Abstract
We report a 57-year-old man with recurrent meningoencephalitis resulting in bouts of altered consciousness, encephalopathy, tremors, focal seizures, and paraparesis. The neurological manifestations were accompanied by fever and leukocytosis in the absence of other systemic manifestations. MRI abnormalities of the brain, brainstem, spinal cord and meninges and CSF pleocytosis and elevated protein were observed. Exhaustive studies failed to reveal an etiology. Brain biopsy revealed nodules of neutrophils and macrophages, but no vasculitis. The lesions were not vasocentric as would be expected with neuro-Behcet’s disease and neuro-Sweet’s disease. The disorder was responsive to high-dose corticosteroid therapy and, ultimately, to anakinra, an IL-1α and IL-1β receptor antagonist.
Keywords: Meningoencephalitis, Neutrophils, Anakinra, CNS inflammation
Introduction
We report an unusual, recurrent, neutrophilic, multifocal inflammatory disease affecting the brain, spinal cord, and leptomeninges accompanied by recurrent fever and leukocytosis in the absence of any other systemic manifestations. Treatment with anakinra, a recombinant interleukin 1 receptor antagonist, appeared to be effective in suppressing recurrences.
Case report
In March 2019, a 57-year-old man became increasingly somnolent. Recent history was pertinent for conjunctivitis and an isolated tongue ulcer. On July 20, 2019, he was admitted to hospital with altered consciousness, inability to recognize family members, and an altered gait. An MRI of the brain (Fig. 1) showed diffuse poorly defined increased FLAIR/T2 signal abnormalities in brain and brainstem without restricted diffusion. CSF analysis (Table 1) showed 390 white blood cells (WBCs)/mL3 (46% lymphocytes), glucose 50 mg/dL, and protein 95 mg/dL. Broad-spectrum antibiotics were initiated. Examination revealed poor attention, orientation to person only, frontal release signs, extremity and head tremors, and diffuse hyperreflexia with normal strength, tone, and sensation. Extensive testing was non-diagnostic, including microbiological studies of blood and CSF; nextGen sequencing of CSF for pathogens; CT scan of chest, abdomen, and pelvis; CSF cytology and flow cytometry studies; paraneoplastic panel; test for anti-NMO and anti-MOG antibodies; serological studies for systemic rheumatic/autoimmune diseases; ophthalmological evaluation; and skin biopsy. Fevers to 39.4 °C were noted. Clinical and radiographic improvement followed treatment with 1000 mg of intravenous methylprednisolone followed by oral glucocorticoids.
