Abstract
The VirE2 single-stranded DNA-binding protein (SSB) of Agrobacterium tumefaciens is required for delivery of T-DNA to the nuclei of susceptible plant cells. By yeast two-hybrid and immunoprecipitation analyses, VirE2 was shown to self-associate and to interact with VirE1. VirE2 mutants with small deletions or insertions of a 31-residue oligopeptide (i31) at the N or C terminus or with an i31 peptide insertion at Leu236 retained the capacity to form homomultimers. By contrast, VirE2 mutants with modifications outside a central region located between residues 320 and 390 retained the capacity to interact with VirE1. These findings suggest the tertiary structure of VirE2 is important for homomultimer formation whereas a central domain mediates formation of a complex with VirE1. The capacity of VirE2 mutants to interact with full-length VirE2 in the yeast Saccharomyces cerevisiae correlated with the abundance of the mutant proteins in A. tumefaciens, suggesting that VirE2 is stabilized by homomultimerization in the bacterium. We further characterized the promoter and N- and C-terminal sequence requirements for synthesis of functional VirE2. A PvirB::virE2 construct yielded functional VirE2 protein as defined by complementation of a virE2 null mutation. By contrast, PvirE or Plac promoter constructs yielded functional VirE2 only if virE1 was coexpressed with virE2. Deletion of 10 or 9 residues from the N or C terminus of VirE2, respectively, or addition of heterologous peptides or proteins to either terminus resulted in a loss of protein function. However, an i31 peptide insertion at Tyr39 had no effect on protein function as defined by the capacity of the mutant protein to (i) interact with native VirE2, (ii) interact with VirE1, (iii) accumulate at abundant levels in A. tumefaciens, and (iv) restore wild-type virulence to a virE2 null mutant. We propose that Tyr39 of VirE2 corresponds to a permissive site for insertion of heterologous peptides or proteins of interest for delivery across kingdom boundaries.
A number of bacterial pathogens have evolved dedicated transport machinery for delivering effector macromolecules to the cytosols of plant and mammalian host cells during infection. One family of transporters, the contact-dependent or type III secretion systems, appears to have evolved by appropriation of a flagellar basal body structure for secretion of protein effectors to eukaryotic cells (1, 30). A second family, here designated as the adapted-conjugation or type IV secretion systems, likely evolved from an ancestral DNA conjugation apparatus (11, 13, 55). Members of the type III secretion systems export effector proteins to target cells (1, 30, 48). Members of the adapted-conjugation secretion family export a variety of substrates, including multisubunit toxin, as in the case of the Bordetella pertussis Ptl system, and nucleoprotein particles, as in the case of the Agrobacterium tumefaciens T-DNA transport system (11, 13, 55). Two notable mechanistic themes have emerged among representatives of the types III and IV secretion systems, the capacity to deliver substrates across kingdom boundaries by way of a presumed transenvelope channel and the requirement for the infecting bacterium to establish direct physical contact with target cells. Precisely how these systems establish productive contact with eukaryotic cells and how these systems recognize and export substrates across the bacterial cell envelope are subjects of intensive investigation in many laboratories.
The A. tumefaciens T-DNA transporter is an especially interesting member of the adapted-conjugation family because of its extreme promiscuity with respect to both the types of substrates it exports and the range of cell types to which it can deliver these substrates (13, 21). During infection of plants, A. tumefaciens uses the T-DNA transporter to deliver a single-stranded (ss) form of T-DNA termed the T strand in association with one or more proteins to plant nuclei (60). The T-DNA transporter also can export the non-self-transmissible IncQ plasmid RSF1010 to plants, most probably also as a ssDNA-protein transfer intermediate (8, 51). Results of genetic studies suggest that in addition to these nucleoprotein substrates, the T-DNA transporter can export two protein substrates, the VirE2 ssDNA-binding protein (SSB) and VirF, independently of any association with T-DNA (7, 14, 45, 52). A. tumefaciens transfers T-DNA to a wide variety of dicotyledenous and monocotyledenous plant species (31, 44). Recent studies also have shown that A. tumefaciens can deliver DNA to the yeast Saccharomyces cerevisiae (9, 10, 43) and a number of filamentous fungi (21). A. tumefaciens also can use the T-DNA transporter to conjugally deliver the IncQ plasmid RSF1010 to agrobacterial recipients (5, 26). This extremely wide host range has prompted speculation that recipient cells are completely passive entities with respect to this DNA transfer event.
We have begun to explore the sequence and protein interaction requirements for export of one of the protein substrates, the ∼60-kDa VirE2 SSB. Previous work has identified several proteins with which VirE2 potentially interacts in A. tumefaciens. First, DNA-binding studies have shown that VirE2 binds highly cooperatively to ssDNA, a property consistent with the prediction that this SSB self-associates (16, 47). Second, the reported stabilizing effects of VirE1 on VirE2 in Escherichia coli (39) and A. tumefaciens (22) and recent genetic evidence indicating that VirE1 is required for VirE2 export from A. tumefaciens (52) suggest that these two proteins interact. Third, both VirE2 and VirD2, the endonuclease responsible for processing the T-DNA from its position on the Ti plasmid, associate with T-DNA (14, 57), and both are exported to plant cells, where nuclear localization sequences (NLSs) present in both proteins are thought to mediate the delivery of the T-DNA to plant nuclei (17, 29). These proteins potentially interact in the context of the VirD2-T-strand–VirE2 nucleoprotein particle, the so-called T complex (60). Finally, VirE2 is dependent on VirB proteins for export (7), suggesting that at some point during the transfer process VirE2 interacts with one or more of these transporter subunits. In this study, we have probed for VirE2 interactions with VirB, VirD, and VirE proteins. We present yeast two-hybrid data indicating VirE2 self-association and interaction with VirE1, and we present biochemical evidence that these interactions occur in A. tumefaciens cells. We have further defined the VirE2 sequence requirements for self-association and interaction with VirE1 by analysis of a large collection of VirE2 insertion and deletion mutants. Our findings support a model depicting VirE2 self-association and interaction with VirE1 as intermediate steps in the export pathway.
MATERIALS AND METHODS
Bacterial and yeast strains.
E. coli DH5α was used for plasmid constructions, and E. coli CC118 (37) was used for transposon mutagenesis. A. tumefaciens A348 is A136 with the octopine-type pTiA6NC plasmid (28). A. tumefaciens LBA4404 is a T-DNA deletion derivative (41). PC1000 is A348 lacking the virB operon (24), KE1 is A348 with a Kanr cassette in place of the virE operon (39), and At12516 is A348 with a Spcr cassette in place of the virE2 gene (27). S. cerevisiae Y190 containing a GAL4-responsive lacZ gene was used for the two-hybrid interaction studies (2).
Bacterial growth and vir gene induction.
Conditions for growth of E. coli and A. tumefaciens have been previously described (25). For analysis of Vir protein content, cells were grown to an optical density at 600 nm (OD600) of 0.5 in MG/L medium (49) with antibiotic selection. A 1-ml culture was pelleted and resuspended at an OD600 of 0.2 in induction medium with 200 μM acetosyringone (AS) (24). Cells were incubated with shaking at 23°C for 18 h to induce vir gene expression. Plasmids were maintained in E. coli and A. tumefaciens by the addition of carbenicillin (50 μg/ml), kanamycin (50 μg/ml), or tetracycline (5 μg/ml) to the growth medium.
Construction of virE1 and virE2 expression plasmids.
Details of plasmid constructions are provided below, and relevant characteristics of plasmids are summarized in Table 1. The wild-type virE2 gene was engineered to carry an NdeI restriction site at its initiation codon as follows. Plasmid pPC705, expressing virE1 and virE2 from Plac, was constructed by introducing an NdeI fragment from pSW108 (54) into pBCSK+.NdeI (6). Plasmid pPC706 is pPC705 with a Kanr gene from pUC4K (Pharmacia) inserted as a HincII fragment into the unique ScaI site. pPC706 and other ColE1 plasmids described in this paper were introduced into A. tumefaciens by ligation to the IncP plasmid pSW172 (12) or the Kanr derivative pXZ151 (pSW172 with the Kanr gene from pUC4K inserted at the unique StuI site), in which case a B (for broad host range) is added to the ColE1 plasmid name, i.e., pPCB706. Plasmid pPC714 is pPC705 with an NdeI restriction site at the virE2 translational start site introduced by oligonucleotide-directed mutagenesis (32) using the oligonucleotide VirE2.NdeI GAAAGATCCATATGCTCACTCC (the NdeI site is underlined). Plasmid pPC725, which expresses virE2 directly downstream of Plac, was created by digestion of pPC714 with SmaI and religation to remove the NdeI site downstream of virE2. The virE1 coding sequence then was removed by NdeI digestion at the engineered NdeI site at the 5′ end of virE2 and at a natural NdeI site at the 5′ end of virE1 followed by plasmid religation. The entire virE2 gene on pPC725 was sequenced to ensure that no other changes were introduced during oligonucleotide-directed mutagenesis. Plasmid pXZ46 expressing virE2 from Plac was constructed by introducing the 1.65-kb NdeI-XhoI fragment from pPC725 into pBSIISK+.NdeI (6). Plasmid pXZ27 expressing virE2 from PvirB was constructed by substituting a 1.6-kb NdeI-XhoI fragment carrying virE2 from pPC725 for a ∼1.5-kb NdeI-XhoI fragment carrying virB1 in pPC914KS+ (6). Plasmid pPC732, expressing virE2 directly downstream of PvirE, was constructed by substituting the 1.6-kb NdeI-XhoI fragment from pPC725 for a ∼2.2-kb NdeI-XhoI fragment in pPC731. Plasmid pPC731, coexpressing virE1 and virE2 from PvirE, was constructed by introducing a ∼2.8-kb XbaI-SmaI fragment from pSW108 into pBSIIKS+ (Stratagene) such that PvirE was oriented opposite Plac. Plasmid pXZ237, coexpressing virE1 and virE2 from Plac, was constructed by introducing a ∼2.2-kb NdeI-XhoI fragment from pPC731 into pBSIIKS+.NdeI (6). Plasmids pXZ235 and pXZ236 were constructed by introducing a frameshift mutation and translation termination codon near the 5′ end of virE1 by SphI digestion, T4 DNA polymerase treatment to make blunt ends, and religation of pPC731 and pXZ237, respectively. These plasmids contain both the virE1 and virE2 coding sequences but synthesize only 6 residues of VirE1 and all of VirE2. Plasmid pXZ425, an IncP plasmid expressing virE2 from PvirB, was constructed by introducing a ∼2.7-kb BamHI-XhoI fragment from pXZ27 into similarly digested pSW172.
