Abstract
Abstract: In anticipation of a phylogenetically revised monograph of Entoloma in Europe, six new species of subgenus Cyanula are described here. Entoloma cistocruentatum is associated with Cistus in Spain, E. dislocatum occurs in montane regions in Catalonia (Spain) and Tuscany (Italy), E. indikon is known from Denmark and three species are mainly distributed in the Nordic countries in Europe: E. calceus, E. perchalybeum and E. praecipuum. Entoloma incarnatofuscescens, from the /Rusticoides clade is neotypified. A fully amended description is given based on molecular evidence, which includes the recently described E. violaceoparkensis and E. klofacianum which became later synonyms.
Citation: Noordeloos ME, Vila J, Jordal JB, Kehlet T, Brandrud TE, Bendiksen E, Moreau P-A, Dondl M, Lorås J, Larsson E, Dima B (2022). Contributions to the revision of the genus Entoloma (Basidiomycota, Agaricales) in Europe: six new species from subgenus Cyanula and typification of E. incarnatofuscescens. Fungal Systematics and Evolution 9: 87–97. doi: 10.3114/fuse.2022.09.06
Keywords: Entolomataceae, ITS barcode, new species, phylogeny, synonymy, taxonomy
INTRODUCTION
This study is part of a large-scale molecular phylogenetic and morphological revision of the /Cyanula clade of the genus Entoloma in Europe to be published in due course (Dima et al. in prep.) and a new, completely revisited monograph of all European species of the /Cyanula clade (Noordeloos in prep.). The /Cyanula clade is here defined in a wide sense, including all clampless, often vividly coloured species, formerly included in subgen. Leptonia but shown to be phylogenetically quite distant from the clamped Leptonia s. str. taxa (Morozova et al. 2014). The material in the present study comes from various sources. In the Nordic countries, much work has been done on Entoloma, in the framework of the Norwegian Entoloma project and studies of the alpine mycota in Sweden resulting in a constant flow of publications in recent years (Brandrud et al. 2018, 2019, Crous et al. 2021, Dima et al. 2021, Haelewaters et al. 2021, Noordeloos et al. 2018, 2020). Jordi Vila and collaborators studied the mycota of Spain, Catalonia (Caballero & Vila 2013, Vila & Caballero 2007, 2009, Vila & Llimona 2010, Vila et al. 2013, 2014, 2021) which yielded some of the proposed new taxa.
The phylogenetic position of the new species described here will be dealt with in depth in a forthcoming study based on a world-wide sampling of the subgenus Cyanula (Dima et al. in prep.).
MATERIAL AND METHODS
Morphology
All collections studied were photographed in the field and attention was paid in observing the surrounding vegetation and putative ecology for each collection based on above-ground observations. The material was described after collecting to document the ephemeral macroscopic characters (especially colours) and dried and stored in the respective fungaria. Microscopic characters were studied with standard light microscopy methods. Spores, basidia and cystidia were observed in squash preparations of small parts of the lamellae in 5 % KOH or 1 % Congo Red in concentrated NH4OH. The pileipellis was examined on a radial section of the pileus in water. Basidiospore dimensions are based on observing 40 spores in side view, while cystidia and basidia dimensions are based on observing at least 10 structures per collection. Basidia were measured excluding sterigmata and the spores excluding hilum. Spore length to width ratio is reported as ‘Q’ and average length to width ratio is reported as ‘Qav’. All studied material is stored in the herbaria of Oslo (O), Gothenburg (GB), or Leiden (L), unless otherwise indicated.
DNA extraction and sequencing
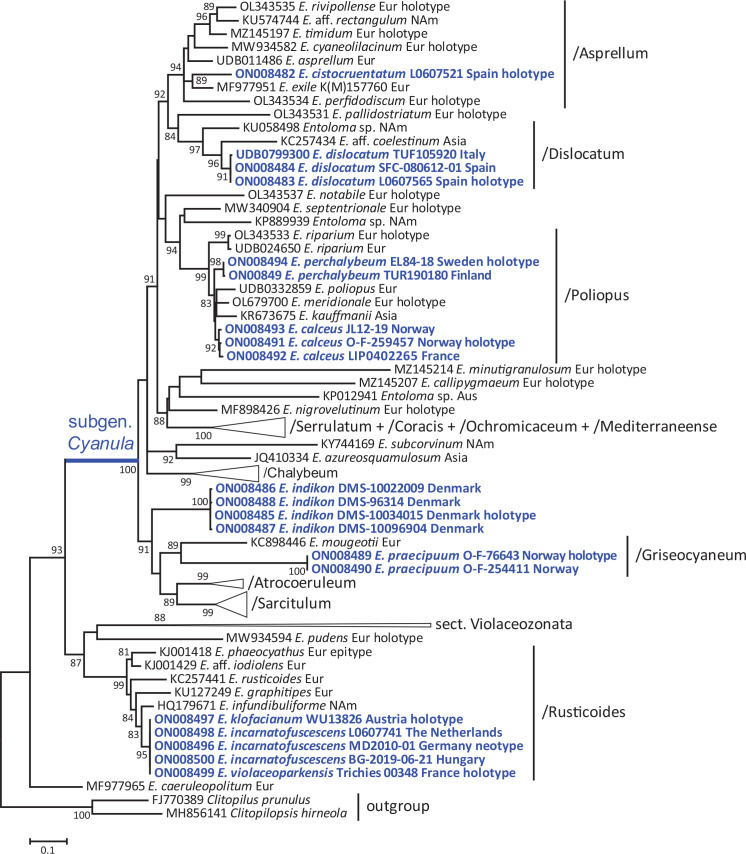
