Abstract
Objective:
The aim of this study was to synthesize evidence available on continuous infusion ketamine versus nonketamine regimens for analgosedation in critically ill patients.
Data sources
A search of MEDLINE, EMBASE, CINAHL, CDSR, and ClinicalTrials.gov was performed from database establishment to November 2021 using the following search terms: critical care, ICU, ketamine, sedation, and anesthesia. All studies included the primary outcome of interest: daily opioid and/or sedative consumption.
Study selection and data extraction
Relevant human studies were considered. Randomized controlled trials (RCT), quasi-experimental studies, and observational cohort studies were eligible. Two reviewers independently screened articles, extracted data, and appraised studies using the Cochrane RoB and ROBINS-I tools.
Data synthesis
A total of 13 RCTs, 5 retrospective, and 1 prospective cohort study were included (2255 participants). The primary analysis of six RCTs demonstrated reduced opioid consumption with ketamine regimens (n = 494 participants, −13.19 µg kg−1 h−1 morphine equivalents, 95% CI −22.10 to −4.28, P = 0.004). No significant difference was observed in sedative consumption, duration of mechanical ventilation (MV), ICU or hospital length of stay (LOS), intracranial pressure, and mortality. Small sample size of studies may have limited ability to detect true differences between groups.
Relevance to patient care and clinical practice
This meta-analysis examining ketamine use in critically ill patients is the first restricting analysis to RCTs and includes up-to-date publication of trials. Findings may guide clinicians in consideration and dosing of ketamine for multimodal analgosedation.
Conclusion
Results suggest ketamine as an adjunct analgosedative has the potential to reduce opioid exposure in postoperative and MV patients in the ICU. More RCTs are required before recommending routine use of ketamine in select populations.
Keywords: analgosedation, benzodiazepine, critical care, ketamine, opioid, sedative
Introduction
Traditional critical care analgesic and sedative drugs require careful selection and titration to balance their efficacy and adverse effect profile as their use can result in extended mechanical ventilation (MV), prolonged stay, and long-term morbidity.1,2 Choice of agent is influenced by a number of factors. 3
Opioids are a mainstay for analgosedation in the ICU 3 but are limited by tolerance, hyperalgesia, reduced blood pressure, and risk of withdrawal 4 or persistent use. 5 Judicious use is favorable amidst a global opioid crisis that affects approximately 36.3 million people. 6 Benzodiazepines, a frequently administered sedative, poses risks of respiratory and cardiovascular depression, delirium, and unintended oversedation from drug accumulation. 1 Nonbenzodiazepine sedatives (propofol, dexmedetomidine) may be preferrable alternatives due to evidence of improved short-term outcomes (ICU length of stay [LOS], duration of MV, and delirium). However, they too are limited by the risk of hypotension, hemodynamic instability, and in the case of dexmedetomidine, cost.2,7,8
Multimodal analgesia can be used to minimize opioid use and optimize analgosedation. 3 Ketamine is an attractive adjunct with both sedative and analgesic properties, quick onset of action, and limited bioaccumulation and rapid recovery. 9 However, ketamine may precipitate psychomimetic adverse effects (e.g., hallucinations and nightmares) and at high doses, can also impact cardiovascular function, increasing blood pressure, heart rate, and arrhythmias.7,9 Due to the dose-dependent adverse effects, ketamine may be used more often as an opioid and sedative sparing agent in conjunction with other medications rather than as a solo agent.
The evidence to support ketamine use in the ICU is growing. It is hypothesized that ketamine administration in the critically ill may reduce opioid and other sedative drug consumption. In this systematic review, our primary objective was to summarize available evidence regarding the impact of continuous ketamine infusion on opioid and sedative drug consumption in adult and pediatric critically ill patients. Our secondary objectives included evaluating the effects of ketamine on the duration of MV, ICU, and hospital LOS, level of sedation and pain, adverse events (e.g., intracranial pressure [ICP] elevation, incidence of delirium), and all-cause mortality.
Methods
Protocol and Registration
This systematic review was designed, conducted, and reported in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines (Appendices Table A1). 10 The protocol is available in the PROSPERO international prospective registry (PROSPERO 2020 CRD42020173693).
Eligibility Criteria
A broad search strategy was set to identify studies that compared continuous infusion ketamine versus any nonketamine-containing analgosedation regimens (e.g., opioids, propofol, dexmedetomidine, and benzodiazepines) in critically ill patients. Randomized controlled trials (RCTs), quasi-experimental studies, and observational cohort studies were eligible; cross-over designs were excluded. Pre-post study designs in which comparisons were made within participants were also excluded due to a time-dependent bias in the critically ill patients where patient status may deteriorate with time making postintervention results difficult to compare with those preintervention. Studies were not limited by language, geographic location, year of publication, or subject age. Only published studies were included. Abstracts and ongoing studies were qualitatively reviewed and excluded from the main analysis.
All eligible studies must have included our primary outcome of interest: daily opioid and/or sedative consumption during ICU or hospital stay. Secondary outcomes were the duration of MV, ICU LOS, hospital LOS, level of sedation, adverse events (e.g., ICP elevation, incidence of delirium), and all-cause mortality.
Information Sources and Search
The following databases were searched from inception until November 19, 2021: MEDLINE (Ovid), EMBASE (Ovid), CINAHL, Cochrane Central Database of Controlled Trials and Systematic Reviews (CDSR), and ClinicalTrials.gov. The search strategy was designed by an experienced medical librarian (CDC) and included concepts for study population, drug, and indication using terms and keywords derived from scoping search and expertise in the subject field (Appendices Table A2 presents the MEDLINE search strategy, including search terms and relevant Medical Subject Headings [MeSH]).
Study Selection, Data Collection Process, and Data Items
Two reviewers (K.C. and D.R.W.) independently screened titles and abstracts of identified studies for inclusion based on above eligibility criteria using Covidence software. Google Translate was used when screening non-English articles. A third reviewer (L.D.B.) resolved discrepancies or undecided cases. Full text was obtained for agreed upon studies and independently screened by two reviewers (K.C. and D.R.W.). Reference lists of relevant studies were screened by to identify other relevant studies (K.C.).
Data were abstracted using a standardized form in Excel by two independent reviewers (K.C. and C.T.). Study characteristics, including author, country, publication year, population, intervention, comparator, randomization, blinding, study drug protocol, funding, ICU type, study design, sample size, sample demographics, follow-up period, inclusion and exclusion criteria, and outcomes, were collected. While study authors were contacted for missing data, no additional information was acquired.
Risk of Bias
Two reviewers (K.C. and C.T.) independently assessed the risk of bias for each included study. The Cochrane Collaboration tool for Assessing Risk of Bias 1 (RoB) was used for RCTs and the Risk of Bias In Nonrandomized Studies—of Interventions (ROBINS-I) tool for cohort studies.11,12 Discrepancies were mediated by a third reviewer (D.R.W.).
Summary Measures and Synthesis of Results
When pooling of outcome data was appropriate, RevMan software was used to conduct meta-analyses (Review Manager [RevMan] Version 5.4, The Cochrane Collaboration, 2020). As per the protocol, only RCTs were combined in the main meta-analysis due to a sufficient number identified. Observational studies were retained for a separate analysis and qualitative purposes. All opioids were converted into morphine equivalents (MEQ), 13 whereas sedatives (benzodiazepines) were included when convertible to midazolam equivalents. Where conversion of opioid doses was required and weight was not reported, a standard of 75 kg average patient weight was assumed. Cumulative opioid doses were divided by measurement time point to estimate dose per kg per hour. All studies reporting benzodiazepine consumption used midazolam, and therefore no conversion was necessary. Statistical heterogeneity was measured using the I2 statistic.
Mean difference (MD) summarized the primary outcome (opioid and sedative consumption) with 95% confidence intervals (CI). Study data were pooled for meta-analyses in a random effects model where outcome measures were comparable. Meta-analyses were performed for morphine and midazolam equivalents consumption, 13 duration of MV, ICU LOS, hospital LOS, ICP, and mortality. All outcomes were summarized using an MD with the exception of mortality using odd ratios with 95% CI. Due to heterogeneity in reporting of mortality, we used the last mortality data reported (hospital mortality4,14-16 and ICU mortality 17 ). RCTs reporting mortality over a shortened defined period (≤5 days) were excluded from this analysis.16,18-23 When needed and to enable meta-analysis, means and standard deviations were estimated using medians and interquartile ranges as previously described. 24 Due to the small number of included studies, an Egger’s test was not performed to assess publication bias. 25
Risk of Bias Across Studies
Within study, selective reporting of outcomes was examined by comparing the a priori outcomes listed in the Methods section with those reported in the Results section. The GRADE framework (Grading of Recommendations, Assessment, Development and Evaluations) was used to rate the quality of evidence 26 for each pooled outcome undergoing meta-analysis by two reviewers (L.D.B./D.R.W.). An overall quality rating is applied (very low, low, moderate, or high) to describe the certainty in the evidence.
