Abstract
Background
Older adults denoted one of the populations that mostly suffered from the consequences of the COVID-19 pandemic. The cost of confinement was paid in terms of social isolation, distance from relatives and friends, lack of social support, and limited access to the healthcare system, which had a negative impact on health of older adults with comorbidities and frailty.
Objectives
The purpose of the present study was to report the consequences of the COVID-19 pandemic on cognitive performances, functional status, and health-related quality of life among frail outpatients, compared to pre-pandemic status.
Method
The current sample was part of a larger sample of frail and pre-frail outpatients, who were first evaluated at the clinic between April and May 2019 and who underwent a first follow-up evaluation between April and May 2020. Those outpatients who have undergone the first follow-up evaluation were contacted between April and May 2021 and were asked to voluntarily participate in a second telephone-based evaluation. Cognitive performances (through Mini Mental State Examination − MMSE), functional independency in basic and instrumental daily activities, physical and mental components of health-related quality of life (SF-12 PCS and SF-12 MCS, respectively) were evaluated and compared to previous evaluations.
Results
Seventy one outpatients (mean age of 80.69 years) completed the present follow-up evaluation. Patients reported significantly lower cognitive performances (mean MMSE 19.37; p < 0.001), lower physical quality of life (mean score 31.69; p < 0.001), and lower mental quality of life (mean score 38.79; p < 0.001) compared to both pre-pandemic baseline and the first follow-up. Moreover, patients showed a significantly reduced independency in basic daily activities (mean score 3.8; p = 0.004), and a significantly reduced independency in managing telephone (p = 0.012) and medications (p = 0.035), compared to baseline.
Conclusions
The COVID-19 pandemic has been a prolonged stressor over time, which has markedly affected health-related quality of life of outpatients, and it can be considered a stressor that might have contributed to the patients' greater cognitive and functional vulnerability.
Keywords: COVID-19, Health-related quality of life, Cognitive functioning, Functional status, Frailty
Introduction
Since it was declared a pandemic in March 2020, the COVID-19 has had a growing negative impact on public health worldwide. Older adults denoted one of the populations that mostly suffered from the consequences of the pandemic, resulting in exposure to the risk of greater symptomatology's severity and to a high risk of adverse outcomes such as hospitalization and mortality [1]. Many strategies have been implemented to contain the spread of the disease and to protect the weakest groups of the population, including older adults; consistently, the institution of national lockdown, quarantine, and maintaining social distance have been the most widespread preventive strategies [2]. Nevertheless, older adults have paid the cost of confinement in terms of social isolation, distance from relatives and friends, lack of social support, and a consequent limited access to the healthcare system. In this context, recent evidence have interestingly highlighted that during the pandemic, older adults needed to readjust their lifestyle habits, by driving less, traveling less, and spending much more time on the computer [3]. These forced conditions (e.g., social isolation, limited access to cognitively stimulating activities) progressively affected older adults' physical and functional status [4], as well as their psychological health, peculiarly characterized by clinically significant psychological distress, in terms of depression and anxiety levels [5].
Remarkably, the cost of these consequences appears exponentially higher in elderly subjects with comorbidities and frailty, therefore more vulnerable to external stressors [6]. Although older adults with frailty have been the subject of a progressively increased interest over the last months, most studies have partially captured the complex consequences of the pandemic on their health status. In this regard, institutionalized patients affected by the SARS-CoV-2 have often been involved in studies, with the aim of emphasizing the importance of frailty assessment in the management of the disease's sequelae. Similarly, clinical outcomes of interest, such as cognitive and functional status and quality of life, have been also evaluated in subjects affected by the SARS-CoV-2, or they have been discussed separately. Therefore, a need that appears partially unmet is the integrated evaluation of the consequences related to living during the COVID-19 pandemic (and not the consequences of the disease per se) on the older adults' health, by jointly assessing their quality of life and their cognitive and functional status.
Consistently with the reported recent literature, we hypothesized that the changes in lifestyle, caused by the pandemic, might have had a negative impact on peculiar sensitive domains, such as cognition, functional independency, and health-related quality of life. Thus, the purpose of the present study was to report the consequences of the COVID-19 pandemic on cognitive performances, functional status, and health-related quality of life among frail outpatients, compared to pre-pandemic status.
