Abstract
Introduction
Coronavirus-2019 disease (COVID-19)-associated acute kidney injury (AKI) and its short and mid-term effect on kidney has been well established in the previous literature, indicating a high number of AKI in hospitalized patients associated with high rates of mortality, followed by high rates of unresolved kidney injury at the time of discharge. However, the long-term impact of AKI and its resulting lack of recovery at the time of discharge has not been investigated. Herein, we sought to explore the possible relationship between AKI and unresolved kidney injury and post-discharge mortality.
Method
In this cohort study, patients hospitalized with COVID-19 who survived until discharge were followed for a median of 9.6 months. AKI during hospitalization based on the staging according to Kidney Disease: Improving Global Outcomes (KDIGO) criteria and kidney injury status at discharge and other comorbidities and mortality during the follow-up period were recorded. The desired association was investigated using Cox proportional hazards regression after adjustment for potential confounders.
Result
Among 1,017 discharged patients, 298 patients (29.3%) experienced AKI during hospitalization according to KDIGO criteria, of whom 178 patients (59.7%) were diagnosed with unresolved kidney injury at the time of discharge. After adjusting for potential confounders, Cox regression indicated that AKI stage 3 (hazard ratio (HR): 4.56, 95% confidence interval (CI): 1.89–10.99, p = 0.001) and unresolved kidney injury at the time of discharge (HR: 2.09, 95% CI: 1.18–3.73, p = 0.011) were significantly associated with mortality during the post-discharge period. Additionally, Kaplan-Meier curves for overall survival indicated an increased risk of mortality in patients with stage 2, stage 3 AKI, and unresolved kidney injury at the time of discharge (p < 0.001).
Conclusion
Overall, it was shown that patients with COVID-19 who develop AKI, mainly stage 2 and 3, and patients with unresolved kidney injury at the time of discharge, were at an increased risk of mortality, even after hospitalization for an extended period of time.
Keywords: Acute kidney injury, Kidney dysfunction, AKI recovery, Mortality, Coronavirus disease 2019
Introduction
Acute kidney injury (AKI) has been recognized as a common complication of the Coronavirus-2019 disease (COVID-19) and is one of the main contributors to COVID-19 mortality [1]. COVID-19-associated AKI and its long-term impact on the kidney, reflected by the higher population of patients without renal recovery at the hospital discharge as well as patients with a substantial decrease in estimated glomerular filtration rate in the after hospital discharge period, compared to AKI in patients without COVID-19 has been well established [2, 3, 4, 5]. However, despite the significant association between COVID-19 associated AKI and mortality, the long-term impact of COVID-19-associated AKI and continued kidney dysfunction on mortality has not been well investigated.
Methods
This study was performed in line with the Helsinki declaration and approved by the Ethics Committee of Tehran University of Medical Sciences (Code: IR.TUMS.VCR.REC.1398.1037). In this prospective cohort study, conducted at Sina Hospital (affiliated with Tehran University of Medical Sciences), a tertiary educational center in Tehran, Iran, all consecutive patients aged ≥18 years old, diagnosed with COVID-19 who were hospitalized between February 16 2020, and December 1, 2020 and survived to discharge were included. The COVID-19 diagnosis was ascertained in patients with positive reverse-transcriptase polymerase-chain-reaction test of respiratory samples for severe acute respiratory syndrome coronavirus 2 or in patients with clinical suspicion for COVID-19 according to the World Health Organization (WHO) interim guidance with a chest computed tomography involvement in favor of COVID-19. Patients with a history of end-stage renal kidney disease and those lost to follow-up were excluded. Medical records, including each patient's characteristics and hospital course, were collected using the hospital's electronic health system. Owing to the unavailability of prehospital patients baseline serum creatinine (SCr) in most of the population, baseline creatinine was imputed according to a modification of diet in renal disease estimated glomerular filtration fraction of 75 mL/min/1.73 m2 as per the Kidney Disease: Improving Global Outcomes AKI guideline, a validated method in line with previous studies utilizing the same mechanism [3, 6]. Patients were followed from the day of discharge (“time zero” or index date) for a median of 9.6 months (interquartile range of 6.4–11.4 months). The data regarding the occurrence and date of mortality among the discharged patients was obtained once from the national organization for civil registration of Iran on February 23, 2021. They were divided based on the presence of AKI at the time of hospitalization and the state of renal recovery at the time of discharge, and the association of respected groups with mortality was explored. AKI and was defined according to the Kidney Disease: Improving Global Outcomes criteria determined as the increase in SCr to ≥1.5 times to 1.9 times of baseline occurred within the previous 7 days or an increase in SCr by ≥0.3 mg/dL (≥26.5 μmol/L) within 48 h as stage 1, 2–2.9 times increase in SCr within 7 days as stage 2, and 3 times or more increase in SCr within 7 days as stage 3. AKI recovery was defined as <0.3 mg/dL (<26.5 μmol/L) difference from the baseline SCr and with less than a 50% increase from the baseline creatinine at the time of discharge.