Results
Study Selection
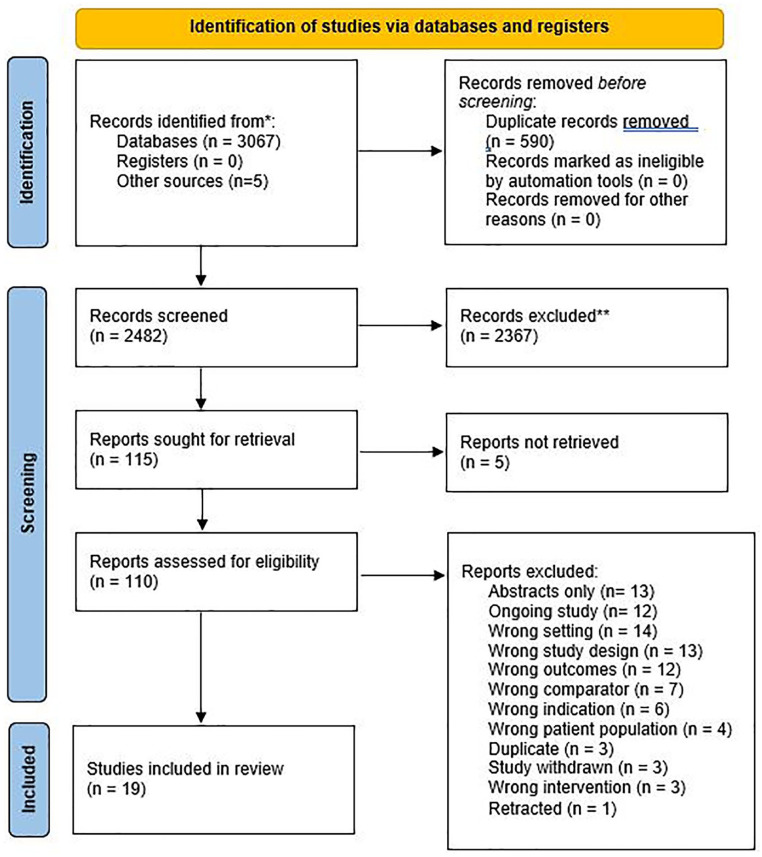
The search yielded 3067 potentially relevant citations of which after removing duplicates and screening titles and abstracts, 110 citations were reviewed in full (Figure 1). However, 19 studies met the inclusion criteria: 13 RCTs4,14,15,17-23,27,28 and 6 observational cohort studies.29-33
Figure 1.
PRISMA 2020 flow diagram for new systematic reviews.
Abbreviation: PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analysis.
Study Characteristics
The characteristics of the included studies and key results are summarized in Tables 1 and 2, respectively. Critically ill patient populations, ketamine regimens, and dosing were heterogeneous (Table 1).
Table 1.
Characteristics of Included Studies Grouped by Date Within Interventions.
| Author, year | Setting | Study type | N | Follow-up period | Intervention | Control | Inclusion criteria |
|---|---|---|---|---|---|---|---|
| Experimental studies | |||||||
| Kolenda et al 17 | Germany, mixed ICU | RCT, unblinded | 24 | Up to 14 days of sedation | Ketamine + midazolam | Fentanyl + midazolam | Patients with moderate or severe head injury, age 16-72 years, analgosedation ≥ 3 days |
| Christ et al 18 | Austria, mixed ICU | RCT, blinding unclear | 25 | 24 hours | Ketamine + midazolam | Sufentanil + midazolam | MV patients with catecholamine-dependent heart failure |
| Kim et al 28 | Korea, mixed ICU | RCT, blinding unclear | 38 | 24 hours | Ketamine + midazolam | Morphine + midazolam | MV patients |
| Bourgoin et al 22 | France, ICU in trauma center | RCT, double-blind | 25 | First 4 days of sedation | Ketamine + midazolam | Sufentanil + midazolam | MV patients with TBI and CT scan indicating significant risk of increased ICP, age 16-75 years |
| Bourgoin et al 27 | France, ICU in trauma center | RCT, blinding unclear | 30 | 24 hours of target sedation, then plasma concentration doubled for 15 minutes | Ketamine + midazolam | Sufentanil + midazolam | Patients with severe TBI requiring ICP monitoring, age 18-75 years |
| Schmittner et al 19 | Germany, mixed ICU | RCT, blinding unclear | 24 | 5 days | S(+)-Ketamine + methohexitone | Fentanyl + methohexitone | Patients with severe TBI or aSAH and ICU ventilation > 12 hours, age > 18 years |
| Quisilema-Cadena et al 15 | Cuba, mixed ICU | RCT, unblinded | 18 | Sep 2014-May 2015 (until discharge) | Ketamine + midazolam | Morphine + midazolam | MV patients |
| Guillou et al 20 | France, surgical ICU | RCT, double-blind | 93 | 48 hours | Ketamine + morphine PCA | Normal saline + morphine PCA | Ventilated patients postabdominal surgery, age > 18 years |
| Minoshima et al 21 | Japan, mixed ICU | RCT, double-blind | 36 | 48 hours | Ketamine + morphine PCA | Normal saline + morphine PCA | Patients undergoing posterior correction surgery for adolescent idiopathic scoliosis, age 10-19 years |
| Anwar et al 23 | The United Kingdom, cardiac ICU | RCT, double-blind | 150 | 48 hours | Ketamine + pregabalin + morphine PCA | Placebo + pregabalin + morphine PCA | Patients postelective cardiac surgery through sternotomy, age 18-80 years |
| Dzierba et al 14 | Dzierba, medical ICU | RCT, unblinded | 20 | Until discharge, death or 7 days | Ketamine + fentanyl/hydromorphone + midazolam | Fentanyl/hydromorphone + midazolam | MV patients receiving ECMO for severe ARDS and requiring deep sedation, age ≥ 18 years |
| Perbet et al 4 | France, mixed ICU | RCT, double-blind | 162 | 90 days | Ketamine + remifentanil + midazolam/propofol | Normal saline + remifentanil + midazolam/propofol | MV patients requiring MV > 24 hours, age ≥ 18 years |
| Amer et al 16 | Saudi Arabia, medical, surgical, and transplant ICUs | RCT, unblinded | 83 | 28 days | Ketamine + fentanyl + propofol | Fentanyl + propofol | Patients requiring MV > 24 hours, age ≥ 18 years |
| Observational studies | |||||||
| Von der Brelie et al 30 | Germany, mixed ICU | Retrospective cohort | 65 | June 2010-Dec 2013 (until discharge) | Ketamine in sedative regimen | No ketamine in sedative regimen | Patients with aSAH requiring sedation |
| Reese et al 31 | The United States, ICU at tertiary center | Retrospective control group and prospective ketamine group | 46 | Jan 2012-April 2015 (until discharge) | Ketamine as primary sedative for 48 hours or less | No ketamine use (historical control) | MV patients with septic shock requiring sedation, age 18-89 years |
| Historical control group: 2010-2011 | |||||||
| Prospective cohort study: 2012-2015 | |||||||
| Park et al 29 | Korea, tertiary PICU | Retrospective cohort | 240 | Jan 2015-Dec 2017 (until discharge) | Ketamine (mostly add-on) + sedative (mostly opioids) | Sedative (mostly opioids) | Patients sedated for ≥ 24 hours |
| Shurtleff et al 32 | The United States, ICU at tertiary center | Retrospective cohort | 79 | Nov 2015-Apr 2017 (up to 12 days) | Ketamine in sedative regimen for at least 6 hours | Propofol | Patients receiving analgosedation for ≥ 6 hours, age ≥ 18 years |
| Jaeger et al 33 | The United States, medical ICU | Retrospective cohort | 172 | Jan 2013-Dec 2018 | Ketamine in sedative regimen | Non-ketamine sedation | MV patients (≥ 24 hours) receiving continuous infusion sedation (≥ 6 hours), age ≥ 18 years |
| Wu et al 34 | Netherlands, medical-surgical ICU | Post hoc subgroup of prospective cohort | 925 | Jul 2016-Feb 2020 | Ketamine in sedative regimen | Non-ketamine sedation | Patients receiving analgosedation and expected to survive ≥ 48 hours |
Abbreviations: ARDS, acute respiratory distress syndrome; aSAH, aneurysmal subarachnoid hemorrhage; ECMO, extracorporeal membrane oxygenation; GCS, Glasgow Coma Score; h, hours; ICP, intracranial pressure; ICU, intensive care unit; MV, mechanical ventilation; PCA, patient-controlled analgesia; PICU, pediatric intensive care unit; RCT, randomized controlled trial; TBI, traumatic brain injury.
