Abstract
Objective
To evaluate the safety and efficacy of COVID-19 vaccines in pregnant women performing an updated meta-analysis.
Methods
We searched PubMed, Cochrane Central, and SCOPUS from inception to March 2022. Outcomes of interest were incidence of adverse maternal, fetal and neonatal consequences pertaining to safety of the vaccines. Secondarily, we analyzed the number of SARS-CoV-2 infections, hospitalization for COVID-19, and admission to the I.C.U. for COVID-19 assessing the effectiveness of vaccines. Results were pooled using a random effects model.
Results
Ten observational studies (n=326,499) analyzing pregnant women were included. Our results suggest that COVID-19 vaccination prevents infection (OR: 0.56, 95% CI: 0.47, 0.67; P = <0.00001) and related hospitalizations (OR: 0.50, 95% CI: 0.31, 0.82; P = 0.006) effectively. It was also observed that vaccination does not change adverse outcomes in pregnancy, namely preeclampsia or eclampsia, stroke (four weeks of delivery), meconium-stained amniotic fluid, spontaneous vaginal delivery, operative vaginal delivery, cesarean delivery, postpartum hemorrhage, and blood transfusions. Furthermore, the vaccine was observed to be protective against neonatal COVID-19 I.C.U. admissions (OR: 0.85; 95% CI: 0.81, 0.90; P = <0.00001).
Conclusion
Our pooled analysis suggests that the COVID-19 vaccination in pregnant women prevents infection effectively and has no adverse outcomes. Future large-scale trials in a randomized fashion are needed to confirm our results.
Key Words: COVID-19, Vaccines, Pregnancy, Safety, Efficacy, Adverse outcomes
Negative outcomes associated with COVID-19 in pregnant patients make vaccination an imperative precaution for this patient population. Pregnant patients were excluded from the randomized clinical trials conducted early in the pandemic to determine the safety and efficacy of COVID-19 vaccination, with limited data being obtained when pregnant patients were unknowingly enrolled in these trials.1 However, recent population-based studies demonstrated the effectiveness of the COVID-19 vaccine in pregnant women which has been established with an appropriate immunologic response observed. Antibody titers in pregnant women were similar to those seen in non-pregnant women and passive immunity to newborns has also been conferred, as antibodies were observed in the umbilical cord blood and breast milk.2 There are still no clear and universal guidelines that enforce the vaccination of pregnant females against COVID-19 and at this point, vaccination strategies for pregnant patients are varying over different countries. There is hesitation amongst healthcare professionals in advising vaccination for pregnant women due to an insufficient amount of data regarding the vaccine's safety profile.3 Furthermore, even in countries where pregnant women are encouraged to get vaccinated against COVID-19, a lower acceptance rate of the vaccine is observed in pregnant versus non-pregnant patients, with safety concerns being documented as the major hurdle toward obtaining vaccines.4
The willingness to get vaccinated amongst pregnant women has changed dramatically over time, with only 29% of pregnant women accepting the vaccine in studies conducted in the first half of 2021 and rising to as high as 77.4% towards the end.5 Furthermore, the rate of vaccine acceptance varies significantly between countries, with the lowest rates seen in Switzerland and Ireland and the highest in China, Qatar, and Italy. Numerous factors encouraged the acceptance of the vaccine, not only including the safety of the vaccine but also appropriate awareness regarding the vaccine, mass vaccination of pregnant women within the country, and trust in public health services.5
Significantly altered outcomes have been seen following a globally adopted vaccination strategy catered to at-risk patient populations. Our meta-analysis aimed to primarily evaluate the safety of the COVID-19 vaccine and to further investigate its efficacy, thus adding relevance to the existing literature to improve the current level of understanding.
METHODS
Data sources and search strategy
We followed the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement6 and the Meta-analysis of Observational Studies in Epidemiology (MOOSE) group for this study.7 The following databases were used to conduct our search: PubMed, Cochrane Library, and SCOPUS. We searched for articles published in each database from inception to March 2022 (the comprehensive search strategy is given in Supplemental Table 1). The reference lists of the retrieved articles and previous meta-analyses were manually reviewed for studies that might be relevant.
Study selection and eligibility criteria
All articles were exported to EndNote Reference Library (version X9; Clarivate Analytics, Philadelphia, Pennsylvania), where duplicates were found and eliminated. Two reviewers (O.M.S. and M.N.) independently reviewed and shortlisted the remaining articles based on their relevance to the eligibility criteria. After reviewing the titles and abstracts of the papers, the whole article was read. A third reviewer (I.H.) was consulted in the event of disagreements. We included observational studies that investigated the safety and efficacy of COVID-19 vaccinations in pregnant women. The following studies were excluded: (a) studies not using COVID-19 vaccination as the exposure; (b) insufficient data to compute the outcomes for the effectiveness and safety of COVID-19 vaccines among pregnant women; (c) duplicate studies or overlapping participants; (d) reviews, editorials, conference papers, case reports, or animal experiments; and (e) studies published in a non-English language.
Data extraction and quality of assessment
The safety of COVID-19 vaccinations was the primary outcome of our study. Patients who received one dose, more than one dose or two doses were considered vaccinated and the ones who did not receive any vaccines before or during pregnancy, or received a dose after delivery were considered unvaccinated. The following data was obtained from the included studies: (1) study characteristics, such as first author, publication year, and study design; (2) study population characteristics, such as sample sizes, age groups, trimester of pregnancy, and locations; (3) types of COVID-19 vaccines, and the number of doses; (4) outcomes related to the safety of COVID-19 vaccines: the incidence of adverse maternal (preeclampsia or eclampsia, stroke, meconium-stained amniotic fluid, spontaneous vaginal delivery, operative vaginal delivery, cesarean delivery, postpartum hemorrhage, abortion, and blood transfusion), fetal (preterm birth < 37 weeks, stillbirth), or neonatal outcomes (5 min Apgar score < 7, small for gestational age (S.G.A.), low birthweight < 2500 g, very low birthweight < 1500 g, neonatal I.C.U. admission) following vaccination; and (5) outcomes for the effectiveness of COVID-19 vaccines: number of SARS-CoV-2 infections, hospitalization for COVID-19, and admission to the I.C.U. for COVID-19. Data extraction was carried out independently by two investigators (I.H. and M.O.K.) using the criteria mentioned above, with conflicts resolved by consensus or with the help of a third investigator (S.A.S.). The Newcastle–Ottawa quality assessment scale was used to assess the risk of bias. With an overall quality score of 9 stars, cohort studies were classed as having a low (7 stars), moderate (5–6 stars), and high (4 stars) risk of bias. Two investigators (S.A.S. and M.N.) independently assessed the quality of the data, and any inconsistencies were resolved by consensus or with the help of a third investigator (I.H.).