Categorical variables were compared using the χ2 or Fischer's exact tests and were presented as numbers (%). Continuous variables with normal distribution were expressed as mean ± standard deviation and compared utilizing the T test. Cox proportional hazards regression was performed to estimate hazard ratios (HRs) with 95% confidence intervals (CIs) for COVID-19 associated AKI and unresolved kidney injury at the time of discharge with mortality in univariate analysis. Additionally, the multivariate analysis was conducted to adjust for potential confounders, including the risk factors that remained significant in univariate analysis. Furthermore, the Kaplan-Meier curve and the log-rank test were used to investigate the association between COVID-19 associated AKI and renal dysfunction at the discharge and all-cause mortality.
Results
A total of 1,192 patients were discharged during the study period. After excluding patients with end-stage renal kidney disease and patients with missing creatinine values, 1,017 patients who survived until discharge entered the final analysis. During the follow-up with a median of 9.6 months, 67 patients (6.5%) incurred death (online suppl. Fig. 1; for all online suppl. material, see www.karger.com/doi/10.1159/000524451). Out of all survived patients at the time of discharge, in-hospital AKI was experienced in 298 patients (29.3%). Among patients with AKI, 178 patients (59.7%) were diagnosed with unresolved kidney injury at the time of discharge. Patients who developed COVID-19 associated AKI were significantly older (59.9 ± 18.1 vs. 56.6 ± 15.2, p = 0.005) and more likely to be male (86.2% vs. 50.2%, p < 0.001) than patients without AKI (Table 1). Furthermore, the development of AKI during hospitalization was significantly higher in patients with comorbidities, including hypertension, diabetes mellitus, dyslipidemia, coronary artery disease, congestive heart failure, cerebrovascular accident, and chronic kidney disease. Due to the exclusion of patients who incurred death during hospitalization, there was no difference in intensive care unit admissions and disease severity between patients with and without AKI. In line with our postulation, not only patients with COVID-19-associated AKI during hospitalization experienced significantly higher rates of all-cause mortality after discharge than patients without AKI (9.4% vs. 5.4%. p = 0.020) but also higher stages of AKI was tied to higher mortality rates after discharge (33.3% vs. 15.2% vs. 5.7% mortality rates in stage 3, 2, and 1, respectively, p < 0.001). Accordingly, patients with stage 2 and 3 AKI were more likely to experience unresolved kidney injury at the time of the hospital discharge than patients with stage 1 AKI (93.5% of patients with stage 2 AKI and 91.7% of patients with stage 3 AKI vs. 49.6% of patients with stage 1 AKI, p < 0.001).
Table 1.
