Supplemental Digital Content is Available in the Text. Patients with chronic back pain with solicitous spouses show enhanced brain responses and pain ratings to painful stimulation in the presence but not the absence of their spouse.
Keywords: Chronic pain, Affective processing, Solicitous spouse, Operant conditioning, Brain response, Pain behavior
Abstract
The experience of pain and pain behaviors is not only determined by physiological but also psychosocial factors. In this context, the learning history of the individual and specifically operant reinforcement related to spouse responses might play an important role. We investigated the effect of a solicitous and habitually pain-reinforcing spouse for the processing of pain in patients with chronic pain. Using multichannel electroencephalography, pain behaviors, and self-reports of pain, we examined 20 patients with chronic back pain (10 with solicitous and 10 with nonsolicitous spouses) and 10 matched healthy controls. The participants received a series of painful and nonpainful electrical stimuli applied to the site of pain (back) and a control area (finger) in the presence vs absence of the spouse. The global field power of the electroencephalogram with a focus in the frontal region was enhanced in patients with chronic back pain who had a solicitous spouse compared to those with a nonsolicitous spouse and the healthy controls. This was specific for the painful stimulation at the back and occurred only in the presence but not the absence of the spouse. Pain ratings of intensity and unpleasantness were also higher in the patients with solicitous spouses when the spouse was present during painful stimulation. These data suggest that significant other responses indicative of operant reinforcement may have a direct effect on the cerebral processing of pain and related pain perception.
1. Introduction
The experience of pain is determined not only by physiological but also by psychosocial factors. Previous research suggests that the presence of a significant other such as a spouse 9 and their behavior 5–7 can have a substantial influence on perceived pain. A supportive spouse and affectionate touch can reduce pain intensity and brain responses to painful stimuli. 21,30,37 However, the influence of the spouse seems to differ depending on acute and chronic states of pain. In patients with chronic pain, solicitous, pain-attentive spouse responses can reinforce and thus augment pain behavior. This was found for both patient-reported 14,18,41 and experimenter-observed 39,44 solicitous responses of spouses. For example, the presence of a habitually solicitous spouse decreased the patient's pain threshold in a cold pressor test by 75%, whereas the presence of a spouse who punished or distracted from pain expressions at home resulted in a 28% increase in pain thresholds of the patients. 12
Fordyce 19 first conceptualized the role of the social context for the development of chronic pain in an operant learning framework, which posits that social responses (e.g. attention for expressions of pain) in the environment of persons with chronic pain may reinforce pain behaviors. 17,35 Thus, through the path of operant conditioning, significant others and especially the spouse's attention and affection can become discriminative cues for pain behaviors.
These behavioral changes can also be mirrored in brain responses. Dowman and Rosenfeld 8 in the rat and Miltner et al. 33 in humans showed that the somatosensory evoked potential elicited by noxious stimulation can be increased or decreased by selectively reinforcing increases or decreases in amplitude. Flor et al. 15 reinforced patients with chronic pain for the increase or decrease of self-reported pain ratings and found a lack of extinction of both self-reported pain levels and the learned brain response (N150 component of the evoked potential of the electroencephalography [EEG]) to increased pain ratings in the patients. However, social reinforcement by a significant other in patients with chronic pain has not been studied on the behavioral and neuronal levels.
This study sought to determine to what extent the presence of a solicitous, pain-reinforcing spouse serves as a discriminative cue for altered processing of painful and nonpainful tactile stimulation on the verbal and brain levels. We assumed that the presence compared with the absence of a solicitous spouse during painful but not innocuous stimulation would lead to enhanced brain and verbal responses in the patients. We did not expect differential modulation of pain responses in patients with nonsolicitous spouses or healthy controls. In addition, we assumed that the effect would be specific for painful stimulation at the site of the clinical pain, for example, the back, but not the finger, in patients with chronic back pain. Finally, we assumed that later EEG components related to affective processing of pain involving frontal brain regions would be most affected by social reinforcement.
2. Materials and methods
2.1. Participants
Twenty patients with chronic back pain and 10 healthy controls matched for age and sex were studied. Patients were selected from a larger group of 120 patients with chronic back pain, recruited through the pain clinic of the cooperating hospital, and self-referred based on media coverage of the project. This larger study provided the opportunity to split participants into 2 groups based on significantly different self-reported solicitous and nonsolicitous partner responses to their pain. The spouses of the selected patients either habitually reinforced (N = 10, values > 2 on the Solicitous Responses Scale and <2 on the Punishing Responses Scale as well as <2 on the distracting responses subscales of the West Haven-Yale Multidimensional Pain Inventory [MPI] 16,27 ), constituting the solicitous group, or punished or ignored pain expressions and encouraged distraction from pain (N = 10, values > 2 on the Punishment and >2 on the Distraction Scales of the MPI), constituting the nonsolicitous group. The sample size was based on these extreme groups. We computed a post hoc power analysis for our main outcome variable global field power, which showed an effect size of 1.1. With an N of 10 per group and an alpha of 0.05, we achieved a sufficient power of 0.96.