TABLE 1.
Plasmids used in this studya
| Plasmid | Relevant characteristics | Source or reference |
|---|---|---|
| Vectors | ||
| pACT2.2 | GAL4AD plasmid | 2 |
| pAS2 | GAL4BD plasmid | 2 |
| pSW172 | Tetr broad-host-range IncP cloning vector | 12 |
| pXZ151 | Kanr pSW172 derivative | This study |
| GAL4 expression constructs | ||
| pXZaE1 | pACT2.2 with virE1 gene | This study |
| pXZaE2 | pACT2.2 with virE2 gene | This study |
| pXZbE1 | pAS2 with virE1 gene | This study |
| pXZbE2 | pAS2 with virE2 gene | This study |
| pSE1111 | pACT2.2 with SNF4 | 2 |
| pSE1112 | pAS2 with SNF1 | 2 |
| pXZbE201 to pXZbE212 | pAS2 with virE2 truncations fused to GAL4BD | This study |
| pXZaE201 to pXZaE212 | pACT2.2 with virE2 truncations fused to GAL4AD | This study |
| pXZ741 to pXZ750 | pACT2.2 with virE2::i31 alleles fused to GAL4AD | This study |
| pXZ751 to pXZ760 | pAS2 with virE2::i31 alleles fused to GAL4BD | This study |
| virE1 and virE2 expression constructs | ||
| pPC725 | pBCSK+.NdeI with virE2; NdeI site at 5′ end | This study |
| pXZ46 | pBSIISK+.NdeI with virE2 expressed from Plac | This study |
| pXZ27 | pBSIIKS+ with virE2 expressed from PvirB | This study |
| pPC731 | pBSIIKS+.NdeI with virE2 expressed from PvirE | This study |
| pXZ425 | pSW172 with virE2 expressed from PvirB | This study |
| pXZ237 | pBSIIKS+.NdeI with virE1 and virE2 expressed from Plac | This study |
| pPC732 | pBSIIKS+.NdeI with virE1 and virE2 coexpressed from PvirE | This study |
| pXZ235 | pPC731 with virE1 containing a translational stop signal at codon 6 and virE2 expressed from PvirE | This study |
| pXZ236 | pXZ237 with virE1 containing a translational stop signal at codon 6 and virE2 expressed from Plac | This study |
| virE2 insertion and deletion plasmids | ||
| pXZ201 to pXZ212 | pBSIIKS+ with virE2 truncations expressed from PvirB | This study |
| pXZ721 to pXZ730 | pBSIIKS+.NdeI with virE2::i31 alleles expressed from Plac | This study |
| pXZ761 to pXZ770 | pBSIIKS+ with virE2::i31 alleles expressed from PvirB | This study |
| pXZ731 | pBSIISK+.NdeI with an out-of-frame i31 insertion at codon 11 of virE2 | This study |
| pXZ738 | pBSIISK+.NdeI with an out-of-frame i31 insertion at codon 468 of virE2 | This study |
| pXZ427 | pXZ425/pXZ761 cointegrate with virE2 and virE2.39::i31 expressed from independent PvirB promoters | This study |
| Hybrid protein constructs | ||
| pXZ234 | pBSIIKS+ with his-virE2 expressed from Plac | This study |
| pXZ238 | pBSIIKS+ with GAL4AD::virE2 expressed from PvirB | This study |
| pCSK100 | pBCSK+ with gfp coding for M2 mutant expressed from Plac | 36 |
| pXZ428 | pED32 IncP plasmid with gfp::virE2 expressed from PvirB | This study |
| pXZ66 | pBKSK+.NdeI with virE2::gfp expressed from Plac | This study |
| pXZ479 | pBSIIKS+ with virE2::gfp expressed from PvirB | This study |
| pBG3 | pBSIIKS+ with virE2::phoA expressed from PvirB | This study |
| pXZ426 | pXZ425/pXZ66 cointegrate with virE2 and virE2::gfp expressed from independent PvirB promoters | This study |
| pXZ168 | pBSIISK+.NdeI with virE1::gfp expressed from Plac | This study |
| pXZB169 | pXZ425/pXZ168 cointegrate with virE2 and virE1::gfp expressed from independent PvirB promoters | This study |
Except where indicated, ColE1 plasmids expressing virE constructs were ligated to IncP plasmid pSW172 or pXZ151 for introduction into A. tumefaciens; the cointegrate plasmid in A. tumefaciens is given the ColE1 plasmid name plus a B (for broad host range).
Plasmids for two-hybrid analysis.
The GAL4 activation domain (AD) and DNA-binding domain (BD) fusion plasmids were constructed by use of the GAL4AD plasmid pACT2.2 and the GAL4BD plasmid pAS2 (2). The fusion junctions were confirmed by sequencing. Plasmid pXZaE1 expressing GAL4AD::virE1 was constructed by introducing a 0.77-kb NdeI-BglII fragment from pPC731 at NdeI and BamHI sites of pACT2.2. Plasmid pXZbE1, expressing GAL4BD::virE1, was constructed by introducing a ∼0.8-kb NdeI-XhoI fragment from pXZaE1 into pAS2. Plasmids pXZaE2 and pXZbE2, expressing GAL4AD::virE2 and GAL4BD::virE2, were constructed by introducing a 1.63-kb NdeI-XhoI fragment from pPC725 into pACT2.2 and pAS2, respectively. Plasmids pSE1111, with SNF4 fused to GAL4AD, and pSE1112, with SNF1 fused to GAL4BD, were used as positive-interaction controls for the two-hybrid screen (2). S. cerevisiae Y190 containing a GAL4-responsive lacZ gene was transformed individually or together with the GAL4AD and -BD plasmids with selection for leucine, tryptophan, or leucine and tryptophan prototrophies, respectively (2, 4).
Construction of virE2 insertion mutations.
In-frame insertions of a 31-residue oligopeptide (i31) were constructed by use of TnlacZ/in according to the method of Manoil and Bailey (38). The initial step of the mutagenesis procedure involved isolation of pXZ46 plasmids harboring the transposon TnlacZ/in. A 0.2-ml aliquot of an E. coli CC118(pXZ46) cell culture grown to stationary phase was mixed with λTnlacZ/in at a multiplicity of 0.1 to 0.3 phage/cell. Cells were incubated for 10 min at 37°C; then 0.8 ml of Luria-Bertani (LB) medium was added, and cells were incubated for 2 h at 30°C with aeration. The culture was plated on LB medium containing ampicillin (100 μg/ml) and chloramphenicol (50 μg/ml) and incubated at 30°C for 2 days to select for transductants. To isolate pXZ46 with the transposon inserted in frame in virE2, plasmid DNA was isolated from several thousand pooled colonies and reintroduced into E. coli DH5α with selection on LB agar minus NaCl and supplemented with 5% sucrose, ampicillin plus chloramphenicol, and 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) at 25 μg/ml. Plasmid DNA from blue, kanamycin-sensitive colonies was subjected to restriction digestion analysis with BamHI (cutting inside the transposon) and XhoI (cutting downstream of virE2) to locate the transposon insertion site. Plasmids harboring the transposon in virE2 were digested with BamHI and religated to excise the majority of the transposon, leaving coding sequence for a total of 31 residues. The exact positions of the i31 peptide insertions were confirmed by sequencing across the relevant regions of virE2 as identified by restriction digestion analysis. Ten plasmids, designated pXZ721 through pXZ730, harbored in-frame insertions fairly evenly spaced along the length of virE2. Two additional plasmids, pXZ731 and pXZ738, harbored out-of-frame insertions immediately after residues Lys13 and Arg468, respectively. To assay for interactions of the in-frame VirE2::i31 mutant proteins with native VirE2 in S. cerevisiae, the ∼1.9-kb NdeI-XhoI fragments carrying the virE2::i31 alleles from these 10 plasmids were subcloned into the GAL4AD plasmid pACT2.2 to create pXZ741 through pXZ750 and into the GAL4BD plasmid pAS2 to create pXZ751 through pXZ760. To assay for protein stability and functionality in A. tumefaciens, the virE2::i31 alleles were substituted for the virB1 gene behind the PvirB promoter of pPC914KS+ to create pXZ761 through pXZ770.
Construction of virE2 deletions.
Plasmid pPC725 served as the source for restriction fragments encoding portions of virE2. Fragments were obtained by digestion with NdeI and the subsequent use of a novel restriction site within the coding sequence. Other virE2 deletion mutants were constructed from the plasmids encoding virE2::i31 alleles described above. Within the 96-bp insertion in each of these alleles is a unique BamHI site. Fragments coding for 5′ or 3′ segments of virE2 were obtained by digestion with NdeI and BamHI or with BamHI and XhoI, respectively. These fragments were introduced into the GAL4 vectors and downstream of PvirB in pPC914KS+ for expression in A. tumefaciens. The in-frame fusion of GAL4 sequences to the virE2 deletion derivatives was confirmed by sequencing.
Construction of hybrid proteins.
