Abstract
Background:
Pediatric and adolescent HIV treatment programs in sub-Saharan Africa have rapidly expanded and evolved over the past decade. Real-world evidence of how implementation of new policies over time has impacted treatment outcomes is lacking, but crucial for guiding implementation of the next phases of the HIV treatment response in children. We examined how treatment outcomes in Zambia’s national pediatric and adolescent HIV treatment programs changed over time as new policies were implemented.
Methods:
We used data from Zambia’s routine electronic health record to analyze ART-naïve children and adolescents living with HIV (CALHIV) between the ages of 0 and 19 years old who newly enrolled in care between January 1, 2011 and March 31, 2019 at 102 facilities supported by the Centre for Infectious Disease Research in Zambia (CIDRZ). We characterize changes in the distribution of the age and sex of new enrollees over time. We use an interrupted time-series design to examine rates of ART initiation, retention in care, time to ART initiation, and first-line ART regimens among new enrollees across different age strata as they changed over time with the adoption of new ART guidelines in 2014 and 2017.
Findings:
Between January 1, 2011 and March 31, 2019, 26,214 ART-naïve CALHIV newly enrolled at one of 102 ART facilities in two provinces in Zambia. Rates of new enrollments increased by 25–35% among children under the age of 15 over time, but by 92.3% among adolescents, with the largest absolute increase among adolescent females. Rates of ART initiation increased steadily and in parallel across all age groups from before the 2014 guidelines to after the 2017 guidelines were implemented (<2y: 42.4% to 81.6%; 2–5y: 39.3% to 82.8%; 5–15y: 49.2% to 86.6%; 15–19y: 52.4% to 86.2%); median time to ART initiation went from 2 to 3 months to same-day initiation during this same time period. Rates of retention on ART 6 months after linkage saw much smaller improvements over time (<2y: 35.4% to 52.0%; 2–5y: 40.2% to 54.4%; 5–15y: 46.7% to 63.4%; 15–19y: 40.1% to 52.7%).
Interpretation:
Improvements in ART initiation occurred largely in parallel across age groups over time despite universal treatment being implemented at different time points for different ages. Although rates of ART initiation reach high levels, retention on ART remained low. This analysis provides a comprehensive examination of how pediatric and adolescent outcomes have evolved over the past decade in Zambia and identifies where more targeted efforts will be needed over the next decade.
Funding:
National Institutes of Health
Keywords: HIV, pediatric, adolescent, treatment outcomes, enrollment, ART initiation, retention in care, guideline changes, universal treatment, interrupted time-series
INTRODUCTION
Despite strong evidence for HIV treatment and prevention, outcomes across the care continuum among children and adolescents living with HIV (CALHIV) and progress in reaching UNAIDS 90-90-90 targets have lagged behind gains seen in adult population1–3. Over the past decade, evolving data has led the World Health Organization (WHO) and national HIV programs to rapidly adapt guidelines on treatment eligibility and treatment regimens over time, most recently culminating in recommendations for universal treatment regardless of age or immune status. These policy changes are aimed at optimizing treatment and prevention, but real-world evidence assessing the implementation of these policies and how they have impacted outcomes among CALHIV over time is lacking but crucial for developing the next phases of the HIV treatment response in children and adolescents.
Assessing the evolution and progress of pediatric and adolescent HIV programs can help identify both the gaps in care that have been successfully addressed while also underscoring those that still need attention across different age groups. Over the past decade, universal ART has gradually been adopted across all age groups, but how this correlates with rates of ART initiation, the selection of age-appropriate regimens, retention in care, and, ultimately, viral suppression is unknown. Programs have also experienced demographic shifts from younger children to more adolescents4. Understanding both the current evolution of treatment programs is needed to inform and prioritize emerging innovations in prevention, testing, and treatment strategies1–3,5–7 for different age groups and types of care gaps. Such rigorous epidemiologic evidence can thus help guide tailored approaches across the care continuum and will enable pediatric and adolescent HIV programs continue to evolve and reach newer 95-95-95 targets for epidemic control.
We leverage data from Zambia’s national pediatric and adolescent HIV program from 2011 to 2019 to examine temporal trends in outcomes and the impact of changes in treatment guidelines across different age groups. Zambia has one of the largest public health HIV care and treatment programs in Africa—approximately 72,000 children and 66,000 adolescents currently living with HIV—and a national electronic health record (EHR), SmartCare, that has allowed the country to monitor its large cohort of individuals over a substantial period of time. We leverage this EHR to examine demographic shifts in the number of CALHIV newly entering HIV care across age groups. We also use an interrupted time series design to examine rates of ART initiation, time to ART initiation, retention in care, and first-line ART regimens as they changed over time with the adoption of new ART guidelines8. This analysis provides a comprehensive examination of how pediatric and adolescent HIV outcomes have evolved over the past decade in Zambia and identifies where more targeted efforts will be needed over the next decade.
METHODS
Patient Population and Setting
We analyzed data from a cohort of ART-naïve CALHIV between the ages of 0 and 19 years old who newly enrolled in care between January 1, 2011 and March 31, 2019. CALHIV were from 102 Zambian Ministry of Health facilities that received technical support from the Centre for Infectious Disease Research in Zambia (CIDRZ), a nongovernmental organization operating across 2 of the 10 provinces in Zambia. Date of enrollment was considered the first visit an individual made to an HIV facility to receive care (i.e., linkage), and we excluded CALHIV who had evidence of previously receiving care at a different facility (i.e., transfer ins). During this time period, Zambia updated its HIV treatment guidelines on February 1, 2014 and then again on January 1, 2017 (appendix p 6). In 2014, universal ART was adopted as policy for children between 2 and 15 years old (it was already in place since 2010 for children less than 2 years old) and any pregnant or breastfeeding woman (i.e., Option B+); additionally, the CD4 eligibility threshold for everyone else was increased from less than 350 cell/μl to less than 500 cells/μl. In 2017, Zambia transitioned to universal ART treatment for all people living with HIV. CALHIV ineligible for ART at the time of enrollment were followed up with clinical evaluations and CD4 counts every 6 months until becoming eligible for ART.
The study was approved by the University of Zambia Biomedical Research Ethics Committee (UNZABREC) and the institutional review board at the Washington University School of Medicine. This research consists of secondary analyses of pre-existing de-identified programmatic data; thus, informed consent was not obtained.
Measurements
Sociodemographic, clinical, facility-level, and visit history measurements were obtained from the national EHR and laboratory systems used in routine HIV care in Zambia. To populate the EHR, providers first complete standardized paper clinical forms during routine patient encounters, and then data clerks enter this information into the electronic database. We queried this database for all individual-level patient sociodemographic characteristics (e.g., age, sex, facility), clinical characteristics (e.g., CD4 counts, WHO Stage, anthropometrics, ART regimen), and longitudinal visit history (e.g., follow-up visits, ART pick-ups, enrollment date, date of ART initiation) data from our cohort. Data capture extended through December 31, 2019 to ensure all individuals had adequate time to observe the retention outcome.
