Supplemental Digital Content is Available in the Text. Improvements in clinical pain measures after interdisciplinary multimodal pain therapy are associated with increased global network efficiency at theta frequencies measured by resting-state electroencephalography in patients with chronic pain.
Keywords: Chronic pain, Interdisciplinary multimodal pain therapy, EEG, Resting state, Oscillations, Connectivity
Abstract
Chronic pain is a major healthcare issue posing a large burden on individuals and society. Converging lines of evidence indicate that chronic pain is associated with substantial changes of brain structure and function. However, it remains unclear which neuronal measures relate to changes of clinical parameters over time and could thus monitor chronic pain and treatment responses. We therefore performed a longitudinal study in which we assessed clinical characteristics and resting-state electroencephalography data of 41 patients with chronic pain before and 6 months after interdisciplinary multimodal pain therapy. We specifically assessed electroencephalography measures that have previously been shown to differ between patients with chronic pain and healthy people. These included the dominant peak frequency; the amplitudes of neuronal oscillations at theta, alpha, beta, and gamma frequencies; as well as graph theory-based measures of brain network organization. The results show that pain intensity, pain-related disability, and depression were significantly improved after interdisciplinary multimodal pain therapy. Bayesian hypothesis testing indicated that these clinical changes were not related to changes of the dominant peak frequency or amplitudes of oscillations at any frequency band. Clinical changes were, however, associated with an increase in global network efficiency at theta frequencies. Thus, changes in chronic pain might be reflected by global network changes in the theta band. These longitudinal insights further the understanding of the brain mechanisms of chronic pain. Beyond, they might help to identify biomarkers for the monitoring of chronic pain.
1. Introduction
Chronic pain is a major healthcare issue that affects approximately a fifth of the adult population and thereby causes a tremendous burden to individuals and society. 7,33 Converging lines of evidence indicate that the brain figures prominently in the susceptibility, development, and maintenance of chronic pain. 3,37 Imaging studies have revealed substantial alterations of brain structure and function in patients suffering from chronic pain. 37 In particular, functional magnetic resonance imaging has shown extended dysfunction of brain networks. 3 Furthermore, graph theory-based analyses have demonstrated a disruption of global network architecture as well as altered local connectivity of network hubs. 4,27,32,38,42,43 Moreover, electroencephalography (EEG) and magnetoencephalography studies have indicated changes of neuronal oscillations at different frequencies. 35,50,52 In particular, slowing of the dominant peak frequency around 10 Hz and increases of neuronal oscillations at theta (4-8 Hz) and alpha (8-13 Hz) frequencies have been reported. 50 Moreover, there is mounting evidence for local and global changes in functional connectivity at theta, alpha, and gamma (30-100 Hz) frequencies. 12,56,60 These abnormalities have mostly been observed by cross-sectional comparisons of brain function between patients suffering from chronic pain and healthy participants. However, whether and how these measures can monitor changes of chronic pain over time, including those related to treatment, remains to be elucidated.
To address this question, we assessed 41 patients with chronic pain longitudinally, directly before and 6 months after interdisciplinary multimodal pain therapy (IMPT). Interdisciplinary multimodal pain therapy is a powerful biopsychosocial treatment approach for chronic pain. It aims at physical reconditioning through medical and behavioral therapy as well as education in structured therapeutic programs. 31 Pre-IMPT and post-IMPT assessments included clinical measures and resting-state EEG recordings. Longitudinal changes of clinical measures were related to longitudinal changes of brain function assessed by EEG using frequentist as well as Bayesian statistics, which facilitates the interpretation of negative findings and effect sizes. 34 We specifically hypothesized that longitudinal changes in clinical measures are reflected by longitudinal changes of EEG measures that have previously been described to differ between patients with chronic pain and healthy participants as well as graph theory-based measures of brain connectivity. The overall goal of this approach was to further the understanding of the brain mechanisms of chronic pain and to aid the development of biomarkers of chronic pain. 14
2. Materials and methods
2.1. Participants
Fifty adult patients suffering from chronic pain participated in the study. All patients had been screened and scheduled for an IMPT program at the Center for Interdisciplinary Pain Medicine of the Technical University of Munich (TUM) between January 2018 and March 2019. The inclusion criteria comprised a clinical diagnosis of chronic pain, apart from primary headache disorders, with pain lasting more than 6 months and a minimum reported average pain intensity ≥2/10 (0 = no pain and 10 = worst imaginable pain) during the past 4 weeks. Further inclusion criteria were the willingness and ability to comply with physical (ie, sufficient mobility to participate in the physiotherapy) and psychological requirements (ie, motivation for behavioral change) of the IMPT program as assessed by a multiprofessional team of physicians, psychologists, and physiotherapists before enrolment in the program. The exclusion criteria were acute changes in the pain condition in the past 3 months because of recent injuries or surgeries as well as concomitant neuropsychiatric diseases apart from depression. Moreover, patients on regular benzodiazepine medication were excluded. Other medication was not restricted, and medication intake was assessed and quantified using the Medication Quantification Scale (MQS). 23 Nine patients were excluded from the study. Two patients were excluded because of surgical interventions for their respective pain condition closely before their scheduled follow-up assessment, another because of a limbic encephalitis in the time between the baseline and follow-up assessment, and another because of poor EEG data quality at the baseline assessment. In addition, 5 patients withdrew from the study for personal reasons. Thus, the final group used for the longitudinal analyses comprised 41 patients (age 51 ± 17 years [mean ± SD], 25 female) (for further details on the patient group, see Table 1 and Supplementary Table 1, available at http://links.lww.com/PAIN/B556). In this group, 26 patients predominantly suffered from chronic back pain, 8 from joint pain, 4 from peripheral neuropathic pain, and 3 from chronic widespread pain. All participants provided written informed consent. The study was approved by the Ethics Committee of the Medical Faculty of the TUM and conducted in accordance with the relevant guidelines and regulations. The study was preregistered under the ClinicalTrials.gov identifier NCT03634670.
Table 1.
Patient group characteristics and comparisons of clinical measures at baseline and follow-up.
| Baseline (mean ± SD) | Follow-up (mean ± SD) | Comparisons (paired sample t tests) | |
|---|---|---|---|
| Age (y) | 51.46 ± 17.26 | — | — |
| Sex (f/m) | 25/16 | — | — |
| Disease duration (y) | 8.44 ±7.08 | — | — |
| MQS | 8.95 ±7.13 | 8.17 ±0.07 | T(df40) = 1.09 P = 0.283 BF10 = 0.29 |
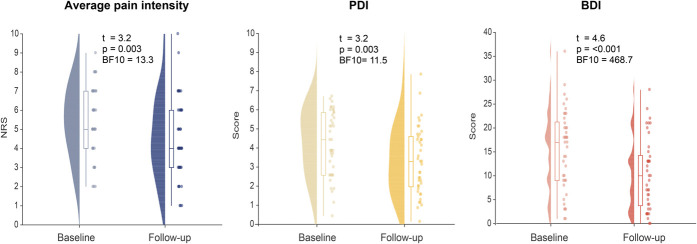
| Avg. pain intensity | 5.41 ± 1.82 | 4.56 ± 2.12 | T(df40) = 3.22 P = 0.003 BF10 = 13.31 |
| PDI | 4.24 ± 1.69 | 3.32 ± 1.76 | T(df40) = 3.16 P = 0.003 BF10 = 11.54 |
| BDI | 15.41 ± 1.32 | 10.29 ± 1.16 | T(df40) = 4.55 P < 0.001 BF10 = 468.68 |
Avg. pain intensity, Average pain intensity in the past 4 weeks (Numerical Rating Scale, NRS 0-10); BDI, Beck Depression Inventory II; BF10, Bayes Factor; f, female, m, male; MQS, Medication Quantification Scale; PDI, Pain Disability Index.
