Abstract
BACKGROUND
The authors recently reported a series of children with vertebral artery (VA) compression during head turning who presented with recurrent posterior circulation stroke. Whether VA compression occurs during head positioning for cranial surgery is unknown.
OBSERVATIONS
The authors report a case of a child with incidental rotational occlusion of the VA observed during surgical head positioning for treatment of an intracranial arteriovenous fistula. Intraoperative angiography showed dynamic V3 occlusion at the level of C2 with distal reconstitution via a muscular branch “jump” collateral, supplying reduced flow to the V4 segment. She had no clinical history or imaging suggesting acute or prior stroke. Sequential postoperative magnetic resonance imaging scans demonstrated signal abnormality of the left rectus capitus muscle, suggesting ischemic edema.
LESSONS
This report demonstrates that rotational VA compression during neurosurgical head positioning can occur in children but may be asymptomatic due to the presence of muscular VA–VA “jump” collaterals and contralateral VA flow. Although unilateral VA compression may be tolerated by children with codominant VAs, diligence when rotating the head away from a dominant VA is prudent during patient positioning to avoid posterior circulation ischemia or thromboembolism.
Keywords: bow hunter’s syndrome, vertebral artery compression, vertebral artery, pediatric stroke, pediatric neurosurgery
ABBREVIATIONS : AVF = arteriovenous fistula, DSA = digital subtraction angiography, MRI = magnetic resonance imaging, RVAO = rotational vertebral arteriopathy/occlusion, VA = vertebral artery
Rotational vertebral arteriopathy/occlusion (RVAO) is a controversial entity with uncertain prevalence in the pediatric population. In adults, a related phenomenon, sometimes called “bow hunter’s syndrome,” presents with symptoms of posterior circulation insufficiency due to dynamic narrowing of the vertebral artery (VA) during head rotation (typically to the contralateral side).1 Pediatric RVAO may present with stuttering or recurrent transient ischemic attacks or strokes, classically in young boys.2,3 Confirmatory diagnosis requires catheter digital subtraction angiography (DSA) with images obtained in the neutral and rotated head position.4 In select cases, surgical untethering of ligamentous bands or bony excrescences with or without fusion of the C1–C2 articulation can alleviate symptoms and prevent further neurological injury.5
In contrast to symptomatic RVAO, many authors have suggested that asymptomatic dynamic VA compression is highly prevalent and may even represent variant normal physiology.6 Cadaveric studies dating to 1927 showed that dynamic compression of the VA is frequent at the level of the axis.7 In early angiographic work, Faris et al. reported temporary occlusion of at least 1 VA in up to 25% of healthy men with head rotation.8 A later study using Doppler ultrasound of V3 revealed hemodynamically significant stenosis or occlusion in 6.3% of healthy control subjects.9 In adults, RVAO is classically associated with vertebrobasilar insufficiency, usually in the presence of contralateral VA atherosclerosis or hypoplasia.1 The pathogenesis of stroke is likely different in children and is more appropriately termed an “arteriopathy” due to chronic or recurrent vessel injury, resulting in artery-to-artery embolic infarcts rather than flow-related ischemia.2,3 The incidence of asymptomatic RVAO is not well defined.
We present a case of RVAO incidentally noted during intraoperative DSA in a young girl undergoing surgical ligation of an intracranial pial arteriovenous fistula (AVF). Flow to the intradural VA was preserved via a VA–VA jump collateral, and the patient had no postoperative neurological symptoms. Transient RVAO due to surgical head positioning has not been reported previously in the literature, to our knowledge, and suggests the importance of careful head positioning for pediatric neurosurgery not only to gain access to the intracranial pathology but also to preserve physiological brain perfusion.
Illustrative Case
At age 8 months, the patient was treated for streptococcal meningitis and bacteremia with multiorgan failure, intracranial abscesses, and anterior skull base osteomyelitis. During this hospitalization, brain magnetic resonance imaging (MRI)/magnetic resonance angiography demonstrated a high-flow vascular malformation in the region of the velum interpositum. No acute intervention was performed because the patient was asymptomatic.
