Abstract
Study Design:
Cross-sectional internet survey of people living with degenerative cervical myelopathy.
Objective:
The purpose of this study was to quantify pain distribution, severity, and interference in persons with degenerative cervical myelopathy.
Methods:
Eighty-two participants with degenerative cervical myelopathy were recruited for this internet survey. This survey utilized the Michigan Body Map and brief pain inventory (BPI) to assess anatomical distribution and severity of pain as well as the patient derived modified Japanese Orthopedic Association scale (p-mJOA) for myelopathic severity and SF-36 for measures of health-related quality of life. Internal consistency was evaluated with Cronbach’s alpha. Pearson’s correlations were assessed with p-mJOA and SF-36. Multivariate analysis of variance was used to determine if history of prior surgery or concomitant pain diagnosis impacted experience of pain.
Results:
Michigan body map distribution and brief pain inventory severity and interference were correlated with p-mJOA and SF-36 scores (p < 0.05). Pain was moderate to severe in 78% of participants. Pain was commonly widespread. Pain scales were sufficiently internally consistent (α > 0.9). History of surgery or other pain diagnosis did not impact experience of pain in myelopathy.
Conclusions:
Pain is commonly identifiable in large areas of the body, is frequently moderate to severe in intensity and impacts quality of life and severity of myelopathy in a cohort of individuals with myelopathy who have pain.
Keywords: cervical, myelopathy, spondylosis, spondylotic, stenosis, disc herniation, ossification posterior longitudinal ligament, degeneration, disability, recovery, questionnaire, pain
Introduction
Degenerative Cervical Myelopathy [DCM] arises when arthritic changes in the cervical spine, compress and injure the spinal cord1,2 causing a slow-motion spinal cord injury. This can cause a range of neurological symptoms, including loss of digital dexterity, balance, sensation, bladder or bowel function but also pain which may represent central neuropathic pain due to spinal cord injury (neuropathic pain is pain arising as a direct consequence of a lesion or disease affecting the somatosensory system 3 in combination with nociceptive pain due to cervical spondylosis which is recognized in ICD-11 as a chronic secondary pain syndrome labeled Chronic secondary musculoskeletal pain. 4
Pain is now a pressing research priority in DCM: our survey of 659 persons with degenerative cervical myelopathy (PwCM), identified that pain was their number 1 recovery priority, independent of baseline functional status. 5 Despite this, pain has been infrequently measured in clinical studies of DCM, and where it has, typically only in reference to the neck and/or arm.6,7 Neck and/or arm pain is reported to affect 30-50% of persons with degenerative cervical myelopathy (PwCM).8-10 While surgical management will typically reduce pain overall in DCM, 11 pain does not diminish in all individuals following surgery. 12 Moreover, in patients with mild DCM, pain was associated with a more severe phenotype. 13
This focus on neck and arm pain may stem from the overlap of DCM with cervical spondylosis (the etiology of DCM) and potential for coexistent radicular nerve root entrapment which causes peripheral neuropathic pain. These conditions, which more often occur without myelopathy, are a common source of neck and arm pain. Additionally, PwCM commonly have other explanations for pain, such as degenerative disease in other joints or regions of the spine 14 or primary chronic pain disorders such as fibromyalgia.15,16 Consequently in its purist form, DCM has often been considered by some a painless condition.
However, the experience in traumatic spinal cord injury [SCI], a close relative of DCM,17-19 should indicate that this is an oversimplification. SCI has long been known to trigger central sensitization due to spinal cord injury with pain experienced in remote anatomical areas 20 resulting in a complex whole body experience of pain in people and animal models. 21 Thus, due to an overlapping, though not identical, pathophysiology of dysfunction and neuropathic pain between SCI and DCM,17-19 some PwCM may likewise have similar expressions of a complex whole body experience of neuropathic pain.
To our knowledge, the broader experience of pain in DCM is uncharacterized. This is clearly important to inform its effective measurement 22 and investigation. To that end, the aim of this paper was to determine the anatomical distribution, severity, impairment due to pain, and neuropathic quality and its impact on quality of life in PwCM who have a painful phenotype. We hypothesized that pain would be spread beyond the neck and upper extremities due to a spinal cord injury related neuropathic component with a consequent impact on function and health-related quality of life. Quality-of-life studies indicate that in addition to the obvious pain-related suffering experienced by patients, neuropathic pain is associated with depression, disordered sleep, and impairments in physical function. 23
Methods
Participants
A survey was developed with input from Pain physicians and piloted among PwCM. The survey was constructed using Survey Monkey (California, USA). On entry into the survey, participants were presented with a description of DCM and asked to confirm their diagnosis (if they answered “no,” survey was discontinued). Patient Reported Outcome Measures [PROMs] were selected to measure each Initiative on Methods, Measurement, and Pain Assessments in Clinical Trials (IMMPACT) domain, 24 including Brief Pain Inventory (BPI) to determine pain intensity and interference, Neck Disability Index and SF-36 to determine the effect of DCM on patient experience, and Michigan Body Map (MBM) to determine its location. The Douleur Neuropathique 4 (DN4) was additionally used to interpret presence of neuropathic pain quality. Demographics including disease severity as measured using the self-reported modified Japanese Orthopaedic Association score [p-mJOA], 25 age, biological sex, length of time with DCM, time since onset of pain, and presence of other pain diagnoses.
Informed consent was sought prior to entry into an open, online survey. Participation was voluntary and advertised using Myelopathy.org, an international non-profit organization dedicated to promoting understanding and awareness of DCM, to help patients, professionals and supporters. This included 2 email calls to the Myelopathy.org Community, and a number of posts through Myelopathy Support, its peer-to-peer support group. Recruitment ran from January to September 2018. Repeat entries from the same IP address were blocked. Ethical approval (application number HBREC.2016.15) was granted by Human Biology Research Ethics Committee of the University of Cambridge, Cambridge, United Kingdom.
