Abstract
Background
The relationship between non-alcoholic fatty liver degree as well as non-alcoholic fatty liver disease (NAFLD) and irritable bowel syndrome (IBS) remains poorly understood. We aimed to investigate the prospective association of non-alcoholic fatty liver degree as well as NAFLD with incident IBS in a large-scale population-based cohort.
Methods
Participants free of IBS, coeliac disease, inflammatory bowel disease, alcoholic liver disease, and any cancer at baseline from the UK Biobank were included. Non-alcoholic fatty liver degree was measured by a well-validated fatty liver index (FLI), with FLI ≥ 60 as an indicator of NAFLD. Primary outcome was incident IBS. Cox proportional hazard model was used to investigate the associated risk of incident IBS.
Results
Among 396,838 participants (mean FLI was 48.29 ± 30.07), 153,203(38.6%) were with NAFLD diagnosis at baseline. During a median of 12.4-year follow-up, 7129 cases of incident IBS were identified. Compared with non-NAFLD, NAFLD patients showed a 13% higher risk of developing IBS (HR = 1.13, 95%CI: 1.05–1.17) after multivariable adjustment. Compared with the lowest, the highest FLI quartile was associated with a significantly increased risk of IBS (HRQ4 VS Q1 = 1.21, 1.13–1.30, Ptrend < 0.001). Specifically, the positive association between non-alcoholic fatty liver degree and IBS was also observed by per SD change of FLI (adjusted HR = 1.08, 1.05–1.10). Further sensitivity analysis and subgroup analysis indicated similar results, with the positive association particularly observed in females, but not in males.
Conclusions
High degree of non-alcoholic fatty liver as well as non-alcoholic fatty liver disease is associated with increased risk of incident IBS. Further studies are warranted to confirm the findings and elucidate the underlying biological mechanisms.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12916-022-02460-8.
Keywords: Non-alcoholic fatty liver disease, Irritable bowel syndrome, Cohort study
Background
Irritable bowel syndrome (IBS) is a common disorder of gut-brain interaction, characterized by recurrent abdominal pain accompanied by altered bowel habits and bloating without any organic lesions [1, 2]. Recent global epidemiological study reported an estimated 10.1% and 4.1% of the population suffered from IBS according to Rome III and IV criteria, respectively [3]. IBS remains a major disorder associated with reduced health-quality of life, leading to considerable medical costs and a significant economic burden to both patients and the whole society [4, 5]. Therefore, it is of high priority to identify contributing factors, particularly modifiable lifestyle risk factors, to help develop targeted prevention strategies. However, to date, such evidence is limited.
Non-alcoholic fatty liver disease (NAFLD) is defined as excessive hepatic steatosis in the absence of specific causes (i.e., alcohol consumption, hepatitis B or C infection) [6]. Approximately 25% of adult population is affected by NAFLD currently, with worsening epidemic in recent decades coinciding with rising IBS incidence [3, 7, 9]. The factors for pathogenesis of NAFLD are not well clarified. Previous findings suggest insulin resistance, metabolic syndrome, obesity as well as inflammation are involved in the process [8–10]. Growing evidence by several animal and vitro experimental studies supports a plausible link between NAFLD and IBS, including shared proinflammatory cytokines (i.e., increased expressions of tumor necrosis factor (TNF)-α, interleukin (IL)-6, IL-8 and IL-1β, decreased levels of IL-10), cross-talk of liver-brain-gut neural arc and gut-liver axis, dysfunction of intestinal microbiota and impaired intestinal barrier as well as intestinal dysmotility [11–17]. Investigation of fatty liver degree and NAFLD associated with IBS risk is an urgent need considering growing disease burden of IBS. However, to the best of our knowledge, there are no prior epidemiological studies examining NAFLD as well as the degree of fatty liver in relation to risk of incident IBS. Whether fatty liver could affect functions of the gastrointestinal tract and further lead to syndromic manifestations typical of IBS remains to be answered yet.
To address these knowledge gaps, we prospectively investigated the association of non-alcoholic fatty liver degree, as well as NAFLD, with risk of incident IBS in a large population-based long-term follow-up UK cohort.
Methods
Study population
This study population was composed of over 500,000 participants from an ongoing large-scale prospective cohort, UK Biobank (UKB). Briefly, participants ranging from 37 to 73 years of age from 22 assessment centers across England, Wales, and Scotland were enrolled between 2006 and 2010. All participants completed baseline questionnaires with anthropometric assessments and reported medical conditions. Details of UKB design were described elsewhere [18]. The study was approved by North West Multicenter Research Ethical Committee, and all participants signed written informed content.
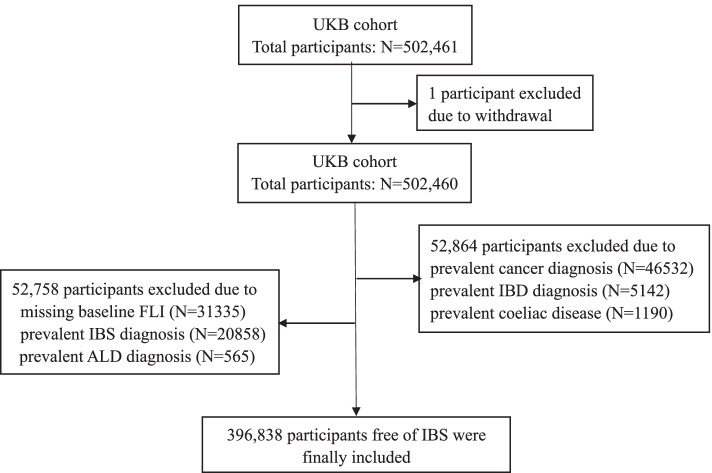
Participants who were free of IBS with an available non-alcoholic fatty liver index at enrollment were included in this study. Those who already had cancer, inflammatory bowel disease (IBD), alcoholic liver disease (ALD), or coeliac disease diagnosis at enrollment were excluded. All diagnoses were identified through International Classification of Disease-10 (ICD-10) codes (Additional file 1: Table S1). Additionally, 1 participant withdrawal from UKB was excluded. Therefore, a total of 396,838 participants were included in the final analysis. Flowchart of participant selection was listed in Fig. 1.
Fig. 1.
