Abstract
Background
Small round cell sarcomas (SRCSs) account for most solid malignancies in the pediatric age group and are a part of group of malignant tumors characterized by heterogenous clinical presentation and overlapping microscopic features of small, round, primitive cells. In addition to the recently established certain genetically defined subset of undifferentiated round cell sarcomas of soft tissue and bone, this group of sarcomas include desmoplastic small round cell tumor, poorly differentiated synovial sarcoma, alveolar rhabdomyosarcoma, mesenchymal chondrosarcoma, and small cell osteosarcoma. Although, those entities share clinical and cytomorphologic features and cannot be unequivocally classified based on clinical presentation and morphology alone. Most of SRCSs characterizes of particular patterns of protein expression or genetic changes and ancillary tests remain necessary to confirm or rule out a specific diagnosis. Subtle but occasionally distinctive cytologic features narrows the number of differential diagnoses and helps to select appropriate ancillary tests necessary for the final diagnosis. Thus, when adequate fine needle aspiration (FNA) biopsy specimen is combined with ancillary tests, a specific histologic diagnosis can be made in almost all cases. However, due to complex cytologic features of SRCS as well as various quality and diversity of FNA smears, there are cases in that cytologic features which do not entirely match the known diagnostic criteria.
Summary
The aim of this review was to summarize cytomorphologic criteria and to present rare and divergent cytological features of SRCSs. Careful assessment of clinical presentation, cytological features, immunohistochemical patterns, and molecular alternations is necessary for an accurate diagnosis. Knowing of rare and divergent microscopic findings that does not fit with the known cytological criteria will help to avoid misdiagnosis.
Key Messages
The role of FNA biopsies diagnosing soft tissue and bone tumors has been increasing because of the ability of ancillary tests to assist in the diagnosis of specific tumors. SRCSs may be diagnosed accurately in cytology specimens. Access to clinical and radiographic presentation, utility of ancillary tests, understanding complexity of cytological features, and awareness of the rare cytologic findings that differ from that of the established diagnostic criteria are essential to make correct diagnosis.
Keywords: Cytology, Fine needle aspiration, Round cell, Sarcoma, Soft tissue
Introduction
Small round cell sarcomas (SRCSs) are a part of larger group of small cell malignant tumors which includes lymphoma, blastemal tumors, and small cell carcinomas. SRCSs are high-grade sarcomas characterized by a predominantly small, round-to-oval, and relatively undifferentiated cells. The representative of this group is Ewing sarcoma (ES), which is characterized by gene fusions including EWSR1 or FUS and ETS transcription factors family, newly described CIC-rearranged sarcoma, sarcoma with BCOR genetic alterations, round cell sarcoma with EWSR1-non-ETS fusions, desmoplastic small round cell tumor (DSRCT), poorly differentiated synovial sarcoma (SS), alveolar rhabdomyosarcoma (RMS), mesenchymal chondrosarcoma, and small cell osteosarcoma. Thus, some of SRCS lack specific cytomorphologic features and are difficult to clarify on morphology alone, most of those entities characterizes of subtle and occasionally distinctive cytologic features [1, 2, 3, 4, 5] (Table 1).
Table 1.
Common and rare, divergent cytologic features of SRCS
| Tumor | Diagnostically important microscopic features | Divergent microscopic features |
|---|---|---|
| ES | Smears patterns and background: Hypercellular smears. Mixture of clustered and dispersed cells. Occasional perivascular arrangement of tumor cells and pseudorosettes. Tigroid background Tumor cells micromorphology: Monomorphous population of small round cells with high N:C ratio, bland nuclear morphology, and inconspicuous nucleoli. Double cell population large light and small dark cells (better appreciated in air-dried smears). Larger cells with cytoplasmic vacuoles that contain glycogen. Occasionally moderately pleomorphic cells, spindle-shaped cells, or cells with rhabdoid morphology | Desmoplasia (eosinophilic connective tissue) in the background. Intranuclear inclusions |
|
| ||
| CIC-rearranged sarcoma | Smears patterns and background: Hypercellular smears. Dispersed cells and loosely cohesive cell clusters and sheets. Perivascular arrangements and traversing capillaries evident in cell sheets. Necrotic debris and myxoid stroma Tumor cells micromorphology: Mild to moderate pleomorphism but more prominent variation in size and shape of nuclei, compared to that of ES. Centrally or eccentrically placed round-to-ovoid, occasional polygonal, angulated, and spindle-shaped nuclei with irregular nuclear membrane, coarse chromatin pattern and variably prominent nucleoli. Majority tumor cells with scant to moderate cytoplasm, occasionally small cytoplasmic vacuoles or poorly preserved cytoplasmic borders creating slender cytoplasmic processes connecting one cell to another |
Desmoplasia (eosinophilic connective tissue) in the background and tigroid background. Cells with rhabdoid morphology |
|
| ||
| Sarcoma with BCOR genetic alterations | Smears patterns and background: Hypercellular smears with dispersed cells and loosely cohesive cell clusters. Sometimes perivascular arrangements, or papillary clusters with vascular cores. Desmoplasia (connective eosinophilic tissue), myxoid stroma, and necrotic debris Tumor cells micromorphology: Round and spindled cells with evident pleomorphism. Double cell population: large, “light” cells and small, dark cells resembling those of ES |
|
|
| ||
| DSRCT | Smears patterns and background: Moderate cellular smears. Loosely cohesive sheets and clusters or less common, tight clusters. Desmoplastic stroma fragments and occasional acinar-like structures or pseudorosettes. Necrosis and apoptosis Tumor cells micromorphology: Uniform or slightly pleomorphic undifferentiated cells with round-to-oval, slightly angulated, irregular nuclei with granular chromatin, inconspicuous nucleoli and scant cytoplasm. Nuclear molding and paranuclear cytoplasmic densities |
Hint of myxoid background matrix |
|
| ||
| Poorly differentiated SS of small cell type |
Smears patterns and background: Hypercellular smears. Mixture of tight clusters, sheets, dispersed small round cells and stripped nuclei. Rarely cells forming rosette-like structures Tumor cells micromorphology: Small round uniform cells. Showing round-to-ovoid nuclei, and scant cytoplasm. Occasional mild nuclear pleomorphism |
Double cell population: large, light cells and small, dark cells resembling those of ES |
|
| ||
| Alveolar RMS | Smears patterns and background: Hypercellular smears. Dispersed cells admixed with naked nuclei and clusters of loosely cohesive cells. Sometimes perivascular arrangement of tumor cells and lacy (tigroid) background Tumor cells micromorphology: Predominantly small- to medium-sized round or ovoid cells with scanty cytoplasm, hyperchromatic nuclei with coarse chromatin, often with large nucleoli. Occasionally Binucleated and multinucleated tumor cells and cells with eccentric nuclei and cytoplasmic densities |
|
|
| ||
| Mesenchymal chondrosarcoma |
Smears patterns and background: Predominantly cohesive cell clusters. Biphasic pattern. Fragments of fibrocartilaginous matrix and variable presentation of fibrillar and myxoid stroma and osteoclasts Tumor cells micromorphology: Tumor cells with sparse or moderate cytoplasm and round-to-oval, occasionally spindled nuclei with coarse chromatin and inconspicuous nucleoli | Desmoplasia (eosinophilic connective tissue) in the background |
|
| ||
| Small cell osteosarcoma | Smears patterns and background: Hypercellular aspirates. A mixture of cohesive fragments and dispersed small- to medium-sized cells. Rare osteoid Tumor cells micromorphology: Small- to medium- sized, slightly pleomorphic cells with high N:C ratios, round, occasionally oval or elongated nuclei, finely granular nuclear chromatin, scant cytoplasm or bare nuclei, fine cytoplasmic vacuoles |
Chondromyxoid matrix |
Fine needle aspiration (FNA) biopsy has been successfully used as an initial diagnostic procedure or as a complement to Trucut core needle biopsy (CNB) for the diagnosis and treatment of sarcomas, in the context of a multidisciplinary approach. Regarding diagnosing of SRCS, the role of cytology has been increasing because of the ability of ancillary tests to assist in the diagnosis of specific tumors.
Despite the name, FNA smears of SRCS may display variable cell population and variety of nuclear and cytoplasmic features such as mixture of smaller, darker, larger, and paler cells, admixture of spindle cells, significant nuclear pleomorphism, cells with cytoplasmic vacuolations or with abundant cytoplasm, and cytoplasmic densities creating rhabdoid-like morphology. In addition, many of those entities characterize of specific architectural patterns such as pseudo-rosettes formations, papillary structures with vascular cores, the presence of “tigroid” or myxoid background, cartilaginous, fibrillary, and osteoid matrix or eosinophilic connective tissue likely associated with desmoplasia, presented intercellular or in the background of the smears. All those microscopic features narrow the number of differential diagnoses and help to select appropriate ancillary tests necessary for the final diagnosis.
Ewing Sarcoma
ES is a primitive small round cell neoplasm in the group of neuroectodermal neoplasms characterized by gene fusions including EWSR1 and ETS transcription factors family. In the World Health Organization (WHO) Classification 2020, ES includes in larger group of undifferentiated SRCS of bone and soft tissue including round cell sarcomas with EWSR1-non-ETS fusions, CIC-rearranger sarcomas, and sarcomas with BCOR genetic alternations [1, 6]. The term peripheral neuroectodermal tumor which was traditionally used when ES displays neuroectodermal differentiation disappeared as both neoplasms share the same molecular profile.
ES is the third most frequent primary bone sarcoma after osteosarcoma and chondrosarcoma and second most common soft tissue and bone malignancies in children and adolescents after osteosarcoma. This neoplasm is rare before age 5 and after age 30 and extremely rare in elderly patients. Approximately, 80% of cases arise in patients younger than 20 years of age. Most common sites are the shafts of the long bones, pelvic bones, ribs, and spine. They may however occur in almost any bone. Sites of extraskeletal ES accounting 10–20% of neoplasm, include various parts of the body (cutaneous, subcutaneous, soft tissue, paraspinal muscles, the retroperitoneum, kidneys, and breasts) [4, 7, 8, 9]. The cytologic criteria of ES have been reported in several publications [4, 5, 8, 10, 11, 12, 13].