Inclusion Criteria and Missing Data
Of visitors with unique IP addresses, n = 124 completed the question on diagnosis of myelopathy. Of these 3 were excluded answering “no.” Of these 121, n = 82 (67.77%) completed p-mJOA scores and were subsequently analyzed. There was no difference in p-mJOA scores between participants who completed the p-mJOA and subsequently discontinued the survey and those who completed the whole survey (T80 = 0.11, P = .91). For correlational analyses, all participants who completed both respective questionnaires were analyzed. For multivariate analyses, only those who completed the whole survey and had no missing data (n = 63) were analyzed. Furthermore, as respondents in our cohort may have other pain diagnoses occurring simultaneously, we meticulously examined the MBM responses from all 35 anatomical regions along with self-reported other pain diagnoses (i.e. shoulder tendinopathy, etc.) and responses to the DN4 to determine whether the respondents’ pain may be related to a condition other than myelopathy. For example, if a respondent hypothetically had a concomitant unilateral extremity pain generator, but a bilateral and neuropathic pain response the respondent was included. Finally, a secondary analysis was performed excluding all individuals who reported any other pain generator regardless if their pain was neuropathic in quality or present in remote locations to the pain generator. These secondary findings were compared to the initial findings. We elected to perform these as separate analyses to not introduce any other potential bias by excluding individuals who may have another painful diagnosis.
Statistical Analysis
Statistical analyses were conducted in SPSS Version 27 (IBM, INC). The primary variables of interest were MBM and BPI severity and BPI interference. Secondary variables of interest were p-mJOA, NDI, SF-36 subscales, age, biological sex, and duration of pain and disease. Descriptive statistics were calculated for each variable. Additionally, percentages of participants reporting neck pain, head pain, trunk pain outside of the neck and head, upper extremity pain, and lower extremity pain were calculated from the MBM data. Percentages of participants categorized as mild, moderate, and severe pain respectively were calculated from the BPI Severity subscale. Internal consistency was determined for MBM, BPI severity, and BPI interference based on Cronbach’s alpha. A Multivariate analysis of variance was used to determine if any measures were different between participants who self-reported other additional pain diagnoses and/or prior history of surgery for DCM. Correlations between measures were assessed using Pearson’s correlations. Alpha was set at p < .05 a priori.
Results
Overall cohort demographics and their perception of pain are summarized in Table 1: Participants were more frequently female (72%) and had undergone surgery (76%). On average, pain had been experienced for ∼8 years and was most commonly located in the upper extremity although also in the trunk and in the neck. Below neck pain, defined as pain in the trunk or either lower extremity, was also a frequent finding (∼88%). Likewise, pain was commonly identified bilaterally in the upper (∼82%) and lower (∼69%) extremities. Pain was frequently (∼78%) moderate-severe as measured using the BPI criteria. Clinically positive neuropathic pain, defined as a DN4 >4, was present in ∼73% of respondents. The MBM(α = 0.90), BPI severity(α = 0.90), and BPI interference(α = 0.92) were all sufficiently, internally consistent. MBM was positively correlated with BPI severity (r = .48, P < .001) and BPI interference (r = .49, P < .001). BPI severity and interference were positively correlated with each other (r = .71, P < .001). Pain was alleviated ∼44% by current therapy.
Table 1.
Descriptives.
| Mean | (St. Dev) | |
|---|---|---|
| Age (y) | 52.93 | (9.10) |
| Sex (M/F) | 18M/64F | |
| Surgery for DCM (Y/N) | 62/20 | |
| Time since Diagnosis (y) | 3.80 | (5.15) |
| Time since Onset (y) | 9.12 | (9.22) |
| Time since Pain Onset (y) | 8.01 | (9.00) |
| p-mJOA | 12.30 | (2.88) |
| BPI | ||
| BPI Severity | 5.12 | (1.92) |
| Mild (%) | 21.95% | |
| Moderate (%) | 50.00% | |
| Severe (%) | 28.05% | |
| BPI Interference | 6.06 | (2.46) |
| BPI Pain Relief (%) | 43.95 | (27.57) |
| Michigan Body Map | 12.9615 | (7.40) |
| Neck (%) | 79.49% | |
| Head (%) | 33.33% | |
| Trunk (%) | 82.05% | |
| UE (%) | 88.46% | |
| LE (%) | 70.51% | |
| Below Neck (%) | 87.8% | |
| Douleur Neuropathique 4 (DN4) |
5.47 | (2.08) |
| %>4 | 81.33 | |
| NDI | 48.17 | (18.84) |
| SF-36 | ||
| PF | 26.20 | (8.98) |
| RP | 43.50 | (8.63) |
| BP | 31.82 | (8.26) |
| GH | 37.51 | (8.70) |
| VT | 33.50 | (6.83) |
| SF | 27.25 | (11.92) |
| RE | 35.77 | (6.43) |
| MH | 36.16 | (9.59) |
| PCS | 35.36 | (5.80) |
| MCS | 35.34 | (8.20) |
Anatomic and Neuropathic Description of Pain Responses from the MBM and the DN4 are briefly summarized in Tab. 1. Detailed descriptions of anatomical location of pain frequencies for each region specified on the MBM are listed in Table 2. Participants generally had pain in at least 1 upper extremity. Either shoulder (∼83%), either upper arm (∼60%), either forearm (∼56%), and either wrist/hand (∼73%) pain were common, but less commonly was either elbow (∼38%) pain. For the lower extremity, individuals frequently had hip(∼55%) and ankle/foot (∼50%) pain but less commonly buttocks (∼43%), groin (∼23%), thigh (∼41%), knee (∼42%), and lower leg (∼46%) pain. Pain in the trunk was more commonly in the upper (∼62%) and lower (∼62%) back and less commonly in the abdomen (∼17%) and pelvis (∼13%). Pain in any segment of the right upper extremity plus any segment of the left upper extremity was identified in 82% of our sample. Likewise, bilateral pain in the lower extremity was identified in 69% of our sample. Of 5 upper extremity segments, pain was identified in 2.38 ± 1.72 and 2.30 ± 1.76 for left and right respectively Similarly, of 7 lower extremity segments, pain was identified in 2.44 ± 2.34 and 2.15 ± 2.00 segments for left and right limbs respectively. For neuropathic quality, the pain was most frequently described as “burning” (∼79%) followed “electric shocks” (56%) and “cold” (32%). The pain was commonly described as anatomically associated (i.e. same area) with “pins and needles” (∼77%), “tingling” (∼75%), “numbness” (76%), but less commonly “itching” (∼25%). Respondents report “reduced sensation to touch” and “reduced sensation to pricking” upon palpation 64% and ∼35% of the time. The pain was made worse by brushing in 28% of respondents.