Total DNA of most of the samples was extracted from 15–30 mg of dry material, using a NucleoSpin Plant II Mini Kit (Macherey-Nagel, Düren, Germany) following the manufacturer’s instructions. Final elutions were done in a total volume of 100 μL elution buffer. The internal transcribed spacer (ITS) was amplified with primers ITS1F (Gardes & Bruns 1993) and ITS4 (White et al. 1990). Polymerase chain reaction (PCR) protocols were following Dima et al. (2016) and Papp & Dima (2018). The successful PCR amplification was checked on a 1 % agarose gel stained with Ethidium bromide. Sanger sequencing was performed by LGC Genomics (Berlin, Germany) with the same primers used in the PCR amplification. Molecular study of some material was performed in the Norwegian Barcode of Life (NorBOL) or followed Alvarado et al. (2015). Chromatograms were checked and edited with the CodonCode Aligner package (CodonCode Corp., Centerville, Massachusetts, USA). Sequence comparison with public and own databases followed Noordeloos et al. (2017). Our dataset is composed of 117 nrDNA ITS sequences, carefully selected after an initial analysis using published and all our unpublished ITS sequences (data not shown). Newly generated sequences were submitted to GenBank. The dataset was aligned in MAFFT online v. 7 (http://mafft.cbrc.jp/alignment/server) choosing the E-INS-I strategy (Katoh & Standley 2013). The alignment was checked and edited in SeaView v. 5 (Gouy et al. 2021). Maximum Likelihood (ML) phylogenetic reconstruction was performed in PhyML v. 3.1 (Guindon et al. 2010) using the non-parametric, Shimodaira-Hasegawa version of the approximate likelihood-ratio test (SH-aLRT) (Anisimova et al. 2011) with the following settings: GTR+I+G model of evolution, gamma distribution of 10 rate categories and tree topology search as SPR. The resulting phylogenetic tree (Fig. 1) was edited in MEGA 7 (Kumar et al. 2016) and Adobe Illustrator CS4.
Fig. 1.
Phylogenetic tree derived from Maximum Likelihood (PhyML) analysis based on nrITS1-5.8S-ITS2 data. ML bootstrap support (BS) values are shown at the nodes (BS > 80 %). Species treated in this study are marked with blue.
RESULTS
Phylogeny
We used a total of 115 Entoloma and two Clitopilus sequences (as outgroup) for our analysis. The ITS alignment comprises 915 characters including gaps. The resulting phylogenetic tree from the PhyML analysis is shown in Fig 1. All of the species described as new to science or typified in this study received high statistical support in our analysis. Altogether 19 ITS barcode sequences were newly generated for this study (Fig. 1).
Taxonomy
Entoloma cistocruentatum Vila, Noordel. & Dima, sp. nov. MycoBank MB 840817 Fig. 2A, D.
Fig. 2.

A, D. Entoloma cistocruentatum, A. Habit in situ. D. Spores. B, E. Entoloma dislocatum. B. Habit in situ. E. Spores. C, F, G. Entoloma indikon. C. Habit in situ. F. Spores. G. Lamella edge. Scale bar = 10 μm. All figures from the respective holotypes.
Etymology: The epithet refers to the morphological similarity with Entoloma cruentatum and the habitat under Cistus.
Basidiocarps collybioid. Pileus 15–20 mm diam, convex to plano-convex, flattened to somewhat depressed at centre, not umbonate; not hygrophanous, not translucently striate or only slightly at margin of younger specimens, very dark blue-black, barely fading when aging; entirely finely fibrillose to subsquamulose, especially with age. Lamellae unequal with few lamellulae, adnate, relatively distant, somewhat thick, pale greyish blue then with pink tinges; with a slightly paler, entire, or somewhat irregular, concolourous edge, particularly in immature specimens. Stipe 25–35 × 3–4 mm, cylindrical, often twisted, concolourous with pileus or paler, finely fibrillose to subsquamulose, especially towards the apex; with white tomentose base. Context pale grey blue. Taste and smell not noted. Basidiospores 8–9.6 × 5.9–6.9 μm, av. 9.0 × 6.4 μm; Q = 1.16–1.62, Qav = 1.4, heterodiametrical, (5–)6(–7)-angled in side view. Basidia 32–38 × 8.5–10 μm; 4-spored, almost cylindrical. Lamella edge fertile. Cheilocystidia absent. Pileipellis a cutis with transitions to a trichoderm, with elements 3.7–9 μm wide. Subpellis with broader cylindrical elements, up to 24 μm. Pigment blue, intracellular, abundant in pileipellis. Clamp-connections absent in all tissues.
Habitat and distribution: In Mediterranean vegetation with Cistus salviifolius and C. monspeliensis, on siliceous soils. Known only from Spain.
Typus: Spain, Catalonia, Selva, Tossa de Mar, Serra d’Aiguafina, alt. 80 m a.s.l., under Cistus salviifolius and C. monspeliensis on siliceous soil, 17 Dec. 2002, X. Llimona, J. Vila & E. Ballesteros (holotype L-0607521, isotype JVG 1021217-2) – ITS sequence, GenBank ON008482.