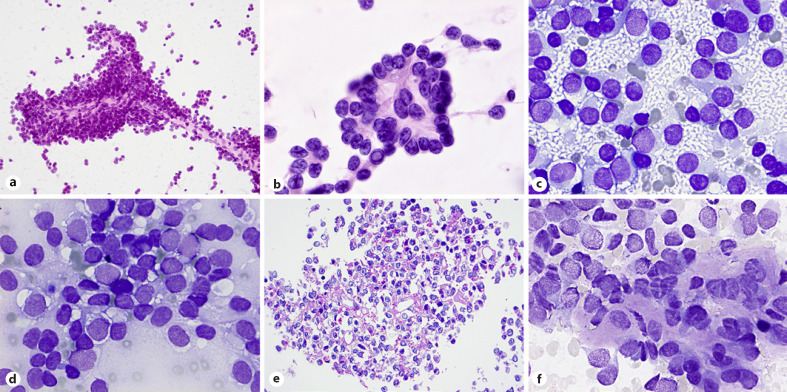
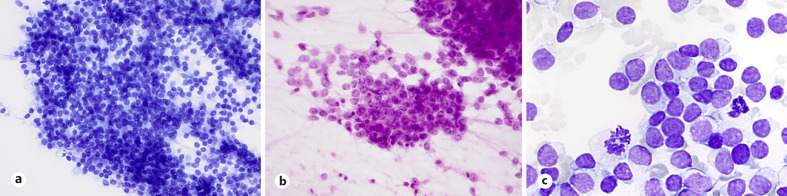
Diagnosis of ES may be suggested from technically satisfactory and preferably both air-dried and wet-fixed smears. Aspirates are commonly hypercellular with dispersed cells admixed with clusters of loosely cohesive cells, occasional perivascular arrangement of tumor cells and rosette-like structures and (Fig. 1a, b). Double-cell population, large light cells with abundant, “thin” cytoplasm with clear spaces or vacuoles, round nuclei with finely chromatin texture, and small dark cells with scant cytoplasm and hyperchromatic nuclei are better appreciated in the air-dried smears so are the “tigroid” background and intracytoplasmic vacuoles (Fig. 1c, d). Cytoplasmic glycogen is associated with cytoplasmic vacuoles and clear spaces (Fig. 1e). Occasionally spindle-shaped cells or cells with rhabdoid morphology may be seen. Cytologic features of ES include uncommon divergent findings such as intranuclear inclusion and eosinophilic connective tissue fragments in the background of the smears (Fig. 1b, f).
Fig. 1.
ES. a, b Loosely cohesive or tight clusters with perivascular arrangement of tumor cells and occasional rosette-like structures (H&E stain). c, d Dual appearances: small dark and large light cells, tigroid background, and intracytoplasmic vacuoles obvious in air-dried smears (MGG stain). e Cytoplasmic glycogen associated with cytoplasmic vacuoles and clear spaces (cell block; PAS stain). b, f Uncommon, divergent findings in smears: intranuclear inclusions and eosinophilic tissue fragments (desmoplasia) in the background of the smears (H&E and MGG stains).
More than 90% of ES show strong, cytoplasmic membrane positivity with CD99 and about 75%, predominantly cases with gene fusions, nuclear staining with the FLI-1, and ERG antibodies. Approximately, 25% of cases express keratin and differentiated subtype (previously peripheral neuroectodermal tumor) expresses neuroendocrine markers [14, 15, 16, 17]. Nuclear marker NKX2.2 is sensitive and moderately specific, while novel marker PAX7 is a sensitive marker for ES but also stains several ES mimics [18, 19, 20].
Majority of ES harbor the chromosomal translocation t(11;22)(q24;q12) involving the EWSR1 gene on chromosome 22 and the FLI-1 gene on chromosome 11, corresponding to EWSR1-FLI1 fusion (85%), or EWSR1-ERG fusion (10%). In the remaining cases EWSR1 or FUS is rearrangement with other ETS family members (like ETV1, ETV4, FEV). Finally, small subset of tumors may show fusions with FUS instead of EWSR1 with ERG or FEV [1, 7, 9, 21, 22, 23].
CIC-Rearranged Sarcoma
CIC-rearranged sarcoma shows slight male predominance, and age range presentation from children to adults with a peak incidence in the third decade of life. Most cases arise in the soft tissue of the trunk and limbs with a small subset of cases affects bones [24, 25, 26] CIC- rearranged sarcoma have highly aggressive course and the reported 5-year overall survival rate is 17%–44% [25, 26].
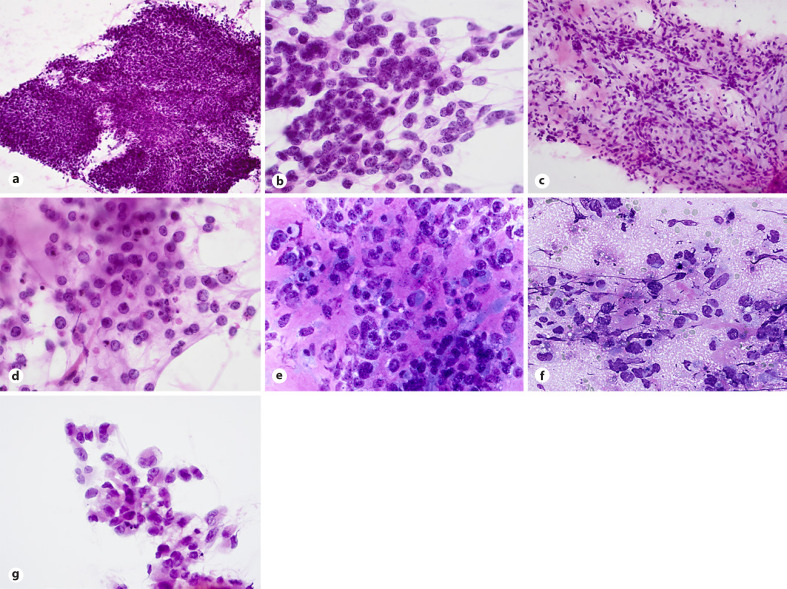
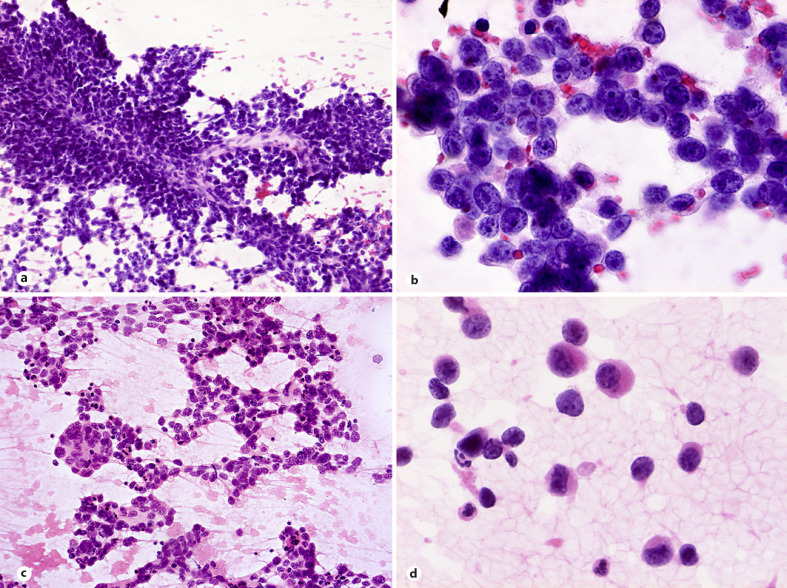
To date, there are only a few reports describing cytological features of these tumors [27, 28, 29, 30, 31]. Smears are usually hypercellular with tumor cells dispersed or arranged in tight (Fig. 2a) or loosely cohesive clusters and sheets. Small capillaries surrounded by the tumor cells or traversing vessels are evident in cell sheets (Fig. 2c). Tumor cells show mild to moderate pleomorphism but more prominent variation in size and shape of nuclei, compared to that of ES. While nuclei in CIC-rearranged sarcoma cases are commonly round-to-ovoid, occasional cells with polygonal, angulated, and spindle-shaped nuclei with irregular nuclear membrane are also seen in smears. Centrally or eccentrically placed nuclei show coarse chromatin pattern, irregular nuclear contours, and variably prominent nucleoli (Fig. 2b). Majority tumor cells show scant to moderate cytoplasm, some with small cytoplasmic vacuoles or poorly preserved cytoplasmic borders creating slender cytoplasmic processes connecting one cell to another (Fig. 2b). Necrotic debris in the background is a frequent finding in smears as is presence of myxoid stroma (Fig. 2d).
Fig. 2.
CIC-rearranged sarcoma. a, b Hypercellular smears with tight clusters or dispersed tumor cells showing centrally or eccentrically placed nuclei with coarse chromatin pattern, small nucleoli and poorly preserved cytoplasmic borders creating slender cytoplasmic processes connecting one cell to another (H&E staining). c, d. Small traversing vessels within cell sheets, apoptosis and myxoid stroma presented in smears (H&E staining). e–g Divergent but distinctive findings include desmoplastic matrix, tigroid background, and cells with a rhabdoid morphology (MGG and H&E stains).
No pseudorosettes are found in published CIC-rearranged sarcoma cases [27, 28, 29, 30, 31], but unpublished data mentioned presence of pseudorosettes in some cases [31]. In addition, not previously reported divergent findings in smears include desmoplastic background matrix, tigroid background and mono-, bi-, and multinucleated cells with eccentrically placed nuclei and cytoplasmic densities creating rhabdoid morphology (Fig. 2e-g).