Materials and Methods
The participants of the present report were older outpatients, referred to the Geriatrics and Multidimensional Evaluation Clinic of the University Hospital in Messina (Italy). The current sample originated from a larger sample of frail and pre-frail outpatients, who were first evaluated at the clinic between April and May 2019 (baseline − t0), and who underwent a first follow-up evaluation (t1) between April and May 2020 (concerning only cognitive performances and health-related quality of life). The details on the sample, as well as the baseline and the first follow-up findings are discussed in a previous study [7]. Those outpatients who underwent the first follow-up were contacted between April and May 2021, and they were asked to voluntarily participate in a second telephone-based evaluation (t2), given the protracted restrictions and the limited access to the clinic.
The telephone-based evaluation performed at t2 included the assessment of cognitive and functional status, health-related quality of life, and the report of negative age-related outcomes. The detailed description of the cognitive assessment's procedure is reported elsewhere [7]. In summary, we administered the validated Italian telephone version of the Mini Mental State Examination (Itel-MMSE) to screen for cognitive functioning; in line with the standard procedure, by using an algorithm, we then converted the score obtained on the Itel-MMSE into the corresponding estimated score of the MMSE, in order to make comparisons. Functional status was assessed through the Basic Activities of Daily Living (BADL) scale and by two items from the Instrumental Activities of Daily Living (IADL) scale, namely managing telephone and managing medications. Health-related quality of life was assessed through the Short Form-12 (SF-12), which provides two synthetic indexes, namely the Physical Component Summary (PCS) and the Mental Component Summary (MCS). For each patient included in the present report, pre-pandemic baseline data (t0) on cognitive performances, functional status, health-related quality of life, and age-related outcomes were retrospectively retrieved from our records.
Statistical Analysis
Data were analyzed through the IBM SPSS.26 statistical software. A post hoc power analysis was performed, by using the G*Power software (version 3.1.9.6); based on an effect size of 0.85, the sample size of 71, and the α error probability of 0.05, we obtained an effective power of 99%. Continuous variables were expressed as mean ± standard deviation; categorical variables were expressed as count and percentages. Because of the relatively small sample size, a nonparametric statistical approach was preferred. The McNemar test for paired samples was used to test the presence of significant differences in dichotomous variables between follow-up and baseline; Friedman's test and Wilcoxon test for paired samples were used to test the presence of significant differences in continuous variables. Values of p < 0.05 were considered statistically significant.
Results
For the purpose of the present study, we were able to contact by phone ninety-three outpatients, among those who underwent the first follow-up evaluation [7] since eleven subjects were no longer traceable. Fourteen subjects did not provide their consent to the current telephonic evaluation; eight deceased subjects were reported. Therefore, the final sample included in the present study consisted of 71 outpatients, with a mean age of 80.7 years and a prevalence of female gender of approximately 70%. The outpatients' frailty status was originally evaluated at baseline, by the calculation of a 35-deficit Frailty Index (FI); FI cutoff values were defined according to the Rockwood's deficit accumulation model [8]. Only two outpatients reported to have been infected by the SARS-CoV-2.
The principal baseline characteristics, which were retrospectively collected, are summarized in Table 1. Differences in age-related outcomes, cognitive performances, functional status, and health-related quality of life between baseline and the current follow-up evaluation are reported in Table 2.
Table 1.
Baseline (t0) sample's clinical characteristics
| Characteristics | All sample (N = 71) |
|---|---|
| Smoking, n (%) | 4 (5.8) |
| Chronic cerebrovascular condition, n (%) | 24 (33.3) |
| Hypertension, n (%) | 66 (94.1) |
| Atrial fibrillation, n (%) | 11 (15.6) |
| Diabetes, n (%) | 30 (43.1) |
| Dyslipidemia, n (%) | 12 (17.6) |
| Hypercholesterolemia, n (%) | 21 (29,4) |
| Thyroid failure, n (%) | 9 (13.7) |
| Chronic obstructive pulmonary disease, n (%) | 7 (9.8) |
| Osteoporosis, n (%) | 20 (29.4) |
| Kidney failure, n (%) | 11 (15.6) |
| Frailty index (FI), mean±SD | 0.24±0.10 |
| Frailty status, n (%) | |
| Frail (FI ≥0.25) | 33 (46.9) |
| Pre-frail (FI 0.08–0.24) | 38 (53.1) |
| Nonfrail (FI <0.08) | 0 (0) |
| Handgrip strength, kg | |
| Male | 23.56±7.08 |
| Female | 15.5±5.49 |
| Gait speed, m/s | 0.66±0.17 |
Gait speed was measured over a 4-m pathway, asking the participant to walk at a usual pace starting from a standing position. Handgrip strength was measured through a hand-held Jamar dynamometer, and it was expressed as the maximum performance based on three measurements of both hands. SD, standard deviation.
