of additional interventions: The following were prohibited: nasal sodium cromolyn; nasal atropine or ipratropium bromide; guaifenesin; oral/intramuscular/intranasal corticosteroids (except oral inhaled corticosteroids for asthma or mild‐strength topical corticosteroids for dermatologic purposes); antihistamines; decongestants; topical, oral or ocular antiinflammatory drugs; nonsteroidal anti‐inflammatory drugs; or topical nasal or oral antifungal agents |
|
| Outcomes |
Outcomes of interest in the review: Primary outcomes: 1. Disease severity symptom score; patients reported individual symptom scores at 16 weeks, measured on a scale of 0 to 3 (0 = none; 1 = mild; 2 = moderate; 3 = severe) for the following symptoms: nasal obstruction, anterior rhinorrhoea, post‐nasal drip, loss of sense of smell 2. Significant adverse effect: epistaxis Secondary outcomes: 3. Endoscopy (nasal polyps size); measured at 16 weeks in both left and right nasal fossa on a scale of 0 to 3 (see inclusion criteria for details) and summed for each nasal fossa to give a total score 4. Adverse effects: local irritation Other outcomes reported by the study:
|
|
| Notes | All patients had a 14‐day, single‐blind placebo run‐in period where signs and symptoms of nasal polyps were evaluated. The compliance rate was similar; 95.5% (MFNS) and 94.8% (placebo) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "At baseline, patients were randomised (1:1 ratio) according to a computer‐generated allocation schedule" Comment: adequate randomisation method |
| Allocation concealment (selection bias) | Low risk | Quote: "Study medication was supplied for double‐blind administration in a treatment kit labelled with study number, subject number, and dosing instructions." Comment: adequate allocation concealment |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "Study medication was supplied for double‐blind administration in a treatment kit labelled with study number, subject number, and dosing instructions." Comment: trial was "double‐blind". Patients in the control group received a "matching placebo nasal spray" |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Comment: there is no information about the blinding of outcome assessors. However, most outcomes were patient‐reported and if there was adequate blinding this should be sufficient. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 25/375 (6.7%) in the intervention group and 37/336 (9.9%) in the control group discontinued treatment. The reasons are provided in the paper and the small numbers are unlikely to affect the results significantly. |
| Selective reporting (reporting bias) | Low risk | Comment: the protocol for this study was available (https://clinicaltrials.gov/ct2/show/NCT01386125). The analysis method, primary outcomes and reporting of adverse events is well described in the protocol and reported as planned within the paper. |
| Other bias | Unclear risk | Comment: there is no information within the paper regarding validation of any of the outcomes used |
AE: adverse event ASA: acetylsalicylic acid BDP: beclomethasone dipropionate CRS: chronic rhinosinusitis CT: computed tomography d: day ENT: ear, nose and throat F: female FESS: functional endoscopic sinus surgery FEV1: forced expiratory volume in one second FP: fluticasone propionate FPND: fluticasone propionate nasal drops INCS: intranasal corticosteroids ITT: intention‐to‐treat M: male MRI: magnetic resonance imaging NSAIDs: non‐steroidal anti‐inflammatory drugs PL: placebo PNIF: peak nasal inspiratory flow RCT: randomised controlled trial RSOM‐31: Rhinosinusitis Outcome Measures‐31 instrument SD: standard deviation UPSIT: University of Pennsylvania Smell Identification Test VAS: visual analogue scale
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| ALA 2015 | POPULATION: children and adults with uncontrolled asthma and CRS. The population did not meet the EPOS definition of CRS. |
| Albu 2010 | DESIGN: perioperative treatment using topical steroids for improvement of surgical outcomes in patients undergoing surgery |
| Bross‐Soriano 2004 | POPULATION: all patients underwent endoscopic polypectomy at the start of the trial |
| Cassano 1996 | POPULATION: all patients had surgery at the start of the trial |
| Chalton 1985 | DURATION: treatment and follow‐up only 4 weeks (betamethasone drops) |
| Cuenant 1986 | POPULATION: chronic allergic or bacterial sinusitis |
| Dijkstra 2004 | POPULATION: treatment started 1 week after FESS (continued for 1 year) |
| Drettner 1982 | POPULATION: treatment started 4 weeks after polypectomy surgery (continued for 3 months) |
| Ehnhage 2009 | DESIGN: perioperative study; patients received intranasal corticosteroids/placebo before FESS, and then carried on intranasal corticosteroids/placebo |
| el Naggar 1995 | STUDY DESIGN: treatment started immediately after intranasal polypectomy; randomised by side of nose |
| Filiaci 2000 | STUDY DESIGN: treatment and follow‐up only 8 weeks (budesonide) |
| Furukido 2005 | INTERVENTION: YAMIK sinus catheter for 4 weeks |
| Gulati 2001 | POPULATION: treatment started 1 week after polypectomy surgery (continued for 3 months) |
| Hartwig 1988 | POPULATION: all patients had polypectomy and treatment started the day after surgery |
| Jankowski 2001 | DURATION: treatment only 8 weeks (budesonide) |
| Jankowski 2009 | DURATION: there was an intranasal corticosteroids (fluticasone) versus placebo comparison only for 8 weeks, subsequently all patients had intranasal corticosteroids (for 6 months) |
| Johansson 2002 | DURATION: treatment and follow‐up only 2 weeks |
| Jorissen 2009 | POPULATION: all participants had FESS at the start of the trial |
| Jurkiewicz 2004 | POPULATION: started treatment after endoscopic polypectomy |
| Kang 2008 | POPULATION: study started directly after FESS |
| Kapucu 2012 | DURATION: follow‐up only 1 month (triamcinolone intra‐polyp steroid injections or nasal spray versus placebo) |
| Karlsson 1982 | POPULATION: included patients immediately after polypectomy |
| Keith 1995 | DURATION: treatment and follow‐up only 4 weeks (budesonide) |
| Lavigne 2002 | INTERVENTION: sinus irrigation with steroids using MAST tube, for 3 weeks |
| Lildholdt 1995 | DURATION: treatment and follow‐up only 4 weeks |
| Malmberg 1988 | POPULATION: all patients had polypectomy within 2 months of the start of the trial |
| Man 2013 | DURATION: treatment and follow‐up was for just 6 weeks. The intervention used was 3 mg of fluticasone in 240 ml of normal saline. |
| Mastalerz 1997 | POPULATION: aspirin‐induced asthma and chronic eosinophilic rhinitis |
| Meltzer 1993 | DURATION: treatment and follow‐up only 7 weeks; patients with maxillary sinusitis had flunisolide spray versus placebo added to amoxicillin/clavulanate |
| Mygind 1975 | DURATION: treatment and follow‐up only 3 weeks |
| Optinose 2012 | OTHER: RCT prematurely ended |
| Passali 2003 | POPULATION: patients started treatment 1 month after surgery |
