Abstract
Background:
Biomarkers are key tools in cancer management. In neuroendocrine tumors (NETs), Chromogranin A (CgA) was considered acceptable as a biomarker. We assessed the clinical efficacy of a multigenomic blood biomarker (NETest) to CgA over a 5-year period.
Methods:
An observational, prospective, cross-sectional, multicenter, multinational, comparative cohort assessment. Cohort #1) NETest evaluation in NETs (1,684) and cancers, benign diseases, controls (731). Cohort #2: (n=1,270): matched analysis of NETest/ CgA in a sub-cohort of NETs (n=922) vs. other diseases and controls (n=348). Disease status assessed by RECIST. NETest measurement: qPCR (ULN: 20), CgA (EuroDiagnostica, ULN: 108ng/mL). Statistics: Mann-Whitney U-test, AUROC, Chi2 and McNemar’s test.
Results:
Cohort #1: NETest diagnostic accuracy was 91% (p<0.0001) and identified pheochromocytomas (98%), small intestine (94%), pancreas (91%), lung (88%), gastric (80%) and appendix (79%). NETest reflected grading: G1: 40±1, G2 (50±1) and G3 (52±1). Loco-regional disease levels were lower (38±1) than metastatic (52±1, p<0.0001). NETest accurately stratified RECIST-assessed disease extent: no disease (21±1), stable (43±2), progressive (62±2) (p<0.0001). NETest concordance with imaging (CT/MRI/68Ga-SSA-PET) 91%. Pre-surgery, all NETs (n=153) were positive (100%). After palliative R1/R2 surgery (n=51) all (100%) remained elevated. After curative R0-surgery (n=102), NETest levels were normal in 81(70%) with no recurrence at 2-years. In the 31 (30%) with elevated levels 25 (81%) recurred within 2 years.
Cohort #2: NETest diagnostic accuracy was 87% and CgA 54% (p<0.0001). NETest was more accurate than CgA for grading (Chi2=7.7, OR=18.5) and metastatic identification (Chi2=180, OR=8.4). NETest identified progressive disease (95%) vs CgA (57%, p<0.0001). Imaging concordance for NETest was 91% vs. CgA (46%) (p<0.0001). Recurrence prediction after surgery was NETest-positive in >94% vs. CgA 11%.
Conclusion:
NETest accurately diagnoses NETs and is an effective surrogate marker for imaging, grade, metastases and disease status compared to CgA. A multigenomic liquid biopsy is an accurate biomarker of NET disease.
Keywords: NETest, biomarker, neuroendocrine tumor, NEN, Chromogranin
INTRODUCTION:
Effective blood biomarkers can provide an easily available, non-invasive, irradiation-free, real-time appreciation of the disease status of a patient [1]. In the past, monoanalytes that assessed single aspects of a disease (e.g., myeloma protein) or secretory products (e.g., insulin) provided useful information. Such tools, however, measure a single aspect of tumor biology. The evolution of both molecular biology and scientific tools to identify and quantify multiple regulatory pathways have facilitated development of multianalyte biomarkers [2]. This has been advanced by mathematical deep-learning strategies. Such strategies in blood have led to the recent consideration of liquid biopsies as real-time, diagnostic and clinical management tools [3].
Neuroendocrine tumor (NET) biomarkers have focused on measurements of secretory products e.g., gastrin, serotonin or chromogranin A (CgA) [4]. Guidelines consider such biomarkers as either not useful or as controversial (Level III evidence) [5]. A recent overview by Caplin et al., emphasized the urgent requirement for novel NET biomarkers given that CgA has a “less-than-ideal diagnostic sensitivity and specificity” [6].
In NETs, a multigenomic blood assay (NETest) is considered as a more accurate biomarker than CgA [7-10]. The assay has been described in detail and independently validated [8, 9, 11]. It comprises a 51-gene expression-based liquid biopsy for NETs that utilizes PCR-technology and multianalyte algorithmic analyses. Output is scored 0-100 with >20 abnormal. Stable disease is 21-40 and progressive 41-100 [10]. A meta-analysis by Oberg et al., concluded the NETest had a ~95% diagnostic accuracy and was an effective (>80%) monitor of treatment efficacy [10]. Their study noted that a multigenomic biomarker assay could provide a significant fiscal advantage in standard medical practice.
The inability to accurately monitor NETs using an effective pan-NET biomarker is associated with substantial financial costs [12]. Here, we report the results of NETest in 1,684 consecutive, prospectively collected NETs assessed over 5-years for diagnostic and surgical utility. Additionally, we examined the NETest directly compared to CgA in a sub-cohort of 922 NETs in whom both biomarker measurements were available.
METHODS:
Study Design:
This is a prospective, multicenter, multinational, cross-sectional study, which comprises 1,684 gastroenteropancreatic, lung and other NETs as well as 256 controls and 475 non-neuroendocrine tumors. We enrolled and evaluated all patients (irrespective of disease extent or pathological grade) over a five-year period (10/1/2015-9/30/2020, study design: Supplemental Table 1). No exclusion criteria were used. Samples were collected for NETest and where requested, for CgA measurements. When multiple samples were collected, only the initial sample was evaluated in this study.
Patient demographics are included in Table 1. Primary outcome was based on a NET diagnosis. Variables included clinical data (diagnosis, grade, staging, imaging, status, surgery effectiveness) and biomarkers (NETest, CgA). Data were collected (Western Institutional Review Board #20150174) and evaluated per STROBE (Strengthening the Reporting of Observational Studies in Epidemiology) guidelines [13]. Evaluations included utility as a diagnostic using area under the receiver curve (AUROC) analyses, correlation with imaging and relationship to tumor organ site, histological grade, clinical status and metastases. In all individuals, a single blood sample per patient was evaluated. For surgical evaluation, two samples were collected, a pre- and post-operative blood samples, and NETest and CgA levels were assessed. This enabled demonstration of the effectiveness of surgical intervention. For matched biomarker assessment, we calculated the relative risk (RR) using the McNemar’s test and odd’s ratio (OR) or undertook Chi2 analyses.
