Abstract
The wider application of gentamicin is limited by potential adverse effects (nephrotoxicity and ototoxicity). The goal of our study was to investigate the effects of chloroquine on biochemical and oxidative stress parameters in gentamicin-induced nephrotoxicity in rats. Animals were randomly divided into 1 of 5 groups. First was Sham group (0.9% NaCl) (n = 8); second group received gentamicin (n = 8); while third (n = 8), fourth (n = 8) and fifth group (n = 8) received gentamicin and chloroquine in a dose of 0.3, 1 and 3 mg/kg, respectively. The urea and creatinine levels were significantly lower in chloroquine treated groups in doses of 0.3 mg/kg and 1 mg/kg (P < 0.001). Total oxidant status and the oxidative stress index showed significantly lower values in all chloroquine treated groups (P < 0.001; P < 0.005). Malondialdehyde was lower in chloroquine treatment in doses of 0.3 mg/kg (P < 0.005) and 3 mg/kg (P < 0.05). Chloroquine treatment markedly reduced the level of superoxide dismutase in doses of 1 mg/kg (P < 0.01) and 3 mg/kg (P < 0.05). Our study showed that chloroquine attenuates gentamicin-induced nephrotoxicity in rats regarding biochemical and oxidative stress parameters.
Keywords: gentamicin-induced nephrotoxicity, chloroquine, oxidative stress, creatinine and urea, rats
Introduction
Gentamicin represents an aminoglycoside antibiotic which acts as a protein synthesis inhibitor through binding to the 30s subunit of bacterial ribosome. This bactericidal antibiotic is very effective against severe infections, especially Gram negative bacteria. However, its wider application in clinical practice is limited by its well known adverse effects.
In the first place, it is nephrotoxicity which, according to some recent data, occurs in almost third of patients who were exposed to gentamicin for more than a week. 1 Additionally, one study showed that even one dose of this antibiotic can lead to acute kidney injury. 2 Gentamicin-induced nephrotoxicity (GIN) represents complex entity with a still unclear pathogenesis. However, it is well known that this nephrotoxicity is dose-dependent3,4 and leads to functional and morphological changes in the kidney such as elevated blood urea and creatinine level in serum, declined glomerular filtration rate (GFR), edema, and acute injury in proximal tubules.5,6
Additionally, recent studies concerning this topic showed that inflammation, oxidative stress and massive production of free radicals provided the basis for development of GIN. 7 Such findings brought different agents with antioxidant activity in the focus of examination as potential therapy for GIN. 8
Some recent studies advocated that an old antimalarial drug - chloroquine may provide beneficial effects on renal function. The potential protective mechanisms in kidney include increase in urine flow rate, glomerular filtration rate, sodium excretion and stimulation of nitric oxide synthase. 9 In addition to the above, this antimalarial drug can affect both glomerular hemodynamics and tubular function. 10 Chloroquine and its derivate hydroxychloroquine -induced alterations in proton fluxes influence the type and chemical reactivity of reactive oxygen species (ROS). 11
Research on chloroquine and hydroxychloroquine has become particularly important in the last two years, in the era of the COVID-19 pandemic. Although this old and well-known antimalarial drug was originally used in protocols against COVID-19 infection, its toxic and adverse effects appear to outweigh its potential benefits. However, it is important to point out that numerous studies on this topic have initiated research on the pleiotropic effects of this agent and its possibilities for the treatment of many other diseases.
Although research on chloroquine is often contradictory, our previous research confirmed significant protection of renal ischemia-reperfusion injury in rats by single dose of chloroquine. 12
Also the importance of oxidative stress and the influence of pharmacological agents were examined in this experimental model in our previous research. 13
Oxidative stress is defined as an imbalance between the increased formation of pro-oxidants and depleted antioxidant mechanisms. When the redox balance becomes disrupted, potentially toxic reactive molecules can produce broad damage to the other biomolecules and cellular homeostasis. 14 Antioxidative defense is achieved by the synergistic action of enzymatic (superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx), glutathione reductase (GR), glutathione-S-transferase (GST) and glucose-6-phosphate dehydrogenase (G6PD)) and non-enzymatic (reduced glutathione, bilirubin, albumin, vitamins C and E, etc.) antioxidants. A significant number of different analytical methods are used for assessment of oxidative stress in biological samples. An insight into the redox status can be made by quantification of individual markers of prooxidation such as lipid peroxides (malondialdehyde-MDA), protein oxidation products (advanced oxidation protein products-AOPP) and markers of DNA damage, in combination with various enzymatic and non-enzymatic antioxidants. 15 The general extent of oxidation can be estimated by assaying total oxidant status (TOS), while total antioxidant status (TAS) considers the mutual effect of enzymatic and non-enzymatic antioxidants. The oxidative stress index (OSI) is derived from TOS and TAS values and it delivers a comprehensive insight into redox status, suggesting the presence or absence of oxidative stress.16,17
The goal of our study was to investigate the effects of chloroquine regarding some biochemical and oxidative stress parameters in an experimental model of gentamicin-induced nephrotoxicity in rats.
