Abstract
Capnocytophaga canimorsus is a Gram-negative zoonotic pathogen capable of causing serious infection following dog or cat bite. Infections often manifest as sepsis, fatal septic shock, gangrene, bacteraemia, meningitis and endocarditis. Here we report a case of C. canimorsus bacteraemia complicated by tricuspid valve infective endocarditis and septic pulmonary emboli.
Keywords: Capnocytophaga canimorsus, infective endocarditis, dog bites, tricuspid valve
Introduction
Capnocytophaga canimorsus is a facultative anaerobic Gram-negative bacilli that belongs to the normal oral flora of healthy dogs and cats [1]. Zoonotic transmission occurs often as a consequence of a bite or scratch and may present as a serious infection manifesting as severe sepsis, gangrene of digits or extremities, high-grade bacteraemia, meningitis, brain abscess, mycotic aneurysm, respiratory tract infection, endocarditis and rare ocular infections [2–4]. Cases of C. canimorsus infection described in the literature show a 28–30 % fatality rate, but it is probable that less severe infections are underreported in the literature and local infections from dog bites may be treated effectively with empirical antibiotics [5–7]. Here we report a patient with C. canimorsus bacteraemia complicated by tricuspid valve infective endocarditis (IE) and bilateral septic pulmonary emboli.
Case description
A man in his sixties with a history of type 2 diabetes mellitus, tobacco use, homelessness, methamphetamine abuse, and no prior history of heart disease or intravenous drug use presented to an outside hospital with fevers, chills, dyspnea on exertion and left sided back pain. His initial laboratory results were notable for a white count: 14.8 and lactic acid: 2.5. Due to the patient’s report of a recent syncopal episode with palpitations, he was subsequently evaluated by echocardiography, which demonstrated a large 1.9×2.9 cm mobile vegetation on the tricuspid valve with severe tricuspid regurgitation. A CT angiogram also performed on admission showed multiple pulmonary emboli with a large pulmonary artery embolus involving the entire right lower lobe pulmonary artery. One of two blood cultures on admission grew out Gram-negative rods, which were sent out for anaerobic culture identification and antimicrobial susceptibility testing provided by Mayo Clinic (test ID: ANAID and MMLSA). He was treated empirically with cefepime and ciprofloxacin for 1 week for Gram-negative rod bacteraemia. Given the large vegetation observed on the tricuspid valve, the pulmonary embolism was presumed to be septic. However, since a thrombotic embolism could not be ruled out, he was started on a continuous heparin drip. He was then transferred to our hospital for evaluation for tricuspid valve replacement.
The patient reported that he had lived with his Rottweiler dog for the past 3.5 years. While he could not recall recent dog bites or his dog licking his wounds, he reported that his dog bit him a couple times approximately 1 year ago. He further reported living in close proximity with his dog, often eating food out of the same bowel.
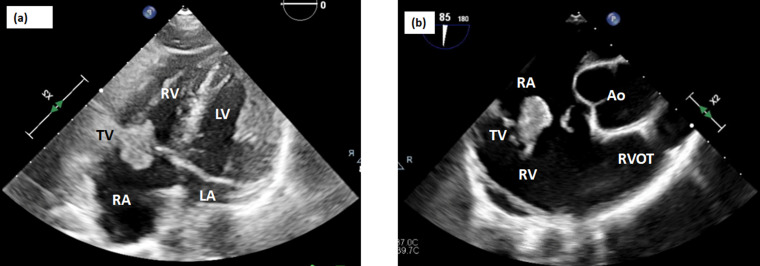
On physical examination, the patient was found to be malnourished and underweight (BMI 14.1 kg m−2), with poor dentition, showing multiple tooth fractures. His lungs were clear to auscultation bilaterally. No heart murmur, chest or back tenderness, joint swelling, tenderness or deformities, or suspicious skin lesions were noted on examination. A repeat transthoracic echocardiogram showed severe tricuspid regurgitation, left ventricular ejection fraction of ~50%, and the presence of a 1.8×2.0 cm mobile vegetation that appeared to be attached to the atrial aspect of the anterior tricuspid leaflet. The vegetation was observed to enter the right ventricle during diastole and obstruct tricuspid leaflet closure during systole. Smaller vegetations were also observed in the right ventricular outflow tract. (Fig. 1a). Carotid ultrasound demonstrated 14–25 % diameter reduction stenosis of the right and left internal carotid arteries. The patient was empirically started on cefepime for Gram-negative rod bacteraemia, but switched to piperacillin/tazobactam 4.5 g every 8 h after two of four blood cultures obtained from his previous hospitalization were identified as C. canimorsus through the previously described outside bacterial identification service provided by Mayo Clinic. Microbial sensitivities could not be obtained due to difficulty in culturing the organism. The decision was made to optimize the patient for future valve surgery, during which time he remained hospitalized for i.v. antibiotics, clearance of blood cultures and planning for tooth extraction to prevent reinfection.