Table 2.
Key Results of Included Studies Grouped by Date Within Interventions.
| Author, year | Ketamine dosing | Main outcomes | Key results |
|---|---|---|---|
| Experimental studies | |||
| Kolenda et al 17 | Ketamine 65 mg kg−1 day−1 (2.7 mg kg−1 h−1) adjusted to clinical requirements | Recovery of motor response, sedation/analgesia, sedative and opioid consumption, MAP, ICP | Median midazolam consumption was similar in the ketamine and fentanyl groups (11.1 vs 10.7 mg kg−1 day−1). Additional sedatives were required in 2 ketamine patients and 4 fentanyl patients. However, 3 patients in the ketamine group and 1 in the fentanyl group died during follow-up. Incidence of persistent ICP (>25 mmHg) was similar in the two groups. |
| Christ et al 18 | Ketamine titrated to RSS = 5 | Hemodynamic parameters, catecholamine requirements, midazolam consumption | Mean midazolam dose was not significantly different in the ketamine versus sufentanil groups (0.12 vs 0.15 mg kg−1 h−1). Comparable sedation was achieved in both groups using ketamine mean 2.5 mg kg−1 h−1 and sufentanil 0.88 µg kg−1 h−1. |
| Kim et al 28 | Unclear | Hemodynamic changes | Mean midazolam consumption was nonsignificantly higher in the ketamine versus morphine group (52.1 vs 46.7 mg day−1). |
| Bourgoin et al 22 | Ketamine 50 µg kg−1 min−1 (3 mg kg−1 h−1), adjusted to ICP and CPP levels | ICP, CPP | Mean midazolam dose was similar in the ketamine and sufentanil groups (1.64 vs 1.63 µg kg−1 min−1). After infusions were stopped, improvement in GCS score was faster in the nonketamine group. However, GCS score was similar at patient recovery. Four patients in the ketamine group and 3 in the comparator died during the study. Mean daily ICP and CPP values were similar. |
| Bourgoin et al 27 | Infusion to plasma concentration 1.0 µg mL−1, adjusted to pain scores | ICP, CPP, MAP, and drug plasma concentrations after increasing dose | Mean BIS was 74 versus 65 in the ketamine and sufentanil groups (nonsignificant). Mean ICP values were similar. |
| Schmittner et al 19 | Ketamine 0.5 mg kg−1 bolus, then titrated to target sedation (maximum 2 mg kg−1 h−1) | MAP, CVP, ICP, and CPP | Mean sedation levels measured by BIS and ICP were similar from days 1 to 5. Persistent ICP (>20 mmHg) was reported in 8 ketamine and 6 fentanyl patients. |
| Quisilema-Cadena et al 15 | Ketamine 0.3 mg kg−1 bolus, then 0.05-0.4 mg kg−1 h−1 increased by 0.05 mg kg−1 h−1 q60 min until adequate sedation achieved | Time to extubation, RASS | Mean midazolam daily dose was not significantly different between the ketamine and morphine group (0.1 vs 0.1 mg kg−1 day−1). |
| Median ketamine dose 0.6 mg kg−1 day−1 (0.025 mg kg−1 h−1) | |||
| Guillou et al 20 | Ketamine 0.5 mg kg−1 bolus, then 2 µg kg−1 min−1 (0.12 mg kg−1 h−1) during the first 24 hours, then 1 µg kg−1 min−1 (0.06 mg kg−1 h−1) | VAS pain scores, opioid consumption | Mean morphine consumption was significantly lower in the ketamine versus placebo group (58 vs 80 mg at 48 hours). |
| Minoshima et al 21 | Ketamine 0.5 mg kg−1 bolus during surgery, then 2 µg kg−1 min−1 (0.12 mg kg−1 h−1) postoperatively for 48 hours | Morphine consumption, pain and sedation scores | Cumulative mean morphine consumption was significantly lower in the ketamine versus placebo group at 24 hours (0.59 vs 0.75 mg kg−1) and 48 hours (0.89 vs 1.16 mg kg−1). Sedation levels were similar at 24 hours after ICU arrival. Hospital LOS were also similar. No delirium nor psychomimetic effects were observed in either group. |
| Anwar et al 23 | Ketamine 0.1 mg kg−1 h−1 for 48 hours postoperatively | Pain at 3 and 6 months, acute pain, opioid use, and analgesic requirements | Median morphine consumption was significantly lower in the ketamine group by 4 mg/day. Sedation scores, ICU LOS, and hospital LOS were similar. |
| Dzierba et al 14 | Ketamine 40 mg bolus, then 5 µg kg−1 min−1 (0.3 mg kg−1 h−1) | Opioid and sedative consumption, renal replacement therapy, and delirium | Median midazolam consumption was not significantly different between ketamine and nonketamine groups (8 vs 6 mg day−1). Median fentanyl consumption was also similar (6 vs 5 mg day−1). The duration of MV, ICU LOS, and hospital LOS was similar. Mortality was equal among the two groups. Delirium was present in 7 patients in the ketamine group and 9 of the control group on the day of waking. |
| Perbet et al 4 | Ketamine 3.3 µg kg−1 min−1 (0.2 mg kg−1 h−1) | Daily opiate consumption, delirium, opioid consumption, and ventilation days | Both mean midazolam and propofol consumption were similar between the ketamine and placebo groups (midazolam 1.4 vs 1.6 mg kg−1 day−1; propofol 31 vs 35 mg kg−1 day−1). Mean reimfentanil consumption was not significantly different (7.9 µg kg−1 h−1 vs 9.3 µg kg−1 h−1). Median duration of MV and ICU stay was similar. ICU mortality was 35% (n = 28) and 43% (n = 35); hospital mortality was 39% (n = 31) and 45% (n = 37). |
| Incidence of delirium was significantly lower in the ketamine group (21% vs 37%, P = 0.03). When present, delirium was also significantly shorter in the ketamine group (mean 2.8 vs 5.3 days, P = 0.005). | |||
| Amer et al 16 | Ketamine 1-2 µg kg−1 min−1 (0.06-0.12 mg kg−1 h−1) | Consent, recruitment, and protocol adherence rate | Both median propofol and fentanyl consumption were similar between ketamine and placebo groups (propofol 28.0 vs 28.4 mg kg−1 at 48 hours; fentanyl 69.6 vs 63.5 µg kg−1 at 48 hours). Percent of patients at 24-hour goal RASS was 67.5% in the ketamine group and 52.4% in the control group (P = 0.24) (at 48 hours, 73.5 and 66.7%, P = 0.70) |
| Observational studies | |||
| Von der Brelie et al 30 | Ketamine up to a maximum of 500 mg h−1 (~6.7 mg kg−1 h−1) | ICP and vasopressor consumption | None of the patients in the ketamine group experienced a critical increase in ICP after administration of ketamine. Mortality and adverse events were similar in the two groups. |
| Reese et al 31 | Ketamine 1-2 mg kg−1 bolus, then 5 µg kg−1 min−1 (0.3 mg kg−1 h−1) with a titration of 2 µg kg−1 min−1 q30 min to adequate sedation | Vasopressor consumption, ketamine consumption, other sedative and analgesic use, and MV days | Total benzodiazepine dose was significantly lower at 24 hours in the ketamine group (10 vs 42 mg), and nonsignificantly at 48 hours (26 vs 75 mg). Cumulative fentanyl dose at 48 hours was significantly lower in the ketamine group (429 vs 2235 µg). |
| Park et al 29 | Median ketamine infusion rate of 8.1 µg kg−1 min−1 (0.49 mg kg−1 h−1) | BP, HR, RR, vasogenic medications, mortality, sedation, and pain scores | In total, 64 patients in the ketamine group vs 120 patients in the nonketamine group received fentanyl. The duration of MV was significantly shorter in the ketamine group (median 17.0 vs 7.5 days). Hospital and ICU LOS were also significantly lower in the ketamine group. |
| Shurtleff et al 32 | Ketamine 5 µg kg−1 min−1, titrated by 5 µg kg−1 min−1 q5 min up to max 25 µg kg−1 min−1; median infusion dose was 7 µg kg−1 min−1 (0.42 mg kg−1 h−1) | Days without delirium or coma | Ketamine was mostly used in patients who failed first-line sedation regimens. No significant difference in delirium was detected. Total midazolam and fentanyl consumption were not significantly different between groups. Median ICU (15 vs 12 days) and hospital LOS (11 vs 8 days) were longer in the ketamine groups. |
| Jaeger et al 33 | Median ketamine infusion rate of 7.9 µg kg−1 min−1 (0.47 mg kg−1 h−1) | % of RASS and pain scores at goal, sedative, and vasopressor consumption | Ketamine was mostly used in patients who failed first-line sedation regimens. No significant difference was found in midazolam nor fentanyl consumption. ICU LOS was longer in the ketamine group (8.8 vs 5.2 days). |
| Wu et al 34 | 0.50 mg kg−1 h−1 in the delirium incidence group and 0.12 mg kg−1 h−1 in the no delirium group | Incident ICU delirium | Ketamine use was greater in patients with delirium (16% vs 0.7%, P < 0.01). Note that a greater proportion of ketamine patients were urgent surgical admissions (54% vs 34%, P = 0.01) and received average IV MEQ dose ≥ 10 mg day−1 (91% vs 75%, P = 0.02). |
Abbreviations: BIS, bispectral index; BP, blood pressure; CPP, cerebral perfusion pressure; CVP, central venous pressure; GCS, Glasgow Coma Scale; HR, heart rate; ICP, intracranial pressure; ICU, intensive care unit; IV, intravenous; LOS, length of stay; MAP, mean arterial pressure; MV, mechanical ventilation; RASS, Richmond Agitation-Sedation Scale; RR, respiratory rate; RSS, Ramsay Sedation Scale; VAS, visual analogue scale.