| Qvarnberg 1992 | POPULATION: did not meet current CRS definitions ‐ "recurrent or chronic maxillary sinusitis" (no nasal polyps) |
| Rotenberg 2011 | POPULATION: all patients underwent endoscopic sinus surgery at the start of the trial |
| Rowe‐Jones 2005 | POPULATION: all patients underwent endoscopic sinus surgery at the start of the trial |
| Ruhno 1990 | DURATION: treatment and follow‐up only 4 weeks |
| Saunders 1999 | DESIGN: perioperative study: patients were given intranasal corticosteroids for 2 weeks before polypectomy |
| Slifirski 2009 | POPULATION: all patients underwent surgery at the start of the trial before randomisation |
| Stjarne 2009 | POPULATION: all patients underwent FESS at the start of the trial |
| Taub 1968 | DESIGN: cross‐over study. 4 weeks of treatment followed by dexamethasone spray/placebo, then switched. Unclear if there was a washout period. Results not presented separately. |
| Toft 1982 | DURATION: treatment only 2 weeks (beclomethasone). A cross‐over study with 1‐week follow‐up. |
| Tos 1998 | DURATION: treatment and follow‐up only 6 weeks (budesonide) |
| Vento 2012 | POPULATION: all patients had surgery around 2 weeks before the treatment started |
| Virolainen 1980 | POPULATION: all patients underwent radical ethmoidectomy surgery 2 days prior to treatment starting |
| Wang 2015 | DURATION: treatment and follow‐up only 14 days (budesonide nebulisation) |
CRS: chronic rhinosinusitis EPOS: European Position Paper on Rhinosinusitis and Nasal Polyps 2012 FESS: functional endoscopic sinus surgery RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
Bachert 2004.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | Conference proceeding: we cannot locate the abstract |
Meln 2004.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | We cannot locate the abstract |
Pisano 2000.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | We cannot locate the abstract |
Riem 2005.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | We cannot locate the abstract |
Ygind 1996.
| Methods | — |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | We cannot locate the full text |
Characteristics of ongoing studies [ordered by study ID]
NCT01013701.
| Trial name or title | 'Compare the effects of fluticasone furoate nasal spray vs placebo in patients with nasal polypoid disease' |
| Methods | Double‐blind, parallel assignment, randomised controlled trial |
| Participants | Adults with nasal polyps |
| Interventions | ‐ Fluticasone furoate ‐ Placebo |
| Outcomes | To evaluate the effect of once daily nasal steroid therapy with fluticasone furoate nasal spray (110 µg/day) in suppressing nasal polyp‐induced symptoms over the course of 16 weeks in patients presenting to the clinic with active nasal polypoid disease |
| Starting date | 2009 |
| Contact information | Peter S. Creticos MD, Johns Hopkins University |
| Notes | ClinicalTrials.gov website indicates that the recruitment status of this study is unknown because the information has not been verified recently. We tried to contact the study authors to find out more information but we did not receive a response. |
NCT01622569.
| Trial name or title | 'Study evaluating the efficacy and safety of intranasal administration of 100, 200, and 400 μg of fluticasone propionate twice a day (bid) using a novel bi directional device in subjects with bilateral nasal polyposis followed by an 8‐week open‐label extension phase to assess safety' |
| Methods | Double‐blind, parallel assignment, randomised controlled trial |
| Participants | Adults with bilateral nasal polyposis |
| Interventions | ‐ Fluticasone propionate 100 μg twice a day ‐ Fluticasone propionate 200 μg twice a day ‐ Fluticasone propionate 400 μg twice a day ‐ Matching placebo For 16 weeks |
| Outcomes | ‐ Reduction of nasal congestion/obstruction symptoms ‐ Reduction in total polyp grade (sum of scores from both nasal cavities) No secondary outcomes were listed in the trial registry entry |
| Starting date | 2013 |
| Contact information | Optinose US Inc. No further details provided. |
| Notes | Study has been listed as completed on the registry website (October 2015). No results are currently available. |
NCT01624662.
| Trial name or title | 'Efficacy and safety study of intranasal administration of 100, 200, and 400 μg of fluticasone propionate twice a day (bid) using a novel bi directional device in subjects with bilateral nasal polyposis followed by an 8‐week open‐label extension phase to assess safety' |
| Methods | Double‐blind, parallel assignment, randomised controlled trial |
| Participants | Adults with bilateral nasal polyposis |
| Interventions | ‐ Fluticasone propionate 100 μg twice a day ‐ Fluticasone propionate 200 μg twice a day ‐ Fluticasone propionate 400 μg twice a day ‐ Matching placebo For 16 weeks |
| Outcomes | ‐ Reduction of nasal congestion/obstruction symptoms ‐ Reduction in total polyp grade (sum of scores from both nasal cavities) No secondary outcomes were listed in the trial registry entry |
| Starting date | 2012 |
| Contact information | Optinose US Inc. No further details provided. |
| Notes | Study has been listed as completed on the registry website (October 2015). No results are currently available. |
Differences between protocol and review
In the protocol, the example given for local irritation was "including sore throat, oral thrush". This has been expanded to include "and other local nasal irritation such as dryness, itchiness etc."
Although we had planned to present data for chronic rhinosinusitis without nasal polyps and chronic rhinosinusitis with nasal polyps in subgroups as a visual comparison, this was not carried out except for the nasal polyps and adverse events outcomes. For all the other outcomes there was no more than one study of patients with chronic rhinosinusitis without nasal polyps available for analysis. We had footnoted this in the forest plots and highlighted it in the write up whenever this occurred.
As part of the discussions about the use of a total symptom score we noted that many papers within the suite of reviews did not present information for all four elements of the EPOS criteria for defining chronic rhinosinusitis (EPOS 2012). In particular, many studies that only included patients with nasal polyps did not present information on facial pressure or pain. We made the decision that where individual symptoms were recorded, they should be presented within the outcome of disease severity symptom score within the paper as this information would be useful for the reader.
Contributions of authors
Lee Yee Chong: scoped, designed and wrote the protocol, screened abstracts, extracted data, conducted the analysis and wrote up the review.
Karen Head: reviewed and edited the protocol, screened abstracts, extracted data, helped to check the analysis and contributed to the writing of the review.
Claire Hopkins: clinical guidance at all stages of project scoping and protocol development, full text screening, data analysis and interpretation of the review. Commented on drafts of the review.
