Abstract
Objective:
A literature review of antiplatelet agents for primary and secondary stroke prevention, including mechanism of action, cost, and reasons for lack of benefit.
Data sources:
Articles were gathered from MEDLINE, Cochrane Reviews, and PubMed databases (1980-2021). Abstracts from scientific meetings were considered. Search terms included ischemic stroke, aspirin, clopidogrel, dipyridamole, ticagrelor, cilostazol, prasugrel, glycoprotein IIb/IIIa inhibitors.
Study selection and data extraction:
English-language original and review articles were evaluated. Guidelines from multiple countries were reviewed. Articles were evaluated independently by 2 authors.
Data synthesis:
An abundance of evidence supports aspirin and clopidogrel use for secondary stroke prevention. In the acute phase (first 21 days postinitial stroke), these medications have higher efficacy for preventing further stroke when combined, but long-term combination therapy is associated with higher hemorrhage rates. Antiplatelet treatment failure is influenced by poor adherence and genetic polymorphisms. Antiplatelet agents such as cilostazol may provide extra benefit over clopidogrel and aspirin, in certain racial groups, but further research in more diverse ethnic populations is needed.
Relevance to patient care and clinical practice:
This review presents the data available on the use of different antiplatelet agents poststroke. Dual therapy, recurrence after initiation of secondary preventative therapy, and areas for future research are discussed.
Conclusions:
Although good evidence exists for the use of certain antiplatelet agents postischemic stroke, there are considerable opportunities for future research to investigate personalized therapies. These include screening patients for platelet polymorphisms that confer antiplatelet resistance and for randomized trials including more racially diverse populations.
Keywords: stroke, antiplatelets, aspirin, clopidogrel, cilostazol, prasugrel, ticagrelor, ticlopidine
Introduction
Stroke is the second most common cause of death worldwide and one of the leading causes of long-term disability globally. 1 Over the last 25 years, there has been a global reduction in the rate of death and age-adjusted stroke prevalence, but overall, the absolute numbers of stroke cases have increased as populations have developed greater longevity. 1 Ischemic stroke is, by far, the most common cause of stroke worldwide, accounting for 10 times more strokes than hemorrhagic strokes in higher income countries, 2 but with much less difference observed in lower income countries. 3 Although the rate of stroke deaths is decreasing, it is believed that up to 50% of stroke-related deaths are attributable to poorly managed modifiable risk factors. 4 Management of hypertension, hypercholesterolemia, diabetes, smoking, and cardiac arrhythmias such as atrial fibrillation all have a considerable evidence base for reducing stroke occurrence and recurrence. 5
The risk of recurrent ischemic stroke events in the first 30 days is high with 1 in 25 people having a recurrent stroke in this time frame. 6 Therefore, treatments employed to reduce this initial risk can have considerable impact on reducing morbidity and mortality. Antiplatelet agents are indicated when the cause of the ischemic stroke is determined to be noncardioembolic antiplatelets modify the risk of further stroke events and reduce the rate of death in this acute period and in the long term. 7
The most used antiplatelet agents worldwide include aspirin, clopidogrel, and dipyridamol. 8 All have high-level evidence for the prevention of stroke recurrence. Unfortunately, there is a population of patients who exhibit “resistance” to these medications (have ischemic events while on an antiplatelet agent), develop adverse effects from use, or develop allergic reactions. 9 Combining antiplatelets is associated with increased risk of bleeding when used for long-term prevention, although this increased risk is often outweighed by decreased stroke recurrence in the short term.10,11 As a result, the treatment provider is faced with a difficult decision about how best to treat a patient with further stroke events who has already received one antiplatelet agent.
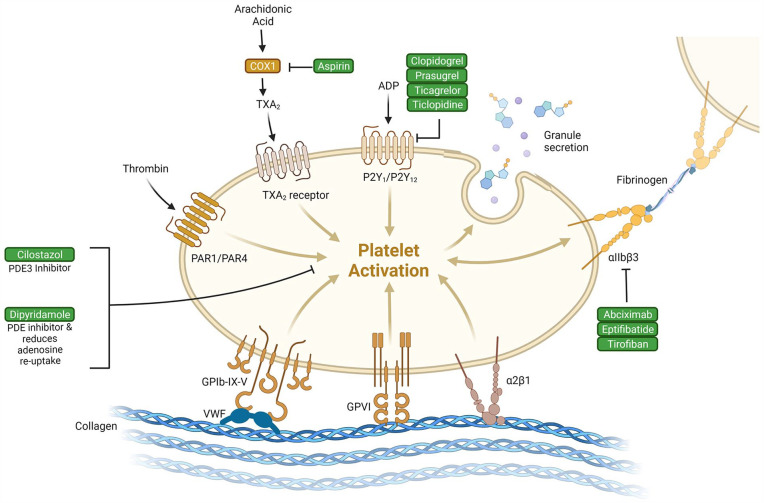
The class of medications that have antiplatelet activity is large with a diverse set of mechanistic actions. This offers the opportunity to utilize alternative antiplatelet agents if a patient has an ischemic stroke while on first-line therapies. The decision about which antiplatelet agent to use when a more commonly used drug has undesired effects is difficult due to limited data comparing antiplatelet agents head-to-head. 10 Figure 1 highlights the different mechanisms attributed to the action of several antiplatelet agents.
Figure 1.
Antiplatelet agents and their mechanisms of action on the platelet. This figure highlights the site of action for multiple antiplatelet agents. This figure highlights how the multiple sites of action may contribute to the increased efficacy when certain antiplatelet agents are combined. Created with BioRender.com.
Abbreviations: ADP, adenosine diphosphate; COX, cyclo-oxygenase; TXA2, thromboxane A2.
In this review article, we have summarized the evidence available for each antiplatelet drug used in the treatment of ischemic stroke. We have then offered suggestions for alternative therapeutic options when faced with recurrent stroke. We conclude with a description of what the future may hold for antiplatelet therapies in ischemic stroke.
Methods of Review
Articles were gathered from MEDLINE, Cochrane Reviews, and PubMed databases. The literature search included all article published between January 1980 and September 2021. Abstracts from scientific meetings were considered. Search terms included ischemic stroke, aspirin, clopidogrel, dipyridamole, ticagrelor, cilostazol, prasugrel, and glycoprotein IIb/IIIa inhibitors. Study selection was limited to English-language original and review articles. Guidelines from America, United Kingdom, Canada, and China were reviewed. When presenting clinical data on each antiplatelet agent, we have focused on randomized control trial (RCT) or systematic review levels of evidence.
Antiplatelet Drugs
Aspirin
Aspirin is the most widely studied antiplatelet agent that is used in the acute phase and in secondary prevention of ischemic stroke, either alone or in combination therapy with other antiplatelet agents. 12 Aspirin is an essential World Health Organization (WHO) medication, generally well tolerated and inexpensive. Aspirin exhibits its effects by irreversibly inhibiting cyclo-oxygenase (COX), which reduces platelet aggregation by inhibiting the synthesis of the procoagulant thromboxane A2 (TXA2).
Multiple studies have demonstrated the efficacy of aspirin in the acute phase of ischemic stroke. 7 A meta-analysis of 40 000 patients from the International Stroke Trial (IST) and Chinese Acute Stroke Trial (CAST) compared aspirin (160-300 mg) with placebo or no medication, for a total of 2 to 4 weeks. 13 Aspirin administration within 48 hours of stroke led to a significant reduction in the overall risk of early recurrent stroke (7 per 1000) and death (4 per 1000), with no significant increase in hemorrhagic stroke or transformation during this timeframe. This corresponds to a 30% odds reduction of recurrent ischemic stroke (number needed to treat [NNT] = 140). 7 These studies provide the main evidence that supports the use of aspirin in the acute setting, including the timeframe at which the risk of recurrent stroke is the highest. 14 The CAST recruited 20 000 patients across 413 Chinese hospitals, whilst IST included a similar number of patients from 36 different countries, around 80% of whom were treated in European Hospitals. The population in the CAST study had half the rate of mortality during inpatient admission compared with IST, which the authors attribute to the exclusion criteria of severe strokes, lower mean age of participants, and the different etiologies of stroke (ie, intracranial vs extracranial disease). 15
In those receiving thrombolysis with alteplase, it is recommended to delay aspirin administration for 24 hours. In the Antiplatelet Therapy in Combination with Recombinant t-PA Thrombolysis in Ischemic Stroke (ARTIS) trial, early aspirin administration was shown to not improve outcomes at 3 months as judged by mRS score of 0 to 2 (relative risk [RR] = 0.94, 95% CI = 0.82-1.09), and increased the risk of intracerebral hemorrhage (ICH) when co-administered with alteplase, compared with no additional treatment (RR = 2.78, 95% CI = 1.01-7.63). 16
The 2009 Antithrombotic Trialists’ collaboration (ATC) meta-analysis included a subset of 6170 patients taking aspirin as a secondary preventative agent following ischemic stroke or TIA. 17 Aspirin compared with placebo showed a reduction of 17% (95% CI = 4%-28%) in any stroke, but with an increase in hemorrhagic stroke (RR = 1.9, 95% CI = 1.06-3.4) and GI bleeding (RR = 2.69, 95% CI = 1.25-5.76) during follow-up periods ranging from 1 to 6 years. 17 There remains net benefit in taking aspirin as a secondary preventative, 18 which holds true when taking age and sex into account. Dosing regimens have been extensively investigated, with the earlier 2002 collaboration finding that a daily dose of 75 to 150 mg of aspirin confers equivalent benefit long-term without the added risk of bleeding of higher doses. 19
Although there is good evidence for the efficacy of aspirin as both an acute treatment for ischemic stroke and secondary prevention, it is not licensed as a primary prevention agent for stroke in the United Kingdom. 20 Although aspirin is not licensed for this indication in the United Kingdom, it is recommended by the American Heart Association and American Stroke Association (AHA/ASA) as a primary preventative agent in cardiovascular disease, including stroke, in patients with a 10-year cardiovascular risk score of above 10% (level IIa evidence). 21 A 2020 meta-analysis of 157 054 participants across 11 studies examined the risk of stroke in patients without cardiovascular disease who were taking 75 to 500 mg of aspirin daily as primary prevention. 22 The studies included patients varying from low to moderate risk of cardiovascular disease, with risk factors including hypertension and diabetes, who were followed up for at least 1 year. Although aspirin was associated with a significant reduction in myocardial infarction, there was no reduction in nonfatal strokes (odds ratio [OR] = 0.94, 95% CI = 0.85-1.04), nor in cardiovascular mortality rates. However, there was an increased risk of hemorrhagic strokes (OR = 1.29, 95% CI = 1.06-1.56, number needed to harm [NNH] = 8333) and of major gastrointestinal bleeds in those taking aspirin (OR = 1.83, 95% CI = 1.43-2.35, NNH = 2040). These data suggest that the place for aspirin treatment in stroke is secondary prevention rather than primary prevention.