Patients with primary chronic musculoskeletal pain of at least 6 months duration were included in this study. The exclusion criteria for patients and controls were use of psychoactive medication (narcotics, opioids, benzodiazepines, tranquilizers, barbiturates, antidepressants, and stimulants) and diagnoses of mental disorders, provided by trained clinical psychologists in structured clinical interviews. Patients with pain related to inflammatory causes, neurological complications, or prior back surgery were also excluded. The 3 groups did not significantly differ in age (F(2,27)= 0.06, n.s.), sex (χ2(2)= 0.48, n.s.), duration (F(2,27)= 1.23, n.s.), and quality (F(2,27)= 0.17, n.s.) of the marital relationship. The 2 pain groups were not significantly different in pain intensity (F (1,18) = 0.93) and duration (F(1,18) = 2.42, n.s.). Table 1 gives an overview of demographic and clinical data of the 3 groups that were studied. Informed written consent was obtained from all participants. The study conformed to the tenets of the Declaration of Helsinki, was approved by the review board of the German Psychological Association and was not preregistered. Data on part of the sample (N = 9 patients and N = 8 controls) were published as an extended abstract. 28
Table 1.
Demographic and clinical characteristics.
| Patients with solicitous spouses | Patients with nonsolicitous spouses | Healthy controls | |
|---|---|---|---|
| Age in y (M, SD) | 44.40 (9.76) | 44.44 (13.35) | 46.10 (15.18) |
| Duration of relationship in years (M, SD) | 19.25 (10.46) | 12.02 (9.09) | 17.972 (13.04) |
| Quality of relationship (0-5, M, SD) | 1.93 (1.08) | 1.65 (1.19) | 1.75 (1.07) |
| Pain intensity (VAS 0-100, M, SD) | 24.50 (21.91) | 33.30 (19.88) | |
| Pain duration in years (M, SD) | 9.47 (4.53) | 14.31 (8.81) | |
| Pain behaviors (0-20, M, SD) | 6.80 (2.47) | 4.00 (1.83) | |
| MPI solicitous responses (0-6, M, SD, min, max) | 4.25 (0.54) (3.60, 5.20) |
1.95 (0.80) (0.40, 3.00) |
|
| MPI punishing responses (0-6, M, SD, min max) | 0.27 (0.38) (0.00, 1.00) |
3.60 (0.73) (2.33, 5.00) |
|
| MPI distracting responses (0-6, M, SD, min, max) | 1.0 (0.63) (0.00, 1.67) |
3.79 (1.00) (2.33, 5.33) |
|
| Sex N, male/female | 4/6 | 5/5 | 5/5 |
| Education | |||
| N < 10 y | 3 | 3 | 7 |
| N > 10 y | 7 | 7 | 3 |
M, mean; min, minimum; Max, maximum; MPI, West Haven-Yale Multidimensional Pain Inventory; SD, standard deviation; VAS, visual analogue scale.
2.2. Psychometric data
The patients participated in a pain interview, where important characteristics of their pain, the impact of the pain on their lives, treatment history, and coping attempts were assessed. 17 The patients' pain behaviors were recorded during this interview using the German version of the Pain Behavior Checklist. 13,26 The patients also completed the German version of the West Haven-Yale Multidimensional Pain Inventory (MPI). 16,27 The MPI assesses pain severity, interference, affective distress, social support, life control, significant other responses (punishing, solicitous, and distracting) to pain behaviors of the patients, and general activity levels of the patients. This measure was used to describe the characteristics of the patients with chronic back pain. The frequency of coping and catastrophizing pain-related self-statements, as measured by the Pain-Related Self-Statement Scale, 10 was recorded to determine the relationship of deficient coping as a factor that might affect pain processing. All participants completed an information sheet on the length of their intimate relationship and the short form of the Dyadic Adjustment Scale. 24
2.3. Painful and nonpainful electrical stimulation and pain ratings
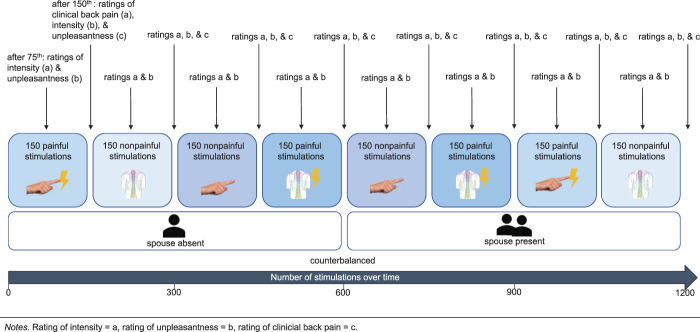
Electrical stimuli were applied either to the finger or to the back, at a painful (midway between pain threshold and pain tolerance) or nonpainful (midway between perception and pain threshold) level, and in counterbalanced order in the presence and the absence of the spouse (Fig. 1). The stimuli were intracutaneously applied using a gold electrode 4 and were presented in the nociceptive and nonnociceptive ranges, individually adapted to the subjects' pain thresholds and automatically delivered by a programmable constant current stimulator in the absence of the experimenter who supervised the study through a 1-way screen and intercom.