Carl Philpott: clinical guidance at all stages of project scoping and protocol development, full text screening, data analysis and interpretation of the review. Contributed to the writing of the review.
Anne GM Schilder: commented on drafts and contributed to the writing of the review.
Martin J Burton: helped to draft the protocol; clinical guidance at all stages of project scoping and protocol development and contributed to the writing of the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
National Institute for Health Research, UK.
Funding to complete a suite of reviews on medical interventions for chronic rhinosinusitis in 2015/2016 (award reference 14/174/03), in addition to infrastructure funding for Cochrane ENT
Declarations of interest
Lee Yee Chong: none known.
Karen Head: none known.
Claire Hopkins: I have received financial support from several companies involved in producing instruments for sinus surgery: Acclarent, Sinusys, Cryolife and Medtronic.
Carl Philpott: I have previously received consultancy fees from the companies Acclarent, Navigant, Aerin Medical and Entellus.
Anne GM Schilder: Professor Anne Schilder is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review. Her evidENT team at UCL is supported by her NIHR Research Professorship award with the remit to develop a UK infrastructure and programme of clinical research in ENT, Hearing and Balance. Her institution has received a grant from GSK for a study on the microbiology of acute tympanostomy tube otorrhoea.
Martin J Burton: Professor Martin Burton is joint Co‐ordinating Editor of Cochrane ENT, but had no role in the editorial process for this review.
New
References
References to studies included in this review
Aukema 2005 {published data only}
- Aukema AAC, Mulder PGH, Fokkens WJ. Treatment of nasal polyposis and chronic rhinosinusitis with fluticasone propionate nasal drops reduces need for sinus surgery. Journal of Allergy and Clinical Immunology 2005;115(5):1017‐23. [DOI] [PubMed] [Google Scholar]
- Aukema AAC, Mulder PGM, Fokkens WJ. Treatment of nasal polyposis and chronic sinusitis with fluticasone propionate nasal drops (Nasules) reduces symptoms and need for FESS. Proceedings of 19th Congress of the European Rhinologic Society; 2002 Jun 16‐19; Ulm, Germany. 2002.
Chur 2013 {published data only}
- Chur V, Small CB, Stryszak P, Teper A. Mometasone furoate nasal spray is safe for the treatment of nasal polyps in pediatric subjects 6‐17 years of age. Journal of Allergy & Clinical Immunology 201;25(2 Suppl 2):AB101. [Google Scholar]
- Chur V, Small CB, Stryszak P, Teper A. Safety of mometasone furoate nasal spray in the treatment of nasal polyps in children. Pediatric Allergy and Immunology 2013;24(1):33‐8. [PUBMED: 23331528] [DOI] [PubMed] [Google Scholar]
Hansen 2010 {published data only}
- Hansen FS, Djupesland PG, Fokkens WJ. Preliminary efficacy of fluticasone delivered by a novel device in recalcitrant chronic rhinosinusitis. Rhinology 2010;48:292‐9. [DOI] [PubMed] [Google Scholar]
Holmberg 1997 {published data only}
- Holmberg K, Juliusson S, Balder B, Smith DL, Richards DH, Karlsson G. Fluticasone propionate aqueous nasal spray in the treatment of nasal polyposis. Annals of Allergy, Asthma, and Immunology 1997;78(3):270‐6. [PUBMED: 9087151] [DOI] [PubMed] [Google Scholar]
Holopainen 1982 {published data only}
- Holopainen E, Grahne B, Malmberg H. Budesonide in the treatment of nasal polyposis. European Journal of Respiratory Diseases 1982;63 Suppl 122:221‐8. [PubMed] [Google Scholar]
Johansen 1993 {published data only}
- Johansen LV, Illum P, Kristensen S, Winther L, Vang PS, Synnerstad B. The effect of budesonide (Rhinocort) in the treatment of small and medium‐sized nasal polyps. Clinical Otolaryngology & Allied Sciences 1993;18(6):524‐7. [DOI] [PubMed] [Google Scholar]
Keith 2000 {published data only}
- Holmström M. Clinical performance of fluticasone propionate nasal drops. Allergy: European Journal of Allergy and Clinical Immunology 1999;54(Suppl 53):21‐5. [DOI] [PubMed] [Google Scholar]
- Keith P, Nieminen J, Hollingworth K, Dolovich J. Efficacy and tolerability of fluticasone propionate nasal drops 400 mug daily compared with placebo for the treatment of bilateral polyposis in adults. Clinical and Experimental Allergy 2000;30(10):1460‐8. [DOI] [PubMed] [Google Scholar]
Lang 1983 {published data only}
- Land DA, McNeill J. Double blind controlled study of effect of topical steroids on nasal polyps. Clinical Otolaryngology 1983;8:139. [Google Scholar]
Lund 1998 {published data only}
- Lund VJ, Flood J, Sykes AP, Richards DH. Effect of fluticasone in severe polyposis. Archives of Otolaryngology ‐‐ Head and Neck Surgery 1998;124(5):513‐8. [PUBMED: 9604976] [DOI] [PubMed] [Google Scholar]
Lund 2004 {published data only}
- Lund V, Black S, Laszloy Z, Schrewlius C, Akerlund A. Budesonide aqueous nasal spray (BANS: Rhinocort AquaTM) is effective as monotherapy in stable patients with chronic rhinosinusitis. Journal of Allergy and Clinical Immunology 2002;109 Suppl 1:AB886. [Google Scholar]
- Lund VJ, Black JH, Szabo LZ, Schrewelius C, Akerlund A. Efficacy and tolerability of budesonide aqueous nasal spray in chronic rhinosinusitis patients. Rhinology 2004;42(2):57‐62. [PubMed] [Google Scholar]
Mosges 2011 {published data only}
- Mosges R, Bachert C, Rudack C, Hauswald B, Klimek L, Spaeth J, et al. Efficacy and safety of mometasone furoate nasal spray in the treatment of chronic rhinosinusitis. Advances in Therapy 2011;28(3):238‐49. [DOI] [PubMed] [Google Scholar]
Parikh 2001 {published data only}
- Parikh A, Scadding GK, Darby Y, Baker RC. Topical corticosteroids in chronic rhinosinusitis: a randomized, double‐blind, placebo‐controlled trial using fluticasone propionate aqueous nasal spray. Rhinology 2001;39(2):75‐9. [PubMed] [Google Scholar]
Penttilla 2000 {published data only}
- Holmström M. Clinical performance of fluticasone propionate nasal drops. Allergy: European Journal of Allergy and Clinical Immunology 1999;54(Suppl 53):21‐5. [DOI] [PubMed] [Google Scholar]
- Penttila M, Holstrom M, Poulsen P, Hollingworth K. The efficacy and tolerability of fluticasone propionate (FP) nasal drops 400fig once daily and twice daily compared with placebo in the treatment of nasal polyposis. Allergy 1998;53 Suppl 43:109. [Google Scholar]