Notes: Entoloma cistocruentatum nests within the /Asprellum clade and is distinctive on account of its very dark blue-black basidiocarps, fibrillose-squamulose stipe, fertile lamella edge and relatively small spores. Entoloma asprellum is very different, usually with a brown, rarely blue, translucently striate pileus and much larger spores. Entoloma chalybeum, in the /Chalybeum clade, it can also have dark blue tones but is differentiated by its microscopy (spores, lamella edge) and habitat on grasslands and the recently described E. caeruleopinophilum, of the same clade, has less dark blue colours, striate pileus and larger spores. It also resembles of E. cruentatum and E. pseudocruentatum because of the blue tinged basidiocarps, small spores and fertile lamella edge. However, both these species are phylogenetically very distant and belong to different clades in subgenus Cyanula (Crous et al. 2021). Entoloma cistocruentatum is morphologically most easily distinguished from E. cruentatum and E. pseudocruentatum by the non-translucently striate pileus and Mediterranean habitat.
Entoloma dislocatum Vila, Dima & Noordel., sp. nov. MycoBank MB 840819 Fig. 2B, E.
Etymology: Named after its remarkable phylogenetic position in an essentially extra-European clade within Cyanula.
Basidiocarps collybioid. Pileus 10–40 mm diam, convex to conico-convex then applanate, sometimes depressed to umbilicate at the centre, not hygrophanous, not translucently striate or up to half the radius in well hydrated specimens, vivid blue (Italian collection) to dark blue or blue grey, almost black at centre, paler, more grey-tinged in old specimens, especially at the margin, finely fibrillose-tomentose, more squamulose towards the centre. Lamellae adnate to subdecurrent, relatively distant and thick, sometimes ventricose, rarely anastomosing, white to pale grey then with pink tinges, rarely with blue tinges (Italian collection), with entire, concolourous edges, or sometimes with grey-blue tones in old specimens. Stipe 25–40(–60) × 3–4 mm, central, cylindrical, concolourous with pileus or paler, glabrous to slightly tomentose or with scattered fibrils, white tomentose at base. Context thin, pale greyish. Taste and smell indistinct. Basidiospores 8.2–10.7 × 5.7–7.6 μm, av. 9.5 × 6.6 μm, Q = 1.27–1.8, Qav = 1.46, heterodiametrical, 6–7(–8)-angled, rarely with a subnodulose aspect in side view. Basidia 27–35 × 12–14 μm, 4-spored, narrowly clavate. Lamella edge sterile to heterogeneous. Cheilocystidia 40–65 × 12–16 μm, subcylindrical to clavate, hyaline or with diffuse blue-grey intracellular pigment. Pileipellis a cutis of cylindrical hyphae, 10.5–16.5 μm wide, with transitions to a trichoderm at the centre and clavate terminal elements, up to 18 μm wide. Pigment grey to blue greyish, intracellular in pileipellis. Clamp-connections absent in all tissues.
Habitat and distribution: Terrestrial in deciduous mixed forest (Fagus sylvatica) on calcareous soil; in mixed deciduous forest (Castanea sativa) on acid soil and in a forest of Quercus ilex, Q. humilis and Pinus pinea, under Hedera helix and Rubus ulmifolius on acid soil. Known from Spain and Italy.
Typus: Spain, Catalonia, Osona, Vidrà, near Collfred, alt. 1 340 m a.s.l., under Fagus sylvatica, on calcareous soil, 17 Sep. 2008, J. Vila & X. Llimona (holotype L-0607565, isotype JVG 1080917-4) – ITS sequence, GenBank ON008483.
Additional materials examined: Italy, Monti Sabatini, Bracciano-Martignano Regional Natural Park (Rome), alt. 530 m a.s.l., on the road through a Castanea sativa forest, 2 Nov. 2020, A. Knijn & A. Ferretti, TUF105920 – ITS sequence, UNITE UDB0799300. Spain, Catalonia, Vallès Oriental, Santa Maria de Martorelles Serra de Marina, alt. 360 m a.s.l., in a forest of Quercus ilex, Q. humilis and Pinus pinea, under Hedera helix and Rubus ulmifolius on acid soil, 12 Jun. 2008, F. Caballero, SFC 080612-01 – ITS sequence, GenBank ON008484.
Notes: At first sight, Entoloma dislocatum is similar to E. chalybeum with its dark blue, opaque pileus and stipe. However, the lamellae lack blue tinges, and the lamella edge is concolourous or vaguely tinged blue, whereas that of E. chalybeum is dark brown to bluish black. Also, the spores of E. dislocatum are distinctly smaller. And finally, the habitat in deciduous forest is different. Both E. dislocatum and the recently published E. pallidostriatum (Vila et al. 2021) do not belong to the /Chalybeum or /Poliopus clades, but take a rather isolated position in a small, but distinct clade with some poorly known extralimital species (Dima et al. in prep.). Knijn et al. (2021) described an unnamed blue Cyanula species from Italy, which corresponds with E. dislocatum after a comparison of the ITS sequences.
Entoloma indikon Kehlet, Noordel. & Dima, sp. nov. MycoBank MB 842932 Fig. 2C, F, G.
Etymology: ινδικ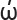 ν = dark blue, referring to the stipe colour. The name derives from the Greek word ‘indikon’ which simply means ‘from India’ or indigo, a blue dye shipped from India to Europe.
ν = dark blue, referring to the stipe colour. The name derives from the Greek word ‘indikon’ which simply means ‘from India’ or indigo, a blue dye shipped from India to Europe.