Figure 1.
Overview of the experimental conditions.
The perception threshold was determined by means of 3 ascending series of electrical stimuli. Subsequently, the stimulation intensity was enhanced continuously until the subject rated between 50 and 60 on a numerical rating scale (NRS, with the extreme points “not perceptible (0)” and “very strong (100)”). A series of 20 stimuli of the determined intensity were delivered and rated after the entire series. If the rating stayed below the 50 to 60 range of the NRS, the actual intensity was set as nonpainful stimulation intensity and maintained. If the rating was higher or lower than the 50 to 60 range, the stimulation intensity was changed until the subjects rated 20 subsequent stimuli between 50 and 60. The procedure for the determination of the painful stimulation was analogous. An ascending series of stimuli were delivered until the subjects indicated that the pain threshold was reached. The stimulation intensity was then fixed when the subjects rated 20 subsequent stimuli between 50 and 60 on a numerical rating scale with the end points not painful and unbearable pain. The sequence of conditions (site [back or finger], pain or no pain, and spouse presence or absence) was counterbalanced.
Each stimulation block was 7.5 minutes long, and the participants were allowed to shift their position in the rating phase after each block. The total experiment including the ratings lasted approximately 90 minutes. In each condition (back painful, back nonpainful, finger painful, finger nonpainful, and each in the presence and absence of the spouse), the patients with solicitous spouses as well as the patients with nonsolicitous spouses and the matched controls without pain were presented with 150 amplitude-modulated electrical stimuli per block (8 blocks total). Thus, there were 1200 stimuli overall. Steady-state stimulation was chosen to simulate a tonic pain stimulus. This type of stimulation causes a continuous dull ache. The carrier frequency was 150 Hz. In each condition, the modulation frequency was 14.7 Hz for two-thirds and 7.4 Hz for the remaining third of the stimuli. 36 The participants were asked to count the less frequent 7.4 Hz stimuli to maintain their attention. The stimulus duration was 683 ms, and the baseline was 217 ms with 10 modulation periods for the 14.7 Hz stimuli and 5 modulation periods for the 7.4 Hz stimuli. Stimulus presentation was randomized with a mean interstimulus interval of 2010 ms (see Fig. 1 for the sequence of the stimulus presentation).
After each 75th trial, the subjects rated the intensity of the electric stimulus (nonpainful stimulation: “How intense was the stimulus?”—NRS with the end points 0 = no perception and 100 = very strong stimulation; painful stimulation: “How painful was the electric stimulus?”—NRS with the end points 0 = no pain and 100 = unbearable pain) and its unpleasantness (painful and nonpainful stimulation: “How unpleasant was the stimulation?”—0 = not at all unpleasant to 100 = very unpleasant). After every 150th trial, the subjects rated the intensity of their current clinical back pain (0 = no pain and 100 = unbearable pain) independent of the stimulus-induced pain.
2.4. Physiological recordings
During the painful or nonpainful stimulation procedure, the EEG was recorded at 86 scalp locations determined according to the extended International 10/20 system. An additional 3 channels served for electro-oculographic, 2 for electromyographic, and 1 for electrocardiographic recordings to assess artifacts. A sampling rate of 1000 Hz was used for all channels. Scalp recordings were monopolar, referenced to linked ears. Electro-oculographic recordings were bipolar. Electroencephalographic and electrooculographic data were recorded using Neuroscan SynAmps amplifiers (MES, Munich, Germany) and sampled at 1000 Hz. Data acquisition was continuous and segmented off line. Impedance of earlobe electrodes was maintained below 2 kΩ, and all other electrode impedances were kept below 5 kΩ. An adhesive impedance cardiographic electrode spanning around the arm was connected with all channels; a second electrode was attached around the chest and the back and connected with the ground electrode. The ground electrodes terminated in the head box of the SynAmps amplifier. The EEG data were corrected for artifacts, rereferenced to average reference, corrected with a baseline of 217 ms, and selectively averaged with experimental condition (group, site, and pain or no pain) and modulation frequency as the selection criterion. Epochs with voltage variation exceeding 50 μV, with marked muscular activity, or with electrooculographic variation exceeding 50 μV were discarded (7% total). For each experimental condition, the 14.7-Hz steady-state response was extracted from the respective average by a fast Fourier transform (FFT) bandpass filter. All FFT bins with the exception of the real components of the 2 center bins bracketing the modulation frequency (14.7 Hz) and the first harmonic of the modulation frequency as well as the 4 immediate neighbor bins of those 2 center bins were zeroed. The center bins were retained, and the neighboring bins were divided by 2. An inverse FFT yielded the filtered time-domain waveforms, that is, the steady-state response. The peak-to-peak amplitude of the response was determined for the latency interval of 250 to 500 ms post stimulus.
