Abstract
Introduction:
Body dysmorphic disorder (BDD) is severe, chronic, and undertreated. Apps could substantially improve treatment access.
Objective:
We provide an initial test of the usability and efficacy of coach-supported app-based cognitive behavioral therapy (CBT) for BDD. The Perspectives app covers core treatment components: psychoeducation, cognitive restructuring, exposure with response prevention, mindfulness, attention retraining, and relapse prevention.
Methods:
A randomized waitlist-controlled trial was conducted. Adults (N = 80) with primary BDD were assigned to 12 weeks of Perspectives or waitlist. Coaches promoted engagement and answered questions via in-app messaging and phone calls. BDD severity was measured at baseline, mid-treatment, and end of treatment by blinded independent evaluators (Yale-Brown Obsessive Compulsive Scale Modified for BDD; BDD-YBOCS). Secondary outcomes included BDD-related insight, depression, quality of life, and functioning.
Results:
App uptake and satisfaction were high. In intent-to-treat analyses, Perspectives app-based CBT was associated with significantly lower BDD-YBOCS severity at end of treatment (M [SD]: 16.8 [7.5]) compared to the waitlist (26.7 [6.2]; p < 0.001, d = 1.44). App-based CBT was associated with greater improvements across all secondary measures, with medium to large effects.
Conclusions:
Perspectives, supported by a bachelor’s-level coach, is an efficacious, scalable treatment for adults with BDD.
Keywords: Body dysmorphic disorder, Cognitive behavioral therapy, Smartphone, Digital health, Clinical trial
Introduction
Body dysmorphic disorder (BDD) is severe, prevalent, and chronic, and characterized by often debilitating preoccupations with perceived appearance flaws [1, 2]. Individuals engage in time-consuming compulsive behaviors, experience significant distress and impairment, and suffer from high incidence of comorbid depression and suicide risk [3, 4].
Fortunately, effective interventions exist. The gold standard is cognitive behavioral therapy (CBT) for BDD [5, 6]. Despite its strong evidence base, most patients do not receive CBT [7, 8]. Therapists familiar with BDD and trained to deliver CBT are limited [7]. Consequently, wait times are often long and treatment inaccessible to many. Access is further limited by barriers like cost, scheduling constraints, and shame [9, 10].
Digital therapies could reduce the access to care gap, as they are scalable and address the above barriers [11]. To date, one program has developed a therapist-guided computer-based CBT for BDD (BDD-NET) [12–14], demonstrating that digital CBT can be safe and effective for BDD. We built on this foundation by adapting treatment to be delivered via smartphone [15]. Apps allow users to flexibly learn and practice evidence-based skills on their own schedule.
In the current study, we tested the efficacy of Perspectives, the first and only app-based treatment for BDD [15]. A prior pilot of Perspectives with therapist support showed high feasibility and acceptability, with large declines in BDD severity at post-treatment and follow-up [15]. With scalability as a priority, we tested Perspectives using bachelor’s-level coaches in place of doctoral-level clinicians. [16, 17]. We hypothesized that adults with BDD would exhibit greater symptom reductions after 12 weeks of Perspectives compared to a waitlist. Secondary aims explored the impact of Perspectives on insight, depression, quality of life, and functioning.
Materials and Methods
This 12-week randomized controlled trial (RCT) compared guided app-based CBT (Perspectives) to a waitlist in a parallel group design (approved by Mass General Brigham IRB). Procedures were regularly reviewed by a data safety monitoring board and external regulatory monitors. Participants provided consent. Eligible participants were recruited nationally (07/2019–03/2021), at least 18 years old, living in the United States, and presenting with primary DSM-5 BDD. Participants taking psychotropic medications were on stable doses for at least 2 months prior to enrollment. See online supplementary materials for full eligibility criteria (for all online suppl. material, see www.karger.com/doi/10.1159/000524628).
Procedure
Random assignment to Perspectives or waitlist was stratified by medication status (see online supplementary materials). Figure 1 shows the flow of participants through the trial.
Fig. 1.