By immunohistochemistry, CIC-rearranged sarcomas express CD99 with diffuse and membranous pattern in approximately 20% of cases, while in most cases, multifocal, patchy pattern or even completely absent expression. Nuclear immunoreactivity for FLI1, WT1, and ETV4 is present in most cases while NKX2.2 and PAX7 are negative [24, 25, 32, 33, 34, 35]. Calretinin and ERG can be positive, while expression of keratin, S-100, and myogenic markers is rarely detected [25, 26]. Two translocations have been described for majority of CIC-DUX4 fusion sarcomas: t(4;19)(q35;q13) and t(10;19)(q26;q13), resulting with CIC-DUX4 fusion [24, 25, 32, 33, 34]. Non-DUX4 partner genes can be rarely detected, including NUTM1, NUTM2A, LEUTX, or FOXO4 [36, 37, 38]. Cases with CIC-NUTM1 express NUT protein [38].
Sarcoma with BCOR Genetic Alterations
BCOR-rearranged sarcomas are uncommon and consist of a group of tumors, characterized by genetic alterations of BCOR gene, most often BCOR-CCNB3. This neoplasm occurs in bone and soft tissue (ratio 1.5:1) in the pelvis, lower limbs, and paraspinal region in children with a male sex predominance and over 90% of patients younger than 20 [39, 40, 41, 42]. Clinical course is similar to ES with reported 5-year overall survival 75% [39, 40, 41]. Other sarcomas with BCOR genetic alterations are undifferentiated round cell sarcoma of infancy and primitive myxoid mesenchymal tumor of infancy characterized by BCOR-ITD and very rare neoplasms with lack of well-established clinicopathological features [43, 44].
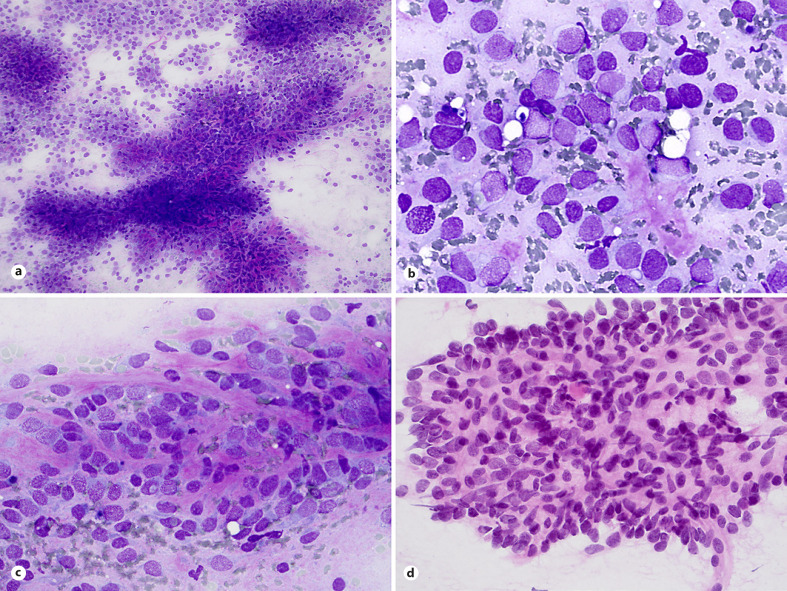
To date, there are only three papers which reported cytological features of BCOR-CCNB3 sarcoma [31, 45, 46]. Smears are usually hypercellular with mixture of dispersed cells and cell sheets and papillary clusters with vascular cores (Fig. 3a) but no rosettes formations. In one cytology report, small tumor cell clusters with thin and delicate vascular cores and tiny vascular fragments were conspicuous findings [45]. Double-cell population, large light cells, and small dark cells with hyperchromatic nuclei remaining smears of ES are common findings (Fig. 3b, c), as is admixture of spindle cells (Fig. 3d). Most nuclei show inconspicuous nucleoli. Smears of BCOR-CCNB3 sarcoma present significant nuclear pleomorphisms and single cells with rhabdoid-like features. Background necrosis, eosinophilic connective tissue (Fig. 3b, c), and myxoid stroma can be found in most cases [31].
Fig. 3.
Sarcoma with BCOR genetic alterations. a Hypercellular smears with cohesive cell sheets of round and spindled cells, dispersed cells, and papillary clusters with vascular cores (MGG stain). b, c Nuclear pleomorphism, double-cell population, large light cells and small dark cells, and eosinophilic connective tissue in the background are common findings (d) as is admixture of spindle cells (MGG and H&E stains).
By immunohistochemistry, BCOR-CCNB3 sarcoma express cyclin B3, BCOR protein, TLE1, and SATB2. Reactivity for CD99 occurs in approximately 50% of cases with staining pattern similar to CIC-rearranged sarcomas [39, 40, 41].
Round Cell Sarcoma with EWSR1-Non-ETS Fusions
Round cell sarcoma with EWSR1-non-ETS fusions is the least common entity of undifferentiated SRCS of bone and soft tissue and is defined as a sarcoma with EWSR1 or FUS fusions involving non-ETS gene family partners [1]. Clinically round cell sarcomas with FUS-NFATC2 and EWSR1-NFATC2 occur in children and adults with striking male predilection while EWSR1-PATZ1 sarcomas occur in broad range of age and affects both sexes equally. FUS-NFATC2 and EWSR1-NFATC2 affect exclusively or mainly bones respectively while most cases of EWSR1-PATZ1 sarcomas arise in the deep soft tissue of chest wall and abdomen, and the brain. To date, only one paper mentioned cytologic description of EWSR1-PATZ1 sarcoma exists describing hypercellular smears of round cells with marked cellular pleomorphism intermixed with small, short spindle cells.
Desmoplastic Small Round Cell Tumor
DSRCT is an aggressive malignant mesenchymal tumor of unknown histogenesis. It is predominantly found in the abdominal cavity in adolescents and young adults with a striking male predominance and peak incidence in the third decade of life. Clinical presentation is often dramatic, with a large intra-abdominal or retroperitoneal/pelvic mass with multiple serosal implants and subsequent metastasis to lymph nodes, liver, and lungs. An extra-abdominal location is rare, with the thorax and para testicular area being the most common sites. The prognosis of DSRCT is extremely poor and the average survival is less than 3 years [1, 47, 48].
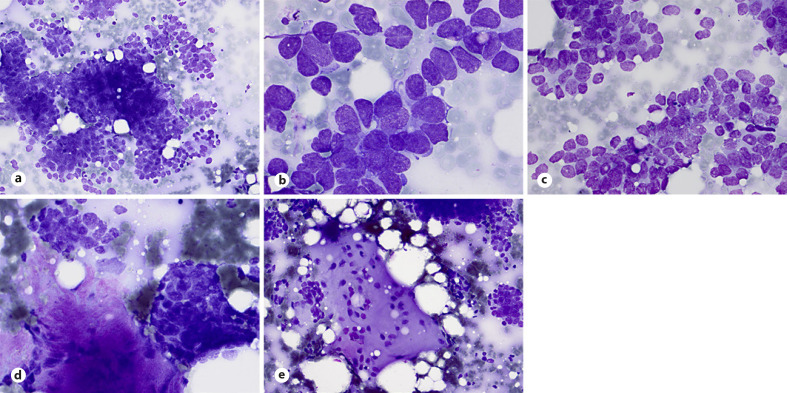
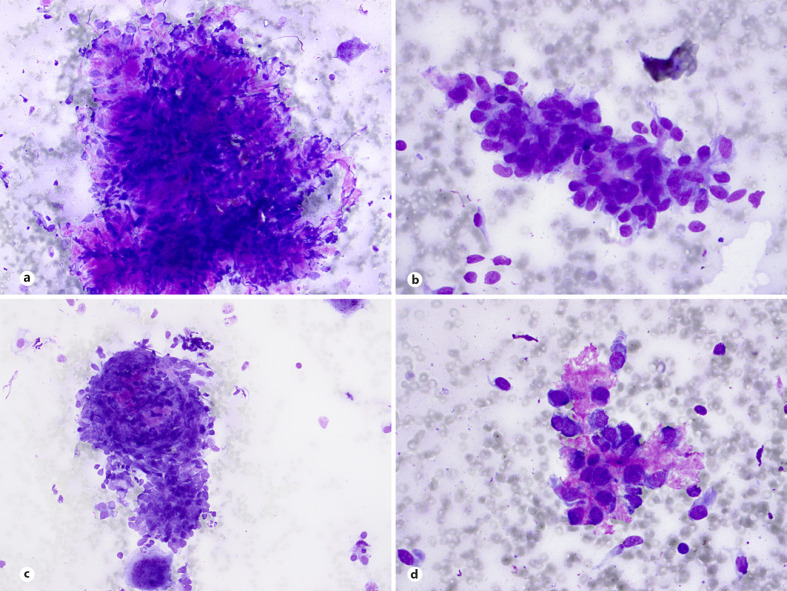
Aspirates show commonly loosely cohesive sheets and clusters or less common, tight clusters of uniform or slightly pleomorphic cells with high N:C ratios; scant or poorly preserved cytoplasm; round-to-oval, occasionally angulated, irregular nuclei with granular chromatin and inconspicuous nucleoli (Fig. 4a, b). Nuclear molding and paranuclear cytoplasmic densities are common findings (Fig. 4b, c). Mitosis, apoptosis, necrosis, and crushing artifacts are common findings in smears [49, 50, 51, 52]. The cytomorphology of DSRCT, though similar to other small blue round cell sarcomas, displays characteristic findings of nests of tumor cells with some cohesiveness retained, associated with desmoplastic stroma and occasional acinar-like structures or pseudorosettes (Fig. 4b, d). Rarely, cytologic features of DSRCT include divergent finding such as myxoid matrix in the background of the smears (Fig. 4e). DSRCT exhibits polyphenotypic differentiation, and neoplastic cells typically express epithelial (keratin and EMA), myogenic (desmin), mesenchymal (vimentin), neural (neuroendocrine), and WT1 markers [49, 53, 54]. DSRCT is characterized by a unique translocation t(11;22)(p13;q12) with EWSRI-WT1 gene fusion [48].
Fig. 4.
DSRCT. a–c Aspirates show commonly loosely cohesive or cohesive clusters of malignant cells with scant and poorly preserved cytoplasm, irregular hyperchromatic nuclei, molding, and occasional acinar-like structures (MGG stain). d Small nests of tumor cells with associated desmoplastic stroma are characteristic (MGG stain). e Rarely, cytologic features of DSRCT include divergent finding such as myxoid matrix in the background of the smears (MGG stain).