2.5. Data analysis
The normal distribution of all outcomes was tested and verified by the Kolmogorov–Smirnov test. Based on the peak-to-peak amplitudes of the steady-state response, the global field power (GFP) of the EEG was computed. For all groups (solicitous, nonsolicitous, and controls without pain) and each experimental condition of stimulation site (back vs finger) and the stimulation level (painful vs nonpainful), the ratio of power in each EEG channel as well as the GFP ratio across channels was computed for the presence vs absence of the spouse. This analysis permitted the determination of the subset of channels where evoked activity synchronized with the stimulation differed between spouse presence and absence. Probability brain maps, which allow an inference as to the brain region generating the activity contrast in question, were derived from the significant analysis of variance contrasts. Source analyses are not possible with this type of analysis. The GFP ratio values related to spouse presence vs absence were submitted to a group (patients with solicitous spouses vs patients with nonsolicitous spouses vs controls) × site (finger vs back) × level (painful vs nonpainful) analysis of variance (ANOVA). To determine the brain area with maximum differentiation of the responses, an ANOVA using the 3 groups, all electrodes, and the back vs finger site was computed. The ratings of pain intensity and unpleasantness were also examined by a repeated measures analysis with group as between factors and site and level as within factors. Pain behaviors were analyzed by a 1-way ANOVA because they were not examined during the painful stimulation but only before the experiment. T tests were used if only 2 groups were compared. Significance levels were Greenhouse–Geisser corrected. Bonferroni-corrected post hoc tests were computed if the effects were significant. Pearson correlations were used to examine whether time since pain onset, pain intensity and interference, affective distress, social support, life control, or coping ability were related to the pain variables. IBM SPSS Statistics for Windows, version 25.0, 25 was used for these analyses.
3. Results
3.1. Higher brain activation in patients with solicitous spouses in the presence of the spouse
A significant group × site × level effect for the global field power ratio (F(2,27) = 9.01, P = 0.001) was observed. Spouse presence yielded a higher brain response only for painful stimulation at the back (F(2,27) = 4.78, P < 0.05) but not for painful stimulation at the control region (finger) or nonpainful stimulation at the back or the finger (all F(2,27)< 2.14, n.s.). Post hoc comparisons showed that the painful stimulation on the back compared with the finger yielded a significantly higher brain response in the patients with solicitous spouses compared with the healthy controls (P < 0.05). The patients with nonsolicitous spouses did not significantly differ from the controls (Figure 2).
Figure 2.
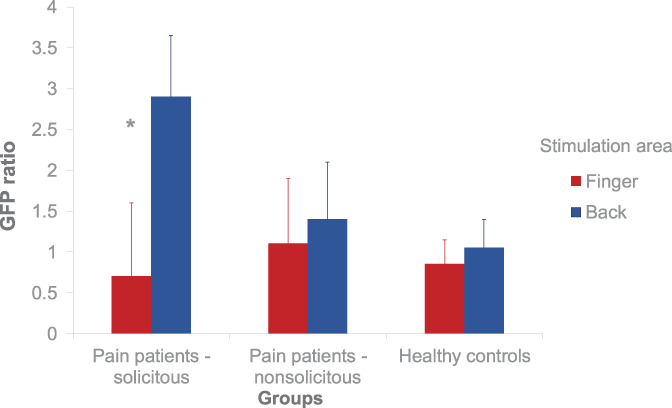
Brain reactivity to painful stimulation. The mean (±SEM) global field power ratio for the 3 groups and the 2 sites in the presence vs the absence of the spouse is shown. *P < 0.05.
The site of the maximal group difference for the significant painful stimulation at the back was limited to the frontocentral region (ANOVA groups × electrodes × site: F(4,112) = 2.7, P = 0.03) (Figure 3), with a small cluster around Cz and Fz showing highly significant differences between groups and conditions (all P< 0.01).
Figure 3.
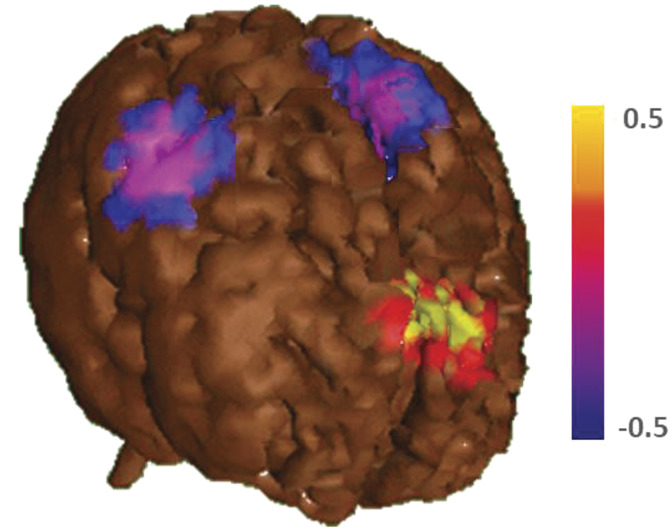
Location of the brain activation. This figure shows the activation related to the finger and back stimulation in the primary somatosensory cortex for all subjects (blue) to indicate the site of activation related to the somatosensory stimulation. In red (P < 0.01) and yellow (P < 0.05), the probability map for the painful back stimulation in the presence vs absence of the spouse is displayed. The main difference for the spouse effect is centered in the frontocentral region, far away from the primary somatosensory processing area.
