Abstract
Hydrophobins are small amphipathic proteins conserved in filamentous fungi. In this review, the properties and functions of Aspergillus hydrophobins are comprehensively discussed on the basis of recent findings. Multiple Aspergillus hydrophobins have been identified and categorized in conventional class I and two non-conventional classes. Some Aspergillus hydrophobins can be purified in a water phase without organic solvents. Class I hydrophobins of Aspergilli self-assemble to form amphipathic membranes. At the air–liquid interface, RolA of Aspergillus oryzae self-assembles via four stages, and its self-assembled films consist of two layers, a rodlet membrane facing air and rod-like structures facing liquid. The self-assembly depends mainly on hydrophobin conformation and solution pH. Cys4–Cys5 and Cys7–Cys8 loops, disulfide bonds, and conserved Cys residues of RodA-like hydrophobins are necessary for self-assembly at the interface and for adsorption to solid surfaces. AfRodA helps Aspergillus fumigatus to evade recognition by the host immune system. RodA-like hydrophobins recruit cutinases to promote the hydrolysis of aliphatic polyesters. This mechanism appears to be conserved in Aspergillus and other filamentous fungi, and may be beneficial for their growth. Aspergilli produce various small secreted proteins (SSPs) including hydrophobins, hydrophobic surface–binding proteins, and effector proteins. Aspergilli may use a wide variety of SSPs to decompose solid polymers.
Keywords: Aspergillus, hydrophobin, self-assembly, biopolymer degradation, small secreted protein
1. Introduction
Hydrophobins are low-molecular-weight (<20 kDa) amphipathic proteins widely conserved in filamentous fungi. In general, the similarity of amino acid sequences among hydrophobins is very low, but hydrophobins have eight conserved Cys residues, four disulfide bonds, and a specific number of amino acid residues between the Cys residues (C-X5–7C-C-X19–39-C-X8–23-C-X5-C-C-X6–18-C-X2–13 or C-X9–10C-C-X11-C-X16-C-X8–9-C-C-X10-C-X6–7) [1,2]. Hydrophobins have β-barrel structures that are similar to each other [2,3,4,5,6]. Some filamentous fungi such as Aspergillus, Penicillium, Trichoderma, extremophilic species, or mycorrhizal fungi have several to over 10 hydrophobin-encoding genes, whereas many filamentous fungi have only a few such genes [7,8,9,10,11]. The expression profiles of multiple hydrophobin genes depend on the growth stage of filamentous fungi and culture conditions, and cellular localization varies among hydrophobins [12,13,14]. Hydrophobins are secreted by filamentous fungi and self-assemble at solid–liquid or air–liquid interfaces to form amphipathic membranes [15,16,17]. Because formation of such membranes reduces interfacial surface tension, hydrophobins contribute to the formation of aerial hyphae and conidia [18,19,20]. Hydrophobins are specifically accumulated inside aerial hyphae, where they associate with lipid-enriched organelles and may affect the structure and increase longevity of aerial hyphae [7]. Secretion of hydrophobins becomes highest at the sporulation phase, when they form a protective coating of rapidly produced spores [7]. Hydrophobins are involved in the water sensing mechanism of spores and are linked to germination [7]. Hydrophobins coat the surfaces of aerial structures and make these surfaces hydrophobic, which contributes to both conidial dispersal [14] and adsorption of pathogenic filamentous fungi on host insects or plants, whose surfaces are hydrophobic [21,22]. Since hydrophobin-coated hyphae and conidia can escape recognition by the immune systems of animals (e.g., insects, mammals) and plants, hydrophobins are thought to contribute to host infection by pathogenic filamentous fungi [14,23,24]. Hydrophobins attached to solid surfaces are able to recruit and immobilize various proteins such as bovine serum albumin, IgG, avidin, glucose oxidase, horseradish peroxidase, and cutinases [25,26,27,28,29]. Hydrophobins are classified into several classes according to their hydropathy patterns, amino acid sequences, and the solubility of their self-assembled membranes [19,30,31,32,33]. Classification and applications of hydrophobins will be addressed in detail in the next section.
The genus Aspergillus belongs to ascomycetes and is a polyphyletic taxon containing many fungi imperfecti [34]. Generally, Aspergilli are highly capable of decomposing solid polymers and have been widely used in the fermentation industry for a long time [35,36]. Currently, Aspergilli are used as the host microorganisms for production of these compounds owing to their high productivity of proteins and primary and secondary metabolites [37,38,39,40]. Aspergilli are used for industrial production of a variety of enzymes, such as amylase, cellulase, glucosidase, hemicellulase, lipase, and phytase from Aspergillus oryzae and Aspergillus niger [41,42,43,44,45,46,47,48,49,50,51,52], and low-molecular-weight compounds such as itaconic acid from Aspergillus terreus [53], citric acid from A. niger [54], and kojic acid from A. oryzae [55]. Aspergilli can infect animals or plants, and are important in the medical, food, and agricultural and livestock fields [39,56,57,58,59,60]. The whole genomes of major Aspergilli have been sequenced [39,61,62]. Genomic DNA sequences of many Aspergilli are available at the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/, accessed on 8 July 2022) and CAoGD (https://nribf21.nrib.go.jp/CAoGD/, accessed on 8 July 2022). New industrially valuable substances are searched by genome mining, and the mechanisms of pathogenicity are also the focus of ongoing studies [63]. The biological functions of Aspergillus hydrophobins have been studied for the last 30 years [24,26,28]. Aspergillus hydrophobins form a coating layer on the surface of the cell wall and are involved in infection of animals [24,64,65]. In 2005, Takahashi et al. [26] found that conidial hydrophobin of A. oryzae was specifically induced when the fungus was grown on polybutylene succinate co-adipate (PBSA) as the sole carbon source. Subsequently, the expression of hydrophobin genes was found to be induced in other filamentous fungi such as Aspergillus nidulans [66], A. niger [67,68], and Trichoderma reesei [69] when these fungi were cultivated on solid polymers of plant origin such as cellulose [67,69] or xylan [69], or on straw [67,68], or steam-exploded sugarcane bagasse [66]. These studies suggest that hydrophobins are also involved in solid polymer degradation by filamentous fungi. Therefore, studying Aspergillus hydrophobins will expand our understanding of solid polymer degradation and utilization, and infection of animals [24,64,65] or plants [59,60] by Aspergilli. Because novel properties have been discovered in Aspergillus hydrophobins, we expect that other properties and biological functions of hydrophobins will be clarified by studying them in Aspergilli.
The characteristics of hydrophobins from Aspergilli differ from those of other hydrophobins; therefore, studying Aspergillus hydrophobins is important for understanding their biological roles. However, no comprehensive analysis of the findings on Aspergillus hydrophobins is available. In this review, the physicochemical properties and biochemical and biological functions of hydrophobins produced by Aspergilli are comprehensively discussed on the basis of recent findings.
2. Classification and Applications of Hydrophobins
2.1. Classification
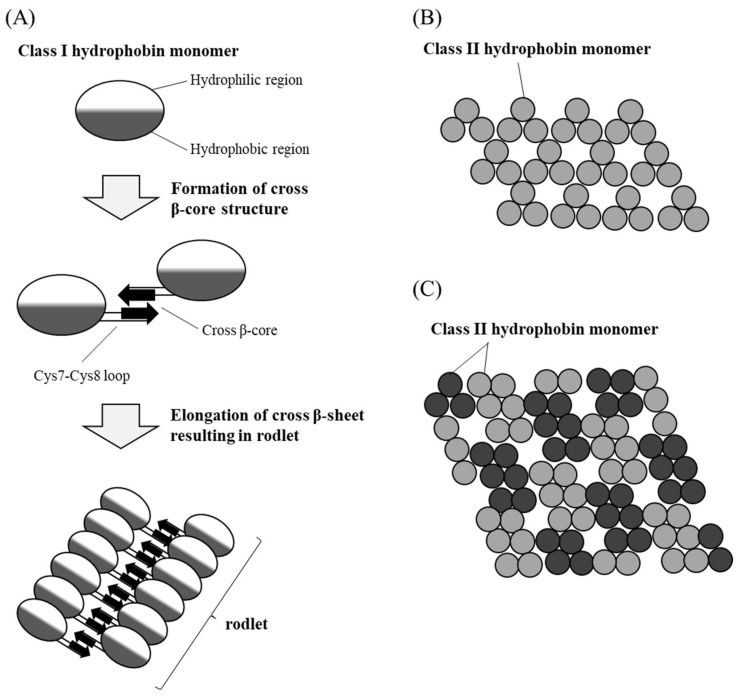
Hydrophobins are classified mainly into classes I and II [19,30,31,32,33]. Class I includes SC3 from Schizophyllum commune [30], EAS from Neurospora crassa [16], RodA from Aspergillus fumigatus (AfRodA) [65], MPG1 from Magnaporthe oryzae [21], DewA from A. nidulans [70], and RolA (HypA) from A. oryzae [26]. Class I hydrophobins are further subdivided into class IA and class IB according to their origin from ascomycetes or basidiomycetes, respectively [10,71]. Class I hydrophobins form self-assembled structures called “rodlets”, which are similar to β-amyloid fibrils [3,20,72]. Rodlets can be solubilized in trifluoroacetic acid, but are barely soluble in HCl, NaOH, sodium dodecyl sulfate, or ethanol [15,73,74]. In vivo, rodlets can be observed on the surface of aerial structures such as hyphae and conidia [1,13,75] (Figure 1). In vitro, rodlets can form on solid surfaces such as mica and highly oriented pyrolytic graphite (HOPG) [3,76,77,78,79]. Loops Cys3–Cys4, Cys4–Cys5, and Cys7–Cys8 are rich in hydrophobic amino acid residues and do not form specific secondary structures [72]. One or more of these loops may be involved in adsorption to solid surfaces and rodlet formation [2,31,76,78]. Particular hydrophobic amino acid residues in the Cys7–Cys8 loop are essential for both the adsorption to solid surfaces and rodlet formation [31]. It is suggested that hydrophobic residues of the Cys7–Cys8 loop of two hydrophobin molecules form a cross-β core and that continuous elongation of a cross-β sheet results in rodlet formation [76,80] (Figure 2A).
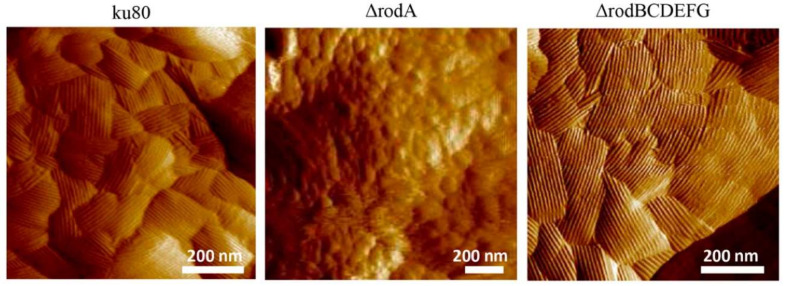
Figure 1.
Structure of the conidial cell wall surface of A. fumigatus ku80, ΔrodA, and ΔrodBCDEFG. Atomic force microscopic images show the presence of rodlets on the surface of ku80 and ΔrodBCDEFG and their absence on that of ΔrodA, in which AfRodA is deleted (Reprinted with permission from [13]. 2017, Journal of Fungi).
Figure 2.
Schematic representation of hydrophobin self-assembly. (A) Class I hydrophobin self-assembles into rodlets (based on [76,80]). (B,C) Self-assembled structure of class II hydrophobin composed of (B) 3- or (C) 6-molecule units (based on [91]).
Class II hydrophobins include HFBI and HFBII from T. reesei [26,69], HFB4 and HFB7 from Trichoderma harzianum [81,82], VDHI from Verticillium dahliae [83], and NC2 from N. crassa [84]. Class II hydrophobins are found in many ascomycetous filamentous fungi such as Trichoderma species [69,81,82,85], Fusarium species [83,86], and Neurospora species [84], but not in Aspergillus species. Class II hydrophobins form self-assembled monolayers that structurally differ from rodlets; these structures can be solubilized in trifluoroacetic acid, HCl, NaOH, sodium dodecyl sulfate, or ethanol [72,87,88]. The self-assembled structures of some class II hydrophobins such as cerato-ulmin can be dissociated through pressure or cooling [88,89]. In vitro, class II hydrophobins form bilayers with hydrophilic domains pointing inward and hydrophobic domains pointing outward, and also form multilayers consisting of stacked bilayers [90]. In general, class II hydrophobin layers show no defined morphology. Atomic force microscopy analysis combined with Monte-Carlo simulation suggests that the self-assembled monolayer of HFBI and HFBII forms a lattice structure in which 3 or 6 molecules are regularly arranged as one unit [91] (Figure 2B,C). Class II hydrophobins have shorter Cys3–Cys4 and Cys7–Cys8 loops than class I hydrophobins, and the hydrophobic region of class II hydrophobins (called “hydrophobic patch”) occupies a smaller part of the molecular surface than that of class I hydrophobins [85]. The Cys3–Cys4 and Cys7–Cys8 loops of class II hydrophobins have random structures; because these loops are short and the proportion of random structures is low, class II hydrophobins do not form rodlets but associate with each other without a large change in their three-dimensional structures to form a regularly aligned self-assembled monolayer [76,78,85]. Molecular dynamics simulation suggests that HFBI adsorbs at the water–oil interface with its hydrophobic patch facing the oil phase without a change in the secondary structure, regardless of the initial orientation [92].
In addition to conventional classes I and II, an intermediate class of hydrophobins, called class III in Aspergilli and pseudo-class I in Trichoderma species, has been reported; the pattern of the number of amino acid residues between the Cys residues in different loops in this class is intermediate between those of classes I and II [13,20,93,94]. No consensus has been reached on whether class III and pseudo-class I are the same class. Hydrophobins of an unknown class that cannot be classified in classes I–III or pseudo-class I have also been reported in Aspergilli [12,95]. Compared to the physicochemical properties, biochemical properties, and biological functions of conventional classes I and II, those of non-conventional classes (class III, pseudo-class I, and unknown class) are still poorly understood, except for hydropathy patterns [20,89]. On the basis of the amino acid sequences, class III hydrophobins are predicted to form rodlets that are similar to those of class I hydrophobins [10].