2.2. Procedures
To longitudinally assess clinical and electrophysiological measures, participants were assessed twice in 2 different sessions: baseline and follow-up. Assessments included the completion of standardized questionnaires and resting-state EEG recordings. The baseline assessment was performed in the week before or within the first 3 days of the IMPT program. The follow-up assessment was performed between 6 and 9 months after the baseline evaluation (Fig. 1).
Figure 1.
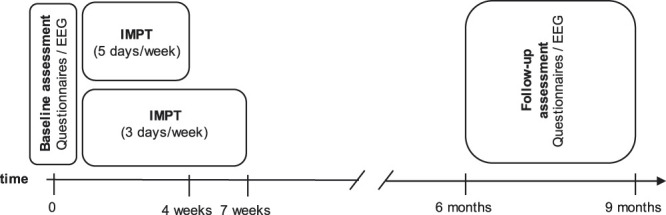
Timeline of procedures. Patients underwent 2 identical assessments, including evaluation of clinical characteristics using questionnaires and resting-state EEG. The baseline assessment was performed in the week before or within the first 3 days of the interdisciplinary multimodal pain therapy program. The follow-up assessment was performed 6 to 9 months later. Interdisciplinary multimodal pain therapy was provided on 20 days over a period of either 4 weeks (5 days per week) or 7 weeks (3 days per week). EEG, electroencephalography.
Patients participated in a standardized IMPT program with 20 days of treatment in a specialized day clinic. Therapy was provided either over a period of 4 weeks, with 5 days of treatment per week, or over 7 weeks, with 3 treatment days per week. Interdisciplinary multimodal pain therapy follows a biopsychosocial concept and uses an integrated and multidisciplinary diagnostic and treatment approach to restore physical as well as emotional performance of patients with chronic pain. 31 To this end, patients are enrolled in structured therapeutic programs comprising medical and behavioral therapy as well as patients' education provided by a multiprofessional team. 31 In this study, IMPT was provided at the group and individual level by an interdisciplinary multiprofessional team of psychologists, physiotherapists, and physicians with a specialization in pain medicine. Groups consisted of up to 8 patients, who underwent the entire IMPT program together. At the group level, patients received 4 treatment sessions per day, 2 with a physiotherapeutic focus, including movement experience, following the Feldenkrais method and 2 educational sessions on relaxation techniques, including progressive muscle relaxation, following the Jacobson method and cognitive–behavioral strategies with a focus on pain coping. At the individual level, patients received 4 consultations with a physician focusing on optimization of pain medication, 4 sessions of counseling with a cognitive–behavioral focus by a psychologist, and 2 physiotherapy sessions focusing on the individual pain problems over the course of the program.
2.3. Clinical measures
Clinical measures were selected following the recommendations for clinical trials and IMPT 17,30,48 to reflect 3 core outcome domains: pain intensity, physical functioning, and emotional functioning. Pain intensity was assessed using a Numerical Rating Scale; patients were asked to rate their average pain throughout the past 4 weeks on a scale from 0 to 10 (0 = no pain and 10 = maximum pain). Physical functioning was assessed with a focus on pain interference using the Pain Disability Index, a simple seven-item questionnaire asking patients to rate their pain-related impairment in the domains “Family/Home responsibilities,” “Recreation,” “Social Activity,” “Occupation,” “Sexual Behavior,” “Self-Care,” as well as “Life-Support Activity” on a scale from 0 to 10 (0 = no impairment and 10 = fully impaired). 57 Single-item scores are averaged resulting in an overall score from 0 to 10. 57 Depressive symptoms were assessed using the Beck Depression Inventory II. 6 Moreover, the medication intake was assessed using the MQS which allows to quantify medication profiles in patients with chronic pain in a single numerical value. 23
2.4. Electroencephalography
2.4.1. Recordings
Brain activity was recorded using resting-state EEG. Participants were instructed to stay in a relaxed and wakeful state, without performing any particular task. In each session, two 5-minute blocks were recorded, 1 with the eyes open and another with the eyes closed. During the eyes-open condition, participants fixated a fixation cross presented centrally on a screen. The order of the blocks was counterbalanced across participants. During data acquisition, participants were comfortably seated and listened to white noise through headphones.
Data were acquired with 64 electrodes (EasyCap, Herrsching, Germany) and a BrainAmp MR plus amplifier (Brain Products, Munich, Germany). The electrode layout included all 10-20 system electrodes; the additional electrodes Fpz, CPz, POz, Oz, Iz, AF3/4, F5/6, FC1/2/3/4/5/6, FT7/8/9/10, C1/2/5/6, CP1/2/3/4/5/6, TP7/8/9/10, P1/2/5/6/7/8, and PO3/4/7/9/10; and 2 electrodes below the outer canthus of each eye. All electrodes were referenced to FCz and grounded at AFz. Simultaneously, muscle activity was recorded with 2 bipolar surface electromyography (EMG) electrode montages placed on the right masseter and neck (semispinalis capitis and splenius capitis) muscles and a BrainAmp ExG MR amplifier (Brain Products). The EMG ground electrode was placed at vertebra C2. All data were sampled at 1000 Hz (0.1 µV resolution) and band-pass filtered between 0.016 and 250 Hz. Impedances were kept below 20 kΩ.
2.4.2. Preprocessing
Preprocessing was performed with the BrainVision Analyzer software v 2.1 (Brain Products) and MATLAB R2016b (Mathworks, Natick, MA). Preprocessing was performed separately for the baseline and follow-up recordings. In the BrainVision Analyzer, data were filtered with zero phase shift Butterworth filters. First, a 1-Hz high pass filter (time constant 0.1591, order 8) was applied to remove low-frequency drifts, followed by a 50-Hz notch filter to remove the electrical noise. After filtering, a first visual scan of the whole recording was performed by an experienced professional and highly contaminated segments were marked as “bad segments.” Next, independent component analysis was performed, 29 and components representing eye movements and muscle artifacts were visually identified based on time courses and topographies. Artifactual components were subtracted from the raw unfiltered EEG time series. Subsequently, a semiautomatic raw data inspection marked signal jumps higher than ±100 µV and their adjacent time intervals (200 ms before and after the jump) as bad segments. Finally, rereferencing to the average reference was performed, and the reference electrode FCz was reconstrued and added to the signal array. Data were exported from the BrainVision Analyzer and further preprocessed in MATLAB with the FieldTrip toolbox (version 20170124). 47 All recordings were preprocessed by the same experienced researcher.
