Abstract
Background:
Prolonged symptoms after COVID-19 are an important concern due to the large numbers affected by the pandemic.
Objectives:
To ascertain the frequency of gastrointestinal (GI) manifestations as part of long GI COVID.
Design:
A systematic review and meta-analysis of studies reporting GI manifestations in long COVID was performed.
Data Sources and Methods:
Electronic databases (Medline, Scopus, Embase, Cochrane Central Register of Controlled Trials, and Web of Science) were searched till 21 December 2021 to identify studies reporting frequency of GI symptoms in long COVID. We included studies reporting overall GI manifestations or individual GI symptoms as part of long COVID. We excluded pediatric studies and those not providing relevant information. We calculated the pooled frequency of various symptoms in all patients with COVID-19 and also in those with long COVID using the inverse variance approach. All analysis was done using R version 4.1.1 using packages ‘meta’ and ‘metafor’.
Results:
A total of 50 studies were included. The frequencies of GI symptoms were 0.12 [95% confidence interval (CI), 0.06–0.22, I2 = 99%] and 0.22 (95% CI, 0.10–0.41, I2 = 97%) in patients with COVID-19 and those with long COVID, respectively. The frequencies of abdominal pain, nausea/vomiting, loss of appetite, and loss of taste were 0.14 (95% CI, 0.04–0.38, I2 = 96%), 0.06 (95% CI, 0.03–0.11, I2 = 98%), 0.20 (95% CI, 0.08–0.43, I2 = 98%), and 0.17 (95% CI, 0.10–0.27, I2 = 95%), respectively, after COVID-19. The frequencies of diarrhea, dyspepsia, and irritable bowel syndrome were 0.10 (95% CI, 0.04–0.23, I2 = 98%), 0.20 (95% CI, 0.06–0.50, I2 = 97%), and 0.17 (95% CI, 0.06–0.37, I2 = 96%), respectively.
Conclusion:
GI symptoms in patients were seen in 12% after COVID-19 and 22% as part of long COVID. Loss of appetite, dyspepsia, irritable bowel syndrome, loss of taste, and abdominal pain were the five most common GI symptoms of long COVID. Significant heterogeneity and small number of studies for some of the analyses are limitations of the systematic review.
Keywords: abdominal pain, diarrhea, gastrointestinal symptoms, long COVID haulers, nausea, post-COVID syndrome, SARS-CoV-2, vomiting
Introduction
COVID-19 has brought forth a multitude of challenges to the health-care systems globally. Apart from the significant morbidity and mortality associated with COVID-19 during the initial phase, there is a growing recognition and concern about the long-term consequences of COVID-19. Described variously as ‘long COVID’, ‘post COVID’, ‘long-haul COVID’, and so on, the condition is not clearly characterized regarding the time of onset and the clinical manifestations. 1 The WHO defines it as a constellation of symptoms which occur 3 months after COVID-19 and last for 2 months or more and do not have an alternative explanation. 2 Centers for Disease Control and Prevention (CDC) has described this condition to occur even after 4 week of COVID-19. 3 Typically the occurrence of fatigue, breathlessness, and cognitive dysfunction is considered the major manifestation of COVID-19 but the WHO definition also allows for gastrointestinal (GI) issues like diarrhea, constipation, acid reflux, abdominal pain, and altered smell/taste as a part of post-COVID-19 symptoms. The WHO definition is based on Delphi consensus while the CDC’s definition is based on input from a panel of provider and researcher experts.
GI and hepatic manifestations of acute COVID-19 are well recognized.4,5 The distribution of angiotensin converting enzyme 2 (ACE-2) receptors in the GI tract, systemic effects of the disease, and use of a multitude of the drugs are believed to result in these manifestations. 6
On the contrary, the GI manifestations of long COVID are not as well recognized. Certain GI symptoms including abdominal pain and diarrhea have been reported with long-COVID syndrome. However, there is a lack of a systematic analysis of the GI manifestations of long COVID and the implications for the patients, health-care workers, and institutions are unclear. It is also unclear as to how these manifestations may vary with respect to various definitions in vogue. These manifestations are, as of now, not included in the standard definition of long COVID. Since these manifestations are likely to affect the quality of life and may result in work-related absences, it is important to characterize the manifestations and their frequency. Therefore, we conducted a systematic review to assess the GI manifestation of ‘long COVID’ and the frequency of these manifestations.
Methods
Search strategy
The present systematic review and meta-analysis was conducted in accordance with the guidance provided by the PRISMA statement. 7 We searched various electronic databases including Medline, Embase, Cochrane Central Register of Controlled Trials, Scopus, Science Citation Index Expanded, and Emerging Sources Citation Index from inception till 20 December 2021. The keywords used for the search were (‘Covid-19 OR SARS-CoV-2 OR coronavirus disease 2019’) AND (‘long covid OR postcovid OR long haul OR sequelae OR persistent symptoms’). Filters for human studies were applied to all database searches except for Cochrane Central Register of Controlled Trials. Results were limited to English language publications. The detailed search strategy is shown in Supplemental Appendix A. The results obtained from all the databases were combined and duplicate studies were removed. Two reviewers (RT and AC) separately screened the title and abstract to select any studies reporting on data about the GI manifestations or liver dysfunction. The studies selected for full-text screen were seen by two authors (SK and VS) for data extraction.
Inclusion criteria
We included studies reporting on frequencies of GI or hepatic manifestations as part of post-COVID/long-COVID syndrome in the adult population. We excluded (1) studies if the number of patients were less than 10, (2) studies reporting the frequency of long COVID in pediatric population as most studies on pediatric population also included multisystem inflammatory syndrome as post-COVID sequelae, (3) studies that report only the multisystem inflammatory syndrome, (4) studies that are non-English, and (5) studies that only report the frequency of changes in taste and smell sensation as part of long-COVID syndrome. We also excluded studies that did not report the relevant outcome data.
Definitions
WHO defines long COVID as post-COVID-19 condition that occurs in individuals with a history of probable or confirmed SARS-CoV-2 infection, usually 3 months from the onset of COVID-19 with symptoms that last for at least 2 months and cannot be explained by an alternative diagnosis. 2
NICE/CDC describe ‘Post-COVID Condition’ as an umbrella term for the wide range of physical and mental health consequences experienced by some patients who are present four or more weeks after SARS-CoV-2 infection, including by patients who had initial mild or asymptomatic acute infection.3,8 Supplemental Table 1 depicts the similarities and differences between the two definitions.
Data extraction
Data were extracted from the included studies by two reviewers (RT and AC) and any disagreement was resolved through discussion involving the other two reviewers (SK and VS). Extracted data included publication details (author and year), place and duration of study, total number of COVID patients, total number of long-COVID patients, total number of long COVID with GI symptoms, and their characteristics including age, gender, comorbidity severity of acute SARS-CoV-2 infection, and frequencies of various GI manifestation (nausea, vomiting, diarrhea, constipation, abdominal pain, loss of appetite, loss of taste, irritable bowel syndrome, dyspepsia). We also extracted the data on mean follow-up time, follow-up mode, term used to refer to long-term effects, total patients with resolution of symptoms, and average time to resolution of symptoms. We also extracted the data reporting the frequency of long COVID with respect to the underlying severity of COVID.