2.2. Applications
Various studies on industrial utilization of the unique physicochemical properties of hydrophobins have been underway since ca. 2000. Some examples are listed in Table 1. The amphipathic property of hydrophobins can be applied to the dispersion of colloids. Coating of the hydrophobic surfaces of HOPG, carbon nanotubes, and Teflon particles with hydrophobins makes them hydrophilic [96,97], which improves dispersibility in aqueous solvents [96,97,98,99]. Hydrophobins also enhance the dispersibility of hydrophobic drugs and inhibit drug crystallization [100,101]. Hydrophobins stabilize emulsions and foams, allowing them to be stored for long periods of time [19,102,103,104]. Hydrophobin HFBII associates with enzymes and prevents their unfolding, improving their thermal stability [105,106]. Because hydrophobin self-assembled structures are highly oriented and stable, hydrophobins fused with other polypeptides such as enzymes, domains that can bind other molecules, or peptides targeting specific cells can be used for displaying the polypeptides on solid surfaces with high orientation and density [72,82,107,108,109,110,111,112]. Hydrophobins attached to solid surfaces can interact with some low-molecular-mass proteins or chemicals [25,26,82,113,114]. Coating solid surfaces with some hydrophobins can prevent bacterial adhesion to these surfaces [115]. Since mammalian immune cells seem to hardly recognize A. fumigatus conidia coated with AfRodA [24], hydrophobins including AfRodA might be used as coating materials that prevent immune recognition of nano-particles used to deliver drugs to target tissues or organs [24,101,116,117].
Table 1.
Expected applications of hydrophobins.
| Application | Hydrophobin | Class | Reference |
|---|---|---|---|
| Modification of the wettability of solid surfaces (e.g., Teflon, glass, mica, resin, and stone) | SC3 | IB | [118] |
| DewA | IA | [119] | |
| HFBI | II | [119,120] | |
| Enhancement of the dispersibility of hydrophobic particles (e.g., graphene, carbon nano tubes, highly oriented pyrolytic graphite, pigments, and minerals) in aqueous solvents | EAS | IA | [98] |
| HGFI | IB | [98] | |
| HFBI | II | [96,97] | |
| HFBII | II | [99] | |
| Enhancement of the dispersibility of hydrophobic drugs and inhibition of drug crystallization | HFBI | II | [100,101] |
| Coating of metal microparticles for medical applications and of drug particles | SC3 | IB | [101] |
| AfRodA | IA | [24] | |
| HFBI | II | [116] | |
| HFBII | II | [117] | |
| Inhibition of bacterial adhesion to solid surfaces | DewA | IA | [115] |
| Immobilization of functional peptides and proteins (e.g., cell adhesion factor, cellulose-binding module, enzymes, histidine tag, and Protein A) on solid surfaces | DewA | IA | [109,111,113] |
| DewB | IA | [111] | |
| HGFI | IB | [27,112] | |
| RolA | IA | [26] | |
| SC3 | IB | [25] | |
| VmhII | IB | [108,110,114] | |
| HFBI | II | [27,107] | |
| HFB4 | II | [82] | |
| HFB7 | II | [82] | |
| Fusion partner for mass production and efficient purification of recombinant enzymes | HFBI | II | [121] |
| Enhancement of thermostability of enzymes | HFBI | II | [105,106] |
| Stabilization of emulsions, bubbles, and foams for long-term storage | SC3 | IB | [19] |
| HFBI | II | [104] | |
| HFBII | II | [102,103] |
3. Characteristics of Hydrophobins from Three Aspergillus Species
Over 50 potential hydrophobins have been identified in Aspergilli [10]. Even more Aspergillus hydrophobins can be predicted from genomic DNA sequences deposited in databases such as those housed at National Center for Biotechnology Information. Among Aspergillus hydrophobins, 14 hydrophobins from three species (A. fumigatus, A. nidulans, and A. oryzae) listed in Table 2 have been characterized [12,13,23,26,64,65,70,95]. Hydrophobins of Aspergillus flavus, A. niger, and Eurotium rubrum (synonym: Aspergillus ruber) have been identified and studied by proteome analysis [122,123], transcriptome analysis [67,68,124], or observation of conidial surface by scanning electron microscopy [125].
Table 2.
Aspergillus hydrophobins that are characterized biochemically.
| Organism | Hydrophobin | Number of Cys | Accession Number * | Class | Length, a.a. | Location | Reference |
|---|---|---|---|---|---|---|---|
| Aspergillus oryzae | RolA (HypA) | 8 | AO090020000588 | I | 151 | [26] | |
| Aspergillus fumigatus | AfRodA | 8 | AFUA_5G09580 | I | 159 | conidia | [65] |
| RodB | 8 | AFUA_1G17250 | I | 140 | conidia | [23] | |
| RodC | 8 | AFUA_8G07060 | I | 155 | conidia | [95] | |
| RodD | 8 | AFUA_5G01490 | unknown | 193 | [95] | ||
| RodE | 8 | AFUA_8G05890 | I | 179 | [95] | ||
| RodF | 9 | AFUA_5G03280 | III | 190 | [13] | ||
| RodG | 8 | AFUA_2G14661 | III | 125 | [13] | ||
| Aspergillus nidulans | AnRodA | 8 | AN8803 | I | 157 | conidia | [64] |
| DewA | 8 | AN8006 | I | 135 | conidia | [70] | |
| DewB | 8 | AN1837 | I | 135 | conidia | [12] | |
| DewC | 8 | AN6401 | unknown | 143 | conidia | [12] | |
| DewD | 8 | AN0940 | unknown | 101 | conidia | [12] | |
| DewE | 8 | AN7539 | unknown | 109 | conidia | [12] |
* Accession numbers are from the National Center for Biotechnology Information (https://www.ncbi.nlm.nih.gov/, accessed on 23 May 2022).
In A. fumigatus, seven hydrophobin genes, AfrodA–G, have been identified [13,23,65,95]. AfRodA, RodB, RodC, and RodE belong to class I, RodF and RodG belong to class III, and the classification of RodD and RodE is controversial [10,93]. AfRodA is well studied, and only AfRodA is shown to be involved in rodlet formation on the conidial surface (Figure 1) [13,23] and immunological inertia of the conidia [13,24]. The immune system is activated when dectin-1 recognize β-glucan of the fungal cell wall, or dectin-2 or -3 recognize α-mannan of the fungal cell wall [126,127]. AfRodA masks dectin-1 and -2-dependent responses and helps fungal cells avoid immune recognition [128]. Some fungal cell wall proteins such as Ywp1, Erg1, and Lrg1 also mask dectin-dependent responses; however, these proteins are not hydrophobins [129,130]. AfrodA is most highly expressed during sporulation, whereas rodB is expressed in the biofilm. The transcription of AfrodA is controlled by the conidial transcriptional factor BrlA, not by AbaA or WetA [64]. AfRodA, RodB, and RodC are located on the conidial surface [13,23]. Atomic force microscopy investigation of the conidial surface has shown that AfRodA self-assembles into rodlets through bilayers [80]. Within the bilayers, the hydrophobic domains of AfRodA face inwards, making the hydrophobic core. A study of AfRodA structure by NMR spectroscopy and atomic force microscopy has shown that hydrophobic amino acid residues in Cys–Cys loops are important for rodlet formation [31]. Substitution of the conserved Cys residues in AfRodA abolishes the AfRodA secretion to the conidial surface and therefore the rodlet layer [31,131], as reported for MPG1 of Magnaporthe grisea [132]. A similar phenomenon has been reported for SC3 of S. commune; disruption of disulfide bonds by a reducing agent and free thiol-blocking reagents abolishes rodlet formation by SC3 [133]. The phenylpropanoid isoeugenol inhibits rodlet formation by AfRodA on the conidial surface by decreasing the AfrodA transcription level and by interacting with the Cys residues of AfRodA [134].
In A. nidulans, six hydrophobin genes, AnrodA and dewA–E, have been identified. The transcription of AnrodA is controlled by the conidial transcriptional factor BrlA [135]. The expression of AnrodA and dewA–C has been detected in conidia, but not in vegetative hyphae [12,135]. The expression of dewD and dewE has been detected both in conidia and hyphae [12]. AnRodA, DewA, and DewB belong to class I, whereas the allocation of DewC–E to a particular class is controversial [12,70]. AnRodA and DewA are well studied, and the structure of DewA has been analyzed by NMR spectroscopy [6]. AnRodA and DewA confer hydrophobicity to the conidial surface, but only AnRodA is involved in rodlet formation on the conidial surface [12,136]. All hydrophobins of A. nidulans contribute to colony hydrophobicity [12,136]. DewA–E are involved in cell wall formation. AnRodA, DewA–E are all localized to conidial surface [12]. However, when DewA and DewB are expressed under the control of the AnRodA promoter and the signal peptide from AnRodA is used for secretion, incomplete rodlets are formed on the conidial surface, suggesting that AnRodA can be substituted with neither DewA nor DewB [12]. The coating layer of DewA on glass surfaces, but not those of DewC–E, is stable against ethanol and SDS [136].
Out of several hydrophobins in A. oryzae, only RolA (AO090020000588) has been biochemically analyzed. The rolA expression patterns and RolA localization are not well characterized. However, RolA is secreted into liquid culture medium when A. oryzae is grown in the presence of biodegradable plastic PBSA [26]. It is suggested that the transcription of the rolA orthologue in A. flavus, a fungus that is considered to have evolved from a common ancestor with A. oryzae [137,138], is controlled by the conidial transcription factor BrlA [124]. Two hydrophobic amino acid residues (Leu137, Leu142) in the Cys7–Cys8 loop of RolA are cooperatively involved in RolA adsorption to solid surfaces such as PBSA [139].
4. Purification of Hydrophobins and Analysis of Self-Assembly at Interface
Most hydrophobins are purified under denaturing conditions and then refolded because they are highly hydrophobic and aggregate easily [18,23,31,58,140,141,142]. Some hydrophobins are purified by two-phase extraction or reverse-phase high performance liquid chromatography (HPLC) by using their amphiphilic properties [23,78,85,109,121,143]. Only some hydrophobins can be purified in water phase without denaturation, refolding, and high concentrations of organic solvents [17,23,26,28,82,90]. Five Aspergillus hydrophobins have been purified (Table 3). RolA, AfRodA, RodB, and AnRodA have been purified in water phase without using organic solvents [26,28,142]. RolA is the only Aspergillus hydrophobin that has been purified via a homologous expression system without an affinity tag, denaturing, refolding, and organic solvents [17,26]. RolA secreted into the medium from A. oryzae rolA-overexpressing strain has been purified by hydrophobic chromatography, anion exchange chromatography, and cation exchange chromatography, without any affinity tag [17,26]. Recombinant AnRodA has been purified from an A. oryzae AnrodA-expressing strain by using the same method as for RolA purification with no affinity tag [28]. AfRodA and RodB secreted into the medium from Pichia pastoris AfrodA– or rodB–expressing strains have been purified by immobilized metal affinity chromatography with a histidine-tag [142].
Table 3.
Procedures for Aspergillus hydrophobin purification.
| Hydrophobin | Origin | Host for Production | Tag | Purification Procedure | Reference |
|---|---|---|---|---|---|
| RolA | Aspergillus oryzae | Aspergillus oryzae | Hydrophobic chromatography (omittable), anion exchange chromatography, and cation exchange chromatography | [17,26] | |
| Escherichia coli | Denaturation and refolding | [148] | |||
| AfRodA | Aspergillus fumigatus | Escherichia col | His-tag | Immobilized metal affinity chromatography (IMAC) | [142] |
| Pichia pastoris | His-tag | IMAC | [142] | ||
| Escherichia coli | His-tag | IMAC and refolding | [31] | ||
| RodB | Aspergillus fumigatus | Aspergillus fumigatus | Rodlet extraction, denaturation, and reverse-phase HPLC | [23] | |
| Pichia pastoris | His-tag | IMAC | [142] | ||
| AnRodA | Aspergillus nidulans | Aspergillus oryzae | Hydrophobic chromatography, anion exchange chromatography, and cation exchange chromatography | [8] | |
| DewA | Aspergillus nidulans | Trichoderma reesei | His-tag | Precipitation, denaturation, and refolding | [141] |
| Escherichia coli | His-tag | IMAC and reverse-phase HPLC | [143] | ||
| Escherichia coli | His-tag | Solubilization of inclusion body and refolding | [113] | ||
| Escherichia coli | His-tag | Aqueous two-phase separation using isopropyl alcohol | [109] |
Rodlet formation by DewA, RolA, and AfRodA has been analyzed in vitro [6,17,31,144]. At high concentrations, DewA forms dimers but no rodlets [6]. DewA monomers are either conformers A (major type) or conformers B (minor type). Conformers B cannot form dimers but form rodlets more rapidly than conformers A [6]. At the solid–liquid or air–liquid interface, RolA self-assembles to form rodlets. RolA self-assembles at the air–liquid interface to form Langmuir films (membranes) via four stages [17]. RolA Langmuir film undergoes a phase transition from a gas film to a liquid-expanded film, then to a liquid-condensed film, and finally to a self-assembled film. The final self-assembled structures of other hydrophobins, for example, HGFI from Grifola frondosa [145] and Vmh2 from Pleurotus ostreatus [146,147], have been analyzed, but the process of their self-assembly has not. RolA Langmuir film at the air–liquid interface is structurally different on its hydrophobic and hydrophilic surfaces: a rodlet membrane faces air and rod-like structures face the liquid [17]. At the solid–liquid interface, the self-assembled structure of RolA differs depending on solid surface properties (hydrophobic or charged) and pH conditions, which is attributed to the involvement of charged amino acid residues in the Cys–Cys loops in self-assembly [144]. In addition, the adsorption of RolA depends mainly on the hydrophobic interaction between the solid surface and RolA in the water phase [144]. The interaction between RolA and solid surfaces is also affected by the zeta potential of RolA and the hydrophobicity of its Cys–Cys loops. The structures of assembled RolA differ according to the amount that is adsorbed on solid surfaces [144]. AfRodA self-assembly on the HOPG surface (a solid–liquid interface) has been characterized [31]. Chimeric AfRodA with the central Cys7–Cys8 loop replaced with that of the class II hydrophobin NC2 of N. crassa is able to form rodlets. AfRodA mutants with the substitution of one or two hydrophobic amino acid residues in the Cys4–Cys5 loop (I114G, L115G) or Cys7–Cys8 loop (L145G, I146G) also form rodlets. These chimeric AfRodA and AfRodA mutants need longer lag time for self-assembly than does wild-type AfRodA. Peptides corresponding to the Cys4–Cys5 or Cys7–Cys8 loops of AfRodA form fibrils. Therefore, both the Cys4–Cys5 and Cys7–Cys8 loops are involved in rodlet formation by AfRodA [31]. Rodlet formation by hydrophobin EAS requires only the Cys7–Cys8 loop [76]. The involvement of the Cys4–Cys5 loop in hydrophobin self-assembly has been reported so far in AfRodA only [31]. Because Leu145 of AfRodA corresponds to Leu137 of RolA [10], corresponding leucine residues in other RodA-like hydrophobins may be involved in both adsorption to solid surfaces and self-assembly.