Fig. 1.
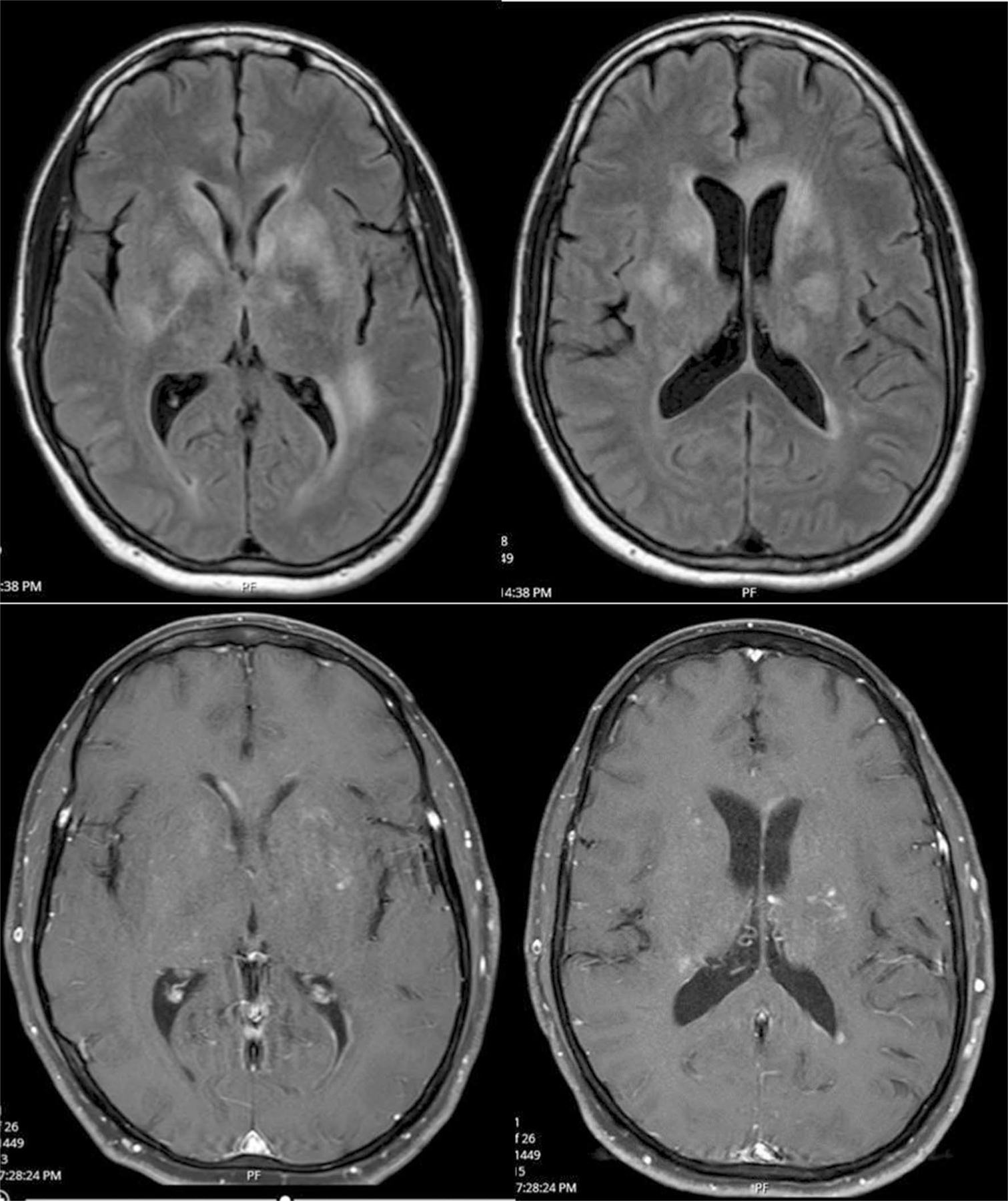
MRI of the brain from July 21, 2019. FLAIR (top) and contrast enhanced images (bottom) from July 22, 2019, showing multiple fluffy hyperintense T2 lesions with contrast enhancement. Lesions were observed in basal ganglia, left genu of the corpus callosum, around the frontal horns, atria of left lateral ventricle, thalami, and cerebral peduncles. There were no diffusion-restricted lesions. Slight enhancement was observed in some lesions as well as of the ependyma of the frontal horns of the lateral ventricles
Table 1.
Table of cerebrospinal fluid results
| 7/20/2019 | 7/24/2019 | 7/29/2019 | 8/9/2019 | 8/19/2019 | 10/23/2019 | 12/11/2019 | 7/27/2019 | |
|---|---|---|---|---|---|---|---|---|
| CSF WBC | 390 | 1300 | 159 | 1260 | 123 | 84 | 6 | 0 |
| CSF RBC | 0 | 0 | 20 | 0 | 28 | 199 | 1 | 4 |
| CSF Differential | 41N/46L/13M | 50N/37L/13M | 20N/70L/9M | 74N/15L/11M | 0N/97L/1M | 1N/84L/13M | 0N/84L/13M | |
| CSF Protein | 95 | 83 | 56 | 141 | 45 | 67 | 34 | 31 |
| CSF Glucose | 50 | 45 | 68 | 76 | 48 | 71 | 59 | |
| CSF OCB | 0 | 0 | 0 |
Readmission was prompted by episodes of speech arrest, worsening tremors, and an inability to ambulate. An EEG demonstrated bilateral intermittent rhythmic delta activity and multifocal epileptiform discharges responding to levetiracetam. MRIs of the cervical and thoracic spine (Fig. 2) revealed discontinuous T2 hyperintense lesions in cervical and thoracic cord with fusiform expansion of the cord and patchy leptomeningeal and intramedullary cord enhancement. Repeat CSF analysis showed 1563 WBCs/mL3 (74% neutrophils) and protein 141 mg/dL. Full-body PET/CT was unremarkable. A left frontal lobe biopsy performed on August 12, 2019 showed gliosis and rare lymphocytes. Clinical and radiographic improvement followed the administration of prednisone 80 mg daily and 5 courses of plasma exchange.
Fig. 2.
