Objective:
The relative usefulness of arterial stiffness parameters on renal function remains controversial. This study aimed to compare the predictive ability of three arterial stiffness parameters at baseline; cardio-ankle vascular index (CAVI), heart–ankle pulse wave velocity (haPWV) and CAVI0, a variant of CAVI that theoretically excludes dependence on blood pressure, for renal function decline in Japanese general population.
Methods:
A total of 27 864 Japanese urban residents without renal impairment at baseline who participated in two to eight consecutive (mean 3.5 ± 1.7 times) annual health examinations were studied.
Results:
During the study period, 6.6% of participants developed renal function decline (estimated glomerular filtration rate <60 ml/min per 1.73 m2), all of whom had relatively high values in all arterial stiffness parameters. In receiver-operating characteristic curve analysis, the discriminatory power for renal function decline showed a decreasing trend of CAVI to haPWV to CAVI0 (C-statistic: 0.740 vs. 0.734 vs. 0.726). The cut-offs were CAVI 8.0, haPWV 7.23 and CAVI0 11.6. In Cox-proportional hazards analysis for increase of each parameter above cut-off or by 1 standard deviation (SD) adjusted for two models of confounders, only CAVI always contributed significantly to renal function decline. Restricted cubic spline regression analysis suggested that CAVI most accurately reflected the risk of renal function decline.
Conclusion:
Increase in arterial stiffness parameters, especially CAVI, may represent a major modifiable risk factor for renal function decline in the general population. Further research is needed to examine whether CAVI-lowering interventions contribute to the prevention of chronic kidney disease.
Keywords: arterial stiffness parameter, cardio-ankle vascular index, cardio-ankle vascular index0, pulse wave velocity, renal function decline
INTRODUCTION
Chronic kidney disease (CKD) is a public health problem characterized by structural and functional abnormalities in the kidney [1]. Diabetes mellitus, hypertension, obesity and smoking are common risk factors that contribute to CKD [2]. Major complications of CKD include anemia, cardiovascular disease (CVD), renal osteopenia, and metabolic acidosis, which are major contributors to increased morbidity and mortality. However, CKD can be detected by routine clinical examination, and treatment can prevent the progression of the disease, and improve survival and quality of life.
Systemic arterial stiffening is a feature of the aging process, and is associated with various atherosclerotic diseases including CKD [3,4]. Increased arterial stiffness reflects lower vascular distensibility, and may be a potential therapeutic target for cardiometabolic complications. In particular, increased arterial stiffness in CKD has been found to be primarily because of decreased renal excretion of vascular toxins, maladaptation of metabolic and hormonal processes and consequently causing premature vascular aging [5]. Furthermore, arterial stiffness may even reflect the upstream pathogenesis of CKD. Pulse wave velocity (PWV), a traditional arterial stiffness parameter, has been reported to predict renal function decline before the onset of advanced renal dysfunction, suggesting that arterial stiffness may be a target for renal protection [6]. However, PWV is essentially affected by blood pressure (BP) at the time of measurement [7,8], and thus, may underestimate the degree of vascular dysfunction caused by CVD risks other than hypertension. In fact, a multicenter longitudinal study performed in a large European population revealed that carotid–femoral PWV was unnaturally more associated with hypertension than with glucose intolerance and dyslipidemia [9]. In addition, the BP-dependent arterial stiffness parameter hampers the assessment of the effects of antihypertensive drugs on vascular function. To overcome this problem, the cardio-ankle vascular index (CAVI) has been established [10,11]. CAVI reflects the stiffness of the whole arterial tree constituting the aorta, femoral artery and tibial artery. This parameter was originally derived from the stiffness parameter β proposed by Hayashi et al. [12] and Kawasaki et al. [13]. The theory of stiffness parameter β was applied to a length of artery using Bramwell–Hill's equation [14]. The fact that CAVI is independent of BP at the time of measurement has been confirmed both theoretically and experimentally [15,16]. Furthermore, CAVI has been reported to be an independent predictor of not only CVD events [17,18] but also renal function decline in Japanese general population [19,20]. Recently, however, Spronck et al. [21] have disputed that CAVI is inherently dependent on BP at the time of measurement, and proposed CAVI0 as a variant form of CAVI that theoretically excludes dependence on BP. Their claim is based on two reasons. First, β of the Kawasaki's equation is not equal to the BP-independent β in Hayashi's equation as reference pressure is substituted by diastolic pressure in Kawasaki's equation. Second, ln (Ps/Pd)/ΔP (Ps, systolic pressure; Pd, diastolic pressure; ΔP, Ps − Pd) in the CAVI formula is approximated by linearization, and thus presents a problem. In the CAVI0 formula proposed by these investigators, only Pd is used in the CAVI0 formula, not both Ps and Pd. However, we have pointed out that this change may have a risk of underestimation by CAVI0 in individuals with elevated Pd[16]. Thus, although the theoretical BP dependence in arterial stiffness parameters has been discussed in depth in recent years, there are insufficient longitudinal clinical studies to determine the superiority or inferiority of those parameters.
In this report, we compare the predictive ability of the three arterial stiffness parameters; haPWV, CAVI and CAVI0, for renal function decline in a large sample from the Japanese general population, and discuss the causes of the differences in ability as well as the significance of arterial stiffness measurement for predicting or preventing renal function decline.
MATERIALS AND METHODS
Participants and design
We performed a retrospective cohort study in Japanese urban residents who underwent annual health examinations between 2010 and 2018.
