Abstract
Study Design:
Systematic review. Surgical decompression for degenerative cervical myelopathy (DCM) is associated with perioperative complications, including difficulty or discomfort with swallowing (dysphagia) as well as changes in sound production (dysphonia). This systematic review aims to (1) outline how dysphagia and dysphonia are defined in the literature and (2) assess the quality of definitions using a novel 4-point rating system.
Methods:
An electronic database search was conducted for studies that reported on dysphagia, dysphonia or other related complications of DCM surgery. Data extracted included study design, surgical details, as well as definitions and rates of surgical complications. A 4-point rating scale was developed to assess the quality of definitions for each complication.
Results:
Our search yielded 2,673 unique citations, 11 of which met eligibility criteria and were summarized in this review. Defined complications included odynophagia (n = 1), dysphagia (n = 11), dysphonia (n = 2), perioperative swelling complications (n = 2), and soft tissue swelling (n = 3). Rates of dysphagia varied substantially (0.0%-50.0%) depending on whether this complication was patient-reported (4.4%); patient-reported using a modified Swallowing Quality of Life questionnaire (43.1%) or the Bazaz criteria (8.8%-50.0%); or diagnosed using an extensive protocol consisting of clinical assessment, a bedside swallowing test, evaluation by a speech and language pathologist and a modified barium swallowing test/fiberoptic endoscopy (42.9%). The reported incidences of dysphonia also ranged significantly from 0.6% to 38.0%.
Conclusion:
There is substantial variability in reported rates of dysphagia and dysphonia due to differences in data collection methods, diagnostic strategies, and definitions. Consolidation of nomenclature will improve evaluation of the overall safety of surgery.
Keywords: degenerative cervical myelopathy, cervical spondylotic myelopathy, surgery, complications, dysphagia; dysphonia
Introduction
Degenerative cervical myelopathy (DCM), a recently coined term encompassing cervical spondylotic myelopathy (CSM), ossification of the posterior longitudinal ligament (OPLL) and other degenerative causes of cord compression, is often treated surgically. 1 The goals of surgery include removing the compressive pathology, increasing the space available for the spinal cord, and stabilizing the spinal column. 2 Surgical decompression can be accomplished anteriorly and/or posteriorly using techniques such as discectomy, corpectomy, laminectomy or laminoplasty.
Although surgery is generally safe and effective, complications occur in approximately 14.1% of cases. 3 These include dysphagia or odynophagia and dysphonia or odynophonia. 4 Reported rates of dysphagia and dysphonia vary substantially in the literature and are often dependent on the method of data collection, study design, and how the complications were defined. Due to this inconsistency, it is difficult to accurately convey surgical risk to patients and appropriately manage their expectations. This information is critical as dysphagia and dysphonia may result in significant physical and psychosocial impairment, malnutrition, increased hospital length of stay, and increased risk of aspiration pneumonia and mortality.5-7 Moreover, these complications are associated with increased anxiety, depression, and social isolation, and reduced quality of life.8,9
This variability in reporting demonstrates a pressing need to standardize definitions for complications associated with DCM surgery, including dysphagia and dysphonia. This information will enable evaluation of the overall safety of surgery, important risk factors, and the impact of complications on recovery rate, patient satisfaction and cost. Furthermore, an accurate assessment of complications will benefit both the patient and surgeon by empowering patient-informed choice, facilitating shared decision-making and enabling a better evaluation of risks and benefits of each procedure. Finally, accurately identifying complications will help prevent adverse events, optimize outcomes, and improve assessments of quality of care. This systematic review aims to (1) outline how dysphagia and dysphonia are defined in the literature, and (2) assess the quality of definitions using a novel 4-point rating system.
Methods
Eligibility Criteria
Studies were included in this review if they examined 10 or more adult patients (≥18 years of age) with DCM (cord compression caused by spondylosis, disc herniation, OPLL, hypertrophy or ossification of the ligamentum flavum (HLF, OLF), instability, subluxation and/or progressive kyphosis). Patients must have been treated surgically and evaluated postoperatively.
Studies were considered for inclusion if they defined 1 or more complications (as opposed to adverse events) related to surgical intervention. For the purpose of this review, a complication was defined as any undesirable medical event affecting a patient that can be attributed to the operation and may or may not be anticipated. In contrast, an adverse event is any untoward medical incidence occurring during a study period that is not related to surgery, but may or may not be related to a patient’s myelopathy. In some studies, it may be difficult to distinguish between an adverse event and a complication.
Studies were excluded if they involved patients with only radiculopathy or non-degenerative causes of myelopathy, such as trauma, neoplasms or rheumatoid arthritis. Case reports, meta-analyses, systematic reviews, editorials, commentaries, and conference proceedings were also excluded.
Information Sources
An electronic search was performed in MEDLINE, MEDLINE in Process, EMBASE and Cochrane Central Register of Controlled Trials for literature published up to and including January 22nd, 2016.
Search
The search strategy was first developed in MEDLINE and then modified for the other 3 databases. The following terms were used to search all databases: (DCM OR CSM OR OPLL) AND surgery AND (adverse events OR complications). Other keywords were also included in the search strategy, including those related to dysphagia and dysphonia. Only studies on humans and written in English were considered for inclusion. Four libraries were used to access the full texts of articles.
Study Selection
All duplicates, conference abstracts, systematic or literature reviews, commentaries, letters, case reports, and studies in other languages were excluded using a reference library. The remaining abstracts and titles were reviewed and sorted by 3 independent investigators (L.T., S.C., M.T.K.) as possibly relevant or irrelevant. The full texts of the articles classified as possibly relevant were examined by a fourth reviewer (S.F.L.). Uncertainty about inclusion was resolved through discussion and consensus. Final decisions were reviewed and approved by the senior author (M.G.F).
