Abstract
Background
Clear-cell renal cell carcinoma (ccRCC) is one of the most common malignant tumors worldwide whose poor prognosis results in a serious disease burden on patients. The changing trend of the long-term relative survival rates (RSRs) of patients with ccRCC was analyzed in this study to evaluate their treatment results over a 15-year period.
Methods
This study is a retrospective study, which assessed and predicted the 1-, 3-, and 5-year survival rates of patients with ccRCC during 2001-2005, 2006-2010, 2011-2015, and 2016-2020 using data extracted from the Surveillance, Epidemiology, and End Results (SEER) database. Period analysis was used in this study to analyze the data from the SEER database and to assess survival differences according to age, sex, race, and socioeconomic status (SES) during the 15-year study period by comparing Kaplan-Meier curves.
Results
During 2001-2015, the 5-year RSR of patients with ccRCC increased from 78.4% to 83.0%, and the generalized linear model predicted that the 5-year RSR increased to 85.7% during 2016-2020. The RSR of patients with ccRCC differed significantly with SES, race, sex, and age. Compared with male patients, the survival advantage of female patients decreased as their age increased. The RSR of all patients with ccRCC was also lower in patients with a lower SES and of black race.
Conclusion
This study found an improvement in the RSR of patients with ccRCC during 2001-2020. Understanding the change trend of the survival rate of patients with ccRCC is helpful to improve the design of clinical trials. It also provides basic data and a scientific basis for evaluating the harm of ccRCC on the health of affected patients and the effect of cancer prevention, and developing cancer prevention plans.
Keywords: clear cell renal cell carcinoma; surveillance, epidemiology, and end results; relative survival rate; period analysis; sex; socioeconomic status
Introduction
Renal cell carcinoma (RCC) is a malignant tumor located in the genitourinary system. Its incidence has soared recently. In 2017, there were about 63 990 new RCC cases worldwide, and about 693 000 people died from it. 1 Studies have found that the incidence of RCC is high in the United States, 2 and it is also increasing, including more than doubling during 1975-2020. 3 Regarding histological subtypes, more than 70% of RCCs are clear-cell renal cell carcinomas (ccRCCs), making it the most common subtype. 4 Studies have shown that in the past two decades, two major changes have taken place in the therapeutic landscape of patients with metastatic renal cell carcinoma (mRCC). The first were the approval of the first TKIs (tyrosine kinase inhibitors) for the treatment of mRCC in 2005 and 2006 and more regulatory approvals followed, and the second was the rapid increase of diverse immunotherapy combinations in recent years, both of which showed better efficacy and improved prognosis than before.5-9 The renewal of surgical methods and the increase of surgical usage have also improved the prognosis of RCC.10,11 It is worth noting that despite new treatment options, accidental discovery is still the most common modality of RCC diagnosis, and the survival rate of patients with advanced RCC remains very low. 3 It is therefore of great importance to further elucidate the epidemiological characteristics of RCC and its subtypes in order to improve its prevention, timely diagnosis, and treatment, which will in turn increase the survival rate of the patients and help to curb the increasing disease burden. Although some recent studies have evaluated the epidemiological characteristics of RCC,1,12,13 studies on the effects of demographic factors on the prognosis of patients with ccRCC are rare. CcRCC accounts for most RCCs, but its epidemiological characteristics are not necessarily consistent with those of RCC as a result of the nonnegligible heterogeneity among different RCC subtypes. Furthermore, even though some studies investigated the relationship between demographic factors and ccRCC prognosis, they paid more attention to how a specific demographic factor such as socioeconomic status (SES), race, or marital status impacted its survival rate.5,14,15 There were few studies that comprehensively analyzed the effects of demographic factors on ccRCC prognosis, and they mostly used traditional cohort analysis or complete analysis for long-term survival analysis. 12 Previous studies have shown that traditional cohort and complete methods provided less up-to-date and accurate estimates of long-term survival, hampering the disclosure of recent improvement in prognosis. 16 In addition, almost none of the above SEER-based studies predicted the future survival rates. Model-based period analysis is an up-to-date approach of survival estimation. 17 Compared with traditional methods, the estimated survival rates of model-based period analysis are closer to the real survival rates, which improves the accuracy and timeliness of survival analysis.16,18 It can also use existing data to predict the future survival rates. 18 This method has been widely used,19,20 but has not been reported for ccRCC.
Therefore, for the first time, we used model-based period analysis to analyze the latest survival data from the Surveillance, Epidemiology, and End Results (SEER) database to comprehensively analyze the survival rate changes and demographic factors of patients with ccRCC since the start of the 21st century, and to predict the survival rate of the disease in order to provide an up-to-date and comprehensive view of the epidemiology and prognosis of ccRCC. We also aimed to reflect the therapeutic effect of ccRCC since the start of the 21st century and analyze the reason for the increased survival rate of affected patients. Patients with ccRCC were also stratified by age, sex, race, and SES to compare their differences in relative survival rate (RSR).
Material and Methods
Database
This study used the SEER data for “18 cohort database [Incidence-SEER 18 Regs Research Data + Hurricane Katrina Impacted Louisiana Cases, Nov 2018 Sub (1975-2016 varying)]” as the data source, which comprises 18 registries that cover about 27.8% of the United States population. The SEER program is the only comprehensive population-based source of information in the United States, providing the most comprehensive data on cancer survival, tumors, demographics, and socioeconomic characteristics, and is considered to represent the quality standard among cancer registrations worldwide. 21
Variables
SEER*STAT software version 8.3.9.1 (https://seer.cancer.gov/, accession number: 15756-Nov2019) was used to identify patients diagnosed during 2001-2015 with ICD-O-3 (third revision of the International Classification of Oncology Diseases) morphological code 8310/3 (clear-cell adenocarcinoma) and anatomical code C64.9 (kidney) from the SEER database. Cases of ccRCC were excluded if they were diagnosed by autopsy or with only death-certificate reports, or had invalid information or a lack of any follow-up information. Finally, 83 825 patients with ccRCC were identified. All patient details were de-identified because SEER data are de-identified and publicly available for research. The cases were stratified by sex (male and female), age at diagnosis (0-49, 50-64, 65-79, and ≥80 years), race (white, black, and other), and SES. SES represented the household poverty rate in the area where the patient resided, and was based on the percentage of households living below the national poverty line in the 2000 United States Census of Population and Housing.22,23 Patients were divided into the four quartiles of the family poverty rate: rich areas (<5.34%), low-poverty areas (≥5.34% and <7.70%), medium-poverty areas (≥7.70% and <12.69%), and high-poverty areas (≥12.69%).22,23
Statistical Analyses
This study is a retrospective study, which applied period analysis based on a generalized linear model, and the RSRs of the three observation periods of 2001-2005, 2006-2010, and 2011-2015 were estimated using the data of the existing complete tumor registration system, and the change trend was analyzed. We also predicted the 1-, 3-, and 5-year RSRs of patients with ccRCC during 2016-2020. This study followed relevant Equator guidelines. In this study, we used RSR rather than absolute (observed) survival rate, which indicates net survival in the situation that cancer is the sole cause of death. 24 The RSR is defined as the absolute survival rate of the target patients divided by the expected survival rate of a comparable group from general population who have demographic data similar to that of the target patients with cancer, such as race, sex, and age.19,24 It can be expressed as: where and represent the absolute survival rate and expected survival rate. When calculating the relative survival rate at 5 years, k is 5. Among them, the expected survival rate was derived from nationwide population life tables for the year 2000 stratified by age, sex and calendar time,19,24 using the Ederer II method.19,24 The point estimation of RSR and its standard error were calculated by Greenwood’s method.17,24 In this study, the overall survival rate was estimated by constructing Kaplan-Meier curves. We used the log-rank test with a significance threshold of .05 to evaluate the difference between these curves. Cox regression analysis was used to determine whether age, sex, race, and SES were independent risk factors. All statistical analyses were performed using the periodR, rms, Hmisc, lattice, survival, Formula, ggplot2, foreign, and survminer packages of R software version 4.0.2 (https://www.r-project.org/).
