Abstract
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease. Not only genetics, but the intestinal environment affected by gut microbiota is also the key to pathogenesis. Besides the occurrence of diabetes, gut microbiota dysbiosis may also contribute to the development of diabetes-related complications. Fecal microbiota transplantation (FMT) is an emerging technique that had shown its potential as a treatment for metabolic disease. Here, we report the first case of T1DM with malnutrition and gastrointestinal symptoms treated with FMT. A 24-year-old T1DM patient suffered from poor blood glucose control, recurrent nausea and vomiting, severe malnutrition, and intractable constipation after insulin treatment. The clinical response of the patients after FMT was well, especially nausea and vomiting were significantly relieved. In addition, constipation, nutritional status, and blood glucose control (fasting blood glucose, HbA1c) gradually improved. A degree of similarity was found in gut microbiota composition between the patient and healthy donor after FMT while it was totally different before the treatment. Furthermore, pathway function analysis of MetaCYC database implies that the potential mechanism of the response of FMT may be driven by specific bacteria involved in several metabolic pathways that need further exploration. To sum up, we believe that the reconstruction of intestinal flora by FMT may be a new choice for the treatment of T1DM patients with malnutrition.
Keywords: fecal microbiota transplantation (FMT), gut microbiota, malnutrition, MetaCYC database, metagenomic sequencing, type 1 diabetes mellitus (T1DM)
Introduction
Type 1 diabetes mellitus (T1DM) is a chronic autoimmune disease characterized by T-cell-mediated destruction of β-cells leading to absolute insulin deficiency. 1 Recently, more and more evidence indicated that genetics is not the only factor in the occurrence and development of T1DM. 2 T1DM is driven by genetic, immune, environmental, and other factors.2,3 The interaction between gut microbiota and the immune system regulating the intestinal environment is one of the key factors. Gut microbiota is not only related to the occurrence of diabetes but also contributes to the development of diabetes-related complications, especially diabetes-related malnutrition. Research had revealed the association between malnutrition and the alteration of the intestinal flora, but the majority of them focused on children or animal experiments. 4 Fecal microbiota transplantation (FMT), a technology of transplanting gut microbiota of healthy people into patients’ intestines to restore the normal function of intestinal flora in the treatment of intestinal5–7 and extra-intestinal diseases,8,9 is also considered as a special ‘organ transplantation’. Its potential for diabetes and malnutrition treatment is worth exploring.
This reported case of T1DM with malnutrition was treated with FMT. And there is a significant improvement shown in blood glucose (Glu) concentration and nutritional status after the FMT treatment.
Case report
A 24-year-old patient was diagnosed with T1DM 1 year ago and the patient’s fasting Glu concentration was not well controlled after a subcutaneous injection of insulin. Since February 2021, the patient repeatedly visited hospitals because of some symptoms of diabetic ketoacidosis, such as abdominal pain, nausea, and vomiting. These symptoms continued after the relevant treatment, and the case was hospitalized in the endocrinology department, on August 14. Then, the patient was transferred to our department on August 27 with a weight of 25.5 kg and a body mass index (BMI) of 11.8 kg/m2 and was diagnosed with type 1 diabetic ketosis with severe malnutrition after the Nutritional Risk Screening (NRS2002) with a score of 4/13. Besides, the patient had a history of depressive disorder and was treated with drugs.
Obvious gastrointestinal symptoms accompanied by intractable constipation are still repeated after conventional treatment, including proton pump inhibitors (PPIs), mosapride, Oryz-Aspergillus enzyme and pancreatin tablet, metoclopramide, and enteral nutrition. Therefore, we decided to treat the patient with FMT with informed consent. A routine examination was conducted to ensure that there are no contraindications of FMT. In order to enhance enteral nutrition supplement and digestive tract therapy in FMT, we performed nasojejunal tube implantation for the patients. The stool came from a 29-year-old healthy donor. The preparation of fecal microbial suspensions is carried out in accordance with the instructions of the automatic microfiltration system (GenFMTer; FMT Medical, Nanjing, China). The screening of healthy donor and the preparation and transplantation of microbial suspensions followed the Nanjing consensus 10 (Supplementary 1). The prepared fecal microbial suspension was stored at a low temperature of −80°C. Then rewarmed and allocated to 200 ml for injection through nasojejunal tube. This process was executed once on the 3rd and 6th of September, respectively. There were no adverse reactions during the FMT treatment.