Table 1.
NET patient demographics
| Site | No. | Age Median range |
Gender (M/F) |
Grade | Mets | IPD | Clinical Status | CgA* | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| G1 | G2 | G3 | TC | AC | ND | No. | % | No. | % | NED/CR | SD | PD | ND | No. | % | ||||
| Appx. | 56 | 42 (18-56) | 14:42 | 28 | 11 | 4** | 13 | 11 | 20% | 23 | 41% | 19 | 31 | 6 | 0 | 26 | 46% | ||
| Colon | 23 | 59.5 (38-83) | 7:16 | 8 | 7 | 1 | 7 | 18 | 78% | 19 | 82% | 2 | 11 | 9 | 1 | 10 | 43% | ||
| CUP | 81 | 62 (25-82) | 38:43 | 11 | 27 | 9 | 34 | 60 | 74% | 75 | 93% | 4 | 32 | 31 | 14 | 41 | 51% | ||
| Duod. | 36 | 57 (30-87) | 20:16 | 17 | 5 | 1 | 13 | 23 | 64% | 30 | 83% | 4 | 21 | 7 | 4 | 25 | 69% | ||
| Stomach | 76 | 60 (11-88) | 24:52 | 45 | 13 | 7 | 11 | 16 | 21% | 25 | 33% | 50 | 15 | 10 | 1 | 56 | 74% | ||
| Lung | 301 | 62 (18-87) | 107:194 | 3 | 142 | 131 | 25 | 129 | 43% | 256 | 85% | 37 | 125 | 123 | 6 | 137 | 44% | ||
| Pancreas | 359 | 58 (15-87) | 179:180 | 105 | 152 | 30 | 72 | 233 | 65% | 309 | 86% | 29 | 133 | 152 | 26 | 253 | 70% | ||
| PPGL | 121 | 36 (10-64) | 58:63 | 10 | 2 | 119 | 35 | 29% | 117 | 97% | 4 | 81 | 36 | 0 | 0 | 0% | |||
| Rectum | 88 | 56 (21-82) | 47:38 | 52 | 18 | 3 | 15 | 29 | 33% | 39 | 44% | 42 | 19 | 20 | 7 | 65 | 74% | ||
| SI | 521 | 62 (22-87) | 257:264 | 272 | 139 | 7 | 103 | 443 | 85% | 464 | 89% | 36 | 254 | 172 | 11 | 296 | 57% | ||
| Others # | 22 | 66.5 (38-77) | 7:15 | 2 | 7 | 3 | 9 | 19 | 86% | 21 | 95% | 1 | 9 | 11 | 1 | 13 | 59% | ||
AC = atypical carcinoid. App. = appendix. CR = complete remission. CUP = carcinoid of unknown primary. Duod. = duodenum. IPD = image-positive disease. ND = no data. NED = no evidence of disease. PD = progressive disease. PPGL = pheochromocytomas and paragangliomas. SD = Stable disease. SI = small intestine. TC = typical carcinoid.
These are included in Cohort 2.
4: 3 were goblet cell carcinoids
including ovarian, pituitary, testicular and thymic NETs.
Clinical data:
Disease status was assessed by clinical review, imaging (CT/MRI or 68Ga-SSA-studies), grade, and metastases. Status (i.e., stable or progressive disease) was determined using RECIST 1.0 (CT/MRI) or 1.1 (NETspot) [14]. Surgical efficacy was evaluated in a subset (n=153) followed for 24 months. Surgery included R0 (n=102), R1 (n=29) and R2 (n=22) resections (Table 2).
Table 2.
Surgical cohort: Clinical and Biomarker Details
| Specifics | R0 | R1 | R2 |
|---|---|---|---|
| No. | 102 | 29 | 22 |
| Age: median (range) | 59 (27-84) | 50 (26-85) | 60 (19-74) |
| Gender: M/F | 49/53 | 15/14 | 12/10 |
| Sites: | |||
| Lung | 26 | 1 | 0 |
| Pancreas | 45 | 7 | 5 |
| Small Bowel | 29 | 16 | 17 |
| Appendix | 0 | 1 | 1 |
| Stomach | 2 | 1 | 0 |
| Duodenum | 0 | 2 | 2 |
| Grade: | |||
| G1 | 40 | 19 | 17 |
| G2 | 32 | 10 | 5 |
| G3 | 3* | 0 | 0 |
| AC | 9 | 1 | 0 |
| TC | 17 | 0 | 0 |
| Staging | |||
| T | |||
| 1 | 38 | 0 | 0 |
| 2 | 34 | 16 | 5 |
| 3 | 20 | 13 | 11 |
| 4 | 10 | 0 | 6 |
| N | |||
| 0 | 55 | 7 | 0 |
| 1 | 44 | 22 | 22 |
| x | 3** | 0 | 0 |
| M | |||
| 0 | 98 | 18 | 10 |
| 1 | 4 | 11 | 12 |
| NETest (D0) Median (range)* | 67 (27-100) | 87 (40-100) | 87 (33-100) |
| NETest. No. positive (%) * | 102 (100%) | 29 (100%) | 22 (100%) |
| CgA (D0) Median (range)** | 55 (16-1158) | 67 (35-156) | 75 (40-158) |
| CgA: No. positive (%) ** | 18 (22%) | 2 (18%) | 3 (33%) |
Measured in Cohort 1
Measured in Cohort 2
Blood collection/send-out:
Whole blood samples (3 ml) were collected for NETest [15] and plasma for CgA. Samples were shipped to a clinically-certified US laboratory (Wren Laboratories CL-0704, CLIA 07D2081388, NYSDOH PFI: 9138) for measurements. All blood tubes were de-identified, and measurements were blinded.