Poorly Differentiated SS of Small Cell Type
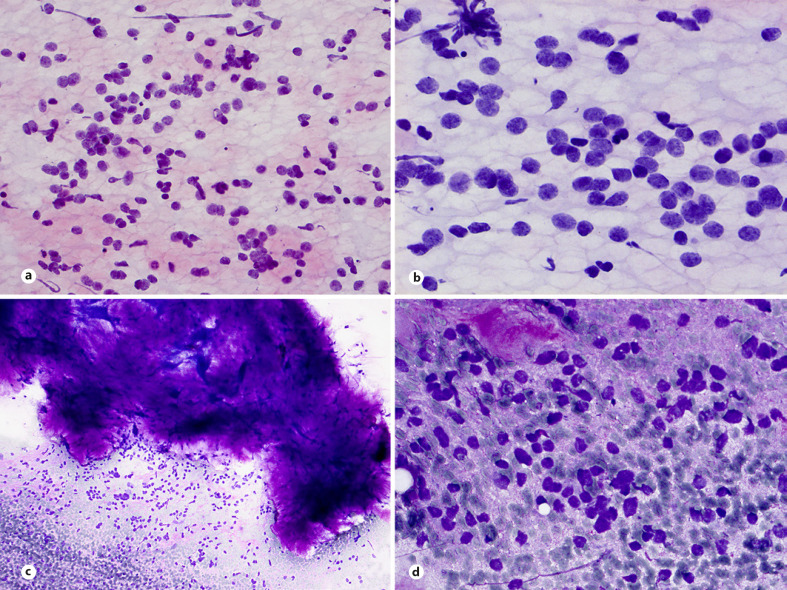
SS is characterized by a pathognomonic translocation t(X;18) which is present in >95% of cases. SS accounts for 5–10% of soft tissue sarcomas and is a highly aggressive sarcoma with common local recurrences and metastases in approximately 50% of cases. SS affects both sexes equally and may occur at any age thus most patients are between the ages of 10 and 40 years. Most SS cases are deep seated and arise in the extremities, trunk, and head and neck region, but they may occur elsewhere in the body, including rare locations in visceral organs, genital tracts, the retroperitoneum, and the mediastinum [1, 55, 56, 57]. Based on their morphology, SS are divided on monophasic, biphasic, or poorly differentiated subtypes, the last divided on three morphologic variants: the small cell variant resembling ES, the spindle cell variant resembling malignant peripheral nerve sheath tumor, and the epithelioid, pleomorphic variant with rhabdoid features [1, 58, 59, 60, 61, 62, 63]. The cytologic criteria of SS have been reported in several publications [58, 59, 60, 62, 63, 64, 65, 66, 67, 68]. FNA smears of SS are usually cellular and in low magnification show a distinctive pattern of tight clusters of bland spindle cells alternating with dispersed cells or bare nuclei. Small cell variant of SS shares cytomorphological features of small round primitive cells with high N:C ratio with those of other malignant SRCSs. The cytologic smears are highly cellular, consisting of a mixture of loose or tight clusters and dispersed uniform and monotonous cells, showing round-to-ovoid nuclei and scant cytoplasm (Fig. 5a, b). Mitotic figures, occasional mild nuclear pleomorphism, and cells forming rosette-like structures may be also observed [58, 59]. Aspirates of morphologic variants of poorly differentiated SS showing double-cell population, large light cells, and small dark cells resembling that of ES (Fig. 5c) may cause diagnostic difficulties and a definitive diagnosis requires immunocytochemical and molecular genetics examinations [60, 61]. Neoplastic cells of SS stain diffusely and strongly for TLE1. Most of the SS stain positively for CD99 and Bcl-2 and are often focally positive for EMA and cytokeratins while focal S100 expression occurs in approximately 40% of cases [1, 57, 63, 66]. SS has a specific and often sole genetic anomaly of the t(X;18)(p11;q11) translocation which creates a SS18/SSX fusion gene [67, 68, 69, 70].
Fig. 5.
Poorly differentiated SS. a, b Cellular smears with loosely cohesive clusters and dispersed cells showing distinctive round cell morphology (MGG and H&E stains). c Double-cell population, large light cells, and small dark cells resembling that of ES is occasional finding in smears (MGG stain).
Alveolar RMS
RMS is a malignant tumor with differentiation towards skeletal muscle and is the most common sarcoma of childhood. Pediatric RMS belongs to the group of neoplasms that are commonly examined by FNAB [2, 5, 71, 72, 73, 74, 75, 76]. The sensitivity and specificity for those tumors exceed 90% and with an adequate sampling and the use of ancillary techniques a diagnosis of a specific histologic subtype is establish in the majority of cases [5, 76, 77]. Four main subtypes of RMS are recognized in the current WHO classification: embryonal, alveolar, spindle cell/sclerosing, and pleomorphic [1]. The embryonal subtype accounts for approximately 60% of cases. Alveolar RMS accounts for about 20% of all pediatric RMS and occurs most frequently in adolescents and young adults between 10 and 25 years of age. Alveolar RMS is a neoplasm composed of a monomorphic population of primitive round cells with skeletal muscle differentiation and belongs to the group of small, round, blue-cell sarcomas. Aspirates are commonly hypercellular with dispersed cells admixed with clusters of loosely cohesive cells and occasionally perivascular arrangement of tumor cells (Fig. 6a). In contrast to embryonal RMS, smears are more uniform with predominantly small- to medium-sized round or ovoid cells with hyperchromatic nuclei, often with large nucleoli (Fig. 6b). Binucleated and multinucleated tumor cells and cells with eccentric nuclei and cytoplasmic densities are frequent findings in smears (Fig. 6c, d).
Fig. 6.
Alveolar RMS. a Loosely cohesive cluster with perivascular arrangement of tumor cells (H&E stain). b Loosely and dispersed, single, small-, and medium-sized cells with hyperchromatic nuclei and prominent nucleoli (H&E stain). c Loosely sheets of medium-sized cells with irregular hyperchromatic nuclei and occasional tumor giant cells (H&E stain). d Rhabdomyoblastic morphology of tumor cells (H&E stain).
Similar to embryonal RMS, tumor cells are immunoreactive with desmin, myogenin, MyoD1, and PAX7. Aberrant expression of keratins, CD99, S100 protein, neuron-specific enolase, and CD56 may present diagnostic challenges and confusion with other small round blue-cell tumors [77, 78]. Cytogenetically, alveolar RMS shows recurrent translocations, with t(2;13)(q35;q14) being the most common and t(1;13)(p36;q14) occurring in fewer cases. These translocations result in chimeric genes encoding fusion proteins with strong transcriptional activation and oncogenic effects. The genes involved are PAX3 located on chromosome 2 and PAX7 on chromosome 1. They fuse with FOXO1 on chromosome 13 resulting in the PAX3/PAX7-FOXO1 fusion genes [79, 80, 81]. The MYCN oncogene in 2p24 can also be amplified and overexpressed in fusion-positive cases [82].
Mesenchymal Chondrosarcoma
Mesenchymal chondrosarcoma is a rare high-grade bimorphic malignant tumor composed of islands of low-grade hyaline cartilage and malignant small round cells. Most cases occur in the second and third decades of life. The craniofacial bones (jawbones), ribs, vertebrae, and the ilium are the most common sites [1, 83]. Approximately, 30% of mesenchymal chondrosarcomas arise primarily in extraskeletal sites, commonly in meninges and somatic soft tissues [1, 84].
The cytologic features of mesenchymal chondrosarcoma have been reported in a few case reports [85, 86, 87, 88, 89]. Small, round monomorphic (ES-like) tumor cells in cohesive clusters, some embedded in a fibrillar matrix, have been described [85, 86, 87, 88, 89, 90] (Fig. 7a). Tumor cells contain sparse or moderate cytoplasm and round-to-oval, occasionally spindled nuclei with coarse chromatin and inconspicuous nucleoli (Fig. 7b). Fragments of fibrocartilaginous matrix and eosinophilic connective tissue, osteoclasts, and myxoid stroma are variable presented in smears (Fig. 7b-d). A biphasic pattern of cartilaginous fragments and small blue round cells support the diagnosis mesenchymal chondrosarcoma in FNA smears. When a biphasic pattern is not obvious and smears only show a small round cell population, the differential diagnosis includes other small cell malignancies and correlation to clinical and radiographic data as well as ancillary techniques is crucial for a correct diagnosis.
Fig. 7.
Mesenchymal chondrosarcoma. a Tight cluster of tumor cells embedded in the fibrocartilaginous matrix and osteoclast-like giant cell in the right upper corner (MGG stain). b, c Cluster of small and medium-sized cells with sparse or poorly preserved cytoplasm, round-to-oval, occasional spindled nuclei and intercellular strands of pinkish-violet matrix resembling osteoid. Osteoclast-like giant cells in the background. (MGGstain). d Group of tumor cells with delicate “lacy” eosinophilic stroma (MGG stain).
The typical immunophenotype of mesenchymal chondrosarcoma is S100 protein positivity in the cartilaginous component and CD99, SOX9, and NKX2.2 positivity in the small cell population. Variable positivity for desmin and negative staining for FLI-1 and CD45 help in distinguishing mesenchymal chondrosarcoma from ES and non-Hodgkin lymphoma. Small cell osteosarcoma may exhibit CD99 membrane positivity in some cases, which may cause a problem in distinguishing it from mesenchymal chondrosarcoma. A recurrent and specific HEY1-NCOA2 gene fusion is found in this subtype of chondrosarcoma and it is notably missing in the other variants of the disease. The pathogenetic mechanism of the fusion is not fully elucidated, but it could upregulate transcription [91]. The IDH1 and IDH2 mutations seen in the conventional chondrosarcomas are usually missing [92].