Results
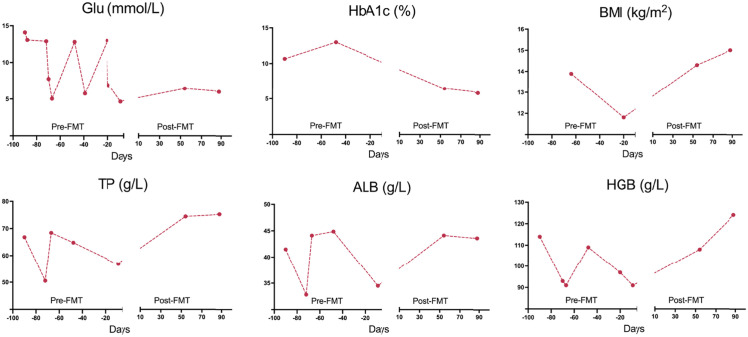
After the FMT treatment, the clinical response of patients was very well, especially nausea and vomiting were significantly relieved, and constipation gradually improved, so digestive drugs were gradually discontinued. Moreover, the patient’s weight and BMI increased gradually (Figure 1). Other clinical indexes, total protein (TP), albumin (ALB), and hemoglobin (HGB) also recovered to a normal level. In terms of glycemic control, Glu and glycosylated hemoglobin (HbA1c) were maintained at a fine level, and the dose of insulin was decreased accordingly. Moreover, fasting C-peptide was recovered to 0.12 nmol/l from an extremely low level.
Figure 1.
Dynamic curves of Glu, HbA1c, BMI, TP, ALB, and HGB.
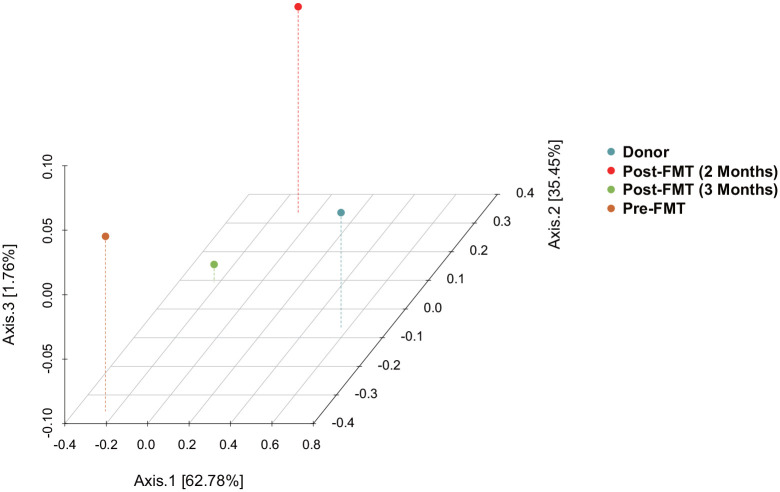
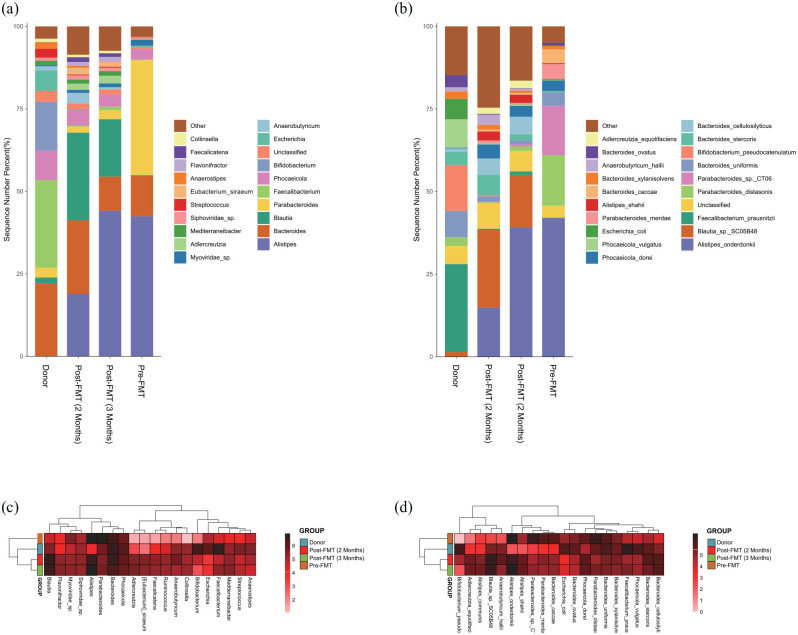
Metagenomic sequencing (Supplementary 2) was performed on fecal samples from the donor and the patient (pre- and post-FMT). The Venn diagram (Figure 2) and principal coordinates analysis (PCoA) analysis (Figure 3) showed the α and β diversity of the gut microbiota among the fecal samples. In contrast to the patient, the fecal microbiota of healthy donor was more abundant in Bacteroides uniformis, Bifidobacterium pseudocatenulatum, Escherichia coli, and Faecalibacterium prausnitzii (Figure 4(b)). Significant changes were found in the distribution of the top 20 flora in relative abundances pre- and post-FMT. At the genus level (Figure 4(c)), the relative abundances of Adlercreutzia, Anaerobutyricum, Anaerostipes, Bacteroides, Bifidobacterium, Blautia, Collinsella, Eubacterium siraeum, Faecalibacterium, Faecalicatena, Flavonifractor, Mediterraneibacter, Phocaeicola, Siphoviridae sp., and Streptococcus increased, while those of Alistipes, Escherichia, Myoviridae sp., and Parabacteroides decreased post-FMT. At the species level (Figure 4(d)), the relative abundances of Adlercreutzia equolifaciens, Alistipes shahii, Anaerobutyricum hallii, Bacteroides cellulosilyticus, Bacteroides stercoris, Bacteroides xylanisolvens, Bifidobacterium pseudocatenulatum, Blautia sp. SC05B48, Faecalibacterium prausnitzii, Phocaeicola dorei, and Phocaeicola vulgatus increased, while those of Alistipes onderdonkii, Bacteroides caccae, Bacteroides ovatus, B. uniformis, Escherichia coli, Parabacteroides distasonis, Parabacteroides merdae, and Parabacteroides sp. CT06 decreased post-FMT.
Figure 2.
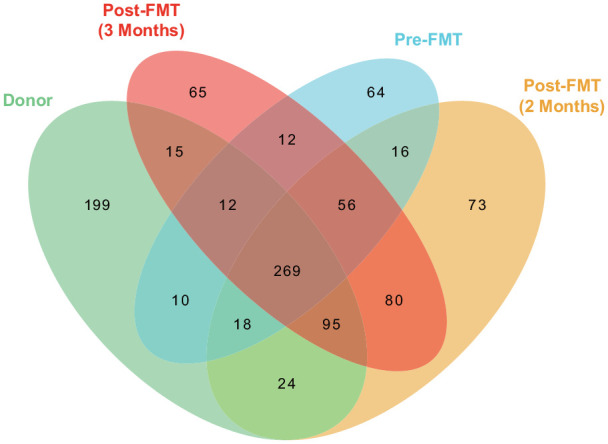
Venn diagram showed all OTUs obtained in fecal samples of donor and receiver (pre- and post-FMT).
Figure 3.
PCoA analysis showed the β diversity among the fecal samples of donor and receiver (pre- and post-FMT).
Figure 4.
The bar plot showed the distribution of the top 20 flora in relative abundance at the genus (a) and species (b) levels. The heatmap showed the clustering results of the top 20 flora in relative abundance at the genus (c) and species (d) levels.