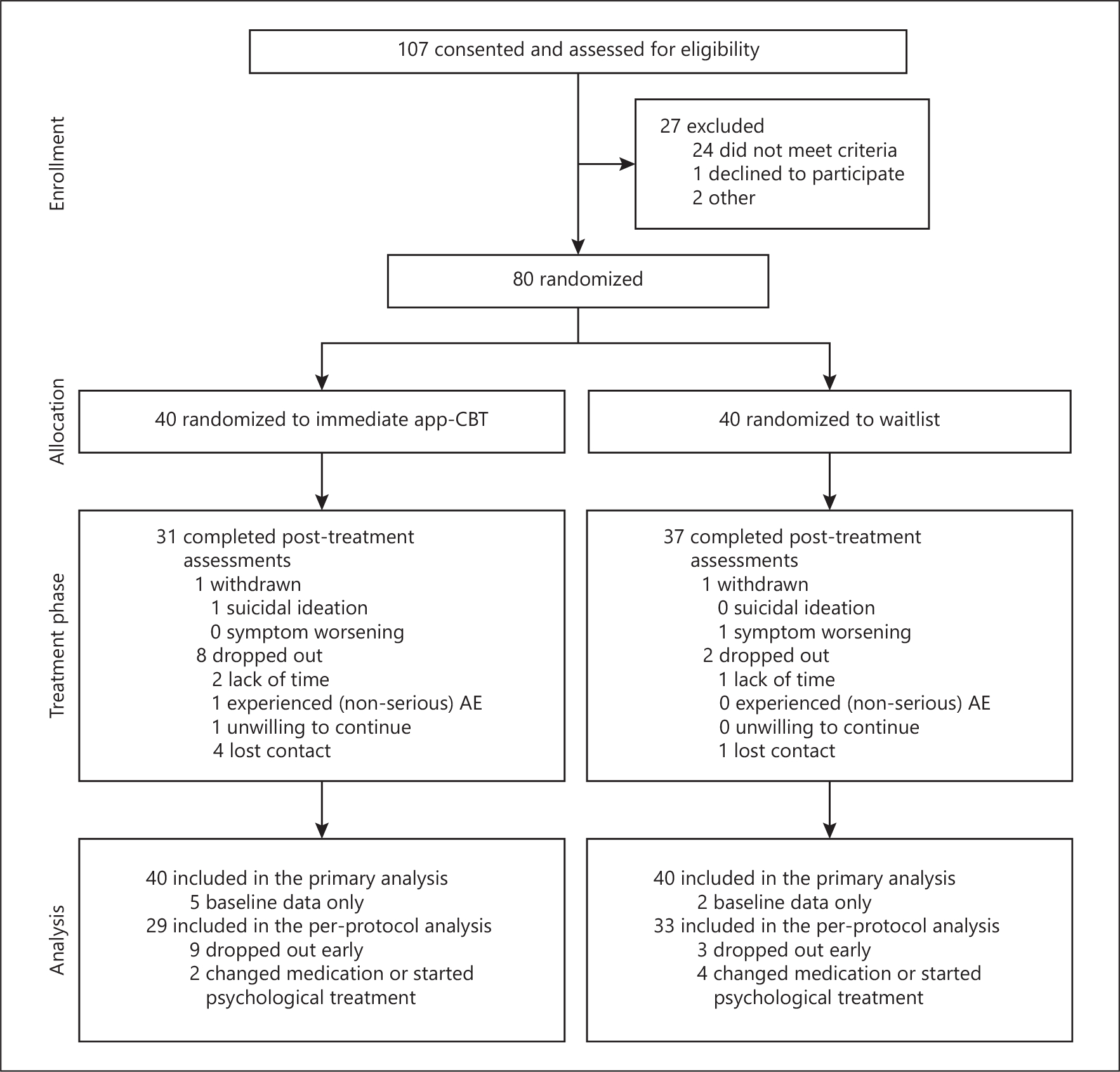
Flow of participants through the 12-week randomized-controlled trial of guided app-based CBT vs. a waitlist condition for people with a primary diagnosis of body dysmorphic disorder.
Treatment
Perspectives provides CBT over 12 weeks, an acceptable and effective duration for in-person, guided computer-delivered, and guided app-based CBT for BDD [12, 15, 18]. For details of app development and beta testing see [15]. Treatment comprises core CBT for BDD modules (for detailed descriptions, see [19–21]) in the following order: (1) psychoeducation; (2) restructuring maladaptive thoughts; (3) exposure (tailored to participant’s goals); (4) response prevention; (5) mindfulness and attentional retraining; (6) values and enhancing self-esteem and self-compassion; (7) relapse prevention.
Coaches.
Each participant was assigned a bachelor’s-level coach. Coaches enhanced motivation and engagement through brief onboarding and mid-treatment calls and asynchronous in-app messaging, providing feedback, recommendations, encouragement, and support for goal setting and personalizing skills. Coaches followed a manual and were supervised by a licensed clinician.
Measures
Assessments occurred at baseline, mid-treatment (week 6), and end of treatment (week 12). Clinician-administered measures were completed via video conference by blinded doctoral-level independent evaluators and included: Mini International Neuropsychiatric Interview (MINI 7.02) [22], Columbia-Suicide Severity Rating Scale (C-SSRS) [23], Yale-Brown Obsessive Compulsive Scale Modified for BDD (BDD-YBOCS) [24], Brown Assessment of Beliefs Scale (BABS) [25], and Clinical Global Impression – Improvement Scale (CGI-I) for BDD and global symptoms [26]. Self-report measures included Quick Inventory of Depressive Symptomatology – Self Report (QIDS-SR) [27], Quality of Life, Enjoyment, and Satisfaction Questionnaire – Short Form (Q-LES-Q-SF) [28], Sheehan Disability Scale (SDS) [29], and CGI-I for BDD [26]. Uptake and satisfaction (Perspectives only) were assessed at mid- and post-treatment using the Client Satisfaction Questionnaire (CSQ) [30]. We measured treatment utilization as time spent on the app and self-report estimates of time practicing treatment skills both on and off the app. See online supplementary materials for details.