Data analysis
The data analysis was done using R version 4.1.1. 9 The packages ‘meta’ and ‘metafor’ were used apart from the base package. 10 For calculation of pooled rates, the estimates were logit transformed and combined using the ‘inverse variance’ approach. The pooled relative risk was estimated using the Mantel–Haenszel method. 11 We performed analyses for frequency of GI manifestations (including individual overall GI long-COVID manifestations and individual symptoms like diarrhea, pain abdomen, dyspepsia, nausea/vomiting, loss of taste, loss of appetite, constipation, and irritable bowel syndrome). Separate analysis was done for GI manifestations of long COVID in the entire subset of COVID-19 and in patients who had long COVID.
Risk of bias assessment
Two investigators made independent assessments of methodological rigor and risk of bias in the included studies. The Joanna Briggs Institute tool for prevalence studies was used to assess the studies that showed the prevalence of GI symptoms. 12 This includes assessment regarding the appropriateness of the included population, and the sampling, description of subjects, and whether the diagnosis of long COVID was assessed appropriately and similarly in all individuals. Publication bias was assessed for the GI manifestations of long COVID in patients with COVID-19 and those with long COVID by using funnel plot and Egger test.
Results
Study selection
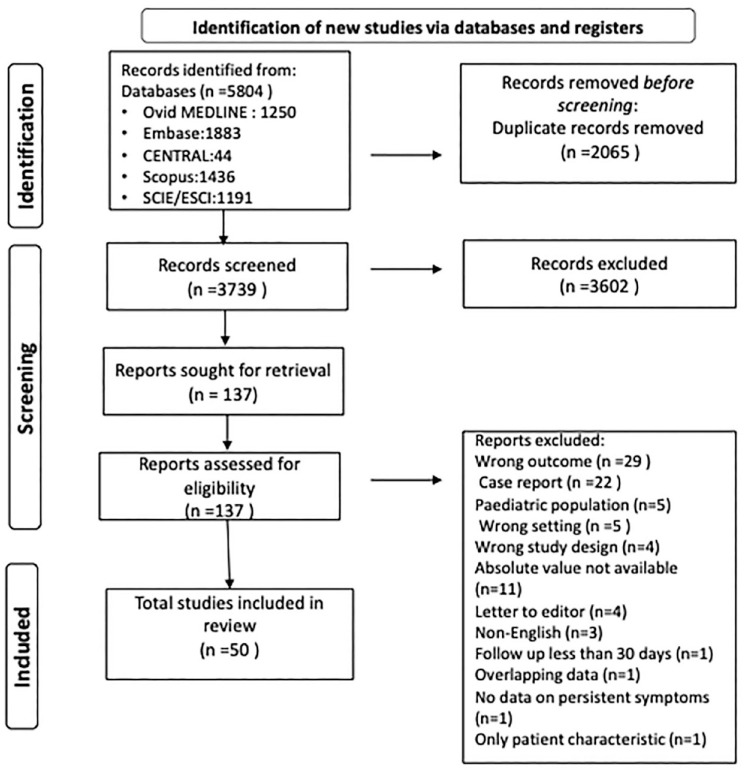
Of the 5804 records identified after database search, 2065 were duplicates. Of the 3739 titles which underwent initial screening, 3602 were removed for various reasons and eventually 137 articles underwent full-text screening. A total of 50 studies were included in the final analysis. Full PRISMA flowchart of study selection is depicted in Figure 1. Table 1 shows the details of the included studies with the study design, type of population, symptoms, duration, and the information provided.13–62 Supplemental Table 2 shows the excluded studies with reasons of exclusion.
Figure 1.
PRISMA flowchart depicting the process of screening and selection of studies.
Table 1.
Table showing the list of included studies with demographic details of included patients.
| Authors | Year | Type | Country | Included patients | Total number of patients | Total number of patients with GI symptoms | Individual GI symptoms | Age and sex | Follow-up time | Mode of follow-up | Term used | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diarrhea | Pain | Nausea/vomiting | Constipation | IBS | LOA | LOT | Cholangiopathy | Gastroparesis | Dyspepsia | |||||||||||
| Adame et al. 13 | 2021 | Abstract | USA | All | Total: 101/long COVID 25 | 21 | 21 | 21 | 21 | – | – | – | – | – | – | – | –/F 20 | 60 days | In person | Long hauler |
| Akinci Ozyurek et al. 14 | 2021 | Retrospective | Turkey | All | Total 315/long COVID 229 | 1 | 1 | – | – | – | – | – | – | – | – | – | –/F158 | 4 weeks | In person | Long COVID |
| Anaya et al. 15 | 2021 | Cross-sectional | Colombia | All | Total 100/long COVID 65 | – | 46 | 24 | – | – | – | 23 | – | – | – | – | Median 49 years/F 53 | Median 219 days | Survey | Post-COVID manifestation |
| Areekal et al. 16 | 2021 | Cross-sectional | India | Hospitalized | Total 335/long COVID 221 | – | 6 | – | – | – | – | 8 | – | – | – | – | –/F 161 | 4 weeks | Telephone | Post-COVID syndrome |
| Augustin et al. 17 | 2021 | Prospective | Germany | Non-hospitalized | Total 353/long COVID 123 | – | 4 | – | – | – | – | – | – | – | – | – | –/– | 7 months | In person | -COVID syndrome |
| Blackett et al. 18 | 2021 | Retrospective | USA | Hospitalized | Total 147/long – | – | 6 | 11 | 6 | 10 | 44 | – | – | – | – | – | –/72 | Median 106 days | Web based | Persistent syndrome |
| Blair et al. 19 | 2021 | Retrospective | USA | All | Total 166/long COVID 118 | – | 4 | 2 | 3 | – | – | – | – | – | – | – | –/– | 4 weeks | Telephone | Chronic COVID 19 syndrome |
| Buttery et al. 20 | 2021 | Cross-sectional | UK | All | Total 1865/long COVID – | – | – | – | – | – | – | 430 | – | – | – | – | –/1440 | 12 weeks | Web based | Long COVID |
| Calogero et al. 21 | 2021 | Abstract | USA | All | Total 12,224/long COVID – | – | – | – | – | – | – | – | – | – | 12 | – | –/– | 161 days | Chart review | Symptoms after COVID-19 |
| Carrillo-Garcia et al. 22 | 2021 | Retrospective | Spain | Hospitalized | Total 165/long COVID 109 | – | – | – | – | – | – | 55 | – | – | – | – | –/– | 3 months | Telephone | Sequelae of COVID |
| Chevinsky et al. 23 | 2021 | Retrospective | USA | Non-hospitalized | Total 74,446/long COVID 44,489 | – | – | 667 | 401 | – | – | – | – | – | – | – | – | 31–120 days | Review | Post-COVID syndrome |
| Chopra et al. 24 | 2021 | Retrospective | India | Non-hospitalized | Total 57/long COVID 25 | – | 1 | – | – | – | – | – | – | – | – | – | Mean 34.9 years/– | 30 days | Telephone | Long COVID |
| Dennis et al. 25 | 2021 | Prospective | UK | All | Total 201/long COVID – | – | 118 | – | – | 108 | – | – | – | – | – | – | Mean 44/142 | 4 weeks | In person | Post-COVID syndrome |
| Faruqui et al. 26 | 2021 | Retrospective | USA | Hospitalized | Total 2047/long COVID – | – | – | – | – | – | – | – | – | 12 | – | – | Mean 58 years/– | 118 days | Chart review | Late complication |
| Faycal et al. 27 | 2021 | Prospective | France | Non hospitalized | Total 429/long COVID 175 | 12/175 | – | – | – | – | – | – | – | – | – | – | Median 41.6 years/F 311 | 30 days | Telephone | Persistent symptoms |
| Fernández-de-Las-Peñas et al. 28 | 2021 | Prospective | Spain | Hospitalized | Total 1969/long COVID 1232 | 133 | 49 | – | – | – | – | – | – | – | – | – | Mean 61 years/F 915 | 8.4 months | Telephone | Post-COVID syndrome |
| Galal et al. 29 | 2021 | Cross-sectional | Egypt | Hospitalized | Total 430/long COVID 370 | – | – | – | – | – | – | 157 | – | – | – | 119 | Mean 37.4 years/F 274 | Mean 176 days | In person | Post-COVID symptoms |
| Galván-Tejada et al. 30 | 2020 | Retrospective | Mexico | All | Total 141/long COVID – | – | – | – | 22 | – | – | – | – | – | – | – | –/– | Mean 36 days | In person | Persistent symptoms |
| Ghoshal et al. 31 | 2021 | Prospective | Bangladesh and India | Hospitalized | Total 280/long COVID – | – | – | – | – | – | 15 | – | – | – | – | 16 | –/– | 1 month | In person/Telephone | Postinfectious symptoms |