Fig. 1.
Transthoracic and transesophageal echocardiogram images. (a) Apical four chamber view and (b) right ventricular inflow–outflow view showing large (~1.8×2 cm) tricuspid valve mobile vegetation attached to the atrial aspect of the anterior tricuspid leaflet. Mass enters the RV during diastole and obstructs tricuspid leaflet closure during systole. LV, left ventricle; RV, right ventricle; LA, left atrium; RA, right atrium; TV, tricuspid valve; Ao, aorta; RVOT, right ventricular outflow tract
On hospital day 20, a transesophageal echocardiograph (Fig. 1b) noted tricuspid valve vegetation with no notable progression in size. Due to increased right ventricular dilatation and pressure (29 mmHG) the patient underwent surgical debridement and tricuspid valve replacement. During the operation, a large 2 cm vegetation, along with several other smaller vegetations, was removed. The patient received a #33 Epic biological tissue valve prosthesis. Pathological evaluation of valve tissue found benign valvular tissue with adherent fibrinous debris with associated acute inflammation, although no bacterial organisms were identified by Gram stain or culture. Serial blood cultures since admission identified no organisms. The patient gradually improved for 1 week after surgery and completed a 20-day course of piperacillin/tazobactam but chose to leave against medical advice without any antibiotics.
The patient returned to the emergency room 1 week later after developing sharp, left-sided flank pain and a fever up to 38.3 °C. Given the incomplete piperacillin/tazobactam course and concern regarding resistance, he was started on meropenem for continued treatment of C. canimorsus infection. A repeat transesophageal echocardiogram was unremarkable and showed no significant echocontrast, thrombus, masses, protruding atheroma, patent foramen ovale, or paravalvular pathology on the tricuspid or native valves. However, the patient developed a worsening cough and was found to have a right lower lobe pulmonary embolism (previously seen on a prior CT scan from outside hospital) requiring anticoagulation, and a right loculated pleural effusion which resolved with chest tube placement. The patient completed an additional 28-day course of meropenem with no clinical evidence of recurrent infection at follow-up after discharge.
Discussion
C. canimorsus is a rare but emerging zoonotic pathogen that is now recognized as the second most frequently identified organism related to dog bite infections after Pasteurella multocida [2]. The increase in frequency associated with C. canimorsus infections has been speculated to be due to an increase in dog/cat owners leading to greater opportunities for animal bites, and enhanced laboratory and recovery techniques [8]. However, consistent identification and isolation of C. canimorsus remains elusive; primarily due to its fastidious, slow- growing nature and requirement for meticulous growth conditions, including enriched agar media and incubation in 5–10 % CO2 [9, 10].
Infective endocarditis caused by C. canimorsus is a rare and poorly understood manifestation, accounting for <2 % of reported C. canimorsus bloodstream infections [10]. To date, only 25 cases of C. canimorsus IE have been reported in the literature since its discovery in 1977 (Table 1). Approximately 80 % of reported cases of C. canimorusis infection were associated with a predisposing condition, such as splenectomy, cirrhosis, alcoholism, lung disease and immunocompromised status [2, 11]. Remarkably, 40 % of C. canimorsus IE cases presented with tricuspid valve IE, which is in contrast to the fact that tricuspid valve IE accounts for only 5–10 % of all IE cases. The present case adds to this body of literature, highlighting that C. canimorsus has an increased predilection for tricuspid valve infection that is independent of other known predisposing factors, such as alcoholism, intravenous drug abuse, cardiac implantable electronic devices, central venous catheters or congenital heart disease. While the exact mechanism that predisposes certain micro-organisms such as C. canimorus to manifest as tricuspid valve IE remains unclear, some have speculated that intrinsic differences in the endothelium of valves may influence adherence to particular heart valves. [12]
Table 1.