Data Analysis
Power Analysis
Pre-COVID-19, the study was powered a priori to detect a BDD-YBOCS group difference of effect size d = 0.90 by week 12, assuming two-sided α = 0.05, 90% power, equal group allocation, 15% dropout, and a sample size of 64 [6, 12, 19]. We increased the sample size to 80 on 12/11/2020 to reflect a 10% decrease in the anticipated effect size from 0.90 to 0.81 due to COVID-19 stressors. Fifty-six participants (70%; n = 28 per group) were subsequently enrolled (after 3/16/2020).
Analyses used our intent-to-treat sample (participants who completed a baseline assessment, n = 80). We repeated the primary outcome analysis with a “per protocol” sample (participants who completed post-treatment assessments and did not initiate prohibited treatment, n = 62; see online supplementary materials).
Primary Analyses
The primary endpoint was intent-to-treat analysis of end of treatment (week 12) difference in BDD-YBOCS scores between treatment and waitlist groups. We computed a hierarchical mixed model (i.e., GLMM) that included time (baseline, mid-point, end of treatment), group, and their interaction as fixed effects, and modeled time as a repeated measure using either an autoregressive (AR1), Toeplitz, compound symmetry, or unstructured covariance matrix, based on best fit determined by AIC and BIC. The main hypothesis test was based on a specific contrast of treatment difference at week 12. Between-group effect sizes were calculated using Cohen’s d. We also conducted a per-protocol analysis and four sensitivity analyses to examine the robustness and validity of findings (see online supplementary materials).
Secondary Outcomes, Dropout, Engagement, and Satisfaction
We used the mixed modeling approach described above for secondary outcomes (BABS, QIDS-SR, SDS, Q-LES-Q). We summarized the percentage of participants in each arm who achieved response (≥30% reduction in BDD-YBOCS score) and remission (BDD-YBOCS score ≤16) at end of treatment [24, 31]. We examined associations between baseline characteristics and dropout (not completing endpoint BDD-YBOCS) using logistic regressions (see online supplementary materials). To quantify treatment engagement, we calculated the mean number of minutes coaches spent on phone calls and chat messages, minutes spent using the app, and self-reported time spent practicing skills. Satisfaction was estimated with the CSQ.
Results
Baseline Characteristics
Participants (n = 80) were recruited from 25 states in the U.S. and predominantly female (67 [84%]), non-Hispanic (70 [88%]), and white (57 [71%]), with a mean age of 27 (SD = 9.6). Table 1 summarizes demographic and clinical characteristics.
Table 1.
Demographic and clinical characteristics of the randomized participants
| Variable | Immediate CBT (n = 40) | Waitlist (n = 40) |
|---|---|---|
|
| ||
| Demographics | ||
| Age, mean (SD) | 27.8 (9.9) | 26.2 (9.5) |
| Sex at birth | ||
| Female | 92.5 (37) | 75.0 (30) |
| Male | 7.5 (3) | 25.0 (10) |
| Gender identity | ||
| Female | 90.0 (36) | 75.0 (30) |
| Male | 7.5 (3) | 25.0 (10) |
| Genderqueer or non-binary | 2.5 (1) | 0.0 (0) |
| Hispanic ethnicitya | 7.5 (3) | 15.0 (6) |
| Race | ||
| White | 82.5 (33) | 60.0 (24) |
| Black | 0.0 (0) | 5.0 (2) |
| Asian/Pacific Islander | 10.0 (4) | 20.0 (8) |
| Other | 7.5 (3) | 15.0 (6) |
| Education | ||
| ≤High school graduate | 20.0 (8) | 15.0 (6) |
| Technical school/some college | 27.5 (11) | 27.5 (11) |
| College graduate | 27.5 (11) | 27.5 (11) |
| Graduate or professional school | 25.0 (10) | 30.0 (12) |
| Marital status | ||
| Single, never married | 75.0 (30) | 70.0 (28) |
| Married | 17.5 (7) | 17.5 (7) |
| Living with partner | 5.0 (2) | 5.0 (2) |
| Divorced/separated | 2.5 (1) | 7.5 (3) |
| Employment | ||
| Full-time (≥35 h/week) | 45.0 (18) | 32.5 (13) |
| Part-time (<35 h/week) | 12.5 (5) | 5.0 (2) |
| Student | 30.0 (12) | 60.0 (24) |
| Unemployed | 5.0 (2) | 0.0 (0) |
| Homemaker | 7.5 (3) | 2.5 (1) |
|
| ||
| Clinical characteristics | ||
| Duration of BDD, years, mean (SD) | 14.0 (9.9) | 12.6 (11.6) |
| Number of body parts of concernc, mean (SD) | 11.2 (6.3) | 11.1 (6.4) |
| Delusional BDD | 10.0 (4) | 10.0 (4) |
