Abstract
Case series
Patients:—
Final Diagnosis: Post-amputation phantom limb pain
Symptoms: Phantom limb pain
Medication: —
Clinical Procedure: —
Specialty: Anesthesiology
Objective:
Unusual or unexpected effect of treatment
Background:
Postamputation phantom and residual limb pain are common and frequently intractable, with few reliably effective treatments. Pulsed nonthermal shortwave (radiofrequency) electromagnetic field therapy is a noninvasive treatment used previously as an adjunct analgesic and wound healing therapy. Its use for postamputation pain remains unexamined.
Case Reports:
Twelve patients with an above or below knee amputation with persistent, intractable phantom and/or residual limb pain unresponsive to multiple previous invasive treatments were provided with a noninvasive, wearable, pulsed electromagnetic field device (RecoveryRx, BioElectronics Corporation, Frederick, MD, USA). Patients used the included dressings to self-apply the 12 cm-diameter ringed antenna to their residual limb and then activated the device, which delivered nonthermal radiofrequency energy continuously for up to 30 days. Of the 12 individuals, 4 (33%) experienced minimal/no change, 7 (58%) rated their phantom and/or residual limb pain as “very much improved” at the conclusion of treatment, and 1 (8%) patient reported “moderate” improvement, using the Patient Global Impression of Change scale. Of the 8 responders, worst and average phantom limb pain improved a mean (SD) of 4.0 (2.9) and 4.2 (1.8) points on the 0 to 10 numeric rating scale, respectively. Worst and average residual limb pain improved 5.4 (3.7) and 3.5 (2.4) points, respectively.
Conclusions:
These cases suggest that pulsed electromagnetic field therapy may be an effective treatment for intractable postamputation pain. Considering the low patient burden of noninvasive, wearable devices, combined with few contraindications and no significant side effects or adverse events, further study with a randomized, controlled trial is warranted.
Keywords: Amputation, Residual Limb Pain, Analgesia, Electromagnetic Fields, Phantom Limb, Pulsed Radiofrequency Treatment, Radiofrequency Therapy
Background
Although estimates vary, 50% to 85% of people with an amputation develop persistent pain, which has a significant negative impact on physical and mental health and substantially decreases overall quality of life. Postamputation pain rarely spontaneously resolves and is frequently resistant to available treatments [1].
One possible alternative is pulsed nonthermal shortwave (radio-frequency) therapy which, over the last 7 decades, has been used to treat acute and chronic pain, decrease inflammation and edema, and hasten wound healing and bone regeneration [2]. The mechanism of action is complex, multifactorial, and only partially understood. The most generally accepted biochemical-based theory involves the promotion of calcium binding to calmodulin, which activates endothelial and neuronal nitric oxide synthase isoforms, producing nitric oxide that has anti-inflammatory and analgesic effects, among other consequences, such as decreasing edema while increasing blood and lymph flow [3]. Biophysical-based theories introduce a role of stochastic resonance, which results in a modulatory effect on the sensory nervous system. More recently, chronic pain has been identified as a syndrome of central sensitization, whereby an increase in synaptic efficacy and decrease in inhibitory pain pathways results in central amplification of previously subthreshold synaptic inputs [4,5].
The original pulsed electromagnetic field machines were large and heavy and required an external power supply [6]. However, in the last few decades, wearable devices that are small, light, battery-powered, and disposable have been developed and are now cleared by the United States Food and Drug Administration, with indications including the treatment of musculoskeletal and postoperative pain and edema. Multiple investigations suggest the analgesic benefits for chronic pain conditions, including knee osteoarthritis and failed back surgery syndrome [7,8], among others [9].
However, it remains unknown whether pulsed shortwave fields will decrease postamputation phantom and/or residual limb pain. Therefore, we report 12 cases with which we explored the possibility of treating postamputation pain with this modality.
Case Reports
The University of California, San Diego, Institutional Review Board (San Diego, CA, USA) waives the review of case reports and short series. Written, informed consent documenting Health Insurance Portability and Accountability Act authorization and allowing for publication of nonidentifying medical information and in situ device imaging in the form of a case series was obtained from all patients.