5. Involvement of Hydrophobins in Solid Polymer Degradation
5.1. Hydrophobin–Cutinase Interactions in A. oryzae and A. nidulans
5.1.1. Aspergillus oryzae
Direct evidence for hydrophobin involvement in the degradation of solid polymers was first reported in A. oryzae in 2005 [26]. This fungus co-expresses RolA and CutL1 when grown on PBSA as a sole carbon source and hydrolyzes the polyester [26,149]. The secreted RolA adsorbs to the PBSA surface [26,139], then it recruits and condenses CutL1 [8,26] (and a CutL1 homologue, CutC [8]), and thus promotes PBSA hydrolysis [8,26] (Figure 3). Cutinases hydrolyze various aliphatic esters such as cutin, PBSA, and triglycerides [149] and are produced by many fungi and bacteria [150,151]. PBSA is structurally similar to cutin, an insoluble wax polyester in the plant protective cuticle [152]. Since both hydrophobin and cutinase are produced by many pathogenic filamentous fungi and promote infection by these fungi [58,150,151,153,154,155,156,157], PBSA degradation via RolA–CutL1 interaction is thought to mimic infection by these fungi [8,26].
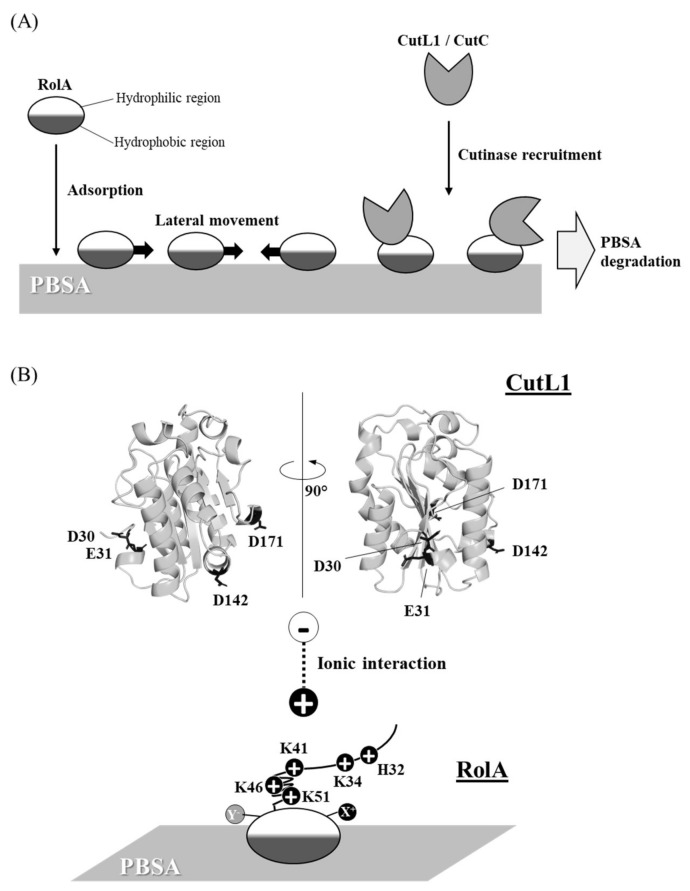
Figure 3.
Schematic model of PBSA–RolA–CutL1 interaction. (A) Adsorption, lateral mobility, and cutinase recruitment by RolA on the PBSA surface (based on [8]). (B) Mechanism of the interaction between RolA and CutL1 (adapted from [8,158]).
Several key characteristics of RolA–CutL1/CutC interaction have been clarified [8,26,158].
(1) It is important that RolA adsorbs to the PBSA surface before CutL1 reaches the surface [26]. The PBSA degradation is only slightly accelerated by simultaneous addition of RolA and CutL1 in comparison with the effect of CutL1 alone. RolA secondary structure changes after its adsorption to a solid surface, and this change is necessary for CutL1 recruitment [26].
(2) The adsorbed RolA moves laterally on the PBSA surface but stops moving when CutL1 is added [26] (Figure 3A). Therefore, RolA may act as an anchor or scaffold to tether CutL1. The RolA molecules that do not interact with CutL1 move randomly to expose the PBSA surface to the recruited CutL1.
(3) The recruitment of CutL1 by RolA attached to solid surfaces is driven by ionic interactions between these proteins [8] (Figure 3B). Their interactions are affected by the protonation state of the side chains of amino acid residues in both RolA and CutL1 in a pH-dependent manner. Addition of NaCl prevents these ionic interactions.
(4) Positively charged N-terminal residues His32 and Lys34 of RolA and negatively charged residues Asp30, Glu31, Asp142, and Asp171 on the hydrophilic surface of CutL1 are critically involved in RolA-dependent CutL1 recruitment via ionic interactions [8,158] (Figure 3B). Chemical modification of these charged residues or their substitution with non-charged residues such as serine markedly weaken the RolA–CutL1 interaction. The interactions between the RolA-H32S/K34S mutant and CutL1-E31S/D142S/D171S mutant, and between wild-type RolA and CutL1-D30S/E31S/D142S/D171S are still stronger than the interaction between the wild-type proteins in the presence of NaCl. Therefore, other charged residues (e.g., Lys41, Lys46, and Lys51 of RolA) or complementarity of the three-dimensional structures of RolA and CutL1 may be involved in the interaction.
It cannot be excluded that the properties of cutinases such as substrate specificity and thermal stability may change due to their interaction with RolA, however, this has not been studied yet.
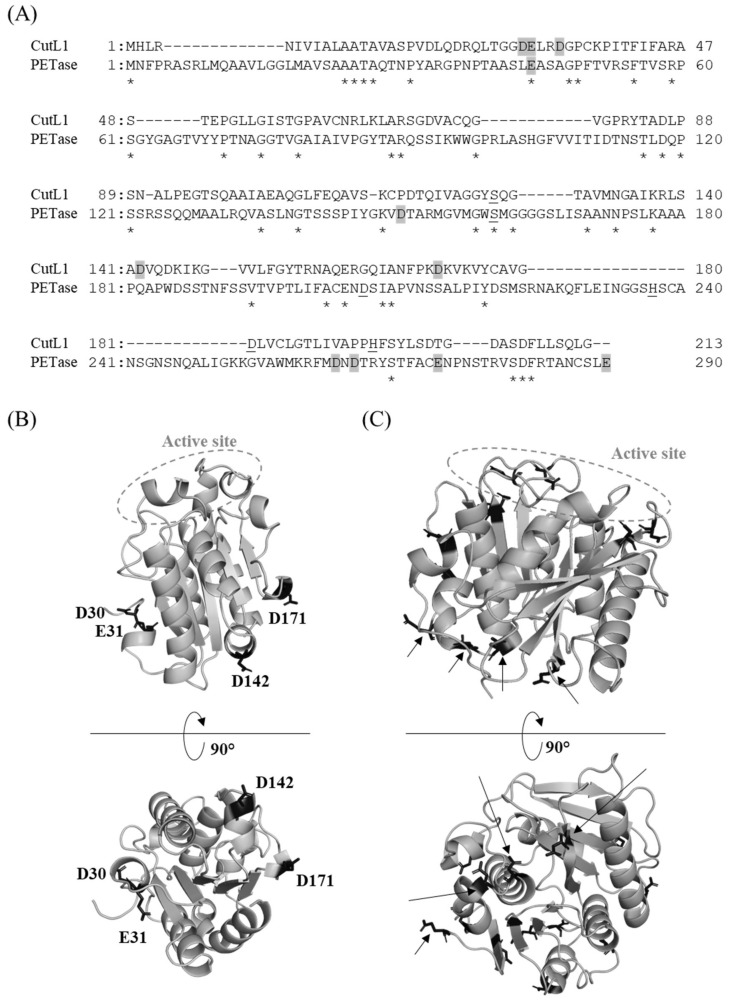
Recently, it has been reported that RolA promotes the degradation of polyethylene terephthalate (PET) by PET-degrading enzyme [148,159], or PETase [160], from the betaproteobacterium Ideonella sakaiensis; both PETase and cutinases are alpha/beta-hydrolases. The estimated molecular weight of PETase (27.6 kDa) is about 40% higher than that of CutL1 (19.7 kDa), and the amino acid sequence identity is very low (19.24%; Figure 4A). However, the three-dimensional structures of PETase (Protein databank ID 5XJH; [161]) and CutL1 (Protein databank ID 3GBS; [162]) are similar and some of the negatively charged residues in both proteins are located on the opposite side of the active site (Figure 4B,C). Therefore, the mechanisms of the RolA–PETase and RolA–CutL1 interactions may be similar. Thus, RolA may interact with and recruit various cutinases and cutinase-like enzymes, and thus enhance the hydrolysis of various aliphatic esters by these enzymes.
Figure 4.
Amino acid sequences and three-dimensional structures of CutL1 and PETase. (A) Alignment of the amino acid sequences. Identical residues are indicated by asterisks. Negatively charged residues of CutL1 that are required for the interaction with RolA and negatively charged residues of PETase that are located on the opposite side of the active site are shaded in gray. Catalytic residues are underlined. (B) Three-dimensional structure of CutL1. Negatively charged residues that are required for the interaction with RolA are shown as black stick models. (C) Three-dimensional structure of PETase. All negatively charged residues are shown as black stick models. Those located on the opposite side of the active site are indicated by arrows.
5.1.2. Aspergillus nidulans
The model Aspergilli A. nidulans has multiple genes encoding both hydrophobins (Table 2) and cutinases [12,70]. Tanaka et al. reported that hydrophobin AnRodA interacts with cutinases Cut1 and Cut2, promoting PBSA degradation [28]. AnRodA, Cut1, and Cut2 are the orthologues of RolA, CutL1, and CutB of A. oryzae, respectively [8,28]. Expression of the cut1 and cut2 genes is induced by lipidic carbon sources such as suberin, cutin, or olive oil [163,164,165]. Expression of the AnrodA gene is induced by steam-exploded sugarcane bagasse [66], which is composed of cellulose, hemicellulose, lignin, and wax ester [166,167]. In such culture, the activity of extracellular polysaccharide-hydrolyzing enzymes (e.g., cellulases or amylases) and the fungal biomass of the ΔAnrodA strain are lower than those of the wild-type strain [66]. Therefore, A. nidulans may use AnRodA for the degradation of not only aliphatic esters but also polysaccharides.
AnRodA interacts with Cut1 and Cut2 via ionic interactions in the same way as RolA interacts with CutL1 and CutC [28]. Interestingly, AnRodA also interacts with CutL1 of A. oryzae via ionic interactions, although the interaction is much weaker than that between RolA and CutL1 [8]. Positively charged residues in the N-terminus of AnRodA (His23, Lys35, and Lys41) are widely spaced, whereas those of RolA (His32, Lys34, and Lys41) are clustered together in their primary structures [28] (Figure 5). Thus, in the RolA–CutL1/CutC and AnRodA–Cut1/Cut2 interactions [26,28], charged amino acid residues may be in more suitable positions on the surfaces of hydrophobins and cutinases than those in the AnRodA–CutL1 interaction.
Figure 5.
N-terminal regions of hydrophobins in the clade containing AnRodA and RolA (as in [8]). Identical residues are indicated by asterisks, and highly conserved residues are indicated by periods. Positively charged residues (Arg, His, and Lys) are shaded in gray. Cys residues are shown in bold.
5.2. Hydrophobin–Cutinase Interactions in Other Fungi
To date, only a few hydrophobin–cutinase interactive combinations have been reported in filamentous fungi, including A. oryzae [8,26] and A. nidulans [28]. Among other filamentous fungi, the combinations of hydrophobins MPG1 (class I) and MHP1 (class II) and the cutinase Cut2 have been reported in the rice blast fungus M. oryzae [29]. However, phylogenetic analysis of hydrophobins and cutinases by Takahashi et al. (Figure 6; [8]) suggests a variety of potential combinations, for example, hydrophobin Pc22g14290 (accession number CAP98717.1)–Cutinase 1 (CAP97019.1) of Penicillium chrysogenum, hydrophobin BCDW1_9126 (EMR82223.1)–cutinase BCDW1_3897 (EMR87444.1) of Botrytis cinerea, and hydrophobin FVG_03685 (EWG41603.1)–Cutinase 3 (EWG55667.1) of Fusarium verticillioides; all accession numbers are from GenBank.
Figure 6.
Phylogenetic analysis of (A) major class I hydrophobins and (B) cutinases and acetylxylan esterases. Acetylxylan esterases are underlined. The cutinases and acetylxylan esterases form the following three groups: (i) ascomycetes cutinases, including all Aspergilli cutinases; (ii) cutinases from other ascomycetes and basidiomycetes; and (iii) acetylxylan esterases and cutinases which show high similarities to acetylxylan esterases. All sequences are from ascomycetes or basidiomycetes (reproduced with permission from [8]. 2015, Molecular Microbiology).
Some class I hydrophobins (Figure 6A) are predicted on the basis of coding sequences only. Many class I hydrophobins, including those of ascomycetes, have multiple positively charged residues in their N-terminal regions upstream of the first Cys residue [8]. Most predicted hydrophobins in the clade containing AnRodA and RolA are from Aspergillus and Penicillium species [8] and have at least three positively charged N-terminal residues in similar positions (Figure 5). Some class I hydrophobins from other clades also have multiple positively charged N-terminal residues, for instance, Hydpt1 of Pisolithus tinctorius (GenBank accession number AAC49307.1; 10 positively charged N-terminal residues), SC6 of S. commune (CAA07545.1; 4 positively charged N-terminal residues), and Vmh1 of P. ostreatus (CAB41405.1; 4 positively charged N-terminal residues) [168]. Most ascomycetous and basidiomycetous filamentous fungi that harbor hydrophobins (Figure 6A) have several cutinases, including those predicted on the basis of coding sequences. Some filamentous fungi also have acetylxylan esterases of the carbohydrate esterase 5 family (Figure 6B), with amino acid sequences highly similar to those of cutinases. Negatively charged residues corresponding to Glu31, Asp142, and Asp171 of CutL1 are highly conserved in many cutinases of ascomycetes, in some cutinases of basidiomycetes, and in some acetylxylan esterases [8]. The ionic interactions of hydrophobins with cutinases may be common at least in Aspergillus and Penicillium species, and possibly in many ascomycetes and in some basidiomycetes [8,28,158].
6. Low-Molecular-Weight Proteins with Properties Similar to Those of Hydrophobins
Low-molecular-weight proteins (<300 amino acid residues) secreted by filamentous fungi, such as hydrophobins, hydrophobic surface–binding proteins (HsbA [21] and HsbA-like proteins) that do not show a specific pattern of conserved Cys residues characteristic of hydrophobins, and effector proteins, are collectively referred to as small secreted proteins (SSPs) [169,170,171]. In Aspergilli, non-hydrophobin SSPs also attach to solid surfaces and recruit hydrolytic enzymes. HsbA (14.4 kDa) from A. oryzae attaches to the PBSA surface in the presence of Ca2+ and recruits CutL1 [172]. Similar to the expression of the rolA and cutl1 genes, that of the hsbA gene is induced by PBSA [26,149,172,173]. The hsbA expression is also induced in solid-state culture with wheat bran [172,174]. Proteins homologous to HsbA and their orthologues are found in A. niger and A. nidulans [67,68,169]. When these fungi are grown in a medium containing wheat straw, or A. nidulans is grown in a medium containing sugarcane bagasse pulp, expression of genes encoding HsbA orthologues is induced [67,68,169]. These observations suggest that HsbA and its orthologues are likely involved in the degradation of solid polymers.