Material and Methods
Animals and Ethical Approval
Experiments were performed on adult, 2 months old, male Wistar rats (n = 40) weighing between 210 and 270 g. Before the beginning and during the experiments, animals were housed in polycarbonate cages (4 per cage). The living conditions were maintained constant at 12 h/12 h light/day cycle, a temperature of 24 ± 1°C, and a humidity of 55 ± 10%. The animals had ad libitum access to food (pellets of standard rodent diet) and water (tap water).
The methodology used in our investigation was approved by the Ethical Commission for the Welfare Protection of Experimental Animals (Medical Faculty, University of Belgrade, 2017– 2379/02). Guidelines from the European Convention for the Protection of Vertebrate Animals Used for Experimental and other Scientific Purposes, Directive 2010/63/EU for animal experiments, the Guide for the Care and Use of Laboratory Animals and Good Laboratory Practice were fully followed. 13
Experimental Groups
Animals were randomly divided into 1 of 5 groups. First was Sham group in which animals were given saline (0.9% NaCl) (n = 8); second group received just gentamicin (n = 8); while third (n = 8), fourth (n = 8) and fifth group (n = 8) received gentamicin and chloroquine in a dose of 0.3, 1 and 3 mg/kg, respectively.
Acute Kidney Damage Induced by Gentamicin
Commercially available gentamicin was used for induction of nephrotoxicity. Gentamicin was used in a 100 mg/kg dose and it was administrated by intraperitoneal route (i.p.). The administration was repeated every day during a period of 7 days. The process of administration was performed in the morning every day at the same time point. The complete methodology is described in our previous paper. 13
Sample Collection
Sample collection was undertaken on a first day post final gentamicine administration. Before sample collection, animals were anesthezied with an intraperitoneal bolus injection of sodium thiopentone (Thiopental®, Nycomed Pharma, Unterschleibheim, Germany) in a dose of 120 mg/kg. Animals were allowed to stabilize and to enter deep anesthesia before the samples were taken. The depth of anesthesia was investigated by pinching the rat tail and the space between the toes with tweezers or by poking these areas with a needle. The absence of responding to both these stimuli was considered as established deep anesthesia.
After establishing of deep anesthesia, the blood samples were obtained by heart puncture. The blood samples were kept for 2 h at the room temperature, in order to extract the serum and to allow samples to clot. After that, collected vessels were centrifuged for 15 minutes at 1000 X g, which led to the final serum separation. Samples were stored at −80°C for further use. After cardiac punition, post mortem, both kidneys were harvested for further investigation. 13
Tissue Preparation
Approximately 5 mm3 of kidney tissues volume was excised, placed in sterile tubes and stored at −80°C until further manipulation. Each tissue specimen was thawed in 500 μL of .5 mol/l sterile phosphate buffer (pH 7.5) and homogenized with a mechanical homogenizer (M-HOG-020, Labec, Marrickville, NSW, Australia) in sterile tubes, immersed in ice cold distilled water during the homogenization process. The homogenates were centrifuged at 8.161 × g for 10 minutes, and the supernatants were aliquoted and stored at −80°C for further analyses. 18
Total Protein Concentration
Total protein in tissue homogenates was determined by the Bradford spectrophotometric method, 19 using a Shimadzu, UV-2600 spectrophotometer (Shimadzu, Kyoto, Japan). The results were expressed as the mean mg proteins per ml of tissue homogenates.
Total Oxidant Status
TOS was determined according to the method of Erel. 16 The assay is based on the oxidation of ferrous to ferric ion in acidic medium in the presence of various oxidant species, and the detection of ferric ion by xylenol orange. Calibration and construction of a standard curve were performed using a hydrogen peroxide concentration gradient (0-100 μmol/l). The absorbance at 560 nm was measured on a spectrophotometer. The results were expressed as the mean micro molar equivalent of hydrogen peroxide per mg of proteins in tissue homogenates [μmol H2O2 Eq./mg protein].