Table 2.
Differences in clinical outcomes, cognitive performances, functional status, and health-related quality of life between baseline (t0) and follow-up (t2)
| Whole sample (N = 71) |
|||
|---|---|---|---|
| baseline (t0) | follow-up (t2) | p value | |
| Presence of caregiver, n (%) | 38 (54.9) | 59 (84.3) | <0.001 |
| Hospitalization in the last year, n (%) | 9 (13.7) | 21 (29.4) | 0.042 |
| Falls in the last year, n (%) | 25 (35,2) | 29 (41.1) | ns |
| Fractures in the last year, n (%) | 4 (5.8) | 9 (13.7) | ns |
| MMSE | 23.39±3.91 | 19.37±4.54 | <0.001 |
| BADL score | 4.45±1.4 | 3.81±1.6 | 0.004 |
| Independency in managing phone, n (%) | 68 (96.1) | 51 (72.5) | 0.012 |
| Independency in managing medications, n (%) | 37 (52.9) | 22 (31.3) | 0.035 |
| PCS | 49.47±9.21 | 31.69±9.52 | <0.001 |
| MCS | 51.04±10.88 | 38.79±14.84 | <0.001 |
The BADL scale's score expresses the number of maintained functions (i.e., the number of activities the subject is able to carry out independently). The BADL score ranges from 0 to 6, with higher scores referring to greater independence. The PCS and the MCS scores range from 0 to 100, with higher scores denoting better health-related quality of life. MMSE, Mini Mental State Examination; BADL, Basic Activities of Daily Life; PCS, Physical Component Summary; MCS, Mental Component Summary; ns, not significant.
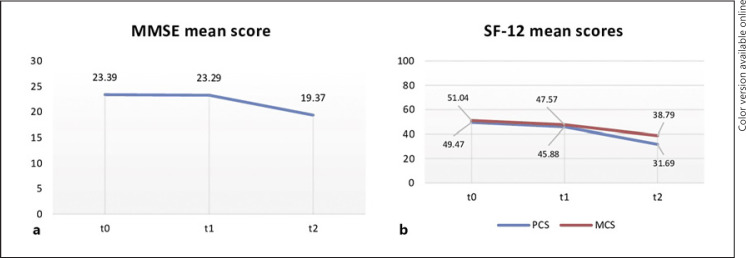
The peculiar changes at t0, t1, and t2 in the MMSE score, as well as in the PCS and MCS scores are also graphically reported in Figure 1 since cognition and health-related quality of life were the only variables in common between the three evaluations.
Fig. 1.
Differences in cognitive performances, as expressed by the mean MMSE score (a), and in quality of life domains, as expressed by the two synthetic components of the SF-12 (b), between the three evaluation times (t0, t1, and t2). The two synthetic components were the PCS and the MCS.
With regard to cognitive performances, patients reported significantly lower scores on the MMSE at t2 (mean 19.37 ± 4.54), compared to the scores obtained at baseline (z = −4.927; p < 0.001) and t1 (z = −3.908; p < 0.001). With regard to the quality of life domains, PCS resulted significantly lower at t2 (mean score 31.69 ± 9.52), compared to the scores obtained at baseline (z = −5.110; p < 0.001) and t1 (z = −5.012; p < 0.001), which indicates a poor physical health. Similarly, patients reported significantly lower MCS scores at t2 (mean score 38.79 ± 14.84), compared to the scores obtained at baseline (z = −3.898; p < 0.001) and t1 (z = −3.210; p < 0.001).