Summary of clinical features and treatment outcomes for patients with infective endocarditis caused by C. canimorsus reported in the literature based on a PubMed literature search 1977–2021 using the search terms ‘Capnocytophaga Canimorsus Endocarditis.’
|
Case |
Age/sex |
Comorbidities/risk factors |
Source of infection |
Valve involved |
Surgery intervention |
Antibiotic used (duration, days) |
Complications |
Outcome |
Reference |
|---|---|---|---|---|---|---|---|---|---|
|
1 |
50/M |
nd |
Dog bite |
AV |
Yes |
nd |
nd |
Died |
[14] |
|
2 |
nd |
nd |
nd |
AV |
Yes |
Penicillin G |
nd |
Cured |
[14] |
|
3 |
nd |
nd |
nd |
MV |
No |
Penicillin G |
nd |
Cured |
[14] |
|
4 |
64/M |
nd |
Dog bite |
TV, AV |
No |
Vancomycin+gentamicin |
nd |
Died |
[15] |
|
5 |
59/F |
Atrial myxoma, CLL, steroid use |
nd |
TV |
Yes |
Cephalothin+gentamicin (14) |
nd |
Died |
[16] |
|
6 |
39/M |
Alcohol abuse |
Dog contact |
MV |
No |
Ampicillin (42)+tobramycin |
Glomerulonephritis |
Cured |
[17] |
|
7 |
24/M |
Heart murmur |
Dog bite |
AV |
No |
Penicillin (28) |
nd |
Cured |
[18] |
|
8 |
47/M |
Alcohol abuse |
Dog contact |
TV |
Yes |
Vancomycin (14)+gentamicin (14)+penicillin (42) |
nd |
Cured |
[19] |
|
9 |
56/M |
nd |
Dog contact |
TV |
No |
Penicillin (42)+gentamicin (NS) |
nd |
Cured |
[20] |
|
10 |
52/M |
Aortic stenosis, pacemaker |
Dog bite |
AV |
No |
Penicillin G, aztreonam (35) |
nd |
Cured |
[21] |
|
11 |
69/F |
COPD |
None |
TV |
No |
Cefuroxime (7)+gentamicin (7),+flucloxacillin (7), penicillin G (42) |
nd |
Cured |
[22] |
|
12 |
63/M |
Aortic valve replacement |
Dog contact |
PAV |
Yes |
Ceftriaxone (28)+gentamicin (28), penicillin G (28) |
Anaemia, CHF |
Cured |
[23] |
|
13 |
41/F |
Rheumatic mitral valve disease |
Dog contact |
MV |
Yes |
Ceftriaxone |
nd |
Cured |
[24] |
|
14 |
42/M |
Alcohol abuse |
Dog bite |
AV |
Yes |
Ceftriaxone +gentamicin |
nd |
Cured |
[25] |
|
15 |
55/M |
COPD, alcohol abuse, i.v. drug user |
Dog |
AV, TV |
Yes |
Meropenem+ciprofloxacin |
nd |
Cured |
[26] |
|
16 |
65/M |
Dislipidemia, aortic stenosis, hypertension |
None |
AV, TV |
Yes |
Ampicillin +gentamicin |
Anaemia, renal insufficiency |
Cured |
[27] |
|
17 |
73/M |
Prosthetic atrial valve, atrial fibrillation, diabetes, renal insufficiency |
Dog contact |
AV |
No |
Meropenem+ciprofloxacin |
Anaemia |
Cured |
[28] |
|
18 |
43/M |
Alcohol abuse |
Lion bite |
AV, MV |
Yes |
Ceftriaxone+gentamicin+vancomycin |
None |
Died |
[29] |
|
19 |
76/F |
ICD |
Dog scratch |
ICD |
Yes |
nd |
nd |
Cured |
[30] |
|
20 |
49/F |
None |
Dog faeces |
TV |
Yes |
Meropenem (42) |
Pulmonary embolism |
Cured |
[31] |
|
21 |
46/M |
None |
Dog bite |
AV |
|
Gentamicin +cefazolin, ceftriaxone (28) |
nd |
Cured |
[9] |
|
22 |
59/F |
None |
Dog contact |
AV |
Yes |
Meropenem+vancomycin+gentamicin, i.v. benzylpenicillin (28) |
nd |
Cured |
[32] |
|
23 |
47/M |
None |
Dog contact |
AV |
Yes |
Meropenem (42) |
nd |
Cured |
[33] |
|
24 |
70/F |
Osteoarthritis, heart murmur, thymoma |
Dog contact |
TV |
Yes |
Benzylpenicillin (14) |
Pulmonary embolism |
Cured |
[34] |
|
25 |
63/M |
ESRD on HD, COPD, nephrectomy, prostate/lung cancer |
Dog contact |
TV |
No |
Piperacillin/tazobactam, amoxicillin/clavulanate, ceftriaxone +gentamicin |
nd |
Cured |
[35] |
M, male; F, female; nd, not documented, AV, aortic valve; MV, mitral valve; TV, tricuspid valve; ICD, implantable cardioverter defibrillator; COPD, chronic obstructive pulmonary disease; ESRD, end stage renal disease; HD, hemodialysis; T2DM, type 2 diabetes mellitus, CLL, chronic lymphocytic leukaemia.