Total Antioxidant Status
TAS was determined according to the method of Erel. 15 The method is based on decoloration of 2,2′-azinobis-(3-ethylbenzothiazoline-6-sulfonic acid radical cation [ABTS(*+)] by various antioxidants. Calibration and construction of standard curve were performed using a Trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid) concentration gradient (0-100 μmol/l). The absorbance at 660 nm was measured spectrophotometrically. The results were expressed as the mean micromolar equivalents of Trolox per mg of proteins tissue homogenates [μmol Trolox Eq./mg protein].
Oxidative Stress Index
OSI was defined as the ratio of TOS to TAS, and it was displayed by arbitrary unit = TOS [μmol H2O2 Eq./mg protein]/TAS [μmol Trolox Eq./mg protein]. 15
Lipid Peroxides
Lipid peroxides were determined according to the thiobarbituric acid reactive substances (TBARS) method, quantifying the total amount of malondialdehyde (MDA). 20 The pink colored chromogen’s absorbance formed by the reaction of 2-thiobarbituric acid with the breakdown products of lipid peroxidation was read at 535 nm. A standard curve constructed by MDA linear concentration gradient (0-100 μmol/l) was used for calibration. The results are expressed as the mean micromolar equivalents of MDA per mg of proteins in tissue homogenates [μmol/mg protein].
Advanced Oxidation Protein Products
The method of Witko-Sarsat was used to determine the AOPP concentration. 21 A linear concentration gradient (0-100 μmol/l) of chloramine-T formed the standard curve used for calibration. The results are expressed as the mean micromolar equivalents of chloramine-T per mg of proteins in tissue homogenates [μmol chloramine-T Eq./mg protein].
Superoxide Dismutase
Superoxide dismutase activity was estimated by using the method of Sun and Zigman, which is based on the measurement of the absorbance change during autooxidation of adrenalin into adrenochrome at 340 nm. 22 Commercial SOD was used as an enzyme standard for calculation of total SOD activity. The results are expressed in units of enzyme activity per mg of proteins in tissue homogenates [U/mg protein].
Catalase
CAT activity was determined by the method of Beers and Sizer. 23 The absorbance change during hydrogen peroxide breakdown by CAT was measured spectrophotometrically at 240 nm. Commercial CAT was used as an enzyme standard for evaluation of the total CAT activity. The results are expressed in units of enzyme activity per mg of proteins in tissue homogenates [U/mg protein].
Statistics
All biochemical measurements were run in duplicates and data were expressed as mean ± SD. Data distribution was examined using the Kolmogorov-Smirnov test. Evaluation of statistical significance was assessed by One-Way ANOVA test, supplemented by Bonferroni post hoc test for estimation of differences between biochemical parameters in different groups. Correlation analysis was performed with Pearson’s correlation test. For all analyses, P values at the level of 0.05 and less were considered statistically significant. All statistical analyses were performed using SPSS20.0 software package (IBM Corp., Armonk, NY).
Results
Biochemical markers of kidney function (urea, creatinine and Na+ level in animal serum)
The serum levels of urea were different among investigated animal groups. The highest concentrations were registered in the group of animals that received gentamicin. In groups that received chloroquine in lowest and medium dose (0.3 mg/kg and 1 mg/kg), urea levels were significantly reduced (Figure 1A, P < 0.001). The group of animals treated with gentamicin + chloroquine 3 mg/kg had a significantly higher urea level than a group of healthy, Sham animals and group treated with lowest dose of chloroquine (Figure 1A, P < 0.001). Additionally, there was statistically significant difference between the group treated with highest (3 mg/kg) and medium dose of chloroquine (1 mg/kg) (Figure 1A, P < 0.01).
Figure 1.
Biochemical markers of kidney function (urea, creatinine and Na+ level in animal serum). A-urea levels, B-creatinine levels, C-sodium levels. Sham group treated only with saline (0.9% NaCl). Gentamicin group treated only with gentamicin, while groups G + C0.3 mg/kg, G + C 1 mg/kg and G + C 3 mg/kg were treated with gentamicin and chloroquine in dose of 0.3, 1 and 3 mg/kg, respectively. Each bar represents mean ± SD.