NETest measurement:
Transcripts (mRNA) were isolated and real-time PCR performed [15-17]. Targets were normalized and quantified (population control) [15-17]. Final results are expressed as an activity index from 0-100 [15-17]. NETest-positive:>20, progressive disease 41-100 [10], abnormally elevated≥80 (predictor of progression) [8].
CgA measurement:
CgA measurement was undertaken using the NEOLISA™ kit (EuroDiagnostica), ULN:108ug/L.
Statistical analysis:
Analyses were performed using Prism 9.0 (GraphPad 9.0.0-Windows, La Jolla, CA, www.graphpad.com) and MedCalc (bvba v19.6, Ostend, Belgium; www.medcalc.org; 2013). Descriptive statistics and intergroup analyses included 2-tailed non-parametric tests (Mann-Whitney U-test), 2-tailed Chi-square (Yates correction), McNemar’s test (matched NETest and CgA samples) and AUROC-analyses. All data are presented as mean±SEM. P<0.05 was considered significant.
Power analysis (NETs and controls, power=0.95, α=0.05) adequate to differentiate NETest scores (dichotomous output, assuming 75% incidence in NETs and 25% incidence in controls) was calculated. Requirements were a minimum of 23 per group (total=46). Overall, 2,415 patients (~50x) were evaluated including 1,684 NETs.
RESULTS:
1. Demographics
1A. NETs:
The study included 1,684 NETs (Table 1). All organ sites including neural tumors were evaluated. Median ages ranged from 36 (pheochromocytomas, paragangliomas [PPGL]) to 62 (lung, small intestine). Genders ranged from almost parity (pancreas 179:180) to female-predominant (appendix: 75% women). Histology was evaluable in 75% (n=1,263). The incidence of metastases was stomach (21%), lung (43%), pancreas (65%) and small intestine (85%). Appendiceal, gastric and rectal NETs were typically collected post-endoscopy or surgery. Individual demographics (staging, size) for these tumors are included in the Supplementary document (Supplementary Table 2).
Image-positive disease (IPD) was identified in appendix (41%), rectal (44%), stomach (33%), lung (85%), pancreas (86%), small intestine (89%) and PPGLs (97%). Clinical status was evaluable in 91% (n=1,536). Matched CgAs were available in 922 NETs (Cohort 2) [controls (n=105), benign disease, CgA (n=131) and non-NET neoplasia (n=112)].
1B: Controls and Non-NETs (n=831):
This comprised controls, benign disease and non-NET malignancies.
Controls (n=256):
Asymptomatic, in good health, none identified malignancy or were being treated for disease. Median age was 37 (range: 20-78) with a gender distribution of 144:111. The ages of controls were significantly (p<0.0001) younger than NETs, individuals with benign diseases and non-NET neoplasia.
Benign Diseases (n=138):
Including gastro-esophageal reflux disease, chronic obstructive pulmonary disease, idiopathic pulmonary fibrosis, chronic pancreatitis, benign pancreatic cysts and hypertension. The median age was 65 (22-87) and predominantly male (92:46).
Non-NET neoplasia (n=337):
Including colon, esophageal, gallbladder, lung (adenocarcinoma, squamous cell cancer), pancreatic, rectal, and small intestinal adenocarcinomas. Median age was 67 (20-88) with a gender distribution of 188:121.
2. NET diagnosis and correlation with imaging
2A. NET Diagnosis:
In Cohort 1 (n=2,415), the NETest diagnosed NETs (accuracy: 91%, AUC: 0.97, p<0.0001) compared to controls and non-NET diseases including neoplastic and benign disease. The NETest was detected in all NET types: 98% pheochromocytomas and paragangliomas, 96% lung, 94% small intestine, 91% pancreas, 80% gastric, 79% appendiceal and 45% rectal. This ranged from 4% in controls to 22% in benign diseases to 36% in other neoplasia (p<0.0001).
In the different tumor types, levels were: pheochromocytomas (80±4), paragangliomas (65±3), small intestinal (50±1), pancreatic (49±1), bronchopulmonary NETs (45±2), appendiceal (34±3), gastric (30±2) and rectal (30±3) (Figure 1A). These were significantly elevated (p<0.001) compared to controls (8±1), benign disease (21±2) and other neoplasia (20±1).
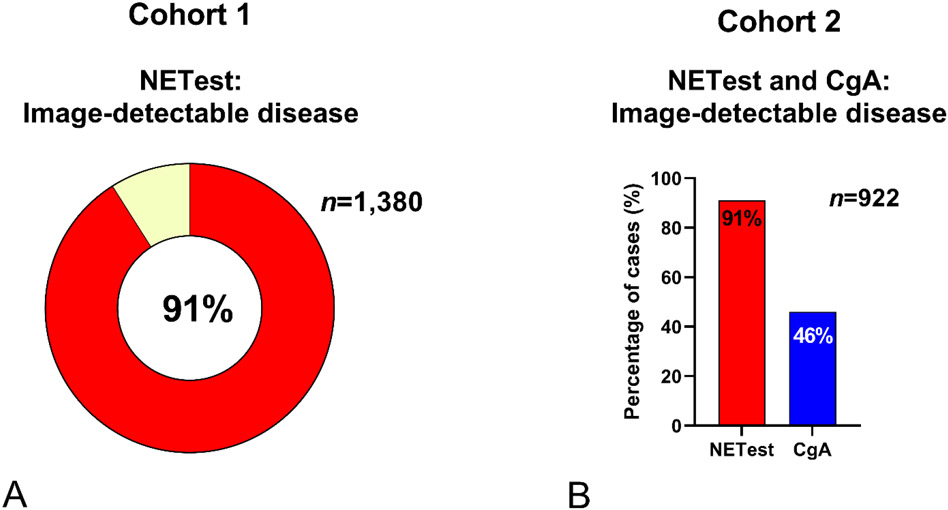
Figure 1. Biomarker diagnostic utility.
1A. Cohort 1 (n=2,415): NETest scores (y-axis) and the percentage positive cases (x-axis) irrespective of disease status (includes CR/NED) for the principal organ sites. Upper limit of normal=20 is expressed by the red dotted line. The majority of NETs (>80%) exhibit an elevated NETest with mean scores >20.