Flowchart of the study population. UKB: UK Biobank; IBS: irritable bowel syndrome; IBD: inflammatory bowel disease; ALD: alcoholic liver disease; FLI: fatty liver index
Assessment of baseline non-alcoholic fatty liver degree and NAFLD
As no imaging, ultrasonography, or histological data regarding fatty liver was available in the large-scale UKB cohort, we used a well-established index, fatty liver index (FLI), to measure the degree of non-alcoholic fatty liver [19]. Briefly, FLI was calculated through four variables including BMI, waist circumstance (WC), triglycerides (TG), and gamma-glutamyltransferase (GGT) using a previously published and validated regression model [19]. It has been proved to be a reliable index with good discrimination performance of liver ultrasonography-determined NAFLD [area under the receiver operator curve, AUROC = 0.85 (95%CI: 0.81–0.88)] and transient elastography-determined NAFLD (AUROC = 0.85), which has been externally validated and widely accepted in a population-based study [19–21]. Meanwhile, the weighted percent-agreement between FLI and transient elastography was as high as 75.11% (95%CI: 75.10%-75.12%) when validated in a nationally representative sample of the western general population rather than a clinical population [21]. We classified FLI according to quartile distribution with the lowest quartile group as the reference group and the other three quartile groups as exposure groups. Moreover, we also used NAFLD diagnosis or not according to a predefined cutoff, with FLI ≥ 60 as an indicator of NAFLD [19]. Participants who had baseline FLI < 60 were considered in the non-exposure group (non-NAFLD group), while others who were diagnosed as NAFLD were considered in the exposure group (NAFLD group). Further, NAFLD patients with BMI < 25 kg/m2 and ≥ 25 kg/m2 would be defined as lean and non-lean NAFLD, respectively. Accordingly, NAFLD patients with BMI ≥ 30 kg/m2 and < 30 kg/m2 would be considered as obese and non-obese NAFLD, separately [22, 23]. Besides, in order to examine the impact of fatty liver measurement on our findings, another well-established index, hepatic steatosis index (HSI), was used to define NAFLD in sensitivity analyses. HSI could be calculated as 8* (serum alanine aminotransferase (ALT)/aspartate aminotransferase (AST) ratio) + BMI (+ 2, if female; + 2, if type 2 diabetes) [24]. An HSI > 36 was defined as an indicator of NAFLD [24].
Ascertainment of outcome
Primary endpoint was incident IBS, which was determined via ICD-10 codes (K58, Additional file 1: Table S1). IBS diagnosis was based on self-report or linkage to primary care and/or hospital admission data with a censoring date of June 2021.
Covariates
Based on epidemiological evidence, some sociodemographic characteristics, health behaviors, and comorbidities at baseline were adjusted as covariates [1, 4, 16, 17]. Potential confounders included age (continuous variable), gender (male or female), ethnicity (white or nonwhite), socioeconomic status, education level, smoking status (never, current, or previous), alcohol drinking (never, current, or previous), type 2 diabetes (Yes or No) and physical activity. Socioeconomic status was based on the Townsend deprivation index, which was calculated immediately prior to participants joining UKB using preceding national census output areas [25]. Townsend deprivation index for socioeconomic deprivation was divided into four quartiles. Education was based on self-report of the highest qualification achieved and classified as university or non-university. Physical activity was self-reported and divided into three levels (high, moderate, and low) based on IPAQ (International Physical Activity Questionnaire).
Statistical analysis
Incidence rate with 95% confidence interval (CI) of IBS was calculated as a number of events per 1000 person-years through Poisson regression. The 12-year cumulative incidence of IBS was calculated by the Kaplan–Meier method. Cox proportional hazard model was conducted to examine the association between fatty liver and incident IBS. The follow-up period started from baseline to the date of first IBS diagnosis or censored at end of the study (June 2021), date of death, or lost-to-follow-up for participants who did not develop IBS. Considering a very small percentage of missing values (0.1–1.2% for all covariates were missing), missing indicators were used.
For FLI quartiles, per standard deviation (SD) change of FLI and diagnosis of NAFLD or not according to predefined cutoff, three multivariable models in addition to univariable analysis were accomplished: model 1, adjusted for age and gender; model 2, additionally adjusted for Townsend deprivation index, education level, ethnicity, smoking status, and alcohol drinking; model 3, additionally adjusted for physical activity and type 2 diabetes. Moreover, restricted cubic spline analysis was conducted to examine the potential non-linear association between baseline FLI and incident IBS, with knots placed at 10th, 50th, and 90th percentiles and the median value of baseline FLI (46.55) as a reference point. Furthermore, subgroup analysis was performed to investigate whether the association between the degree of non-alcoholic fatty liver as well as NAFLD and IBS varied by age (< 45 years, 45-64 years, ≥ 65 years), gender, alcohol drinking, and smoking status. Effect modification was also detected by adding interaction terms of each stratified variable (i.e., age, gender, alcohol drinking, smoking status) and non-alcoholic fatty liver exposure (i.e., FLI quartiles, per SD change of FLI, diagnosis of NAFLD or not). Further analyses were conducted to investigate the association between NAFLD type (lean/non-lean, non-obese/obese NAFLD) and risk of IBS.
In order to assess the robustness of the results, several sensitivity analyses were conducted. Firstly, we excluded participants who had an IBS diagnosis within 1 or 2 years after recruitment respectively, in order to avoid detection bias. Secondly, to rule out the influence of alcohol intake on the non-alcoholic fatty liver during the whole follow-up period, incident ALD cases after baseline were further excluded. Thirdly, the competing risk model by considering lost-to-follow-up and death as competing events were conducted, since those participants might develop IBS thereafter. Fourthly, participants who had hepatitis B/C virus seropositivity were excluded. Fifthly, we additionally adjusted psychologic disorder including depression and anxiety as confounders. Finally, an age-matched (1:1 matching, ± 2 years) cohort was generated as the new dataset to further investigate the association between NAFLD and IBS.
Additionally, sensitivity analyses were conducted by using HSI [diagnosis of NAFLD or not according to predefined cutoff (HSI > 36), per SD change] via adjusted model 3, with additional similar analyses by excluding incident IBS cases within 1 or 2 years after baseline, excluding incident ALD cases, excluding participants with hepatitis B/C virus seropositivity or performing competing risk model.
A two-tailed P value < 0.05 was considered to be statistically significant. All analyses were conducted using SAS software Version 9.4 and R version 4.0.2 (forestplot, tableone, ggplot2, and survival packages).
Results
Baseline characteristics
Of all 396,838 participants, 47.8% were male. The mean (SD) age was 56.22 (8.11) years at enrollment. The mean FLI was 48.29 ± 30.07. Overall, 153,203(38.6%) participants had a NAFLD diagnosis (FLI ≥ 60) before or at enrollment. Participants in the highest quartile of FLI were more likely to be male, have a lower education level, a lower level of socioeconomic deprivation, higher BMI and WC, a higher level of TG, GGT, and ALT and a higher proportion of prevalent diabetes (Table 1). Baseline characteristics of the study cohort according to the diagnosis of NAFLD or not were listed in Additional file 1: Table S2. Median follow-up period was 12.4 years (interquartile range: 11.6–13.1 years).
Table 1.