The included RCTs were generally small (range N = 18-162) compared with cohort studies (N = 46-925 subjects). Seven RCTs compared the use of ketamine in combination with a sedative (6 midazolam, 1 methohexitone) to an opioid with sedative in adult head-injured patients17,19,22,27 or MV patients.15,18,28 Three RCTs compared ketamine in combination with morphine patient-controlled analgesia (PCA) to placebo with morphine PCA following major surgery (adolescents 21 or adults20,23). Three RCTs compared ketamine in combination with both an opioid and sedative to the same regimen without ketamine in MV adults.4,14,16 The sedation regimens in the 6 cohort studies were less clear, as patients in the control group received usual care. Subjects in the comparator group generally received ketamine as an add-on, where traditional sedatives or analgesics were not sufficient to maintain adequate sedation. Patient demographics also varied among the cohort studies, with inclusion criteria ranging from any patients who received prolonged sedation (≥ 6 hours) to patients receiving MV, or with specific diagnoses, such as subarachnoid hemorrhage or septic shock. RCTs were conducted in France, Germany, Austria, Korea, Cuba, Japan, the United Kingdom, the United States, and Saudi Arabia. Observational studies were conducted in Korea, Germany, the United States, and the Netherlands.
Seven RCTs were excluded from pooling data for opioid consumption due to lack of reporting as ketamine was used to replace an opioid in the regimen.15,17-19,22,27,28 Seven RCTs were excluded from pooling data for midazolam consumption: (1) two were missing appropriate data,17,27 (2) four did not include midazolam in the analgosedative regimen,19-21,23 and (3) one only used midazolam as an alternative to propofol (eg. allergy). 16 Here, 7 RCTs did not report length of MV due to a non-MV study population17,19-23,27 and 3 due to a lack of reporting on the outcome.15,18,28 Despite all 13 RCTs taking place in the ICU population, 7 did not report ICU LOS as an outcome17-21,27,28 and 1 study only reported on ICU LOS for both treatment groups combined. 15 Similarly, 8 of the 13 RCTs did not report hospital LOS.4,15,17-20,22,27
Ketamine dosing strategies varied widely among the studies. Some studies (n = 6, 32%) employed a bolus of ketamine prior to continuous infusion.14,15,19-21,31 Continuous infusions ranged from 0.025 15 to 3.0 mg kg−1 h−122 with the lower end of the dose range generally used for PCA.
Risk of Bias
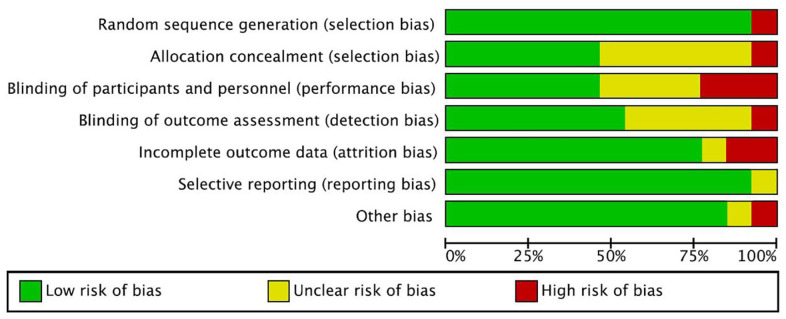
The risk of bias was scored as high in 5 of the 13 RCTs. Three studies were due to lack of blinding,14,16,17 1 due to exclusion of patients who reached maximum sedative doses, 19 and 1 due to concerns of missing outcome data, bias in measurement of the outcome, and in selection of the reported results 22 (Figures A1 and A2). Three studies were assessed with low risk of overall bias due to appropriate RCT design and complete reporting.4,21,23 Five studies were noted to have some concerns primarily due to allocation concealment and blinding not explicitly reported.15,18,19,27,28
Quality assessment of observational cohort studies using ROBINS-I indicated high risk of bias in all 6 studies. An immortal time bias was noted in observational studies; patients in the treatment arm entered the treatment group at later points during analgosedation as ketamine was typically used as an adjunct when traditional regimens failed to maintain adequate sedation. In addition, a selection bias exists in the retrospective cohort studies whereby ketamine was mainly added as an adjunctive analgosedative when traditional regimens were insufficient.29,30
Synthesis of Results
Heterogeneity across studies was high, and in our main analysis, only data from RCTs were pooled. However, observational studies were pooled for hypothesis generating purposes and guiding future research. Meta-analyses were not performed on the following outcomes due to lack of outcome data or factors that made pooling inappropriate: sedation levels, pain, and adverse events. Outcomes are described qualitatively and in Tables 1 and 2.
Opioid consumption
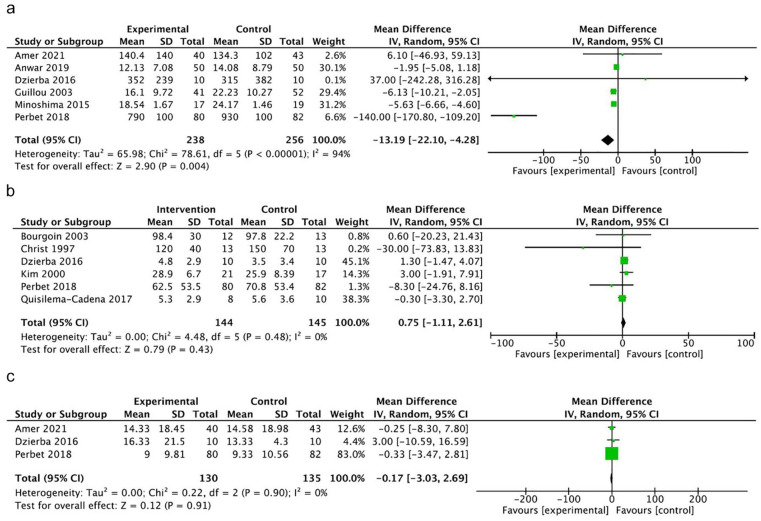
Overall, 6 RCTs reporting opioid consumption were pooled with 494 participants (Figure 2a).4,14,16,20,21,23 Opioid consumption was 13.19 µg kg−1 h−1 MEQ less (MD, 95% CI −22.10 to −4.28, P = 0.004, very low certainty) in the ketamine group. An I2 of 94% suggests high heterogeneity across the studies, likely owing to the range of target study populations. Three of the studies enrolled postoperative patients in the ICU receiving morphine PCA (with or without adjunctive continuous infusion ketamine) all with similar dosing regimens.20,21,23 Another study focused on patients on MV and ECMO—sample size was small (n = 20) and the study was weighted only 0.1% in the meta-analysis. 14 Two studies focused on MV patients—one reported a relatively large reduction in opioid consumption 4 and the other an increase in the ketamine group. 16
Figure 2.