Fusions to the N terminus of VirE2 were constructed as follows. Plasmid pPC730, expressing a polyhistidine tag sequence fused to the 5′ end of virE2, was constructed by introducing the virE2 coding sequence as an NdeI-XhoI fragment from pPC725 into pET15b (Novagen). Plasmid pXZ234, expressing his-virE2 from PvirB, was constructed by substituting the ∼1.8-kb NcoI-XhoI fragment from pPC730 for the NcoI-XhoI fragment carrying virB1 in pPC914KS+.NcoI. Plasmid pXZ238, expressing GAL4AD fused to the 5′ end of virE2, was constructed by PCR amplification of the GAL4AD::virE2 chimeric gene, using pXZaE2 as a template and the oligonucleotides GA5 (5′-AAGAGATCTAACCATGGATAAAGCGGAATTAATT) and ADHT (5′-TGCCGGTAGAGGTGTGGTCA) as primers, such that an NcoI site (underlined) was introduced at the translational start site. The amplified NcoI-XhoI fragment encoding the chimeric gene was substituted for virB1 in pPC914KS+.NcoI (19). Plasmid pXZ64, expressing an allele for the M2 mutant of green fluorescence protein (GFP) fused to the 5′ end of virE2, was constructed by introducing a ∼1.7-kb NdeI (made blunt ended with the Klenow fragment of DNA polymerase)-XhoI fragment from pPC725 (see above) into pWM678 digested with SmaI and XhoI. Plasmid pWM678, a gift from W. Margolin, is a derivative of pGZ with gfp lacking its stop codon expressed from Plac (36). Plasmid pXZ428, expressing gfp::virE2 from PvirB, was constructed by introducing a ∼2.3-kb XbaI-XhoI fragment from pXZ64 into similarly digested pED32 (53). Plasmid pCSK100 expresses wild-type gfp from Plac (36).
Fusions to the C terminus of VirE2 were constructed as follows. Plasmid pXZ24, expressing phoA fused to the 3′ end of virE2, was constructed by first introducing an XbaI site immediately upstream of the virE2 stop codon by PCR amplification with pPC725 as a template and the −48 reverse primer of pBluescript and E2FU (5′-AATCTAGAAAGCTGTTGACGCTTTGGC [the XbaI site is underlined]) as primers. Plasmid pXZ23, expressing virE2 lacking its stop codon, was constructed by introducing the amplified ∼1.7-kb NdeI-XbaI fragment into pBKSK+.NdeI (6). Plasmid pXZ24, expressing virE2::phoA from Plac, was constructed by introducing the ∼1.4-kb XbaI-KpnI fragment from pUI1156 (19) into pXZ23. Plasmid pBG3, expressing virE2::phoA from PvirB, was constructed by substitution of a ∼3.1-kb NdeI-KpnI fragment from pXZ24 for an NdeI-KpnI fragment carrying virB1 in pPC914KS+. Plasmid pXZ66, expressing gfp fused to the 3′ end of virE2, was constructed by substituting a 0.72-kb XbaI-KpnI fragment coding for the M2 GFP mutant from pWM678 for the phoA gene of similarly digested pXZ24. A gfp stop codon is located just upstream of the KpnI site (36). Plasmid XZ479, expressing virE2.533::gfp from PvirB, was constructed by substituting a ∼2.06-kb MluI-KpnI fragment from pXZ66 coding for the last two-thirds of virE2 fused to gfp for this segment of virE2 fused to phoA in pBG3.
VirE1 fused at its C terminus to GFP was constructed by first introducing an XbaI site immediately upstream of the virE1 stop codon by PCR amplification with pXZ237 as a template and the −48 reverse primer of pBluescript and E1FU (5′-TGGTCTAGATCCTTCTGACCAGCAAG [the XbaI site is underlined]) as primers. Plasmid pXZ168, expressing full-length virE1 fused to gfp, was constructed by introducing the PCR product as an NdeI-XbaI fragment into pBSIIKS+.NdeI and then introducing gfp carried on a 0.7-kb XbaI-KpnI fragment from pWM678 at the 3′ end of virE1.
β-Galactosidase activity assays.
Nitrocellulose replicas of patched colonies were assayed for β-galactosidase activity by freeze fracturing cells in liquid nitrogen and incubating the fractured cells on Whatman filter paper soaked in buffer containing X-Gal (2). Quantitative assays were performed according to the procedure of Bai and Elledge (2).
Protein analysis.
Proteins were resolved by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) as previously described (25). VirE proteins were visualized by SDS-PAGE followed by transfer of proteins to nitrocellulose membranes and development of the immunoblot with goat anti-rabbit antibodies conjugated to alkaline phosphatase. For analysis of Vir protein content, 1 ml of AS-induced culture adjusted to an OD600 of 0.5 was pelleted by centrifugation, resuspended in 50 μl of H2O plus 50 μl of protein sample buffer, and immediately boiled for 3 min. Volumes of sample buffer were adjusted so that all samples represented equivalent numbers of cells per milliliter. Conditions for preparation and lysis of spheroplasts and for immunoprecipitation have been described previously (14). Immunoprecipitates were examined by SDS-PAGE and immunostaining of blots with anti-VirE2 (14), anti-i31 peptide antiserum (kindly supplied by C. Manoil), or an anti-GFP antiserum (kindly supplied by W. Margolin).
Virulence assays.
A. tumefaciens strains were tested for virulence on uniformly wounded Kalanchoe daigremontiana leaves as previously described (59). Controls for the tumorigenesis assays included coinoculation of the same leaf with the wild-type strain A348 and the avirulent strain A136. Assays were repeated at least five times for each strain on separate leaves. All strains were grown identically prior to inoculation of plants, approximately the same numbers of cells were inoculated, and leaves used in the assays were of approximately the same age. Tumors were photographed 4 to 5 weeks after inoculation.
RESULTS
VirE2 complex formation in S. cerevisiae.
VirE2 showed a capacity to self-associate as evident by yeast two-hybrid analysis (Fig. 1). S. cerevisiae Y190 cells carrying pXZaE2 and pXZbE2, expressing GAL4AD::VirE2 and GAl4BD::VirE2, respectively, exhibited high levels of β-galactosidase activity (∼335 Miller units). By contrast, strains carrying either plasmid alone, or strains carrying pXZaE2 and vector plasmid pACT2.2 or pXZbE2 as well as vector plasmid pAS2 (data not shown) displayed low β-galactosidase activities (<2 Miller units). The previously demonstrated interaction between GALBD::SNF1 and GALAD::SNF4 served as a positive control for these experiments (2). Cells carrying the corresponding plasmids, pSE1112 and pSE1111, exhibited β-galactosidase activities of ∼265 Miller units.
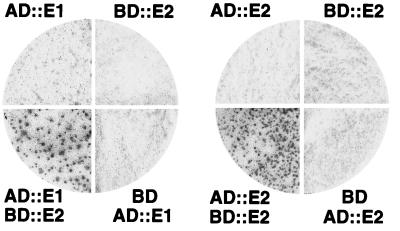
FIG. 1.
VirE2 self-association and interaction with VirE1 as determined with the yeast two-hybrid assay (2, 4). At least 75 yeast colonies per quadrant were replica plated onto nitrocellulose and assayed for β-galactosidase activity. Strains carrying pXZbE2 (BD::E2) and pXZaE1 (AD::E1) or pXZaE2 (AD::E2) displayed high levels of β-galactosidase activity.
We screened for interactions between VirE2 and full-length versions of other Vir proteins that potentially interact with VirE2, including VirB1 through VirB11, VirD1, VirD2, VirD4, and VirE1. VirE2 reproducibly displayed a strong interaction with only VirE1 among these proteins, as evidenced by the high β-galactosidase activities (361 Miller units) of yeast cells carrying pXZaE1 and pXZbE2 (Fig. 1). S. cerevisiae carrying only pXZaE1 or pXZaE1 and the vector pAS2 displayed background β-galactosidase activities, demonstrating the specificity of the VirE1-VirE2 interaction. However, Y190 cells carrying only pXZbE1 exhibited high levels of β-galactosidase activity, suggesting that VirE1 self-activates lacZ expression when fused to the GAL4BD.
VirE2 complex formation in A. tumefaciens.
We next tested whether VirE2 interacts with itself and with VirE1 in A. tumefaciens cells. VirE2 self-association was assessed by cosynthesis of native VirE2 and a VirE2.533::GFP hybrid protein that exerts trans-dominant effects in merodiploid cells (Fig. 2). Anti-VirE2 antiserum precipitated VirE2 from extracts of wild-type strain A348 (lane 1), as well as A348(pCSKB100) (lane 2), without coprecipitating native GFP. Conversely, the anti-GFP antiserum precipitated GFP from extracts of A348(pCSKB100) without coprecipitating VirE2 (lane 3). These results establish that VirE2 does not bind nonspecifically to GFP. The anti-GFP antiserum coprecipitated both VirE2.533::GFP and VirE2 from extracts of A348(pXZB43) (lane 4). In addition, these antisera coprecipitated both proteins from extracts of LBA4404(pXZB43), a T-DNA deletion strain (lane 5); KE1(pXZ426), a virE operon deletion mutant (lane 7); and PC1000(pXZB43), a virB operon deletion mutant (data not shown). Together, these findings show that the presumptive VirE2.533::GFP-VirE2 complex forms independently of an association with T strands, VirE1, or VirB proteins.
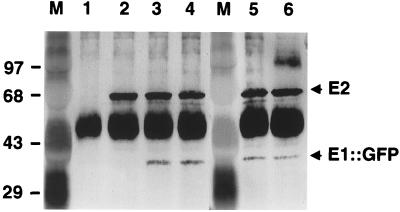
FIG. 2.
VirE2 self-association in A. tumefaciens as determined by immunoprecipitation analysis. Immunoprecipitates were electrophoresed through SDS–12.5% (lanes 1 to 7) or SDS–10% (lanes 8 to 11) polyacrylamide gels, and blots were developed with anti-VirE2 (top panel) or anti-GFP (bottom panel) antiserum as previously described (25). Material was precipitated from extracts of wild-type A348 (lane 1) and A348(pCSKB100) (lane 2) with anti-VirE2 antiserum. Material was precipitated from extracts of A348(pCSKB100) (lane 3), A348(pXZB43) (lane 4), LBA4404(pXZB43) (lane 5), KE1(pXZB43) (lane 6), and KE1(pXZB426) (lane 7) with anti-GFP antiserum. Material was precipitated from extracts of wild-type A348 (lane 8), A348(pXZB761) (lane 9), KE1(pXZB761) (lane 10), and KE1(pXZ427) (lane 11) with anti-i31 peptide antiserum. The various forms of VirE2 are indicated at the right. Some proteolyis of VirE2::GFP is evident. The heavy staining band at ∼45 kDa is due to immunoreactivity of immunoglobulin G (IgG) heavy chain present in the immunoprecipitates. M, molecular mass markers from Bio-Rad (top panel) or Gibco-BRL (bottom panel), with sizes (in kilodaltons) indicated at left.