| Gold et al. 32 | 2021 | Prospective | Greece | All | Total 185/Long 56 | – | – | 7 | – | – | – | – | – | – | – | – | –/– | 1 month | Survey | Long COVID |
| Hossain et al. 33 | 2021 | Prospective | Bangladesh | All | Total 2198/long COVID 356 | – | – | – | – | – | – | 12 | – | – | – | – | Mean 38.7 years/F 607 | 12 weeks | – | Long COVID |
| Islam et al. 34 | 2021 | Cross-sectional | Bangladesh | All | Total 1002/long COVID 200 | – | 127 | – | – | – | – | – | – | – | – | – | Mean 34.7 years/F 401 | 30 days | Survey | Long COVID |
| Nayagam et al. 35 | 2021 | Retrospective | UK | Hospitalized | Total 564/long COVID – | – | – | – | – | – | – | – | – | 24 | – | – | Median 67.7 years/F 258 | 60 days | In person | Persistent symptoms |
| Jones et al. 36 | 2021 | Retrospective | UK | All | Total3151/ long COVID 310 | – | 196 | 196 | – | – | – | 294 | – | – | – | – | Mean 52.1 year/F 224 | 4 weeks | Online survey | Long COVID |
| Karaarslan et al. 37 | 2021 | Prospective | Turkey | Hospitalized | Total 300/long COVID 216 | – | 4 | – | – | – | – | 31 | 45 | – | – | – | Mean 53 year/F 121 | 1 month | Telephone | Persistent symptoms |
| Klein et al. 38 | 2021 | Prospective | Israel | Mild | Total 103/long COVID 47 | – | 1 | 1 | 1 | – | – | – | 8 | – | – | – | Mean 35 years/F 39 | 6 months | Telephone | Long-lasting effect |
| Kozak et al. 39 | 2021 | Retrospective | Canada | All | Total 223/long COVID 62 | 19 | – | – | – | – | – | 3 | 10 | – | – | – | 49.1 years/F 38 | >90 days | In person | Long COVID |
| Leth et al. 40 | 2021 | Prospective | Denmark | Hospitalized | Total 49/long COVID 47 | 15 | 4 | 5 | 4 | – | – | 2 | 15 | – | – | – | Median 58 years/F 28 | Median 128 days | Telephone and in person | Persistent symptoms |
| Liang et al. 41 | 2020 | Prospective | China | Hospitalized | Total 76/long COVID – | – | 20 | – | – | – | – | – | – | – | – | – | 41.3 years/F 55 | 3 months | In person | Persistent symptoms |
| Lombardo et al. 42 | 2021 | Prospective | Italy | All | Total 303/long COVID 244 | 35 | – | – | – | – | – | – | – | – | – | – | Median 53 years/F 165 | Median 12.2 months | Phone | Long-term complication |
| Marasco et al. 43 | 2021 | Prospective | Multi-center | Hospitalized | Total 489/long COVID – | – | 37 | 47 | 41 | 67 | – | – | – | – | – | – | 50.6 years/F – | 30 days | Questionnaires | Persistent symptoms |
| Messin et al. 44 | 2021 | Retrospective | France | All | Total 74/long COVID 53 | – | 3 | – | – | – | – | – | 8 | – | – | – | 54.7 years/F30 | 6 months | Telephone | Persistent symptoms |
| Mohamed-Hussein et al. 45 | 2021 | Cross-sectional | Egypt | All | Total 262/long COVID 157 | 123 | – | – | – | – | – | – | – | – | – | – | – | 12 weeks | Clinic or phone | Long COVID |
| Noviello et al. 46 | 2021 | Prospective | Italy | All | Total 164/long COVID – | – | 29 | – | – | – | 43 | – | – | – | – | – | Median 44.1 years/F 66 | Median 4.8 months | Web based | Persistent symptoms |
| Rank et al. 47 | 2021 | Prospective | Germany | Mild | Total 83/long COVID 51 | – | – | – | – | – | – | – | 18 | – | – | – | – | –/– | Questionaries | Long-term symptoms |
| Rizvi et al. 48 | 2021 | Retrospective | USA | Hospitalized | Total 17,462/long COVID – | 404 | 214 | – | – | – | – | – | – | – | – | – | Median 66 years/F 336 | 6 months | In person | GI sequelae |
| Saigal et al. 49 | 2021 | Prospective | UK | Hospitalized | Total 643/long COVID – | 54 | – | – | – | – | – | – | – | – | – | – | 62.3 years/F 245 | Median 63 days | Virtual | Long COVID |
| Scherlinger et al. 50 | 2021 | Prospective | France | All | Total –/long COVID 30 | – | 9 | – | 3 | – | – | – | 3 | – | – | – | Median 40 years/F 18 | Median 152 days | In person | Long COVID |
| Shang et al. 51 | 2021 | Prospective | China | Severe | Total 796/long COVID 441 | 87 | – | – | – | – | – | – | – | – | – | – | – | 6 months | Phone | Sequelae of COVID |
| Shoosanglertwijit et al. 52 | 2021 | Prospective | Thailand | Hospitalized | Total –/long COVID 87 | 11 | – | 2 | – | 4 | – | – | – | – | – | 2 | –/– | 6 months | – | Postinfectious FGID |
| Salmon-Ceron et al. 53 | 2021 | Prospective | France | All | Total –/long COVID 70 | 17 | 12 | 3 | 6 | – | – | – | – | – | – | – | Median 45 years/F 55 | 2 months | In person | Prolonged COVID symptoms |
| Suárez-Robles et al. 54 | 2020 | Prospective | France | Hospitalized | Total –/long COVID 134 | – | – | – | – | – | – | 36 | 29 | – | – | – | 58.53 years/F72 | 3 months | Telephone | Residual symptoms |
| Taquet et al. 55 | 2021 | Retrospective | UK | All | Total 273,618/long COVID 155,962 | 42,630 | – | – | – | – | – | – | – | – | – | – | 46.3 years/F152157 | 6 months | – | Long COVID |
| Tiwari et al. 56 | 2021 | Cross-sectional | Nepal | Non- critical | Total 132/long COVID 66 | – | 1 | – | – | – | – | 9 | 1 | – | – | – | 36 years/F 28 | 2 months | Telephone or in person | Persistent symptoms |
| Tosato et al. 57 | 2021 | Cross-sectional | Italy | Hospitalized | Total 165/long COVID 137 | – | 32 | – | – | – | – | 63 | 53 | – | – | – | 73 years/F 53 | 25–109 days | – | Persistent symptoms |
| Vayner et al. 58 | 2021 | Abstract | USA | All | Total 90/ long COVID - | – | – | – | 2 | – | – | – | 10 | – | – | – | 49.5 years/– | 1 months | Telephone | Residual symptoms |
| Vélez et al. 59 | 2021 | Retrospective | USA | All | Total 200/long COVID – | 79 | – | – | – | – | 21 | – | – | – | – | 77 | 46 years/F 60 | 6 months | Telephone | Post-COVID syndrome |
| Weng et al. 60 | 2021 | Prospective | China | Hospitalized | Total 117/long COVID - | 52 | 17 | 8 | 21 | – | – | 28 | – | – | – | – | –/– | 90 days | Telephone | Long-term sequelae |
| Zhang et al. 61 | 2021 | Retrospective | China | All | Total 2433/long COVID 1095 | – | 18 | – | 5 | – | – | 20 | 35 | – | – | – | Median 60 years/ F 1228 | 1 year | Telephone | Postinfectious symptoms |
| Zhou et al. 62 | 2021 | Prospective | China | HCW | Total 15/long COVID 12 | – | 3 | – | – | – | – | 3 | – | – | – | – | Median 29 years/F 12 | 3 months | In person | Persistent symptoms |
F, females; GI, gastrointestinal; HCW, health-care workers; IBS, irritable bowel syndrome; LOA, loss of appetite; LOT, loss of taste.