| Primary body part of concernd | ||
| Face | 47.5 (19) | 30.0 (12) |
| Skin | 37.5 (15) | 25.0 (10) |
| Nose | 22.5 (9) | 37.5 (15) |
| Body build | 22.5 (9) | 15.0 (6) |
| Hair | 17.5 (7) | 15.0 (6) |
| Jaw | 12.5 (5) | 20.0 (8) |
| Legs | 15.0 (6) | 7.5 (3) |
| Weight | 12.5 (5) | 10.0 (4) |
| Eyes | 12.5 (5) | 7.5 (3) |
| Breasts | 15.0 (6) | 5.0 (2) |
| Cheeks | 5.0 (2) | 12.5 (5) |
| Butt | 10.0 (4) | 7.5 (3) |
| Head | 5.0 (2) | 10.0 (4) |
| Teeth | 10.0 (4) | 2.5 (1) |
| Current psychiatric comorbidities (DSM-5)d | ||
| Social anxiety disorder | 37.5 (15) | 22.5 (9) |
| Major depressive disorder/episode | 27.5 (11) | 25.0 (10) |
| Generalized anxiety disorder | 22.5 (9) | 22.5 (9) |
| Obsessive compulsive disorder | 10.0 (4) | 12.5 (5) |
| Agoraphobia | 10.0 (4) | 10.0 (4) |
| Post-traumatic stress disorder | 2.5 (1) | 10.0 (4) |
| Other | 12.5 (5) | 12.5 (5) |
| Number of comorbid psychiatric disorders | ||
| None | 32.5 (13) | 35.0 (14) |
| 1 | 35.0 (14) | 35.0 (14) |
| 2 | 20.0 (8) | 20.0 (8) |
| 3+ | 12.5 (5) | 10.0 (4) |
| Current psychotropic medicationd | ||
| None | 72.5 (29) | 62.5 (25) |
| Serotonin reuptake inhibitor | 22.5 (9) | 27.5 (11) |
| Non-SRI antidepressant | 5.0 (2) | 5.0 (2) |
| Antipsychotic | 2.5 (1) | 0.0 (0) |
| Other psychotropic medicationb | 10.0 (4) | 20.0 (8) |
Data are presented as % (n), except where indicated as mean (SD).
n = 1 missing (white race; ethnicity assumed to be non-Hispanic for calculations).
Includes benzodiazepines; anticonvulsants not included.
Body parts of concern include all parts of concern, not just body parts of primary concern.
Percentage sums may exceed 100% as participants could report more than one body part of primary concern, more than one diagnosis, or be on more than one stable psychotropic medication.
Primary Outcome
In the primary intent-to-treat analysis, BDD severity (BDD-YBOCS) at end of treatment was significantly lower in Perspectives compared to the waitlist (Table 2), with a large effect (p < 0.001, d = 1.44). This difference was also detectable in the per-protocol sample (n = 62; d = 1.49) and all sensitivity analyses (d range: 0.74 to 1.09; see online supplementary materials). Response rates for assessment completers at end of treatment were 68% (21/31) in Perspectives and 14% (5/37) in the waitlist. Full or partial remission was observed in 52% (16/31) in Perspectives and 8% (3/37) in the waitlist.
Table 2.
Symptom severity, quality of life, and functioning over time by treatment group in the intent-to-treat sample (n = 80)
| Outcome measure | App-based CBT (n = 40) |
Waitlist (n = 40) |
Est. mean group difference M [95% CI] | p | Effect size (group difference) d [95% CI] | ||
|---|---|---|---|---|---|---|---|
| M (SD) | n | M (SD) | n | ||||
|
| |||||||
| BDD-YBOCS | |||||||
| Baseline | 29.9 (4.0) | 40 | 30.9 (4.8) | 40 | – | – | – |
| Week 6 | 23.8 (6.9) | 35 | 27.1 (6.0) | 38 | – | – | – |
| Week 12 | 16.8 (7.5) | 31 | 26.7 (6.2) | 37 | −10.1 [−13.3, −7.0] | <0.001 | −1.44 [−1.99, −0.91] |
| Within-group effect size | −2.26 [−2.93, −1.58] | −0.75 [−1.03, −0.47] | |||||
| BABS | |||||||
| Baseline | 15.1 (3.2) | 40 | 14.5 (3.4) | 40 | – | – | – |
| Week 6 | 12.3 (5.2) | 35 | 13.5 (4.8) | 38 | – | – | – |
| Week 12 | 8.3 (5.0) | 30 | 13.2 (4.9) | 37 | −4.8 [−7.1, −2.6] | <0.001 | −1.00 [−1.51, −0.48] |
| Within-group effect size | −1.67 [−2.18, −1.15] | −0.29 [−0.53, −0.06] | |||||
| QIDS-SR | |||||||
| Baseline | 11.1 (4.4) | 40 | 11.4 (4.0) | 40 | – | – | – |
| Week 6 | 10.1 (4.8) | 34 | 10.1 (4.7) | 36 | – | – | – |
| Week 12 | 7.1 (4.5) | 28 | 10.3 (4.2) | 36 | −3.3 [−5.3, −1.2] | 0.002 | −0.74 [−1.26, −0.23] |
| Within-group effect size | −0.91 [−1.39, −0.42] | −0.28 [−0.66, 0.11] | |||||
| SDS | |||||||
| Baseline | 16.0 (6.6) | 40 | 17.1 (6.8) | 40 | – | – | – |
| Week 6 | 11.1 (6.4) | 34 | 13.2 (7.2) | 37 | – | – | – |
| Week 12 | 7.6 (6.8) | 28 | 13.5 (7.3) | 37 | −5.9 [−9.2, −2.5] | <0.001 | −0.82 [−1.34, −0.31] |
| Within-group effect size | −1.25 [−1.78, −0.72] | −0.51 [−0.81, −0.21] | |||||
| Q-LES-Q-SF | |||||||
| Baseline | 52.7 (16.3) | 40 | 48.3 (10.2) | 40 | – | – | – |
| Week 6 | 54.0 (16.4) | 34 | 52.1 (12.2) | 37 | – | – | – |
