Abstract
Human exposure to carcinogenic volatile organic compounds (VOCs), such as benzene, from hand sanitizers is a topic of current concern. In light of the heavy use of hand sanitizers during the COVID-19 pandemic, determination of exposure to toxicants present in these products deserves attention. The US Food and Drug Administration (FDA) had set an interim limit for benzene in alcohol-based hand sanitizers at 2000 parts-per-billion (ppb). We determined the concentrations of and exposure to three VOCs namely, benzene, toluene and styrene, in 200 hand sanitizers using high-resolution gas chromatography coupled with high-resolution mass spectrometry (HRGC-HRMS). Benzene, toluene and styrene were found in 31%, 25% and 32%, respectively, of the samples analyzed at mean concentrations of 395 (range: 0.181–22,300), 164 (range: 0.074–20,700) and 61.3 ng/g (range: 0.082–4200 ng/g), respectively. Benzene was found at concentrations > 2000 ng/g (above the FDA interim limit) in 5% of the samples, representing 9 brands. The mean potential dermal exposure doses (DEDs) to benzene (children/teenagers: 34.6; adults: 24.7 ng/kg-bw/d) were higher than those for toluene (children/teenagers: 14.4; adults: 10.3 ng/kg-bw/d) and styrene (children/teenagers: 5.37; adults: 3.83 ng/kg-bw/d) in the 200 hand sanitizers analyzed. The estimated cancer risk from exposure to benzene in children/teenagers and adults from hand sanitizer use (at an estimated usage rate of 5 g/day) was greater than the one-in-a-million risk benchmark (1.0 × 10−6) for 10% and 9% of the samples, respectively. To the best of our knowledge, this is the first study to determine both the concentrations of and exposure risks to benzene, toluene and styrene present in hand sanitizers.
Keywords: Benzene, Hand sanitizers, Volatile organic compounds, Cancer risk, Exposure
1. Introduction
Amidst the global coronavirus pandemic that began in 2019 (COVID-19), the production and use of hand sanitizers to help control viral spread have increased significantly (Leslie et al., 2021; Berardi et al., 2020). Alcohol-based hand sanitizers typically contain, as active antimicrobial agents, ethanol or isopropanol (or sometimes n-propanol) at concentrations of 60–95% (v/v), produced either from the fermentation of biomass (natural) or from petroleum-based sources (synthetic). Due to the heavy demand for hand sanitizers coupled with shortages of raw materials such as ethanol, use of industrial-grade alcohols was permitted interim in many countries. Hand sanitizer manufacturers used different feedstock that affected the quality of the end products and the type of impurities they contain (Tse et al., 2021). Besides methanol and acetaldehyde, some volatile organic compounds (VOCs) such as benzene have been found in hand sanitizers (Valisure, 2021). The International Agency for Research on Cancer (IARC) classifies benzene as a known human carcinogen (ATSDR, 2007a, 2007b; WHO, 2010a). Similarly, other VOCs such as toluene and styrene are also expected to be present in petroleum-derived alcohols. The United States Environmental Protection Agency (EPA) classifies toluene and styrene as hazardous pollutants (EPA, 2017), and the World Health Organization classifies styrene as a possible (Group 2B) human carcinogen (WHO, 2010a). The occurrence of and exposure to VOCs such as toluene and styrene present in hand sanitizers are unknown.
Exposure to benzene causes leukemia and other non-cancerous effects such as neurological, cardiovascular, gastrointestinal, reproductive and renal toxicities (Pal et al., 2022; CDC, 2021; Li et al., 2021; ATSDR, 2007a, 2007b; Lamm, 2006; Jones et al., 1991). Toluene is sequestered in fatty tissues and brain, and exposure can result in renal toxicity, myocardial infarction, cardiac arrhythmias, depression and reproductive toxicities (ATSDR, 2017; Li et al., 2021). Exposure to styrene is linked to respiratory, hematological, neurological, endocrine, reproductive and central nervous system toxicity (ATSDR, 2010; Li et al., 2021).
Concern exists about exposure to VOCs from hand sanitizers. Methanol intoxication has been reported in individuals who ingest alcohol-based hand sanitizers (CDC, 2020a, 2020b). For benzene, the FDA issued an interim limit of 2000 ng/g in June of 2020 in alcohol-based hand sanitizers (US FDA, 2020), which was then withdrawn in the December of 2021. The FDA also issued an import alert in 2021 for 231 hand sanitizer products, 83% of which were manufactured in Mexico and 7% in China (Valisure, 2021). A few studies have previously reported occurrence of benzene (Lin et al., 2020; Lim et al., 2014; Hudspeth et al., 2022), toluene (Lin et al., 2020; Lim et al., 2014; Steinemann et al., 2021) and/or styrene (Lin et al., 2020; Steinemann et al., 2021) in consumer products such as hand wash soaps, disinfectants, sunscreens, fresheners, cleaners, glues, marking pens and hygiene products. However, those earlier studies were predominantly qualitative and little is known about the concentrations of and exposure to benzene, toluene and styrene present in hand sanitizers or the risks associated with such exposure. In this study, we determined the concentrations and profiles of these three VOCs in 200 commercially available hand sanitizers representing 121 brands, using high-resolution gas chromatography–high-resolution mass spectrometry (HRGC-HRMS). Furthermore, we assessed exposure doses and non-carcinogenic (for benzene, toluene and styrene) and carcinogenic risks (for benzene) from the use of hand sanitizers.