- Penttila M, Poulsen P, Hollingworth K, Holmstrom M. Dose‐related efficacy and tolerability of fluticasone propionate nasal drops 400 microg once daily and twice daily in the treatment of bilateral nasal polyposis: a placebo‐controlled randomized study in adult patients. Clinical and Experimental Allergy 2000;30(1):94‐102. [PUBMED: 10606936] [DOI] [PubMed] [Google Scholar]
Small 2005 {published data only}
- Small CB, Hernandez J, Reyes A, Schenkel E, Damiano A, Stryszak P, et al. Efficacy and safety of mometasone furoate nasal spray in nasal polyposis. Journal of Allergy and Clinical Immunology 2005;116(6):1275‐81. [PUBMED: 16337459] [DOI] [PubMed] [Google Scholar]
Stjarne 2006 {published data only}
- Stjarne P, Blomgren K, Caye‐Thomasen P, Salo S, Soderstrom T. The efficacy and safety of once‐daily mometasone furoate nasal spray in nasal polyposis: a randomized, double‐blind, placebo‐controlled study. Acta Oto‐Laryngologica 2006;126(6):606‐12. [DOI] [PubMed] [Google Scholar]
Stjarne 2006a {published data only}
- Stjarne P, Mosges R, Jorissen M, Passali D, Bellussi L, Staudinger H, et al. A randomized controlled trial of mometasone furoate nasal spray for the treatment of nasal polyposis. Archives of Otolaryngology ‐‐ Head and Neck Surgery 2006;132(2):179‐85. [DOI] [PubMed] [Google Scholar]
Vlckova 2009 {published data only}
- Vlckova I, Navrátil P, Kana R, Pavlicek P, Chrbolka P, Djupesland PG. Effective treatment of mild‐to‐moderate nasal polyposis with fluticasone delivered by a novel device. Rhinology 2009;47(4):419‐26. [DOI] [PubMed] [Google Scholar]
Zhou 2015 {published data only}
- A randomized, double‐blind, placebo‐controlled, multicenter study to evaluate the efficacy and safety of 200 mcg bid mometasone furoate nasal spray (MFNS) in the treatment of nasal polyps. www.clinicaltrials.gov/show/NCT01386125 (accessed 10 March 2016).
- Zhou B, He G, Liang J, Cheng L, Mehta A, Liu S, et al. Mometasone furoate nasal spray in the treatment of nasal polyposis in Chinese patients: a double‐blind, randomized, placebo‐controlled trial. International Forum of Allergy & Rhinology. 2016;6(1):88‐94. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
ALA 2015 {published data only}
- American Lung Association‐Asthma Clinical Research Centers Writing Committee, Dixon AE, Castro M, Cohen RI, Gerald LB, Holbrook JT, et al. Efficacy of nasal mometasone for the treatment of chronic sinonasal disease in patients with inadequately controlled asthma. Journal of Allergy and Clinical Immunology 2015;135(3):701‐9e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Albu 2010 {published data only}
- Albu S, Gocea A, Mitre I. Preoperative treatment with topical corticoids and bleeding during primary endoscopic sinus surgery. Otolaryngology ‐ Head and Neck Surgery 2010;143(4):573‐8. [DOI] [PubMed] [Google Scholar]
Bross‐Soriano 2004 {published data only}
- Bross‐Soriano D, Arrieta‐Gomez JR, Prado‐Calleros H. Infections after endoscopic polypectomy using nasal steroids. Otolaryngology ‐ Head and Neck Surgery 2004;130(3):319‐22. [DOI] [PubMed] [Google Scholar]
Cassano 1996 {published data only}
- Cassano P, Marini F, Indraccolo AS, Curatoli FP. Corticosteroid therapy in the prevention of recurrent post‐surgical nasal polyposis. Acta Otorhinolaryngologica Italica 1996;16(4):334‐8. [PubMed] [Google Scholar]
Chalton 1985 {published data only}
- Chalton R, Mackay I, Wilson R, Cole P. Double blind, placebo controlled trial of betamethasone nasal drops for nasal polyposis. British Medical Journal 1985;291(6498):788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalton R, Mackay IS, Wilson R, Cole P. A double blind, placebo controlled trial of betamethasone nasal drops in the treatment of nasal polyposis. Oto‐Rhino‐Laryngological Research Society (ORS). Abstracts of the meeting held at the Institute of Laryngology and Otology, London October 1985. Clinical Otolaryngology and Allied Sciences 1986;11:389. [Google Scholar]
Cuenant 1986 {published data only}
- Cuenant G, Stipon JP, Plante‐Longchamp G. Efficacy of endonasal neomycin‐tixocortol pivalate irrigation in the treatment of chronic allergic and bacterial sinusitis. ORL: Journal of Oto‐Rhino‐Laryngology 1986;48(4):226‐32. [DOI] [PubMed] [Google Scholar]
Dijkstra 2004 {published data only}
- Dijkstra MD, Ebbens FA, Poublon RML, Fokkens WJ. Fluticasone propionate aqueous nasal spray does not influence the recurrence rate of chronic rhinosinusitis and nasal polyps 1 year after functional endoscopic sinus surgery. Clinical and Experimental Allergy 2004;34(9):1395‐400. [DOI] [PubMed] [Google Scholar]
- Dijkstra MD, Poublon RML, Fokkens WJ. The role of topical steroids after functional endoscopic sinus surgery for chronic sinusitis and/or nasal polyps. Clinical Otolaryngology and Allied Sciences 2001;26(4):341‐2. [Google Scholar]
Drettner 1982 {published data only}
- Drettner B, Ebbesen A, Nilsson M. Prophylactive treatment with flunisolide after polypectomy. Rhinology 1982;20(3):149‐58. [PubMed] [Google Scholar]
Ehnhage 2009 {published data only}
- Ehnhage A, Olsson P, Kolbeck KG, Skedinger M, Dahlen B, Alenius M, et al. Functional endoscopic sinus surgery improved asthma symptoms as well as PEFR and olfaction in patients with nasal polyposis. Allergy 2009;64(5):762‐9. [DOI] [PubMed] [Google Scholar]
- Olsson P, Ehnhage A, Nordin S, Stjarne P. Quality of life is improved by endoscopic surgery and fluticasone in nasal polyposis with asthma. Rhinology 2010;48(3):325‐30. [DOI] [PubMed] [Google Scholar]
el Naggar 1995 {published data only}
- Naggar M, Kale S, Aldren C, Martin F. Effect of Beconase nasal spray on olfactory function in post‐nasal polypectomy patients: a prospective controlled trial. Journal of Laryngology and Otology 1995;109(10):941‐4. [DOI] [PubMed] [Google Scholar]
Filiaci 2000 {published data only}
- Filiaci F, Passali D, Puxeddu R, Schrewelius C. A randomized controlled trial showing efficacy of once daily intranasal budesonide in nasal polyposis. Rhinology 2000;38(4):185‐90. [PubMed] [Google Scholar]
Furukido 2005 {published data only}
- Furukido K, Takeno S, Ueda T, Yajin K. Cytokine profile in paranasal effusions in patients with chronic sinusitis using the YAMIK sinus catheter with and without betamethasone. European Archives of Oto‐Rhino‐Laryngology 2005;262(1):50‐4. [DOI] [PubMed] [Google Scholar]
Gulati 2001 {published data only}