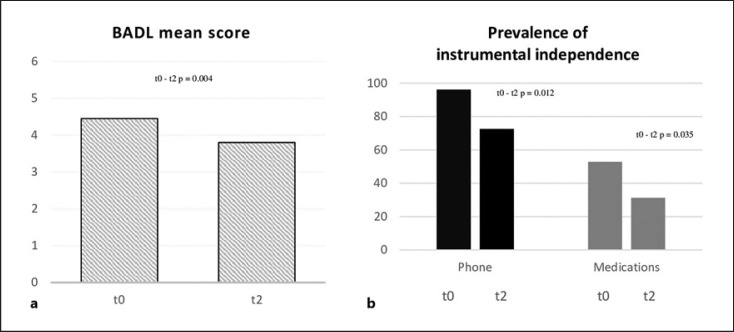
Functional status has been evaluated at baseline, and it was re-evaluated only in the current follow-up. Patients at t2 resulted less independent in managing basic daily activities (mean BADL score = 3.8 ± 1.6), compared to baseline (z = −2.846; p = 0.004). Similarly, the prevalence of patients resulting independent in managing telephone and medications was lower at t2, compared to baseline (p = 0.012; p = 0.035, respectively). Changes in functional status between baseline and the current follow-up are graphically summarized in Figure 2.
Fig. 2.
Changes in functional status between baseline (t0) and the current follow-up (t2), in terms of the mean BADL score (a) and prevalence of independent patients in managing phone and medications (b).
Discussion and Conclusion
An evaluation of elderly outpatients was carried out between April and May 2021; thus, over 1 year after the COVID-19 outbreak and approximately 2 year after the pre-pandemic baseline evaluation. The first evidence was the marked worsening of cognitive performances and of physical and mental components of health-related quality of life, compared to the baseline, as well as compared to a previous follow-up evaluation, which was carried out only 2 months after the pandemic outbreak in 2020. The present report additionally highlighted that compared to the pre-pandemic baseline, patients were progressively less independent in carrying out basic and instrumental daily activities; this evidence was consistently accompanied by the significant increase in the number of patients who needed support from a caregiver, compared to the baseline.
The investigated sample of outpatients faced the COVID-19 pandemic starting from an initial condition of pre-frailty or frailty [8]. Frailty denotes a condition of increased vulnerability to external stressors, and it is acknowledged as a predictor of age-related negative outcomes [9]; therefore, it is reasonable to consider the pandemic as a stressor that might have contributed to patients' cognitive and functional increased vulnerability. As a noteworthy aggravating circumstance, the pandemic has been a prolonged stressor over time, which has markedly affected health-related quality of life and psychological well-being.
We acknowledge some limitations that need to be overcome in further studies. First, this is a monocentric study including a relatively small sample of elderly outpatients, which precluded to make deeper inferences and comparisons; for instance, it precluded to investigate differences between patients who exhibited a worsening and those who did not. In addition, the predominant prevalence of females did not allow us to explore possible gender differences. Furthermore, we did not evaluate frailty status at t2; nonetheless, we evaluated outpatients' functional status, by reporting their reduced daily independency. The use of the Itel-MMSE might have been a source of bias; nonetheless, we converted its score into the MMSE equivalent, following the standard test procedure [7], in order to make the comparisons consistent. Eventually, the use of self-reported measures of functional status should be acknowledged as a further limitation. Despite these limitations, our findings appear in line with the need to investigate the impact of the pandemic on the most representative health-related domains, such as cognitive status, functional independency, and quality of life, which were jointly assessed. In accordance with our purpose, we have highlighted how the marked worsening involved each aforementioned domain, without distinction, especially in comparison with the pre-pandemic health status of our sample. As stated in the Introduction, the majority of studies have mostly described the negative effects of the SARS-CoV-2 infection and of its sequelae, on different age-related outcomes. This report instead sought to focus on the cognitive, functional, and psychological negative consequences of living during the pandemic, rather than on the effects of the disease itself.
In the context of the current pandemic, a patient-centered evaluation approach appears increasingly necessary, in order to capture the complexity of elderly physical, cognitive, and psychological health, and to outline weaknesses and resilience factors in facing such a dramatic contingency [10]. Because of the current limited access to healthcare services, the implementation of remote monitoring and consultations for frail outpatients have been suggested as a promising and helpful care strategy [11]; similarly, remotely delivered clinical psychological support has been recently recommended, in order to reduce psychological distress due to the pandemic among older adults [12]. Because of the multifactorial negative impact of pandemic on older adults' health, it is necessary the implementation of multidisciplinary strategies and interventions, in order to promote the progressive rapprochement of elderly patients to the healthcare system, as well as to social relationships in safety.