2. Materials and methods
2.1. Sample collection
A convenience sample size of two hundred hand sanitizers were purchased online (n = 27), in retail stores (n = 169) and from dispensers located in public places (such as restaurants and hospitals, n = 4) in New York State from 13 April 2021 to 22 July 2021. A complete list of hand sanitizers analyzed in this study is presented in Table S1. Information regarding brand name, lot number, location of purchase, alcohol content, size and country of manufacture was taken from product labels (Table S1). The hand sanitizer samples represented 121 brands (1 to 9 samples per brand) and were alcohol-based, except two samples, which were benzalkonium-based. The inactive and active ingredients present in the hand sanitizers analyzed are presented in Table S2.
2.2. Chemicals and reagents
Analytical standards of benzene, toluene and styrene of purities ≥ 99.9%, ≥99.9% and ≥ 98.0%, respectively, as well as three corresponding isotopically labeled internal standards (ISs: benzene-d6 [purity: ≥99.0%], toluene-d8 [≥99.0%] and styrene-d8 [≥98.0%]), were purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC-grade acetonitrile (≥99.9%) and water were purchased from J.T. Baker (Center Valley, PA, USA).
2.3. Analysis of Benzene, toluene and styrene
Benzene, toluene and styrene contents were determined in hand sanitizers by following a method described by the FDA with slight modifications (US FDA, 2020). Briefly, 0.3 g of hand sanitizer was weighed into a 15-mL polypropylene (PP) tube (Falcon™, Fisher Scientific, Waltham, MA, USA) and 4.95 mL of acetonitrile and 50 μL of 1000 ng/mL internal standard (IS) mixture (in acetonitrile) were added. The sample mixture was shaken in a reciprocal shaker (Eberbach Corp., Ann Arbor, MI, USA) at 280 oscillations/min for 10 min. The supernatant was transferred into a new PP tube, and a 1-mL aliquot of the supernatant was transferred into a glass vial for HRGC-HRMS analysis.
Identification and quantification of target analytes were accomplished using an Agilent 7890A high-resolution gas chromatograph (Agilent Technologies, Santa Clara, CA, USA) coupled with a JEOL JMS-800D Ultra FOCUS™ high-resolution mass spectrometer (JEOL USA, Inc., Peabody, MA, USA). Chromatographic separation was accomplished using a DB-Select 624 Ultra Inert column (30 m length, 0.250 mm inner diameter, 1.40 μm film thickness; Agilent Technologies, Santa Clara, CA, USA). Helium was used as the carrier gas. Two microliters of the sample extracts were injected in the pulsed split mode (50:1 split ratio with pulsed pressure of 25 psi for 0.5 min and split flow rate of 50 mL/min). The carrier gas flow was maintained at 1 mL/min. The inlet temperature was set at 250 °C. The column oven temperature was programmed as follows: held at 40 °C for 5 min, increased to 240 °C at a rate of 30 °C/min, and then held for 4 min. The equilibration time was 0.5 min and the total run time was 15.667 min. The MS source temperature was 230 °C. An electron impact positive ionization selected ion monitoring (SIM) was used for compound acquisition. The ions were monitored at m/z 78.0464 for benzene, m/z 84.0841 benzene-d6, m/z 92.0620 for toluene, m/z 100.1123 for toluene-d8, m/z 104.0620 for styrene and m/z 112.1123 for styrene-d8. Polyfluorokerosene was used as the reference compound for calibration of the HRMS.
Quantification of the target analytes was achieved using an isotope dilution method, by taking the ratio of the absolute response of each native analyte to that of the corresponding isotope-labeled internal standard. Peak integration, calibration and quantitation were performed using DioK software (JEOL USA, Inc., Peabody, MA, USA).