Additional analysis of pathways and functions on the MetaCYC database was performed (Table 1). A total of 29 pathways associated with 20 functions were found altered post-FMT. They were ANAGLYCOLYSIS-PWY, COA-PWY-1, PEPTIDOGLYCANSYN-PWY, PANTO-PWY, PWY0-1319, PWY-1042, PWY-2942, PWY-3841, PWY5097, PWY-5659, PWY-5667, PWY-5686, PWY-5695, PWY-6121, PWY-6122, PWY-6123, PWY-6124, PWY-6151, PWY-6277, PWY-6385, PWY-6386, PWY-6387, PWY-6609, PWY66-400, PWY-7219, PWY-7220, PWY-7282, PYRIDOXSYN-PWY, and VALSYN-PWY.
Table 1.
MetaCYC database pathway function analysis.
| Functions (altered post-FMT) | Pathway IDs | Principal bacteria |
|---|---|---|
| 4-Amino-2-methyl-5-phosphomethylpyrimidine biosynthesis | PWY-7282 | Bacteroides ovatus/xylanisolvens |
| 5-Aminoimidazole ribonucleotide biosynthesis | PWY-6121 PWY-6122 PWY-6277 |
Alistipes onderdonkii
Clostridium bolteae Eggerthella lenta Eubacterium siraeum |
| Adenine and adenosine salvage III | PWY-6609 |
Alistipes shahii
Bacteroides caccae/ovatus/xylanisolvens Odoribacter splanchnicus Ruminococcus gnavus |
| Adenosine deoxyribonucleotides de novo biosynthesis II | PWY-7220 | Bacteroides cellulosilyticus |
| Adenosine ribonucleotides de novo biosynthesis | PWY-7219 |
Alistipes onderdonkii/shahii
Bacteroides caccae/cellulosilyticus/ovatus/uniformis/vulgatus Parabacteroides distasonis/merdae Ruminococcus gnavus |
| CDP-diacylglycerol biosynthesis | PWY-5667 PWY0-1319 |
Bacteroides caccae/ovatus/stercoris/uniformis
Parabacteroides distasonis/merdae |
| Coenzyme A biosynthesis | COA-PWY-1 |
Bacteroides caccae/ovatus/uniformis/vulgatus
Eubacterium siraeum Megasphaera elsdenii Parabacteroides distasonis |
| Folate transformations | PWY-3841 |
Bacteroides caccae/ovatus/uniformis/vulgatus
Parabacteroides distasonis/merdae |
| GDP-mannose biosynthesis | PWY-5659 |
Alistipes onderdonkii/shahii
Bacteroides ovatus/xylanisolvens Eubacterium siraeum |
| Glycolysis | ANAGLYCOLYSIS-PWY PWY-1042 PWY66-400 |
Alistipes shahii
Bacteroides caccae/ovatus Odoribacter splanchnicus |
| Inosine-5’-phosphate biosynthesis | PWY-6123 PWY-6124 |
Bacteroides ovatus |
| L-lysine biosynthesis | PWY-2942 PWY5097 |
Bacteroides caccae/cellulosilyticus /ovatus/stercoris/uniformis/vulgatus
Parabacteroides distasonis/merdae |
| L-valine biosynthesis | VALSYN-PWY |
Bacteroides caccae/cellulosilyticus/massiliensis/stercoris/vulgatus
Eubacterium siraeum Parabacteroides distasonis |
| Peptidoglycan biosynthesis | PEPTIDOGLYCANSYN-PWY PWY-6385 |
Alistipes onderdonkii
Bacteroides ovatus/uniformis/vulgatus Parabacteroides distasonis/merdae |
| Phosphopantothenate biosynthesis | PANTO-PWY |
Bacteroides caccae/cellulosilyticus/ovatus /stercoris/uniformis/vulgatus
Parabacteroides distasonis/merdae |
| Pyridoxal 5’-phosphate biosynthesis | PYRIDOXSYN-PWY | Bacteroides caccae/ovatus/stercoris/uniformis |
| S-adenosyl-L-methionine cycle I | PWY-6151 |
Bacteroides massiliensis/stercoris/uniformis/vulgatus
Eubacterium hallii |
| UDP-N-acetylmuramoyl-pentapeptide biosynthesis | PWY-6386 PWY-6387 |
Alistipes onderdonkii
Bacteroides ovatus/uniformis/vulgatus Parabacteroides distasonis |
| UMP biosynthesis | PWY-5686 |
Alistipes onderdonkii
Bacteroides caccae/ovatus/stercoris/uniformis Collinsella aerofaciens Eubacterium siraeum Parabacteroides distasonis/merdae |
| Urate biosynthesis/inosine 5’-phosphate degradation | PWY-5695 |
Alistipes shahii
Bacteroides caccae/ovatus/stercoris/vulgatus Megasphaera elsdenii Parabacteroides distasonis |
Discussion