The influence of spouse presence was the higher, the longer the pain problem had persisted (correlation of the peak brain response and time since pain onset for the patients with pain: r(18) = 0.52, P < 0.05) but was unrelated to pain severity, pain-related interference, affective distress, or life control as assessed by the MPI and coping ability as assessed by the Pain-Related Self-Statement Scale (all rs(18)< 0.24, n.s.).
3.2. Higher habitual and acute pain and unpleasantness ratings in patients with solicitous spouses in the presence of the spouse
For the ratings during the stimulation, the overall ANOVAs (group × site × level) were significant for both pain intensity (F(2,27)= 9.35, P < 0.05) and unpleasantness (F(2,27)= 3.46, P< 0.05) as well as habitual pain ratings (F(1,18)= 4.34, P = 0.05). Spouse presence led to significant increases in the perceived pain intensity of the electrical stimuli (M = 59.80 and SD = 14.92 in the presence and M = 52.25 and SD= 15.92 in the absence of the spouse; t(9) = 2.39, P< 0.05; Figure 4), the unpleasantness of the stimuli (M = 61.40, SD = 11.59 in the presence and M = 54.47, SD = 9.50 in the absence of the spouse; t(9) = 3.12, P < 0.05), and the clinical back pain ratings (presence: M = 34.00, SD = 23.91 and absence: M = 31.25, SD = 21.45; t(9) = 1.83, P = 0.05) in the group with solicitous spouses but not the group with nonsolicitous spouses and the pain-free control group. No significant correlations with the MPI variables or time since onset were present.
Figure 4.
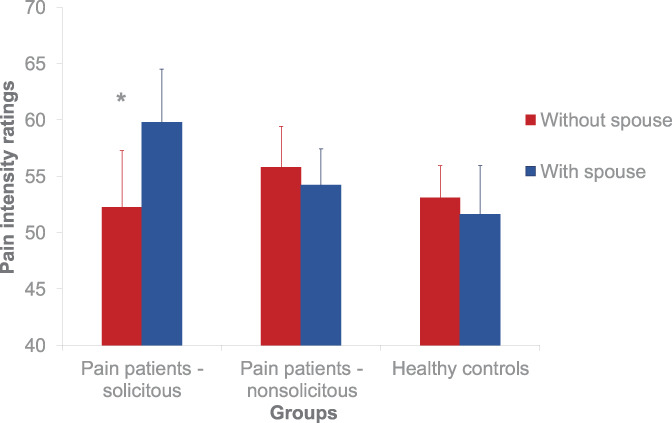
Pain intensity ratings. The figure shows the mean ratings of the painful stimulation for the 3 groups (healthy, patients with a solicitous spouse, and patients with a nonsolicitous spouse) and the 2 conditions (with and without spouse). *P < 0.05.
4. Discussion
In this study, we have shown that pain intensity and unpleasantness ratings and pain-related brain activation patterns were significantly higher in patients with solicitous partners in the presence vs absence of the spouse.
These data suggest that the mere presence of a solicitous spouse serves as a discriminative cue for enhanced cerebral processing of acute pain stimuli in patients with chronic pain who are habitually reinforced for the expression of pain behaviors. The global field power of the EEG, an indicator of the brain's response to the steady-state stimuli that were applied to simulate a tonic pain stimulus, was almost 3 times higher in the presence compared with the absence of the spouse when the site of pain was stimulated in a painful manner, and the spouse was reinforcing pain behaviors in the home environment. This spouse effect was found neither in patients whose spouses showed punishing or distracting (here termed nonsolicitous) responses to the pain nor in the pain-free controls. This result adds to a growing literature on the important effects of learning and memory processes on the experience of pain. 1,17,32
In relation to brain activity, the location of the differential effects in the frontocentral region suggests that this activity may originate from the anterior cingulate and might thus be more related to affective than sensory-discriminative pain processing. 38 A detailed source analysis was not possible with this type of stimulation; thus, this assumption needs to be verified in further research. These data are, however, in accordance with findings by Singer et al. 40 showing that empathy toward the pain experience of another person is also mediated by the anterior cingulate. These data also are in accordance with studies that sought to separate emotional and sensory components of pain and found differential activity in either the anterior cingulate (unpleasantness focus) or the somatosensory cortex (sensory focus). 23,38 Notably, they support the hypothesis of a shift in functional brain activity during continuous ratings of fluctuations in spontaneously occurring back pain from brain regions related to nociception to more emotion-related circuitry in the transition process from acute to chronic pain. 22
The differential association of frontocentral brain activity in response to tonic pain stimuli is in accordance with previous data showing an increase in cerebral responding in patients with chronic pain over time. 2,11 These data suggest that abnormal brain responses to painful stimulation develop over time and may later be a prime contributor to chronic pain. Here, this notion was supported by the responsiveness of both acute and habitual pain ratings as well as unpleasantness estimates to spouse presence in the couples with frequent reinforcement of pain behaviors.