In MATLAB, data were segmented into 2-second epochs with 0.5-second overlap. A 2-second epoch length was chosen to balance the stationarity of the signals and the number of samples for lower frequencies (down to 4 Hz). 13,59 The only exception was for the computation of the sensorimotor peak frequency, in which the data were segmented into 5-second epochs with 0.5-second overlap to increase the frequency resolution. 21 Epochs that contained ‘bad segments’ marked in the BrainVision Analyzer were discarded. Finally, all data were downsampled to 250 Hz in FieldTrip with the piecewise cubic hermite interpolating polynomial method to improve performance.
2.4.3. Analysis
Differences in brain activity between patients with chronic pain and healthy participants have been described regarding the dominant peak frequency as well as local and global power in the theta, alpha, beta, and gamma frequency bands. The most consistent findings are a slowing of the dominant peak frequency and increases of power in the theta and alpha band. 50 Moreover, there is mounting evidence for local and global changes in functional connectivity at theta, alpha, and gamma (30-100 Hz) frequencies. 12,56,60 We therefore analyzed the dominant peak frequency; oscillatory brain activity (power) at theta (4-8 Hz), alpha (8-13 Hz), beta (14-30 Hz), and gamma (60-100 Hz) frequencies; as well as local and global network measures of functional connectivity. The analyses were focused on the eyes closed EEG data because they are less prone to artifacts and show more stable characteristics over time. 16
2.4.3.1. Dominant peak frequency
As no standard for the computation of peak frequencies exists, we used the 3 most frequently used approaches: local maximum, center of gravity (CoG), and local CoG (sensorimotor). For the first 2 approaches, the power spectrum was computed with the 2-second epoched data between 1 and 100 Hz using a fast Fourier transformation with Slepian multitapers. Frequency smoothing was 1 Hz, and frequency resolution was 0.5 Hz. Before power computation, a band-stop filter between 45 and 55 Hz was applied to remove line noise.
In the first approach, peak frequency was determined on the average power spectrum across all epochs and channels as the frequency with the highest local maximum (larger than its 2 neighboring samples) of power amplitude in the frequency range 6 to 14 Hz. 5 In the second approach, the peak frequency was computed as the CoG of the average power spectrum across all epochs and channels in the range 6 to 14 Hz. Specifically, it was computed as the frequency-weighted sum of the averaged power spectrum in the specified frequency range divided by the sum of the power spectrum in the specified frequency range. 5,36 In the third approach, the power spectrum was computed with the 5-second epoched data between 1 and 100 Hz using a fast Fourier transformation with Hanning multitapers. Frequency resolution was 0.2 Hz. The local sensorimotor CoG at electrodes C3, Cz, and C4 was computed on single epochs in the range from 9 to 11 Hz. Peak frequency was the average across epochs and channels of the computed CoGs. 21
2.4.3.2. Amplitudes of neuronal oscillations (power)
The amplitudes of neuronal oscillations (power) at theta, alpha, beta, and gamma frequencies were analyzed in source space. To this end, linearly constrained minimum variance beamforming was used to project the band-pass filtered data of each frequency band from electrode space into source space for each participant and session. This was performed with a combination of predefined FieldTrip functions 47 and custom-written code as previously described. 56 Spatial filters for every recording and frequency band were computed based on the covariance matrices of the band-pass filtered data as a lead field matrix. A 3-dimensional grid with a 1-cm resolution covering the brain was defined, resulting in a total of 2020 voxels in the brain. The lead field was constructed for each voxel using a realistically shaped 3-shell boundary-element volume conduction model based on the template Montreal Neurological Institute brain. A regularization parameter of 5% of the covariance matrix was used, and the dipole orientation of most variance was chosen using singular value decomposition. Next, preprocessed and band-pass filtered EEG data of each patient were projected through the individual spatial filter to extract the time series and power of neuronal activity in each frequency band for each voxel. In addition, relative power at each frequency band was calculated by normalizing power values at the frequency band with the sum of all power values in the frequency range 1 to 100 Hz.
2.4.3.3. Network measures of functional connectivity
Functional connectivity for each participant, session, and frequency band was computed in source space using the debiased weighted phase lag index. 62 The debiased weighted phase lag index is a well-established phase-based measure of functional connectivity which is well reproducible 24 and insensitive to volume conduction and field spread effects. 62 Connectivity matrices were computed for each frequency bin and subsequently averaged across the respective frequency band. Connectivity matrices were thresholded to the 5%, 10%, and 20% strongest connections and binarized to reduce the computational load and facilitate interpretation. 20 Graph theory-based measures were used to reduce the high dimensionality of the connectivity matrices and condense the information to single parameters that could be tracked longitudinally and used for correlation analyses. According to graph theory, networks are collections of nodes and edges connecting the nodes. In the present context, nodes were defined as voxels and edges as thresholded functional connectivity values between voxels. Two local graph theory measures, assessing the connectivity of certain nodes, and 3 global measures, indicating the organization of the whole network, were computed. Local measures were the degree and the local clustering coefficient (CC). The degree indicates the number of connections to all other nodes of the network, and the local CC indicates the number of connections to neighboring nodes. Global graph measures were the global CC (gCC), the global efficiency (gEff), and the small-worldness (S). 1 The gCC is the average of the local CC of all nodes and thus regarded as a measure of segregation in functional networks. The gEff is the inverse of the average shortest pathlength and thus represents a measure of integration in functional networks. The small-worldness (S) provides a measure of the balance of functional integration and segregation by comparing the gCC and gEff of the network with the gCC and gEff of a random network with the same number of vertices and edges. More formally . 28 Thus, it can quantify the balance of segregation and integration and hence the effectiveness of communication in a functional network. 28,53,64 All graph measures were calculated in the theta, alpha, beta, and gamma band, based on the binary connectivity matrix with the brain connectivity toolbox 2019-03-03 release. 53
2.5. Statistical analyses
To statistically assess potential changes in clinical measures from the baseline to follow-up, frequentist and Bayesian statistics were performed using JASP (JASP Team 2021, version 0.9.2) and MATLAB with the FieldTrip toolbox. In Bayesian hypothesis testing, the Bayes factor (BF10) provides a continuous measure to quantify the evidence in favor of or against a certain hypothesis. It allows to interpret the significance of negative findings (“absence of evidence vs evidence of absence”) and to estimate effect sizes. 34 A BF10 > 1 indicates more evidence for the alternative hypothesis, whereas a BF10 < 1 indicates that evidence against the alternative hypothesis prevails. A BF10 > 3 and > 10 is commonly regarded as moderate and strong evidence in favor of the alternative hypothesis, respectively, whereas a BF10 < 0.33 and < 0.1 is regarded as moderate and strong evidence against the alternative hypothesis, respectively. Differences in clinical measures between baseline and follow-up were assessed using frequentist and Bayesian paired sample t tests.