Ethics approval and consent to participate
The protocol of the study was prepared in accordance with the Declaration of Helsinki, and this study was approved by the Institutional Review Board and Ethics Committee of Sakura Hospital, School of Medicine, Toho University (No. S20091). Written informed consent for the examinations was obtained from the participants, and informed consent to participate in this study was obtained by opt-out method.
Data collection
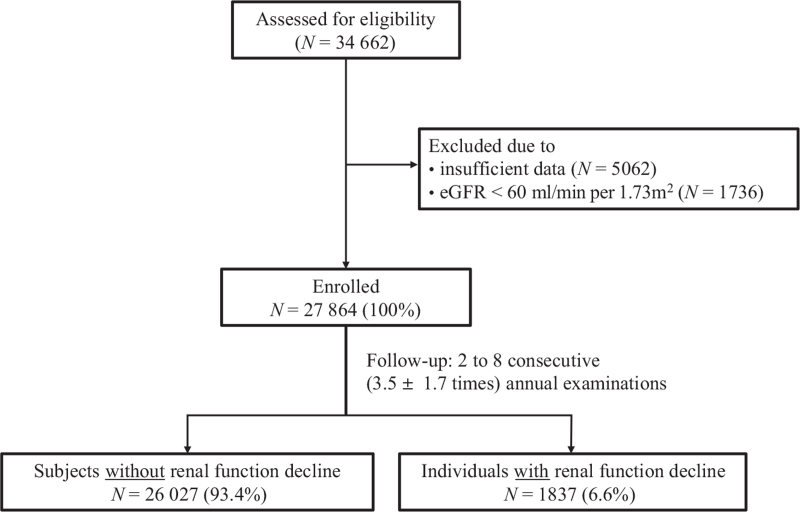
The population-based sample used in this retrospective cohort analysis consisted of 34 662 Japanese residing in major cities nationwide, who participated in the annual CVD and cancer screening program organized by the Japan Health Promotion Foundation, and had undergone two to eight consecutive annual examinations between 2010 and 2018. The participants were volunteers who were not paid and were not recruited for this study (unlike individuals of a clinical trial). Of the 34 662 individuals who were assessed for eligibility, those with inadequate data (N = 5062) and those with estimated glomerular filtration rate (eGFR) less than 60 ml/min per 1.73 m2 at baseline (N = 1736) were excluded. Finally 27 864 individuals who participated in a mean of 3.5 ± 1.7 consecutive annual examinations were enrolled in the study (Fig. 1).
FIGURE 1.
Flow diagram of study participants.
All parameters were assessed using standardized methods. Height and body weight were measured, and BMI was calculated as follows: body weight (kg) divided by square of height (m). Blood samples were collected from the antecubital vein in the morning after 12 h of fasting to measure fasting plasma glucose (FPG), triglycerides and high-density lipoprotein cholesterol (HDL-C). Low-density lipoprotein-cholesterol (LDL-C) was calculated using Friedewald formula: LDL-C = total cholesterol (TC) − (HDL-C) − (TG/5).
The eGFR was calculated by the following equation from the Japanese Society of Nephrology [22]:
eGFR (ml/min per 1.73 m2) = 194 × creatinine−1.094 × age−0.287 (×0.739 if female).
Renal function decline was defined as eGFR less than 60 ml/min per 1.73 m2, corresponding to GFR category 3a or worse [23].
Spot urine samples were collected and used for urinalysis by dipstick method. Urinalysis results were recorded as (−), (±), (1+), (2+) and (3+). Proteinuria was defined as urinary protein (1+) or above, which corresponds to a urine protein level of 30 mg/dl or higher.
Current smoking and habitual alcohol consumption status were determined by a questionnaire. Habitual alcohol consumption was defined as daily drinking. Whether the individuals were receiving current treatment for each metabolic disorder (hypertension, diabetes mellitus and dyslipidemia) was also investigated.
Measurement of arterial stiffness parameters and blood pressure
All the arterial stiffness parameters were automatically calculated using VaSera VS-1500 (Fukuda Denshi Co Ltd, Tokyo, Japan).
The CAVI was calculated by the following formula [10]:
CAVI = a{2ρ × ln (Ps/Pd)/ΔP × PWV2} + b, where s is SBP; Pd is (DBP; ΔP is Ps − Pd; ρ is blood density; PWV is cardio-ankle pulse wave velocity, and a and b are constants.
Additionally, CAVI was converted to CAVI0 by the following formula [24]:
CAVI0 = (CAVI − b)/a × (Ps/Pd − 1)/ln (Ps/Pd) − ln(Pd/Pref), where Pref is reference pressure = 100 mmHg.
Moreover, haPWV was calculated by the following formula [25]:
haPWV = Lha/(Thb + Tba), where Lha is the arterial path length from the aortic annulus to the midpoint of the right ankle cuff estimated by the individuals’ height; Thb is the time interval between the commencement of the second heart sound and the dicrotic notch on the right brachial arterial pressure wave; and Tba is the ’foot-to-foot’ time interval between brachial and posterior-tibial arterial pressure waves.
The cuffs were wrapped around the upper arms and ankles of an individual in supine position with the head in the midline position. Examination was started after 5 min of rest. When detecting pulse waves in the upper arms and ankles with cuffs, a low cuff pressure of 30–50 mmHg was used to minimize the influence of cuff pressure on hemodynamics. BP was measured from the upper arm cuffs.
We compared the ability of these three arterial stiffness parameters at baseline to predict renal function decline.