Data Extraction and Synthesis
The following data were extracted from each article if available: title, author, year, study design, number of patients, diagnosis, type of surgery, follow-up period/average length of follow-up, and type, definition, incidence, onset, and duration of complications.
Surgical complications were classified into 4 categories: biomechanical or hardware-related, pain or discomfort, neurological, and dysphagia/dysphonia. This review summarizes current definitions for dysphagia, dysphonia and other related complications.
Rating of Individual Definitions
A novel 4-point rating system was created to evaluate the quality of definitions: “COMP,” Clinical finding, Objective criteria, Modality and Point in time. A single point was granted for each of the following:
The complication is linked to a clinical finding and is described qualitatively;
The modality of identifying the complication is described (X-ray, magnetic resonance imaging (MRI), computed tomography (CT), lab, patient-reported, etc.);
The clinical finding is described using a quantitative measurement or has been categorized based on objective criteria. If the complication was described quantitatively, a point for criterion (A) was automatically awarded;
The time frame of evaluation was indicated (days, weeks, months following surgery).
This rating scale was developed and modified through consensus among the authors. Each criterion was selected based on trends in current definitions. This 4-point scale assigns substantial weight to definitions that include numerical measurements. Inter-rater reliability of scoring was 90.9% (10/11). Table 1 uses dysphagia as an example to illustrate how definitions are rated using this system.
Table 1.
An Example Demonstrating How Definitions Are Scored Using a 4-Point Rating System.
| Definition | Criterion A | Criterion B | Criterion C | Criterion D |
|---|---|---|---|---|
| Difficulty swallowing | ✓ | |||
| Patient-reported and laryngoscope-confirmed difficulty swallowing | ✓ | ✓ | ||
| Patient-reported and laryngoscope-confirmed difficulty swallowing; mild defined as rare swallowing discomfort for solids, moderate as occasional swallowing discomfort for specific solid foods and severe as frequent swallowing discomfort for solids and rare discomfort with liquids | ✓ | ✓ | ✓ | |
| Patient-reported and laryngoscope-confirmed difficulty swallowing within 30 days of surgery; mild defined as rare swallowing discomfort for solids, moderate as occasional swallowing discomfort for specific solid foods and severe as frequent swallowing discomfort for solids and rare discomfort with liquids | ✓ | ✓ | ✓ | ✓ |
Reporting
This systematic review was formatted using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement. 10 For the purpose of this review, it was not necessary to evaluate the risk of bias of each individual article or assess the strength of the overall body of evidence. Instead, we rated the quality of each definition using the 4-point rating system described above.
Results
Study Selection
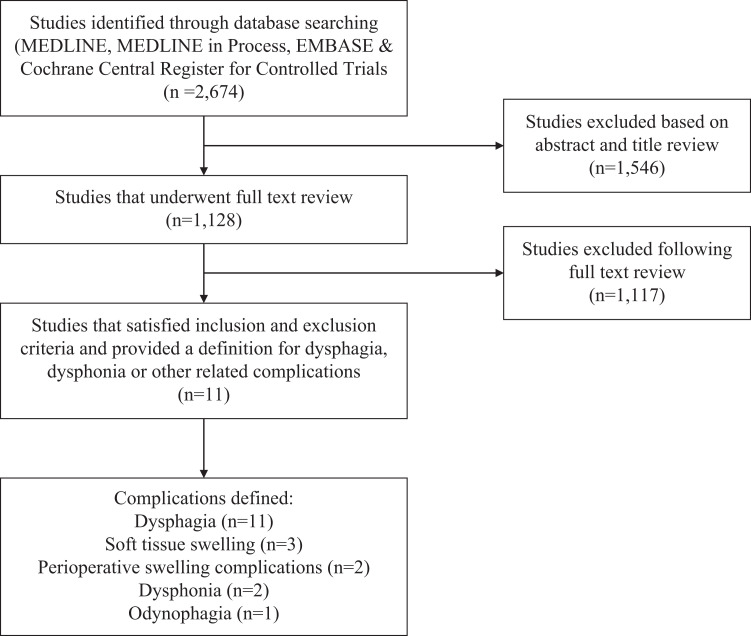
The literature search yielded 2,674 unique citations. Of these, 1,546 articles were excluded based on abstract and title review. A total of 1,128 studies underwent full text review, 1,117 of which were excluded. Common reasons for exclusion were that the study (1) included patients with only radiculopathy or with myelopathy secondary to trauma, tumor or rheumatoid arthritis, (2) did not discuss complications, and (3) did not define reported complications. A total of 11 articles satisfied our inclusion criteria. Figure 1 displays the search and study selection process.
Figure 1.
An overview of the search and study selection process.
Study Characteristics
The 11 included articles were published between 2008 and 2016. Two studies (18%) were prospective and 9 were retrospective (82%) (Table 2). Sample sizes ranged from 14 to 477 patients.
Table 2.