Table 2.
Anatomical Descriptive Data. Detailed Michigan Body Map Results.
| Total | Op | NOp/NS | NOp/S | |
|---|---|---|---|---|
| Head | 33.33 | 33.30 | 38.50 | 31.60 |
| Face | 15.38 | 11.10 | 15.40 | 18.40 |
| Rt jaw | 12.82 | 14.80 | 15.40 | 10.50 |
| Lt jaw | 12.82 | 14.80 | 15.40 | 10.50 |
| Neck | 79.49 | 85.20 | 84.60 | 73.70 |
| Upper back | 61.54 | 70.40 | 46.20 | 60.50 |
| Lower back | 61.54 | 74.10 | 46.20 | 57.90 |
| Rt chest/breast | 21.79 | 25.90 | 23.10 | 18.40 |
| Lt chest/breast | 28.21 | 33.30 | 23.10 | 26.30 |
| Abdomen | 16.67 | 18.50 | 7.70 | 18.40 |
| Pelvis | 12.82 | 18.50 | 7.70 | 10.50 |
| Rt shoulder | 62.82 | 74.10 | 79.60 | 50.00 |
| Rt upper arm | 42.31 | 55.60 | 46.20 | 31.60 |
| Rt elbow | 28.21 | 29.60 | 30.80 | 26.30 |
| Rt lower arm | 41.03 | 37.00 | 30.80 | 47.40 |
| Rt wrist/hand | 58.97 | 66.70 | 53.80 | 55.30 |
| Lt shoulder | 61.54 | 63.00 | 53.80 | 63.20 |
| Lt upper arm | 44.87 | 48.10 | 38.50 | 44.70 |
| Lt elbow | 30.77 | 33.30 | 15.40 | 34.20 |
| Lt lower arm | 43.59 | 44.40 | 15.40 | 52.60 |
| Lt wrist/hand | 60.26 | 63.00 | 46.20 | 63.20 |
| Rt hip | 32.05 | 29.60 | 46.20 | 28.90 |
| Rt buttocks | 35.90 | 48.10 | 23.10 | 31.60 |
| Rt groin | 15.38 | 22.20 | 15.40 | 10.50 |
| Rt upper leg | 29.49 | 33.30 | 23.10 | 28.90 |
| Rt knee | 33.33 | 37.00 | 15.40 | 36.80 |
| Rt lower leg | 30.77 | 18.50 | 7.70 | 47.40 |
| Rt ankle/foot | 41.03 | 40.70 | 15.40 | 50.00 |
| Lt hip | 26.92 | 22.20 | 15.40 | 34.20 |
| Lt buttocks | 35.90 | 40.70 | 15.40 | 39.50 |
| Lt groin | 17.95 | 18.50 | 15.40 | 18.40 |
| Lt upper leg | 37.18 | 33.30 | 23.10 | 44.70 |
| Lt knee | 39.74 | 40.70 | 7.70 | 50.00 |
| Lt lower leg | 43.59 | 37.00 | 15.40 | 57.90 |
| Lt ankle/foot | 46.15 | 48.10 | 15.40 | 55.30 |
Abbreviations: Op, other pain; Op/NS, other pain/no surgery; Op/S, other pain with history of surgery.
Association of Pain With DCM Severity, Function and Quality of Life
Correlations between primary and secondary variables of interest are presented in Table 3. MBM, BPI Severity, and BPI Interference were moderately associated with NDI scores and moderately to strongly with individual subscale and PCS summary scale scores on the SF-36. SF-36 General Health was only associated with BPI interference. Correlations were always negative indicating that a higher (worse) score on MBM, BPI severity, and BPI interference were associated with a lower (worse) score on SF-36 scales. Correlations with SF-36 were generally stronger for severity and interference than MBM.
Table 3.
Correlations in the Total Cohort With and Without Any Other Pain Between Michigan Body Map, BPI Severity, and BPI Interference with Age, Duration Measures, and SF-36 Quality of Life.