Similarly to urea, creatinine was also increased in gentamicin group. The creatinine level was significantly lower in the groups that received chloroquine in lowest and medium dose (0.3 mg/kg and 1 mg/kg) (Figure 1B, P < 0.001). The creatinine values were significantly higher in a group of animals simultaneously treated with gentamicin and chloroquine in highest dose (3 mg/kg) when compared to the group group of healthy, Sham animals and group treated with lowest dose of chloroquine (Figure 1B, P < 0.001). Additionally, there was a statistically significant difference between the group treated with highest (3 mg/kg) and medium dose of chloroquine (1 mg/kg) (Figure 1B, P < 0.05).
The serum sodium level was unchanged and there were almost identical sodium levels between investigated groups (Figure 1C).
Oxidative Stress Markers
An insight into the oxidant/antioxidant events has been made by examination of overall and individual indicators of prooxidation and antioxidation processes. Figure 2. demonstrates the TOS level, together with MDA and AOPP concentration which represent biochemical markers of oxidative kidney damage in different experimental groups. It is clear that the gentamicin treated rats exhibited the highest levels of TOS, while the chloroquine treated rats (in all doses) had the significantly lower values of TOS, even lower than in Sham group of animals (Figure 2A, P < 0.001).
Figure 2.
Oxidative stress markers of prooxidation in kidney tissue of experimental animals. A-TOS levels; B-MDA amount; C-AOPP concentration. Sham group treated only with saline (.9% NaCl). Gentamicin group treated only with gentamicin, while groups G + C 0.3 mg/kg, G + C 1 mg/kg and G + C 3 mg/kg were treated with gentamicin and chloroquine in dose of 0.3, 1 and 3 mg/kg, respectively. Each bar represents mean ± SD.
Regarding the MDA concentration, rats treated with gentamicin had highest extent of lipid peroxidation, while chloroquine treatment in lowest and highest dose (0.3 and 3 mg/kg) markedly reduced the production of lipid peroxides (Figure 2B, P < 0.005 and P < 0.05, respectively). All experimental groups had similar AOPP concentration, showing no significant difference among them (Figure 2C, P > 0.05).
TAS levels were markedly elevated in the Sham control group, while other experimental groups did not differ significantly (Figure 3A), which suggests its outflow in response to oxidative stress caused by gentamicin treatment. The gentamicin treated animals had highest augmentation of SOD activity, when compared to the other groups. Chloroquine treatment in medium and highest dose (1 and 3 mg/kg) significantly reduced SOD activity compared to the gentamicin group (Figure 3B, P < 0.01 and P < 0.05, respectively). There is no apparent difference between groups in CAT activity (Figure 3C, P > 0.05).
FIgure 3.
Oxidative stress markers of antioxidative protection in kidney tissue of experimental animals. A-TAS levels; B-SOD activity; C-CAT activity. Sham group treated only with saline (.9% NaCl). Gentamicin group treated only with gentamicin, while groups G + C .3 mg/kg, G + C 1 mg/kg and G + C 3 mg/kg were treated with gentamicin and chloroquine in dose of .3, 1 and 3 mg/kg, respectively. Each bar represents mean ± SD.
The OSI showed that overall redox status is imbalanced and that the extensive renal oxidative stress exists in gentamicin treated animals (Figure 4). The same figure clearly shows that chloroquine reduced the oxidative stress in all 3 applied doses (Figure 4, P < 0.005) and brought it to the physiological level, since there was no significant difference between all treated and Sham group of animals (Figure 4, P > 0.05).
Figure 4.
OSI levels in kidney tissue of different experimental groups. Sham group treated only with saline (0.9% NaCl). Gentamicin group treated only with gentamicin, while groups G + C 0.3 mg/kg, G + C 1 mg/kg and G + C 3 mg/kg were treated with gentamicin and chloroquine in dose of 0.3, 1 and 3 mg/kg, respectively. Each bar represents mean ± SD.
Multiple significant correlations with strong association between TOS and MDA in gentamicin treated group (r = 0.921, P < 0.01) and chloroquine treated groups were noticed in all applied doses - 0.3, 1 and 3 mg/kg (r = 0.986, P < 0.01; r = 0.873, P < 0.05; r = 0.982, P < 0.05, respectively) (Figure 5).
Figure 5.
Correlation between TOS level and MDA amount in different experimental groups. A-animals treated with 0.3 mg/kg of chloroquine; B-animals treated with 1 mg/kg of chloroquine; C-animals treated with 3 mg/kg of chloroquine; D-animals treated only with gentamicin.
Although the overall AOPP levels are not increased significantly in any group, there is a strong positive correlation between its concentration and TOS levels in gentamicin treated rats (r=0.961, P < 0.01) (Figure 6A). In the same group, the marked negative correlation between TAS and AOPP was showed (r=0.964, P < 0.01 ) (Figure 6B).