A nonthermal, pulsed shortwave (radiofrequency) device (Figure 1; Model 088, BioElectronics, Frederick, MD, USA) was offered to 12 adults who had intractable phantom limb pain following a transfemoral or transtibial amputation with a pain intensity of at least 3 on a numeric pain scale from 0 to 10 at least daily for more than 1 year (Table 1). All patients had not responded to multiple additional noninvasive, pharmacologic, and device-specific interventions (eg, meditation, transcutaneous electrical nerve stimulation, and spinal cord stimulation) and experienced 2 to 34 years of intractable phantom and/or residual limb pain.
Figure 1.

A wearable, pulsed shortwave device with a pulse generator and flexible 12-cm diameter antenna. The unit is secured with an included cotton-based kinesiology tape (black bandage in image). The single control is an on/off button on the back of the pulse generator, and the green light emitting diode indicates the unit is functioning.
Table 1.
Anthropometric patient characteristics.
| All patients (n=12) | Responders (n=8) | Non-responders (n=4) | |
|---|---|---|---|
| Age (years) | 66 (7) | 67 (5) | 63 (9) |
| Female sex (#) | 3 (25%) | 2 (25%) | 1 (25%) |
| Height (cm) | 175 (8) | 174 (7) | 178 (10) |
| Weight (kg) | 69 (24) | 77 (25) | 84 (37) |
| Body mass index (kg/m2) | 25 (8) | 25 (7) | 26 (11) |
| Right sided amputation (#) | 5 (42%) | 5 (63%) | 1 (25%) |
| Below knee amputation (#) | 7 (58%) | 4 (50%) | 2 (50%) |
| Years since original amputation | 11 (10) | 9 (11) | 14 (6) |
| Experiences phantom limb pain (#) | 11 (92%) | 7 (88%) | 4 (100%) |
| Experiences residual limb pain (#) | 10 (83%) | 7 (88%) | 3 (75%) |
Data presented as mean (SD) or number of patients (percentage).
Each of the 12 patients was provided 1 device by mail. After recording a history and baseline pain levels by telephone, instructions on activating, self-applying, and caring for the device were provided. Patients were contacted once within the first week and then weekly for 5 weeks. Patients were asked to use the device as much as possible with a goal of 24 h/day for 30 days, although we did not collect information regarding the actual duration of use each day. The only limitation was that the device could not be submerged in water.
The initial location of device placement varied somewhat among individuals since each had a unique amputation location, history of nerve resection, and location of phantom and residual limb pain. However, in general, when patients were not using a prosthesis, they placed the ring antenna around the distal end of their residual limb, much like a ring on a finger. If the ring could not fit around the residual limb, the device was simply positioned at the end of the limb and held in place with either the included dressing (Figure 1) or a shrinker sock. When using a prosthesis, patients moved the device to either the quadriceps (pain in the femoral nerve distribution) or hamstring muscles (pain in the sciatic nerve distribution). Most moved the device to multiple locations to optimize the analgesic response. Residual limb pain was defined as painful sensations located in the portion of the limb still physically present. Phantom limb pain was defined as painful sensations experienced where there was no longer a portion of the limb.
By Days 7 to 14, four patients (33%) experienced minimal/ no change, 7 (58%) rated their phantom and/or residual limb pain as “very much improved”, and 1 patient (8%) reported “moderate” improvement, using the Patient Global Impression of Change scale. These assessments did not change for the remainder of treatment, and the groups of 4 and 8 patients were labeled “nonresponders” and “responders”, respectively. Of the 8 responders, worst and average phantom limb pain improved a mean (SD) of 4.0 (2.9) and 4.2 (1.8) points on the 0 to 10-point numeric rating scale, respectively (Figures 2, 3). Three patients experienced a complete resolution of phantom limb pain during use. Worst and average residual limb pain improved 5.4 (3.7) and 3.5 (2.4) points, respectively (Figures 4, 5). Two patients experienced a complete resolution of residual limb pain during use. Of the 8 responders, 5 patients reported at baseline nightly awakenings due to pain, and these individuals all had complete resolution of sleep disturbances by the end of the treatment period.
Figure 2.