Statistical Analysis
We sought to examine how Zambia’s national pediatric and adolescent HIV treatment programs have changed over time. First, we describe how the distribution of the age and sex of CALHIV newly enrolling in care changed over time. Second, we sought to examine changes in patient outcomes in Zambia’s national pediatric and adolescent HIV treatment programs over time, with specific focus on the impact of implemented new HIV treatment guidelines. To do so, we used an interrupted time-series design to examine how outcomes among newly enrolling CALHIV have changed over time in relation to these guidelines changes8. Among all newly enrolling CALHIV, we examined ART initiation within 3 months of enrollment, time from enrollment to ART initiation, and retention in care on ART at 6 months (defined as having initiated ART and making at least one visit between 3- and 9- months post-enrollment after). Among those individuals who initiated ART, we also examined the proportion initiated on either an efavirenz-, nevirapine-, or lopinavir/ritonavir-based regimen. We also examined scale-up of dolutegravir-based regimens across age groups in supplemental analysis as it was only introduced in mid-2018 in Zambia.
We considered that the implementation of new guidelines would be associated with an initial rapid (but not immediate) effect on patient outcomes during the first 90 days after guidelines were rolled out, followed by a return to a new baseline secular trend after the 90-day transition period up until the next treatment guidelines were implemented. Thus, statistically, we modelled individual-level patient outcomes as a function of their date of enrollment using linear splines, stratifying by age group. We modelled a change of slope during the first 90 days after guidelines were implemented in 2014 and 2017 (i.e., to account for the rapid transition period where guidelines were being fully implemented) followed by another change of slope after the transition period to establish the new baseline secular trend during the remainder of the guideline period8. We applied established methods using Poisson regression with robust variances to model binary outcomes (i.e., ART initiation within 3 months, retention on ART at 6 months, and ART regimens). For time to ART initiation, we used median regression (i.e., quantile regression estimating the 50th percentile) to estimate the median time to ART initiation among all newly enrolling CALHIV. This approach estimating the median allowed us to account for individuals who never initiated ART (in contrast to using linear regression to estimate the mean). From these models, we then estimated marginal estimates (i.e., absolute percentages of ART initiation/retention or median time to ART) with 95% confidence interval and also estimated the change in outcomes from a baseline immediately prior to 2014 and 2017 guideline rollout compared to 90, 180, 365, and 730 days after guideline rollout.
Additional methodologic details on study setting, measurements, and statistical methods are presented in the appendix (p 2).
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
RESULTS
Between January 1, 2011 and March 31, 2019, 26,214 ART naïve CALHIV newly enrolled at one of 102 ART facilities in two provinces in Zambia (Figure 1) (4,234 <2 year olds [16.2%], 3,038 2–5 year olds [11.6%], 8,128 5–15 year olds [31.0%], and 10,814 15–19 year olds [41.3%]). Among children below the age of fifteen entering care, sex was evenly distributed (<2 year olds 53.1% female, 2–5 year olds 50.5% female, 5–15 year old 55.5% female), but adolescents between 15 and 19 years old skewed heavily towards being female (82.6%) (Table 1). A majority of children were from Lusaka as opposed to Western province.
Figure 1: Patient Flowchart.
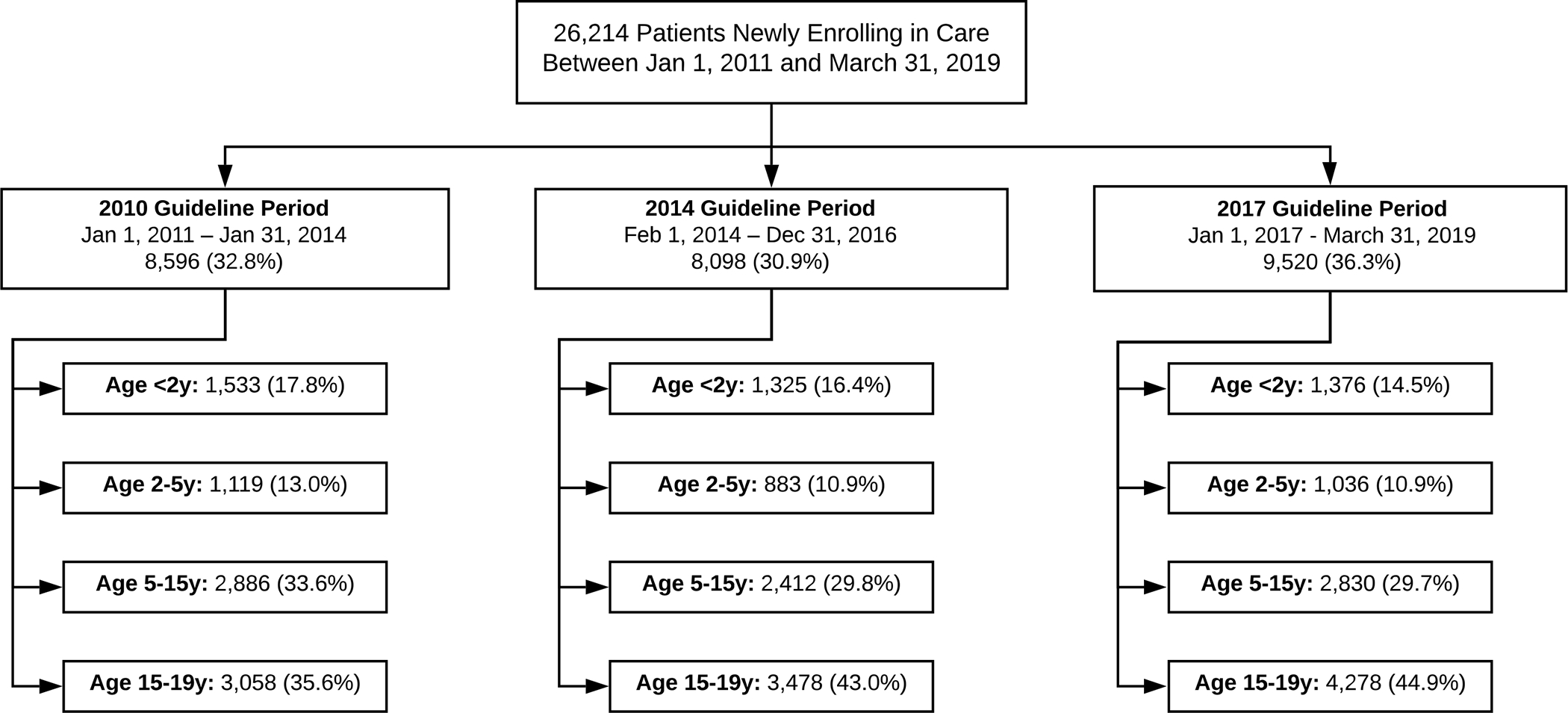
Table 1.