A further interesting aspect of this study was the limitation of the differential spouse effects to painful stimulation compared with nonpainful stimulation and the site of pain (back vs finger). This further underlines the assumption that these effects are based on learning and related to the expression of pain behaviors in the context of pain episodes. This is in line with the finding that the magnitude of the brain activation in the presence vs absence of the spouse was significantly positively correlated with time since onset of the chronic pain problem. The differential pain intensity and unpleasantness as well as the habitual pain ratings were not related to time since pain onset, suggesting that the physiological measures may be more sensitive than the self-report responses.
The difference between the 3 study groups suggests strong dyadic-based and couple-based effects on the conditioning mechanisms in chronic pain and confirms the assumption that the inclusion of the spouse in the treatment of chronic pain may have beneficial effects. 42 Indeed, including the partner into therapy has been reported beneficial for different mental and physical disorders, 3 and chronic pain in particular. 45 Most patients with chronic pain live in a couple relationship and couples often rearrange their daily routines and way of life to adapt to the pain. 31 Partners engage in solicitous behavior for a variety of reasons, most prominently to reduce the patient's immediate distress—and also because in the beginning—during the acute pain phase; this very behavior was indeed helpful. Both the affected couples and clinicians alike face challenges to break the vicious cycle of concerned attention and increased pain. Based on this, therapy aims to sensitively analyze the couple's interaction behavior, develop alternatives and train the partner to distract the patient from the pain, to reinforce pain-incompatible behavior, and elaborate scenarios that improve relationship quality. 43
This study has several limitations. First, the data are based on a small selected sample and need to be confirmed by studies with a larger number of patients. The use of EEG recordings with tonic frequency-modulated pain stimuli did not permit an analysis of the brain sources of the spouse-related activation. Further research should use functional imaging methods that can also evaluate activation in subcortical brain regions to determine with greater specificity the brain regions that are involved in the “spouse” effect. In addition, the immediate effects of supportive and solicitous spouse behaviors in association with relationship quality, the patient's level of depression, and the partner's empathic accuracy might allow for more refined analyses of the conditioning effects in a dyadic context. This also includes the dyadic assessment of associated brain–brain interactions using a hyperscanning approach, 29 where the responses of both the patients and the spouses could be assessed and compared. For pain, electroencephalogram-based hyperscanning has been used to document that better brain-to-brain coupling was related to higher analgesia induced by social touch in a healthy study group. 20 Moreover, different aspects of spouse responses, such as solicitous vs supportive behaviors as well as negative affect of the spouse, 34 should be addressed in this context. We also failed to assess the behavioral responses to the painful stimulation in the patients and pain-free group during the experiment. Gender effects should also be targeted; however, our sample size did not permit this analysis.
In summary, the present data suggest that partner interaction and the spouse's solicitous behavior may play a reinforcing role not only on the behavioral and verbal level but also in the central nervous system response to painful stimuli in chronic pain. Thereby, the study adds important information on the brain mechanisms underlying the role of social factors in the development of chronic pain and may provide insights for mechanistic treatment approaches.
Conflict of interest statement
The authors have no conflicts of interest to declare.
Supplemental video content
A video abstract associated with this article can be found at http://links.lww.com/PAIN/B554.
Acknowledgements
The authors dedicate this article to the late Werner Lutzenberger, who helped to develop the experimental design, and also thank Bärbel Knost for help with data acquisition. The data are available on reasonable request from HF. This work was supported by the Deutsche Forschungsgemeinschaft (Fl 156/41-1—Koselleck award to H. Flor and SFB1158/B03 to F. Nees and H. Flor and SFB1158/B02 to B. Ditzen).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
Contributor Information
Frauke Nees, Email: frauke.nees@zi-mannheim.de.
Beate Ditzen, Email: Beate.Ditzen@med.uni-heidelberg.de.