Four years later, at age 6, the patient underwent cerebral angiography demonstrating a non-galenic, pial AVF of the internal cerebral vein. After multidisciplinary review at the University of California, San Francisco, cerebrovascular center, endovascular treatment was undertaken,10 significantly reducing the shunt fraction. Two days later, the patient returned for surgical ligation with intraoperative angiography.
To assess for interval change in the AVF prior to craniotomy, we obtained an angiogram with the patient supine with her head neutral prior to cranial fixation. Left VA injection (Fig. 1A, Video 1) showed normal opacification of the VA. The patient was then positioned and prepared for craniotomy. A gel roll bump was placed under the patient’s left shoulder, and her head was turned approximately 30° from the horizon and was approximately 60° relative to the shoulders, making her head parallel to the horizon. The sagittal plane was parallel to the floor with the vertex tilted down to facilitate gravity retraction of the right cerebral hemisphere for a right parasagittal interhemispheric transcallosal surgical approach. The patient’s head was then fixed in 3-point pin fixation with a radiolucent head holder. Craniotomy and clip ligation of the AVF were performed, followed by intraoperative angiography to confirm complete fistula disconnection. Intraoperative DSA of the left VA injection, performed to confirm complete resection, showed occlusion of the V3 segment of the left VA (Fig. 1B, Video 2) with late filling of V4 via VA–VA collateral flow from a muscular branch (Fig. 1C). The occlusion was promptly recognized as position related, and the patient’s head was gently rotated approximately 15° toward neutral and resecured in the head holder. A repeat injection in this position showed normal opacification of the VA and posterior circulation with no residual AVF. Postsurgical MRI (Fig. 2) showed no acute infarct but revealed well-circumscribed T2/fluid-attenuated inversion recovery hyperintensity in the left rectus capitus muscle. No residual fistula was observed, and the patient recovered well from surgery with no neurological deficit. Review of previous MRI and computed tomography imaging of the skull base and craniocervical junction revealed no significant bony asymmetry or structural variants (i.e., arcuate foramen, segmental fusion anomalies). Follow-up neuroimaging 5 days later showed persistent rectus capitus signal abnormality and no delayed cerebral ischemia. The patient had no neck tenderness or difficulty with head rotation.
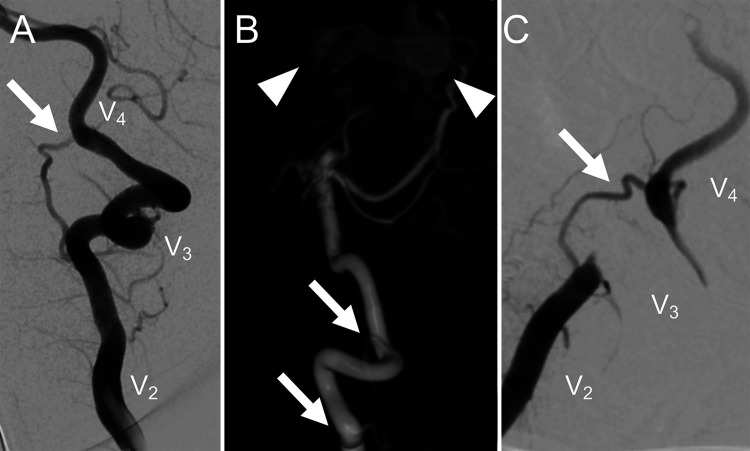
FIG. 1.
Left VA injection (A) prior to head rotation for craniotomy. Normal flow is seen in V2, V3, and V4 segments, and a prominent muscular branch (white arrow) is noted. 3D DSA of left VA injection (B) shows a multihole pial AVF (white arrowheads) and demonstrates that the presumed muscular branch (white arrows) connects the V2 and V4 segments. Intraoperative left VA injection with head rotation for the transcallosal approach (C) shows occlusion of the V3 segment with “jump” collateral filling of V4 via the collateral branch (white arrow).