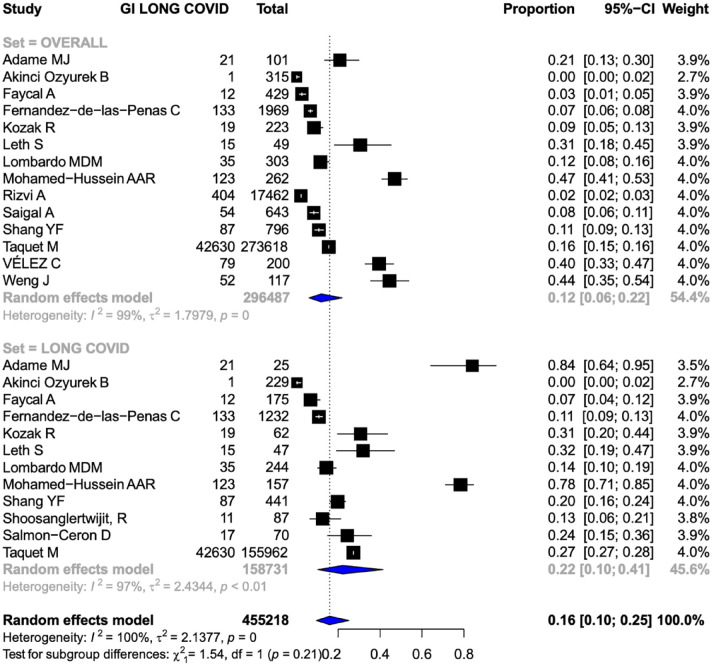
Frequency of GI long COVID
The overall frequency of GI symptoms was reported in 14 studies involving 296,487 patients.13,14,27,28,39,40,42,45,48,49,51,55,59,60 The frequency of overall GI symptoms in patients with COVID-19 was 0.12 [95% confidence interval (CI), 0.06–0.22, I2 = 99%] (Figure 2). The frequency of GI symptoms in long COVID was reported in 12 studies (158,731 patients).13,14,27,28,39,40,42,45,51,52,53,55 The frequency of GI symptoms in patients with long COVID was 0.22 (95% CI, 0.10–0.41, I2 = 97%) (Figure 2).
Figure 2.
Forest plots showing the pooled frequency of GI manifestations of long COVID in patients with COVID-19 (Upper Forest plot) and patients with long COVID (Lower Forest Plot). GI, gastrointestinal.
The frequency of GI long-COVID symptoms in patients with severe COVID-19 was 0.13 (95% CI, 0.04–0.34, I2 = 99%) (five studies, 19,067 patients)40,48,49,51,60 (Supplemental Figure 1). The frequency was 0.14 (95% CI, 0.05–0.34, I2 = 98%) in studies reporting mixed disease severity (severe as well as non-severe disease)13,14,39,42,45,55,59 (Supplemental Figure 1). Similarly, the frequency of GI manifestations in patients having long COVID after severe COVID infection was 0.20 (95% CI, 0.12–0.32, I2 = 71%)40,51,52 (Supplemental Figure 2). The frequency of long GI COVID in patients having long COVID after mixed severe disease was 0.21 (95% CI, 0.05–0.56, I2 = 97%)14,39,42,45,53,55 (Supplemental Figure 2).
We also assessed the frequency of GI manifestations as per the different definitions of long COVID. The frequency of GI manifestations in patients with COVID-19 as per WHO definition was 0.18 (95% CI, 0.08–0.36, I2 = 99%)39,40,42,45,48,51,59,60 (Supplemental Figure 3). The frequency of GI manifestations in patients with long COVID as per WHO definition was 0.28 (95% CI, 0.15–0.46, I2 = 97%)39,40,42,45,51,52,55 (Supplemental Figure 3). The frequency of GI manifestations in patients with COVID-19 as per NICE/CDC definition was 0.14 (95% CI, 0.06–0.28, I2 = 99%)13,14,39,40,42,45,48,49,51,59,60 (Supplemental Figure 4). The frequency of GI manifestations in patients with long COVID as per NICE/CDC definition was 0.26 (95% CI, 0.11–0.50, I2 = 96%)13,14,39,40,42,45,51,52,53,55 (Supplemental Figure 4).