Results
Baseline Characteristics of Patients With ccRCC
Table 1 lists the number of cases identified in the SEER database during the three observation periods. During 2001-2015, 83 825 patients with ccRCC were identified, and the number of patients continued to increase over the 15-year study period, from 17 822 in the first 5 years to 29 167 in the second 5 years (63.7% increase), and then to 36 836 in the third 5 years (26.3% increase). The number of patients with ccRCC in each stratified subgroup also showed a similar growth trend, and the growth rates were similar among the different subgroups. During the 3 observation periods, the distributions of the numbers of cases in different ages, sexes, races, and SESs were relatively stable, with at least 1000 cases in each classification subgroup. The age at the ccRCC diagnosis was mostly between 50 and 79 years (comprising 75.7% of the population). There were more male than female patients (51 962 vs 31 863), and there were far more white than black patients (71 999 vs 6138). The distribution of the number of patients in each SES group was relatively balanced. However, there was also a positive correlation with the degree of poverty (17 751, 19 138, 22 861, and 24 075 patients in rich, and low-, medium-, and high-poverty areas, respectively) (Table 1).
Table 1.
Number of cases of clear cell renal cell carcinoma in different observation periods.
| Category | 2001-2005 | 2006-2010 | 2011-2015 |
|---|---|---|---|
| Whole population | 17 822 (21.3%) | 29 167 (34.8%) | 36 836 (43.9%) |
| Sex | |||
| Male | 10 935 (61.4%) | 17 985 (61.7%) | 23 042 (62.6%) |
| Female | 6887 (38.6%) | 11 182 (38.3%) | 13 794 (37.4%) |
| Race | |||
| White | 15 518 (87.1%) | 25 041 (85.8%) | 31 440 (85.3%) |
| Black | 1213 (6.8%) | 2178 (7.5%) | 2747 (7.5%) |
| Other | 1091 (6.1%) | 1948 (6.7%) | 2649 (7.2%) |
| Age(y) | |||
| 0-49 | 3143 (17.6%) | 5102 (17.5%) | 6199 (16.8%) |
| 50-64 | 7014 (39.4%) | 11 762 (40.3%) | 14 905 (40.5%) |
| 65-79 | 6312 (35.4%) | 10 107 (34.7%) | 13 356 (36.3%) |
| 80+ | 1353 (7.6%) | 2196 (7.5%) | 2376 (6.4%) |
| SES | |||
| Rich | 3811 (21.4%) | 6308 (21.6%) | 7632 (20.7%) |
| Low poverty | 4264 (23.9%) | 6647 (22.8%) | 8227 (22.3%) |
| Medium poverty | 4615 (25.9%) | 7922 (27.2%) | 10 324 (28.0%) |
| High poverty | 5132 (28.8%) | 8290 (28.4%) | 10 653 (28.9%) |
Notes: Not all columns round to 100% due to rounding.
Abbreviation: y, year; SES, socioeconomic status
Cox Regression Analysis Results of Survival Time in Patients With ccRCC
We applied univariate and multivariate Cox regression analyses to the survival data of patients with ccRCC diagnosed during 2001-2015 in order to determine the relationship between each factor and the prognosis of patients. The results indicated that age, sex, race, and SES were independent risk factors (P < .01 for all variables; Table 2).
Table 2.
Summary of Cox regression Analysis of Survival time of patients with clear cell renal cell carcinoma in 18 SEER sites from 2001 to 2015.
| Univariate | Multivariate | |||||
|---|---|---|---|---|---|---|
| Variable | 95% CI | HR | P | 95%CI | HR | P |
| Age (y) | ||||||
| N | 1.0 | 1.0 | ||||
| N+1 | 1.043-1.045 | 1.044 | <.001 | 1.044-1.046 | 1.045 | <.001 |
| Sex | ||||||
| Male | 1.0 | 1.0 | ||||
| Female | .817-.859 | .837 | <.001 | .768-.808 | .788 | <.001 |
| Race | ||||||
| White | 1.0 | 1.0 | ||||
| Black | 1.050-1.149 | 1.098 | <.001 | 1.115-1.222 | 1.167 | <.001 |
| Other | .855-.946 | .900 | <.001 | .864-.956 | .909 | <.001 |
| SES | ||||||
| Rich | 1.0 | 1.0 | ||||
| Low-poverty | 1.020-1.097 | 1.058 | <.01 | 1.022-1.100 | 1.060 | <.01 |
| Medium-poverty | 1.070-1.149 | 1.109 | <.001 | 1.074-1.153 | 1.113 | <.001 |
| High-poverty | 1.157-1.240 | 1.198 | <.001 | 1.152-1.235 | 1.193 | <.001 |
Notes: Age is a continuous variable. N represents age.
Abbreviations: 95% CI, 95% confidence interval; HR, hazard risk; y, year; SES, socioeconomic status.