Figure 6.
Correlation between TOS level and AOPP amount (A) and TAS level and AOPP amount (B) in gentamicin treated animals.
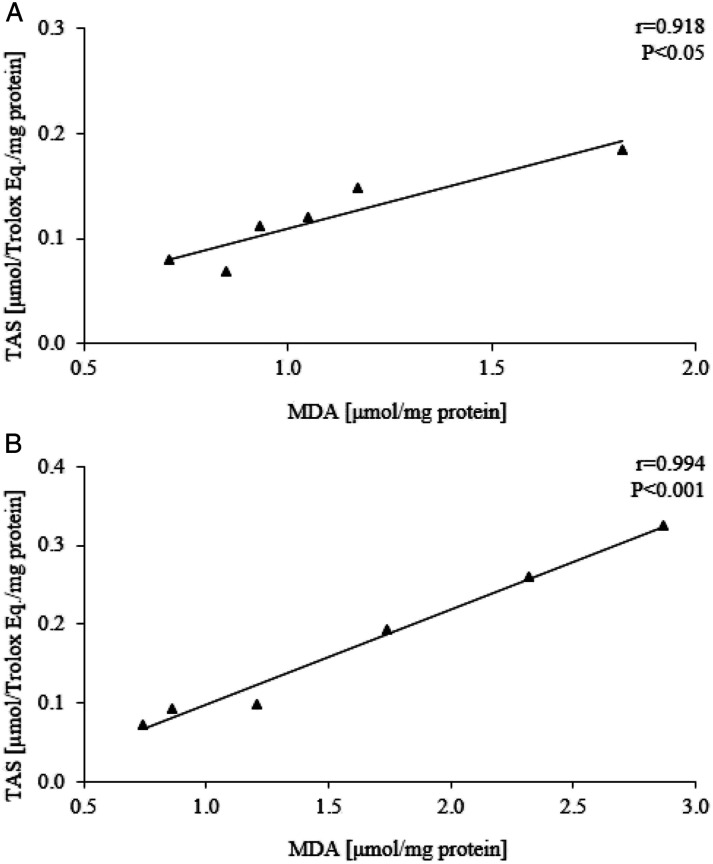
A chloroquine treated animals with 0.3 and 1 mg/kg establish a competent TAS response to lipid peroxidation, which is evidenced by significant positive correlations presented in Figure 7. Since these strong associations are not present in Sham control, they implicate a corrective effect of chloroquine on cellular redox homeostasis.
Figure 7.
Correlations between TAS level and MDA concentration in group of animals treated with 0.3 mg/kg (A) and 1 mg/kg (B) of chloroquine.
Discussion
Our study confirmed that the administration of gentamicin at the dose of 100 mg/kg/day for 7 days i.p., successfully induced nephrotoxicity in all treated animals. This finding is in agreement with some previous reports.13,24,25
Additionally, gentamicin-treated rats produced an increase in parameters of renal oxidative stress (TOS, OSI, MDA and SOD activity). Some earlier reports showed that overproduction of ROS represents the most important pathways in the process of gentamicin-induced nephrotoxicity.
Although the pathogenesis of this clinical entity is still unclear, oxidative stress is considered to be linked with depletion of proximal renal tubules’ antioxidant potential which causes lipid peroxidation, protein oxidation, apoptosis induction and cause tubular damages.26,27
Additionally, ROS play a crucial role in the progression of inflammation via NF-κB (Nuclear factor kappa B) activation which further enhance synthesis of inducible nitric oxide synthase (iNOS), cytokines - TNF-α (Tumor necrosis factor – alpha) and IL-6 (interleukin 6) and other proinflammatory markers. 28
Some recent evidence suggests that oxidative stress in gentamicin-induced nephrotoxicity happens by apoptosis through mitochondrial dysfunction. 29 Several studies showed that gentamicin amplifies the generation of ROS in the kidney.
This is the reason why many substances with antioxidant properties have been tested in previous years in this model.30-32
The effects of chloroquine on gentamicin-induced nephrotoxicity in our experimental model showed some different effects in relation to the administered dose. For example, the lowest dose of applied chloroquine provided the most significant protection regarding biochemical markers of kidney damage. In the group where the highest dose of chloroquine was applied, the urea and creatinine levels were slightly lower than those in the gentamicin group, but still significantly higher than in the Sham group.