Basidiocarps collybioid. Pileus 10–35 mm diam, campanulate to convex then plano-convex with acute or slightly flattened to truncate centre, with straight, later somewhat crenulate margin, medium to dark reddish brown with darker, blackish brown centre (“eye”), vaguely translucently striate when fresh, then distinctly and deeply translucently striate, minutely granulose all over at first, later covered with fine fibrillose patches, more or less glabrous towards margin. Lamellae L= about 36, l = 3–5, adnate-emarginate to deeply emarginate, ventricose, whitish or creamy-pink, then brownish pink, with slightly irregular, concolourous edge. Stipe 30–70 × 2–4 mm, cylindrical or gradually broadened towards base, rather pale blue to dark blue-violaceous all over, not glabrous but with scattered whitish longitudinal fibrils, base white tomentose. Basidiospores 8.5–10(–11) × 6.5–8.4 μm, av. 9.5–9.6 × 7.0–7.4 μm, Q = 1.1–1.45, Qav = 1.3–1.35, heterodiametrical to subisodiametrical, 5–6 angled in sideview with pronounced angles. Basidia 34–50 × 9.5–12.5 μm, 4-spored, clavate. Lamella edge heterogeneous to entirely sterile, made up of scattered or densely packed hyphae with tufts of subcylindrical to fusiform terminal elements, 6–15 μm wide, without or with pale blue, intracellular pigment. Pileipellis a cutis with transitions to a trichoderm, with clavate terminal elements, 100–140 × 20–50 μm. Pigment brown, intracellular. Stipitipellis a cutis of cylindrical hyphae, 4.5–12 μm, wide, with clusters of clavate to subcylindrical terminal elements (“caulocystidia”). Clamp-connections absent in all tissues.
Habitat and distribution: Terrestrial in damp humus under Alnus and Frangula. Only known from two localities in central Sjælland, Denmark, including the type locality, where it has been observed during several years.
Typus: Denmark, Sjælland, Lejre, Helvigstrup Skov, terrestrial in damp humus, with Alnus glutinosa, Frangula alnus and ferns in undergrowth, 14 Sep. 2019, T. Kehlet (holotype DMS-10034015, C) – ITS sequence, GenBank ON008485.
Additional materials examined: Denmark, Sjælland, Lejre, Helvigstrup Skov, 10 Sep. 2019, T. Kehlet, DMS-10022009 (C) – ITS sequence, GenBank ON008486; ibid., 19 Sep. 2020, T. Kehlet, DMS-10096904 (C) – ITS sequence, GenBank ON008487; Sjælland, Strandskov, near Englerup, 12 Jun. 2010, T. Læssøe, DMS-96314 (C) – ITS sequence, GenBank ON008488.
Notes: Entoloma indikon is a sister species of E. phaeodiscum (Vila & Caballero 2007) from which it differs in colour of the pileus and stipe, the pileus often becoming deeply translucently striate, the more distinctly fibrillose stipe surface and the heterogeneous to sterile lamella edge. Both species share small, iso- to heterodimetrical spores and belong to the small /Phaeodiscum clade, sister to the /Griseocyaneum clade.
Entoloma praecipuum J.B. Jordal, Noordel. & Dima, sp. nov. MycoBank: MB 842929 Fig. 3A–D.
Fig. 3.

A–D. Entoloma praecipuum. A. Habit in situ. B. Spores. C. Lamella edge. D. Pileipellis (all from holotype). E–H. Entoloma calceus. E, F. Habit in situ. G. Spores. H. Lamella edge (E from PAM00092901, all other figures from holotype). Scale bar = 10 μm (spores); 20 μm (lamella edges); 40 μm (pileipellis).
Etymology: praecipuus (Lat.) = special, extraordinary.
Basidiocarps collybioid. Pileus 10–25 mm broad, campanulate to convex, expanding with age with umbilicate center and undulating margin, not distinctly hygrophanous, deeply translucently striate when moist, very dark sepia at centre, moderately dark brown on limb and paler brown at margin and between the striae, rather smooth when young, then innately fibrillose breaking up in scattered, irregular and relatively coarse squamules with age. Lamellae rather crowded, deeply emarginate, ventricose, initially pale grey then pale pink with coarsely serrate, concolourous edge. Stipe 50–70 × 2–5 mm, cylindrical, gradually broadened towards base, almost white, hyaline, covered in white, innate fibrils lengthwise, very brittle and easily splitting lengthwise. Smell and taste not known. Basidiospores 7.5–10(–10.5) × 5.0–7.0 μm, av. 8.5–10 × 5.5-6.0 μm, Q = 1.0–1.3, Qav = 1.1–1.15, subisodiamatrical to shortly heterodiametrical, mostly 5, rarely 6-angled in side-view, with fairly sharp angles. Basidia 20–27 × 7–10 μm, 4-spored, clampless. Lamella edge sterile with densely clustered, subcylindrical-flexuous cheilocystidia, septate, with terminal elements of 20–34 × 5–11 μm, without pigment. Hymenophoral trama regular, made up of cylindrical to inflated hyphae, 4–15 μm wide. Pileipellis a cutis to a trichoderm of cylindrical to inflated hyphae, 10–20 μm wide with clavate terminal elements, up to 25 μm wide. Pigment pale brown, intracellular in pileipellis. Brilliant granules scarce in trama of pileus and lamellae. Stipitipellis a cutis of narrow, cylindrical hyphae, 4–11 μm wide, with a few loose terminal endings, especially at apex. Clamp-connections absent in all tissues.
Habitat and distribution: In semi-natural grasslands, rich in Hygrocybe and Entoloma species. So far only known from the type locality and a nearby locality, both in Norway.
Typus: Norway, Møre og Romsdal, Sunndal, Jordalsgrend, Jordalsøra, in semi-natural meadow, southern boreal zone, 30 Jul. 2020, J.B. Jordal (holotype O-F-76643; isotype L-0607467) – ITS sequence, GenBank ON008489.