1B. Cohort 2 (n=1,270): Relationship between the percentage positive cases and the calculated relative risk (versus the controls, benign and malignant diseases: n=348) for the principal sites. NETest (red circles) and CgA (blue circles). Pancreatic (P), small bowel (SB), CUP and lung (BP – bronchopulmonary) exhibited relative risks of >3 for the NETest. CgA exhibited lower performance metrics especially for rectal (R), lung (BP) and pancreatic (P) NETs (all with <40% positive samples, RR<1.5). Dotted lines reflect a 50% cut-off for test positive (horizontal line) and a RR of 1.0 (vertical line).
BD = Benign disease. BP = bronchopulmonary (lung). CON = control. CR = complete remission. CUP = carcinoid of unknown primary. G = gastric. IVD = in vitro diagnostic. NED = no evidence of disease. NEO = other neoplasia. P = pancreatic. R = rectal. SB = small bowel.
In a sub-analysis of the significance values of the appendiceal (p<0.05), gastric (p<0.001) and rectal (p<0.0001) NETs, NETest levels were identified to significantly (r=0.98) correlate with tumor stage and size (Supplementary Figure 1 and Supplementary Table 3).
In Cohort 2 (n=1,260), the NETest (87%) was a significantly more accurate diagnostic than CgA (54%, p<0.0001, McNemar: 254, OR=8.6; 95%CI: 5.2-9.3). An evaluation of individual tumor organ sites identified a better relative risk (RR) for diagnosis (compared to non-NETs: n=388) than CgA (Figure 1B). Analysis and comparison of the different organ sites identified that CUPs, small intestinal, pancreatic and lung NETs (>80% NETest-positive) exhibited RR>3.0. In comparison, CgA was positive in 30-70% and the RR ranged 1.5-2. Gastric NETs exhibited similar metrics, irrespective of biomarker tested.
2B. Imaging:
In Cohort 1 (n=1,684 NETs), 1,380 (82%) exhibited image-detectable disease using CT/MRI/68Ga-SSA-PET. NETest levels in image-detectable disease were 51±1. In those with no detectable disease, levels were 21±1 (p<0.0001). O imaging concordance was 91% (Figure 2A).
Figure 2. NETest versus CgA: Imaging utility.
2A. Cohort 1 (n=1,684). NETest was elevated in image-positive disease (n=1,380) in 91%.
2B. Cohort 2 (n=922). NETest-positive 91% positive compared to CgA elevated in 46%. NETest is significantly more accurate than CgA (p<0.0001).
In Cohort 2 (n=922 NETs), image-detectable disease was NETest-positive in 91% compared to 46% CgA-positives (Chi2=232, OR=9.1) (Figure 2B). In the surgical sub-group analysis (R1 image-negative cohort, n=81), the NETest was positive in 64/81 (79%) compared to CgA in 25% (p<0.0001).
3. Correlation with clinical parameters: disease status, histological grade and metastasis
Cohort 1 (n=1,684):
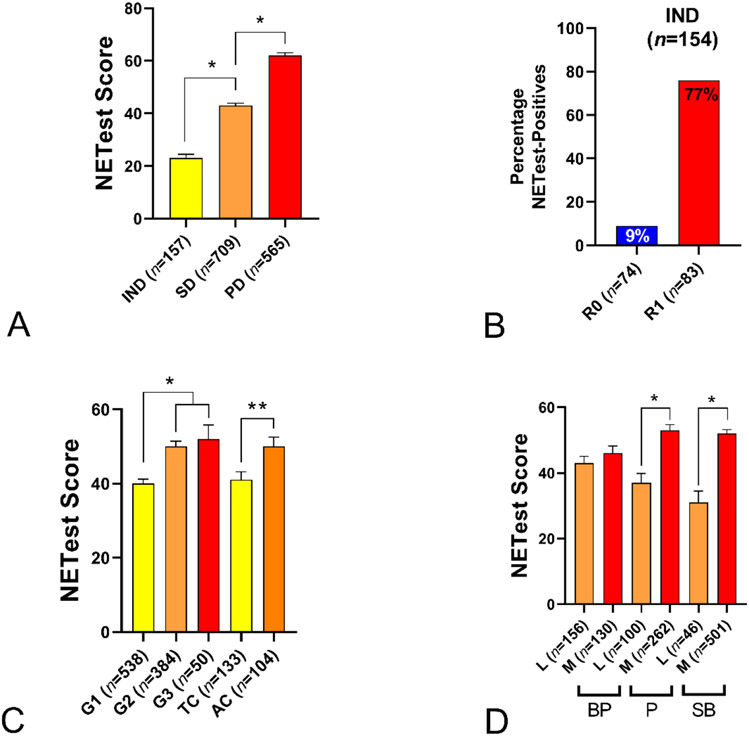
The NETest stratified image-negative (21±1) from stable (43±1) from progressive disease (62±1) (p<0.0001) (Figure 3A). In the image-negative cohort (n=157), 83 Supplementary Table 4. The NETest was positive in 64 (77%). In the “true” negatives (image-negative and no evidence of disease per histopathology: n=74), the NETest was positive in 7 (9%, score: 56±8) (Figure 3B). A sub-analysis of the data identified the NETest accurately correlated (83%) with microscopic disease irrespective of tumor site (Supplementary Table 4).
Figure 3. Correlations with clinical parameters.
3A. Clinical Disease status. NETest scores were significantly elevated in those with SD vs. IND and in PD vs. SD. (1-way ANOVA: F = 191.3, *p<0.01).
3B Image Negative Disease. In the IND cohort with microscopic disease (R1) the percentage positive NETest was 76% versus 9% in those with R0 resection (Chi2=64, p<0.0001).
3C. Grading. NETest scores were significantly elevated in G2 and G3 tumors compared to G1. In BPNETs the AC group NETest levels were similar to G2 and G3 and were elevated compared to TC (not different to G1) *p<0.01, **p<0.05.