- Gulati SK, Sharma K, Kaur Shergill G, Kumar R. Prophylactic budesonide nasal spray after polypectomy. Indian Journal of Otolaryngology and Head and Neck Surgery 2001;53(3):207‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hartwig 1988 {published data only}
- Hartwig S, Linden M, Laurent C, Vargo AK, Lindqvist N. Budesonide nasal spray as prophylactic treatment after polypectomy (a double blind clinical trial). Journal of Laryngology and Otology 1988;102(2):148‐51. [DOI] [PubMed] [Google Scholar]
Jankowski 2001 {published data only}
- Jankowski R, Schrewelius C, Bonfils P, Saban Y, Gilain L, Prades JM, et al. Efficacy and tolerability of budesonide aqueous nasal spray treatment in patients with nasal polyps. Archives of Otolaryngology ‐‐ Head and Neck Surgery 2001;127(4):447‐52. [DOI] [PubMed] [Google Scholar]
Jankowski 2009 {published data only}
- Jankowski R, Klossek JM, Attali V, Coste A, Serrano E. Long‐term study of fluticasone propionate aqueous nasal spray in acute and maintenance therapy of nasal polyposis. Allergy 2009;64(6):944‐50. [DOI] [PubMed] [Google Scholar]
Johansson 2002 {published data only}
- Johansson L, Holmberg K, Melen I, Stierna P, Bende M. Sensitivity of a new grading system for studying nasal polyps with the potential to detect early changes in polyp size after treatment with a topical corticosteroid (budesonide). Acta Oto‐Laryngologica 2002;122(1):49‐53. [DOI] [PubMed] [Google Scholar]
Jorissen 2009 {published data only}
- Jorissen M, Bachert C. Effect of corticosteroids on wound healing after endoscopic sinus surgery. Rhinology 2009;47:280‐6. [DOI] [PubMed] [Google Scholar]
- Jorissen M, Bachert C. Effect of postoperative mometasone furoate nasal spray on wound healing following endoscopic sinus surgery. World Allergy Organization Journal. XXth World Allergy Organization Congress & VIIth Asia Pacific Congress of Allergology, Asthma and Clinical Immunology, Bangkok, Thailand. 2007; Vol. S318:Abstract No. 997.
Jurkiewicz 2004 {published data only}
- Jurkiewicz D, Zielnik‐Jurkiewicz B, Wojdas A. Effectiveness of fluticasone propionate in nasal polyps treatment. International Review of Allergology and Clinical Immunology 2004;10(1):22‐4. [Google Scholar]
Kang 2008 {published data only}
- Kang IG, Yoon BK, Jung JH, Cha HE, Kim ST. The effect of high‐dose topical corticosteroid therapy on prevention of recurrent nasal polyps after revision endoscopic sinus surgery. American Journal of Rhinology 2008;22(5):497‐501. [DOI] [PubMed] [Google Scholar]
Kapucu 2012 {published data only}
- Kapucu B, Cekin E, Erkul BE, Cincik H, Gungor A, Berber U. The effects of systemic, topical, and intralesional steroid treatments on apoptosis level of nasal polyps. Otolaryngology ‐ Head and Neck Surgery 2012;147(3):563‐7. [DOI] [PubMed] [Google Scholar]
Karlsson 1982 {published data only}
- Karlsson G, Rundcrantz H. A randomized trial in intranasal beclomethasone dipropionate after polypectomy. Rhinology 1982;20(3):144‐8. [PubMed] [Google Scholar]
Keith 1995 {published data only}
- Keith PK, Conway M, Evans S, Edney P, Jennings B, Andersson B, et al. A double‐blind comparison of intranasal budesonide dry powder vs placebo in nasal polyposis. Journal of Allergy and Clinical Immunology 1995;95(1):204. [Google Scholar]
Lavigne 2002 {published data only}
- Lavigne F, Cameron L, Renzi PM, Planet JF, Christodoulopoulos P, Lamkioued B, et al. Intrasinus administration of topical budesonide to allergic patients with chronic rhinosinusitis following surgery. Laryngoscope 2002;112(5):858‐64. [DOI] [PubMed] [Google Scholar]
Lildholdt 1995 {published data only}
- Lildholdt T, Rundcrantz H, Bende M, Larsen K. Glucocorticoid treatment for nasal polyps. The use of topical budesonide powder, intramuscular betamethasone, and surgical treatment. Archives of Otolaryngology ‐‐ Head and Neck Surgery 1997;123(6):595‐600. [DOI] [PubMed] [Google Scholar]
- Lildholdt T, Rundcrantz H, Lindqvist N. Efficacy of topical corticosteroid powder for nasal polyps: a double‐blind, placebo‐controlled study of budesonide. Clinical Otolaryngology and Allied Sciences 1995;20(1):26‐30. [DOI] [PubMed] [Google Scholar]
Malmberg 1988 {published data only}
- Malmberg H, Holopainen E, Pukander J, Virolainen E, Haye R, Lorentzen P, et al. A one year placebo controlled study of beclomethasone dipropionate (BDP) in the management of nasal polyposis following polypectomy. European Respiratory Journal 1988;1 Suppl 2:202s. [Google Scholar]
Man 2013 {published data only}
- Man LX, Farhood Z, Luong A, Fakhri S, Feldman RM, Orlander PR, et al. The effect of intranasal fluticasone propionate irrigations on salivary cortisol, intraocular pressure, and posterior subcapsular cataracts in postsurgical chronic rhinosinusitis patients. International Forum of Allergy & Rhinology 2013;3(12):953‐7. [DOI] [PubMed] [Google Scholar]
Mastalerz 1997 {published data only}
- Mastalerz L, Milewski M, Duplaga M, Nizankowska E, Szczeklik A. Intranasal fluticasone propionate for chronic eosinophilic rhinitis in patients with aspirin‐induced asthma. Allergy 1997;52(9):895‐900. [DOI] [PubMed] [Google Scholar]
Meltzer 1993 {published data only}
- Meltzer EO, Orgel HA, Backhaus JW, Busse WW, Druce HM, Metzger WJ, et al. Intranasal flunisolide spray as an adjunct to oral antibiotic therapy for sinusitis. Journal of Allergy and Clinical Immunology 1993;92(6):812‐23. [DOI] [PubMed] [Google Scholar]
Mygind 1975 {published data only}
- Mygind N. Beclomethasone dipropionate in intranasal treatment [Le dipropionate de beclomethasone en traitement intranasal]. La Nouvelle Presse Médicale 1977;6(15):1319‐21. [PubMed] [Google Scholar]
- Mygind N, Pedersen CB, Prytz S, Sorensen H. Treatment of nasal polyps with intranasal beclomethasone dipropionate aerosol. Clinical Allergy 1975;5(2):159‐64. [DOI] [PubMed] [Google Scholar]
- Mygind N, Pedersen CB, Sorensen H. Treatment of nasal polyps. Ugeskrift for Laeger 1976;734(9):548‐52. [PubMed] [Google Scholar]
Optinose 2012 {published data only}
- A double blind, randomised, parallel group, placebo‐controlled study to investigate the efficacy and tolerability of intranasal fluticasone propionate delivered by the OptiNose device in adult patients with bilateral nasal polyposis. www.clinicaltrialsregister.eu/ctr‐search/trial/2006‐006340‐60/CZ (accessed 10 March 2016).