In the present case, we were unable to identify any sites of inoculum, yet C. canimorsus infection was consistent with our patient’s history of close contact with his pet Rottweiler dog. Furthermore, we were unable to verify C. canimorsus by Gram stain and culture on the resected tricuspid tissue. However the observation of fibrinous debris with associated acute inflammation remains consistent with C. canimorsus IE, and there are no better alternative explanation for these findings. The difficulty of culturing C. canimorsus in vitro makes determining sensitivities challenging, as observed in the present case. Previous studies have shown that C. canimorsus is sensitive to imipnem, clindamycin, linezolid and tetracyclines [13]. Here we reported successful resolution of infection through a combination of piperacillin/tazobactam (20 days) and meropenem (28 days) due to initial noncompliance.
Conclusion
The presented case highlights the importance of vigilant management and awareness of C. canimorsus infection. Given the high case fatality rate for patients with C. canimorsus IE and the difficulty of organism identification, a high clinical index of suspicion in patients with animal contact and early intervention with antibiotics such as piperacillin/tazobactam or meropenem is required to prevent the poor outcomes associated with this infection.
Funding information
The authors received no specific grant from any funding agency.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Consent to publish
Written informed consent for publication of clinical details and clinical images was obtained from the patient.
Footnotes
Abbreviations: Ao, aorta; AV, aortic valve; CLL, chronic lymphocytic leukaemia; COPD, chronic obstructive pulmonary disease; ESRD, end stage renal disease; F, female; HD, hemodialysis; ICD, implantable cardioverter defibrillator; IE, infective endocarditis; LA, left atrium; LV, left ventricle; M, male; MV, mitral valve; ND, not documented; RA, right atrium; RV, right ventricle; RVOT, right ventricular outflow tract; T2DM, type 2 diabetes mellitus; TV, tricuspid valve.