2.4. Quality assurance and quality control (QA/QC)
Two hundred hand sanitizer samples were analyzed in six batches, each comprising 25–40 samples. Analysts took care not to use products that many introduce sources of contamination (including hand lotions or sanitizers) before and during the analysis. Powder-free, Purple Nitrile™ gloves (Kimtech™; Kimberly-Clark Corporation, Neenah, WI, USA) were worn by analysts during sample preparation and analysis. Acetonitrile was used as the procedural blank (n = 19) to check for contamination arising from laboratory materials and solvents. The mean absolute amounts of benzene and toluene found in procedural blanks were 1.19 ng/mL (range = 1.54–4.23 ng/mL) and 32.4 ng/mL (range = 26.8–37.1 ng/mL), respectively (Figure S1). Styrene was not found in procedural blanks. The mean concentrations of benzene and toluene found in procedural blanks were subtracted from concentrations measured in samples. High absolute levels of toluene were carefully monitored and only those samples with responses at least twice higher than in blanks were reported as detectable values. A hand sanitizer sample was fortified with target analytes at concentrations of 10 and 100 ng/mL and analyzed through the entire procedure. Recoveries of benzene, toluene and styrene in the fortified sample were in the ranges of 80–120% (mean: 106%), 70–130% (mean: 80.3%) and 80–120% (mean: 104%), respectively. Acetonitrile was injected after every 10 samples to monitor for sample-to-sample carryover of target analytes. No carryover was observed for any of the target analytes. A midpoint calibration standard (100 ng/mL) was injected after every 10 samples to monitor instrumental drift in sensitivity. A 10-point, linear, non-forced-through-zero standard calibration curve was prepared at a concentration range of 1–1000 ng/mL (regression coefficient > 0.99 for each analyte) for quantification. Select samples were analyzed in duplicate by inserting them in multiple batches, and the relative standard deviation (RSD) of duplicate analysis ranged from 2.67% to 20.5% for benzene, 4.05% to 9.39% for toluene and 1.44% to 22.8% for styrene. The limits of detection (LODs) of benzene, toluene and styrene were 0.114, 0.105 and 0.116 ng/g, respectively. For the calculation of mean and median concentrations, analyte concentrations below the LOD were substituted with the value LOD divided by the square root of 2.
2.5. Exposure and risk assessments
It should be noted that 98% of the hand sanitizers analyzed in this study were alcohol-based. Alcohol is a known skin penetration enhancer which renders the cell membrane to become highly permeable to the chemicals present in hand sanitizers (Mahmood et al., 2020; Lachenmeier, 2008). Hence, we limited our exposure calculation to dermal pathway. Furthermore, studies have reported dermal permeation of neat benzene or benzene vapors was low (<5%) but in the presence of alcohol that value can be enhanced (Williams et al., 2011; Kezic and Nielsen, 2009; Wester and Maibach, 2000; Susten et al., 1985). In the absence of relevant dermal permeation rates for benzene co-exposed with alcohols, we used a conservative value of 100% in our exposure calculation.
The potential dermal exposure doses (DED, ng/kg-bw/d) were calculated for benzene, toluene and styrene using the following Eq. (1) (EPA, 2011):
| (1) |
where C is the concentration (ng/g) of the target analyte in each hand sanitizer sample, A is the average amount of hand sanitizer applied per day (g/day) and BW is the body weight (kg) (Table S3). The hand sanitizer usage rate was reported to be 5 g/day (Choi et al., 2021). The average body weights of children/teenagers and adults were defined to be 57 and 80 kg, respectively (ATSDR, 2016). Concentrations measured in each sample was used to calculate DED and to assess exposure frequency distribution.
The non-carcinogenic risks from dermal exposure to benzene, toluene and styrene were calculated as a hazard quotient (HQ) using the following Eq. (2) (EPA, 2011; ATSDR, 2016; Lin et al., 2020):
| (2) |
where DED is the potential dermal exposure dose (ng/kg-bw/d) and RfD is the reference dose (ng/kg-bw/d) (Table S3).The RfDs of benzene, toluene and styrene were reported to be 4 X 103, 8 X 104 and 2 X 105 ng/kg-bw/d, on the basis of decreased lymphocyte count, increased kidney weight and hematological effects, respectively (Qin et al., 2019; Lim et al., 2014; Chaiklieng et al., 2019). A HQ value > 1 is suggestive of potential health risks.
The cancer risk (CR) from dermal exposure to benzene was calculated using the following Eq. (3) (EPA, 2011; ATSDR, 2016; Lim et al., 2014):
| (3) |
where DED is the potential dermal exposure dose (ng/kg-bw/d) and DSF is the dermal cancer slope factor (kg-bw/d/ng) for benzene (Table S3). Since toluene and styrene have no known carcinogenicity in humans, cancer risks were not calculated for these two compounds.
A model calculation of the determination of DED, HQ and CR for benzene is shown in the Supplementary Text 1 (Text S1).
2.6. Data analysis
Statistical analysis was performed using SPSS 19.0 (SPSS Inc., Chicago, IL, USA), Excel Spreadsheet 2019 (Microsoft Office Professional Plus, Redmond, WA, USA) and GraphPad Prism 9.1.1 (GraphPad Software, San Diego, CA, USA). The normality of measured data was tested by Shapiro-Wilk test (p > 0.05 was considered normal). Because many of the variables were not normally distributed, Mann-Whitney test and Kruskal-Wallis test were used in the comparison of analyte concentrations between various categories. Spearman correlation analysis was performed to examine relationship between benzene, toluene and styrene concentrations and heat maps were generated. The significance level was set at p < 0.05 for all statistical analysis.