Baseline characteristics according to baseline fatty liver index in the UK Biobank cohort
| Characteristic | Total (N = 396,838) | Quartile 1 (N = 98,371) | Quartile 2 (N = 99,289) | Quartile 3 (N = 99,714) | Quartile 4 (N = 99,464) | P value |
|---|---|---|---|---|---|---|
| Age (years)a | 56.22 ± 8.11 | 54.22 ± 8.21 | 56.63 ± 8.10 | 57.19 ± 7.98 | 56.83 ± 7.82 | < 0.001 |
| Gender | < 0.001 | |||||
| Male | 189,759 (47.8) | 18,005 (18.3) | 44,415 (44.7) | 60,465 (60.6) | 66,874 (67.2) | |
| Female | 207,079 (52.2) | 80,366 (81.7) | 54,874 (55.3) | 39,249 (39.4) | 32,590 (32.8) | |
| Ethnicity | < 0.001 | |||||
| Non-White | 22,788 (5.7) | 4840 (4.9) | 6078 (6.1) | 6445 (6.5) | 5425 (5.5) | |
| White | 372,582 (93.9) | 93,243 (94.8) | 92,834 (93.5) | 92,871 (93.1) | 93,634 (94.1) | |
| Unknown | 1468 (0.4) | 288 (0.3) | 377 (0.4) | 398 (0.4) | 405 (0.4) | |
| Education level | < 0.001 | |||||
| Non-university | 261,356 (65.9) | 56,972 (57.9) | 63,886 (64.3) | 67,902 (68.1) | 72,596 (73.0) | |
| University | 130,800 (33.0) | 40,552 (41.2) | 34,307 (34.6) | 30,466 (30.6) | 25,475 (25.6) | |
| Unknown | 4682 (1.2) | 847 (0.9) | 1096 (1.1) | 1346 (1.3) | 1393 (1.4) | |
| Townsend deprivation index | ||||||
| Mean (SD) | − 1.30 (3.09) | − 1.51 (2.97) | − 1.46 (3.02) | − 1.35 (3.08) | − 0.90 (3.24) | < 0.001 |
| Q1(≤ − 3.63) | 99,950 (25.2) | 26,497 (26.9) | 26,241 (26.4) | 25,483 (25.6) | 21,729 (21.8) | < 0.001 |
| Q2(− 3.63 to − 2.12) | 99,303 (25.0) | 25,220 (25.6) | 25,605 (25.8) | 25,311 (25.4) | 23,167 (23.3) | |
| Q3(− 2.12–0.58) | 99,259 (25.0) | 24,667 (25.1) | 24,710 (24.9) | 24,739 (24.8) | 25,143 (25.3) | |
| Q4 (> 0.58) | 97,832 (24.7) | 21,869 (22.2) | 22,617 (22.8) | 24,047 (24.1) | 29,299 (29.5) | |
| Unknown | 494 (0.1) | 118 (0.1) | 116 (0.1) | 134 (0.1) | 126 (0.1) | |
| Smoking status | < 0.001 | |||||
| Never | 218,022 (54.9) | 61,575 (62.6) | 57,310 (57.7) | 52,689 (52.8) | 46,448 (46.7) | |
| Previous | 135,124 (34.1) | 27,564 (28.0) | 31,237 (31.5) | 35,645 (35.7) | 40,678 (40.9) | |
| Current | 41,725 (10.5) | 8894 (9.0) | 10,288 (10.4) | 10,857 (10.9) | 11,686 (11.7) | |
| Unknown | 1967 (0.5) | 338 (0.3) | 454 (0.5) | 523 (0.5) | 652 (0.7) | |
| Alcohol drinking | < 0.001 | |||||
| Never | 17,303 (4.4) | 4221 (4.3) | 4224 (4.3) | 4365 (4.4) | 4493 (4.5) | |
| Previous | 13,388 (3.4) | 3058 (3.1) | 2957 (3.0) | 3270 (3.3) | 4103 (4.1) | |
| Current | 365,177 (92.0) | 90,914 (92.4) | 91,874 (92.5) | 91,812 (92.1) | 90,577 (91.1) | |
| Unknown | 970 (0.2) | 178 (0.2) | 234 (0.2) | 267 (0.3) | 291 (0.3) | |
| IPAQ | < 0.001 | |||||
| Low | 59,637 (15.0) | 10,788 (11.0) | 12,741 (12.8) | 15,232 (15.3) | 20,876 (21.0) | |
| Moderate | 131,111 (33.0) | 33,043 (33.6) | 32,875 (33.1) | 33,318 (33.4) | 31,875 (32.0) | |
| High | 131,356 (33.1) | 37,193 (37.8) | 35,528 (35.8) | 32,338 (32.4) | 26,297 (26.4) | |
| Unknown | 74,734 (18.8) | 17,347 (17.6) | 18,145 (18.3) | 18,826 (18.9) | 20,416 (20.5) | |
| BMI | < 0.001 | |||||
| < 18.5 kg/m2 | 1896 (0.5) | 1858 (1.9) | 31 (0.0) | 7 (0.0) | 0 (0.0) | |
| 18.5–24.9 kg/m2 | 124,057 (31.3) | 79,531 (80.8) | 35,633 (35.9) | 8257 (8.3) | 636 (0.6) | |
| 25.0–29.9 kg/m2 | 171,674 (43.3) | 16,904 (17.2) | 59,704 (60.1) | 68,734 (68.9) | 26,332 (26.5) | |
| ≥ 30 kg/m2 | 99,211 (25.0) | 78 (0.1) | 3921 (3.9) | 22,716 (22.8) | 72,496 (72.9) | |
| Diabetes | 10,014 (2.5) | 437 (0.4) | 1159 (1.2) | 2281 (2.3) | 6137 (6.2) | < 0.001 |
| WC (cm)a | 90.47 (13.43) | 75.31 (6.29) | 86.02 (6.01) | 94.10 (6.20) | 106.24 (9.84) | < 0.001 |
| TG (mg/dL)a | 154.40(91.02) | 90.82 (33.99) | 127.80(51.69) | 168.45(73.41) | 229.75(115.16) | < 0.001 |
| GGT (U/L)b | 26.40 (18.60, 41.00) | 17.30 (14.10, 22.20) | 23.00 (18.00, 31.10) | 30.60 (23.00, 43.50) | 44.30 (30.80, 69.10) | < 0.001 |
| ALT (U/L)b | 20.25 (15.47, 27.57) | 15.50 (12.60, 19.20) | 18.60 (15.00, 23.50) | 22.40 (17.70, 29.00) | 28.10 (21.20, 38.20) | < 0.001 |
| AST (U/L)b | 24.40 (21.00, 28.90) | 22.50 (19.60, 26.10) | 23.70 (20.70, 27.50) | 25.00 (21.60, 29.20) | 27.00 (22.90, 32.70) | < 0.001 |
| FLIa | 48.29 (30.07) | 10.76 (4.98) | 32.52 (7.66) | 61.12 (8.53) | 88.26 (6.97) | < 0.001 |
| FLI ≥ 60 | 153,203 (38.6) | 0 (0.0) | 0 (0.0) | 53,739 (53.9) | 99,464 (100.0) | < 0.001 |
| HSIa | 35.60 (5.87) | 30.21 (2.78) | 33.42 (3.10) | 36.48 (3.54) | 42.25 (5.42) | < 0.001 |
| HSI > 36 | 163,136 (41.3) | 2012 (2.1) | 19,663 (19.9) | 52,180 (52.5) | 89,281 (90.3) | < 0.001 |
Note: Numbers are n (%) unless otherwise stated
IPAQ International Physical Activity Questionnaire, BMI body mass index, WC waist circumstance, TG triglycerides, GGT gamma-glutamyltransferase, ALT alanine aminotransferase, AST aspartate aminotransferase, FLI fatty liver index, HSI hepatic steatosis index
adisplayed as mean ± standard deviation
bdisplayed as median (interquartile range)
Baseline non-alcoholic fatty liver and risk of incident IBS
During a total of 4,776,162 person-years’ follow-up, 7129 cases of incident IBS were identified. Cumulative incidence rate of IBS was 1.49 (95%CI: 1.46–1.53) per 1000 person-years. The 12-year cumulative incidence of IBS was 1.8% (95%CI: 1.7–1.9%), 1.7% (1.6–1.8%), and 1.8% (1.7–1.9%) in quartile 2, 3 and 4 groups versus 1.9% (1.8–2.0%) in the lowest quartile group. Cox proportional hazard regression model with restricted cubic spline indicated baseline FLI was linearly associated with risk of IBS (P = 0.383, Additional file 2: Fig. S1). Fatty liver was associated with a 21% risk increase of IBS (HRQ4 VS Q1 = 1.21, 95%CI: 1.13–1.30, Ptrend < 0.001) according to fully adjusted model 3 (Table 2). Meanwhile, compared with the lowest quartile group (Q1), both FLI Q2 (adjusted HR = 1.12, 1.05–1.20) and Q3 (adjusted HR = 1.17, 1.10–1.26) groups were associated with a significantly higher risk of IBS.