3D. Disease Extent. NETest scores were significantly elevated in metastatic vs. localized disease. (*p<0.0001).
AC = atypical carcinoid. BP = bronchopulmonary. IND = image-negative disease. P = Pancreas. PD = progressive disease. SB = small bowel. SD = stable disease. TC = typical carcinoid. Mean±SEM.
NETest levels reflected grading: G1 40±1, G2 50±1, and G3 52±4 (Kruskal-Wallis statistic 49.4, p<0.0001) (Figure 3C). A separate evaluation of the NETest and Ki67 index identified a significant correlation (r=0.16, p<0.0001, Supplementary Figure 2).
NETest scores were significantly higher in metastatic (52±1, p<0.0001) than localized disease (38±1). Mean levels in individual tumor types were: gastric (40±7vs. 26±3, p=0.07), lung (46±2 vs. 43±2, p=0.06), pancreas (53±2 vs. 37±3, p<0.0001), small bowel (52±1 vs. 31±4, p<0.0001) and PPGL (72±4 vs. 61±4, p=0.02) (Figure 3D). A sub-analysis of appendiceal, gastric and rectal NETs, confirmed Stage IV disease to exhibit significantly (p<0.05) elevated levels versus Stage I disease (Supplementary Figure 1 and Table 2).
Cohort 2 (n=922):
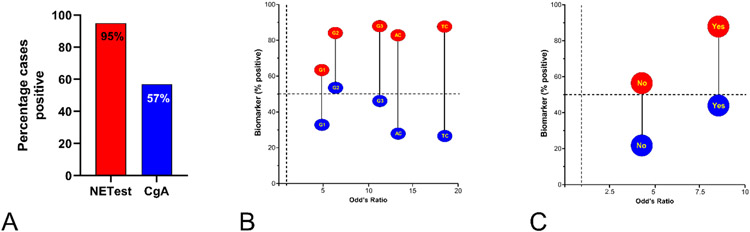
The percentage with a positive NETest and progressive disease was 95% compared to CgA (57%, p<0.0001) (Figure 4A). For stable disease, the values were 78% and 35%, respectively (p<0.0001). For grading, the NETest was more accurate than CgA with individual grades exhibiting McNemar’s Chi2 OR ranging from 5.03 (G1) to 18.5 (TC) (Figure 4B). For metastatic detection, the NETest was more accurate with an OR=8.4. Metastatic disease was 87% NETest-positive compared to CgA (48%-positive) (Figure 4C).
Figure 4. Cohort #2 NETest/CgA Correlation with clinical parameters (n=922).
4A. Progressive disease. NETest 95% positive was compared CgA 57% (p<0.0001).
4B. Grade analysis by McNemar test of the relationship between the percentage positivity and Odd’s ratio for grades and NETest/CgA. The OR ranged was from 5.03 for G1 tumors (NETest positive: 63% vs. CgA-positive 33%) to 18.5 (TC: NETest positive 87% vs. 27% for CgA). Overall, the NETest (red circles) is 5-20x more accurate than CgA (blue circles) for grade.
4C. Metastasis assessment using the McNemar’s test. For no metastases, the OR was 3.9. NETest was elevated in 60% vs. 20% for CgA. For metastases, the OR was 8.4 (87% elevated NETest, 47% elevated CgA). Overall, the NETest (red circles) is 8x more accurate than CgA (blue circles) for identifying metastasis.
AC = atypical carcinoid. BP = bronchopulmonary. CR = complete remission. NED = no evidence of disease. P = Pancreas. PD = progressive disease. SB = small bowel. SD = stable disease. TC = typical carcinoid. Mean±SEM.
Dotted lines reflect 50% who are biomarker positive (horizontal line, 4B-C) and an OR of 1.0 (vertical line: 4B-C).
4. NETest and Surgery
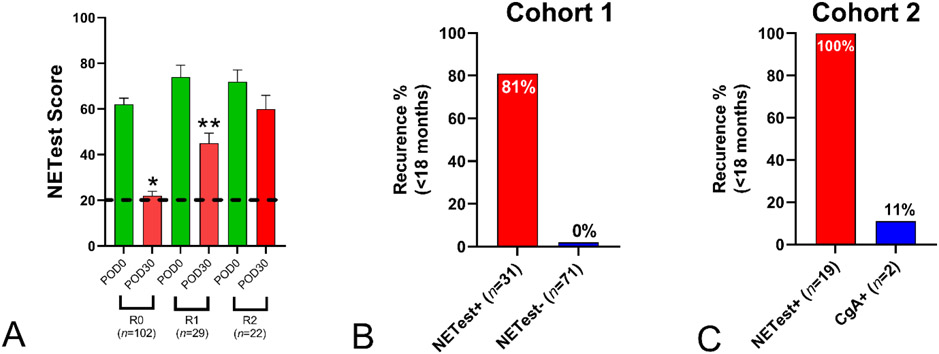
Pre-surgical NETest levels were elevated (68±3) in all 153 (100%). After R2 surgery, there was no significant decrease (60±6, p=NS) while R1 (45±4, p=0.001) and R0 (22±2, p<0.0001) were associated with significant decreases (Figure 5A). All R2 and R1 surgeries were NETest-positive at POD30 compared to 31 (31%) of the R0 group. In the R0 group (n=71) with a normal NETest none (0%) recurred while 25 (81%) with an elevated level recurred within 24 months (Chi2=17.1, p<0.0001, Figure 5B).
Figure 5. NETest and Prediction of Surgical Recurrence.
5A. Surgical strategies. R0 and R1 surgery significantly decreased NETest levels (respectively *p<0.0001, **p<0.002). R0 NETest levels were significantly decreased (p<0.0001) compared to R1. R2 resection failed to decrease NETest levels
5B. Cohort 1, R0 percentage recurrence rate within 24 months. Of the 71 with a normal NETest, recurrence was 0% (0/71). Of the 31 with elevated NETest levels 25 (81%) recurred.