RSR Change in Patients With ccRCC
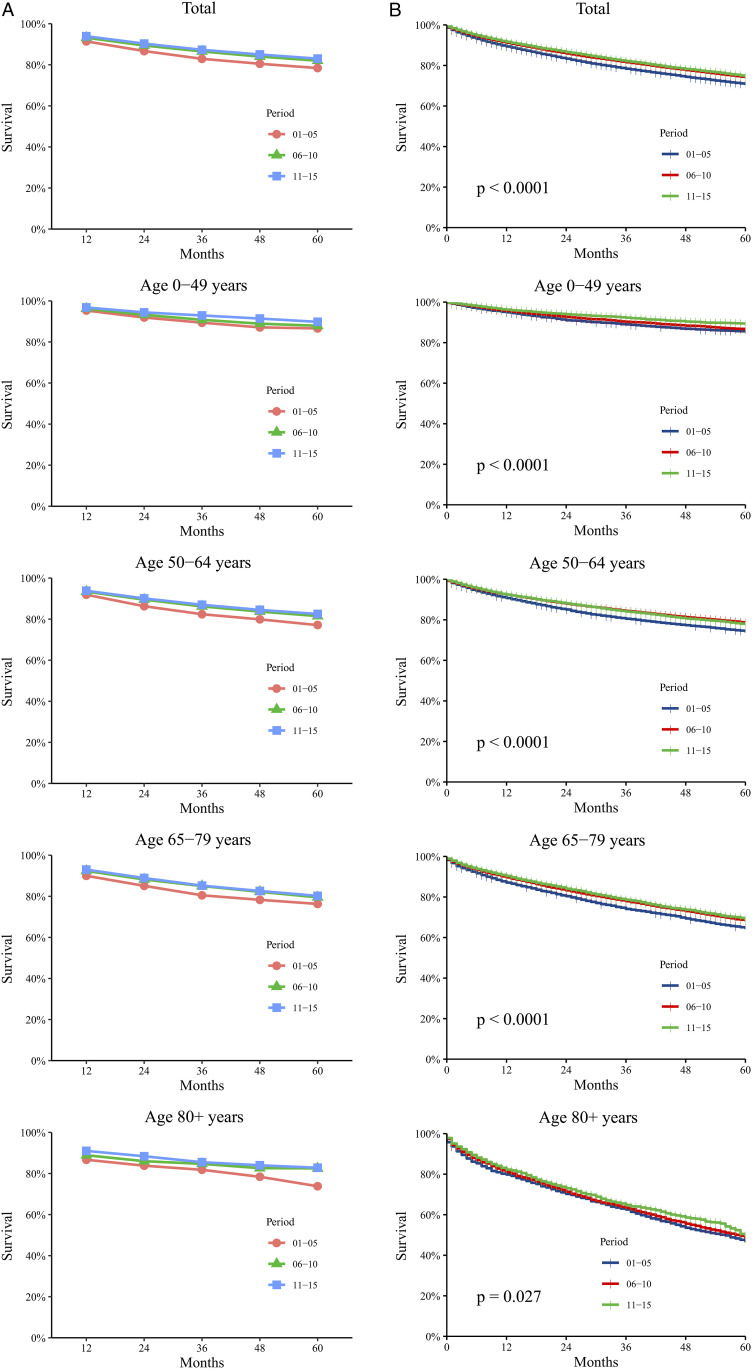
There were 83 825 patients with ccRCC diagnosed during 2001-2015. The RSRs of these patients increased at varying rates within the 15 years, and the 5-year RSR increased from 78.4% and 82.0% to 83.0%. The 5-year RSR of the second 5 years was 4.6% higher than that of the first 5 years, which was significantly higher than the 1.2% increase from the second 5 years to the third 5 years, and the 3-year RSR also showed a similar growth trend. At the same time, the RSRs of patients in all age groups had similar upward trends (Figure 1A and Supplemental Table S1). As age increased, the RSR of patients with ccRCC decreased significantly. The 5-year RSR of patients aged 0-49 years was 89.8%, while that of patients aged ≥80 years decreased to 82.8% between 2011 and 2015. In the first two 5-year periods, the RSR of patients in different age groups also had the same trend. Kaplan-Meier survival analysis indicated that the survival time of patients with ccRCC in all age groups showed a significant growth trend during the 15-year study period. At the same time, the survival time of patients showed a significant downward trend as age increased (Figure 1B).
Figure 1.
Trends in 5-year relative survival rates (A) and Kaplan-Meier survival analyses (B) for patients with ccRCC from 18 SEER original sites from 2001 to 2015. Data are shown by age group (total and age 0-49, 50-64, 65-79 and 80+ years) and calendar period.
The generalized linear model predicted that the 1-, 3-, and 5-year RSRs of all patients with ccRCC during 2016-2020 were 94.8%, 88.1%, and 85.7%, respectively, which were higher than those during the 15-year study period (Supplemental Table S1), indicating that the prognosis of patients with ccRCC will improve further in the future. The predicted RSRs of each age group during 2016-2020 are listed in Supplemental Table S1.
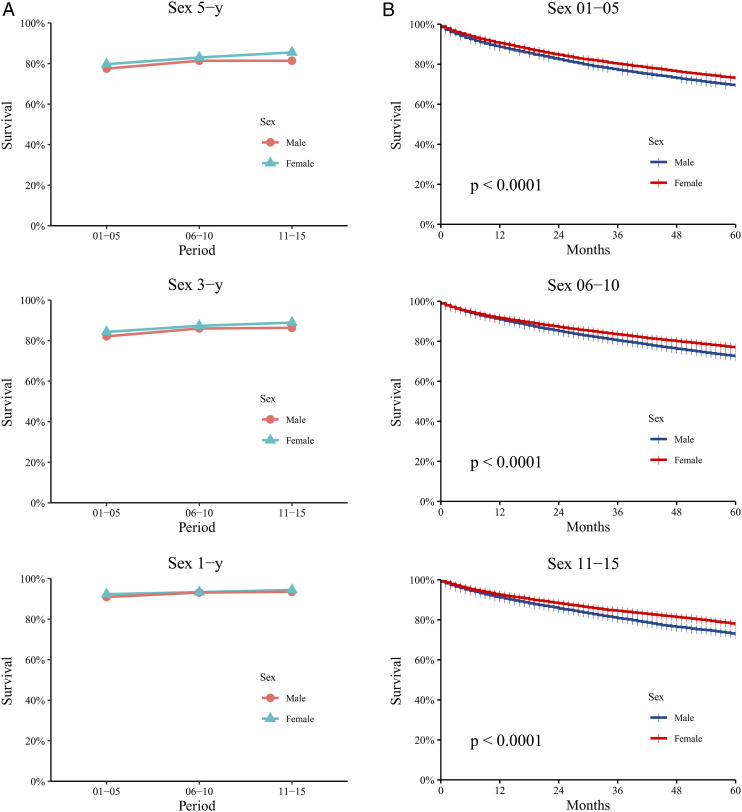
During these 15 years, the RSRs of both male and female patients increased, with each of the 1-, 3-, and 5-year RSRs being higher in females than in males.
The 5-year RSRs of male and female patients increased from 77.5% to 81.4% and from 79.7% to 85.5%, respectively (Figure 2A and Supplemental Table S2). The P value was calculated using Kaplan-Meier survival analysis, which indicated significant differences in survival times between males and females during the 15-year study period (P<.0001 during 2001-2005, 2006-2010, and 2011-2015; Figure 2B).
Figure 2.