Baseline patient characteristics by guideline exposure and age group, n = 26,214
| <2 years | 2–5 years | 5–15 years | 15–19 years | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 2010 Guidelines (n = 1533) | 2014 Guidelines (n = 1325) | 2017 Guidelines (n = 1376) | 2010 Guidelines (n = 1119) | 2014 Guidelines (n = 883) | 2017 Guidelines (n = 1036) | 2010 Guidelines (n = 2886) | 2014 Guidelines (n = 2412) | 2017 Guidelines (n = 2830) | 2010 Guidelines (n = 3058) | 2014 Guidelines (n = 3478) | 2017 Guidelines (n = 4278) | |
| Male Sex, n (%) | 703 (45.9) | 643 (48.5) | 641 (46.6) | 542 (48.4) | 442 (50.1) | 521 (50.3) | 1294 (44.8) | 1064 (44.1) | 1260 (44.5) | 547 (17.9) | 578 (16.6) | 754 (17.6) |
| Median Age, years (IQR) | 1.1 (0.6, 1.5) | 1.1 (0.6, 1.6) | 1.0 (0.4, 1.5) | 3.2 (2.5, 4.1) | 3.2 (2.5,4.1) | 3.3 (2.6, 4.1) | 9.5 (7.1, 12.5) | 9.9 (7.5,12.2) | 9.9 (7.4, 12.4) | 18.5 (17.2,19.3) | 18.5 (17.3,19.4) | 18.6 (17.5,19.4) |
| Median CD4 count at enrollment, cells/μL (IQR) | 974 (576, 1475) | 1032 (648,1519) | 1091 (677,1526) | 809 (499,1199) | 723 (423,1133) | 711 (430,1061) | 484 (286, 747) | 439 (226, 689) | 457 (261,723) | 368 (224, 541) | 356 (212, 534) | 369 (226, 545) |
| Median CD4 percent at enrollment, (IQR) | 19.8 (13.5, 28.6) | 19.5 (14.1,25.5) | 20.0 (15.0, 25.5) | 22.5 (14.4, 31.0) | 19.3 (12.0, 27.4) | 19.7 (12.3, 27.1) | 19.0 (11.9, 26.8) | 16.7 (10.0, 26.1) | 20.1 (13.2, 29.2) | 19.4 (13.3, 26.2) | 19.0 (11.9, 26.2) | 21.2 (14.0, 29.2) |
| WHO stage at enrollment, n (%) | ||||||||||||
| I | 51 (43.2) | 25 (21.6) | 11 (31.4) | 42 (53.0) | 9 (14.0) | 8 (33.0) | 206 (59.5) | 89 (44.5) | 75 (65.2) | 1518 (62.4) | 1821 (68.0) | 2474 (79.0) |
| II | 8 (6.8) | 2 (1.7) | 1 (2.9) | 3 (4.0) | 5 (8) | 0 (0.0) | 52 (15.0) | 19 (9.5) | 7 (6.1) | 337 (13.8) | 393 (14.7) | 329 (10.5) |
| III | 54 (45.8) | 83 (71.6) | 23 (65.7) | 29 (37) | 48 (76) | 16 (67) | 82 (23.7) | 88 (44.0) | 33 (28.7) | 532 (21.9) | 421 (15.7) | 314 (10.0) |
| IV | 5 (4.2) | 6 (5.2) | 0 (0.0) | 5 (6.0) | 1 (2.0) | 0 (0.0) | 6 (17) | 4 (2.0) | 0 (0.0) | 47 (19) | 44 (16) | 14 (0.4) |
| Median Weight/BMI- for-Ageb z-score at enrollment, (IQR) | −1.8 (−3.0, −0.6) | −1.6 (−2.7, −0.4) | −1.2 (−2.5, −0.1) | −2.0 (−3.0, −0.9) | −1.9 (−3.0, −0.9) | −1.6 (−2.5, −0.7) | −0.8 (−1.7, 0.1) | −1.0 (−2.0, −0.1) | −0.7 (−1.7, 0.2) | −0.4 (−1.3, 0.4) | −0.2 (−1.2, 0.6) | −0.1 (−1.0, 0.6) |
| Province, n (%) | ||||||||||||
| Lusaka | 1319 (86.0) | 1137 (85.8) | 1247 (90.6) | 955 (85.3) | 742 (84.0) | 903 (87.2) | 2594 (89.9) | 2097 (86.9) | 2427 (85.8) | 2770 (90.6) | 3083 (88.6) | 3813 (89.1) |
| Western | 214 (14.0) | 188 (14.2) | 129 (9.4) | 164 (14.7) | 141 (16.0) | 133 (12.8) | 292 (10.1) | 315 (13.1) | 403 (14.2) | 288 (9.4) | 395 (11.4) | 465 (10.9) |
| ART initiated within 3 months, n (%) | 628 (41.0) | 855 (64.5) | 1083 (78.7) | 473 (42.3) | 612 (69.3) | 840 (81.2) | 1291 (44.7) | 1709 (70.9) | 2411 (85.2) | 1370 (44.8) | 2541 (73.1) | 3786 (88.5) |
| Median Days to ART initiationc, (IQR) | 42 (14, 203) | 6 (0, 37) | 0 (0, 1) | 45 (14,294) | 9 (0, 42) | 0 (0, 1) | 43 (14, 322) | 14 (0, 45) | 0 (0, 1) | 28 (0,210) | 7 (0, 34) | 0 (0, 0) |
| Median CD4 count at ART initiationc cells/μL (IQR) | 1034 (615, 1530) | 1095 (675, 1618) | 1060 (580, 1500) | 810 (495, 1181) | 708 (412, 1049) | 718 (451, 1062) | 446 (285, 720) | 418 (225, 651) | 458 (261,728) | 326 (198, 483) | 337 (206, 494) | 369 (229, 542) |
| Median CD4 percent at ART initiationc (IQR) | 20.7 (14.2, 31.0) | 20.6 (14.6, 26.1) | 21.4 (15.8, 28.7) | 24.5 (14.8, 33.7) | 19.5 (11.0, 27.4) | 20.5 (13.5, 27.3) | 18.6 (12.2, 26.6) | 16.5 (10.0, 25.7) | 20.6 (13.4, 29.3) | 18.0 (12.2, 25.5) | 18.5 (11.7, 25.7) | 21.2 (14.2, 29.2) |
| First ART Regimenc, n (%) | ||||||||||||
| EFV-based | 57 (5.8) | 97 (9.4) | 52 (4.4) | 121 (14.8) | 139 (18.8) | 173 (19.3) | 696 (32.3) | 1251 (61.4) | 1764 (69.7) | 1200 (62.5) | 2552 (94.3) | 3320 (92.8) |
| LPV-based | 70 (7.1) | 547 (52.8) | 927 (79.0) | 19 (2.3) | 277 (37.5) | 577 (64.5) | 31 (1.4) | 88 (4.3) | 359 (14.2) | 21 (1.1) | 35 (1.3) | 53 (1.5) |
| NVP-based | 746 (76.0) | 289 (27.9) | 47 (4.0) | 614 (75.2) | 245 (33.2) | 49 (5.5) | 1297 (60.2) | 575 (28.2) | 156 (6.2) | 588 (30.6) | 72 (2.7) | 32 (0.9) |
| DTG-based | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (0.1) | 0 (0) | 0 (0) | 37 (1.5) | 0 (0) | 0 (0) | 113 (3.2) |
| Other | 108 (11.0) | 103 (9.9) | 148 (12.6) | 63 (7.7) | 77 (10.4) | 95 (10.6) | 132 (6.1) | 125 (6.1) | 215 (8.5) | 110 (5.7) | 47 (1.7) | 61 (1.7) |
Weight-for-age z-score calculated for those <5 years old, BMI-for-age z-score calculated for those 5–25 years old.
Only among in individuals who initiated ART. Abbreviations: EFV – Efavirenz, NVP – Nevirapine, LPV – lopinavir, DTG - dolutegravir
Among children less than 15 years old, the absolute number of newly enrolling children per year remained relatively stable prior to 2017, with 25–35% increases across age groups after 2017 (Figures 1 and 2). In contrast, the absolute number of newly enrolling 15–19 year olds per year has continued to grow steadily over time (92.3% increase between the 2011 and 2017 guidelines periods), largely driven by increase in the number of female adolescents newly entering care. Thus, adolescents have made up a greater proportion of newly enrolling children over time, while the proportion made up of <2 year olds, 2–5 year olds, and 5–15 year olds has been decreasing. Despite changing patterns in the number of patients newly enrolling, particularly among adolescents, the sex distribution remained stable over time across all age groups (Figure 2).