Table 2.
Risk of IBS according to Quartiles of baseline fatty liver index
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | P for trend | |
|---|---|---|---|---|---|
| No. of participants | 98,371 | 99,289 | 99,714 | 99,464 | |
| No. of incident IBS | 1882 | 1797 | 1701 | 1749 | |
| Follow-up, person-years | 1,192,803 | 1,197,965 | 1,199,435 | 1,185,959 | |
| Follow-up, years | |||||
| Mean ± SD | 12.1 ± 1.8 | 12.1 ± 1.8 | 12.0 ± 1.9 | 11.9 ± 2.1 | |
| Median (IQR) | 12.4 (11.7–13.1) | 12.4 (11.6–13.1) | 12.4 (11.6–13.1) | 12.3 (11.6–13.1) | |
| Hazard ratio for incident IBS (95%CI, P value) | |||||
| Adjusted model 1 | Reference | 1.15 (1.08–1.23) | 1.24 (1.16–1.33) | 1.36 (1.27–1.46) | < 0.001 |
| Adjusted model 2 | Reference | 1.13 (1.06–1.21) | 1.20 (1.12–1.28) | 1.27 (1.19–1.37) | < 0.001 |
| Adjusted model 3 | Reference | 1.12 (1.05–1.20) | 1.17 (1.10–1.26) | 1.21 (1.13–1.30) | < 0.001 |
Note: Adjusted model 1: Age and gender were adjusted; Adjusted model 2: Townsend deprivation index, education level, ethnicity, smoking status, and alcohol drinking were additionally adjusted; Adjusted model 3: IPAQ (International Physical Activity Questionnaire), and type 2 diabetes were additionally adjusted; P for trend was calculated by using median value (10.5, 32.2, 61.2, and 88.6) of each fatty liver index quartile
IBS irritable bowel syndrome, SD standard deviation, IQR inter-quartile range
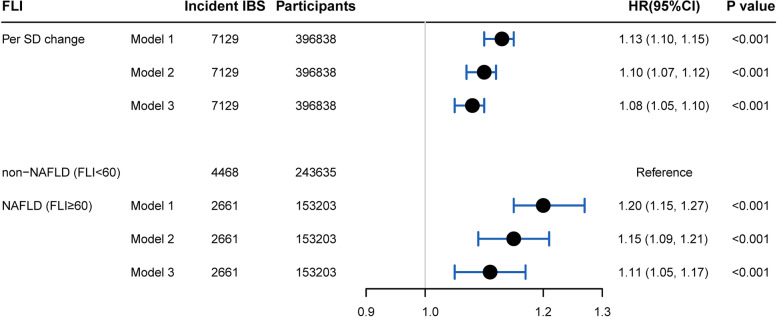
Furthermore, totally, 2661(1.52 per 1000 person-years) and 4468 (1.45 per 1000 person-years) incident IBS occurred in NAFLD and non-NAFLD groups, respectively. Although 12-year cumulative incidence of IBS seemed similar between NAFLD (1.8%, 1.7–1.8%) and non-NAFLD (1.8%, 1.8–1.9%) via the Kaplan–Meier method, NAFLD patients showed a 13% higher risk of developing IBS (HR = 1.13, 1.05–1.17) after multivariable adjustment compared with non-NAFLD (Fig. 2). Moreover, either lean, non-lean, non-obese, or obese NAFLD patients had an increased risk of incident IBS, with a HR of 1.22 (95%CI: 0.92–1.60), 1.11 (1.05–1.17), 1.14 (1.06–1.22), and 1.09 (1.03–1.16), respectively (Additional file 2: Fig. S2). Additionally, an 8% increased risk was associated with per SD change of FLI (Fig. 2).
Fig. 2.
The association between baseline FLI and incident IBS. Note: All adjusted HRs were calculated by adjusting the following covariates: age, gender, Townsend deprivation index, education level, ethnicity, smoking status, alcohol drinking, IPAQ (International Physical Activity Questionnaire), and type 2 diabetes. IBS: irritable bowel syndrome; FLI: fatty liver index; CI: confidence interval; HR: hazard ratio
Subgroup analysis
In subgroup analysis, the increased IBS risk associated with FLI quartiles was generally observed across age, gender, alcohol drinking, and smoking subgroups, except for age ≥ 65 years old, male, and previous alcohol drinking subgroups (Fig. 3A). Moreover, we observed significant interactions across age/gender and FLI quartiles (P for interaction 0.003 for age and 0.001 for gender).
Fig. 3.