References
- [1]. Apkarian AV, Baliki MN, Geha PY. Towards a theory of chronic pain. Prog Neurobiol 2009;87:81–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Baliki MN, Apkarian AV. Nociception, pain, negative moods, and behavior selection. Neuron 2015;87:474–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Baucom DH, Fischer MS, Corrie S, Worrell M, Boeding SE. Treating relationship distress and psychopathology in couples: a cognitive-behavioural approach. 1st ed. New York City: Routledge, 2019:636–45. [Google Scholar]
- [4]. Bromm B, Meier W. The intracutaneous stimulus: a new pain model for algesimetric studies, Methodol. Find Exp Clin Pharmacol 1984;6:405–10. [PubMed] [Google Scholar]
- [5]. Burns JW, Gerhart J, Post KM, Smith DA, Porter LS, Buvanendran A, Fras AM, Keefe FJ. Spouse criticism/hostility toward partners with chronic pain: the role of spouse attributions for patient control over pain behaviors. J Pain 2018;19:1308–17. [DOI] [PubMed] [Google Scholar]
- [6]. Burns JW, Peterson KM, Smith DA, Keefe FJ, Porter LS, Schuster E, Kinner E. Temporal associations between spouse criticism/hostility and pain among patients with chronic pain: a within-couple daily diary study. PAIN 2013;154:2715–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7]. Cano A, Tankha H. Spousal criticism and hostility in response to pain: what is the alternative?. PAIN 2018;159:1–2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8]. Dowman R, Rosenfeld PJ. Operant conditioning of somatosensory evoked potential (SEP) amplitude in rats. I. Specific changes in SEP amplitude and a naloxone-reversible somatotopically specific change in facial nociception. Brain Res 1985;333:201–12. [DOI] [PubMed] [Google Scholar]
- [9]. Eisenberger NI, Master SL, Inagaki TK, Taylor SE, Shirinyan D, Lieberman MD, Naliboff BD. Attachment figures activate a safety signal-related neural region and reduce pain experience. Proc Natl Acad Sci 2011;108:11721–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Flor H, Behle DJ, Birbaumer N. Assessment of pain-related cognitions in chronic pain patients. Behav Res Ther 1993;31:63–73. [DOI] [PubMed] [Google Scholar]
- [11]. Flor H, Braun C, Elbert T, Birbaumer N. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett 1997;224:5–8. [DOI] [PubMed] [Google Scholar]
- [12]. Flor H, Breitenstein C, Birbaumer N, Fürst M. A psychophysiological analysis of spouse solicitousness towards pain behaviors, spouse interaction, and pain perception. Behav Ther 1995;26:255–72. [Google Scholar]
- [13]. Flor H, Heimerdinger K. Erfassung des Schmerzverhaltens [Assessment of pain behaviors]. In: Geissner E, Jungnitsch G, editors. Psychologie des Schmerzes. Diagnose und Therapie. [Psychology of pain: assessment and treatment]. Weinheim: Psychologie Verlags Union, 1992. pp. 99–105. [Google Scholar]
- [14]. Flor H, Kerns RD, Turk DC. The role of spouse reinforcement, perceived pain, and activity levels of chronic pain patients. J Psychosom Res 1987;31:251–9. [DOI] [PubMed] [Google Scholar]
- [15]. Flor H, Knost B, Birbaumer N. The role of operant conditioning in chronic pain: an experimental investigation. PAIN 2002;95:111–18. [DOI] [PubMed] [Google Scholar]
- [16]. Flor H, Rudy TE, Birbaumer N, Streit B, Schugens MM. Zur Anwendbarkeit des West Haven–Yale Multidimensional Pain Inventory im deutschen Sprachraum [Applicability of the West Haven–Yale Multidimensional Pain Inventory in German speaking countries]. Schmerz 1990;4:82–7. [DOI] [PubMed] [Google Scholar]
- [17]. Flor H, Turk DC. Chronic pain: an integrated biobehavioral approach. Seattle: IASP Press, 2011. p. [Google Scholar]
- [18]. Flor H, Turk DC, Rudy TE. Relationship of pain impact and significant other reinforcement of pain behaviors: the mediating role of gender, marital status and marital satisfaction. PAIN 1989;38:45–50. [DOI] [PubMed] [Google Scholar]
- [19]. Fordyce WE. Behavioral methods for chronic pain and illness. St Louis: Mosby, 1976. [Google Scholar]
- [20]. Goldstein P, Weissman-Fogel I, Dumas G, Shamay-Tsoory SG. Brain-to-brain coupling during handholding is associated with pain reduction. Proc Natl Acad Sci 2018;115:E2528–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21]. Grinevich V, Charlet A. Oxytocin: pain relief in skin. PAIN 2017;158:2061–3. [DOI] [PubMed] [Google Scholar]
- [22]. Hashmi JA, Baliki MN, Huang L, Baria AT, Torbey S, Hermann KM, Schnitzer TJ, Apkarian AV. Shape shifting pain: chronification of back pain shifts brain representation from nociceptive to emotional circuits. Brain 2013;136:2751–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23]. Hofbauer RK, Rainville P, Duncan GH, Bushnell MC. Cortical representation of the sensory dimension of pain. J Neurophysiol 2001;86:402–11. [DOI] [PubMed] [Google Scholar]