Correlation analyses were performed to relate individual changes in clinical measures to individual changes in brain function. Changes in clinical measures were correlated to changes in EEG measures that have previously been shown to differ between patients with chronic pain and healthy participants (ie, individual peak frequency as well as absolute and relative power in theta, alpha, beta, and gamma bands) 50 and to changes in graph theory measures of brain networks. 8 For all analyses, changes in clinical as well as brain measures were calculated by subtracting the baseline from follow-up values of the respective measure.
Pearson correlations between changes in clinical measures and changes in peak frequencies and global graph measures were assessed using partial correlations, controlling for sex and changes in the MQS using SPSS (IBM SPSS Statistics, version 25.0, Armonk, NY). The corresponding Bayes factor for each r value was calculated using JASP, as recently described (https://osf.io/sxjhw/). Specifically, the Bayesian correlation tool of the Summary statistics module in JASP was used. The sample size value was obtained by subtracting the number of controlling variables from the actual sample size (n-k).
Cluster-based permutation tests as implemented in FieldTrip 44,47 were used to assess correlations between changes in clinical measures and changes in local brain measures, ie, total and relative power and local graph measures computed voxel-wise for each frequency band. Cluster-based permutation tests were performed on the t values of correlations between voxel-wise changes in EEG measures and the changes in clinical measures. 44 Clusters of neighboring voxels, whose t statistic exceeded a critical threshold of P = 0.05, were selected and t values within each cluster were summed up resulting in cluster-level test statistics. The original cluster-level test statistic was then compared with a reference distribution of maximum cluster t value sums obtained by randomly shuffling clinical and EEG measures across the group and recalculating the cluster-level test statistic. Finally, this comparison resulted in a P value defined by the proportion of permutations in which the cluster-level test statistic exceeded the actually observed maximum cluster-level test statistic derived from the data. The reference distribution was generated through 10,000 randomizations.
The significance level for all frequentist statistical tests was set to 0.05 two-tailed. A sensitivity analysis using G*Power 19 showed that the present sample size of 41, given an α error probability of 0.05 and a power of 0.8, was sufficient to detect medium to strong effect sizes of rho (ρ) = 0.42 for correlation analyses. For all analyses performed for the 4 frequency bands (correlations with power and graph theory-based measures), correction for multiple comparisons across the 4 frequency bands was performed using the Bonferroni method. 26
2.6. Data availability
Raw and preprocessed EEG data as well as clinical data from the baseline and follow-up assessments in BIDS format 49 together with the custom-written analysis code are available at osf.io/a8bpx/.
3. Results
3.1. Clinical measures
We assessed 3 clinical outcome measures which reflect the domains pain intensity (average pain intensity during the past 4 weeks, Numerical Rating Scale 0-10), physical functioning (Pain Disability Index), and emotional wellbeing (Becks Depression Inventory). 17,30,48 All measures were assessed before and 6 to 9 months after IMPT. At the follow-up after IMPT, we found strong evidence for a reduction of pain intensity (T(df40) = 3.20, P = 0.003, BF10 = 13.31), disability (T(df40) = 3.16, P = 0.003, BF10 = 11.54), and severity of depressive symptoms (T(df40) = 4.55, P < 0.001, BF10 = 468.68). Figure 2 and Table 1 show an overview of the data and detailed statistical results, respectively.
Figure 2.
Clinical measures at baseline and follow-up. Measures of average pain intensity on the Numerical Rating Scale (NRS) as well as scores for pain-related disability (Pain Disability Index [PDI]) and depression (Beck Depression Inventory II [BDI]) at baseline and follow-up are depicted. Raincloud plots show unmirrored violin plots displaying the probability density function of the data, boxplots, and individual data points. Boxplots depict the sample median as well as first (Q1) and third quartiles (Q3). Whiskers extend from Q1 to the smallest value within Q1 −1.5* interquartile range (IQR) and from Q3 to the largest values within Q3 +1.5* IQR.
3.2. Correlations between clinical measures and dominant peak frequencies
We first correlated changes in pain intensity, pain-related disability, and depression with changes of the dominant peak frequency. We pursued 3 different approaches to assess the dominant peak frequency. Pearson partial correlations, controlling for sex and medication, of changes in clinical measures with changes in peak frequencies from all 3 approaches showed moderate evidence against a relationship between clinical measures and the dominant peak frequency (all P values > 0.05, 0.2 < BF10 < 0.41). Table 2 shows the detailed results and test statistics.
Table 2.
Correlations of changes in clinical measures and dominant peak frequency measures.
| Δ Avg. pain intensity | Δ PDI | Δ BDI | |
|---|---|---|---|
| Δ absolute ([mean±SD] 0.35 ±1.27 Hz) | r = 0.20, P = 0.22, BF10 = 0.41 | r = −0.11, P = 0.52, BF10 = 0.25 | r = 0.16, P = 0.33, BF10 = 0.32 |
| Δ CoG ([mean±SD] 0.08 ±0.38 Hz) | r = −0.13, P = 0.45, BF10 = 0.27 | r = −0.20, P = 0.24, BF10 = 0.41 | r = 0.03, P = 0.86, BF10 = 0.20 |
| Δ sensorimotor ([mean±SD] 0.01 ±0.09 Hz) | r = 0.03, P = 0.84, BF10 = 0.20 | r = 0.07, P = 0.69, BF10 = 0.22 | r = 0.17, P = 0.32, BF10 = 0.33 |
Correlations were performed as partial correlation analyses, controlling for sex and Δ medication. Δ = follow-up values—baseline values; Absolute = frequency correspondent to the local maximum in the average power spectrum in the range from 6 to 14 Hz; Avg. pain intensity, Average pain intensity in the past 4 weeks (Numerical Rating Scale, NRS 0-10); BDI, Beck Depression Inventory II; BF10, Bayes Factor; CoG, frequency correspondent to the center of gravity of the power spectrum in the 6 to 14 Hz range; Sensorimotor = local CoG of the power spectrum at electrodes C3, Cz, and C4 in the range from 9 to 11 Hz; PDI, Pain Disability Index.
3.3. Correlations between clinical measures and amplitudes of neuronal oscillations
We next correlated changes in clinical measures with changes in the average amplitude of the absolute and relative power spectrum in source space in the theta, alpha, beta, and gamma frequency bands. Cluster-based permutation tests on Pearson correlations were computed. Correction for multiple comparisons was performed across the 4 frequency bands with the Bonferroni method. No significant cluster associated with changes in pain intensity, pain-related disability, or depression was found (all corrected P values > 0.05). Supplementary Table 2 and Supplementary Figure 1 (available at http://links.lww.com/PAIN/B556) show the detailed statistical results and an overview of the correlation t maps, respectively.
3.4. Correlations between clinical measures and network measures of functional connectivity
We finally correlated changes in clinical measures with changes of local and global graph theory-based network measures in the theta, alpha, beta, and gamma frequency bands. We computed the graph theory measures on the connectivity matrices thresholded to the 5%, 10%, and 20% strongest connections. We primarily report results for the 10% threshold. The results did not significantly differ when using the 5% and 20% thresholds (see Supplementary Tables 3 and 4, available at http://links.lww.com/PAIN/B556).