A Summary of Definitions Used for Dysphagia, Dysphonia and Other Related Complications.
| Complication | Definition | Author | Score | Study design* | Timing of evaluation# | Surgical approach | Patients available | # of Events | Incidence |
|---|---|---|---|---|---|---|---|---|---|
| Dysphagia | Patients were asked about swallowing difficulties; classified as painful swallowing (odynophagia) and/or trouble swallowing solids and/or liquids. Severe dysphagia was defined as coughing out swallowed food/sensation of food getting stuck in throat/preferential spitting out of saliva | Bapat et al (2008) | 3 (A, B, C) | P | 0-1 M; 1-3 M; >3M | Anterior and/or Posterior | 129 | 52† 34‡ |
40.3%† 26.4%‡ |
| Patient reported difficulty regarding liquid or solid deglutition | Tetreault et al (2016) | 2 (A, B) | P | <30D | Anterior and/or Posterior | 477 | 21 | 4.4% | |
| Solid or dry food gets stuck when swallowing | Lin (2012) | 1 (A) | R | 24-M§ | Anterior | 120 | 9 | 7.5% | |
| Yu (2014) | R | 3, 6, 12, 18, 24 M (29.2 M) | Anterior | 248 | 17 | 6.9% | |||
| Bazaz criteria: Mild (rare swallowing discomfort for solids), moderate (occasional dysphagia for certain solid foods), or severe (frequent dysphagia for solids and rarely for liquids) | Pourtaheri et al (2013) | 3 (A, B, C) | R | 2-, 6-W, 6 M, last follow-up (4.5Y) | Anterior | 24 | 8, 3, 1& |
33.3%, 12.5%, 4.2%& | |
| Pourtaheri et al (2015) | R | 2-, 6-W, 6 M, last follow-up | Anterior | 37 | 8, 3, 1& |
21.6%, 8.1%, 2.7& |
|||
| Song (2012) | R | 6 W, 3-, 6-, 9-, 12-, 18-, 24-M, yearly; (89.9 M)*† | Anterior | 40 | 6 | 15.0% | |||
| Wang (2015) | R | 3D, 1W | Anterior | 57 | 5*‡ | 8.8%*‡ | |||
| Wang (2014) | R | 2 W, 3-, 6 -M | Anterior | 47 | 7, 2, NA& |
14.9%, 4.3%, NA& | |||
| (1) Bazaz’s criteria, (2) the Swallowing Quality of Life questionnaire was also completed by the patients pre- and postoperatively. A 14-item portion from this questionnaire (total score, 70) was used to assess the degree of dysphagia. | Yang et al (2012) | 3 (A, B, C) | R | 48 H, 2-, 6-, 12-M# |
Anterior | 51 | 22, 8, 0, 0** |
43.1%, 15.7%, 0%, 0%** |
|
| Initial dysphagia screening for all patients was performed using a 2-part protocol: (Part 1) evaluation of case history/clinical presentation; and (Part 2) a bedside swallowing test using 90 mL of water. If the patient met any of the criteria specified in Part 1, experienced coughing or demonstrated change in quality of voice in Part 2, a formal swallow evaluation by a speech language pathologist (SLP) was requested. The patient must pass both parts of the screening in order for the results to be considered negative for dysphagia. SLP evaluation included trials of ice chips, thin and thick liquids, and puree, soft and hard solids, with assessment of oral containment, bolus formation, oral transit, swallowing reflex, laryngeal elevation, voice quality changes, and cough/signs of aspiration. Based on the results, modified barium swallowing or fiberoptic endoscopic evaluation of swallowing was recommended for further evaluation | Chen et al (2013) | 3 (A, B, C) | R | (21.5 M) | Anterior-Posterior | 14 | 6 | 42.9% | |
| Dysphonia | Patient who reported dysphonia underwent indirect laryngoscope to detect vocal cord paralysis | Bapat et al (2008) | 2 (A, B) | P | 0-1 M; 1-3 M; >3M | Anterior and/or Posterior | 129 | 49 | 38.0% |
| Changes or difficulty in vocal sound production reported by the patient | Tetreault et al (2016) | 2 (A, B) | P | <30D | Anterior and/or Posterior | 477 | 3 | 0.6% | |
| Soft Tissue Swelling Complications | Visible swelling of the surgical site, swallowing dysfunction, and/or breathing difficulties leading to: (1) a delay in discharge during the index surgical hospitalization, (2) otolaryngological consultation as an outpatient, (3) a premature return to the office or to the emergency department after hospital discharge, (4) readmission for observation and medical management of swelling without surgical intervention, or (5) readmission for incision and drainage of the surgical site for actual or threatened airway compromise (occurring within 6 weeks of the procedure) | Pourtaheri et al (2013) | 4 (A, B, C, D) | R | <6W | Anterior | 24 | 0 | 0.0% |
| Pourtaheri et al (2015) | R | <6W | Anterior | 37 | 0 | 0.0% | |||
| The distance from the front of the spine at C2 and C3 to the posterior edge of the trachea was measured on lateral radiographs; the difference between preoperative and 2-week postoperative values was computed | Pourtaheri et al (2013) | 4 (A, B, C, D) | R | 2W | Anterior | 24 | NA% | NA% | |
| Pourtaheri et al (2015) | R | 2W | Anterior | 37 | NA% | NA% | |||
| The thickness of the prevertebral soft tissues at C2-C7 was measured on lateral radiographs. At plated levels, the thickness of the prevertebral soft tissue was measured from the front of the plate | Yang et al (2012) | 3 (A, B, C) | R | 48 H, 2-, 6-, 12-M | Anterior | 51 | NA% | NA% |
Abbreviations: P = prospective, R = retrospective.
#Time of diagnosis or onset or period of evaluation ((H = hour(s); D = day(s); W = week(s); M = month(s); Y = year(s); (…) = mean follow up).
† Total number of events and incidence of odynophagia across all time points assessed (0-1 M, 1-3 M and >3 M).
‡Total number of events and incidence of dysphagia across all time points assessed (0-1 M, 1-3 M and >3 M).
§Patients were followed for 24-months; however, all cases of dysphagia resolved within 1 month except for 1 case with graft dislodgement.