Forest plots of comparison. (a) Mean morphine equivalent dose (ME) (µg kg−1 h−1). (b) Mean midazolam dose (µg kg−1 h−1). (c) Mean duration of MV (days).
Abbreviations: CI, confidence interval; IV, intravenous; MV, mechanical ventilation.
Pooling of the 3 observational studies reporting adequate data revealed a significant reduction of opioids (MD −26.53 µg kg−1 h−1 ME, 95% CI −50.95 to −2.11, P = 0.03, very low certainty) (Figure A3).31-33 This is in alignment with results from meta-analysis of the RCTs.
Sedative consumption
All studies reporting benzodiazepine consumption used midazolam.4,14-16,18,22,28 One study was excluded from meta-analysis, as midazolam was used only as an alternative to propofol for reasons, such as an allergy. 16 Meta-analysis of mean midazolam dose across the 6 RCTs with 289 participants4,14,15,18,22,28 demonstrated no difference between groups treated with and without ketamine (MD 0.75 mg kg−1 h−1, 95% CI −1.11 to 2.61, P = 0.43, very low certainty) (Figure 2b). An I2 of 0% suggests minimal heterogeneity.
Duration of MV
Mean duration of MV was reported in 3 RCTs with 265 participants.4,14,16 No difference between ketamine and nonketamine groups was identified (MD −0.17 days, 95% CI −3.03 to 2.69, P = 0.91, very low certainty) (Figure 2c). An I2 of 0% suggests minimal heterogeneity. The duration of MV was significantly longer in one cohort study 29 and comparable in the remaining where reported,31,32,34 but data were not pooled due to bias. Patients typically received ketamine following the failure of first-line regimens in achieving goal analgosedation—therefore, those who received ketamine were more likely to be on MV longer.
ICU and hospital LOS
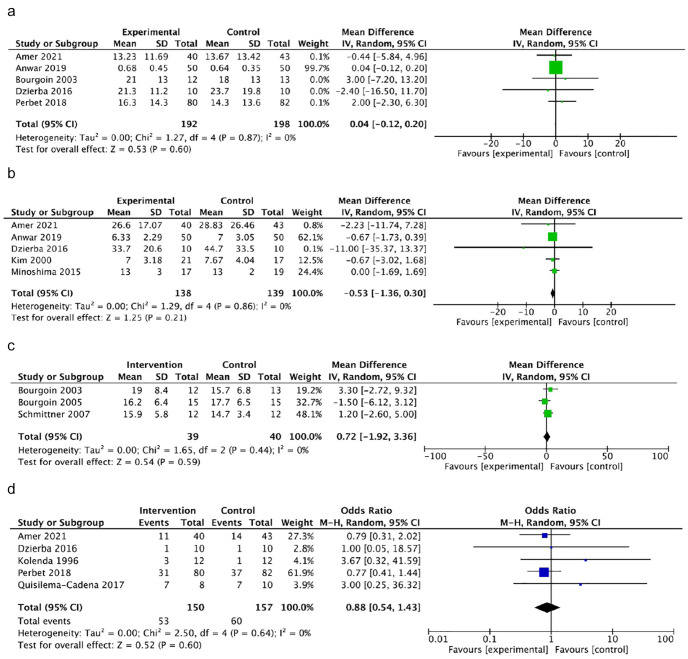
Meta-analysis of mean ICU LOS across 390 patients in 5 studies4,14,16,22,23 demonstrated no difference between the ketamine and nonketamine groups (MD 0.04 days, 95% CI −0.12 to 0.20, P = 0.60, low certainty) (Figure A4a). An I2 of 0% suggests minimal heterogeneity. Similarly, no significant difference in hospital LOS was observed across the 277 patients in 5 studies14,16,21,23,28 (MD −0.53 days, 95% CI −1.36 to 0.30, P = 0.21, low certainty) (Figure A4b). An I2 of 0% suggests minimal heterogeneity. Hospital LOS29,32 and ICU LOS29,32,33 were longer in several individual cohort studies (P < 0.05), but data were not pooled due to bias.
Intracranial pressure
ICP elevation was described as an outcome in 3 RCTs of brain injury patients.19,22,27 Meta-analysis of 79 participants across the 3 RCTs demonstrated no significant difference with ketamine administration (MD 0.72 mmHg, 95% CI −1.92 to 3.36, P = 0.59, low certainty) (Figure A4c). An I2 of 0% suggests minimal heterogeneity.
Mortality
Mortality was comparable between groups in RCTs.4,14-17 Meta-analysis of 307 patients across the 5 RCTs demonstrated no significant difference with ketamine administration (odds ratio 0.88, 95% CI 0.54-1.43, P = 0.60, low certainty) (Figure A4d). Cohort studies demonstrated a nonsignificant association of increased mortality in the ketamine group, possibly owing to selection bias whereby ketamine was administered as an adjunctive analgosedative when first-line sedation regimens were inadequate.29-34
Other outcomes described qualitatively
Qualitative descriptions of outcomes and ketamine doses are further detailed in Table 2. Sedation levels were comparable between treatment groups. In the majority of studies (5 RCTs4,14-16,21 and 6 observational studies29-34), sedation evaluation used the Richmond Agitation-Sedation Scale (RASS). A nurse was reported to perform these assessments in 3 of the RCTs4,16,21 and 3 observational studies,32-34 while the remaining were unclear.14,15,29-31 Another 4 RCTs used the Ramsay Sedation Scale (RSS)—one measured by a nurse, 19 another by a blinded observer, 20 and the remaining unclear.18,28 Anwar et al used a sedation score based on a Likert scale, 23 Bourgoin et al a behavioral pain scale, 27 and Bourgoin et al clinical judgment during endotracheal suction. 22 The remaining study did not report assessing sedation. 17 Of the study designs that allowed comparison of methohexitone and propofol consumption, no difference between ketamine and nonketamine groups was found.4,16,19 Sedation results were not pooled as different measures were used across the trials at varying time points.
A variety of tools were used to measure pain (behavioral pain scale,4,27 bispectral index, 19 visual analogue scale, 20 numerical rating scale,4,21,23 a handheld pressure algometer and monofilament von Frey fibers, 23 critical care pain observation tool,16,32 face pain scale or Face-Leg-Activity-Cry-Consolability score, 29 self-reported pain scores, 33 nonverbal pain scale 33 ). In the majority of studies, the assessor was unidentified, while three reported nurse measurement,4,21,32 and one a blinded observer. 20 Pain scores were comparable between groups where reported (7 RCTs4,16,19-21,23,27 and 3 observational studies29,32,33). However, in the observational study by Shurtleff et al, a greater percentage of patients in the ketamine group achieved target pain scores (99% vs 91%, P = 0.04). 32
All but 3 studies18,28,29 reported the occurrence of adverse events. Reports of adverse events were generally similar between ketamine and morphine groups. Two studies reported hypotension was experienced in a greater proportion of the nonketamine group (ketamine n = 2, 9% vs morphine group n = 5, 29%; 28 n = 2, 25% vs n = 7, 70% 15 ). One RCT and one cohort study reported greater vasopressor use in the nonketamine groups (ketamine n = 6, 50% vs sufentanil n = 8, 62%; 22 ketamine n = 6, 35% vs n = 18, 62% nonketamine, 31 P > 0.05). In the study of postoperative cardiac patients, diplopia occurred more often in the ketamine plus pregabalin group versus pregabalin alone (number needed to harm 4.5 vs 6.3). 23 A significantly lower incidence of delirium was also observed in ketamine groups across several studies. Perbet et al reported a significant reduction of delirium in the ketamine group (n = 17, 21% versus n = 30, 37%, P = 0.03). 4 This observation is echoed with a nonsignificant reduced incidence in the RCT of ECMO patients (n = 7, 70% vs n = 9, 90%) 14 and retrospective cohort of general ICU patients (n = 29, 74% vs n = 34, 85%). 32 In contrast, an observational study reported ketamine use was more likely in patients who experienced delirium versus those who did not (n = 54, 16% versus n = 4, 0.7%, P < 0.01). 16
Discussion
The results of our systematic review and meta-analysis suggest ketamine may have benefits as an analgosedative in the ICU. Ketamine was found to decrease opioid consumption in the ICU, with no evidence of effect on sedation consumption.