Characteristics of the discharged patients
| No AKI (n = 719; 70.7%) |
AKI |
p value* | p value+ | ||||
|---|---|---|---|---|---|---|---|
| total (n = 298; 29.3%) |
stage 1 (n = 228; 22.4%) |
stage 2 (n = 46; 4.5%) |
stage 3 (n = 24; 2.4%) |
||||
| Demographics and comorbidities, n (%) | |||||||
| Age, years | 56.6±15.2 | 59.9±18.1 | 58.4±18.5 | 65.1 ±16.1 | 64.9±15.9 | 0.005 | 0.0271 |
| ≥65 years | 234 (32.5) | 133 (44.6) | 96 (42.1) | 21 (45.7) | 16 (66.7) | <0.001 | 0.070 |
| Male gender | 361 (50.2) | 257 (86.2) | 204 (89.5) | 38 (82.6) | 15 (62.5) | <0.001 | 0.0013 |
| Hypertension | 277 (38.5) | 150 (50.3) | 107 (46.9) | 29 (63.0) | 14 (58.3) | 0.001 | 0.098 |
| Diabetes | 188 (26.1) | 103 (34.6) | 67 (29.4) | 23 (50.0) | 13 (54.2) | 0.007 | 0.0031 |
| Dyslipidemia | 192 (26.7) | 105 (35.2) | 73 (32.0) | 22 (47.8) | 10 (41.7) | 0.006 | 0.097 |
| Coronary artery disease | 121 (16.8) | 84 (28.2) | 67 (29.4) | 11 (23.9) | 6 (25.0) | <0.001 | 0.706 |
| CHF | 18 (2.5) | 18 (6.0) | 12 (5.3) | 3 (6.5) | 3 (12.5) | 0.005 | 0.363 |
| CVA | 20 (2.8) | 16 (5.4) | 10 (4.4) | 4 (8.7) | 2 (8.3) | 0.042 | 0.396 |
| CKD | 5 (0.7) | 32 (10.7) | 13 (5.7) | 11 (23.9) | 8 (33.3) | <0.001 | <0.0011 |
| Chronic respiratory disease | 46 (6.4) | 16 (5.4) | 14 (6.1) | 2 (4.3) | 0 (0.0) | 0.533 | 0.423 |
| Malignancy | 32 (4.5) | 6 (2.0) | 2 (0.9) | 3 (6.5) | 1 (4.2) | 0.062 | 0.0331 |
| Tobacco smoking | 78 (10.7) | 47 (15.8) | 37 (16.2) | 5 (10.9) | 5 (20.8) | 0.030 | 0.514 |
| Laboratory data | |||||||
| Baseline creatinine, mg/dL | 1.06±0.17 | 0.96±0.13 | 0.94±0.13 | 0.99±0.12 | 1.05±0.16 | <0.001 | <0.0011, 3 |
| Admission creatinine, mg/dL | 0.96±0.17 | 1.80±1.18 | 1.38±0.26 | 2.27±0.46 | 4.91±2.19 | <0.001 | <0.0011, 2, 3 |
| Peak creatinine, mg/dL | 0.99±0.16 | 1.88±1.21 | 1.43±0.25 | 2.37±0.38 | 5.25±1.98 | <0.001 | <0.0011, 2, 3 |
| Discharge creatinine, mg/dL | 0.96±0.16 | 1.73±1.11 | 1.37±0.26 | 2.17±0.58 | 4.27±2.50 | <0.001 | <0.0011, 2, 3 |
| COVID-19 hospitalization and follow-up, n (%) | |||||||
| Severe disease | 463 (64.4) | 193 (64.8) | 147 (64.5) | 31 (67.4) | 15 (62.5) | 0.911 | 0.904 |
| ICU admission | 38 (5.3) | 19 (6.4) | 11 (4.8) | 5 (10.9) | 3 (12.5) | 0.491 | 0.136 |
| Length of stay, days | 5 (3–8) | 5 (4–9) | 5 (4–8) | 7.5 (4.75–11) | 9.5 (5–12) | 0.037 | <0.0011 |
| Unresolved kidney injury | 0 (0.0) | 178 (59.7) | 113 (49.6) | 43 (93.5) | 22 (91.7) | <0.001 | <0.0011 |
| Mortality during follow-up | 39 (5.4) | 28 (9.4) | 13 (5.7) | 7 (15.2) | 8 (33.3) | 0.020 | <0.0011, 2, 3 |
AKI, acute kidney injury; CHF, congestive heart failure; CKD, chronic kidney disease; CVA, cerebrovascular accident; ICU, intensive care unit.
Comparison between patients with and without AKI.
Comparison between patients with three stages of AKI. The numbers in superscript indicate the AKI stage with statistically different values based on post-hoc analysis and after Bonferroni correction.
Cox proportional hazards regression was used to assess the potential association of COVID-19 associated AKI stages during hospitalization and post-discharge all-cause mortality (Table 2). Univariate analysis revealed that AKI stage 2 (HR: 2.83, 95% CI: 1.26–6.34, p = 0.011) and 3 (HR: 7.35, 95% CI: 3.43–15.74, p < 0.001) during hospital course were significantly associated with all-cause mortality after discharge. Additionally, after multivariate analysis to adjust for possible confounders, AKI stage 3 (HR: 4.56, 95% CI: 1.89–10.99, p = 0.001) remained significant as a predictor of all-cause mortality after hospital discharge.