Not all patients respond to aspirin. Aspirin treatment failure can be described clinically, in the form of recurrent vascular events such as TIA, stroke and myocardial infarction (MI), as well as biochemically, as measured by elevated TXA2 levels and rapid platelet plug formation. Despite the 10-day platelet turnover time required to restore TXA2 levels post-COX inhibition, aspirin treatment failure is thought to occur with a frequency of 12.9%, as defined by vascular events during aspirin treatment. 23 The heterogeneity in response to aspirin can be explained by several mechanisms, including poor medication adherence, poor absorption, drug interactions, insufficient dosing, and alternative pathways of platelet activation, including COX polymorphisms and the possible upregulation of COX-2 expression during periods of inflammation, which bypasses inhibition by aspirin to produce TXA2.23,24 Genetic differences in other components of thrombotic pathways are thought to contribute to aspirin resistance, examples being polymorphisms of platelet membrane glycoproteins, the P2Y1 gene, and von Willebrand Factor. 25 However, the most common cause of aspirin treatment failure has been shown to be poor adherence. 26
Clopidogrel
Clopidogrel is a second generation thienopyridine antiplatelet agent. It is a prodrug that is metabolized to its active form by the hepatic cytochrome P450 system. The active metabolite is an irreversible inhibitor of the P2Y12 class of adenosine diphosphate (ADP) receptors on the surface of platelets, preventing ADP-mediated activation of the downstream glycoprotein IIb/IIIa complex, resulting in reduced platelet aggregation. Genetic polymorphisms in the enzymes involved in clopidogrel metabolism contribute to variation in response to clopidogrel between individuals. 27
Clopidogrel is licensed for the management of ischemic stroke in both the acute phase if patients are known to be aspirin allergic and as long-term secondary prevention. Clopidogrel loading at a dose of 300 or 600 mg is recommended as the acute treatment,11,28 followed by 75 mg daily long-term. 29 A 300 mg loading dose of clopidogrel in patients with moderate to severe stroke (National Institutes of Health Stroke Scale [NIHSS] ≥4) within 6 hours of admission has been shown to be safe and efficacious, with one retrospective study (n = 1011) showing no difference in poor functional outcomes as defined by mRS >2 on discharge (OR = 0.71, 95% CI = 0.46-1.09), and lower rates of neurological worsening as defined by an NIHSS increase ≥2 in any 24-hour period, although the latter was no longer statistically significant after adjusting for baseline NIHSS. 28
The CAPRIE trial first compared clopidogrel monotherapy with aspirin monotherapy for prevention of vascular events (ischemic stroke, myocardial infarction, or vascular death) in 19 185 patients with established atherosclerotic vascular disease. 30 This included 6431 patients with recent ischemic stroke. Across all patients, clopidogrel lead to an 8.7% relative risk reduction (RRR), 95% CI = 0.3%-16.5%, P = 0.043, in vascular events compared with aspirin alone, without an increase in adverse events. However, for the subset of patients with previous ischemic stroke, there was no significant difference in vascular events for patients treated with clopidogrel compared with aspirin (RRR = 7.3%, 95% CI = −5.7% to 18.7%, P = 0.26). Moreover, no significant difference was seen between aspirin and clopidogrel for prevention of ischemic stroke in both the overall cohort and the previous stroke cohort. 30 Following this, the MATCH trial compared dual antiplatelet therapy (DAPT) with aspirin plus clopidogrel, to clopidogrel monotherapy for secondary prevention in 7599 patients with recent ischemic stroke or high-risk TIA with ≥1 additional vascular risk factor. In patients treated with DAPT for 18 months, no significant reduction in stroke was found and there was a trend toward more major bleeding events, although this trend was not statistically significant. 29 Therefore, when summarizing the results of the MATCH, CHANCE, and POINT trials, the window of benefit for DAPT in preventing recurrent ischemic strokes would appear to be only in the acute poststroke phase.
In summary, the consensus among international guidelines is that after the initial poststroke phase (21 days), clopidogrel alone (or aspirin alone) is as effective and safer than DAPT for secondary prevention of ischemic stroke.
Dipyridamole
Dipyridamole is an antiplatelet drug that has been shown to have multiple mechanisms of action, including inhibiting cAMP-phosphodiesterase, blocking the reuptake and breakdown of adenosine by platelets, and enhancing PGI2 biosynthesis. 31
The drug has been shown in multiple RCTs and meta-analyses to reduce the risk of further vascular events in patients with previous ischemic stroke.32-37 Several trials have shown that dipyridamole alone can reduce the rate of vascular events in patients who have had a previous ischemic stroke,38,39 but when combined with low-dose aspirin, this effect is greater than when the drugs are given separately. 36
Interestingly, and typified by the Cochrane meta-analysis by De Schryver et al, while dipyridamole reduces vascular events compared with control (RR = 0.88, 95% CI = 0.81-0.95), it does not appear to have an effect on the incidence of vascular deaths poststroke.32,36,37 Subgroup analysis of patients who have had a stroke was performed in this meta-analysis to identify whether this group benefited to a greater or lesser extent from dipyridamole and aspirin combination therapy than stroke-naive patients, but the drug appears to have equal effect on all subgroups of ischemic stroke. 33
When comparing dipyridamole and aspirin against clopidogrel for secondary prevention of vascular events poststroke, a single-center UK-based study found that in the first year after stroke, dipyridamole and aspirin therapy have a greater effect at reducing further vascular events (study size n = 3572). 34 Clopidogrel has been shown to be the better therapy at reducing vascular events after 1 year, albeit in a retrospective cohort analysis. 34 Although aspirin combined with dipyridamole has not been shown to be inferior to clopidogrel in preventing further stroke events, the Prevention Regimen for Effectively Avoiding Second Strokes international (PRoFESS), a multicenter trial (n = 20 332) showed a higher likelihood for major hemorrhage in patients treated with both aspirin and dipyridamole (4.1% vs 3.6%, hazard ratio [HR] = 1.15, 95% CI = 1.00-1.32).32,37 This effect is seen after the initial acute stroke phase as detailed in the clopidogrel section of this review.
The propensity to develop headaches while taking dipyridamole is another factor to consider when using this drug as stroke secondary prevention. Up to 40% of patients taking dipyridamole report headaches, 40 with up to 5.9% of patients in the PRoFESS trial stopping the drug for this reason. 32
In summary, dipyridamole in combination with aspirin remains a good treatment for the secondary prevention of ischemic stroke, but the increased risk of bleeding and headaches that are associated with the medication indicates that in current practice, the medication is used less frequently.
Prasugrel
Prasugrel is a third-generation thienopyridine and a prodrug which converts to an active metabolite, R-138727, which irreversibly inhibits the platelet P2Y12 receptor. 41 This prevents the binding of ADP and prevents activation of the glycoprotein IIb/IIIa complex. 41 Unlike clopidogrel, loss of function polymorphisms in CYP2C19 and CYP2C9 are not associated with reduced pharmaco-availability of the active metabolite of prasugrel. 42
The initial evidence for prasugrel was established through large multicenter clinical trials in acute coronary syndromes (ACS). The TRITON-TIMI-38 study, a phase 3 randomized clinical trial, compared clopidogrel (300 mg loading dose and 75 mg daily maintenance dose) versus prasugrel (60 mg loading dose and 10 mg daily maintenance dose) in patients with ACS undergoing percutaneous coronary intervention (PCI), n = 13 608. 43 While prasugrel therapy was associated with reduced rates of myocardial infarction and stent thrombosis compared with clopidogrel (7.4% vs 9.7%, HR = 0.76, 95% CI = 0.67-0.85), there was a higher rate of nonfatal (1.1% vs 0.9%, HR = 1.25, 95% CI = 0.87-1.81) and fatal (0.4% vs 0.1%; HR = 4.19, 95% CI = 1.58-11.11) bleeding events over a follow-up period of 6 to 15 months. 44 Subgroup analysis indicated that individuals who had at least one of either a history of stroke or TIA were ≥75 years of age or weighed <60 kg had higher rates of bleeding and consequently either no net benefit or a net harm from prasugrel. 43 As such, prasugrel is currently contraindicated in individuals with a history of stroke or TIA. In contrast to this, TRILOGY-ACS (n = 7243) found that a dose of prasugrel (10 mg/day in those weighing ≥ 60 kg or 5 mg/day in those weighing <60 kg) given for greater than 12 months in patients aged 75 years or above, medically treated for ACS and without prior stroke or TIA, was associated with a lower risk of ischemic stroke compared with clopidogrel therapy (75 mg/day). 45 However, this was an underpowered study with a low overall number of strokes.