Despite the high frequency of analgosedation in the ICU, practice patterns indicate choice, combination, and dosing of conventional sedatives and opioids remain highly variable. 35 Opioids are limited by tolerance, hyperalgesia, increased risk of withdrawal, and propensity to reduce blood pressure 4 ; propofol by hypotension and hemodynamic instability 7 ; benzodiazepines by risk of respiratory and cardiovascular depression, delirium, and unintended oversedation from drug accumulation 1 ; and dexmedetomidine by hypotension, bradycardia, and cost.1,8 Choice of sedative and regimens is heavily dependent on local practices and clinical judgment. Ketamine has been a subject of interest in several studies and systematic reviews9,36 for its distinct profile and positive hemodynamic effects. To our knowledge, the only other meta-analysis of adjunctive ketamine for analgosedation in critically ill patients was conducted by Manasco et al. 36 The meta-analysis revealed that ketamine was associated with a reduction in propofol infusion rate but had no impact on fentanyl or midazolam. The capture of studies in our meta-analysis, which excluded pre-post study designs, did not provide sufficient data to analyze propofol use. Similarly, we report no difference in midazolam mean dose. However, in contrast to our findings, Manasco et al did not find a significant reduction in fentanyl (−21.5 µg h−1, P = 0.11). 36 This may be owing to several differences: (1) a focus on MV patients where our review included non-MV patients receiving morphine PCAs; (2) analyzing only observational studies (limited by studies using fentanyl) where our analysis included RCTs (opioids converted to MEQ); and (3) inclusion of pre-post study designs which may have introduced a bias where patients received ketamine later in their ICU stay along with escalation of opioids doses. Together, the results suggest a potential opioid-sparing effect of ketamine that may be further elucidated through more RCTs.
While the pooled opioid-sparing effect of ketamine was small, this may be partly explained by the small sample size and identified heterogeneity. Daily cumulative doses of morphine using a PCA for postoperative analgosedation are relatively low compared with opioid administration in MV ICU populations. Heavier weighting of these postoperative PCA studies20,21,23 heavily impacted the results by reducing overall MD in opioid consumption. Our review also sought to detect any signal of adverse effects in critically ill populations at the ketamine doses used for analgosedation. While all studies generally reported comparable or null psychomimetic effects, results of delirium were inconsistent.4,34
Strengths of this systematic review include an extensive search, broad inclusion criteria, meta-analysis limited to RCTs, and inclusion of new studies16,34 not captured in previous reviews. However, results of the meta-analysis should be interpreted with caution due to the heterogeneous nature of the ICU patient populations identified in the studies, ketamine dosing (timing and strength), outcome measures, and various paired drug combinations (sedatives or opioids through infusion or PCA in combination with ketamine). While we recognize the limitations of combining various ICU populations in analysis (e.g. ventilated and nonventilated), all trials represented critically ill ICU patients in pain and increase the generalizability of the findings. By including non-MV patients, likely to have reduced opioid exposure, our results are biased toward the null. Additionally, the available literature does not permit us to determine the effect of ketamine alone. Further limitations include a high or moderate risk of bias due to study structure and reporting in 11 of the 14 RCTs14-22,27,28 and all observational studies included.29-34 The use of ketamine in observational studies only when first-line sedatives were inadequate to achieve goal sedation creates an immortal time bias and selection bias. Patients received ketamine at a later point during their stay following failure of first-line regimens, creating a bias toward patients with longer LOS in the ketamine group. Taking this into consideration along with the number of RCTs identified, observational studies were excluded from meta-analysis. Additionally, 6 of the RCTs had relatively short follow-up periods (≤ 48 h).18,20,21,23,27,28 However, 10 of the 19 RCTs and cohort studies were small (N ≤ 50).14,15,17-19,21,22,27,28,31 Small sample sizes may have underpowered studies to detect adverse effects or a stronger opioid-sparing effect.
Additional RCTs exploring ketamine as an adjunct analgosedation agent to reduce opioid requirements would be valuable. Its potential benefit in reducing iatrogenic drug withdrawal and discharge on addictive substances warrants further study in well-designed trials, employing adequate randomization, blinding (ICU staff and data collection) and powered to detect a difference. Treatment groups using a placebo comparator and controlled dosing regimens of adjunctive ketamine and opioid would allow more definitive elucidation of ketamine as an opioid-sparing agent. Additionally, sparsely reported psychomimetic effects and conflicting results of delirium underline the need for better understanding of potential adverse effects from future trials.
Relevance to Patient Care and Clinical Practice
A current opioid crisis in developed countries is highlighted by the wave of opioid-involved overdoses presenting to emergency departments and overdose-related deaths. This has implications for health care professionals in acute care settings as excessive prescribing during hospitalization and discharge may lead to chronic use.5,37 Limiting opioid overprescribing while minimizing undertreatment of pain is a delicate balance, and the evidence base for alternatives and adjuncts, such as ketamine, is limited.
Results of our meta-analysis suggest ketamine as an adjunct analgosedative has the potential to limit opioid exposure, presenting an additional alternative to traditional regimens that would benefit from further study.
Conclusion
Our findings suggest ketamine in critically ill patients has the potential to have an opioid-sparing effect in postoperative and MV patients in the ICU. While small, the potential for opioid dose reduction warrants further investigation. Further understanding of agents capable of offering analgesia without extended opioid exposure is particularly critical amidst the opioid crisis experienced in many developed nations.
Acknowledgments
The authors thank P Pechlivanoglou (University of Toronto, Toronto) and L Abrahamyan (University of Toronto, Toronto) for their guidance during the conduct of the systematic review.
Appendix
Table A1.
Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) Guidelines.
| Section and topic | Item # | Checklist item | Location where item is reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Title |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for Abstracts checklist. | Abstract |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Introduction |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | Introduction |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Methods |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Methods |
| Search strategy | 7 | Present the full search strategies for all databases, registers, and websites, including any filters and limits used. | Methods |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | Methods |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | Methods |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g. for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | Methods |
| 10b | List and define all other variables for which data were sought (e.g. participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | Methods | |
| Study risk of bias assessment | 11 | Specify the methods used to assess risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | Methods |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g. risk ratio, MD) used in the synthesis or presentation of results. | Methods |
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g. tabulating the study intervention characteristics and comparing against the planned groups for each synthesis [item #5]). | Methods |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | Methods | |
| 13c | Describe any methods used to tabulate or visually display results of individual studies and syntheses. | Methods | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | Methods | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g. subgroup analysis, meta-regression). | NA | |
| 13f | Describe any sensitivity analyses conducted to assess robustness of the synthesized results. | NA | |
| Reporting bias assessment | 14 | Describe any methods used to assess risk of bias due to missing results in a synthesis (arising from reporting biases). | Methods |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | Methods |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Results |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | Results | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Results |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Results |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g. confidence/credible interval), ideally using structured tables or plots. | Results |
| Results of syntheses | 20a | For each synthesis, briefly summarize the characteristics and risk of bias among contributing studies. | Results |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g. confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | Results | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | Results | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | Results | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | Results Figures A1 and A2 |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | Results (GRADE assessment) |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | Discussion |
| 23b | Discuss any limitations of the evidence included in the review. | Discussion | |
| 23c | Discuss any limitations of the review processes used. | Discussion | |
| 23d | Discuss implications of the results for practice, policy, and future research. | Discussion Relevance to Patient Care and Clinical Practice |
|
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | Methods |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | Methods | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | NA | |
| Support | 25 | Describe sources of financial or nonfinancial support for the review, and the role of the funders or sponsors in the review. | Funding |
| Competing interests | 26 | Declare any competing interests of review authors. | Declaration of interests |
| Availability of data, code, and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | NA |
Abbreviation: NA, not applicable.
Table A2.