Table 2.
Risk factors of post-discharge mortality based on cox proportional hazard regression
| Univariate analysis |
Multivariate analysis (AKI staging) |
Multivariate analysis (unresolved kidney injury) |
|||||||
|---|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | p value | HR | 95% CI | p value | HR | 95% CI | p value | |
| Unresolved kidney injury AKI | 2.929 | 1.785–4.806 | <0.001 | − | − | − | 2.099 | 1.181–3.731 | 0.011 |
| Stage 1 | 1.042 | 0.556–1.952 | 0.898 | 0.955 | 0.477–1.909 | 0.897 | − | − | − |
| Stage 2 | 2.836 | 1.268–6.342 | 0.011 | 2.103 | 0.899–4.918 | 0.086 | − | − | − |
| Stage 3 | 7.354 | 3.434–15.748 | <0.001 | 4.560 | 1.891–10.995 | 0.001 | − | − | − |
| Age ≥65 years | 5.157 | 3.004–8.853 | <0.001 | 4.052 | 2.239–7.331 | <0.001 | 4.056 | 2.237–7.354 | <0.001 |
| Male gender | 1.411 | 0.842–2.364 | 0.191 | 1.154 | 0.644–2.067 | 0.629 | 1.038 | 0.589–1.828 | 0.896 |
| Hypertension | 1.555 | 0.963–2.513 | 0.071 | 0.928 | 0.511–1.687 | 0.808 | 0.895 | 0.497–1.611 | 0.713 |
| Diabetes | 1.225 | 0.736–2.040 | 0.434 | 0.728 | 0.403–1.312 | 0.291 | 0.826 | 0.468–1.458 | 0.512 |
| Dyslipidemia | 1.804 | 1.110–2.932 | 0.017 | 1.223 | 0.645–2.316 | 0.536 | 1.142 | 0.601–2.169 | 0.684 |
| Coronary artery disease | 1.570 | 0.923–2.671 | 0.096 | 0.898 | 0.466–1.731 | 0.750 | 0.800 | 0.414–1.546 | 0.508 |
| CHF | 4.662 | 2.307–9.423 | <0.001 | 2.611 | 1.191–5.724 | 0.017 | 2.859 | 1.310–6.236 | 0.008 |
| CVA | 2.846 | 1.230–6.586 | 0.015 | 1.713 | 0.679–4.319 | 0.253 | 1.505 | 0.589–3.846 | 0.392 |
| CKD | 2.724 | 1.177–6.303 | 0.019 | 1.257 | 0.472–3.344 | 0.646 | 1.331 | 0.499–3.552 | 0.567 |
| Chronic respiratory disease 1.250 | 0.502–3.109 | 0.631 | 0.763 | 0.290–2.006 | 0.584 | 0.665 | 0.252–1.754 | 0.411 | |
| Malignancy | 7.642 | 4.169–14.009 | <0.001 | 6.724 | 3.457–13.079 | <0.001 | 7.174 | 3.732–13.791 | <0.001 |
| Tobacco smoking | 2.416 | 1.377–4.240 | 0.002 | 2.043 | 1.088–3.834 | 0.026 | 2.109 | 1.135–3.920 | 0.018 |
AKI, acute kidney injury; CHF, congestive heart failure; CI, confidence interval; CKD, chronic kidney disease; CVA, cerebrovascular accident; HR, hazard ratio.
Univariate analysis indicated that patients unable to achieve kidney recovery at discharge were significantly at a higher risk of mortality after the time of discharge (HR: 2.92, 95% CI: 1.78–4.80, p < 0.001). Interestingly, after adjustment for other variables, unresolved kidney injury was significantly associated with post-discharge all-cause mortality compared to patients who recovered from kidney injury at the time of discharge (HR: 2.09, 95% CI: 1.18–3.73, p = 0.011).