Patients with ccRCC in 18 SEER original sites were divided into gender groups for 1-year, 3-year and 5-year relative survival trend analyses and Kaplan-Meier survival analyses every five years (A, B). Data are shown by gender (male and female) and calendar period.
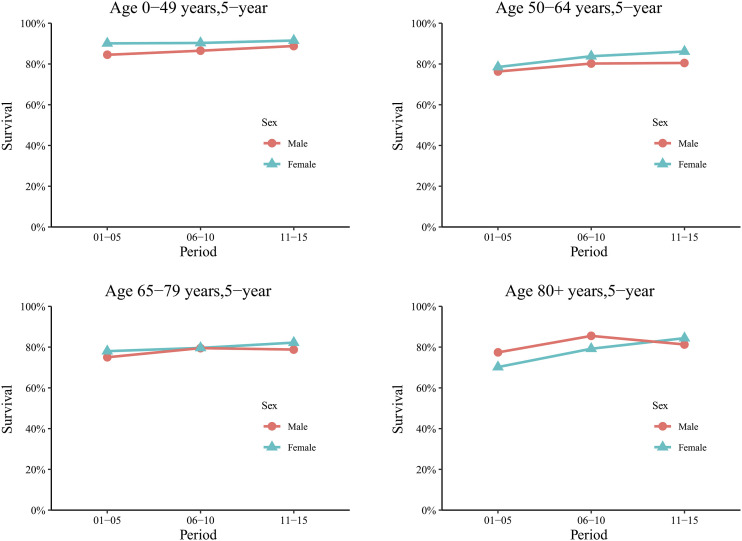
As age increased, the survival advantage of female patients over male patients reduced, and even became a disadvantage. Among patients aged 0-49 years, females had a significantly higher 5-year RSR than males in each of the 5-year periods (90.1% vs 84.5% during 2001-2005, 90.3% vs 86.5% during 2006-2010, and 91.5% vs 88.8% during 2011-2015). Similarly, among those aged 50-64 years, the 5-year RSR was higher for females than for males during each of the 5-year periods, but the difference had already decreased slightly. The survival advantage for females aged 65-79 years decreased further to become no longer significant (78% vs 75% during 2001-2005, 79.6% vs 79.5% during 2006-2010, and 82.2% vs 78.8% during 2011-2015). For patients aged ≥80 years, although the 5-year RSR of female patients still slightly exceeded that of male patients during 2011-2015 (84.4% vs 81.3%), there was a significantly reversed relationship between 2001-2005 and 2006-2010 (77.4% in males vs 70.2% in females during 2001-2005, and 85.5% in males vs 79.2% in females during 2006-2010; Figure 3 and Supplemental Table S3). For the 1- and 3-year RSRs, the sex-induced survival difference also indicated a similar decrease or even a reversal as age increased (Supplemental Figure S1 and Supplemental Table S3).
Figure 3.
Trends in 5-year relative survival rates according to sex for patients with ccRCC from 18 SEER original sites from 2001 to 2015. Data are shown by sex (male and female), age group (ages 0-49, 50-64, 65-79, and 80+ years) and calendar period.
The predicted 5-year RSRs for male and female patients during 2016-2020 were 84.0% and 88.6%, respectively, and the predicted 1- and 3-year RSRs are also listed in Supplemental Table S2 (Supplemental Table S2). These were all higher than those for the 15-year study period (2001-2015).
During the 15-year study period, the RSRs of white and black patients improved, with their 5-year RSRs increasing from 78.8% and 67.8% in the first 5 years to 82.9% and 81.9% in the last 5 years, respectively. During 2001-2005, the 5-year RSR of white patients significantly exceeded that of black patients (78.8% vs 67.8%). The survival advantage of white over black patients decreased in the second 5 years (82.4% vs 75.9%) and became very weak in the third 5 years (82.9% vs 81.9%). The 3-year RSR gap between white and black patients similarly narrowed over time (Figure 4A and Supplemental Table S4). Significant differences were also found between the survival times of white and black patients during 2001-2006 and 2006-2010 using Kaplan-Meier survival analysis (P<.01), while no significant difference was found during 2011-2015 (P>.05; Figure 4C). The predicted 5-year RSRs for white and black patients during 2016-2020 were 85.5% and 90.3%, respectively, and the predicted 1- and 3-year RSRs are listed in Supplemental Table S4, which all increased over the 15-year study period (Supplemental Table S4).
Figure 4.
One-year, 3-year and 5-year relative survival rates and Kaplan-Meier survival analyses according to race (A, C) and SES/county-level poverty rates (B, D) for patients with ccRCC from 18 SEER original sites from 2001 to 2015. Data are shown by race (white and black) and SES/county-level poverty rates (rich, low-poverty, medium-poverty and high-poverty) and calendar period.
Among the four SES groups, the RSR was almost always highest for the rich group and lowest for the high-poverty group. Although the RSRs of all SES groups improved during 2001-2015, the survival gap between the four SES groups in the first 5 years was much larger than that of the last 5 years. For example, during 2001-2005, the RSRs of rich and low-, medium-, and high-poverty groups were 83.6%, 81.3%, 78.0%, and 72.9%, respectively, giving differences between them of 2.3%, 3.3%, and 5.1%, but during 2006-2010 these differences decreased to −.4%, 2.9%, and 1.2%, respectively (RSRs of 83.6%, 84.0%, 81.1%, and 79.9% in the rich, low-, medium-, and high-poverty groups, respectively). The 3-year RSR gap among SES groups also similarly decreased over time, but the change was relatively small (Figure 4B and Supplemental Table S5). The distribution of different SESs among patients of different races cannot be ignored. Compared with black patients, more white patients belonged to rich and low-poverty areas (45.2% vs 24.2%), while there were fewer white patients in high-poverty areas than black patients (27.4% vs 46.8%; Supplemental Figure S2 and Supplemental Table S6). Significant differences were found between the survival times of the four SES groups in the three 5-year periods using Kaplan-Meier survival analysis (P<.01; Figure 4D). The predicted 5-year RSRs for the four SES groups during 2016-2020 were 85.4%, 85.8%, 85.6%, and 85.9%, respectively, indicating an upward trend compared with the previous 15 years (Supplemental Table S5). Supplemental Table S5 lists the predicted 1- and 3-year RSRs.
Discussion
As one of the most common and harmful malignant tumors worldwide, the harm of kidney cancer should not be underestimated in the United States, where the estimated number of new cases of kidney and pelvis cancer ranks it 6th among males and 10th among females. 25 Patients with RCC often have a poor prognosis, which has become a serious threat to public health. 13 As ccRCC is the most common histological type of RCC, 4 accurate analysis and prediction of the survival rate of patients with ccRCC is very important for treatments and nursing care.