Figure 2: Distribution of Newly Enrolling Children and Adolescents by Age, Sex, and Time.
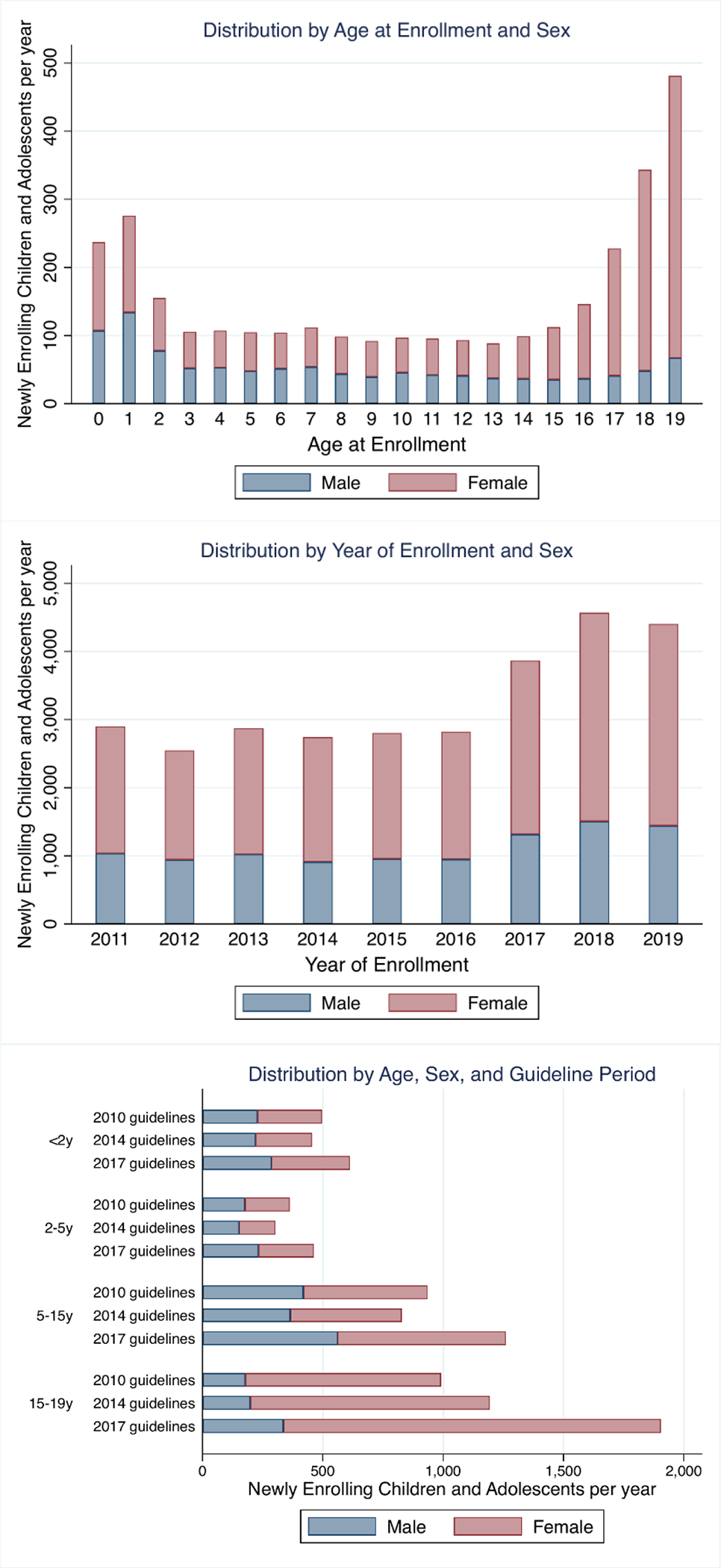
Numbers of newly enrolling children and adolescents are standardized to represent rate of new enrollees per year for periods where partial years are included.
Among less than 2 year olds, ART initiation by 3 months increased, first rapidly then steadily, after implementation of the 2014 guidelines despite universal ART being policy since 2010 (Figure 3, appendix p 8). Rates of ART initiation mostly stabilized around 75–80% by the time the 2017 guidelines were implemented with only small improvements after these guidelines were introduced. Retention on ART at 6 months steadily increased through both the 2010 and 2014 guideline period until stabilizing around 50% during the 2017 guideline period (Figure 3, appendix p 8). During the 2010 guidelines period, children were most frequently initiated on a nevirapine-based regimen, but this shifted to predominantly lopinavir-based regimens by the end of the 2014 guideline period (~80%) (Figure 4, appendix p 10).
Figure 3: Interrupted-Time-Series Analysis of ART Initiation and Retention in Care over Time.
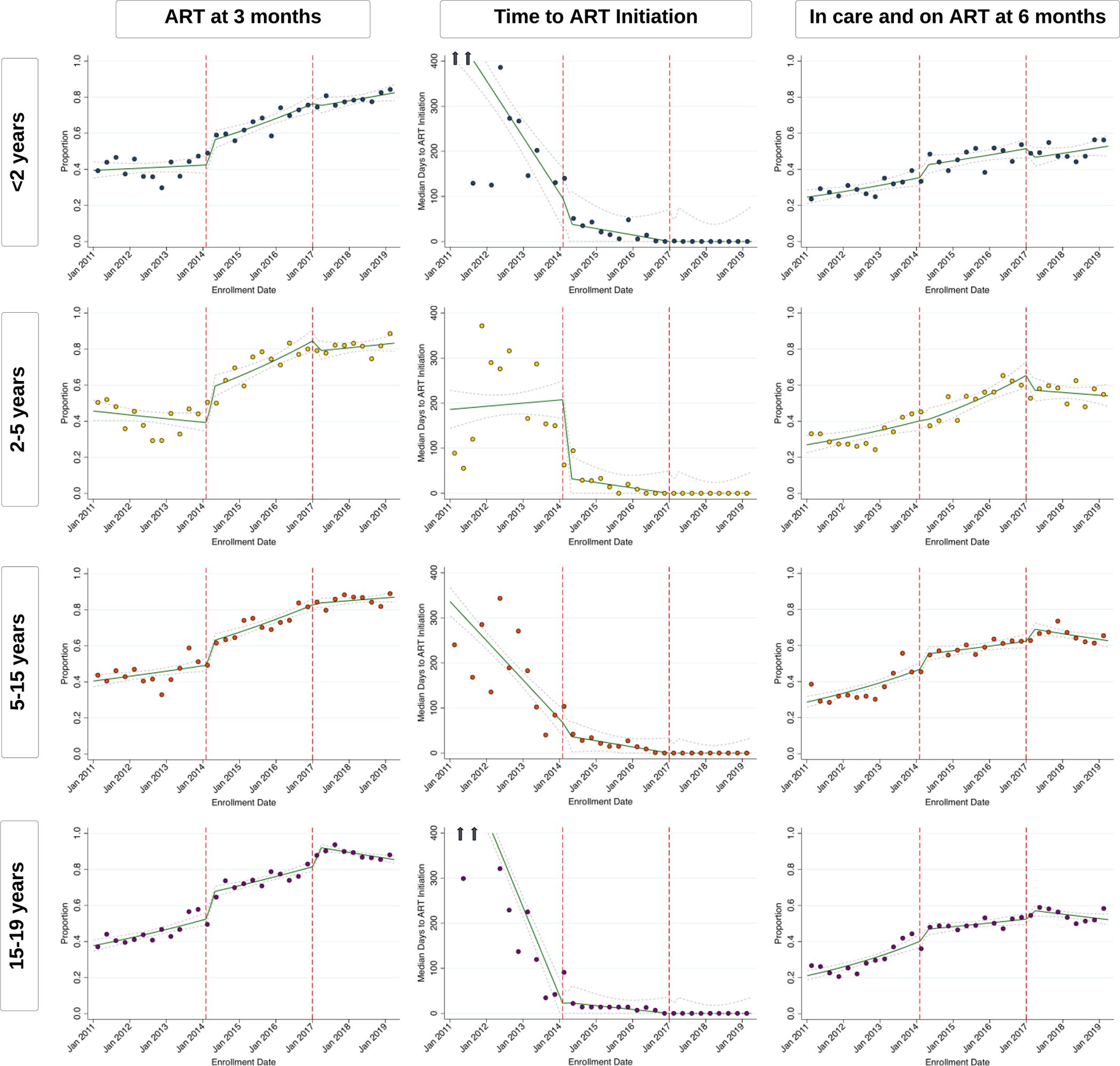
Green lines represent point estimates with confidence intervals (dashed grey lines) from interrupted-time-series models. Each scatter point represents observed individual-level data binned and averaged over 3-month quarters. For ART initiation by 3 months and In Care on ART by 6 months, lines and scatter plots represent the proportion of individuals who enrolled in care on a particular day with that outcome, and, for time to ART initiation, it represents the median days to ART initiation; arrows indicate the presence of estimates beyond the y-axis scale. The denominator for each outcome is all individuals newly enrolling care regardless of whether they initiated ART. Dashed red lines indicate the time that new guidelines were implemented.