Statistical analysis
We conducted all statistical analyses using RevMan (version 5.3; Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014). We used Odds Ratio (O.R.) with 95 % confidence intervals (C.I.) to calculate the dichotomous data as meaningful effect measures for the safety and effectiveness of COVID-19 vaccines based on clinical outcomes. The random-effects model and the Higgins I² statistic were used to analyze and evaluate heterogeneity, respectively. We considered an I² of < 50%, 50-75%, and > 75% indicating low, moderate, and high heterogeneity, respectively. The statistical significance level was considered for hypothesis testing at a p-value <0.05. In case of heterogeneity > 50%, we performed sensitivity analysis by using the leave-one-out analysis to identify the study causing heterogeneity. Furthermore, subgroup analyses were conducted based on adjusted/ unadjusted data, location, type of vaccine, and trimester of first dose vaccine. We decided not to perform any funnel plot asymmetry tests because Cochrane guidelines do not recommend them when fewer than ten papers are included in the analysis (which is the case for all outcomes in our study). In such instances, the test's power is insufficient to distinguish between random and actual asymmetry.8
RESULTS
Basic characteristics
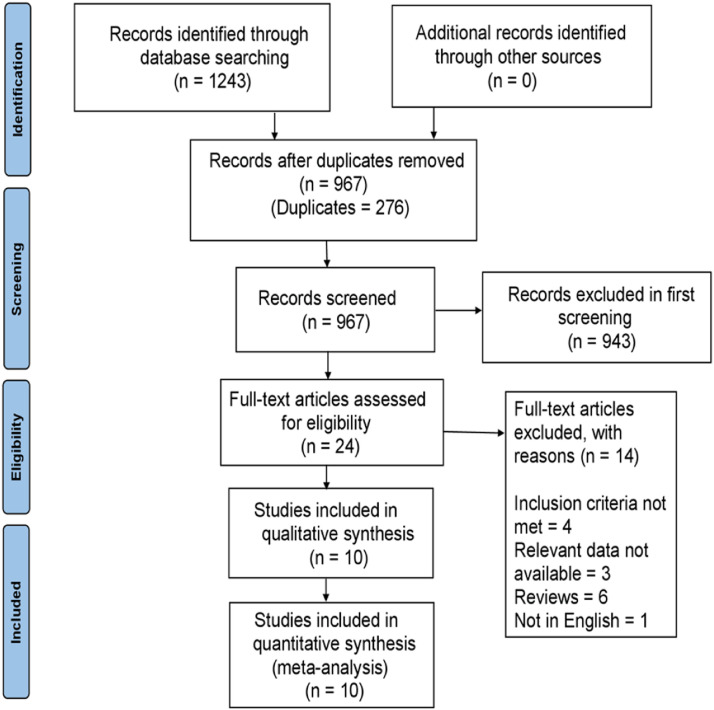
The initial search identified 1243 potential articles up to March 2022. Initially, 276 duplicate articles were removed, following the exclusion of 976 articles after reading the title and abstract. In the remaining 24 articles, 14 were excluded after the full-text screening, four did not meet the inclusion criteria, three did not provide sufficient data, six were reviews, and one was not in the English language - leaving behind ten articles to be included in our meta-analysis. The details of this process are highlighted in the PRISMA flowchart in Figure 1 . The Quality assessment was conducted according to the Newcastle–Ottawa quality assessment scale (Supplemental Table 2), and all studies included were of good quality (≥ 7 stars).
Fig 1.
Flow diagram of the study search and selection process.
The final ten observational cohort studies included in our meta-analysis gave us a pool of 326,499 pregnant women to work with (79,460 vaccinated and 247,039 unvaccinated). The details of the data provided in the studies and the participant's essential demographic characteristics are presented in Table 1 . We assessed a diverse set of outcomes throughout pregnancy and after.
Table 1.
Characteristics of included studies