Small Cell Osteosarcoma
Small cell osteosarcoma, a rare histological subtype of osteosarcoma, accounts for less than 1% of all cases of osteosarcoma [93, 94]. The cytology of rare small cell osteosarcoma has been only briefly described [95, 96, 97] Aspirates from rare small cell osteosarcomas are hypercellular and tumor cells resemble those of ES. A distinction between small cell osteosarcoma and ES based on cytologic findings alone is difficult. The main cytologic feature of small cell osteosarcoma is a mixture of cohesive fragments and dispersed small- to medium-sized cells with scant cytoplasm and bare nuclei (Fig. 8a, b). Fine cytoplasmic vacuoles were reported. Tumor cells are slightly pleomorphic with round, occasionally oval or elongated nuclei, finely granular chromatin, and a high nuclear/cytoplasmic ratio. Identification of osteoid matrix is the key to the diagnosis but can be difficult in smears [94, 95, 96]. In addition, osteoid in FNA smears may display different appearance and can be difficult to distinguish it from eosinophilic desmoplastic tissue or cartilaginous and fibrillary matrix presented in smears of other small round cell neoplasm (Fig. 8c, d).
Fig. 8.
Small-cell osteosarcoma. a, b Dispersed small- to medium-sized tumor cells with scanty cytoplasm, rounded nuclei with granular chromatin and moderate anisonucleosis resembling those of ES (H&E and MGG staining). c Chondromyxoid matrix and dispersed slightly pleomorphic cells. Subsequent histologic section disclosed areas of cartilage in small cell osteosarcoma (MGG stain). d Dispersed tumor cells and small fragment of eosinophilic tissue (MGG stain).
Summary and Conclusion
Cytologic differential diagnosis of SRCS is broad and may be difficult due to their undifferentiated or poorly differentiated micromorphology. Main differentials are discussed above. Sarcomas with a round cell pattern examined frequently by FNA biopsy are ES family and alveolar RMS, while poorly differentiated SS, DSRCT mesenchymal chondrosarcoma, and small cell osteosarcoma are less common.
Differential diagnosis of undifferentiated round cell sarcomas includes other SRCSs [10, 14, 31, 45] but also non-sarcomatous malignancies such as lymphoblastic lymphoma and small cell or neuroendocrine carcinoma. Poorly differentiated SS of small cell type showing double-cell population, resembling that of ES characterizes by specific immunoprofile compared to the ES such as TLE1, EMA, and keratins positivity. Poorly differentiated SS can also mimic BCOR-CCNB3 sarcoma because coexpression of PAX7 and BCOR and occasional expression of TLE1 in the last. EMA and keratins positivity and specific gen fusions are helpful in differential diagnosis.
Cytologic features of ES and other newly described entities of this group such are CIC-rearranged and sarcoma with BCOR genetic alterations may closely mimic alveolar RMS that constitute the main differential diagnosis in FNA smears [31, 45]. Some specific cytologic features described above and immunoprofile of RMS, mainly nuclear myogenic markers such as MYOD1 and myogenin as well as specific gene fusions allows to distinguish these entities. Other differential diagnosis of alveolar RMS includes embryonal RMS, poorly differentiated SS and precursor lymphoblastic lymphoma, aberrant expression of keratins, CD99, S100 protein, neuron-specific enolase, and CD56 in alveolar RMS may present diagnostic challenges. Like other small round blue-cell neoplasms, the correct diagnosis of alveolar RMS requires ancillary techniques such as immunocytochemistry and molecular genetics examinations [4, 71].
DSRCT commonly affects young patients and shows predilection to abdominal cavity. Smears display frequently desmoplastic stroma fragments [49, 50, 51, 52]. DSRCT exhibits polyphenotypic differentiation, but majority of cases show both keratins and desmin positivity [49, 53, 54]. Typical clinical presentation in addition to a unique translocation t(11;22)(p13;q12) with EWSRI-WT1 gene fusion [48, 49] helps to distinguish DSRCT from other small blue round cell tumors and small cell carcinomas.
A biphasic pattern of cartilaginous fragments and small blue round cells in smears of mesenchymal chondrosarcoma support the diagnosis in FNA smears. Mesenchymal chondrosarcoma is a differential diagnostic problem when a biphasic pattern is not obvious in those cases where the small cell population predominates and fragments of chondroid tissue are difficult to find. When smears only show a small round cell population, the differential diagnosis includes other small cell malignancies, and correlations to clinical and radiographic data as well as ancillary techniques are necessary for a correct diagnosis. The immunophenotype of mesenchymal chondrosarcoma includes S100 protein positivity in the cartilaginous component and CD99 and SOX9 positivity in the small cell population. NKX3.1 is a useful immunohistochemical marker in differentiating mesenchymal chondrosarcoma from its histological mimics [98]. Negative staining for FLI-1 and CD45 help in distinguishing mesenchymal chondrosarcoma from ES and non-Hodgkin lymphoma. Smears of small cell osteosarcoma exhibit slightly pleomorphic cells with round- or spindle-shaped nuclei, finely granular chromatin, and a high nuclear/cytoplasmic ratio, resembling that of ES. Clinical information and presence of osteoid is very helpful in the diagnosis. In many cases, it is difficult to find convincing osteoid in smears and to distinguish it from eosinophilic connective tissue. Small cell osteosarcoma may exhibit CD99 membrane positivity in some cases, which may cause a problem in distinguishing it from mesenchymal chondrosarcoma [85, 86, 87, 88, 89, 90]. SATB2 immunoexpression helps to distinguish small cell osteosarcoma from their mimickers but this marker is also positive in BCOR-CCNB3 sarcomas and in many chondrosarcomas [99].
The advantages of soft tissue and bone FNA biopsy are good sensitivity and specificity, a low morbidity and reduced time to diagnosis, the use of ROSE to triage soft tissue and bone lesions for further workup and the low cost/benefit ratio. The role of cytology has been increasing because of the ability of ancillary tests to assist in the diagnosis of specific entities.
The FNA biopsy ideally should be the first biopsy method in the examination of a suspected recurrence or metastasis of a previously treated SRCS followed by CNB or excision biopsy when necessary. Dependent on local expertise and the site of the lesion, FNAB can be used for the evaluation of primary SRCS by itself or simultaneously with CNB or followed by CNB or subsequent open biopsy when necessary. Ancillary tests are essential for the accurate determination of the “line of differentiation” or histopathological subtype of SRCS and the methods used on histopathological specimens are also applicable to cytopathological material. Immunocytochemistry and molecular genetic tests on FNA biopsy material can be used to confirm or rule out a specific diagnosis as particular genetic changes haves been described in many types of SRCS. In summary, FNA biopsy for diagnosis of SRCSs can be recommended as the cytologic examination of these neoplasms gives excellent results both in the evaluation of recurrent and metastatic tumors and primary sarcomas.
Conflict of Interest Statement
The author has no conflicts of interest to declare.
Funding Sources
There are no funding sources.
References
- 1.The WHO Classification of Tumours Editorial Board . WHO classification of tumours soft tissue and bone tumours. 5th ed. Lyon: IARC Press; 2020. [Google Scholar]
- 2.Das DK. Fine-needle aspiration (FNA) cytology diagnosis of small round cell tumors: value and limitations. Indian J Pathol Microbiol. 2004 Jul;47((3)):309–318. [PubMed] [Google Scholar]
- 3.Rajwanshi A, Srinivas R, Upasana G. Malignant small round cell tumors. J Cytol. 2009 Jan;26((1)):1–10. doi: 10.4103/0970-9371.54861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pohar Marinšek Ž. Round cell sarcomas. In: Klijanienko J, Pohar Marinšek Ž, Domanski HA, editors. Small volume biopsy in pediatric tumors. Springer International Publishing; 2018. pp. 137–169. [Google Scholar]
- 5.Pohar-Marinšek Ž. Difficulties in diagnosing small round cell tumours of childhood from fine needle aspiration cytology samples. Cytopathology. 2008;19((2)):67–79. doi: 10.1111/j.1365-2303.2008.00555.x. [DOI] [PubMed] [Google Scholar]
- 6.Kallen ME, Hornick JL. The 2020 WHO classification: what's new in soft tissue tumor pathology? Am J Surg Pathol. 2021;45((1)):1–23. doi: 10.1097/PAS.0000000000001552. [DOI] [PubMed] [Google Scholar]