We further tested for an interaction between VirE2 and VirE2.39::i31, a functional VirE2 derivative that carries an insertion of a 31-residue oligopeptide, i31, immediately after Tyr39 (see below). Anti-i31 peptide antiserum precipitated VirE2.39::i31 from KE1(pXZB761) extracts (lane 10) and did not precipitate VirE2 from wild-type A348 extracts (lane 8), demonstrating the specificity of the anti-i31 antiserum for the VirE2.i31 derivative. This antiserum coprecipitated both VirE2.39::i31 and VirE2 from extracts of A348(pXZB761) (lane 9), KE1(pXZ427) (lane 11), and PC1000 and LBA4404 carrying plasmid pXZ427 (data not shown), confirming that homomultimeric complexes form independently of T strands, VirE1, or VirB proteins.
The interaction of VirE2 with VirE1 was assessed by cosynthesis of native VirE2 and a functional VirE1::GFP hybrid protein (58). Anti-VirE2 antibodies coprecipitated VirE1::GFP and VirE2 from extracts of A348(pXZB168) (Fig. 3, lane 3). Anti-VirE2 antibodies also coprecipitated VirE1::GFP and VirE2 from extracts of LBA4404(pXZB168), KE1(pXZB169), and PC1000(pXZB168) (lanes 4 to 6), demonstrating that heteromultimerization also occurs independently of T-DNA, VirE1, and the VirB proteins. As controls, anti-VirE2 antibodies precipitated VirE2 from extracts of A348 (lane 2) and did not precipitate VirE1::GFP from KE1(pXZB168) cell extracts (lane 1). It should be noted that VirE1 was not detected in the precipitates because the blot was codeveloped with only anti-VirE2 and anti-GFP antisera. Considerably more VirE2 than VirE1::GFP appears to be present in the precipitates, even with the use of anti-GFP antiserum to precipitate the heteromultimer (data not shown). However, further studies are needed to evaluate whether these findings are a reflection of stoichiometric differences in a single complex or the presence of different complexes in vivo or instead result from a difference in antibody affinities or other experimental conditions.
FIG. 3.
VirE2 interaction with a functional VirE1::GFP hybrid protein in A. tumefaciens as determined by immunoprecipitation analysis. Immunoprecipitates were electrophoresed through SDS–12.5% polyacrylamide gels, and blots were developed with anti-VirE2 antiserum and then redeveloped with anti-GFP antiserum. Material was precipitated from KE1(pXZB168) (lane 1), A348 (lane 2), A348(pXZB168) (lane 3), LBA4404(pXZB168) (lane 4), KE1(pXZB169) (lane 5), and PC1000(pXZB168) (lane 6) with anti-VirE2 antiserum. The positions of VirE2 and VirE1::GFP are indicated at the right. The heavy staining band at ∼45 kDa is due to immunoreactivity of immunoglobulin G heavy chain present in the immunoprecipitates. M, molecular mass markers from Gibco-BRL, with sizes (in kilodaltons) indicated at the left.
Regions of VirE2 required for homomultimer formation.
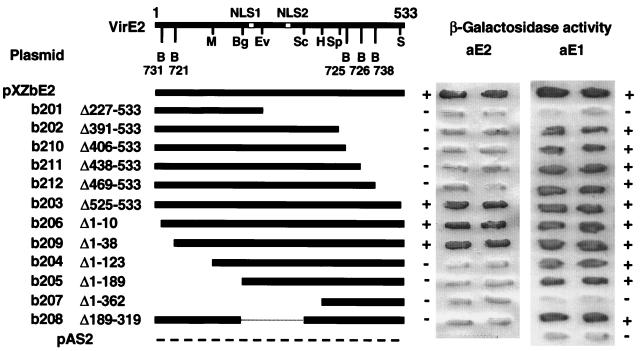
Restriction fragments coding for portions of virE2 were fused to the GAL4BD coding sequence in pAS2 to create plasmids pXZbE201 through pXZbE212 and to the GAL4AD coding sequence in pACT2.2 to create plasmids pXZaE201 through pXZaE212. S. cerevisiae Y190 strains carrying these plasmids individually or together with pXZaE2 or pXZbE2 were assayed for β-galactosidase activity. Figure 4 shows that only the GAL4BD fusions to VirE2 with relatively small N- or C-terminal deletions, 10 or 38 residues at the N terminus or 9 residues at the C terminus, retained the two-hybrid interaction with GAL4AD::VirE2. The VirE2 mutants with larger N- or C-terminal deletions or an internal deletion failed to interact with full-length VirE2. Yeast strains carrying plasmids pXZbE201 to pXZbE212 in the absence of pXZaE2 displayed background levels of β-galactosidase activity showing that the VirE2 truncations did not self-activate transcription from the PGal promoter. Assays with the pXZaE201 to -212 plasmid series showed a similar profile; only VirE2 truncation mutants lacking the 10 or 38 N-terminal residues or 9 C-terminal residues interacted with full-length VirE2 (data not shown). Most of the VirE2 deletion derivatives showed a yeast two-hybrid interaction with VirE1, demonstrating that these derivatives are stable and have the potential for complex formation in the yeast nucleus (Fig. 4) (see below).
FIG. 4.
Deletion analysis to define sequence requirements for VirE2 self-association and interaction with VirE1. Derivatives of VirE2 fused to the GAL4 binding domain in plasmid pAS are depicted schematically at the left. The structure of VirE2 (533 amino acids), with the positions of the two nuclear localization sequences (NLS1 and NLS2) and restriction sites used for subcloning, is presented at the top. Some restriction fragments were subcloned from the pXZ7XX plasmids listed that carry virE2::i31 alleles, as described in the text. Restriction sites are as follows: B, BamHI; M, MluI; Bg, BglII; Ev, EcoRV; Sc, SacI; H, HindIII; Sp, SspI; and S, SalI. At right are β-galactosidase activities of patched yeast colonies carrying the pXZbE2 plasmids listed with either pXZaE2 (AD::E2) or pXZaE1 (AD::E1). +, β-galactosidase activity evident; −, background levels of β-galactosidase activity. Activities of two representative, independently transformed colony patches are shown.
We attempted to further delineate a region of VirE2 required for self-interaction by constructing a series of mutants with insertions of a 31-residue heterologous peptide, i31 (Fig. 5). The goal of this study was to disrupt the interaction interface(s) responsible for VirE2 homomultimerization by insertion of a heterologous oligopeptide. We selected TnlacZ/in for i31 insertional mutagenesis because transposon mutagenesis permitted construction of i31 insertions randomly along the VirE2 peptide sequence and because properties of i31—its large size and overall hydrophilicity—are ideally suited for disruption of protein-protein interaction domains. This approach has proven successful for identifying domains required for multimerization as well as permissive sites of monomeric and multimeric proteins (34, 38, 40). Alleles for the VirE2 insertion mutants were cloned into pACT2.2 to create pXZ741 through pXZ750 and into pAS2 to create pXZ751 through pXZ760. Figure 5 shows that yeast strains carrying plasmids pXZ751 through pXZ760 together with pXZaE2 displayed an overall pattern of β-galactosidase activity resembling that observed with the VirE2 deletion mutants. Mutants with i31 insertions near the N terminus, at residue 39 or 84, or the C terminus, at residue 529, retained the capacity for interaction with VirE2, whereas mutants with other internal insertions did not interact. The one exception was VirE2.236::i31, which in the yeast two-hybrid system showed an interaction with VirE2, albeit slightly reduced in its affinity compared to the VirE2-VirE2 interaction. The insertion mutants exhibited the same interaction patterns when fused to GAL4AD and assayed for interaction with GAL4BD::VirE2. Yeast transformed with the pXZ741 through pXZ750 or pXZ751 through pXZ760 plasmids alone or together with the complementary vector plasmid, pAS2 or pACT2.2, respectively, exhibited low β-galactosidase activities, demonstrating that the VirE2 insertion mutants failed to self-activate transcription by binding directly to a GAL4 domain or the PGal promoter (data not shown).
FIG. 5.
i31 peptide insertion analysis to define sequence requirements for VirE2 self-association and interaction with VirE1. VirE2::i31 derivatives fused to that GAL4 binding domain in plasmid pAS2 are depicted schematically at the left. Sites of i31 insertions are denoted by small vertical lines. The structure of VirE2 (533 amino acids), with the positions of its NLSs, are shown at the top. At right are β-galactosidase activities of patched yeast colonies carrying one of the pXZbE2 plasmids listed with either pXZaE2 (AD::E2) or pXZaE1 (AD::E1). +, β-galactosidase activity evident; −, background levels of β-galactosidase activity. Activities of two representative, independently transformed colony patches are shown.
The results of these two-hybrid studies suggest that the first 38 and last 9 residues of VirE2 are dispensible for self-association. Furthermore, insertion of the i31 peptide at residue 84 or 236 does not disrupt the capacity of VirE2 to multimerize. These findings support a model in which VirE2 dimerization or assembly of higher-order homoligomers is dependent on proper folding of the nearly full-length protein.
Localization of a domain required for VirE1 interaction.