Statement of Ethics
Each procedure completed in this study was in accordance with the ethical standards of our institutional research committee and with the 1964 Helsinki Declaration and its later amendments. Written informed consent was collected for all the participants. This study protocol was reviewed and approved by the Committee of the University Hospital of Messina (Prot. 23/19).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author Contributions
Study concept and design: Alberto Sardella and Giorgio Basile. Acquisition of data: Emanuele Chiara and Federica Bellone. Analysis and interpretation of data: Alberto Sardella, Angela Alibrandi, Antonino Catalano, and Vittorio Lenzo. Drafting of the manuscript: Alberto Sardella. Critical revision of the manuscript for important intellectual content: Maria C. Quattropani and Giorgio Basile.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available on request by the corresponding author, without undue reservation.
References
- 1.Onder G, Rezza G, Brusaferro S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA. 2020;323((18)):1775–6. doi: 10.1001/jama.2020.4683. [DOI] [PubMed] [Google Scholar]
- 2.Rossi R, Socci V, Talevi D, Mensi S, Niolu C, Pacitti F, et al. COVID-19 pandemic and lockdown measures impact on mental health among the general population in Italy. Front Psychiatry. 2020;11:790. doi: 10.3389/fpsyt.2020.00790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Leese MI, Bernstein JPK, Dorociak KE, Mattek N, Wu CY, Beattie Z, et al. Older adults' daily activity and mood changes detected during the COVID-19 pandemic using remote unobtrusive monitoring technologies. Innov Aging. 2021;5((4)):igab032. doi: 10.1093/geroni/igab032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee EPX, Man REK, Gan TLA, Fenwick EK, Aravindhan A, Ho KC, et al. The longitudinal psychological, physical activity and financial impact of a COVID-19 lockdown on older adults in Singapore: the PIONEER-COVID Population-Based Study. Int J Geriatr Psychiatry. 2021;37((1)) doi: 10.1002/gps.5645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaninotto P, Iob E, Demakakos P, Steptoe A. Immediate and longer-term changes in the mental health and well-being of older adults in England during the COVID-19 pandemic. JAMA Psychiatry. 2022;79((2)):151–9. doi: 10.1001/jamapsychiatry.2021.3749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Morley JE, Vellas B. Editorial: COVID-19 and older adults. J Nutr Health Aging. 2020;24((4)):364–5. doi: 10.1007/s12603-020-1349-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Quattropani MC, Sardella A, Morgante F, Ricciardi L, Alibrandi A, Lenzo V, et al. Impact of cognitive reserve and premorbid IQ on cognitive and functional status in older outpatients. Brain Sci. 2021;11((7)):824. doi: 10.3390/brainsci11070824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Song X, Mitnitski A, Rockwood K. Prevalence and 10-year outcomes of frailty in older adults in relation to deficit accumulation: frailty prevalence and outcome. J Am Geriatr Soc. 2010;58((4)):681–7. doi: 10.1111/j.1532-5415.2010.02764.x. [DOI] [PubMed] [Google Scholar]
- 9.Hubbard RE, Maier AB, Hilmer SN, Naganathan V, Etherton-Beer C, Rockwood K. Frailty in the face of COVID-19. Age Ageing. 2020;49((4)):499–500. doi: 10.1093/ageing/afaa095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bianchetti A, Bellelli G, Guerini F, Marengoni A, Padovani A, Rozzini R, et al. Improving the care of older patients during the COVID-19 pandemic. Aging Clin Exp Res. 2020;32((9)):1883–8. doi: 10.1007/s40520-020-01641-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gareri P, Fumagalli S, Malara A, Mossello E, Trevisan C, Volpato S, et al. Management of older outpatients during the COVID-19 pandemic: the GeroCovid Ambulatory Study. Gerontology. 2022;68((4)):412–7. doi: 10.1159/000516969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gorenko JA, Moran C, Flynn M, Dobson K, Konnert C. Social isolation and psychological distress among older adults related to COVID-19: a narrative review of remotely-delivered interventions and recommendations. J Appl Gerontol. 2021;40((1)):3–13. doi: 10.1177/0733464820958550. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available on request by the corresponding author, without undue reservation.