| Record number | First author | Published time | Study design | Location | Sample size |
Vaccine type | No. of dose | Median age | Median gestational age | Risk of bias | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Vaccinated | Unvaccinated | ||||||||||
| 1 | Theiler RN9 | 2021 | Retrospective cohort study | USA | 140 | 1862 | BNT162b2 + Moderna + adenovirus vector vaccine | ≥1 | 31.8 | Last trimester | Low |
| 2 | Butt AA10 | 2021 | Prospective cohort study | Qatar | 407 | 407 | BNT162b2 + Moderna | 2 | 32 | Early Trimester | Low |
| 3 | Dagan N11 | 2021 | Retrospective cohort study | Israel | 10861 | 10861 | BNT162b2 | 2 | 30 | - | Low |
| 4 | Blakeway H3 | 2021 | Retrospective cohort study | England | 140 | 1188 | BNT162b2 + Moderna + adenovirus vector vaccine | ≥1 | 35 | Last trimester | Low |
| 5 | Goldshtein I12 | 2021 | Retrospective cohort study | Israel | 7530 | 7530 | BNT162b2 | ≥1 | 31.1 | - | Low |
| 6 | Rottenstreich M13 | 2021 | Retrospective cohort study | Israel | 712 | 1063 | BNT162b2 | ≥1 | 29.5 | Last trimester | Low |
| 7 | Wainstock T14 | 2021 | Retrospective cohort study | Israel | 913 | 3486 | BNT162b2 | ≥1 | 28.2 | Last trimester | Low |
| 8 | Goldshtein I15 | 2022 | Prospective cohort study | Israel | 7591 | 16697 | BNT162b2 | ≥1 | 31.61 | Second trimester | Low |
| 9 | Magnus MC16 | 2022 | Retrospective cohort study | Norway and Sweden | 28506 | 129015 | BNT162b2 + Moderna + AZD1222 | ≥1 | 31 | Third trimester | Low |
| 10 | Fell DB17 | 2022 | Retrospective cohort study | Canada | 22660 | 74930 | BNT162b2 + Moderna + AZD1222 | ≥1 | 31.9 | Third trimester | Low |
Safety of COVID-19 vaccination
Pregnancy-related outcomes
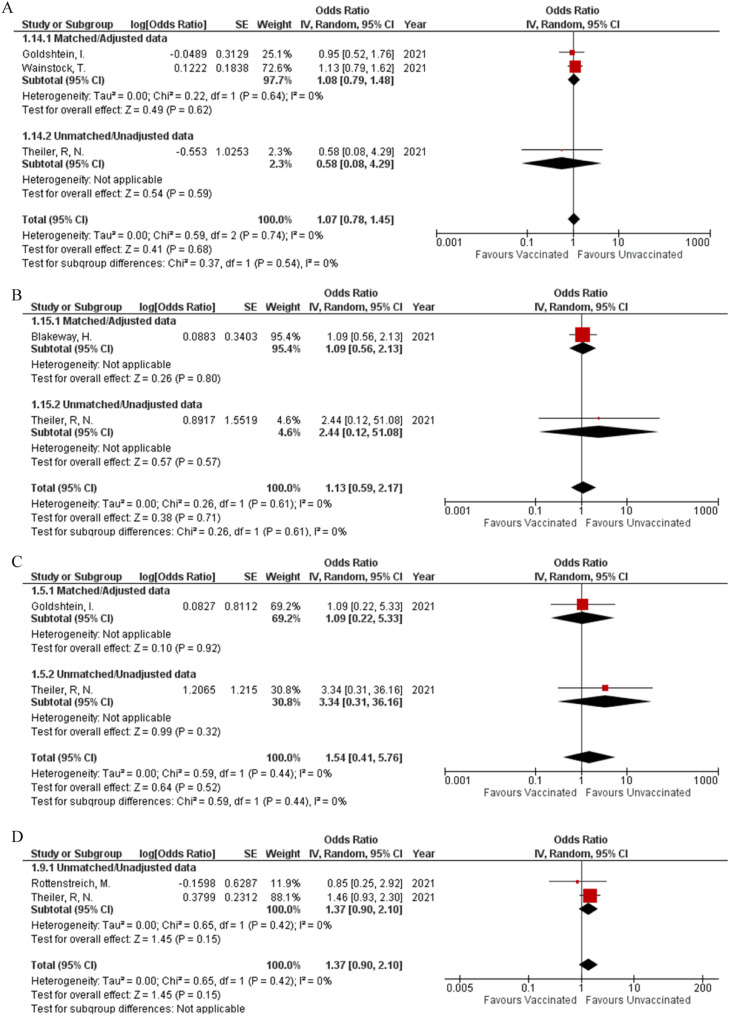
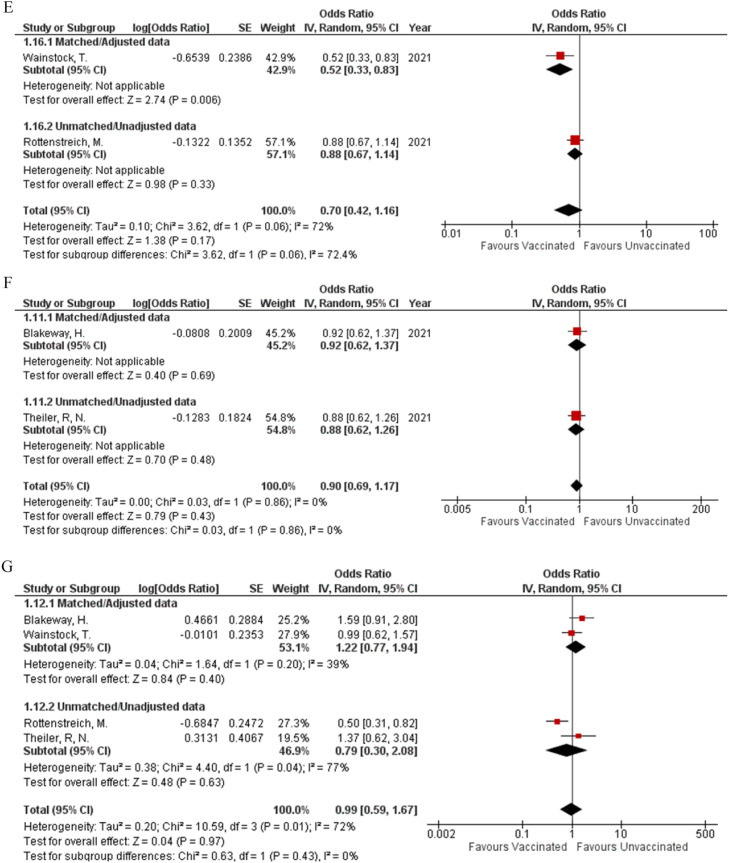
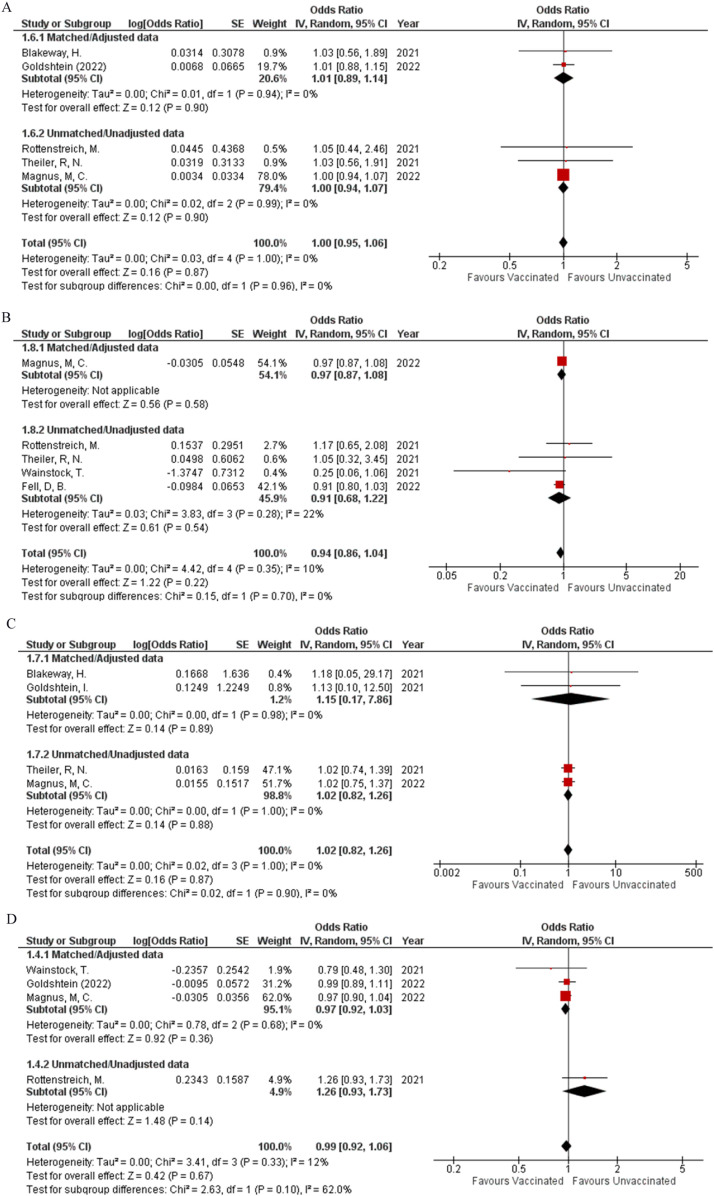
The incidence of preeclampsia (OR: 1.07; 95% CI: 0.78, 1.45; P = 0.68; Fig 2 A) was reported by three studies, while the occurrence of stroke (OR: 1.13; 95% CI: 0.59, 2.17; P = 0.71; Fig 2B) within four weeks, before or after delivery was reported by two studies. Integrated analysis showed no difference in the occurrence of both events regardless of vaccination status respectively, both with minimum heterogeneity (I² = 0%). Two studies were found to report the event of abortion (OR: 1.54; 95% CI: 0.41, 5.76; P = 0.52; Fig 2C) (I² = 0%) and the requirement of blood transfusion (OR: 1.37; 95% CI: 0.90, 2.10; P = 0.15; Fig 2D) (I² = 0%). Vaccination status did not significantly affect the frequency at which these events occurred between the two study groups. No pregnancy-related outcomes showed any statistical difference in their matched and unmatched subgroups.