Effector protein is a generic term for multiple protein groups that promote infection by phytopathogenic filamentous fungi and their growth by enabling the fungi to avoid the plant immune response or by damaging plant tissues [175,176,177,178,179,180,181]. Effector proteins have been found in phytopathogenic filamentous fungi at first; however, orthologs of effector proteins also have been found in ectomycorrhizal and saprobic fungi [177,178,182,183]. Hydrophobins and some effector proteins (e.g., the phytotoxin cerato-platanin) have similar physicochemical and biochemical properties such as high hydrophobicity, strong foam formation, self-assembly at the air–liquid interface, and localization on the fungal cell wall [184,185,186,187,188], but have unrelated amino acid sequences [175,186,189,190]. Contrary to the phytotoxicity of effector proteins such as cerato-platanin, hydrophobin toxicity has not been reported. Hydrophobins form hydrophobic protective coating on the surface of the fungal cell wall, and hydrophobins and hydrophobin-coated hyphae and conidia evade recognition by the immune systems of host plants [21,22,23,24]. Although the evasion mechanism has not been well elucidated, the functions of protective coating formation and plant immune response avoidance are common between hydrophobins and some effector proteins [179,180]. The expression of hydrophobin genes is induced in filamentous fungi by solid polymers of plant origin [66,67,68,69]. Therefore, hydrophobins are considered as effector proteins [33,180,191,192,193]. Some other studies suggest that the HsbA-like proteins of M. oryzae are also effector proteins because their genes are strongly up-regulated during appressorium development, which is strongly related to host infection [192,194].
In Aspergilli, the number of SSP-encoding genes varies greatly among species [169], and the SSP secretion pattern depends on the plant-derived polymer provided as a carbon source. For example, when the same plant-derived polymer (sugarcane bagasse pulp or wheat bran) is used, one group of HsbA orthologues, which includes HsbA of A. oryzae, is barely secreted, whereas another group is secreted on sugarcane bagasse pulp in A. flavus and on wheat bran in Aspergillus clavatus, A. niger, and A. terreus [169]. RolA, HsbA, and effector proteins are widely conserved among these Aspergilli [169]. Thus, Aspergilli may decompose plant polymers through the interaction of various SSPs with various polymer-degrading enzymes. The differences in the SSP expression profiles among species suggest that SSP production is optimized in Aspergilli in response to specific solid polymers and environmental conditions, such as salt concentration, pH, and oxidative stress, to decompose the available solid polymers.
7. Conclusions
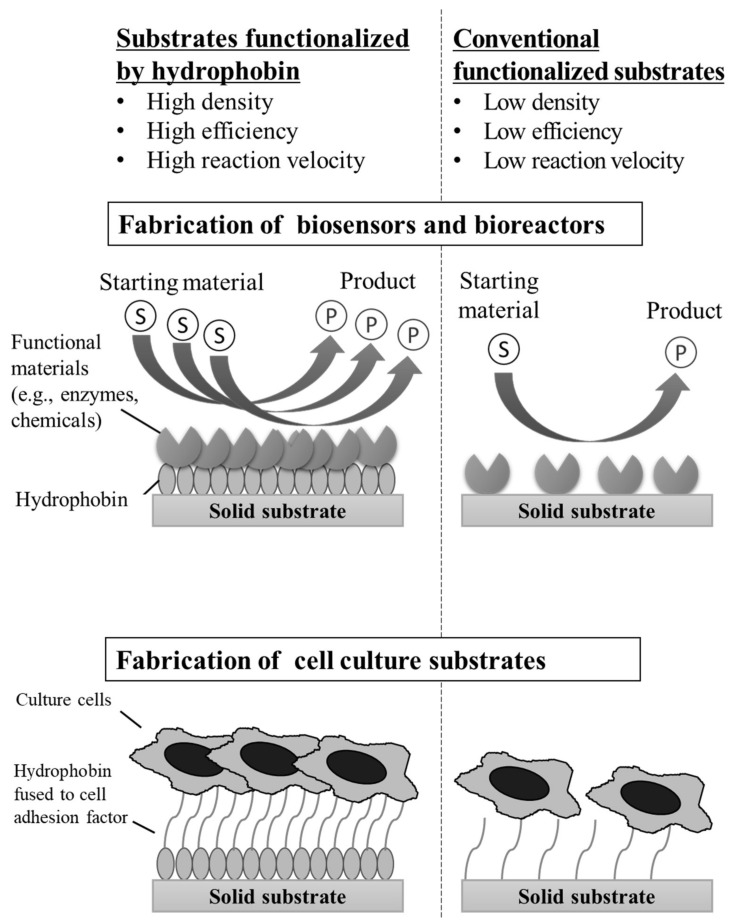
Hydrophobins, low-molecular-weight amphipathic proteins, are widely conserved in filamentous fungi and are localized on the surface of the cell wall. Hydrophobins self-assemble at interfaces and form amphipathic membranes. Class I hydrophobins self-assemble into β-amyloid-like structures called rodlets. Aspergilli have multiple class I hydrophobins. Self-assembly of class I hydrophobins of Aspergilli depends on factors such as hydrophobin conformation, pH of the solution, and the physicochemical properties (e.g., hydrophobicity and functional group) of the solid surface. The Cys4–Cys5 and Cys7–Cys8 loops, four disulfide bonds, and eight conserved Cys residues are all important for the self-assembly of RodA-like class I hydrophobins. The Cys7–Cys8 loop is also important for the adsorption of RodA-like hydrophobins to solid surfaces. Among class I hydrophobins, some Aspergillus hydrophobins such as RolA, AfRodA, RodB, and AnRodA can be purified in water phase without using organic solvents. In addition to class I hydrophobins, non-conventional class hydrophobins (class III and unknown class) but no class II hydrophobins have been found in Aspergilli. The physicochemical properties, biochemical properties, and biological functions of non-conventional class hydrophobins are poorly understood, but these hydrophobins may also be important for Aspergilli. Hydrophobins are beneficial for filamentous fungus growth. For example, RolA and RodA-like hydrophobins interact with cutinases to promote the degradation of aliphatic polyesters. This unique mechanism, first discovered in A. oryzae, appears to be generally conserved in Aspergillus and Penicillium species that possess these hydrophobins. It is necessary to further study the mechanism by which self-assembled structures of hydrophobins on solid polymers recruit hydrolytic enzymes and promote hydrolysis of the polymers beneath the hydrophobin self-assembled structures. To the best of our knowledge, the recruitment of enzymes by non-RodA-like hydrophobins of Aspergilli has not been reported but seems plausible because some fungal hydrophobins other than those from Aspergilli also recruit enzymes. The ability of hydrophobins to interact with a variety of enzymes allows the enzymes to be exploited as “functionalized substrates”; other proteins or compounds can be fixed to a solid substrate on which hydrophobins are adsorbed. This concept may be applicable to the fabrication of biosensors, cell culture substrates, and bioreactors for material degradation or conversion (Figure 7). Aspergilli produce various SSPs including hydrophobins, HsbA, HsbA-like proteins, and effector proteins depending on species and culture conditions. Hydrophobins and HsbA interact with polymer-degrading enzymes, recruiting them and thus enhancing solid polymer degradation. Some biochemical properties and biological functions are common between hydrophobins and effector proteins, hydrophobins and HsbA/HsbA-like proteins, and HsbA/HsbA-like proteins and effector proteins. Therefore, Aspergilli may use a wide variety of SSPs to decompose and utilize solid polymers. Further studies from the physicochemical, biochemical, and genetic viewpoints are necessary for understanding the biological roles of Aspergillus SSPs.
Figure 7.
Schematic diagram of hypothetical applications of hydrophobins: fabrication of biosensors, cell culture substrates, and bioreactors for material degradation or conversion. Left part of each panel: solid substrates functionalized by hydrophobins. Right part of each panel: conventional functionalized substrates in which functional materials are immobilized on the solid surface via a chemical reagent.
Author Contributions
Conceptualization, T.T.; investigation, T.T. and Y.T.; writing—original draft, T.T., Y.T., and K.A.; writing—review and editing, T.T., Y.T., A.Y., and K.A.; funding acquisition, K.A.; supervision, K.A. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was partly supported by a Grant-in-Aid for Scientific Research (B) from the Japan Society for the Promotion of Science (grant number 17H03787 to K.A.), a Grant-in-Aid for Japan Society for the Promotion of Science Fellows (grant number 18J11900 to Y.T.), and the Institute for Fermentation, Osaka, Japan (grant number K-2019-002 to A.Y.). T.T. is also supported by the Institute for Fermentation, Osaka, Japan (grant number K-2021-008 to Ken-ichi Kusumoto at Osaka University).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kershaw M.J., Talbot N.J. Hydrophobins and Repellents: Proteins with Fundamental Roles in Fungal Morphogenesis. Fungal Genet. Biol. 1998;23:18–33. doi: 10.1006/fgbi.1997.1022. [DOI] [PubMed] [Google Scholar]
- 2.Kwan A.H.Y., Winefield R.D., Sunde M., Matthews J.M., Haverkamp R.G., Templeton M.D., Mackay J.P. Structural basis for rodlet assembly in fungal hydrophobins. Proc. Natl. Acad. Sci. USA. 2006;103:3621–3626. doi: 10.1073/pnas.0505704103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morris V.K., Linser R., Wilde K.L., Duff A.P., Sunde M., Kwan A.H. Solid-State NMR Spectroscopy of Functional Amyloid from a Fungal Hydrophobin: A Well-Ordered β-Sheet Core Amidst Structural Heterogeneity. Angew. Chem. Int. Ed. 2012;51:12621–12625. doi: 10.1002/anie.201205625. [DOI] [PubMed] [Google Scholar]
- 4.Pille A., Kwan A., Cheung I., Hampsey M., Aimanianda V., Delepierre M., Latgé J.-P., Sunde M., Guijarro J.I. 1H, 13C and 15N resonance assignments of the RodA hydrophobin from the opportunistic pathogen Aspergillus fumigatus. Biomol. NMR Assign. 2014;9:113–118. doi: 10.1007/s12104-014-9555-1. [DOI] [PubMed] [Google Scholar]
- 5.Rey A.A., Hocher A., Kwan A.H., Sunde M. Backbone and sidechain 1H, 13C and 15N chemical shift assignments of the hydrophobin MPG1 from the rice blast fungus Magnaporthe oryzae. Biomol. NMR Assign. 2012;7:109–112. doi: 10.1007/s12104-012-9394-x. [DOI] [PubMed] [Google Scholar]
- 6.Morris V.K., Kwan A.H., Sunde M. Analysis of the Structure and Conformational States of DewA Gives Insight into the Assembly of the Fungal Hydrophobins. J. Mol. Biol. 2013;425:244–256. doi: 10.1016/j.jmb.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 7.Cai F., Zhao Z., Gao R., Chen P., Ding M., Jiang S., Fu Z., Xu P., Chenthamara K., Shen Q., et al. The pleiotropic functions of intracellular hydrophobins in aerial hyphae and fungal spores. PLoS Genet. 2021;17:e1009924. doi: 10.1371/journal.pgen.1009924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Takahashi T., Tanaka T., Tsushima Y., Muragaki K., Uehara K., Takeuchi S., Maeda H., Yamagata Y., Nakayama M., Yoshimi A., et al. Ionic interaction of positive amino acid residues of fungal hydrophobin RolA with acidic amino acid residues of cutinase CutL1. Mol. Microbiol. 2015;96:14–27. doi: 10.1111/mmi.12915. [DOI] [PubMed] [Google Scholar]
- 9.Rineau F., Lmalem H., Ahren D., Shah F., Johansson T., Coninx L., Ruytinx J., Nguyen T.T.H., Grigoriev I., Kuo A., et al. Comparative genomics and expression levels of hydrophobins from eight mycorrhizal genomes. Mycorrhiza. 2017;27:383–396. doi: 10.1007/s00572-016-0758-4. [DOI] [PubMed] [Google Scholar]
- 10.Jensen B.G., Andersen M.R., Pedersen M.H., Frisvad J.C., Søndergaard I. Hydrophobins from Aspergillus species cannot be clearly divided into two classes. BMC Res. Notes. 2010;3:344. doi: 10.1186/1756-0500-3-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Krijgsheld P., Bleichrodt R., van Veluw G., Wang F., Müller W., Dijksterhuis J., Wösten H. Development in Aspergillus. Stud. Mycol. 2013;74:1–29. doi: 10.3114/sim0006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grünbacher A., Throm T., Seidel C., Gutt B., Röhrig J., Strunk T., Vincze P., Walheim S., Schimmel T., Wenzel W., et al. Six Hydrophobins Are Involved in Hydrophobin Rodlet Formation in Aspergillus nidulans and Contribute to Hydrophobicity of the Spore Surface. PLoS ONE. 2014;9:e94546. doi: 10.1371/journal.pone.0094546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Valsecchi I., Dupres V., Stephen-Victor E., Guijarro J.I., Gibbons J., Beau R., Bayry J., Coppee J.-Y., Lafont F., Latgé J.-P., et al. Role of Hydrophobins in Aspergillus fumigatus. J. Fungi. 2017;4:2. doi: 10.3390/jof4010002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cai F., Gao R., Zhao Z., Ding M., Jiang S., Yagtu C., Zhu H., Zhang J., Ebner T., Mayrhofer-Reinhartshuber M., et al. Evolutionary compromises in fungal fitness: Hydrophobins can hinder the adverse dispersal of conidiospores and challenge their survival. ISME J. 2020;14:2610–2624. doi: 10.1038/s41396-020-0709-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Vocht M.L., Scholtmeijer K., van der Vegte E.W., de Vries O.M., Sonveaux N., Wösten H.A., Ruysschaert J.-M., Hadziioannou G., Wessels J.G., Robillard G.T. Structural Characterization of the Hydrophobin SC3, as a Monomer and after Self-Assembly at Hydrophobic/Hydrophilic Interfaces. Biophys. J. 1998;74:2059–2068. doi: 10.1016/S0006-3495(98)77912-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackay J., Matthews J., Winefield R., Mackay L.G., Haverkamp R., Templeton M. The Hydrophobin EAS Is Largely Unstructured in Solution and Functions by Forming Amyloid-Like Structures. Structure. 2001;9:83–91. doi: 10.1016/S0969-2126(00)00559-1. [DOI] [PubMed] [Google Scholar]
- 17.Terauchi Y., Tanaka T., Mitsuishi M., Yabu H., Yoshimi A., Nantani K., Abe K. Analysis of the self-assembly process of Aspergillus oryzae hydrophobin RolA by Langmuir–Blodgett method. Biosci. Biotechnol. Biochem. 2019;84:678–685. doi: 10.1080/09168451.2019.1706443. [DOI] [PubMed] [Google Scholar]