References
- 1.Zajkowska J, Król M, Falkowski D, Syed N, Kamieńska A. Capnocytophaga canimorsus – an underestimated danger after dog or cat bite – review of literature. Przegl Epidemiol. 2016;70:289–295. [PubMed] [Google Scholar]
- 2.Butler T. Capnocytophaga canimorsus: an emerging cause of sepsis, meningitis, and post-splenectomy infection after dog bites. Eur J Clin Microbiol Infect Dis. 2015;34:1271–1280. doi: 10.1007/s10096-015-2360-7. [DOI] [PubMed] [Google Scholar]
- 3.Hästbacka J, Hynninen M, Kolho E. Capnocytophaga canimorsus bacteremia: clinical features and outcomes from a Helsinki ICU cohort. Acta Anaesthesiol Scand. 2016;60:1437–1443. doi: 10.1111/aas.12752. [DOI] [PubMed] [Google Scholar]
- 4.Chesdachai S, Tai DBG, Yetmar ZA, Misra A, Ough N, et al. The characteristics of Capnocytophaga infection: 10 years of experience. Open Forum Infect Dis. 2021;8:fab175. doi: 10.1093/ofid/ofab175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pers C, Gahrn-Hansen B, Frederiksen W. Capnocytophaga canimorsus septicemia in Denmark, 1982-1995: review of 39 cases. Clin Infect Dis. 1996;23:71–75. doi: 10.1093/clinids/23.1.71. [DOI] [PubMed] [Google Scholar]
- 6.Kullberg BJ, Westendorp RG, Meinders AE. Purpura fulminans and symmetrical peripheral gangrene caused by Capnocytophaga canimorsus (formerly DF-2) septicemia--A complication of dog bite. Medicine (Baltimore) 1991;70:287–292. doi: 10.1097/00005792-199109000-00001. [DOI] [PubMed] [Google Scholar]
- 7.Brenner DJ, Hollis DG, Fanning GR, Weaver RE. Capnocytophaga canimorsus sp. nov. (formerly CDC group DF-2), a cause of septicemia following dog bite, and C. cynodegmi sp. nov., a cause of localized wound infection following dog bite. J Clin Microbiol. 1989;27:231–235. doi: 10.1128/jcm.27.2.231-235.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Janda JM, Graves MH, Lindquist D, Probert WS. Diagnosing Capnocytophaga canimorsus infections. Emerg Infect Dis. 2006;12:340–342. doi: 10.3201/eid1202.050783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sakai J, Imanaka K, Kodana M, Ohgane K, Sekine S, et al. Infective endocarditis caused by Capnocytophaga canimorsus; a case report. BMC Infect Dis. 2019;19:927. doi: 10.1186/s12879-019-4492-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sandoe JAT. Capnocytophaga canimorsus endocarditis. J Med Microbiol. 2004;53:245–248. doi: 10.1099/jmm.0.05274-0. [DOI] [PubMed] [Google Scholar]
- 11.Griego RD, Rosen T, Orengo IF, Wolf JE. Dog, cat, and human bites: a review. J Am Acad Dermatol. 1995;33:1019–1029. doi: 10.1016/0190-9622(95)90296-1. [DOI] [PubMed] [Google Scholar]
- 12.Frontera JA, Gradon JD. Right-side endocarditis in injection drug users: review of proposed mechanisms of pathogenesis. Clin Infect Dis. 2000;30:374–379. doi: 10.1086/313664. [DOI] [PubMed] [Google Scholar]
- 13.Jolivet-Gougeon A, Sixou JL, Tamanai-Shacoori Z, Bonnaure-Mallet M. Antimicrobial treatment of Capnocytophaga infections. Int J Antimicrob Agents. 2007;29:367–373. doi: 10.1016/j.ijantimicag.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 14.Butler T, Weaver RE, Ramani TK, Uyeda CT, Bobo RA, et al. Unidentified gram-negative rod infection. A new disease of man. Ann Intern Med. 1977;86:1–5. doi: 10.7326/0003-4819-86-1-1. [DOI] [PubMed] [Google Scholar]
- 15.Shankar PS, Scott JH, Anderson CL. Atypical endocarditis due to gram-negative bacillus transmitted by dog bite. South Med J. 1980;73:1640–1641. doi: 10.1097/00007611-198012000-00031. [DOI] [PubMed] [Google Scholar]
- 16.Worthington M, Gleason T, Pandian NG, Daly B. Tricuspid valve myxoma infected with dysgonic fermenter-2. South Med J. 1984;77:241–242. doi: 10.1097/00007611-198402000-00027. [DOI] [PubMed] [Google Scholar]
- 17.Archer SL. Dysgonic fermenter 2 infection resulting in chronic glomerulonephritis. Can Med Assoc J. 1985;132:657–660. [PMC free article] [PubMed] [Google Scholar]
- 18.Newton NL, Sharma B. Acute myocardial infarction associated with DF-2 bacteremia after a dog bite. Am J Med Sci. 1986;291:352–354. doi: 10.1097/00000441-198605000-00012. [DOI] [PubMed] [Google Scholar]