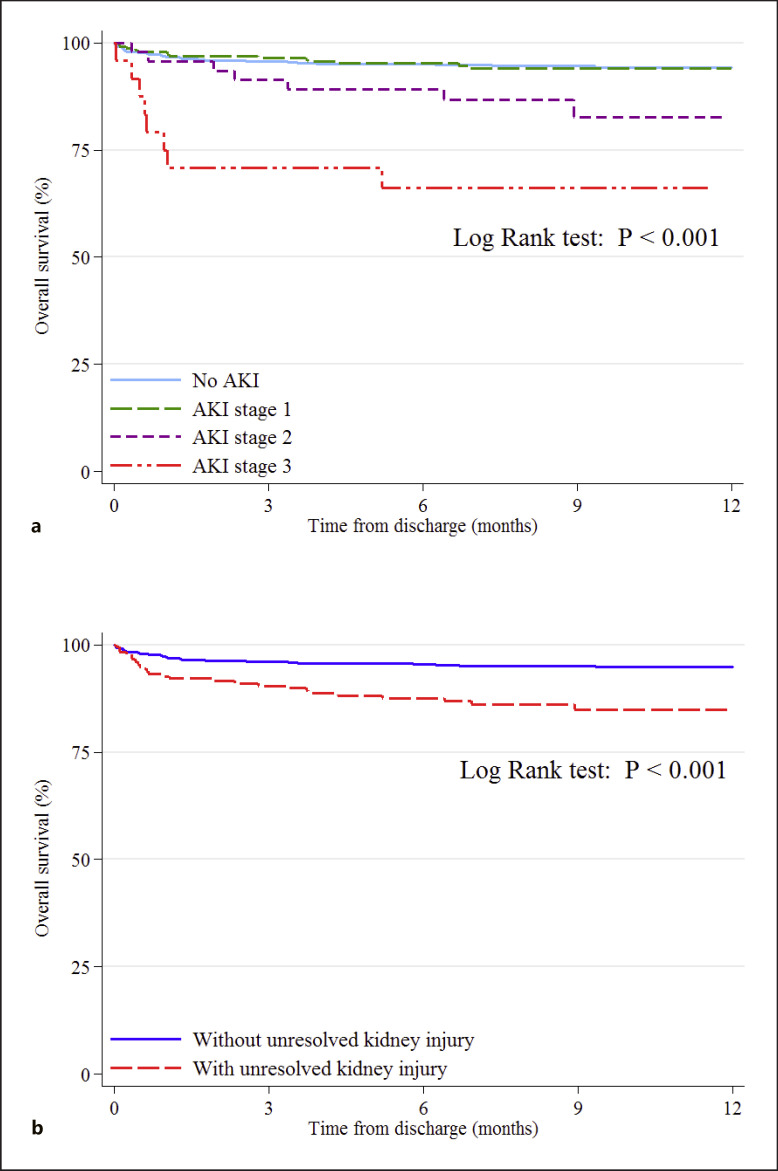
Implementing the Kaplan-Meier curves for overall survival of discharged patients with COVID-19 indicated that although patients with stage 1 AKI did not experience higher mortality rates, patients with stage 2 and 3 had declined the chance of survival during the 9.6 month follow-up time, with stage 3 AKI in a higher risk of mortality than AKI stage 2 (Fig. 1a), which was supplemented by log-rank test (p < 0.001). Furthermore, patients with no kidney recovery at the time of discharge were at increased risk of mortality during the study period (Fig. 1b), supported by a log-rank test (p < 0.001).
Fig. 1.
Kaplan-Meier curves for overall survival of discharged patients with COVID-19 regarding AKI and its staging (a), and unresolved kidney injury (b).
Discussion
In conclusion, we set out to show the potential long-term impact of the COVID-19-associated AKI and its resulting kidney dysfunction at the time of recovery on the post-discharge mortality during a median of 9.6 months. The persistence of kidney injury after COVID-19-associated AKI has been reported at controversial rates, including as high as 32% of patients with no recovery at a median of 21 days after hospital discharge to as low as 9% in the median time of 4 months [3, 7]. However, the high risk of continued kidney dysfunction has been established in the literature [8, 9]. Given the results provided by this study, it seems vital to pay greater attention to patients with COVID-19-associated AKI, in particular patients with higher stages of AKI and patients with unresolved kidney dysfunction at the time of discharge, irrespective of the kidney state in the subsequent follow-ups.
Although this is the first study to investigate the long-term effect of AKI in COVID-19 on mortality, to the best of our knowledge, the following limitations should be acknowledged. First, due to the strains on the health care system, individuals' follow-up to renal function tests could not be provided. Second, this is a single-center study, and interpretation of its results should be made with caution. Last, the specific cause of death in each patient during the study period was not available.
Statement of Ethics
The Ethics Committee of Tehran University of Medical Sciences approved the protocol of this study (Code: IR.TUMS.VCR.REC.1398.1037). A written consent form was depicted from all participants after explaining the aims of the study.