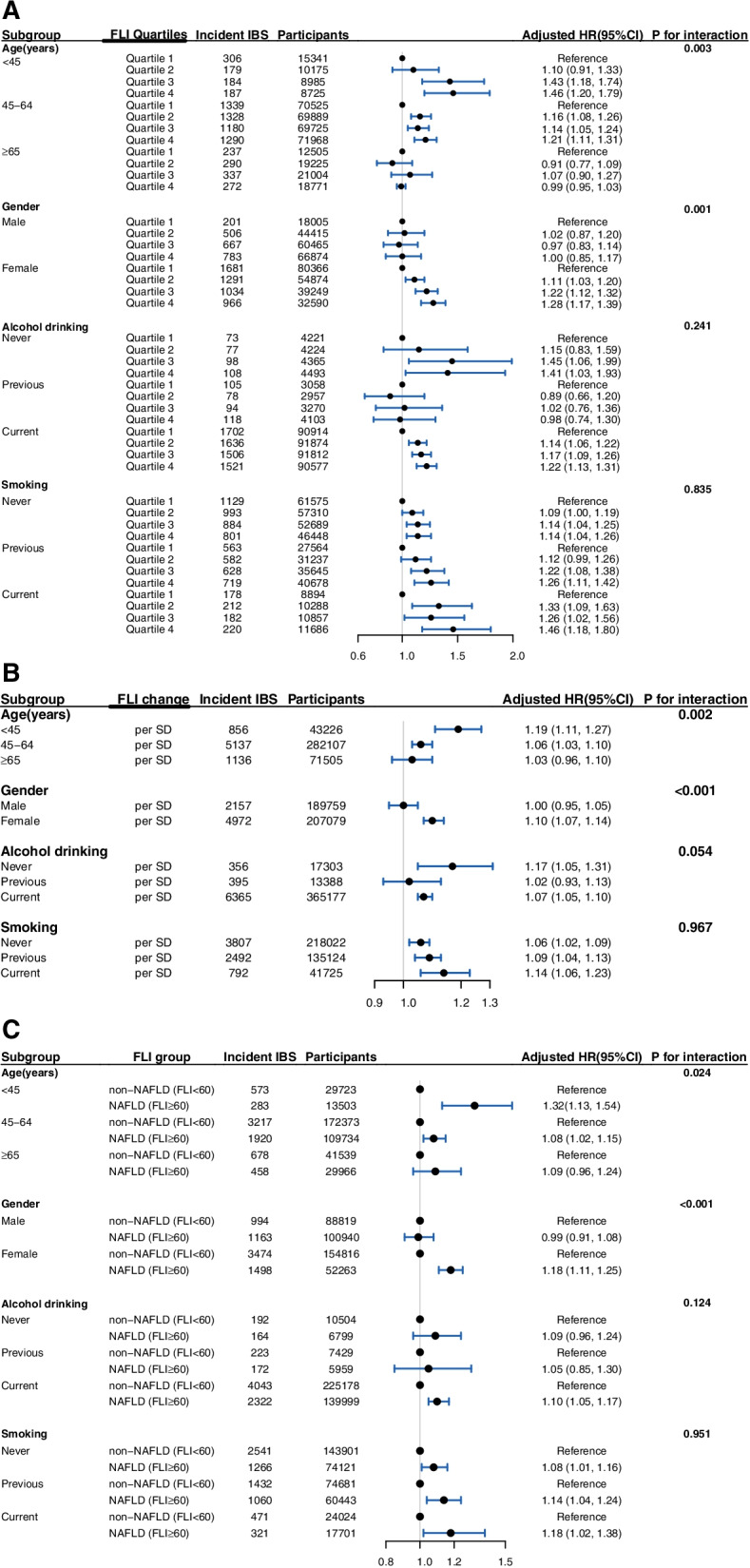
Subgroup analysis for the association between baseline FLI and incident IBS. A According to FLI quartiles. B According to FLI per SD change. C According to the diagnosis of NAFLD or not by the predefined cutoff of FLI. All adjusted HRs were calculated by adjusting the following covariates: age, gender, Townsend deprivation index, education level, ethnicity, smoking status, alcohol drinking, IPAQ (International Physical Activity Questionnaire) and type 2 diabetes; IBS: irritable bowel syndrome; FLI: fatty liver index; CI: confidence interval; HR: hazard ratio
Similarly, consistent subgroup findings were observed when using per SD change and diagnosis of NAFLD or not by a predefined cutoff of FLI (Fig. 3B, C). Significant modification effects by age and gender were both detected when using per SD change (P for interaction 0.002 for age and < 0.001 for gender) and diagnosis of NAFLD or not (P for interaction 0.024 for age and < 0.001 for gender). The increased IBS risk was observed in females but not in males.
Sensitivity analysis
Results of sensitivity analysis by quartiles, per SD change and diagnosis of NAFLD or not according to a predefined cutoff of FLI were similar to the main analysis, when excluding incident IBS cases within 1 year or 2 years after baseline, excluding incident ALD cases, excluding participants with hepatitis B/C virus seropositivity, performing competing risk model, additional adjusting depression and anxiety, or with the age-matched cohort as a dataset (Table 3, Additional file 1: Table S3). Moreover, results of sensitivity analysis by HSI, either treated as per SD change or diagnosis of NAFLD or not, were all consistent with principal findings (Additional file 1: Table S4).
Table 3.
Sensitivity analysis regarding the risk of IBS according to Quartiles of baseline fatty liver index
| FLI Quartiles | No. of IBS | No. of participants | Adjusted HR (95%CI) | P value | P for trend |
|---|---|---|---|---|---|
| Sensitivity analysis 1: excluding IBS participants diagnosed within 1 year after baseline (N = 396,184) | |||||
| Quartile 1 | 1691 | 98,180 | Reference | < 0.001 | |
| Quartile 2 | 1659 | 99,151 | 1.15 (1.08–1.24) | < 0.001 | |
| Quartile 3 | 1552 | 99,565 | 1.19 (1.11–1.28) | < 0.001 | |
| Quartile 4 | 1573 | 99,288 | 1.21 (1.12–1.31) | < 0.001 | |
| Sensitivity analysis 2: excluding IBS participants diagnosed within 2 years after baseline (N = 395,546) | |||||
| Quartile 1 | 1509 | 97,998 | Reference | ||
| Quartile 2 | 1495 | 98,987 | 1.16 (1.08–1.25) | < 0.001 | < 0.001 |
| Quartile 3 | 1405 | 99,418 | 1.20 (1.11–1.30) | < 0.001 | |
| Quartile 4 | 1428 | 99,143 | 1.22 (1.13–1.32) | < 0.001 | |
| Sensitivity analysis 3: excluding incident alcoholic liver disease participants after baseline (N = 395,775) | |||||
| Quartile 1 | 1878 | 98,306 | Reference | ||
| Quartile 2 | 1793 | 99,161 | 1.12 (1.05–1.20) | 0.001 | < 0.001 |
| Quartile 3 | 1696 | 99,496 | 1.18 (1.10–1.26) | < 0.001 | |
| Quartile 4 | 1737 | 98,812 | 1.21 (1.13–1.30) | < 0.001 | |
| Sensitivity analysis 4: competing risk model (N = 396,838, No. of competing events = 24,742) | |||||
| Quartile 1 | 1882 | 98,371 | Reference | ||
| Quartile 2 | 1797 | 99,289 | 1.13 (1.05–1.20) | 0.001 | < 0.001 |
| Quartile 3 | 1701 | 99,714 | 1.18 (1.10–1.26) | < 0.001 | |
| Quartile 4 | 1749 | 99,464 | 1.21 (1.12–1.30) | < 0.001 | |
| Sensitivity analysis 5: excluding HBV or HCV antigen-positive participants after baseline (N = 396,613) | |||||
| Quartile 1 | 1877 | 98,310 | Reference | ||
| Quartile 2 | 1796 | 99,235 | 1.13 (1.05, 1.20) | < 0.001 | < 0.001 |
| Quartile 3 | 1701 | 99,665 | 1.18 (1.10, 1.26) | < 0.001 | |
| Quartile 4 | 1749 | 99,403 | 1.21 (1.13, 1.30) | < 0.001 | |
| Sensitivity analysis 6: additionally adjusted psychologic disorder including depression and anxiety (N = 396,838) | |||||
| Quartile 1 | 1882 | 98,371 | Reference | ||
| Quartile 2 | 1797 | 99,289 | 1.11 (1.03–1.18) | 0.003 | < 0.001 |
| Quartile 3 | 1701 | 99,714 | 1.14 (1.07–1.23) | < 0.001 | |
| Quartile 4 | 1749 | 99,464 | 1.16 (1.08–1.24) | < 0.001 | |
Note: All adjusted HRs were calculated by adjusting the following covariates: age, gender, Townsend deprivation index, education level, ethnicity, smoking status, alcohol drinking, IPAQ (International Physical Activity Questionnaire) and type 2 diabetes. P for trend was calculated by using the median value of each fatty liver index Quartile (10.5, 32.2, 61.2, and 88.6 for sensitivity analysis 1, 2, 4, 5, and 6; 10.5, 32.2, 61.2, and 88.5 for sensitivity analysis 3)
IBS irritable bowel syndrome, HR hazard ratio, CI confidence interval
Discussion
In this prospective cohort study with a long-term follow-up of 0.4 million adults, participants with the highest quartile of the fatty liver index had a 21% increased risk of IBS occurrence. Participants with NAFLD diagnosis had a 13% higher risk of developing IBS. The positive association was particularly observed in females, but not in males.