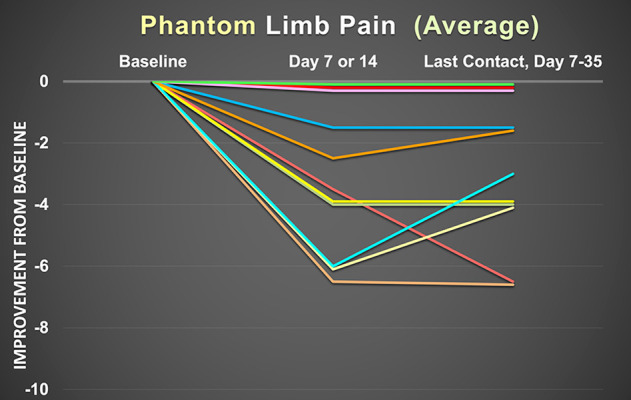
Improvement in average phantom pain as measured with the 0 to 10 numeric rating pain scale for the previous 24 h prior to data collection. Each line represents 1 patient. Of the 12 patients in this report, 1 patient did not experience phantom limb pain at baseline, and therefore there are only 11 patients represented in this graph.
Figure 3.

Improvement in worst (maximum) phantom pain as measured with the 0 to 10 numeric rating pain scale for the previous 24 h prior to data collection. Each line represents 1 patient. Of the 12 patients in this report, 1 patient did not experience phantom limb pain at baseline, and therefore there are only 11 patients represented in this graph.
Figure 4.

Improvement in average residual limb pain as measured with the 0 to 10 numeric rating pain scale for the previous 24 h prior to data collection. Each line represents 1 patient. Of the 12 patients in this report, 2 patients did not experience residual limb pain at baseline, and therefore there are only 10 patients represented in this graph.
Figure 5.

Improvement in worst residual limb pain as measured with the 0 to 10 numeric rating pain scale for the previous 24 h prior to data collection. Each line represents 1 patient. Of the 12 patients in this report, 2 patients did not experience residual limb pain at baseline, and therefore there are only 10 patients represented in this graph.
The 4 patients who did not respond to the treatment removed their device after 7 to 35 days. In contrast, all 8 responders used their device for at least 35 days. Although this specific device is marketed as providing 720 h (30 days) of treatment, patients extended this duration by turning off their units for a few hours each day and/or purchasing additional over-the-counter devices. We were therefore unable to assess any possible post-treatment residual analgesic benefit. Additionally, 3 patients acknowledged using their device on other parts of their body in which they experienced pain, with resolution of pain at those locations (and no pain increase in the amputated limb during the interim). All responders planned to continue using pulsed shortwave therapy indefinitely.
None of the 12 patients reported an increase in supplemental analgesic requirements over their baseline during the treatment period. Following battery exhaustion, the disposable, single-use devices were discarded.
Discussion
The cases of this report suggest that pulsed shortwave therapy may be an effective treatment for intractable postamputation pain. The degree of analgesia provided was unexpected by the healthcare providers as well as the patients. Of course, we had no control group, and therefore how much of the anal-gesia was due to a placebo effect remains unknown. However, a strong placebo effect for a majority of the 12 patients seems improbable considering all had experienced 2 to 34 years of intractable postamputation pain and received multiple noninvasive (eg, mirror therapy), pharmacologic (eg, gabapentin), and device-specific (eg, spinal cord stimulator) interventions in the interim, without relief. Indeed, many patients first expressed skepticism owing to the simplicity of a self-administered device and subsequent incredulity that a noninvasive, equivalent over-the-counter product provided effective analgesia when so many other interventions had previously failed.
Although a randomized, masked, sham-controlled trial is required to validate and quantify any analgesic benefits of pulsed shortwave therapy, its characteristics suggest an extraordinary potential to treat pain: it is noninvasive and nonpharmacologic, can be self-applied in less than 1 min with a simple dressing or tape, has a relatively low cost, with over-the-counter devices cleared for use, and has no identified adverse effects and few contraindications [9]. In addition, the currently available devices are lighter and thinner than many surgical bandages, can be applied to nearly any part of the body, function through clothing or bandages, once initiated require no intervention by patient or provider, have a duration of up to 30 days (unlimited duration using serial devices), and have no potential for misuse, dependence, and diversion. Nevertheless, clinical adoption appears to be limited by a lack of widely disseminated systematic evidence, a historically limited understanding of the mechanism of action, and “a wide variety of unsubstantiated claims that are used for marketing purposes” [10].