| MBM | Severity | Interference | |||||
|---|---|---|---|---|---|---|---|
| r | P | r | P | r | P | ||
| Age | −.01 | .94 | .07 | .55 | .04 | .76 | |
| NDI | .48 | <.001 | .49 | <.001 | .71 | <.001 | |
| DN4 Total Score | .39 | .001 | .24 | .06 | .30 | .02 | |
| Time Since Diagnosis | .08 | .44 | .07 | .44 | .00 | .98 | |
| Time Since Onset Symptoms | −.01 | .92 | .06 | .60 | −.01 | .96 | |
| Time Since Onset of Pain | .04 | .71 | .06 | .59 | −.01 | .96 | |
| SF-36 | |||||||
| PF | −.50 | <.001 | −.52 | <.001 | −.65 | <.001 | |
| RP | −.19 | .12 | −.35 | .01 | −.32 | .01 | |
| BP | −.51 | <.001 | −.76 | <.001 | −.79 | <.001 | |
| GH | −.11 | .38 | −.19 | .13 | −.28 | .02 | |
| VT | −.35 | .01 | −.33 | .01 | −.47 | <.001 | |
| SF | −.48 | <.001 | −.62 | <.001 | −.69 | <.001 | |
| RE | −.48 | <.001 | −.64 | <.001 | −.73 | <.001 | |
| MH | −.12 | .34 | −.32 | .01 | −.41 | .001 | |
| PCS | −.51 | <.001 | −.62 | .01 | −.65 | <.001 | |
| MCS | −.29 | .02 | −.45 | <.001 | −.56 | <.001 | |
Abbreviations: PF, physical function; RP, role physical; BP, bodily pain; GH, general health; VT, vitality; SF, social functioning; RE, role emotional; MH, mental health; PCS, physical component summary; MCS, mental component summary.
Bold indicates P < .05.
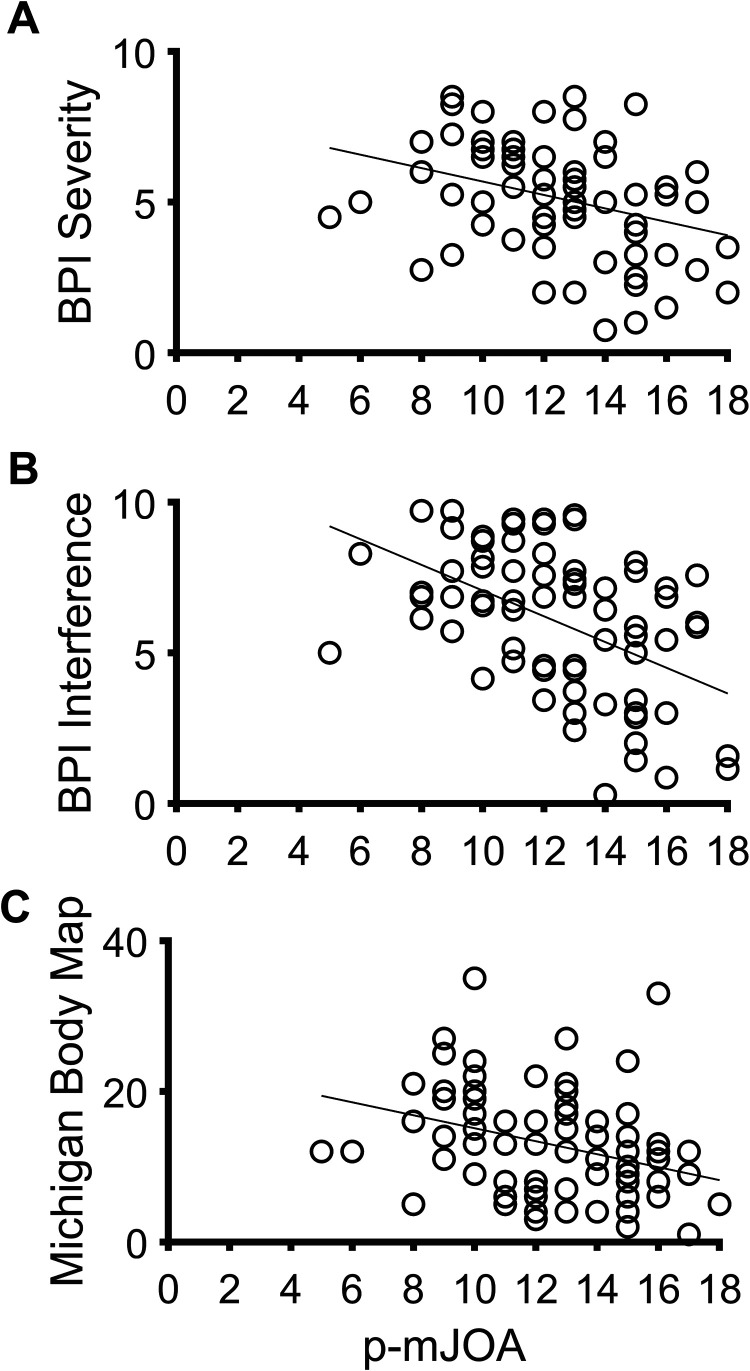
MBM and BPI scores were not associated with age, time since diagnosis, time since onset of myelopathic symptoms, or time since onset of pain. However there were weak to moderate, negative correlations with p-mJOA, an indicator of DCM disease severity (Figure 1).
Figure 1.
Correlations between the p-mJOA with A) BPI Severity (r = −.24, P = .03), B) BPI Interference (r = −.40, P < .001), and C) Michigan Body Map (r = −.28, P = .01).
Compounding Pain Conditions and History of Surgery
A Multivariate Analysis of Variance was calculated for age, time since diagnosis, time since myelopathy onset, time since pain onset, BPI severity, BPI interference, p-mJOA, NDI, and all SF-36 subscales and component summaries by presence of other pain diagnoses. These outcome measures were not different between participants with additional pain diagnoses (λ = 0.81, F18,42 = 0.55, P = .91), between those who had not had surgery (λ = 0.83, F18,42 = 0.74, P = .96), or the interaction of other pain diagnoses and history of surgery (λ = 0.74, F18,42 = 0.82, P = .66). The most common concomitant pain diagnosis was back pain 15/26 with other pain (due to low back pain, spondylolysis, scoliosis, etc.) All of these individuals additionally had upper extremity pain, 13/15 had neck pain, 13/15 had lower extremity pain, and 11/15 had DN4 >4. The second most frequent pain category were other orthopedic diagnoses (arthritis, shoulder pathology, etc.) at 8/26 with other pain. Of these, 6/8 had upper extremity pain, 5/8 had lower extremity pain, 7/8 had neck pain, 4/7 had DN4 >4 (one did not complete the DN4). Notably, all these individuals reported pain remote to their alternative pain generator such as leg pain in a person with shoulder pathology. Two individuals reported fibromyalgia. Unsurprisingly, pain was widespread in these individuals and 1 individual had DN4 >4 with the other not completing the DN4. One additional respondent reported possible low B12 with pain in several limbs and DN4 >4.