- 7.Grunewald TGP, Cidre-Aranaz F, Surdez D, Tomazou EM, De Álava E, Kovar H, et al. Ewing sarcoma. Nat Rev Dis Prim. 2018;4((1)):5. doi: 10.1038/s41572-018-0003-x. [DOI] [PubMed] [Google Scholar]
- 8.Domanski HA, Walther CS. Ewing sarcoma. Monogr Clin Cytol. 2017;22:161–164. doi: 10.1159/000475108. [DOI] [PubMed] [Google Scholar]
- 9.de Alava E. Ewing sarcoma, an update on molecular pathology with therapeutic implications. Surg Pathol Clin. 2017;10((3)):575–585. doi: 10.1016/j.path.2017.04.001. [DOI] [PubMed] [Google Scholar]
- 10.Klijanienko J, Couturier J, Bourdeaut F, Fréneaux P, Ballet S, Brisse H, et al. Fine-needle aspiration as a diagnostic technique in 50 cases of primary Ewing sarcoma/peripheral neuroectodermal tumor. Institut curie's experience. Diagn Cytopathol. 2012;40((1)):19–25. doi: 10.1002/dc.21491. [DOI] [PubMed] [Google Scholar]
- 11.Dahl I, ÅKerman M, Angervall L. Ewing's sarcoma of bone: a Correlative Cytological and Histological Study of 14 cases. Acta Pathol Microbiol Scand Ser A Pathol. 1986;94((6)):363–669. [PubMed] [Google Scholar]
- 12.Sahu K, Pai RR, Khadilkar UN. Fine needle aspiration cytology of the Ewing's sarcoma family of tumors. Acta Cytol. 2000;44((3)):332–336. doi: 10.1159/000328474. [DOI] [PubMed] [Google Scholar]
- 13.Sanati S, Lu DW, Schmidt E, Perry A, Dehner LP, Pfeifer JD. Cytologic diagnosis of Ewing sarcoma/peripheral neuroectodermal tumor with paired prospective molecular genetic analysis. Cancer. 2007;111((3)):192–199. doi: 10.1002/cncr.22692. [DOI] [PubMed] [Google Scholar]
- 14.Fellinger EJ, Garin-Chesa P, Triche TJ, Huvos AG, Rettig WJ. Immunohistochemical analysis of Ewing's sarcoma cell surface antigen p30/32(MIC2) Am J Pathol. 1991;139((2)):317–325. [PMC free article] [PubMed] [Google Scholar]
- 15.Scotlandi K, Serra M, Manara MC, Benini S, Sarti M, Maurici D, et al. Immunostaining of the p30/32(MIC2) antigen and molecular detection of EWS rearrangements for the diagnosis of Ewing's sarcoma and peripheral neuroectodermal tumor. Hum Pathol. 1996;27((4)):408–416. doi: 10.1016/s0046-8177(96)90115-x. [DOI] [PubMed] [Google Scholar]
- 16.Hornick JL. Novel uses of immunohistochemistry in the diagnosis and classification of soft tissue tumors. Mod Pathol. 2014;27((Suppl 1)):S47. doi: 10.1038/modpathol.2013.177. [DOI] [PubMed] [Google Scholar]
- 17.Rossi S, Orvieto E, Furlanetto A, Laurino L, Ninfo V, Dei Tos AP. Utility of the immunohistochemical detection of FLI-1 expression in round cell and vascular neoplasm using a monoclonal antibody. Mod Pathol. 2004;17((5)):547–552. doi: 10.1038/modpathol.3800065. [DOI] [PubMed] [Google Scholar]
- 18.Shibuya R, Matsuyama A, Nakamoto M, Shiba E, Kasai T, Hisaoka M. The combination of CD99 and NKX2.2, a transcriptional target of EWSR1-FLI1, is highly specific for the diagnosis of Ewing sarcoma. Virchows Arch. 2014;465((5)):599–605. doi: 10.1007/s00428-014-1627-1. [DOI] [PubMed] [Google Scholar]
- 19.Russell-Goldman E, Hornick JL, Qian X, Jo VY. NKX2.2 immunohistochemistry in the distinction of Ewing sarcoma from cytomorphologic mimics: diagnostic utility and pitfalls. Cancer Cytopathol. 2018;126((11)):942–949. doi: 10.1002/cncy.22056. [DOI] [PubMed] [Google Scholar]
- 20.Toki S, Wakai S, Sekimizu M, Mori T, Ichikawa H, Kawai A, et al. PAX7 immunohistochemical evaluation of Ewing sarcoma and other small round cell tumours. Histopathology. 2018 Oct;73((4)):645–652. doi: 10.1111/his.13689. [DOI] [PubMed] [Google Scholar]
- 21.Ginsberg JP, De Alava E, Ladanyi M, Wexler LH, Kovar H, Paulussen M, et al. EWS-FLI1 and EWS-ERG gene fusions are associated with similar clinical phenotypes in Ewing's sarcoma. J Clin Oncol. 1999;17((6)):1809–1814. doi: 10.1200/JCO.1999.17.6.1809. [DOI] [PubMed] [Google Scholar]
- 22.Chen S, Deniz K, Sung YS, Zhang L, Dry S, Antonescu CR. Ewing sarcoma with ERG gene rearrangements: a Molecular Study focusing on the prevalence of FUS-ERG and common pitfalls in detecting EWSR1-ERG fusions by FISH. Genes Chromosomes Cancer. 2016;55((4)):340–349. doi: 10.1002/gcc.22336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Antonescu C. Round cell sarcomas beyond Ewing: emerging entities. Histopathology. 2014;64((1)):26–37. doi: 10.1111/his.12281. [DOI] [PubMed] [Google Scholar]
- 24.Choi EYK, Thomas DG, McHugh JB, Patel RM, Roulston D, Schuetze SM, et al. Undifferentiated small round cell sarcoma with t(4;19)(q35;q13.1) CIC-DUX4 fusion a novel highly aggressive soft tissue tumor with distinctive histopathology. Am J Surg Pathol. 2013;37((9)):1379–1386. doi: 10.1097/PAS.0b013e318297a57d. [DOI] [PubMed] [Google Scholar]
- 25.Yoshida A, Goto K, Kodaira M, Kobayashi E, Kawamoto H, Mori T, et al. CIC-rearranged sarcomas: a Study of 20 Cases and Comparisons with Ewing Sarcomas. Am J Surg Pathol. 2016;40((3)):313–323. doi: 10.1097/PAS.0000000000000570. [DOI] [PubMed] [Google Scholar]
- 26.Antonescu CR, Owosho AA, Zhang L, Chen S, Deniz K, Huryn JM, et al. Sarcomas with CIC rearrangements are a distinct pathologic entity with aggressive outcome. Am J Surg Pathol. 2017;41((7)):941–949. doi: 10.1097/PAS.0000000000000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chebib I, Jo VY. Round cell sarcoma with CIC-DUX4 gene fusion: discussion of the distinctive cytomorphologic, immunohistochemical, and molecular features in the differential diagnosis of round cell tumors. Cancer Cytopathol. 2016;124((5)):350–361. doi: 10.1002/cncy.21685. [DOI] [PubMed] [Google Scholar]
- 28.Tang S, Dodd LG. CIC-DUX4 sarcoma diagnosed by fine-needle aspiration cytology: a case report. Diagn Cytopathol. 2018;46((11)):958–963. doi: 10.1002/dc.24027. [DOI] [PubMed] [Google Scholar]
- 29.Kajtár B, Tornóczky T, Kálmán E, Kuzsner J, Hogendoorn PCW, Szuhai K. CD99-positive undifferentiated round cell sarcoma diagnosed on fine needle aspiration cytology, later found to harbour a CIC-DUX4 translocation: a recently described entity. Cytopathology. 2014;25((2)):129–132. doi: 10.1111/cyt.12079. [DOI] [PubMed] [Google Scholar]
- 30.Ito M, Ishikawa M, Kitajima M, Narita J, Hattori S, Endo O, et al. A case report of CIC-rearranged undifferentiated small round cell sarcoma in the cerebrum. Diagn Cytopathol. 2016;44((10)):828–832. doi: 10.1002/dc.23520. [DOI] [PubMed] [Google Scholar]
- 31.Gajdzis P, Pierron G, Klijanienko J. Cytology of undifferentiated round-cell sarcomas of bone and soft tissue: Ewing sarcoma or not ewing sarcoma, that is the question. Acta Cytol. 2021 Aug 25;:1–12. doi: 10.1159/000518146. [DOI] [PubMed] [Google Scholar]
- 32.Specht K, Sung YS, Zhang L, Richter GH, Fletcher CD, Antonescu CR. Distinct transcriptional signature and immunoprofile of CIC-DUX4 fusion-positive round cell tumors compared to EWSR1-rearranged ewing sarcomas: further evidence toward distinct pathologic entities. Genes Chromosomes Cancer. 2014;53((7)):622–633. doi: 10.1002/gcc.22172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Machado I, Yoshida A, Morales MGN, Abrahão-Machado LF, Navarro S, Cruz J, et al. Review with novel markers facilitates precise categorization of 41 cases of diagnostically challenging, “undifferentiated small round cell tumors.” A clinicopathologic, immunophenotypic and molecular analysis. Ann Diagn Pathol. 2018;34:1–12. doi: 10.1016/j.anndiagpath.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 34.Hung YP, Fletcher CD, Hornick JL. Evaluation of ETV4 and WT1 expression in CIC-rearranged sarcomas and histologic mimics. Mod Pathol. 2016;29((11)):1324–1334. doi: 10.1038/modpathol.2016.140. [DOI] [PubMed] [Google Scholar]
- 35.Le Guellec S, Velasco V, Pérot G, Watson S, Tirode F, Coindre JM. ETV4 is a useful marker for the diagnosis of CIC-rearranged undifferentiated round-cell sarcomas: a Study of 127 Cases Including Mimicking Lesions. Mod Pathol. 2016 Dec;29((12)):1523–1531. doi: 10.1038/modpathol.2016.155. [DOI] [PubMed] [Google Scholar]
- 36.Sugita S, Arai Y, Tonooka A, Hama N, Totoki Y, Fujii T, et al. A novel CIC-FOXO4 gene fusion in undifferentiated small round cell sarcoma: a genetically distinct variant of Ewing-like sarcoma. Am J Surg Pathol. 2014 Nov;38((11)):1571–6. doi: 10.1097/PAS.0000000000000286. [DOI] [PubMed] [Google Scholar]
- 37.Sugita S, Arai Y, Aoyama T, Asanuma H, Mukai W, Hama N, et al. NUTM2A-CIC fusion small round cell sarcoma: a genetically distinct variant of CIC-rearranged sarcoma. Hum Pathol. 2017;65:225–230. doi: 10.1016/j.humpath.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Le Loarer F, Pissaloux D, Watson S, Godfraind C, Galmiche-Rolland L, Silva K, et al. Clinicopathologic features of CIC-NUTM1 sarcomas, a new molecular variant of the family of CIC-fused sarcomas. Am J Surg Pathol. 2019;43((2)):268–276. doi: 10.1097/PAS.0000000000001187. [DOI] [PubMed] [Google Scholar]