5C. Cohort 2, R0 percentage recurrence rate within 24 months. All nineteen (100%) with an elevated NETest recurred compared to 11% (2/19) with an elevated CgA.
Mean±SEM. Black dotted line = upper limit of normal. POD0 = pre-operative NETest score; POD30 = post-operative day 30 NETest score.
In the surgical subgroup of Cohort 2 (n=122), all had an elevated NETest preoperatively compared to 28% (n=34) for CgA (p<0.0001). In R0 (n=69), 19 (28%), had elevated post-operative NETest compared to 5 (7%) for CgA. All 19 (100%) with elevated NETests recurred compared to 11% with elevated CgA (p<0.0001, Chi2=15.5).
DISCUSSION:
This study demonstrates that a blood-based, multigenomic signature is an effective diagnostic for NETs and has clinical utility in management since it is concordant with imaging, accurately stratifies disease status (stable vs. progressive) and predicts tumor recurrence after surgery. It significantly outperformed CgA in all clinical parameters.
Study strengths include a real-time, multicenter, multinational experience of the NETest in clinical date-to-day usage. The centers involved were ENETs Centers of Excellence and assessments of disease consistent with the published standards of the group. All samples were deidentified and analyzed blind by a central USA CLIA-approved laboratory. This study is highly-powered (50x higher enrollment than required) to reject the null hypothesis and demonstrate the NETest is more effective biomarker than CgA.
Study limitations include heterogeneity of imaging studies, no centralized pathological review (that in ~25%, histopathology was not available) and dependence on individual centers to provide clinical information. It should be noted that a study design limitation was that we did not evaluate the NETest as either a continuous monitor or as a prognostic for overall survival. Separate studies addressing these questions are ongoing. We anticipate substantial follow-up will be required to adequately evaluate survival and assess the impact of monitoring and prognostic analysis.
Circulating biomarkers are important in oncology management. In NETs, monoanalytes e.g., secretory amines and peptides like CgA were of value in the past. Measurement, however, reflected functionality and ~50% of tumors are “non-functional” without evaluable markers [4]. A further limitation is little information about biological or clinical behavior is provided [2]. Recent European and USA consensus statements conclude that CgA is controversial and has little benefit [5, 18].
The NETest is a next-generation, NET-multigenomic blood biomarker developed by transcriptomic analysis using deep learning strategies to specifically identify tumors with a neuroendocrine genotype [7]. This was developed to capture the NET molecular signature [2] and provide real-time information regarding the biological status of a tumor. The amalgam of 51 NETest genes have been confirmed as bona fide neuroendocrine markers in a large The Cancer Genome Atlas (TCGA) dataset of >10,000 samples [11].
In this study, the NETest diagnosed NET disease with an accuracy >91% compared to CgA (<50%). NETest blood levels correlated significantly with other clinical parameters including imaging, tumor grade, the Ki-67 index, tumor size, AJCC staging, the detection of metastatic disease, and disease status. The area where NETest and CgA were comparable was gastric NETs (RR for NETest 2.0 vs. 2.5 for CgA). We interpret this to reflect that gastric NETs are a pan-mucosal disease and occur against a background of antral G cell hyperplasia. Elevated CgA levels have two sources in gastric NETs; diffuse mucosal ECL cell hyperplasia based on gastrin-drive and antral G-cell proliferation and secretion. Of note, Stage I tumors (T1N0M0) were NETest positive in 26/46 (57%) of cases. We consider this consistent with detection by the NETest of mucosal ECL cell hyperplasia.
Further evidence of NETest utility was provided by surgical data. This cohort provides decisive surgical and pathological evidence of tumor presence or removal and definitively identifies whether a blood biomarker represents tumor presence or absence. In preoperative assessments, the NETest was 100% elevated compared to CgA (28%). Tumor removal as judged by pathological criteria (R0/R1/R2) resulted in normalization of NETest levels in the R0 group as opposed to continued elevated levels after R1/R2 surgery (known residual disease). In 30% of the R0 group, where the NETest failed to normalize, 74% had post-operative radiological-recurrence within 12-months and this was 84% by 24-months. At 24-months, 0% of those with normal NET levels had recurred. Post-operatively, CgA had no relationship to tumor resection or recurrence. Recent ESMO guidelines (2020) noted the NETest might have clinical utility as a marker defining complete surgical removal [18]. Our current study confirms this and demonstrates that a post-operative NETest can be used to accurately (94%) predict disease recurrence. Although guidelines query CgA usage, it is reimbursed and is currently used on an ad hoc basis for surgery follow-up [19].
Small, microscopic or minimal residual disease (MRD, <2-3mm) cannot be detected by current imaging modalities including 68Ga-PET-SSA [20]. Previous reports have demonstrated that the NETest can identify image-negative disease [21]. We note that ~80% of image-negative subjects with R1 disease have a positive NETest score. Given advances in detection technology, it seems likely that “molecular” evidence of non-imageable disease is a NET reality that will need further consideration as therapeutic paradigms evolve.
As a diagnostic, the NETest was >91% accurate for detection of NETs in comparison with healthy controls and other benign and malignant diseases. This is consistent with reports by Oberg et al. [10] and Malczewska and colleagues [22] who assessed NETest diagnostic accuracy in large cohorts and reported values of 94-95%.
The consideration of the NETest in other disease states including different cancers is relevant since neuroendocrine-like differentiation (NELD) has been reported in diverse neoplasia. These include breast, esophagus, colon and prostate cancers with incidences ranging from 5% (breast) to 51% (colorectal adenocarcinomas). An examination of a 10,224 TCGA tumor database by Chen et al., demonstrated that NETest gene expression and NELD at a tissue level was evident in ~ 4% of all tumors [11]. They noted that up to 32% of colon and lung cancers exhibited the NETest signature [11]. In our current study, ~35% of the neoplastic group had NETest scores >20. Individual tumor types ranged from 32% lung (mean NETest=18) to 47% in pancreas (mean NETest=23). This is consistent with blood-based detection of NELD-associated tumors expected in the non-NET neoplasia cohort. The biological and therapeutic implications of evidence of neuroendocrine elements in diverse neoplasia requires further consideration. In prostate cancer it is already considered a critical component in determining therapy and prognosis [23].