To ensure our findings were not driven by those with other pain diagnoses or history of surgery for DCM, subgroup analyses were performed on those without other pain and no surgery (n = 13 overall, n = 9 for correlations with SF-36) and those without pain with previous surgery for DCM(n = 38 overall, n = 36 for durations measures, n = 35 for SF-36). Results of subgroup correlations can be found in Table 4. For brevity, only the SF-36 PCS and SF-36 MCS are reported in the subgroup analysis. The findings of these analyses are largely consistent with the overall analysis. Notable differences are 1) DN4 was more strongly associated with MBM and BPI in those without surgery, 2) duration of myelopathic symptoms and pain were associated with BPI in those without surgery, and 3) MCS was more strongly associated with MBM and BPI in those who have had surgery.
Table 4.
Correlations in Sub-cohort Without Any Other Pain and Further Sub-divided by History of Surgery. Analyses Were Between Michigan Body Map, BPI Severity, and BPI Interference with Age, Neck Disability Index, Douleur Neuropathique 4, Duration Measures, and SF-36 Quality of Life.
| No History of Surgery | History of Surgery | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MBM | Severity | Interference | MBM | Severity | Interference | |||||||
| r | p | r | p | r | p | r | p | r | p | r | p | |
| Age | −.18 | .57 | −.40 | .17 | −.39 | .19 | −.04 | .81 | −.04 | .83 | −.10 | .56 |
| NDI | .82 | <.001 | .87 | <.001 | .77 | .003 | .52 | .001 | .50 | .002 | .73 | <.001 |
| DN4 | .66 | .014 | .66 | .02 | .51 | .08 | .28 | .09 | .22 | .18 | .17 | .31 |
| Time Since Diagnosis | .20 | .51 | .40 | .17 | .25 | .41 | .14 | .41 | .03 | .85 | −.08 | .65 |
| Time Since Onset Symptoms | .22 | .47 | .69 | .01 | .63 | .02 | .16 | .34 | .16 | .33 | .08 | .62 |
| Time Since Onset of Pain | .23 | .46 | .70 | .008 | .65 | .02 | .32 | .053 | .21 | .21 | .15 | .38 |
| SF-36 | ||||||||||||
| PCS | −.85 | <.001 | −.67 | .048 | −.74 | .02 | −.47 | .004 | −.61 | <.001 | −.62 | <.001 |
| MCS | −.41 | .27 | −.51 | .166 | −.56 | .12 | −.34 | .046 | −.50 | .002 | −.62 | <.001 |
Abbreviations: MBM, Michigan Body Map; BPI, brief pain inventory; NDI, neck disability index; DN4, Douleur Neuropathique 4; PCS, physical component summary; MCS, mental component summary.
Bold indicates P < .05.
Discussion
The aim of this study was to describe the experience of pain in a cohort of PwCM who experience pain. Participants described pain, across multiple regions of their body, and not just restricted to the neck or arm. The 2 most frequent areas of pain were neck and either hand which could be attributed to mechanical and radiculopathic pain respectively. However, the commonness of pain bilaterally in the upper extremities, the frequency of neuropathic pain qualities, and the spread of pain to multiple regions of the arm rather than limited to a single dermatomal area suggest these are unlikely to be solely the cause of pain generation for most of our cohort. Pain was moderate to severe, was sub optimally controlled by current treatments and negatively influenced quality of life. A weak, negative correlation with disease severity was demonstrated. There was high internal consistency between BPI and MBM subdomains.
Pain in DCM Can Be a Complex Whole-Body Experience, and Is Currently Underestimated by Routine Assessments
In our sample, pain was experienced throughout the body with a moderate to severe magnitude. This included remote areas such as the torso and legs. Notably, the broad anatomical distribution of pain was associated with severity which in fibromyalgia and pelvic pain is believed to be related to central sensitization of pain. 26 This is in keeping with the experience more broadly of spinal cord injury, 27 where pain is common (affecting up to 85%), often severe, 28 and is not restricted to the level of injury, with “below-level” pain even experienced in complete injuries. 29 Indeed “below-level” pain assessed grossly in our sample as pain in the trunk or lower extremities, was very common (∼88%) substantiating our hypothesis of spinal cord related neuropathic pain. This is relevant as DCM is potentially the most common cause of spinal cord related neuropathic pain syndromes27,30even with only a subset of individuals with DCM having a painful phenotype. Moreover, some recent animal models of DCM concluded that neuropathic pain in DCM may be mechanistically similar to SCI such as increased activated microglia and infiltrating macrophages in the spinal cord. 18 Currently DCM trials have almost exclusively relied on the VAS scales, typically of the neck or arm, to measure pain.6,7 This will have overlooked pain experienced in this series and may contribute to its under-recognition.
Pain Was Weakly Associated With Disease Severity, but Not Duration
In our sample, pain weakly and negatively correlated with disease severity as measured using the p-mJOA. However, duration of symptoms did not. In traumatic spinal cord injury, injury severity typically does not correlate with pain 31 but the proportion of individuals reporting neuropathic pain increased over the first 12 months from injury.32,33
The etiology and natural history of pain following spinal cord injury is unclear. 34 Moreover, the trend of increasing neuropathic pain with time in SCI has been linked to central and peripheral sensitization. 34 Consequently the difference in trends between DCM and SCI most likely reflects our study: the p-mJOA contains reference to pain, while PwCM in this series were reporting symptoms on average 8 years after the onset of pain, which may be beyond this sensitization period.