- 18.Wosten H., de Vries O., Wessels J. Interfacial Self-Assembly of a Fungal Hydrophobin into a Hydrophobic Rodlet Layer. Plant Cell. 1993;5:1567–1574. doi: 10.2307/3869739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wösten H., Schuren F., Wessels J. Interfacial self-assembly of a hydrophobin into an amphipathic protein membrane mediates fungal attachment to hydrophobic surfaces. EMBO J. 1994;13:5848–5854. doi: 10.1002/j.1460-2075.1994.tb06929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ball S.R., Kwan A.H., Sunde M. Hydrophobin Rodlets on the Fungal Cell Wall. Curr. Top. Microbiol. Immunol. 2019;425:29–51. doi: 10.1007/82_2019_186. [DOI] [PubMed] [Google Scholar]
- 21.Talbot N.J., Ebbole D.J., Hamer J.E. Identification and characterization of MPG1, a gene involved in pathogenicity from the rice blast fungus Magnaporthe grisea. Plant Cell. 1993;5:1575–1590. doi: 10.1105/tpc.5.11.1575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leger R.J.S., Staples R.C., Roberts D.W. Cloning and regulatory analysis of starvation-stress gene, ssgA, encoding a hydrophobin-like protein from the entomopathogenic fungus, Metarhizium anisopliae. Gene. 1992;120:119–124. doi: 10.1016/0378-1119(92)90019-L. [DOI] [PubMed] [Google Scholar]
- 23.Paris S., Debeaupuis J.-P., Crameri R., Carey M., Charlès F., Prévost M.C., Schmitt C., Philippe B., Latgé J.P. Conidial Hydrophobins of Aspergillus fumigatus. Appl. Environ. Microbiol. 2003;69:1581–1588. doi: 10.1128/AEM.69.3.1581-1588.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Aimanianda V., Bayry J., Bozza S., Kniemeyer O., Perruccio K., Elluru S.R., Clavaud C., Paris S., Brakhage A.A., Kaveri S.V., et al. Surface hydrophobin prevents immune recognition of airborne fungal spores. Nat. Cell Biol. 2009;460:1117–1121. doi: 10.1038/nature08264. [DOI] [PubMed] [Google Scholar]
- 25.Corvis Y., Walcarius A., Rink R., Mrabet N.T., Rogalska E. Preparing Catalytic Surfaces for Sensing Applications by Immobilizing Enzymes via Hydrophobin Layers. Anal. Chem. 2005;77:1622–1630. doi: 10.1021/ac048897w. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi T., Maeda H., Yoneda S., Ohtaki S., Yamagata Y., Hasegawa F., Gomi K., Nakajima T., Abe K. The fungal hydrophobin RolA recruits polyesterase and laterally moves on hydrophobic surfaces. Mol. Microbiol. 2005;57:1780–1796. doi: 10.1111/j.1365-2958.2005.04803.x. [DOI] [PubMed] [Google Scholar]
- 27.Wang Z., Lienemann M., Qiau M., Linder M.B. Mechanisms of Protein Adhesion on Surface Films of Hydrophobin. Langmuir. 2010;26:8491–8496. doi: 10.1021/la101240e. [DOI] [PubMed] [Google Scholar]
- 28.Tanaka T., Nakayama M., Takahashi T., Nanatani K., Yamagata Y., Abe K. Analysis of the ionic interaction between the hydrophobin RodA and two cutinases of Aspergillus nidulans obtained via an Aspergillus oryzae expression system. Appl. Microbiol. Biotechnol. 2016;101:2343–2356. doi: 10.1007/s00253-016-7979-5. [DOI] [PubMed] [Google Scholar]
- 29.Pham C.L.L., Rey A., Lo V., Soulès M., Ren Q., Meisl G., Knowles T.P.J., Kwan A.H., Sunde M. Self-assembly of MPG1, a hydrophobin protein from the rice blast fungus that forms functional amyloid coatings, occurs by a surface-driven mechanism. Sci. Rep. 2016;6:25288. doi: 10.1038/srep25288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wessels J., De Vries O., Asgeirsdottir S.A., Schuren F. Hydrophobin Genes Involved in Formation of Aerial Hyphae and Fruit Bodies in Schizophyllum. Plant Cell. 1991;3:793–799. doi: 10.2307/3869273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Valsecchi I., Lai J.I., Stephen-Victor E., Pillé A., Beaussart A., Lo V., Pham C.L., Aimanianda V., Kwan A., Duchateau M., et al. Assembly and disassembly of Aspergillus fumigatus conidial rodlets. Cell Surf. 2019;5:100023. doi: 10.1016/j.tcsw.2019.100023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Quarantin A., Hadeler B., Kröger C., Schäfer W., Favaron F., Sella L., Martínez-Rocha A.L. Different Hydrophobins of Fusarium graminearum Are Involved in Hyphal Growth, Attachment, Water-Air Interface Penetration and Plant Infection. Front. Microbiol. 2019;10:751. doi: 10.3389/fmicb.2019.00751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wösten H.A.B. Hydrophobins: Multipurpose Proteins. Annu. Rev. Microbiol. 2001;55:625–646. doi: 10.1146/annurev.micro.55.1.625. [DOI] [PubMed] [Google Scholar]
- 34.Levetin E., Horner W.E., Scott J.A., Barnes C., Baxi S., Chew G.L., Grimes C., Kennedy K., Larenas-Linnemann D., Miller J.D., et al. Taxonomy of Allergenic Fungi. J. Allergy Clin. Immunol. Pr. 2016;4:375–385. doi: 10.1016/j.jaip.2015.10.012. [DOI] [PubMed] [Google Scholar]
- 35.Machida M., Yamada O., Gomi K. Genomics of Aspergillus oryzae: Learning from the History of Koji Mold and Exploration of Its Future. DNA Res. 2008;15:173–183. doi: 10.1093/dnares/dsn020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kusumoto K.-I., Yamagata Y., Tazawa R., Kitagawa M., Kato T., Isobe K., Kashiwagi Y. Japanese Traditional Miso and Koji Making. J. Fungi. 2021;7:579. doi: 10.3390/jof7070579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gouka R.J., Punt P.J., Hondel C.A.M.J.J.V.D. Efficient production of secreted proteins by Aspergillus: Progress, limitations and prospects. Appl. Microbiol. Biotechnol. 1997;47:1–11. doi: 10.1007/s002530050880. [DOI] [PubMed] [Google Scholar]
- 38.Punt P.J., van Biezen N., Conesa A., Albers A., Mangnus J., Hondel C.V.D. Filamentous fungi as cell factories for heterologous protein production. Trends Biotechnol. 2002;20:200–206. doi: 10.1016/S0167-7799(02)01933-9. [DOI] [PubMed] [Google Scholar]
- 39.Galagan J.E., Calvo S.E., Cuomo C., Ma L.-J., Wortman J.R., Batzoglou S., Lee S.-I., Baştürkmen M., Spevak C.C., Clutterbuck J., et al. Sequencing of Aspergillus nidulans and comparative analysis with A. fumigatus and A. oryzae. Nature. 2005;438:1105–1115. doi: 10.1038/nature04341. [DOI] [PubMed] [Google Scholar]
- 40.Li Q., Lu J., Zhang G., Liu S., Zhou J., Du G., Chen J. Recent advances in the development of Aspergillus for protein production. Bioresour. Technol. 2022;348:126768. doi: 10.1016/j.biortech.2022.126768. [DOI] [PubMed] [Google Scholar]
- 41.Carlsen M. Growth and α-amylase production by Aspergillus oryzae during continuous cultivations. J. Biotechnol. 1996;45:81–93. doi: 10.1016/0168-1656(95)00147-6. [DOI] [Google Scholar]
- 42.Swetha S., Nampoothiri K., Soccol C., Pandey A., Dhanya G. Alpha amylase production by Aspergillus oryzae employing solid–state fermentation. J. Sci. Ind. Res. 2007;66:621–626. [Google Scholar]
- 43.Balakrishnan M., Jeevarathinam G., Kumar S.K.S., Muniraj I., Uthandi S. Optimization and scale-up of α-amylase production by Aspergillus oryzae using solid-state fermentation of edible oil cakes. BMC Biotechnol. 2021;21:1–11. doi: 10.1186/s12896-021-00686-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mikai S., Konomi J., Sato Y., Era M., Ninomiya J., Morita H. Simultaneous Increase of Glucoamylase and α-Amylase Production in Submerged Co-culture of Aspergillus and Rhizopus Strains. Jpn. J. Food Eng. 2015;16:111–121. doi: 10.11301/jsfe.16.111. [DOI] [Google Scholar]
- 45.Contesini F.J., Calzado F., Madeira J.V., Rubio M.V., Zubieta M.P., de Melo R.R., Gonçalves T.A. Aspergillus lipases: Biotechnological and industrial application. In: Mérillon J.-M., Ramawat K.G., editors. Fungal Metabolites. Springer International Publishing; Cham, Switzerland: 2017. pp. 639–666. [DOI] [Google Scholar]
- 46.Ohnishi K., Yoshida Y., Sekiguchi J. Lipase production of Aspergillus oryzae. J. Ferment. Bioeng. 1994;77:490–495. doi: 10.1016/0922-338X(94)90116-3. [DOI] [Google Scholar]
- 47.Shinkawa S., Mitsuzawa S. Feasibility study of on-site solid-state enzyme production by Aspergillus oryzae. Biotechnol. Biofuels. 2020;13:31. doi: 10.1186/s13068-020-1669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van Peij N.N.M.E., Gielkens M.M.C., de Vries R.P., Visser J., de Graaff L.H. The Transcriptional Activator XlnR Regulates Both Xylanolytic and Endoglucanase Gene Expression in Aspergillus niger. Appl. Environ. Microbiol. 1998;64:3615–3619. doi: 10.1128/AEM.64.10.3615-3619.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mrudula S., Murugammal R. Production of cellulose by Aspergillus niger under submerged and solid state fermentation using coir waste as a substrate. Braz. J. Microbiol. 2011;42:1119–1127. doi: 10.1590/S1517-83822011000300033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Adefisoye S., Sakariyau A. Production of Glucoamylase by Aspergillus niger in Solid State Fermentation. Adv. Biol. Res. 2018;2:7–11. [Google Scholar]
- 51.Coutinho P.M., Reilly P.J. Glucoamylase structural, functional, and evolutionary relationships. Proteins: Struct. Funct. Bioinform. 1997;29:334–347. doi: 10.1002/(SICI)1097-0134(199711)29:3<334::AID-PROT7>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 52.Shafique S., Bajwa R., Shafique S. Screening of Aspergillus niger and A. flavus strains for extra cellular alpha-amylase activity. Pak. J. Bot. 2009;41:897–905. [Google Scholar]
- 53.Kautola H., Vassilev N., Linko Y.-Y. Continuous itaconic acid production by immobilized biocatalysts. J. Biotechnol. 1990;13:315–323. doi: 10.1016/0168-1656(90)90079-Q. [DOI] [PubMed] [Google Scholar]
- 54.Berovič M. Scale-up of citric acid fermentation by redox potential control. Biotechnol. Bioeng. 1999;64:552–557. doi: 10.1002/(SICI)1097-0290(19990905)64:5<552::AID-BIT5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 55.Terabayashi Y., Sano M., Yamane N., Marui J., Tamano K., Sagara J., Dohmoto M., Oda K., Ohshima E., Tachibana K., et al. Identification and characterization of genes responsible for biosynthesis of kojic acid, an industrially important compound from Aspergillus oryzae. Fungal Genet. Biol. 2010;47:953–961. doi: 10.1016/j.fgb.2010.08.014. [DOI] [PubMed] [Google Scholar]
- 56.Wösten H.A., van Wetter M.-A., Lugones L.G., van der Mei H.C., Busscher H.J., Wessels J.G. How a fungus escapes the water to grow into the air. Curr. Biol. 1999;9:85–88. doi: 10.1016/S0960-9822(99)80019-0. [DOI] [PubMed] [Google Scholar]
- 57.van Wetter M.-A., Wosten H.A.B., Wessels J.G.H. SC3 and SC4 hydrophobins have distinct roles in formation of aerial structures in dikaryons of Schizophyllum commune. Mol. Microbiol. 2000;36:201–210. doi: 10.1046/j.1365-2958.2000.01848.x. [DOI] [PubMed] [Google Scholar]
- 58.Talbot N.J., Kershaw M.J., Wakley G.E., de Vries O., Wessels J., Hamer J.E. MPG1 Encodes a Fungal Hydrophobin Involved in Surface Interactions during Infection-Related Development of Magnaporthe grisea. Plant Cell. 1996;8:985–999. doi: 10.2307/3870210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cohen Y., Shulhani R., Rot Y., Zemach H., Belausov E., Grinberg-Baran M., Borenstein M., Pivonia S., Ezra D., Shtienberg D. Aspergillus niger, the causal agent of black mould disease in date fruits, infects and colonizes flowers and young fruitlets. Plant Pathol. 2021;70:1195–1208. doi: 10.1111/ppa.13358. [DOI] [Google Scholar]
- 60.Dolezal A.L., Shu X., Obrian G.R., Nielsen D., Woloshuk C.P., Boston R.S., Payne G.A. Aspergillus flavus infection induces transcriptional and physical changes in developing maize kernels. Front. Microbiol. 2014;5:384. doi: 10.3389/fmicb.2014.00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nierman W.C., Pain A., Anderson M.J., Wortman J.R., Kim H.S., Arroyo J., Berriman M., Abe K., Archer D.B., Bermejo C., et al. Genomic sequence of the pathogenic and allergenic filamentous fungus Aspergillus fumigatus. Nature. 2005;438:1151–1156. doi: 10.1038/nature04332. [DOI] [PubMed] [Google Scholar]
- 62.Sato A., Oshima K., Noguchi H., Ogawa M., Takahashi T., Oguma T., Koyama Y., Itoh T., Hattori M., Hanya Y. Draft Genome Sequencing and Comparative Analysis of Aspergillus sojae NBRC4239. DNA Res. 2011;18:165–176. doi: 10.1093/dnares/dsr009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.He B., Tu Y., Jiang C., Zhang Z., Li Y., Zeng B. Functional Genomics of Aspergillus oryzae: Strategies and Progress. Microorganisms. 2019;7:103. doi: 10.3390/microorganisms7040103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Stringer M.A., Dean R.A., Sewall T.C., Timberlake E.W. Rodletless, a new Aspergillus developmental mutant induced by directed gene inactivation. Genes Dev. 1991;5:1161–1171. doi: 10.1101/gad.5.7.1161. [DOI] [PubMed] [Google Scholar]