There are several contention issues with the above studies, including the dose of prasugrel used and the dangers of extrapolating results from ACS trials to ischemic stroke. As such, the PRASTRO-I study in Japan randomized 3753 patients aged <75 and weighing >50 kg with noncardioembolic stroke to either prasugrel (3.75 mg/day) or clopidogrel (75 mg/day) for 96 to 104 weeks. 46 While there were a similar number of ischemic strokes, myocardial infarcts, and hemorrhagic strokes between the 2 treatment groups, the noninferiority of prasugrel to clopidogrel was not demonstrated. The PRASTRO-II study investigated 2 different doses of prasugrel (2.5 mg/day or 3.75 mg/day) versus clopidogrel (50 mg/day) in Japanese patients aged ≥75 and weighing ≤50 kg) and found no significant differences in the rates of ischemic stroke, myocardial infarction, vascular death, or major bleeding. 47 As such, there is not enough evidence to support the use of prasugrel instead of using clopidogrel in individuals with a history of ischemic stroke or TIA.
Ticagrelor
Ticagrelor exerts its potent antiplatelet activity by reversibly binding to and inhibiting the platelet adenosine diphosphate P2Y12 receptor. Its antithrombotic effects are well established in the management of patients with ACS. 48 However, in some of the earlier ACS randomized controlled trials, the effect on stroke risk was mixed. In the PLATO study that randomized 18 624 patients with moderate to high-risk ACS undergoing PCI, to either ticagrelor or clopidogrel, ticagrelor was associated with reduced death from all vascular causes (HR = 0.84; CI = 0.77-0.92; P ≤ 0.001). 49 This was attributed mainly to reduced myocardial infarction, but not to a reduction in stroke risk. In fact, patients in the ticagrelor arm experienced a nonsignificantly higher rate of stroke (1.5% vs 1.3%, HR = 1.17; CI = 0.91-1.52; P = 0.22) and fatal intracranial hemorrhage in particular (0.1% vs 0.01%, P = 0.02). 49
These concerns, however, were not born out in RCTs in stroke patients. The SOCRATES study randomized 13 199 patients with mild acute ischemic stroke (AIS) (NIHSS < 5) or high-risk TIA (ABCD 2 score > 4) to either high-dose aspirin (300 mg loading, 100 mg maintenance) or ticagrelor (180 mg loading, 90 mg twice daily maintenance) for 90 days. 50 Ticagrelor was nonsuperior to aspirin monotherapy for the primary endpoint of first occurrence of a composite of vascular endpoints, for example, stroke, MI, death (7.5% aspirin vs 6.7% ticagrelor, HR = 0.89; 95% CI = 0.78-1.01; P = 0.07), but importantly was not found to be associated with increased risk of hemorrhagic stroke (0.3% vs 0.2% respectively). The subsequent THALES study 51 investigated the combination of ticagrelor and aspirin with aspirin alone in this same population (n = 11 016). They found the combination of ticagrelor and aspirin was associated with reduced rates of stroke or death within 30 days compared with aspirin alone (5.5% vs 6.6%; HR = 0.83, 95% CI = 0.71-0.96; P = 0.02; NNT = 91) but slightly higher rates of major bleeding events (0.5% vs 0.1%, HR = 3.99, 95% CI = 1.74-9.14, P = 0.001) and intracranial hemorrhage (0.4% vs 0.1%, HR = 3.33, 95% CI = 1.34-8.28, P = 0.01). Ticagrelor has also been associated with both bradycardia and increased dyspnea when compared with other antiplatelet agents, which also need to be considered when using this medication. 52 No head-to-head trials with clopidogrel either alone or in combination with aspirin have been undertaken, but due to the lack of CYP2C19-mediated activity, ticagrelor may have benefits in clopidogrel-resistant populations. Currently, the drug is not licensed in the United States or United Kingdom.
Ticlopidine
Ticlopidine is a thienopyridine derivative prodrug, like clopidogrel. Its active metabolite selectively and irreversibly inhibits the ADP-binding site on the P2Y12 receptor and thus ADP-induced platelet aggregation. 53 There have been 3 RCTs looking at ticlopidine in the prevention of stroke in patients with a recent TIA or minor stroke. The Canadian American Ticlopidine Study (CATS), n = 1072, compared 500 mg/day ticlopidine with placebo and found an RRR with ticlopidine for stoke, MI, and vascular death of 30.2% (95% CI = 7.5%-48.3%, P = 0.006) at 3 years in those randomized to ticlopidine. 54 In this study, no comparison was made with aspirin. The Ticlopidine Aspirin Stroke Study (TASS), n = 3069, compared 500 mg/day ticlopidine with 1300 mg/day aspirin and found a 21% risk reduction in the development of fatal and nonfatal stroke with ticlopidine compared with aspirin at 3 years (95% CI = 4%-38%). 55 Subgroup analysis of the TASS study showed a 60.8% reduction in fatal and nonfatal stroke risk with ticlopidine in nonwhite patients. 56 In light of these findings, a subsequent study compared 500 mg/day ticlopidine with 650 mg/day aspirin in black patients, given the high stroke burden and underrepresentation of this population in clinical trials. 57 However, the study was stopped after 6.5 years, as futility analysis showed that ticlopidine had a less than 1% chance of being superior to aspirin. A recent population-based cohort study in Taiwan (n = 2585) found that patients with ischemic stroke who were treated with 100 mg/d aspirin had lower rates of recurrent stroke at 3-year follow-up compared with those treated with 75 mg/day clopidogrel (2.03% vs 2.55%, HR = 2.27, 95% CI = 1.02-5.07). About 200 mg/day of ticlopidine was found to be noninferior to aspirin (1.48% vs 2.03%, HR = 0.62, 95% CI = 0.08-4.86). 58
Common adverse effects of ticlopidine include diarrhea (23.6% in CATS and 20.4% in TASS) and rash (17.0% in CATS and 11.9% in TASS). Furthermore, ticlopidine is associated with the development of severe but reversible neutropenia (0.03% in CATS and 0.9% in TASS). At the lower dose used in the cohort study in Taiwan, no patients developed neutropenia, suggesting that this effect may be dose-related. Nevertheless, patients on ticlopidine require regular blood monitoring, particularly in the first few months of treatment. 56
Ticlopidine is an effective drug for the prevention of ischemic stroke. However, its use is limited by potentially severe hematological adverse effects. Therefore, ticlopidine is currently not licensed or recommended for use in ischemic stroke within the United Kingdom, United States, or most of mainland Europe. Further studies using lower doses of ticlopidine are warranted. In particular, studies focussing on a broader range of nonwhite populations, for example East Asian populations, where clopidogrel, but not ticlopidine, 59 is known to be less effective due to higher carrier rates of the CYP2C19 loss-of-function allele. 60
Cilostazol
Cilostazol is a selective inhibitor of phosphodiesterase 3, which increases the activation of intracellular cAMP and thereby inhibits platelet aggregation. As well as the action on platelet aggregation, cilostazol also dilates blood vessels. 61 It is assumed to have a weak antiplatelet effect in acute stages of stroke treatment, but the combined vasodilatory and antiplatelet effect is thought to be the underlying mechanism leading to long-term stroke prevention. 62
There are 2 large trials with large cohorts that have studied the effect of cilostazol in ischemic stroke management, PICASSO and CSPS.com. In the CSPS.com trial, 1879 Japanese patients with high-risk noncardioembolic ischemic stroke were enrolled between 8 and 180 days after stroke. Combination of cilostazol with aspirin or clopidogrel reduced the annual incidence of recurrent ischemic stroke by half compared with monotherapy (2.2% dual therapy with cilostazol vs 4.5% monotherapy, HR = 0.49, 95% CI = 0.31-0.76), without increasing the annual risk of severe or life-threatening bleeding (0.6% dual vs 0.9% monotherapy, HR = 0.66, 95% CI = 0.27-1.60). 63 The PICASSO trial (n = 1534) showed cilostazol may be beneficial for ischemic stroke patients with multiple cerebral microbleeds and those patients in which small vessel disease contributes to their risk of stroke. 63 Cilostazol reduced further strokes in mild (5 vs 16 events; HR = 0.36, 95% CI = 0.13-0.97, P = 0.04) and moderate (16 vs 32 events; HR = 0.50, 95% CI = 0.29-0.92, P = 0.03) white matter changes, which were suggestive of small vessel disease. 63 A meta-analysis of five studies using cilostazol highlighted that in patients with multiple cerebral microbleeds and white matter changes, the relative risk of recurrent stroke in the cilostazol group was significantly lower than in the aspirin group (RR = 0.66, 95% CI = 0.54-0.81). It has also been shown that cilostazol combination therapy (with either aspirin or clopidogrel) results in lower relative risk of recurrent stroke when compared with aspirin or clopidogrel alone (RR = 0.50, 95% CI = 0.35-0.72). 64 In trials that have focused on Asian populations, cilostazol combination therapy (with either aspirin or clopidogrel) has been shown to be effective in long-term secondary stroke prevention (n = 10 225; OR = 0.61, 95% CI = 0.52-0.72, P < 0.0001). 65
There are a few limitations of the cilostazol clinical trials. Most studies were conducted in East Asia enrolling a predominantly East Asian population. 66 Consequently, the absence of evidence for an effect in Western populations is the likely explanation for why cilostazol is not approved by the American Food and Drug Administration, the UK Medicines and Healthcare products Regulatory Agency, or the European Medicines Agency. Future research should focus on expanding the RCT level evidence for this drug to other racial groups and patients with small vessel disease, as the drug appears to have an increased benefit in this subgroup of stroke patients. 65 Interestingly, in an RCT trial investigating the use of cilostazol for the treatment of lacunar stroke (a stroke subtype mainly caused by small vessel disease without specific treatment guidelines), the use of cilostazol in combination with isosorbide mononitrate was well tolerated, evidenced by the fact that 64% of trial participants achieved a full dose 87% achieved a half dose of isosorbide mononitrate. 67 This trial only contained 57 participants though so further larger RCT are needed to confirm this finding.