To localize the region of VirE2 involved in complex formation with VirE1, yeast strains carrying one of the pXZbE201 to pXZbE212 plasmids and pXZaE1 were assayed for β-galactosidase activity. Figure 4 shows that VirE2 exhibited interaction in the yeast two-hybrid system when 142 residues at the C terminus were deleted (VirE2Δ391–533) but lost the capacity to undergo complex formation when the C-terminal 306 residues were deleted (VirE2Δ227–533). Conversely, VirE2 showed interaction in yeast two-hybrid analyses when the first 189 residues were deleted (VirE2Δ1–189) but lost the capacity to multimerize when the first 362 residues were deleted (VirE2Δ1–362). These findings localized a VirE1 interaction domain in the central region of VirE2, between residues 189 and 391. The failure of VirE2Δ227–533 to show an interaction with VirE1 in the two-hybrid system further suggests that residues 189 to 227 are not sufficient for mediating complex formation. Furthermore, the capacity of the internal-deletion mutant VirE2Δ189–319 to interact suggests that a VirE1 interaction interface most probably is localized between residues 320 and 390. These truncated VirE2–GAL4BD fusions failed to activate transcription from PGal when synthesized in the absence of an interactive GALAD fusion protein (data not shown).
Interestingly, all of the i31 insertion mutants, including those with insertions at residues 236 and 331, showed an interaction with VirE1 in the yeast two-hybrid system (Fig. 5). Therefore, a VirE1 interaction domain likely is located between residues 331 and 391, although it is possible that such a domain exists between residues 320 and 331. It also is conceivable that residues 320 to 390 of VirE2 are necessary but not sufficient for complex formation; for example, this region may induce proper protein folding to expose an interaction interface formed by discontinuous residues of VirE2.
virE2 expression from PvirB, but not PvirE or Plac, complements a virE2 null mutation.
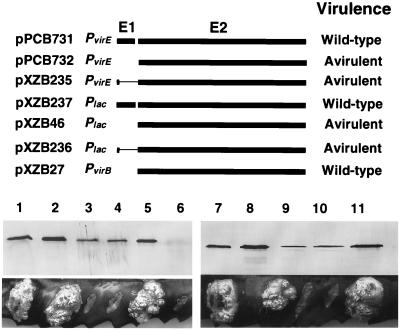
Before evaluating the functionality of the VirE2 mutants in A. tumefaciens, we examined the effect of promoter context on synthesis of functional VirE2 protein. At12516(pXZB27) carrying PvirB::virE2 accumulated VirE2 at higher levels than A348 (Fig. 6). Furthermore, At12516(pXZB27) displayed wild-type virulence in plant tumorigenesis assays, showing that virE2 expression from PvirB on an IncP replicon fully complements the virE2 null mutation (Fig. 6). By contrast, both At12516(pXZB732) expressing Plac::virE2 and At12516(pPCB732) expressing PvirE::virE2 accumulated VirE2 at appreciably lower levels than A348 (Fig. 6). These strains were avirulent in tumorigenesis assays (Fig. 6).
FIG. 6.
Promoter and virE1 sequence requirements for synthesis of functional VirE2. The PvirB, Plac, and PvirE promoters were used to drive expression of virE2 or virE1 and virE2, and the Plac and PvirE promoters were used to drive expression of virE1 containing a translation stop signal at codon 6 and all of virE2. At12516 cells carrying these plasmids were assayed for VirE2 protein abundance by immunoblot analysis and for virulence by inoculation on K. daigremontiana. Samples of protein extracts normalized for total protein content on a per-cell equivalent were electrophoresed through SDS–12.5% polyacrylamide gels, and blots were developed with anti-VirE2 antiserum. The strains used were A348 (lane 1), At12516(pPCB731) (lane 2), At12516(pPCB732) (lane 3), At12516(pXZB235) (lane 4), At12516(pXZB27) (lane 5), At12516 (lane 6), A348 (lane 7), At12516(pXZB237) (lane 8), At12516(pXZB46) (lane 9), At12516(pXZB236) (lane 10), and At12516(pXZB27) (lane 11). Virulence assays of each strain show oncogenic proliferation clearly distinguishable from sites of inoculation that did not proliferate. Results of virulence assays of At12516 cells harboring these plasmids are summarized at the right.
The virE1 and virE2 coding sequences are separated by 4 bp in the virE operon. To assess whether translational coupling is necessary for synthesis of abundant levels of VirE2 from Plac or PvirE, we engineered translational stop signals near the 5′ end of virE1 in plasmids pPCB731 and pXZB237. At12516 carrying the resulting plasmids, pXZB235 and pXZB236, synthesize only 6 residues of VirE1 and all of VirE2. These strains accumulated VirE2 at lower levels than the corresponding strains coexpressing intact virE1 and virE2 from PvirE or Plac (Fig. 6). At12516(pXZB235) and At12516(pXZB236) also were avirulent (Fig. 6). These findings are consistent with the notion that translational coupling serves to enhance the efficiency of virE2 expression. It should be noted that virE1 and virE2 coexpression from the same promoter is not an absolute requirement for synthesis of functional forms of VirE1 and VirE2, since At12516(pXZB27) expressing virE1 from its native position on the Ti plasmid and virE2 from PvirB on an IncP replicon accumulates abundant levels of VirE2 and exhibits wild-type virulence. Presently, we cannot account for the phenotypic differences resulting from virE2 expression from PvirB, PvirE, and Plac. One possibility supported by previous work (12, 20, 49) is that PvirB is a stronger promoter than these other promoters in A. tumefaciens. This increased promoter activity may permit synthesis of a critical threshold level of VirE2 in the absence of translational coupling. Although further studies are needed to more rigorously test this model, our results add to an existing body of evidence suggesting that VirE2 must be abundantly synthesized to mediate T-DNA delivery to plant cells (7, 17, 33, 52). For this reason, all virE2 constructs described below are expressed from PvirB.
Identification of a permissive site at residue 39.
The functionality of the VirE2 insertion mutants was assessed by introduction of the plasmid series pXZ761 to pXZ770 into the A348 and virE2 null-mutant backgrounds. Figure 7A shows a general pattern of mutant protein accumulation that correlates with the capacity of these mutants to interact with full-length VirE2 in S. cerevisiae. That is, mutant proteins with insertions at the N-terminal Tyr39 or Leu84, the internal Leu236, or the C-terminal Ser529 residue accumulated in both A. tumefaciens hosts at levels comparable to native VirE2 synthesized from the Ti plasmid in A348 cells. By contrast, the remaining insertion mutants that failed to self-interact in S. cerevisiae accumulated at lower levels in A. tumefaciens. Protein instability was particularly evident for mutants with i31 insertions at residues Ser147, Met184, and Gly331. This correlation between the capacity to multimerize in S. cerevisiae and protein stability in A. tumefaciens suggests that dimerization or homomultimerization enhances VirE2 stability in A. tumefaciens. Conversely, the mutant proteins may be degraded prior to complex formation.
FIG. 7.
Steady-state abundance and functionality of VirE2::i31 insertion mutants in A. tumefaciens. A348 and At12516 cells carrying these plasmids were assayed for VirE2 protein abundance by immunoblot analysis. (A) Blots were prepared as described in the legend to Fig. 6 with protein samples from plasmid-carrying A348 (top panel) or At12516 (bottom panel) cells. Strains used were as follows: wild type, A348 (top panel) and At12516(pXZB27) (bottom panel); and ΔE2, At12516 (top and bottom panels); the numbers refer to the VirE2 residue immediately preceding the i31 insertion. The small arrow denotes the position of native VirE2. The large arrowhead denotes VirE2::i31 mutants, which migrated to different positions in SDS-polyacrylamide gels. (B) The functionality of the i31 insertion mutants was assessed by inoculation of strains on K. daigremontiana leaves (left leaf, A348 merodiploids coexpressing wild-type virE2 and the indicated virE2::i31 allele; right leaf, At12516 expressing the indicated virE2::i31 allele). The allele for VirE2.39::i31 restored virulence of At12516 cells to wild-type levels. The tumor at the top left was induced by inoculation with wild-type A348, and the tumor at the bottom right was induced by inoculation with At12516(pXZ27). The wound site at the top right shows the avirulence of At12516.
Interestingly, most of the mutant proteins appeared to accumulate at higher levels in At12516 cells than in A348 cells. The differences in protein levels were most pronounced for the mutants with insertions at residues 147, 184, and 331. A348 cells synthesize native VirE2, whereas At12516 cells do not. Thus, it is possible that VirE2 outcompetes the mutant proteins for interaction with another factor that promotes VirE2 stabilization in the A348 background. In At12516 cells lacking VirE2, this factor would be available for interaction with and partial stabilization of the VirE2 insertion mutants. One candidate for such a stabilizing factor is VirE1, which is consistent with previous evidence for stabilizing effects of VirE1 on VirE2 in E. coli (39) and A. tumefaciens (22).
A348 and At12516 cells carrying the pXZ761 to pXZ770 plasmid series were assayed for the capacity to induce the formation of tumors on plants (Fig. 7B). A348 strains carrying these plasmids exhibited wild-type virulence, demonstrating that the corresponding virE2 alleles are recessive. Most of the At12516 strains carrying these plasmids were avirulent, showing that i31 mutants are nonfunctional. It is of considerable interest, however, that At12516(pXZ761) displayed completely wild-type virulence (Fig. 7B). On the basis of these findings, we define Tyr39 as a permissive site of VirE2.
VirE2 cannot accommodate large N- or C-terminal deletions or extensions.
We next tested whether VirE2 can accommodate deletions or extensions at its extremities. At12516(pXZ206) and At12516(pXZ203) displayed attenuated virulence, as shown by induction of small tumors on approximately one-half of the inoculated wound sites (data not shown). VirE2 therefore retains some function after deletions of 10 N- or 9 C-terminal residues. Other VirE2 truncation derivatives with larger N- or C-terminal deletions failed to complement the virE2 null mutation in virulence assays. Next, we fused full-length VirE2 at its N terminus to a ∼2-kDa His tag sequence, the GAL4AD domain, alkaline phosphatase, or GFP and at its C terminus to alkaline phosphatase or GFP (Table 1). At12516 cells expressing alleles for each of these proteins were avirulent, showing that VirE2 cannot accommodate large N- or C-terminal extensions without loss of function (data not shown).