Statistical analysis
The SPSS software (version 27.0.1; IBM, Armonk, New York, USA) or EZR (version 1.54, Saitama Medical Center, Jichi Medical University, Saitama, Japan) [26] was used for statistical analyses. All data are expressed as median [interquartile range (IQR)]. Mann–Whitney U test or Fisher's exact test was performed to examine differences in baseline characteristics between individuals with and those without renal function decline. Spearman's product-moment correlation coefficient (ρ) was used to examine the relationship between clinical variables. Sensitivity and specificity of arterial stiffness parameters to predict renal function decline were analyzed using conventional receiver-operating-characteristic (ROC) curves. The ROC curve and Youden's J Index [J is defined as the maximum of (sensitivity + specificity − 1) to provide the cut-off point in the ROC curve] [27] were generated to evaluate the discriminatory power and select the cut-off value for an arterial stiffness parameter to predict renal function decline. Concordance statistics (C-statistics) that reflect the discriminatory power of three arterial stiffness parameters to predict renal function decline were compared. Additionally, we calculated the continuous net reclassification improvement (NRI) and integrated discrimination improvement (IDI) to compare the contribution of the parameters to renal function decline. Cox-proportional hazards analysis was performed to identify the contribution of the variables to renal function decline, and the result is expressed as hazard ratio with 95% confidence interval (95% CI). Restricted cubic splines were used to detect the nonlinear dependency of the relationship of the risk of renal function decline and arterial stiffening, using five knots at prespecified locations according to the percentiles of the distribution for each arterial stiffness parameter: the 5th, 27.5th, 50th, 72.5th and 95th percentiles (using package ’rms’ for R version 3.6). The analyses were carried out using R with ‘rms’ package. In all comparisons, two-sided P values less than 0.05 were considered statistically significant.
RESULTS
Comparison of baseline clinical and biochemical characteristics in individuals with and without renal function decline during the study period
Of the 27 864 participants, 1837 (6.6%) developed renal function decline during the study period. Table 1 compares the baseline clinical characteristics of participants who did and those who did not develop renal function decline. Individuals with renal function decline were found to have higher male ratio, age, BMI, BP, CAVI, haPWV, CAVI0, FPG, LDL-C, triglycerides, creatinine, frequency of proteinuria and frequencies of current treatment for hypertension/diabetes/dyslipidemia; and lower HDL-C and eGFR.
TABLE 1.
Comparison of baseline clinical and biochemical characteristics in individuals with and those without renal function decline during the study period
| Individuals without renal function decline | Individuals with renal function decline | ||
| Variables | (N = 26 027) | (N = 1837) | P value |
| Male sex (%) | 44.2 | 47.1 | 0.018∗ |
| Age (years) | 45 (36–56) | 61 (52–68) | <0.001 |
| Height (m) | 1.62 (1.56–1.70) | 1.61 (1.55–1.68) | <0.001 |
| BMI (kg/m2) | 21.9 (19.9–24.3) | 22.9 (20.8–24.9) | <0.001 |
| SBP (mmHg) | 116 (107–127) | 126 (113–138) | <0.001 |
| DBP (mmHg) | 72 (65–80) | 78 (70–86) | <0.001 |
| CAVI | 7.5 (6.9–8.2) | 8.4 (7.7–9.1) | <0.001 |
| haPWV | 6.89 (6.36–7.61) | 7.84 (7.14–8.54) | <0.001 |
| CAVI0 | 10.7 (9.7–12.0) | 12.5 (11.0–14.2) | <0.001 |
| FPG (mg/dl) | 85 (80–91) | 89 (84–97) | <0.001 |
| LDL-C (mg/dl) | 121 (100–144) | 130 (109–150) | <0.001 |
| HDL-C (mg/dl) | 67 (56–81) | 63 (53–77) | <0.001 |
| TG (mg/dl) | 78 (55–117) | 96 (69–139) | <0.001 |
| Creatinine (mg/dl) | 0.68 (0.59–0.81) | 0.77 (0.69–0.92) | <0.001 |
| eGFR (ml/min per 1.73 m2) | 82.5 (74.6–92.0) | 65.6 (62.6–69.6) | <0.001 |
| Proteinuria (%) | 5.0 | 7.4 | <0.001∗ |
| Current smoking (%) | 35.1 | 32.9 | 0.061∗ |
| Habitual alcohol drinking (%) | 34.2 | 33.1 | 0.331∗ |
| Receiving treatment for | |||
| Hypertension (%) | 5.4 | 22.8 | <0.001∗ |
| Diabetes mellitus (%) | 3.9 | 6.8 | <0.001∗ |
| Dyslipidemia (%) | 6.0 | 15.1 | <0.001∗ |
Data are presented as median (interquartile range). Mann–Whitney U test and ∗Fisher's exact test were used to compare individuals who did and those who did not develop renal function decline defined as eGFR less than 60 ml/min per 1.73 m2 during the study period. CAVI, cardio-ankle vascular index; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; haPWV, heart–ankle pulse wave velocity; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; TG, triglyceride.