Glycoprotein IIb/IIIa Inhibitors
The glycoprotein IIb/IIIa inhibitors (GP2b3ais), abciximab, eptifibatide, and tirofiban have been evaluated for use in the acute period after ischemic stroke. 68 The GP2b3a family of drugs work to inhibit the platelet cell surface glycoprotein IIb/IIIa receptor that stops platelet aggregation by inhibiting fibrinogen molecule binding. 69 In general, the drugs are short-acting and administered intravenously, making them less appealing for long-term use. 70 The GP2b3ais were initially used in the treatment of AIS due to the large body of evidence of reduced mortality when used to treat myocardial infarction. 71
The GP2b3ai with the greatest amount of evidence for use in AIS is abciximab. Unfortunately, most trials show that the use of abciximab in AIS dramatically increases the risk of symptomatic intracranial hemorrhage when given as an adjunct to thrombolysis.72,73 In fact, the Abciximab in Emergency Treatment of Stroke Trial (AbESTT-II), n = 808, was stopped early due to the increased risk of symptomatic or fatal intracranial hemorrhage within 5 days of enrolment (5.5% of abciximab-treated vs 0.5% placebo-treated in the primary cohort; P = 0.002). 72 Evidence from meta-analyses of GP2b3ai also suggests an increased bleeding risk when used in AIS, but these studies are biased toward the effect of abciximab, as this drug has the greatest number of RCTs. 73 The combined approach to lysis utilizing eptifibatide and recombinant tissue-type plasminogen activator (CLEAR) stroke study (n = 94) evaluated eptifibatide in combination with tissue plasminogen activator (tPA) and showed that there is no increased risk of intracranial hemorrhage compared with tPA alone, which was also confirmed by the higher dose regimen CLEAR-ER trial, n = 126 (OR = 0.15, 95% CI = 0.01–1.40, P = 0.053).74,75 There have been calls for a large-scale trial involving eptifibatide as an adjunct to thrombolysis, but this has yet to be performed. 76
RCTs that have investigated tirofiban use in AIS suggest that it may have a benefit similar to that seen with aspirin if administered within the first 6 hours. 77 A lower mortality rate has been found at 5 months when tirofiban is given in AIS as compared with placebo (2.3% vs 8.7%, OR = 4.05, 95% CI = 1.1-14.9), 78 although no evidence of functional improvement was seen in the tirofiban group. A recent meta-analysis has suggested that there is an increased risk of ICH in people older than the age of 70, those with an NIHSS score greater than 15 (RR = 1.77, 95% CI = 1.14-2.73) or those who have been given a dose of 10 mg or greater of tirofiban (RR = 1.46, 95% CI = 1.07-1.99). 68 This may limit the use of tirofiban clinically.
Currently, the AHA/ASA does not support the use of GP2b3ai for AIS. 8 Further studies could be considered for the drugs eptifibatide and tirofiban, but trial design should consider their effectiveness in the acute setting when compared with other antiplatelets such as aspirin and clopidogrel, and how age and stroke severity may be contraindications to their use.
The following table summarizes the data comparing different antiplatelet agents on their effect on secondary stroke prevention. The NNT and cost per tablet of each drug are described (see Table 1).
Table 1.
Summary of Antiplatelet Agents and Their Effect on Secondary Stroke Prevention.
| Drug | Details | NNT | Dose | N | Cost as defined by the BNF |
|---|---|---|---|---|---|
| Aspirin | Aspirin vs placebo Vascular events within 2 years in patients with TIA/stroke |
40 | Aspirin 25 mg BD | 6602 | £0.0032 per 75 mg tablet |
| Aspirin vs placebo Recurrent stroke within 2-4 weeks in patients with ischemic stroke |
140 | Aspirin 160-300 mg | 40 850 | ||
| Dipyridamole | Dipyridamole vs placebo Recurrent stroke within 2 years in patients with TIA/stroke |
42 | Dipyridamole 200 mg MR BD | 6602 | £0.17 per 200 mg tablet |
| Dipyridamole + aspirin vs aspirin Vascular events within (mean) 2.6 years in patients with TIA/stroke |
37 | Aspirin 30-990 mg Dipyridamole 150-400 mg |
7612 | ||
| Thienopyridines: Clopidogrel and ticlopidine | Clopidogrel/ticlopidine vs aspirin Vascular events within (average) 2 years in patients with TIA/stroke |
100 (not sig) a | Clopidogrel 75 mg or ticlopidine 200-500 mg Aspirin 325-1300 mg |
11 649 | £0.04 per 75 mg tablet |
| Clopidogrel + aspirin vs aspirin Reduction in all nonfatal recurrent stroke (ARR 1.9%) in DAPT group. High-risk TIA/mild stroke patients, followed up for 90 days |
53 | Clopidogrel 75 mg (loaded 300-600 mg) Aspirin 50-325 mg |
10 301 | ||
| Prasugrel | Prasugrel vs clopidogrel Vascular events after treatment for 6-15 months in patients with moderate to high-risk ACS undergoing PCI Subgroup analysis of those with TIA/stroke showed prasugrel has greater bleeding risk and no net benefit |
46 | Prasugrel 10 mg (loaded 60 mg) Clopidogrel 75 mg (loaded 300 mg) |
13 608 | £0.19 per 5 mg tablet |
| Ticagrelor | Ticagrelor vs ticagrelor + aspirin Stroke or death within 30 days in patients with TIA/stroke |
91 | Ticagrelor 90 mg BD + aspirin 75-100 mg | 11 016 | £0.98 per 90 mg tablet |
| Ticagrelor vs aspirin Vascular events within 90 days in patients with high-risk TIA/nonsevere stroke. Ticagrelor not superior to aspirin. |
125 (not sig) | Ticagrelor 90 mg BD or aspirin 100 mg | 13 199 | ||
| Cilostazol | Cilostazol vs aspirin Recurrent stroke within follow-up of between 3 months and 5 years of patients with TIA/stroke |
76 | Cilostazol 200 mg daily Aspirin 81-300 mg |
5681 | £0.12 per 100 mg tablet |
| GP IIb/IIIa inhibitors: Abciximab, eptifibatide, tirofiban |
No evidence for vascular events in stroke population, but secondary outcomes of stroke recurrence available in 2 trials | Abciximab: NA Eptifibatide: £13.61 per 20 mg vial Tirofiban: £159 per 12.5 mg solution |
Vascular events: Nonfatal stroke, nonfatal MI, and vascular death.
Abbreviations: ARR, Absolute Risk Reduction; ACS, acute coronary syndrome; BD, twice daily; BNF, British national formulary; CI, confidence intervals; DAPT, dual antiplatelet therapy; GP, glycoprotein; MI, myocardial infarction; MR, modified release; NA, not available; NNT, numbers needed to treat; OR, odds ratio; PCI, percutaneous coronary intervention; RCT, randomized controlled trial; TIA, transient ischemic attack.
The reduction in odds of vascular events in thienopyridine versus aspirin not statistically significant in the stroke population (OR = 0.94, 95% CI = 0.85-1.03), but was in the overall high-risk vascular population (OR = 0.92, 95% CI = 0.85-0.99), n = 26 255 Clopidogrel and ticlopidine subgroups performed similarly.
Stroke While on Antiplatelet Therapy
In noncardioembolic stroke and TIA, the guidelines recommend aspirin, either alone or in combination with dipyridamole, or clopidogrel. 79 However, no antiplatelet agent was 100% effective in preventing recurrent cerebrovascular events in the clinical trials. Furthermore, the phenomenon of antiplatelet resistance has been well-described. 9
While a meta-analysis of observational studies found evidence in favor of switching to an entirely new antiplatelet combination after a recurrent event, with a reduced incidence of cardiovascular events on follow-up, 80 there are no randomized controlled trials to guide clinicians.
There are also several other possibilities that need to be explored if a patient has a stroke despite first-line antiplatelet therapy. First, it is vital to ensure that the patient is adherent with the antiplatelet as prescribed. 26 Second, a concurrent medication review may reveal interacting drugs that need to be removed. For example, omeprazole can adversely affect clopidogrel metabolism, so if a proton pump inhibitor (PPI) is needed, lansoprazole or an alternative PPI should be prescribed instead. 81 Third, antiplatelet treatment failure should prompt the physician to search for cardioembolic causes of stroke that could respond to anticoagulants rather than antiplatelet agents. Finally, there may be ways to optimize secondary prevention rather than switching antiplatelets, for example, increasing the dose of statin or improving blood pressure and/or blood glucose control. Lifestyle modifications should also be aggressively managed in patients who have had a stroke, as there is strong evidence from the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) study that physical activity prevents further vascular events. 82 In patients who performed regular physical activity such as walking, the odds ratio of a further vascular event (recurrent stroke, myocardial infarction, or vascular death) was 0.6 (95% CI = 0.4-0.8) compared with those who did not.
An important consideration is that a proportion of patients have genetic polymorphisms rendering their platelets resistant to the effects of certain antiplatelets. Several point-of-care testing (POCT) kits are now available to detect these polymorphisms, but the testing kits themselves require further validation before incorporation into randomized trials. 9 Until then, it is unclear how POCT can be used to guide the first choice of antiplatelet, or later switching of antiplatelets in the face of recurrent events.