Additionally, significant protection of kidney damage in this experimental model was shown in all chloroquine-treated groups concerning some oxidative stress parameters that indicate the overall extent of oxidation and comprehensive insight into redox status (TOS, TAS and OSI). All chloroquine treated animals with 0.3 and 1 mg/kg establish a competent TAS response to lipid peroxidation, which is evidenced by significant positive correlations presented in Figure 7. Since these strong associations are not present in Sham control, they implicate a corrective effect of chloroquine on cellular redox homeostasis. Beside this, MDA as a very sensitive, individual marker of prooxidation showed protective effects in this experimental model in lowest (0.3 mg/kg) and highest dose (3 mg/kg), while SOD, as very important antioxidant defense marker displayed beneficial effects on kidney injury in medium and highest dose (1 mg/kg and 3 mg/kg). On the other hand, the use of chloroquine did not have any significant effect on CAT and AOPP values.
Our results suggest that the effects of chloroquine could be dose-dependent in the experimental model of gentamicin-induced nephrotoxicity. This finding is consistent with some of our previous research concerning the effects of chloroquine on kidney damage. 12
Chloroquine, as an old antimalarial drug possesses numerous pleiotropic effects suh as inhibition of autophagy, lysosomal protein degradation and macrophage activation, decreases the level of proinflammatory cytokines and other mediators. 33
Also, some recent research suggests the importance of chloroquine in reducing oxidative stress in kidney pathology. Mo Kang et al. referred that chloroquine can induce AMP-activated protein kinase (AMPK) -α phosphorylation and improved mitochondrial fragmentation which may be very important in development in oxidative stress. Additionally, they explained that this antimalarial drug declined inflammatory markers, such as NF-κB, IL-6, and TNF-α, reduced mitochondrial ROS production and decreased endoplasmic reticulum (ER) stress. 34
However, despite being effective in range of diseases, clinical use of chloroquine was always under scrutiny because it has narrow safety margin and showed wide range of adverse effects including cardiac and neurological disorders, retinopathy and ototoxicity. For example, several studies advocate that hepatotoxicity caused by chloroquine is mainly due to its oxidative potential.35-37
Also, there are studies that emphasize the role of chloroquine as a prooxidative agent in kidney. Giovanella et al. determined that this drug may promote oxidative stress by decreasing non-enzymatic and enzymatic antioxidant defenses, changing the redox state and stimulating oxidative damage to DNA in brain and kidney of rats. 38 However, it should be emphasized that much higher doses of chloroquine have been examined in these studies ranging from human therapeutic equivalent of 360 mg/kg body weight to as high as 2000 mg/kg body weight. Chloroquine appears to exhibit many adverse effects at these doses, including triggering oxidative stress. It was recently published that chloroquine induces endothelial injury through lysosomal dysfunction and oxidative stress. These findings also may contribute to the failure of chloroquine as therapy for COVID-19. 39
However, our study showed for the first time that chloroquine (in low doses) possesses protective effects on biochemical and oxidative stress parameters in an experimental model of gentamicin-induced nephrotoxicity.
Future experiments should include a wider range of doses of chloroquine and its examination on some more specific markers of inflammation (such as NF-κB, TNF-α, IL-1, IL-6 etc.), some other markers of oxidative stress as well as novel markers of kidney injury - KIM-1 (Kidney injury molecule–1), NGAL (Neutrophil gelatinase-associated lipocalin) etc. in order to provide more accurate explanation of nephroprotective effects of chloroquine in this experimental model. Also, it could be important to examine the effects of hydroxychloroquine in this experimental model of gentamicin-induced nephrotoxicity, as a less toxic derivative of chloroquine. Such and similar studies could have clinical significance in reducing the adverse effects of gentamicin therapy.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Ministry of Education, Science and Technological Development of the Republic of Serbia (Project No. 175023).