3. Results and discussion
3.1. Concentrations of Benzene, toluene and styrene
We detected benzene in 31% hand sanitizer samples at a mean concentration of 395 ng/g (range: 0.181–22,300 ng/g) (Table 1). Ten hand sanitizers (5%) from nine brands had benzene concentrations above 2000 ng/g, the interim limit set by the FDA (Table S4). The highest benzene concentration of 22,300 ng/g (>10 times the FDA interim limit) was found in a sample from South Korea. Our results are in line with an investigation conducted by Valisure, a Connecticut-based pharmacy product testing company, that had previously reported the occurrence of benzene in 44 (17%) of 260 hand sanitizer samples they analyzed (Valisure, 2021). The reported benzene concentrations in those samples ranged from 100 to 16,100 ng/g (Valisure, 2021).
Table 1.
Concentrations (ng/g) and dermal exposure dose (ng/kg-bw/d) of benzene, toluene and styrene in hand sanitizers marketed in the United States (n = 200).
| Statistic | Benzene |
Toluene |
Styrene |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration (ng/g) |
DED Children/Teenagers (ng/kg-bw/d) |
DED Adults (ng/kg- bw/d) |
Concentration (ng/g) |
DED Children/Teenagers (ng/kg-bw/d) |
DED Adults (ng/kg- bw/d) |
Concentration (ng/g) |
DED Children/Teenagers (ng/kg-bw/d) |
DED Adults (ng/kg- bw/d) |
|
| Minimum | 0.081 | 0.007 | 0.005 | 0.074 | 0.007 | 0.005 | 0.082 | 0.007 | 0.005 |
| 25% Percentile | 0.081 | 0.007 | 0.005 | 0.074 | 0.007 | 0.005 | 0.082 | 0.007 | 0.005 |
| Median | 0.081 | 0.007 | 0.005 | 0.074 | 0.007 | 0.005 | 0.082 | 0.007 | 0.005 |
| 75% Percentile | 29.0 | 2.52 | 1.79 | 0.074 | 0.007 | 0.005 | 51.3 | 4.50 | 3.21 |
| Maximum | 22,300 | 1960 | 1390 | 20,700 | 1820 | 1290 | 4200 | 368 | 263 |
| Mean | 395 | 34.6 | 24.7 | 164 | 14.4 | 10.3 | 61.3 | 5.37 | 3.83 |
| S.D. | 1980 | 174 | 124 | 1480 | 130 | 92.5 | 309 | 27.1 | 19.3 |
| SEM | 140 | 12.3 | 8.78 | 105 | 9.18 | 6.54 | 21.8 | 1.91 | 1.37 |
SD: Standard deviation, SEM: Standard error of mean, DED: Dermal exposure dose. Values below the limit of detection are substituted with the value LOD divided by the square root of 2.
We found toluene and styrene in 25% and 32% of the samples analyzed, respectively, at mean concentrations of 164 (range: 0.074–20,700 ng/g) and 61.3 ng/g (range: 0.082–4200 ng/g), respectively (Table 1). The highest concentrations of toluene and styrene were found in hand sanitizers from Turkey (20,700 ng/g) and China (4200 ng/g), respectively (Table S4). There are no FDA limits on toluene and styrene in hand sanitizers.
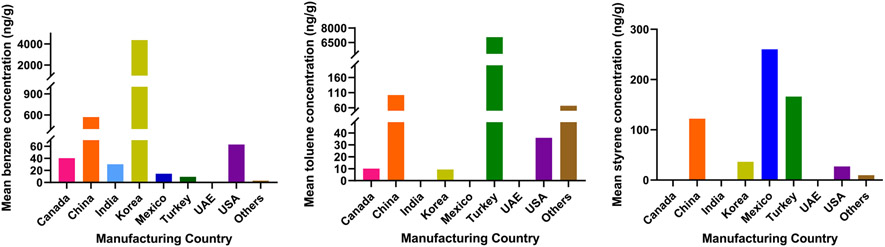
The hand sanitizers (n = 200) analyzed in this study were grouped based on their country of origin: Canada (n = 7), China (n = 67), India (n = 2), Korea (n = 8), Mexico (n = 3), Turkey (n = 3), the United Arab Emirates (n = 1) and the USA (n = 84) (Figure S2). Information regarding country of origin was not available for 25 samples (categorized as “Others”). Even though the number of samples analyzed for each country was small, benzene concentrations varied significantly by country of origin (p < 0.05). Hand sanitizers from Korea and China contained significantly higher concentrations of benzene than those from other countries (p < 0.05). The mean concentrations of benzene in hand sanitizers from Korea (4370 ng/g; range: 0.081–22,300 ng/g) were the highest, which was followed by those from China (570 ng/g; range: 0.081–9840 ng/g) (Fig. 1 and S3). Toluene concentrations did not vary significantly among the countries (p > 0.05), whereas styrene concentrations did vary significantly (p < 0.05), according to country of origin. The mean concentrations of toluene in hand sanitizers from Turkey (7050 ng/g; range: 0.074–20,700 ng/g) were the highest, followed by those from China (102 ng/g; range: 0.074–2190 ng/g). The mean concentrations of styrene in hand sanitizers from Mexico (260 ng/g; range: 0.082–779 ng/g) were the highest, followed by those from Turkey (166 ng/g; range: 0.082–425 ng/g). Nevertheless, the concentrations of styrene in hand sanitizers from China were significantly higher than those found in other countries (p < 0.05). It is also worth to mention that even within a country, concentrations of benzene, toluene and styrene in hand sanitizers varied by several orders of magnitude (Figure S3).