DISCUSSION
Definition of the translocation-competent forms of substrates exported via the T-DNA transporter is of paramount importance in developing a detailed mechanistic understanding of this transport system. One substrate, the T-DNA, is composed of the T strand bound at its 5′ end to the VirD2 endonuclease (50, 56, 57). VirE2 SSB coats the length of the ssDNA, but it is not yet established whether VirE2 assembles with the VirD2-T strand in the bacterium or in the plant cell (7, 13, 14, 52). A second substrate, VirE2 SSB, can be exported independently of the VirD2–T-strand particle (7, 42, 52). The present study of the VirE2 SSB was initiated with a goal of defining the nature of VirE2 complexes in A. tumefaciens. Results of our investigations have shown that (i) VirE2 self-associates and interacts with VirE1 in the heterologous yeast host and in A. tumefaciens; (ii) almost the entire VirE2 protein sequence is required for self-assembly, whereas residues between 320 and 390 mediate formation of a complex with VirE1; (iii) there is an intriguing promoter context requirement for synthesis of functional VirE2; and (iv) VirE2 can tolerate a peptide insertion near its 5′ end but not fusions of heterologous proteins at either terminus.
VirE2 homomultimer formation in A. tumefaciens.
Our studies of VirE2 self-interaction confirm previous reports of this interaction by Baron et al. (3) and Deng et al. (22) by the use of the yeast two-hybrid system. We further showed that VirE2 coprecipitates with the functional VirE2.39::i31 derivative and with a VirE2.533::GFP fusion protein from A. tumefaciens cell extracts (Fig. 2), strongly suggesting that VirE2 homomultimerization is a physiologically relevant event. Recent genetic studies in our laboratory provide additional support for the notion that VirE2 self-association is necessary for the export of both T-DNA and VirE2. We have found that the virE2.533::gfp allele displays negative dominance, as shown by the strongly attenuated virulence phenotype of virE2/virE2.533::gfp merodiploid strains (58). Furthermore, negative dominance can be suppressed by virE2 overexpression (58). These findings may imply that the VirE2.533::GFP::VirE2 complexes recovered in our immunoprecipitation studies (Fig. 2) fail to interact productively with the T-DNA transport system. VirE2 overproduction might then serve to titrate the hybrid protein, permitting export of enough wild-type protein for restoration of virulence.
Of further interest, virE2.533::gfp also exerts negative dominance in mixed-infection experiments. These experiments typically involve coinfection of plant tissues with two A. tumefaciens strains, a T-DNA deletion mutant and a virE2 mutant. Although both strains are avirulent, coinoculation results in restoration of virulence and the formation of plant tumors (42). A model designed to account for this phenomenon is that the T-DNA deletion mutant exports VirE2 and the virE2 mutant exports the VirE2–T-strand particle to the same plant cell, where these molecules assemble as the oncogenic T complex. virE2.533::gfp was found to suppress virulence when expressed in either the virE2 mutant or the T-DNA deletion mutant during a mixed infection (58). These results suggest that VirE2.533::GFP inhibits the export of both VirE2 by the T-DNA deletion mutant and the VirD2–T-strand particle by the virE2 mutant. By contrast, virE2.533::gfp does not inhibit virB-dependent transfer of the IncQ plasmid RSF1010 between bacterial cells (58). VirE2 is not required for IncQ plasmid transfer to bacterial recipients (5, 26). We therefore propose a model in which the hybrid protein somehow prevents the export of substrates with which it directly interacts in the bacterium. We have not been able to detect GFP fluorescence transfer to plant cells during the infection process. Therefore, it is unlikely that VirE2::GFP manifests its phenotypic effects after export to the eukaryotic host.
Biochemical studies with purified VirE2 protein have supplied independent evidence of VirE2 oligomerization. Renaturation of urea-solubilized VirE2 obtained from E. coli inclusion bodies was found to result in the spontaneous assembly of tetramers (47), an oligomeric structure common to E. coli SSB and other prokaryotic and eukaryotic SSBs (35, 46). Furthermore, in a recent study, Deng et al. (22) demonstrated interactions between VirE2 derivatives tagged with glutathione S-transferase or polyhistidine. Given that these homomultimers assemble in solution, it is likely that VirE2 dimers or higher-order oligomers also correspond to the stabilized form of VirE2 in vivo. Consistent with this prediction, our studies of VirE2.i31 mutants showed that there is a general correlation between VirE2 stability in A. tumefaciens and the capacity of these derivatives to interact with full-length VirE2 in S. cerevisiae (Fig. 5 and 7). Thus, reminiscent of components of numerous other dimeric and multimeric protein complexes, including the T-DNA transporter itself (13), newly synthesized VirE2 monomers may be stabilized by multimerization in A. tumefaciens cells.
VirE2 is one of the most abundant Vir proteins, representing as much as 0.1% of the total cellular protein in induced A. tumefaciens cells (15). Although there are no definitive data establishing that VirE2 must accumulate at such high levels for infecting strains to display wild-type virulence, our studies have shown that virE2 expression from the PvirB, PvirE, and Plac promoters results in VirE2 accumulation at different levels, and only cells accumulating high levels of VirE2 display wild-type virulence (Fig. 6). In addition, recent mixed-infection experiments have shown that competing substrates, such as the RSF1010 transfer intermediate (7, 51) or the Osa protein encoded by the IncW plasmid pSA (33), exert negative effects on virulence, presumably by interfering with VirE2 export. The available data therefore suggest that virulence is attenuated by perturbations at the level of virE2 gene expression or VirE2 protein export, both of which effectively diminish the amount of VirE2 delivered to plant cells.
VirE2 displays strong positive cooperativity for ssDNA binding, and VirE2-ssDNA complexes form extremely rapidly and are very stable under physiological conditions (16, 47). Thus, it seems highly likely that VirE2 rapidly associates with free T strands, which have been shown previously (50, 56) to accumulate at high levels upon induction of bacterial cells. VirE2 homomultimerization therefore most probably corresponds to an intermediate step in the T-DNA export pathway. VirE2 binding to the VirD2–T-strand particle might facilitate export of the nucleoprotein particle by providing requisite protein-protein contacts with the T-DNA transporter. Additionally, VirE2 binding might confer a structure on the T strand which is necessary for its passage through the mating channel, a possibility supported by electron microscopy studies showing that the binding of VirE2 to ssDNA results in elongation and thinning of the DNA (16). VirE2 homomultimerization also most probably is obligatory for VirE2 export under conditions in which this SSB is exported independently of T-DNA. A model suggesting that VirE2 is cotranslationally exported during the infection process has been presented. We believe that the capacity of VirE2 to form homomultimers in solution and in vivo, its accumulation to high levels in induced bacterial cells, its high affinity for ssDNA, and its capacity to interact with VirE1 (see below) are incompatible with such a model.
Our yeast two-hybrid studies showed that almost the entire VirE2 protein sequence is necessary for homomultimerization. Interestingly, an i31 insertion at Tyr 236 did not inhibit self-association. This finding is consistent with results of a previous study which showed that linker-insertion mutants with two heterologous residues inserted at positions 213 and 256 accumulated at wild-type levels and that the position 213 insertion mutant retained most of its function, as assessed by virulence assay (23). By contrast, mutant proteins with 2-residue insertions at positions 10, 94, 378, and 472 were unstable and nonfunctional (23). Thus, a central region of VirE2, identified by insertion mutations at residues 213, 236, and 256, is dispensible for protein stability and probably also VirE2 homomultimer formation as demonstrated for the 236::i31 mutant (Fig. 4) and as implied for the position 213 mutant on the basis of its functionality (23). The central region of VirE2 carries two NLS sequences that are thought to be required for targeting to the plant nuclei (17, 29). This region most probably is surface exposed to permit formation of productive contacts with plant nuclear targeting factors, as opposed to being buried in a VirE2 dimerization interface.
Interaction of VirE2 with VirE1.
Recent progress has been made toward assigning a function for the ∼7.5-kDa VirE1 protein in the infection process. First, results from mixed-infection experiments suggest that VirE1 contributes in some way to the export of VirE2 (52). An avirulent mutant lacking virE1 and synthesizing abundant levels of VirE2 failed to induce tumor formation when coinoculated on plants with a virE2 null mutant, which was interpreted as evidence of a role for VirE1 in the export of VirE2. Additional findings led to the proposal that VirE1 specifically mediates VirE2 export while being dispensable for export of the VirD2–T-strand particle (52).
Deng et al. (22) recently showed an interaction between VirE1 and VirE2 in the yeast two-hybrid system. Biochemical studies utilizing epitope-tagged VirE1 and VirE2 proteins provided further evidence for this interaction (22). Our yeast two-hybrid studies showed similar results (Fig. 1), and our immunoprecipitation studies provided evidence for VirE1-VirE2 complex formation in A. tumefaciens cells (Fig. 2). Deng et al. (22) observed a sixfold-stronger interaction between VirE2 and VirE1 than between two VirE2s. However, our quantitative β-galactosidase assays suggest that both interactions occur with comparable affinity in S. cerevisiae. The difference in these experimental findings might be due to our use of the GAL4 system versus the use of the LexA system by Deng et al. (22).
Deng et al. (22) further showed that cells expressing virE2 from the Ptac promoter accumulated low levels of VirE2 protein in the absence of virE1 coexpression, which they interpreted as evidence for a stabilizing activity of VirE1 on VirE2. While we cannot exclude the possibility of a role for VirE1 in enhancing VirE2 stability, results of our promoter studies strongly suggest that VirE2 accumulation at abundant levels depends at least in part on cotranslation of virE1 and virE2 when either the Plac or PvirE promoter is used to drive gene expression. However, for an unknown reason, cotranslation is not required to achieve abundant levels of VirE2 protein when the PvirB promoter is used to drive virE2 gene expression. We have found that both the virE2 mutant At12516 and the virE operon mutant KE1 accumulate abundant levels of VirE2 upon expression of virE2 from the PvirB promoter carried on plasmid pXZB27 (58) (Fig. 6). Because KE1(pXZB27) cells lack the virE1 gene, these findings establish that neither the cotranslation of virE1 and virE2 nor the synthesis of VirE1 protein is absolutely essential for accumulation of abundant levels of VirE2 in A. tumefaciens.