*†Patients were evaluated at these time points; however, all cases of dysphagia resolved spontaneously in 6 months.
&Total number of events and incidence of mild, moderate and severe dysphagia at 2-weeks.
*‡Three patients were diagnosed with dysphagia 3 days after the operation; 2 patients were diagnosed with dysphagia 1-week after the operation.
** Total number of events and incidence of dysphagia at 48-hours, 2-, 6- and 12-months, respectively.
%The mean distance of soft tissue swelling was provided and not the incidence of this complication.
Complication Definitions
The complications defined in the reviewed studies included odynophagia (n = 1), 11 dysphagia (n = 11),11-21 dysphonia (n = 2),11,12 perioperative swelling complications (n = 2)15,21 and soft tissue swelling (n = 3).15,19,21 Table 2 summarizes the study design, timing of follow-up, surgical approach, and definitions of complications of each included study. Table 3 displays the reported incidences of odynophagia, dysphagia, dysphonia, and other swelling complications.
Table 3.
Mean Incidence of Dysphagia, Dysphonia and Other Related Complications at Final Follow-Up.
| Complication | Definitions/studies with incidences | Mean incidence at final follow up (range) | Number of patients evaluated |
|---|---|---|---|
| Dysphagia | 6/11 | (0-42.9%) | 1244 |
| Dysphonia | 2/2 | (0.6-38.0%) | 606 |
Soft Tissue Swelling
|
1/2 2/3 |
0% NA* |
61 112 |
* The mean distance of soft tissue swelling was provided and not the incidence of this complication.
Odynophagia or Dysphagia
Eleven studies provided 6 definitions of dysphagia.11-21 Qualitative definitions included (1) trouble swallowing solids and/or liquids (rating = 3, incidence = 26.4%), 11 (2) difficulty regarding liquid or solid deglutition (rating = 2, incidence = 4.4%), 12 and (3) sticking of solid or dry foods when swallowing (rating = 1, incidence = 6.9-7.5%).13,14 Two of these studies specified that patients were questioned about their swallowing difficulties.11,12
The severity of dysphagia was defined or categorized in 7 studies11,15-19,21; these definitions received 3 points on our rating scale. The Bazaz scale (Table 4) was the most commonly used system.15-19,21 This system classifies severity of dysphagia based on patient-reported episodes of difficulty swallowing liquids and solids. The categories include no difficulty, mild difficulty (no difficulty with liquids, rare difficulty with solids), moderate difficulty (no or rare difficulty with liquids, occasional difficulty with specific solid foods such as bread or meat), and severe difficulty (difficulty with liquids, frequent difficulty with solids in most situations). 22 Three of these studies reported incidence as well as severity of dysphagia15,17,21; rates ranged from 14.9% to 33.3% for mild, 4.3% to 12.5% for moderate and 2.7% to 4.2% for severe dysphagia. In a study by Bapat et al, severe dysphagia was defined as either coughing out swallowed food, the sensation of food getting stuck in the throat, or preferential spitting out of saliva. 11
Table 4.
Other Scales Used to Evaluate Dysphagia.
| Dysphagia scale | Description |
|---|---|
| American Speech-Language-Hearing Association (ASHA) Swallowing Scale (Lewis-Mesiongale-Boehm Swallowing Scale) |
|
| Dysphagia Handicap Index | Self-rated severity of dysphagia on a 7-point interval scale from 1 or “normal” to 7 or “severe” with 4 indicating moderate swallowing problems. |
| Dysphagia Numeric Rating Scale | Patient-reported swallowing difficulty, rated from 0 to 10 (similar to Visual Analog Pain Scale) 0: No swallowing difficulty 1 to 3: Rare episodes of swallowing difficulty 4 to 6: Occasional swallowing difficulty with solid food, but no difficulty with liquids 7 to 10: Swallowing difficulty with solids and liquids |
| Dysphagia Outcome Severity Scale | 7-point scale that incorporates functional levels of independence, nutrition, and diet: Full per-oral nutrition: Normal diet Level 7: Normal in all situations Normal diet; no strategies or extra time needed Level 6: Within functional limits/modified independence Normal diet, functional swallow; patient may have mild oral or pharyngeal delay, retention or trace epiglottal undercoating but independently and spontaneously compensates/clears; may need extra time for meal; no aspiration or penetration across consistencies Full per-oral nutrition: Modified diet and/or independence: Level 5: Mild dysphagia: Distant supervision, may need one diet consistency restricted May exhibit 1 or more of the following: aspiration of thin liquids only but with strong reflexive cough to clear completely; airway penetration midway to cords with 1 or more consistency or to cords with 1 consistency but clears spontaneously; retention in pharynx that is cleared spontaneously; mild oral dysphagia with reduced mastication and/or oral retention that is cleared spontaneously Level 4: Mild-moderate dysphagia: Intermittent supervision/cueing, 1 or 2 consistencies restricted May exhibit 1 or more of the following: retention in pharynx cleared with cue; retention in the oral cavity that is cleared with cue; aspiration with 1 consistency, with weak or no reflexive cough (or airway penetration to the level of the vocal cords with cough with 2 consistencies or airway penetration to the level of the vocal cords without cough with 1 consistency) Level 3: Moderate dysphagia: Total assist, supervision, or strategies, 2 or more diet consistencies restricted May exhibit 1 or more of the following: moderate retention in pharynx, cleared with cue; moderate retention in oral cavity, cleared with cue; airway penetration to the level of the vocal cords without cough with 2 or more consistencies (or aspiration with 2 consistencies, with weak or no reflexive cough or aspiration with 1 consistency, no cough and airway penetration to cords with 1, no cough) Non-oral nutrition necessary Level 2: Moderately severe dysphagia: Maximum assistance or use of strategies with partial per oral-nutrition only (tolerates at least one consistency safely with total use of strategies) May exhibit 1 or more of the following: severe retention in pharynx, unable to clear or needs multiple cues; severe oral stage bolus loss or retention, unable to clear or needs multiple cues; aspiration with 2 or more consistencies, no reflexive cough, weak volitional cough (or aspiration with 1 or more consistency, no cough and airway penetration to cords with 1 or more consistency, no cough) Level 1: Severe dysphagia: Unable to tolerate any per-oral nutrition safely. May exhibit 1 or more of the following: severe retention in pharynx, unable to clear; severe oral stage bolus loss or retention, unable to clear; silent aspiration with 2 or more consistencies, non-functional volitional cough; or unable to achieve swallow |