Fig. 1.
Mean concentrations of benzene (ng/g), styrene (ng/g) and toluene (ng/g) measured in hand sanitizer samples (n = 200) stratified according to the country of manufacture. (Samples for which the place of manufacture was unavailable are categorized as Others).
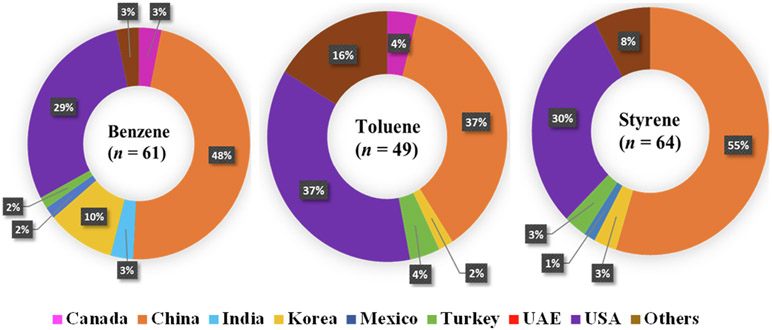
Benzene, toluene and styrene were found in 48%, 37% and 55%, respectively, of the hand sanitizers from China and 29%, 37%, and 30%, respectively, of those from the USA (Fig. 2). The study by Valisure in 2021 reported similar results, finding benzene in 50% samples from China, 34% samples from the USA, 11% from Korea, 2% from Australia and 2% from Mexico (Valisure, 2021). We calculated the percentage of hand sanitizer samples containing benzene, toluene and styrene for each country (Figure S4). Although the sample size was small per country, benzene was found in 100%, 75% and 43% of hand sanitizers from India, Korea and China, respectively. Toluene was found in 67%, 29% and 27% of hand sanitizers from Turkey, Canada and China, respectively. Styrene was found in 67%, 52% and 33% of hand sanitizers from Turkey, China and Mexico, respectively. The major sources of benzene, toluene and styrene are impurities in alcohols that are used as active ingredients. Among 200 samples analyzed, two were non-alcohol based (benzalkonium chloride was the active ingredient) hand sanitizers originating from Canada and Turkey. Benzene was not found in either of the samples. However, toluene concentration of 20,700 ng/g was found in the sample from Turkey, whereas the sample from Canada contained neither toluene nor styrene.
Fig. 2.
Percentage of hand sanitizer samples containing benzene, toluene and styrene according to the country of origin. For 25 hand sanitizers (categorized as Others), information on the country of manufacture was unavailable. The n represents the total number of hand sanitizers containing benzene, toluene and styrene, respectively.
3.2. Exposure and risk assessments
The calculated DEDs to benzene, toluene and styrene from hand sanitizers ranged from 0.007 to 1956 (mean = 34.6), 0.007 to 1816 (mean = 14.4) and 0.007 to 368 (mean = 5.37) ng/kg-bw/d, respectively, for children/teenagers, whereas those for adults ranged from 0.005 to 1394 (mean = 24.7), 0.005 to 1294 (mean = 10.4) and 0.005 to 263 (mean = 3.83), respectively (Table 1 and S4). Slightly higher exposure doses in children/teenagers than adults are explained by the smaller bodyweights of the former. A study estimated average exposure dose to benzene of 320 μg/day (for a person weighing 70 Kg, it would be 4570 ng/kg-bw/d), in the United States with indoor air inhalation accounting for > 90% of the exposure (Wallace, 1996; WHO, 2010b). The mean dermal exposure doses of benzene calculated from hand sanitizers were two orders of magnitude lower. Similarly, styrene exposure dose (total) in the United States was reported to range from 260 to 780 ng/kg-bw/d, in 2000 (inhalation was the dominant pathway), and dermal exposure doses calculated in our study were two orders of magnitude lower.