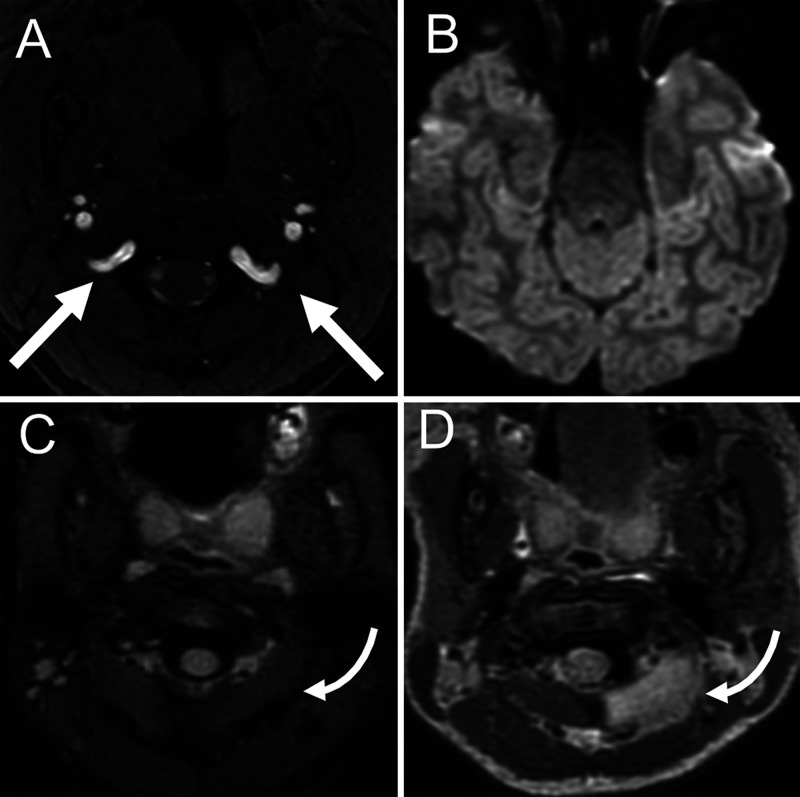
FIG. 2.
Preintervention time-of-flight magnetic resonance angiography (A) shows codominance of the V3 segments of the VA (white arrows). Postsurgical diffusion-weighted MRI (B) showed no acute ischemia following documented left V3 VA occlusion. Fluid-attenuated inversion recovery MRI before surgery (C) and after surgery (D) shows onset of focal edema of the left rectus capitus musculature on the left (curved arrows). Note: C is a fat-suppressed image; D is a non–fat-suppressed image.
VIDEO 1. Clip showing preoperative left VA DSA with the head in neutral position demonstrating normal filling of the V3 segment. Click here to view.
VIDEO 2. Clip showing intraoperative left VA DSA with the head rotated to the right demonstrating nonfilling of the V3 segment. Flow within a muscular branch (artery of Salmon) is redirected to the distal VA as a “jump” collateral. Click here to view.
Discussion
This report describes the incidental detection of RVAO due to craniocervical positioning in neurological surgery in a child. Postoperative imaging showed no posterior circulation ischemia, confirming the adequacy of the contralateral VA and ipsilateral muscular branch collateral flow. Although the possibility of RVAO during surgical head positioning has been raised by others,11,12 this is the first case, to our knowledge, that documents angiographically “dynamic” occlusion before and after rotation of the head for neurosurgical positioning.