| Functional Oral Intake Scale | Tube Dependent (levels 1-3)
|
| Modified Swallowing-Quality of Life Survey | Patient questionnaire with a symptom scale and 10 quality of life domains: burden, food selection, eating duration, eating desire, fear, sleep, fatigue, communication, mental health and social functioning. The format of each question varies throughout the instrument. |
| Dysphagia short questionnaire | An 18-point questionnaire (lower score reflects milder symptoms) that requires patients to report on their ability to swallow, incorrect swallowing, lump feeling, involuntary weight loss and pneumonia. |
| MD Anderson Dysphagia Inventory | Patients are required to answer strongly agree, agree, no opinion, disagree or strongly degree to 20 questions related to the emotional, functional and physical consequences of swallowing difficulties. |
| Bazaz’s Criteria | None: No problems with liquids, no problems with solids Mild: No problems with liquids, rare problems with solids Moderate: No or rare problems with liquids, occasional problems with solids Severe: No or rare problems with liquids, frequent problems with solids |
| Dysphagia Management Staging Scale | Nondysphagia: competent oral, pharyngeal and esophageal management of all food categories Mild: feeding and swallowing disorder is compensated for using a single strategy: diet restrictions, medications or adaptive feeding/swallowing strategies. Person maintains satisfactory nutrition, hydration and respiratory function Moderate: feeding and swallowing disorder can be managed with a combination of 2 or more strategies strategies: diet restrictions, medications and adaptive feeding/swallowing strategies. Person maintains satisfactory nutrition, hydration and respiratory function Severe: feeding and swallowing disorder can be managed with a combination of 2 or more strategies strategies: diet restrictions, medications and adaptive feeding/swallowing strategies. Problems related to nutrition, hydration and respiratory function persist Profound: Non-oral feeding is required for supplemental or total nutrition |
In a study by Yang et al, patients completed a modified version of the Swallowing Quality of Life Questionnaire both pre- and postoperatively. 19 On a five-point scale, each patient chose (1) “almost always,” (2) “often,” (3) “sometimes,” (4) “hardly ever” or (5) “never” with regard to the following 14 signs of dysphagia: coughing, choking when eating food, choking when taking liquids, having thick saliva or phlegm, gagging, drooling, problems chewing, having excess saliva or phlegm, having to clear throat, food sticking in throat, food sticking in mouth, food or liquid dribbling out of mouth, food or liquid coming out of nose, and coughing food or liquid out of mouth when it gets stuck. 23 The questionnaire is scored from 14 to 70, with a lower score indicating an increased degree of dysphagia. This definition received 3 points on our rating scale. The reported incidence of dysphagia in this study was 43.1% at 48-hours.
An extensive evaluation of dysphagia was completed in a study by Chen et al (rating = 3 points). 20 Initial dysphagia screening followed a 2-part protocol that consisted of evaluating a patient's history and clinical presentation, and performing a bedside swallowing test using 90 mL of water. Relevant features of a clinical presentation included presence of a brainstem stroke, decreased level of consciousness, difficulty/sitting upright, shortness of breath, slurred speech, facial weakness/droop, cognitive deficits, pneumonia, weak cough, hoarse voice, wet/gurgly sounding voice, drooling or wet cough. For the bedside swallowing test, relevant observations included coughing while drinking water or within 1 minute afterward, and wet or hoarse vocal quality following test. If the patient met any of these criteria, a formal evaluation by a speech language pathologist was requested; this included (1) trials of ice chips, thin and thick liquids, puree, soft and hard solids, (2) assessment of oral containment, bolus formation, oral transit, swallow reflex, laryngeal elevation, voice quality changes and coughs or signs of aspiration. A modified barium swallowing or fiberoptic endoscopic evaluation of swallowing was further recommended when necessary. The reported incidence of dysphagia in this study was 42.9%.
A single study by Bapat et al classified odynophagia as painful swallowing. The reported incidence was 40.3%.
Dysphonia
A single study defined dysphonia as changes or difficulty in vocal sound production reported by the patient (rating = 2, incidence = 0.6%). 12 A second study specified that patients who reported dysphonia underwent indirect laryngoscopy to detect vocal cord paralysis (rating = 2, incidence = 36.5%). 11
Swelling Complications
Other related complications included perioperative swelling (rating = 4) and soft tissue swelling. Two studies by Pourtaheri et al defined swelling complications as visible swelling of the surgical site, swallowing dysfunction, and/or breathing difficulties leading to (1) a delay in discharge during the index surgical hospitalization, (2) otolaryngological consultation as an outpatient, (3) a premature return to the office or to the emergency department after hospital discharge, (4) readmission for observation and medical management of swelling without surgical intervention, or (5) readmission for incision and drainage of the surgical site for actual or threatened airway compromise.15,21 No swelling complications were reported in either study.