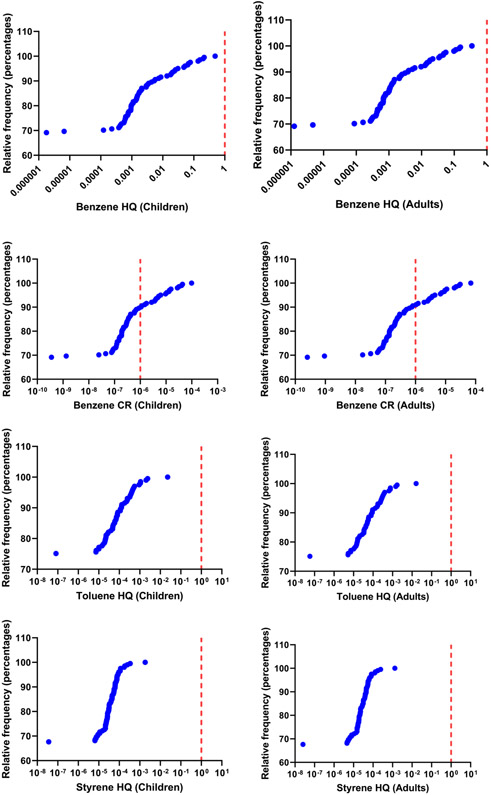
The non-carcinogenic risks of benzene, toluene and styrene were calculated as HQs based on the reference doses (RfD) of the EPA (Qin et al., 2019; Lim et al., 2014; Chaiklieng et al., 2019) (Tables S3 and S6). All HQ values were < 1 even for the samples that contained the highest concentrations of target analytes (Fig. 3), which suggested a lack of potential non-carcinogenic risks at the daily usage rate of 5 g/day.
Fig. 3.
Frequency distribution curves for exposure risks associated to benzene, toluene and styrene present in hand sanitizer samples. HQ refers to hazard quotients for non-carcinogenic risk; CR stands for carcinogenic risk. The red line indicates the threshold value for HQ or CR.
The cancer risk (CR) represents the upper-bound excess lifetime risk estimated to result from continuous exposure to an agent over a lifetime, and risk-specific doses are derived from the slope factor to estimate the dose associated with a specific risk level, for example, a one-in-a-million (i.e., 1.0 × 1−6) increased lifetime risk (Qin et al., 2019). The CR values estimated for benzene from mean concentrations (Lin et al., 2020; Lim et al., 2014) in hand sanitizers were 1.73 × 1CT−5 (range: 3.55 × 10−10 to 9.78 × 10−5) for children/teenagers and 1.23 × 10−5 (range: 2.53 × 10−10 to 6.97 × 10−5) for adults. Of the 200 samples analyzed, benzene concentrations in 20 (10%) and 18 (9%) hand sanitizers, respectively, for children/teenagers and adults, exceeded the acceptable benchmark risk level that is often considered by regulatory agencies in the United States to pose the de minimus risk (1.0 × 10−6) (Fig. 3 and Table S5).
The concentrations of benzene and styrene in hand sanitizers (ρ = 0.204, p < 0.05) were weakly correlated, whereas no correlation was found between benzene and toluene (p > 0.05) or toluene and styrene (p > 0.05) (Figure S5). Concentrations of benzene and toluene did not vary according to alcohol content (p > 0.05), whereas the concentrations of styrene (p < 0.05) varied significantly, depending on the alcohol content of hand sanitizers (Figure S6). These results suggest that benzene, toluene and styrene are derived from not only active but also inactive ingredients used in hand sanitizers.
Studies reporting the occurrence of benzene, toluene and styrene in hand sanitizers are meager, and our study provides the first quantitative evidence of both occurrence and associated exposure risks. Another strength of this study is the utilization of HRGC-HRMS for the detection of select VOCs, that is highly sensitive and selective. However, the study has few limitations. First, the analysis is based on convenience sample of hand sanitizers and the number of samples analyzed for each country was small. Second, uncertainties exist in potential exposure and risk assessments, which include dermal permeation factors and daily usage rates. Nevertheless, as mentioned above, dermal permeation rates of benzene in the presence of an alcohol can be enhanced when applied on the skin. Our exposure calculation may be considered conservative, for the dermal pathway. Inhalation is the major pathway of exposure to many VOCs including benzene. Even though dermal exposure to target VOCs from hand sanitizers is below the threshold values for toxic effects, other routes of exposure would augment risks. Demographic factors that affect exposure were not described in this study.
4. Conclusions
To our knowledge this is the first study to determine the concentrations of and dermal exposure to benzene, toluene and styrene in hand sanitizers marketed in the United States. We found benzene at elevated concentrations (>2000 ng/g) in 5% of hand sanitizers analyzed. Benzene exposure from 20 of the 200 hand sanitizers analyzed in this study would increase the EPA’s benchmark for the de minimus cancer risk (>1 × 10−6) in children/teenagers and adults. This study provides important baseline information for further epidemiological studies of exposure to VOCs from hand sanitizers during the COVID pandemic and in general.
Supplementary Material
Acknowledgements
The research reported here was supported, in part, by the US National Institute of Environmental Health Sciences (NIEHS) under award number U2CES026542 (KK). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIEHS.