Figure 4: Interrupted-Time-Series Analysis of ART Regimens over Time.
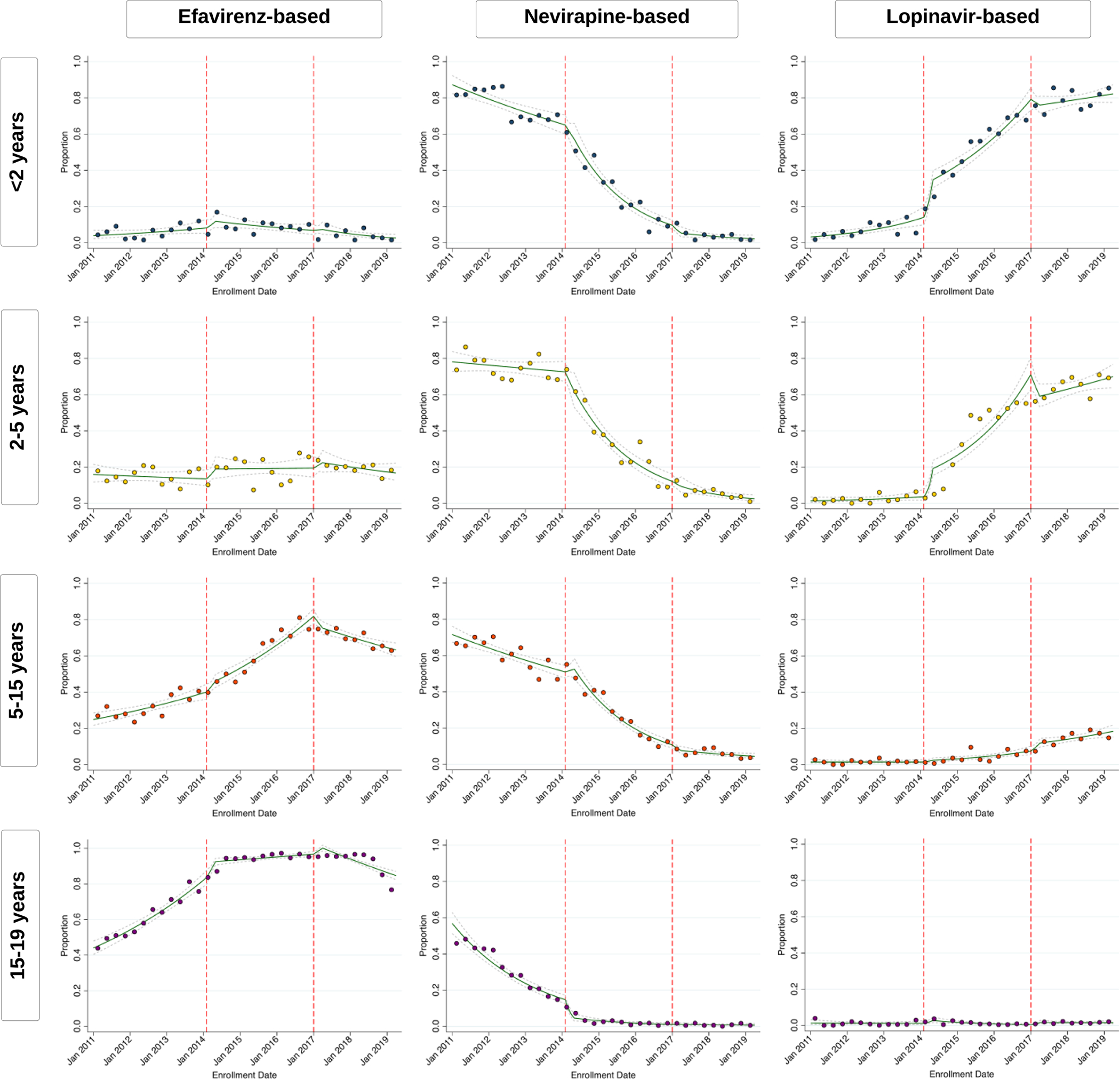
Green lines represent point estimates with confidence intervals (dashed grey lines) from interrupted-time-series models. Each scatter point represents observed individual-level data binned and averaged over 3-month quarters. Lines and scatter plots represent the proportion of individuals who were started on a particular ART regimen based on their data of enrollment. The denominator for ART regimen newly enrolling individuals who are also known to have initiated ART. Dashed red lines indicate the time that new guidelines were implemented.
Among both the 2–5 year old and 5–15 year old group, ART initiation by 3 months sharply increased within the first 90 days of implementation of 2014 guidelines (when universal treatment was implemented for both these age groups), but continued to steadily increase through the 2014 guideline period until stabilizing around 80–85% during the 2017 guideline period (Figure 3, appendix p 8). Similarly, retention on ART at 6 months steadily increased through the 2010 and 2014 guideline periods, but remained relatively steady after implementation of the 2017 guidelines (around 55% for 2–5 year olds and 65% for 5–15 year olds). Both age groups shifted from being initiated on nevirapine-based regimens to either lopinavir-based regimens (for 2–5 year olds) or efavirenz-based regimens (for 5–15 year olds) during the 2014 guideline period (Figure 4, appendix p 10). Since its introduction in mid-2018, 5–15 year olds have now also slowly started transitioning to dolutegravir-based regimens (appendix p 12).