Three studies defined soft tissue swelling on lateral radiographs using the following criteria: (1) the distance from the front of the spine at C2 and C3 to the posterior edge of the trachea15,21; and (2) the thickness of the prevertebral soft tissues at C2-C7 (measured from the front of the plate at plated levels). 19 The definition used by Pourtaheri et al scored 4 points on our rating scale as it specified that swelling was evaluated at 2-weeks following surgery.
Discussion
Complications are the most commonly used proxy for surgical quality of care. Unfortunately, there are no standardized criteria for defining or classifying complications following DCM surgery. This knowledge gap prevents an accurate evaluation of the safety of surgery, the identification of important risk factors and an assessment of the impact of complications on patient recovery, satisfaction and cost of care. Furthermore, without this information, it is challenging to predict and prevent adverse events, optimize patient status preoperatively and encourage shared decision-making among the surgeon, patient, and their family. This systematic review provides an overview of current definitions of dysphagia, dysphonia and other swelling-related complications. Based on our results, dysphagia should be evaluated using a combination of a dysphagia scale (e.g. dysphagia outcome severity scale), assessment by a speech language pathologist and, if deemed clinically appropriate, a barium-swallowing test. Diagnosis of dysphonia should be based on patient-reported changes in voice production as well as indirect laryngoscopy. Three additional reviews will summarize definitions used for biomechanical- or hardware-related, pain, and neurological complications. Together, these summaries will guide the development of a classification system for the reporting of surgical complications.
Dysphagia is one of the most common complications following anterior decompression of the cervical spine; in fact, some surgeons believe it to be an inevitable consequence of surgery.24,25 Patients with dysphagia suffer from dysfunction of the complex neurological sequence associated with swallowing and/or the structures involved in the swallowing process. 26 Dysfunction can occur during any of the 3 phases of swallowing, including the oral preparatory and transport phase (sucking, chewing and moving food or liquid into throat), the pharyngeal phase (starting the swallowing reflex, moving food down the throat, and closing off the airway to prevent aspiration and choking), and the esophageal phase (relaxing and tightening the openings at the top and bottom of the esophagus and moving food from the esophagus into the stomach). Dysphagia has important consequences as it may lead to social isolation, malnutrition, and delayed postoperative recovery.5-9 Furthermore, this complication can significantly impair a patient’s quality of life and increase the risk of aspiration pneumonia and mortality. 26
Rates of dysphagia are highly variable across studies and often depend on definitions, assessment tools, methods of data collection and timing of postoperative evaluation. Specifically, Anderson and Arnold reported that rates are often higher in prospective studies and with patient self-reports compared to rates collected retrospectively through chart review. 26 Furthermore, Danto et al suggested that interviewing the patient regarding symptoms of dysphagia yields a higher incidence than depending on patient self-reports. 27 Swallowing ability and the extent of dysfunction can also be objectively assessed using physiologic instruments such as a barium swallowing test. 28 The results of these tests, however, do not necessarily correlate with patient symptoms, and are often inadequate for complete diagnosis, as dysphagia is a subjective sensation of swallowing disturbance. 26
The Bazaz criteria are often used to qualify the severity of dysphagia. 22 According to Skeppholm et al, however, there are several limitations to this scale: (1) it is clinician-administered, which may introduce bias, (2) it has not been validated, (3) its categories may be too broad to distinguish patients with varying severities, and (4) it ascribes more importance to swallowing solids than liquids. 29 Given these limitations, other strategies should be used to evaluate severity. Of note, the Dysphagia Outcome and Severity Scale is an easy-to-use, 7-point scale that incorporates a patient’s independence, nutrition, and diet (Table 4). 30 This scale also focuses on characteristics of impaired swallowing, including the degree of bolus loss; oral retention and the ability to compensate with or without cueing; pharyngeal retention (in valleculae and/or pyriform sinuses) and the ability to clear retention with or without cueing; and penetration-aspiration. Severity may also be classified based on results from a bedside swallowing assessment and a modified barium swallowing test. The categories of this assessment include (1) mild; delayed bolus control and transport or mild stasis without laryngeal penetration, (2) moderate; poor oral transport, pharyngeal stasis with all consistencies, laryngeal penetration or mild aspiration with only one consistency, and (3) severe; substantial aspiration or inability to swallow. 31
Table 4 provides a summary of relevant, available tools. Furthermore, both clinical and laboratory assessments are critical for diagnosing dysphagia and identifying its cause. 26 Physical examination includes the evaluation of oral reflexes and sensation, saliva management, level of arousal, motor function of face, lips, tongue, palate and larynx, and cranial nerve function (sensory components of cranial nerves V, IX and X, and motor components of cranial nerves V, VII, X, XI and XII). Instruments used to evaluate dysphagia include (1) cervical radiographs to exclude structurally-induced dysphagia (e.g. graft dislodgement, retropharyngeal abscess, postoperative edema or hematoma); (2) videofluoroscopy to visualize the oral cavity, pharynx and esophagus; determine the presence, severity and timing of aspiration; and assess impairment of the swallowing mechanism; (3) endoscopy to evaluate the esophagus; (4) ultrasound to observe movement of swallowing structures; and (5) electromyography to analyze electric activity of swallowing muscles. 26 These tests can be used to distinguish between the types of dysphagia, differentiate between a functional disorder and a structural problem, determine the risk of aspiration, and evaluate the mechanics of swallowing.