Ovid MEDLINE Search Strategy: Epub Ahead of Print, In-Process and Other Nonindexed Citations, Ovid MEDLINE Daily and Ovid MEDLINE 1946-Present (Original Search February 2020 Before Update in November 2021).
| # | Searches | Results |
|---|---|---|
| 1 | Critical Care/ | 51 016 |
| 2 | intensive care units/ or burn units/ or coronary care units/ or intensive care units, pediatric/ or recovery room/ or respiratory care units/ | 67 942 |
| 3 | (ICU or ICUs or PICU or PICUs or SICU or SICUs or CCU or CCUs).tw, kf,kw. | 62 411 |
| 4 | ([ or critical or acute] adj3 care).tw, kf,kw. | 190 858 |
| 5 | ([ or coronary or heart] adj3 (unit$1 or center$1 or center$1)).tw, kf,kw. | 12 640 |
| 6 | (respiratory adj3 [unit$1 or center$1 or center$1]).tw, kf,kw. | 3692 |
| 7 | ([ or surger$] adj3 (unit$1 or center$1 or center$1)).tw, kf,kw. | 21 708 |
| 8 | (burn adj3 [unit$1 or center$1 or center$1]).tw, kf,kw. | 4184 |
| 9 | Ketamine/ | 12 186 |
| 10 | (ketamine$ or calipsol$ or calypsol$ or kalipsol$ or ketalar$ or ketanest$ or ketaset$).tw, kf,kw. | 18 220 |
| 11 | “Hypnotics and Sedatives”/ | 28 858 |
| 12 | Deep Sedation/ or Conscious Sedation/ | 9700 |
| 13 | Anesthesia, Intravenous/ or Anesthesia/ or “Anesthesia and Analgesia”/ | 72 342 |
| 14 | Anesthetics/ or Anesthetics, General/ | 23 073 |
| 15 | Sedat$.tw, kf,kw. | 59 329 |
| 16 | ($sedation or $sedations or $sedate or $sedates or $sedatory or $sedative or $sedatives).tw, kf,kw. | 55 125 |
| 17 | (anesthe$ or anaesthe$).tw, kf,kw. | 380 063 |
| 18 | 1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 | 273 211 |
| 19 | 9 or 10 | 19 673 |
| 20 | 11 or 12 or 13 or 14 or 15 or 16 or 17 | 453 017 |
| 21 | 18 and 19 and 20 | 418 |
| 22 | animals/ not humans/ | 4 640 038 |
| 23 | 21 not 22 | 387 |
Abbreviations: CCU, critical care unit; ICU, intensive care unit; PICU, pediatric intensive care unit; SICU, surgical intensive care unit.
Figure A1.
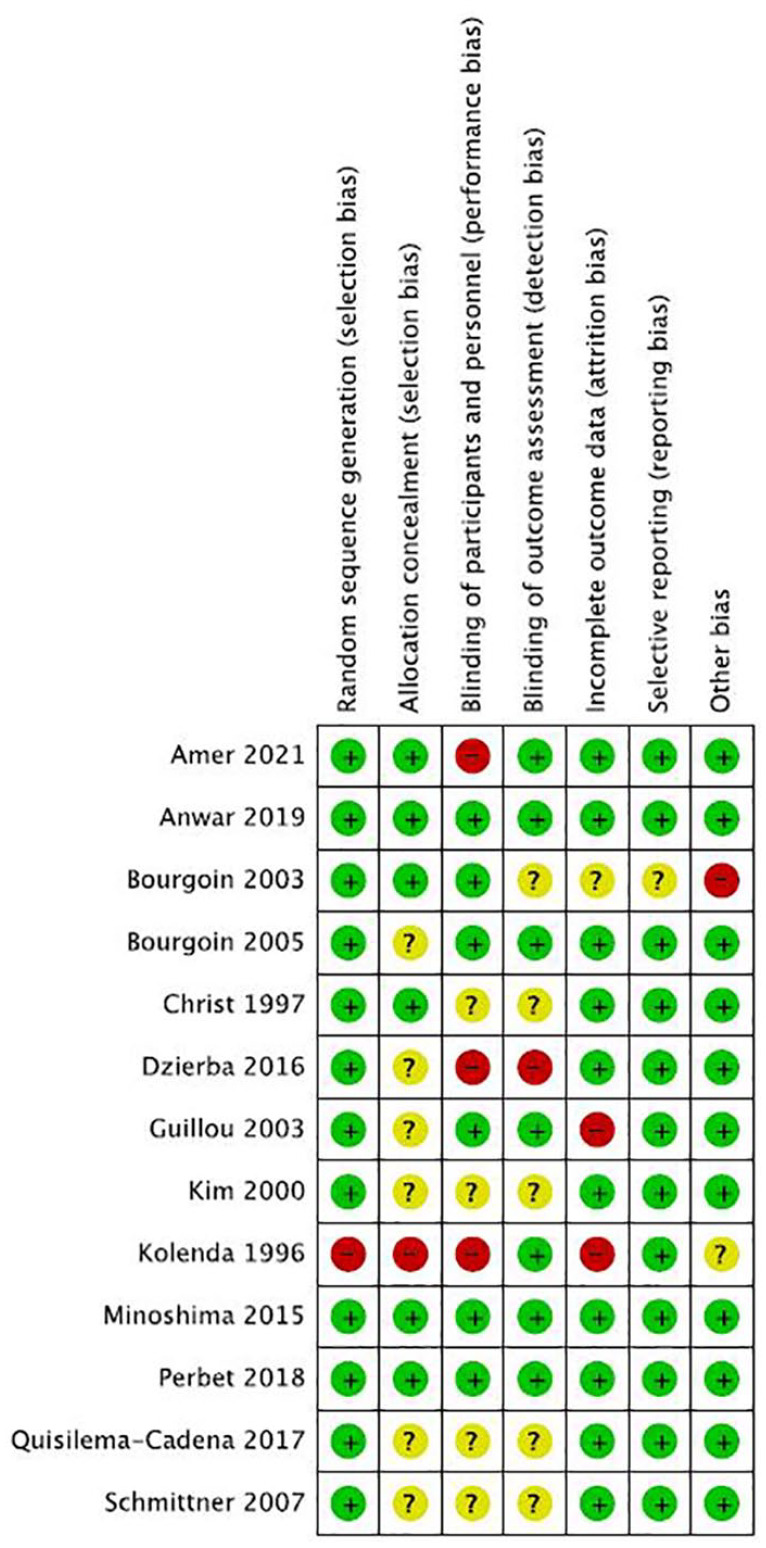
Bias assessment of included RCTs using the Cochrane RoB 1 tool (n = 13).
Abbreviations: RCT, randomized controlled trials; RoB 1, risk of bias 1.
Figure A2.
Breakdown of bias of included RCTs using the Cochrane RoB 1 tool (n = 13).
Abbreviations: RCT, randomized controlled trials; RoB 1, risk of bias 1.
Figure A3.
Forest plot comparison of opioid consumption across observational studies.
Abbreviations: CI, confidence interval; IV, intravenous.
Figure A4.
Forest plot of comparison across RCTs: (a) Mean length of ICU stay (days), (b) Mean length of hospital stay (days), (c) Intracranial pressure (ICP, mmHg), and (d) Mortality.
Abbreviations: CI, confidence interval; IV, intravenous; ICU, intensive care unit; ICP, intracranial pressure; RCT, randomized controlled trials.
Footnotes
Author Contributions: KC is the first reviewer for study inclusion, data extraction, quality assessment, and writer; LDB contributed in clinical and research guidance for the conduct of systematic review and he is the third reviewer resolving discrepancies between the first and second reviewer; CT is the second reviewer for data extraction and quality assessment; HW participated in interpretation of data, manuscript review, and guidance; CDC contributed in guidance for building and translating literature search and update of literature search; DRW contributed in clinical and research guidance for conduct of systematic review, analysis, and second reviewer for study inclusion.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: D.R.W. is supported by a Fonds de recherche du Québec-Santé (FRQ-S) clinical scientist career grant.
ORCID iD: Katalina Chan  https://orcid.org/0000-0003-0638-8352
https://orcid.org/0000-0003-0638-8352
References
- 1. Wang JG, Belley-Cote E, Burry L, et al. Clonidine for sedation in the critically ill: a systematic review and meta-analysis. Crit Care. 2017;21:75. doi: 10.1186/s13054-017-1610-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Burry L, Rose L, McCullagh IJ, Fergusson DA, Ferguson ND, Mehta S. Daily sedation interruption versus no daily sedation interruption for critically ill adult patients requiring invasive mechanical ventilation. Cochrane Database Syst Rev. 2014;2014:CD009176. doi: 10.1002/14651858.CD009176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46:e825-e873. doi: 10.1097/CCM.0000000000003299. [DOI] [PubMed] [Google Scholar]
- 4. Perbet S, Verdonk F, Godet T, et al. Low doses of ketamine reduce delirium but not opiate consumption in mechanically ventilated and sedated ICU patients: a randomised double-blind control trial. Anaesth Crit Care Pain Med. 2018;37:589-595. doi: 10.1016/j.accpm.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 5. Wunsch HA-O, Hill AD, Fu L, et al. New opioid use after invasive mechanical ventilation and hospital discharge. Am J Respir Crit Care Med. 2020;202:568-575. doi: 10.1164/rccm.201912-2503OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. World Health Organization. Opioid overdose. Accessed December 1, 2021. https://www.who.int/news-room/fact-sheets/detail/opioid-overdose.