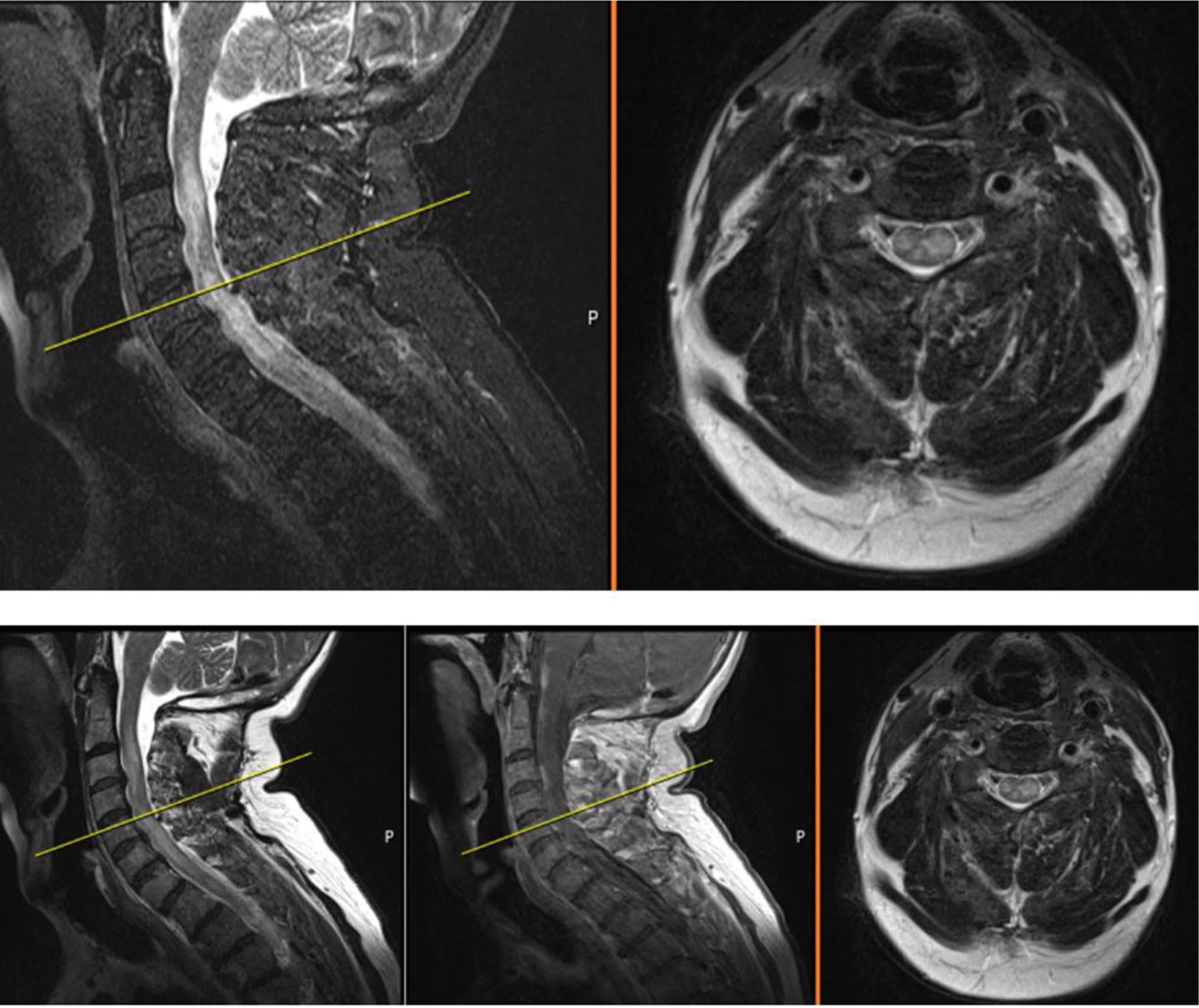
MRI of cervical spine from August 8, 2019. Sagittal T2 (left), sagittal contrast enhanced (center) and axial (T2) cervical spine MRI showing confluent signal hyperintensities within an expanded spinal cord with leptomeningeal enhancement
Following subsequent prednisone taper, neurological deterioration with fever and leukocytosis led to read-mission on October 21, 2019. High-dose intravenous methylprednisolone was reinitiated. A T10 vertebral body biopsy performed for a vertebral body abnormality on MRI was unremarkable. Repeat brain and meningeal biopsies from the left parietal lobe performed on November 7, 2019 revealed nodules of neutrophils and macrophages and reactive gliosis consistent with a predominantly neutrophilic inflammatory process (Fig. 3). There were no granulomas or vasculitis and no evidence of malignancy or infection despite exhaustive testing which included Broad Range Bacteria PCR sequencing (Mayo Clinic). A cytokine multiplex study of the CSF showed a low IL-2R of < 4 pg/mL (normal 95–851 pg/mL) though all other cytokines were normal. On March 18, 2020, anakinra 100 mg subcutaneously daily was initiated. Prednisone 60 mg/day was gradually tapered to 10 mg daily. Dapsone 25 mg twice daily was later added.