Observations
The clinical entity of RVAO is a poorly understood etiology of childhood stroke. It has even been postulated as a mechanism of the sudden infant death syndrome.13 The V3 segment of the VA comprises 4 distinct loops and is anchored laterally by a fibrous periosteal ring of the atlas and medially by the dural ring at the foramen magnum. Redundancy of V3 loops has been posited as an etiology for RVAO.14 Arnautović et al. analogized V3 to the cavernous internal carotid artery and suggested that its surrounding venous plexus may in part serve to protect against mechanical impingement by adjacent bony elements.11 It has been shown that, in addition to pure rotation, hyperextension15 and traction forces16 may exacerbate RVAO. It follows that positioning the head of an anesthetized patient in a neurosurgical head frame could generate extraphysiological forces predisposing to RVAO. This is a particularly important consideration for pediatric neurosurgery because the craniocervical ligaments in children are estimated to have 10% of the stiffness of those in adults.17 For unclear reasons, case series of symptomatic RVAO in children show a striking predilection for boys; therefore, our case demonstrates that this phenomenon also occurs in girls.2,3
The suboccipital artery of Salmon (muscular branch of V3) is a relatively consistent VA branch that nourishes the upper cervical paraspinal muscles.18 This branch is known to communicate with ascending cervical and occipital artery branches, rendering it a potential hazard during endovascular therapy. This case shows that this branch can function as a VA–VA collateral when V3 is mechanically compromised. In this patient, codominance of the VA system enabled compensatory supply to the basilar artery from the right VA as well.
Lessons
Although intraoperative angiography is infrequently performed given the ready availability of high-resolution noninvasive imaging, we increasingly employ it at our institution for pediatric patients with complex cerebrovascular disease to reduce the need for reoperation after postoperative angiography. It is critical that neuroangiographers recognize the position-dependent appearance and transient nature of RVAO and not mistake it for iatrogenic dissection. Although antithrombotics are typically recommended for secondary stroke prevention after symptomatic RVAO in children,2,3 intraoperative heparinization would be contraindicated in the setting of craniotomy and likely would be unnecessary if there were no evidence of artery-to-artery embolus.
In summary, this case report describes asymptomatic and incidental RVAO during cranial surgery in a young girl. This finding is likely underrecognized clinically as well as in the literature due to the limited use of intraoperative angiography. Preservation of VA flow may be achieved from the contralateral VA or the ipsilateral muscular artery of Salmon (VA–VA collateral). In cases of VA dominance, such intraoperative occlusion could result in cerebral infarction; thus, we advise diligent review of VA anatomy prior to cranial surgery in children whose head will be rotated or extended contralateral to their dominant VA.
Disclosures
Dr. Hetts has received grants from Stryker Neurovascular, fees from MicroVention Terumo, personal fees from Johnson & Johnson, grants from Siemens Healthineers, and nonfinancial support from Penumbra for work outside the current work.
Author Contributions
Conception and design: Caton, Halbach, Hetts. Acquisition of data: Narsinh, Roland. Analysis and interpretation of data: Caton, Narsinh, Roland, Halbach, Fox, Fullerton, Hetts. Drafting the article: Caton, Halbach. Critically revising the article: Narsinh, Baker, Abla, Roland, Halbach, Fox, Fullerton, Hetts. Reviewed submitted version of manuscript: Caton, Narsinh, Abla, Roland, Fox, Fullerton, Hetts. Approved the final version of the manuscript on behalf of all authors: Caton. Administrative/technical/material support: Hetts. Study supervision: Hetts.
Supplemental Information
Videos
Video 1. https://vimeo.com/481719185.
Video 2. https://vimeo.com/481719191.