| Week 12 | 66.5 (16.4) | 28 | 55.2 (12.4) | 37 | 11.8 [4.9, 18.6] | 0.001 | 0.79 [0.28, 1.30] |
| Within-group effect size | 0.84 [0.41, 1.27] | 0.66 [0.28, 0.93] | |||||
BDD-YBOCS, Yale-Brown Obsessive Compulsive Scale Modified for BDD; BABS, Brown Assessment of Beliefs Scale; QIDS-SR, Quick Inventory of Depressive Symptomatology – Self Report; SDS, Sheehan Disability Scale; Q-LES-Q-SF, Quality of Life, Enjoyment, and Satisfaction Questionnaire – Short Form. Within-group effect sizes were calculated as differences from pre- to post-treatment (baseline to week 12) divided by the standard deviations of raw scores and using raw means for completers only. Effect sizes for between-group differences at week 12 are Cohen’s d based on raw means. Estimated mean group differences and p values are model-based estimates from intent-to-treat analyses.
Secondary Outcomes
End of treatment group differences favoring Perspectives in the intent-to-treat sample were also present in insight (p < 0.001, d = 1.00), depressive symptoms (p = 0.002, d = 0.74), functional impairment (p < 0.001, d = 0.83), and quality of life (p = 0.001, d = 0.77) (Table 2).
Dropout was 23% (9/40) in Perspectives and 8% (3/40) in the waitlist (OR [95% CI]: 3.53 [0.79, 22.01], p = 0.11). No baseline demographic or clinical characteristics were associated with dropout (all ps > 0.05). In Perspectives, coaches spent a mean of 26.9 min (SD = 10.9) speaking on the phone per participant across 2.1 (SD = 0.8) phone calls, and 1.5 min (SD = 1.3) per participant per week via chat. Participants self-reported using the app or practicing skills a median 60 min per week (range: 2–420); 28% (SD = 16) was on the app. Participants spent an average 130.2 (SD = 77.2) min in the app on 30.4 (SD = 14.3) days. End-of treatment CSQ scores (25.9±5.5) suggest that Perspectives was satisfactory. Overall, 86% were very (14/28) or mostly (10/28) satisfied and 89% (25/28) would recommend Perspectives. Though promising, it is possible that some displeased participants did not complete the questionnaire and/or withdrew prematurely. Adverse events and concomitant treatments are described in online supplementary materials; there were no serious adverse events.
Discussion
Findings support that Perspectives, a guided app-based CBT for BDD, is an effective, satisfactory treatment for BDD. In a 12-week RCT, compared to a waitlist, adults using Perspectives with light coach support experienced greater improvements in BDD severity, insight, depression, quality of life, and functioning, with large effects.
Two-thirds (68%) of individuals receiving Perspectives were responders and over half exhibited symptom remission. This response rate exceeds two of three trials of 12-week psychologist-guided internet-based CBT (BDD-NET), which range from 47% to 54% [12, 13]; although not direct comparisons, these and the present trial had highly similar eligibility criteria, sample sizes, baseline severity, and demographic features. Response rate also exceeds past 12-week face-to-face CBT trials, which range from 40% to 54% [5, 18]. However, comparisons are cautious as some samples were more severe (e.g., prevalence of delusional BDD and unemployment) [18]. The only trials with higher response rates include a 24-week face-to-face trial (83.3%–84.6%) [6] and the small pilots of BDD-NET (82%) [14] and Perspectives (90%) [15].
Results are important as demand for psychotherapy far exceeds availability of clinicians [32]. Perspectives offers a scalable, accessible solution. Implementing app-based treatment – even with coach support – would reduce clinician time required from an estimated minimum 600 min per patient to under 60 min. Digital treatments are expected to reduce disparities across healthcare by mitigating practical and attitudinal barriers that disproportionately impact racial and ethnic minority patients [33]. Apps can be used regardless of location, scheduling constraints, or comfort with face-to-face interventions. Perspectives breaks content into small chunks intended to be accessed briefly, frequently, and throughout day-to-day life; this format may be preferable for some and enhance learning and generalization [34, 35].