Consideration of Dual Antiplatelet Therapy
As discussed previously in this article, there have been numerous studies comparing DAPT with single antiplatelet regimes for various timeframes. The use of DAPT within the first 21 days poststroke does offer additional benefit for patients with mild stroke or high-risk TIA. 83 Again, the largest body of evidence comes for the use of clopidogrel and aspirin in combination. A recent AHA/ASA meta-analysis of dual versus single antiplatelet therapy for stroke prevention in patients with ischemic stroke or TIA concluded that short-duration DAPT (up to 90 days) initiated soon after the index event reduced the risk of recurrent stroke (RR = 0.68, 95% CI = 0.55-0.83), with no significant increase in major bleeding. In contrast, long-term DAPT increased the risk of major bleeding with no reduction in recurrent stroke risk. 84 The role of triple antiplatelet therapy has also been examined. The TARDIS trial (n = 3096), which was stopped early on safety and futility grounds, found that 30 days of an intensive antiplatelet regime (aspirin, clopidogrel, and dipyridamole) carried significantly higher bleeding risks, with no commensurate reduction in stroke recurrence, compared with standard therapy of clopidogrel or aspirin and dipyridamole. 85
Aspirin and dipyridamole have been shown to have efficacy as long-term secondary prevention, but in the acute setting, DAPT comprised of aspirin and clopidogrel remains the combination of choice. The PRoFESS trial examined long-term DAPT (aspirin and dipyridamole) versus clopidogrel and found no difference in stroke recurrence rates, but higher rates of bleeding in the DAPT group. 32 While ticagrelor is not licensed for stroke in the United Kingdom, the THALES study found that DAPT with aspirin resulted in fewer strokes at 30 days compared with aspirin alone but came with a slightly higher bleeding risk. 51 Interestingly, in a recent network meta-analysis, the use of aspirin and clopidogrel (HR = 0.74, 95% CI = 0.65-0.84, n = 5517) or aspirin and ticagrelor (HR = 0.79, 95% CI = 0.68-0.91, n = 5853) was compared with aspirin alone (n = 10,722) and found that both DAPT regimes were superior to aspirin alone at preventing recurrent stroke or death up to 90 days post treatment initiation (https://pubmed.ncbi.nlm.nih.gov/34870698/). This again though was at the expense of an increased risk of major hemorrhage.
The current consensus is that DAPT is appropriate in the acute phase (defined as the first 30 days poststroke) postischemic stroke, especially when initiated promptly, but there appears to be no benefit in continuing this further. 83 Guidelines from several countries, including the United States, United Kingdom, and China, recommend commencing DAPT with aspirin plus clopidogrel within 24 hours of minor stroke (defined as NIHSS ≤3) or high-risk TIA (defined as ABCD 2 ≥ 4, the ABCD 2 score being an estimate of stroke risk after TIA based on patients age, blood pressure, clinical features, duration of symptoms, and presence of diabetes) and continuing for 21 days.86-88 This is largely based on the CHANCE and POINT trials that showed that DAPT with aspirin plus clopidogrel for up to 21 days leads to a significant reduction in recurrent ischemic stroke at 90 days compared with aspirin monotherapy (RR = 0.70, 95% CI = 0.61-0.8, NNT = 53), n = 10 301.11,89 Further work could investigate using several different combinations of antiplatelet agents which have not been trialed to date.
Using Antiplatelets and Anticoagulants in Combination
In certain situations, anticoagulant therapy for secondary prevention of stroke is thought to be beneficial over antiplatelet agents. One such scenario would be the use of anticoagulants poststroke in patients who have atrial fibrillation (AF). 90 The combination of both anticoagulants and antiplatelet agents is considered in patients with stroke in the context of preexisting cardiovascular disease, such as coronary artery disease in the presence of AF. In this situation, the patient is likely to be started on anticoagulants to treat the AF poststroke, but the evidence for continuing, stopping, or adding an antiplatelet agent when coronary artery disease also exists is less clear. In elderly cohorts (n = 10 093), it has been shown that there is a small increased risk of bleeding within 90 days of discharge in patients on both antiplatelets and anticoagulants (1.3% on only warfarin vs 1.9% on warfarin + antiplatelet, OR = 1.46, 95% CI = 0.998-2.12), 91 but these data are in the context of patients diagnosed with AF not who have been started on new therapies after stroke. In the GARFIELD-AF cohort study (n = 24 436), it was suggested that when patients are treated with a combination of both drugs, there is not overall benefit on all-cause mortality for these patients (adjusted HR = 1.22, 95% CI = 0.98-1.51), suggesting that the risks of increased bleeding are not outweighed by the benefits. 92 Evidence from the use of combination therapy in patients who have unstable coronary artery disease may suggest that short-term antiplatelets improve patient outcome, but the benefit is reduced when the drugs are used more long-term. 93 In this scenario, clopidogrel is thought to be the most efficacious antiplatelet to use in combination with anticoagulation. 94 When combination therapy has been investigated as a comparator to antiplatelet agents for all causes of stroke, no additional benefit was seen of using combination therapy, except when low-dose unfractionated heparin and aspirin were used in combination (OR = 0.75, 95% CI = 0.56-1.03), but this interaction warrants further investigation before firm conclusions can be made as reduction seen in recurrent stroke risk was not significant. 95 It has also been investigated if the use of heparin in addition to standard therapy improves outcome in the first 6 months poststroke, and again no additional benefit was observed. 96
When generalizing to the ischemic stroke population, there appears to be little benefit of combination therapy. The caveat to this though may be that in certain situations when a patient presents with stroke and unstable coronary artery disease, the use of combinations of antiplatelets and anticoagulant drugs may provide extra benefit. Currently, there is no RCT level evidence of the benefits of combination therapy poststroke assessing this particular group and is an important area for future research to investigate.
Relevance to Patient Care and Clinical Practice
Several strategies could be considered to improve the use of antiplatelet agents in the treatment of stroke in future research trials and clinical practice. It appears that in the vast majority of cases, the long-term secondary prevention of stroke is best managed using either clopidogrel or aspirin. In the acute setting, there are several issues that potentially could be improved using alternative antiplatelet agents. When considering these issues, the application of a personalized medicine approach to the treatment of stroke with antiplatelets should be considered.
There is a large body of evidence to suggest that certain platelet genetic polymorphisms can render patients resistant to treatment with either clopidogrel or aspirin. Given the strong evidence base for the use of aspirin and clopidogrel in AIS and in secondary prevention, screening for these polymorphisms upon initial stroke presentation could be considered when personalizing the approach to antiplatelet use. These results could inform whether to consider combination therapy, switch antiplatelet agent, or increase the aspirin dose, although these options must be balanced against the risk of bleeding, drug adverse effects, and the possibility of resistance to other antiplatelet medications. The greatest risk of recurrent stroke is within the first 30 days of the initial event. Therefore, in future clinical settings, as genetic phenotyping becomes more accessible, this may be done as part of the initial stroke assessment. This would allow the stroke physician to then make a more informed decision as to which antiplatelet agent to use in cases of clopidogrel or aspirin resistance.
Biochemical response to aspirin and clopidogrel can be measured through in vitro tests such as platelet function analysis (PFA) tests, light transmission aggregometry, and by in vivo quantification of thromboxane metabolites. However, there is poor correlation between the different assay results in each individual subject. 24 The variety of proposed mechanisms and lack of consensus regarding best screening modality means that there is no current single test to reliably determine which patients are likely to experience aspirin treatment failure, thereby requiring clinical judgment about ongoing treatment strategy if aspirin treatment failure were to occur currently.
Within the acute setting, decisions about the use of certain antiplatelets in combination with thrombolysis/thrombectomy and which combination of antiplatelets to use as part of DAPT could be reviewed as part of future clinical trials. There is evidence that certain antiplatelet agents may improve outcome when used in conjunction with thrombolysis in the acute setting (eptifibatide, glycoprotein IIb/IIIa inhibitor, potentially being one of these drugs). When considering the use of DAPT in the initial secondary prevention of stroke, it should also be considered that not all combinations of antiplatelets have been researched, and so better combinations may still be found. However, it is important to remember that there appears to be more risk than benefit to extending DAPT beyond 21 days. Most of the research for the use of clopidogrel and aspirin as part of DAPT focuses on Western and Chinese populations; therefore, it may be that patients of different ethnicities respond better to other combinations of antiplatelets in this acute phase.
Race should also be considered when planning future long-term therapy trials for the secondary prevention of stroke. There is evidence already that stroke type, incidence, and risk factors differ among ethnic groups; 97 therefore, secondary prevention of stroke is likely to be confounded by this. Although there is very good evidence for the use of clopidogrel and aspirin in the long-term prevention of stroke, there is a signal that other antiplatelets may have additional benefits to nonwhite populations (cilostazol is a potential example of this in Japanese populations). Ticagrelor and prasugrel do not rely on the activity of CYP2C19 and therefore may also have increased benefits over clopidogrel in East Asian populations known to have a higher carrier frequency of the CYP2C19 loss-of-function allele. 60 Antiplatelet stroke research, like many other areas of clinical research, should try to develop clinical trial level evidence in populations around the world that are not a majority white. This will help to develop a personalized medicine approach to the treatment of stroke which will benefit all stroke patients, but also potentially help to address the racial disparities unfortunately seen in stroke care. 98
Finally, strategies aimed at improving patient adherence to prescribed drugs should also be considered. One of the largest factors affecting the efficacy of antiplatelet agents in the secondary prevention of stroke is patient nonadherence with medications. 26 It has been suggested the noncompliance with the use of antiplatelet agents can be as high as 35% twelve months postischemic stroke. There are several factors that are thought to affect this, including being older than the age of 70 years old, already taking multiple medications (>4 drugs), coming from a lower social economic class and being from a more rural community. 99 In English-speaking communities, having a poorer proficiency in English has been associated with reduced antiplatelet adherence, as has having multiple medical comorbidities. 100 Strategies aimed at improving awareness of the side effects of antiplatelet agents, endowing patients with more knowledge about why they are taking the drug, and having access to appropriate medication counseling have been shown to be factors that can be addressed to improve patient antiplatelet adherence. 101
Conclusion
Antiplatelet agents remain one of the most efficacious and best researched secondary preventive measures for the treatment of stroke. The use of both clopidogrel and aspirin is well established in both the acute and secondary prevention settings and is the basis of most clinical guidelines around the world. Although there is already good evidence for the appropriate use of antiplatelets postischemic stroke, future research may focus on how to personalize the approach to antiplatelet prescription. This may take the form of screening patients for platelet polymorphisms that confer antiplatelet resistance or extending randomized controlled trial level evidence to be more encompassing of the diverse racial populations that are affected by stroke.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: S.M.B. is supported by an NIHR Clinical Lectureship in Neurology.