Spearman's product-moment correlation coefficient matrix
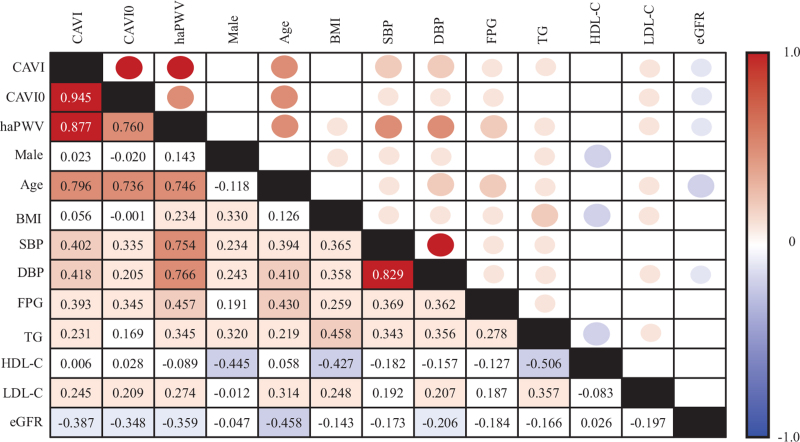
The correlation between clinical variables and arterial stiffness parameters at baseline are presented in Fig. 2. In simple linear regression analyses, CAVI corrected positively and very strongly with CAVI0 and with haPWV, and CAVI0 correlated strongly with haPWV. All three arterial stiffness parameters correlated positively with age, BP, FPG, triglycerides and LDL-C; and negatively with eGFR. Especially, haPWV showed the strongest correlation with BP, followed by CAVI and CAVI0, in that order.
FIGURE 2.
Spearman's product-moment correlation coefficient matrix. Spearman's correlation coefficients are presented in appropriate cells in the matrix. The results are color-coded according to the degree of correlation.
Comparison of association between arterial stiffness parameters and renal function decline
ROC curve was generated to assess the discriminatory power and select the cut-off value of each arterial stiffness parameter for renal function decline. All three arterial stiffness parameters had C-statistics over 0.7, and therefore, had good discriminatory power. The cut-off values of CAVI, haPWV and CAVI0 for predicting renal function decline were 8.0, 7.23 and 11.6, respectively (Table 2a). These cut-offs were used in the subsequent Cox-proportional hazards analyses presented in Table 3. The discriminatory powers of the three parameters were then compared using C-statistic comparison, NRI and IDI (Table 2b). Results of the analyses showed that CAVI was most strongly associated with renal function decline, followed by haPWV and CAVI0, in that order. Only in the C-statistic comparison between haPWV and CAVI0 was there no significant difference.
TABLE 2.
Comparison of associations of arterial stiffness parameters with renal function decline
| (a) | |||||
| C-statistics (95% CI) | P value | Cut-off | Sensitivity | Specificity | |
| CAVI | 0.740 (0.729–0.751) | <0.001 | 8.0 | 0.672 | 0.690 |
| haPWV | 0.734 (0.722–0.775) | <0.001 | 7.23 | 0.726 | 0.632 |
| CAVI0 | 0.726 (0.714–0.738) | <0.001 | 11.6 | 0.657 | 0.688 |
| (b) | |||||
| P value for C-statistics | NRI (95% CI) | P value for NRI | IDI (95% CI) | P value for IDI | |
| CAVI vs. haPWV | 0.038 | 0.159 (0.112–0.206) | <0.001 | 0.004 (0.002–0.006) | <0.001 |
| CAVI vs. CAVI0 | <0.001 | 0.477 (0.430–0.523) | <0.001 | 0.007 (0.006–0.009) | <0.001 |
| haPWV vs. CAVI0 | 0.095 | 0.201 (0.154–0.248) | <0.001 | 0.003 (0.004–0.006) | 0.023 |
(a) Discriminatory powers and cut-off values of arterial stiffness parameters for renal function decline. Youden index was used to select the optimum cut-off point for each arterial stiffness parameter. (b) Comparisons of discriminatory power between arterial stiffness parameters for renal function decline. CAVI, cardio-ankle vascular index; CI, confidence interval; haPWV, heart–ankle pulse wave velocity; IDI, integrated discrimination improvement; NRI, net reclassification improvement.
TABLE 3.
Adjusted hazard ratios of arterial stiffness parameters for renal function decline
| CAVI | haPWV | CAVI0 | ||||
| ≥8.0 | 1SD increase | ≥7.23 | 1SD increase | ≥11.6 | 1SD increase | |
| Model 1 | 1.182 | 1.115 | 1.120 | 1.144 | 1.147 | 1.023 |
| (1.010–1.383)∗ | (1.039–1.197)∗ | (0.946–1.325) | (1.050–1.246)∗ | (0.985–1.336) | (0.968–1.080) | |
| Model 2 | 1.188 | 1.108 | 1.238 | 1.115 | 1.151 | 1.023 |
| (1.043–1.353)∗ | (1.041–1.179)∗ | (1.088–1.409)∗ | (1.054–1.179)∗ | (1.014–1.306)∗ | (0.974–1.074) | |
Hazards ratios (95% confidence interval) estimated using Cox-proportional hazards analyses are shown. Renal function decline is defined as eGFR less than 60 ml/min per 1.73 m2 during the study period. Model 1: confounders include age, sex, BMI, proteinuria, SBP, FPG and HDL-C. Model 2: confounders include age, sex, BMI, proteinuria, and treatments for hypertension, diabetes and dyslipidemia. CAVI, cardio-ankle vascular index; haPWV, heart–ankle pulse wave velocity; SD, standard deviation.
P less than 0.05.