References
- 1. Kuether TA, Nesbit GM, Clark WM, et al. Rotational vertebral artery occlusion: a mechanism of vertebrobasilar insufficiency. Neurosurgery. 1997;41(2):427–433. doi: 10.1097/00006123-199708000-00019. [DOI] [PubMed] [Google Scholar]
- 2. Fox CK, Fullerton HJ, Hetts SW, et al. Single-center series of boys with recurrent strokes and rotational vertebral arteriopathy. Neurology. 2020;95(13):e1830–e1834. doi: 10.1212/WNL.0000000000010416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rollins N, Braga B, Hogge A, et al. Dynamic arterial compression in pediatric vertebral arterial dissection. Stroke. 2017;48(4):1070–1073. doi: 10.1161/STROKEAHA.116.016236. [DOI] [PubMed] [Google Scholar]
- 4. Barton JW, Margolis MT. Rotational obstructions of the vertebral artery at the atlantoaxial joint. Neuroradiology. 1975;9(3):117–120. doi: 10.1007/BF00332957. [DOI] [PubMed] [Google Scholar]
- 5. Zaidi HA, Albuquerque FC, Chowdhry SA, et al. Diagnosis and management of bow hunter’s syndrome: 15-year experience at barrow neurological institute. World Neurosurg. 2014;82(5):733–738. doi: 10.1016/j.wneu.2014.02.027. [DOI] [PubMed] [Google Scholar]
- 6. Toole JF, Tucker SH. Influence of head position upon cerebral circulation. Studies on blood flow in cadavers. Arch Neurol. 1960;2(6):616–623. doi: 10.1001/archneur.1960.03840120022003. [DOI] [PubMed] [Google Scholar]
- 7. De Kleyn A, Nieuwenhuyse P. Schwindelanfalle und Nystagmus bei einer bestimmten Stellung des Kopfes Acta Otolaryngol 1927. 11 1 155 157 [Google Scholar]
- 8. Faris AA, Poser CM, Wilmore DW, et al. Radiologic visualization of neck vessels in healthy men. Neurology. 1963;13(5):386–396. doi: 10.1212/wnl.13.5.386. [DOI] [PubMed] [Google Scholar]
- 9. Arnetoli G, Amadori A, Stefani P, et al. Sonography of vertebral arteries in De Kleyn’s position in subjects and in patients with vertebrobasilar transient ischemic attacks. Angiology. 1989;40(8):716–720. doi: 10.1177/000331978904000805. [DOI] [PubMed] [Google Scholar]
- 10. Hetts SW, Keenan K, Fullerton HJ, et al. Pediatric intracranial nongalenic pial arteriovenous fistulas: clinical features, angioarchitecture, and outcomes. AJNR Am J Neuroradiol. 2012;33(9):1710–1719. doi: 10.3174/ajnr.A3194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Arnautović KI, al-Mefty O, Pait TG, et al. The suboccipital cavernous sinus. J Neurosurg. 1997;86(2):252–262. doi: 10.3171/jns.1997.86.2.0252. [DOI] [PubMed] [Google Scholar]
- 12. George B, Laurian C. Surgical approach to the whole length of the vertebral artery with special reference to the third portion. Acta Neurochir (Wien) 1980;51(3-4):259–272. doi: 10.1007/BF01406753. [DOI] [PubMed] [Google Scholar]
- 13. Pamphlett R, Raisanen J, Kum-Jew S. Vertebral artery compression resulting from head movement: a possible cause of the sudden infant death syndrome. Pediatrics. 1999;103(2):460–468. doi: 10.1542/peds.103.2.460. [DOI] [PubMed] [Google Scholar]
- 14. Hardin CA, Poser CM. Rotational obstruction of the vertebral artery due to redundancy and extraluminal cervical fascial bands. Ann Surg. 1963;158(1):133–137. doi: 10.1097/00000658-196307000-00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Okawara S, Nibbelink D. Vertebral artery occlusion following hyperextension and rotation of the head. Stroke. 1974;5(5):640–642. doi: 10.1161/01.str.5.5.640. [DOI] [PubMed] [Google Scholar]
- 16. Brown BStJ, Tatlow WFT. Radiographic studies of the vertebral arteries in cadavers. Effects of position and traction on the head. Radiology. 1963;81(1):80–88. doi: 10.1148/81.1.80. [DOI] [PubMed] [Google Scholar]
- 17. Phuntsok R, Mazur MD, Ellis BJ, et al. Development and initial evaluation of a finite element model of the pediatric craniocervical junction. J Neurosurg Pediatr. 2016;17(4):497–503. doi: 10.3171/2015.8.PEDS15334. [DOI] [PubMed] [Google Scholar]
- 18. Tubbs RS, Shah NA, Sullivan BP, et al. Surgical anatomy and quantitation of the branches of the V2 and V3 segments of the vertebral artery. Laboratory investigation. J Neurosurg Spine. 2009;11(1):84–87. doi: 10.3171/2009.3.SPINE08683. [DOI] [PubMed] [Google Scholar]