Additional material examined: Norway, Møre og Romsdal, Sunndal, Jordalsgrend, Jordalsvøttu, in semi-natural meadow, southern boreal zone, 28 Aug. 1994, G. Gaarder & J.B. Jordal, O-F-254411 – ITS sequence, GenBank ON008490.
Notes: Entoloma precipuum belongs to the /Griseocyaneum clade, where is takes a rather isolated position (data not shown). It differs from most species in this clade by the less distinctly squamulose, but rather innately fibrillose, translucently striate pileus, complete lack of blue tinges and the sterile lamella edge. Entoloma kedrovense from the Russian Far East (Noordeloos & Morozova 2008) is somewhat similar but has a squamulose pileus and larger spores.
Entoloma calceus Noordel., Bendiksen, Brandrud, P.-A. Moreau & Vila, sp. nov. MycoBank MB 842930 Fig. 3E–H.
Misapplied name: Entoloma atromarginatum (Romagn. & J. Favre) Zschiesch. sensu Moreau in Noordeloos, 2004: 1333 (photo).
Etymology: From Latin calceus = shoe, referring to the shape of the lake at the type-locality: “Skotjern”, which means lake in shape of a shoe.
Basidiocarps collybioid. Pileus 10–25(–30) mm diam, conico-campanulate then hemispherical, with small but usually marked depression, sometimes truncate-conical to convex, initially not hygrophanous and hardly translucently striate, becoming translucently striate-grooved, entirely blackish-blue to deep violaceus blue at first, then fading to pale mouse-grey, sometimes however, retaining the blackish-blue colour at centre, finally discolouring whitish to pale pinkish with age. Lamellae about 35 reaching stipe, 1–2 series of lamellae, rather distant, ventricose almost free, remaining white for a relatively long time, then pale pink; edge serrulate, concolourous with sides or black especially towards margin of pileus. Stipe 60–80 × 1–2 mm, smooth, deep blue when young, later hyaline grey-blue, paler at apex, white towards base. Context very thin and brittle, deep bluish when young. Smell none. Basidiospores (10–)10.9–12.5(–14) × (7.5–)8–9.5 μm, av. 10.5–11.8 × 7.9–8.8 μm, Q = (1.2–)1.3–1.6, heterodiametrical, with 6–7 angles. Basidia 22–30 × 8.5–10.5 μm, cylindrical, mostly 4-spored (a few 2-spored, generating macrospores up to 13.8 × 9.6 μm), clampless. Lamella edge sterile of the serrulatum type, made of an alternance of clusters of cheilocystidia 7–15 μm broad, some septate, clavate to lageniform, some irregularly shaped almost tibiiform, issued from radially arranged hyphae, more abundant towards insertion of stipe, with or without dark brown intracellular pigment. Hymenophoral trama made of parallel, mostly slender hyphae 3.5–9 μm wide, cylindrical to inflate towards septa, smooth, pale, mixed with numerous vascular hyphae 5–6 μm wide, strongly branched and forming a reticulum. Pileipellis a cutis with transitions to a trichoderm of somewhat gelatinized cylindrical hyphae 3.5–5.5 μm wide, with clavate terminal elements, 10–25 μm wide. Pigment dark blue, intracellular in pileipellis. Stipitipellis a simple cutis without hairs or caulocystidia. Stipititrama with numerous lactiferous hyphae. Clamp-connections absent.
Habitat and distribution: Terrestrial, sphagnophilous, in mires, fens and peat-bogs in boreal-montane biomes, such as a swamp area in Picea abies forest, and in a few with Carex rostrata. Also found in pioneer vegetation in an inundated zone of hydropower plant. Known from Norway and France.
Typus: Norway, Oppland, Lunner, Skotjernfjellet og Snellingsrøysene Nature Reserve, in swamp Picea abies forest margin along small mire stripe (7 m broad, dominated by Carex rostrata in Sphagnum sp. (cf. russowii), 580 m a.s.l., 11 Aug. 2018, E. Bendiksen & T.E. Brandrud, TEB 051-18 (holotype O-F-259457) – ITS sequence, GenBank ON008491.
Additional material examined: France, Isère, Séchilienne, lac Luitel, floating peat bog, attached to Sphagnum magellanicum, 29 Sep. 2000, P.-A. Moreau, PAM00092901 (LIP 0402265) – ITS sequence, GenBank ON008492. Norway, Nordland, Hattfjelldal, Røsvassholmen, in Picea abies forest with short-grown and sparse vegetation in the water regulation zone, 22 Aug. 2019, J. Lorås, JL12-19 – ITS sequence, GenBank ON008493.
Notes: Entoloma calceus is an attractive species, with initially bright, deep violaceous blue pileus and stipe. The pileus, however, changes dramatically with age to mouse-grey and becomes slightly translucently striate. The lamella edge has a structure similar to that of E. serrulatum and can be pigmented or not. Our current studies in subgen. Cyanula, to be published in due course (Dima et al. in prep.) make clear, that a coloured lamella is not of great diagnostic value in Cyanula. The collection from France (PAM00092901) has a blackish-brown lamella edge contrasting with the pale colours of pileus and stipe and was accordingly identified as E. atromarginatum (Noordeloos 2004). This taxon, described from peat bogs in the French Jura (Romagnesi & Favre 1938), was described and illustrated as a pale brown species, which may resemble discoloured specimens of E. calceus (Fig. 3E). Our original identification of the French collections differed mainly by larger spores and persistent light blue colours; this pale colour could have escaped J. Favre’s colour-blind eyes. Our observations of the lectotype (M.E. N.) confirm the spore dimensions provided by Romagnesi & Favre (12–15 × 7–9 μm); E. calceus has distinctly shorter spores, 10.5–11.8 × 7.9–8.8 μm. The lectotype of R. atromarginatus appeared unsuitable for the extraction of DNA and no modern collection strictly matching the protologue is known to us at this time.