Local network characteristics were assessed using the local clustering coefficient (CC) and the degree. These 2 local graph measures were calculated voxel-wise, and their changes were correlated with changes in pain intensity, pain-related disability, and depression using cluster-based permutation statistics. Cluster-based permutation tests were used to assess statistical significance of correlations (see Supplementary Table 3 for detailed statistical results, available at http://links.lww.com/PAIN/B556). After the Bonferroni correction across the 4 frequency bands, 1 significant cluster indicating a positive relationship between changes in pain-related disability and changes in the local degree in the beta band was found (p [corr./uncorr.] = 0.028/0.007). The cluster was located in the brainstem and cerebellum (see Supplementary Figure 2, available at http://links.lww.com/PAIN/B556). No other significant cluster was found (all corrected P values >0.05).
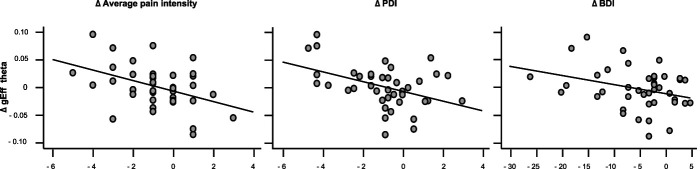
Global network characteristics were assessed using the global CC (gCC), the gEff, and the small-worldness (S) (see Supplementary Fig. 3 for an overview of the data, available at http://links.lww.com/PAIN/B556). Pearson partial correlations, controlling for sex and medication, between changes in clinical measures and changes in global graph measures were computed for each frequency band. Bonferroni corrections were performed across the 4 frequency bands. We found strong evidence for a negative correlation of changes in gEff in the theta band with changes in pain intensity (r = −0.56, p [corr./uncorr] = <0.001/<0.001, BF10= 133.5) and pain-related disability (r = −0.46, p [corr./uncorr.] = 0.012/0.003, BF10= 13.6) and inconclusive evidence for a correlation with changes in depression (r = −0.33, p [corr./uncorr.] = 0.132/0.033, BF10= 1.78). Figure 3 shows a graphical representation of these correlations, and Table 3 shows the detailed statistical results. No other significant correlations of changes in global graph measures in any frequency band with changes in the clinical measures were found (Table 3, remaining corrected P values >0.05, 0.2 < BF10 <1.78).
Figure 3.
Correlations of changes in theta band global efficiency with changes in clinical measures. Bivariate correlations of changes in the graph theory-based measure global efficiency (gEff) in the theta band with changes in average pain intensity, pain-related disability (Pain Disability Index [PDI]), and depression (Beck Depression Inventory II [BDI]) are depicted. Δ = change = follow-up values—baseline values.
Table 3.
Partial correlations of changes in clinical measures and global graph theory-based network measures controlled for sex and change in medication.
| Δ Avg. pain intensity | Δ PDI | Δ BDI | |
|---|---|---|---|
| Δ gCC | |||
| Theta | r = 0.20, P = 0.23, BF10 = 0.41 | r = 0.23, P = 0.17 BF10 = 0.52 | r = 0.17, P = 0.32, BF10 = 0.33 |
| Alpha | r = 0.08, P = 0.65, BF10 = 0.22 | r = −0.10, P = 0.54, BF10 = 0.24 | r = 0.02, P = 0.91, BF10 = 0.20 |
| Beta | r = 0.22, P = 0.19, BF10 = 0.48 | r = 0.13, P = 0.43, BF10 = 0.27 | r = 0.10, P = 0.56, BF10 = 0.10 |
| Gamma | r = −0.15, P = 0.36, BF10 = 0.30 | r = −0.16, P = 0.33, BF10 = 0.32 | r = −0.03, P = 0.85, BF10 = 0.24 |
| Δ gEff | |||
| Theta | r = −0.56***, P <0.001, BF10 = 133.5 | r = −0.46*, P = 0.003, BF10 = 13.6 | r = −0.34, P = 0.03 BF10 = 1.78 |
| Alpha | r = −0.34, P = 0.03, BF10 = 1.78 | r = −0.29, P = 0.07 BF10 = 0.76 | r = −0.04, P = 0.83, BF10 = 0.21 |
| Beta | r = −0.09, P = 0.58, BF10 = 0.23 | r = 0.01, P = 0.93, BF10 = 0.20 | r = 0.02, P = 0.92, BF10 = 0.20 |
| Gamma | r = 0.07, P = 0.67, BF10 = 0.22 | r = 0.17, P = 0.31, BF10 = 0.33 | r = 0.09, P = 0.58, BF10 = 0.23 |
| Δ S | |||
| Theta | r = −0.06, P = 0.70, BF10 = 0.21 | r = 0.01, P = 0.93 BF10 = 0.20 | r = 0.002, P = 0.99, BF10 = 0.20 |
| Alpha | r = −0.07, P = 0.69, BF10 = 0.22 | r = −0.19, P = 0.24, BF10 = 0.38 | r = 0.01, P = 0.96, BF10 = 0.20 |
| Beta | r = 0.16, P = 0.33, BF10 = 0.32 | r = 0.15, P = 0.38, BF10 = 0.30 | r = 0.11, P = 0.49, BF10 = 0.25 |
| Gamma | r = −0.11, P = 0.51, BF10 = 0.25 | r = −0.07, P = 0.67, BF10 = 0.22 | r = −0.01, P = 0.94, BF10 = 0.20 |
Correlations were calculated as partial correlation analyses, controlling for sex and Δ in medication. Δ = follow-up values—baseline values; Avg. pain intensity, Average pain intensity in the past 4 weeks (Numerical Rating Scale, NRS 0-10); BDI, Beck Depression Inventory II; BF10, Bayes Factor; gCC, global Clustering Coefficient; gEff, global Efficiency; S, small-worldness; PDI, Pain Disability Index.
Table shows uncorrected p values; Asterisks indicate the level of significance of correlations after correcting for multiple comparisons across the 4 frequency bands: *P < 0.05, ***P < 0.001.
4. Discussion
In this study, we longitudinally assessed clinical and resting-state EEG measures in 41 patients with chronic pain undergoing IMPT. The results showed that pain intensity, pain-related disability, and depression were significantly reduced at follow-up 6 to 9 months after IMPT. 22,31 These changes were not correlated with changes in measures of brain activity which have previously been shown to differ between patients with chronic pain and healthy participants. We however found that the decreases of pain intensity and disability were associated with increases of gEff at theta frequencies. Thus, this graph theory-based network measure, which reflects the global ease of communication in the brain, should be further evaluated for its potential to monitor changes of chronic pain over time.