Data also reveal that more work is needed. Although long waitlists or no treatment are the reality for many with BDD, this waitlist control design should be regarded as an initial indication that app-based CBT can be beneficial. However, positive expectations, contact with a coach, or other non-CBT elements could have contributed to outcomes. Thus, next steps include testing Perspectives against an active control, in real-world settings, and in more clinically (e.g., general appearance concerns or mild or subthreshold BDD symptoms) and demographically diverse samples [36, 37]. These steps are needed to more rigorously establish its efficacy and effectiveness. Follow-up adaptive or dismantling designs would allow us to determine the most potent and personalized elements for patients. Although many skills should generalize to other concerns, Perspectives does not explicitly address comorbidities (e.g., depression, substance use). Notably, participants in Perspectives did exhibit declines in depressive symptoms. It remains an empirical question whether such complexity requires more hands-on support.
Psychiatry is moving towards personalized, stepped care. Digital tools are critical, particularly for common and undertreated conditions like BDD. Apps can be widely and inexpensively disseminated, conserving expensive and limited clinician time for individuals unlikely to respond or not responding to digital approaches. Furthermore, developers can implement new tools and adapt content with up-to-date evidence quickly, outpacing dissemination of new research to individual clinicians. Perspectives offers an inimitable foundation in this effort to provide high-quality, evidence-based care for BDD.
Supplementary Material
Acknowledgments
The authors wish to thank Joshua Curtiss, PhD, Angela Fang, PhD, Ryan Jacoby, PhD, Berta Summers, PhD, Rachel Vanderkruik, PhD, Anne Chosak, PhD, Rebecca Berger-Gutierrez, BS, Clare Beatty, BA, Julia Carrellas, BA, Ilana Ladis, BA, Zoe Laky, BA, Sarah Miller, BA, Anna Schwartzberg, BA, Abigail Szkutak, BA, Emma Wolfe, BA, Margaret Hall, BA, and Barbara Rosemberg, MHA for performing study ratings and/or providing coaching and/or administrative support. The authors also wish to thank their Data and Safety Monitoring Board members: Drs. Stefan Hofmann, Jedidiah Siev, and Kiara Timpano.
Funding Sources
Research was supported by Koa Health (formerly Telefonica Alpha). Investigators from MGH (S.W., H.W., and J.L.G.) developed the Perspectives app in collaboration with technologists and designers from Koa Health via a user-centered, iterative design approach. The investigators from MGH were responsible for the study design and for the execution of the study; authors from Koa Health supported the technical activities related to the development and deployment of the app, and provided funding for the project. Koa Health had no role in the recruitment of participants and had no access to data during the trial. The sponsor (Koa Health) was able to make minor edits to the draft of the paper. Dr. Weingarden was supported in part by the National Institute Of Mental Health of the National Institutes of Health under Award Number K23MH119372 (Weingarden). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflict of Interest Statement
Dr. Wilhelm is a presenter for the Massachusetts General Hospital Psychiatry Academy in educational programs supported through independent medical education grants from pharmaceutical companies; she has received royalties from Elsevier Publications, Guilford Publications, New Harbinger Publications, Springer, and Oxford University Press. Dr. Wilhelm has also received speaking honoraria from various academic institutions and foundations, including the International Obsessive Compulsive Disorder Foundation, the Tourette Association of America and the Centers for Disease Control and Prevention. In addition, she received payment from the Association for Behavioral and Cognitive Therapies for her role as Associate Editor for the Behavior Therapy journal, as well as from John Wiley & Sons, Inc. for her role as Associate Editor on the journal Depression & Anxiety. Dr. Wilhelm has also received honoraria from One-Mind for her role in PsyberGuide Scientific Advisory Board. Dr. Wilhelm is also on the Scientific Advisory Board for Koa Health, Inc. and for Noom, Inc. Dr. Wilhelm has received research and salary support from Koa Health, Inc. Additionally, Dr. Wilhelm has a consulting agreement with Noom, Inc. Dr. Weingarden receives salary support from Koa Health (formerly Telefónica Alpha, Inc) and is a presenter for the Massachusetts General Hospital Psychiatry Academy in educational programs supported through independent medical education grants from pharmaceutical companies. Dr. Greenberg has received salary support from Koa Health (formerly Telefónica Alpha, Inc) and is a presenter for the Massachusetts General Hospital Psychiatry Academy in educational programs supported through independent medical education grants from pharmaceutical companies. Dr. Hoeppner has received salary support from Koa Health. Dr. Snorrason has received salary support from Koa Health. Dr. Bernstein has received salary support from Koa Health. Dr. McCoy has received research funding from the Brain and Behavior Research Foundation, National Institute of Mental Health, National Institute of Nursing Research, National Human Genome Research Institute, and Koa Health. Dr. Harrison is Founder/CEO of Koa Health, a digital mental health company that collaborated with Dr. Wilhelm and her team at MGH to build Perspectives. Dr Harrison also serves on the WHO Roster of Experts for Digital Health, sits on the Board of EMPOWER (a non-profit organization promoting the training of community health workers to provide mental healthcare), and is a member of the Expert Panel for implementing the Wellcome Trust’s mental health strategy.