ORCID iDs: Marharyta Kamarova  https://orcid.org/0000-0001-8733-0050
https://orcid.org/0000-0001-8733-0050
Hamish Patel  https://orcid.org/0000-0001-6313-0185
https://orcid.org/0000-0001-6313-0185
Simon M. Bell  https://orcid.org/0000-0002-2781-6478
https://orcid.org/0000-0002-2781-6478
References
- 1. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16(11):877-897. doi: 10.1016/S1474-4422(17)30299-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Andersen Klaus K, Olsen Tom S, Dehlendorff C, Kammersgaard Lars P. Hemorrhagic and ischemic strokes compared. Stroke. 2009;40(6):2068-2072. doi: 10.1161/STROKEAHA.108.540112. [DOI] [PubMed] [Google Scholar]
- 3. Poon MT, Bell SM, Al-Shahi Salman R. Epidemiology of intracerebral haemorrhage. Front Neurol Neurosci. 2015;37:1-12. doi: 10.1159/000437109. [DOI] [PubMed] [Google Scholar]
- 4. Avan A, Digaleh H, Di Napoli M, et al. Socioeconomic status and stroke incidence, prevalence, mortality, and worldwide burden: an ecological analysis from the Global Burden of Disease Study 2017. BMC Med. 2019;17(1):191. doi: 10.1186/s12916-019-1397-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Boehme AK, Esenwa C, Elkind MSV. Stroke risk factors, genetics, and prevention. Circ Res. 2017;120(3):472-495. doi: 10.1161/CIRCRESAHA.116.308398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hankey GJ. Long-term outcome after ischaemic stroke/transient ischaemic attack. Cerebrovasc Dis. 2003;16(suppl 1):14-19. doi: 10.1159/000069936. [DOI] [PubMed] [Google Scholar]
- 7. Sandercock PA, Counsell C, Tseng MC, Cecconi E. Oral antiplatelet therapy for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;2014(3):CD000029. doi: 10.1002/14651858.CD000029.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Powers WJ, Rabinstein AA, Ackerson T, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2019;50(12):e344-e418. doi: 10.1161/STR.0000000000000211. [DOI] [PubMed] [Google Scholar]
- 9. Topçuoglu MA, Arsava EM, Ay H. Antiplatelet resistance in stroke. Expert Rev Neurother. 2011;11(2):251-263. doi: 10.1586/ern.10.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Malloy RJ, Kanaan AO, Silva MA, Donovan JL. Evaluation of antiplatelet agents for secondary prevention of stroke using mixed treatment comparison meta-analysis. Clin Ther. 2013;35(10):1490-1500. doi: 10.1016/j.clinthera.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 11. Johnston SC, Easton JD, Farrant M, et al. Clopidogrel and aspirin in acute ischemic stroke and high-risk TIA. N Engl J Med. 2018;379(3):215-225. doi: 10.1056/nejmoa1800410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Naqvi IA, Kamal AK, Rehman H. Multiple versus fewer antiplatelet agents for preventing early recurrence after ischaemic stroke or transient ischaemic attack. Cochrane Database Syst Rev. 2020;8(8):CD009716. doi: 10.1002/14651858.CD009716.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chen ZM, Sandercock P, Pan HC, et al. Indications for early aspirin use in acute ischemic stroke: a combined analysis of 40 000 randomized patients from the Chinese acute stroke trial and the international stroke trial. On behalf of the CAST and IST collaborative groups. Stroke. 2000;31(6):1240-1249. doi: 10.1161/01.str.31.6.1240. [DOI] [PubMed] [Google Scholar]
- 14. Hillen T, Coshall C, Tilling K, Rudd AG, McGovern R, Wolfe CD. Cause of stroke recurrence is multifactorial: patterns, risk factors, and outcomes of stroke recurrence in the South London Stroke Register. Stroke. 2003;34(6):1457-1463. doi: 10.1161/01.STR.0000072985.24967.7F. [DOI] [PubMed] [Google Scholar]
- 15. CAST: randomised placebo-controlled trial of early aspirin use in 20,000 patients with acute ischaemic stroke. CAST (Chinese Acute Stroke Trial) Collaborative Group. Lancet. 1997;349(9066):1641-1649. doi: 10.1016/S0140-6736(97)04010-5. [DOI] [PubMed] [Google Scholar]
- 16. Zinkstok SM, Roos YB. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet. 2012;380(9843):731-737. doi: 10.1016/S0140-6736(12)60949-0. [DOI] [PubMed] [Google Scholar]
- 17. Baigent C, Blackwell L, Collins R, et al. Aspirin in the primary and secondary prevention of vascular disease: collaborative meta-analysis of individual participant data from randomised trials. Lancet. 2009;373(9678):1849-1860. doi: 10.1016/S0140-6736(09)60503-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hackam DG, Spence JD. Antiplatelet therapy in ischemic stroke and transient ischemic attack. Stroke. 2019;50(3):773-778. doi: 10.1161/STROKEAHA.118.023954. [DOI] [PubMed] [Google Scholar]
- 19. Antithrombotic Trialists’ Collaboration. Collaborative meta-analysis of randomised trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71-86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. NICE. Primary prevention of CVD—management—antiplatelet treatment. CKS, NICE; 2020. https://cks.nice.org.uk/topics/antiplatelet-treatment/management/primary-prevention-of-cvd/ [Google Scholar]
- 21. Meschia JF, Bushnell C, Boden-Albala B, et al. Guidelines for the primary prevention of stroke: a statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2014;45(12):3754-3832. doi: 10.1161/STR.0000000000000046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Judge C, Ruttledge S, Murphy R, et al. Aspirin for primary prevention of stroke in individuals without cardiovascular disease-A meta-analysis. Int J Stroke. 2020;15(1):9-17. doi: 10.1177/1747493019858780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Martin CP, Talbert RL. Aspirin resistance: an evaluation of current evidence and measurement methods. Pharmacotherapy. 2005;25(7):942-953. doi: 10.1592/phco.2005.25.7.942. [DOI] [PubMed] [Google Scholar]
- 24. Shahid F, Chahal CA, Akhtar MJ. Aspirin treatment failure: is this a real phenomenon? a review of the aetiology and how to treat it. JRSM Short Rep. 2013;4(4):30. doi: 10.1177/2042533313475576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Wang TH, Bhatt DL, Topol EJ. Aspirin and clopidogrel resistance: an emerging clinical entity. European Heart Journal. 2005;27(6):647-654. doi: 10.1093/eurheartj/ehi684. [DOI] [PubMed] [Google Scholar]
- 26. Schwartz KA, Schwartz DE, Barber K, Reeves M, De Franco AC. Non-compliance is the predominant cause of aspirin resistance in chronic coronary arterial disease patients. J Transl Med. 2008;6:46. doi: 10.1186/1479-5876-6-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhang YJ, Li MP, Tang J, Chen XP. Pharmacokinetic and pharmacodynamic responses to clopidogrel: evidences and perspectives. Int J Environ Res Public Health. 2017;14(3):301. doi: 10.3390/ijerph14030301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Shaban A, Monlezun DJ, Rincon N, Tiu J, Valmoria M, Martin-Schild S. Safety and efficacy of acute clopidogrel load in patients with moderate and severe ischemic strokes. Stroke Res Treat. 2016;2016:8915764. doi: 10.1155/2016/8915764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Diener HC, Bogousslavsky J, Brass LM, et al. Aspirin and clopidogrel compared with clopidogrel alone after recent ischaemic stroke or transient ischaemic attack in high-risk patients (MATCH): randomised, double-blind, placebo-controlled trial. Lancet. 2004;364(9431):331-337. doi: 10.1016/S0140-6736(04)16721-4. [DOI] [PubMed] [Google Scholar]
- 30. A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). CAPRIE Steering Committee. Lancet. 1996;348(9038):1329-1339. doi: 10.1016/S0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 31. Harker LA, Kadatz RA. Mechanism of action of dipyridamole. Thromb Res Suppl. 1983;4:39-46. doi: 10.1016/0049-3848(83)90356-0. [DOI] [PubMed] [Google Scholar]
- 32. Sacco RL, Diener H-C, Yusuf S, et al. Aspirin and extended-release dipyridamole versus clopidogrel for recurrent stroke. N Engl J Med. 2008;359(12):1238-1251. doi: 10.1056/NEJMoa0805002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Halkes PH, Gray LJ, Bath PM, et al. Dipyridamole plus aspirin versus aspirin alone in secondary prevention after TIA or stroke: a meta-analysis by risk. J Neurol Neurosurg Psychiatry. 2008;79(11):1218-1223. doi: 10.1136/jnnp.2008.143875. [DOI] [PubMed] [Google Scholar]
- 34. Barlas RS, Loke YK, Mamas MA, et al. Effect of antiplatelet therapy (aspirin + dipyridamole versus clopidogrel) on mortality outcome in ischemic stroke. Am J Cardiol. 2018;122(6):1085-1090. doi: 10.1016/j.amjcard.2018.05.043. [DOI] [PubMed] [Google Scholar]