4.1. Power and peak frequency measures
Previous cross-sectional studies comparing patients with chronic pain with healthy participants have described differences in various EEG measures of brain activity. 50 These particularly include a slowing of the dominant peak frequency of the EEG, 50 which also correlates with pain sensitivity in healthy human participants. 21 Moreover, previous studies showed differences of oscillations between patients and healthy participants at various frequencies but most consistently increases of theta and alpha oscillations in patients with chronic pain. 9,11,15,18,35,39,50,54,55,63,65 These alterations have been related to the concept of thalamocortical dysrhythmia. 40 This concept considers increased theta activity and subsequent slowing of the dominant peak frequency to result from thalamic bursts driven by abnormal nociceptive input. 40,41,54 However, slowing of the dominant peak frequency and increases of theta oscillations in chronic pain were not always found. 56 The present longitudinal data from a patient group with mixed chronic pain entities extend these investigations by showing that the dominant peak frequency and neuronal oscillations at theta to gamma frequencies do not track changes in chronic pain over time.
4.2. Functional connectivity measures
Our results showed a positive relationship between changes in pain-related disability and changes in local degree in the beta band in the cerebellum. However, this relationship was found for only one but not the other clinical measures. Moreover, it is unclear how reliably EEG can detect brain signals from cerebellar sources. 2 Thus, the functional significance of this finding remains unclear. Further analyses showed that decreases in pain intensity and pain-related disability after IMPT were associated with increases of gEff in the theta band. Thus far, only a few cross-sectional studies have compared local and global brain network measures between patients with chronic pain and healthy participants. The results are not consistent yet. Considering local connectivity, changes were observed at different locations (prefrontal cortex, 56 anterior cingulate cortex, 60 somatosensory areas, 12,60 default mode network, 12,61 and salience network 61 ), in different graph measures 10 (local clustering, local efficiency, and degree), and at different frequency bands (theta, alpha, beta, and gamma). Considering global connectivity, decreases at theta 12 and gamma frequencies 56 were found. These heterogeneous cross-sectional findings are complemented and extended by the present longitudinal findings, which point towards a positive relationship between improvements of chronic pain and increases of global network efficiency at theta frequencies. Overall, these results are compatible with an important role of neuronal oscillations and connectivity at theta frequencies in chronic pain. Moreover, because the gEFF reflects the brain's ability to efficiently combine information from specialized brain areas, this might be interpreted as an enhanced ease of communication between different brain regions 53 related to successful treatment. Furthermore, the relationships of clinical changes to a global network measure suggest that global network functioning plays an important role in chronic pain.
4.3. Implications
The present EEG approach might help to develop an easily accessible biomarker of pain because EEG is broadly available, cost-effective, and potentially mobile. 14,51 However, pain biomarkers can have many different functions, such as indicating susceptibility and providing information on diagnosis and prognosis. 14 Longitudinal approaches such as the present one might be particularly relevant for a biomarker that can monitor chronic pain over longer periods of time and in response to treatment. The present results suggest that global network measures at theta frequencies might be particularly relevant for such a monitoring biomarker. However, further longitudinal studies in larger cohorts of patients are needed to confirm these findings. Beyond, the present findings might help to identify targets for noninvasive brain stimulation techniques and biofeedback. 25 For example, it has been shown that transcranial alternating current stimulation over the prefrontal cortex at theta frequencies can modulate functional integration and hub capacity of the anterior cingulate cortex as well as the affective state in healthy participants. 46 Such approaches might also be able to normalize aberrant local connectivity and global network functioning in chronic pain.
4.4. Limitations
Several limitations have to be considered when interpreting the present results. First, aberrant neuronal oscillations and connectivity patterns occur in various neuropsychiatric disorders. 45,58 It thus remains unclear whether the observed changes are specific to chronic pain. Second, EEG is confined by a lower spatial resolution, especially regarding deeper sources. This might foster spurious connectivity, especially in the cerebellum. 2 Hence, there is a high probability that the local cluster observed in this region is an artifact. Moreover, this study uses a very fine-grained parcellation that might be prone to spurious interactions including the common drive effect. Future studies might therefore evaluate the influence of different parcellations on connectivity matrices. Third, patients in this study suffered from different pain entities but predominantly from chronic back pain. Thus, results do not necessarily generalize to other chronic pain conditions, and future studies should use larger patient cohorts that allow for subgroup analyses and detection of smaller effects. Fourth, a part of the patients took antidepressants, opioids, and other centrally acting medication that might potentially influence brain function. However, medication intake as assessed by MQS scores was unchanged from the baseline to follow-up and was additionally used as a controlling variable for correlation analyses. Fifth, owing to the lack of a control group, clinical and neuronal changes in the present cohort cannot be specifically attributed to IMPT. However, the effectiveness of IMPT has been demonstrated before, 31 and its assessment has not been the scope of the present work. Instead, the main goal of this study was to identify EEG measures which are suited to longitudinally track changes of chronic pain including those related to treatment. 14
5. Conclusion
In conclusion, the present findings indicate that EEG measures of brain activity which can differentiate between patients with chronic pain and healthy participants do not necessarily track longitudinal changes of chronic pain. Instead, we found preliminary and tentative evidence that global network function at theta frequencies might be helpful to longitudinally monitor chronic pain.
Conflict of interest statement
T.R Tölle received consulting fees from Almirall Hermal, AOP Orphan, Benkitt Rekinser, Bionest Partners, Grünenthal, Hexal, Indivior, Kaia Health, Lilly, Medscape Mundipharma, MSD, Novartis, Pfizer, Recordati Pharma, Sanofi-Aventis, and TAD Pharma; all not related to the present work. The remaining authors have no conflicts of interest to declare.
Appendix A. Supplemental digital content
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B556.
Acknowledgements
The authors thank all patients for their participation in the study and the team of the TUM Center for Interdisciplinary Pain Medicine, especially Christine Berger and Vesna Dolanjski. Moreover, the authors thank Simon Kucharsky as well as Bernhard Haller for statistical advice. The study was supported by the Deutsche Forschungsgemeinschaft (PL 321/10-2, PL321/11-2, and PL321/13-1) and the TUM School of Medicine Clinician Scientist Program (KKF).
Footnotes
Sponsorships or competing interests that may be relevant to content are disclosed at the end of this article.
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal's Web site (www.painjournalonline.com).
Contributor Information
Henrik Heitmann, Email: henrik.heitmann@tum.de.
Cristina Gil Ávila, Email: cristina.gil@tum.de.
Moritz M. Nickel, Email: moritz.nickel@tum.de.
Son Ta Dinh, Email: son.ta.dinh@tum.de.
Elisabeth S. May, Email: elisabethsusanne.may@tum.de.
Laura Tiemann, Email: laura.tiemann@tum.de.
Vanessa D. Hohn, Email: vanessa.hohn@tum.de.
Thomas R. Tölle, Email: thomas.toelle@tum.de.