- 65.Thau N., Monod M., Crestani B., Rolland C., Tronchin G., Latgé J.P., Paris S. Rodletless mutants of Aspergillus fumigatus. Infect. Immun. 1994;62:4380–4388. doi: 10.1128/iai.62.10.4380-4388.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Brown N.A., Ries L.N.A., Reis T.F., Rajendran R., dos Santos R.A.C., Ramage G., Riaño-Pachón D.M., Goldman G.H. RNAseq reveals hydrophobins that are involved in the adaptation of Aspergillus nidulans to lignocellulose. Biotechnol. Biofuels. 2016;9:1–17. doi: 10.1186/s13068-016-0558-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Delmas S., Pullan S.T., Gaddipati S., Kokolski M., Malla S., Blythe M.J., Ibbett R., Campbell M., Liddell S., Aboobaker A., et al. Uncovering the Genome-Wide Transcriptional Responses of the Filamentous Fungus Aspergillus niger to Lignocellulose Using RNA Sequencing. PLoS Genet. 2012;8:e1002875. doi: 10.1371/journal.pgen.1002875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pullan S.T., Daly P., Delmas S., Ibbett R., Kokolski M., Neiteler A., van Munster J.M., Wilson R., Blythe M.J., Gaddipati S., et al. RNA-sequencing reveals the complexities of the transcriptional response to lignocellulosic biofuel substrates in Aspergillus niger. Fungal Biol. Biotechnol. 2014;1:3. doi: 10.1186/s40694-014-0003-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nakari-Setala T., Aro N., IlmeN M., Munoz G., Kalkkinen N., Penttila M. Differential Expression of the Vegetative and Spore-Bound Hydrophobins of Trichoderma reesei Cloning and Characterization of the Hfb2 Gene. JBIC J. Biol. Inorg. Chem. 1997;248:415–423. doi: 10.1111/j.1432-1033.1997.00415.x. [DOI] [PubMed] [Google Scholar]
- 70.Stringer M.A., Timberlake W.E. DewA encodes a fungal hydrophobin component of the Aspergillus spore wall. Mol. Microbiol. 1995;16:33–44. doi: 10.1111/j.1365-2958.1995.tb02389.x. [DOI] [PubMed] [Google Scholar]
- 71.Gandier J.-A., Langelaan D.N., Won A., O’Donnell K., Grondin J.L., Spencer H.L., Wong P., Tillier E., Yip C., Smith S., et al. Characterization of a Basidiomycota hydrophobin reveals the structural basis for a high-similarity Class I subdivision. Sci. Rep. 2017;7:srep45863. doi: 10.1038/srep45863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wösten H.A., de Vocht M.L. Hydrophobins, the fungal coat unravelled. Biochim. Biophys. Acta (BBA)-Rev. Biomembr. 2000;1469:79–86. doi: 10.1016/S0304-4157(00)00002-2. [DOI] [PubMed] [Google Scholar]
- 73.Wessels J.G.H., De Vries O.M.H., Asgeirsdottir S., Springer J. The thn mutation of Schizophyllum commune, which suppresses formation of aerial hyphae, affects expression of the Sc3 hydrophobin gene. J. Gen. Microbiol. 1991;137:2439–2445. doi: 10.1099/00221287-137-10-2439. [DOI] [PubMed] [Google Scholar]
- 74.Lo V.C., Ren Q., Pham C.L.L., Morris V.K., Kwan A.H., Sunde M. Fungal Hydrophobin Proteins Produce Self-Assembling Protein Films with Diverse Structure and Chemical Stability. Nanomaterials. 2014;4:827–843. doi: 10.3390/nano4030827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Cole G.T., Sekiya T., Kasai R., Yokoyama T., Nozawa Y. Surface ultrastructure and chemical composition of the cell walls of conidial fungi. Exp. Mycol. 1979;3:132–156. doi: 10.1016/S0147-5975(79)80025-0. [DOI] [Google Scholar]
- 76.Macindoe I., Kwan A.H., Ren Q., Morris V.K., Yang W., Mackay J.P., Sunde M. Self-assembly of functional, amphipathic amyloid monolayers by the fungal hydrophobin EAS. Proc. Natl. Acad. Sci. USA. 2012;109:E804–E811. doi: 10.1073/pnas.1114052109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yang W., Ren Q., Wu Y.-N., Morris V.K., Rey A.A., Braet F., Kwan A.H., Sunde M. Surface functionalization of carbon nanomaterials by self-assembling hydrophobin proteins. Biopolymers. 2012;99:84–94. doi: 10.1002/bip.22146. [DOI] [PubMed] [Google Scholar]
- 78.Niu B., Gong Y., Gao X., Xu H., Qiao M., Li W. The functional role of Cys3–Cys4 loop in hydrophobin HGFI. Amino Acids. 2014;46:2615–2625. doi: 10.1007/s00726-014-1805-0. [DOI] [PubMed] [Google Scholar]
- 79.Wang X., Song D., Wang B., Yang J., Ge L., Zhao L., Xu H., Qiao M. A mutant of hydrophobin HGFI tuning the self-assembly behaviour and biosurfactant activity. Appl. Microbiol. Biotechnol. 2017;101:8419–8430. doi: 10.1007/s00253-017-8577-x. [DOI] [PubMed] [Google Scholar]
- 80.Zykwinska A., Pihet M., Radji S., Bouchara J.-P., Cuenot S. Self-assembly of proteins into a three-dimensional multilayer system: Investigation of the surface of the human fungal pathogen Aspergillus fumigatus. Biochim. Biophys. Acta (BBA)-Proteins Proteom. 2014;1844:1137–1144. doi: 10.1016/j.bbapap.2014.03.001. [DOI] [PubMed] [Google Scholar]
- 81.Neuhof T., Dieckmann R., Druzhinina I.S., Kubicek C.P., Nakari-Setälä T., Penttilä M., von Döhren H. Direct identification of hydrophobins and their processing in Trichoderma using intact-cell MALDI-TOF MS. FEBS J. 2007;274:841–852. doi: 10.1111/j.1742-4658.2007.05636.x. [DOI] [PubMed] [Google Scholar]
- 82.Espino-Rammer L., Ribitsch D., Przylucka A., Marold A., Greimel K.J., Acero E.H., Guebitz G.M., Kubicek C.P., Druzhinina I.S. Two Novel Class II Hydrophobins from Trichoderma spp. Stimulate Enzymatic Hydrolysis of Poly(Ethylene Terephthalate) when Expressed as Fusion Proteins. Appl. Environ. Microbiol. 2013;79:4230–4238. doi: 10.1128/AEM.01132-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Klimes A., Dobinson K.F. A hydrophobin gene, VDH1, is involved in microsclerotial development and spore viability in the plant pathogen Verticillium dahliae. Fungal Genet. Biol. 2006;43:283–294. doi: 10.1016/j.fgb.2005.12.006. [DOI] [PubMed] [Google Scholar]
- 84.Ren Q., Kwan A.H., Sunde M. Solution structure and interface-driven self-assembly of NC2, a new member of the Class II hydrophobin proteins. Proteins Struct. Funct. Bioinform. 2013;82:990–1003. doi: 10.1002/prot.24473. [DOI] [PubMed] [Google Scholar]
- 85.Hakanpää J., Szilvay G., Kaljunen H., Maksimainen M., Linder M., Rouvinen J. Two crystal structures of Trichoderma reesei hydrophobin HFBI--The structure of a protein amphiphile with and without detergent interaction. Protein Sci. 2006;15:2129–2140. doi: 10.1110/ps.062326706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Minenko E., Vogel R.F., Niessen L. Significance of the class II hydrophobin FgHyd5p for the life cycle of Fusarium graminearum. Fungal Biol. 2014;118:385–393. doi: 10.1016/j.funbio.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 87.Linder M.B., Szilvay G., Nakari-Setälä T., Penttilä M.E. Hydrophobins: The protein-amphiphiles of filamentous fungi. FEMS Microbiol. Rev. 2005;29:877–896. doi: 10.1016/j.femsre.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 88.Hektor H.J., Scholtmeijer K. Hydrophobins: Proteins with potential. Curr. Opin. Biotechnol. 2005;16:434–439. doi: 10.1016/j.copbio.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 89.Scholtmeijer K., Wessels J.G.H., Wösten H.A.B. Fungal hydrophobins in medical and technical applications. Appl. Microbiol. Biotechnol. 2001;56:1–8. doi: 10.1007/s002530100632. [DOI] [PubMed] [Google Scholar]
- 90.Basheva E.S., Kralchevsky P.A., Danov K.D., Stoyanov S.D., Blijdenstein T.B.J., Pelan E.G., Lips A. Self-Assembled Bilayers from the Protein HFBII Hydrophobin: Nature of the Adhesion Energy. Langmuir. 2011;27:4481–4488. doi: 10.1021/la2001943. [DOI] [PubMed] [Google Scholar]
- 91.Magarkar A., Mele N., Abdel-Rahman N., Butcher S., Torkkeli M., Serimaa R., Paananen A., Linder M., Bunker A. Hydrophobin Film Structure for HFBI and HFBII and Mechanism for Accelerated Film Formation. PLOS Comput. Biol. 2014;10:e1003745. doi: 10.1371/journal.pcbi.1003745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu H., Yang S., Chen Z., Xu Z., Quan X., Zhou J. Orientation and Conformation of Hydrophobin at the Oil–Water Interface: Insights from Molecular Dynamics Simulations. Langmuir. 2022;38:6191–6200. doi: 10.1021/acs.langmuir.2c00614. [DOI] [PubMed] [Google Scholar]
- 93.Littlejohn K.A., Hooley P., Cox P. Bioinformatics predicts diverse Aspergillus hydrophobins with novel properties. Food Hydrocoll. 2012;27:503–516. doi: 10.1016/j.foodhyd.2011.08.018. [DOI] [Google Scholar]
- 94.Seidl-Seiboth V., Gruber S., Sezerman U., Schwecke T., Albayrak A., Neuhof T., Von Doehren H., Baker S., Kubicek C.P. Novel Hydrophobins from Trichoderma Define a New Hydrophobin Subclass: Protein Properties, Evolution, Regulation and Processing. J. Mol. Evol. 2011;72:339–351. doi: 10.1007/s00239-011-9438-3. [DOI] [PubMed] [Google Scholar]
- 95.Beauvais A., Schmidt C., Guadagnini S., Roux P., Perret E., Henry C., Paris S., Mallet A., Prévost M.-C., Latgé J.P. An extracellular matrix glues together the aerial-grown hyphae of Aspergillus fumigatus. Cell. Microbiol. 2007;9:1588–1600. doi: 10.1111/j.1462-5822.2007.00895.x. [DOI] [PubMed] [Google Scholar]
- 96.Laaksonen P., Kainlauri M., Laaksonen T., Shchepetov A., Jiang H., Ahopelto J., Linder M.B. Interfacial Engineering by Proteins: Exfoliation and Functionalization of Graphene by Hydrophobins. Angew. Chem. Int. Ed. 2010;49:4946–4949. doi: 10.1002/anie.201001806. [DOI] [PubMed] [Google Scholar]
- 97.Wang X., Wang H., Huang Y., Zhao Z., Qin X., Wang Y., Miao Z., Chen Q., Qiao M. Noncovalently functionalized multi-wall carbon nanotubes in aqueous solution using the hydrophobin HFBI and their electroanalytical application. Biosens. Bioelectron. 2010;26:1104–1108. doi: 10.1016/j.bios.2010.08.024. [DOI] [PubMed] [Google Scholar]
- 98.Wang Z., Huang Y., Li S., Xu H., Linder M.B., Qiao M. Hydrophilic modification of polystyrene with hydrophobin for time-resolved immunofluorometric assay. Biosens. Bioelectron. 2010;26:1074–1079. doi: 10.1016/j.bios.2010.08.059. [DOI] [PubMed] [Google Scholar]
- 99.Heinonen H., Laaksonen P., Linder M.B., Hentze H.-P. Engineered Hydrophobin for Biomimetic Mineralization of Functional Calcium Carbonate Microparticles. J. Biomater. Nanobiotechnology. 2014;05:1–7. doi: 10.4236/jbnb.2014.51001. [DOI] [Google Scholar]
- 100.Sallada N., Li Y., Berger B., Lamm M.S. Engineered Hydrophobin as a Crystallization Inhibitor for Flufenamic Acid. ACS Appl. Bio Mater. 2021;4:6441–6450. doi: 10.1021/acsabm.1c00612. [DOI] [PubMed] [Google Scholar]
- 101.Akanbi M.H.J., Post E., Meter-Arkema A., Rink R., Robillard G.T., Wang X., Wösten H.A., Scholtmeijer K. Use of hydrophobins in formulation of water insoluble drugs for oral administration. Colloids Surf. B Biointerfaces. 2010;75:526–531. doi: 10.1016/j.colsurfb.2009.09.030. [DOI] [PubMed] [Google Scholar]
- 102.Cox A.R., Aldred D.L., Russell A.B. Exceptional stability of food foams using class II hydrophobin HFBII. Food Hydrocoll. 2009;23:366–376. doi: 10.1016/j.foodhyd.2008.03.001. [DOI] [Google Scholar]
- 103.Tchuenbou-Magaia F.L., Norton I., Cox P. Hydrophobins stabilised air-filled emulsions for the food industry. Food Hydrocoll. 2009;23:1877–1885. doi: 10.1016/j.foodhyd.2009.03.005. [DOI] [Google Scholar]
- 104.Niu B., Wang D., Yang Y., Xu H., Qiao M. Heterologous expression and characterization of the hydrophobin HFBI in Pichia pastoris and evaluation of its contribution to the food industry. Amino Acids. 2011;43:763–771. doi: 10.1007/s00726-011-1126-5. [DOI] [PubMed] [Google Scholar]
- 105.Mokhtari-Abpangoui M., Lohrasbi-Nejad A., Zolala J., Torkzadeh-Mahani M., Ghanbari S. Improvement Thermal Stability of d-Lactate Dehydrogenase by Hydrophobin-1 and in Silico Prediction of Protein–Protein Interactions. Mol. Biotechnol. 2021;63:919–932. doi: 10.1007/s12033-021-00342-7. [DOI] [PubMed] [Google Scholar]
- 106.Lohrasbi-Nejad A., Torkzadeh-Mahani M., Hosseinkhani S. Hydrophobin-1 (HFB1) promotes thermostability of firefly luciferase. FEBS J. 2016;283:2494–2507. doi: 10.1111/febs.13757. [DOI] [PubMed] [Google Scholar]
- 107.Kurppa K., Hytönen V.P., Nakari-Setälä T., Kulomaa M.S., Linder M.B. Molecular engineering of avidin and hydrophobin for functional self-assembling interfaces. Colloids Surf. B Biointerfaces. 2014;120:102–109. doi: 10.1016/j.colsurfb.2014.05.010. [DOI] [PubMed] [Google Scholar]
- 108.Piscitelli A., Pennacchio A., Longobardi S., Velotta R., Giardina P. Vmh2 hydrophobin as a tool for the development of “self-immobilizing” enzymes for biosensing. Biotechnol. Bioeng. 2016;114:46–52. doi: 10.1002/bit.26049. [DOI] [PubMed] [Google Scholar]
- 109.Ahn S.-O., Lim H.-D., You S.-H., Cheong D.-E., Kim G.-J. Soluble Expression and Efficient Purification of Recombinant Class I Hydrophobin DewA. Int. J. Mol. Sci. 2021;22:7843. doi: 10.3390/ijms22157843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sorrentino I., Carrière M., Jamet H., Stanzione I., Piscitelli A., Giardina P., Le Goff A. The laccase mediator system at carbon nanotubes for anthracene oxidation and femtomolar electrochemical biosensing. Analyst. 2022;147:897–904. doi: 10.1039/d1an02091a. [DOI] [PubMed] [Google Scholar]