- 7. Smischney NJ, Hoskote SS, Gallo de Moraes A, et al. Ketamine/propofol admixture (ketofol) at induction in the critically ill against etomidate (KEEP PACE trial): study protocol for a randomized controlled trial. Trials. 2015;16:177. doi: 10.1186/s13063-015-0687-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lexicomp. DexmedeTOMIDine (lexi-drugs). Accessed August 1, 2021. http://online.lexi.com.myaccess.library.utoronto.ca/lco/action/doc/retrieve/docid/patch_f/6715?cesid=0icuYwNDqMD&searchUrl=%2Flco%2Faction%2Fsearch%3Fq%3DdexmedeTOMIDine%26t%3Dname%26va%3Ddexmede.
- 9. Patanwala AE, Martin JR, Erstad BL. Ketamine for analgosedation in the intensive care unit: a systematic review. J Intensive Care Med. 2017;32(6):387-395. doi: 10.1177/0885066615620592. [DOI] [PubMed] [Google Scholar]
- 10. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Higgins JPT, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sterne JA, Hernan MA, Reeves BC, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016;355:i4919. doi: 10.1136/bmj.i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Compendium of pharmaceuticals and specialties. Opioids. Accessed August 1, 2021. https://www-e-therapeutics-ca.myaccess.library.utoronto.ca/search.
- 14. Dzierba AL, Brodie D, Bacchetta M, et al. Ketamine use in sedation management in patients receiving extracorporeal membrane oxygenation. Intensive Care Med. 2016;42(11):1822-1823. [DOI] [PubMed] [Google Scholar]
- 15. Quisilema-Cadena JM, Cordero-Escobar I, Gonzalez-Hernandez O. Comparacion de dose esquemas de sedoanalgesia en el paciente critico ventilado en el hospital. Rev Mex Anestesiol. 2017;40:155-161. [Google Scholar]
- 16. Amer MA-O, Maghrabi K, Bawazeer M, et al. Adjunctive ketamine for sedation in critically ill mechanically ventilated patients: an active-controlled, pilot, feasibility clinical trial. J Intensive Care. 2021;9:54. doi: 10.1186/s40560-021-00569-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kolenda H, Gremmelt A, Rading S, Braun U, Markakis E. Ketamine for analgosedative therapy in intensive care treatment of head-injured patients. Acta Neurochir (Wien). 1996;138(10):1193-1199. [DOI] [PubMed] [Google Scholar]
- 18. Christ G, Mundigler G, Merhaut C, et al. Adverse cardiovascular effects of ketamine infusion in patients with catecholamine-dependent heart failure. Anaesth Intensive Care. 1997;25(3):255-259. [DOI] [PubMed] [Google Scholar]
- 19. Schmittner MD, Vajkoczy SL, Horn P, et al. Effects of fentanyl and S(+)-ketamine on cerebral hemodynamics, gastrointestinal motility, and need of vasopressors in patients with intracranial pathologies: a pilot study. J Neurosurg Anesthesiol. 2007;19(4):257-262. [DOI] [PubMed] [Google Scholar]
- 20. Guillou N, Tanguy M, Seguin P, Branger B, Campion J-P, Malledant Y. The effects of small-dose ketamine on morphine consumption in surgical intensive care unit patients after major abdominal surgery. Anesth Analg. 2003;97(3):843-847. [DOI] [PubMed] [Google Scholar]
- 21. Minoshima R, Kosugi S, Nishimura D, et al. Intra- and postoperative low-dose ketamine for adolescent idiopathic scoliosis surgery: a randomized controlled trial. Acta Anaesthesiol Scand. 2015;59(10):1260-1268. doi: 10.1111/aas.12571. [DOI] [PubMed] [Google Scholar]
- 22. Bourgoin A, Albanese J, Wereszczynski N, Charbit M, Vialet R, Martin C. Safety of sedation with ketamine in severe head injury patients: comparison with sufentanil. Crit Care Med. 2003;31(3):711-717. [DOI] [PubMed] [Google Scholar]
- 23. Anwar S, Cooper J, Rahman J, Sharma C, Langford R. Prolonged perioperative use of pregabalin and ketamine to prevent persistent pain after cardiac surgery. Anesthesiology. 2019;131(1):119-131. doi: 10.1097/ALN.0000000000002751. [DOI] [PubMed] [Google Scholar]
- 24. Wan X, Wang W, Liu J, Tong T. Estimating the sample mean and standard deviation from the sample size, median, range and/or interquartile range. BMC Med Res. 2014;14:135. doi: 10.1186/1471-2288-14-135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557-560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Cochrane Training. Introduction to GRADE. Accessed August 1, 2021. https://training.cochrane.org/introduction-grade.
- 27. Bourgoin A, Albanese J, Leone M, Sampol-Manos E, Viviand X, Martin C. Effects of sufentanil or ketamine administered in target-controlled infusion on the cerebral hemodynamics of severely brain-injured patients. Crit Care Med. 2005;33:1109-1113. [DOI] [PubMed] [Google Scholar]
- 28. Kim TH, Lim CM, Shim TS, et al. Comparison of the efficacy between ketamine and morphine on sedation and analgesia in patients with mechanical ventilation. Korean J Crit Care Med. 2000;15:82-87. [Google Scholar]
- 29. Park S, Choi AY, Park E, et al. Effects of continuous ketamine infusion on hemodynamics and mortality in critically ill children. PLoS One. 2019;14(10):e0224035. doi: 10.1371/journal.pone.0224035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Von der Brelie C, Seifert M, Rot S, et al. Sedation of patients with acute aneurysmal subarachnoid hemorrhage with ketamine is safe and might influence the occurrence of cerebral infarctions associated with delayed cerebral ischemia. World Neurosurg. 2017;97:374-382. doi: 10.1016/j.wneu.2016.09.121. [DOI] [PubMed] [Google Scholar]
- 31. Reese JM, Sullivan VF, Boyer NL, Mount CA. A non-comparative prospective pilot study of ketamine for sedation in adult septic shock. Mil Med. 2018;183:e409-e13. doi: 10.1093/milmed/usy121. [DOI] [PubMed] [Google Scholar]
- 32. Shurtleff V, Radosevich JJ, Patanwala AE. Comparison of ketamine- versus nonketamine-based sedation on delirium and coma in the intensive care unit. J Intensive Care Med. 2020;35:536-541. doi: 10.1177/0885066618767619. [DOI] [PubMed] [Google Scholar]
- 33. Jaeger M, Attridge RL, Neff LA, Gutierrez GC. Safety and effectiveness of sedation with adjunctive ketamine versus nonketamine sedation in the medical intensive care unit. J Pharm Pract. 2020;34:850-856. doi: 10.1177/0897190020925932. [DOI] [PubMed] [Google Scholar]
- 34. Wu TT, Ko S, Kooken R, van den Boogaard M, Devlin JW. Exploring ketamine analgosedation use and its effect on incident delirium in critically ill adults. Crit Care Explor. 2021;3:e0544. doi: 10.1097/CCE.0000000000000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Burry LD, Williamson DR, Perreault MM, et al. Analgesic, sedative, antipsychotic, and neuromuscular blocker use in Canadian intensive care units: a prospective, multicentre, observational study. Can J Anaesth. 2014;61(7):619-630. doi: 10.1007/s12630-014-0174-1. [DOI] [PubMed] [Google Scholar]
- 36. Manasco AT, Stephens RJ, Yaeger LH, Roberts BW, Fuller BM. Ketamine sedation in mechanically ventilated patients: a systematic review and meta-analysis. J Crit Care. 2020;56:80-88. doi: 10.1016/j.jcrc.2019.12.004. [DOI] [PubMed] [Google Scholar]
- 37. Erstad BL. Implications of the opioid epidemic for critical care practice. J Am Coll Clin Pharm. 2019;2:161-166. [Google Scholar]