Given the rising incidence of NAFLD globally during recent decades, our results may partially explain the current upward trend of IBS [3, 7, 8]. An epidemiological projection indicates there would be an expected increase of nearly 120 million people living with IBS between 2020 and 2040 worldwide [26]. Non-alcoholic fatty liver degree as well as NAFLD might be etiologically associated with IBS occurrence. If confirmed by future studies, the findings may have profound public health significance for the prevention of IBS. An estimated 7.3% (95% CI, 4.2–10.3%) of all IBS cases would be avoided if all UKB cohort members decreased their baseline FLI by more than 27. Particularly, approximately 10.3% (95% CI, 6.3–14.3%) of all IBS cases among women would have been avoided if baseline FLI decreased by more than 27 among study participants. Hence, if applied to the current general population, a considerable amount of health resources and medical cost related to IBS would have been saved.
The biological mechanisms for the positive association of a high degree of non-alcoholic fatty liver and NAFLD with incident IBS remain to be fully elucidated. Since NAFLD pathogenesis has been mainly considered as liver fat accumulation and subsequent hepatic inflammation based on “two-hit” theory, hepatic fat accumulation and inflammation along with immune system activation are hypothetically implicated in IBS development [16, 17, 27]. Several studies discovered similar trends of shared proinflammatory cytokines in both IBS and NAFLD, including increased expressions of TNF-α, IL-6, IL-8, and IL-1β, as well as decreased levels of IL-10 in vitro, animal models, and human studies [28–32]. Although the underlying mechanism was unclear, those cytokines have been reported to play important roles via Toll-like receptors (TLR) in the development of IBS symptoms, including the effect on peripheral and central nervous systems to develop hypersensitivity and gut hypomotility (TNF-α), stimulation of gut submucosal neurons (IL-6), intestinal barrier integrity (TNF-α, IL-6) and maintenance of gut homeostasis (IL-1β, IL-6, IL-10) [16, 17, 29].
Furthermore, the interaction between gut and liver primarily through the portal vein and biliary tract has attracted increasing attention recently, the so-called gut-liver axis [33, 34]. It has been reported bile salts and antimicrobial molecules are transported from the liver to the intestinal lumen via the biliary tract to maintain gut eubiosis by controlling unrestricted bacterial overgrowth [13, 33]. As diseased fatty liver could not effectively inhibit the overgrowth of bacteria, harmful microbial by-products could not be removed timely, which may further aggravate gut dysbiosis. Increasing evidence has revealed the involvement of intestinal dysbiosis in IBS pathogenesis [35, 36]. Alternation of gut microbiota (i.e., increased Clostridium and decreased lactobacilli with bifidobacterial) was associated with impaired intestinal permeability, impaired intestinal motility, and visceral hypersensitivity, which may contribute to the development of IBS symptoms [33–36].
Additionally, communication between gut and liver has been reported further transmitted from liver to brain via the autonomic nervous system, indicating an involvement of liver-brain-gut neural arc [13–15]. Recent experimental evidence demonstrated novel vago-vagal liver-brain-gut reflex arc mediated the differentiation of colonic peripheral regulatory T cells (pTreg cells), which could maintain immune homeostasis and thereby prevent excessive inflammatory response [14]. A mouse study revealed hepatic vagal sensory afferent nerves could reduce colonic pTreg cell pool through activation of muscarinic acetylcholine receptors once left vagal sensory afferents from liver to brainstem was disrupted, leading to disturbance of intestinal barrier and further susceptibility of IBS [14]. Despite recent advances in understanding of liver-brain-gut interaction, more investigation is needed to further clarify related potential mechanisms.
Interestingly, a positive association between non-alcoholic fatty liver and risk of IBS was observed in females rather than males in our study. Despite IBS is developed predominantly in females, the sex-gender difference in incident IBS still remains largely unknown. Recent data suggested interaction of trace aminergic signaling and sex hormones, especially female reproductive hormones, may play a critical role in IBS genesis [37, 38]. Disturbance of trace aminergic system might result in altered colonic ion secretion, hyperreactivity of the immune system, and fluctuations of 5-hydroxytryptamine (5-HT) levels, thereby leading to disruption of the gut microbiome, and mucosal immunity, all of which are implicated as etiological factors in IBS pathogenesis [37, 38]. Further studies are needed to confirm our findings and elucidate possible mechanisms.
To the best of our knowledge, there are no studies highlighting the link between non-alcoholic fatty liver degree as well as NAFLD and incident IBS. Based on the well-designed, large-scaled population-based cohort with the longest follow-up to date, non-alcoholic fatty liver degree measured in different approaches (i.e., NAFLD or not, quartiles, per SD change) was examined in detail and all were consistently observed to associate with increased risk of IBS. Moreover, multiple important lifestyle confounders, including age, gender, alcohol, smoking, physical activity, and socioeconomic status, were thoroughly adjusted. Additionally, various sensitivity analyses by accounting for protopathic bias and misclassification bias, and further substantial subgroup analyses were conducted, verifying the robustness of the results.
Several limitations should be considered. Firstly, NAFLD was measured by estimated indices in this study, rather than the gold diagnostic criteria including hepatic image or histology from liver biopsy due to unavailable data, which might lead to potential measurement error. However, FLI has shown excellent performance with transient elastography determined NAFLD and has been widely accepted as a reasonable substitute for obtaining population estimates [20, 21]. Meanwhile, our sensitivity analysis considering HSI as a measurement of NAFLD [24] indicated the robustness of principal findings. In addition, risk estimates would be attenuated and toward to the null association even if this non-differential measurement error existed, which instead supported our positive associations. Secondly, residual confounders cannot be ruled out since some potential covariates, either unmeasured or unknown, may confound the association between NAFLD and IBS, although we have carefully controlled for numerous potential confounders. Thirdly, IBS was identified according to ICD-10 codes in primary care or hospitalized medical records, rather than via structured questionnaire (i.e., Rome III or IV scale) or interview. Since some IBS cases in the community may not take medical consultation, a proportion of IBS cases in this large population-based cohort might remain undiagnosed, leading to an underestimation of the IBS incidence rate. However, underdiagnosis of IBS would exist in both exposure (i.e., NAFLD or quartile 2–4 groups) and non-exposure groups (i.e., non-NAFLD or quartile 1 group). Thus, the association would be underestimated under the circumstances of probably non-differential misclassification bias, which would in turn support our positive findings. Fourthly, non-alcoholic fatty liver was only assessed at baseline. Thus, a change of fatty liver degree during the follow-up could not be obtained. Accordingly, the association between changes in NAFLD and the risk of IBS could not be evaluated. Finally, we were not able to further examine the association between NAFLD and the development of different IBS subtypes due to the unavailability of such data in UKB. Future cohort studies are needed to elucidate associated risk with IBS subtypes.