In this review, only 2 studies were identified that defined dysphonia.11,12 The reported incidences ranged from 0.63% to 36.5% depending on definitions, the timing of postoperative evaluation, sample sizes and inclusion criteria. For example, the study by Tetreault et al had a larger sample size than the study by Bapat et al and included patients treated both anteriorly and posteriorly. Dysphonia, however, is typically a complication of anterior surgery; as a result, the rate reported by Tetreault et al would likely be higher in a cohort of only anteriorly treated patients. Dysphonia is most commonly caused by damage to the recurrent laryngeal nerve, which is susceptible to stretch injury or transection in almost all cervical procedures, but can also result from injury to the superior laryngeal nerve during operations involving the upper cervical region near C3-C4. 32 The variability of reported rates in this review indicates a need to standardize definitions of this complication. Consolidating nomenclature will reduce subjectivity in the reporting of complications, enhance accuracy when evaluating the safety of surgery and enable physicians to better convey risks to their patients.
This review represents the first part of a larger effort to develop guidelines for reporting surgical complications in DCM surgery. This process will require the compilation of results from the current body of literature as well as professional opinion. Before the development of this classification system, we suggest that future research on the safety of surgery report the following factors: definition of the complication studied, method of data collection (e.g. nurse, surgeon, research coordinator), duration of follow-up, rates and cause of mortality, grading system used to evaluate severity, readmission or reoperation rates and percentage lost to follow-up. This knowledge will allow for more accurate reporting of complications, facilitate improved interpretation of results on surgical safety, and enable integration of larger national and international databases through common data elements.
Limitations
This review focused specifically on patients with DCM and excluded those with radiculopathy, tumors, infection or trauma. However, these etiologies may also be treated with anterior cervical spine surgery. Thus, as a result of our strict inclusion and exclusion criteria, our review may not have fully captured all measures used to assess postoperative dysphagia. However, this study is part of a larger project (RECODE-DCM) that aims to develop a minimum dataset and standardize definitions (including surgical complications) in exclusively DCM.
Conclusions
Reported incidences of dysphagia, dysphonia and other swelling-related complications vary widely in DCM surgery. There is a pressing need to standardize definitions and develop guidelines for accurately reporting surgical complications. In the interim, we suggest that authors define complications in accordance with our 4-point rating scale.
Acknowledgments
MGF wishes to acknowledge the Gerry and Tootsie Halbert Chair in Neural Repair and Regeneration as well as the DeZwirek Family Foundation.
Footnotes
Authors’ Note: Lindsay Tetreault, MD, PhD, and Stefan F. Lange, MD, are co-authors.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Jefferson R. Wilson, MD, PhD  https://orcid.org/0000-0001-5965-0305
https://orcid.org/0000-0001-5965-0305
Benjamin M. Davies, MBChBH, BScH  https://orcid.org/0000-0003-0591-5069
https://orcid.org/0000-0003-0591-5069
Michael G. Fehlings, MD, PhD  https://orcid.org/0000-0002-5722-6364
https://orcid.org/0000-0002-5722-6364
References
- 1.Nouri A, Tetreault L, Singh A, Karadimas SK, Fehlings MG. Degenerative cervical myelopathy: epidemiology, genetics, and pathogenesis. Spine (Phila Pa 1976). 2015;40(12):E675–E693. [DOI] [PubMed] [Google Scholar]
- 2.Lawrence BD, Shamji MF, Traynelis VC, et al. Surgical management of degenerative cervical myelopathy: a consensus statement. Spine (Phila Pa 1976). 2013;38(22 suppl 1):S171–S172. [DOI] [PubMed] [Google Scholar]
- 3.Fehlings MG, Tetreault LA, Kurpad S, et al. Change in functional impairment, disability, and quality of life following operative treatment for degenerative cervical myelopathy: a systematic review and meta-analysis. Global Spine J. 2017;7(3 suppl):53S–69S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lawrence BD, Jacobs WB, Norvell DC, Hermsmeyer JT, Chapman JR, Brodke DS. Anterior versus posterior approach for treatment of cervical spondylotic myelopathy: a systematic review. Spine (Phila Pa 1976). 2013;38(22 suppl 1):S173–S182. [DOI] [PubMed] [Google Scholar]
- 5.Ferraris VA, Ferraris SP, Moritz DM, Welch S. Oropharyngeal dysphagia after cardiac operations. Ann Thorac Surg. 2001;71(6):1792–1795; discussion 1796. [DOI] [PubMed] [Google Scholar]
- 6.Gee E, Lancaster E, Meltzer J, Mendelsohn AH, Benharash P. A targeted swallow screen for the detection of postoperative dysphagia. Am Surg. 2015;81(10):979–982. [DOI] [PubMed] [Google Scholar]
- 7.Ochoa JB. Nutrition assessment and intervention in the patient with dysphagia: challenges for quality improvement. Nestle Nutri Inst Workshop Ser. 2012;72:77–83. [DOI] [PubMed] [Google Scholar]
- 8.Nguyen NP, Frank C, Moltz CC, et al. Impact of dysphagia on quality of life after treatment of head-and-neck cancer. Int J Radiat Oncol Biol Phys. 2005;61(3):772–778. [DOI] [PubMed] [Google Scholar]