ORCID iDs
Branislava Medic Brkic https://orcid.org/0000-0001-7012-6766
Dragana Srebro https://orcid.org/0000-0002-9680-0840
Katarina Savic Vujovic https://orcid.org/0000-0002-4701-6291
References
- 1.Al-Kuraishy HM, Al-Gareeb AI, Al-Naimi MS. Renoprotective effect of irbesartan in a rat model of gentamicin-induced nephrotoxicity: Role of oxidative stress. J Lab Physicians. 2019;11(3):200-205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Azzam SI, Abdul-Razzak KK, Jarada MW. The nephroprotective effects of pioglitazone and glibenclamide against gentamicin-induced nephrotoxicity in rats: A comparative study. J Chemother. 2010;22(2):88–91. [DOI] [PubMed] [Google Scholar]
- 3.Balakumar P, Chakkarwar V, Kumar V, Jain A, Reddy J, Singh M. Experimental models for nephropathy. Journal of the Renin-Angiotensin-Aldosterone System. 2008;9(4): 189–195. [DOI] [PubMed] [Google Scholar]
- 4.Tavafi M, Ahmadvand H. Effect of rosmarinic acid on inhibition of gentamicin induced nephrotoxicity in rats. Tissue Cell. 2011;43(6): 392–397. [DOI] [PubMed] [Google Scholar]
- 5.Rodrigues F, Prata M, Oliveira I. Gingerol fraction from Zingiber officinale protects against gentamicin-induced nephrotoxicity. Antimicrob Agents Chemother. 2014;58(4): 1872–1878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moreira M, Nascimento M, Bozzo T. Ascorbic acid reduces gentamicin-induced nephrotoxicity in rats through the control of reactive oxygen species. Clinical Nutrition. 2014;33(2): 296–301. [DOI] [PubMed] [Google Scholar]
- 7.Al-Kuraishy HM, Al-Gareeb AL, Rasheed HA. Antioxidant and anti-inflammatory effects of curcumin contribute into attenuation of acute gentamicin-induced nephrotoxicity in rats. Asian J Pharm Clin Res. 2019;12:466–468. [Google Scholar]
- 8.Talebi A, Karimi A, Ouguerram K, et al. Lack of nephroprotective efficacy of Althaea officinalis flower extract against gentamicin renal toxicity in male rats. Int J Prev Med. 2014;5:1360–1363. [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed MH, Ashton N, Balment RJ. The effect of chloroquine on renal function and vasopressin secretion: a nitric oxide-dependent effect. J Pharmacol Exp Ther. 2003;304:156‐161. [DOI] [PubMed] [Google Scholar]
- 10.Ahmed MH, Ashton N, Balment RJ. Renal function in a rat model of analgesic nephropathy: effect of chloroquine. J Pharmacol Exp Ther. 2003;305:123–130. [DOI] [PubMed] [Google Scholar]
- 11.Klouda CB, Stone WL. Oxidative Stress, Proton Fluxes, and Chloroquine/Hydroxychloroquine Treatment for COVID-19. Antioxidants. 2020;21(9):894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Todorovic Z, Medic B, Basta-Jovanovic G, Radojevic Skodric S, Stojanovic R, Rovcanin B, et al. Acute pretreatment with chloroquine attenuates renal I/R injury in rats. PLoS One. 2014;9(3):e92673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Medić B, Stojanović M, Rovčanin B, et al. Pioglitazone attenuates kidney injury in an experimental model of gentamicin-induced nephrotoxicity in rats. Sci Rep. 2019;9(1):13689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rovcanin B, Medic B, Kocic G, Cebovic T, Ristic M, Prostran M. Molecular dissection of renal ischemia-reperfusion: Oxidative stress and cellular events. Curr Med Chem 2016;23:1965-1980. [DOI] [PubMed] [Google Scholar]
- 15.Halliwell B, Whiteman M. Measuring reactive species and oxidative damage in vivo and in cell culture: how should you do it and what do the results mean? Br J Pharmacol. 2004;142:231-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin Biochem. 2005;38:1103-1111. [DOI] [PubMed] [Google Scholar]
- 17.Erel O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin Biochem. 2004;37:277-285. [DOI] [PubMed] [Google Scholar]
- 18.Pecinová A, Drahota Z, Nůsková H, Pecina P, Houštěk J. Evaluation of basic mitochondrial functions using rat tissue homogenates. Mitochondrion. 2011;11:722-728. [DOI] [PubMed] [Google Scholar]
- 19.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248-254. [DOI] [PubMed] [Google Scholar]
- 20.Varshney R, Kale RK. Effects of calmodulin antagonists on radiation-induced lipid peroxidation in microsomes. Int J Radiat Biol. 1990;58:733-743. [DOI] [PubMed] [Google Scholar]