The safety of pulsed, nonthermal electromagnetic fields has been investigated and confirmed by a plethora of government agencies and independent societies [9,11,12]. For example, the Institute for Electrical and Electronics Engineers Standards for Radio Frequency Electromagnetic Field Exposure concluded that “there are no adverse health effects that are not thermally related” [11]. Over the last quarter-century, pulsed electromagnetic field devices have delivered over 3 million treatments, without reports of adverse effects or significant adverse events [10]. While no significant adverse events have been associated with pulsed electromagnetic field therapy [11], there are contra-indications, including pregnancy, use in an area of preexisting malignancy, placement within 6” of an existing implanted pulse generator (eg, cardiac pacer), and use in children less than 17 years of age [9].
Conclusions
The presented cases suggest that pulsed shortwave therapy may be an effective treatment for intractable postamputation pain. Considering the low patient burden of noninvasive wearable devices combined with few contraindications and no significant adverse effects or adverse events, further investigation with a randomized, controlled trial is warranted.
Acknowledgments
The authors would like to thank Sree Koneru, PhD (BioElectronics Corporation, Frederick, MD, and Meraqui Medical, Fremont, CA) and Kenneth J. McLeod, PhD (Department of Systems Science and Industrial Engineering and the Clinical Science and Engineering Research Laboratory, Binghamton University, Binghamton, NY) for reviewing the introduction, methods, and discussion sections for technical accuracy.
Footnotes
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors who confirm that the images are original with no duplication and have not been previously published in whole or in part.
References:
- 1.Hsu E, Cohen SP. Postamputation pain: Epidemiology, mechanisms, and treatment. J Pain Res. 2013;6:121–36. doi: 10.2147/JPR.S32299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guo L, Kubat NJ, Nelson TR, Isenberg RA. Meta-analysis of clinical efficacy of pulsed radio frequency energy treatment. Ann Surg. 2012;255(3):457–67. doi: 10.1097/SLA.0b013e3182447b5d. [DOI] [PubMed] [Google Scholar]
- 3.Pilla AA. Nonthermal electromagnetic fields: From first messenger to therapeutic applications. Electromagn Biol Med. 2013;32(2):123–36. doi: 10.3109/15368378.2013.776335. [DOI] [PubMed] [Google Scholar]
- 4.Woolf CJ. Central sensitization: implications for the diagnosis and treatment of pain. Pain. 2011;152(3 Suppl.):S2–S15. doi: 10.1016/j.pain.2010.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Latremoliere A, Woolf CJ. Central sensitization: A generator of pain hyper-sensitivity by central neural plasticity. J Pain. 2009;10(9):895–926. doi: 10.1016/j.jpain.2009.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenn JE. Effect of pulsed electromagnetic energy (Diapulse) on experimental hematomas. Can Med Assoc J. 1969;100(5):251–54. [PMC free article] [PubMed] [Google Scholar]
- 7.Bagnato GL, Miceli G, Marino N, et al. Pulsed electromagnetic fields in knee osteoarthritis: A double blind, placebo-controlled, randomized clinical trial. Rheumatology (Oxford) 2016;55(4):755–62. doi: 10.1093/rheumatology/kev426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harper WL, Schmidt WK, Kubat NJ, Isenberg RA. An open-label pilot study of pulsed electromagnetic field therapy in the treatment of failed back surgery syndrome pain. Int Med Case Rep J. 2015;8:13–22. doi: 10.2147/IMCRJ.S73068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guo L, Kubat NJ, Isenberg RA. Pulsed radio frequency energy (PRFE) use in human medical applications. Electromagn Biol Med. 2011;30(1):21–45. doi: 10.3109/15368378.2011.566775. [DOI] [PubMed] [Google Scholar]
- 10.Gaynor JS, Hagberg S, Gurfein BT. Veterinary applications of pulsed electromagnetic field therapy. Res Vet Sci. 2018;119:1–8. doi: 10.1016/j.rvsc.2018.05.005. [DOI] [PubMed] [Google Scholar]
- 11.Committee on M, Radiation COMAR technical information statement: expert reviews on potential health effects of radiofrequency electromagnetic fields and comments on the bioinitiative report. Health Phys. 2009;97(4):348–56. doi: 10.1097/HP.0b013e3181adcb94. [DOI] [PubMed] [Google Scholar]
- 12.Ahlbom A, Green A, Kheifets L, et al. ICNIRP (International Commission for Non-Ionizing Radiation Protection) Standing Committee on Epidemiology Epidemiology of health effects of radiofrequency exposure. Environ Health Perspect. 2004;112(17):1741–54. doi: 10.1289/ehp.7306. [DOI] [PMC free article] [PubMed] [Google Scholar]