Given the selection of genes specific to neural and endocrine tumor tissue, the NETest assay detected all NETs irrespective of organ of origin. Nervous system tumors that exhibit neuroendocrine features e.g., pheochromocytomas and paragangliomas, expressed high levels of NETest genes and were ~100% detectable. This is consistent with their “neuroendocrine” genotype. This is supported by the TCGA study that confirmed numerous (n=21) NETest genes in nervous system-derived tumors including meningiomas and glioblastomas [11].
In separate reports, van Treijen et al. [8], and Liu et al. [9], described the accuracy of the NETest to differentiate stable from progressive disease as between 84-96%. Our study confirmed the NETest as an accurate disease status biomarker and identified that progressive disease exhibited significantly elevated scores. In all NET types examined, metastatic disease was associated with elevated scores (compared to localized disease). This is consistent with increased “omic-cluster expression” in metastatic disease and its association with a worse prognosis [24]. This relationship was also reflected in histopathological grading where an increasing grade correlated with elevated NETest blood levels. Grading, as determined by Ki67 (a monoanalyte with proliferative biological associations), has proven useful in stratifying lesions particularly at the low and high ends of the neoplastic spectrum. The relationship to a multigenomic PCR might therefore be predicted. Our assessment of 877 GEP-NETs confirmed a weak (r=0.16-0.19) but statistically significant relationship. The clinical associations of the NETest support that tumor biology changes are reflected in a blood measurement and the assay captures alterations in tumor activity (including proliferation) during the evolution of NET disease.
It is of interest as to whether the NETest could be a surrogate for imaging. The correlation between NETest and image-positive disease (CT/MRI and 68Ga-PET-CT) was 91%. This is similar to other studies that identified associations of 86%-95% [8, 9, 22]. The NETest was also positive in ~80% of image-negative but microscopic disease-positive disease. The NETest was positive in 71 of 154 image-negative disease subjects. Sixty-four (90%) of these also exhibited MRD.
68Ga-PET-CT is highly sensitive since it identifies somatostatin receptors on tumors. This also has theranostic implications and is used for peptide receptor radionuclide therapy (PRRT) [3]. Identification of receptors does not predict treatment responsiveness since presence of a target does not connate radio-sensitivity [3]. Indeed, ~30% of PRRT-treated patients are non-responders. PRRT is costly and has well-described renal, hematological and bone marrow toxicity [3]. Specific genes identified in the NETest predict PRRT efficacy with ~95% accuracy [25]. This provides a basis to identify individuals in whom PRRT-treatment will likely be successful. We anticipate that a similar approach can be undertaken with the NETest to develop predictors for other NET treatments such as SSAs, everolimus and sunitinib.
Since there is high concordance with imaging, using the NETest to reduce diagnostic radiation exposure has been proposed [9]. One study reported a decrease in CT usage in ~40%[9]. While this study was not designed to assess health economic issues, there appears to be fiscal advantages to using an accurate biomarker. A blood test which costs ~$500, provides an objective evaluation and decreases imaging modalities associated with radiation exposure (cost $2,000-$7,000) seems an attractive consideration for management. This is especially relevant in NET disease that often has a long, indolent course.
While evaluation of a novel strategy has scientific interest, an assessment of its clinical relevance by comparison to a commonly used clinical strategy (CgA) has practical relevance. Small studies have previously proposed the NETest to be more effective than blood CgA measurement [6-9, 26]. The largest NET/control group included 253 subjects with matched NETest/CgA samples [26]. Our study (n=1,270) includes other neoplasia and benign diseases and provides a significantly more substantial and diverse clinical evaluation. The large sample size negates any issues related to Type II errors.
This study, in a substantially powered and diverse NET cohort, enrolled over a 5-year time-period, demonstrated the NETest provides an accurate, non-invasive strategy to real-time assess disease status and surgical treatment efficacy. The consideration of an accurate, non-invasive biomarker to identify disease that can be used as a surrogate, or provide synergistic information with imaging, has attractive clinical and health economic possibilities.
Supplementary Material
Key messages:
Blood-based multianalyte biomarkers are effective, non-invasive, real-time tools (liquid biopsy) for cancer management.
Chromogranin (CgA) is no longer considered an effective NET biomarker in national cancer guidelines.
The NETest is a 51-marker gene liquid biopsy that is >90% accurate for NET diagnosis.
NETest concordance with imaging, grade, metastases and disease status significantly outperforms CgA (p<0.0001).
NETest after surgery predicts residual disease and recurrence with >94% accuracy compared to CgA: 11%.
Footnotes
Disclosures
The authors have declared no conflicts of interest.