Fig 2.
Forest plots for safety of COVID-19 Vaccines (Adverse pregnant, fetal or neonatal outcomes). A: Preeclampsia or Eclampsia. B: Stroke. C: Abortion. D: Blood transfusion. E: Meconium-stained amniotic fluid. F: Spontaneous vaginal delivery. G: Operative vaginal delivery. H: Cesarean delivery. I: Postpartum hemorrhage
Outcomes related to delivery
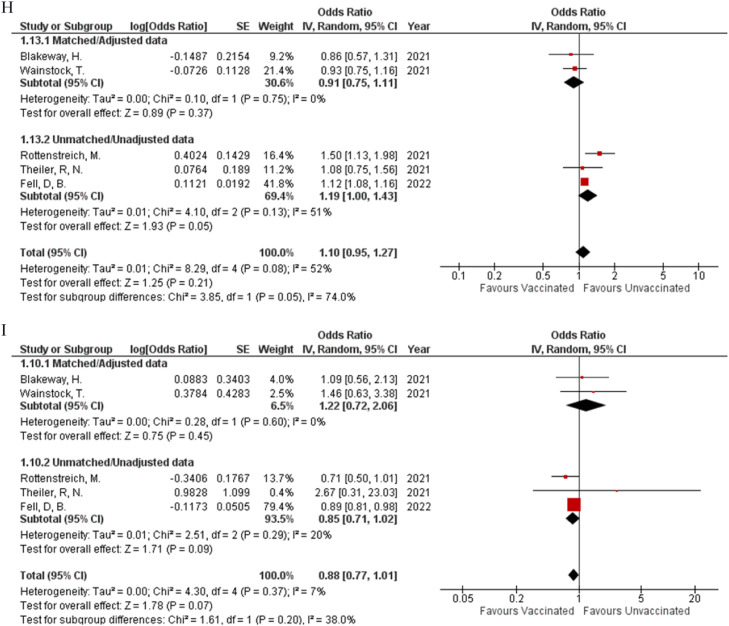
Two studies assessed meconium-stained amniotic fluid (OR: 0.70; 95% CI: 0.42, 1.16; P = 0.17; Fig 2E) (I² = 72%) and spontaneous vaginal delivery (OR: 0.90; 95% CI: 0.69, 1.17; P = 0.43; Fig 2F) (I² = 0%) with neither of them having any difference in the rate of occurrence between vaccinated and unvaccinated participants. Sensitivity analysis on meconium-stained amniotic fluid could not be performed to reduce heterogeneity due to the presence of only two studies. Four studies provided data to assess the occurrence of operative vaginal delivery, and pooled analysis showed that no group had a significantly higher or lower probability for the outcome to occur based on their vaccination status (OR: 0.99; 95% CI: 0.59, 1.67; P = 0.97; Fig 2G) (I² = 72%). By performing sensitivity analysis, heterogeneity was brought down to 0% by removing Rottenstreich et al. (OR: 1.22; 95% CI: 0.88, 1.70; P = 0.22; Supplemental Figure 1A), while the effect remained the same. Five studies were found to report cesarean delivery (OR: 1.10; 95% CI: 0.95, 1.27; P = 0.21; Fig 2H) (I² = 52%) and postpartum hemorrhage (OR: 0.88; 95% CI: 0.77, 1.01; P = 0.07; Fig 2I) (I² = 7%). After performing the sensitivity analysis for cesarean delivery (OR: 1.06; 95% CI: 0.95, 1.18; P = 0.28; Supplemental Figure 1B) (I² = 25%), it was noted that neither of the outcomes was likely to significantly increase or decrease with a positive vaccination status. No outcomes related to delivery showed any statistical difference between their matched and unmatched subgroups.
Fetal outcomes
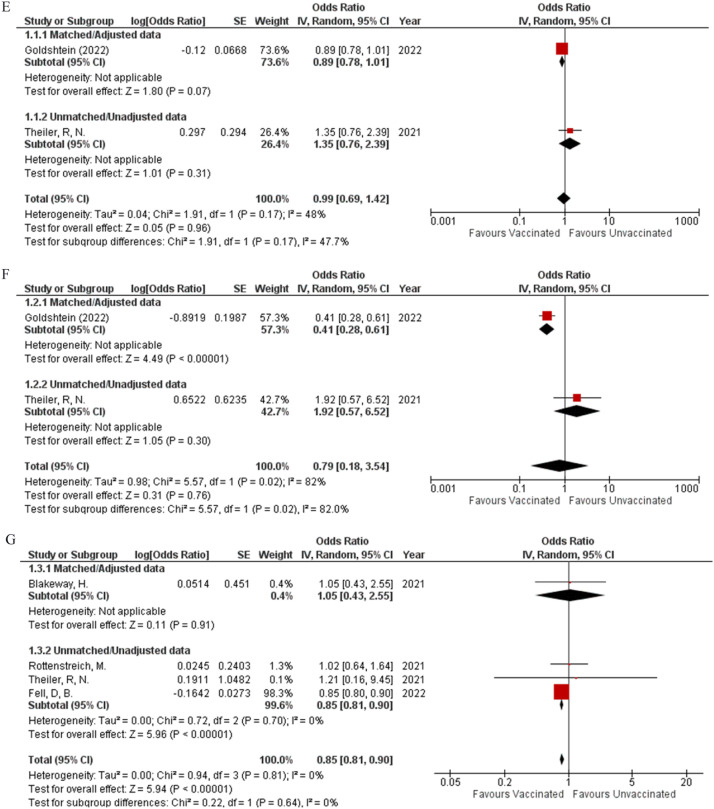
When comparing fetal outcomes there was no statistical difference noted between vaccinated pregnant women and their child being born preterm (OR: 1; 95% CI: 0.95, 1.06; P = 0.87; Fig 3 A) (I² = 0%) or having a 5-minute APGAR score less than 7 (OR: 0.94; 95% CI: 0.86, 1.04; P = 0.22; Fig 3B) (I² = 10%) as compared to unvaccinated pregnant women, when collective analysis was done with data gathered from five studies. The events of having a stillbirth (OR: 1.02; 95% CI: 0.82, 1.26; P = 0.87; Fig 3C) (I² = 0%) and the baby being small for gestational age (OR: 0.99; 95% CI: 0.92, 1.06; P = 0.67; Fig 3D) (I² = 12%) were also not affected by the mother's vaccination status as extrapolated from data retrieved from four studies. The pregnant mother's vaccination status was also unlikely to alter the outcomes of the baby having a low birth weight (OR: 0.99; 95% CI: 0.69, 1.42; P = 0.96; Fig 3E) (I² = 48%) or very low birth weight (OR: 0.79; 95% CI: 0.18, 3.54; P = 0.76; Fig 3F) (I² = 82%) as calculated from the combined analysis from data reported in two studies. Additionally, there was a significant difference in the matched and unmatched data subgroups (P = 0.02). Four studies reported data about Neonatal ICU admission and combining their results showed that the vaccine played a protective role in significantly decreasing the neonatal ICU admission rates (OR: 0.85; 95% CI: 0.81, 0.90; P = <0.00001; Fig 3G) (I² = 0%). Apart from very low birth weight, none of the other fetal outcomes had any statistical difference between their matched and unmatched subgroups.