Gut microbiota plays an important role in human health and disease. Compared with the gut microbiota of healthy people, the abundance of butyric acid-producing anaerobic bacteria and firmicutes/bacteroides ratio of T1DM patients decreased,11,12 which was consistent with our metagenomics results. De Groot et al. 13 have found that FMT treatment can improve Glu control in patients with T1DM and retain the function of residual β cells. The glucose concentration of our patient fluctuated from 4.66 to 14.10 mmol/l before FMT, and it was controlled at an average of 6.23 mmol/l after the treatment. These improvements in glucose metabolism might owe to the alteration in B. ovatus which was confirmed over-abundance in T1DM children as compared with healthy controls. 14 Cinek et al. 15 have found that the imbalance within the Bacteroides genus might be one of the key factors in T1DM. But some species, such as B. caccae and Bacteroides vulgatus, were found to increase in T1DM women in pregnancy while they were proved to decrease in islet autoimmunity children and inversely associated with it,15,16 their roles in T1DM are still ambiguous. The results of our case were in line with the pregnant women study. These three species of Bacteroides were involved in 19 functions alteration post-FMT. Among them, pyridoxal 5’-phosphate that could protect islet beta-cell from streptozotocin-induced dysfunction might be the key pathway (PYRIDOXSYN-PWY) that benefits diabetes improvement. 17 But this specific mechanism still needs further exploration to illustrate.
Besides diabetes, our patient was also complicated with severe malnutrition. The application of FMT treatment in malnutrition was still relatively rare, only one case of weight gain after FMT was reported. 18 In our case, the improvement in the condition of nutrition is markedly reflected in the increase in BMI, TP, ALB, and HGB. The patient also came back from malnutrition-led amenorrhea after FMT. In the gut microbiota changes part, two bacteria came to our notice. Blautia is a kind of intestinal flora that had a positive correlation with weight. 19 We observed a significant increase in Blautia after FMT. Parabacteroides, a probiotic enriched in the patient’s flora before FMT and decreased after FMT. Evidence showed that P. distasonis improves lipid metabolism and decreases weight gain by elevating the level of succinate. 20 Other research found that succinate-fed mice had a higher basic energy consumption. 21 These implied that the over-abundance of P. distasonis might turn it into a pathogenic bacterium, which led to malnutrition in our case.
In addition, the patient had been hospitalized for depressive disorder and had taken duloxetine previously. And our results were surprised to find changes in depression-related flora, such as A. onderdonkii, B. uniformis, and P. diatasonis.22–25 Fortunately, the depression did not recur during the follow-up.
Conclusion
In summary, FMT treatment was successfully performed in a T1DM patient with malnutrition in this case. This means a new choice for the treatment of these diseases was now offered. Further research on its mechanism still needs to be done. Despite this being a case report, our results are highly suggestive for exploring the association between gut microbiota and metabolic diseases.
Supplemental Material
Supplemental material, sj-docx-1-taj-10.1177_20406223221117449 for Fecal microbiota transplantation treatment for type 1 diabetes mellitus with malnutrition: a case report by Yan-Chun Xie, Xu-Bin Jing, Xiang Chen, Ling-Zi Chen, Shao-Hui Zhang and Xian-Bin Cai in Therapeutic Advances in Chronic Disease
Acknowledgments
None.
Footnotes
ORCID iD: Xian-Bin Cai  https://orcid.org/0000-0002-3967-8666
https://orcid.org/0000-0002-3967-8666
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yan-Chun Xie, Department of Gastroenterology, The First Affiliated Hospital of Shantou University Medical College, Shantou, P.R. China.