- 111.Fokina O., Fenchel A., Winandy L., Fischer R. Immobilization of LccC Laccase from Aspergillus nidulans on Hard Surfaces via Fungal Hydrophobins. Appl. Environ. Microbiol. 2016;82:6395–6402. doi: 10.1128/AEM.01413-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Puspitasari N., Lee C.-K. Class I hydrophobin fusion with cellulose binding domain for its soluble expression and facile purification. Int. J. Biol. Macromol. 2021;193:38–43. doi: 10.1016/j.ijbiomac.2021.10.089. [DOI] [PubMed] [Google Scholar]
- 113.Boeuf S., Throm T., Gutt B., Strunk T., Hoffmann M., Seebach E., Mühlberg L., Brocher J., Gotterbarm T., Wenzel W., et al. Engineering hydrophobin DewA to generate surfaces that enhance adhesion of human but not bacterial cells. Acta Biomater. 2012;8:1037–1047. doi: 10.1016/j.actbio.2011.11.022. [DOI] [PubMed] [Google Scholar]
- 114.Longobardi S., Gravagnuolo A.M., Rea I., De Stefano L., Marino G., Giardina P. Hydrophobin-coated plates as matrix-assisted laser desorption/ionization sample support for peptide/protein analysis. Anal. Biochem. 2014;449:9–16. doi: 10.1016/j.ab.2013.11.021. [DOI] [PubMed] [Google Scholar]
- 115.Weickert U., Wiesend F., Subkowski T., Eickhoff A., Reiss G. Optimizing biliary stent patency by coating with hydrophobin alone or hydrophobin and antibiotics or heparin: An in vitro proof of principle study. Adv. Med. Sci. 2011;56:138–144. doi: 10.2478/v10039-011-0026-y. [DOI] [PubMed] [Google Scholar]
- 116.Valo H.K., Laaksonen P., Peltonen L.J., Linder M.B., Hirvonen J.T., Laaksonen T.J. Multifunctional Hydrophobin: Toward Functional Coatings for Drug Nanoparticles. ACS Nano. 2010;4:1750–1758. doi: 10.1021/nn9017558. [DOI] [PubMed] [Google Scholar]
- 117.Sarparanta M., Bimbo L.M., Rytkönen J., Mäkilä E., Laaksonen T.J., Laaksonen P., Nyman M., Salonen J., Linder M.B., Hirvonen J., et al. Intravenous Delivery of Hydrophobin-Functionalized Porous Silicon Nanoparticles: Stability, Plasma Protein Adsorption and Biodistribution. Mol. Pharm. 2012;9:654–663. doi: 10.1021/mp200611d. [DOI] [PubMed] [Google Scholar]
- 118.Scholtmeijer K., Janssen M.I., Gerssen B., de Vocht M.L., van Leeuwen B.M., van Kooten T.G., Wösten H.A.B., Wessels J.G.H. Surface Modifications Created by Using Engineered Hydrophobins. Appl. Environ. Microbiol. 2002;68:1367–1373. doi: 10.1128/AEM.68.3.1367-1373.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Winandy L., Schlebusch O., Fischer R. Fungal hydrophobins render stones impermeable for water but keep them permeable for vapor. Sci. Rep. 2019;9:6264. doi: 10.1038/s41598-019-42705-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Qin M., Wang L.-K., Feng X.-Z., Yang Y.-L., Wang R., Wang C., Yu L., Bin Shao A., Qiao M.-Q. Bioactive Surface Modification of Mica and Poly(dimethylsiloxane) with Hydrophobins for Protein Immobilization. Langmuir. 2007;23:4465–4471. doi: 10.1021/la062744h. [DOI] [PubMed] [Google Scholar]
- 121.Linder M.B., Qiao M., Laumen F., Selber K., Hyytiä T., Nakari-Setälä T., Penttilä M.E. Efficient Purification of Recombinant Proteins Using Hydrophobins as Tags in Surfactant-Based Two-Phase Systems. Biochemistry. 2004;43:11873–11882. doi: 10.1021/bi0488202. [DOI] [PubMed] [Google Scholar]
- 122.Mohammed M.R.S., Balamurgan M.K., Amrathlal R.S., Kannan P., Jayapal J.M., Namperumalsamy V.P., Prajna L., Dharmalingam K. Dataset for the spore surface proteome and hydrophobin A/RodA proteoforms of A. flavus. Data Brief. 2019;23:103817. doi: 10.1016/j.dib.2019.103817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Ramirez-Garcia A., Pellon A., Buldain I., Antoran A., Arbizu-Delgado A., Guruceaga X., Rementeria A., Hernando F.L. Proteomics as a Tool to Identify New Targets Against Aspergillus and Scedosporium in the Context of Cystic Fibrosis. Mycopathologia. 2017;183:273–289. doi: 10.1007/s11046-017-0139-3. [DOI] [PubMed] [Google Scholar]
- 124.Yang K., Geng Q., Song F., He X., Hu T., Wang S., Tian J. Transcriptome Sequencing Revealed an Inhibitory Mechanism of Aspergillus flavus Asexual Development and Aflatoxin Metabolism by Soy-Fermenting Non-Aflatoxigenic Aspergillus. Int. J. Mol. Sci. 2020;21:6994. doi: 10.3390/ijms21196994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van Veluw G., Teertstra W., de Bekker C., Vinck A., van Beek N., Muller W., Arentshorst M., van der Mei H., Ram A., Dijksterhuis J., et al. Heterogeneity in liquid shaken cultures of Aspergillus niger inoculated with melanised conidia or conidia of pigmentation mutants. Stud. Mycol. 2013;74:47–57. doi: 10.3114/sim0008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Lee D.H., Kim H.W. Innate immunity induced by fungal β-glucans via dectin-1 signaling pathway. Int. J. Med. Mushrooms. 2014;16:1–16. doi: 10.1615/IntJMedMushr.v16.i1.10. [DOI] [PubMed] [Google Scholar]
- 127.Chaliha C., Rugen M.D., Field R.A., Kalita E. Glycans as Modulators of Plant Defense Against Filamentous Pathogens. Front. Plant Sci. 2018;9 doi: 10.3389/fpls.2018.00928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Carrion S.D.J., Leal S.M., Ghannoum M.A., Aimanianda V., Latgé J.-P., Pearlman E. The RodA Hydrophobin on Aspergillus fumigatus Spores Masks Dectin-1– and Dectin-2–Dependent Responses and Enhances Fungal Survival In Vivo. J. Immunol. 2013;191:2581–2588. doi: 10.4049/jimmunol.1300748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Granger B.L. Accessibility and contribution to glucan masking of natural and genetically tagged versions of yeast wall protein 1 of Candida albicans. PLoS ONE. 2018;13:e0191194. doi: 10.1371/journal.pone.0191194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hameed S., Hans S., Singh S., Dhiman R., Monasky R., Pandey R., Thangamani S., Fatima Z. Revisiting the Vital Drivers and Mechanisms of β-Glucan Masking in Human Fungal Pathogen, Candida albicans. Pathogens. 2021;10:942. doi: 10.3390/pathogens10080942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Valsecchi I., Stephen-Victor E., Wong S.S.W., Karnam A., Sunde M., Guijarro J.I., De Francisco B.R., Krüger T., Kniemeyer O., Brown G.D., et al. The Role of RodA-Conserved Cysteine Residues in the Aspergillus fumigatus Conidial Surface Organization. J. Fungi. 2020;6:151. doi: 10.3390/jof6030151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Kershaw M.J., Thornton C.R., Wakley G.E., Talbot N.J. Four conserved intramolecular disulphide linkages are required for secretion and cell wall localization of a hydrophobin during fungal morphogenesis. Mol. Microbiol. 2005;56:117–125. doi: 10.1111/j.1365-2958.2005.04547.x. [DOI] [PubMed] [Google Scholar]
- 133.de Vocht M.L., Reviakine I., Wösten H.A.B., Brisson A., Wessels J.G.H., Robillard G.T. Structural and Functional Role of the Disulfide Bridges in the Hydrophobin SC3. J. Biol. Chem. 2000;275:28428–28432. doi: 10.1074/jbc.M000691200. [DOI] [PubMed] [Google Scholar]
- 134.Gupta L., Sen P., Bhattacharya A.K., Vijayaraghavan P. Isoeugenol affects expression pattern of conidial hydrophobin gene RodA and transcriptional regulators MedA and SomA responsible for adherence and biofilm formation in Aspergillus fumigatus. Arch. Microbiol. 2022;204:214. doi: 10.1007/s00203-022-02817-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Breakspear A., Momany M. Aspergillus nidulans Conidiation Genes dewA, fluG, and stuA Are Differentially Regulated in Early Vegetative Growth. Eukaryot. Cell. 2007;6:1697–1700. doi: 10.1128/EC.00189-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Winandy L., Hilpert F., Schlebusch O., Fischer R. Comparative analysis of surface coating properties of five hydrophobins from Aspergillus nidulans and Trichoderma reesei. Sci. Rep. 2018;8:12033. doi: 10.1038/s41598-018-29749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Chang P.-K., Ehrlich K.C. What does genetic diversity of Aspergillus flavus tell us about Aspergillus oryzae? Int. J. Food Microbiol. 2010;138:189–199. doi: 10.1016/j.ijfoodmicro.2010.01.033. [DOI] [PubMed] [Google Scholar]
- 138.Watarai N., Yamamoto N., Sawada K., Yamada T. Evolution of Aspergillus oryzae before and after domestication inferred by large-scale comparative genomic analysis. DNA Res. 2019;26:465–472. doi: 10.1093/dnares/dsz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tanaka T., Tanabe H., Uehara K., Takahashi T., Abe K. Involvement of hydrophobic amino acid residues in C7–C8 loop of Aspergillus oryzae hydrophobin RolA in hydrophobic interaction between RolA and a polyester. Biosci. Biotechnol. Biochem. 2014;78:1693–1699. doi: 10.1080/09168451.2014.932684. [DOI] [PubMed] [Google Scholar]
- 140.Song D., Gao Z., Zhao L., Wang X., Xu H., Bai Y., Zhang X., Linder M.B., Feng H., Qiao M. High-yield fermentation and a novel heat-precipitation purification method for hydrophobin HGFI from Grifola frondosa in Pichia pastoris. Protein Expr. Purif. 2016;128:22–28. doi: 10.1016/j.pep.2016.07.014. [DOI] [PubMed] [Google Scholar]
- 141.Schmoll M., Seibel C., Kotlowski C., Vendt F.W.G., Liebmann B., Kubicek C.P. Recombinant production of an Aspergillus nidulans class I hydrophobin (DewA) in Hypocrea jecorina (Trichoderma reesei) is promoter-dependent. Appl. Microbiol. Biotechnol. 2010;88:95–103. doi: 10.1007/s00253-010-2710-4. [DOI] [PubMed] [Google Scholar]
- 142.Pedersen M.H., Borodina I., Moresco J.L., Svendsen W.E., Frisvad J.C., Søndergaard I. High-yield production of hydrophobins RodA and RodB from Aspergillus fumigatus in Pichia pastoris. Appl. Microbiol. Biotechnol. 2011;90:1923–1932. doi: 10.1007/s00253-011-3235-1. [DOI] [PubMed] [Google Scholar]
- 143.Morris V.K., Ren Q., Macindoe I., Kwan A.H., Byrne N., Sunde M. Recruitment of Class I Hydrophobins to the Air:Water Interface Initiates a Multi-step Process of Functional Amyloid Formation. J. Biol. Chem. 2011;286:15955–15963. doi: 10.1074/jbc.M110.214197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Terauchi Y., Nagayama M., Tanaka T., Tanabe H., Yoshimi A., Nanatani K., Yabu H., Arita T., Higuchi T., Kameda T., et al. Adsorption kinetics and self-assembled structures of Aspergillus oryzae hydrophobin RolA on hydrophobic and charged solid surfaces. Appl. Environ. Microbiol. 2022 doi: 10.1128/aem.02087-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yu L., Zhang B., Szilvay G.R., Sun R., Jänis J., Wang Z., Feng S., Xu H., Linder M.B., Qiao M. Protein HGFI from the edible mushroom Grifola frondosa is a novel 8 kDa class I hydrophobin that forms rodlets in compressed monolayers. Microbiology. 2008;154:1677–1685. doi: 10.1099/mic.0.2007/015263-0. [DOI] [PubMed] [Google Scholar]
- 146.Houmadi S., Rodriguez R.D., Longobardi S., Giardina P., Fauré M.C., Giocondo M., Lacaze E. Self-Assembly of Hydrophobin Protein Rodlets Studied with Atomic Force Spectroscopy in Dynamic Mode. Langmuir. 2012;28:2551–2557. doi: 10.1021/la2028093. [DOI] [PubMed] [Google Scholar]
- 147.Houmadi S., Ciuchi F., De Santo M.P., De Stefano L., Rea I., Giardina P., Armenante A., Lacaze E., Giocondo M. Langmuir−Blodgett Film of Hydrophobin Protein from Pleurotus ostreatus at the Air−Water Interface. Langmuir. 2008;24:12953–12957. doi: 10.1021/la802306r. [DOI] [PubMed] [Google Scholar]
- 148.Puspitasari N., Tsai S.-L., Lee C.-K. Fungal Hydrophobin RolA Enhanced PETase Hydrolysis of Polyethylene Terephthalate. Appl. Biochem. Biotechnol. 2020;193:1284–1295. doi: 10.1007/s12010-020-03358-y. [DOI] [PubMed] [Google Scholar]
- 149.Maeda H., Yamagata Y., Abe K., Hasegawa F., Machida M., Ishioka R., Gomi K., Nakajima T. Purification and characterization of a biodegradable plastic-degrading enzyme from Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2005;67:778–788. doi: 10.1007/s00253-004-1853-6. [DOI] [PubMed] [Google Scholar]
- 150.Purdy R.E., Kolattukudy P.E. Hydrolysis of plant cuticle by plant pathogens. Purification, amino acid composition, and molecular weight of two isoenzymes of cutinase and a nonspecific esterase from Fusarium solani f. pisi. Biochemistry. 1975;14:2824–2831. doi: 10.1021/bi00684a006. [DOI] [PubMed] [Google Scholar]
- 151.Sweigard J.A., Chumley F.G., Valent B. Cloning and analysis of CUT1, a cutinase gene from Magnaporthe grisea. Mol. Gen. Genet. 1992;232:174–182. doi: 10.1007/BF00279994. [DOI] [PubMed] [Google Scholar]
- 152.Fang X., Qiu F., Yan B., Wang H., Mort A.J., Stark R.E. NMR studies of molecular structure in fruit cuticle polyesters. Phytochemistry. 2001;57:1035–1042. doi: 10.1016/S0031-9422(01)00106-6. [DOI] [PubMed] [Google Scholar]