Passali 2003 {published data only}
- Passali D, Bernstein JM, Passali FM, Damiani V, Passali GC, Bellussi L. Treatment of recurrent chronic hyperplastic sinusitis with nasal polyposis. Archives of Otolaryngology ‐‐ Head and Neck Surgery 2003;129(6):656‐9. [DOI] [PubMed] [Google Scholar]
Qvarnberg 1992 {published data only}
- Qvarnberg Y, Kantola O, Salo J, Toivanen M, Valtonen H, Vuori E. Influence of topical steroid treatment on maxillary sinusitis. Rhinology 1992;30(2):103‐12. [PubMed] [Google Scholar]
Rotenberg 2011 {published data only}
- Rotenberg BW, Zhang I, Arra I, Payton KB. Postoperative care for Samter's triad patients undergoing endoscopic sinus surgery: a double‐blinded, randomized controlled trial. Laryngoscope 2011;121:2702–5. [DOI] [PubMed] [Google Scholar]
Rowe‐Jones 2005 {published data only}
- Rowe‐Jones JM, Medcalf M, Durham SR, Richards DH, Mackay IS. Functional endoscopic sinus surgery: 5 year follow up and results of a prospective, randomised, stratified, double‐blind, placebo controlled study of postoperative fluticasone propionate aqueous nasal spray. Rhinology 2005;43(1):2‐10. [PubMed] [Google Scholar]
Ruhno 1990 {published data only}
- Ruhno J, Andersson B, Denburg J, Anderson M, Hitch D, Lapp P, et al. A double‐blind comparison of intranasal budesonide with placebo for nasal polyposis. Journal of Allergy and Clinical Immunology 1990;86(6 Pt 1):946‐53. [DOI] [PubMed] [Google Scholar]
Saunders 1999 {published data only}
- Saunders MW, Wheatley AH, George SJ, Lai T, Birchall MA. Do corticosteroids induce apoptosis in nasal polyp inflammatory cells? In vivo and in vitro studies. Laryngoscope 1999;109(5):785‐90. [DOI] [PubMed] [Google Scholar]
Slifirski 2009 {published data only}
- Slifirski J, Rosiek‐Biegus M, Girek M, Fal A. Different effect of topical steroids in nasal polyps' treatment depending on the polyp's cytology. Allergy 2009;64:131. [Google Scholar]
Stjarne 2009 {published data only}
- Stjarne P, Olsson P, Alenius M. Use of mometasone furoate to prevent polyp relapse after endoscopic sinus surgery. Archives of Otolaryngology ‐‐ Head and Neck Surgery 2009;135(3):296‐302. [DOI] [PubMed] [Google Scholar]
Taub 1968 {published data only}
- Taub SJ. A double‐blind labeled study of dexamethasone nasal aerosol vs. placebo in the treatment of nasal polyposis. Journal of Allergy 1968;41(1):10‐3. [DOI] [PubMed] [Google Scholar]
- Taub SJ. Dexamethasone nasal aerosol vs. placebo in the treatment of nasal polyposis. Eye, Ear, Nose & Throat Monthly 1968;47(8):392‐5. [PubMed] [Google Scholar]
Toft 1982 {published data only}
- Toft A, Wihl JA, Toxman J, Mygind N. Double‐blind comparison between beclomethasone dipropionate as aerosol and as powder in patients with nasal polyposis. Clinical Allergy 1982;12(4):391‐401. [DOI] [PubMed] [Google Scholar]
- Wihl JA. Topical corticosteroids and nasal reactivity. European Journal of Respiratory Diseases. Supplement 1982;122:205‐10. [PubMed] [Google Scholar]
Tos 1998 {published data only}
- Tos M, Svendstrup F, Arndal H, Orntoft S, Jakobsen J, Borum P, et al. Efficacy of an aqueous and a powder formulation of nasal budesonide compared in patients with nasal polyps. American Journal of Rhinology 1998;12(3):183‐9. [DOI] [PubMed] [Google Scholar]
Vento 2012 {published data only}
- Vento SI, Blomgren K, Hytonen M, Simola M, Malmberg H. Prevention of relapses of nasal polyposis with intranasal triamcinolone acetonide after polyp surgery: a prospective double‐blind, placebo‐controlled, randomised study with a 9‐month follow‐up. Clinical Otolaryngology 2012;37(2):117‐23. [DOI] [PubMed] [Google Scholar]
Virolainen 1980 {published data only}
- Virolainen E, Puhakka H. The effect of intranasal beclomethasone dipropionate on the recurrence of nasal polyps after ethmoidectomy. Rhinology 1980;18(1):9‐18. [PubMed] [Google Scholar]
Wang 2015 {published data only}
- NCT02024659. Effects and safety of budesonide inhalation suspension via transnasal nebulization in eosinophilic chronic rhinosinusitis with nasal polyps. clinicaltrials.gov/show/NCT02024659 (access 19 February 2016).