Conclusions
In summary, in this large-scale prospective cohort study of the UK population, a high degree of non-alcoholic fatty liver as well as non-alcoholic fatty liver disease was associated with an increased risk of incident IBS. However, these findings are needed to be confirmed by further well-designed prospective cohort studies and trials in the diverse ethnic population. Future animal and experimental research are also warranted to elucidate the underlying biological mechanisms.
Supplementary Information
Additional file 1. Table S1. ICD codes and Field ID definingdiseases in UKB. Table S2. Baseline characteristicsaccording to diagnosis of NAFLD or not by predefined cutoff of FLI in UKBiobank cohort. Table S3.Sensitivity analysis regarding risk of IBS according to per SD change anddiagnosis of NAFLD or not by predefined cutoff of FLI. Table S4. Sensitivityanalysis regarding risk of IBS according to per SD change and diagnosis ofNAFLD or not by predefined cutoff of baseline hepatic steatosis index.
Additional file 2. FigureS1. Restricted cubic splinefor the association of baseline fatty liver index with incident IBS. Figure S2. Risk of incident IBSassociated with NAFLD type (lean, non-obese and obese NAFLD).
Acknowledgements
This research has been conducted using the UK Biobank Resource under application number [74444].
Glossary
Abbreviations
- 5-HT
5-Hydroxytryptamine
- ALD
Alcoholic liver disease
- ALT
Serum alanine aminotransferase
- AST
Aspartate aminotransferase
- AUROC
Area under the receiver operator curve
- CI
Confidence interval
- FLI
Fatty liver index
- GGT
Gamma-glutamyltransferase
- HSI
Hepatic steatosis index
- IBD
Inflammatory bowel disease
- IBS
Irritable bowel syndrome
- ICD-10
International Classification of Disease-10
- IL
Interleukin
- IPAQ
International Physical Activity Questionnaire
- NAFLD
Non-alcoholic fatty liver disease
- SD
Standard deviation
- TG
Triglycerides
- TLR
Toll-like receptors
- TNF
Tumor necrosis factor
- UKB
UK Biobank
- WC
Waist circumstance
Authors’ contributions
SSW and STZ1 designed the study. SSW drafted the manuscript. SSW analyzed the data. ZRY and CZY revised the manuscript. SSW, ZRY, SL, QZ, STZ1, CZY, and STZ2 interpreted the results, incorporated comments for the co-authors, and finalized the manuscript. All authors read and approved the final manuscript.
Funding
This work was funded by the National Natural Science Foundation of China (No. 82070550).
Availability of data and materials
All data relevant to the study were using the UK Biobank Resource under application number [74444]. No additional data available.
Declarations
Ethics approval and consent to participate
The UKB study was approved by the North West Multicenter Research Ethical Committee (21/NW/0157), and all participants or their proxy respondents provided written informed consent.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ford AC, Sperber AD, Corsetti M, Camilleri M. Irritable bowel syndrome. Lancet. 2020;396(10263):1675–1688. doi: 10.1016/S0140-6736(20)31548-8. [DOI] [PubMed] [Google Scholar]
- 2.Lacy BE, Pimentel M, Brenner DM, Chey WD, Keefer LA, Long MD, et al. ACG Clinical Guideline: Management of Irritable Bowel Syndrome. Am J Gastroenterol. 2021;116(1):17–44. doi: 10.14309/ajg.0000000000001036. [DOI] [PubMed] [Google Scholar]
- 3.Sperber AD, Bangdiwala SI, Drossman DA, Ghoshal UC, Simren M, Tack J, et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology. 2021;160(1):99–114.e3. doi: 10.1053/j.gastro.2020.04.014. [DOI] [PubMed] [Google Scholar]
- 4.Frändemark Å, Törnblom H, Jakobsson S, Simrén M. Work productivity and activity impairment in irritable bowel syndrome (IBS): a multifaceted problem. Am J Gastroenterol. 2018;113(10):1540–1549. doi: 10.1038/s41395-018-0262-x. [DOI] [PubMed] [Google Scholar]
- 5.Peery AF, Crockett SD, Murphy CC, Lund JL, Dellon ES, Williams JL, et al. Burden and cost of gastrointestinal, liver, and pancreatic diseases in the United States: update 2018. Gastroenterology. 2019;156(1):254–272.e11. doi: 10.1053/j.gastro.2018.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi K, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: Practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67(1):328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 7.Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69(4):896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 8.Younossi ZM. Non-alcoholic fatty liver disease-A global public health perspective. J Hepatol. 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 9.Dietrich P, Hellerbrand C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Pract Res Clin Gastroenterol. 2014;28(4):637–653. doi: 10.1016/j.bpg.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 10.Younossi Z, Anstee QM, Marietti M, Hardy T, Henry L, Eslam M, et al. Global burden of NAFLD and NASH: trends, predictions, risk factors and prevention. Nat Rev Gastroenterol Hepatol. 2018;15(1):11–20. doi: 10.1038/nrgastro.2017.109. [DOI] [PubMed] [Google Scholar]
- 11.Porras D, Nistal E, Martínez-Flórez S, González-Gallego J, García-Mediavilla MV, Sánchez-Campos S. Intestinal Microbiota Modulation in Obesity-Related Non-alcoholic Fatty Liver Disease. Front Physiol. 2018;9:1813. doi: 10.3389/fphys.2018.01813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Higarza SG, Arboleya S, Gueimonde M, Gómez-Lázaro E, Arias JL, Arias N. Neurobehavioral dysfunction in non-alcoholic steatohepatitis is associated with hyperammonemia, gut dysbiosis, and metabolic and functional brain regional deficits. PLoS ONE. 2019;14(9):e0223019. doi: 10.1371/journal.pone.0223019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding JH, Jin Z, Yang XX, Lou J, Shan WX, Hu YX, et al. Role of gut microbiota via the gut-liver-brain axis in digestive diseases. World J Gastroenterol. 2020;26(40):6141–6162. doi: 10.3748/wjg.v26.i40.6141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Teratani T, Mikami Y, Nakamoto N, Suzuki T, Harada Y, Okabayashi K, et al. The liver-brain-gut neural arc maintains the T reg cell niche in the gut. Nature. 2020;585(7826):591–596. doi: 10.1038/s41586-020-2425-3. [DOI] [PubMed] [Google Scholar]
- 15.Matsubara Y, Kiyohara H, Teratani T, Mikami Y, Kanai T. Organ and brain crosstalk: The liver-brain axis in gastrointestinal, liver, and pancreatic diseases. Neuropharmacol. 2022;205:108915. doi: 10.1016/j.neuropharm.2021.108915. [DOI] [PubMed] [Google Scholar]