- 39.Pierron G, Tirode F, Lucchesi C, Reynaud S, Ballet S, Cohen-Gogo S, et al. A new subtype of bone sarcoma defined by BCOR-CCNB3 gene fusion. Nat Genet. 2012;44((4)):461–466. doi: 10.1038/ng.1107. [DOI] [PubMed] [Google Scholar]
- 40.Cohen-Gogo S, Cellier C, Coindre JM, Mosseri V, Pierron G, Guillemet C, et al. Ewing-like sarcomas with BCOR-CCNB3 fusion transcript: a Clinical, Radiological and Pathological Retrospective Study from the Société Française des Cancers de L'Enfant. Pediatr Blood Cancer. 2014;61((12)):2191–2198. doi: 10.1002/pbc.25210. [DOI] [PubMed] [Google Scholar]
- 41.Kao YC, Owosho AA, Sung YS, Zhang L, Fujisawa Y, Lee JC, et al. BCOR: CCNB3 fusion positive sarcomas − a clinicopathologic and molecular analysis of 36 cases with comparison to morphologic spectrum and clinical behavior of other round cell sarcomas. Am J Surg Pathol. 2018;42((5)):604–615. doi: 10.1097/PAS.0000000000000965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Puls F, Niblett A, Marland G, Gaston CLL, Douis H, Mangham DC, et al. BCOR-CCNB3 (Ewing-like) sarcoma: a clinicopathologic analysis of 10 cases, in comparison with conventional Ewing sarcoma. Am J Surg Pathol. 2014;38((10)):1307–1318. doi: 10.1097/PAS.0000000000000223. [DOI] [PubMed] [Google Scholar]
- 43.Kao YC, Sung YS, Zhang L, Huang SC, Argani P, Chung CT, et al. Recurrent BCOR internal tandem duplication and YWHAE - NUTM2B fusions in soft tissue undifferentiated round cell sarcoma of infancy: overlapping genetic features with clear cell sarcoma of kidney. Am J Surg Pathol. 2016;40((8)):1009–1020. doi: 10.1097/PAS.0000000000000629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Antonescu CR, Kao YC, Xu B, Fujisawa Y, Chung C, Fletcher CDM, et al. Undifferentiated round cell sarcoma with BCOR internal tandem duplications (ITD) or YWHAE fusions: a Clinicopathologic and Molecular Study. Mod Pathol. 2020;33((9)):1669–1677. doi: 10.1038/s41379-020-0557-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gajdzis P, Laé M, Pierron G, Brisse HJ, Orbach D, Fréneaux P, et al. Fine-needle aspiration features of BCOR-CCNB3 sarcoma. Am J Clin Pathol. 2020;153((3)):315–321. doi: 10.1093/ajcp/aqz159. [DOI] [PubMed] [Google Scholar]
- 46.Watabe S, Kikuchi Y, Mukaiyama J, Kato T, Sato K, Imanishi J, et al. Cytological features of BCOR-CCNB3 sarcoma: comparison with Ewing sarcoma and synovial sarcoma. Cytopathology. 2021 Nov;32((6)):771–778. doi: 10.1111/cyt.13034. [DOI] [PubMed] [Google Scholar]
- 47.Gerald WL, Miller HK, Battifora H, Miettinen M, Silva EG, Rosai J. Intra-abdominal desmoplastic small round-cell tumor. Report of 19 cases of a distinctive type of high-grade polyphenotypic malignancy affecting young individuals. Am J Surg Pathol. 1991 Jun;15((6)):499–513. [PubMed] [Google Scholar]
- 48.Mello CA, Campos FAB, Santos TG, Silva MLG, Torrezan GT, Costa FD, et al. Desmoplastic small round cell tumor: a review of main molecular abnormalities and emerging therapy. Cancers. 2021 Jan 28;13((3)):498. doi: 10.3390/cancers13030498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klijanienko J, Colin P, Couturier J, Lagacé R, Fréneaux P, Pierron G, et al. Fine-needle aspiration in desmoplastic small round cell tumor: a report of 10 new tumors in 8 patients with clinicopathological and molecular correlations with review of the literature. Cancer Cytopathol. 2014;122:386–393. doi: 10.1002/cncy.21415. [DOI] [PubMed] [Google Scholar]
- 50.Ferlicot S, Coué O, Gilbert E, Beuzeboc P, Servois V, Klijanienko J, et al. Intraabdominal desmoplastic small round cell tumor: report of a case with fine needle aspiration, cytologic diagnosis and molecular confirmation. Acta Cytol. 2001 Jul-Aug;45((4)):617–621. doi: 10.1159/000327875. [DOI] [PubMed] [Google Scholar]
- 51.Leça LB, Vieira J, Teixeira MR, Monteiro P. Desmoplastic small round cell tumor: diagnosis by fine-needle aspiration cytology. Acta Cytol. 2012;56((5)):576–580. doi: 10.1159/000338523. [DOI] [PubMed] [Google Scholar]
- 52.Zeppa P, Lepore M, Vetrani A, Palombini L. Occult lymph node metastasis from desmoplastic small round cell tumor diagnosed by fine needle aspiration cytology. A case report. Acta Cytol. 2003 May-Jun;47((3)):501–505. doi: 10.1159/000326559. [DOI] [PubMed] [Google Scholar]
- 53.Dave B, Shet T, Chinoy R. Desmoplastic round cell tumor of childhood: can cytology with immunocytochemistry serve as an alternative for tissue diagnosis? Diagn Cytopathol. 2005 Jun;32((6)):330–335. doi: 10.1002/dc.20244. [DOI] [PubMed] [Google Scholar]
- 54.Sharma S, Kamala R, Nair D, Ragavendra TR, Mhatre S, Sabharwal R, et al. Round cell tumors: classification and immunohistochemistry. Indian J Med Paediatr Oncol. 2017 Jul-Sep;38((3)):349–353. doi: 10.4103/ijmpo.ijmpo_84_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bergh P, Meis-Kindblom JM, Gherlinzoni F, Berlin O, Bacchini P, Bertoni F, et al. Synovial sarcoma: identification of low and high risk groups. Cancer. 1999 Jun 15;85((12)):2596–2607. doi: 10.1002/(sici)1097-0142(19990615)85:12<2596::aid-cncr16>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 56.Gazendam AM, Popovic S, Munir S, Parasu N, Wilson D, Ghert M. Synovial sarcoma: a clinical review. Curr Oncol. 2021 May 19;28((3)):1909–1920. doi: 10.3390/curroncol28030177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Thway K, Fisher C. Synovial sarcoma: defining features and diagnostic evolution. Ann Diagn Pathol. 2014 Dec;18((6)):369–380. doi: 10.1016/j.anndiagpath.2014.09.002. [DOI] [PubMed] [Google Scholar]
- 58.Silverman JF, Landreneau RJ, Sturgis CD, Raab SS, Fox KR, Jasnosz KM, et al. Small-cell variant of synovial sarcoma: fine-needle aspiration with ancillary features and potential diagnostic pitfalls. Diagn Cytopathol. 2000 Aug;23((2)):118–123. doi: 10.1002/1097-0339(200008)23:2<118::aid-dc11>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 59.Kwon MS. Aspiration cytology of pulmonary small cell variant of poorly differentiated synovial sarcoma metastatic from the tongue: a case report. Acta Cytol. 2005 Jan-Feb;49((1)):92–96. doi: 10.1159/000326103. [DOI] [PubMed] [Google Scholar]
- 60.Hummel P, Yang GC, Kumar A, Cohen JM, Winkler B, Melamed J, et al. PNET-like features of synovial sarcoma of the lung: a pitfall in the cytologic diagnosis of soft-tissue tumors. Diagn Cytopathol. 2001 Apr;24((4)):283–288. doi: 10.1002/dc.1060. [DOI] [PubMed] [Google Scholar]
- 61.Akerman M, Domanski HA. The complex cytological features of synovial sarcoma in fine needle aspirates, an analysis of four illustrative cases. Cytopathology. 2007 Aug;18((4)):234–240. doi: 10.1111/j.1365-2303.2007.00458.x. [DOI] [PubMed] [Google Scholar]
- 62.Pelmus M, Guillou L, Hostein I, Sierankowski G, Lussan C, Coindre JM. Monophasic fibrous and poorly differentiated synovial sarcoma: immunohistochemical reassessment of 60 t(X;18)(SYT-SSX)-positive cases. Am J Surg Pathol. 2002 Nov;26((11)):1434–1440. doi: 10.1097/00000478-200211000-00005. [DOI] [PubMed] [Google Scholar]
- 63.Akerman M, Ryd W, Skytting B, Scandinavian Sarcoma Group Fine-needle aspiration of synovial sarcoma: criteria for diagnosis − retrospective reexamination of 37 cases, including ancillary diagnostics. A Scandinavian Sarcoma Group Study. Diagn Cytopathol. 2003 May;28((5)):232–238. doi: 10.1002/dc.10265. [DOI] [PubMed] [Google Scholar]
- 64.Kilpatrick SE, Teot LA, Stanley MW, Ward WG, Savage PD, Geisinger KR. Fine-needle aspiration biopsy of synovial sarcoma. A cytomorphologic analysis of primary, recurrent, and metastatic tumors. Am J Clin Pathol. 1996 Dec;106((6)):769–775. doi: 10.1093/ajcp/106.6.769. [DOI] [PubMed] [Google Scholar]
- 65.Klijanienko J, Caillaud JM, Lagacé R, Vielh P. Cytohistologic correlations in 56 synovial sarcomas in 36 patients: the institut curie experience. Diagn Cytopathol. 2002 Aug;27((2)):96–102. doi: 10.1002/dc.10151. [DOI] [PubMed] [Google Scholar]
- 66.Kottu R, Prayaga AK. Synovial sarcoma with relevant immunocytochemistry and special emphasis on the monophasic fibrous variant. J Cytol. 2010 Apr;27((2)):47–50. doi: 10.4103/0970-9371.70736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Srinivasan R, Gautam U, Gupta R, Rajwanshi A, Vasistha RK. Synovial sarcoma: diagnosis on fine-needle aspiration by morphology and molecular analysis. Cancer. 2009 Apr 25;117((2)):128–136. doi: 10.1002/cncy.20006. [DOI] [PubMed] [Google Scholar]