References
- [1]. Achard S, Delon-Martin C, Vertes PE, Renard F, Schenck M, Schneider F, Heinrich C, Kremer S, Bullmore ET. Hubs of brain functional networks are radically reorganized in comatose patients. Proc Natl Acad Sci U S A 2012;109:20608–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2]. Andersen LM, Jerbi K, Dalal SS. Can EEG and MEG detect signals from the human cerebellum? Neuroimage 2020;215:116817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3]. Baliki MN, Apkarian AV. Nociception, pain, negative moods, and behavior selection. Neuron 2015;87:474–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4]. Barroso J, Wakaizumi K, Reis AM, Baliki M, Schnitzer TJ, Galhardo V, Apkarian AV. Reorganization of functional brain network architecture in chronic osteoarthritis pain. Hum Brain Mapp 2021;42:1206–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5]. Bazanova OM, Vernon D. Interpreting EEG alpha activity. Neurosci Biobehav Rev 2014;44:94–110. [DOI] [PubMed] [Google Scholar]
- [6]. Beck AT, Steer RA, Brown G. Manual for the Beck Depression Inventory-II. San Antonio: Psychological Corporation, 1996. [Google Scholar]
- [7]. Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life, and treatment. Eur J Pain 2006;10:287–333. [DOI] [PubMed] [Google Scholar]
- [8]. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci 2009;10:186–98. [DOI] [PubMed] [Google Scholar]
- [9]. Camfferman D, Moseley GL, Gertz K, Pettet MW, Jensen MP. Waking EEG cortical markers of chronic pain and sleepiness. Pain Med 2017;18:1921–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10]. Case M, Shirinpour S, Vijayakumar V, Zhang H, Datta Y, Nelson S, Pergami P, Darbari DS, Gupta K, He B. Graph theory analysis reveals how sickle cell disease impacts neural networks of patients with more severe disease. Neuroimage Clin 2019;21:101599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11]. Case M, Shirinpour S, Zhang H, Datta YH, Nelson SC, Sadak KT, Gupta K, He B. Increased theta band EEG power in sickle cell disease patients. J Pain Res 2018;11:67–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12]. Choe MK, Lim M, Kim JS, Lee DS, Chung CK. Disrupted resting state network of fibromyalgia in theta frequency. Sci Rep 2018;8:2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13]. Chu CJ, Kramer MA, Pathmanathan J, Bianchi MT, Westover MB, Wizon L, Cash SS. Emergence of stable functional networks in long-term human electroencephalography. J Neurosci 2012;32:2703–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14]. Davis KD, Aghaeepour N, Ahn AH, Angst MS, Borsook D, Brenton A, Burczynski ME, Crean C, Edwards R, Gaudilliere B, Hergenroeder GW, Iadarola MJ, Iyengar S, Jiang Y, Kong JT, Mackey S, Saab CY, Sang CN, Scholz J, Segerdahl M, Tracey I, Veasley C, Wang J, Wager TD, Wasan AD, Pelleymounter MA. Discovery and validation of biomarkers to aid the development of safe and effective pain therapeutics: challenges and opportunities. Nat Rev Neurol 2020;16:381–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15]. de Vries M, Wilder-Smith OH, Jongsma ML, van den Broeke EN, Arns M, van Goor H, van Rijn CM. Altered resting state EEG in chronic pancreatitis patients: toward a marker for chronic pain. J Pain Res 2013;6:815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16]. Duan W, Chen X, Wang YJ, Zhao W, Yuan H, Lei X. Reproducibility of power spectrum, functional connectivity and network construction in resting-state EEG. J Neurosci Methods 2021;348:108985. [DOI] [PubMed] [Google Scholar]
- [17]. Dworkin RH, Turk DC, Farrar JT, Haythornthwaite JA, Jensen MP, Katz NP, Kerns RD, Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J, Immpact. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. PAIN 2005;113:9–19. [DOI] [PubMed] [Google Scholar]
- [18]. Fallon N, Chiu Y, Nurmikko T, Stancak A. Altered theta oscillations in resting EEG of fibromyalgia syndrome patients. Eur J Pain 2017;22:49–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19]. Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behav Res Methods 2009;41:1149–60. [DOI] [PubMed] [Google Scholar]
- [20]. Fornito A, Zalesky A, Bullmore ET. Fundamentals of Brain Network Analysis. San Diego: Academic Press, 2016. [Google Scholar]
- [21]. Furman AJ, Prokhorenko M, Keaser ML, Zhang J, Chen S, Mazaheri A, Seminowicz DA. Sensorimotor peak alpha frequency is a reliable biomarker of prolonged pain sensitivity. Cereb Cortex 2020;30:6069–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22]. Gatchel RJ, McGeary DD, McGeary CA, Lippe B. Interdisciplinary chronic pain management: past, present, and future. Am Psychol 2014;69:119–30. [DOI] [PubMed] [Google Scholar]
- [23]. Harden RN, Weinland SR, Remble TA, Houle TT, Colio S, Steedman S, Kee WG. Medication Quantification Scale Version III: update in medication classes and revised detriment weights by survey of American Pain Society Physicians. J Pain 2005;6:364–71. [DOI] [PubMed] [Google Scholar]
- [24]. Hardmeier M, Hatz F, Bousleiman H, Schindler C, Stam CJ, Fuhr P. Reproducibility of functional connectivity and graph measures based on the phase lag index (PLI) and weighted phase lag index (wPLI) derived from high resolution EEG. PLoS One 2014;9:e108648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25]. Hohn VD, May ES, Ploner M. From correlation towards causality: modulating brain rhythms of pain using transcranial alternating current stimulation. Pain Rep 2019;4:e723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26]. Holm S. A simple sequentially rejective multiple test procedure. Scand J Statist 1979;6:65–70. [Google Scholar]
- [27]. Huang S, Wakaizumi K, Wu B, Shen B, Wu B, Fan L, Baliki MN, Zhan G, Apkarian AV, Huang L. Whole-brain functional network disruption in chronic pain with disk herniation. PAIN 2019;160:2829–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28]. Humphries MD, Gurney K. Network “small-world-ness”: a quantitative method for determining canonical network equivalence. PLoS One 2008;3:e0002051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29]. Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, Sejnowski TJ. Removing electroencephalographic artifacts by blind source separation. Psychophysiology 2000;37:163–78. [PubMed] [Google Scholar]
- [30]. Kaiser U, Kopkow C, Deckert S, Neustadt K, Jacobi L, Cameron P, De Angelis V, Apfelbacher C, Arnold B, Birch J, Bjarnegard A, Christiansen S, Williams CdCA, Gossrau G, Heinks A, Huppe M, Kiers H, Kleinert U, Martelletti P, McCracken L, de Meij N, Nagel B, Nijs J, Norda H, Singh JA, Spengler E, Terwee C, Peter T, Vlaeyen JWS, Wandrey H, Neugebauer E, Sabatowski R, Schmitt J. Developing a core outcome-domain set to assessing effectiveness of interdisciplinary multimodal pain therapy: the VAPAIN consensus statement on core outcome-domains. PAIN 2017;159:673–83. [DOI] [PubMed] [Google Scholar]
- [31]. Kaiser U, Treede RD, Sabatowski R. Multimodal pain therapy in chronic noncancer pain-gold standard or need for further clarification? PAIN 2017;158:1853–9. [DOI] [PubMed] [Google Scholar]