Xu-Bin Jing, Department of Gastroenterology, The First Affiliated Hospital of Shantou University Medical College, Shantou, P.R. China.
Xiang Chen, Departments of Health Care Center, The First Affiliated Hospital of Shantou University Medical College, Shantou, P.R. China.
Ling-Zi Chen, Department of Gastroenterology, The First Affiliated Hospital of Shantou University Medical College, Shantou, P.R. China.
Shao-Hui Zhang, Department of Gastroenterology, The First Affiliated Hospital of Shantou University Medical College, Shantou, P.R. China.
Xian-Bin Cai, Department of Gastroenterology, The First Affiliated Hospital of Shantou University Medical College, 57 Changping Road, Shantou 515041, Guangdong, P.R. China.
Declarations
Ethics approval and consent to participate: All methods were performed in accordance with the provisions of the Declaration of Helsinki of 1975. The Committee of The First Affiliated Hospital of Shantou University Medical College approved the study (approval no. 2019090). Written informed consent was obtained from the patient for treatment before FMT.
Consent for publication: Written informed consent was obtained from the patient for publication of the case and any accompanying data.
Author contributions: Yan-Chun Xie: Data curation; Formal analysis; Visualization; Writing – original draft.
Xu-Bin Jing: Conceptualization; Resources; Supervision; Writing – review & editing.
Xiang Chen: Formal analysis; Visualization; Writing – review & editing.
Ling-Zi Chen: Methodology; Writing – review & editing.
Shao-Hui Zhang: Methodology; Writing – review & editing.
Xian-Bin Cai: Conceptualization; Funding acquisition; Project administration; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The Science and Technology Planning Project of Shantou City (grant no. 190430145264400), the General Program of the Natural Science Foundation of Guangdong Province, China (grant no. 2019A1515010917), and the Medical Scientific Research Foundation of Guangdong Province, China (grant no. A2019275), and the 2020 Li Ka Shing Foundation Cross-Disciplinary Research Grant (grant no. 2020LKSFG07D).
Competing interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Availability of data and materials: The raw data were available in the NCBI Bioproject database with an ID of PRJNA849709.
References
- 1. DiMeglio LA, Evans-Molina C, Oram RA. Type 1 diabetes. The Lancet 2018; 391: 2449–2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Durazzo M, Ferro A, Gruden G. Gastrointestinal microbiota and type 1 diabetes mellitus: the state of art. J Clin Med 2019; 8: 1843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ilonen J, Lempainen J, Veijola R. The heterogeneous pathogenesis of type 1 diabetes mellitus. Nat Rev Endocrinol 2019; 15: 635–650. [DOI] [PubMed] [Google Scholar]
- 4. Fluitman KS, De Clercq NC, Keijser BJF, et al. The intestinal microbiota, energy balance, and malnutrition: emphasis on the role of short-chain fatty acids. Expert Rev Endocrinol Metab 2017; 12: 215–226. [DOI] [PubMed] [Google Scholar]
- 5. Surawicz CM, Brandt LJ, Binion DG, et al. Guidelines for diagnosis, treatment, and prevention of Clostridium difficile infections. Am J Gastroenterol 2013; 108: 478–498; quiz 499. [DOI] [PubMed] [Google Scholar]
- 6. Johnsen PH, Hilpüsch F, Cavanagh JP, et al. Faecal microbiota transplantation versus placebo for moderate-to-severe irritable bowel syndrome: a double-blind, randomised, placebo-controlled, parallel-group, single-centre trial. Lancet Gastroenterol Hepatol 2018; 3: 17–24. [DOI] [PubMed] [Google Scholar]