Lastly, among 15–19 year olds, both the 2014 and 2017 guidelines led to sharp increases in ART initiation during the first 90 days after guidelines were implemented (Figure 3, appendix p 8). After the 2014 guidelines, rates of ART initiation continued to increase until 2017, but after implementation of the 2017 guidelines, rates remained stable around 85–90% after the first 90 days. Retention on ART followed a similar pattern with rates plateauing around 55% after universal treatment was implemented in this age group (i.e., after the 2017 guidelines). Zambia’s national treatment program almost fully transitioned to initiating adolescents on efavirenz-based regiments prior to the 2014 guidelines being implemented (Figure 4, appendix p 10), but is now also rapidly shifting to dolutegravir-based regimens since its introduction (appendix p 12).
Across all age groups, the median time to ART initiation was frequently greater than 3 months prior to the 2014 guideline period, but steadily decreased over time to around 2 weeks during the 2014 guidelines period and to same-day initiation after the 2017 guidelines were implemented (Figure 3, appendix p 8).
Secular time (i.e., guideline periods) and the facility a patient enrolled were the factors most associated with ART initiation and retention on ART across all age groups (appendix p 13). Facility explained a high degree of the total variability in patient outcomes among children less than 15 years old, but accounted for significantly less of variability in outcomes among adolescents. Additionally, being from Western Province was associated with similar incidence of ART initiation but trended towards decreased retention in care across all age strata. A documented enrollment CD4 count was also strongly associated with increased rates of retention in care across age strata.
DISCUSSION
We used an interrupted time series design to assess temporal trends and the impact of national policy changes on ART initiation and retention in care in Zambia’s pediatric and adolescent HIV program. Overall, these findings help to understand the programmatic successes with regards to ART initiation and retention in care, whilst also highlighting the substantial advancement in HIV prevention and treatment outcomes that will be needed over the next decade and beyond.
Over the past decade—but particularly between 2014 and 2017—there were large advances in ART initiation, time to ART initiation, and retention in care. This coincides with the period of rapid expansion of Zambia’s pediatric ART program where substantial efforts were being made to reach 90-90-90 targets. Outcomes appear to reach a plateau after this period and around the time when universal treatment for all individuals living with HIV was implemented. This may reflect the point at which the Zambian treatment program became saturated and programmatic scale-up and expansion was no longer sufficient to reach the more peripheral pediatric populations. Those children “left behind” and who are still not initiating ART even with expanded access and universal ART eligibility likely require targeted, innovative programs in non-standard forums including community distribution points and other differentiated service delivery models1–3,5,6. Although rates of and time to ART initiation improved substantially, rates of retention in care continued to lag behind with only 50–60% of those linking to care being in care and on ART six months later, which is consistent with prior evidence9–11. In our analysis, the facility one attended was significantly associated with treatment outcomes so addressing heterogeneity in the quality of care and the experience of children and adolescents across facilities—and engaging them in the planning of new activities—is essential12–15. For young children, family-centered care models allow both parents and children to receive HIV care at the same place and time1,16, while adolescents—who experience new set of challenges as they are transitioning to adulthood12,13,17—may benefit from tailored youth-friendly spaces, peer-education, addressing stigma, and integrating care with other services such as family planning1,2,5,6,13. Many post-partum women are also mobile so programs also need to develop pathways for smooth transitions for both mothers and their babies18,19. Thus, despite the substantial gains made over the past decade, achieving sustained and lifelong treatment success in the pediatric populations still requires significant investment in the development, evaluation, and scale-up of innovative treatment strategies for this population, which Zambia has already begun to do.
We found that improvements in ART initiation occurred largely in parallel across age groups despite the fact that policies for universal treatment were implemented at different times for different age groups. For example, children less than 2 years old were universally eligible for ART beginning in 2010, but real-world implementation lagged behind with rates of ART initiation substantially improving only after 2014 (but also plateauing after the 2017 guidelines were implemented). This trend was similar in the 2- to 5- and 5–15 year old age groups—who had universal treatment implemented 2014—as we all as the 5–19 year old group—who had universal treatment in 2017. In contrast, we did identify very rapid and concordant shifts in the first-line ART regimens being used once policies changed (including early evidence of the rapid transition to dolutegravir-based regimens after its introduction in mid-2018). These findings suggest a few important insights regarding the relationship between adoption of the policies and whether/when they are sufficient to ensure widespread implementation. Rapid shifts in the first-line ART regimen prescribing may have been due to concomitant changes in the national ART supply chain, which could have limited availability of alternative regimens and greatly facilitated guideline-concordant prescribing across facilities. On the other hand, improvements in ART initiation practices—where decisions are primarily made at the provider- and clinic-level—may have also necessitated changes to the organizational climate that were not accomplished with policy changes alone. In particular, expansion of Zambia’s HIV treatment program, concurrent efforts to improve care quality, and increases in monitoring over the past decade may have been greater drivers of improved outcomes over time as opposed to specific policy changes for particular age groups. Indeed, implementation strategies such as these (e.g., practice facilitation and audit-and-feedback) have both been shown to be extremely effective at improving the uptake of guidelines in real-world settings20,21. Ultimately, these findings highlight the importance of considering robust implementation strategies to accompany policy changes to ensure that there is rapid and complete uptake.
Lastly, we also note several important trends in the demographics of Zambia’s pediatric and adolescent HIV program. Most notable are the dramatic increases in the number of adolescent girls entering HIV care. Adolescent girls have a much higher incidence of HIV compared to male peers22–24, and the demographic shifts in HIV programs have been previously documented4,25. The burden of this problem however, is also likely underestimated as adolescents living with HIV are least likely to know their status26; ZAMPHIA estimates indicate that only 40.6% of adolescents in Zambia are aware of their HIV status and only 30.7% overall are virally suppressed9. Targeted multi-sectoral HIV prevention programs for adolescents such as PEPFAR’s DREAM program are likely important for stemming this emerging shift in HIV incidence7. Additionally, newer innovations such as HIV self-testing and distribution among peers27–30 and PrEP5,22,31 need to also be integrated within existing programs. We also noted that the number of new children linking to care in age groups below 15 declined between 2014 and 2017, but then had a slight uptick after 2017. This is likely due to the overall declines in vertical transmission4,32 accompanied by more recent improvements in early infant diagnoses (EID) and implementation of birth testing in Zambia in 2017, which would lead to increased identification of children who vertically acquired HIV. Nevertheless, a substantial number of children still present at later ages, so scale-up of birth testing and EID (including follow-up up through 24 months so as not to miss children who acquire HIV through breastfeeding) and timely linkage to care remains an ongoing challenge32,33.
Our study has several limitations. First, inherent limitations in our data sources precluded us from capturing more detailed outcomes among CALHIV who became lost to follow-up, including whether they had transferred between facilities or had died. These transfers—as well as errors in record entry—may mean that the absolute percentages of retention in care in this study are actually underestimated. Still, the characteristics and distribution of children newly entering care as well as their outcomes are largely consistent with previous studies from sub-Saharan Africa4,10,11, thus suggesting that there are no major biases in the temporal trends that we found. Second, it is possible that some CALHIV who appeared to be newly enrolling in care may actually have transferred from another facility or attempted to pose as a newly diagnosed patient. Although there is no unique national patient identifier in Zambia that would readily allow us to identify these instances, our cohort building process included steps that excluded all individuals who had evidence of previously receiving care elsewhere. Third, we were only able to assess CALHIV once they linked to care and were not able to assess any changes at earlier stages in the HIV care cascade, including changes in HIV incidence, testing, and linkage to care after diagnosis. Fourth, there was substantial missingness with regards to CD4 counts, WHO staging, and anthropometric measurements. Lastly, we compared outcomes in our ITS analyses to a baseline from immediately prior to the new guidelines being adopted, but this assessment therefore does not incorporate previous secular trends. Although not typical for ITS analyses8, we felt this comparison represented a more plausible counterfactual compared to assuming that the pre-guideline trend would continue indefinitely.