Fig 3.
Forest plots for safety of COVID-19 Vaccines (Adverse pregnant, fetal or neonatal outcomes). A: Preterm birth (<37 weeks). B: 5 min APGAR score <7. C: Stillbirth. D: Small for gestational age. E: Low birthweight <2500g). F: Very low birth weight (<1500g). G: Neonatal ICU admissions
We performed subgroup analyses on the basis of location (Israel, USA, Canada, Norway, Sweden, and England), trimester (first, second and third), and type of vaccines (mRNA-273, BNT162b2, AZD1222).
Country wise
In Canada, the incidence of caesarean delivery was seen to be significantly more (OR: 1.12; 95% CI: 1.08, 1.16; P = <0.00001) and postpartum hemorrhage was noted to occur significantly less (OR: 0.89; 95% CI: 0.81, 0.98; P = 0.02). Preterm birth was observed to be significantly lower in Israel (OR: 0.87; 95% CI: 0.76, 0.99; P = 0.03) (I² = 0%) and Norway (OR: 0.81; 95% CI: 0.71, 0.92; P = 0.001). Stillbirth was observed to occur at a lower rate in Sweden (OR: 0.71; 95% CI: 0.52, 0.99; P = 0.04) and Norway (OR: 0.42; 95% CI: 0.20, 0.91; P = 0.03). Israel was the country noted to have a significantly lower occurrence of the infants having very low birth weight (<1500g) (OR: 0.41; 95% CI: 0.28, 0.61; P = <0.00001), while Canada was the country that showed a significant decrease in neonatal ICU admissions (OR: 0.85; 95% CI: 0.80, 0.90; P = <0.00001). The analysis is represented in Supplemental Figure 2.
Trimester
When performing subgroup analysis according to the Trimester (First, Second and Third), we found a significant decrease in preterm births when patients were vaccinated in the third trimester (OR: 0.70; 95% CI: 0.63, 0.77; P = <0.00001). The analysis is represented in Supplemental Figure 3.
Type of vaccines
Subgroup analysis with classification according to the type of vaccination used showed a significant decrease in preterm births, particularly when the Pfizer BionTech162b2 (OR: 0.91; 95% CI: 0.86, 0.97; P = 0.006) and the Moderna mRNA1273 (OR: 0.83; 95% CI: 0.73, 0.94; P = 0.003) vaccines were used. The analysis is represented in Supplemental Figure 4.
Efficacy of COVID-19 vaccination
SARS-Cov-2 Infections
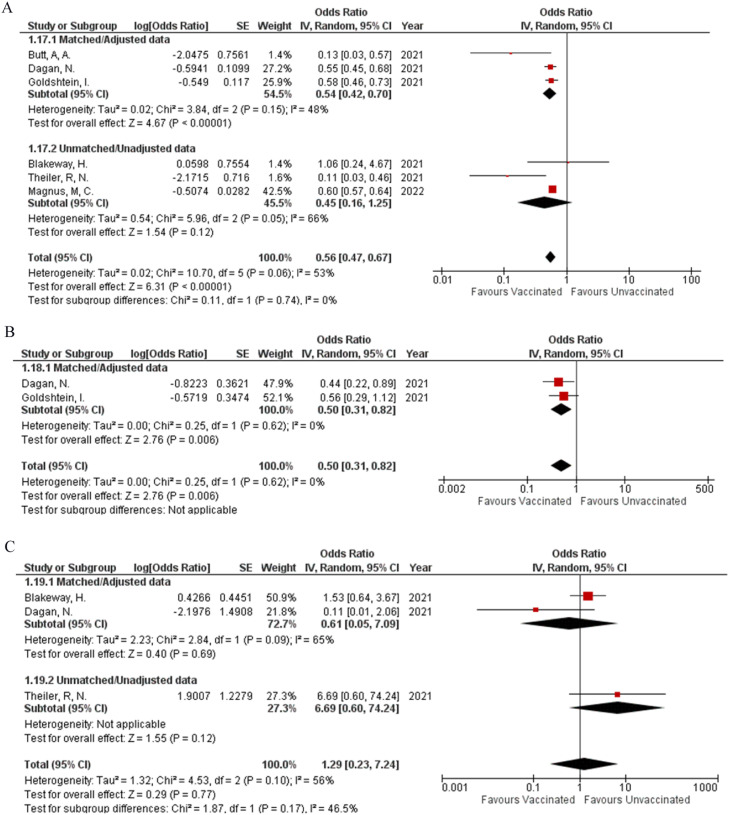
Six studies were included in analyzing the efficacy of the COVID-19 vaccine. Receiving the vaccine was shown to decrease the chances of getting infected with the virus (OR: 0.56; 95% CI: 0.47, 0.67; P = <0.00001; Fig 4 A); however, the heterogeneity was high at 53%. Performing the sensitivity analysis by removing Theiler et al., the heterogeneity was brought down to an acceptable level of 25%, with the effect still being significant (OR: 0.58; 95% CI: 0.52, 0.65; P = <0.00001; Supplemental Figure 2C).
Fig 4.
Forest plots for effectiveness of COVID-19 Vaccines. A: SARS-CoV-2 infections. B: COVID-19 related hospitalization. C: COVID-19 related ICU admissions.
COVID-19 related hospitalizations
COVID-19 related hospitalizations were also significantly reduced after receiving the vaccine (OR: 0.50; 95% CI: 0.31, 0.82; P = 0.006; Fig 4B) (I² = 0%) when analyzing data from two studies.
When considering COVID-19 related ICU admissions the vaccine failed to produce a significant impact (OR: 1.29; 95% CI: 0.23, 7.24; P = 0.77; Fig 4C) as assessed from 3 studies with high heterogeneity at 56%. When adjusting for heterogeneity by removing Dagan et al an acceptable level of 21% was achieved; however, the effect remained insignificant (OR: 2.05; 95% CI: 0.65, 6.50; P = 0.22; Supplemental Figure 2D).