Entoloma perchalybeum Noordel., J.B. Jordal & Dima, sp. nov. MycoBank MB 842931 Fig. 4.
Fig. 4.

Entoloma perchalybeum. A, B. Habit in situ. C. Spores. D. Cheilocystidia. E. Pileipellis (all from holotype). Scale bar = 10 μm (spores, cystidia); 20 μm (pileipellis).
Etymology: per (Lat.) = resembling, referring to the likeness with E. chalybeum.
Basidiocarps collybioid. Pileus 10–25 mm diam, hemispherical to convex, finally expanded, with blunt, subumbilicate centre, not hygrophanous, initially not translucently striate, but becoming deeply translucently striate to centre with age, very dark blackish blue and entirely tomentose at first, then paler between the striate and at margin, more purplish brown, with fine, dark blue, pointed squamules all over. Lamellae moderately distant, adnate, white with blue tinge, with entire, concolourous edge. Stipe 20–40 × 2–4 mm, cylindrical, dark blue like pileus, smooth, polished, base white tomentose. Basidiospores 9.0–12.5 × 6.4–9.2 μm; average 10.1–10.8 × 7.4–7.9 μm, Q = 1.2–1.5; Qav. = 1.20–1.37, heterodiametrical, with 6–7 rather blunt angles. Basidia 25–36 × 9.5–12.5 μm, subclavate, remarkably constricted, round base, 4-spored. Lamella edge sterile. Cheilocystidia 25–58 × 5.5–19 μm, cylindrical, subclavate, hyaline (type). Hymenophoral trama of cylindrical hyphae 3.5–15 μm wide. Pileipellis a cutis of cylindrical, inflated hyphae, 9–35 μm wide, terminal cells 30–50 × 12–16 μm, short clavate, brown intracellular pigment in ammonia. Pigment brown grey, intracellular vacuolar and granular (in ammonia). Stipitipellis a cutis of cylindrical hyphae, 3.5–9 μm wide, pale brown intracellular pigment, hyaline, surface with some short terminal cells 25–40 × 11–17 μm. Clamp-connections absent in all tissues.
Habitat and distribution: Terrestrial in groups, calciphilous, in alpine heaths and boreal, damp mixed forest with Picea abies, Pinus sylvestris, Betula spp. and Alnus incana. Subarctic, found in Northern Fennoscandia only.
Typus: Sweden, Pite Lappmark, Arjeplog, west of Nuorta Krapesvarre, in rich alpine vegetation, low alpine zone, 12 Aug. 2018, J.B. Jordal, E. Larsson & J. Vauras (holotype GB-0209474, isotype L-0608235) – ITS sequence, GenBank ON008494.
Additional material examined: Finland, Regio kuusamoensis, Kuusamo, Valtavaara Nature Reserve, damp forest with Picea abies, Pinus sylvestris, Betula spp. and Alnus incana, 30 Aug. 2005, J. Vauras, TUR190180 – ITS sequence, GenBank ON008495.
Notes: Entoloma perchalybeum shares many characters with E. chalybeum, particularly when fresh on account of the very similar colour, non-translucently striate, squamulose pileus, polished stipe and blue tinge in the lamellae, but the pileus becomes quickly translucently striate when maturing (cf. Entoloma lazulinum). Both E. chalybeum and E. lazulinum usually have brown instead of blue lamella edges and furthermore are phylogenetically very distant (Dima et al. in prep.).
Neotypification and emendation of Entoloma incarnatofuscescens
Entoloma incarnatofuscescens (Britzelm.) Noordel., Persoonia 12: 461. 1985. MB 104243 Fig. 5.
Fig. 5.

Entoloma incarnatofuscescens. Habit, spores and pileipellis. A, B and D. Neotype. C. Holotype of E. violaceoparkensis. E. L-0607741. F. Holotype of E. klofacianum. Scale bars = 10 μm.
Basionym: Agaricus incarnatofuscescens Britzelm., Ber. naturhist. Augsburg 8: 6. 1894.
Synonyms: Leptonia incarnatofuscescens (Britzelm.) Sacc., Syll. Fung. 11: 47. 1895.
Entoloma klofacianum Noordel. et al., Öst. Z. Pilzk. 4: 128. 1995.
Entoloma violaceoparkensis Noordel. & Trichies, in Noordeloos, Entoloma s.l., Fungi Europaei vol. 5 (Saronno) 5a: 1120. 2004.
? Rhodophyllus leptonipes Kühner & Romagn., Rev. Mycol. (Paris) 19: 6. 1954.