This study demonstrated that the RSR of patients with ccRCC increased gradually during the three 5-year observation periods from 2001 to 2015 and during the prediction period from 2016 to 2020. The improvement in the RSR of patients with ccRCC may benefit from advances in diagnosis and treatment techniques, such as the adoption of new risk assessment models, updates to surgery, and the application of new targeted drugs and immunotherapy.5-9,26 Notably, the 5-year RSR of patients during 2006-2010 was significantly higher than that during 2001-2005 (4.6%), while that of patients during 2011-2015 was still higher than that during 2006-2010, but less significantly (1.2%). The 3-year survival rates also had a similar growth trend. On December 20, 2005 and January 26, 2006, the tyrosine kinase inhibitors (TKIs) sorafenib and sunitinib malate, respectively, were approved by the United States Food and Drug Administration because they had great advantages over other adjuvant therapies in ccRCC treatment.27-29 This period happened to be at the junction of the first two 5-year observation periods. The significant increase in the 3- and 5-year RSRs we found in the first two 5-year periods therefore may reflect the excellent therapeutic efficacy of TKIs and the revolutionary changes they brought. However, despite the adoption of multiple treatment modes, no significant progress was made to improve the prognosis of patients with ccRCC during the 15-year study period, and drug intolerance and many side effects are still inevitable.5,27,30,31 This highlights the urgency of continuing to develop new and more specific treatments to improve the prognosis of ccRCC.
The analysis performed in this study also indicated that the survival rate of patients was affected to varying degrees by age, sex, race, and SES during the 15-year study period.
Our results indicate that the RSR of patients with ccRCC increased in all age groups during 2001-2015. At the same time, the RSR of patients showed a significant downward trend as age increased. There are previous reports of age being an independent prognostic factor for patients with ccRCC.13,32 Reasons for why young patients with ccRCC have a better prognosis than old patients may include the tumors of young patients being more localized, smaller, having lower metastasis rates, and lower grades and stages.13,32,33 The age difference may also be because elderly patients are less likely to receive surgery, radiotherapy, or chemotherapy, have a lack of care due to a loss of the spouse, and have more chronic diseases.32,34
We observed that the 1-, 3-, and 5-year RSRs of female patients with ccRCC were higher than those of male patients during 2001-2015. This sex difference has been reported previously.33,35,36 The related studies have indicated that this difference in survival between sexes may be because female patients have a higher probability of accidental renal cancer detection, their tumors tending to have slower progression and lower grades and stages, and their decisions on treatment differing from those of males.33,35,37 Sex-specific mutations of BAP1 and other genes, which are particularly obvious in ccRCC, may also be an underlying reason. 36
We also observed that the RSR of patients with ccRCC improved over time for both sexes. It was particularly interesting that grouping patients by age revealed that the RSR of female patients was significantly higher than that of male patients in the younger age groups (0-49 and 50-64 years old). However, this difference was very small in the older age group (65-79 years old), and was even reversed in the oldest age group (≥80 years old). Micheli et al analyzed the EUROCARE-4 database, and obtained similar results. 38 This finding suggests that the survival advantage of female patients before menopause is mostly caused by biological rather than cultural factors. Many studies have investigated the relationship between the risk of hormone-related factors and RCC and its molecular biological mechanisms,39-43 and estrogen is thought to play a protective role in RCC progression.40,42,43 Our findings reflected this viewpoint and further suggested the effect of sex hormone levels on the prognosis of patients with ccRCC.
Our data indicated that the 1-, 3-, and 5-year RSRs of white patients with ccRCC were higher than those of black patients during 2001-2015. According to previous studies, the survival difference between black and white patients with ccRCC was related to various factors such as genetic factors, phenotypic factors, intratumoral signal transduction pathways, immune-related pathways, responsiveness to targeted therapy, opportunities for nephrectomy, and renal complications.44-50
We also found that the survival advantage of white patients over black patients continued to decrease over time. This suggested that the difference in RSR between races was not limited to genetic differences. Similarly, rich and low-poverty groups had higher RSRs, and the survival gap between SES subgroups also narrowed over time. When classified by SES, more white patients are classified as low-poverty or rich, while more black patients are classified as high-poverty. These SES distribution differences between black and white patients may help to explain the similar survival differences between races and SES groups, due to how SES can significantly affect cancer prognoses.14,51-53 Patients in areas of high poverty tend to have lower household income, are more likely to live in counties with lower health insurance coverage, scarce health-care resources, poor health environment, and are considered to have more health-risk behaviors, all of which may eventually lead to higher cancer mortality,48,54-56 which also occur among black patients.44,51,57-59 Our results indicate that both between different races and among SES subgroups, the RSR of patients increased steadily and were similar over time, which may be due to improvements in medical resources, policies, and insurance.60,61
Our study timely and accurately updated the survival estimates of patients with ccRCC, and more comprehensively and accurately reflected the overall status and dynamic trend of the long-term survival rate of patients with ccRCC in the United States since the 21st century. It also helped to predict future survival trends. Meanwhile, this study may contribute to the design of health care policies and management to balance differences in survival conditions among age groups, sexes, races and SES groups and to improve patient survival.
On the overall level, this study reflected the improvement of the RSRs of patients with ccRCC caused by multiple factors in the diagnosis and treatment of ccRCC, providing a panoramic viewpoint. However, it could not accurately reflect and compare the prognosis improvement caused by specific treatment strategy. Therefore, with many different potential first-line options likely available for patients in the future,5-9 further clinical trials need to be carried out to directly compare the specific treatment strategies of ccRCC to define the best treatment strategy in particular patient subpopulations. Meanwhile, it will become increasingly important to identify more reliable predictive biomarkers and increase our understanding of tumor microenvironment.5,6
In addition, studies have shown that, in addition to basic demographic factors, other factors such as tumor stage, tumor grade and surgery also affected the prognosis of patients with ccRCC.11,12 Therefore, it is necessary to conduct further studies including these factors.
Limitations
This study had some limitations. First, its retrospective design meant that it might have been affected by selection, information, and mixed biases, such as under-registration, misclassification of cases within and among countries, and variations in SES. Second, the results can only reflect the selected SEER areas and are not appropriate to generalize to other geographical locations. Third, some potential important factors such as radiotherapy, chemotherapy, certain biological indicators and certain cultural factor are lacked in the SEER database. It is therefore difficult to directly assess the link between changes in treatment and improvements in survival outcomes, as well as the impact of other potential factors on survival. Fourth, because the key point of this study is to explore the effects of age, sex, race and SES on RSRs in patients with ccRCC, this study did not include other factors such as tumor stage, tumor grade, and marital status and further analysis by stratification of these variables, which may lead to the RSR differences between patients.
Conclusion
In conclusion, our analysis of cancer data in the SEER database found that the RSR of patients with ccRCC improved over the 15-year study period. We observed a survival advantage in female patients, as well as a significant relationship between this survival advantage and the age of female patients. The difference in RSRs between white and black patients and that between patients with different SES also indicated similar decreasing trends over time, suggesting the relationship between these two factors. In the future it is necessary to obtain a better understanding of the molecular mechanism of ccRCC occurrence and development, develop novel and effective antineoplastic drugs, and increase the use of imaging examinations. Combined with improvements in medical insurance and the health-care system, this will further improve the survival rate of ccRCC.