Footnotes
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Credit authorship contribution statement
Vineet Kumar Pal: Methodology, Data curation, Formal analysis, Writing - original draft. Sunmi Lee: Sample collection, Instrumental analysis, Review of draft manuscript. Mrudula Naidu: Collecting product information, Review of draft manuscript. Conner Lee: Sample preparation, Review of draft manuscript. Kurunthachalam Kannan: Conceptualization, Funding acquisition, Supervision, Writing - review & editing.
Appendix A. Supplementary material
Supplementary data to this article can be found online at https://doi.org/10.1016/j.envint.2022.107449.
Data availability
Data will be made available on request.
References
- ATSDR, 2007a. Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Benzene. https://www.atsdr.cdc.gov/toxprofiles/tp3.pdf. Access date: 10 November 2021. [PubMed] [Google Scholar]
- ATSDR, 2007b. Agency for Toxic Substances and Diseases Registry (ATSDR). Public health statement for benzene. https://www.atsdr.cdc.gov/ToxProfiles/tp3-c1-b.pdf. Access date: 10 November 2021. [Google Scholar]
- ATSDR, 2010. Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Styrene, https://www.atsdr.cdc.gov/toxprofiles/tp53.pdf. Access date: 20 December 2021. [PubMed] [Google Scholar]
- ATSDR, 2016. Agency for Toxic Substances and Disease Registry (ATSDR). Exposure Dose Guidance for Body Weight, https://www.atsdr.cdc.gov/pha-guidance/resources/ATSDR-EDG-Body-Weight-508.pdf. Access date: 04 May 2022. [Google Scholar]
- ATSDR, 2017. Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Toluene, https://www.atsdr.cdc.gov/toxprofiles/tp56.pdf. Access date: 20 December 2021. [Google Scholar]
- Berardi A, Perinelli DR, Merchant HA, Bisharat L, Basheti IA, Bonacucina G, Cespi M, Palmieri GF, 2020. Hand sanitizers amid CoViD-19: A critical review of alcohol-based products on the market and formulation approaches to respond to increasing demand. Int. J. Pharm 584, 119431 10.1016/j.ijpharm.2020.119431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2020a. Centrals for Disease Control and Prevention (CDC). Health Advisory. Health Advisory, https://emergency.cdc.gov/han/2020/han00434.asp. Access date: 5 August 2021. [Google Scholar]
- CDC, 2020b. Centers for Disease Control and Prevention (CDC). Serious Adverse Health Events, Including Death, Associated with Ingesting Alcohol-Based Hand Sanitizers Containing Methanol — Arizona and New Mexico, May–June 2020. https://www.cdc.gov/mmwr/volumes/69/wr/mm6932e1.htm. Access date: 20 September 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CDC, 2021. Centers for Disease Control and Prevention (CDC). Drug facts about benzene. https://emergency.cdc.gov/agent/benzene/basics/facts.asp. Access date: 4 April 2021. [Google Scholar]
- Chaiklieng S, Suggaravetsiri P, Autrup H, 2019. Risk Assessment on Benzene Exposure among Gasoline Station Workers. Int. J. Environ. Res. Public Health 16, 2545. 10.3390/ijerph16142545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi K, Sim S, Choi J, Park C, Uhm Y, Lim E, Kim AY, Yoo SJ, Lee Y, 2021. Changes in Handwashing and Hygiene Product Usage Patterns in Korea before and after the Outbreak of COVID-19. Environ. Sci. Eur 33 (1), 79. 10.1186/s12302-021-00517-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudspeth A, Zenzola N, Kucera K, Wu Q, Light D, 2022. Independent Sun Care Product Screening for Benzene Contamination. Environ Health Perspect 130, 037701. 10.1289/EHP10386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A, Anderson D, Parke DV, 1991. The toxicity of benzene and its metabolism and molecular pathology in human risk assessment. Occup. Environ. Med 48, 437–444. 10.1136/oem.48.7.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kezic S, Nielsen JB, 2009. Absorption of chemicals through compromised skin. Int. Arch. Occup. Environ. Health. 82, 677–688. 10.1007/s00420-009-0405-x. [DOI] [PubMed] [Google Scholar]
- Lachenmeier DW, 2008. Safety evaluation of topical applications of ethanol on the skin and inside the oral cavity. J. Occup. Med. Toxicol 3, 26. 10.1186/1745-6673-3-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm SH, Grünwald HW, 2006. Benzene Exposure and Hematotoxicity. Science 312 (5776), 998–999. [DOI] [PubMed] [Google Scholar]
- Leslie RA, Zhou SS, Macinga DR, 2021. Inactivation of SARS-CoV-2 by commercially available alcohol-based hand sanitizers. Am. J. Infect. Control 49, 401–402. 10.1016/j.ajic.2020.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li AJ, Pal VK, Kannan K, 2021. A review of environmental occurrence, toxicity, biotransformation and biomonitoring of volatile organic compounds. Environ. Chem. Ecotoxicol 3, 91–116. 10.1016/j.enceco.2021.01.001. [DOI] [Google Scholar]