- 35. Diener HC, Cunha L, Forbes C, Sivenius J, Smets P, Lowenthal A. European Stroke Prevention Study. 2. Dipyridamole and acetylsalicylic acid in the secondary prevention of stroke. J Neurol Sci. 1996;143(1-2):1-13. doi: 10.1016/s0022-510x(96)00308-5. [DOI] [PubMed] [Google Scholar]
- 36. De Schryver EL, Algra A, van Gijn J. Dipyridamole for preventing stroke and other vascular events in patients with vascular disease. Cochrane Database Syst Rev. 2007;3:CD001820. doi: 10.1002/14651858.CD001820.pub3. [DOI] [PubMed] [Google Scholar]
- 37. Diener HC, Sacco RL, Yusuf S, et al. Effects of aspirin plus extended-release dipyridamole versus clopidogrel and telmisartan on disability and cognitive function after recurrent stroke in patients with ischaemic stroke in the Prevention Regimen for Effectively Avoiding Second Strokes (PRoFESS) trial: a double-blind, active and placebo-controlled study. Lancet Neurol. 2008;7(10):875-884. doi: 10.1016/S1474-4422(08)70198-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Halkes PH, van Gijn J, Kappelle LJ, Koudstaal PJ, Algra A. Aspirin plus dipyridamole versus aspirin alone after cerebral ischaemia of arterial origin (ESPRIT): randomised controlled trial. Lancet. 2006;367(9523):1665-1673. doi: 10.1016/S0140-6736(06)68734-5. [DOI] [PubMed] [Google Scholar]
- 39. Second European Stroke Prevention Study. ESPS-2 Working Group. J Neurol. 1992;239(6):299-301. doi: 10.1007/BF00867583. [DOI] [PubMed] [Google Scholar]
- 40. de Vos-Koppelaar NC, Kerkhoff H, de Vogel EM, Zock E, Dieleman HG. The effect of a slower than standard dose escalation scheme for dipyridamole on headaches in secondary prevention therapy of strokes: a randomized, open-label trial (DOSE). Cerebrovasc Dis. 2014;37(4):285-289. doi: 10.1159/000360751. [DOI] [PubMed] [Google Scholar]
- 41. Wiviott SD, Antman EM, Braunwald E. Prasugrel. Circulation. 2010;122(4):394-403. doi: 10.1161/CIRCULATIONAHA.109.921502. [DOI] [PubMed] [Google Scholar]
- 42. Brandt JT, Close SL, Iturria SJ, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost. 2007;5(12):2429-2436. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 43. Serebruany VL, Alberts MJ, Hanley DF. Prasugrel in the poststroke cohort of the TRITON Trial: the clear and present danger. Cerebrovasc Dis. 2008;26(1):93-94. doi: 10.1159/000138337. [DOI] [PubMed] [Google Scholar]
- 44. Wiviott SD, Braunwald E, McCabe CH, et al. Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2007;357(20):2001-2015. doi: 10.1056/NEJMoa0706482. [DOI] [PubMed] [Google Scholar]
- 45. Chin CT, Neely B, Magnus Ohman E, et al. Time-varying effects of prasugrel versus clopidogrel on the long-term risks of stroke after acute coronary syndromes: results from the TRILOGY ACS trial. Stroke. 2016;47(4):1135-1139. doi: 10.1161/STROKEAHA.115.012454. [DOI] [PubMed] [Google Scholar]
- 46. Ogawa A, Toyoda K, Kitagawa K, et al. Comparison of prasugrel and clopidogrel in patients with non-cardioembolic ischaemic stroke: a phase 3, randomised, non-inferiority trial (PRASTRO-I). Lancet Neurol. 2019;18(3):238-247. doi: 10.1016/S1474-4422(18)30449-6. [DOI] [PubMed] [Google Scholar]
- 47. Kitagawa K, Toyoda K, Kitazono T, et al. Safety and efficacy of prasugrel in elderly/low body weight Japanese patients with ischemic stroke: randomized PRASTRO-II. Cerebrovasc Dis. 2020;49(2):152-159. doi: 10.1159/000506825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bonaca MP, Bhatt DL, Cohen M, et al. Long-term use of ticagrelor in patients with prior myocardial infarction. N Engl J Med. 2015;372(19):1791-1800. doi: 10.1056/NEJMoa1500857. [DOI] [PubMed] [Google Scholar]
- 49. Wallentin L, Becker RC, Budaj A, et al. Ticagrelor versus clopidogrel in patients with acute coronary syndromes. N Engl J Med. 2009;361(11):1045-1057. doi: 10.1056/NEJMoa0904327. [DOI] [PubMed] [Google Scholar]
- 50. Johnston SC, Amarenco P, Albers GW, et al. Ticagrelor versus aspirin in acute stroke or transient ischemic attack. N Engl J Med. 2016;375(1):35-43. doi: 10.1056/NEJMoa1603060. [DOI] [PubMed] [Google Scholar]
- 51. Johnston SC, Amarenco P, Denison H, et al. Ticagrelor and aspirin or aspirin alone in acute ischemic stroke or TIA. N Engl J Med. 2020;383(3):207-217. doi: 10.1056/NEJMoa1916870. [DOI] [PubMed] [Google Scholar]
- 52. Zhang N, Xu W, Li O, Zhang B. The risk of dyspnea in patients treated with third-generation P2Y12 inhibitors compared with clopidogrel: a meta-analysis of randomized controlled trials. BMC Cardiovasc Disord. 2020;20(1):140. doi: 10.1186/s12872-020-01419-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Flores-Runk P, Raasch RH. Ticlopidine and antiplatelet therapy. Ann Pharmacother. 1993;27(9):1090-1098. doi: 10.1177/106002809302700915. [DOI] [PubMed] [Google Scholar]
- 54. Gent M, Blakely JA, Easton JD, et al. The Canadian American Ticlopidine Study (CATS) in thromboembolic stroke. Lancet. 1989;1(8649):1215-1220. doi: 10.1016/s0140-6736(89)92327-1. [DOI] [PubMed] [Google Scholar]
- 55. Hass WK, Easton JD, Adams HP, Jr, et al. A randomized trial comparing ticlopidine hydrochloride with aspirin for the prevention of stroke in high-risk patients. Ticlopidine Aspirin Stroke Study Group. N Engl J Med. 1989;321(8):501-507. doi: 10.1056/NEJM198908243210804. [DOI] [PubMed] [Google Scholar]
- 56. Weisberg LA. The efficacy and safety of ticlopidine and aspirin in non-whites: analysis of a patient subgroup from the Ticlopidine Aspirin Stroke Study. Neurology. 1993;43(1):27-31. doi: 10.1212/wnl.43.1_part_1.27. [DOI] [PubMed] [Google Scholar]
- 57. Gorelick PB, Richardson D, Kelly M, et al. Aspirin and ticlopidine for prevention of recurrent stroke in black patients: a randomized trial. Jama. 2003;289(22):2947-2957. doi: 10.1001/jama.289.22.2947. [DOI] [PubMed] [Google Scholar]
- 58. Wong YS, Tsai CF, Hsu YH, Ong CT. Efficacy of aspirin, clopidogrel, and ticlopidine in stroke prevention: a population-based case-cohort study in Taiwan. PLoS One. 2020;15(12):e0242466. doi: 10.1371/journal.pone.0242466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Maeda A, Ando H, Asai T, et al. Differential impacts of CYP2C19 gene polymorphisms on the antiplatelet effects of clopidogrel and ticlopidine. Clin Pharmacol Ther. 2011;89(2):229-233. doi: 10.1038/clpt.2010.268. [DOI] [PubMed] [Google Scholar]
- 60. Scott SA, Sangkuhl K, Stein CM, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for CYP2C19 genotype and clopidogrel therapy: 2013 update. Clin Pharmacol Ther. 2013;94(3):317-323. doi: 10.1038/clpt.2013.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Kimura Y, Tani T, Kanbe T, Watanabe K. Effect of cilostazol on platelet aggregation and experimental thrombosis. Arzneimittelforschung. 1985;35(7A):1144-1149. [PubMed] [Google Scholar]
- 62. Goto S. Cilostazol: potential mechanism of action for antithrombotic effects accompanied by a low rate of bleeding. Atheroscler Suppl. 2005;6(4):3-11. doi: 10.1016/j.atherosclerosissup.2005.09.002. [DOI] [PubMed] [Google Scholar]
- 63. Kim BJ, Kwon SU, Park JH, et al. Cilostazol versus aspirin in ischemic stroke patients with high-risk cerebral hemorrhage: subgroup analysis of the PICASSO trial. Stroke. 2020;51(3):931-937. doi: 10.1161/STROKEAHA.119.023855. [DOI] [PubMed] [Google Scholar]
- 64. Kim SM, Jung JM, Kim BJ, Lee JS, Kwon SU. Cilostazol mono and combination treatments in ischemic stroke: an updated systematic review and meta-analysis. Stroke. 2019;50(12):3503-3511. doi: 10.1161/STROKEAHA.119.026655. [DOI] [PubMed] [Google Scholar]
- 65. McHutchison C, Blair GW, Appleton JP, et al. Cilostazol for secondary prevention of stroke and cognitive decline: systematic review and meta-analysis. Stroke. 2020;51(8):2374-2385. doi: 10.1161/STROKEAHA.120.029454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Tan CH, Wu AG, Sia C-H, et al. Cilostazol for secondary stroke prevention: systematic review and meta-analysis. Stroke Vasc Neurol. 2021;6:410-423. doi: 10.1136/svn-2020-000737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Blair GW, Appleton JP, Flaherty K, et al. Tolerability, safety and intermediary pharmacological effects of cilostazol and isosorbide mononitrate, alone and combined, in patients with lacunar ischaemic stroke: the LACunar Intervention-1 (LACI-1) trial, a randomised clinical trial. EClinicalMedicine. 2019;11:34-43. doi: 10.1016/j.eclinm.2019.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Zhu X, Cao G. Safety of glycoprotein IIb-IIIa inhibitors used in stroke-related treatment: a systematic review and meta-analysis. Clin Appl Thromb Hemost. 2020;26: 1-11. doi: 10.1177/1076029620942594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Lippi G, Montagnana M, Danese E, Favaloro EJ, Franchini M. Glycoprotein IIb/IIIa inhibitors: an update on the mechanism of action and use of functional testing methods to assess antiplatelet efficacy. Biomark Med. 2011;5(1):63-70. doi: 10.2217/bmm.10.119. [DOI] [PubMed] [Google Scholar]