Based on the results of their in vitro studies and the identification of physical similarities, Deng et al. (22) proposed that VirE1 might function by a mechanism analogous to that of the Syc protein “bodyguards” of the type III secretion pathways. The Syc proteins are postulated to bind internal domains of cognate effector proteins to prevent premature interactions with other, coexported proteins in the bacterial cytoplasm (30). Our analyses of a large collection of VirE2::i31 mutants and VirE2 deletion derivatives permitted localization of a putative VirE1 interaction domain between residues 320 and 390 of VirE2. Interestingly, this region of VirE2 is important for self-association, and it also is located between the NLS sequences (Fig. 4) and a C-terminal region required for ssDNA binding (23). Thus, the binding of VirE1 to the central region of VirE2 could modulate a myriad of interactions, including the interaction of VirE2 with itself, T-DNA, the T-DNA transporter, coexported proteins, or even nuclear targeting proteins in the plant cell. Deng et al. (22) proposed that VirE1 prevents VirE2 self-association in vivo, implying that VirE2 homomultimers are dead-end complexes. On the basis of the genetic and biochemical data summarized above, we favor a model depicting VirE2 homomultimers as critical intermediate complexes in the T-DNA and VirE2 export pathways. A definition of precisely how VirE1 might configure VirE2 oligomers for export by the T-DNA transporter awaits further study.
Functionality of VirE2 hybrid proteins.
VirE2 is an excellent candidate for manipulation as a delivery system for proteins or peptides of interest to plant cells because of its demonstrated association with T strands in plant cells and its importance in T-complex trafficking to plant nuclei. That VirE2 also can move to plant nuclei independently of T-DNA raises the further possibility that VirE2 hybrid proteins can be delivered to plant nuclei for purposes unrelated to T-DNA transfer per se. Toward development of VirE2 as a protein delivery system, we assessed the functionality of VirE2 mutants and hybrid proteins. Our results showed that the first 10 and last 9 residues of VirE2 are required for wild-type protein function. Moreover, fusions of heterologous proteins or peptides to either terminus abolished VirE2 function. Based on these findings, we suggest that it is impractical to base the development of a protein delivery system on the generation of fusions to either end of VirE2.
However, the VirE2.39::i31 mutant protein displays all of the properties of the wild-type protein in its capacity to (i) dimerize with VirE2 in S. cerevisiae and interact with VirE2 in A. tumefaciens, (ii) interact with VirE1 in S. cerevisiae and A. tumefaciens, and (iii) accumulate to abundant levels in A. tumefaciens. Most importantly, the virE2.39::i31 allele restores full virulence to a virE2 null mutant, and it is phenotypically silent when coexpressed with wild-type virE2 in merodiploid cells. We propose that a domain near the N terminus, as defined by the Tyr39::i31 mutation, corresponds to a novel region of VirE2 into which heterologous sequences can be inserted without disruption of protein function. Whether a protein delivery system based on insertion at this permissive site is universally applicable for all proteins or domains of interest awaits further investigation. Nevertheless, our present findings strongly indicate that this site will tolerate insertions of at least small proteins or protein domains of interest.
ACKNOWLEDGMENTS
We thank S. Elledge for providing the yeast strains and protocols for the two-hybrid studies and C. Manoil for the necessary constructs, antibodies, and protocols for the i31 insertional mutagenesis studies. We thank W. Margolin for GFP constructs and antibodies. We also thank Brenda Graf for excellent technical help and other members of this laboratory for helpful discussions. We thank Gene Nester for sharing results prior to publication.
This work was supported by NIH grant GM48746.
REFERENCES
- 1.Anderson D M, Schneewind O. Type III machines of gram-negative pathogens: injecting virulence factors into host cells and more. Curr Opin Microbiol. 1999;2:18–24. doi: 10.1016/s1369-5274(99)80003-4. . (Review.) [DOI] [PubMed] [Google Scholar]
- 2.Bai C, Elledge S J. Gene identification using the yeast two-hybrid system. Methods Enzymol. 1996;273:331–347. doi: 10.1016/s0076-6879(96)73029-x. [DOI] [PubMed] [Google Scholar]
- 3.Baron C, Thorstenson Y R, Zambryski P C. The lipoprotein VirB7 interacts with VirB9 in the membranes of Agrobacterium tumefaciens. J Bacteriol. 1997;179:1211–1218. doi: 10.1128/jb.179.4.1211-1218.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartel P L, Fields S. The yeast two-hybrid system. Oxford, United Kingdom: Oxford University Press; 1997. [Google Scholar]
- 5.Beijersbergen A, Dulk-Ras A D, Schilperoort R A, Hooykaas P J J. Conjugative transfer by the virulence system of Agrobacterium tumefaciens. Science. 1992;256:1324–1327. doi: 10.1126/science.256.5061.1324. [DOI] [PubMed] [Google Scholar]
- 6.Berger B R, Christie P J. Genetic complementation analysis of the Agrobacterium tumefaciens virB operon: virB2 through virB11 are essential virulence genes. J Bacteriol. 1994;176:3646–3660. doi: 10.1128/jb.176.12.3646-3660.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Binns A, Beaupre C, Dale E. Inhibition of VirB-mediated transfer of diverse substrates from Agrobacterium tumefaciens by the IncQ plasmid RSF1010. J Bacteriol. 1995;177:4890–4899. doi: 10.1128/jb.177.17.4890-4899.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchanan-Wollaston V, Passiatore J E, Cannon F. The mob and oriT mobilization functions of a bacterial plasmid promote its transfer to plants. Nature. 1987;328:172–175. [Google Scholar]
- 9.Bundock P, den Dulk-Ras A, Beijersbergen A, Hooykaas P J. Trans-kingdom T-DNA transfer from Agrobacterium tumefaciens to Saccharomyces cerevisiae. EMBO J. 1995;14:3206–3214. doi: 10.1002/j.1460-2075.1995.tb07323.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bundock P, Hooykaas P J. Integration of Agrobacterium tumefaciens T-DNA in the Saccharomyces cerevisiae genome by illegitimate recombination. Proc Natl Acad Sci USA. 1996;93:15272–15275. doi: 10.1073/pnas.93.26.15272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burns D L. Biochemistry of type IV secretion. Curr Opin Microbiol. 1999;2:25–29. doi: 10.1016/s1369-5274(99)80004-6. . (Review.) [DOI] [PubMed] [Google Scholar]
- 12.Chen C-Y, Winans S C. Controlled expression of the transcriptional activator gene virG in Agrobacterium tumefaciens by using the Escherichia coli lac promoter. J Bacteriol. 1991;173:1139–1144. doi: 10.1128/jb.173.3.1139-1144.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christie P J. The Agrobacterium tumefaciens T-complex transport apparatus: a paradigm for a new family of multifunctional transporters in eubacteria. J Bacteriol. 1997;179:3085–3094. doi: 10.1128/jb.179.10.3085-3094.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christie P J, Ward J E, Winans S C, Nester E W. The Agrobacterium tumefaciens virE2 gene product is a single-stranded-DNA-binding protein that associates with T-DNA. J Bacteriol. 1988;170:2659–2667. doi: 10.1128/jb.170.6.2659-2667.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Citovsky V, DeVos G, Zambryski P. Single-stranded DNA binding protein encoded by the virE locus of Agrobacterium tumefaciens. Science. 1988;240:501–504. doi: 10.1126/science.240.4851.501. [DOI] [PubMed] [Google Scholar]
- 16.Citovsky V, Wong M L, Zambryski P. Cooperative interaction of Agrobacterium VirE2 protein with single-stranded DNA: implications for the T-DNA transfer process. Proc Natl Acad Sci USA. 1989;86:1193–1197. doi: 10.1073/pnas.86.4.1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Citovsky V, Zupan J, Warnick D, Zambryski P. Nuclear localization of Agrobacterium VirE2 protein in plant cells. Science. 1992;256:1802–1805. doi: 10.1126/science.1615325. [DOI] [PubMed] [Google Scholar]
- 18.Dang T A, Zhou X-R, Graf B, Christie P J. Dimerization of the Agrobacterium tumefaciens VirB4 ATPase and the effect of ATP-binding cassette mutations on assembly and function of the T-DNA transporter. Mol Microbiol. 1999;32:1239–1253. doi: 10.1046/j.1365-2958.1999.01436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dang T A T, Christie P J. The VirB4 ATPase of Agrobacterium tumefaciens is a cytoplasmic membrane protein exposed at the periplasmic surface. J Bacteriol. 1997;179:453–462. doi: 10.1128/jb.179.2.453-462.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Das A, Stachel S, Ebert P, Allenza P, Montoya A, Nester E. Promoters of Agrobacterium tumefaciens Ti-plasmid. Nucleic Acids Res. 1986;14:1355–1364. doi: 10.1093/nar/14.3.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Groot M J A, Bundock P, Hooykaas P J J, Beijersbergen A G M. Agrobacterium tumefaciens-mediated transformation of filamentous fungi. Nat Biotechnol. 1998;16:839–842. doi: 10.1038/nbt0998-839. [DOI] [PubMed] [Google Scholar]
- 22.Deng W, Chen L, Peng W-T, Liang X, Sekiguchi S, Gordon M P, Nester E W. VirE1 is a specific molecular chaperone for the exported single-stranded-DNA-binding protein VirE2 in Agrobacterium. Mol Microbiol. 1999;31:1795–1808. doi: 10.1046/j.1365-2958.1999.01316.x. [DOI] [PubMed] [Google Scholar]