In conclusion, we identified significant improvements in the rates of ART initiation and retention in care among CALHIV over time, although retention on ART at 6 months still remained quite low even in the most recent period. Improvements across age groups occurred steadily and largely in parallel despite differences in ART guidelines, suggesting somewhat limited impact of the adoption of new policies themselves and the importance of longitudinal efforts in scaling-up Zambia’s pediatric and adolescent program for all age groups. Additionally, we noted important demographic shifts among children now entering HIV care. This study provides important new information on the temporal trends and improvements in Zambia’s pediatric and adolescent HIV program and highlights key gaps along the care continuum that demand urgent attention going forward. Closing these gaps and making progress towards achieving 95-95-95 in children and adolescents will require differentiated HIV prevention and treatment strategies that target their unique social and developmental contexts.
Supplementary Material
RESEARCH IN CONTEXT.
Evidence before this study
We searched PubMed for studies that assessed pediatric outcomes of HIV treatment programs in resource-limited settings and the impact of changes to national treatment guidelines on these outcomes. We used the following combinations of search terms without any language or date restrictions: “HIV”, “pediatric”, “children”, “adolescents”, “treatment outcomes”, “enrollment”, “ART initiation”, “retention”, “universal treatment, “test and treat”, and “guideline changes”. We identified relevant English-language clinical trials and cohort studies published between 2010 and 2021 from this PubMed search. Additional references were identified by manually searching the citation lists of relevant manuscripts. We identified several cohort studies that described poor rates of ART initiation and retention in care among children, with a range of estimates depending on the setting and specific age group studied. Most studies only examined outcomes cross-sectionally and did not assess how treatment outcomes were changing over time. Among studies that did assess changes over time, almost all were performed prior to 2015 and do not assess outcomes during the contemporary era of HIV treatment and its associated innovations in treatment. Only one study examined outcomes up through 2017, but this was only among children less than 5 years old and it was restricted to those who had initiated ART and whose care status remained known over the follow-up period (i.e., did not consider those who were lost from care). No studies assessed how changes in treatment guidelines impacted outcomes across different age groups. Additionally, we did identify more contemporary studies that showed how demographics of pediatric and adolescent HIV are changing over time with increasing number of adolescents enrolling in HIV care, driven primarily by increasing incidence in adolescent females. These studies did not assess treatment outcomes, however. There is thus limited information of HIV treatment outcomes including ART initiation and retention in care among children and adolescents living with HIV during the test-and-treat era and how changes to treatment eligibility guidelines over the past decade have impacted these outcomes.
Added value of this study
We used an interrupted times series design to evaluate how treatment outcomes in Zambia’s national pediatric and adolescent HIV treatment programs changed over time as new policies were implemented. We assessed the distribution of the age and sex of new enrollees, rates of ART initiation, retention in care, time to ART initiation, and first-line ART regimens among new enrollees across different age strata as they changed over time with the adoption of new ART guidelines in 2014 and 2017. The study design allowed us to examine the real-world impacts of implementing new HIV treatment guidelines—which instituted universal HIV treatment at different times across different age groups—on both shorter- and longer-term longitudinal patient outcomes, and how these impacts varied across age groups. These results quantify the progress made in improving pediatric and adolescent HIV outcomes in one of the largest longitudinal studies to date (approximately 25,000 patients in 102 facilities across two provinces in Zambia) and identifies where more targeted efforts will be needed in this large national treatment program over the next decade.
Implications of all the available evidence
Evidence suggests that rates of ART initiation and retention in care have steadily improved over time, but are likely beginning to plateau after universal treatment across all age groups was implemented. Across age strata, improvement largely occurred in parallel even though universal treatment was implemented at different time points for each age group, suggesting that improvements may have been associated with overall treatment scale-up in Zambia over the past decade rather than specific changes in guidelines. Rates of ART initiation reached high levels, but retention in care on ART at 6 months is lagging behind. Additionally, there have been large increases in the number of adolescent females entering HIV care, likely corresponding to the significant increases in HIV incidence seen in this group, but only small increases across other demographics. Overall, these findings help to understand the successes pediatric and adolescent HIV programs have had in increasing rates of ART initiation and retention in care, but also highlight the substantial advancements in HIV prevention and treatment outcomes that will be needed over the next decade to reach the 95-95-95 goals and beyond.
Acknowledgements:
We want to thank all staff at the Centre for Infectious Diseases Research in Zambia (CIDRZ). We also thank the Zambian Ministry of Health, Center for Disease Control, and PEPFAR for developing the health systems and electronic medical record necessary to conduct this research.
Financial Support:
This work was supported by the National Institutes of Health (KL2 TR002346 to AM and K24 AI134413 to EHG). This project has also been supported by the President’s Emergency Plan for AIDS Relief (PEPFAR) through the Centers for Disease Control and Prevention (CDC). The funders had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Footnotes
Declarations of Interests: This work was supported by the National Institutes of Health (KL2 TR002346 to AM and K24 AI134413 to EHG). IS, MWM, TS, and CBM report support from the United States President’s Emergency Plan for AIDS Relief/Centers for Disease Control and Prevention during the conduct of this study. IS, CBM, and EHG report grants from the Bill and Melinda Gates Foundation outside the submitted work. EHG reports educational grants from Viiv Healthcare outside the submitted work. All other authors declare no competing interests. The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Data Availability Statement:
The Government of Zambia allows data sharing when applicable local conditions are satisfied. To request data access, contact the CIDRZ Ethics and Compliance Committee chair/Chief Scientific Officer, Dr. Roma Chilengi (Roma.Chilengi@cidrz.org), or the Secretary to the Committee/Head of Research Operations, Ms. Hope Mwanyungwi (Hope. Mwanyungwi@cidrz.org), mentioning the intended use for the data.