- 16.Scalera A, Di Minno MN, Tarantino G. What does irritable bowel syndrome share with non-alcoholic fatty liver disease? World J Gastroenterol. 2013;19(33):5402–5420. doi: 10.3748/wjg.v19.i33.5402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Purssell H, Whorwell PJ, Athwal VS, Vasant DH. Non-alcoholic fatty liver disease in irritable bowel syndrome: More than a coincidence? World J Hepatol. 2021;13(12):1816–1827. doi: 10.4254/wjh.v13.i12.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sudlow C, Gallacher J, Allen N, Beral V, Burton P, Danesh J, et al. UK biobank: an open access resource for identifying the causes of a wide range of complex diseases of middle and old age. PLoS Med. 2015;12:e1001779. doi: 10.1371/journal.pmed.1001779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liu Z, Suo C, Zhao R, Yuan H, Jin L, Zhang T, et al. Genetic predisposition, lifestyle risk, and obesity associate with the progression of nonalcoholic fatty liver disease. Dig Liver Dis. 2021;53(11):1435–1442. doi: 10.1016/j.dld.2021.07.009. [DOI] [PubMed] [Google Scholar]
- 21.Jones GS, Alvarez CS, Graubard BI, McGlynn KA. Agreement Between the Prevalence of Nonalcoholic Fatty Liver Disease Determined by Transient Elastography and Fatty Liver Indices. Clin Gastroenterol Hepatol. 2022;20(1):227–229.e2. doi: 10.1016/j.cgh.2020.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ye Q, Zou BY, Yeo YH, Li J, Huang DQ, Wu Y, et al. Global prevalence, incidence, and outcomes of non-obese or lean non-alcoholic fatty liver disease: a systematic review and meta-analysis. Lancet Gastroenterol Hepatol. 2020;5(8):739–752. doi: 10.1016/S2468-1253(20)30077-7. [DOI] [PubMed] [Google Scholar]
- 23.Papatheodoridi M, Cholongitas E. Diagnosis of non-alcoholic fatty liver disease (NAFLD): current concepts. Curr Pharm Des. 2018;24(38):4574–4586. doi: 10.2174/1381612825666190117102111. [DOI] [PubMed] [Google Scholar]
- 24.Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42(7):503–508. doi: 10.1016/j.dld.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 25.Townsend P. Deprivation. J. Soc Policy. 1987;16(02):125. doi: 10.1017/S0047279400020341. [DOI] [Google Scholar]
- 26.Black CJ, Ford AC. Global burden of irritable bowel syndrome: trends, predictions and risk factors. Nat Rev Gastroenterol Hepatol. 2020;17(8):473–486. doi: 10.1038/s41575-020-0286-8. [DOI] [PubMed] [Google Scholar]
- 27.Day CP, James OF. Steatohepatitis: a tale of two “hits”? Gastroenterology. 1998;114(4):842–845. doi: 10.1016/S0016-5085(98)70599-2. [DOI] [PubMed] [Google Scholar]
- 28.Bennet SM, Polster A, Törnblom H, Isaksson S, Capronnier S, Tessier A, et al. Global Cytokine Profiles and Association With Clinical Characteristics in Patients With Irritable Bowel Syndrome. Am J Gastroenterol. 2016;111(8):1165–1176. doi: 10.1038/ajg.2016.223. [DOI] [PubMed] [Google Scholar]
- 29.Ivashkin V, Poluektov Y, Kogan E, Shifrin O, Sheptulin A, Kovaleva A, et al. Disruption of the pro-inflammatory, anti-inflammatory cytokines and tight junction proteins expression, associated with changes of the composition of the gut microbiota in patients with irritable bowel syndrome. PLoS ONE. 2021;16(6):e0252930. doi: 10.1371/journal.pone.0252930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Choghakhori R, Abbasnezhad A, Hasanvand A, Amani R. Inflammatory cytokines and oxidative stress biomarkers in irritable bowel syndrome: Association with digestive symptoms and quality of life. Cytokine. 2017;93:34–43. doi: 10.1016/j.cyto.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 31.Panera N, Corte CD, Crudele A, Stronati L, Nobili V, Alisi A. Recent advances in understanding the role of adipocytokines during non-alcoholic fatty liver disease pathogenesis and their link with hepatokines. Expert Rev Gastroenterol Hepatol. 2016;10(3):393–403. doi: 10.1586/17474124.2016.1110485. [DOI] [PubMed] [Google Scholar]
- 32.Hou X, Yin S, Ren R, Liu S, Yong L, Liu Y, et al. Myeloid-Cell-Specific IL-6 Signaling Promotes MicroRNA-223-Enriched Exosome Production to Attenuate NAFLD-Associated Fibrosis. Hepatology. 2021;74(1):116–132. doi: 10.1002/hep.31658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brescia P, Rescigno M. The gut vascular barrier: a new player in the gut-liver-brain axis. Trends Mol Med. 2021;27(9):844–855. doi: 10.1016/j.molmed.2021.06.007. [DOI] [PubMed] [Google Scholar]
- 34.Tripathi A, Debelius J, Brenner DA, Karin M, Loomba R, Schnabl B, et al. The gut-liver axis and the intersection with the microbiome. Nat Rev Gastroenterol Hepatol. 2018;15(7):397–411. doi: 10.1038/s41575-018-0011-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh R, Zogg H, Wei L, Bartlett A, Ghoshal UC, Rajender S, et al. Gut Microbial Dysbiosis in the Pathogenesis of Gastrointestinal Dysmotility and Metabolic Disorders. J Neurogastroenterol Motil. 2021;27(1):19–34. doi: 10.5056/jnm20149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhao L, Yang W, Chen Y, Huang F, Lu L, Lin C, et al. A Clostridia-rich microbiota enhances bile acid excretion in diarrhea-predominant irritable bowel syndrome. J Clin Invest. 2020;130(1):438–450. doi: 10.1172/JCI130976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kim YS, Kim N. Sex-Gender Differences in Irritable Bowel Syndrome. J Neurogastroenterol Motil. 2018;24(4):544–558. doi: 10.5056/jnm18082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pretorius L, Smith C. The trace aminergic system: a gender-sensitive therapeutic target for IBS? J Biomed Sci. 2020;27(1):95. doi: 10.1186/s12929-020-00688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1. Table S1. ICD codes and Field ID definingdiseases in UKB. Table S2. Baseline characteristicsaccording to diagnosis of NAFLD or not by predefined cutoff of FLI in UKBiobank cohort. Table S3.Sensitivity analysis regarding risk of IBS according to per SD change anddiagnosis of NAFLD or not by predefined cutoff of FLI. Table S4. Sensitivityanalysis regarding risk of IBS according to per SD change and diagnosis ofNAFLD or not by predefined cutoff of baseline hepatic steatosis index.
Additional file 2. FigureS1. Restricted cubic splinefor the association of baseline fatty liver index with incident IBS. Figure S2. Risk of incident IBSassociated with NAFLD type (lean, non-obese and obese NAFLD).
Data Availability Statement
All data relevant to the study were using the UK Biobank Resource under application number [74444]. No additional data available.