- 7. Costello SP, Hughes PA, Waters O, et al. Effect of fecal microbiota transplantation on 8-week remission in patients with ulcerative colitis: a randomized clinical trial. JAMA 2019; 321: 156–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bajaj JS, Kassam Z, Fagan A, et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: a randomized clinical trial. Hepatology 2017; 66: 1727–1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vendrik KEW, Ooijevaar RE, de Jong PRC, et al. Fecal microbiota transplantation in neurological disorders. Front Cell Infect Microbiol 2020; 10: 98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Group FMT-S. Nanjing consensus on methodology of washed microbiota transplantation. Chin Med J 2020; 133: 2330–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Leiva-Gea I, Sanchez-Alcoholado L, Martin-Tejedor B, et al. Gut microbiota differs in composition and functionality between children with type 1 diabetes and MODY2 and healthy control subjects: a case-control study. Diabetes Care 2018; 41: 2385–2395. [DOI] [PubMed] [Google Scholar]
- 12. Giongo A, Gano KA, Crabb DB, et al. Toward defining the autoimmune microbiome for type 1 diabetes. ISME J 2011; 5: 82–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. De Groot P, Nikolic T, Pellegrini S, et al. Faecal microbiota transplantation halts progression of human new-onset type 1 diabetes in a randomised controlled trial. Gut 2021; 70: 92–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Mokhtari P, Metos J, Anandh Babu PV. Impact of type 1 diabetes on the composition and functional potential of gut microbiome in children and adolescents: possible mechanisms, current knowledge, and challenges. Gut Microbes 2021; 13: 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cinek O, Kramna L, Lin J, et al. Imbalance of bacteriome profiles within the Finnish Diabetes Prediction and Prevention study: parallel use of 16S profiling and virome sequencing in stool samples from children with islet autoimmunity and matched controls. Pediatr Diabetes 2017; 18: 588–598. [DOI] [PubMed] [Google Scholar]
- 16. Roth-Schulze AJ, Penno MAS, Ngui KM, et al. Type 1 diabetes in pregnancy is associated with distinct changes in the composition and function of the gut microbiome. Microbiome 2021; 9: 167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kiran SG, Dorisetty RK, Umrani MR, et al. Pyridoxal 5’ phosphate protects islets against streptozotocin-induced beta-cell dysfunction – in vitro and in vivo. Exp Biol Med 2011; 236: 456–465. [DOI] [PubMed] [Google Scholar]
- 18. Alang N, Kelly CR. Weight gain after fecal microbiota transplantation. Open Forum Infect Dis 2015; 2: ofv004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zhao T, Zhan L, Zhou W, et al. The effects of erchen decoction on gut microbiota and lipid metabolism disorders in Zucker diabetic fatty rats. Front Pharmacol 2021; 12: 647529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wang K, Liao M, Zhou N, et al. Parabacteroides distasonis alleviates obesity and metabolic dysfunctions via production of succinate and secondary bile acids. Cell Rep 2019; 26: 222–235.e225. [DOI] [PubMed] [Google Scholar]
- 21. De Vadder F, Kovatcheva-Datchary P, Zitoun C, et al. Microbiota-produced succinate improves glucose homeostasis via intestinal gluconeogenesis. Cell Metab 2016; 24: 151–157. [DOI] [PubMed] [Google Scholar]
- 22. Gomez-Nguyen A, Basson AR, Dark-Fleury L, et al. Parabacteroides distasonis induces depressive-like behavior in a mouse model of Crohn’s disease. Brain Behav Immun 2021; 98: 245–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Zhang Y, Fan Q, Hou Y, et al. Bacteroides species differentially modulate depression-like behavior via gut-brain metabolic signaling. Brain Behav Immun 2022; 102: 11–22. [DOI] [PubMed] [Google Scholar]
- 24. Strandwitz P, Kim KH, Terekhova D, et al. GABA-modulating bacteria of the human gut microbiota. Nat Microbiol 2019; 4: 396–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Parker BJ, Wearsch PA, Veloo ACM, et al. The genus Alistipes: gut bacteria with emerging implications to inflammation, cancer, and mental health. Front Immunol 2020; 11: 906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-taj-10.1177_20406223221117449 for Fecal microbiota transplantation treatment for type 1 diabetes mellitus with malnutrition: a case report by Yan-Chun Xie, Xu-Bin Jing, Xiang Chen, Ling-Zi Chen, Shao-Hui Zhang and Xian-Bin Cai in Therapeutic Advances in Chronic Disease