Fig. 3.
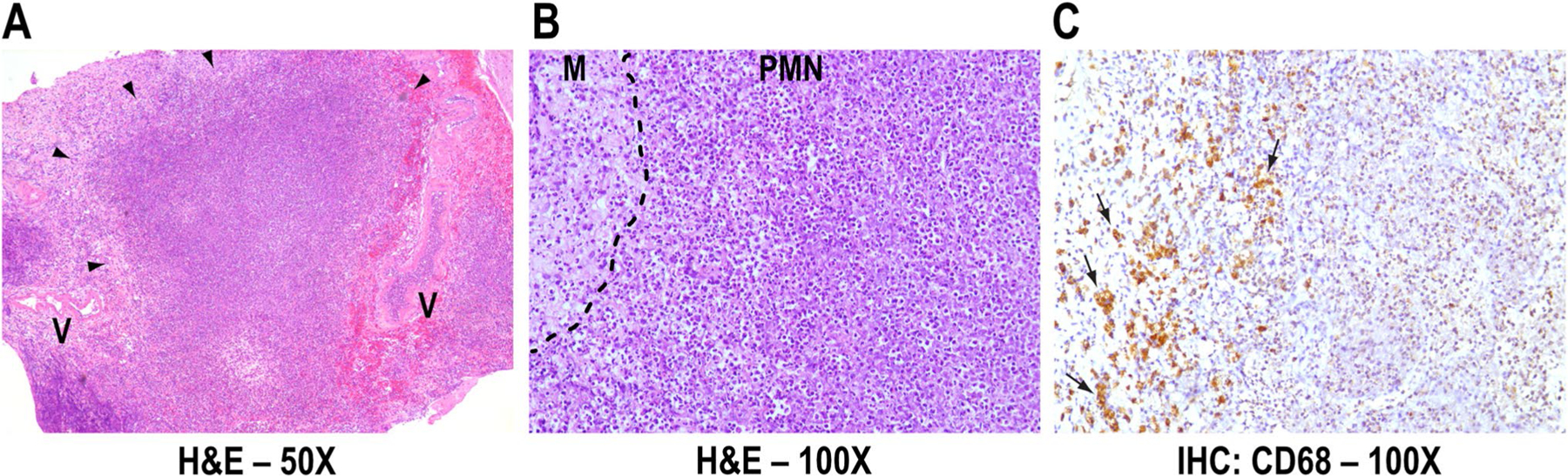
Neuropathology. Representative leptomeningeal inflammatory nodule (arrowheads) (a), composed of neutrophils/polymorphonu-clear leukocytes (PMN) surrounded by macrophages (M) (b), which express Cluster of Differentiation 68 glycoprotein (CD68, brown, arrows) (c). Vessel walls (V) are intact (a). H&E Hematoxylin and Eosin, IHC Immunohistochemistry, × magnification
Subsequent serial imaging studies of brain and spine showed significant improvement. By February 18, 2020, the brain showed no new enhancing or diffusion-restricted lesions. Cervical and thoracic spine imaging showed resolution of the spinal cord lesions. As of October 14, 2020, his cognitive function and language and speech were normal; head and bilateral upper extremity tremors were mild, and his gait and tandem were normal.
Discussion
We describe an unusual, recurrent, subacute neutrophilic disorder confined to the central nervous system. To the best of our knowledge, it matches no previously described disorder. Despite extensive studies, there was no evidence of infection, demyelination, granulomatous disease, or paraneoplastic or neoplastic disease.