Supplemental Material
Supplemental Material for Analysis and Prediction of the Survival Trends of Patients with Clear-Cell Renal Cell Carcinoma: A Model-Based Period Analysis, 2001-2015 by Sicong Du, BD, Yu Zhong, MD, Shuai Zheng, MD, and Jun Lyu, PhD in Cancer Control.
Supplemental Material for Analysis and Prediction of the Survival Trends of Patients with Clear-Cell Renal Cell Carcinoma: A Model-Based Period Analysis, 2001-2015 by Sicong Du, BD, Yu Zhong, MD, Shuai Zheng, MD, and Jun Lyu, PhD in Cancer Control.
Supplemental Material for Analysis and Prediction of the Survival Trends of Patients with Clear-Cell Renal Cell Carcinoma: A Model-Based Period Analysis, 2001-2015 by Sicong Du, BD, Yu Zhong, MD, Shuai Zheng, MD, and Jun Lyu, PhD in Cancer Control.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethical Approval: All procedures performed in the present study were in accordance with the principles outlined in the 1964 Helsinki Declaration and its later amendments.
Informed Consent: This study was exempted from obtaining informed consents and review and approval by the institutional research committee of Zhongshan School of Medicine of Sun Yat-sen University because SEER research data is publicly available and all patient data are de-identified.
Data Availability: The data sets generated and/or analyzed during the current study are available in the SEER database (https://seer.cancer.gov/).
Supplemental Material: Supplemental material for this article is available online.
ORCID iDs
Sicong Du https://orcid.org/0000-0003-1214-9323
References
- 1.Zhang SL, Sun HT, Li ZM, et al. A real-world 1:1 propensity-matched study revealed unmarried status was independently associated with worse survival for patients with renal clear cell carcinoma. J Cancer. 2019;10(16):3767-3777. doi: 10.7150/jca.31744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.King SC, Pollack LA, Li J, King JB, Master VA. Continued increase in incidence of renal cell carcinoma, especially in young patients and high grade disease: United States 2001 to 2010. J Urol. 2014;191(6):1665-1670. doi: 10.1016/j.juro.2013.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Padala SA, Barsouk A, Thandra KC, et al. Epidemiology of Renal Cell Carcinoma. World J Oncol. 2020;11(3):79-87. doi: 10.14740/wjon1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F. International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol. 2015;67(3):519-530. doi: 10.1016/j.eururo.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Goebell PJ, Ivanyi P, Bedke J, et al. Consensus paper: current state of first- and second-line therapy in advanced clear-cell renal cell carcinoma. Future Oncol. 2020;16(29):2307-2328. doi: 10.2217/fon-2020-0403. [DOI] [PubMed] [Google Scholar]
- 6.Gill DM, Hahn AW, Hale P, Maughan BL. Overview of Current and Future First-Line Systemic Therapy for Metastatic Clear Cell Renal Cell Carcinoma. Curr Treat Options Oncol. 2018;19(1):6. doi: 10.1007/s11864-018-0517-1. [DOI] [PubMed] [Google Scholar]
- 7.Rizzo A, Mollica V, Santoni M, et al. Impact of Clinicopathological Features on Survival in Patients Treated with First-line Immune Checkpoint Inhibitors Plus Tyrosine Kinase Inhibitors for Renal Cell Carcinoma: A Meta-analysis of Randomized Clinical Trials. Eur Urol Focus. 2022;8(2):514-521. doi: 10.1016/j.euf.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Massari F, Rizzo A, Mollica V, et al. Immune-based combinations for the treatment of metastatic renal cell carcinoma: a meta-analysis of randomised clinical trials. Eur J Cancer. 2021;154:120-127. doi: 10.1016/j.ejca.2021.06.015. [DOI] [PubMed] [Google Scholar]
- 9.Mollica V, Santoni M, Matrana MR, et al. Concomitant Proton Pump Inhibitors and Outcome of Patients Treated with Nivolumab Alone or Plus Ipilimumab for Advanced Renal Cell Carcinoma. Targeted Oncol. 2022;17(1):61-68. doi: 10.1007/s11523-021-00861-y. [DOI] [PubMed] [Google Scholar]
- 10.Xiao WJ, Zhu Y, Dai B, Zhang HL, Ye DW. Assessment of survival of patients with metastatic clear cell renal cell carcinoma after radical cytoreductive nephrectomy versus no surgery: a seer analysis. Int Braz J Urol. 2015;41(2):288-295. doi: 10.1590/S1677-5538.IBJU.2015.02.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhanghuang C, Wang J, Zhang Z, et al. A Web-Based Prediction Model for Cancer-Specific Survival of Elderly Patients With Clear Cell Renal Cell Carcinoma: A Population-Based Study. Front Public Health. 2021;9:833970. doi: 10.3389/fpubh.2021.833970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feng X, Zhang LN, Tu WZ, Cang SD. Frequency, incidence and survival outcomes of clear cell renal cell carcinoma in the United States from 1973 to 2014: A SEER-based analysis. Med UK Ed. 2019;98(31):e16684. doi: 10.1097/MD.0000000000016684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhang G, Wu Y, Zhang J, et al. Nomograms for predicting long-term overall survival and disease-specific survival of patients with clear cell renal cell carcinoma. OncoTargets Ther. 2018;11:5535-5544. doi: 10.2147/OTT.S171881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zheng Y, Sun Y, Yang H, Liu J, Xing L, Sun Y. The role of income disparities on survival in metastatic clear cell renal cell carcinoma in the targeted therapy era. Eur J Health Econ. 2020;21(8):1223-1233. doi: 10.1007/s10198-020-01223-7. [DOI] [PubMed] [Google Scholar]
- 15.Jivanji D, Jamieson S, Mallory C, et al. The Association Between Race and 5-year Survival in Patients With Clear Cell Renal Cell Carcinoma: A Cohort Study. Urology. 2021;148:185-191. doi: 10.1016/j.urology.2020.10.055. [DOI] [PubMed] [Google Scholar]
- 16.Jiang X, Wang L, Cheng Y, Tang H, Chen T. Assessment of long-term survival of cancer patients using cancer registry data from eastern China: Period analysis is superior to traditional methods. Int J Cancer. 2020;147(4):996-1005. doi: 10.1002/ijc.32866. [DOI] [PubMed] [Google Scholar]
- 17.Holleczek B, Gondos A, Brenner H. periodR - an R package to calculate long-term cancer survival estimates using period analysis. Methods Inf Med. 2009;48(2):123-128. doi: 10.3414/ME0563. [DOI] [PubMed] [Google Scholar]