Emended description: Basidiocarps omphalioid. Pileus 5–25(–40) mm broad, campanulate or conical then convex or plano-convex with involute then deflexed margin, usually with distinctly umbilicate centre to funnel-shaped, more rarely with slight depression or with small umbo, weakly to distinctly hygrophanous, when moist usually deeply translucently striate, rarely not, pinkish brown, yellowish brown to reddish brown, darker at centre and on striae, sometimes blue or violaceous-brown, slightly to distinctly pallescent on drying to greyish brown, minutely squamulose at centre, fibrillose towards margin or minutely tomentose-squamulose all over. Lamellae distant to moderately crowded, adnate or emarginate with decurrent tooth, then decurrent, triangular to segmentiform, sometimes veined on sides, pale grey or brown then pinkish brown, rarely with bluish tinge, with concolourous or slightly darker edge. Stipe 15–70 × 1–3 mm, cylindrical or compressed, often with bulbous base, dark to medium dark blue-grey, steel blue or violaceous-brown, smooth, glabrous, polished or with scattered longitudinal fibrils, white tomentose at base. Context brown in cortex of pileus, blue-grey in cortex of stipe, inner parts almost white. Smell none or slightly farinaceous. Basidiospores 7.5–10.5(–11) × 6.0–8.5 μm, Q = 1.0–1.45(–1.6), Qav = 1.15–1.4, iso- to heterodiametrical, (4–) 5–7 angled in side view. Basidia 20–43 × 7–12 μm, clampless or rarely clamped. Lamella edge fertile. Cystidia absent. Pileipellis a trichoderm of strongly inflated clavate to spheropedunculate elements, up to 30 μm wide. Pileitrama regular, made up of strongly inflated hyphae, 4–20 μm wide. Pigment parietal, probably also pale intracellular in pileipellis; also rarely minutely encrusting in lower parts of pileipellis. Brilliant granules sparse. Clamp-connections absent or scarcely present.
Habitat and distribution: Terrestrial, saprotrophic, often on bare, preferably loamy, nutrient rich, damp soil in mixed forest, parks and gardens. Widespread and probably fairly common all over Europe.
Typus: Germany, Bayern, Kleinhartpenning, Hackensee, 16 Aug. 2010, M. Dondl (neotype MD 2010-01 (L), designated here) – MycoBank MBT 10005752, ITS sequence, GenBank ON008496.
Additional materials examined: Austria, Styria, Bad Gleichenberg, Kurpark (MTB 9161/1), 31 Aug. 1994, W. Klofac, WU 13826, holotype of E. klofacianum – ITS sequence, GenBank ON008497. Germany, Bayern, 86316 Friedberg, 12 Aug. 2019, T. Laschner, L-0607741 – ITS sequence, GenBank ON008498. France, Moselle, Moyeuvre-Petite, 8 Aug. 2002, G. Trichies 00348, holotype of E. violaceoparkensis (L-0607466) – ITS sequence, GenBank ON008499. Hungary, Mecsek Mts, Kárász, 21 Jun. 2019, G. Benkő & K. Fábrics, BG-2019-06-19 (ELTE) – ITS sequence, GenBank ON008500.
Notes: This tiny, omphalinoid species, which in its typical form is easy to recognise on morphological characters, is often taken for a species of Cyanula because of the steel-blue, polished stipe, appears to belong to the /Rusticoides clade, which is rather distantly related to Cyanula. It appears to be rather variable in colour. Besides the normally pinkish brown to yellow brown pileus, also variants with blue and violaceous tinges are now included in this species as became clear from the molecular genetic study of E. violaceoparkensis and E. klofacianum. The shape of the spores varies considerably from subisodiametrical to distinctly heterodiametrical, often within one basidiocarp and includes also the variant with predominantly 4–5 angled, subisodiametrical spores, described as E. klofacianum. Clamp-connections are usually not found but can be present in the hymenium or pileipellis. Intracellular pigment is dominant, but sometimes also slight incrustations are found in the lower part of the pileipellis and in pileitrama. Rhodophyllus leptonipes probably represents a later synonym, based on the morphology (Kühner & Romagnesi 1954), but the lectotype (PC) appeared unsuitable for DNA extraction to confirm it.
Acknowledgments
The Kits van Waveren Foundation (Naturalis, Leiden, The Netherlands) provided funding for some of the sequencing. Gábor Benkő, Krisztina Fábrics, Thomas Læssøe, Thomas Laschner, Anton Hausknecht, Gerard Trichies, Jukka Vauras, Øyvind Weholt and Karl Wehr supplied us with valuable material for this study. The French specimens of Entoloma calceus were collected by P.-A. Moreau in the protected area “Réserve Naturelle du lac Luitel” thanks to the collaboration of its curator Carole Desplanques (ONF Isère, France). The work of Bálint Dima was supported by the ELTE Institutional Excellence Program 2020 financed by the National Research, Development and Innovation Office of Hungary (TKP2020-IKA-05).
Footnotes
Conflict of interest: The authors declare that there is no conflict of interest.