REFERENCES
- 1.Penazzato M, Amzel A, Abrams EJ, et al. Pediatric Treatment Scale-Up: The Unfinished Agenda of the Global Plan. JAIDS Journal of Acquired Immune Deficiency Syndromes 2017; 75. [DOI] [PubMed] [Google Scholar]
- 2.Enane LA, Davies MA, Leroy V, et al. Traversing the cascade: urgent research priorities for implementing the ‘treat all’ strategy for children and adolescents living with HIV in sub-Saharan Africa. J Virus Erad 2018; 4(Suppl 2): 40–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iyun V, Technau K-G, Vinikoor M, et al. Variations in the characteristics and outcomes of children living with HIV following universal ART in sub-Saharan Africa (2006–17): a retrospective cohort study. The Lancet HIV 2021; 8(6): e353–e62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maskew M, Bor J, MacLeod W, Carmona S, Sherman GG, Fox MP. Adolescent HIV treatment in South Africa’s national HIV programme: a retrospective cohort study. Lancet HIV 2019; 6(11): e760–e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hosek S, Pettifor A. HIV Prevention Interventions for Adolescents. Current HIV/AIDS reports 2019; 16(1): 120–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casale M, Carlqvist A, Cluver L. Recent Interventions to Improve Retention in HIV Care and Adherence to Antiretroviral Treatment Among Adolescents and Youth: A Systematic Review. AIDS Patient Care and STDs 2019; 33(6): 237–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chimbindi N, Birdthistle I, Floyd S, et al. Directed and target focused multi-sectoral adolescent HIV prevention: Insights from implementation of the ‘DREAMS Partnership’ in rural South Africa. J Int AIDS Soc 2020; 23 Suppl 5(Suppl 5): e25575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bernal JL, Cummins S, Gasparrini A. Interrupted time series regression for the evaluation of public health interventions: a tutorial. Int J Epidemiol 2017; 46(1): 348–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zambia Population-based HIV Impact Assessment (ZAMPHIA) 2016: First Report. Zambia Ministry of Health. Lusaka, Zambia, December 2017. [Google Scholar]
- 10.Abuogi LL, Smith C, McFarland EJ. Retention of HIV-Infected Children in the First 12 Months of Anti-Retroviral Therapy and Predictors of Attrition in Resource Limited Settings: A Systematic Review. PloS one 2016; 11(6): e0156506–e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McNairy ML, Lamb MR, Carter RJ, et al. Retention of HIV-infected children on antiretroviral treatment in HIV care and treatment programs in Kenya, Mozambique, Rwanda, and Tanzania. Journal of acquired immune deficiency syndromes (1999) 2013; 62(3): e70–e81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ritchwood TD, Ba A, Ingram L, et al. Community perspectives of South African adolescents’ experiences seeking treatment at local HIV clinics and how such clinics may influence engagement in the HIV treatment cascade: a qualitative study. AIDS Care 2020; 32(1): 83–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zanoni BC, Sibaya T, Cairns C, Haberer JE. Barriers to Retention in Care are Overcome by Adolescent-Friendly Services for Adolescents Living with HIV in South Africa: A Qualitative Analysis. AIDS Behav 2019; 23(4): 957–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Asuquo SE, Tahlil KM, Muessig KE, et al. Youth engagement in HIV prevention intervention research in sub-Saharan Africa: a scoping review. Journal of the International AIDS Society 2021; 24(2): e25666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Day S, Kapogiannis BG, Shah SK, et al. Adolescent participation in HIV research: consortium experience in low and middle-income countries and scoping review. Lancet HIV 2020; 7(12): e844–e52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khumalo PN, Katirayi L, Ashburn K, et al. ‘There are no more secrets’: acceptability of a family-centered model of care for HIV positive children in Eswatini. BMC health services research 2020; 20(1): 951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanyon C, Seeley J, Namukwaya S, et al. “Because we all have to grow up”: supporting adolescents in Uganda to develop core competencies to transition towards managing their HIV more independently. Journal of the International AIDS Society 2020; 23(S5): e25552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips TK, Clouse K, Zerbe A, Orrell C, Abrams EJ, Myer L. Linkage to care, mobility and retention of HIV-positive postpartum women in antiretroviral therapy services in South Africa. J Int AIDS Soc 2018; 21 Suppl 4(Suppl Suppl 4): e25114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clouse K, Vermund SH, Maskew M, et al. Mobility and Clinic Switching Among Postpartum Women Considered Lost to HIV Care in South Africa. J Acquir Immune Defic Syndr 2017; 74(4): 383–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gagliardi AR, Alhabib S, and the members of the Guidelines International Network Implementation Working G. Trends in guideline implementation: a scoping systematic review. Implementation Science 2015; 10(1): 54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pantoja T, Opiyo N, Lewin S, et al. Implementation strategies for health systems in low-income countries: an overview of systematic reviews. Cochrane Database Syst Rev 2017; 9(9): Cd011086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Abdool Karim Q, Baxter C, Birx D. Prevention of HIV in Adolescent Girls and Young Women: Key to an AIDS-Free Generation. JAIDS Journal of Acquired Immune Deficiency Syndromes 2017; 75. [DOI] [PubMed] [Google Scholar]
- 23.Birdthistle I, Tanton C, Tomita A, et al. Recent levels and trends in HIV incidence rates among adolescent girls and young women in ten high-prevalence African countries: a systematic review and meta-analysis. The Lancet Global Health 2019; 7(11): e1521–e40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Oliveira T, Kharsany AB, Gräf T, et al. Transmission networks and risk of HIV infection in KwaZulu-Natal, South Africa: a community-wide phylogenetic study. Lancet HIV 2017; 4(1): e41–e50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zanoni BC, Archary M, Buchan S, Katz IT, Haberer JE. Systematic review and meta-analysis of the adolescent HIV continuum of care in South Africa: the Cresting Wave. BMJ Glob Health 2016; 1(3): e000004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ayieko J, Chamie G, Balzer L, et al. Mobile, Population-wide, Hybrid HIV Testing Strategy Increases Number of Children Tested in Rural Kenya and Uganda. The Pediatric infectious disease journal 2018; 37(12): 1279–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eshun-Wilson I, Jamil MS, Witzel TC, et al. A Systematic Review and Network Meta-analyses to Assess the Effectiveness of Human Immunodeficiency Virus (HIV) Self-testing Distribution Strategies. Clin Infect Dis 2021; 73(4): e1018–e28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Amstutz A, Kopo M, Lejone TI, et al. “If it is left, it becomes easy for me to get tested”: Use of oral self-tests and community health workers to maximize the potential of home-based HIV testing among adolescents in Lesotho. Journal of the International AIDS Society 2020; 23(S5): e25563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pettifor A, Lippman SA, Kimaru L, et al. HIV self-testing among young women in rural South Africa: A randomized controlled trial comparing clinic-based HIV testing to the choice of either clinic testing or HIV self-testing with secondary distribution to peers and partners. EClinicalMedicine 2020; 21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hatzold K, Gudukeya S, Mutseta MN, et al. HIV self-testing: breaking the barriers to uptake of testing among men and adolescents in sub-Saharan Africa, experiences from STAR demonstration projects in Malawi, Zambia and Zimbabwe. Journal of the International AIDS Society 2019; 22(S1): e25244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Celum CL, Delany-Moretlwe S, Baeten JM, et al. HIV pre-exposure prophylaxis for adolescent girls and young women in Africa: from efficacy trials to delivery. J Int AIDS Soc 2019; 22 Suppl 4(Suppl Suppl 4): e25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chi BH, Mbori-Ngacha D, Essajee S, et al. Accelerating progress towards the elimination of mother-to-child transmission of HIV: a narrative review. J Int AIDS Soc 2020; 23(8): e25571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jani IV, De Schacht C. Innovations and challenges in early infant diagnosis of HIV. Curr Opin HIV AIDS 2019; 14(1): 55–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The Government of Zambia allows data sharing when applicable local conditions are satisfied. To request data access, contact the CIDRZ Ethics and Compliance Committee chair/Chief Scientific Officer, Dr. Roma Chilengi (Roma.Chilengi@cidrz.org), or the Secretary to the Committee/Head of Research Operations, Ms. Hope Mwanyungwi (Hope. Mwanyungwi@cidrz.org), mentioning the intended use for the data.