Adjusted hazard ratio of each arterial stiffness parameter for renal function decline
In the next analysis, the contribution of each arterial stiffness parameter (increase above cut-off or by 1 SD) to renal function decline was assessed using Cox-proportional hazards analysis (Table 3). Among the clinical variables that showed a significant difference in the comparison shown in Table 1, those factors that did not show collinearity were selected as confounders. Model 1 included age, sex, BMI, proteinuria, SBP, FPG and HDL-C as confounders. In model 2, in addition to age, sex, BMI and proteinuria, treatments for hypertension, diabetes and dyslipidemia replaced SBP, FPG and HDL-C in model 1. In this analysis, an increase of CAVI above the cut-off point or by 1 SD contributed significantly and independently to renal function decline in both models 1 and 2. On the other hand, an increase of haPWV above the cut-off point did not contribute to renal function decline in model 1. As for the CAVI0, only an increase of CAVI0 above the cut- off point contribute to renal function decline in model 2.
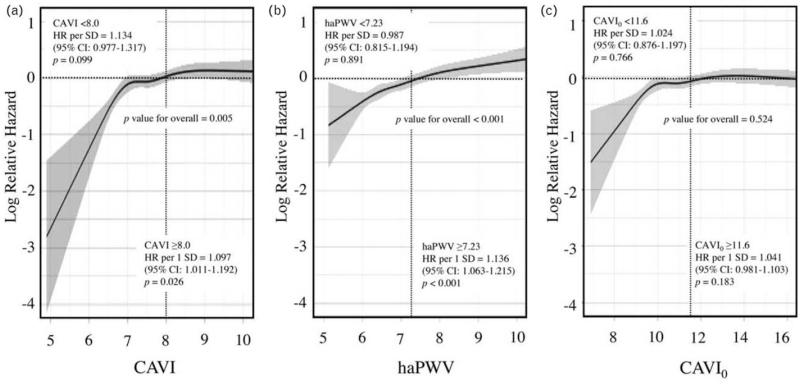
Restricted cubic spline for renal function decline
Restricted cubic splines of the three arterial stiffness parameters for renal function decline are shown in Fig. 3. As the arterial stiffness parameter is affected by metabolic disorders and their treatments, only sex and age were included as confounders in this analysis. CAVI0 showed no significant linear relationship with renal function decline, for the overall data and also for the data above and below the cut-off point (Fig. 3c). On the other hand, CAVI (Fig. 3a) and haPWV (Fig. 3b) showed a significant positive linear relationship with renal function decline overall, and especially for the data above the respective cut-off points. Below the cut-off value (8.0), the risk of renal function decline tended to be reduced as CAVI decreased (P = 0.099). On the other hand, the spline of haPWV was the most linear of the three parameters, but the range of relative hazard was narrower compared with the other two parameters, and lower haPWV was not significantly associated with lower risk of renal function decline (P = 0.891).
FIGURE 3.
Restricted cubic splines for renal function decline. Association between each arterial stiffness parameter and renal function decline is presented as hazard ratio (solid line) and 95% CI (shaded area). Renal function decline was defined as eGFR less than 60 ml/min per 1.73 m2 during the study period. Results from Cox-proportional hazards model of renal function decline were modeled using restricted cubic splines with five knots (the 5th, 27.5th, 50th, 72.5th and 95th percentiles), adjusted for age and sex. Median value of risk for renal function decline was considered the reference (hazard ratio = 1). The x-axis is expressed as the mean ± 2SD range of each arterial stiffness parameter. CI, confidence interval; eGFR, estimated glomerular filtration rate; SD, standard deviation.
DISCUSSION
In the present study, 6.6% of participants developed renal function decline during the study period, and all arterial stiffness parameters showed elevated values in individuals with renal function decline. In ROC curve analysis, CAVI had the strongest discriminatory power for renal function decline, followed by haPWV and CAVI0, in that order. In four types of Cox-proportional models for renal function decline adjusted for confounders, CAVI0 was extracted as a significant contributor in one and haPWV in three, whereas CAVI was a significant contributor in all models. Restricted cubic splines suggest that CAVI may be the most accurate index among the three in reflecting the risk of renal function decline. The novelty of this retrospective cohort study is the comparison by various approaches of the abilities of three different arterial stiffness parameters in predicting renal impairment. In summary, haPWV, a remarkable BP-dependent arterial stiffness parameter, was more predictive of renal function decline than CAVI0, which has been proposed to completely eliminate BP dependence in theory. However, CAVI was most strongly associated with renal function decline compared with haPWV and CAVI0.
Although the present study showed that both CAVI and haPWV were predictors of renal function decline in Cox-proportional hazards analyses, area under the curve in ROC analysis, NRI and IDI revealed that CAVI had better discriminatory power for renal function decline compared with haPWV and CAVI0. Specifically, predictive performance of haPWV for renal function decline was lower compared with CAVI, despite many individuals with high haPWV showed relatively increased BP at the time of measurement. This result is consistent with our previous study showing that CAVI was more strongly associated than haPWV with mortality in hemodialysis patients [28]. The relatively weak predictive performance of haPWV for renal function decline compared with CAVI may be because of the underestimation of atherogenic risk factors other than elevated BP.
The NRI of CAVI relative to haPWV or CAVI0 shown in Table 2b represents net increase in the proportion of individuals assigned a more appropriate risk for renal function decline under CAVI, respectively. The percentage of individuals with renal function decline correctly reclassified by CAVI was 15.9% (95% CI 11.2–20.6%) compared with haPWV, and 47.7% (95% CI 43–52.3%) compared with CAVI0. Continuous NRI used in the present study defines any change in predicted probability for renal function decline as either upward or downward movement, whereas IDI considers a weight for each movement and is equal to the difference in discrimination slopes. Resultantly, improvement in classification accuracy of CAVI was mitigated by IDI, whereas it remained statistically significant. However, the result of ROC analysis, NRI and IDI does not necessarily guarantee the more effective clinical relevance of CAVI, and large-scale prospective cohort studies are needed in the future.