Statement of Ethics
Research was conducted ethically in accordance with the World Medical Association Declaration of Helsinki. Participants provided electronic, written informed consent prior to the initiation of any study procedures. Study protocol was reviewed and approved by the Mass General Brigham Institutional Review Board, approval number 2017P000293.
Trial registration: ClinicalTrials.gov NCT04034693.
Data Availability Statement
The data that support the findings of this study are not publicly available due to their sensitive nature. Data sharing may be possible upon reasonable request to the corresponding author.
References
- 1.American Psychological Association. Diagnostic and statistical manual of mental disorders. 5th ed. Washington, DC: The Association; 2013. [Google Scholar]
- 2.Phillips KA, Menard W, Quinn E, Didie ER, Stout RL. A 4-year prospective observational follow-up study of course and predictors of course in body dysmorphic disorder. Psychol Med. 2013. May;43(5):1109–17. [DOI] [PubMed] [Google Scholar]
- 3.Frare F, Perugi G, Ruffolo G, Toni C. Obsessive–compulsive disorder and body dysmorphic disorder: a comparison of clinical features. Eur Psychiatry. 2004. Aug;19(5):292–8. [DOI] [PubMed] [Google Scholar]
- 4.Phillips KA, Menard W, Fay C, Pagano ME. Psychosocial functioning and quality of life in body dysmorphic disorder. Compr Psychiatry. 2005;46(4):254–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harrison A, Fernández de la Cruz L, Enander J, Radua J, Mataix-Cols D. Cognitive-behavioral therapy for body dysmorphic disorder: a systematic review and meta-analysis of randomized controlled trials. Clin Psychol Rev. 2016. Aug;48:43–51. [DOI] [PubMed] [Google Scholar]
- 6.Wilhelm S, Phillips KA, Greenberg JL, O’Keefe SM, Hoeppner SS, Keshaviah A, et al. Efficacy and posttreatment effects of therapist-delivered cognitive behavioral therapy vs supportive psychotherapy for adults with body dysmorphic disorder: a randomized clinical trial. JAMA Psychiatry. 2019. Apr;76(4):363–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marques L, Weingarden HM, LeBlanc NJ, Wilhelm S. Treatment utilization and barriers to treatment engagement among people with body dysmorphic symptoms. J Psychosom Res. 2011. Mar;70(3):286–93. [DOI] [PubMed] [Google Scholar]
- 8.Schulte J, Schulz C, Wilhelm S, Buhlmann U. Treatment utilization and treatment barriers in individuals with body dysmorphic disorder. BMC Psychiatry. 2020. Feb;20(1):69–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fang A, Matheny NL, Wilhelm S. Body dysmorphic disorder. Psychiatr Clin North Am. 2014. Sep;37(3):287–300. [DOI] [PubMed] [Google Scholar]
- 10.Weingarden H, Renshaw KD, Wilhelm S, Tangney JP, Dimauro J. Anxiety and shame as risk factors for depression, suicidality, and functional impairment in body dysmorphic disorder and obsessive compulsive disorder. J Nerv Ment Dis. 2016. Nov;204(11):832–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilhelm S, Weingarden H, Ladis I, Braddick V, Shin J, Jacobson NC. Cognitive-behavioral therapy in the digital age: presidential address. Behav Ther. 2020. Jan;51(1):1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enander J, Andersson E, Mataix-Cols D, Lichtenstein L, Alström K, Andersson G, et al. Therapist guided internet based cognitive behavioural therapy for body dysmorphic disorder: single blind randomised controlled trial. BMJ. 2016. Feb;4:352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gentile AJ, La Lima C, Flygare O, Enander J, Wilhelm S, Mataix-Cols D, et al. Internet-based, therapist-guided, cognitive-behavioural therapy for body dysmorphic disorder with global eligibility for inclusion: an uncontrolled pilot study. BMJ Open. 2019. Mar;9(3):e024693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enander J, Ivanov VZ, Andersson E, Mataix-Cols D, Ljótsson B, Rück C. Therapist-guided, Internet-based cognitive-behavioural therapy for body dysmorphic disorder (BDD-NET): a feasibility study. BMJ Open. 2014. Sep;4(9):e005923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilhelm S, Weingarden H, Greenberg JL, McCoy TH, Ladis I, Summers BJ, et al. Development and pilot testing of a cognitive-behavioral therapy digital service for body dysmorphic disorder. Behav Ther. 2020. Jan;51(1):15–26. [DOI] [PubMed] [Google Scholar]
- 16.Baumeister H, Reichler L, Munzinger M, Lin J. The impact of guidance on internet-based mental health interventions: a systematic review. Internet Interv. 2014;1(4):205–15. [Google Scholar]