- 18.Brenner H, Hakulinen T. Up-to-date and precise estimates of cancer patient survival: model-based period analysis. Am J Epidemiol. 2006;164(7):689-696. doi: 10.1093/aje/kwj243. [DOI] [PubMed] [Google Scholar]
- 19.Li Y, Feng A, Zheng S, Chen C, Lyu J. Recent Estimates and Predictions of 5-Year Survival in Patients with Gastric Cancer: A Model-Based Period Analysis. Cancer Control. 2022;29:10732748221099227. doi: 10.1177/10732748221099227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shao Z, Zheng S, Chen C, Lyu J. Evaluation and Prediction Analysis of 3- and 5-Year Survival Rates of Patients with Cecal Adenocarcinoma Based on Period Analysis. Int J Gen Med. 2021;14:7317-7327. doi: 10.2147/IJGM.S334071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yang J, Li Y, Liu Q, et al. Brief introduction of medical database and data mining technology in big data era. J Evid Base Med. 2020;13(1):57-69. doi: 10.1111/jebm.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Krieger N, Chen JT, Waterman PD, Rehkopf DH, Subramanian SV. Race/ethnicity, gender, and monitoring socioeconomic gradients in health: a comparison of area-based socioeconomic measures--the public health disparities geocoding project. Am J Publ Health. 2003;93(10):1655-1671. doi: 10.2105/ajph.93.10.1655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Krieger N, Chen JT, Waterman PD, Soobader MJ, Subramanian SV, Carson R. Geocoding and monitoring of US socioeconomic inequalities in mortality and cancer incidence: does the choice of area-based measure and geographic level matter?: the Public Health Disparities Geocoding Project. Am J Epidemiol. 2002;156(5):471-482. doi: 10.1093/aje/kwf068. [DOI] [PubMed] [Google Scholar]
- 24.Wu WT, Li YJ, Feng AZ, et al. Data mining in clinical big data: the frequently used databases, steps, and methodological models. Mil Med Res. 2021;8(1):44. doi: 10.1186/s40779-021-00338-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siegel RL, Miller KD, Jemal A. Cancer statistics. CA A Cancer J Clin. 2018;68(1):7-30. doi: 10.3322/caac.21442. [DOI] [PubMed] [Google Scholar]
- 26.Tegos T, Tegos K, Dimitriadou A, Dimitriadis G. Current and emerging first-line systemic therapies in metastatic clear-cell renal cell carcinoma. J BUON. 2019;24(4):1340-1353. [PubMed] [Google Scholar]
- 27.Zhao J, Zhu Y, Zhang C, et al. Sorafenib or sunitinib as postoperative adjuvant therapy for Chinese patients with locally advanced clear cell renal cell carcinoma at high risk for disease recurrence. Urol Oncol. 2013;31(8):1800-1805. doi: 10.1016/j.urolonc.2012.04.019. [DOI] [PubMed] [Google Scholar]
- 28.Muselaers CH, Stillebroer AB, Desar IM, et al. Tyrosine kinase inhibitor sorafenib decreases 111In-girentuximab uptake in patients with clear cell renal cell carcinoma. J Nucl Med. 2014;55(2):242-247. doi: 10.2967/jnumed.113.131110. [DOI] [PubMed] [Google Scholar]
- 29.Pilskog M, Bostad L, Edelmann RJ, Akslen LA, Beisland C, Straume O. Tumour cell expression of interleukin 6 receptor alpha is associated with response rates in patients treated with sunitinib for metastatic clear cell renal cell carcinoma. J Pathol Clin Res. 2018;4(2):114-123. doi: 10.1002/cjp2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wei Y, Chen X, Ren X, et al. Identification of MX2 as a Novel Prognostic Biomarker for Sunitinib Resistance in Clear Cell Renal Cell Carcinoma. Front Genet. 2021;12:680369. doi: 10.3389/fgene.2021.680369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Armesto M, Marquez M, Arestin M, et al. Integrated mRNA and miRNA Transcriptomic Analyses Reveals Divergent Mechanisms of Sunitinib Resistance in Clear Cell Renal Cell Carcinoma (ccRCC). Cancers. 2021;13(17):4401. doi: 10.3390/cancers13174401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yue G, Deyu L, Lianyuan T, et al. Clinical features and prognostic factors of patients with metastatic renal cell carcinoma stratified by age. Aging (Albany NY). 2021;13(6):8290-8305. doi: 10.18632/aging.202637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aron M, Nguyen MM, Stein RJ, Gill IS. Impact of gender in renal cell carcinoma: an analysis of the SEER database. Eur Urol. 2008;54(1):133-140. doi: 10.1016/j.eururo.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 34.Eguchi T, Bains S, Lee MC, et al. Impact of Increasing Age on Cause-Specific Mortality and Morbidity in Patients With Stage I Non-Small-Cell Lung Cancer: A Competing Risks Analysis. J Clin Oncol. 2017;35(3):281-290. doi: 10.1200/JCO.2016.69.0834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fukushima H, Saito K, Yasuda Y, et al. Female Gender Predicts Favorable Prognosis in Patients With Non-metastatic Clear Cell Renal Cell Carcinoma Undergoing Curative Surgery: Results From the International Marker Consortium for Renal Cancer (INMARC). Clin Genitourin Cancer. 2020;18(2):111-116. doi: 10.1016/j.clgc.2019.10.027. [DOI] [PubMed] [Google Scholar]
- 36.Ricketts CJ, Linehan WM. Gender Specific Mutation Incidence and Survival Associations in Clear Cell Renal Cell Carcinoma (CCRCC). PLoS One. 2015;10(10):e0140257. doi: 10.1371/journal.pone.0140257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tsui KH, Shvarts O, Smith RB, Figlin R, de Kernion JB, Belldegrun A. Renal cell carcinoma: prognostic significance of incidentally detected tumors. J Urol. 2000;163(2):426-430. doi: 10.1016/s0022-5347(05)67892-5. [DOI] [PubMed] [Google Scholar]
- 38.Micheli A, Ciampichini R, Oberaigner W, et al. The advantage of women in cancer survival: an analysis of EUROCARE-4 data. Eur J Cancer. 2009;45(6):1017-1027. doi: 10.1016/j.ejca.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 39.Wang K, Sun Y, Tao W, Fei X, Chang C. Androgen receptor (AR) promotes clear cell renal cell carcinoma (ccRCC) migration and invasion via altering the circHIAT1/miR-195-5p/29a-3p/29c-3p/CDC42 signals. Cancer Lett. 2017;394:1-12. doi: 10.1016/j.canlet.2016.12.036. [DOI] [PubMed] [Google Scholar]
- 40.He D, Li L, Zhu G, et al. ASC-J9 suppresses renal cell carcinoma progression by targeting an androgen receptor-dependent HIF2alpha/VEGF signaling pathway. Cancer Res. 2014;74(16):4420-4430. doi: 10.1158/0008-5472.CAN-13-2681. [DOI] [PubMed] [Google Scholar]