Clinical manifestations of long COVID
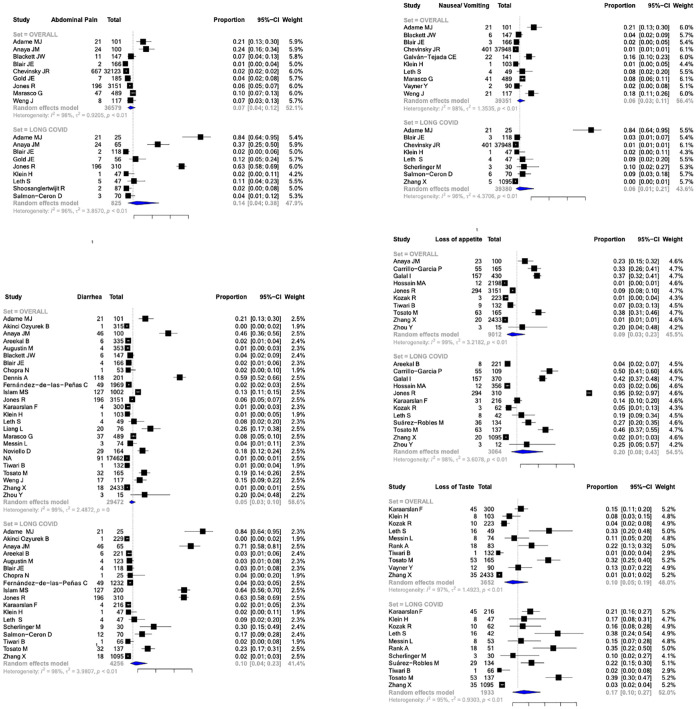
The frequency of abdominal pain as a part of long GI COVID was 0.07 (95% CI, 0.04–0.12, I2 = 98%) in patients with COVID-19 infection while it was 0.14 (95% CI, 0.04–0.38, I2 = 96%) in patients having long COVID13,15,18,19,23,32,36,38,40,43,52,53,60 (Figure 3). The frequency of nausea/vomiting as a part of long GI COVID was 0.06 (95% CI, 0.03–0.11, I2 = 98%) in patients with COVID-19 infection while it was 0.06 (95% CI, 0.01–0.21, I2 = 96%) in patients having long COVID13,18,19,23,30,38,40,43,50,53,58,60,61 (Figure 3). The frequency of loss of appetite as a part of long GI COVID was 0.09 (95% CI, 0.03–0.23, I2 = 99%) in patients with COVID-19 infection while it was 0.20 (95% CI, 0.08–0.43, I2 = 98%) in patients having long COVID.15,16,22,29,33,36,37,39,40,54,57,61,62 The frequency of loss of taste as a part of long GI COVID was 0.10 (95% CI, 0.05–0.19, I2 = 97%) in patients with COVID-19 infection while it was 0.17 (95% CI, 0.10–0.27, I2 = 95%) in patients having long COVID37–40,44,47,50,54,56,57,58,61 (Figure 3). The frequency of diarrhea as a part of long GI COVID was 0.05 (95% CI, 0.03–0.10, I2 = 99%) in patients with COVID-19 infection while it was 0.10 (95% CI, 0.04–0.23, I2 = 98%) in patients having long COVID13–19,24,25,28,34,36–38,40,41,43,44,46,50,53,56,57,60,61,62 (Figure 3).
Figure 3.
Forest plots depicting the pooled frequencies of various GI symptoms (abdomen pain, diarrhea, nausea/vomiting, loss of appetite, loss of taste) in COVID 19 and long COVID. GI, gastrointestinal.
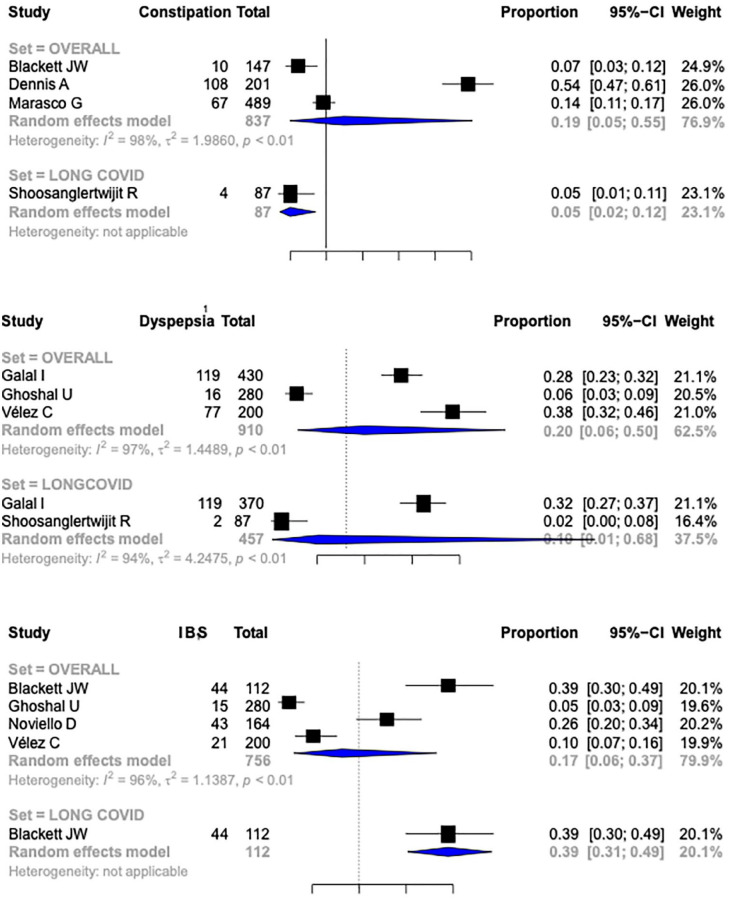
The frequency of constipation as a part of long GI COVID was 0.19 (95% CI, 0.05–0.55, I2 = 98%) in patients with COVID-19 infection (Figure 4). There was only one study reporting frequency of constipation in patients with long COVID.18,25,43,52 The frequency of dyspepsia as a GI manifestation of long COVID-19 was reported by three studies (910 patients of COVID-19).29,31,59 The frequency of dyspepsia after long COVID was 0.20 (95% CI, 0.06–0.50, I2 = 97%)29,52 (Figure 4). Only two studies reported frequency of dyspepsia in long COVID. Four studies (756 patients) reported the frequency of irritable bowel syndrome (IBS) after COVID-19 infection.18,31,46,59 The pooled rate of IBS after COVID-19 was 0.17 (95% CI, 0.06–0.37, I2 = 96%) (Figure 4). Only one study reported the frequency of IBS among patients with long COVID. 18 Only a few studies reported GI symptoms at multiple time points during the follow-up (Supplemental Table 3).
Figure 4.
Forest plots depicting the pooled frequencies of various GI manifestations (constipation, dyspepsia, and irritable bowel syndrome) in COVID 19 and long COVID. GI, gastrointestinal.
A single study reported about 12 patients (11 males) showing cholangiopathic changes as delayed manifestation in patients who had recovered from severe COVID. 26 This was characterized by both biochemical abnormality (elevated alkaline phosphatase) and radiological abnormality (changes in Magnetic resonance cholangiopancreatography). Another report suggested the presence of gastroparesis in 12 patients as confirmed by a positive gastric-emptying study done for suggestive symptoms. 21
Heterogeneity
Since there was significant heterogeneity in overall estimates of GI manifestations in long COVID. We performed multiple subgroup analysis. Based on the duration of follow-up (<3 months, >3 months), there were no significant differences in frequency of GI symptoms between these two groups. The frequency of GI symptoms in studies reporting a follow-up of less than 3 months was 0.11 (95% CI, 0.02–0.38, I2 = 98%) while for studies with more than 3 months of follow-up it was 0.12 (95% CI, 0.06–0.22, I2 = 100%)13,14,27,28,39,40,42,45,48,49,51,55,59,60 (Supplemental Figure 5). Subgroup analysis on the basis of mode of follow-up suggested a slightly higher frequency of symptoms with telephonic follow-up [0.14 (95% CI, 0.06–0.31, I2 = 98%)] as compared to in person follow-up [0.04 (95% CI, 0.01–0.19, I2 = 98%)]13,14,27,28,39,40,42,45,48,49,51,55,59,60 (Supplemental Figure 6). The frequency of GI symptoms in America was 0.12 (95% CI, 0.03–0.36, I2 = 99%), in Europe was 0.07 (95% CI, 0.03–0.17, I2 = 97%) whereas only two studies reported frequencies from Asia and one from Africa (Supplemental Figure 7).13,14,27,28,39,40,42,45,48,49,51,55,59,60 On the basis of study type, the frequency of GI symptoms was 0.08 (95% CI, 0.02–0.26, I2 = 100%) for retrospective studies while it was 0.12 (95% CI, 0.06–0.24, I2 = 97%) for prospective studies13,14,27,28,39,40,42,45,48,49,51,55,59,60 (Supplemental Figure 8).