- Lim SK, Shin HS, Yoon KS, Kwack SJ, Um YM, Hyeon JH, Kwak HM, Kim JY, Kim TH, Kim YJ, Roh TH, Lim DS, Shin MK, Choi SM, Kim HS, Lee BM, 2014. Risk Assessment of Volatile Organic Compounds Benzene, Toluene, Ethylbenzene, and Xylene (BTEX) in Consumer Products. J. Toxicol. Environ. Health, Part A 77, 1502–1521. 10.1080/15287394.2014.955905. [DOI] [PubMed] [Google Scholar]
- Lin N, Ding N, Meza-Wilson E, Manuradha Devasurendra A, Godwin C, Kyun Park S, Batterman S, 2020. Volatile organic compounds in feminine hygiene products sold in the US market: A survey of products and health risks. Environ. Int 144, 105740 10.1016/j.envint.2020.105740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahmood A, Eqan M, Pervez S, Alghamdi HA, Tabinda AB, Yasar A, Brindhadevi K, Pugazhendhi A, 2020. COVID-19 and frequent use of hand sanitizers; human health and environmental hazards by exposure pathways. Sci. Total Environ 742, 140561 10.1016/j.scitotenv.2020.140561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pal VK, Li AJ, Zhu H, Kannan K, 2022. Diurnal variability in urinary volatile organic compound metabolites and its association with oxidative stress biomarkers. Sci. Total Environ 818, 151704 10.1016/j.scitotenv.2021.151704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin P, Cao F, Lu S, Li L, Guo X, Zhao B, Wan Z, Bi B, 2019. Occurrence and health risk assessment of volatile organic compounds in the surface water of Poyang Lake in March 2017. RSC Adv. 9, 22609–22617. 10.1039/C9RA02450F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinemann A, Nematollahi N, Rismanchi B, Goodman N, Kolev SD, 2021. Pandemic products and volatile chemical emissions. Air Qual Atmos Health 14, 47–53. 10.1007/s11869-020-00912-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Susten AS, Dames BL, Burg JR, Niemeier RW, 1985. Percutaneous penetration of benzene in hairless mice: An estimate of dermal absorption during tire-building operations. Am. J. Ind. Med 7 (4), 323–335. [DOI] [PubMed] [Google Scholar]
- Tse TJ, Nelson FB, Reaney MJT, 2021. Analyses of Commercially Available Alcohol-Based Hand Rubs Formulated with Compliant and Non-Compliant Ethanol. Int. J. Environ. Res. Public Health 18, 3766. 10.3390/ijerph18073766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA, 2011. EPA, Exposure Factors Handbook. Exposure Factors Handbook Edition Final Report 2011. Chapter 7. https://cfpub.epa.gov/ncea/risk/recordisplay.cfm?deid=236252Access Access date: 12 December 2021. [Google Scholar]
- US EPA, United States Environmental Protection Agency, 2017. Initial list of hazardous air pollutants with modifications, https://www.epa.gov/haps/initial-list-hazardous-air-pollutants-modifications Access date: January 30, 2018.
- US FDA, 2020. Temporary Policy for Preparation of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency (COVID-19). https://www.fda.gov/drugs/coronavirus-covid-19-drugs/hand-sanitizers-covid-19. Access date: 12 December 2021. [Google Scholar]
- Valisure, 2021.Valisure Citizen Petition on Hand Sanitizer Products Containing Benzene Contamination and Other Significant Issues. https://assets-global.websitefiles.com/6215052733f8bb8fea016220/626fee4c78a2e4769ba2b7fe_FDA-2021-P-0338-0001_attachment_1.pdf. Access date: 15 May 2021.
- Wallace L, 1996. Environmental exposure to benzene: an update. Environ. Health Perspect 104 (suppl 6), 1129–1136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wester RC, Maibach HI, 2000. Benzene percutaneous absorption: Dermal exposure relative to other benzene sources. Int. J. Occup. Environ. Health 6, 122–126. 10.1179/oeh.2000.6.2.122. [DOI] [PubMed] [Google Scholar]
- WHO, 2010a. World Health Organization (WHO). IARC monographs on the evaluation of carcinogenic risks to humans, https://monographs.iarc.who.int/wp-content/uploads/2018/06/mono93.pdf. Access date: 15 November 2021. [Google Scholar]
- WHO, 2010b. World Health Organization (WHO). WHO guidelines for indoor air quality: selected pollutants. WHO Regional Office for Europe, Copenhagen, Denmark, (http://www.euro.who.int/pubrequest). Access date: June 30, 2022. [Google Scholar]
- Williams PRD, Sahmel J, Knutsen J, Spencer J, Bunge AL, 2011. Dermal absorption of benzene in occupational settings: Estimating flux and applications for risk assessment. Crit. Rev. Toxicol 41, 111–142. 10.3109/10408444.2010.530224. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.