- 41.Ha YS, Lee GT, Modi P, et al. Increased Expression of Androgen Receptor mRNA in Human Renal Cell Carcinoma Cells is Associated with Poor Prognosis in Patients with Localized Renal Cell Carcinoma. J Urol. 2015;194(5):1441-1448. doi: 10.1016/j.juro.2015.03.078. [DOI] [PubMed] [Google Scholar]
- 42.Yu CP, Ho JY, Huang YT, et al. Estrogen inhibits renal cell carcinoma cell progression through estrogen receptor-beta activation. PLoS One. 2013;8(2):e56667. doi: 10.1371/journal.pone.0056667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stone L. Kidney cancer: Androgen receptor--a new target in renal cell carcinoma? Nat Rev Urol. 2014;11(8):425. doi: 10.1038/nrurol.2014.159. [DOI] [PubMed] [Google Scholar]
- 44.Rose TL, Deal AM, Krishnan B, et al. Racial disparities in survival among patients with advanced renal cell carcinoma in the targeted therapy era. Cancer. 2016;122(19):2988-2995. doi: 10.1002/cncr.30146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krishnan B, Rose TL, Kardos J, Milowsky MI, Kim WY. Intrinsic Genomic Differences Between African American and White Patients With Clear Cell Renal Cell Carcinoma. JAMA Oncol. 2016;2(5):664-667. doi: 10.1001/jamaoncol.2016.0005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Freedman BI, Kopp JB, Langefeld CD, et al. The apolipoprotein L1 (APOL1) gene and nondiabetic nephropathy in African Americans. J Am Soc Nephrol. 2010;21(9):1422-1426. doi: 10.1681/ASN.2010070730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Paulucci DJ, Sfakianos JP, Skanderup AJ, et al. Genomic differences between black and white patients implicate a distinct immune response to papillary renal cell carcinoma. Oncotarget. 2017;8(3):5196-5205. doi: 10.18632/oncotarget.14122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zini L, Perrotte P, Capitanio U, et al. Race affects access to nephrectomy but not survival in renal cell carcinoma. BJU Int. 2009;103(7):889-893. doi: 10.1111/j.1464-410X.2008.08119.x. [DOI] [PubMed] [Google Scholar]
- 49.Appel LJ, Wright JT, Jr., Greene T, et al. Intensive blood-pressure control in hypertensive chronic kidney disease. N Engl J Med. 2010;363(10):918-929. doi: 10.1056/NEJMoa0910975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hofmann JN, Corley DA, Zhao WK, et al. Chronic kidney disease and risk of renal cell carcinoma: differences by race. Epidemiology. 2015;26(1):59-67. doi: 10.1097/EDE.0000000000000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Du XL, Fang S, Coker AL, et al. Racial disparity and socioeconomic status in association with survival in older men with local/regional stage prostate carcinoma: findings from a large community-based cohort. Cancer. 2006;106(6):1276-1285. doi: 10.1002/cncr.21732. [DOI] [PubMed] [Google Scholar]
- 52.Kumachev A, Trudeau ME, Chan KK. Associations among socioeconomic status, patterns of care and outcomes in breast cancer patients in a universal health care system: Ontario's experience. Cancer. 2016;122(6):893-898. doi: 10.1002/cncr.29838. [DOI] [PubMed] [Google Scholar]
- 53.Woods LM, Rachet B, Coleman MP. Origins of socio-economic inequalities in cancer survival: a review. Ann Oncol. 2006;17(1):5-19. doi: 10.1093/annonc/mdj007. [DOI] [PubMed] [Google Scholar]
- 54.Zhang SL, Zhang ZY, Liu ZJ, et al. A real-world study of socioeconomic factors with survival in adults aged 18-64 years with renal cell carcinoma. Future Oncol. 2019;15(21):2503-2515. doi: 10.2217/fon-2018-0827. [DOI] [PubMed] [Google Scholar]
- 55.Danzig MR, Weinberg AC, Ghandour RA, Kotamarti S, McKiernan JM, Badani KK. The association between socioeconomic status, renal cancer presentation, and survival in the United States: a survival, epidemiology, and end results analysis. Urology. 2014;84(3):583-589. doi: 10.1016/j.urology.2014.05.024. [DOI] [PubMed] [Google Scholar]
- 56.O'Connor JM, Sedghi T, Dhodapkar M, Kane MJ, Gross CP. Factors Associated With Cancer Disparities Among Low-Medium-and High-Income US Counties. JAMA Netw Open. 2018;1(6):e183146. doi: 10.1001/jamanetworkopen.2018.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mafolasire A, Yao X, Nawaf C, et al. Racial disparities in renal cell carcinoma: a single-payer healthcare experience. Cancer Med. 2016;5(8):2101-2108. doi: 10.1002/cam4.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lin J, Zahm SH, Shriver CD, Purdue M, McGlynn KA, Zhu K. Survival among Black and White patients with renal cell carcinoma in an equal-access health care system. CCC (Cancer Causes Control). 2015;26(7):1019-1026. doi: 10.1007/s10552-015-0594-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lipworth L, Tarone RE, McLaughlin JK. Renal cell cancer among African Americans: an epidemiologic review. BMC Cancer. 2011;11:133. doi: 10.1186/1471-2407-11-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sommers BD, Blendon RJ, Orav EJ, Epstein AM. Changes in Utilization and Health Among Low-Income Adults After Medicaid Expansion or Expanded Private Insurance. JAMA Intern Med. 2016;176(10):1501-1509. doi: 10.1001/jamainternmed.2016.4419. [DOI] [PubMed] [Google Scholar]
- 61.Betancourt JR, Renfrew MR. Unequal treatment in the US: lessons and recommendations for cancer care internationally. J Pediatr Hematol Oncol. 2011;33(suppl 2):S149-S153. doi: 10.1097/MPH.0b013e318230dfea. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Material for Analysis and Prediction of the Survival Trends of Patients with Clear-Cell Renal Cell Carcinoma: A Model-Based Period Analysis, 2001-2015 by Sicong Du, BD, Yu Zhong, MD, Shuai Zheng, MD, and Jun Lyu, PhD in Cancer Control.
Supplemental Material for Analysis and Prediction of the Survival Trends of Patients with Clear-Cell Renal Cell Carcinoma: A Model-Based Period Analysis, 2001-2015 by Sicong Du, BD, Yu Zhong, MD, Shuai Zheng, MD, and Jun Lyu, PhD in Cancer Control.
Supplemental Material for Analysis and Prediction of the Survival Trends of Patients with Clear-Cell Renal Cell Carcinoma: A Model-Based Period Analysis, 2001-2015 by Sicong Du, BD, Yu Zhong, MD, Shuai Zheng, MD, and Jun Lyu, PhD in Cancer Control.