- 21.Witko-Sarsat V, Friedlander M, Capeillere-Blandin C, et al. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304-1313. [DOI] [PubMed] [Google Scholar]
- 22.Sun M, Zigman S. Determination of superoxide dismutase in erythrocytes using the method of adrenaline autooxidation. Anal Biochem 1978;90:81-89. [DOI] [PubMed] [Google Scholar]
- 23.Beers RF, Sizer IW. A spectrophotometric method for measuring the breakdown of hydrogen peroxide by catalase. J Biol Chem. 1952;195:133-140. [PubMed] [Google Scholar]
- 24.Babaeenezhad E, Hadipour Moradi F, Rahimi Monfared S, et al. D-Limonene Alleviates Acute Kidney Injury Following Gentamicin Administration in Rats: Role of NF-κB Pathway, Mitochondrial Apoptosis, Oxidative Stress, and PCNA. Oxid Med Cell Longev. 2021;2021:6670007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abd-Elhamid T, Elgamal D, Ali S. Reno-protective effects of ursodeoxycholic acid against gentamicin-induced nephrotoxicity through modulation of NF-κB, eNOS and caspase-3 expressions. Cell Tissue Res. 2018;374(2):367‐387. [DOI] [PubMed] [Google Scholar]
- 26.Al-Kuraishy HM, Al-Gareeb AI, Al-Maiahy TJ. Concept and connotation of oxidative stress in preeclampsia. J Laboratory Physicians. 2018;10:276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Al-Kuraishy HM, Al-Gareeb AI, Al-Nami MS. Irbesartan attenuates gentamicin-induced nephrotoxicity in rats through modulation of oxidative stress and endogenous antioxidant capacity. Int J Prev Med. 2020;11:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lopez-Novoa J, Quiros Y, Vicente L, Morales A, Lopez-Hernandez F. New insights into the mechanism of aminoglycoside nephrotoxicity: an integrative point of view. Kidney Int. 2011;79(1):33‐45. [DOI] [PubMed] [Google Scholar]
- 29.Vysakh A, Abhilash S, Kuriakose J, Midhun S, Jyothis M, Latha M. Protective effect of Rotula aquatica Lour against gentamicin induced oxidative stress and nephrotoxicity in Wistar rats. Biomed Pharmacother. 2018;106:1188‐1194. [DOI] [PubMed] [Google Scholar]
- 30.Abdelrahman R. Protective effect of apocynin against gentamicin-induced nephrotoxicity in rats. Hum Exp Toxicol. 2017;37(1):27‐37. [DOI] [PubMed] [Google Scholar]
- 31.Beshay O, Ewees M, Abdel-Bakky M, Hafez S, Abdelrehim A, Bayoumi A. Resveratrol reduces gentamicin-induced EMT in the kidney via inhibition of reactive oxygen species and involving TGF-β/Smad pathway. Life Sci. 2020;258:118178. [DOI] [PubMed] [Google Scholar]
- 32.Erseçkin V, Mert H, İrak K, Yildirim S, Mert N. Nephroprotective effect of ferulic acid on gentamicin-induced nephrotoxicity in female rats. Drug Chem Toxicol. 2020;43:1‐7. [DOI] [PubMed] [Google Scholar]
- 33.Ouyang Q, Huang Z, Wang Z, Chen X, Ni J, Lin L. Effects of pristane alone or combined with chloroquine on macrophage activation, oxidative stress, and TH1/TH2 skewness. J Immunol Res. 2014;2014:613136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kang JM, Lee HS, Kim J, et al. Beneficial effect of chloroquine and amodiaquine on type 1 diabetic tubulopathy by attenuating mitochondrial Nox4 and endoplasmic reticulum stress. J Korean Med Sci. 2020;35(36):e305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pari L, Murugavel P. Protective effect of α-lipoic acid against chloroquine-induced hepatotoxicity in rats. J Appl Toxicol. 2004;24(1):21‐26. [DOI] [PubMed] [Google Scholar]
- 36.Farombi EO, Shyntum YY, Emerole GO. Influence of chloroquine treatment and Plasmodium falciparum malaria infection on some enzymatic and non-enzymatic antioxidant defense indices in humans. Drug Chem Toxicol. 2003;26(1):59‐71. [DOI] [PubMed] [Google Scholar]
- 37.Kumar Mishra S, Singh P, Rath SK. Protective effect of quercetin on chloroquine-induced oxidative stress and hepatotoxicity in mice. Malar Res Treat. 2013;2013:141734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giovanella F, Ferreira GK, de Prá SD, et al. Effects of primaquine and chloroquine on oxidative stress parameters in rats. An Acad Bras Cienc. 2015;87(2):1487-1496. [DOI] [PubMed] [Google Scholar]
- 39.Gregório P, da Cunha RS, Biagini G, et al. Chloroquine may induce endothelial injury through lysosomal dysfunction and oxidative stress. Toxicol Appl Pharmacol. 2021;414:115412. [DOI] [PMC free article] [PubMed] [Google Scholar]