CNS inflammatory diseases with neutrophil preponderance include neuro-Behçet’s Disease (NBD) and neuro-Sweet Disease (NSD). Other than isolated conjunctivitis and a tongue ulcer at presentation, our patient had no other manifestations to suggest either. Though “isolated neuro-Behcet’s disease” [1] has been reported that diagnosis was presumptive and established without pathological study. Our case does not meet the diagnostic criteria for Behcet’s disease [2]. He had neither recurrent oral or genital aphthous lesions nor uveitis. HLA typing revealed B35 and B50; neither strongly associated with Behcet’s disease. Diagnostic criteria for Sweet’s syndrome [3] were not met either and he had neither drug exposure nor underlying malignancy. Additionally, a vasculitic or vasocentric inflammatory component expected with both NBD and NSD [4, 5] was lacking.
The predominantly neutrophilic inflammatory response suggested that anakinra, an IL-1α and IL-1β receptor antagonist, may prove useful therapeutically. While approved for use in rheumatoid arthritis and neonatal-onset multisystem inflammatory disease, it appears to be useful for a variety of other inflammatory disorders [6–10]. To date, anakinra has been successful in suppressing the disorder.
Funding
There were no sources of funding for this case report.
Footnotes
Compliance with ethical standards
Conflicts of interest The authors report no conflicts of interest relevant to this manuscript.
Ethical statement The subject of this case report and his family have provided expressed written consent for its publication.
Informed consent The authors have obtained informed consent from the patient to describe his illness.
References
- 1.Saracino D, Allegorico L, Barbarulo AM, Pollo B, Giaccone G, D’Amico A, D’Incerti L, Bugiani O, Di Iorio G, Sampaolo S et al. (2018) Neuro-Behcet’s disease presenting as an isolated progressive cognitive and behavioral syndrome. Neurocase 24(5–6):238–241 [DOI] [PubMed] [Google Scholar]
- 2.International Team for the Revision of the International Criteria for Behcet’s D (2014) The International Criteria for Behcet’s Disease (ICBD): a collaborative study of 27 countries on the sensitivity and specificity of the new criteria. J Eur Acad Dermatol Venereol 28(3):338–347 [DOI] [PubMed] [Google Scholar]
- 3.von den Driesch P (1994) Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol 31(4):535–556 (quiz 557–560) [DOI] [PubMed] [Google Scholar]
- 4.Arai Y, Kohno S, Takahashi Y, Miyajima Y, Tsutusi Y (2006) Autopsy case of neuro-Behcet’s disease with multifocal neutrophilic perivascular inflammation. Neuropathology 26(6):579–585 [DOI] [PubMed] [Google Scholar]
- 5.Charlson R, Kister I, Kaminetzky D, Shvartsbeyn M, Meehan SA, Mikolaenko I (2015) CNS neutrophilic vasculitis in neuro-Sweet disease. Neurology 85(9):829–830 [DOI] [PubMed] [Google Scholar]
- 6.Bettiol A, Silvestri E, Di Scala G, Amedei A, Becatti M, Fiorillo C, Lopalco G, Salvarani C, Cantarini L, Soriano A et al. (2019) The right place of interleukin-1 inhibitors in the treatment of Behcet’s syndrome: a systematic review. Rheumatol Int 39(6):971–990 [DOI] [PubMed] [Google Scholar]
- 7.Giacomelli R, Sota J, Ruscitti P, Campochiaro C, Colafrancesco S, Dagna L, Iacono D, Iannone F, Lopalco G, Sfriso P et al. (2021) The treatment of adult-onset Still’s disease with anakinra, a recombinant human IL-1 receptor antagonist: a systematic review of literature. Clin Exp Rheumatol 39(1):187–195 [DOI] [PubMed] [Google Scholar]
- 8.Hennig S, Bayegan K, Uffmann M, Thalhammer F, Winkler S (2012) Pneumonia in a patient with familial Mediterranean fever successfully treated with anakinra—case report and review. Rheumatol Int 32(6):1801–1804 [DOI] [PubMed] [Google Scholar]
- 9.Kone-Paut I, Cimaz R, Herberg J, Bates O, Carbasse A, Saulnier JP, Maggio MC, Anton J, Piram M (2018) The use of interleukin 1 receptor antagonist (anakinra) in Kawasaki disease: a retrospective cases series. Autoimmun Rev 17(8):768–774 [DOI] [PubMed] [Google Scholar]
- 10.Swart JF, Barug D, Mohlmann M, Wulffraat NM (2010) The efficacy and safety of interleukin-1-receptor antagonist anakinra in the treatment of systemic juvenile idiopathic arthritis. Expert Opin Biol Ther 10(12):1743–1752 [DOI] [PubMed] [Google Scholar]