Risk of bias analysis
Few studies had concern regarding description of the selected sample with lack of clear details. Most of the studies had included appropriate statistical analysis and appropriate methods of assessment of GI symptoms as well as long COVID (Supplemental Table 4). Few studies did not clearly report the demographic information at the presenting site. On the contrary, almost all studies reported an appropriate sample frame to address the target population. As the Joanna Briggs Institute guidance suggests against using a score cutoff for quality assessment, we also did not score the studies. The visual impression of the funnel plots (Supplemental Figures 9 and 10) and the Egger test did not suggest any publication bias. The t statistic for the overall COVID analysis and long-COVID analysis was −0.78 (p = 0.45210 and −1.05 (p = 0.3230).
Discussion
While the clinical manifestations of acute COVID-19 are in the form of a systemic disease with pulmonary and extrapulmonary manifestations, the long-COVID syndrome has largely been described to have systemic, neuropsychiatric, pulmonary, and cardiac manifestations. 63 In fact, initially the manifestations of long COVID including brain fog were met with a denial but now the syndrome is well recognized thanks to advocacy by the patients.64–66 However, the understanding of the entire spectrum of manifestations of long COVID is evolving. Multiple reports have ascribed various GI manifestations to long COVID; however, a systematic assessment of the GI manifestations and frequency has not been previously reported. In this systematic review of 50 studies, we found that loss of taste, loss of appetite, abdominal pain, nausea and vomiting, and diarrhea were encountered in a subset of patients while constipation was one of the most common manifestations. Overall, GI symptoms as part of long COVID occurred in around 12% of patients with acute COVID-19. Further, a significant number of patients developed dyspepsia and irritable bowel syndrome as a sequelae to COVID-19. These findings would suggest that GI symptoms are an important accompaniment of long COVID. Our analysis suggests that the GI manifestations of long COVID are not related to severity of underlying COVID-19 and could occur in those with mild initial disease.
The mechanisms behind the GI manifestations occurring as a part of post-COVID syndrome are not completely understood. The manifestations during acute COVID-19 are believed to be related to the increased expression of ACE-2 expression on the small bowel mucosa which may result in intestinal infection by the virus. Prolonged shedding of virions from the GI tract is recognized and could be responsible for some of the GI manifestations of long COVID. 67 Interestingly, presence of coronavirus-like particles has been reported long back in patients with tropical sprue and the diarrhea was explained by enterocyte damage caused by the virus. 68 It would be worthwhile to evaluate whether patients with diarrhea and IBS-like presentation after COVID-19 have enterocyte damage resulting from SARS-CoV-2 infection. Postinfectious IBS is a well-recognized condition and the occurrence of IBS after COVID-19 may also be similar to this variant of IBS. 69
Gut microbiome profile might also have a role in long-term complications of COVID-19. The susceptibility of the microbiota to viral antigens as well as pro-inflammatory cytokines might have a crucial role in long GI manifestations. Patients with post-COVID-19 syndrome were found to have increased levels of Ruminococcus gnavus and Bacteroides vulgatus and decreased levels of Faecalibacterium prausnitzii. 70 The same study also showed gut dysbiosis to have a role in neuropsychiatric as well as respiratory symptoms of post-acute COVID syndrome. However, it is unclear if the changes in gut microbiota play a role in causation of GI manifestations of long COVID. Also, the role of manipulation of gut microbiota profile in prevention or management of post-COVID GI manifestations is unclear.
The strengths of the study include we compiled all the data available on prevalence and symptomatology of GI long COVID for guiding clinicians in the pandemic. This would help define the contours of this new entity. We also compared the long-term outcomes after severe COVID-19 as compared to non-severe disease. There are certain limitations to the study. First, most of the included studies were retrospective. Second, the impact of various strains of SARS-CoV-2 on long COVID could not be analyzed as there were no strain-specific studies. Third, the symptoms were mainly subjective in patients with COVID-19 infection on follow-up. Further, a quantitative analysis could not be done for some symptoms (like constipation) because of the small number of reports. Also, most of the analyses demonstrated significant heterogeneity and although the subgroup analyses were performed, the heterogeneity was still significant.
Conclusion
In the present systematic review, GI symptoms as part of long COVID were seen in 12% of patients with acute COVID and 22% of long COVID. Loss of appetite, dyspepsia, irritable bowel syndrome, loss of taste, and abdominal pain were the five most common GI symptoms of long COVID. The odds of having GI manifestations of long COVID among patients with severe versus non-severe disease were not statistically different. Future studies should look at the societal impact, prevention, and treatment of long COVID including the GI manifestations.
Supplemental Material
Supplemental material, sj-docx-1-tag-10.1177_17562848221118403 for Gastrointestinal manifestations of long COVID: A systematic review and meta-analysis by Arup Choudhury, Raseen Tariq, Anuraag Jena, Elissa Kinzelman Vesely, Siddharth Singh, Sahil Khanna and Vishal Sharma in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-2-tag-10.1177_17562848221118403 for Gastrointestinal manifestations of long COVID: A systematic review and meta-analysis by Arup Choudhury, Raseen Tariq, Anuraag Jena, Elissa Kinzelman Vesely, Siddharth Singh, Sahil Khanna and Vishal Sharma in Therapeutic Advances in Gastroenterology
Acknowledgments
None.
Footnotes
ORCID iDs: Sahil Khanna  https://orcid.org/0000-0002-7619-8338
https://orcid.org/0000-0002-7619-8338
Vishal Sharma  https://orcid.org/0000-0003-2472-3409
https://orcid.org/0000-0003-2472-3409
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Arup Choudhury, Department of Gastroenterology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Raseen Tariq, Division of Gastroenterology, Department of Medicine, Mayo Clinic, Rochester, MN, USA.
Anuraag Jena, Department of Gastroenterology, Postgraduate Institute of Medical Education and Research, Chandigarh, India.
Elissa Kinzelman Vesely, Mayo Clinic, Rochester, MN, USA.
Siddharth Singh, Division of Gastroenterology and Biomedical Informatics, University of California San Diego, La Jolla, CA, USA.
Sahil Khanna, Division of Gastroenterology, Department of Medicine, Mayo Clinic, Rochester, MN, USA.
Vishal Sharma, Department of Gastroenterology, Postgraduate Institute of Medical Education and Research, Sector 12, Chandigarh 160012, India.
Declarations
Ethics approval and consent to participate: Not applicable because this is a systematic review of already published literature and no patients were recruited for this work.
Consent for publication: Not applicable.
Author contribution(s): Arup Choudhury: Data curation; Validation; Writing – original draft.
Raseen Tariq: Data curation; Validation; Writing – original draft.
Anuraag Jena: Validation; Writing – review & editing.
Elissa Kinzelman Vesely: Data curation.
Siddharth Singh: Validation; Writing – review & editing.
Sahil Khanna: Conceptualization; Supervision; Validation; Writing – review & editing.
Vishal Sharma: Conceptualization; formal analysis; Methodology; Supervision; Writing – original draft; Writing – review & editing.
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: The data used for the systematic review is from previously published works and is publicly available.
References
- 1. Nabavi N. Long covid: how to define it and how to manage it. BMJ 2020; 370: m3489. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization. A clinical case definition of post COVID-19 condition by a Delphi consensus, 6 October 2021, https://www.who.int/publications/i/item/WHO-2019-nCoV-Post_COVID-19_condition-Clinical_case_definition-2021.1 (2021, accessed 23 March 2022).