References
- 1.Perera FP, Weinstein IB. Molecular epidemiology: recent advances and future directions. Carcinogenesis 2000; 21: 517–524. [DOI] [PubMed] [Google Scholar]
- 2.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011; 144: 646–674. doi: 10.1016/j.cell.2011.1002.1013. [DOI] [PubMed] [Google Scholar]
- 3.Bodei L, Schöder H, Baum RP et al. Molecular profiling of neuroendocrine tumours to predict response and toxicity to peptide receptor radionuclide therapy. Lancet Oncol 2020; 21: e431–e443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oberg K, Modlin IM, De Herder W et al. Consensus on biomarkers for neuroendocrine tumour disease. Lancet Oncol. 2015; 16: e435–e446. doi: 4 10.1016/S1470-2045(1015)00186-00182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shah MH, Kulke MH, Goldner WS et al. NCCN Guidelines: Neuroendocrine and Adrenal Tumors, Version 3.2018 -Sept 11. In. 2018; 693–702. doi: 6 10.6004/jnccn.2018.0056. [DOI] [PubMed] [Google Scholar]
- 6.Caplin ME, Ratnayake GM. Diagnostic and therapeutic advances in neuroendocrine tumours. Nat Rev Endocrinol 2020. [DOI] [PubMed] [Google Scholar]
- 7.Modlin I, Drozdov I, Kidd M. The Identification of gut neuroendocrine tumor disease by multiple synchronous transcript analysis in blood. Plos One 2013; e63364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Treijen MJC, van der Zee D, Heeres BC et al. Blood Molecular Genomic analysis predicts the disease course of GEP NET patients: a validation study of the predictive value of the NETest®. Neuroendocrinology 2020; 3: 000509091. [DOI] [PubMed] [Google Scholar]
- 9.Liu E, Paulson S, Gulati A et al. Assessment of NETest Clinical utility in a US Registry-based study. The Oncologist 2019; 24: 783–790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oberg K, Califano A, Strosberg JR et al. A meta-analysis of the accuracy of a neuroendocrine tumor mRNA genomic biomarker (NETest) in blood. Ann Oncol. 2020; 31: 202–212. doi: 2 10.1016/j.annonc.2019.1011.1003. Epub 2019 Dec 1020. [DOI] [PubMed] [Google Scholar]
- 11.Chen F, Zhang Y, Gibbons DL et al. Pan-Cancer Molecular Classes Transcending Tumor Lineage Across 32 Cancer Types, Multiple Data Platforms, and over 10,000 Cases. Clin Cancer Res. 2018; 24: 2182–2193. doi: 21 10.1158/1078-0432.CCR-2117-3378. Epub 2018 Feb 2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strosberg J, Casciano R, Stern L et al. United States-based practice patterns and resource utilization in advanced neuroendocrine tumor treatment. World J Gastroenterol. 2013; 19: 2348–2354. doi: 23 10.3748/wjg.v2319.i2315.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.von Elm E, Altman DG, Egger M et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Lancet 2007; 370: 1453–1457. [DOI] [PubMed] [Google Scholar]
- 14.Eisenhauer EA, Therasse P, Bogaerts J et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009; 45: 228–247. doi: 2 10.1016/j.ejca.2008.1010.1026. [DOI] [PubMed] [Google Scholar]
- 15.Pavel M, Jann H, Prasad V et al. NET Blood Transcript Analysis Defines the Crossing of the Clinical Rubicon: When Stable Disease Becomes Progressive. Neuroendocrinology 2017; 104: 170–182. [DOI] [PubMed] [Google Scholar]
- 16.Filosso P, Kidd M, Roffinella M et al. The utility of blood neuroendocrine gene transcript measurement in the diagnosis of bronchopulmonary neuroendocrine tumors (BPNET) and as a tool to evaluate surgical resection and disease progression. European J Cardiothoracic Surgery 2018; 53: 631–639. [DOI] [PubMed] [Google Scholar]
- 17.Cwikla JB, Bodei L, Kolasinska-Cwikla A et al. Circulating transcript analysis (NETest) in GEP-NETs treated with Somatostatin Analogs defines Therapy. J Clin Endocrinol Metab 2015; 100: E1437–1445. [DOI] [PubMed] [Google Scholar]
- 18.Pavel M, Öberg K, Falconi M et al. Gastroenteropancreatic neuroendocrine neoplasms: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020; 31: 844–860. doi: 8 10.1016/j.annonc.2020.1003.1304. Epub 2020 Apr 1016. [DOI] [PubMed] [Google Scholar]
- 19.Lamarca A, Clouston H, Barriuso J et al. Follow-Up Recommendations after Curative Resection of Well-Differentiated Neuroendocrine Tumours: Review of Current Evidence and Clinical Practice. J Clin Med 2019; 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Capdevila J, Casanovas O, Salazar R et al. Translational research in neuroendocrine tumors: pitfalls and opportunities. Oncogene. 2017; 36: 1899–1907. doi: 18 10.1038/onc.2016.1316. Epub 2016 Sep 1819. [DOI] [PubMed] [Google Scholar]
- 21.Malczewska A, Bodei L, Kidd M, Modlin IM. Blood mRNA Measurement (NETest) for Neuroendocrine Tumor Diagnosis of Image-Negative Liver Metastatic Disease. J Clin Endocrinol Metab. 2019; 104: 867–872. doi: 8 10.1210/jc.2018-01804. [DOI] [PubMed] [Google Scholar]
- 22.Malczewska A, Witkowska M, Wojcik-Giertuga M et al. Prospective Evaluation of the NETest as a liquid biopsy for Gastroenteropancreatic and Bronchopulmonary Neuroendocrine Tumours: An ENETS Centre of Excellence Experience Neuroendocrinology 2020; (in press). [DOI] [PubMed] [Google Scholar]
- 23.Yadav KK, Shameer K, Readhead B et al. Systems Medicine Approaches to Improving Understanding, Treatment, and Clinical Management of Neuroendocrine Prostate Cancer. Curr Pharm Des 2016; 22: 5234–5248. [DOI] [PubMed] [Google Scholar]
- 24.Kidd M, Kitz A, Drozdov I, Modlin I. Neuroendocrine Tumor Omic Gene Cluster Analysis Amplifies the Prognostic Accuracy of the NETest. Neuroendocrinology 2020; May 11. doi: 10.1159/000508573. [DOI] [PubMed] [Google Scholar]
- 25.Bodei L, Kidd MS, Singh A et al. PRRT Genomic Signature in Blood for Prediction of 177Lu-octreotate Efficacy. EJNMMI 2018; 45: 1155–1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Treijen MJC, Korse CM, van Leeuwaarde RS et al. Blood Transcript Profiling for the Detection of Neuroendocrine Tumors: Results of a Large Independent Validation Study. Front Endocrinol (Lausanne). 2018; 9:740.: 10.3389/fendo.2018.00740. eCollection 02018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.