The superiority of CAVI compared with the other two parameters identified in the present study seems to be inconsistent to the findings of Spronck et al. [29] compared the same three arterial stiffness parameters in 154 individuals. They reported that the predictive ability of the composite endpoint [death (n = 21) and heart failure hospitalization (n = 18)] showed a decreasing trend of haPWV to CAVI to CAVI0, and the investigators attributed these results to the increasing amount of BP correction of the parameters. However, from their hazard ratio data, as the 95% CIs of the three indices overlapped, it is not necessarily valid to determine the superiority of the arterial stiffness parameters based on the slight differences in hazard ratio. Furthermore, the composite endpoint used in their study is not necessarily related to vascular dysfunction. In addition, the number of endpoints examined was small, and the confounders used in the Cox-proportional hazards analysis did not include age and sex ratio.
As for the relationship between CAVI and BP measurement, the independence of CAVI on BP at the time of measurement has been confirmed in vivo in human individuals. Shirai et al. [15] demonstrated that administration of metoprolol, a selective β1-blocker, did not change CAVI while the drug decreased BP, whereas administration of doxazosin, a selective α1-blocker, induced decreases in both BP and CAVI. Furthermore, Mestanik et al. [30] reported that the cold pressor test transiently increased CAVI but did not change CAVI0. The cold pressor test is known to induce arterial smooth muscle contraction through sympathetic activation [31]. Accordingly, these data indicate that while CAVI is unaffected by acute BP fluctuation, it is influenced by short-term arterial smooth muscle contraction, while CAVI0 does not change by arterial smooth muscle contraction. In other words, unlike CAVI0, CAVI indicates not only organic stiffness but also functional stiffness.
Spronck et al. [21] have proposed CAVI0 as a variant of CAVI that theoretically corrects even more strongly for the dependence on BP. CAVI and CAVI0 are based on the stiffness index β and a wave equation derived from Newton's second law. The major difference between CAVI and CAVI0 is that CAVI employs β over a range of diastolic to systolic pressures, whereas CAVI0 employs β at only diastolic pressure. This difference in structural formula may induce a divergence between CAVI and CAVI0. Indeed, CAVI values of Japanese hypertensive individuals were higher than those of normotensive individuals in both men and women but CAVI0 values in young hypertensive women was significantly lower than those of young normotensive women; showing unexplainable data [16]. Also, in simple and multiple regression analysis, CAVI0 showed a negative relationship with Pd. We sought explanations for these unnatural results. There is no problem with the CAVI0 formula itself. Both CAVI and CAVI0 are calculated from haPWV, the value of which differs depending on the BP at the stage of the cardiac cycle when measurement is made (Pd to Ps). CAVI0 is based on PWV at Pd, from the investigators’ assumption that foot-to-foot haPWV corresponds to PWV at Pd[21]. However, CAVI has been proven and verified to correspond to PWV measured at the mid-point BP (Pm) between Pd and Ps[16,32], and not at Pd. Using only Pd and not Pm in CAVI0 created incongruous measurements in some participants populations. Furthermore, a recent review published by Giudici et al. [33] suggested that use of Pd in CAVI0 resulted in unexpected findings: negative correlation between CAVI0 and Pd and the lower CAVI0 in young hypertensive women compared with normotensive individuals. However, while they admitted that the haPWV-relevant BP was not Pd, they calculated it to be much closer to Pd than to Pm. They estimated the haPWV-relevant BP to be 0.91 × Pd + 0.09 × Ps. To calculate this, they assumed that the pulse wave transit time from the heart to brachium (tb) and the foot-to-foot time difference between the brachial pulse wave and ankle pulse wave (tba) to be 56 and 228 ms, respectively. However, their figures are far different from the actual measured values of tb (81.78 ± 11.26 ms) and tba (90.27 ± 14.65 ms) (data of Japan Health Promotion Foundation; values of tb and tba recorded from 13 444 individuals between November 2005 and March 2007). Therefore, the formula for estimating haPWV-relevant BP is incorrect, and there is no basis for the claim that the BP is almost Pd. Also, they claim that the negative correlation with BP is because of high pulse pressure among the individuals with lower Pd, who are supposed to have stiffer blood vessels [29]. However, a negative relationship is observed even in healthy people with pulse pressure less than 50 mmHg [34]. The negative relationship between CAVI0 and BP cannot be explained by high pulse pressure, but is just because of the incorrect values. However, although the clinical significance of CAVI0 is questionable, it provides valuable opportunity to review the mathematical meaning and deepen the discussions in this field.
Increased CAVI reflects not only organic lesion in the macrovessels but also microcirculatory disorders induced by inflammation and oxidative stress [11,19]. Therefore, vascular aging indicated by elevated CAVI may lead to renal function decline via damage in the microvasculature. On the other hand, previous study has shown the possibility that appropriate treatments and behavior modification may decrease CAVI [11]. In addition to the management of metabolic disorders, other approaches including smoking cessation [35], treatment of periodontitis [36], improvement of sleep duration [37] and continuous positive airway pressure for obstructive sleep apnea [38] may contribute to the improvement of CAVI. Therefore, the risk of renal function decline in individuals with high CAVI may also be reduced through reduction of CAVI by therapeutic approaches appropriate to the underlying cause.
A limitation of the present study is that our findings may not be generalized to other ethnic groups. Further research is needed to address this issue and to confirm whether therapeutic approaches to reduce CAVI decrease the risk of developing end-stage renal disease and CVDs.