- 3. CDC. Long COVID or post-COVID conditions, https://www.cdc.gov/coronavirus/2019-ncov/long-term-effects/index.html (2021, accessed 23 March 2022).
- 4. Cheung KS, Hung IFN, Chan PPY, et al. Gastrointestinal manifestations of SARS-CoV-2 infection and virus load in fecal samples from a Hong Kong cohort: systematic review and meta-analysis. Gastroenterology 2020; 159: 81–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kumar-M P, Mishra S, Jha DK, et al. Coronavirus disease (COVID-19) and the liver: a comprehensive systematic review and meta-analysis. Hepatol Int 2020; 14: 711–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ni W, Yang X, Yang D, et al. Role of angiotensin-converting enzyme 2 (ACE2) in COVID-19. Crit Care 2020; 24: 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021; 372: n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Venkatesan P. NICE guideline on long COVID. Lancet Respir Med 2021; 9: 129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Balduzzi S, Rücker G, Schwarzer G. How to perform a meta-analysis with R: a practical tutorial. Evid Based Ment Health 2019; 22: 153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suurmond R, van Rhee H, Hak T. Introduction, comparison, and validation of meta-essentials: a free and simple tool for meta-analysis. Res Synth Methods 2017; 8: 537–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kuritz SJ, Landis JR, Koch GG. A general overview of Mantel-Haenszel methods: applications and recent developments. Annu Rev Public Health 1988; 9: 123–160. [DOI] [PubMed] [Google Scholar]
- 12. Munn Z, Moola S, Lisy K, et al. Methodological guidance for systematic reviews of observational epidemiological studies reporting prevalence and cumulative incidence data. Int J Evid Based Healthc 2015; 13: 147–153. [DOI] [PubMed] [Google Scholar]
- 13. Adame MJ, Nadda KM, Seashore J. Late sequelae of COVID-19 infection in patients without comorbidities. Am J Respir Crit Care Med 2021; 203: A3821. [Google Scholar]
- 14. Akinci Ozyurek B, Sahin Ozdemirel T, Akkurt ES, et al. What are the factors that affect post COVID 1st month’s continuing symptoms? Int J Clin Pract 2021; 75: e14778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Anaya JM, Rojas M, Salinas ML, et al. Post-COVID syndrome. A case series and comprehensive review. Autoimmun Rev 2021; 20: 102947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Areekal B, Sukumaran ST, Andrews AM, et al. Persistence of symptoms after acute COVID-19 infection-an experience from a tertiary care centre in South India. J Clin Diagn Res 2021; 15: LC05–LC08. [Google Scholar]
- 17. Augustin M, Schommers P, Stecher M, et al. Post-COVID syndrome in non-hospitalised patients with COVID-19: a longitudinal prospective cohort study. Lancet Reg Health Eur 2021; 6: 100122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Blackett JW, Li J, Jodorkovsky D, et al. Prevalence and risk factors for gastrointestinal symptoms after recovery from COVID-19. Neurogastroenterol Motil 2022; 34: e14251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blair JE, Gotimukul A, Wang F, et al. Mild to moderate COVID-19 illness in adult outpatients: characteristics, symptoms, and outcomes in the first 4 weeks of illness. Medicine (Baltimore) 2021; 100: e26371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Buttery S, Philip KEJ, Williams P, et al. Patient symptoms and experience following COVID-19: results from a UK-wide survey. BMJ Open Respir Res 2021; 8: e001075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Calogero C, Chauhan K, Chalikonda, et al. S3282 evaluation of delayed gastric emptying in patients with a prior SARS-CoV-2 infection: a single center review. Am J Gastroenterol 2021; 116: S1352. [Google Scholar]
- 22. Carrillo-Garcia P, Garmendia-Prieto B, Cristofori G, et al. Health status in survivors older than 70 years after hospitalization with COVID-19: observational follow-up study at 3 months. Eur Geriatr Med 2021; 12: 1091–1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chevinsky JR, Tao G, Lavery AM, et al. Late conditions diagnosed 1-4 months following an initial coronavirus disease 2019 (COVID-19) encounter: a matched-cohort study using inpatient and outpatient administrative data-United States, 1 March-30 June 2020. Clin Infect Dis 2021; 73(Suppl. 1): S5–S16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Chopra N, Chowdhury M, Singh AK, et al. Clinical predictors of long COVID-19 and phenotypes of mild COVID-19 at a tertiary care centre in India. Drug Discov Ther 2021; 15: 156–161. [DOI] [PubMed] [Google Scholar]
- 25. Dennis A, Wamil M, Alberts J, et al. Multiorgan impairment in low-risk individuals with post-COVID-19 syndrome: a prospective, community-based study. BMJ Open 2021; 11: e048391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Faruqui S, Okoli FC, Olsen SK, et al. Cholangiopathy after severe COVID-19: clinical features and prognostic implications. Am J Gastroenterol 2021; 116: 1414–1425. [DOI] [PubMed] [Google Scholar]
- 27. Faycal A, Ndoadoumgue AL, Sellem B, et al. Prevalence and factors associated with symptom persistence: a prospective study of 429 mild COVID-19 outpatients. Infect Dis Now 2022; 52: 75–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fernández-de-Las-Peñas C, Martín-Guerrero J, Navarro-Pardo E, et al. Gastrointestinal symptoms at the acute COVID-19 phase are risk factors for developing gastrointestinal post-COVID symptoms: a multicenter study. Intern Emerg Med 2021; 17: 583–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Galal I, Hussein AARM, Amin MT, et al. Determinants of persistent post-COVID-19 symptoms: value of a novel COVID-19 symptom score. Egypt J Bronchology 2021; 15: 10. [Google Scholar]
- 30. Galván-Tejada CE, Herrera-García CF, Godina-González S, et al. Persistence of COVID-19 symptoms after recovery in Mexican population. Int J Environ Res Public Health 2020; 17: 9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ghoshal UC, Ghoshal U, Rahman MM, et al. Post-infection functional gastrointestinal disorders following coronavirus disease-19: a case-control study. J Gastroenterol Hepatol 2022; 37: 489–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gold JE, Okyay RA, Licht WE, et al. Investigation of long COVID prevalence and its relationship to Epstein-Barr virus reactivation. Pathogens 2021; 10: 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hossain MA, Hossain KMA, Saunders K, et al. Prevalence of long COVID symptoms in Bangladesh: a prospective inception cohort study of COVID-19 survivors. BMJ Glob Health 2021; 6: e006838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Islam MS, Ferdous MZ, Islam US, et al. Treatment, persistent symptoms, and depression in people infected with COVID-19 in Bangladesh. Int J Environ Res Public Health 2021; 18: 1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nayagam JS, Jeyaraj R, Mitchell T, et al. Patterns and prediction of liver injury with persistent cholestasis in survivors of severe SARS-CoV-2 infection. J Infect 2021; 82: e11–e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Jones R, Davis A, Stanley B, et al. Risk predictors and symptom features of long COVID within a broad primary care patient population including both tested and untested patients. Pragmat Obs Res 2021; 12: 93–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Karaarslan F, Demircioğlu Güneri F, Kardeş S. Postdischarge rheumatic and musculoskeletal symptoms following hospitalization for COVID-19: prospective follow-up by phone interviews. Rheumatol Int 2021; 41: 1263–1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Klein H, Asseo K, Karni N, et al. Onset, duration and unresolved symptoms, including smell