- 153.Kolattukudy P.E., Podila G.K., Mohan R. Molecular basis of the early events in plant–fungus interaction. Genome. 1989;31:342–349. doi: 10.1139/g89-052. [DOI] [Google Scholar]
- 154.St Leger R.J., Joshi L., Roberts D.W. Adaptation of proteases and carbohydrases of saprophytic, phytopathogenic and entomopathogenic fungi to the requirements of their ecological niches. Microbiology. 1997;143:1983–1992. doi: 10.1099/00221287-143-6-1983. [DOI] [PubMed] [Google Scholar]
- 155.Chen S., Su L., Chen J., Wu J. Cutinase: Characteristics, preparation, and application. Biotechnol. Adv. 2013;31:1754–1767. doi: 10.1016/j.biotechadv.2013.09.005. [DOI] [PubMed] [Google Scholar]
- 156.Ball S.R., Pham C.L.L., Lo V., Morris V.K., Kwan A.H., Sunde M. Formation of Amphipathic Amyloid Monolayers from Fungal Hydrophobin Proteins. Methods Mol. Biol. 2019;2073:55–72. doi: 10.1007/978-1-4939-9869-2_4. [DOI] [PubMed] [Google Scholar]
- 157.Fan C.-Y., Köller W. Diversity of cutinases from plant pathogenic fungi: Differential and sequential expression of cutinolytic esterases by Alternaria brassicicola. FEMS Microbiol. Lett. 1998;158:33–38. doi: 10.1111/j.1574-6968.1998.tb12796.x. [DOI] [Google Scholar]
- 158.Terauchi Y., Kim Y.-K., Tanaka T., Nanatani K., Takahashi T., Abe K. Asp30 of Aspergillus oryzae cutinase CutL1 is involved in the ionic interaction with fungal hydrophobin RolA. Biosci. Biotechnol. Biochem. 2017;81:1363–1368. doi: 10.1080/09168451.2017.1321952. [DOI] [PubMed] [Google Scholar]
- 159.Puspitasari N., Tsai S.-L., Lee C.-K. Class I hydrophobins pretreatment stimulates PETase for monomers recycling of waste PETs. Int. J. Biol. Macromol. 2021;176:157–164. doi: 10.1016/j.ijbiomac.2021.02.026. [DOI] [PubMed] [Google Scholar]
- 160.Yoshida S., Hiraga K., Takehana T., Taniguchi I., Yamaji H., Maeda Y., Toyohara K., Miyamoto K., Kimura Y., Oda K. A bacterium that degrades and assimilates poly(ethylene terephthalate) Science. 2016;351:1196–1199. doi: 10.1126/science.aad6359. [DOI] [PubMed] [Google Scholar]
- 161.Joo S., Lee S., Kim K. Structural insight into molecular mechanism of poly(ethylene terephthalate) degradation. FEBS J. 2017;284:309. doi: 10.1038/s41467-018-02881-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Liu Z., Gosser Y., Baker P.J., Ravee Y., Lu Z., Alemu G., Li H., Butterfoss G.L., Kong X.-P., Gross R., et al. Structural and Functional Studies of Aspergillus oryzae Cutinase: Enhanced Thermostability and Hydrolytic Activity of Synthetic Ester and Polyester Degradation. J. Am. Chem. Soc. 2009;131:15711–15716. doi: 10.1021/ja9046697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Castro-Ochoa L.D., Peña-Montes C., González-Canto A., Alva-Gasca A., Esquivel-Bautista R., Navarro-Ocaña A., Farrés A. ANCUT2, an Extracellular Cutinase from Aspergillus nidulans Induced by Olive Oil. Appl. Biochem. Biotechnol. 2012;166:1275–1290. doi: 10.1007/s12010-011-9513-7. [DOI] [PubMed] [Google Scholar]
- 164.Martins I., Hartmann O.D., Alves P.C., Martins C., Garcia H., Leclercq C.C., Ferreira R., He J., Renaut J., Becker J.D., et al. Elucidating how the saprophytic fungus Aspergillus nidulans uses the plant polyester suberin as carbon source. BMC Genom. 2014;15:613. doi: 10.1186/1471-2164-15-613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bermúdez-García E., Peña-Montes C., Martins I., Pais J., Pereira C.S., Sánchez S., Farrés A. Regulation of the cutinases expressed by Aspergillus nidulans and evaluation of their role in cutin degradation. Appl. Microbiol. Biotechnol. 2019;103:3863–3874. doi: 10.1007/s00253-019-09712-3. [DOI] [PubMed] [Google Scholar]
- 166.Rezende C.A., de Lima M.A., Maziero P., Deazevedo E.R., Garcia W., Polikarpov I. Chemical and morphological characterization of sugarcane bagasse submitted to a delignification process for enhanced enzymatic digestibility. Biotechnol. Biofuels. 2011;4:54. doi: 10.1186/1754-6834-4-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Qi G., Peng F., Xiong L., Lin X., Huang C., Li H., Chen X., Chen X. Extraction and characterization of wax from sugarcane bagasse and the enzymatic hydrolysis of dewaxed sugarcane bagasse. Prep. Biochem. Biotechnol. 2016;47:276–281. doi: 10.1080/10826068.2016.1224246. [DOI] [PubMed] [Google Scholar]
- 168.Tagu D., Nasse B., Martin F. Cloning and characterization of hydrophobins-encoding cDNAs from the ectomycorrhizal Basidiomycete Pisolithus tinctorius. Gene. 1996;168:93–97. doi: 10.1016/0378-1119(95)00725-3. [DOI] [PubMed] [Google Scholar]
- 169.Valette N., Benoit-Gelber I., Di Falco M., Wiebenga A., De Vries R.P., Gelhaye E., Morel-Rouhier M. Secretion of small proteins is species-specific within Aspergillus sp. Microb. Biotechnol. 2016;10:323–329. doi: 10.1111/1751-7915.12361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Zhao Z., Cai F., Gao R., Ding M., Jiang S., Chen P., Pang G., Chenthamara K., Shen Q., Akcapinar G.B., et al. At least three families of hyphosphere small secreted cysteine-rich proteins can optimize surface properties to a moderately hydrophilic state suitable for fungal attachment. Environ. Microbiol. 2021;23:5750–5768. doi: 10.1111/1462-2920.15413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Feldman D., Yarden O., Hadar Y. Seeking the Roles for Fungal Small-Secreted Proteins in Affecting Saprophytic Lifestyles. Front. Microbiol. 2020;11:455. doi: 10.3389/fmicb.2020.00455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Ohtaki S., Maeda H., Takahashi T., Yamagata Y., Hasegawa F., Gomi K., Nakajima T., Abe K. Novel Hydrophobic Surface Binding Protein, HsbA, Produced by Aspergillus oryzae. Appl. Environ. Microbiol. 2006;72:2407–2413. doi: 10.1128/AEM.72.4.2407-2413.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Garrido S.M., Kitamoto N., Watanabe A., Shintani T., Gomi K. Functional analysis of FarA transcription factor in the regulation of the genes encoding lipolytic enzymes and hydrophobic surface binding protein for the degradation of biodegradable plastics in Aspergillus oryzae. J. Biosci. Bioeng. 2012;113:549–555. doi: 10.1016/j.jbiosc.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 174.Maeda H., Sano M., Maruyama Y., Tanno T., Akao T., Totsuka Y., Endo M., Sakurada R., Yamagata Y., Machida M., et al. Transcriptional analysis of genes for energy catabolism and hydrolytic enzymes in the filamentous fungus Aspergillus oryzae using cDNA microarrays and expressed sequence tags. Appl. Microbiol. Biotechnol. 2004;65:74–83. doi: 10.1007/s00253-004-1608-4. [DOI] [PubMed] [Google Scholar]
- 175.de Wit P.J.G.M., Mehrabi R., van den Burg H.A., Stergiopoulos I. Fungal effector proteins: Past, present and future. Mol. Plant Pathol. 2009;10:735–747. doi: 10.1111/j.1364-3703.2009.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Dodds P.N., Rafiqi M., Gan P.H.P., Hardham A.R., Jones D., Ellis J.G. Effectors of biotrophic fungi and oomycetes: Pathogenicity factors and triggers of host resistance. N. Phytol. 2009;183:993–1000. doi: 10.1111/j.1469-8137.2009.02922.x. [DOI] [PubMed] [Google Scholar]
- 177.Koeck M., Hardham A.R., Dodds P.N. The role of effectors of biotrophic and hemibiotrophic fungi in infection. Cell. Microbiol. 2011;13:1849–1857. doi: 10.1111/j.1462-5822.2011.01665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Plett J.M., Kemppainen M., Kale S.D., Kohler A., Legué V., Brun A., Tyler B.M., Pardo A.G., Martin F. A Secreted Effector Protein of Laccaria bicolor Is Required for Symbiosis Development. Curr. Biol. 2011;21:1197–1203. doi: 10.1016/j.cub.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 179.Lo Presti L., Lanver D., Schweizer G., Tanaka S., Liang L., Tollot M., Zuccaro A., Reissmann S., Kahmann R. Fungal Effectors and Plant Susceptibility. Annu. Rev. Plant Biol. 2015;66:513–545. doi: 10.1146/annurev-arplant-043014-114623. [DOI] [PubMed] [Google Scholar]
- 180.Tanaka S., Kahmann R. Cell wall–associated effectors of plant-colonizing fungi. Mycologia. 2021;113:247–260. doi: 10.1080/00275514.2020.1831293. [DOI] [PubMed] [Google Scholar]
- 181.Fukada F., Rössel N., Münch K., Glatter T., Kahmann R. A small Ustilago maydis effector acts as a novel adhesin for hyphal aggregation in plant tumors. N. Phytol. 2021;231:416–431. doi: 10.1111/nph.17389. [DOI] [PubMed] [Google Scholar]
- 182.Saloheimo M., Paloheimo M., Hakola S., Pere J., Swanson B., Nyyssönen E., Bhatia A., Ward M., Penttilä M. Swollenin, a Trichoderma reesei protein with sequence similarity to the plant expansins, exhibits disruption activity on cellulosic materials. JBIC J. Biol. Inorg. Chem. 2002;269:4202–4211. doi: 10.1046/j.1432-1033.2002.03095.x. [DOI] [PubMed] [Google Scholar]
- 183.Guzmán-Guzmán P., Alemán-Duarte M.I., Delaye L., Herrera-Estrella A., Olmedo-Monfil V. Identification of effector-like proteins in Trichoderma spp. and role of a hydrophobin in the plant-fungus interaction and mycoparasitism. BMC Genet. 2017;18:16. doi: 10.1186/s12863-017-0481-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Carresi L., Pantera B., Zoppi C., Cappugi G., Oliveira A.L., Pertinhez T.A., Spisni A., Scala A., Pazzagli L. Cerato-platanin, a phytotoxic protein from Ceratocystis fimbriata: Expression in Pichia pastoris, purification and characterization. Protein Expr. Purif. 2006;49:159–167. doi: 10.1016/j.pep.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 185.Sbrana F., Bongini L., Cappugi G., Fanelli D., Guarino A., Pazzagli L., Scala A., Vassalli M., Zoppi C., Tiribilli B. Atomic force microscopy images suggest aggregation mechanism in cerato-platanin. Eur. Biophys. J. 2007;36:727–732. doi: 10.1007/s00249-007-0159-x. [DOI] [PubMed] [Google Scholar]
- 186.Pazzagli L., Cappugi G., Manao G., Camici G., Santini A., Scala A. Purification, Characterization, and Amino Acid Sequence of Cerato-platanin, a New Phytotoxic Protein from Ceratocystis fimbriata f. sp. platani. J. Biol. Chem. 1999;274:24959–24964. doi: 10.1074/jbc.274.35.24959. [DOI] [PubMed] [Google Scholar]
- 187.Frischmann A., Neudl S., Gaderer R., Bonazza K., Zach S., Gruber S., Spadiut O., Friedbacher G., Grothe H., Seidl-Seiboth V. Self-assembly at Air/Water Interfaces and Carbohydrate Binding Properties of the Small Secreted Protein EPL1 from the fungus Trichoderma atroviride. J. Biol. Chem. 2013;288:4278–4287. doi: 10.1074/jbc.M112.427633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gaderer R., Bonazza K., Seidl-Seiboth V. Cerato-platanins: A fungal protein family with intriguing properties and application potential. Appl. Microbiol. Biotechnol. 2014;98:4795–4803. doi: 10.1007/s00253-014-5690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Ebaccelli I. Cerato-platanin family proteins: One function for multiple biological roles? Front. Plant Sci. 2015;5:769. doi: 10.3389/fpls.2014.00769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Müller O., Schreier P.H., Uhrig J.F. Identification and characterization of secreted and pathogenesis-related proteins in Ustilago maydis. Mol. Genet. Genom. 2007;279:27–39. doi: 10.1007/s00438-007-0291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Casarrubia S., Daghino S., Kohler A., Morin E., Khouja H.-R., Daguerre Y., Veneault-Fourrey C., Martin F.M., Perotto S., Martino E. The Hydrophobin-Like OmSSP1 May Be an Effector in the Ericoid Mycorrhizal Symbiosis. Front. Plant Sci. 2018;9:546. doi: 10.3389/fpls.2018.00546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Shimizu M., Nakano Y., Hirabuchi A., Yoshino K., Kobayashi M., Yamamoto K., Terauchi R., Saitoh H. RNA-Seq of in planta -expressed Magnaporthe oryzae genes identifies MoSVP as a highly expressed gene required for pathogenicity at the initial stage of infection. Mol. Plant Pathol. 2019;20:1682–1695. doi: 10.1111/mpp.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mwape V.W., Mobegi F.M., Regmi R., Newman T.E., Kamphuis L.G., Derbyshire M.C. Analysis of differentially expressed Sclerotinia sclerotiorum genes during the interaction with moderately resistant and highly susceptible chickpea lines. BMC Genom. 2021;22:333. doi: 10.1186/s12864-021-07655-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Soanes D.M., Chakrabarti A., Paszkiewicz K.H., Dawe A.L., Talbot N.J. Genome-wide Transcriptional Profiling of Appressorium Development by the Rice Blast Fungus Magnaporthe oryzae. PLOS Pathog. 2012;8:e1002514. doi: 10.1371/journal.ppat.1002514. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.