When performing the subgroup analysis based on location, there was a significant decrease noted in COVID-19 infection after receiving the vaccination in all locations except England (OR: 1.06; 95% CI: 0.24, 4.68; P = 0.94); however, there was no significance noted when subgroup analysis was performed to assess COVID-19 related hospitalizations.
DISCUSSION
Our meta-analysis was conducted to determine the safety and effectiveness of COVID-19 vaccination in pregnant patients. We included ten observational studies in our analysis, which encompassed data from England, the U.S.A., Israel, Norway, Sweden, Qatar, and Canada, with a total of 326,499 pregnant patients under consideration. The Pfizer BNT162b2, Moderna mRNA1273, adenovirus vector vaccine, and AZD1222 were included. While the Centre for Disease Control and Prevention (C.D.C.) urges pregnant females to ensure complete vaccination against COVID-19, the WHO currently recommends the vaccination of pregnant females under the circumstance that the benefits are greater than the possible risks.18 , 19 Through these recommendations, numerous countries have begun urging their pregnant citizens to obtain the COVID-19 vaccine. However, there is a gap between the current rate of vaccination and the desired rate, owing to doubts regarding the safety of the available vaccines.3 Our meta-analysis reinforced the lack of adverse outcomes in association with COVID-19 vaccination during and after pregnancy for females and neonates and reiterated the effectiveness of the former as well.
Indicators of adverse pregnancy outcomes such as ‘preeclampsia’ or ‘eclampsia’, ‘stroke within four weeks of delivery’, ‘operative vaginal delivery’, ‘cesarean delivery’, ‘postpartum hemorrhage’, ‘blood transfusions’, ‘meconium-stained amniotic fluid’ and ‘abortion’ were used to determine the safety profile of COVID-19 vaccination. Our study revealed that there were no adverse pregnancy outcomes in vaccinated females in comparison to the unvaccinated. Current guidelines being followed regarding vaccination of pregnant females enforce the administration of the Inactivated Influenza vaccine and the Inactivated Tetanus Diphtheria Pertussis vaccine, with live vaccines such as the Measles Mumps Rubella vaccine and the Varicella vaccine being contraindicated in pregnancy.20 The type of vaccine plays a major role in its respective safety profile, with live vaccines being contraindicated due to the increased risk of perinatal infection and congenital birth defects- while inactivated vaccines do not pose such risks to the mother or fetus.21 The Pfizer Biontech and Moderna vaccines are mRNA vaccines that use the SARS CoV 2 spike protein as the target antigen, thus inducing a cell-mediated and humoral antibody response by activating CD4 T cells, CD8 cells and B cells. The efficacy of the mRNA vaccines has been established through relevant clinical trials in non-pregnant patients, and the adverse effects associated with them were minimal.22 , 23 Similar to influenza and the Tdap vaccines, which are inactivated and have a favorable safety profile in pregnancy, the mRNA vaccines are also demonstrating a lack of negative pregnancy outcomes which can be attributed to the nature of the vaccine and the processing of the mRNA within the host cells.24
Concern regarding the risk of spontaneous abortions followed by immunization exists, and the former has been identified as an outcome that needs to be examined closely when determining the safety of vaccines being administered during pregnancy. Fortunately, this meta-analysis stressed on there being no difference in the incidence of abortion between vaccinated and unvaccinated women (OR: 1.54; 95% CI: 0.41, 5.76; P = 0.07; Fig 2C) - encouraging health professionals and patients alike to recommend and take the vaccine respectively.25 This was also true for operative vaginal status (OR: 0.99; 95% CI: 0.59, 1.67; P = 0.97; Fig 2G) and caesarean deliveries (OR: 1.10; 95% CI: 0.95, 1.27; P = 0.21; Fig 2H) where no significant difference was observed. However, our study demonstrated heterogeneity for operative vaginal and cesarean delivery, though our overall results did not differ after sensitivity analysis. This heterogeneity can be attributed to a retrospective cohort study conducted by Rottenstreich et al., who reported an increased number of elective cesarean deliveries and a lower number of vacuum-assisted vaginal deliveries in women who received two doses of the vaccine. However, in their study, women who received two doses of the vaccine were older, had previous cesarean deliveries, and underwent treatment for infertility. The patient populations listed are already at an increased risk for cesarean delivery, thus proving to be a possible reason for their findings.13
Our analysis revealed that negative fetal outcomes such as ‘preterm birth’ - (OR: 1; 95% CI: 0.95, 1.06; P = 0.87; Fig 3A), ‘5 min Apgar score< 7’- (OR: 0.94; 95% CI: 0.86, 1.04; P = 0.22; Fig 3B) and ‘stillbirth’ - (OR: 1.02; 95% CI: 0.82, 1.26; P = 0.87; Fig 3C) show no significant difference between vaccinated and unvaccinated pregnant females. COVID-19 has been shown to significantly increase the risk of preterm birth and stillbirth, possibly due to an aggressive systemic inflammatory response and placental hypoperfusion. Our meta-analysis determined that not only will vaccination decrease the risk of COVID-19 infection, but it also has no impact on the rate of preterm birth and stillbirth- thus leading to an overall decrease of these two negative outcomes in pregnant females.26 Amongst neonatal outcomes included, we found that the incidence of neonatal ICU admission decreased in the vaccinated group (OR: 0.85; 95% CI: 0.81, 0.90; P = <0.00001; Fig 3G). Magnus et al. found a lower rate of neonatal admissions in women vaccinated within the third trimester and attributed this to increased awareness regarding perinatal and neonatal care amongst women who opt for the vaccine.16
In the subgroup analysis of adverse pregnancy outcomes based on location, the vaccinated group in Canada demonstrated a higher incidence of caesarean delivery (OR: 1.12; 95% CI: 1.08, 1.16; P = <0.00001; Supplemental Figure 2C) and a lower incidence of postpartum hemorrhage (OR: 0.89; 95% CI: 0.81, 0.98; P = 0.02; Supplemental Figure 2D). The relevant study included 97950 individuals, with 22660 receiving the COVID-19 vaccine - a much higher number of participants in comparison to the groups from the other locations included, possibly resulting in such findings.17 The SARS CoV 2 virus has been shown to result in obstetric complications such as postpartum hemorrhage, possibly due to a progressive coagulopathy resulting in the consumption of clotting factors. The group in Canada had a high number of vaccinated individuals, preventing infection and the decreased development of such obstetric complications in that population.27 , 28 Among fetal outcomes, preterm birth decreased after vaccination in Israel (OR: 0.87; 95% CI: 0.76, 0.99; P = 0.03; Supplemental Figure 2E) and Norway (OR: 0.81; 95% CI: 0.71, 0.92; P = 0.001; Supplemental Figure 2E), in comparison to USA and Sweden which showed no difference while the incidence of stillbirth decreased in Norway (OR: 0.42; 95% CI: 0.20, 0.91; P = 0.03; Supplemental Figure 2F) and Sweden (OR: 0.71; 95% CI: 0.52, 0.99; P = 0.04; Supplemental Figure 2F) with no change in the groups from the other locations. This could be due to the low number of stillbirths noted by Magnus et al. in comparison to the other studies, coming to only 0.2% of all included births.16