- Wang C, Lou H, Wang X, Wang Y, Fan E, Li Y, et al. Effect of budesonide transnasal nebulization in patients with eosinophilic chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology 2015;135(4):922‐9.e6. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Bachert 2004 {published data only}
- Bachert C, Bertrand B, Claes J, Clement PAR, Daele J, Demanez J‐P, et al. Efficacy of budesonide powder treatments in nasal polyposis. Proceedings of the 3rd International Consensus Conference on Nasal Polyposis; 2004 Apr 23‐25; Brussels, Belgium. 2004.
Meln 2004 {published data only}
- Meln I, Johansson L, Holmberg K, Stierna P, Bende M. Results from treatment with topical steroids. Proceedings of the 3rd International Consensus Conference on Nasal Polyposis; 2004 Apr 23‐25; Brussels, Belgium. 2004:335.
Pisano 2000 {published data only}
- Pisano G, Tricarico D, Ascione E, Varricchio AM. Management of nasal polyposis: efficacy of intranasal corticosteroid with hypertonic solution. Proceedings of XVIII Congress of European Rhinologic Society and XIX International Symposium on Infection and Allergy of the Nose; 2000 Jun 25‐29; Barcelona, Spain. 2000:AB414.
Riem 2005 {published data only}
- Riem L. Mometasone fuorate for the treatment of nasal polyps. Medizinische Welt 2005;56:224. [Google Scholar]
Ygind 1996 {published data only}
- Ygind N, Lindholdt T. Nasal polyps treatment: medical management. Allergy and Asthma Proceedings 1996;17(5):275‐82. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
NCT01013701 {published data only}
- Compare the effects of fluticasone furoate nasal spray vs placebo in patients with nasal polypoid disease. https://clinicaltrials.gov/ct2/show/NCT01013701 (accessed 10 March 2016).
NCT01622569 {published data only}
- NCT01622569. Study evaluating the efficacy and safety of intranasal administration of 100, 200, and 400 μg of fluticasone propionate twice a day (bid) using a novel bi directional device in subjects with bilateral nasal polyposis followed by an 8‐week open‐label extension phase to assess safety. https://clinicaltrials.gov/show/NCT01622569 (accessed 27 February 2016).
NCT01624662 {published data only}
- NCT01624662. Efficacy and safety study of intranasal administration of 100, 200, and 400 μg of fluticasone propionate twice a day (bid) using a novel bi directional device in subjects with bilateral nasal polyposis followed by an 8‐week open‐label extension phase to assess safety. https://clinicaltrials.gov/show/NCT01624662 (accessed 27 February 2016).
Additional references
Balk 2012
- Balk EM, Earley A, Patel K, Trikalinos TA, Dahabreh IJ. Empirical assessment of within‐arm correlation imputation in trials of continuous outcomes [Internet]. Report No.: 12(13)‐EHC141‐EF. AHRQ Methods for Effective Health Care. Rockville (MD): Agency for Healthcare Research and Quality, 2012. [PubMed] [Google Scholar]
Benninger 2003
- Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, et al. Adult chronic rhinosinusitis: definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngology ‐ Head and Neck Surgery 2003;129(Suppl 3):S1‐32. [DOI] [PubMed] [Google Scholar]
Cho 2012
- Cho SH, Hong SJ, Han B, Lee SH, Suh L, Norton J, et al. Age‐related differences in the pathogenesis of chronic rhinosinusitis. Journal of Allergy and Clinical Immunology 2012;129(3):858‐60.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2016a
- Chong LY, Head K, Hopkins C, Philpott C, Burton MJ, Schilder AGM. Different types of intranasal steroids for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011993.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Chong 2016b
- Chong LY, Head K, Hopkins C, Philpott C, Glew S, Scadding G, et al. Saline irrigation for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011995.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Cohen 1988
- Cohen J. Statistical Power Analysis in the Behavioral Sciences. 2nd Edition. Hillsdale (NJ): Lawrence Erlbaum Associates, Inc., 1988. [Google Scholar]
DeMarcantonio 2011
- DeMarcantonio MA, Han JK. Nasal polyps: pathogenesis and treatment implications. Otolaryngologic Clinics of North America 2011;44(3):685‐95, ix. [DOI] [PubMed] [Google Scholar]
Ebbens 2010
- Ebbens FA, Toppila‐Salmi SK, Renkonen JA, Renkonen RL, Mullol J, Drunen CM, et al. Endothelial L‐selectin ligand expression in nasal polyps. Allergy 2010;65(1):95‐102. [DOI] [PubMed] [Google Scholar]
Ebbens 2011
- Ebbens FA, Toppila‐Salmi S, Groot EJ, Renkonen J, Renkonen R, Drunen CM, et al. Predictors of post‐operative response to treatment: a double blind placebo controlled study in chronic rhinosinusitis patients. Rhinology 2011;49(4):413‐9. [DOI] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple, graphical test. BMJ (Clinical research ed.) 1997;315(7109):629‐34. [PUBMED: 9310563] [DOI] [PMC free article] [PubMed] [Google Scholar]
EPOS 2012
- Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinology. Supplement 2012;50 Suppl 23:1‐298. [PubMed] [Google Scholar]
Fokkens 2007
- Fokkens W, Lund V, Mullol J. European Position Paper on Rhinosinusitis and Nasal Polyps 2007. Rhinology 2007;45(Suppl 20):1‐139. [PubMed] [Google Scholar]
Gliklich 1995
- Gliklich RE, Metson R. The health impact of chronic sinusitis in patients seeking otolaryngologic care. Otolaryngology ‐ Head and Neck Surgery 1995;113(1):104‐9. [DOI] [PubMed] [Google Scholar]
Grobler 2008
- Grobler A, Weitzel EK, Buele A, Jardeleza C, Cheong YC, Field J, et al. Pre‐ and postoperative sinus penetration of nasal irrigation. Laryngoscope 2008;118(11):2078‐81. [DOI] [PubMed] [Google Scholar]
Hamilos 2000
- Hamilos DL. Chronic sinusitis. Journal of Allergy and Clinical Immunology 2000;106:213‐27. [DOI] [PubMed] [Google Scholar]
Handbook 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Harvey 2009