- [24]. Hunsley J, Pinsent C, Lefebvre M, James-Tanner S, Vito D. Construct validity of the short forms of the dyadic adjustment scale. Fam Relat 1995;44:231–7. [Google Scholar]
- [25]. IBM Corp. IBM SPSS Statistics for Windows. Armonk: IBM Corp, 2020. [Google Scholar]
- [26]. Kerns RD, Haythornthwaite J, Rosenberg R, Southwick S, Giller EL, Jacob MC. The pain behavior check list (PBCL): factor structure and psychometric properties. J Behav Med 1991;14:155–67. [DOI] [PubMed] [Google Scholar]
- [27]. Kerns RD, Turk DC, Rudy TE. The West Haven-Yale multidimensional pain inventory (WHYMPI). PAIN 1985;23:345–56. [DOI] [PubMed] [Google Scholar]
- [28]. Knost B, Flor H, Birbaumer N. Schmerzverhalten, Partnerreaktionen und somatosensorisch evozierte Potentiale chronischer Schmerzpatienten bei akuten Schmerztests [Pain behaviors, spouse responses and somatosensory evoked potentials of chronic pain patients in acute pain tests]. Z für Klinische Psychol Psychotherapie 1999;28:242–7. [Google Scholar]
- [29]. Koike T, Tanabe HC, Sadato N. Hyperscanning neuroimaging technique to reveal the “two-in-one” system in social interactions. Neurosci Res 2015;90:25–32. [DOI] [PubMed] [Google Scholar]
- [30]. Kreuder A-K, Wassermann L, Wollseifer M, Ditzen B, Eckstein M, Stoffel-Wagner B, Hennig J, Hurlemann R, Scheele D. Oxytocin enhances the pain-relieving effects of social support in romantic couples. Hum Brain Mapp 2019;40:242–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31]. Leonard MT, Cano A, Johansen AB. Chronic pain in a couples context: a review and integration of theoretical models and empirical evidence. J Pain 2006;7:377–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32]. Meulders A. Fear in the context of pain: lessons learned from 100 years of fear conditioning research. Behav Res Ther 2020;131:103635. [DOI] [PubMed] [Google Scholar]
- [33]. Miltner W, Larbig W, Braun C. Biofeedback of somatosensory event-related potentials: can individual pain sensations be modified by biofeedback-induced self-control of event-related potentials?. PAIN 1988;35:205–13. [DOI] [PubMed] [Google Scholar]
- [34]. Nah S, Martire LM, Zhaoyang R. Perceived patient pain and spousal caregivers' negative affect: the moderating role of spouse confidence in patients' pain management. J Aging Health 2020;32:1282–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Newton-John TR. How significant is the Significant Other in patient coping in chronic pain?. Pain Manag 2013;3:485–93. [DOI] [PubMed] [Google Scholar]
- [36]. Noss RS, Boles CD, Yingling CD. Steady-state analysis of somatosensory evoked potentials. Electroencephalogr Clin Neurophysiol 1996;100:453–61. [PubMed] [Google Scholar]
- [37]. Pfeifer A-C, Schroeder-Pfeifer P, Schneider E, Schick M, Heinrichs M, Bodenmann G, Ehlert U, Herpertz SC, Läuchli S, Eckstein M, Ditzen B. Oxytocin and positive couple interaction affect the perception of wound pain in everyday life. Mol Pain 2020;16:1744806920918692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38]. Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science 1997;277:968–71. [DOI] [PubMed] [Google Scholar]
- [39]. Romano JM, Turner JA, Friedman LS, Bulcroft RA, Jensen MP, Hops H. Observational assessment of chronic pain patient-spouse behavioral interactions. Behav Ther 1991;22:549–67. [Google Scholar]
- [40]. Singer T, Seymour B, O'Doherty J, Kaube H, Dolan RJ, Frith CD. Empathy for pain involves the affective but not sensory components of pain. Science 2004;303:1157–62. [DOI] [PubMed] [Google Scholar]
- [41]. Stroud MW, Thorn BE, Jensen MP, Boothby JL. The relation between pain beliefs, negative thoughts, and psychosocial functioning in chronic pain patients. PAIN 2000;84:347–52. [DOI] [PubMed] [Google Scholar]
- [42]. Tankha H, Caño A, Dillaway H. “Now I have hope”: rebuilding relationships affected by chronic pain. Fam Syst Health J Collab Fam Healthc 2020;38:51–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Thieme K, Flor H, Turk DC. Psychological pain treatment in fibromyalgia syndrome: efficacy of operant behavioural and cognitive behavioural treatments. Arthritis Res Ther 2006;8:R121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Turner JA, Jensen MP, Romano JM. Do beliefs, coping, and catastrophizing independently predict functioning in patients with chronic pain? PAIN 2000;85:115–25. [DOI] [PubMed] [Google Scholar]
- [45]. Wirick DM, Teufel-Prida LA. Chronic lower back pain: cognitive behavioral therapy with family therapy interventions. Fam J 2018;26:86–9. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
A video abstract associated with this article can be found at http://links.lww.com/PAIN/B554.