REFERENCES
- Alvarado P, Moreno G, Vizzini A, et al. (2015). Atractosporocybe, Leucocybe and Rhizocybe: three new clitocyboid genera in the Tricholomatoid clade (Agaricales) with notes on Clitocybe and Lepista. Mycologia 107: 123–136. [DOI] [PubMed] [Google Scholar]
- Anisimova M, Gil M, Dufayard J-F, et al. (2011). Survey of branch support methods demonstrates accuracy, power and robustness of fast likelihood-based approximation schemes. Systematic Biology 60: 685–699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandrud TE, Bendiksen E, Jordal JB, et al. (2019). On some Entoloma species (Tricholomatinae, Basidiomycota) little known or new to Norway. Agarica 39: 31–52. [Google Scholar]
- Brandrud TE, Bendiksen E, Jordal JB, et al. (2018). Entoloma species of the rhodopolioid clade (subgenus Entoloma; Tricholomatinae, Basidiomycota) in Norway. Agarica 38: 21–46. [Google Scholar]
- Caballero F, Vila J. (2013). Entoloma nuevos o interesantes de la Peninsula Ibérica (3). Adiciones y correcciones. In: Vila J. et al. Studies in Entoloma. Fungi non delineati 66: 63–85 + 136–145. Ed. Candusso, Alassio. [Google Scholar]
- Crous PW, Cowan DA, Maggs-Kölling G, et al. (2021). Fungal Planet description sheets: 1182–1283. Persoonia 46: 313–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dima B, Lindström H, Liimatainen K, et al. (2016). Typification of Friesian names in Cortinarius sections Anomali, Spilomei and Bolares, and description of two new species from northern Europe. Mycological Progress 15: 903–919. [Google Scholar]
- Dima B, Brandrud TE, Corriol G, et al. (2021). Fungal Systematics and Evolution: FUSE 7. Sydowia 73: 271–340. [Google Scholar]
- Gardes M, Bruns TD. (1993). ITS primers with enhanced specificity for basidiomycetes—application to the identification of mycorrhizae and rusts. Molecular Ecology 2(2): 113–118. [DOI] [PubMed] [Google Scholar]
- Gouy M, Tannier E, Comte N, et al. (2021). Seaview Version 5: a multiplatform software for multiple sequence alignment, molecular phylogenetic analyses and tree reconciliation. Methods in Molecular Biology 2231: 241–260. [DOI] [PubMed] [Google Scholar]
- Guindon S, Dufayard JF, Lefort V, et al. (2010). New algorithms and methods to estimate Maximum-Likelihood phylogenies: assessing the performance of PhyML 3.0. Systematic Biology 59: 307–321. [DOI] [PubMed] [Google Scholar]
- Haelewaters D, Dima B, Abdel-Hafiz BII, et al. (2020): Fungal Systematics and Evolution 6. Sydowia 72: 231–356. [Google Scholar]
- Katoh K, Standley DM. (2013). MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Molecular Biology and Evolution 30: 772–780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knijn A, Ferretti A, Saar I. (2021). Rare findings from the chestnut forest of Monte Rocca Romana (Latium, Italy). Italian Journal of Mycology 50: 78–91. [Google Scholar]
- Kühner R, Romagnesi H. (1954). Compléments à la ’Flore Analytique’. I. Espéces nouvelles ou critiques de Rhodophyllus. Revue de Mycologie 19(1): 3–46. [Google Scholar]
- Kumar S, Stecher G, Tamura K. (2016). MEGA7: Molecular Evolutionary Genetics Analysis Version 7.0 for Bigger Datasets. Molecular Biology and Evolution 33: 1870–1874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morozova OV, Noordeloos ME, Vila J. (2014). Entoloma subgenus Leptonia in boreal-temperate Eurasia: towards a phylogenetic species concept. Persoonia 32: 141–169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noordeloos ME. (2004). Entoloma s.l. (suppl.). Fungi Europaei vol. 5A, Ed. Candusso, Alassio. [Google Scholar]
- Noordeloos ME, Morozova OV. (2010). New and noteworthy Entoloma species from the Primorsky Territory, Russian Far East. Mycotaxon 112: 231–255. [Google Scholar]
- Noordeloos ME, Dima B, Weholt Ø, et al. (2017). Entoloma chamaemori a new, small-spored, boreal Entoloma species, with isolated phylogenetic position. Phytotaxa 298: 289–295. [Google Scholar]
- Noordeloos ME, Lorås J, Eidissen SE, et al. (2020). Three new Entoloma species of the Cyanula clade from (sub)alpine habitats in Northern Norway and Sweden. Sydowia 73: 185–196. [Google Scholar]
- Noordeloos ME, Weholt Ø, Bendiksen E, et al. (2018). Entoloma aurorae-borealis sp. nov. and three rare Entoloma species in the Sinuatum clade (subg. Entoloma) from northern Europe. Sydowia 70: 199–210. [Google Scholar]
- Papp V, Dima B. (2018). New systematic position of Aurantiporus alborubescens (Meruliaceae, Basidiomycota), a threatened old-growth forest polypore. Mycological Progress 17: 319–332 [Google Scholar]
- Romagnesi H, Favre J. (1938). Quelques Rhodophylles nouveaux ou rares des hauts-marais jurassiens. Revue de Mycologie 3: 60–77. [Google Scholar]
- Vila J, Caballero F. (2007). Entoloma nuevos o interesantes de la Península Ibérica. Fungi non Delineati 38. Ed. Candusso, Alassio. [Google Scholar]
- Vila J, Caballero F. (2009). Entoloma nuevos o interesantes de la Península Ibérica (2). Fungi non Delineati. Pars XLV. Ed. Candusso, Alassio. [Google Scholar]
- Vila J, Carbó J, Caballero F, et al. (2013). A first approach to the study of the genus Entoloma subgenus Nolanea sensu lato using molecular and morphological data. Fungi non Delineati. 65: 3–62, 93–135 (iconography). Ed. Candusso, Alassio. [Google Scholar]
- Vila J, Caballero F, Carbó J, et al. (2014). Preliminary morphologic and molecular study of the Entoloma rusticoides group (Agaricales - Basidiomycota). Revista Catalana de Micologia 35: 65–99. [Google Scholar]
- Vila J, Llimona X. (2010). Noves dades sobre el component fúngic de les comunitats de Cistus de Catalunya. III. Addicions, correccions i claus d’identificació. Revista Catalana Micologia 31: 103–137. [Google Scholar]
- Vila J, Noordeloos ME, Reschke K, et al. (2021). New species of the genus Entoloma (Basidiomycota, Agaricales) from Southern Europe. Österreichische Zeitschrift für Pilzkunde 29: 123–153. [Google Scholar]
- White TJ, Bruns T, Lee S, et al. (1990). Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics In: PCR protocols: a guide to methods and applications (Innis MA, Gelfand DH, Sninsky JJ, et al., eds). Academic Press, New York, USA: 315–322. [Google Scholar]