- Harvey RJ, Debnath N, Srubiski A, Bleier B, Schlosser RJ. Fluid residuals and drug exposure in nasal irrigation. Otolaryngology ‐ Head and Neck Surgery 2009;141(6):757‐61. [DOI] [PubMed] [Google Scholar]
Hastan 2011
- Hastan D, Fokkens WJ, Bachert C, Newson RB, Bislimovska J, Bockelbrink A, et al. Chronic rhinosinusitis in Europe ‐ an underestimated disease. A GA2LEN study. Allergy 2011;66(9):1216‐23. [DOI] [PubMed] [Google Scholar]
Head 2016a
- Head K, Chong LY, Hopkins C, Philpott C, Burton MJ, Schilder AGM. Short‐course oral steroids alone for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011991.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Head 2016b
- Head K, Chong LY, Hopkins C, Philpott C, Schilder AGM, Burton MJ. Short‐course oral steroids as an adjunct therapy for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011992.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Head 2016c
- Head K, Chong LY, Piromchai P, Hopkins C, Philpott C, Schilder AGM, et al. Systemic and topical antibiotics for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2016, Issue 4. [DOI: 10.1002/14651858.CD011994.pub2] [DOI] [PMC free article] [PubMed] [Google Scholar]
Jaeschke 1989
- Jaeschke R, Singer J, Guyatt GH. Measurement of health status. Ascertaining the minimal clinically important difference. Controlled clinical trials 1989;10(4):407‐15. [PUBMED: 2691207] [DOI] [PubMed] [Google Scholar]
Kalish 2012
- Kalish L, Snidvongs K, Sivasubramaniam R, Cope D, Harvey RJ. Topical steroids for nasal polyps. Cochrane Database of Systematic Reviews 2012, Issue 12. [DOI: 10.1002/14651858.CD006549.pub2] [DOI] [PubMed] [Google Scholar]
Kern 2008
- Kern RC, Conley DB, Walsh W, Chandra R, Kato A, Tripathi‐Peters A, et al. Perspectives on the etiology of chronic rhinosinusitis: an immune barrier hypothesis. American Journal of Rhinology 2008;22(6):549‐59. [DOI] [PMC free article] [PubMed] [Google Scholar]
Keswani 2012
- Keswani A, Chustz RT, Suh L, Carter R, Peters AT, Tan BK, et al. Differential expression of interleukin‐32 in chronic rhinosinusitis with and without nasal polyps. Allergy 2012;67(1):25‐32. [DOI] [PMC free article] [PubMed] [Google Scholar]
Larsen 2004
- Larsen P, Tos M. Origin of nasal polyps: an endoscopic autopsy study. Laryngoscope 2004;114(4):710‐9. [DOI] [PubMed] [Google Scholar]
McNally 1997
- McNally PA, White MW, Kaliner MA. Sinusitis in an allergists' office: analysis of 2000 consecutive cases. Allergy and Asthma Proceedings 1997;18:169‐75. [DOI] [PubMed] [Google Scholar]
Mullol 2009
- Mullol J. Corticosteroid treatment in chronic rhinosinusitis: the possibilities and the limits. Immunology and Allergy Clinics of North America 2009;29(4):657‐68. [DOI] [PubMed] [Google Scholar]
Ragab 2004
- Ragab SM, Lund VJ, Scadding G. Evaluation of the medical and surgical treatment of chronic rhinosinusitis: a prospective, randomised, controlled trial. Laryngoscope 2004;114(5):923‐30. [DOI] [PubMed] [Google Scholar]
Ragab 2010
- Ragab SM, Lund VJ, Scadding G, Saleh HA, Khalifa MA. Impact of chronic rhinosinusitis therapy on quality of life: a prospective randomized controlled trial. Rhinology 2010;48(3):305‐11. [DOI] [PubMed] [Google Scholar]
Revicki 2008
- Revicki D, Hays RD, Cella D, Sloan J. Recommended methods for determining responsiveness and minimally important differences for patient‐reported outcomes. Journal of Clinical Epidemiology 2008;61(2):102‐9. [PUBMED: 18177782] [DOI] [PubMed] [Google Scholar]
RevMan 2014 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Snidvongs 2011
- Snidvongs K, Kalish L, Sacks R, Craig JC, Harvey RJ. Topical steroid for chronic rhinosinusitis without polyps. Cochrane Database of Systematic Reviews 2011, Issue 8. [DOI: 10.1002/14651858.CD009274] [DOI] [PubMed] [Google Scholar]
Spector 1998
- Spector SL, Bernstein IL, Li JT, Berger WE, Kaliner MA, Schuller DE, et al. Parameters for the diagnosis and management of sinusitis. Journal of Allergy and Clinical Immunology 1998;102(6 Pt 2):S107‐44. [DOI] [PubMed] [Google Scholar]
Tan 2011
- Tan BK, Li QZ, Suh L, Kato A, Conley DB, Chandra RK, et al. Evidence for intranasal antinuclear autoantibodies in patients with chronic rhinosinusitis with nasal polyps. Journal of Allergy and Clinical Immunology 2011;128(6):1198‐206.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Thomas 2013
- Thomas WW 3rd, Harvey RJ, Rudmik L, Hwang PH, Schlosser RJ. Distribution of topical agents to the paranasal sinuses: an evidence‐based review with recommendations. International Forum of Allergy and Rhinology 2013;3(9):691‐703. [DOI] [PubMed] [Google Scholar]
Tomassen 2011
- Tomassen P, Zele T, Zhang N, Perez‐ Novo C, Bruaene N, Gevaert P, et al. Pathophysiology of chronic rhinosinusitis. Proceedings of the American Thoracic Society 2011;8(1):115‐20. [DOI] [PubMed] [Google Scholar]
van Drunen 2009
- Drunen CM, Reinartz SM, Wigman J, Fokkens W. Inflammation in chronic rhinosinusitis and nasal polyposis. Immunology and Allergy Clinics of North America 2009;29(4):621‐9. [DOI] [PubMed] [Google Scholar]
Zhang 2008
- Zhang N, Zele T, Perez‐Novo C, Bruaene N, Holtappels G, DeRuyck N, et al. Different types of T‐effector cells orchestrate mucosal inflammation in chronic sinus disease. Journal of Allergy and Clinical Immunology 2008;122(5):961‐8. [DOI] [PubMed] [Google Scholar]
Zhang 2009
- Zhang XH, Lu X, Long XB, You XJ, Gao QX, Cui YH, et al. Chronic rhinosinusitis with and without nasal polyps is associated with decreased expression of glucocorticoid‐induced leucine zipper. Clinical and Experimental Allergy 2009;39(5):647‐54. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Chong 2015
- Chong LY, Head K, Hopkins C, Philpott C, Burton MJ. Intranasal steroids versus placebo or no intervention for chronic rhinosinusitis. Cochrane Database of Systematic Reviews 2015, Issue 12. [DOI: 10.1002/14651858.CD011996] [DOI] [PMC free article] [PubMed] [Google Scholar]