and taste changes, in mild COVID-19 infection: a cohort study in Israeli patients. Clin Microbiol Infect 2021; 27: 769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kozak R, Armstrong SM, Salvant E, et al. Recognition of long-COVID-19 patients in a Canadian tertiary hospital setting: a retrospective analysis of their clinical and laboratory characteristics. Pathogens 2021; 10: 1246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Leth S, Gunst JD, Mathiasen V, et al. Persistent symptoms in patients recovering from COVID-19 in Denmark. Open Forum Infect Dis 2021; 8: ofab042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Liang L, Yang B, Jiang N, et al. Three-month follow-up study of survivors of coronavirus disease 2019 after discharge. J Korean Med Sci 2020; 35: e418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lombardo MDM, Foppiani A, Peretti GM, et al. Long-term coronavirus disease 2019 complications in inpatients and outpatients: a one-year follow-up cohort study. Open Forum Infect Dis 2021; 8: ofab384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Marasco G, Cremon C, Barbaro MR, et al. Prevalence of gastrointestinal symptoms in severe acute respiratory syndrome coronavirus 2 infection: results of the prospective controlled multinational GI-COVID-19 study. Am J Gastroenterol 2022; 117: 147–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Messin L, Puyraveau M, Benabdallah Y, et al. COVEVOL: natural evolution at 6 months of COVID-19. Viruses 2021; 13: 2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mohamed-Hussein AAR, Amin MT, Makhlouf HA, et al. Non-hospitalised COVID-19 patients have more frequent long COVID-19 symptoms. Int J Tuberc Lung Dis 2021; 25: 732–737. [DOI] [PubMed] [Google Scholar]
- 46. Noviello D, Costantino A, Muscatello A, et al. Functional gastrointestinal and somatoform symptoms five months after SARS-CoV-2 infection: a controlled cohort study. Neurogastroenterol Motil 2022; 34: e14187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Rank A, Tzortzini A, Kling E, et al. One year after mild COVID-19: the majority of patients maintain specific immunity, but one in four still suffer from long-term symptoms. J Clin Med 2021; 10: 3305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Rizvi A, Patel Z, Liu Y, et al. Gastrointestinal sequelae 3 and 6 months after hospitalization for coronavirus disease 2019. Clin Gastroenterol Hepatol 2021; 19: 2438–2440.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Saigal A, Naidu S, Shah A, et al. S54 ‘Long-COVID’: the need for multi-disciplinary working. Thorax 2021; 76: A33–A34. [Google Scholar]
- 50. Scherlinger M, Felten R, Gallais F, et al. Refining “Long-COVID” by a prospective multimodal evaluation of patients with long-term symptoms attributed to SARS-CoV-2 infection. Infect Dis Ther 2021; 10: 1747–1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Shang YF, Liu T, Yu JN, et al. Half-year follow-up of patients recovering from severe COVID-19: analysis of symptoms and their risk factors. J Intern Med 2021; 290: 444–450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Shoosanglertwijit R, Pitisuttithum P, Patcharatrakul T, et al. Predictors of viral shedding in fecal samples among Coronavirus disease 2019 (COVID-19) patients and subsequent functional gastrointestinal disorders (FGIDs). J Gastroenterol Hepatol 2021; 36: 67–68. [Google Scholar]
- 53. Salmon-Ceron D, Slama D, De Broucker T, et al. Clinical, virological and imaging profile in patients with prolonged forms of COVID-19: a cross-sectional study. J Infect 2021; 82: e1–e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Suárez-Robles M, Iguaran-Bermúdez MDR, García-Klepizg JL, et al. Ninety days post-hospitalization evaluation of residual COVID-19 symptoms through a phone call checklist. Pan Afr Med J 2020; 37: 289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Taquet M, Dercon Q, Luciano S, et al. Incidence, co-occurrence, and evolution of long-COVID features: a 6-month retrospective cohort study of 273,618 survivors of COVID-19. PLoS Med 2021; 18: e1003773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tiwari B, Ghimire M, Bhatta G, et al. Persistent symptoms in non-critical COVID-19 patients at two months follow-up in a district hospital: a descriptive cross-sectional study. JNMA J Nepal Med Assoc 2021; 59: 550–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Tosato M, Carfì A, Martis I, et al. Prevalence and predictors of persistence of COVID-19 symptoms in older adults: a single-center study. J Am Med Dir Assoc 2021; 22: 1840–1844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vayner Y, Lessen S, Shah R, et al. Residual symptom burden in adult COVID-19 survivors at one, three, and six months after COVID-19 illness. Am J Respir Crit Care Med 2021; 203: A3851. [Google Scholar]
- 59. Vélez C, Paz M, Silvernale C, et al. Factors associated with chronic de novo post-coronavirus disease gastrointestinal disorders in a metropolitan US county. Clin Gastroenterol Hepatol 2022; 20: e1488–e1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Weng J, Li Y, Li J, et al. Gastrointestinal sequelae 90 days after discharge for COVID-19. Lancet Gastroenterol Hepatol 2021; 6: 344–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Zhang X, Wang F, Shen Y, et al. Symptoms and health outcomes among survivors of COVID-19 infection 1 year after discharge from hospitals in Wuhan, China. JAMA Netw Open 2021; 4: e2127403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Zhou Y, Zhang J, Zhang D, et al. Linking the gut microbiota to persistent symptoms in survivors of COVID-19 after discharge. J Microbiol 2021; 59: 941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Mehandru S, Merad M. Pathological sequelae of long-haul COVID. Nat Immunol 2022; 23: 194–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Alwan NA. The teachings of Long COVID. Commun Med 2021; 1: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. McCorkell L, Assaf GS, Davis HE, et al. Patient-led research collaborative: embedding patients in the Long COVID narrative. Pain Rep 2021; 6: e913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Callard F, Perego E. How and why patients made Long Covid. Soc Sci Med 2021; 268: 113426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Wu Y, Guo C, Tang L, et al. Prolonged presence of SARS-CoV-2 viral RNA in faecal samples. Lancet Gastroenterol Hepatol 2020; 5: 434–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Baker SJ, Mathan M, Mathan VI, et al. Chronic enterocyte infection with coronavirus. One possible cause of the syndrome of tropical sprue? Dig Dis Sci 1982; 27: 1039–1043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Settanni CR, Ianiro G, Ponziani FR, et al. COVID-19 as a trigger of irritable bowel syndrome: a review of potential mechanisms. World J Gastroenterol 2021; 27: 7433–7445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Liu Q, Mak JWY, Su Q, et al. Gut microbiota dynamics in a prospective cohort of patients with post-acute COVID-19 syndrome. Gut 2022; 71: 544–552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tag-10.1177_17562848221118403 for Gastrointestinal manifestations of long COVID: A systematic review and meta-analysis by Arup Choudhury, Raseen Tariq, Anuraag Jena, Elissa Kinzelman Vesely, Siddharth Singh, Sahil Khanna and Vishal Sharma in Therapeutic Advances in Gastroenterology
Supplemental material, sj-docx-2-tag-10.1177_17562848221118403 for Gastrointestinal manifestations of long COVID: A systematic review and meta-analysis by Arup Choudhury, Raseen Tariq, Anuraag Jena, Elissa Kinzelman Vesely, Siddharth Singh, Sahil Khanna and Vishal Sharma in Therapeutic Advances in Gastroenterology