- 17.Johnston L, Titov N, Andrews G, Spence J, Dear BF. A RCT of a transdiagnostic internet-delivered treatment for three anxiety disorders: examination of support roles and disorder-specific outcomes. JAMA Psychiatry. 2021;78:361–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Veale D, Anson M, Miles S, Pieta M, Costa A, Ellison N. Efficacy of cognitive behaviour therapy versus anxiety management for body dysmorphic disorder: a randomised controlled trial. Psychother Psychosom. 2014;83:341–53. [DOI] [PubMed] [Google Scholar]
- 19.Wilhelm S, Phillips KA, Didie E, Buhlmann U, Greenberg JL, Fama JM, et al. Modular cognitive-behavioral therapy for body dysmorphic disorder: a randomized controlled trial. Behav Ther. 2014. May;45(3):314–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wilhelm S, Phillips KA, Fama JM, Greenberg JL, Steketee G. Modular cognitive-behavioral therapy for body dysmorphic disorder. Behav Ther. 2011;42(4):624–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilhelm S, Phillips K, Steketee G. A cognitive behavioral treatment manual for body dysmorphic disorder. New York: Guilford Press; 2013. [Google Scholar]
- 22.Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(20):22–33. [PubMed] [Google Scholar]
- 23.Posner K, Brent D, Lucas C, Gould M, Stanley B, Brown G, et al. Columbia-suicide severity rating scale (C-SSRS). New York, NY: Columbia University Medical Center; 2008. p. 10. [Google Scholar]
- 24.Phillips KA, Hollander E, Rasmussen SA, Aronowitz BR, DeCaria C, Goodman WK. A severity rating scale for body dysmorphic disorder: development, reliability, and validity of a modified version of the yale-brown obsessive compulsive scale. Psychopharmacol Bull. 1997;33(1):17–22. [PubMed] [Google Scholar]
- 25.Eisen JL, Phillips KA, Baer L, Beer DA, Atala KD, Rasmussen SA. The Brown assessment of beliefs scale: reliability and validity. Am J Psychiatry. 1998. Jan;155(1):102–8. [DOI] [PubMed] [Google Scholar]
- 26.Guy W ECDEU assessment manual for psychopharmacology. US Department of Health, Education, and Welfare, Public Health Service, Alcohol, Drug Abuse, and Mental Health Administration, National Institute of Mental Health, Psychopharmacology Research Branch, Division of Extramural Research Programs; 1976. [Google Scholar]
- 27.Rush AJ, Trivedi MH, Ibrahim HM, Carmody TJ, Arnow B, Klein DN, et al. The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry. 2003. Sep;54(5):573–83. [DOI] [PubMed] [Google Scholar]
- 28.Endicott J, Nee J, Harrison W, Blumenthal R. Quality of life enjoyment and satisfaction questionnaire: a new measure. Psychopharmacol Bull. 1993;29(2):321–6. [PubMed] [Google Scholar]
- 29.Sheehan DV, Harnett-Sheehan K, Raj BA. The measurement of disability. Int Clin Psychopharmacol. 1996. Jun;11 Suppl 3:89–95. [DOI] [PubMed] [Google Scholar]
- 30.Attkisson C, Zwick R. The client satisfaction questionnaire: psychometric properties and correlations with service utilization and psychotherapy outcome. Eval Program Plann. 1982;5(3):233–7. [DOI] [PubMed] [Google Scholar]
- 31.de la Cruz LF, Enander J, Rück C, Wilhelm S, Phillips KA, Steketee G, et al. Empirically defining treatment response and remission in body dysmorphic disorder. Psychol Med. 2021. Jan;51(1):83–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cavanagh K Geographic inequity in the availability of cognitive behavioural therapy in England and Wales: a 10-year update. Behav Cogn Psychother. 2014. Jul;42(4):497–501. [DOI] [PubMed] [Google Scholar]
- 33.Ramos G, Chavira DA. Use of technology to provide mental health care for racial and ethnic minorities: evidence, promise, and challenges. Cogn Behav Pract. 2022;29(1):15–40. [Google Scholar]
- 34.Jost NS, Jossen SL, Rothen N, Martarelli CS. The advantage of distributed practice in a blended learning setting. Educ Inf Technol. 2021;26(3):3097–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weingarden H, Matic A, Calleja RG, Greenberg JL, Harrison O, Wilhelm S. Optimizing smartphone-delivered cognitive behavioral therapy for body dysmorphic disorder using passive smartphone data: initial insights from an open pilot trial. JMIR mHealth uHealth. 2020. Jun;8(6):e16350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Koran LM, Abujaoude E, Large MD, Serpe RT. The prevalence of body dysmorphic disorder in the United States adult population. CNS Spectr. 2008. Apr;13(4):316–22. [DOI] [PubMed] [Google Scholar]
- 37.Boroughs MS, Krawczyk R, Thompson JK. Body dysmorphic disorder among diverse racial/ethnic and sexual orientation groups: prevalence estimates and associated factors. Sex Roles. 2010;63(9–10):725–37. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are not publicly available due to their sensitive nature. Data sharing may be possible upon reasonable request to the corresponding author.