The Experience of Pain, and Its Severity, Substantially Reduced Quality of life
DCM is associated with a substantial reduction in quality of life, which remains low despite surgical treatment: Oh et al 35 demonstrated that SF-36 scores among PwCM were lower than many other chronic diseases, such as heart and lung diseases. Despite these observations, it is unclear what drives the reduced quality of life in DCM, with previous studies showing no correlation with spinal cord white matter integrity for example. 36
In our sample, we demonstrated an association with pain. Comparing our sample characteristics to Oh et al 35 we observed similar impairments in physical component quality of life, but greater impairments in mental health, across a cohort with equivalent disease severity.
Do the MBM and BPI Have a Role in Assessment of PwCM?
Recent work 5 has identified that recovery of pain is the number 1 priority in and therefore accurate characterization will be essential to future studies. While our findings cannot be confidently generalized to all PwCM, it is clear at least a subset experience pain beyond that captured by conventional assessments.
In research at least, additional instruments will therefore be required. Our experience here with the MBM and BPI is promising, with both tools showing high internal consistency in this series. The establishment of a core measurement set for DCM outcomes, is an objective of the AO Spine RECODE-DCM initiative 22 and once the core outcomes have been established, instruments will be selected. It is noted that the core measurement set for pain following traumatic spinal cord injury includes the BPI, but not the MBM 37 although there are noted limitations. 38
At this stage, the implications for a broader characterization of pain in clinical care remains unclear. While of clear value for symptom management, the role of pain in surgical decision making for example is unclear: current international guidelines on DCM treatment advocate management based on mJOA thresholds though pain may be an important factor in mild DCM.13,39 The mJOA is a compound score of motor, sensory and bladder function, with pain only considered in the arm or hand. In traumatic spinal cord injury, the anatomical distribution and severity of pain has been associated with impaired descending pain inhibition and increased temporal summation (spinal hyperexcitability of pain processing).40,41 Moreover quantitative pain testing via contact heat evoked potentials, pain inhibition and temporal summation have demonstrated potential for discriminating between control and individuals with spinal cord, predicting future declines in function in PwCM, and future spinal cord related pain.40-43 Therefore it is likely that BPI, MBM, as well as quantitative pain testing (e.g. mechanical/thermal allodynia, contact heat evoked potentials, temporal summation, etc.), while not tested in this study, would improve clinical decision making in managing PwCM, but substantial research in this domain remains to be performed.
Limitations
This study used an internet survey, advertised through the Myelopathy.org (Cambridge, UK) community, to study perspectives on pain. Consequently, there is potential for a selection bias that should be considered when generalizing results. However, the objective of this study was to explore the broader perception of pain in a cohort of PwCM reporting pain and not to generalize our findings to all PwCM. The consistent prevalence of findings across participants, suggest at least for a subset of DCM, these are relevant. Moreover, the cohort demographics (aside from gender) aligned closely with Oh et al. 35 Further, due to the nature of this study as an internet survey, we were unable to independently confirm a diagnosis of DCM. However, a substantial majority of respondents reported a history of surgery for DCM.
An additional limitation is that we were did not collect what specific treatments individuals were receiving for their pain (i.e. anticonvulsant agents, anxiolytic agents, physiotherapy, etc.) to correlate against reported pain relief values. Spinal cord related pain syndromes often receive limited benefit from current therapies, and thus is unlikely to have masked effects such as pain location or experience. 27 An evaluation of treatment response was beyond the scope of our present investigation, but should be considered in future studies, to better guide management for PwCM.
The ICD-11 classification of Chronic pain 4 (considered a disease rather than merely a symptom) indicates that pain severity, pain-related interference (as measured using the BPI) and pain-related distress should be assessed. In addition problematic cognitive (e.g., catastrophizing, excessive worry), emotional (e.g., fear, anger), behavioral (e.g., avoidance) and/or social factors (e.g., work, relationships) that accompany the chronic pain should also be assessed. Psychosocial factors may contribute to the cause, the maintenance and/or the exacerbation of the pain and/or associated disability and/or when the chronic pain results in negative psychobehavioral consequences (e.g., demoralization, hopelessness, avoidance, withdrawal). Future research should delineate the impact of these additional concepts on the experience of pain, myelopathic severity, and health related quality of life. Determining clinical utility of instruments to assess these further constructs will aid in improving clinical outcomes.
Conclusions
The pain experience of PwCM who do report pain is frequently moderate-severe, widespread, interfering and of neuropathic quality. This pain is commonly long term and sub-optimally alleviated by current pain treatments. The severity of and anatomical distribution of pain is weakly correlated with myelopathy severity. Future therapies which better address pain may therefore result in improved quality of life for PwCM who have pain.
Acknowledgments
MRNK is supported by a NIHR Clinician Scientist Award. BMD is supported by a Royal College of Surgeons (England) Research Fellowship.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This report is independent research arising from a Clinician Scientist Award, CS-2015-15-023, supported by the National Institute for Health Research. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the National Institute for Health Research or the Department of Health and Social Care.