In the subgroup analysis based on the trimester in which the first dose was given on neonatal outcomes, a decrease in preterm birth was seen when the former was given in the third trimesters (OR: 0.70; 95% CI: 0.63, 0.77; P = <0.00001; Supplemental Figure 3B), while no significant difference was seen for small for gestational age births. However, this can be attributed to the fact that only 3.9% of the vaccinated group were those who received their first dose in the first trimester, thus leading to a low overall number of events.16 The Royal College of Obstetricians and Gynaecologists have suggested that pregnant women should preferably take their COVID-19 vaccine after the first trimester, provided they are not at an increased risk for infection with the virus due to the fetus being more susceptible to the development of adverse outcomes during that window. Nevertheless, several studies including ours have not shown a significant change in neonatal adverse outcomes, post-vaccination in the first trimester.29 When the subgroup analysis was done based on the type of vaccine, the Pfizer BionTech162b2 (OR: 0.91; 95% CI: 0.86, 0.97; P = 0.006; Supplemental Figure 4C) and the Moderna mRNA1273 (OR: 0.83; 95% CI: 0.73, 0.94; P = 0.003; Supplemental Figure 4C) vaccines decreased the incidence of preterm births amongst vaccinated females, in comparison to the AstraZeneca vaccine which showed no significant difference between the vaccinated and unvaccinated. The vaccine's protective effect against infection in pregnant females and thus decrease in negative outcomes after infection, such as preterm birth, has been documented thus explaining the BNT162b2 and mRNA1273 vaccines’ leading to a decrease in the incidence of preterm births.30
Our analysis reinforced the effectiveness of the COVID-19 vaccine as our results showed a decrease in the number of SARS CoV 2 infections in vaccinated pregnant females (OR: 0.56, 95% CI: 0.47, 0.67, P = <0.00001; Fig 4A), along with a decrease in COVID-19 hospitalization (OR: 0.50, 95% CI: 0.31, 0.82; P = 0.006; Fig 4B) in those infected, when compared to unvaccinated pregnant females. When investigating the vaccine's immunologic response, a significant rise in IgG, IgM, and IgA antibody titers was measured in vaccinated pregnant females, with a sustained IgG response seen two weeks after immunization. Furthermore, vaccination induced a more robust immunologic response in comparison to that seen after infection with the virus.31 Despite adverse pregnancy and fetal outcomes associated with SARS CoV 2 infection, several studies have revealed that COVID-19 vaccine uptake in pregnant females is not reaching the desired level, with women of older age, those undergoing fertility treatment, and those with higher education levels or socioeconomic status receiving the vaccine more readily in comparison to those who do not fit these criteria. 9 , 13 Skjefte et al. explored the degree of vaccine acceptance amongst pregnant females and determined that a majority will be willing to get immunized against the SARS-CoV-2 virus provided a greater than 90% effectiveness rate is established- which has now been established through numerous clinical trials.32
Heterogeneity was seen for SARS CoV 2 infection during or after pregnancy and COVID-19 related I.C.U. admission, though no significant difference was noted after sensitivity analysis. Though Theiller et al. did establish a significant decrease in COVID-19 infection rate in vaccinated pregnant females, the heterogeneity in our results is due to this study, possibly due to vaccine efficacy observed for a short time as most females received the vaccination in the third trimester.9 The rate of COVID-19 ICU admission did not differ amongst the vaccinated and unvaccinated groups. However, the heterogeneity in our results can be attributed to the study conducted by Dagan et al., who did not find enough relevant events to draw a conclusion.11 Based on location, the population of vaccinated pregnant females from England showed no difference in SARS CoV 2 infection rates in comparison to the unvaccinated group on subgroup analysis, while all other studies included showed a significant difference. In the relevant study conducted by Blakeway et al., women with pre-pregnancy diabetes mellitus were more prone to take the vaccine. However, this group is also more susceptible to infection including COVID-19, thus being one of many possible risks the participants of the English study were exposed to, leading to the disparity between it and other included studies.3
Our analysis had several limitations. Only observational studies were included in our study, with no randomized clinical trials. Our participants received either one dose, more than one or two doses, which were all taken as one and the same. We were not able to perform a subgroup analysis based on the number of doses received. The subgroup analysis performed based on trimester was limited to the first dose of the vaccine, instead of two doses. Furthermore, the subgroup analysis performed on the type of vaccine could not assess all the outcomes owing to limited available data. Another limitation was that few studies reported unmatched data which can result in confounding bias, thus leading to variability in baseline characteristics and outcome measures. We choose OR analysis over risk analysis to report data pooled through the included studies which may exaggerate the association but this was done to maximize the included sample size and minimize confounding bias. In addition, we included ten studies, all of them did not report all the outcomes we assessed, not allowing us to perform publication bias. Another limitation of our paper was that the included studies lacked data on the variabilities in response to therapies or vaccinations with respect to different COVID-19 variants and sub-variants, therefore, we were not able to perform a subgroup analysis based on the aforementioned.
CONCLUSIONS
Our study clearly outlined the lack of significant adverse pregnancy, fetal and neonatal outcomes after the COVID-19 vaccine. These results were seen regardless of the type of vaccine and the trimester in which females receive the vaccination. Furthermore, our study reinforced the decrease in COVID-19 infection rate and decrease in COVID-related hospitalizations in pregnant females. Clear guidelines are needed to help healthcare professionals in advising and implementing the vaccination of pregnant females against COVID-19. Pregnant patients also require the assurance needed to readily take the vaccine. Our meta-analysis can act as a reference for healthcare professionals and policymakers, for the prompt and much-needed vaccination of pregnant females against the SARS-CoV-2 virus.
Financial disclosure
None of the authors has a financial interest in any of the procedures, devices, or products mentioned in this manuscript.
Ethical statement
The study conforms to the Declaration of Helsinki.
IRB approval
No IRB approval required for this manuscript as no human subjects were involved.
Footnotes
Conflict of interest: No authors report any conflicts.
Funding:None.
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.ajic.2022.08.014.