Conflict of Interest Statement
The authors declare no conflict of interest.
Funding Sources
This study was not funded by any agency.
Author Contributions
Azar Hadadi: methodology, investigation, writing − original draft, visualization, and critically revising the manuscript. Hossein Farrokhpour: investigation, writing − original draft, data curation, methodology, writing − original draft, visualization, and critically revising the manuscript. Sina Rashedi: methodology, investigation, writing − original draft, methodology, writing − original draft, visualization, analysis, data curation, and critically revising the manuscript. Samira Kafan: validation, methodology, writing − original draft, visualization, and reviewing the revised version. Mehran Sotoudehnia: investigation, writing − original draft, visualization, and reviewing the revised version. Hormat Rahimzadeh: validation, investigation, data curation, writing − review & editing, and reviewing the revised version. Seidzia Tabatabaei: conceptualization, methodology, supervision, writing − review & editing, critically revising the manuscript. Effat Razeghi and Ziba Aghsaeifard: conceptualization, methodology, supervision, writing − review & editing, and reviewing the revised version. All the authors who contributed to this study approved the final version of the manuscript to be published and agreed to be responsible for all aspects of the work.
Data Availability Statement
Data used to generate this study are available upon reasonable request to the corresponding.
Acknowledgment
We appreciate the Research Development Centre of Sina Hospital for its intellectual supports.
Azar Hadadi, Hossein Farrokhpour, and Sina Rashedi contributed equally to this work and shared co-first authorship.
References
- 1.Fu EL, Janse RJ, de Jong Y, van der Endt VHW, Milders J, van der Willik EM, et al. Acute kidney injury and kidney replacement therapy in COVID-19: a systematic review and meta-analysis. Clin Kidney J. 2020;13((4)):550–63. doi: 10.1093/ckj/sfaa160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rahimzadeh H, Kazemian S, Rahbar M, Farrokhpour H, Montazeri M, Kafan S, et al. The risk factors and clinical outcomes associated with acute kidney injury in patients with COVID-19: data from a large cohort in Iran. Kidney Blood Press Res. 2021;46((5)):620–8. doi: 10.1159/000517581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chan L, Chaudhary K, Saha A, Chauhan K, Vaid A, Zhao S, et al. AKI in hospitalized patients with COVID-19. J Am Soc Nephrol. 2021;32((1)):151–60. doi: 10.1681/ASN.2020050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nugent J, Aklilu A, Yamamoto Y, Simonov M, Li F, Biswas A, et al. Assessment of acute kidney injury and longitudinal kidney function after hospital discharge among patients with and without COVID-19. JAMA Netw Open. 2021;4((3)):e211095. doi: 10.1001/jamanetworkopen.2021.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowe B, Cai M, Xie Y, Gibson AK, Maddukuri G, Al-Aly Z. Acute kidney injury in a national cohort of hospitalized US veterans with COVID-19. Clin J Am Soc Nephrol. 2021;16((1)):14–25. doi: 10.2215/CJN.09610620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Závada J, Hoste E, Cartin-Ceba R, Calzavacca P, Gajic O, Clermont G, et al. A comparison of three methods to estimate baseline creatinine for RIFLE classification. Nephrol Dial Transplant. 2010;25((12)):3911–8. doi: 10.1093/ndt/gfp766. [DOI] [PubMed] [Google Scholar]
- 7.Zhang NH, Cheng YC, Luo R, Zhang CX, Ge SW, Xu G. Recovery of new-onset kidney disease in COVID-19 patients discharged from hospital. BMC Infect Dis. 2021;21((1)):397. doi: 10.1186/s12879-021-06105-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fisher M, Neugarten J, Bellin E, Yunes M, Stahl L, Johns TS, et al. AKI in hospitalized patients with and without COVID-19: a Comparison Study. J Am Soc Nephrol. 2020;31((9)):2145–57. doi: 10.1681/ASN.2020040509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lee JR, Silberzweig J, Akchurin O, Choi ME, Srivatana V, Lin J, et al. Characteristics of acute kidney injury in hospitalized COVID-19 patients in an urban academic medical center. Clin J Am Soc Nephrol. 2021;16((2)):284–6. doi: 10.2215/CJN.07440520. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data used to generate this study are available upon reasonable request to the corresponding.