ORCID iD: Timothy Boerger, MS, ATC  https://orcid.org/0000-0003-1587-3704
https://orcid.org/0000-0003-1587-3704
Oliver Mowforth, BA, MB, BChir1  https://orcid.org/0000-0001-6788-745X
https://orcid.org/0000-0001-6788-745X
References
- 1.Davies BM, Mowforth OD, Smith EK, Kotter MR. Degenerative cervical myelopathy. BMJ. 2018;360:k186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Badhiwala JH, Ahuja CS, Akbar MA, et al. Degenerative cervical myelopathy—update and future directions. Nat Rev Neurol. 2020;16(2):108–124. doi:10.1038/s41582-019-0303-0 [DOI] [PubMed] [Google Scholar]
- 3.Treede RD, Jensen TS, Campbell JN, et al. Neuropathic pain: redefinition and a grading system for clinical and research purposes. Neurology. 2008;70(18):1630–1635. doi:10.1212/01.wnl.0000282763.29778.59 [DOI] [PubMed] [Google Scholar]
- 4.Treede RD, Rief W, Barke A, et al. Chronic pain as a symptom or a disease: the IASP classification of chronic pain for the international classification of diseases (ICD-11). Pain. 2019;160(1):19–27. doi:10.1097/j.pain.0000000000001384 [DOI] [PubMed] [Google Scholar]
- 5.Davies B, Mowforth O, Sadler I, et al. Recovery priorities in degenerative cervical myelopathy: a cross-sectional survey of an international, online community of patients. BMJ Open. 2019;9(10):e031486. doi:10.1136/bmjopen-2019-031486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies BM, McHugh M, Elgheriani A, et al. Reported outcome measures in degenerative cervical myelopathy: a systematic review. PLoS One. 2016;11(8):e0157263. doi:10.1371/journal.pone.0157263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies BM, McHugh M, Elgheriani A, et al. The Reporting of study and population characteristics in degenerative cervical myelopathy: a systematic review. PLoS One. 2017;12(3):e0172564. doi:10.1371/journal.pone.0172564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hilton B, Tempest-Mitchell J, Davies B, Kotter M. Assessment of degenerative cervical myelopathy differs between specialists and may influence time to diagnosis and clinical outcomes. PLoS One. 2018;13(12):e0207709. doi:10.1371/journal.pone.0207709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thakar S, Rajshekhar V. Evaluation of pain as a preference-based health status measure in patients with cervical spondylotic myelopathy undergoing central corpectomy. Acta Neurochir (Wien). 2012;154(2):335–340. doi:10.1007/s00701-011-1229-5 [DOI] [PubMed] [Google Scholar]
- 10.Kimura A, Shiraishi Y, Inoue H, Endo T, Takeshita K. Predictors of persistent axial neck pain after cervical laminoplasty. Spine (Phila Pa 1976). 2018;43(1):10–15. doi:10.1097/brs.0000000000002267 [DOI] [PubMed] [Google Scholar]
- 11.Fehlings MG, Tetreault LA, Kurpad S, et al. Change in functional impairment, disability, and quality of life following operative treatment for degenerative cervical myelopathy: a systematic review and meta-analysis. Global Spine J. 2017;7(3 Suppl):53S–69S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang SJ, Jiang SD, Jiang LS, Dai LY. Axial pain after posterior cervical spine surgery: a systematic review. Eur Spine J. 2011;20(2):185–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Badhiwala JH, Hachem LD, Merali Z, et al. Predicting outcomes after surgical decompression for mild degenerative cervical myelopathy: moving beyond the MJOA to identify surgical candidates. Neurosurgery. 2020;86(4):565–573. doi:10.1093/neuros/nyz160 [DOI] [PubMed] [Google Scholar]
- 14.Overley SC, Kim JS, Gogel BA, Merrill RK, Hecht AC. Tandem spinal stenosis: a systematic review. JBJS Rev. 2017;5(9):e2. doi:10.2106/jbjs.rvw.17.00007 [DOI] [PubMed] [Google Scholar]
- 15.Heffez DS, Ross RE, Shade-Zeldow Y, et al. Clinical evidence for cervical myelopathy due to Chiari malformation and spinal stenosis in a non-randomized group of patients with the diagnosis of fibromyalgia. Eur Spine J. 2004;13(6):516–523. doi:10.1007/s00586-004-0672-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heffez DS, Ross RE, Shade-Zeldow Y, et al. Treatment of cervical myelopathy in patients with the fibromyalgia syndrome: outcomes and implications. Eur Spine J. 2007;16(9):1423–1433. doi:10.1007/s00586-007-0366-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Baptiste DC, Fehlings MG. Pathophysiology of cervical myelopathy. Spine J. 2006;6(6 Suppl):190s–197s. doi:10.1016/j.spinee.2006.04.024 [DOI] [PubMed] [Google Scholar]
- 18.Takeura N, Nakajima H, Watanabe S, Honjoh K, Takahashi A, Matsumine A. Role of macrophages and activated microglia in neuropathic pain associated with chronic progressive spinal cord compression. Scientific Reports. 2019;9(1):15656.doi:10.1038/s41598-019-52234 -1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dolan RT, Butler JS, O’Byrne JM, Poynton AR. Mechanical and cellular processes driving cervical myelopathy. World J Orthop. 2016;7(1):20–29. doi:10.5312/wjo.v7.i1.20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulsebosch CE, Xu GY, Perez-Polo JR, Westlund KN, Taylor CP, McAdoo DJ. Rodent model of chronic central pain after spinal cord contusion injury and effects of gabapentin. J Neurotrauma. 2000;17(12):1205–1217. doi:10.1089/neu.2000.17.1205 [DOI] [PubMed] [Google Scholar]
- 21.Kramer JL, Minhas NK, Jutzeler CR, Erskine EL, Liu LJ, Ramer MS. Neuropathic pain following traumatic spinal cord injury: models, measurement, and mechanisms. J Neurosci Res. 2017;95(6):1295–1306. doi:10.1002/jnr.23881 [DOI] [PubMed] [Google Scholar]