In conclusion, elevated arterial stiffness parameters, especially CAVI, may represent a major modifiable risk factor for renal function decline in the general population. Further research is needed to examine whether CAVI-lowering interventions contribute to prevention of chronic kidney disease.
ACKNOWLEDGEMENTS
The authors would like to thank all the staff members in our departments who contributed to this study. Additionally, we are grateful to Mr. Shinichi Tsuda and Mr. Koji Takahashi (Fukuda Denshi Co., Ltd., Tokyo, Japan) for valuable advice on statistical analysis and discussion.
Authors’ contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; gave final approval of the version to be published; and agree to be accountable for all aspects of the work.
Availablility of data and materials
The data that support the findings of this study are not publicly available because they contain information that could compromise the privacy of research participants. Further enquiries may be directed to the corresponding author.
Conflicts of interest
There are no conflict of interest.
Footnotes
Abbreviations: BP, blood pressure; CAVI, cardio-ankle vascular index; CKD, chronic kidney disease; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; FPG, fasting plasma glucose; haPWV, heart–ankle pulse wave velocity; HDL-C, high-density lipoprotein-cholesterol; IDI, integrated discrimination improvement; IQR, interquartile range; LDL-C, low-density lipoprotein cholesterol; NRI, net reclassification improvement; Pd, diastolic pressure; Ps, systolic pressure; ROC, receiver-operating-characteristic; SD, standard deviation; ΔP, Ps, −Pd
REFERENCES
- 1.Andrew S, Levey AS, Coresh J. Chronic kidney disease. Lancet 2012; 379:165–180. [DOI] [PubMed] [Google Scholar]
- 2.Onozaki A, Nagayama D, Azuma N, Sugai K, Shitara E, Sakai T, et al. Relation of maximum lifetime body mass index with age at hemodialysis initiation and vascular complications in Japan. Obes Facts 2021; 14:550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zieman SJ, Melenovsky V, Kass DA. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol 2005; 25:932–943. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell GF, Hwang SJ, Vasan RS, Larson MG, Pencina MJ, Hamburg NM, et al. Arterial stiffness and cardiovascular events: the Framingham Heart Study. Circulation 2010; 121:505–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zanoli L, Lentini P, Briet M, Castellino P, House AA, London GM, et al. Arterial stiffness in the heart disease of CKD. J Am Soc Nephrol 2019; 30:918–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fountoulakis N, Thakrar C, Patel K, Viberti G, Gnudi L, Karalliedde J. Increased arterial stiffness is an independent predictor of renal function decline in patients with type 2 diabetes mellitus younger than 60 years. J Am Heart Assoc 2017; 6:e004934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kokubo Y, Watanabe M, Higashiyama A, Nakao YM, Kusano K, Miyamoto Y. Development of a basic risk score for incident atrial fibrillation in a Japanese general population - the Suita study. Circ J 2017; 81:1580–1588. [DOI] [PubMed] [Google Scholar]
- 8.Shirai K, Song M, Suzuki J, Kurosu T, Oyama T, Nagayama D, et al. Contradictory effects of β1- and α1- aderenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI)–CAVI independent of blood pressure. J Atheroscler Thromb 2011; 18:49–55. [DOI] [PubMed] [Google Scholar]
- 9.Topouchian J, Labat C, Gautier S, Bäck M, Achimastos A, Blacher J, et al. Effects of metabolic syndrome on arterial function in different age groups: the Advanced Approach to Arterial Stiffness study. J Hypertens 2018; 36:824–833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shirai K, Utino J, Otsuka K, Takata M. A novel blood pressure-independent arterial wall stiffness parameter; cardio-ankle vascular index (CAVI). J Atheroscler Thromb 2006; 13:101–107. [DOI] [PubMed] [Google Scholar]
- 11.Saiki A, Ohira M, Yamaguchi T, Nagayama D, Shimizu N, Shirai K, Tatsuno I. New horizons of arterial stiffness developed using cardio-ankle vascular index (CAVI). J Atheroscler Thromb 2020; 27:732–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayashi K, Handa H, Nagasawa S, Okumura A, Moritake K. Stiffness and elastic behavior of human intracranial and extracranial arteries. J Biomech 1980; 13:175–184. [DOI] [PubMed] [Google Scholar]
- 13.Kawasaki T, Sasayama S, Yagi S, Asakawa T, Hirai T. Non-invasive assessment of the age related changes in stiffness of major branches of the human arteries. Cardiovasc Res 1987; 21:678–687. [DOI] [PubMed] [Google Scholar]
- 14.Bramwell JC, Hill AV. The velocity of the pulse wave in man. Proc R Soc London Series B 1926; 93:298–306. [Google Scholar]
- 15.Shirai K, Song M, Suzuki J, Kurosu T, Oyama T, Nagayama D, et al. Contradictory effects of beta1- and alpha1- aderenergic receptor blockers on cardio-ankle vascular stiffness index (CAVI)–CAVI independent of blood pressure. J Atheroscler Thromb 2011; 18:49–55. [DOI] [PubMed] [Google Scholar]