- [32]. Kaplan CM, Schrepf A, Vatansever D, Larkin TE, Mawla I, Ichesco E, Kochlefl L, Harte SE, Clauw DJ, Mashour GA, Harris RE. Functional and neurochemical disruptions of brain hub topology in chronic pain. PAIN 2019;160:973–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33]. Kennedy J, Roll JM, Schraudner T, Murphy S, McPherson S. Prevalence of persistent pain in the U.S. adult population: new data from the 2010 national health interview survey. J Pain 2014;15:979–84. [DOI] [PubMed] [Google Scholar]
- [34]. Keysers C, Gazzola V, Wagenmakers EJ. Using Bayes factor hypothesis testing in neuroscience to establish evidence of absence. Nat Neurosci 2020;23:788–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35]. Kisler LB, Kim JA, Hemington KS, Rogachov A, Cheng JC, Bosma RL, Osborne NR, Dunkley BT, Inman RD, Davis KD. Abnormal alpha band power in the dynamic pain connectome is a marker of chronic pain with a neuropathic component. Neuroimage Clin 2020;26:102241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36]. Klimesch W, Schimke H, Pfurtscheller G. Alpha frequency, cognitive load and memory performance. Brain Topogr 1993;5:241–51. [DOI] [PubMed] [Google Scholar]
- [37]. Kuner R, Flor H. Structural plasticity and reorganisation in chronic pain. Nat Rev Neurosci 2017;18:113. [DOI] [PubMed] [Google Scholar]
- [38]. Larkin TE, Kaplan CM, Schrepf A, Ichesco E, Mawla I, Harte SE, Mashour GA, Clauw DJ, Harris RE. Altered network architecture of functional brain communities in chronic nociplastic pain. Neuroimage 2021;226:117504. [DOI] [PubMed] [Google Scholar]
- [39]. Lim M, Kim JS, Kim DJ, Chung CK. Increased low- and high-frequency oscillatory activity in the prefrontal cortex of fibromyalgia patients. Front Hum Neurosci 2016;10:111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40]. Llinas R, Urbano FJ, Leznik E, Ramirez RR, van Marle HJ. Rhythmic and dysrhythmic thalamocortical dynamics: GABA systems and the edge effect. Trends Neurosci 2005;28:325–33. [DOI] [PubMed] [Google Scholar]
- [41]. Llinas RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci U S A 1999;96:15222–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42]. Mano H, Kotecha G, Leibnitz K, Matsubara T, Nakae A, Shenker N, Shibata M, Voon V, Yoshida W, Lee M, Yanagida T, Kawato M, Rosa MJ, Seymour B. Classification and characterisation of brain network changes in chronic back pain: a multicenter study. Wellcome Open Res 2018;3:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43]. Mansour A, Baria AT, Tetreault P, Vachon-Presseau E, Chang PC, Huang L, Apkarian AV, Baliki MN. Global disruption of degree rank order: a hallmark of chronic pain. Sci Rep 2016;6:34853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44]. Maris E, Oostenveld R. Nonparametric statistical testing of EEG- and MEG-data. J Neurosci Methods 2007;164:177–90. [DOI] [PubMed] [Google Scholar]
- [45]. Mouraux A, Iannetti GD. The search for pain biomarkers in the human brain. Brain 2018;141:3290–307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46]. Onoda K, Kawagoe T, Zheng H, Yamaguchi S. Theta band transcranial alternating current stimulations modulates network behavior of dorsal anterior cingulate cortex. Sci Rep 2017;7:3607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47]. Oostenveld R, Fries P, Maris E, Schoffelen JM. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput Intell Neurosci 2011;2011:156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48]. Patel KV, Amtmann D, Jensen MP, Smith SM, Veasley C, Turk DC. Clinical outcome assessment in clinical trials of chronic pain treatments. Pain Rep 2021;6:e784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49]. Pernet CR, Appelhoff S, Gorgolewski KJ, Flandin G, Phillips C, Delorme A, Oostenveld R. EEG-BIDS, an extension to the brain imaging data structure for electroencephalography. Sci Data 2019;6:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50]. Pinheiro ES, Queiros FC, Montoya P, Santos CL, Nascimento MA, Ito CH, Silva M, Nunes Santos DB, Benevides S, Miranda JG, Sa KN, Baptista AF. Electroencephalographic patterns in chronic pain: a systematic review of the literature. PLoS One 2016;11:e0149085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51]. Ploner M, May ES. Electroencephalography and magnetoencephalography in pain research - current state and future perspectives. PAIN 2018;159:206–11. [DOI] [PubMed] [Google Scholar]
- [52]. Ploner M, Sorg C, Gross J. Brain rhythms of pain. Trends Cogn Sci 2017;21:100–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53]. Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage 2010;52:1059–69. [DOI] [PubMed] [Google Scholar]
- [54]. Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain 2006;129:55–64. [DOI] [PubMed] [Google Scholar]
- [55]. Stern J, Jeanmonod D, Sarnthein J. Persistent EEG overactivation in the cortical pain matrix of neurogenic pain patients. Neuroimage 2006;31:721–31. [DOI] [PubMed] [Google Scholar]
- [56]. Ta Dinh S, Nickel MM, Tiemann L, May ES, Heitmann H, Hohn VD, Edenharter G, Utpadel-Fischler D, Tolle TR, Sauseng P, Gross J, Ploner M. Brain dysfunction in chronic pain patients assessed by resting-state electroencephalography. PAIN 2019;160:2751–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57]. Tait RC, Pollard CA, Margolis RB, Duckro PN, Krause SJ. The Pain Disability Index: psychometric and validity data. Arch Phys Med Rehabil 1987;68:438–41. [PubMed] [Google Scholar]
- [58]. Uhlhaas PJ, Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron 2012;75:963–80. [DOI] [PubMed] [Google Scholar]
- [59]. van Diessen E, Numan T, van Dellen E, van der Kooi AW, Boersma M, Hofman D, van Lutterveld R, van Dijk BW, van Straaten EC, Hillebrand A, Stam CJ. Opportunities and methodological challenges in EEG and MEG resting state functional brain network research. Clin Neurophysiol 2015;126:1468–81. [DOI] [PubMed] [Google Scholar]
- [60]. Vanneste S, De Ridder D. Chronic pain as a brain imbalance between pain input and pain suppression. Brain Commun 2021;3:fcab014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61]. Vanneste S, Ost J, Van Havenbergh T, De Ridder D. Resting state electrical brain activity and connectivity in fibromyalgia. PLoS One 2017;12:e0178516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62]. Vinck M, Oostenveld R, van Wingerden M, Battaglia F, Pennartz CM. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage 2011;55:1548–65. [DOI] [PubMed] [Google Scholar]
- [63]. Vuckovic A, Hasan MA, Fraser M, Conway BA, Nasseroleslami B, Allan DB. Dynamic oscillatory signatures of central neuropathic pain in spinal cord injury. J Pain 2014;15:645–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64]. Watts DJ, Strogatz SH. Collective dynamics of 'small-world' networks. Nature 1998;393:440–2. [DOI] [PubMed] [Google Scholar]
- [65]. Zhou R, Wang J, Qi W, Liu FY, Yi M, Guo H, Wan Y. Elevated resting state gamma oscillatory activities in electroencephalogram of patients with post-herpetic neuralgia. Front Neurosci 2018;12:750. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental digital content associated with this article can be found online at http://links.lww.com/PAIN/B556.