- 22.Davies BM, Khan DZ, MOWFORTH OD, et al. RE-CODE DCM (Research objectives and common data elements for degenerative cervical myelopathy): a consensus process to improve research efficiency in DCM, through establishment of a standardized dataset for clinical research and the definition of the research priorities. Global Spine J. 2019;9(1 Suppl):65s–76s. doi:10.1177/2192568219832855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen MP, CHODROFF MJ, Dworkin RH. The impact of neuropathic pain on health-related quality of life: review and implications. Neurology. 2007;68(15):1178–1182. doi:10.1212/01.wnl.0000259085.61898.9e [DOI] [PubMed] [Google Scholar]
- 24.Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106(3):337–345. doi:10.1016/j.pain.2003.08.001 [DOI] [PubMed] [Google Scholar]
- 25.Rhee JM, Shi WJ, Cyriac M, et al. The P-MJOA: a patient-derived, self-reported outcome instrument for evaluating cervical myelopathy: comparison with the MJOA. Clin Spine Surg. 2018;31(2):E115–E120. doi:10.1097/bsd.0000000000000591 [DOI] [PubMed] [Google Scholar]
- 26.Kutch JJ, ICHESCO E, Hampson JP, et al. Brain signature and functional impact of centralized pain: a multidisciplinary approach to the study of chronic pelvic pain (MAPP) network study. Pain. 2017;158(10):1979–1991. doi:10.1097/j.pain.0000000000001001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nakajima H, Uchida K, Takayasu M, USHIDA T. A nationwide survey of spinal cord-related pain syndrome in Japan: clinical characteristics and treatment. Spine Surg Relat Res. 2019;3(4):319–326. doi:10.22603/ssrr.2018-0096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siddall PJ. Management of neuropathic pain following spinal cord injury: now and in the future. Spinal Cord. 2009;47(5):352–359. doi:10.1038/sc.2008.136 [DOI] [PubMed] [Google Scholar]
- 29.Shiao R, Lee-Kubli CA. Neuropathic pain after spinal cord injury: challenges and research perspectives. Neurotherapeutics. 2018;15(3):635–653. doi:10.1007/s13311-018-0633-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamashita T, Takahashi K, Yonenobu K, Kikuchi S. Prevalence of neuropathic pain in cases with chronic pain related to spinal disorders. J Orthop Sci. 2014;19(1):15–21. doi:10.1007/s00776-013-0496-9 [DOI] [PubMed] [Google Scholar]
- 31.Marcondes BF, Sreepathi S, Markowski J, et al. Pain severity and mobility one year after spinal cord injury: a multicenter, cross-sectional study. Eur J Phys Rehabil Med. 2016;52(5):630–636. [PubMed] [Google Scholar]
- 32.Hagen EM, Rekand T. Management of neuropathic pain associated with spinal cord injury. Pain Ther. 2015;4(1):51–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Finnerup NB, Norrbrink C, Norrbrink C, et al. Phenotypes and predictors of pain following traumatic spinal cord injury: a prospective study. J pain. 2014;15(1):40–48. doi:10.1016/j.jpain.2013.09.008 [DOI] [PubMed] [Google Scholar]
- 34.Hulsebosch CE, Hains BC, Crown ED, Carlton SM. Mechanisms of chronic central neuropathic pain after spinal cord injury. Brain Res Rev. 2009;60(1):202–213. doi:10.1016/j.brainresrev.2008.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oh T, Lafage R, Lafage V, et al. Comparing quality of life in cervical spondylotic myelopathy with other chronic debilitating diseases using the short form survey 36-health survey. World Neurosurg. 2017;106:699–706. doi:10.1016/j.wneu.2016.12.124 [DOI] [PubMed] [Google Scholar]
- 36.Jones JG, Cen SY, Lebel RM, Hsieh PC, Law M. Diffusion tensor imaging correlates with the clinical assessment of disease severity in cervical spondylotic myelopathy and predicts outcome following surgery. AJNR Am J Neuroradiol. 2013;34(2):471–478. doi:10.3174/ajnr.A3199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bryce TN, Budh CN, Cardenas DD, et al. Pain after spinal cord injury: an evidence-based review for clinical practice and research: report of the national institute on disability and rehabilitation research spinal cord injury measures meeting. J Spinal Cord Med. 2007;30(5):421–440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cohen ML, Kisala PA, Dyson-Hudson TA, Tulsky DS. Measuring pain phenomena after spinal cord injury: development and psychometric properties of the SCI-QOL pain interference and pain behavior assessment tools. J Spinal Cord Med. 2018;41(3):267–280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fehlings MG, Tetreault LA, Riew KD, et al. A clinical practice guideline for the management of patients with degenerative cervical myelopathy: recommendations for patients with mild, moderate, and severe disease and NONMYELOPATHIC patients with evidence of cord compression. Global Spine J. 2017;7(3 Suppl):70S–83S.doi:10.1177/2192568217701914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gruener H, Zeilig G, Laufer Y, Blumen N, Defrin R. Differential pain modulation properties in central neuropathic pain after spinal cord injury. Pain. 2016;157(7):1415–1424. doi:10.1097/j.pain.0000000000000532 [DOI] [PubMed] [Google Scholar]
- 41.Albu S, Gomez-Soriano J, Avila-Martin G, Taylor J. Deficient conditioned pain modulation after spinal cord injury correlates with clinical spontaneous pain measures. Pain. 2015;156(2):260–272. doi:10.1097/01.j.pain.0000460306.48701.f9 [DOI] [PubMed] [Google Scholar]
- 42.Jutzeler CR, Ulrich A, Huber B, Rosner J, Kramer JLK, Curt A. Improved diagnosis of cervical spondylotic myelopathy with contact heat evoked potentials. J Neurotrauma. 2017;34(12):2045–2053. doi:10.1089/neu.2016.4891 [DOI] [PubMed] [Google Scholar]
- 43.Gruener H, Zeilig G, Gaidukov E, et al. Biomarkers for predicting central neuropathic pain occurrence and severity after spinal cord injury: results of a long-term longitudinal study. Pain. 2020;161(3):545–556. doi:10.1097/j.pain.0000000000001740 [DOI] [PubMed] [Google Scholar]