- 23.Dombek P, Ream W. Functional domains of Agrobacterium tumefaciens single-stranded DNA-binding protein VirE2. J Bacteriol. 1997;179:1165–1173. doi: 10.1128/jb.179.4.1165-1173.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandez D, Dang T A T, Spudich G M, Zhou X-R, Berger B R, Christie P J. The Agrobacterium tumefaciens virB7 gene product, a proposed component of the T-complex transport apparatus, is a membrane-associated lipoprotein exposed at the periplasmic surface. J Bacteriol. 1996;178:3156–3167. doi: 10.1128/jb.178.11.3156-3167.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fernandez D, Spudich G M, Zhou X-R, Christie P J. The Agrobacterium tumefaciens VirB7 lipoprotein is required for stabilization of VirB proteins during assembly of the T-complex transport apparatus. J Bacteriol. 1996;178:3168–3176. doi: 10.1128/jb.178.11.3168-3176.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fullner K J. Role of Agrobacterium virB genes in transfer of T complexes and RSF1010. J Bacteriol. 1998;180:430–434. doi: 10.1128/jb.180.2.430-434.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fullner K J, Nester E W. Temperature affects the T-DNA transfer machinery of Agrobacterium tumefaciens. J Bacteriol. 1996;178:1498–1504. doi: 10.1128/jb.178.6.1498-1504.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Garfinkel D J, Simpson R B, Ream L W, White F F, Gordon M P, Nester E W. Genetic analysis of crown gall: fine structure map of the T-DNA by site-directed mutagenesis. Cell. 1981;27:143–153. doi: 10.1016/0092-8674(81)90368-8. [DOI] [PubMed] [Google Scholar]
- 29.Howard E A, Zupan J R, Citovsky V, Zambryski P C. The VirD2 protein of A. tumefaciens contains a C-terminal bipartite nuclear localization signal: implications for nuclear uptake of DNA in plant cells. Cell. 1992;68:109–118. doi: 10.1016/0092-8674(92)90210-4. [DOI] [PubMed] [Google Scholar]
- 30.Hueck C J. Type III protein secretion systems in bacterial pathogens of animals and plants. Microbiol Mol Biol Rev. 1998;62:379–433. doi: 10.1128/mmbr.62.2.379-433.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ishida Y, Saito H, Ohta S, Hiei Y, Komari T, Kumashiro T. High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat Biotechnol. 1996;14:745–750. doi: 10.1038/nbt0696-745. [DOI] [PubMed] [Google Scholar]
- 32.Kunkel T A, Bebenek K, McClary J. Efficient site-directed mutagenesis using uracil-containing DNA. Methods Enzymol. 1991;204:125–139. doi: 10.1016/0076-6879(91)04008-c. [DOI] [PubMed] [Google Scholar]
- 33.Lee L-Y, Gelvin S B, Kado C I. pSa causes oncogenic suppression of Agrobacterium by inhibiting VirE2 protein export. J Bacteriol. 1999;181:186–196. doi: 10.1128/jb.181.1.186-196.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lippincott J, Traxler B. MalFGK complex assembly and transport and regulatory characteristics of MalK insertion mutants. J Bacteriol. 1997;179:1337–1343. doi: 10.1128/jb.179.4.1337-1343.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lohman T M, Ferrari M F. Escherichia coli single-stranded DNA-binding protein: multiple DNA-binding modes and cooperativities. Annu Rev Biochem. 1994;63:527–570. doi: 10.1146/annurev.bi.63.070194.002523. [DOI] [PubMed] [Google Scholar]
- 36.Ma X, Ehrhardt D W, Margolin W. Colocalization of cell division proteins FtsZ and FtsA to cytoskeletal structures in living Escherichia coli cells by using green fluorescent protein. Proc Natl Acad Sci USA. 1996;93:12998–3003. doi: 10.1073/pnas.93.23.12998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manoil C. Analysis of membrane protein topology using alkaline phosphatase and β-galactosidase gene fusions. Methods Cell Biol. 1991;34:61–75. doi: 10.1016/s0091-679x(08)61676-3. [DOI] [PubMed] [Google Scholar]
- 38.Manoil C, Bailey J. A simple screen for permissive sites in proteins: analysis of Escherichia coli lac permease. J Mol Biol. 1997;267:250–263. doi: 10.1006/jmbi.1996.0881. [DOI] [PubMed] [Google Scholar]
- 39.McBride K E, Knauf V C. Genetic analysis of the virE operon of the Agrobacterium Ti plasmid pTiA6. J Bacteriol. 1988;170:1430–1437. doi: 10.1128/jb.170.4.1430-1437.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nelson B D, Traxler B. Exploring the role of integral membrane proteins in ATP-binding cassette transporters: analysis of a collection of MalG insertion mutants. J Bacteriol. 1998;180:2507–2514. doi: 10.1128/jb.180.9.2507-2514.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ooms G, Hooykaas P J J, Van Veen R J M, Van Beelen P, Regensburg-Tunik R, Schilperoort R A. Octopine Ti-plasmid deletion mutants of Agrobacterium tumefaciens with emphasis on the right side of the T-region. Plasmid. 1982;7:15–19. doi: 10.1016/0147-619x(82)90023-3. [DOI] [PubMed] [Google Scholar]
- 42.Otten L, De Greve H, Leemans J, Hain R, Hooykaas P, Schell J. Restoration of virulence of vir region mutants of Agrobacterium tumefaciens strain B6S3 by coinfection with normal and mutant Agrobacterium strains. Mol Gen Genet. 1984;195:159–163. [Google Scholar]
- 43.Piers K L, Heath J D, Liang X, Stephens K M, Nester E W. Agrobacterium tumefaciens-mediated transformation of yeast. Proc Natl Acad Sci USA. 1996;93:1613–1618. doi: 10.1073/pnas.93.4.1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Raineri D M, Bottino P, Gordon M P, Nester E W. Agrobacterium-mediated transformation of rice (Oryza sativa L.) Bio/Technology. 1990;8:33–38. [Google Scholar]
- 45.Regensburg T A, Hooykaas P J. Transgenic N. glauca plants expressing bacterial virulence gene virF are converted into hosts for nopaline strains of A. tumefaciens. Nature. 1993;363:69–71. doi: 10.1038/363069a0. [DOI] [PubMed] [Google Scholar]
- 46.Ruvola P P, Keating K M, Williams K R, Chase J W. Single-stranded DNA binding proteins (SSBs) from prokaryotic transmissible plasmids. Proteins Struct Funct Genet. 1991;9:120–134. doi: 10.1002/prot.340090206. [DOI] [PubMed] [Google Scholar]
- 47.Sen P, Pazour G J, Anderson D, Das A. Cooperative binding of Agrobacterium tumefaciens VirE2 protein to single-stranded DNA. J Bacteriol. 1989;171:2573–2580. doi: 10.1128/jb.171.5.2573-2580.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sory M-P, Cornelius G R. Translocation of a hybrid YopE-adenylate cyclase from Yersinia enterocolitica into HeLa cells. Mol Microbiol. 1994;14:583–594. doi: 10.1111/j.1365-2958.1994.tb02191.x. [DOI] [PubMed] [Google Scholar]
- 49.Stachel S E, An G, Flores C, Nester E W. A Tn3lacZ transposon for the random generation of beta-galactosidase gene fusions: application to the analysis of gene expression in Agrobacterium. EMBO J. 1985;4:891–898. doi: 10.1002/j.1460-2075.1985.tb03715.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stachel S E, Timmerman B, Zambryski P. Activation of Agrobacterium tumefaciens vir gene expression generates multiple single-stranded T-strand molecules from the pTiA6 T-region: requirement for 5′ virD gene products. EMBO J. 1987;6:857–863. doi: 10.1002/j.1460-2075.1987.tb04831.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Stahl L E, Jacobs A, Binns A N. The conjugal intermediate of plasmid RSF1010 inhibits Agrobacterium tumefaciens virulence and VirB-dependent export of VirE2. J Bacteriol. 1998;180:3933–3939. doi: 10.1128/jb.180.15.3933-3939.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sundberg C, Meek L, Carroll K, Das A, Ream W. VirE1 protein mediates export of the single-stranded DNA-binding protein VirE2 from Agrobacterium tumefaciens into plant cells. J Bacteriol. 1996;178:1207–1212. doi: 10.1128/jb.178.4.1207-1212.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward J E J, Dale E M, Christie P J, Nester E W, Binns A N. Complementation analysis of Agrobacterium tumefaciens Ti plasmid virB genes by use of a vir promoter expression vector: virB9, virB10, and virB11 are essential virulence genes. J Bacteriol. 1990;172:5187–5199. doi: 10.1128/jb.172.9.5187-5199.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Winans S, Allenza P, Stachel S, McBride K, Nester E. Characterization of the virE operon of the Agrobacterium Ti plasmid pTiA6. Nucleic Acids Res. 1987;15:825–837. doi: 10.1093/nar/15.2.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winans S C, Burns D L, Christie P J. Adaptation of a conjugal transfer system for the export of pathogenic macromolecules. Trends Microbiol. 1996;4:64–68. doi: 10.1016/0966-842X(96)81513-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yanofsky M F, Porter S G, Young C, Albright L M, Gordon M P, Nester E W. The virD operon of Agrobacterium tumefaciens encodes a site-specific endonuclease. Cell. 1986;47:471–477. doi: 10.1016/0092-8674(86)90604-5. [DOI] [PubMed] [Google Scholar]
- 57.Young C, Nester E W. Association of the VirD2 protein with the 5′ end of T strands in Agrobacterium tumefaciens. J Bacteriol. 1988;170:3367–3374. doi: 10.1128/jb.170.8.3367-3374.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhou, X.-R., and P. J. Christie. Unpublished data.
- 59.Zhou X-R, Christie P J. Suppression of mutant phenotypes of the Agrobacterium tumefaciens VirB11 ATPase by overproduction of VirB proteins. J Bacteriol. 1997;179:5835–5842. doi: 10.1128/jb.179.18.5835-5842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zupan J, Zambryski P. The Agrobacterium DNA transfer complex. Crit Rev Plant Sci. 1997;16:279–295. [Google Scholar]