- 9.Garcia-Peris P, Paron L, Velasco C, et al. Long-term prevalence of oropharyngeal dysphagia in head and neck cancer patients: impact on quality of life. Clin Nutr. 2007;26(6):710–717. [DOI] [PubMed] [Google Scholar]
- 10.Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bapat MR, Chaudhary K, Sharma A, Laheri V. Surgical approach to cervical spondylotic myelopathy on the basis of radiological patterns of compression: prospective analysis of 129 cases. Eur Spine J. 2008;17(12):1651–1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tetreault L, Tan G, Kopjar B, et al. Clinical and surgical predictors of complications following surgery for the treatment of cervical spondylotic myelopathy: results from the multicenter, prospective AOSpine international study of 479 patients. Neurosurgery. 2016;79(1):33–44. [DOI] [PubMed] [Google Scholar]
- 13.Lin Q, Zhou X, Wang X, Cao P, Tsai N, Yuan W. A comparison of anterior cervical discectomy and corpectomy in patients with multilevel cervical spondylotic myelopathy. Eur Spine J. 2012;21(3):474–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yu S, Li F, Yan N, Yuan C, He S, Hou T. Anterior fusion technique for multilevel cervical spondylotic myelopathy: a retrospective analysis of surgical outcome of patients with different number of levels fused. PLoS One. 2014;9(3):e91329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pourtaheri S, Emami A, Hwang K, et al. Cervical corpectomy with ultra-low-dose rhBMP-2 in high-risk patients: 5-year outcomes. Orthopedics. 2013;36(12):931–935. [DOI] [PubMed] [Google Scholar]
- 16.Song KJ, Lee KB, Song JH. Efficacy of multilevel anterior cervical discectomy and fusion versus corpectomy and fusion for multilevel cervical spondylotic myelopathy: a minimum 5-year follow-up study. Eur Spine J. 2012;21(8):1551–1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang ZD, Zhu RF, Yang HL, et al. The application of a zero-profile implant in anterior cervical discectomy and fusion. J Clin Neurosci. 2014;21(3):462–466. [DOI] [PubMed] [Google Scholar]
- 18.Wang Z, Zhu R, Yang H, et al. Zero-profile implant (Zero-p) versus plate cage benezech implant (PCB) in the treatment of single-level cervical spondylotic myelopathy. BMC Musculoskelet Disord. 2015;16:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang L, Gu Y, Liang L, et al. Stand-alone anchored spacer versus anterior plate for multilevel anterior cervical diskectomy and fusion. Orthopedics. 2012;35(10):e1503–1510. [DOI] [PubMed] [Google Scholar]
- 20.Chen CJ, Saulle D, Fu KM, Smith JS, Shaffrey CI. Dysphagia following combined anterior-posterior cervical spine surgeries clinical article. J Neurosurg Spine. 2013;19(3):279–287. [DOI] [PubMed] [Google Scholar]
- 21.Pourtaheri S, Hwang K, Faloon M, et al. Ultra-low-dose recombinant human bone morphogenetic protein-2 for 3-level anterior cervical diskectomy and fusion. Orthopedics. 2015;38(4):241–245. [DOI] [PubMed] [Google Scholar]
- 22.Bazaz R, Lee MJ, Yoo JU. Incidence of dysphagia after anterior cervical spine surgery: a prospective study. Spine (Phila Pa 1976). 2002;27(22):2453–2458. [DOI] [PubMed] [Google Scholar]
- 23.Siska PA, Ponnappan RK, Hohl JB, Lee JY, Kang JD, Donaldson WF, III. Dysphagia after anterior cervical spine surgery: a prospective study using the swallowing-quality of life questionnaire and analysis of patient comorbidities. Spine (Phila Pa 1976). 2011;36(17):1387–1391. [DOI] [PubMed] [Google Scholar]
- 24.Campbell PG, Yadla S, Malone J, et al. Early complications related to approach in cervical spine surgery: single-center prospective study. World Neurosurg. 2010;74(2-3):363–368. [DOI] [PubMed] [Google Scholar]
- 25.Kalb S, Reis MT, Cowperthwaite MC, et al. Dysphagia after anterior cervical spine surgery: incidence and risk factors. World Neurosurg. 2012;77(1):183–187. [DOI] [PubMed] [Google Scholar]
- 26.Anderson KK, Arnold PM. Oropharyngeal dysphagia after anterior cervical spine surgery: a review. Global Spine J. 2013;3(4):273–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Danto J, DiCapua J, Nardi D, et al. Multiple cervical levels: increased risk of dysphagia and dysphonia during anterior cervical discectomy. J Neurosurg Anesthesiol. 2012;24(4):350–355. [DOI] [PubMed] [Google Scholar]
- 28.Riley LH, III, Vaccaro AR, Dettori JR, Hashimoto R. Postoperative dysphagia in anterior cervical spine surgery. Spine (Phila Pa 1976). 2010;35(9 suppl):S76–S85. [DOI] [PubMed] [Google Scholar]
- 29.Skeppholm M, Ingebro C, Engstrom T, Olerud C. The dysphagia short questionnaire: an instrument for evaluation of dysphagia: a validation study with 12 months’ follow-up after anterior cervical spine surgery. Spine (Phila Pa 1976). 2012;37(11):996–1002. [DOI] [PubMed] [Google Scholar]
- 30.O’Neil KH, Purdy M, Falk J, Gallo L. The Dysphagia Outcome and Severity Scale. Dysphagia. 1999;14(3):139–145. [DOI] [PubMed] [Google Scholar]
- 31.Ott DJ, Hodge RG, Pikna LA, Chen MY, Gelfand DW. Modified barium swallow: clinical and radiographic correlation and relation to feeding recommendations. Dysphagia. 1996;11(3):187–190. [DOI] [PubMed] [Google Scholar]
- 32.Chen CC, Huang YC, Lee ST, Chen JF, Wu CT, Tu PH. Long-term result of vocal cord paralysis after anterior cervical disectomy. Eur Spine J. 2014;23(3):622–626. [DOI] [PMC free article] [PubMed] [Google Scholar]