- 70. Iversen A, Galatius S, Jensen JS. The optimal route of administration of the glycoprotein IIb/IIIa receptor antagonist abciximab during percutaneous coronary intervention; intravenous versus intracoronary. Curr Cardiol Rev. 2008;4(4):293-299. doi: 10.2174/157340308786349480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Boersma E, Harrington RA, Moliterno DJ, et al. Platelet glycoprotein IIb/IIIa inhibitors in acute coronary syndromes: a meta-analysis of all major randomised clinical trials. Lancet. 2002;359(9302):189-198. doi: 10.1016/S0140-6736(02)07442-1. [DOI] [PubMed] [Google Scholar]
- 72. Adams HP, Jr, Effron MB, Torner J, et al. Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT-II). Stroke. 2008;39(1):87-99. doi: 10.1161/STROKEAHA.106.476648. [DOI] [PubMed] [Google Scholar]
- 73. Ciccone A, Motto C, Abraha I, Cozzolino F, Santilli I. Glycoprotein IIb-IIIa inhibitors for acute ischaemic stroke. Cochrane Database Syst Rev. 2014;3:CD005208. doi: 10.1002/14651858.CD005208.pub3. [DOI] [PubMed] [Google Scholar]
- 74. Pancioli AM, Broderick J, Brott T, et al. The combined approach to lysis utilizing eptifibatide and rt-PA in acute ischemic stroke: the CLEAR stroke trial. Stroke. 2008;39(12):3268-3276. doi: 10.1161/STROKEAHA.108.517656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Adeoye O, Knight WA, Khoury J, et al. A matched comparison of eptifibatide plus rt-PA versus rt-PA alone in acute ischemic stroke. J Stroke Cerebrovasc Dis. 2014;23(5):e313-e315. doi: 10.1016/j.jstrokecerebrovasdis.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Adeoye O, Sucharew H, Khoury J, et al. Combined approach to lysis utilizing eptifibatide and recombinant tissue-type plasminogen activator in acute ischemic stroke-full dose regimen stroke trial. Stroke. 2015;46(9):2529-2533. doi: 10.1161/STROKEAHA.115.010260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Torgano G, Zecca B, Monzani V, et al. Effect of intravenous tirofiban and aspirin in reducing short-term and long-term neurologic deficit in patients with ischemic stroke: a double-blind randomized trial. Cerebrovasc Dis. 2010;29(3):275-281. doi: 10.1159/000275503. [DOI] [PubMed] [Google Scholar]
- 78. Siebler M, Hennerici MG, Schneider D, et al. Safety of tirofiban in acute ischemic stroke: the SaTIS trial. Stroke. 2011;42(9):2388-2392. doi: 10.1161/STROKEAHA.110.599662. [DOI] [PubMed] [Google Scholar]
- 79. Greving JP, Diener HC, Reitsma JB, et al. Antiplatelet therapy after noncardioembolic stroke. Stroke. 2019;50(7):1812-1818. doi: 10.1161/STROKEAHA.118.024497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Lee M, Saver JL, Hong KS, Rao NM, Wu YL, Ovbiagele B. Antiplatelet regimen for patients with breakthrough strokes while on aspirin: a systematic review and meta-analysis. Stroke. 2017;48(9):2610-2613. doi: 10.1161/STROKEAHA.117.017895. [DOI] [PubMed] [Google Scholar]
- 81. Allen C, Dunn SP, Macaulay TE, Mukherjee D. Clopidogrel-proton pump inhibitor interaction: a primer for clinicians. Cardiovasc Hematol Disord Drug Targets. 2010;10(1):66-72. doi: 10.2174/187152910790780078. [DOI] [PubMed] [Google Scholar]
- 82. Turan TN, Nizam A, Lynn MJ, et al. Relationship between risk factor control and vascular events in the SAMMPRIS trial. Neurology. 2017;88(4):379-385. doi: 10.1212/WNL.0000000000003534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Prasad K, Siemieniuk R, Hao Q, et al. Dual antiplatelet therapy with aspirin and clopidogrel for acute high risk transient ischaemic attack and minor ischaemic stroke: a clinical practice guideline. BMJ. 2018;363:k5130. doi: 10.1136/bmj.k5130. [DOI] [PubMed] [Google Scholar]
- 84. Brown DL, Levine DA, Albright K, et al. Benefits and risks of dual versus single antiplatelet therapy for secondary stroke prevention: a systematic review for the 2021 guideline for the prevention of stroke in patients with stroke and transient ischemic attack. Stroke. 2021;52(7):e468-e479. doi: 10.1161/STR.0000000000000377. [DOI] [PubMed] [Google Scholar]
- 85. Bath PM, Woodhouse LJ, Appleton JP, et al. Antiplatelet therapy with aspirin, clopidogrel, and dipyridamole versus clopidogrel alone or aspirin and dipyridamole in patients with acute cerebral ischaemia (TARDIS): a randomised, open-label, phase 3 superiority trial. Lancet. 2018;391(10123):850-859. doi: 10.1016/S0140-6736(17)32849-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Powers WJ, Rabinstein AA, Ackerson T, et al. 2018 Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2018;49(3):e46-e110. doi: 10.1161/STR.0000000000000158. [DOI] [PubMed] [Google Scholar]
- 87. Stroke Foundation. Clinical guidelines for stroke management 2017. Accessed December 31, 2021. https://informme.org.au/guidelines/clinical-guidelines-for-stroke-management.
- 88. Wang Y, Liu M, Pu C. 2014 Chinese guidelines for secondary prevention of ischemic stroke and transient ischemic attack. Int J Stroke. 2017;12(3):302-320. doi: 10.1177/1747493017694391. [DOI] [PubMed] [Google Scholar]
- 89. Wang Y, Wang Y, Zhao X, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369(1):11-19. doi: 10.1056/NEJMoa1215340. [DOI] [PubMed] [Google Scholar]
- 90. Altavilla R, Caso V, Bandini F, et al. Anticoagulation after stroke in patients with atrial fibrillation. Stroke. 2019;50(8):2093-2100. doi: 10.1161/STROKEAHA.118.022856. [DOI] [PubMed] [Google Scholar]
- 91. Shireman TI, Howard PA, Kresowik TF, Ellerbeck EF. Combined anticoagulant–antiplatelet use and major bleeding events in elderly atrial fibrillation patients. Stroke. 2004;35(10):2362-2367. doi: 10.1161/01.STR.0000141933.75462.c2. [DOI] [PubMed] [Google Scholar]
- 92. Fox KAA, Velentgas P, Camm AJ, et al. Outcomes associated with oral anticoagulants plus antiplatelets in patients with newly diagnosed atrial fibrillation. JAMA Netw Open. 2020;3(2):e200107. doi: 10.1001/jamanetworkopen.2020.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Barnes GD. Combining antiplatelet and anticoagulant therapy in cardiovascular disease. Hematology. 2020;2020(1):642-648. doi: 10.1182/hematology.2020000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Asencio LA, Huang JJ, Alpert JS. Combining antiplatelet and antithrombotic therapy (triple therapy): what are the risks and benefits? Am J Med. 2014;127(7):579-585. doi: 10.1016/j.amjmed.2014.02.030. [DOI] [PubMed] [Google Scholar]
- 95. Berge E, Sandercock P. Anticoagulants versus antiplatelet agents for acute ischaemic stroke. Cochrane Database Syst Rev. 2002;4:CD003242. doi: 10.1002/14651858.CD003242. [DOI] [PubMed] [Google Scholar]
- 96. Whiteley WN, Adams HP, Jr, Bath PM, et al. Targeted use of heparin, heparinoids, or low-molecular-weight heparin to improve outcome after acute ischaemic stroke: an individual patient data meta-analysis of randomised controlled trials. Lancet Neurol. 2013;12(6):539-545. doi: 10.1016/S1474-4422(13)70079-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97. Sacco RL. Stroke disparities: from observations to actions. Stroke. 2020;51(11):3392-3405. doi: 10.1161/STROKEAHA.120.030428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Levine DA, Duncan PW, Nguyen-Huynh MN, Ogedegbe OG. Interventions targeting racial/ethnic disparities in stroke prevention and treatment. Stroke. 2020;51(11):3425-3432. doi: 10.1161/STROKEAHA.120.030427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Kim SJ, Kwon OD, Choi HC, Lee E-J, Cho B, Yoon DH. Prevalence and associated factors of premature discontinuation of antiplatelet therapy after ischemic stroke: a nationwide population-based study. BMC Neurol. 2021;21(1):349. doi: 10.1186/s12883-021-02384-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Palacio AM, Vidot DC, Tamariz LJ, et al. Can we identify minority patients at risk of nonadherence to antiplatelet medication at the time of coronary stent placement? J Cardiovasc Nurs. 2017;32(6):522-529. doi: 10.1097/JCN.0000000000000387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Pithara C, Pufulete M, Johnson TW, Redwood S. Patient perspectives of nuisance bleeding and adherence to dual antiplatelet therapy: a qualitative study. Open Heart. 2020;7(2):e001405. doi: 10.1136/openhrt-2020-001405. [DOI] [PMC free article] [PubMed] [Google Scholar]