Appendix. SUPPLEMENTARY MATERIALS
Reference
- 1.Wang EW, Parchem JG, Atmar RL, Clark EH. SARS-CoV-2 vaccination during pregnancy: a complex decision. Open Forum Infect Dis. 2021;8:ofab180. doi: 10.1093/ofid/ofab180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gray KJ, Bordt EA, Atyeo C, et al. COVID-19 vaccine response in pregnant and lactating women: a cohort study. medRxiv. 2021 doi: 10.1101/2021.03.07.21253094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Blakeway H, Prasad S, Kalafat E, et al. COVID-19 vaccination during pregnancy: coverage and safety. Am J Obstet Gynecol. 2022;226 doi: 10.1016/j.ajog.2021.08.007. 236.e1-236.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Skirrow H, Barnett S, Bell S, et al. Women's views on accepting COVID-19 vaccination during and after pregnancy, and for their babies: a multi-methods study in the UK. BMC Pregnancy Childbirth. 2022;22:33. doi: 10.1186/s12884-021-04321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Januszek SM, Faryniak-Zuzak A, Barnaś E, et al. The approach of pregnant women to vaccination based on a COVID-19 systematic review. Medicina (Kaunas) 2021;57:977. doi: 10.3390/medicina57090977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hutton B, Salanti G, Caldwell DM, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 7.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis of observational studies in epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 8.Higgins JPT., Thomas J, Chandler J, et al. Cochrane handbook for systematic reviews of interventions version 6.0 (updated July 2019) Cochrane. 2019 www.training.cochrane.org/handbook Accessed September 21, 2022. [Google Scholar]
- 9.Theiler RN, Wick M, Mehta R, Weaver AL, Virk A, Swift M. Pregnancy and birth outcomes after SARS-CoV-2 vaccination in pregnancy. Am J Obstet Gynecol MFM. 2021;3 doi: 10.1016/j.ajogmf.2021.100467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butt AA, Chemaitelly H, Al Khal A, et al. SARS-CoV-2 vaccine effectiveness in preventing confirmed infection in pregnant women. J Clin Invest. 2021;131 doi: 10.1172/JCI153662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dagan N, Barda N, Biron-Shental T, et al. Effectiveness of the BNT162b2 mRNA COVID-19 vaccine in pregnancy. Nat Med. 2021;27:1693–1695. doi: 10.1038/s41591-021-01490-8. [DOI] [PubMed] [Google Scholar]
- 12.Goldshtein I, Nevo D, Steinberg DM, et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326:728–735. doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rottenstreich M, Sela HY, Rotem R, Kadish E, Wiener-Well Y, Grisaru-Granovsky S. Covid-19 vaccination during the third trimester of pregnancy: rate of vaccination and maternal and neonatal outcomes, a multicentre retrospective cohort study. BJOG. 2022;129:248–255. doi: 10.1111/1471-0528.16941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wainstock T, Yoles I, Sergienko R, Sheiner E. Prenatal maternal COVID-19 vaccination and pregnancy outcomes. Vaccine. 2021;39:6037–6040. doi: 10.1016/j.vaccine.2021.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Goldshtein I, Steinberg DM, Kuint J, et al. Association of BNT162b2 COVID-19 vaccination during pregnancy with neonatal and early infant outcomes. JAMA Pediatr. 2022;176:470–477. doi: 10.1001/jamapediatrics.2022.0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Magnus MC, Örtqvist AK, Dahlqwist E, et al. Association of SARS-CoV-2 vaccination during pregnancy with pregnancy outcomes. JAMA. 2022;327:1469–1477. doi: 10.1001/jama.2022.3271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fell DB, Dhinsa T, Alton GD, et al. Association of COVID-19 vaccination in pregnancy with adverse peripartum outcomes. JAMA. 2022;327:1478–1487. doi: 10.1001/jama.2022.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hunter M, Moodley J, Moran N. Perspectives on COVID-19 vaccination for pregnant women in South Africa. Afr J Prim Health Care Fam Med. 2021;13:e1–e3. doi: 10.4102/phcfm.v13i1.2998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Annexes to the recommendations for use of the Pfizer–BioNTech vaccine BNT162b2 against COVID-19. (2022, January 21). World Health Organization. Accessed September 21, 2022. https://www.who.int/publications/i/item/WHO-2019-nCoV-vaccines-SAGE-recommendation-BNT162b2-GRADE-ETR-annexes
- 20.Guidelines for Vaccinating Pregnant Women. (2016, August) Centers for Disease Control and Prevention. Accessed September 21, 2022. https://www.cdc.gov/vaccines/pregnancy/hcp-toolkit/guidelines.html?CDC_AA_refVal=https%3A%2F%2Fwww.cdc.gov%2Fvaccines%2Fpregnancy%2Fhcp%2Fguidelines.html
- 21.Edwards JM, Watson N, Focht C, et al. Group B streptococcus (GBS) colonization and disease among pregnant women: a historical cohort study. Infect Dis Obstet Gynecol. 2019;2019 doi: 10.1155/2019/5430493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Golob JL, Lugogo N, Lauring AS, Lok AS. SARS-CoV-2 vaccines: a triumph of science and collaboration. JCI Insight. 2021;6 doi: 10.1172/jci.insight.149187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baden LR, El Sahly HM, Essink B, et al. COVE Study Group Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park JW, Lagniton PNP, Liu Y, Xu RH. mRNA vaccines for COVID-19: what, why and how. Int J Biol Sci. 2021;17:1446–1460. doi: 10.7150/ijbs.59233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kharbanda EO, Haapala J, DeSilva M, et al. Spontaneous abortion following COVID-19 vaccination during pregnancy. JAMA. 2021;326:1629–1631. doi: 10.1001/jama.2021.15494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wei SQ, Bilodeau-Bertrand M, Liu S, Auger N. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ. 2021;193:E540–E548. doi: 10.1503/cmaj.202604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hcini N, Maamri F, Picone O, et al. Maternal, fetal and neonatal outcomes of large series of SARS-CoV-2 positive pregnancies in peripartum period: a single-center prospective comparative study. Eur J Obstet Gynecol Reprod Biol. 2021;257:11–18. doi: 10.1016/j.ejogrb.2020.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalsar P, Datta S, Kalsar A, Kipkoech Kirui B, Kanyike AM. Severe postpartum hemorrhage in an asymptomatic COVID-19 patient: a call to be on guard. Int Med Case Rep J. 2021;14:683–687. doi: 10.2147/IMCRJ.S334249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Coronavirus (COVID-19), pregnancy and women's health. Royal college of obstetricians and gynecologists. Accessed April 18, 2022. https://www.rcog.org.uk/guidance/coronavirus-covid-19-pregnancy-and-women-s-health/
- 30.Ma Y, Deng J, Liu Q, Du M, Liu M, Liu J. Effectiveness and safety of COVID-19 vaccine among pregnant women in real-world studies: a systematic review and meta-analysis. Vaccines (Basel) 2022;10:246. doi: 10.3390/vaccines10020246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gray KJ, Bordt EA, Atyeo C, et al. Coronavirus disease 2019 vaccine response in pregnant and lactating women: a cohort study. Am J Obstet Gynecol. 2021;225 doi: 10.1016/j.ajog.2021.03.023. 303.e1-303.e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Skjefte M, Ngirbabul M, Akeju O, et al. COVID-19 vaccine acceptance among pregnant women and mothers of young children: results of a survey in 16 countries. Eur J Epidemiol. 2021;36:197–211. doi: 10.1007/s10654-021-00728-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.