- 68.Zhang Y, Wessman S, Wejde J, Tani E, Haglund F. Diagnosing synovial sarcoma by fine-needle aspiration cytology and molecular techniques. Cytopathology. 2019 Sep;30((5)):504–509. doi: 10.1111/cyt.12736. [DOI] [PubMed] [Google Scholar]
- 69.Ladanyi M, Antonescu CR, Leung DH, Woodruff JM, Kawai A, Healey JH, et al. Impact of SYT-SSX fusion type on the clinical behavior of synovial sarcoma: a Multi-Institutional Retrospective Study of 243 patients. Cancer Res. 2002 Jan 1;62((1)):135–140. [PubMed] [Google Scholar]
- 70.Panagopoulos I, Mertens F, Isaksson M, Limon J, Gustafson P, Skytting B, et al. Clinical impact of molecular and cytogenetic findings in synovial sarcoma. Genes Chromosomes Cancer. 2001 Aug;31((4)):362–372. doi: 10.1002/gcc.1155. [DOI] [PubMed] [Google Scholar]
- 71.Klijanienko J, Caillaud JM, Orbach D, Brisse H, Lagacé R, Vielh P, et al. Cyto-histological correlations in primary, recurrent and metastatic rhabdomyosarcoma: the institut curie's experience. Diagn Cytopathol. 2007 Aug;35((8)):482–487. doi: 10.1002/dc.20662. [DOI] [PubMed] [Google Scholar]
- 72.Pohar-Marinsek Z, Anzic J, Jereb B. Topical topic: value of fine needle aspiration biopsy in childhood rhabdomyosarcoma − twenty-six years of experience in Slovenia. Med Pediatr Oncol. 2002 Jun;38((6)):416–420. doi: 10.1002/mpo.10075. [DOI] [PubMed] [Google Scholar]
- 73.Akhtar M, Ali MA, Bakry M, Hug M, Sackey K. Fine-needle aspiration biopsy diagnosis of rhabdomyosarcoma: cytologic, histologic, and ultrastructural correlations. Diagn Cytopathol. 1992;8((5)):465–474. doi: 10.1002/dc.2840080507. [DOI] [PubMed] [Google Scholar]
- 74.Kilpatrick SE, Ward WG, Chauvenet AR, Pettenati MJ. The role of fine-needle aspiration biopsy in the initial diagnosis of pediatric bone and soft tissue tumors: an institutional experience. Mod Pathol. 1998 Oct;11((10)):923–928. [PubMed] [Google Scholar]
- 75.de Almeida M, Stastny JF, Wakely PE, Jr, Frable WJ. Fine-needle aspiration biopsy of childhood rhabdomyosarcoma: reevaluation of the cytologic criteria for diagnosis. Diagn Cytopathol. 1994;11((3)):231–236. doi: 10.1002/dc.2840110308. [DOI] [PubMed] [Google Scholar]
- 76.Willén H, Akerman M, Carlén B. Fine needle aspiration (FNA) in the diagnosis of soft tissue tumours; a review of 22 years experience. Cytopathology. 1995 Aug;6((4)):236–247. doi: 10.1111/j.1365-2303.1995.tb00475.x. [DOI] [PubMed] [Google Scholar]
- 77.Rekhi B, Gupta C, Chinnaswamy G, Qureshi S, Vora T, Khanna N, et al. Clinicopathologic features of 300 rhabdomyosarcomas with emphasis upon differential expression of skeletal muscle specific markers in the various subtypes: a single institutional experience. Ann Diagn Pathol. 2018 Oct;36:50–60. doi: 10.1016/j.anndiagpath.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 78.Morotti RA, Nicol KK, Parham DM, Teot LA, Moore J, Hayes J, et al. Children's oncology group. An immunohistochemical algorithm to facilitate diagnosis and subtyping of rhabdomyosarcoma: the children's oncology group experience. Am J Surg Pathol. 2006 Aug;30((8)):962–968. doi: 10.1097/00000478-200608000-00005. [DOI] [PubMed] [Google Scholar]
- 79.Gautam U, Srinivasan R, Rajwanshi A, Bansal D, Marwaha RK, Vasishtha RK. Reverse transcriptase-polymerase chain reaction as an ancillary molecular technique in the diagnosis of small blue round cell tumors by fine-needle aspiration cytology. Am J Clin Pathol. 2010 Apr;133((4)):633–645. doi: 10.1309/AJCPPJJ0PY4XZOEC. [DOI] [PubMed] [Google Scholar]
- 80.Kilpatrick SE, Bergman S, Pettenati MJ, Gulley ML. The usefulness of cytogenetic analysis in fine needle aspirates for the histologic subtyping of sarcomas. Mod Pathol. 2006 Jun;19((6)):815–819. doi: 10.1038/modpathol.3800598. [DOI] [PubMed] [Google Scholar]
- 81.Barr FG. Gene fusions involving PAX and FOX family members in alveolar rhabdomyosarcoma. Oncogene. 2001 Sep 10;20((40)):5736–5746. doi: 10.1038/sj.onc.1204599. [DOI] [PubMed] [Google Scholar]
- 82.Barr FG, Duan F, Smith LM, Gustafson D, Pitts M, Hammond S, et al. Genomic and clinical analyses of 2p24 and 12q13-q14 amplification in alveolar rhabdomyosarcoma: a report from the children's oncology group. Genes Chromosomes Cancer. 2009 Aug;48((8)):661–672. doi: 10.1002/gcc.20673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Vencio EF, Reeve CM, Unni KK, Nascimento AG. Mesenchymal chondrosarcoma of the jaw bones: Clinicopathologic Study of 19 Cases. Cancer. 1998 Jun 15;82((12)):2350–2355. doi: 10.1002/(sici)1097-0142(19980615)82:12<2350::aid-cncr8>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 84.Xu J, Li D, Xie L, Tang S, Guo W. Mesenchymal chondrosarcoma of bone and soft tissue: a systematic review of 107 patients in the past 20 years. PLoS One. 2015 Apr 7;10((4)):e0122216. doi: 10.1371/journal.pone.0122216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trembath DG, Dash R, Major NM, Dodd LG. Cytopathology of mesenchymal chondrosarcomas: a report and comparison of four patients. Cancer. 2003 Aug 25;99((4)):211–216. doi: 10.1002/cncr.11300. [DOI] [PubMed] [Google Scholar]
- 86.González-Cámpora R, Otal Salaverri C, Gomez Pascual A, Hevia Vazquez A, Galera Davidson H. Mesenchymal chondrosarcoma of the retroperitoneum. Report of a case diagnosed by fine needle aspiration biopsy with immunohistochemical, electron microscopic demonstration of S-100 protein in undifferentiated cells. Acta Cytol. 1995 Nov-Dec;39((6)):1237–1243. [PubMed] [Google Scholar]
- 87.Jaetli V, Gupta S. Mesenchymal chondrosarcoma of maxilla: a rare case report. Med Oral Patol Oral Cir Bucal. 2011 Jul 1;16((4)):e493–6. doi: 10.4317/medoral.16.e493. [DOI] [PubMed] [Google Scholar]
- 88.Misra V, Singh PA. Cytodiagnosis of extraosseous mesenchymal chondrosarcoma of meninges: a case report. Acta Cytol. 2008 May-Jun;52((3)):366–368. doi: 10.1159/000325525. [DOI] [PubMed] [Google Scholar]
- 89.Doria MI, Jr, Wang HH, Chinoy MJ. Retroperitoneal mesenchymal chondrosarcoma. Report of a case diagnosed by fine needle aspiration cytology. Acta Cytol. 1990 Jul-Aug;34((4)):529–532. [PubMed] [Google Scholar]
- 90.Domanski HA, Walther CS. Ewing sarcoma. Monogr Clin Cytol. 2017;22:157–158. doi: 10.1159/000475108. [DOI] [PubMed] [Google Scholar]
- 91.Wang L, Motoi T, Khanin R, Olshen A, Mertens F, Bridge J, et al. Identification of a novel, recurrent HEY1-NCOA2 fusion in mesenchymal chondrosarcoma based on a genome-wide screen of exon-level expression data. Genes Chromosomes Cancer. 2012 Feb;51((2)):127–139. doi: 10.1002/gcc.20937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Amary MF, Bacsi K, Maggiani F, Damato S, Halai D, Berisha F, et al. IDH1 and IDH2 mutations are frequent events in central chondrosarcoma and central and periosteal chondromas but not in other mesenchymal tumours. J Pathol. 2011 Jul;224((3)):334–343. doi: 10.1002/path.2913. [DOI] [PubMed] [Google Scholar]
- 93.Nakajima H, Sim FH, Bond JR, Unni KK. Small cell osteosarcoma of bone. Review of 72 cases. Cancer. 1997 Jun 1;79((11)):2095–2106. doi: 10.1002/(sici)1097-0142(19970601)79:11<2095::aid-cncr6>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 94.Edeiken J, Raymond AK, Ayala AG, Benjamin RS, Murray JA, Carrasco HC. Small-cell osteosarcoma. Skeletal Radiol. 1987;16((8)):621–628. doi: 10.1007/BF00357110. [DOI] [PubMed] [Google Scholar]
- 95.Bishop JA, Shum CH, Sheth S, Wakely PE, Jr, Ali SZ. Small cell osteosarcoma: cytopathologic characteristics and differential diagnosis. Am J Clin Pathol. 2010 May;133((5)):756–761. doi: 10.1309/AJCPO07VGDZCBRJF. [DOI] [PubMed] [Google Scholar]
- 96.Uma K, Cherian G, Nayak V, Patil S. Small cell osteosarcoma of the mandible: case report and review of its diagnostic aspects. J Oral Maxillofac Pathol. 2011 Sep;15((3)):330–334. doi: 10.4103/0973-029X.86713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ali RH, Lee CH, Hayes MM. Metastatic small cell osteosarcoma to the liver: a diagnostic pitfall for fine-needle aspiration cytology. Diagn Cytopathol. 2014 Feb;42((2)):161–164. doi: 10.1002/dc.22894. [DOI] [PubMed] [Google Scholar]
- 98.Syed M, Mushtaq S, Loya A, Hassan U. NKX3.1 a useful marker for mesenchymal chondrosarcoma: an Immunohistochemical Study. Ann Diagn Pathol. 2021 Feb;50:151660. doi: 10.1016/j.anndiagpath.2020.151660. [DOI] [PubMed] [Google Scholar]
- 99.Machado I, Navarro S, Picci P, Llombart-Bosch A. The utility of SATB2 immunohistochemical expression in distinguishing between osteosarcomas and their malignant bone tumor mimickers, such as Ewing sarcomas and chondrosarcomas. Pathol Res Pract. 2016 Sep;212((9)):811–816. doi: 10.1016/j.prp.2016.06.012. [DOI] [PubMed] [Google Scholar]