- 19.Niefield S, Young EJ. Native valve endocarditis caused by dysgonic fermenter type 2 bacilli. Am J Med Sci. 1988;296:69–70. doi: 10.1097/00000441-198807000-00013. [DOI] [PubMed] [Google Scholar]
- 20.Andersen HK, Pedersen M. Infective endocarditis with involvement of the tricuspid valve due to Capnocytophaga canimorsus . Eur J Clin Microbiol Infect Dis. 1992;11:831–832. doi: 10.1007/BF01960884. [DOI] [PubMed] [Google Scholar]
- 21.Decoster H, Snoeck J, Pattyn S. Capnocytophaga canimorsus endocarditis. Eur Heart J. 1992;13:140–142. doi: 10.1093/oxfordjournals.eurheartj.a060035. [DOI] [PubMed] [Google Scholar]
- 22.Kooter AJ, Derks A, Vasmel WLE. Rapidly progressive tricuspid valve endocarditis caused by Capnocytophaga canimorsus infection in an immunocompetent host. Clin Microbiol Infect. 1999;5:173–175. doi: 10.1111/j.1469-0691.1999.tb00533.x. [DOI] [PubMed] [Google Scholar]
- 23.Ngaage DL, Kotidis KN, Sandoe JA, Unnikrishnan Nair R. Do not snog the dog: infective endocarditis due to Capnocytophaga canimorsus . Eur J Cardiothorac Surg. 1999;16:362–363. doi: 10.1016/s1010-7940(99)00131-1. [DOI] [PubMed] [Google Scholar]
- 24.Frigiola A, Badia T, Lovato R, Cogo A, Fugazzaro MP, et al. Infective endocarditis due to Capnocytophaga canimorsus . Ital Heart J. 2003;4:725–727. [PubMed] [Google Scholar]
- 25.Wareham DW, Michael JS, Warwick S, Whitlock P, Wood A, et al. The dangers of dog bites. J Clin Pathol. 2007;60:328–329. doi: 10.1136/jcp.2006.037671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hayani O, Higginson LAJ, Toye B, Burwash IG. Man’s best friend? Infective endocarditis due to Capnocytophaga canimorsus. Can J Cardiol. 2009;25:e130–2. doi: 10.1016/s0828-282x(09)70076-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Coutance G, Labombarda F, Pellissier A, Legallois D, Hamon M, et al. Capnocytophaga canimorsus endocarditis with root abscess in a patient with a bicuspid aortic valve. Heart Int. 2009;4:e5. doi: 10.4081/hi.2009.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jalava-Karvinen P, Grönroos JO, Tuunanen H, Kemppainen J, Oksi J, et al. Capnocytophaga canimorsus: a rare case of conservatively treated prosthetic valve endocarditis. APMIS. 2018;126:453–456. doi: 10.1111/apm.12839. [DOI] [PubMed] [Google Scholar]
- 29.Barry M. Double native valve infective endocarditis due to Capnocytophaga canimorsus: first reported case caused by a lion bite. Case Rep Infect Dis. 2018;2018:4821939. doi: 10.1155/2018/4821939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Squire G, Hetherington S. First reported case of lead-related infective endocarditis secondary to Capnocytophaga canimorsus: “Dog Scratch” endocarditis. BMJ Case Rep. 2020;13:e233783. doi: 10.1136/bcr-2019-233783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardoso RM, Rodrigues J, Garcia D, Campos ID. Tricuspid valve endocarditis due to Capnocytophaga canimorsus . BMJ Case Rep. 2019;12:12. doi: 10.1136/bcr-2019-233721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.McNicol M, Yew P, Beattie G, Loughlin L. A case of Capnocytophaga canimorsus endocarditis in A non-immunosuppressed host: the value of 16S PCR for diagnosis. Access Microbiol. 2021;3:000235. doi: 10.1099/acmi.0.000235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sri A, Droscher E, De Palma R. Capnocytophaga canimorsus and infective endocarditis-making a dog’s dinner of the aortic valve: a case report. Eur Heart J Case Rep. 2021;5:ytab278. doi: 10.1093/ehjcr/ytab278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindén S, Gilje P, Tham J, Lindstedt S, Rasmussen M. Capnocytophaga canimorsus tricuspid valve endocarditis. IDCases. 2021;24:e01083. doi: 10.1016/j.idcr.2021.e01083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oluyombo R, Tsiouli E, Gunda SS. Case report of Capnocytophaga canimorsus septicemia with infective endocarditis in A hemodialysis patient: A not widely known dog pathogen. Hemodial Int. 2021;25:E22– E25. doi: 10.1111/hdi.12917. [DOI] [PubMed] [Google Scholar]