- 16.Shirai K, Suzuki K, Tsuda S, Shimizu K, Takata M, Yamamoto T. Comparison of cardio–ankle vascular index (CAVI) and CAVI0 in large healthy and hypertensive populations. J Atheroscler Thromb 2019; 26:603–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sato Y, Nagayama D, Saiki A, Watanabe R, Watanabe Y, Imamura H, et al. Cardio-ankle vascular index is independently associated with future cardiovascular events in outpatients with metabolic disorders. J Atheroscler Thromb 2016; 23:596–605. [DOI] [PubMed] [Google Scholar]
- 18.Miyoshi T, Ito H, Shirai K, Horinaka S, Higaki J, Yamamura S, et al. CAVI-J (Prospective Multicenter Study to Evaluate Usefulness of Cardio-Ankle Vascular Index in Japan) investigators. Predictive value of the cardio-ankle vascular index for cardiovascular events in patients at cardiovascular risk. J Am Heart Assoc 2021; 10:e020103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Itano S, Yano Y, Nagasu H, Tomiyama H, Kanegae H, Makino H, et al. Association of arterial stiffness with kidney function among adults without chronic kidney disease. Am J Hypertens 2020; 33:1003–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nagayama D, Fujishiro K, Tsuda S, Watanabe Y, Yamaguchi T, Suzuki K, et al. Enhanced prediction of renal function decline by replacing waist circumference with ‘A Body Shape Index (ABSI)’ in diagnosing metabolic syndrome: a retrospective cohort study in Japan. Int J Obes (Lond) 2022; 46:564–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Spronck B, Avolio AP, Tan I, Butlin M, Reesink KD, Delhaas T. Arterial stiffness index beta and cardio-ankle vascular index inherently depend on blood pressure but can be readily corrected. J Hypertens 2017; 35:98–104. [DOI] [PubMed] [Google Scholar]
- 22.Matsuo S, Imai E, Horio M, Yasuda Y, Tomita K, Nitta KY, et al. Revised equations for estimated GFR from serum creatinine in Japan. Am J Kidney Dis 2009; 53:982–992. [DOI] [PubMed] [Google Scholar]
- 23.Chapter 1: definition and classification of CKD. Kidney Int Suppl (2011) 2013; 3:19–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spronck B, Mestanik M, Tonhajzerova I, Jurko A, Tan I, Butlin M, Avolio AP, et al. Easy conversion of cardio-ankle vascular index into CAVI0: influence of scale coefficients. J Hypertens 2019; 37:1913–1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tomoto T, Sugawara J, Hirasawa A, Imai T, Maeda S, Ogoh S. Impact of short-term training camp on arterial stiffness in endurance runners. J Physiol Sci 2015; 65:445–449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kanda Y. Investigation of the freely available easy-to-use software ‘EZR’ for medical statistics. Bone Marrow Transplant 2013; 48:452–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Youden WJ. Index for rating diagnostic tests. Cancer 1950; 3:32–35. [DOI] [PubMed] [Google Scholar]
- 28.Murakami K, Inayama E, Itoh Y, Tuchiya S, Iwasaki M, Tamura N, et al. The Role of cardio-ankle vascular index as a predictor of mortality in patients on maintenance hemodialysis. Vasc Health Risk Manag 2021; 17:791–798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Spronck B, Obeid MJ, Paravathaneni M, Gadela NV, Singh G, Magro CA, et al. Predictive ability of pressure-corrected arterial stiffness indices: comparison of pulse wave velocity, cardio-ankle vascular index (CAVI), and CAVI0. Am J Hypertens 2021. hpab168.doi: 10.1093/ajh/hpab168 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mestanik M, Spronck B, Jurko A, Mestanikova A, Jurko T, Butlin M, et al. P135 assessment of novel blood pressure corrected cardio-ankle vascular index in response to acute blood pressure changes. Artery Res 2019; 25:S173. [Google Scholar]
- 31.Lamotte G, Boes CJ, Low PA, Coon EA. The expanding role of the cold pressor test: a brief history. Clin Auton Res 2021; 31:153–155. [DOI] [PubMed] [Google Scholar]
- 32.Takahashi K, Yamamoto T, Tsuda S, Maruyama M, Shirai K. The background of calculating CAVI: lesson from the discrepancy between CAVI and CAVI(0). Vasc Health Risk Manag 2020; 16:193–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Giudici A, Khir AW, Reesink KD, Delhaas T, Spronck B. Five years of cardio-ankle vascular index (CAVI) and CAVI0: how close are we to a pressure-independent index of arterial stiffness? J Hypertens 2021; 39:2128–2138. [DOI] [PubMed] [Google Scholar]
- 34.Takahashi K, Yamamoto T, Tsuda S, Maruyama M. Shirai K, Asmar R, Orimo H. CAVI behind the formula. Cardio-ankle vascular index: overview & clinical application. Tokyo: Compass Co., Ltd; 2021. 33–38. [Google Scholar]
- 35.Noike H, Nakamura K, Sugiyama Y, Iizuka T, Shimizu K, Takahashi M, et al. Changes in cardio-ankle vascular index in smoking cessation. J Atheroscler Thromb 2010; 17:517–525. [DOI] [PubMed] [Google Scholar]
- 36.Hayashida H, Saito T, Kawasaki K, Kitamura M, Furugen R, Iwasaki T, et al. Association of periodontitis with carotid artery intima-media thickness and arterial stiffness in community-dwelling people in Japan: the Nagasaki Islands study. Atherosclerosis 2013; 229:186–191. [DOI] [PubMed] [Google Scholar]
- 37.Morita N, Kambayashi I, Okuda T, Oda S, Takada S, Nakajima T, et al. Inverse relationship between sleep duration and cardio-ankle vascular index in children. J Atheroscler Thromb 2017; 24:819–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tomita Y, Kasai T. Relationship between cardio-ankle vascular index and obstructive sleep apnea. Rev Cardiovasc Med 2020; 21:353–363. [DOI] [PubMed] [Google Scholar]